Abstract
Rationale:
Ameloblastoma is generally characterized as a benign tumor originating in odontogenic epithelium. However, few cases of metastatic malignant ameloblastoma have also been reported. Due to the low incidence of malignant ameloblastoma, there is no established treatment regimen. To explore effective treatment for malignant ameloblastoma, we reported this case study.
Patients concerns:
This report described a case of a 28-year-old malignant ameloblastoma female patient with multiple metastasis (brain and lung).
Diagnoses:
The patient presented ameloblastoma of the left mandible in 2012. Three years later, local recurrence and brain metastasis was observed during a follow-up examination. Five years later, malignant ameloblastoma was detected by imaging and immunohistochemistry in the bilateral multiple pulmonary nodules and mediastinal lymph nodes.
Interventions:
The patient was initially treated with tumor resection. Three years later after local recurrence and brain metastasis, she was accepted the extensive mandibulectomy supplemented with brain stereotactic body radiotherapy (SBRT). When diagnosed with pulmonary metastasis, the patient received combined chemotherapy regimen of MAID (mesna, adriamycin, ifosfamide and dacarbazine) for 6 cycles.
Outcomes:
The efficacy evaluation was partial remission (PR) after the 6 cycles of MAID. The last patient follow-up was July 24th 2018, and no evidence of progression was observed. The progression-free survival (PFS) of the patient was more than 9 months.
Lessons:
Surgical resection is the optimal treatment for locally recurrent ameloblastoma. SBRT may be an effective treatment for unresectable oligometastasis of malignant ameloblastoma. Finally, combined chemotherapy of MAID showed encouraging effects in the management of metastatic malignant ameloblastoma.
Keywords: adriamycin, chemotherapy, dacarbazine, ifosfamide, MAID, mesna, metastatic malignant ameloblastoma
1. Introduction
Ameloblastoma is generally characterized as a benign tumor arising from odontogenic epithelium. The median age of diagnosis is 36 years. More than 80% ameloblastomas originate in the mandible.[1] In 2016, the fourth edition of WHO classified ameloblastoma into 2 types: the unicystic ameloblastoma and the extra osseous/peripheral ameloblastoma.[2] This was simplified compared with the four types of 2005 WHO classification.[3] Ameloblastoma grows slowly with local invasiveness. The highest recurrence type, with a rate between 60% and 80%, is solid/multicystic ameloblastoma according to 2005 WHO classification.[4] The main treatment for ameloblastoma is surgery, including both curettage and radical surgery.[5]
Although ameloblastoma has benign biological characteristics, distant metastases can occur in cases of malignant ameloblastoma. The most likely metastatic location of malignant ameloblastoma is the lung.[6] Due to its low incidence rate, there is no standard therapy for metastatic ameloblastoma. Here, we reported a case of malignant ameloblastoma with lung and brain metastases.
2. Case presentation
A 28-year-old female with a history of ameloblastoma of the left mandible underwent local tumor curettage in 2012. Either local recurrence or distant metastasis had not been found until 2015. Brain metastasis and local recurrence were confirmed by magnetic resonance imaging (MRI) and contrast computer tomography (CT). The patient received left extensive mandibulectomy and brain stereotactic body radiotherapy (SBRT) at the dose of 24.4Gy. The disease was stable until bilateral multiple pulmonary nodules and mediastinal lymph nodes were identified by chest CT in November 2017. After admitting the patient into our hospital, malignant ameloblastoma was detected in the pulmonary nodule biopsy by Hematoxylin and eosin (H&E) staining and immunohistochemistry (Figs. 1 and 2). We performed whole exon detection for metastatic lesion specimens by next generation sequencing (NGS). The detection included somatic mutation, germline mutation, microsatellite instability and tumor mutation load. A total of 7 somatic mutations were revealed including BRAF (c.1799T>A, p.V600E EX15 41.2%), MYCN (c.131>T, p.P44L EX2 40.2%), MLL2(c.3045C[2>1], p.L1016∗fs∗1 EX11 35.9%), ARIDIA(c.3715+1G>A IVS14 34.5%), MLL2(c.6392C[5>6], p.A2133Rfs∗22 EX31 33.5%), RUNX1(c.364G[4>6], p.D123Gf∗11 EX5 31.5%) and ASXL1(c.1927G[8>9], p.G646Wfs∗12 EX13 29.8%). Germline mutations were not detected. Microsatellite status was stable with low tumor mutation load (7.0 Muts/Mb).
