Abstract
The natural histories of intramural hematoma (IMH) and penetrating atherosclerotic ulcer (PAU) are highly variable as they may progress to aneurysm formation, rupture, or dissection, or even resolve, in the specific case of IMH. Imaging plays an increasingly important role in clinical and surgical management of IMH and PAU. In contrast to ulcer-like projections, images of intramural blood pools have not been widely reported in CT studies of patients with IMH. Understanding the imaging characteristics and the natural course of each of these entities would help clinicians and surgeons to identify patients at greatest risk for bad prognosis and may improve outcomes. This paper discusses the pathophysiology of these entities, the controversies regarding their natural history, and the prognostic factors that should be identified in CT scans.
Keywords: acute aortic syndrome, intramural aortic hematoma, aortic hematoma, penetrating ulcers of aorta
INTRODUCTION
The majority of publications consider intramural hematomas (IMH) and penetrating atherosclerotic ulcers (PAU) of the aorta to be variants of classical dissection, within the generic classification of acute aortic syndrome. The Stanford classification is used to guide management of all three clinical conditions.1 However, the pathophysiology and natural history of IMH and PAU are the subject of a great deal of debate and in many aspects are not related to classical dissection.
An IMH is defined as a hematoma of the aorta wall with no evidence of a media-intima entry rupture. However, in many cases, small intimal tears can be found during open procedures or using modern high-resolution imaging methods. Rupture of the vasa vasorum (VV) as cause of IMH is the subject of debate, and there is considerable evidence that these ruptures are a secondary phenomenon rather than the original cause of the IMH.2
A PAU is defined as erosion of the intima and the internal elastic membrane, with subsequent penetration of blood into the tunica media via atherosclerotic plaques. Clinical series in the literature cover small numbers of patients with PAU and IMH, and the natural history of these pathologies is the subject of much debate.2
Both entities occur in older patients than classical dissection, which has implications for initial clinical and radiological presentation and for the natural history of these aortic wall injuries.
CHANGES TO THE AORTA WALL IN THE ELDERLY
To better understand the clinical and radiological presentation of PAU, IMH, and classical dissection, we must take into account the structural changes to the aorta that occur with aging. Compared with classical dissection, PAU and IMH occur in older patients who, consequently, have different parietal abnormalities. With aging, spaces appear between the elastic lamellae, caused by loss of the fine elastic fibers that join them, and fibrosis of the tunica media can be observed. These changes result in thickening and stiffening of the wall, which is exacerbated by arterial hypertension and the appearance of calcifications.
Elastin is synthesized by smooth muscle cells (SMC), which, in turn, are scarce in the major elastic arteries and reduce in number with aging. In addition to apoptosis of a considerable number of SMC, they separate from each other and their phenotype modifies to a state of senescence. The lost elasticity of the aorta results in rigidity, dilatation, stretching, and tortuosity.3 - 5
The correlation between tropoelastin synthesis by SMC and mRNA levels also changes. Expression of tropoelastin on the wall reduces with each decade of life beyond 60 years, which is the equivalent of a 94% reduction in elastogenic regenerative potential, when extrapolated to 40 years of age.6 - 8
These aging-related histological changes are observed with greater intensity in the external third of the tunica and are considered a fundamental part of the pathophysiologic mechanisms of aortic dissection and of IMH.9 , 10
INTRAMURAL HEMATOMA AND THE ROLE OF THE VASA VASORUM
The adventitial VV are present in the large elastic vessels with more than 29 lamellae, and are thus found in greater numbers in the ascending aorta, the aortic arch, and the descending aorta, and in smaller quantities in the abdominal aorta.
The relationship between rupture of VV and IMH was first established in 1920 by Krukenberg11 and, since then, has been accepted as a possible cause of IMH. Intramural hematomas in the absence of PAU tend to be larger, sometimes compromising the ascending aorta, aortic arch, and descending aorta. The majority of publications accept the cause and effect relationship between rupture of VV and IMH, but there is scant evidence to confirm this interpretation. It seems difficult to explain that these small vessels, with low intraluminal pressure, can dissect large portions of the aorta and, in some cases, evolve to rupture of the artery wall. This mechanism is also unable to explain why the hematoma in IMH secondary to PAU tends to be limited, despite the existence of direct communication between the lumen and tunica media. Furthermore, Park et al. detected a 73% rate of intimal rupture among patients with type A IMH (in the ascending aorta) treated with surgery in whom a preoperative tomography did not detect any kind of defect.12
In other words, a significant proportion of the IMH cases classified as type A using tomography were in fact cases of dissection with a small entry orifice that had sealed spontaneously. It is possible that these small intima-media tears are even more common, because, in cases of distal rupture with retrograde extension of the hematoma to the aortic arch and ascending aorta, these lesions are also erroneously classified as Stanford type A. It is no surprise, therefore, that intimal tears are not found during surgery in some cases (Figure 1).
