Abstract
Objectives:
To evaluate whether transcatheter aortic valve replacement (TAVR) with the SAPIEN 3 valve (S3-TAVR) results in improved quality of life (QoL) compared with previous generation TAVR devices or surgical aortic valve replacement (SAVR).
Background:
In patients with severe aortic stenosis (AS) at intermediate surgical risk (IR), TAVR using the SAPIEN-XT valve (XT-TAVR) results in similar QoL compared with SAVR. Compared with SAPIEN-XT, the SAPIEN-3 valve offers a lower delivery profile and modifications to reduce paravalvular regurgitation.
Methods:
Between February and December 2014, 1078 IR patients with severe AS were treated with S3-TAVR in the PARTNER 2-S3i trial. QoL was assessed at baseline, 1 month, and 1 year using the Kansas City Cardiomyopathy Questionnaire (KCCQ), SF-36, and EQ-5D. We compared QoL outcomes of S3-TAVR patients with the SAVR and XT-TAVR arms of the PARTNER 2A trial using propensity score stratification to adjust for differences between the treatment groups.
Results:
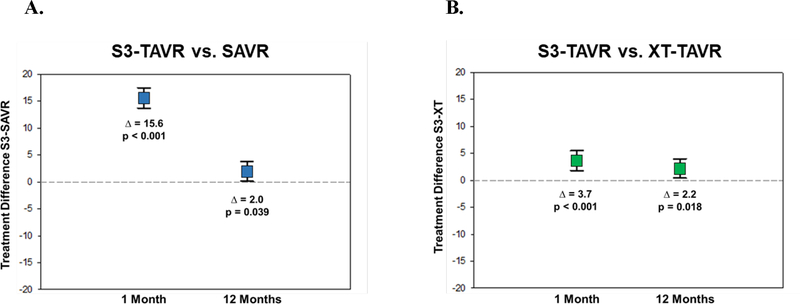
Over 1 year, S3-TAVR was associated with substantial improvements in QoL compared with baseline. At 1 month, S3-TAVR was associated with better QoL than either SAVR or XT-TAVR (adjusted differences in KCCQ-Overall Summary score 15.6 and 3.7 points respectively; p<0.001). At 1 year, the differences in QoL between S3-TAVR and both SAVR and XT-TAVR were reduced but remained statistically significant (adjusted differences 2.0 and 2.2 points, respectively; p<0.05). Similar results were seen for generic QoL outcomes.
Conclusions:
Among IR patients with severe AS, S3-TAVR resulted in improved QoL at both 1 month and 1 year compared with both XT-TAVR and SAVR.
Keywords: Transcatheter Aortic Valve Replacement, Quality of Life, Intermediate Surgical Risk
CONDENSED ABSTRACT
Whether transcatheter aortic valve replacement (TAVR) using the newer generation SAPIEN 3 valve (S3-TAVR) results in improved quality of life (QoL) compared with TAVR using the SAPIEN-XT valve (XT-TAVR) or surgical aortic valve replacement (SAVR) is unknown. Using propensity score stratification to adjust for differences between the treatment groups, we compared QoL outcomes of S3-TAVR patients enrolled in the PARTNER 2-S3i trial with the SAVR and XT-TAVR arms of the PARTNER 2A trial. At both 1 month and 1 year follow-up, S3-TAVR was associated with better disease-specific and generic QoL than either SAVR or XT-TAVR.
INTRODUCTION
Over the last decade, multiple trials have demonstrated that transcatheter aortic valve replacement (TAVR) is associated with comparable survival and quality of life outcomes compared with surgical aortic valve replacement (SAVR) in patients with severe, symptomatic aortic stenosis (AS) at high surgical risk (1–4). As TAVR has been embraced by the cardiology community, the use of these devices has expanded to lower risk patients with promising results. Recently, the PARTNER (Placement of AoRTic TraNscathetER Valve) 2 Cohort A trial demonstrated similar rates of death or disabling stroke as well as similar quality of life at 2 years in patients at intermediate surgical risk treated with either SAVR or TAVR using the second generation SAPIEN XT valve (XT-TAVR) (5,6).
While the PARTNER 2A trial was underway, a third-generation balloon-expandable TAVR system, SAPIEN-3 (Edwards Lifesciences, Irvine, CA, USA), was developed. Compared with SAPIEN-XT, SAPIEN-3 offers a lower profile delivery system as well as an outer skirt, designed to reduce paravalvular regurgitation. When TAVR using the SAPIEN-3 valve (S3-TAVR) was compared with SAVR in a propensity adjusted analysis of intermediate risk patients, S3-TAVR was found to be associated with reduced rates of death and stroke at 1 year (7). However, many elderly patients who are candidates for TAVR care at least as much about quality of life as they do about duration of life (8). As such, it is important to understand whether differences in clinical outcomes as well as differences in procedure-related complications (such as paravalvular regurgitation, bleeding, atrial fibrillation, or permanent pacemaker implantation), might lead to differences in health status between alternative valve replacement procedures. To address this gap in knowledge, we used data from the PARTNER 2A randomized trial and SAPIEN-3 intermediate risk registry to compare health status outcomes among patients with severe AS at intermediate surgical risk treated with S3-TAVR vs. either SAVR or XT-TAVR.
METHODS
Study Design and Population
The design of the PARTNER 2A randomized trial and the SAPIEN 3 intermediate risk registry, including inclusion and exclusion criteria (which were identical for the 2 trials), study procedures, and follow-up protocols, have been described previously (5,7). Briefly, these studies enrolled patients with severe, symptomatic AS at intermediate surgical risk (defined as a predicted risk of 30-day mortality between 4% and 8%, based on either the Society of Thoracic Surgeons (STS) mortality risk score or clinical assessment by a multidisciplinary heart team). In the PARTNER 2A trial, patients were stratified according to available access route (transfemoral vs. transthoracic) and randomized 1:1 to undergo either TAVR using the SAPIEN-XT valve (XT-TAVR) or SAVR. In the SAPIEN-3 intermediate risk (S3i) registry, patients underwent TAVR using the SAPIEN-3 valve (S3-TAVR) via either a transfemoral or transthoracic approach, as appropriate. Both the PARTNER 2A trial and S3i registry were approved by the institutional review board at each site, and written informed consent was obtained from all patients.
