Abstract
The aim of this study was to analyze the relationship between the percent of euploid embryo and the tolerance of embryo biopsy in preimplantation genetic screening (PGS).
PubMed and trial registers were searched for clinical studies that patients were randomized to the PGS group or the control group from 1995 to October 2017. The patients of advanced maternal age, repeated implantation failure, and good prognosis with or without PGS in randomized controlled trials (RCTs) were collected.
Original data from 9 RCT studies comparing in-vitro fertilization with and without PGS including 1642 patients were obtained and they were divided into 3 subgroups according to the percent of euploid embryo. PGS significantly increased live birth babies per embryo transferred (risk ratio: 2.98, 95% confidence interval: 1.54–5.75) in ≤30% of euploid embryo subgroups and but in other 2 groups, PGS has no effect. Significant negative correlation was found between the percent of euploid embryo and the tolerance of embryo biopsy in PGS (r = 0.80, P = 0.010)
The tolerance of embryo biopsy in PGS was associated negatively with the percent of euploid embryo. There was a beneficial effect when PGS was used in the patients with the lowest percent of euploid embryo.
Keywords: embryo biopsy, euploid embryo, meta-analysis, PGS, systematic review
1. Introduction
There are higher probability that aneuploidies in pregnancies occur in the women of advanced maternal age (AMA), with a history of recurrent miscarriage or repeated implantation failure (RIF), and with a partner with low sperm quality.[1–5] The aneuploidy may result in miscarriage, stillbirth, or the birth of a child with chromosomal disorder such as Down syndrome.[6,7] Preimplantation genetic screening (PGS) evaluates 1 or 2 cells from day 3 embryos created through IVF and discovered the chromosome abnormality embryos, which can screen for euploid embryos and remove aneuploid ones and in theory can increase live births rate.[8] However, some articles reported PGS was not helpful for live birth rate or disadvantageous and the biopsy and mosaicism of embryo are the major reasons.[9,10]
In biopsy, an embryo is taken out of the incubator for a couple of minutes, made a hole in the zona pellucida via mechanical dissection, acidic Tyrode solution or a laser and ≥1cells aspirated from this embryo. Those additional operations could be harmful for an embryo.[11] The chromosomal mosaicism is the phenomenon that not all cells in an embryo have the same chromosomal content, with mosaicism rates varying from 15% to >90%, which is highly relevant for the efficacy of PGS.[12–14] With female age increasing, the frequency of abnormal chromosome and mosaicism becomes higher, as well as the embryo quality becomes poorer. Similarly, their tolerance of additional operations perhaps decreases. The damage of embryos after additional operations perhaps surpass the beneficial effect of PGS with the decreasing percent of euploid embryo, which leads to no effect of PGS in the total process. AMA and RIF of aneuploidy rates were 20% to 50% in these articles[14–22] and there was not a reference value. However, there has not been some related data to analyze the relationships between the percent of euploid embryo and the tolerance of embryo biopsy in PGS.
2. Method
2.1. Search strategy and data extraction
We performed a literature search in PubMed and databases for registration of randomized controlled trials (RCTs) from 1995 to October 2015 with the following terms: (preimplantation genetic screening OR aneuploidy screening OR preimplantation testing OR embryo screening) and Randomized Controlled Trial. An RCT about PGS used to screen the euploid embryo and not for AMA, RIF, or the general population will be selected.
2.2. Eligibility criteria
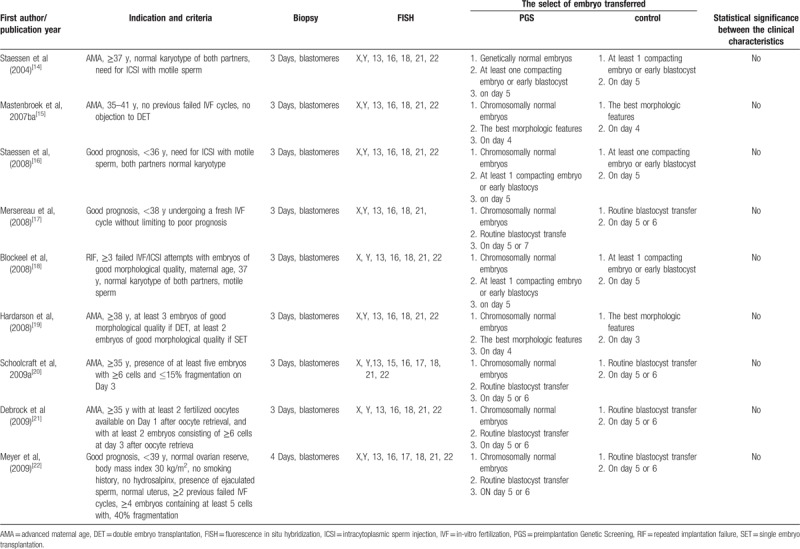
We collected randomized clinical trials of women undergoing in vitro fertilization, receiving embryo transfers with previous PGS compared with women without PGS. Main outcomes of interest for the review were those related with the percent of euploid embryo, live birth rates per transfer, and live birth babies per embryo; live birth babies per embryo and embryo tolerance after PGS in theory were assessed as the theoretical calculation of data (Table 1).[14–22] We assessed trials’ methodological quality paying special attention to the generation of the randomization sequence, the allocation concealment adequacy, the blinding of investigators, patients and outcome assessment, and the reporting of follow-up.
