Abstract
Endometrial cancer is the most common gynecologic cancer in women today. It is surgically staged, and while surgery is the primary treatment modality, the identification of disease extent—in particular extrauterine spread—prior to surgery is important to optimize treatment decision making. Ultrasound and MRI are useful for evaluating the extent of local disease, while CT and PET are used for detecting lymph node or distant metastases. Diffusion-weighted MRI has also been used for detecting small metastatic deposits in lymph nodes and omentum. Extrauterine soft tissue involvement can be detected by ultrasound, CT, MRI, and PET. Recently, intraoperative visualization techniques, such as sentinel lymph node mapping, are increasingly used to avoid extensive surgical staging without compromising treatment. Imaging is also used for planning adjuvant treatment and detection of postoperative residual disease in high-risk patients, monitoring and detecting recurrent disease, and in post-treatment surveillance of asymptomatic patients with high risk of relapse.
Keywords: Endometrial cancer, MRI, PET, Radiology, Ultrasound
1. Background
Endometrial cancer is the most common gynecologic cancer seen in women today [1]. It is more prevalent in high-resource countries, but its incidence is rising in low-resource countries as a result of rising obesity and improved longevity. Historically, endometrial cancer is classified according to histologic subtype, but recently—as a result of the Cancer Genome Atlas (TCGA)—a molecular-based classification has been advocated owing to its superior prognostication [2].
Endometrial cancer is curable, especially in the early stages. Endometrioid histology has better prognosis than nonendometrioid histologies. Surgery is the mainstay of treatment. Adjuvant radiotherapy and systemic chemotherapy play a role in selected cases. Accurate mapping of the extent of cancer spread is important for appropriate application of local and/or regional treatment. Although endometrial cancer is surgically staged, the identification of disease extent—in particular extrauterine spread—prior to surgery is important to optimize treatment planning. This has been facilitated by noninvasive medical imaging technologies including ultrasound, X-ray, computed tomography (CT), magnetic resonance imaging (MRI), positron emission tomography (PET), and, increasingly, co-registered images such as PET-CT or PET-MRI. More recently, intraoperative visualization techniques, such as sentinel lymph node (SLN) mapping are being used to avoid extensive surgical staging without compromising treatment.
The aim of the present article is to describe the practical application of imaging in the routine diagnostic assessment, staging, treatment selection, adjuvant treatment planning, and post-treatment surveillance of endometrial cancer patients.
2. Clinical presentation and work-up
Most endometrial cancer occurs in postmenopausal women over the age of 50 years (median age of 63 years) who commonly present with vaginal bleeding. Less commonly the presentation is associated with vaginal discharge, while abdominal distension and pain are usually associated with advanced disease.
Evaluation of a woman suspected to have endometrial cancer involves detailed history-taking including identifying risk factors, and physical examination including pelvic examination. Pelvic examination involves estimation of the size and mobility of the uterus, detection of any vaginal extension of pathology, and direct inspection of the genital tract to exclude other common local causes of bleeding. First-line imaging is a pelvic ultrasound. This should begin with transabdominal ultrasound to assess the pelvic organs, followed by transvaginal ultrasound to assess endometrial thickness and any extension of abnormality in the cervix. Endometrial thickness greater than 4 mm in a postmenopausal woman is highly suspicious of an endometrial pathology, warranting further evaluation that can be done with endometrial sampling. In a meta-analysis reviewing the accuracy of endometrial sampling devices, the Pipelle endometrial sampler (Pipelle de Cornier, Paris, France) was found to be highly sensitive for the detection of endometrial carcinoma, with detection rates of 99.6% and 91% for post- and premenopausal women respectively, and 81% for atypical endometrial hyperplasia [3].
When office endometrial sampling is not possible, for example in severe cervical stenosis or virgin patients, hysteroscopy with dilatation and curettage (D&C) is the next investigation of choice. Hysteroscopy with D&C is indicated if ultrasound indicates the presence of endometrial polyps that may contain premalignant or malignant cells that could be missed by endometrial sampling alone, or to obtain more tissue if the diagnosis of neoplasia from office biopsy is in question. Hysteroscopy D&C should also be considered in women with bleeding who are taking tamoxifen [4].
3. Staging and treatment selection
Once a diagnosis of endometrial cancer has been established, the treatment of choice is total hysterectomy with bilateral salpingo-oophorectomy and surgical staging in the majority of cases. Lymph node status assessment is required for accurate staging and for treatment planning.
The detection of lymph node metastases in surgically staged patients varies between 8% and 33% depending on the quality of node dissection, pathologic assessment protocols, histologic subtype, and the clinical stage [5–8]. Increasing lymph node involvement is associated with depth of myometrial invasion and histologic grade of tumor. The incidence of nodal metastases in patients with less than and greater than 50% myometrial invasion was 5% and 18%, respectively [5,9]. Knowledge of lymph node status assists in deciding adjuvant treatment and in radiotherapy planning. A systematic pelvic and para-aortic lymphadenectomy is associated with increased morbidity, more so in combination with radiotherapy when the incidence of serious complications can be up to 26% [10,11]. Thus, routine systematic pelvic and para-aortic lymphadenectomy for staging purposes is not recommended [12]. Staging lymphadenectomy can be avoided in endometrioid adenocarcinoma histology where the FIGO grade is 1 or 2 and the tumor is limited to the inner half of the myometrium, cervical stroma is not involved and more recently, these cases are staged with the SLN mapping algorithm [13]. The SLN mapping algorithm for staging has replaced lymphadenectomy in many practices worldwide and can be applied to almost all cases of apparent uterine-confined endometrial cancer regardless of FIGO grade or histotype [14]. Following a normal peritoneal survey, any suspicious lymph node should be removed and sent for histologic examination. In the absence of SLN mapping, myometrial invasion can be identified preoperatively using MRI to evaluate the need for lymphadenectomy [15]. Where MRI is not available, intraoperative frozen section of the suspected region of the myometrium can be used to determine myometrial invasion in patients with grade 1–2 histology [16].
The aim of successful endometrial cancer surgery, when possible, is definitive treatment or, if surgery alone is not curative, to remove gross disease and accurately stage the patient in order to direct postoperative adjuvant therapies.
In the majority of patients with grade 1 and 2 endometrioid histology, preoperative imaging will not significantly change the basic management or prognosis of these patients. However, imaging is helpful in patients with suspected extrauterine disease where additional surgery is required for its removal or for planning of adjuvant treatment. It is desirable to know the following information prior to the surgery:
Histology and grade.
Myometrial invasion (in select cases where fertility preservation or ovarian preservation is considered).
Presence of resectable gross disease outside the uterus (enlarged ovary, nodes, and omental disease).
