Abstract
The efficacy of sorafenib in combination with transarterial chemoembolization (TACE) or multiple-line therapies in patients with advanced hepatocellular carcinoma (HCC) remains unclear. This study aimed to investigate the overall survival (OS) of patients with advanced HCC in response to different combination therapies.
We analyzed the treatment and OS of 401 patients with Barcelona clinic liver cancer stage C HCC between 2012 and 2017. Mortality was analyzed using multivariate Cox regression, and OS was analyzed by the Kaplan–Meier method.
The mean age was 59 years and males were predominant. During a median follow-up time of 8.6 months (range, 1–80 months), 346 (86.2%) patients died. In the multivariate Cox regression analysis, primary tumor size ≥5 cm, serum alpha-fetoprotein ≥200, and serum albumin ≥3.5 were significantly associated with mortality. In addition, compared with sorafenib alone, multiple-line treatments with sorafenib and multiple-line treatments without sorafenib yielded significantly decreased mortality. In the Kaplan–Meier analysis, sorafenib with TACE, multiple-line treatments with sorafenib, third-line treatments with sorafenib, and multiple-line treatments without sorafenib yielded a significantly better median OS than sorafenib alone.
Sorafenib with concurrent multiple-line therapies significantly improved OS. These combination therapies will provide important information for immunotherapy combination with locoregional therapies in advanced HCC.
Keywords: advanced stage, hepatocellular carcinoma, immunotherapy, overall survival, sorafenib
1. Introduction
Hepatocellular carcinoma (HCC) is the fifth most common cancer but the second leading cause of cancer-related death worldwide.[1–3] HCC has a high correlation with viral- and alcohol-related cirrhosis[4] and is the second leading cause of cancer-related death in Taiwan.[5] Performing ultrasound and monitoring alpha-fetoprotein (AFP) in patients with an elevated risk of HCC is suggested,[6–9] but only a small percentage of HCCs are diagnosed during routine monitoring. Therefore, a minority of HCC patients are considered for surgical resection.[10,11] Additionally, most HCC patients are diagnosed at an intermediate or advanced stage, and one-third of patients are diagnosed with advanced stage HCC.[12,13]
Patients with macrovascular invasion (MVI) and/or extrahepatic spread (EHS) are classified as Barcelona clinic liver cancer (BCLC) stage C, and sorafenib is the only suggested therapy for BCLC stage C patients.[7,14] Some studies have demonstrated that sorafenib increases overall survival (OS) in patients with MVI and/or EHS.[8,15,16] However, the clinical prognosis and median OS of most patients after sorafenib treatment remain unsatisfactory. In attempts to improve the effects of sorafenib, several studies of subsequent or combination therapies with radiofrequency ablation (RFA),[17] radiotherapy,[18] hepatic artery infusion chemotherapy (HAIC),[19] and systemic therapy[20] have been performed. In addition, some reports have demonstrated that in BCLC stage C patients, the combination of sorafenib with transarterial chemoembolization (TACE) can improve OS compared with that obtained from sorafenib or TACE monotherapy.[21,22] Recently, studies have shown that compared with administration of either sorafenib or TACE alone, the coadministration of conventional TACE and sorafenib increases the time to progression but does not increase OS in BCLC stage B/C patients.[23–26] However, the effect of sorafenib in combination with TACE or other multiple-line therapies is still controversial and needs to be studied. Hence, this study aimed to identify clinical factors predicting OS. Moreover, we investigated the OS in patients with advanced HCC who underwent sorafenib monotherapy or sorafenib with concurrent multiple-line therapies.
2. Materials and methods
2.1. Patient enrollment
We retrospectively enrolled 401 BCLC stage C HCC patients with MVI or/and EHS between 2012 and 2017 at E-Da Hospital, I-Shou University, Kaohsiung, Taiwan. The study was approved by the Ethics Committee of E-Da Hospital (EMRP-107-121), and all of the participants supplied informed consent. Patients were diagnosed with HCC based on at least 1 typical HCC image or histological confirmation according to the recommendations of the AASLD.[14] OS was defined the range from the time of inclusion to the time of the last follow-up or death, and follow-up was conducted until July 2018. Antiviral therapy was defined as nucleoside analogs administered to patients with hepatitis B virus (HBV) or Peg-interferon or direct-acting antiviral agents administered to patients with hepatitis C virus (HCV) according to the guidelines of the Taiwan Association for the Study of the Liver.
2.2. Treatment
Patients received 1 or more treatments, which were classified as sorafenib, surgical resection, RFA, TACE, radiotherapy, HAIC, and best supportive care (BSC). A total of 56.8% of patients initially received the standard dose (800 mg/d) of sorafenib, and 43.2% of patients initially received lower than standard doses. First-line, second-line, third-line, and fourth-line treatments were defined as therapies administered to patients who received 1 treatment, 2 treatments, 3 treatments, and 4 treatments, respectively. Multiple-line treatment was defined as the concurrent administration of 2 or more treatments. For sorafenib-based treatments, the treatments were categorized as sorafenib alone and as second-line, third-line, and fourth-line treatments with sorafenib.