Table 1.
Characteristics of studies included in a systematic review and meta-analysis of RCTs.
2.3. Data acquisition
Two authors (XJ and CZ) screened the electronic searches for eligible articles by reading the title and abstract. As the controversy occurred, the third author (RZ) must check the articles. The ineligible articles were confirmed and the reasons were signed in it. Two authors (XJ and RZ) read the eligible articles in detail and find the outcomes. If necessary, we contacted the corresponding author of a report in attempt to retrieve missing data.
2.4. Outcome measures
The primary aim of this study was to analyze the relationships between the percent of euploid embryo and the tolerance of embryo biopsy in PGS. The percent of euploid embryo were firstly obtained. Live birth rate per transfer is as the effect of PGS. As seen in Figure 1, there are 2 ideal situations and we can calculate live birth rate per embryo and embryo tolerance after PGS in theory. The details are shown as follows:
Figure 1.
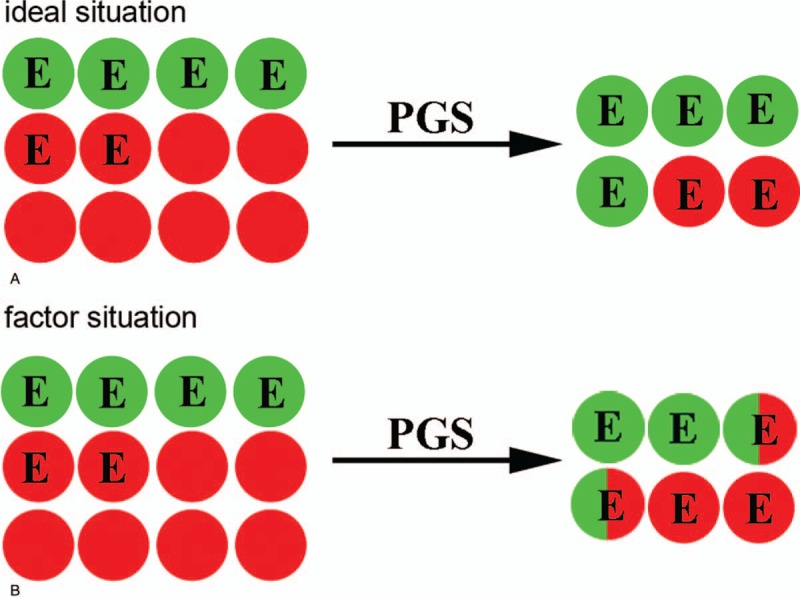
Two situations of PGS, live birth embryo marked by green rounds and other marked by red one. E is the abbreviation for euploid embryo. (A) Ideal situation: all euploid embryos can be successfully screened out and the embryos are not damaged by addition operations and misdiagnosis does not occur. (B) Factor situation: the embryo is damaged by additional operations, which leads the live birth embryos to destroy and not to be selected. PGS = preimplantation genetic screening.
2 ideal situations of live birth rate per embryo is 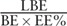 and
and 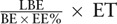 , respectively, where LBE indicates live birth embryo; BE indicates biopsy embryo; EE% indicates percent of euploid embryo; and ET indicates embryo tolerance after PGS.
, respectively, where LBE indicates live birth embryo; BE indicates biopsy embryo; EE% indicates percent of euploid embryo; and ET indicates embryo tolerance after PGS.