Presence of unresectable disease. The possibility of finding extrauterine disease is higher in poorly differentiated endometrioid adenocarcinoma and nonendometrioid carcinoma. In patients with serous histology, extrauterine disease may be present in almost 50% of all cases.
Ultrasound and MRI are used for evaluating the extent of local disease, while CT and PET are used for detecting lymph node or distant metastases [17]. Diffusion-weighted MRI (DWI-MRI) has also been used for detecting small metastatic deposits in lymph nodes and omentum. Extrauterine soft tissue involvement can be detected by transabdominal ultrasound, CT, MRI, and PET. Apart from preoperative staging, imaging is also used for planning adjuvant treatment and for detection of postoperative residual disease in high-risk patients (CT/PET-CT), monitoring and detecting recurrent disease (ultrasound, CT, MRI, and PET/PET-CT) and in post-treatment surveillance of asymptomatic patients with high risk of relapse (PET/PET-CT).
3.1. Low-resource regions where medical imaging is not available
In low-resource conditions, it is reasonable to proceed with total hysterectomy and bilateral salpingo-oophorectomy once the patient has been evaluated by clinical pelvic examination and the histologic diagnosis established. In the vast majority of patients with endometrioid carcinoma and well- to moderately differentiated histology with invasion limited to the inner half of the myometrium or no myometrial invasion, namely FIGO Stage 1A, total abdominal hysterectomy and bilateral salpingo-oophorectomy are usually curative. All other types or stages of endometrial cancer may have locoregionally advanced disease where lymph node sampling is recommended. In poorly differentiated histology or where examination under anesthesia suggests extrauterine disease, a chest X-ray—if available—should be performed. In the absence of SLN mapping, gross examination of myometrial invasion in a cut hysterectomy specimen intraoperatively can be used to guide lymph node sampling. Such gross examination of myometrial invasion correctly identified disease in 89% (64/72) of patients [18]. A pathological review of the surgical specimen should be carried out and, if indicated and where possible, patients should be referred for further treatment.
3.2. Medium-resource regions where ultrasound and CT may be available
Ultrasonography plays a central role in the management of patients with gynecological disorders. It is widely available and is a relatively inexpensive imaging tool. In certain circumstances, its diagnostic accuracy may match that of MRI.
3.2.1. Ultrasound
The uterine cavity and endometrium as well as the adnexa can be examined in great detail using transvaginal ultrasound. Transrectal ultrasound can also provide similar information and is useful in elderly patients with vaginal stenosis. Transabdominal ultrasound should be used in conjunction with transvaginal ultrasound if the uterus is large, and to avoid missing large ovarian or tubal pathology.
Ultrasound examination of the uterus should be carried out systematically in a stepwise fashion [19]. Using a transvaginal ultrasound probe, the cervix should be examined in a sagittal plane for tumor invasion into the cervical stroma. The medial parametria should also be examined, followed by the entire body of the uterus from cornu to cornu in a sagittal plane, and from the cervix to the fundus in an axial plan. Tumor size should then be measured and, if possible, Gordon’s ratio measured at the area of deepest myometrial invasion [19].
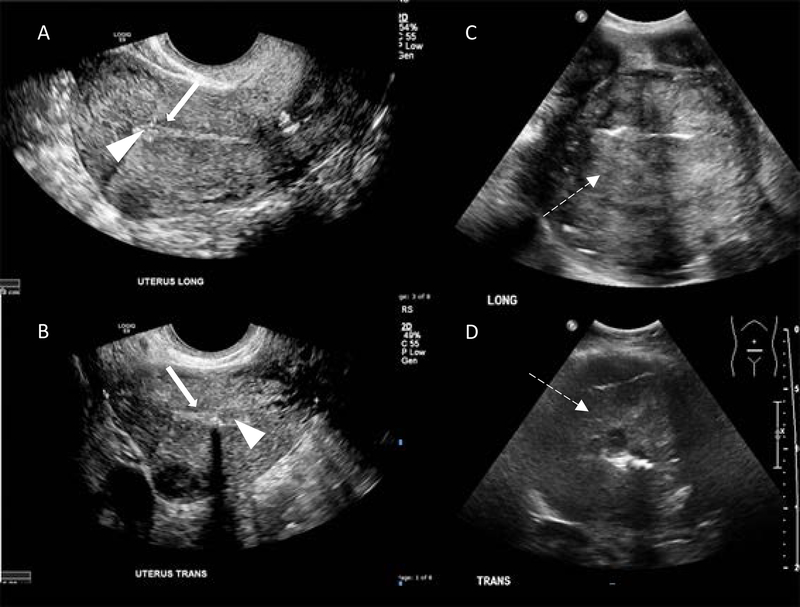
Normal endometrium in a postmenopausal woman is smooth, usually less than 1 mm thick, and has a thin hypoechoic layer between it and the myometrium (Figure 1A and 1B). Although ultrasound can detect altered endometrium, it is not specific and histologic confirmation is always required. There is a higher likelihood of endometrial cancer with increasing thickness of the endometrium and postmenopausal status. In general, a cut-off value of 4–5 mm is indicative of a carcinoma, with 96% sensitivity and 61% accuracy based on a meta-analysis by Smith-Bindman et al. [20]. Furthermore, the heterogeneous echogenicity caused by areas of hemorrhage and necrosis (Figure 1C and 1D) and an irregular endometrial myometrial interphase that denotes myometrial invasion by cancer supports the diagnosis of endometrial cancer. Transvaginal ultrasound may also detect cervical stromal invasion.
Figure 1.
Ultrasound scans of a normal uterus. Transvaginal ultrasound scans of (A) sagittal plane (long) and (B) axial plane (trans) with normal, thin, uniform echogenic endometrium (arrowheads) and subendometrial hypoechoic halo (solid arrows). Transabdominal ultrasound scans of endometrial carcinoma in sagittal plane (C) and axial plane (D). Note heterogeneous echogenicity due to hemorrhage and necrosis (dashed arrows).
Transabdominal ultrasound can provide additional information, mostly related to extrauterine disease.