2.3. Statistical analysis
Numerical data are expressed as the mean (ranges), and categorical data are described using numbers (percentages). Continuous variables were categorized based on the mean values or the limit of normal ranges in Cox regression models. Comparisons of continuous data were performed using the Pearson χ2 test, and categorical variables were analyzed using the Fisher exact test as appropriate. OS was calculated through a Kaplan–Meier analysis and was presented as the median and 95% confidence interval (CI). Cox proportional hazards regression analysis of OS in HCC patients was performed. A P-value of <.05 indicated statistical significance. All statistical analyses were conducted with Statistics Package for Social Science (SPSS) software (version 23.0, SPSS, Inc, Chicago, IL).
3. Results
3.1. Patient demographic data
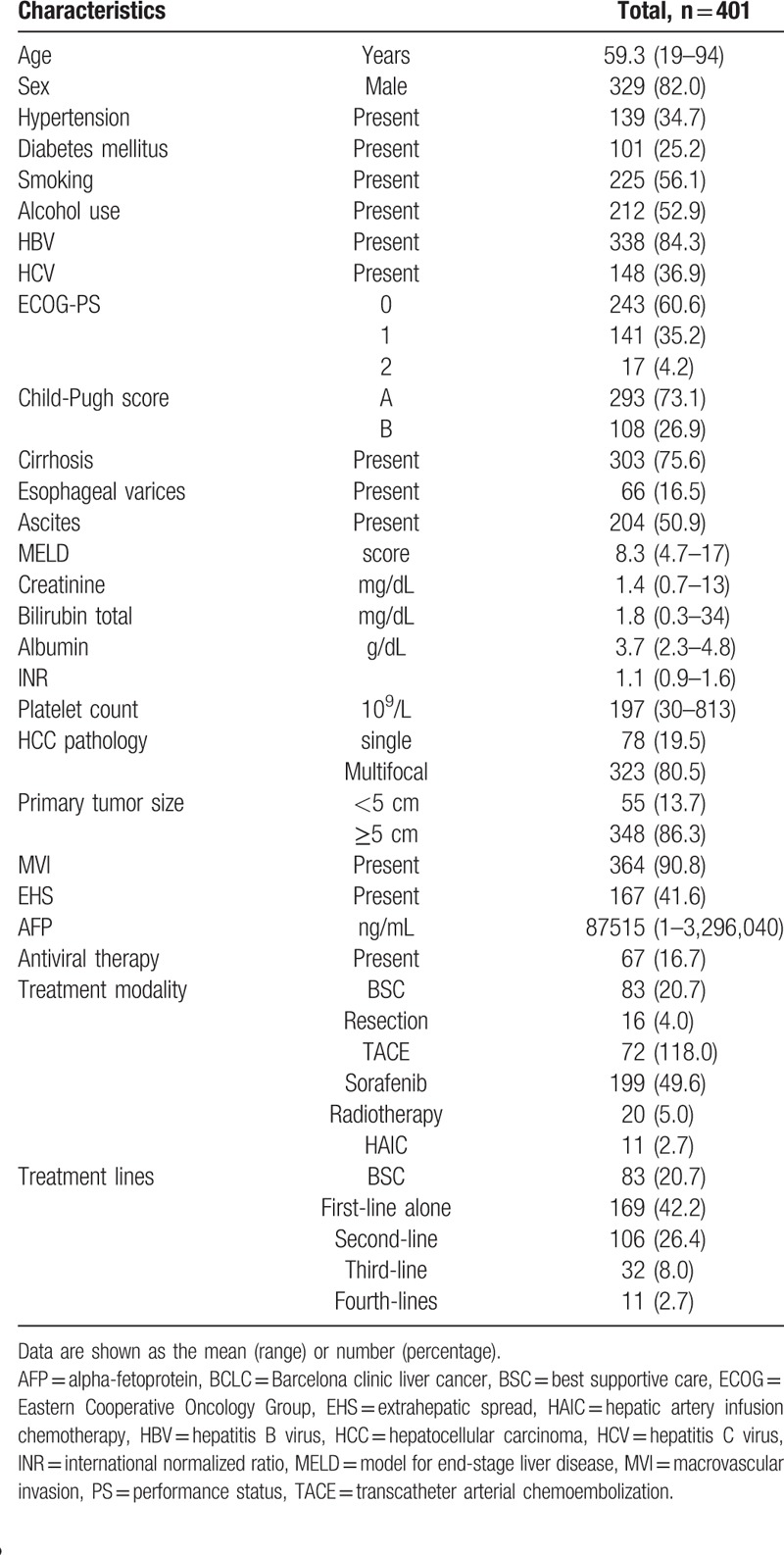
The demographic data and clinical characteristics of 401 BCLC stage C patients are listed in Table 1. The mean age was 59 years, and males were predominant. In addition, 84.3% of the patients reported a history of HBV, 36.9% reported a history of HCV, and 52.9% reported alcohol use. Additionally, 16.7% of patients had received antiviral therapy. Approximately 73.1% of patients were Child-Pugh class A, and 75.6% had cirrhosis. Patients frequently exhibited large and multifocal HCCs. Patients with MVI and EHS accounted for 90.8% and 41.6% of the participants, respectively.