Measurement of Health Status
Health status was evaluated at baseline, 1 month, and 12 months. Both disease-specific and generic health status measures were used, since disease-specific instruments allow for a more sensitive assessment of changes in health status in a particular patient population. Disease-specific health status was assessed using the Kansas City Cardiomyopathy Questionnaire (KCCQ). The KCCQ capture 5 key domains of health status in heart failure patients (physical function, social function, symptoms, self-efficacy and knowledge, and quality of life [defined as the discrepancy between a desired state of health and actual state of health]), and is scored from 0 to 100, with higher scores indicating better health status (9). The individual scales of the KCCQ may be converted into a single overall summary score (KCCQ-OS), which has been shown to correlate with important clinical outcomes including hospitalization, healthcare costs, and death in heart failure populations (10,11). Among patients with heart failure, small, moderate, and large clinical improvements correspond to changes in the KCCQ-OS of approximately 5, 10 and 20 points respectively (12). Work by our group has demonstrated the reliability and validity of this instrument for patients with aortic stenosis (13).
Generic health status was evaluated using the Medical Outcomes Study Short-Form-36 (SF-36) questionnaire and the EuroQOL (EQ-5D). The SF-36 assesses 8 dimensions of health status and has been validated in patients with cardiovascular disease as well as in the general population (14–16). The SF-36 also provides physical and mental component summary scales, which are scored such that the US population mean is 50 with a standard deviation of 10 with higher scores representing better health status. Minimum clinically important differences on the SF-36 summary scales have been determined to be ~2 points (17).
The EQ-5D is a multi-attribute health status classification system that assesses 5 dimensions of general health using a 3-level scale. For the purposes of the PARTNER 2 randomized trial and its associated registries, these responses were transformed into preference-based utility weights using validated population sampling methods (18). These utilities range from 0 to 1, with 0 representing death and 1 representing ideal health.
Statistical Analysis
The primary analytic cohort included only patients who underwent the assigned treatment. Baseline characteristics were compared between cohorts using Student t-tests for continuous variables and chi-square tests for categorical variables. Within the S3-TAVR population, health status scores at 1 month and 12 months were compared with baseline using paired t tests. Of note, the baseline comparator at each time point consisted of only patients who had a health status assessment performed at that time point; as such, these paired comparisons are less susceptible to survivor bias caused by attrition of sicker patients over time.
Although the inclusion and exclusion criteria were identical for the PARTNER 2A trial and the S3i registry, there were minor differences in the clinical characteristics of the two cohorts. As such, we employed propensity score methodology to adjust for baseline differences between treatment groups (19). First, a logistic regression model was developed using 22 pre-specified baseline characteristics to calculate the propensity score for each patient. The STS mortality risk score was not included in this propensity score, as this score is regularly re-estimated by STS and therefore changes over time. Instead, we included many of the factors that comprise the STS risk score, in order to directly adjust for differences in these variables. We also did not include access site in the propensity score or stratify the analysis by access site. This was necessary as access site is inherent to the specific procedure (i.e., more patients are eligible for transfemoral access with S3-TAVR vs. XT-TAVR owing to differences in the diameter of the device delivery system); as such, it would have been inappropriate to include this covariate in our risk-adjustment model. A similar logistic regression model was developed for the S3-TAVR vs. XT-TAVR comparison. Patients were then partitioned into quintiles based on their propensity scores, and variable balance was evaluated within each quintile to assess the adequacy of the propensity model. The propensity score methodology and model used in this analysis was identical to that used previously to compare clinical outcomes between S3-TAVR and SAVR (7) and to support FDA approval of the SAPIEN-3 valve. Health status scores at each time point were then compared between the cohorts using analysis of covariance with adjustment for baseline health status and stratification by propensity score quintile.
In order to provide context for these comparisons, categorical analyses using previously described endpoints that incorporate both health status and survival (20,21) were also performed. A favorable outcome was defined as being alive with a KCCQ-OS score of ≥ 60 (roughly equivalent to NYHA Class II) without a 10 point or greater decrease from baseline. An excellent outcome was defined as being alive with a KCCQ-OS score of ≥ 75 (roughly equivalent to NYHA Class I) without a 10 point or greater decrease from baseline. The association between valve type and these binary outcomes was compared using propensity quintile-stratified logistic regression. Rates of substantial improvement (defined as >20 point increase in KCCQ-OS) and moderate improvement (defined as >10 point increase in KCCQ-OS) among surviving patients were also compared between treatment groups using a similar approach. Pre-procedural variables associated with a favorable outcome at 1 year in the S3-TAVR population were identified using a multivariable logistic regression model with backward stepwise selection (p < 0.10 for retention).
Finally, we performed informal mediation analyses to explore whether differences in complications between the groups might explain any observed differences in health status. For these analyses, we first performed univariate analyses to identify procedure-related complications associated with impaired 1-year health status. Complications that were considered included disabling stroke, disabling/life-threatening bleeding, major vascular complications, acute kidney injury, new atrial fibrillation, new pacemaker implantation, and moderate or severe aortic regurgitation. Variables that were identified as significant correlates of 1-year health status were then added to the original propensity adjusted models (along with age, sex, and STS risk score) to assess whether the observed treatment effect was attenuated.
All statistical analyses were performed using SAS software, version 9.3 (SAS institute INC., Cary NC). A 2-sided p-value of <0.05 was considered statistically significant with no correction for multiple comparisons.
RESULTS
Study Population
The PARTNER 2A trial randomized 1011 patients to undergo XT-TAVR and 1021 patients to undergo SAVR. Of these patients, 996 underwent XT-TAVR and 944 underwent SAVR. A total of 1078 patients were enrolled in the S3i registry, of whom 1077 underwent the assigned procedure. Baseline health status was available for 2829 patients (926 XT-TAVR; 854 SAVR; 1049 S3-TAVR), which formed our primary analytic cohort.
Baseline characteristics are shown in Tables 1A and 1B. Overall, the patients were elderly (mean age 81 years), and 55–60% were male. Up to 18% of patients had a history of myocardial infarction, between 24 and 28% had undergone prior coronary artery bypass surgery, and ~30% had chronic lung disease. After propensity score stratification, the treatment groups were similar, although several patient characteristics remained slightly imbalanced between the groups. This imbalance likely reflects characteristics that were defined differently between the 2 studies (e.g., STS risk score, eligibility for transfemoral access) and therefore excluded from the propensity model. As expected given the different iliofemoral sizing requirements of the SAPIEN–XT and SAPIEN-3 devices, there was a higher rate of transfemoral access eligibility in the S3-TAVR group (88.2% vs. 76.9% in XT-TAVR vs. 76.8% in SAVR; p<0.001)..