3.2.2. Computed tomography
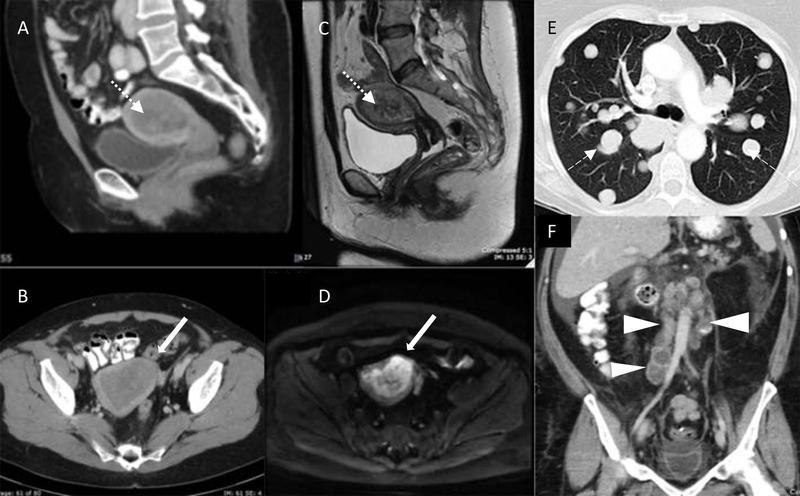
CT is less helpful in investigating abnormalities within the uterus. Figures 2A–D show the difference in image quality and the soft tissue definition between CT (Figure 2A and B) and MRI (Figure 2C and D). On CT, a gross suspicious endometrial mass may be seen as a hypodense lesion, or an enlarged endometrial cavity that often cannot be distinguished from benign lesions. However, CT has better multiplanar spatial resolution that is useful in visualizing the entire pelvic and abdominal cavity for enlarged nodes (Figure 2F) and gross soft tissue masses, as well as distant metastases in the lungs (Figure 2E).
Figure 2.
Computed tomography (A: sagittal plane, B: axial plane) and MRI (C: sagittal T2, D: axial diffusion-weighted) in the same patient with endometrial carcinoma distending the endometrial cavity (dotted arrows) and deep myometrial invasion (solid arrows). E: Axial CT chest with multiple “cannonball” pulmonary metastases (dashed arrows). F: Coronal CT abdomen demonstrating multiple para-aortic necrotic lymph node metastases (arrowheads)
In the absence of MRI or PET, CT should be requested if extrauterine disease is suspected in patients with high-grade histology, deep myometrial invasion, or a large uterus. Frequently, CT is ordered as a baseline investigation prior to histologic diagnosis of endometrial cancer. The use of intravenous contrast is helpful in identifying vascular structures and soft tissue metastases, and distinguishing lymph nodes from small bowel loops. A lymph node with a maximum short axis diameter of greater than or equal to 10 mm is regarded as suspicious of metastatic disease. Nonetheless, the overall reported sensitivity in detecting nodal metastasis is only about 40%. In a study involving measurement of 125 positive and 160 negative pelvic lymph nodes in 32 consecutive patients with node-positive endometrial cancer, there was a positive correlation between the size of the positive lymph node and the size of the metastasis (P<0.01) [21]. Whereas 68 of 125 (54%) positive lymph nodes measured less than 10 mm in maximum short axis diameter, 46 of 160 (29%) negative lymph nodes measured more than 10 mm in maximum short axis diameter [21]. It was observed that patients with single metastatic nodes tend to have tumor deposits in nodes less than 10 mm. In patients with multiple positive nodes, 85% of patients had at least one positive node greater than 10 mm [8]. In node-positive endometrial cancer patients the incidence of a single positive node was 25%–40% and multiple nodes were found in 60%–75% of patients [8]. Therefore, CT should correctly detect more than 40% of node-positive patients—higher than sensitivity and specificity studies would suggest. Additionally, CT scan of the chest, abdomen, and pelvis as a preoperative investigation is useful for excluding unexpected anatomy that may result in modification of planned surgery.
3.3. High-resource countries where modern imaging is available
In countries where health care is streamlined and well-resourced, patients often present to the primary healthcare provider with symptoms prior to referral to a specialist or subspecialist. Transvaginal ultrasound is typically scheduled and, following a suspected diagnosis of endometrial hyperplasia or cancer, patients are referred to a gynecologist for further management and workup. Patients undergo office Pipelle biopsy or examination under anesthesia and D&C to obtain endometrial tissue samples. Once the histologic diagnosis of endometrial cancer is established, a CT or MRI scan is requested following which a patient may have total hysterectomy, bilateral salpingo-oophorectomy, and surgical staging. Commonly, following histologic diagnosis, patients are referred to gynecologic oncology specialist multidisciplinary clinics. Here histology and any available imaging are reviewed. In some centers, all confirmed cases of endometrial cancer will have pelvic MRI prior to surgery. In other centers, CT of the chest, abdomen, and pelvis is requested if prior scan is not available. All pretreatment imaging and pathology is reviewed at multidisciplinary meetings and individual patient management plans are formulated. Diagnostic information related to ultrasound and CT has been discussed in previous sections. Here we describe the role of MRI in endometrial cancer.
3.3.1. Magnetic resonance imaging
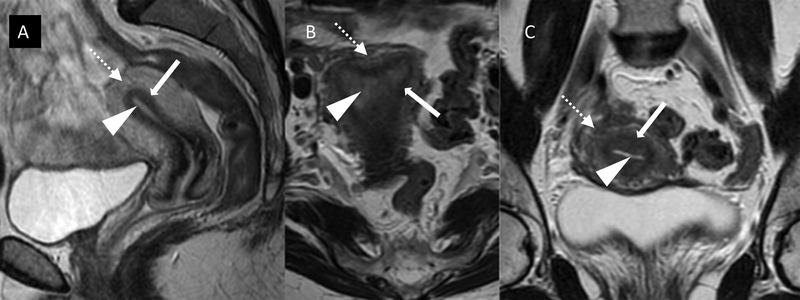
Where available, MRI is the imaging modality of choice for the anatomical study of the pelvis and abdomen. MRI is best suited to detect and evaluate endometrial cancer within the endometrial cavity; tumor infiltration into myometrium, endocervix, and gross extension into the parametria; and other pelvic tumor deposits. On T2-weighted images, endometrial cancer usually appears of intermediate signal intensity. Unlike normal endometrium it does not have a uniform high signal owing to the presence of higher cellularity, necrosis, and hemorrhage within the tumor. The surrounding myometrium is composed of two distinct layers. The inner myometrial layer or “junctional zone” abuts the endometrium and appears as a low signal band, while the outer myometrium is more variable in appearance but usually of intermediate signal (Figure 3). Post intravenous contrast, the innermost myometrial layer enhances uniformly during the early dynamic phase as a continuous line or “subendometrial stripe.” Disruption of the subendometrial stripe or frank breach of the junctional zone is indicative of myometrial invasion. An intact junctional zone and a continuous band of early subendometrial enhancement exclude deep myometrial invasion.
Figure 3.
Normal zonal anatomy on T2-weighted MRI in (A) sagittal, (B) axial, and (C) coronal planes demonstrating high signal (bright) endometrium (arrowheads), low signal (darker) junctional zone or inner myometrium (solid white arrows), and intermediate signal (grey) outer myometrium (dotted arrows).