Table 1.
Demographic data and clinical features of 401 HCC patients.
3.2. Prognostic factors associated with mortality
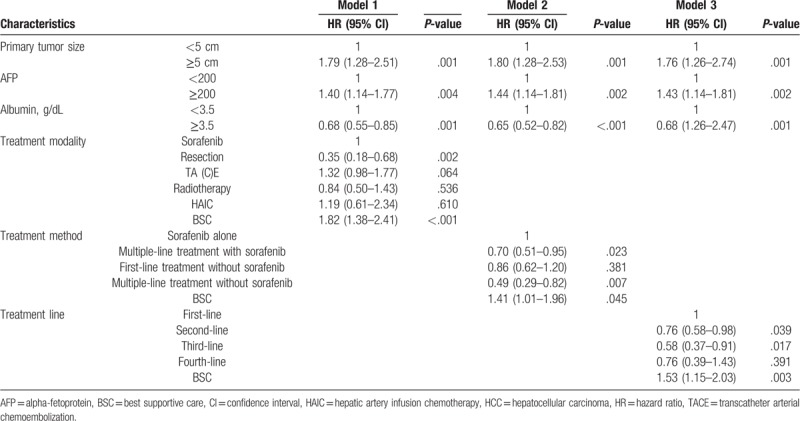
During a median follow-up time of 8.6 months (range, 1–80 months), 346 (86.2%) patients died. The prognostic factors of mortality were analyzed in univariate analyses and are listed in Table 2. Mortality was significantly correlated with serum albumin, primary tumor size, serum AFP, treatment modality, treatment method and treatment line. In the multivariate Cox regression analysis, 3 models were used due to different characteristics for treatments, the results of which are presented in Table 3. In model 1, primary tumor size ≥5 cm (hazard ratio [HR]: 1.80; 95% CI: 1.28–2.53; P = .001) and serum AFP ≥200 (HR: 1.44; 95% CI: 1.14–1.81; P = .002) were significantly correlated with increased mortality. In addition, serum albumin ≥3.5 (HR: 0.65; 95% CI: 0.52–0.82; P < .001) was significantly correlated with decreased mortality. Furthermore, compared with sorafenib, resection (HR: 0.35; 95% CI: 0.18–0.58; P = .002) had a significantly stronger correlation with decreased mortality. Finally, BSC (HR: 1.82; 95% CI: 1.38–2.41; P < .001) was significantly associated with increased mortality. In model 2, primary tumor size ≥5 cm (HR: 1.76; 95% CI: 1.26–2.74; P = .001) and serum AFP ≥200 (HR: 1.44; 95% CI: 1.14–1.81; P = .002) were remarkably correlated with increased mortality. In addition, serum albumin ≥3.5 (HR: 0.68; 95% CI: 0.55–0.85; P = .001) was remarkably correlated with decreased mortality. Furthermore, compared with sorafenib alone, multiple-line treatments with sorafenib (HR: 0.70; 95% CI: 0.51–0.95; P = .023) and multiple-line treatments without sorafenib (HR: 0.49; 95% CI: 0.29–0.82; P = .007) had significantly stronger associations with decreased mortality. Finally, BSC (HR: 1.41; 95% CI: 1.01–1.96) was remarkably correlated with increased mortality. In model 3, primary tumor size ≥5 cm (HR: 1.79; 95% CI: 1.28–2.51; P = .002) and serum AFP ≥200 (HR: 1.40; 95% CI: 1.14–1.77; P = .001) were remarkably correlated with increased mortality. In addition, serum albumin ≥3.5 (HR: 0.68; 95% CI: 0.55–0.85; P = .001) was significantly associated with decreased mortality. Furthermore, compared with first-line treatment, second-line treatments (HR: 0.76; 95% CI: 0.58–0.98; P = .039) and third-line treatments (HR: 0.58; 95% CI: 0.37–0.91; P = .017) were significantly associated with decreased mortality. Finally, BSC (HR: 1.53; 95% CI: 1.15–2.03; P = .003) was significantly correlated with increased mortality.
Table 2.
Univariate analysis of prognostic factors for mortality.