2.5. Statistical analysis
We calculated risk ratios (RRs) with 95% confidence intervals (CIs) for each individual trial. Eight RCTs were divided into 3 subgroups according to different percent of euploid embryo. The fixed-effect model was used to combine data for each indication separately and to combine all included studies. Statistical heterogeneity between results of studies was examined by inspecting the scatter in the data points on the graphs and the overlap of CIs, and by checking the I2 statistic. A value of ≥50% was considered to indicate substantial heterogeneity. In case of substantial heterogeneity, the random-effects model was used instead of the fixed-effect model. Data were analyzed according to the intention-to-treat principle. Revman Software (Version 5, The Cochrane Collaboration) was used to combine data for meta-analysis. The correlation analyses between sex ratios and implantation rate and between sex ratios and some clinical characteristics were performed by Pearson correlation coefficient.
3. Result
3.1. Study exclusions and inclusions
The literature search produced a list of 534 reports and after reading the title and abstracts, 495 studies were excluded. We obtained 39 full articles that were screened further and 15 RCT studies for PGS were potentially eligible (Fig. 1). All trials used fluorescence in situ hybridization (FISH) to classify embryo as euploid or aneuploidy, and CCS was excluded. After detailed analyzing and assessing the above RCTs, 9 studies were selected in the systematic review. Two reports were excluded because they were quasirandomized and the treatment options were on the basis of the couples decision.[23,24] Two studies provided insufficient data to assess the methodological quality, for example, the percent of euploid embryo.[25,26] There were overlapping data between 2 trails[20,27]; the early publication date was excluded. We hypothesize the percent of euploid embryo was the same between the PGS and control group because there was no statistical difference in age, the number of embryo transfers, oocytes retrieved, fertilization rate, and some baseline characteristics. One trial was excluded because the criterion of RIF and AMA was different with other trails.[28] The characteristics and quality features of the selected studies are shown in the Tables 1 and 2. There are 9 trials about PGS that are divided into 3 main groups, such as 4 studies to women of AMA, 3 studies to good prognosis patients, and 1 study to RIF.
Table 2.
Clinic outcomes of 8 RCTs.
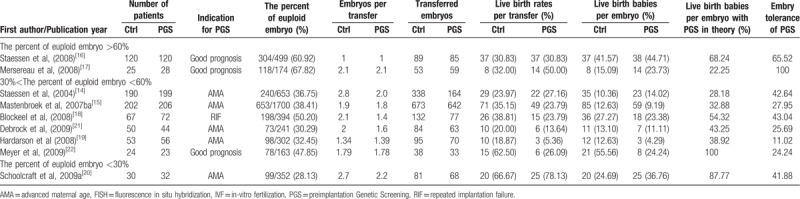
3.2. The different percent of euploid embryo
In the PGS groups of 9 RCTs, total 4478 embryos were successfully to biopsy on the cleavage stage and FISH was used to analyze the chromosome of 1–2 blastomeres; 41.56% (1861/4478) of embryos were chromosomally normal (euploid). The percent of euploid embryo were 35.77% (1163/3251, 28.13% to 38.41%), 50.25% (198/394), and 59.80% (500/836, 47.85% to 67.82%), respectively in AMA, RIF, and good prognosis. In this article, we divided the 9 trails into 3 subgroups via the percent of euploid embryos instead of the indication of PGS (Fig. 2).
Figure 2.
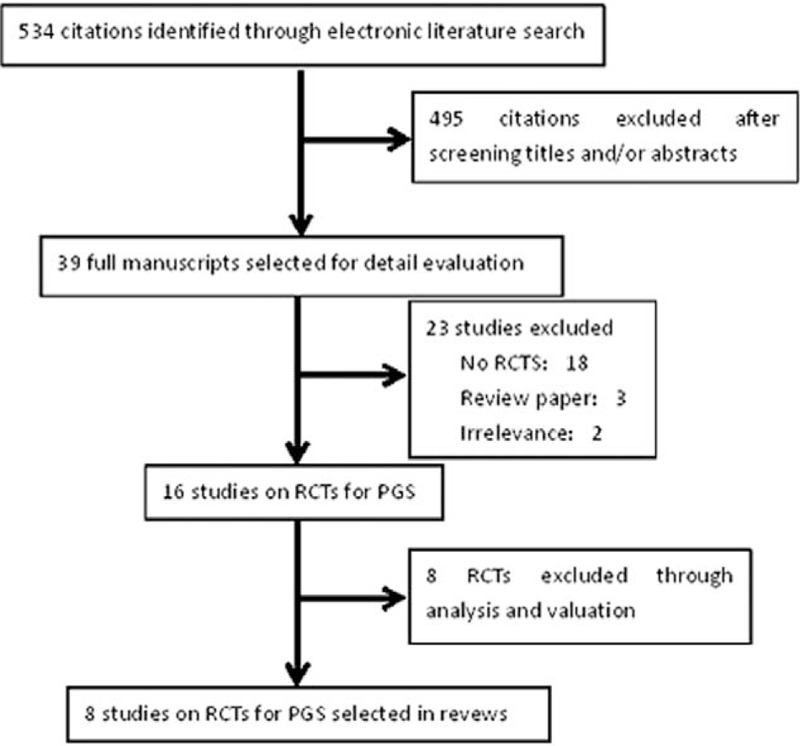
Flow chart of search for RCTs on PGS. PGS = preimplantation genetic screening, RCT = randomized controlled trial.