Figure 1.
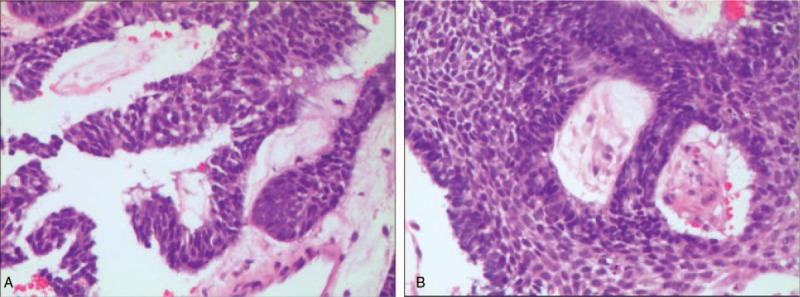
(a and b). Hematoxylin and eosin (H&E) staining of ameloblastoma lung metastasis tissues under microscopically (original magnification×200). Microscopic cell morphology was in accordance with ameloblastoma cell.
Figure 2.
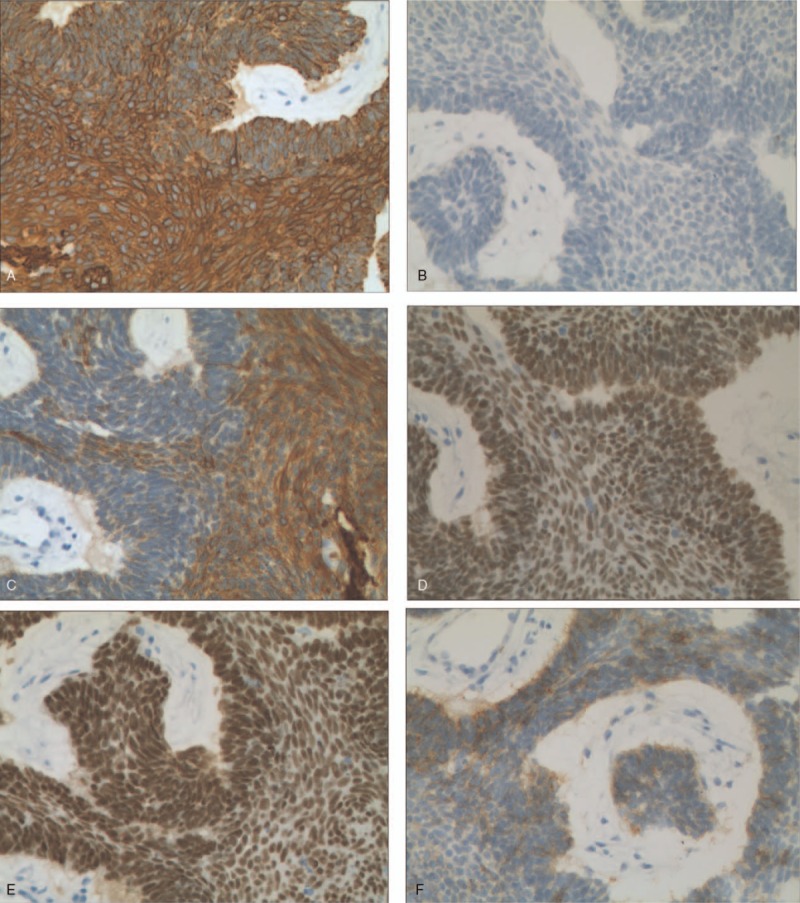
(a–f). Immunohistochemistry staining of ameloblastoma lung metastasis tissue (original magnification×200). The positive of CK(a), P40(d), P63(e), the weakly focally positive of CK5/6(c), CD56(f) and the negative of TTF-1(b).