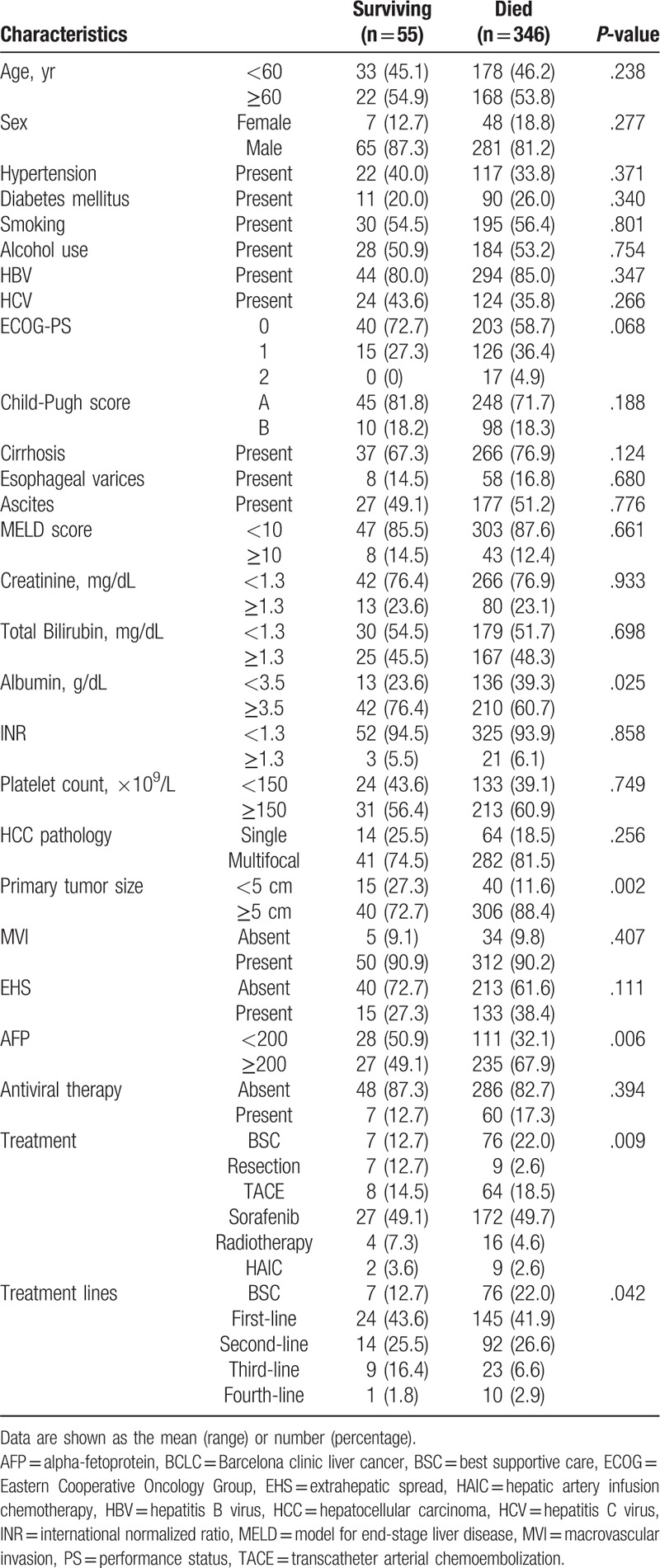
Table 3.
Multivariate Cox regression analysis of prognostic factors for mortality.
3.3. Treatment analysis
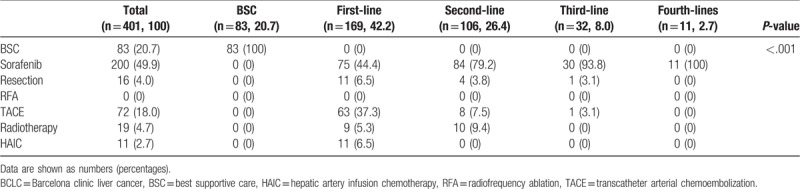
The treatment modality and lines are shown in Table 4. Of the treatment modalities, sorafenib (49.9%) was most frequently employed, followed by BSC (20.7%), transarterial therapies (18.0%), radiotherapy (6.7%), and resection (6.0). Of the treatment lines, first-line therapies (42.7%) were the most commonly used management, followed by second-line therapies (26.4%), BSC (20.7%), third-line therapies (8.0%), and fourth-line therapies (2.7%). In addition, sorafenib was the most common used management in the first-, second-, third-, and fourth-line treatment.
Table 4.
Treatment modality and lines.
3.4. OS of patients undergoing different treatments
Assessment of sorafenib-based treatments indicated that sorafenib alone (8.8 months, 95% CI: 6.1–9.8, P < .001) yielded a remarkably longer median OS than BSC (4.3 months, 95% CI: 3.2–4.8) (Fig. 1A). In addition, sorafenib combined with TACE (14.2 months, 95% CI: 11–16, P = .048), multiple-line treatments with sorafenib (12.6 months, 95% CI: 8.0–15, P = .015), and third-line treatments with sorafenib (16.6 months, 95% CI: 3.9–28, P = .009) resulted in a remarkably longer median OS than sorafenib alone (7.5 months, 95% CI: 5.3–8.6) (Fig. 1B–D). The third-line treatments with sorafenib had the highest median OS (16.6 months), and among the third-line treatments, most of the treatments yielded a median OS concomitant with that of sorafenib, TACE, and radiotherapy.