3.3. The ideal effect of PGS
PGS can detect abnormal copy numbers of chromosomes, or aneuploidies and select euploid embryos to transfer, so in theory it can improve the clinic outcomes. The ideal effect of PGS is shown in Figure 1. In fact, the damage of biopsy can cause the loss of the viable embryo, which is shown in Figure 2. In Table 2, we can calculate live birth rate per embryo and embryo tolerance after PGS in theory
3.4. The live birth rate per embryo
There were 9 RCTs (2731 participants) which reported the live birth, multiple pregnancies, and the number of transfer embryos, so we can calculate live birth rates per embryo We observed a trend toward increase in the live embryos rate via the pooled data analysis (n = 2844, RR = 0.97; 95% CI, 0.82–1.15; P = .76), whereas there was no significant change and there was substantial heterogeneity between studies (I2 = 69%).
Two trails (286 participants) were included in “the percent of euploid embryo >60%” subgroup as shown in Figure 2. The live birth rate per embryo was 36.1% (52 of 144) and 31.6% (45 of 142), respectively, in the control and PGS group and there was no significant change (P = .33). It is the meta-analysis (n = 286, RR = 1.17, 95% CI: 0.85–1.60) and there was no significant heterogeneity (I2 = 0%, P = .38).
Six trials (1498 participants) were included in the “30%<the percent of euploid embryo<60%” subgroup. The live birth rate per embryo was 11.25% (118/1049) and 14.26% (194/1360), respectively in the control and PGS group. PGS significantly decreased live birth rate per embryo (n = 1498; RR = 0.80, 95%, CI: 0.80–0.98).
Single trial (149 participants) was included when evaluating the subgroup “the percent of euploid embryo<30%”, which showed a significant difference in live birth rate per embryo between the PGS group (36.8%, 25/68) and the control group (12.3%, 10/81) (n = 149; RR = 2.98, 95%, CI: 1.54–5.75) (Fig. 2). This date indicated that when the percent of euploid embryo was <30%, PGS could increase live birth rate per embryo ().
Figure 3.
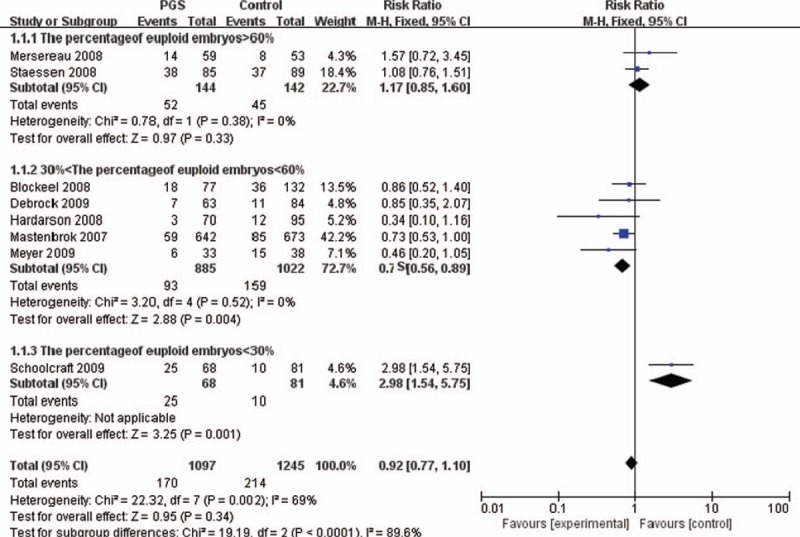
The effect of PGS on the live birth rate per embryo in different percent of euploid embryo. CI = confidence interval, PGS = preimplantation genetic screening
3.5. Embry tolerance after PGS
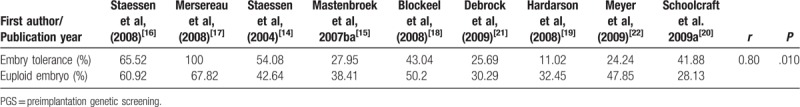
With age, the quality and euploid of embryo decrease and the chromosome mosaicism increases, which weaken the embryos to tolerance of additional operations. In this article, we combined 9 RCTs to analyze the relationship between the percent of euploid embryo and embryo tolerance after PGS (Table 3).[14–22] Here, we investigated there was a positive correlation between them (r = 0.80, P = .010). Although the date was not from the same laboratory, the results provided some message that the embryos’ damage should occur in the PGS. The result needs larger sample analysis to further verify.