The patient received combined chemotherapy including adriamycin (50 mg/m2), ifosfamide (7500 mg/m2), and dacarbazine (1000 mg/m2), which were administered intravenously for 72 hours every 3 weeks. Mesna (2500 mg/m2) was also administered intravenously for 96 hours every 3 weeks. According to RECIST 1.1 criteria, the objective response was partial remission (PR) after 2 cycles of chemotherapy. The lung metastases reduced over 90% compared with the base line after 6 cycles of chemotherapy (Fig. 3). The most common treatment related adverse events included neutropenia, leukopenia, anemia, acratia and nausea. In addition to grade 3 neutropenia, no other grade 3/4 adverse events were observed during chemotherapy.
Figure 3.
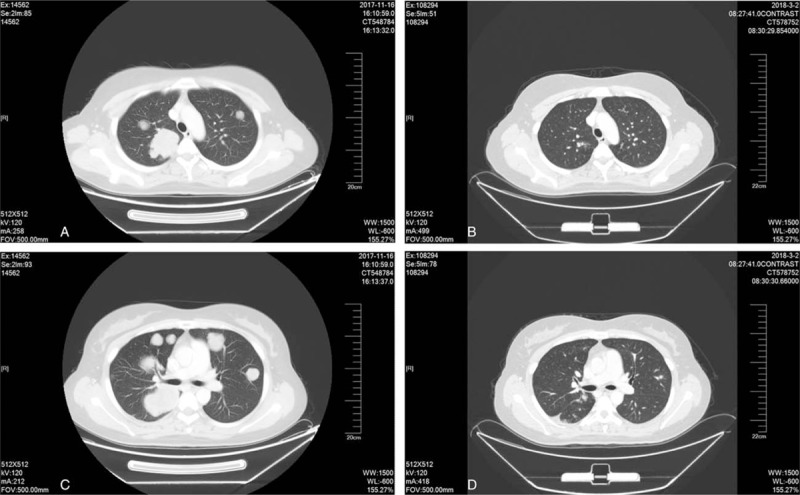
(a–d). Contrast computerized tomography (CT) scans show the multiple lung metastases of the initial presentation from November 2017 (a and c) and the 6 cycles of MAID chemotherapy treatment from March 2018 (b and d), the pulmonary metastases have reduced over 90%.
3. Discussion
Ameloblastoma is a rare odontogenic tumor. There is no significant difference in incidence rate between gender, territory and race.[1] The incidence rate of malignant ameloblastoma is 1.79 per 10 million person/year and the overall survival is 17.6 years from Surveillance, Epidemiology and End-Results (SEER) database.[7] For cases of locoregional ameloblastoma, the advised primary treatment is surgery including radical and conservation surgery.[5] Radical surgery has lower local recurrence rate than conservation surgery.[8] Kunze et al demonstrated that the lung was the organ with the highest metastatic rate of ameloblastoma followed by pleura, lymph nodes, bone, brain, kidney, and liver.[9] When distant metastasis occurred, more aggressive treatments could be applied. Chemotherapy, radiotherapy and surgery are the methods reported in the related literature. Due to the low incidence of ameloblastoma, retrospective and prospective large sample clinical studies have not been conducted. As such, most papers regarding the treatment of ameloblastoma are case reports.[10] Therefore, there is no standard therapy for metastatic ameloblastoma.