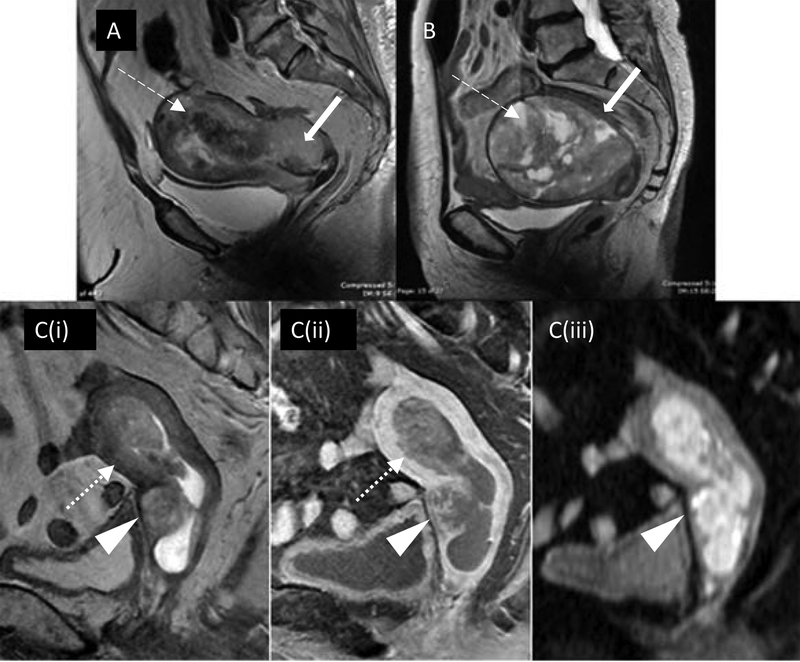
Depth of myometrial invasion is related to the incidence of nodal metastasis. The incidence of nodal metastasis in patients with less than 50% and greater than 50% myometrial invasion was reported to be 5% and 18%, respectively [5]. A study comparing presurgical MRI images with histology showed the value of MRI in predicting the presence and depth of myometrial invasion (Figure 4) and the potential utility of MRI in presurgical prognostication of patients who may be suitable for more conservative treatment [15].
Figure 4.
Deep myometrial invasion on MRI: (A) Sagittal T2 demonstrating obvious deep myoinvasion with heterogeneous tumor obliterating the normal uterine zonal anatomy (dashed arrow) and invading cervical stroma (solid arrow). (B) Pitfall of MRI myometrial invasion assessment: large polypoid tumor stretches and thins the myometrium (dashed arrow) with age-related loss of normal zonal anatomy (solid arrow), pathology confirming no evidence of deep myoinvasion. (C) Multiparametric MRI improving staging accuracy: equivocal depth of myoinvasion and cervical stromal involvement on sagittal T2 (Ci). Dynamic T1 fat saturated post contrast (Cii) shows disruption of subendometrial stripe at anterior midbody of uterus (dotted arrow) confirming myoinvasion. Cervical mucosal enhancement preserved posteriorly but disrupted anteriorly (arrowhead) confirming stromal invasion. Diffusion-weighted imaging (Ciii) highlights tumor extent and deep cervical stromal invasion but absence of bladder wall invasion (arrowhead).
The sensitivity of MRI to distinguish superficial from deep myometrial invasion varies from nearly 60%–88% [22], and is limited in some situations such as very superficial invasion in premenopausal women or a large polypoid tumor extruding into the cervical canal [23,24], as well as in technique and image quality. The combination of T2-weighted and dynamic contrast-enhanced magnetic resonance images offers high accuracy for staging endometrial cancer in the range of 83%–91% (personal communication, KN, May 2018). Endometrial cancer is best examined in the sagittal plane, providing longitudinal views of the uterus and cervix as well as surrounding structures such as bladder, rectum, and loops of bowel.
T2-weighted MRI is the key sequence for evaluating myometrial invasion as it depicts the uterine zonal anatomy, with the intermediate-signal-intensity tumor well delineated against the low-signal-intensity junctional zone.
A minimum of at least two T2-weighted sequences in the sagittal, axial oblique, or coronal oblique orientation (short and long axis of the uterine body) of the pelvis and one T1-weighted enhanced sequence acquired at 2 minutes ± 30 seconds after intravenous contrast injection is recommended.
If cervical invasion is suspected, additional slice orientation perpendicular to the axis of the endocervical canal is recommended [25]. The presence of an intact enhancing cervical mucosa excludes cervical stromal invasion [26].
Multiparametric MRI including thin-section (3 mm) high-resolution multiplanar T2-weighted images and simple modifications, such as the addition of double oblique T2-weighted, diffusion-weighted, and dynamic (30, 60, 120 seconds, and 4 minutes) contrast-enhanced images, improves staging and treatment planning in patients with endometrial carcinoma [26].
Often these preoperative images are helpful not only in planning the primary surgery, but also during deliberation of postoperative adjuvant radiotherapy for the presence of diverticula or any other variation in normal anatomy.