Table 3.
The relationship between the percent of euploid embryo and embryo tolerance after PGS.
4. Discussion
A biopsy of PGS is not a noninvasive examination and those additional operations for the embryos make them damage. In the process of biopsy, those additional operations such as taking an embryo out of the incubator, making a hole in the zona pellucid and aspirating one more cell and so on often occur.[8] Only embryo enduring those additional operations has the opportunity grow as a healthy live baby. It is a consensus that biopsy can influence the efficiency of PGS.[11,29] In medicine, a side effect occurs along with diagnosis and treatment, for example, drug therapy and radiodiagnosis. Gonal-F of main effects is stimulating follicle develop and its side effect is ovarian cyst and ovarian hyperstimulation syndrome (OHSS). PGS is also a method of diagnosis and treatment and its side effect exists naturally which is caused by biopsy, failure rate, and embryo mosaicism.[10] The biopsy is an additional and noninvasive operation. The age-related decline in fertility is attributable to both a decrease in conception rates and an increase in pregnancy loss rates.[30,31] This decline begins at around age 30, and accelerates after age 35, such that fertility is close to zero by the time a woman reaches age 45.[31] The percentage of aneuploid embryos is increased with AMA, which was the main reason of implantation failure and miscarriage. With the decreasing percent of euploid embryo, the tolerance of additional operations also decreases. However, the effect of biopsy alone on pregnancy rates has never been properly studied.
The indication of PGS is the high aneuploid rates in the transferred embryos. With higher aneuploid rates, the PGS efficiency is higher. Up to now, there was no article that reported the relationship between the percent of aneuploid embryos and the effect of PGS. All researchers paid much attention to the groups, AMA or RIF, who perhaps produced higher percent of aneuploid embryos.[10] However, the aneuploid rate was 30% to 50% in AMA, according to 8 articles included in this study, whereas in good prognosis patients, it is about 60%, and not all of AMAs have high aneuploid rates, so there was a cross-section between AMA and good prognosis patients in the aneuploid rate. So, it is unreasonable that AMA and RIF are regarded as the indications of PGS. Here, we first concentrate our attention on the percent of aneuploid embryos and PGS of efficiency. The subgroup was performed via the percent of euploid embryos. When the percent of euploid embryos is at the lowest level (<30%), there is a beneficial effect of PGS on live birth rates per embryo. But the percent of euploid embryos is difficult to foreknow based on AMA and RIF.
In a conclusion, with the decreasing percent of euploid embryo, embryo tolerance after PGS also decreased, which influenced the effect of PGS. However, the percent of euploid embryo is from 28.3% to 50.2% and is difficult to assess in AMA and RIF before biopsy, so PGS is used with caution. This finding was helpful to better understand the effect of PGS in different percent of euploid embryo.
Author contributions
Conceptualization: Chenggui Zhao.
Data curation: Chenggui Zhao, Wei Xu, Rui Zhang.
Formal analysis: Chenggui Zhao, Wei Xu.
Funding acquisition: Chenggui Zhao.
Investigation: Chenggui Zhao.
Methodology: Xinglu Jiang, Chenggui Zhao, Wei Xu.
Project administration: Xinglu Jiang.
Resources: Wei Xu, Rui Zhang.
Software: Chenggui Zhao, Wei Xu, Rui Zhang.