Figure 1. Angiotomography showing intramural hematoma (IMH) compromising the ascending aorta (A) in a 78-year-old patient with acute chest pain. In (B) the site of distal dissection can be seen close to the origin of the celiac trunk. In (C) complete resorption of the IMH by the ascending aorta has occurred within 30 days of placement of an endoprosthesis in the descending aorta, without compression of the true lumen distally (D). This case demonstrates why some IMH classified as Stanford type A are actually IMH originating in the descending aorta with retrograde progression of the hematoma.

More recently, higher-resolution tomography has increasingly demonstrated small intimal injuries and secondary lesions similar to ulcers (ulcer like projections) or intramural blood pools in serial examinations for follow-up of IMH, which are very often interpreted erroneously.
Chronic hypertension is a condition that is present in the majority of patients and is associated with occlusion of VV with neovascularization, increased arterial stiffness, and accelerated atherosclerosis. During a hypertensive crisis, medial ischemia can be aggravated by vasoconstriction of the VV. The most internal portions of the media continue to be fed by diffusion from the arterial lumen. This set of factors results in two regions in the tunica media with distinct characteristics: a more internal, and more elastic portion and a more rigid external portion. The difference in elastic modulus between these two regions sets up a difference in shear force at the interface.13 - 19 In the case of classical dissection, the tendency is for the true lumen to be compressed, because its external portion is more rigid. This change to the elastic modulus can also explain why the false lumen (less elastic and thinner) expands over time.
These altered mechanical elastic characteristics have been demonstrated experimentally in an animal model by resection of the adventitia to exclude the VV and ligature of the intercostal arteries (from which the VV originate in the descending aorta), provoking ischemia in the more external portions of the tunica media.20 , 21 After 2 weeks, it was observed that degradation of the elastic fibers had occurred in the external portion of the tunica media and spontaneous dissection took place in some of the animals. This experiment was replicated later with additional data collection after 4 and 8 weeks.22 This study demonstrated that, as the weeks passed, ischemia of the artery wall caused the extent of tunica media degeneration to increase, involving more internal portions. Thus, when there was advanced ischemia of the artery wall, the majority of the tunica media developed fibrosis. One interpretation of these findings is that progressive occlusion of the VV with aging, aggravated by hypertension and by the related atherosclerosis, can lead to diffuse degeneration of the tunica media. In these cases, when the intima ruptures, the cleavage plane is more external (adjacent to the adventitia) and the mural hematoma tends not to compress the true lumen. This could partially explain why, in many IMH, although rupture of the intima and large scale hematoma along the aorta can be seen, in the majority of cases, significant compression of the true lumen or occlusion of visceral branches are not observed. These findings may also explain why the mural hematoma that occurs in many cases of PAU is limited: the degenerated tunica media with extensive fibrosis does not allow the hematoma to propagate.