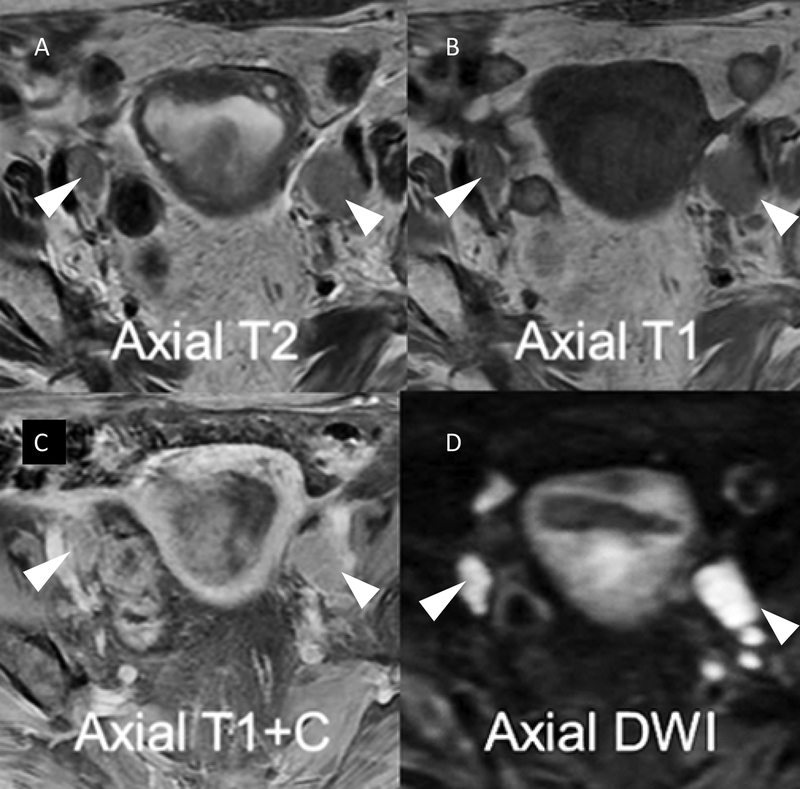
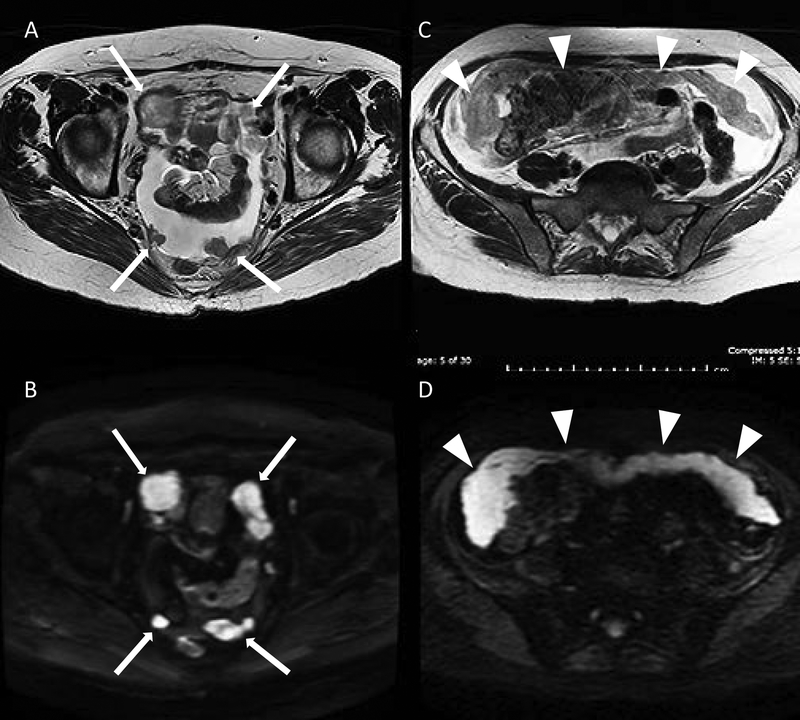
Metastatic node detection with MRI is similar to a high-quality CT scan with variable sensitivity ranging from 38% to 89% and specificity ranging from 78% to 99% [26]. However, DWI-MRI increasingly routine in many centers, and higher field strength 3T MRI may significantly enhance the sensitivity of detecting metastatic lymph nodes (Figure 5) by combining the size of node and relative apparent diffusion coefficient values [27]. Incorporating morphologic features of nodal involvement—best seen at high-resolution T2-weighted MRI—include internal heterogeneity, spiculated nodal margins, necrosis, and signal intensity comparable to that in the primary tumor, improves the accuracy of evaluation in patients with rectal cancer and may be applicable to those with endometrial carcinoma [26]. DWI-MRI is also sensitive in detecting early invasive extrauterine and omental disease (Figure 6).
Figure 5.
Lymph node evaluation on MRI is enhanced by the combination of high resolution T2 (A) and T1(B) anatomical imaging and functional sequences including post contrast T1 fat saturated (C) and diffusion-weighted imaging increasing nodal conspicuity and improving morphological assessment (arrowheads).
Figure 6.
Peritoneal metastatic nodular deposits (solid arrows) demonstrated on axial T2 (A) and diffusion-weighted imaging (B) sequences in the same patient. Omental cake (arrowheads) on axial T2: (C) intermediate signal: grey and prominent restricted diffusion on diffusion-weighted imaging; (D): high signal: bright.
MRI is useful for observing extrauterine disease and any other structural anatomical detail that may influence the extent of primary surgery.
3.3.2. Positron emission tomography–computed tomography
PET-CT is usually employed in the study of metastatic lymph nodes. It has high specificity in detecting metastatic nodes; however, its sensitivity is only modest and depends on the size of metastatic deposit [28]. The detection rate of PET-CT was only 12% in metastatic lesions measuring 4 mm or less, but 100% when the deposit was 10 mm or larger [29]. It is not routinely employed in assessing intrauterine disease although it can detect depth of myometrial invasion and extension into the endocervix reasonably well. In advanced stages, PET-CT was helpful in detecting metastatic deposits in the ovary and omentum, and distant spread. In general, if the baseline MRI or CT scan suggests enlarged nodes and if one believes that removal of all positive nodes during primary surgery will offer a survival advantage, then PET-CT may be helpful in detecting the most caudal metastatic lymph node, therefore guiding the extent of nodal clearance in that patient. Conversely, if PET-CT shows extensive disease where surgery is unlikely to be curative, then a simple (palliative) hysterectomy may be appropriate as the best immediate symptomatic measure, while the remainder of the disease can be managed using palliative hormone therapy, chemotherapy and radiotherapy alone or in combination.
4. Sentinel lymph node mapping
Once the diagnosis of endometrial cancer is established and no contraindication is found on preliminary clinical examination and imaging, primary surgery is planned. Surgery for endometrial cancer can range from a minimum of peritoneal assessment and washings, hysterectomy (abdominal, laparoscopic, or robotic) with bilateral salpingo-oophorectomy, to systematic lymphadenectomy, omentectomy, and removal of any extrauterine disease, if present.
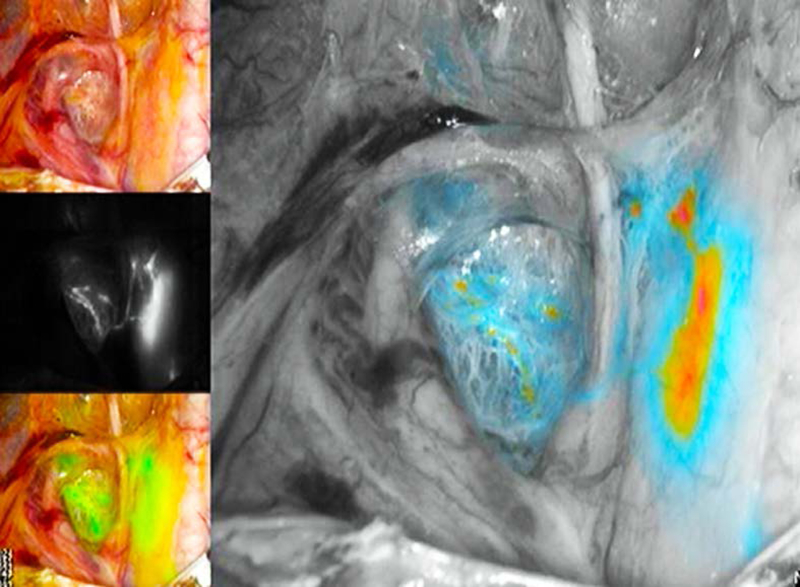
SLN mapping involves injection of a colored dye or radioisotope tracer at both the 9 and 3 o’clock positions or at four sites in all four quadrants of the cervix (Figure 7). Methylene blue was used previously, but now indocyanine green is the preferred choice. A combination of superficial (1–3 mm) and deep (1–2 cm) cervical injection is adequate. SLN mapping can effectively evaluate pelvic nodal status with 85.1% to 98.1% sensitivity, with negative predictive value of up to 99.8% in clinically Stage 1 endometrial cancer patients [30]. Such a high level of accuracy resulted from using a well-defined “SLN surgical algorithm” that was developed at the Memorial Sloan Kettering Cancer Center and adopted in the 2014 NCCN Guidelines [31]. The SLN algorithm involves: (1) removal of SLN and suspicious lymph nodes regardless of mapping; (2) if bilateral SLN is not identified then side-specific pelvic and common iliac node dissection is carried out; and (3) uterine serosal and peritoneal evaluation and washings are carried out.