Table 1A:
Baseline Characteristics of the S3-TAVR and SAVR Cohorts
| S3-TAVRN = 1068 | SAVRN = 936 | P-ValueBefore Adjustment | P-ValueAfter Adjustment | |
|---|---|---|---|---|
| Clinical Characteristics | ||||
| Age (years) | 81.9 ± 6.6 | 81.6 ± 6.7 | 0.283 | 0.834 |
| Male (%) | 61.5 | 54.7 | 0.002 | 0.867 |
| STS Risk Score | 5.3 ± 1.3 | 5.8 ± 1.9 | < 0.001 | < 0.001 |
| Prior MI (%) | 16.1 | 17.7 | 0.330 | 0.939 |
| Prior PCI (%) | 32.1 | 26.9 | 0.011 | 0.882 |
| Prior CABG (%) | 28.0 | 25.5 | 0.214 | 0.966 |
| Cerebrovascular Disease (%) | 9.1 | 10.3 | 0.374 | 0.986 |
| Peripheral Artery Disease (%) | 28.3 | 31.9 | 0.073 | 0.911 |
| Atrial Fibrillation (%) | 35.7 | 34.8 | 0.692 | 0.957 |
| Creatinine > 2 mg/dl (%) | 7.6 | 5.4 | 0.054 | 0.704 |
| COPD (%) | 30.0 | 30.0 | 0.992 | 0.933 |
| Aortic Valve Area (cm2) | 0.7 ± 0.2 | 0.7 ± 0.2 | 0.443 | 0.876 |
| Mean Aortic Valve Gradient (mmHg) | 46.1 ± 12.6 | 44.7 ± 12.5 | 0.014 | 0.010 |
| Transfemoral Access (%) | 88.2 | 76.8 | < 0.001 | < 0.001 |
| Baseline Health Status | ||||
| KCCQ Overall Summary | 53.6 ± 21.7 | 52.9 ± 21.3 | 0.510 | 0.738 |
| KCCQ Physical Limitations | 56.1 ± 24.2 | 55.8 ± 24.4 | 0.774 | 0.923 |
| KCCQ Total Symptoms | 58.0 ± 22.4 | 57.8 ± 22.1 | 0.818 | 0.988 |
| KCCQ Quality of Life | 48.1 ± 23.9 | 46.6 ± 23.3 | 0.165 | 0.921 |
| KCCQ Social Limitation | 51.2 ± 29.9 | 50.8 ± 29.8 | 0.763 | 0.921 |
| SF-36 Physical Summary | 36.3 ± 8.7 | 36.0 ± 8.7 | 0.427 | 0.847 |
| SF-36 Mental Summary | 47.8 ± 11.4 | 47.7 ± 11.7 | 0.901 | 0.201 |
| EQ-5D Utilities | 0.75 ± 0.16 | 0.74 ± 0.17 | 0.076 | 0.738 |
Abbreviations: STS – Society of Thoracic Surgeons; MI – Myocardial infarction; PCI – percutaneous coronary interventions; CABG – coronary artery bypass grafting; NYHA – New York Heart Association Class; LV – left ventricle; KCCQ – Kansas City Cardiomyopathy Questionnaire
Table 1B:
Baseline Characteristics of the S3-TAVR and XT-TAVR Cohorts
| S3-TAVR N = 1068 | XT-TAVR N = 974 | P-Value Before Adjustment | P-Value After Adjustment | |
|---|---|---|---|---|
| Clinical Characteristics | ||||
| Age (years) | 81.9 ± 6.6 | 81.6 ± 6.7 | 0.230 | 0.970 |
| Male (%) | 61.5 | 54.4 | 0.001 | 0.810 |
| STS Risk Score | 5.3 ± 1.3 | 5.8 ± 2.1 | < 0.001 | < 0.001 |
| Prior MI (%) | 16.1 | 18.2 | 0.215 | 0.953 |
| Prior PCI (%) | 32.1 | 27.0 | 0.011 | 0.740 |
| Prior CABG (%) | 28.0 | 23.7 | 0.027 | 0.868 |
| Cerebrovascular Disease (%) | 9.1 | 10.3 | 0.365 | 0.960 |
| Peripheral Artery Disease (%) | 28.3 | 27.6 | 0.740 | 0.950 |
| Atrial Fibrillation (%) | 35.7 | 30.6 | 0.014 | 0.022 |
| Creatinine > 2 mg/dl (%) | 7.6 | 4.9 | 0.013 | 0.366 |
| COPD (O2 Dependent) (%) | 30.0 | 31.2 | 0.551 | 0.928 |
| Aortic Valve Area (cm2) | 0.7 ± 0.2 | 0.7 ± 0.2 | 0.866 | 0.307 |
| Mean Aortic Valve Gradient (mmHg) | 46.1 ± 12.6 | 45.0 ± 13.3 | 0.056 | 0.026 |
| Transfemoral Access (%) | 88.2 | 76.9 | < 0.001 | < 0.001 |
| Baseline Health Status | ||||
| KCCQ Overall Summary | 53.6 ± 21.7 | 53.3 ± 21.8 | 0.784 | 0.606 |
| KCCQ Physical Limitations | 56.1 ± 24.2 | 55.6 ± 24.3 | 0.677 | 0.771 |
| KCCQ Total Symptoms | 58.0 ± 22.4 | 58.2 ± 22.6 | 0.872 | 0.355 |
| KCCQ Quality of Life | 48.1 ± 23.9 | 46.9 ± 23.7 | 0.248 | 0.591 |
| KCCQ Social Limitation | 51.2 ± 29.9 | 52.1 ± 30.5 | 0.553 | 0.221 |
| SF-36 Physical Summary | 36.3 ± 8.7 | 36.1 ± 8.9 | 0.596 | 0.614 |
| SF-36 Mental Summary | 47.8 ± 11.4 | 48.8 ± 11.2 | 0.056 | 0.874 |
| EQ-5D Utilities | 0.75 ± 0.16 | 0.74 ± 0.16 | 0.656 | 0.606 |
Abbreviations: STS – Society of Thoracic Surgeons; MI – Myocardial infarction; PCI – percutaneous coronary interventions; CABG – coronary artery bypass grafting; NYHA – New York Heart Association Class; LV – left ventricle; KCCQ – Kansas City Cardiomyopathy Questionnaire
All 3 groups had evidence of significantly impaired health status at baseline (Tables 1A and 1B) with mean KCCQ-OS scores of ~53, which corresponds to NYHA Class III symptoms. The mean SF-36 Physical Summary score was ~36 for all groups, which is ~1.5 standard deviations below the population mean, and the mean SF-36 Mental Summary score was ~48. There were no significant differences in baseline health status between the 3 groups either before or after propensity stratification.