Supervision: Xinglu Jiang, Chenggui Zhao.
Validation: Rui Zhang.
Writing – original draft: Xinglu Jiang, Chenggui Zhao, Wei Xu.
Writing – review & editing: Xinglu Jiang, Chenggui Zhao, Wei Xu.
Footnotes
Abbreviations: AMA = advanced maternal age, BE = biopsy embryo, CI = confidence intervals, EE% = percent of euploid embryo, ET = embryo tolerance after PGS, FISH = fluorescence in situ hybridization, LBE = live birth embryo, PGS = preimplantation genetic screening, RCTs = randomized controlled trials, RIF = repeated implantation failure, RR = risk ratios.
XJ and CZ contributed equally to this work.
The authors report no conflicts of interest.
This work was supported by Medical Science and Technology Development Foundation of Nanjing Department of Health (YKK17281).
Ethical approval: This article does not contain any studies with human participants or animals performed by any of the authors.
References
- [1].Nagvenkar P, Zaveri K, Hinduja I. Comparison of the sperm aneuploidy rate in severe oligozoospermic and oligozoospermic men and its relation to intracytoplasmic sperm injection outcome. Fertil Steril 2005;84:925–31. [DOI] [PubMed] [Google Scholar]
- [2].Ramasamy R, Scovell JM, Kovac JR, et al. Fluorescence in situ hybridization detects increased sperm aneuploidy in men with recurrent pregnancy loss. Fertil Steril 2015;103:906–9. e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Sullivan AE, Silver RM, LaCoursiere DY, et al. Recurrent fetal aneuploidy and recurrent miscarriage. Obstet Gynecol 2004;104:784–8. [DOI] [PubMed] [Google Scholar]
- [4].Voullaire L, Collins V, Callaghan T, et al. High incidence of complex chromosome abnormality in cleavage embryos from patients with repeated implantation failure. Fertil Steril 2007;87:1053–8. [DOI] [PubMed] [Google Scholar]
- [5].Waltman LA, Eckel-Passow JE, Sharma RG, et al. Advanced maternal age in polyploidy with concurrent aneuploidy. Am J Med Genet A 2013;161A:1200–2. [DOI] [PubMed] [Google Scholar]
- [6].Reddy UM, Page GP, Saade GR, et al. Karyotype versus microarray testing for genetic abnormalities after stillbirth. N Engl J Med 2012;367:2185–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Ricke RM, van Deursen JM. Aneuploidy in health, disease, and aging. J Cell Biol 2013;201:11–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].De Vos A, Van Steirteghem A. Aspects of biopsy procedures prior to preimplantation genetic diagnosis. Prenat Diagn 2001;21:767–80. [DOI] [PubMed] [Google Scholar]
- [9].Los FJ, Van Opstal D, van den Berg C. The development of cytogenetically normal, abnormal and mosaic embryos: a theoretical model. Hum Reprod Update 2004;10:79–94. [DOI] [PubMed] [Google Scholar]
- [10].Mastenbroek S, Twisk M, van der Veen F, et al. Preimplantation genetic screening: a systematic review and meta-analysis of RCTs. Hum Reprod Update 2011;17:454–66. [DOI] [PubMed] [Google Scholar]
- [11].Tarin JJ, Conaghan J, Winston RM, et al. Human embryo biopsy on the 2nd day after insemination for preimplantation diagnosis: removal of a quarter of embryo retards cleavage. Fertil Steril 1992;58:970–6. [DOI] [PubMed] [Google Scholar]
- [12].Taylor TH, Gitlin SA, Patrick JL, et al. The origin, mechanisms, incidence and clinical consequences of chromosomal mosaicism in humans. Hum Reprod Update 2014;20:571–81. [DOI] [PubMed] [Google Scholar]
- [13].van Echten-Arends J, Mastenbroek S, Sikkema-Raddatz B, et al. Chromosomal mosaicism in human preimplantation embryos: a systematic review. Hum Reprod Update 2011;17:620–7. [DOI] [PubMed] [Google Scholar]
- [14].Staessen C, Platteau P, Van Assche E, et al. Comparison of blastocyst transfer with or without preimplantation genetic diagnosis for aneuploidy screening in couples with advanced maternal age: a prospective randomized controlled trial. Hum Reprod 2004;19:2849–58. [DOI] [PubMed] [Google Scholar]