Figure 7.
Example of sentinel lymph node mapping with indocyanine green cervical injection using the PINPOINT system. The right external iliac sentinel lymph node is seen medial to the external iliac vessels, and ventral to the internal iliac vessels. Also demonstrated are lymphatic trunks from the right paracervix crossing over the obliterated umbilical ligament on the way to the sentinel lymph node.
The SLN algorithm is now commonly applied as a surgical staging strategy in all histologies and grades of apparent uterine-confined endometrial cancer. With this approach, all patients will at least have bilateral pelvic nodes sampled and evaluated by a strict pathological protocol to help exclude extrauterine disease and increase precision in the prescription of adjuvant therapy. Comparison of staging outcomes from the SLN algorithm versus traditional lymphadenectomy has been reported by Ducie et al. [14], with encouraging results.
In clinically advanced stages of endometrial cancer where extrauterine disease is suspected on clinical examination or preliminary pelvic ultrasound, CT or MRI, establishing the extent of disease by PET/CT prior to surgery may greatly assist in deciding the extent of surgery over a standard staging laparotomy.
5. Imaging in planning adjuvant radiotherapy
Currently, the indications for postoperative adjuvant radiotherapy are [32]:
Patients with grade 1 and 2 histology with deep myometrial invasion and where lymph nodes were not evaluated.
Patients with grade 3, clear cell, or serous histology, irrespective of myometrial invasion, where lymph nodes were not evaluated.
All stage 3 and 4 patients.
All node-positive patients and grade 3 histologies with deep myometrial invasions are recommended to receive external beam pelvic radiotherapy (EBRT) or abdominopelvic radiotherapy, the so-called extended field radiotherapy (EFRT).
In the era of conformal radiotherapy, as a minimum, CT images with or without contrast in the treatment position are used for radiation planning. Often, enlarged nodes are treated with an additional radiation dose (nodal boost) for potentially better control of the disease. It can be difficult to delineate the “at risk” nodal target volume and determine the upper level of the radiation therapy field where only involved nodes were removed without the complete node dissection, unless this was a sentinel node. Similarly, in high-risk patients where nodes were not evaluated, the use of PET-CT, either fused with the planning CT on the treatment planning system or a planning PET-CT in the radiotherapy position is very useful. In the postoperative radiotherapy setting, the use of PET can result in modification of the treatment plan as additional disease becomes evident in up to 35% of patients [33].
Occasionally, in poorly differentiated and high-grade histology that may have had a close resection margin, postoperative soft tissue recurrences are seen in the interval between surgery and radiotherapy. Some of these patients may require an extra radiotherapy boost requiring interstitial brachytherapy. In such cases a pre-radiotherapy pelvic MRI is very useful for identifying the tumor requiring additional radiotherapy and its relation with surrounding normal tissues.
6. Imaging in surveillance and follow-up
The routine use of imaging in surveillance following initial treatment is not indicated. However, radiological imaging should be considered where a potentially salvageable recurrent disease is suspected clinically or in the post-therapeutic restaging of selected patients at high risk for relapse.
MRI can assist in identifying the extent of local recurrence and appropriateness for surgical resection and radiotherapy. PET–CT has the additional benefit of determining if the extent of recurrence is localized or disseminated. A long interval between the primary treatment and isolated recurrence is usually associated with a better prognosis.
In a retrospective analysis of 36 women previously treated for endometrial cancer, Belhocine et al. [34] found the sensitivity and specificity of PET–CT to be 96% and 78%, respectively. These authors reported that the use of fluorodeoxyglucose (FDG)-PET confirmed recurrence in 88% of the cases, which was useful in localizing the site of disease, and it detected asymptomatic recurrence in 12%. In 35% of patients with confirmed recurrence, additional metastatic sites were detected, significantly altering management. PET has more specificity, sensitivity, and accuracy compared with conventional imaging or tumor markers.
7. Imaging and treatment of recurrent endometrial cancer
Patients with lymphovascular space invasion and lymph node metastases are at high risk of recurrence [35]. Eighty-seven percent of recurrences are seen by three years from the time of primary surgery [36]. Twenty-five percent of all recurrences were solitary vaginal vault recurrences and approximately 60% had distant failure with or without locoregional recurrence [37].
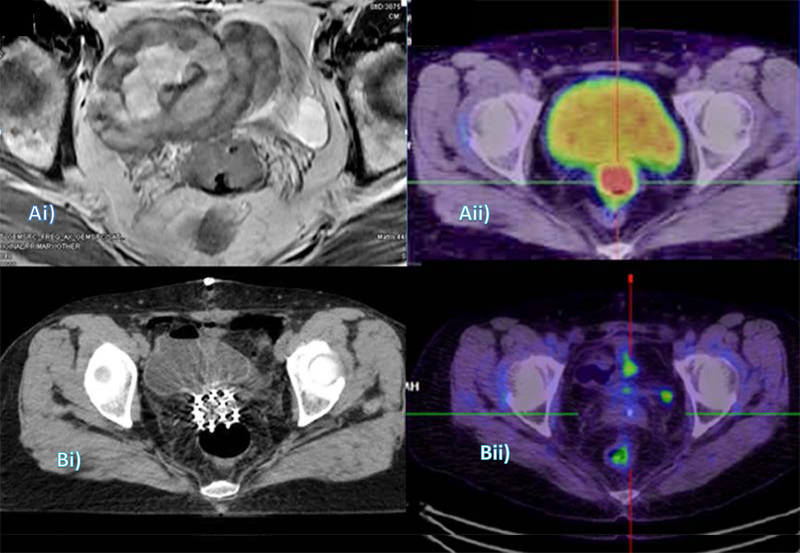
Solitary vaginal vault relapses can be successfully salvaged by surgery or combination of EBRT and intracavitary or interstitial brachytherapy [38,39]. The rate of successful salvage is substantially improved and treatment complication minimized by proper patient selection using PET/CT to exclude asymptomatic multiple metastases. MRI is particularly helpful in visualizing the treatment volume and its relationship to surrounding normal anatomical structures (Figure 8).