Within-Group Comparisons
Health status data were available for 97.3% and 95.4% of patients at 1 month and 1 year respectively in the S3-TAVR group, for 95.4% and 92.1% at 1 month and 1 year respectively in the XT-TAVR group, and for 87.8% and 85.4% at 1 month and 1 year respectively in the SAVR group (Supplementary Table A). Baseline characteristics were generally similar for patients with and without 1 year health status data (Supplementary Table B).
Compared with baseline, patients treated with S3-TAVR demonstrated substantial improvements in health status at both 1 month and 1 year follow-up (Table 2). At 1 month, S3-TAVR patients experienced an increase of 19.1 points in the KCCQ-OS score from baseline (p<0.001), and this improvement was sustained at 1 year (mean change from baseline 23.1 points; p<0.001). Significant health status benefits were also seen on the SF-36 physical summary scale and mental summary scale with 1-year improvements of 5.1 points and 3.9 points respectively (p<0.001 for both comparisons).
Table 2:
Changes in Disease Specific and Generic Health Status after S3-TAVR
| N | Paired Difference vs. Baseline (95% CI) | p-value | |
|---|---|---|---|
| KCCQ Overall Summary | |||
| 30 Days | 1009 | 19.1 (17.7, 20.4) | < 0.001 |
| 1 Year | 907 | 23.1 (21.8, 24.9) | < 0.001 |
| KCCQ Physical Limitations | |||
| 30 Days | 963 | 13.7 (12.2, 15.2) | < 0.001 |
| 1 Year | 854 | 15.1 (13.4, 16.9) | < 0.001 |
| KCCQ Total Symptoms | |||
| 30 Days | 1009 | 16.3 (15.0, 17.7) | < 0.001 |
| 1 Year | 907 | 18.2 (16.0, 19.7) | < 0.001 |
| KCCQ Quality of Life | |||
| 30 Days | 1008 | 26.7 (25.0, 28.3) | < 0.001 |
| 1 Year | 903 | 32.9 (31.0, 34.7) | < 0.001 |
| KCCQ Social Limitation | |||
| 30 Days | 885 | 18.8 (16.7, 21.0) | < 0.001 |
| 1 Year | 779 | 26.6 (24.2, 29.0) | < 0.001 |
| SF-36 Physical Summary | |||
| 30 Days | 991 | 4.8 (4.2, 5.3) | < 0.001 |
| 1 Year | 885 | 5.1 (4.5, 5.8) | < 0.001 |
| SF-36 Mental Summary | |||
| 30 Days | 991 | 3.6 (2.9, 4.3) | < 0.001 |
| 1 Year | 885 | 3.9 (3.1, 4.7) | < 0.001 |
| EQ-5D Utilities | |||
| 30 Days | 1003 | 0.065 (0.054, 0.076) | < 0.001 |
| 1 Year | 902 | 0.050 (0.038, 0.062) | < 0.001 |
Abbreviations: KCCQ – Kansas City Cardiomyopathy Questionnaire
Between-Group Comparisons
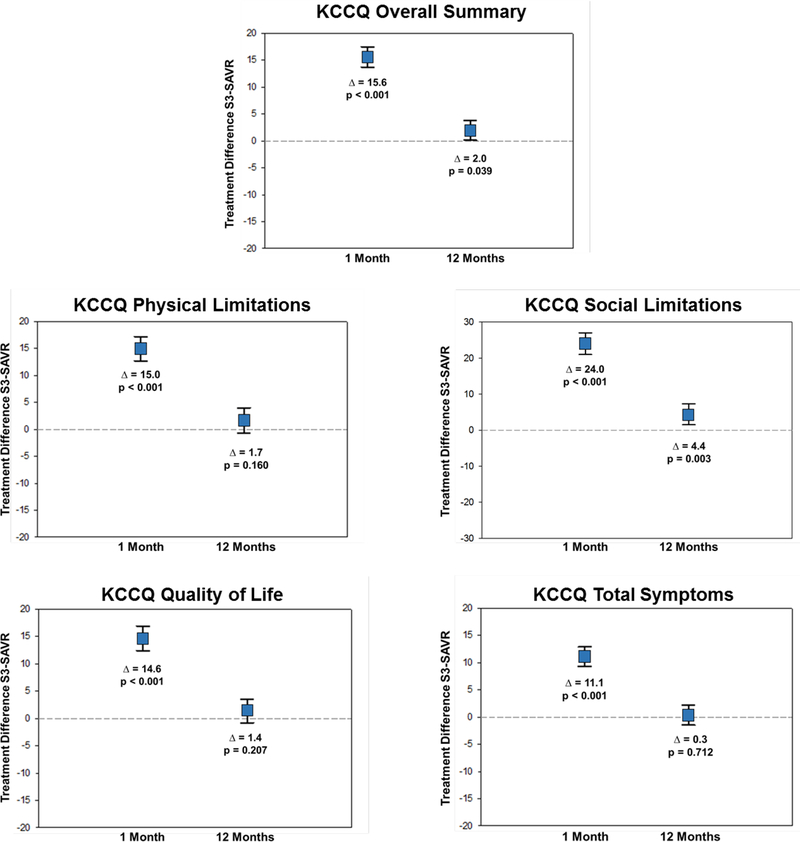
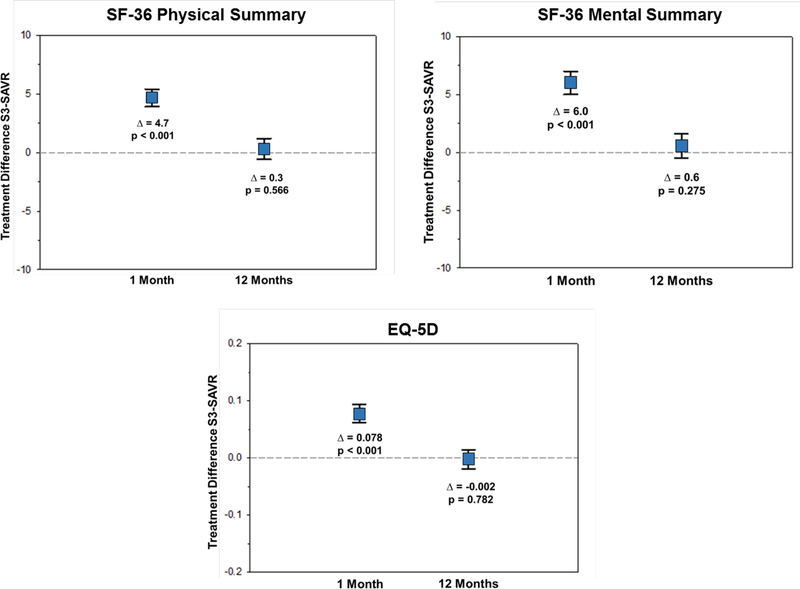
In propensity stratified analyses comparing S3-TAVR vs. SAVR, there was significantly greater health status improvement at 1 month across all KCCQ scales (mean adjusted difference in KCCQ-OS 14.6 points; 95% CI 13.7 to 17.5; p<0.001) (Figure 1A). Similar early benefits of S3-TAVR vs. SAVR were seen in the SF-36 physical summary scale (mean adjusted difference 4.7 points; 95% CI 3.9 to 5.4; p<0.001) and the SF-36 mental summary scale (mean adjusted difference 6.0 points; 95% CI 5.0 to 7.0; p<0.001) (Figure 1B). At 1 year, there remained a significant health status benefit with S3-TAVR vs. SAVR as measured by the KCCQ-OS (mean adjusted difference 2.0 points; 95% CI 0.1 to 3.8; p=0.039), however there were no significant differences between S3-TAVR and SAVR for the generic health status instruments (Supplementary Table C).