Recently, Osada et al. studied histopathological changes in patients with aortic dissection and confirmed the presence of degeneration and occlusion of the VV, associated with degradation of the elastic fibers and accumulation of extracellular matrix in the external third of the tunica media where dissection occurred.23
In contrast to a widely-held belief, compression of the true lumen cannot be explained by pressure differences alone, since experimental studies have demonstrated that neither systolic pressure nor pulse pressure are higher in the false lumen. It is clear that other factors, such as the size of the intimal tear and presence or absence of large reentry orifices with considerable retrograde flow, play important roles in compression of the true lumen.24 - 26
In the specific case of IMH, it has been demonstrated experimentally that peak stress on the tunica media (peak wall stress) is much greater than is observed in an artery without hematoma, which might explain progression to rupture in the direction of the lumen or frank dissection.27
THE PATHOPHYSIOLOGIC SIGNIFICANCE OF ULCER LIKE PROJECTIONS AND BLOOD POOLS IN IMH
In the case of a dissection with a small intimal-medial tear and no distal reentry to the true lumen, formation of the IMH may later be followed by sealing of the entry orifice. The appearance on X-ray would thus be an IMH with no detectable entry. In this case, the tunica media is subjected to very high stress, which has been demonstrated experimentally.27 Since the IMH develops in the more external portions of the media, it can progress with rupture of the origin of intercostal arteries, causing retrograde flow to the wall thus creating blood pools that are detected at the external surface of the hematoma. Blood pools can also be created by small intimal ruptures, appearing now in the internal surface of the hematoma. Other possibilities are: complete resorption of the hematoma, more significant ruptures in the direction of the lumen (creating ulcer like projections) or even dissection, formation of a pseudoaneurysm or frank rupture into the thoracic cavity (Figure 2).
Figure 2. In (A), an ulcer like projection is shown. In (B), blood pools are arrowed. In (C), another ulcer like projection is shown, with adjacent pleural effusion. In (D), the relationship between a blood pool and an intercostal artery is shown.

Serial analysis of IMH patients with images typical of blood pools and ulcer like projections demonstrate that these injuries have completely different natural histories.28 Analysis of serial CT scans of patients with IMH shows that the blood pools that are present at onset or that appear over time are not associated with complications in the majority of cases. Wu et al. conducted a logistic regression analysis that identified the following factors as related to complications: aortic diameter exceeding 45 mm, location in the ascending aorta, and ulcer like projections.28
Prognosis was worse in the specific case of ulcer like projections (rupture into the intima) that were present at onset or that appeared during follow-up.29 - 31 Formation of pseudoaneurysm and progression to dissection or rupture occurred in 31% to 70% of cases and were manifest with greatest frequency in the ascending aorta.
There is a great deal of discussion in relation to descriptions of the natural history of these lesions and interpretations of CT images, since some publications, such as a recent European guideline and a 2018 UpToDate publication, state that IMH can progress to PAU or do not differentiate between PAU and ulcer like projections.32 , 33 As we have seen, by definition, PAU is characterized by erosion of the intima and media from atheromatous plaques. In contrast, ulcer like projections and some blood pools appear at onset or during progression of IMH and are caused by rupture into the lumen and, as such, develop in the opposite direction to PAU. Additionally, each of these lesions has its own specific clinical significance and course.