Figure 8.
Use of imaging in diagnosis, treatment planning, and restaging of recurrent endometrial cancer: (Ai) MRI (axial) shows the vaginal vault mass encased within loops of bowel; and (Aii) Corresponding slice on pre-radiotherapy PET-CT, performed to confirm isolated recurrence. (Bi) Radiotherapy planning CT with interstitial implant needles in situ; and (Bii) Six-month post-treatment restaging PET-CT.
Isolated para-aortic recurrences, although infrequent, are salvageable. Twenty-two of the 29 such recurrences could be salvaged, resulting in median survival of 3.5 (1–15) years [40].
8. Conclusion
The application of imaging in the management of endometrial cancer has become routine. It has resulted in better matching of patients with the appropriate treatment modality or a combination of treatment regimens, resulting in improved locoregional control and reduction in treatment-related morbidity. Imaging has also helped in determining the best salvage strategy for patients with recurrent endometrial cancer.
Synopsis:
Imaging in management of endometrial cancer has resulted in better matching of patients with the appropriate treatment modality or a combination of treatment regimens, resulting in improved locoregional control and reduction in treatment-related morbidity.
Footnotes
Conflict of interest
The authors have no conflicts of interest to declare.
References
- [1].Siegel RL, Miller KD, Jemal A. Cancer statistics, 2018. CA Cancer J Clin 2018;68(1):7–30. [DOI] [PubMed] [Google Scholar]
- [2].Talhouk A, McAlpine JN. New classification of endometrial cancers: the development and potential applications of genomic-based classification in research and clinical care. Gynecol Oncol Res Pract 2016;3:14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Dijkhuizen FP, Mol BW, Brolmann HA, Heintz AP. The accuracy of endometrial sampling in the diagnosis of patients with endometrial carcinoma and hyperplasia: a meta-analysis. Cancer 2000;89:1765–1772. [PubMed] [Google Scholar]
- [4].Abdaal A, Mushtaq Y, Khasati L, Moneim J, Khan F, Ahmed H, et al. Post-menopausal bleeding - Is transvaginal ultrasound a useful first-line investigation in tamoxifen users? Post Reprod Health 2018:2053369118755190. [DOI] [PubMed] [Google Scholar]
- [5].Creasman WT, Morrow CP, Bundy BN, Homesley HD, Graham JE, Heller PB. Surgical pathologic spread patterns of endometrial cancer. A Gynecologic Oncology Group Study. Cancer 1987;60(8 Suppl):2035–2041. [DOI] [PubMed] [Google Scholar]
- [6].ASTEC study group, Kitchener H, Swart AM, Qian Q, Amos C, Parmar MK. Efficacy of systematic pelvic lymphadenectomy in endometrial cancer (MRC ASTEC trial): a randomised study. Lancet 2009;373:125–136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Todo Y, Takeshita S, Okamoto K, Yamashiro K, Kato H. Implications of para-aortic lymph node metastasis in patients with endometrial cancer without pelvic lymph node metastasis. J Gynecol Oncol 2017;28:e59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Ayhan A, Tuncer ZS, Tuncer R, Yuce K, Kucukali T. Tumor status of lymph nodes in early endometrial cancer in relation to lymph node size. Eur J Obstet Gynecol Reprod Biol. 1995;60:61–63. [DOI] [PubMed] [Google Scholar]
- [9].Chi DS, Barakat RR, Palayekar MJ, Levine DA, Sonoda Y, Alektiar K, et al. The incidence of pelvic lymph node metastasis by FIGO staging for patients with adequately surgically staged endometrial adenocarcinoma of endometrioid histology. Int J Gynecol Cancer. 2008;18:269–273. [DOI] [PubMed] [Google Scholar]
- [10].Lewandowski G, Torrisi J, Potkul RK, Holloway RW, Popescu G, Whitfield G, et al. Hysterectomy with extended surgical staging and radiotherapy versus hysterectomy alone and radiotherapy in stage I endometrial cancer: A comparison of complication rates. Gynecol Oncol 1990:36:401–404. [DOI] [PubMed] [Google Scholar]
- [11].Tinga DJ, Timmer PR, Bouma J, Aalders JG: Prognostic significance of single versus multiple lymph node metastases in cervical carcinoma stage IB. Gynecol Oncol 1990;39:175–180. [DOI] [PubMed] [Google Scholar]
- [12].Frost JA, Webster KE, Bryant A, Morrison J. Lymphadenectomy for the management of endometrial cancer. Cochrane Database Syst Rev 2017;10:CD007585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Zahl Eriksson AG, Ducie J, Ali N, McGree ME, Weaver AL, Bogani G, et al. Comparison of a sentinel lymph node and a selective lymphadenectomy algorithm in patients with endometrioid endometrial carcinoma and limited myometrial invasion. Gynecol Oncol 2016;140:394–399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Ducie JA, Eriksson AGZ, Ali N, McGree ME, Weaver AL, Bogani G, et al. Comparison of a sentinel lymph node mapping algorithm and comprehensive lymphadenectomy in the detection of stage IIIC endometrial carcinoma at higher risk for nodal disease. Gynecol Oncol 2017;147:541–548. [DOI] [PubMed] [Google Scholar]
- [15].Cade TJ, Quinn MA, McNally OM, Neesham D, Pyman J, Dobrotwir A. Predictive value of magnetic resonance imaging in assessing myometrial invasion in endometrial cancer: is radiological staging sufficient for planning conservative treatment? Int J Gynecol Cancer 2010;20:1166–1169. [DOI] [PubMed] [Google Scholar]
- [16].Mariani A, Webb MJ, Keeney GL, Haddock MG, Calori G, Podratz KC. Low-risk corpus cancer: is lymphadenectomy or radiotherapy necessary? Am J Obstet Gynecol 2000;182:1506–1519. [DOI] [PubMed] [Google Scholar]
- [17].Epstein E, Blomqvist L. Imaging in endometrial cancer. Best Pract Res Clin Obstet Gynaecol 2014;28:721–739. [DOI] [PubMed] [Google Scholar]