Figure 1A. Adjusted Between-Group Differences in Disease Specific Health Status Between S3-TAVR and SAVR.
Data points represent the adjusted difference in mean health status score between the treatment groups at each time point. Error bars denote 95% confidence intervals.
Figure 1B. Adjusted Between-Group Differences in Generic Health Status Between S3-TAVR and SAVR.
Data points represent the adjusted difference in mean health status score between the treatment groups at each time point. Error bars denote 95% confidence intervals.
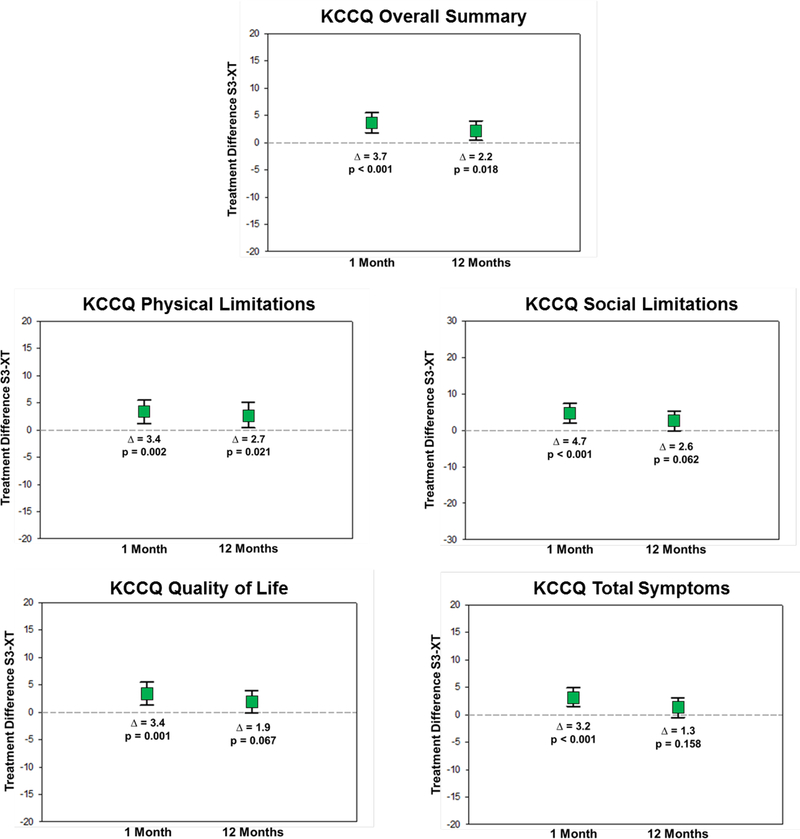
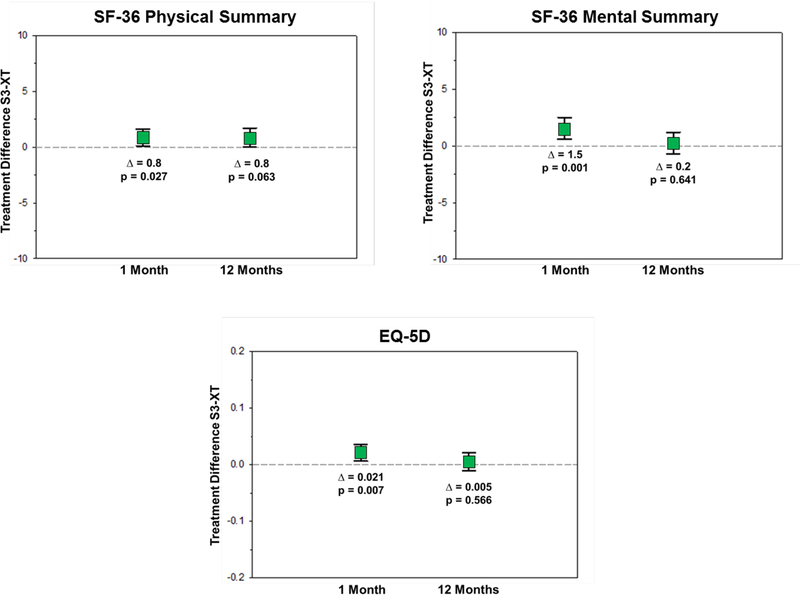
When S3-TAVR was compared with XT-TAVR, there was evidence of modestly greater health status improvement at 1 month with a mean adjusted KCCQ-OS difference of 3.7 points (95% CI 1.8 to 5.5; p<0.001) and similar benefits across most of the KCCQ domains (Figure 2A). Small, but significant, benefits of S3-TAVR were also seen in the SF-36 physical summary scale (mean adjusted difference 0.8 points; 95% CI 0.1 to 1.6; p=0.027) and the SF-36 mental summary scale (mean adjusted difference 1.5 points; 95% CI 0.6 to 2.5; p=0.001) (Figure 2B). At 1 year, there remained a significant health status benefit with S3-TAVR on several disease specific scales (mean adjusted KCCQ-OS difference 2.2 points; 95% CI 0.4 to 4.0; p=0.018); however, there were no significant differences between S3-TAVR and XT-TAVR for most of the generic health status instruments (Supplementary Table D). Results were virtually identical when missing health status outcomes were imputed using multiple imputation (data not shown).
Figure 2A. Adjusted Between-Group Differences in Disease Specific Health Status Between S3-TAVR and XT-TAVR.
Data points represent the adjusted difference in mean health status score between the treatment groups at each time point. Error bars denote 95% confidence intervals.
Figure 2B. Adjusted Between-Group Differences in Generic Health Status Between S3-TAVR and XT-TAVR.