PENETRATING ATHEROSCLEROTIC ULCER
Penetrating atherosclerotic ulcer of the aorta was first described by Shennan in 1934.34 As with IMH, PAU of the aorta is considered a variant of classical dissection, but with distinct clinical presentation and course.
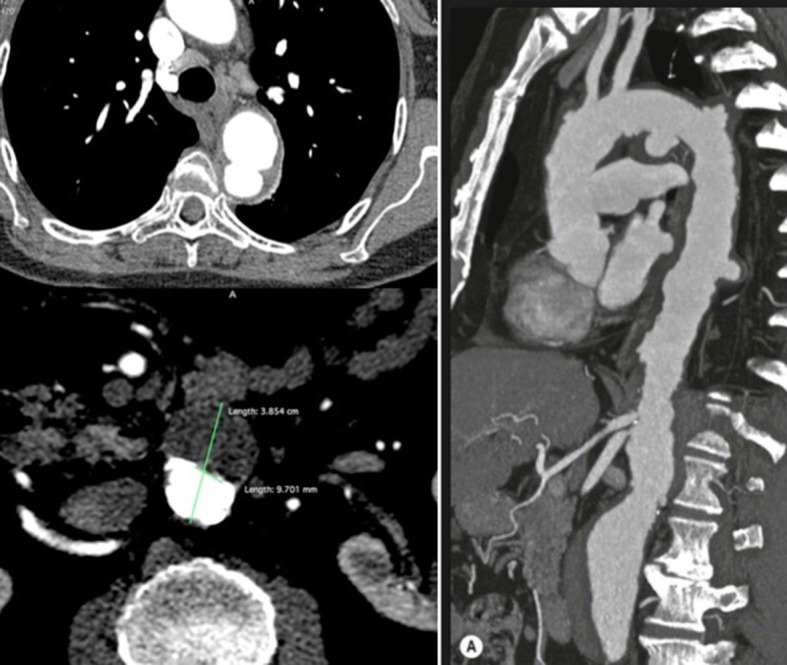
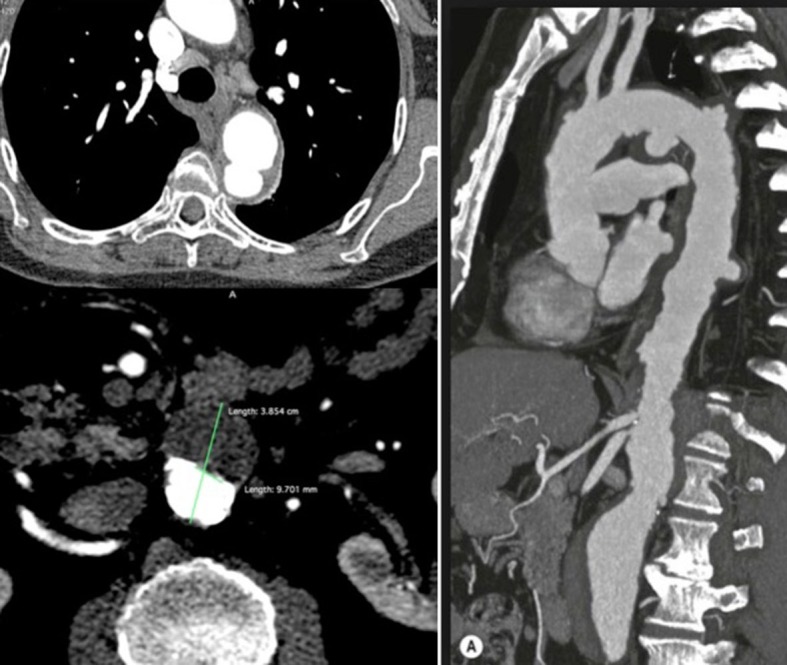
Ulcerations of the thoracic aorta are relatively common in the elderly population, particularly after the seventh decade of life, but their true prevalence is yet to be determined (Figure 3). As with patients with IMH of the aorta, the majority of these patients are hypertensive. Deep ulcerations of the aorta wall can trigger symptoms similar to classical dissection and can occur in conjunction with secondary IMH (Figure 3). Ulcerations occur almost exclusively in the descending thoracic aorta, are frequently multiple, and can vary in size and depth; while abdominal aorta involvement is less common. Progression to rupture or frank dissection is associated with elevated morbidity and mortality.
Figure 3. An 82-year-old patient with multiple penetrating ulcers of the aortic arch and descending aorta, associated with an abdominal aortic aneurysm. Measurements of the depth or size of the necks of ulcers are not reliable signs for indication of endovascular treatment.
Natural history
The natural history of PAU of the aorta is not well known, even though PAU is one of the spectra of acute aortic syndrome.35 , 36
As is well explained by Ganaha et al.,37 there is confusion in the literature with relation to the behavior of PAU. It exhibits more aggressive behavior in symptomatic patients,38 , 39 in contrast with a more benign course in oligosymptomatic and, particularly, asymptomatic patients.40 , 41 Persistent or recurrent chest pain despite aggressive antihypertensives treatment, increased pleural effusion, and acute symptoms are negative prognostic factors, associated with greater risk of progression to aortic dissection or rupture. As is the case with IMH, ulcers that occur in the ascending aorta and the proximal portion of the descending aorta are linked with a larger number of complications. Possible explanations for this behavior include greater hemodynamic aggression to the artery wall at these points and predominance of elastin over collagen in the tunica media of the proximal aorta.42 Penetrating ulcers are responsible for around 2-7% of cases of acute aortic syndrome, with progression of the disease in both symptomatic and asymptomatic patients, justifying the need for control with serial imaging exams.43 , 44
Open or endovascular treatment is justified for symptomatic patients. However, in patients with asymptomatic PAU, it appears that conservative treatment is more appropriate, because there are not yet well-defined criteria that justify interventional treatment, whether for the thoracic or for the abdominal segments.43 , 45 Among these patients, the extent of penetration into the tunica media and the size of the neck of the injury are of questionable value as criteria for indicating surgery, as was demonstrated in the largest clinical series in the literature.43 In asymptomatic patients, indications for open or endovascular surgery are restricted to cases with evidence of pseudoaneurysm formation.