- [15].Mastenbroek S, Twisk M, van Echten-Arends J, et al. In vitro fertilization with preimplantation genetic screening. N Engl J Med 2007;357:9–17. [DOI] [PubMed] [Google Scholar]
- [16].Staessen C, Verpoest W, Donoso P, et al. Preimplantation genetic screening does not improve delivery rate in women under the age of 36 following single-embryo transfer. Hum Reprod 2008;23:2818–25. [DOI] [PubMed] [Google Scholar]
- [17].Mersereau JE, Pergament E, Zhang X, et al. Preimplantation genetic screening to improve in vitro fertilization pregnancy rates: a prospective randomized controlled trial. Fertil Steril 2008;90:1287–9. [DOI] [PubMed] [Google Scholar]
- [18].Blockeel C, Schutyser V, De Vos A, et al. Prospectively randomized controlled trial of PGS in IVF/ICSI patients with poor implantation. Reprod Biomed Online 2008;17:848–54. [DOI] [PubMed] [Google Scholar]
- [19].Hardarson T, Hanson C, Lundin K, et al. Preimplantation genetic screening in women of advanced maternal age caused a decrease in clinical pregnancy rate: a randomized controlled trial. Hum Reprod 2008;23:2806–12. [DOI] [PubMed] [Google Scholar]
- [20].Schoolcraft WB, Katz-Jaffe MG, Stevens J, et al. Preimplantation aneuploidy testing for infertile patients of advanced maternal age: a randomized prospective trial. Fertil Steril 2009;92:157–62. [DOI] [PubMed] [Google Scholar]
- [21].Debrock S, Melotte C, Spiessens C, et al. Preimplantation genetic screening for aneuploidy of embryos after in vitro fertilization in women aged at least 35 years: a prospective randomized trial. Fertil Steril 2010;93:364–73. [DOI] [PubMed] [Google Scholar]
- [22].Meyer LR, Klipstein S, Hazlett WD, et al. A prospective randomized controlled trial of preimplantation genetic screening in the “good prognosis” patient. Fertil Steril 2009;91:1731–8. [DOI] [PubMed] [Google Scholar]
- [23].Gianaroli L, Magli MC, Ferraretti AP, et al. Preimplantation diagnosis for aneuploidies in patients undergoing in vitro fertilization with a poor prognosis: identification of the categories for which it should be proposed. Fertil Steril 1999;72:837–44. [DOI] [PubMed] [Google Scholar]
- [24].Gianaroli L, Magli MC, Munne S, et al. Will preimplantation genetic diagnosis assist patients with a poor prognosis to achieve pregnancy? Hum Reprod 1997;12:1762–7. [DOI] [PubMed] [Google Scholar]
- [25].Werlin L, Rodi I, DeCherney A, et al. Preimplantation genetic diagnosis as both a therapeutic and diagnostic tool in assisted reproductive technology. Fertil Steril 2003;80:467–8. [DOI] [PubMed] [Google Scholar]
- [26].Jansen RP, Bowman MC, de Boer KA, et al. What next for preimplantation genetic screening (PGS)? Experience with blastocyst biopsy and testing for aneuploidy. Hum Reprod 2008;23:1476–8. [DOI] [PubMed] [Google Scholar]
- [27].Stevens J, Wale P, Surrey ES, et al. Is aneuploidy screening for patients aged 35 or over beneficial? A prospective randomized trial. Fertil Steril 2004;82:S249.15363742 [Google Scholar]
- [28].Rubio C, Bellver J, Rodrigo L, et al. Preimplantation genetic screening using fluorescence in situ hybridization in patients with repetitive implantation failure and advanced maternal age: two randomized trials. Fertil Steril 2013;99:1400–7. [DOI] [PubMed] [Google Scholar]
- [29].Hardy K, Martin KL, Leese HJ, et al. Human preimplantation development in vitro is not adversely affected by biopsy at the 8-cell stage. Hum Reprod 1990;5:708–14. [DOI] [PubMed] [Google Scholar]
- [30].Stoop D, Cobo A, Silber S. Fertility preservation for age-related fertility decline. Lancet 2014;384:1311–9. [DOI] [PubMed] [Google Scholar]
- [31].American College of O, Gynecologists Committee on Gynecologic P, Practice Committee of the American Society for Reproductive M. Female age-related fertility decline. Committee Opinion No. 589. Obstet Gynecol 2014;123:719–21. [DOI] [PubMed] [Google Scholar]