- [18].Traen K, Holund B, Mogensen O. Accuracy of preoperative tumor grade and intraoperative gross examination of myometrial invasion in patients with endometrial cancer. Acta Obstet Gynecol Scand. 2007;86:739–741. [DOI] [PubMed] [Google Scholar]
- [19].Leone FP, Timmerman D, Bourne T, Valentin L, Epstein E, Goldstein SR, et al. Terms, definitions and measurements to describe the sonographic features of the endometrium and intrauterine lesions: a consensus opinion from the International Endometrial Tumor Analysis (IETA) group. Ultrasound Obstet Gynecol 2010;35:103–112. [DOI] [PubMed] [Google Scholar]
- [20].Smith-Bindman R, Kerlikowske K, Feldstein VA, Subak L, Scheidler J, Segal M, et al. Endovaginal ultrasound to exclude endometrial cancer and other endometrial abnormalities. JAMA 1998;280:1510–1517. [DOI] [PubMed] [Google Scholar]
- [21].Reich O, Winter R, Pickel H, Tamussino K, Haas J, Petru E. Does the size of pelvic lymph nodes predict metastatic involvement in patients with endometrial cancer? Int J Gynecol Cancer 1996;6:445–447. [Google Scholar]
- [22].Cabrita S, Rodrigues H, Abreu R, Martins M, Teixeira L, Marques C, et al. Magnetic resonance imaging in the preoperative staging of endometrial carcinoma. Eur J Gynaecol Oncol 2008;29:135–137. [PubMed] [Google Scholar]
- [23].Sanjuan A, Escaramis G, Ayuso JR, Roman SM, Torne A, Ordi J, et al. Role of magnetic resonance imaging and cause of pitfalls in detecting myometrial invasion and cervical involvement in endometrial cancer. Arch Gynecol Obstet. 2008;278:535–539. [DOI] [PubMed] [Google Scholar]
- [24].Chung HH, Kang SB, Cho JY, Kim JW, Park NH, Song YS, et al. Accuracy of MR imaging for the prediction of myometrial invasion of endometrial carcinoma. Gynecol Oncol 2007;104:654–659. [DOI] [PubMed] [Google Scholar]
- [25].Kinkel K, Forstner R, Danza FM, Oleaga L, Cunha TM, Bergman A, et al. Staging of endometrial cancer with MRI: guidelines of the European Society of Urogenital Imaging. Eur Radiol 2009;19:1565–1574. [DOI] [PubMed] [Google Scholar]
- [26].Rauch GM, Kaur H, Choi H, Ernst RD, Klopp AH, Boonsirikamchai P, et al. Optimization of MR imaging for pretreatment evaluation of patients with endometrial and cervical cancer. Radiographics 2014;34:1082–1098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Lin G, Ho KC, Wang JJ, Ng KK, Wai YY, Chen YT, et al. Detection of lymph node metastasis in cervical and uterine cancers by diffusion-weighted magnetic resonance imaging at 3T. J Magn Reson Imaging 2008;28:128–135. [DOI] [PubMed] [Google Scholar]
- [28].Narayan K, Hicks RJ, Jobling T, Bernshaw D, McKenzie AF. A comparison of MRI and PET scanning in surgically staged loco- regionally advanced cervical cancer: potential impact on treatment. Int J Gynecol Cancer 2001;11:263–271. [DOI] [PubMed] [Google Scholar]
- [29].Kitajima K, Murakami K, Yamasaki E, Kaji Y, Sugimura K. Accuracy of integrated FDG-PET/contrast-enhanced CT in detecting pelvic and paraaortic lymph node metastasis in patients with uterine cancer. Eur Radiol 2009;19:1529–1536. [DOI] [PubMed] [Google Scholar]
- [30].Abu-Rustum NR. Update on sentinel node mapping in uterine cancer: 10-year experience at Memorial Sloan–Kettering Cancer Center. J Obstet Gynaecol Res. 2014;40:327–334. [DOI] [PubMed] [Google Scholar]
- [31].Barlin JN, Khoury-Collado F, Kim CH, Leitao MM Jr, Chi DS, Sonoda Y, et al. The importance of applying a sentinel lymph node mapping algorithm in endometrial cancer staging: beyond removal of blue nodes. Gynecol Oncol 2012;125:531–535. [DOI] [PubMed] [Google Scholar]
- [32].Meyer LA, Bohlke K, Powell MA, Fader AN, Franklin GE, Lee LJ, et al. Postoperative Radiation Therapy for Endometrial Cancer: American Society of Clinical Oncology Clinical Practice Guideline Endorsement of the American Society for Radiation Oncology Evidence-Based Guideline. J Clin Oncol 2015;33:2908–2913. [DOI] [PubMed] [Google Scholar]
- [33].Simcock B, Narayan K, Drummond E, Bernshaw D, Wells E, Hicks RJ. The Role of Positron Emission Tomography/Computed Tomography in Planning Radiotherapy in Endometrial Cancer. Int J Gynecol Cancer. 2015;25:645–649. [DOI] [PubMed] [Google Scholar]
- [34].Belhocine T, De Barsy C, Hustinx R, Willems-Foidart J. Usefulness of (18)F-FDG PET in the post-therapy surveillance of endometrial carcinoma. Eur J Nucl Med Mol Imaging. 2002;29:1132–1139. [DOI] [PubMed] [Google Scholar]
- [35].Narayan K, Khaw P, Bernshaw D, Mileshkin L, Kondalsamy-Chennakesavan S. Prognostic significance of lymphovascular space invasion and nodal involvement in intermediate- and high-risk endometrial cancer patients treated with curative intent using surgery and adjuvant radiotherapy. Int J Gynecol Cancer 2012;22:260–266. [DOI] [PubMed] [Google Scholar]
- [36].Sohaib SA, Houghton SL, Meroni R, Rockall AG, Blake P, Reznek RH. Recurrent endometrial cancer: patterns of recurrent disease and assessment of prognosis. Clin Radiol 2007;62:28–34; discussion 35–36. [DOI] [PubMed] [Google Scholar]
- [37].Sorbe B, Juresta C, Ahlin C. Natural history of recurrences in endometrial carcinoma. Oncol Lett 2014;8:1800–1806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Sorbe B, Soderstrom K. Treatment of vaginal recurrences in endometrial carcinoma by high-dose-rate brachytherapy. Anticancer Res 2013;33:241–247. [PubMed] [Google Scholar]
- [39].Hardarson HA, Heidemann LN, dePont Christensen R, Mogensen O, Jochumsen KM. Vaginal vault recurrences of endometrial cancer in non-irradiated patients - Radiotherapy or surgery. Gynecol Oncol Rep 2015;11:26–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Narayan K, Lin MY, Bernshaw D, Khaw P, Kondalsamy-Chennakesvan S: Tailoring Adjuvant Radiotherapy in Endometrial Cancer. Indian Journal of Gynecologic Oncology 2017;15:45. [Google Scholar]