Clinical status
The majority of ulcers that occur in elderly and hypertensive patients do not penetrate deeply into the tunica media and do not cause symptoms. Among these patients, episodes of microembolization may occur and trigger diagnosis. However, indication of endoprosthesis deployment in these situations is questionable, since ulcerations can occur in many different segments of the descending and abdominal aorta, which would require coverage of large extensions of the thoracoabdominal aorta, with obvious risks.
Pathophysiology
In PAU, the atheromatous plaque extends deep into the artery wall, breaching the internal elastic membrane and penetrating the tunica media. When this penetration occurs, the tunica media is exposed to pulsating arterial flow, which can cause hemorrhage and IMH. In a Mayo Clinic series, 80% of cases of PAU were accompanied by IMH,46 but more recent series have reported much lower frequencies of associated IMH. This raises the question of whether many of these ulcerations might not actually have been ulcer like projections that developed during the course of an IMH and were incorrectly described as PAU.
Imaging methods
A diagnosis of PAU of the aorta is established by anatomic criteria using CT, which shows a localized area of atheromatous plaque with focal ulceration and thickening of the aorta wall. The classic description on computed tomography is of a saccular, contrast-filled image that penetrates the aorta wall, surrounded by an IMH.
CONCLUSIONS
There are few publications in the literature that deal with the pathophysiology of IMH and PAU, which causes confusion in relation to clinical and radiological interpretation of these injuries and of many aspects of their natural history and their true prevalence in the population. A number of different experimental studies have helped improve interpretation of clinical and radiological findings. The likelihood of prospective studies being undertaken in the near future is very small, and so the best course is to construct well-maintained registers, such as the International Registry of Aortic Dissection (IRAD), which has been accumulating information on acute aortic syndrome for two decades.47 It should be recognized that there are no well-established reporting standards for radiology or long-term medical follow-up with regard to IMH or PAU. These deficiencies should be corrected by our specialty societies in the form of specific registers, to promote better understanding of the changes affecting the aorta wall that are responsible both for the genesis and for the course of PAU and IMH. As Jean Martin Charcot (1825-1893) stated in De l'éxpectation en médecine 48: “Disease is very old and nothing about it has changed. It is we who change, as we learn to recognize what was formerly imperceptible”.
Footnotes
How to cite: Pereira AH. Intramural hematoma and penetrating atherosclerotic ulcers of the aorta: uncertainties and controversies. J Vasc Bras. 2019;18: e20180119. https://doi.org/10.1590/1677-5449.180119
Financial support: None.
The study was carried out at Hospital de Clínicas de Porto Alegre (HCPA), Universidade Federal do Rio Grande do Sul (UFRGS), Porto Alegre, RS, Brazil.
REFERENCES
- 1.Daily PO, Trueblood HW, Stinson EB, Wuerflein RD, Shumway NE. Management of acute aortic dissections. Ann Thorac Surg. 1970;10(3):237–247. doi: 10.1016/S0003-4975(10)65594-4. [DOI] [PubMed] [Google Scholar]
- 2.Sundt TM. Intramural hematoma and penetrating atherosclerotic ulcer of the aorta. Ann Thorac Surg. 2007;83(2):835–834. doi: 10.1016/j.athoracsur.2006.11.019. [DOI] [PubMed] [Google Scholar]