Data points represent the adjusted difference in mean health status score between the treatment groups at each time point t. Error bars denote 95% confidence intervals. t
Categorical Analyses
The results of categorical analyses are summarized in Tables 3 and 4. At 1 month follow-up, the adjusted proportion of surviving patients who experienced a substantial (≥20 point) improvement in the KCCQ-OS was significantly greater with S3-TAVR compared with both SAVR (46.6% vs. 26.4%, p<0.001) and XT-TAVR (46.6% vs. 40.9%, p=0.015) (Table 3). At 1 year, the benefits of S3-TAVR over both SAVR and XT-TAVR were attenuated, but still persisted. Specifically, there remained an absolute 5% difference in the proportion of patients with a substantial improvement in health status, indicating a number needed to treat of ~20 patients for 1 additional patient to have a substantial improvement in health status with S3-TAVR compared with either SAVR or XT-TAVR. When health status outcomes was combined with survival status, S3-TAVR demonstrated significantly higher adjusted rates of favorable outcomes at 1 year compared with SAVR (75.5% vs. 70.0%, p=0.018) and with XT-TAVR (75.5% vs. 68.3%, p=0.001) (Table 4).
Table 3:
Risk-Adjusted Rates of Moderate or Substantial Improvement over Time after S3-TAVR, XT-TAVR, and SAVR
| S3-TAVR | SAVR | p-value | S3-TAVR | XT-TAVR | p-value | |
|---|---|---|---|---|---|---|
| 1 Month | ||||||
| Substantial improvement*, % | 46.6 | 26.4 | < 0.001 | 46.6 | 40.9 | 0.015 |
| Moderate or substantial improvement†, % | 67.2 | 40.9 | < 0.001 | 67.0 | 60.6 | 0.005 |
| 1 Year | ||||||
| Substantial improvement*, % | 54.6 | 49.0 | 0.032 | 55.0 | 50.6 | 0.080 |
| Moderate or substantial improvement†, % | 71.5 | 68.1 | 0.154 | 71.4 | 68.3 | 0.178 |
Defined as increase in the Kansas City Cardiomyopathy Questionnaire Overall Summary Score Scale of > 20 points
Defined as increase in the Kansas City Cardiomyopathy Questionnaire Overall Summary Score Scale of > 10 points
Abbreviations: KCCQ – Kansas City Cardiomyopathy Questionnaire
Table 4:
Rates of Favorable and Excellent Outcome at 1 Year after after S3-TAVR, XT-TAVR, and SAVR
| S3-TAVR vs. SAVR | S3-TAVR vs. XT-TAVR | |||||
|---|---|---|---|---|---|---|
| S3-TAVR (N = 972) | SAVR (N = 713) | P-Value | S3-TAVR (N = 972) | XT-TAVR N = 835) | P-Value | |
| Favorable Outcome * | 75.5% | 70.0% | 0.018 | 75.5% | 68.3% | 0.001 |
| Excellent Outcome † | 59.6% | 53.2% | 0.008 | 59.6% | 54.7% | 0.058 |
Defined as combination of survival and KCCQ-OS score > 60 in the absence of a decline in KCCQ-OS score of ≥ 10 pts from baseline
Defined as combination of survival and KCCQ-OS score > 75 in the absence of a decline in KCCQ-OS score of ≥ 10 pts from baseline
Abbreviations: KCCQ – Kansas City Cardiomyopathy Questionnaire
Pre-procedural characteristics that were independently associated with a favorable outcome at 1 year after S3-TAVR are shown in Supplementary Table E. Higher baseline KCCQ-OS score was associated with greater likelihood of a favorable 1-year outcome. On the other hand, older age, oxygen-dependent chronic obstructive pulmonary disease, diabetes, prior stroke and poor performance on the 15-foot walk test (e.g. > 7 seconds) were all associated with a reduced likelihood of a favorable outcome at 1 year.
Mediation Analyses
Because the differences in 1-year health status between S3-TAVR and both SAVR and XT-TAVR were somewhat unexpected, we performed additional analyses to assess whether differential rates in peri-procedural complications (Supplementary Table F) contributed to the persistent health status benefits seen at 1 year with S3-TAVR. Specifically, among patients treated with S3-TAVR, there were lower rates of disabling/life-threatening bleeding, acute kidney injury, disabling stroke, and new atrial fibrillation compared with both SAVR and XT-TAVR and lower rates of major vascular complications and moderate/severe aortic regurgitation compared with XT-TAVR. When complications were added to the models, the estimated benefits of S3-TAVR vs. SAVR and XT-TAVR were attenuated and no longer statistically significant (S3-TAVR vs. SAVR: mean adjusted KCCQ-OS difference 0.9 points, 95% CI −1.3 to 3.1, p=0.427; S3-TAVR vs. XT-TAVR: mean adjusted KCCQ-OS difference 1.5 points, 95% CI −0.2 to 3.3, p=0.091), thus suggesting that the observed differences in long-term health status were primarily driven by differences in peri-procedural complications between the alternative valve replacement strategies.
DISCUSSION
This is the first study to formally evaluate the effects of a 3rd-generation TAVR device (SAPIEN-3) on health status in patients with severe AS at intermediate surgical risk. In this population, patients treated with S3-TAVR experienced substantial improvement in quality of life within 1 month of treatment, and these benefits were durable through 1 year of follow-up. Furthermore, the health status benefits conferred by S3-TAVR were both substantial and clinically important, with over 70% of surviving patients demonstrating evidence of at least a moderately large clinical improvement (defined as an increase > 10 points in the KCCQ-OS score) at 1 year. When S3-TAVR was compared with SAVR in propensity score adjusted analyses, S3-TAVR was associated with significantly better health status at 1 month. At 1 year, there was evidence of continued, albeit attenuated, benefit of S3-TAVR over SAVR. When S3-TAVR was compared with XT-TAVR, analyses demonstrated a small, but significant, benefit of S3-TAVR on disease-specific health status at both 1 month and 1 year.
Our findings of significant health status benefit at 1 month with S3-TAVR compared with both SAVR and XT-TAVR are consistent with prior studies. The early health status benefit of TAVR over SAVR has been well-documented in several trials comparing TAVR and SAVR in patients at both high surgical risk as well as intermediate surgical risk (1,2,6,22), and reflects the more rapid recovery that would be expected with a less invasive procedure, which does not require a full sternotomy and cardiopulmonary bypass. While the finding of an early health status benefit of S3-TAVR over XT-TAVR may seem surprising since both procedures utilize a similar less invasive approach, the observed difference is likely related to the smaller sheath size required for S3-TAVR, thereby allowing for greater use of transfemoral access as compared with XT-TAVR. Indeed, prior studies have clearly demonstrated that the early health status benefit seen with TAVR over SAVR is limited to those patients who are treated via a transfemoral approach and that no such advantage is observed in patients treated with a transthoracic approach (1,2,6).