A retrospective study confirmed that radiotherapy followed by palliative surgery could control the local recurrence of ameloblastoma.[11] In this study, gene screening revealed that radiotherapy increased the sensitivity to BRAF inhibitors in patients with BRAF gene mutations. As for recurrent ameloblastoma with skull metastasis, radiation therapy also had a better disease control with a disease progression free survival (PFS) of 28 months.[12] However, another study suggested that patients with metastatic ameloblastoma could only benefit from surgery instead of radiation therapy and chemotherapy.[13] Stereotactic body radiotherapy (SBRT) is a technique that has risen in popularity in recent years. Several studies demonstrated that patients of non-small cell lung cancer (NSCLC) who had oligometastasis including brain, bone, lung, lymphatic, or adrenal gland and received SBRT owned the median PFS of 11.2 to 13.7 months and lower local recurrence rate.[14–16] The female patient in our study exhibited local recurrence and a single brain metastasis. She underwent extended maxillary resection combined with SBRT for the brain metastasis with the PFS of 24.0 months.
If multiple metastases are confirmed, local treatments will not control the disease effectively, thus systemic therapy should be the primary focus. In previous studies, the effects of several combination chemotherapy regimens were reported. Secondary regimens include doxorubicin with cisplatin,[17] or paclitaxel with carboplatin[18,19] and triple combined therapy (doxorubicin combined with cisplatin and cyclophamide)[20] showed encouraging efficacy and safety. However, due to the lack of large sample randomized comparative clinical trials, high grade evidence is not available. MAID (mesna, adriamycin, ifosfamide, and dacarbazine) regimen achieved a better outcome in advanced sarcoma with the PFS of more than one year and the ORR of 27% to 35%.[21,22] The most frequent adverse events were grade 3 or 4 hematological toxicities. Meanwhile, a perspective study conducted with patients diagnosed with advanced pulmonary pleomorphic carcinoma treated with MAID had a median PFS and an OS of 2.8 months and 8.7 months, respectively.[23] Therefore, we tried MAID as the first-line chemotherapy for the patient. Fortunately, she achieved PR after 2 cycles. After 6 cycles of MAID chemotherapy regimen, the lung metastases reduced over 90% compared with the base line. The last patient follow-up was July 24th 2018. At that time, imaging results showed no evidence of disease progression. The progression-free survival (PFS) of the patient was more than 9 month. Thus, it appears that MAID may be an effective regimen with good tolerance in patients with metastatic ameloblastoma and further investigation is deserved.
In general, the BRAF V600E mutation is the most aberration in ameloblastoma. Vemurafenib, a BRAF inhibitor, showed promising response in ameloblastoma cells in vitro.[24,25] The whole exon gene sequencing result of the present case is consistent with these studies. In addition, the RAS gene mutation rate is 20% in metastatic ameloblastoma.[26] The overexpression of vascular endothelial growth factor (VEGF) occurs in both benign and malignant ameloblastoma.[27] Although ameloblastoma originates from dental epithelium and epidermal growth factor receptor (EGFR) expression is positive in normal oral mucosa, the EGFR mutation rate was extremely low.[28] At present, no data has shown that EGFR could be used as a target for the treatment of ameloblastoma. In summary, VEGF, TIMP-2, MMP-14 mRNA, FGF, and TGF-β may be potential targets for recurrence and metastasis of malignant ameloblastoma in the future.[29]
4. Conclusion
Due to the low incidence of metastatic malignant ameloblastoma, there is no standard treatment strategy. Surgical resection is the optimal treatment for local recurrence malignant ameloblastoma. According to the literature we reviewed and the case we reported, SBRT may be an effective treatment for unresectable oligometastasis of ameloblastoma, and MAID chemotherapy regimen also showed encouraging efficacy in metastatic malignant ameloblastoma. With further development of methods enabling the detection tumor-specific gene signatures, targeted therapy may be worth further consideration.
Author contributions
Danyang Li drafted the manuscript; Ying Liu designed and critically revised the manuscript, providing language help; Danyang Li, Shuning Xu and Lei Qiao cared the patient and collected the date of the case; Miaomiao Sun and Lifeng Wang selected the figures.
Conceptualization: Ying Liu.
Data curation: Danyang Li, Shuning Xu, Miaomiao Sun, Lei Qiao, Lifeng Wang.
Project administration: Shuning Xu, Lei Qiao.