- 3.Davidson JM, Hill KE, Alford JL. Developmental changes in collagen and elastin biosynthesis in the porcine aorta. Dev Biol. 1986;118(1):103–111. doi: 10.1016/0012-1606(86)90077-1. [DOI] [PubMed] [Google Scholar]
- 4.Selmin O, Volpin D, Bressan GM. Changes of cellular expression of mRNA for tropoelastin in the intraembryonic arterial vessels of developing chick revealed by in situ hybridization. Matrix. 1991;11(5):347–358. doi: 10.1016/S0934-8832(11)80206-4. [DOI] [PubMed] [Google Scholar]
- 5.Wang M, Zhang J, Jiang LQ, et al. Proinflammatory profile within the grossly normal aged human aortic wall. Hypertension. 2007;50(1):219–227. doi: 10.1161/HYPERTENSIONAHA.107.089409. [DOI] [PubMed] [Google Scholar]
- 6.Bruce MC, Honaker CE. Transcriptional regulation of tropoelastin expression in rat lung fibroblasts: Changes with age and hyperoxia. Am J Physiol. 1998;274(6):L940–50. doi: 10.1152/ajplung.1998.274.6.L940. [DOI] [PubMed] [Google Scholar]
- 7.Heinz A, Jung MC, Duca L, et al. Degradation of tropoelastin by matrix metalloproteinases – cleavage site specificities and release of matrikines. FEBS J. 2010;277(8):1939–1956. doi: 10.1111/j.1742-4658.2010.07616.x. [DOI] [PubMed] [Google Scholar]
- 8.Fritze O, Romero B, Schleicher M, et al. Age-Related changes in the elastic tissue of the human aorta. J Vasc Res. 2012;49(1):77–86. doi: 10.1159/000331278. [DOI] [PubMed] [Google Scholar]
- 9.Schlatmann TJM, Becker AE. Histologic changes in the normal aging aorta: implications for dissecting aortic aneurysm. Am J Cardiol. 1977;39(1):13–20. doi: 10.1016/S0002-9149(77)80004-0. [DOI] [PubMed] [Google Scholar]
- 10.Schlatmann TJM, Becker AE. Pathogenesis of dissecting aneurysm of aorta: comparative histopathologic study of significance of medial changes. Am J Cardiol. 1977;39(1):21–26. doi: 10.1016/S0002-9149(77)80005-2. [DOI] [PubMed] [Google Scholar]
- 11.Krukenberg E. Beitrage zur frage des aneurysma dissecans. Beitr Pathol Anat Allg Pathol. 1920;67:329–351. [Google Scholar]
- 12.Park KH, Lim C, Choi JH, et al. Prevalence of aortic intimal defect in surgically treated acute type A intramural hematoma. Ann Thorac Surg. 2008;86(5):1494–1500. doi: 10.1016/j.athoracsur.2008.06.061. [DOI] [PubMed] [Google Scholar]
- 13.Heistad DD, Marcus ML, Law EG, Armstrong ML, Ehrhardt JC, Abboud FM. Regulation of blood flow to the aortic media in dogs. J Clin Invest. 1978;62(1):133–140. doi: 10.1172/JCI109097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Marcus ML, Heistad DD, Armstrong ML, Abboud FM. Effects of chronic hypertension on vasa vasorum in the thoracic aorta. Cardiovasc Res. 1985;19(12):777–781. doi: 10.1093/cvr/19.12.777. [DOI] [PubMed] [Google Scholar]
- 15.Ohhira A, Ohhashi T. Effects of aortic pressure and vasoactive agents on the vascular resistance of the vasa vasorum in canine isolated thoracic aorta. J Physiol. 1992;453(1):233–245. doi: 10.1113/jphysiol.1992.sp019226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Larson EW, Edwards WD. Risk factors for aortic dissection: a necropsy study of 161 cases. Am J Cardiol. 1984;53(6):849–855. doi: 10.1016/0002-9149(84)90418-1. [DOI] [PubMed] [Google Scholar]
- 17.Roberts WC. Aortic dissection: anatomy, consequences and causes. Am Heart J. 1981;101(2):195–214. doi: 10.1016/0002-8703(81)90666-9. [DOI] [PubMed] [Google Scholar]
- 18.Olivetti G, Melissari M, Marchetti G, Anversa P. Quantitative structural changes of the rat thoracic aorta in early spontaneous hypertension. Tissue composition, and hypertrophy and hyperplasia of smooth muscle cells. Circ Res. 1982;51(1):19–26. doi: 10.1161/01.RES.51.1.19. [DOI] [PubMed] [Google Scholar]