The more unexpected finding of our study was that treatment with S3-TAVR resulted in statistically significant health status improvements not only at 1 month, but also at 1 year when compared with both SAVR and XT-TAVR. While these differences were numerically small (~2 points on the KCCQ-OS scale for both SAVR and XT-TAVR), these changes may still reflect meaningful changes from a population perspective. Although a 5 point difference in the KCCQ-OS scale has been shown to be clinically meaningful to an individual patient, it is important to recognize that the mean difference in health status over a population comprises many different individual values. For example, if 40% of patients saw a 5 point improvement in their KCCQ-OS score with a specific intervention and 60% of patients saw no improvement, this would still be considered a clinically meaningful improvement by 40% of the individuals in the study, but would only result in a 2 point mean difference for the overall study population. Indeed, the 5% absolute difference in the proportion of S3-TAVR patients who experienced a substantial improvement in health status (i.e., >20 point increase in KCCQ-OS from baseline) as compared with either SAVR or XT-TAVR at 1 year provides direct evidence that the small difference in health status observed at a population level may indeed represent meaningful differences for some individuals.
There are several possible explanations for the observed health status benefits of S3-TAVR compared with both SAVR and XT-TAVR at 1 year. First, there were differences in several procedure-related complications between the treatment modalities (e.g., atrial fibrillation; vascular complication; paravalvular leak; stroke), which could have affected health status in the long term. This hypothesis is supported by the results of informal mediation analyses, which demonstrated that when post-procedural complications were included in the model, there was attenuation of the health status benefit seen with S3-TAVR compared with both SAVR and XT-TAVR. These findings suggest that at least part of the 1-year health status benefit seen with S3-TAVR over SAVR and XT-TAVR relates to differing frequencies of post-procedural clinical events. Second, it is possible that the increased use of transfemoral access (which has been associated with improved early recovery after TAVR (2,6)) with S3-TAVR compared with XT-TAVR led to carryover health status benefits in these patients. Ideally, a randomized controlled trial would be needed to confirm the superiority in health status benefits of S3-TAVR over SAVR and XT-TAVR and to fully identify the mechanism by which superiority was achieved.
Study Limitations
The results of our study should be interpreted in light of several limitations. Firstly, this was a non-randomized study, although it is important to note that the PARTNER 2A trial and S3i registry used identical inclusion and exclusion criteria, thereby resulting in similar patient characteristics between the studies. In addition, we used a pre-specified propensity score to adjust for differences between the groups. Nevertheless, despite these efforts to reduce confounding, it is possible that the observed differences in health status were driven by differences in patient characteristics that were not controlled for by statistical methods. That said, the results of our mediation analyses suggest that differences in peri-procedural complications may explain much of the observed differences in health status between the groups at 1 year, thereby increasing our confidence that the health status differences we observed were, in fact, due to differences in the treatment modalities and not due to patient differences. Secondly, quality of life assessments were only available through 1 year follow-up. Thus, the durability of these results beyond 1 year is unknown. Lastly, these findings only apply to patients with severe AS at intermediate surgical risk with similar baseline characteristics as the study population and should not be generalized to patients who are dissimilar to the analytic cohort.
Conclusions
In conclusion, among patients with severe AS at intermediate surgical risk, S3-TAVR resulted in improved health status at 1 month compared with both SAVR and XT-TAVR. At 1 year, there were also modest improvements in health status observed with S3-TAVR compared with both SAVR and XT-TAVR that appeared to be driven primarily by lower rates of peri-procedural complications seen with S3-TAVR. Further studies are needed to evaluate the durability of health status benefits with S3-TAVR in intermediate risk patients as well as to assess the effects of S3-TAVR in a low risk surgical population.
Supplementary Material
Central Illustration. Adjusted Between-Group Differences in Kansas City Cardiomyopathy Overall Summary Scale Between S3-TAVR and SAVR and S3-TAVR and XT-TAVR.
Adjusted between-group differences in Kansas City Cardiomyopathy Overall Summary Scale between S3-TAVR and SAVR (Panel A) and S3-TAVR and XT-TAVR (Panel B). Error bars denote 95% confidence intervals. The p values represent difference between mean health status score of the treatment groups at each time point.
CLINICAL PERSPECTIVES.
What is Known:
The SAPIEN-3 valve is the newest commercially available balloon-expandable TAVR prosthesis and provides several design improvements over earlier generation TAVR prostheses, including a lower delivery profile and an outer skirt aimed at reducing paravalvular regurgitation. While prior studies have demonstrated that TAVR with the SAPIEN-3 valve is associated with reduced rates of death and stroke as compared with SAVR, the effect of SAPIEN-3 on patient-reported outcomes is unknown.
What is New:
We found that TAVR with the SAPIEN-3 valve was associated with significant health status benefits at 1 month and 1 year compared with both SAVR and TAVR with the earlier generation SAPIEN-XT valve. Mediation analyses suggested that the observed long-term quality of life benefits of SAPIEN 3 compared with TAVR using the SAPIEN-XT valve or SAVR may be due, in part, to differences in procedure-related complications associated with the different treatment groups.
What is Next:
Further studies are needed to evaluate the durability of health status benefits with SAPIEN-3 in this population.
Acknowledgments
Funding: The PARTNER 2 clinical trial ( NCT 01314313) and quality of life sub-study was funded by a research grant from Edwards LifeSciences, Inc. Dr. Arnold is supported by a Career Development Grant Award (K23 HL116799) from the National Heart, Lung, and Blood Institute.
ABBREVIATIONS
- AS
Aortic stenosis
- KCCQ
Kansas City Cardiomyopathy Questionnaire
- QoL
Quality of life
- SAVR
Surgical aortic valve replacement
- TAVR
Transcatheter aortic valve replacement
Footnotes
Relationship with Industry:
Suzanne J. Baron: Consulting income from Edwards Lifesciences and St. Jude Medical Inc; Travel reimbursement from Medtronic.
Vinod H. Thourani: Research grants and personal fees from Edwards Lifesciences, Medtronic, St. Jude Medical, Sorin Medical, Boston Scientific Inc, Abbott Vascular and Directflow Medical.
Susheel Kodali: Holds equity in Thubrikar Aortic valve Inc; Research grant support from Edwards Lifesciences and St. Jude Medical;
Suzanne V. Arnold: none
Elizabeth Magnuson: none
Kaijun Wang: none
Augusto D Pichard: consulting income from Edwards Lifesciences
Vasilis Babaliaros: Research grants and consulting fees for Edwards Lifesciences, Medtronic, St. Jude Medical, Boston Scientific, Directflow Medical and Abbott Vascular
Isaac George: Consulting income from Edwards Lifesciences and Medtronic.