Figure 1.
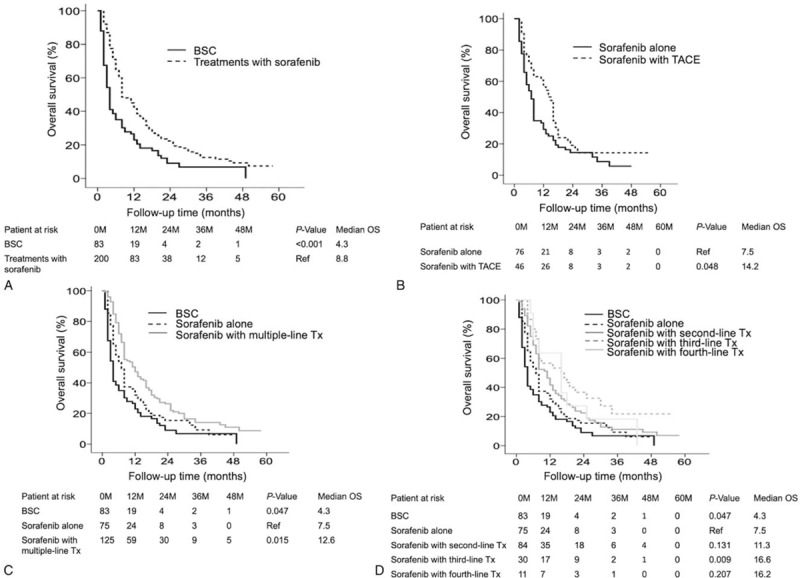
OS of patients with BCLC C stage HCC who underwent different sorafenib-based therapies, as assessed by Kaplan–Meier analysis. Treatment with sorafenib yielded a significantly better median OS than BSC (P < .001) (A). Sorafenib with concurrent TACE had a significantly better median OS than sorafenib alone (P = .048) (B). Sorafenib with multiple-line treatments resulted in a significantly better median OS than sorafenib alone (P < .015) (C). Sorafenib with third-line treatments yielded a significantly better median OS than sorafenib alone (P < .009) (D). BCLC = Barcelona clinic liver cancer, BSC = best supportive care, HCC = hepatocellular carcinoma, OS = overall survival, TACE = transarterial chemoembolization.
Of the combination therapies, multiple-line treatments (12.3 months, 95% CI: 9.3–14.6) resulted in a remarkably longer median OS than either first-line treatment (8.6 months, 95% CI: 6.8–9.2, P = .03) or BSC (4.3 months, 95% CI: 3.2–4.8, P < .001) (Fig. 2A), and third-line treatments (17.2 months, 95% CI: 8.7–25) had a remarkably longer median OS than first-line treatment (8.6 months, 95% CI: 6.8–9.2, P = .015) (Fig. 2B). Therapies with (8.8 months, 95% CI: 6.1–9.8) and without sorafenib (10.3 months, 95% CI: 6.9–13) yielded a similar median OS, but both OS periods were longer than that of BSC (4.3 months, 95% CI: 3.2–4.8, P < .001) (Fig. 2C). Multiple-line treatments with or without sorafenib (12.6 months; 95% CI: 8.0–15, P = .015 and 14.1 months: 95% CI: 9.7–27, P = .013, respectively) yielded a remarkably longer median OS than sorafenib alone (7.5 months; 95% CI: 5.3–8.6) (Fig. 2D).
Figure 2.