- 19.Pereira AH. Rupture of vasa vasorum and intramural hematoma of the aorta: A changing paradigma. J Vasc Bras. 2010;9(2):57–60. doi: 10.1590/S1677-54492010000200008. [DOI] [Google Scholar]
- 20.Stefanadis C, Vlachopoulos C, Karayannacos P, et al. Effect of vasa vasorum flow on structure and function of the aorta in experimental animals. Circulation. 1995;91(10):2669–2678. doi: 10.1161/01.CIR.91.10.2669. [DOI] [PubMed] [Google Scholar]
- 21.Angouras D, Sokolis DP, Dosios T, et al. Effect of impaired vasa vasorum flow on the structure and mechanics of the thoracic aorta: implications for the pathogenesis of aortic dissection. Eur J Cardiothorac Surg. 2000;17(4):468–473. doi: 10.1016/S1010-7940(00)00382-1. [DOI] [PubMed] [Google Scholar]
- 22.Fagundes A, Pereira AH, Corrêa RK, Oliveira MT, Rodriguez R. Effects of removal of the adventitia of the descending aorta and structural alterations in the tunica media in pigs. Rev Col Bras Cir. 2012;39(2):133–138. doi: 10.1590/S0100-69912012000200009. [DOI] [PubMed] [Google Scholar]
- 23.Osada H, Kyogoku M, Matsuo T, Kanemitsu N. Histopathological evaluation of aortic dissection: A comparison of congenital versus acquired aortic wall weakness. Interact Cardiovasc Thorac Surg. 2018;27(2):277–283. doi: 10.1093/icvts/ivy046. [DOI] [PubMed] [Google Scholar]
- 24.Tsai TT, Schlicht MS, Khanafer K, et al. Tear size and location impacts false lumen pressure in an ex vivo model of chronic type B aortic dissection. J Vasc Surg. 2008;47(4):844–851. doi: 10.1016/j.jvs.2007.11.059. [DOI] [PubMed] [Google Scholar]
- 25.Khanafer K, Berguer R. Fluid-structure interaction analysis of Turbulent pulsatile flow within a layered aortic wall as related to aortic dissection. J Biomech. 2009;42(16):2642–2648. doi: 10.1016/j.jbiomech.2009.08.010. [DOI] [PubMed] [Google Scholar]
- 26.Rudenick PA, Segers P, Pineda V, et al. Lumen Flow Patterns and their Relation with Morphological and Biomechanical Characteristics of Chronic Aortic Dissections. Computational Model Compared with Magnetic Resonance Imaging Measurements. PLoS One. 2017;12(1):e0170888. doi: 10.1371/journal.pone.0170888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bukač M, Alber M. Multi-component model of intramural hematoma. J Biomech. 2017;50:42–49. doi: 10.1016/j.jbiomech.2016.11.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wu MT, Wang YC, Huang YL, et al. Intramural blood pools accompanying aortic intramural hematoma: CT appearance and natural course. Radiology. 2011;258(3):705–713. doi: 10.1148/radiol.10101270. [DOI] [PubMed] [Google Scholar]
- 29.Jang YM, Seo JB, Lee YK, et al. Newly developed ulcer-like projection (ULP) in aortic intramural haematoma on follow-up CT: is it different from the ULP seen on the initial CT? Clin Radiol. 2008;63(2):201–206. doi: 10.1016/j.crad.2007.07.020. [DOI] [PubMed] [Google Scholar]
- 30.Lee CW, Kang JW, Lee HJ, Lim TH. MDCT evaluation of intimal defects in intramural hematoma of the aorta: initial findings and follow-up. Int J Cardiovasc Imaging. 2010;26(S2) Suppl 2:295–302. doi: 10.1007/s10554-010-9709-x. [DOI] [PubMed] [Google Scholar]
- 31.Bosma MS, Quint LE, Williams DM, Patel HJ, Jiang Q, Myles JD. Ulcerlike projections developing in noncommunicating aortic dissections: CT findings and natural history. AJR. 2009;193(3):895–905. doi: 10.2214/AJR.08.2073. [DOI] [PubMed] [Google Scholar]
- 32.Riambau V, Böckler D, Brunkwall J, Cao P, Chiesa R. Management of descending thoracic aorta diseases clinical practice guidelines of the European Society for Vascular Surgery (ESVS) Eur J Vasc Endovasc Surg. 2017;53(1):4–52. doi: 10.1016/j.ejvs.2016.06.005. [DOI] [PubMed] [Google Scholar]