D. Craig Miller: Consulting income from Abbott Vascular, St. Jude Medical Inc, and Medtronic.
Murat Tuzcu: none
Kevin Greason: none
Howard C. Herrmann: Research grant support from Abbott Vascular, Bayer, Edwards Lifesciences, Boston Scientific Inc, Medtronic and St. Jude Medical Inc; Consulting income from Edwards Lifesciences
Craig R. Smith
Martin B. Leon: none
David J. Cohen: Research grant support from Edwards Lifesciences, Medtronic, Boston Scientific, and Abbott Vascular; Consulting income from Edwards Lifesciences and Medtronic
REFERENCES
- 1.Arnold SV, Reynolds MR, Wang K et al. Health Status After Transcatheter or Surgical Aortic Valve Replacement in Patients With Severe Aortic Stenosis at Increased Surgical Risk: Results From the CoreValve US Pivotal Trial. JACC Cardiovasc Interv 2015;8:1207–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Reynolds MR, Magnuson EA, Wang K et al. Health-related quality of life after transcatheter or surgical aortic valve replacement in high-risk patients with severe aortic stenosis: results from the PARTNER (Placement of AoRTic TraNscathetER Valve) Trial (Cohort A). J Am Coll Cardiol 2012;60:548–58. [DOI] [PubMed] [Google Scholar]
- 3.Smith CR, Leon MB, Mack MJ et al. Transcatheter versus surgical aortic-valve replacement in high-risk patients. N Engl J Med 2011;364:2187–98. [DOI] [PubMed] [Google Scholar]
- 4.Reardon MJ, Adams DH, Kleiman NS et al. 2-Year Outcomes in Patients Undergoing Surgical or Self-Expanding Transcatheter Aortic Valve Replacement. J Am Coll Cardiol 2015;66:113–21. [DOI] [PubMed] [Google Scholar]
- 5.Leon MB, Smith CR, Mack MJ et al. Transcatheter or Surgical Aortic-Valve Replacement in Intermediate-Risk Patients. N Engl J Med 2016;374:1609–20. [DOI] [PubMed] [Google Scholar]
- 6.Baron SJ, Arnold SV, Wang K et al. Health Status Benefits of Transcatheter vs Surgical Aortic Valve Replacement in Patients With Severe Aortic Stenosis at Intermediate Surgical Risk: Results From the PARTNER 2 Randomized Clinical Trial. JAMA Cardiol 2017. [DOI] [PMC free article] [PubMed]
- 7.Thourani VH, Kodali S, Makkar RR et al. Transcatheter aortic valve replacement versus surgical valve replacement in intermediate-risk patients: a propensity score analysis. Lancet 2016;387:2218–25. [DOI] [PubMed] [Google Scholar]
- 8.Lewis EF, Johnson PA, Johnson W, Collins C, Griffin L, Stevenson LW. Preferences for quality of life or survival expressed by patients with heart failure. J Heart Lung Transplant 2001;20:1016–24. [DOI] [PubMed] [Google Scholar]
- 9.Green CP, Porter CB, Bresnahan DR, Spertus JA. Development and evaluation of the Kansas City Cardiomyopathy Questionnaire: a new health status measure for heart failure. J Am Coll Cardiol 2000;35:1245–55. [DOI] [PubMed] [Google Scholar]
- 10.Soto GE, Jones P, Weintraub WS, Krumholz HM, Spertus JA. Prognostic value of health status in patients with heart failure after acute myocardial infarction. Circulation 2004;110:546–51. [DOI] [PubMed] [Google Scholar]
- 11.Kosiborod M, Soto GE, Jones PG et al. Identifying heart failure patients at high risk for near-term cardiovascular events with serial health status assessments. Circulation 2007;115:1975–81. [DOI] [PubMed] [Google Scholar]
- 12.Spertus J, Peterson E, Conard MW et al. Monitoring clinical changes in patients with heart failure: a comparison of methods. Am Heart J 2005;150:707–15. [DOI] [PubMed] [Google Scholar]
- 13.Arnold SV, Spertus JA, Lei Y et al. Use of the Kansas City Cardiomyopathy Questionnaire for monitoring health status in patients with aortic stenosis. Circ Heart Fail 2013;6:61–7. [DOI] [PubMed] [Google Scholar]
- 14.Ware JE Jr., Sherbourne CD The MOS 36-item short-form health survey (SF-36). I. Conceptual framework and item selection. Med Care 1992;30:473–83. [PubMed] [Google Scholar]
- 15.Kiebzak GM, Pierson LM, Campbell M, Cook JW. Use of the SF36 general health status survey to document health-related quality of life in patients with coronary artery disease: effect of disease and response to coronary artery bypass graft surgery. Heart Lung 2002;31:207–13. [DOI] [PubMed] [Google Scholar]
- 16.Failde I, Ramos I. Validity and reliability of the SF-36 Health Survey Questionnaire in patients with coronary artery disease. J Clin Epidemiol 2000;53:359–65. [DOI] [PubMed] [Google Scholar]
- 17.Ware JKM, Bjorner JB, Turner-Bowkes DM, Gandek B, Maruish ME. Determining important differences in scores. User’s Manual for the SF-36v2 Health Survery Lincoln, RI: Quality Metric Incorporated, 2007. [Google Scholar]
- 18.Shaw JW, Johnson JA, Coons SJ. US valuation of the EQ-5D health states: development and testing of the D1 valuation model. Med Care 2005;43:203–20. [DOI] [PubMed] [Google Scholar]
- 19.D’Agostino RB Jr. Propensity score methods for bias reduction in the comparison of a treatment to a non-randomized control group. Stat Med 1998;17:2265–81. [DOI] [PubMed] [Google Scholar]
- 20.Arnold SV, Reynolds MR, Lei Y et al. Predictors of poor outcomes after transcatheter aortic valve replacement: results from the PARTNER (Placement of Aortic Transcatheter Valve) trial. Circulation 2014;129:2682–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Arnold SV, Spertus JA, Lei Y et al. How to define a poor outcome after transcatheter aortic valve replacement: conceptual framework and empirical observations from the placement of aortic transcatheter valve (PARTNER) trial. Circ Cardiovasc Qual Outcomes 2013;6:591–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Reardon MJ, Van Mieghem NM, Popma JJ et al. Surgical or Transcatheter Aortic-Valve Replacement in Intermediate-Risk Patients. N Engl J Med 2017;376:1321–1331. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.