Validation: Ying Liu.
Writing – original draft: Danyang Li.
Writing – review & editing: Danyang Li.
Footnotes
Abbreviations: CT = computer tomography, EGFR = epidermal growth factor receptor, H&E = hematoxylin and eosin, MAID = mesna, adriamycin, ifosfamide and dacarbazine, MRI = magnetic resonance imaging, NGS = next generation sequencing, NSCLC = non-small cell lung cancer, ORR = objective response rate, OS = overall survival, PFS = progression free survival, PR = partial remission, RECIST = response evaluation criteria in solid tumors, SBRT = stereotactic body radiotherapy, VEGF = vascular endothelial growth factor.
Informed written consent was obtained from the patient for publication of this case report and accompanying images.
The authors have no conflicts of interest to disclose.
References
- [1].Reichart PA, Philipsen HP, Sonner S. Ameloblastoma: biological profile of 3677 cases. Eur J Cancer B Oral Oncol 1995;31B:86–99. [DOI] [PubMed] [Google Scholar]
- [2].Wright JM, Vered M. Update from the 4th Edition of the World Health Organization classification of head and neck tumours: odontogenic and maxillofacial bone tumors. Head Neck Pathol 2017;11:68–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Barnes L, Eveson J, Reichart PA, Sidransky D. World Health Organization Classification of Tumours: Head and Neck Tumours. Lyon: IARC Press; 268–95. [Google Scholar]
- [4].Pogrel MA, Montes DM. Is there a role for enucleation in the management of ameloblastoma? Int J Oral Maxillofac Surg 2009;38:807–12. [DOI] [PubMed] [Google Scholar]
- [5].Sehdev MK, Huvos AG, Strong EW, et al. Proceedings: Ameloblastoma of maxilla and mandible. Cancer 1974;33:324–33. [DOI] [PubMed] [Google Scholar]
- [6].Henderson JM, Sonnet JR, Schlesinger C, et al. Pulmonary metastasis of ameloblastoma: case report and review of the literature. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 1999;88:170–6. [DOI] [PubMed] [Google Scholar]
- [7].Rizzitelli A, Smoll NR, Chae MP, et al. Incidence and survival of malignant ameloblastoma. PLoS One 2015;10:e0117789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Hammarfjord O, Roslund J, Abrahamsson P, et al. Surgical treatment of recurring ameloblastoma, are there options? Br J Oral Maxillofac Surg 2013;51:762–6. [DOI] [PubMed] [Google Scholar]
- [9].Kunze E, Donath K, Luhr HG, et al. Biology of metastasizing ameloblastoma. Pathol Res Pract 1985;180:526–35. [DOI] [PubMed] [Google Scholar]
- [10].Van Dam SD, Unni KK, Keller EE. Metastasizing (malignant) ameloblastoma: review of a unique histopathologic entity and report of Mayo Clinic experience. J Oral Maxillofac Surg 2010;68:2962–74. [DOI] [PubMed] [Google Scholar]
- [11].Kennedy WR, Werning JW, Kaye FJ, et al. Treatment of ameloblastoma and ameloblastic carcinoma with radiotherapy. Eur Arch Otorhinolaryngol 2016;273:3293–7. [DOI] [PubMed] [Google Scholar]
- [12].Huang CM, Chen JY, Chen CH, et al. Radiotherapy for a repeatedly recurrent ameloblastoma with malignant transformation. Head Neck 2014;36:E1–3. [DOI] [PubMed] [Google Scholar]
- [13].Ricard AS, Majoufre-Lefebvre C, Siberchicot F, et al. A multirecurrent ameloblastoma metastatic to the lung. Rev Stomatol Chir Maxillofac 2010;111:98–100. [DOI] [PubMed] [Google Scholar]