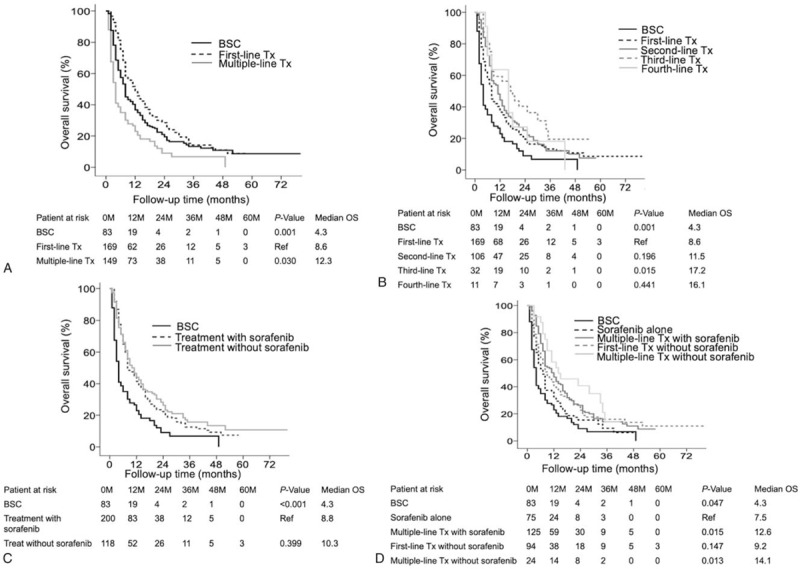
OS of patients with BCLC C stage HCC who underwent multiple-line therapies, as assessed by Kaplan–Meier analysis. Multiple-line treatments yielded a significantly better median OS than first-line treatment (P < .001) (A). Third-line treatments resulted in a significantly better median OS than first-line treatment (P = .015) (B). Treatments with or without sorafenib had a similar median OS (P < .001) (C). Multiple-line treatments with or without sorafenib yielded a significantly better median overall survival than sorafenib alone (P = .013) (D). BCLC = Barcelona clinic liver cancer, HCC = hepatocellular carcinoma, OS = overall survival.
4. Discussion
Sorafenib is the only recommended treatment for patients with BCLC stage C HCC.[7,14] Although sorafenib promotes OS in HCC patients with MVI and/or EHS, the clinical prognosis is still unsatisfactory.[8] In addition, sorafenib in combination with TACE or locoregional therapies may increase OS in patients with advanced HCC.[17,18,22,24] Our study demonstrated that primary tumor size ≥5 cm, serum AFP ≥200, and serum albumin <3.5 were significantly associated with increased mortality. In addition, compared with BSC, sorafenib alone prolonged the OS in patients with BCLC stage C HCC. Furthermore, compared with sorafenib alone, sorafenib with TACE, multiple-line treatments with sorafenib, third-line treatments with sorafenib, and multiple-line treatments without sorafenib significantly increased the median OS. Therefore, sorafenib with concurrent multiple-line therapies significantly improved the OS in patients with BCLC stage C.
The effectiveness of sorafenib in combination with TACE versus sorafenib alone in increasing the OS of patients with BCLC stage C disease is controversial. One study showed that compared with sorafenib alone, sorafenib with concurrent TACE improved the OS in patients with BCLC stage C HCC.[21] However, some studies have shown that compared with sorafenib alone, sorafenib with concurrent TACE did not promote OS in BCLC stage C patients.[23–25] Our study demonstrated that sorafenib with concurrent TACE significantly improved the median OS from 7.5 months to 14.2 months (P = .048) in BCLC stage C HCC. These results are consistent with those of a previous study.[21] Sorafenib in combination with TACE seems to provide better OS if patients have well-preserved liver function and can tolerate these combination therapies. Moreover, many studies on BCLC stage C HCC have shown that compared with sorafenib alone, sorafenib in combination with TACE promotes the tumor response rate, progression-free survival, and time to progression.[23–27]
A randomized controlled trial in a Western country demonstrated that in HCC patients with portal vein thrombosis, sorafenib combined with RFA results in better OS than sorafenib alone.[17] Our study showed that compared with sorafenib alone, sorafenib in combination with multiple-line therapies, including surgical resection, TACE, RFA, radiotherapy or HAIC, resulted in a significantly higher median OS. Furthermore, third-line treatments with sorafenib had the highest median OS (16.6 months). This finding implies that combining sorafenib with locoregional therapy promotes the tumor response and increases OS. The possible reason is that locoregional therapies such as TACE block the feeding vessel, which results in tumor hypoxia. Sorafenib suppresses tumor cell neoangiogenesis and delays tumor progression in HCC patients. In addition, local treatments, including surgical resection, TACE, or RFA, can induce the overproduction of vascular endothelial growth factor (VEGF), which may aggravate the tumor or metastases. Hence, sorafenib may enhance the treatment results by decreasing VEGF overexpression when sequentially administered after local therapies.
Our study showed that multiple-line therapies without sorafenib yielded a better OS (14.2 months) than other therapies. In some cases, patients were not administered sorafenib because of patient intolerance or refusal to take the drug. Several studies have demonstrated that surgical resection significantly favors survival in HCC patients with major vascular invasion, including those with invasion into the portal and hepatic veins.[28–30] RFA and TACE can increase the partial response of tumors, and HAIC should be considered a treatment option for patients with advanced HCC.[31] Radiotherapy may decrease tumor progression and offer survival benefits in HCC patients with portal vein thrombosis.[32,33] The combination of RFA, TACE, HAIC, and radiotherapy can increase the tumor response and delay disease progression. In some cases, these therapies can serve as a bridge to surgical resection. The combination of these therapies should be considered as preoperative treatment modalities and should improve OS in advanced HCC.