- 33.Manning W, Je Black JH. Overview of acute aortic dissection and other acute aortic syndromes. UpToDate. 2018 [Google Scholar]
- 34.Shennan T. Dissecting aneurysms. London: Medical Research Council; 1934. (Special Report Series). [Google Scholar]
- 35.von Kodolitsch Y, Csosz SK, Koschyk DH, et al. Intramural hematoma of the aorta: predictors of progression to dissection and rupture. Circulation. 2003;107(8):1158–1163. doi: 10.1161/01.CIR.0000052628.77047.EA. [DOI] [PubMed] [Google Scholar]
- 36.Vilacosta I, Aragoncillo P, Cañadas V, San Román JA, Ferreirós J, Rodríguez E. Acute aortic syndrome: a new sight to an old conundrum. Heart. 2009;95(14):1130–1139. doi: 10.1136/hrt.2008.153650. [DOI] [PubMed] [Google Scholar]
- 37.Ganaha F, Miller DC, Sugimoto K, et al. The prognosis of Aortic Intramural Hematoma: The Prognosis of aortic Intramural hematoma with and without Penetrating Atherosclerotic ulcer: a clinical and radiologic analysis. Circulation. 2002;106(3):342–348. doi: 10.1161/01.CIR.0000022164.26075.5A. [DOI] [PubMed] [Google Scholar]
- 38.Stanson AW, Kazmier FJ, Hollier LH, et al. Penetrating atherosclerotic ulcers of the thoracic aorta: natural history and clinicopathologic correlations. Ann Vasc Surg. 1986;1(1):15–23. doi: 10.1016/S0890-5096(06)60697-3. [DOI] [PubMed] [Google Scholar]
- 39.Coady MA, Rizzo JA, Hammond GL, Pierce JG, Kopf GS, Elefteriades JA. Penetrating ulcer of the thoracic aorta: what is it? How do we recognize it? How do we manage it? J Vasc Surg. 1998;27(6):1006–1016. doi: 10.1016/S0741-5214(98)70003-5. [DOI] [PubMed] [Google Scholar]
- 40.Harris JA, Bis KG, Glover JL, Bendick PJ, Shetty A, Brown OW. Penetrating atherosclerotic ulcers of the aorta. J Vasc Surg. 1994;19(1):90–99. doi: 10.1016/S0741-5214(94)70124-5. [DOI] [PubMed] [Google Scholar]
- 41.Quint LE, Williams DM, Francis IR, et al. Ulcer like lesions of the aorta: imaging features and natural history. Radiology. 2001;218(3):719–723. doi: 10.1148/radiology.218.3.r01mr24719. [DOI] [PubMed] [Google Scholar]
- 42.Borst HG, Heinemann MK, Stone CD. Surgical treatment of aortic dissection. New York: Churchill Livingstone Inc; 1996. Pathogenesis. pp. 47–54. [Google Scholar]
- 43.Nathan DP, Boonn W, Lai E, et al. Presentation, complications, and natural history of penetrating atherosclerotic ulcer disease. J Vasc Surg. 2012;55(1):10–15. doi: 10.1016/j.jvs.2011.08.005. [DOI] [PubMed] [Google Scholar]
- 44.Pauls S, Orend KH, Sunder-Plassmann L, et al. Endovascular repair of symptomatic penetrating atherosclerotic ulcer of the thoracic aorta. Eur J Vasc Endovasc Surg. 2007;34(1):66–73. doi: 10.1016/j.ejvs.2006.12.029. [DOI] [PubMed] [Google Scholar]
- 45.Georgiadis GS, Antoniou GA, Georgakarakos EI, et al. Surgical or endovascular therapy of abdominal penetrating aortic ulcers and their natural history: A systematic review. J Vasc Interv Radiol. 2013;24(10):1437–1449. doi: 10.1016/j.jvir.2013.05.067. [DOI] [PubMed] [Google Scholar]
- 46.Cho KR, Stanson AW, Potter DD, Cherry KJ, Schaff HV, Sundt TM., 3rd Penetrating atherosclerotic ulcer of the descending thoracic aorta and arch. J Thorac Cardiovasc Surg. 2004;127(5):1393–1399. doi: 10.1016/j.jtcvs.2003.11.050. [DOI] [PubMed] [Google Scholar]
- 47.Hagan PG, Nienaber CA, Isselbacher EM, et al. The International Registry of Dissection (IRAD): New Insights into an old disease. JAMA. 2000;283(7):897–903. doi: 10.1001/jama.283.7.897. [DOI] [PubMed] [Google Scholar]
- 48.Charcot JM. De l’éxpectation en médecine. Paris: Librairie de Germer; 1847. [Google Scholar]