- [14].Fleckenstein J, Petroff A, Schäfers HJ, et al. Long-term outcomes in radically treated synchronous vs. metachronous oligometastatic non-small-cell lung cancer. BMC Cancer 2016;16:348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Collen C, Christian N, Schallier D, et al. Phase II study of stereotactic body radiotherapy to primary tumor and metastatic locations in oligometastatic nonsmall-cell lung cancer patients. Ann Oncol 2014;25:1954–9. [DOI] [PubMed] [Google Scholar]
- [16].Buglione M, Pedretti S, Gipponi S, et al. The treatment of patients with 1-3 brain metastases: is there a place for whole brain radiotherapy alone, yet? A retrospective analysis. Radiol Med 2015;120:1146–52. [DOI] [PubMed] [Google Scholar]
- [17].Amzerin M, Fadoukhair Z, Belbaraka R, et al. Metastatic ameloblastoma responding to combination chemotherapy: case report and review of the literature. J Med Case Rep 2011;5:491–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Grunwald V, Le Blanc S, Karstens JH, et al. Metastatic malignant ameloblastoma responding to chemotherapy with paclitaxel and carboplatin. Ann Oncol 2001;12:1489–91. [DOI] [PubMed] [Google Scholar]
- [19].Ghiam A, Al Zaharani A, Feld R. A case of recurrent metastatic ameloblastoma and hypercalcaemia successfully treated with carboplatin and paclitaxel: long survival and prolonged stable disease. Ecancermedicalscience 2013;7:323–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Ramadas K, Jose CC, Subhashini J, et al. Pulmonary metastases from ameloblastoma of the mandible treated with cisplatin, adriamycin, and cyclophosphamide. Cancer 1990;66:1475–9. [DOI] [PubMed] [Google Scholar]
- [21].Fayette J, Penel N, Chevreau C, et al. Phase III trial of standard versus dose-intensified doxorubicin, ifosfamide and dacarbazine (MAID) in the first-line treatment of metastatic and locally advanced soft tissue sarcoma. Invest New Drugs 2009;27:482–9. [DOI] [PubMed] [Google Scholar]
- [22].Ogura K, Goto T, Imanishi J, et al. Neoadjuvant and adjuvant chemotherapy with modified mesna, adriamycin, ifosfamide, and dacarbazine (MAID) regimen for adult high-grade non-small round cell soft tissue sarcomas. Int J Clin Oncol 2013;18:170–6. [DOI] [PubMed] [Google Scholar]
- [23].Lee J, Jung HA, Kim Y, et al. Efficacy of mesna, doxorubicin, ifosfamide, and dacarbazine (MAID) in patients with advanced pulmonary pleomorphic carcinoma. Lung Cancer 2018;122:160–4. [DOI] [PubMed] [Google Scholar]
- [24].Diniz MG, Gomes CC, Guimarães BV, et al. Assessment of BRAFV600E and SMOF412E mutations in epithelial odontogenic tumours. Tumour Biol 2015;36:5649–53. [DOI] [PubMed] [Google Scholar]
- [25].Brown NA, Rolland D, Mchugh JB, et al. Activating FGFR2-RAS-BRAF mutations in ameloblastoma. Clin Cancer Res 2014;20:5517–26. [DOI] [PubMed] [Google Scholar]
- [26].Brown NA, Betz BL. Ameloblastoma: a review of recent molecular pathogenetic discoveries. Biomark Cancer 2015;7Suppl 2:19–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Kumamoto H, Ohki K, Ooya K. Association between vascular endothelial growth factor (VEGF) expression and tumor angiogenesis in ameloblastomas. J Oral Pathol Med 2002;31:28–34. [DOI] [PubMed] [Google Scholar]
- [28].Vered M, Shohat I, Buchner A. Epidermal growth factor receptor expression in ameloblastoma. Oral Oncol 2003;39:138–43. [DOI] [PubMed] [Google Scholar]
- [29].Zhong Y, Guo W, Wang L, et al. Molecular markers of tumor invasiveness in ameloblastoma: an update. Ann Maxillofac Surg 2011;1:145–9. [DOI] [PMC free article] [PubMed] [Google Scholar]