Our study has several limitations. First, there were significant gender differences in this investigation, but we will enroll more female subjects and reduce the bias. Second, patients may have accepted multimodal sequential therapies and different treatment modalities, which would affect survival. The optimal use and timing of these combination therapies need to be further studied. Third, we did not mention the duration or side effects of sorafenib due to the retrospective nature of this study.
5. Conclusion
The primary tumor size, serum AFP, and serum albumin were significantly associated with mortality. In addition, sorafenib with TACE, multiple-line treatments with sorafenib, third-line treatments with sorafenib, and multiple-line treatments without sorafenib resulted in a significantly better median OS than sorafenib alone. Sorafenib with concurrent multiple-line therapies significantly improved survival in advanced HCC and was demonstrated to have a manageable efficacy in advanced HCC patients with well-preserved liver function. In the future, sorafenib in combination with locoregional therapies will provide important information for immunotherapy combinations with locoregional therapies in advanced HCC.
Author contributions
Conceptualization: Chih-Wen Lin.
Data curation: Pojen Hsiao, Yaw-Sen Chen, Chia-Chang Hsu.
Formal analysis: Pojen Hsiao, Kun-Chou Hsieh, Yaw-Sen Chen, Chia-Chang Hsu.
Investigation: Gin-Ho Lo, Yu-Chan Li, Chih-Wen Lin.
Methodology: Gin-Ho Lo, Yu-Chan Li, Pei-Min Hsieh, Kun-Chou Hsieh, Hung-Yu Lin.
Project administration: Chih-Wen Lin.
Resources: Chih-Wen Lin.
Software: Tsung-Chin Wu, Jen-Hao Yeh.
Supervision: Chih-Wen Lin.
Validation: Tsung-Chin Wu, Jen-Hao Yeh.
Visualization: Gin-Ho Lo, Tsung-Chin Wu, Jen-Hao Yeh.
Writing – original draft: Pojen Hsiao, Yaw-Sen Chen, Chih-Wen Lin.
Writing – review and editing: Pojen Hsiao, Kun-Chou Hsieh, Yaw-Sen Chen, Chih-Wen Lin.
Chih-Wen Lin orcid: 0000-0003-2344-5056.
Footnotes
Abbreviations: AFP = alpha-fetoprotein, BCLC = Barcelona clinic liver cancer, BSC = best supportive care, CI = confidence interval, EHS = extrahepatic spread, HAIC = hepatic artery infusion chemotherapy, HBV = hepatitis B virus, HCC = hepatocellular carcinoma, HCV = hepatitis C virus, HR = hazard ratio, MVI = macrovasular invasion, OS = overall survival, RFA = radiofrequency ablation, TACE = transarterial chemoembolization, VEGF = vascular endothelial growth factor.
PH and K-CH contributed equally to this work.
This study was partly supported by grants from the Ministry of Science and Technology (MOST 105-2314-B-650-004-MY) and E-Da Hospital (EDAHP107040, EDAHP107041, and EDAHP107064).
The authors have no conflicts of interest to disclose.
References
- [1].Torre LA, Bray F, Siegel RL, et al. Global cancer statistics, 2012. CA Cancer J Clin 2015;65:87–108. [DOI] [PubMed] [Google Scholar]
- [2].Bertuccio P, Turati F, Carioli G, et al. Global trends and predictions in hepatocellular carcinoma mortality. J Hepatol 2017;67:302–9. [DOI] [PubMed] [Google Scholar]
- [3].Yang JD, Mohamed EA, Aziz AO, et al. Characteristics, management, and outcomes of patients with hepatocellular carcinoma in Africa: a multicountry observational study from the Africa Liver Cancer Consortium. Lancet Gastroenterol Hepatol 2017;2:103–11. [DOI] [PubMed] [Google Scholar]
- [4].Lin CW, Lin CC, Mo LR, et al. Heavy alcohol consumption increases the incidence of hepatocellular carcinoma in hepatitis B virus-related cirrhosis. J Hepatol 2013;58:730–5. [DOI] [PubMed] [Google Scholar]
- [5].Lu SN, Wang JH, Su CW, et al. Management consensus guideline for hepatocellular carcinoma: 2016 updated by the Taiwan Liver Cancer Association and the Gastroenterological Society of Taiwan. J Formos Med Assoc 2018;117:381–403. [DOI] [PubMed] [Google Scholar]
- [6].Poon D, Anderson BO, Chen LT, et al. Management of hepatocellular carcinoma in Asia: consensus statement from the Asian Oncology Summit 2009. Lancet Oncol 2009;10:1111–8. [DOI] [PubMed] [Google Scholar]
- [7].Galle PR, Forner A, Llovet JM, et al. EASL Clinical Practice Guidelines: Management of hepatocellular carcinoma. J Hepatol 2018;69:182–236. [DOI] [PubMed] [Google Scholar]
- [8].Cheng AL, Guan Z, Chen Z, et al. Efficacy and safety of sorafenib in patients with advanced hepatocellular carcinoma according to baseline status: subset analyses of the phase III Sorafenib Asia-Pacific trial. Eur J Cancer 2012;48:1452–65. [DOI] [PubMed] [Google Scholar]
- [9].Toyoda H, Kumada T, Tada T, et al. Changes in highly sensitive alpha-fetoprotein for the prediction of the outcome in patients with hepatocellular carcinoma after hepatectomy. Cancer Med 2014;3:643–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Davila JA, Morgan RO, Richardson PA, et al. Use of surveillance for hepatocellular carcinoma among patients with cirrhosis in the United States. Hepatology 2010;52:132–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Dam Fialla A, Schaffalitzky de Muckadell OB, Touborg Lassen A. Incidence, etiology and mortality of cirrhosis: a population-based cohort study. Scand J Gastroenterol 2012;47:702–9. [DOI] [PubMed] [Google Scholar]
- [12].Zhang BH, Yang BH, Tang ZY. Randomized controlled trial of screening for hepatocellular carcinoma. J Cancer Res Clin Oncol 2004;130:417–22. [DOI] [PubMed] [Google Scholar]
- [13].Mittal S, Kanwal F, Ying J, et al. Effectiveness of surveillance for hepatocellular carcinoma in clinical practice: a United States cohort. J Hepatol 2016;65:1148–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Bruix J, Sherman M. American Association for the Study of Liver D. Management of hepatocellular carcinoma: an update. Hepatology 2011;53:1020–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Sohn W, Paik YH, Cho JY, et al. Sorafenib therapy for hepatocellular carcinoma with extrahepatic spread: treatment outcome and prognostic factors. J Hepatol 2015;62:1112–21. [DOI] [PubMed] [Google Scholar]
- [16].Bruix J, Cheng AL, Meinhardt G, et al. Prognostic factors and predictors of sorafenib benefit in patients with hepatocellular carcinoma: analysis of two phase III studies. J Hepatol 2017;67:999–1008. [DOI] [PubMed] [Google Scholar]
- [17].Giorgio A, Merola MG, Montesarchio L, et al. Sorafenib combined with radio-frequency ablation compared with sorafenib alone in treatment of hepatocellular carcinoma invading portal vein: a western randomized controlled trial. Anticancer Res 2016;36:6179–83. [DOI] [PubMed] [Google Scholar]
- [18].Chen SW, Lin LC, Kuo YC, et al. Phase 2 study of combined sorafenib and radiation therapy in patients with advanced hepatocellular carcinoma. Int J Radiat Oncol Biol Phys 2014;88:1041–7. [DOI] [PubMed] [Google Scholar]
- [19].Kudo M, Ueshima K, Yokosuka O, et al. Sorafenib plus low-dose cisplatin and fluorouracil hepatic arterial infusion chemotherapy versus sorafenib alone in patients with advanced hepatocellular carcinoma (SILIUS): a randomised, open label, phase 3 trial. Lancet Gastroenterol Hepatol 2018;3:424–32. [DOI] [PubMed] [Google Scholar]
- [20].Abou-Alfa GK, Niedzwieski D, Knox JJ, et al. Phase III randomized study of sorafenib plus doxorubicin versus sorafenib in patients with advanced hepatocellular carcinoma (HCC): CALGB 80802 (Alliance). J Clin Oncol 2016;34:4003. [Google Scholar]
- [21].Varghese J, Kedarisetty C, Venkataraman J, et al. Combination of TACE and sorafenib improves outcomes in BCLC stages B/C of hepatocellular carcinoma: a single centre experience. Ann Hepatol 2017;16:247–54. [DOI] [PubMed] [Google Scholar]
- [22].Hu H, Duan Z, Long X, et al. Sorafenib combined with transarterial chemoembolization versus transarterial chemoembolization alone for advanced-stage hepatocellular carcinoma: a propensity score matching study. PLoS One 2014;9:e966201–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Park JW, Kim YJ, Kim DY, et al. Sorafenib with or without concurrent transarterial chemoembolization in patients with advanced hepatocellular carcinoma: a phase III STAH trial. J Hepatol 2019;70:684–91. [DOI] [PubMed] [Google Scholar]
- [24].Wu FX, Chen J, Bai T, et al. The safety and efficacy of transarterial chemoembolization combined with sorafenib and sorafenib mono-therapy in patients with BCLC stage B/C hepatocellular carcinoma. BMC Cancer 2017;17:6451–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Liu L, Chen H, Wang M, et al. Combination therapy of sorafenib and TACE for unresectable HCC: a systematic review and meta-analysis. PLoS One 2014;9:e911241–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Li L, Zhao W, Wang M, et al. Transarterial chemoembolization plus sorafenib for the management of unresectable hepatocellular carcinoma: a systematic review and meta-analysis. BMC Gastroenterol 2018;18:1381–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Jun CH, Yoon JH, Cho E, et al. Barcelona clinic liver cancer-stage C hepatocellular carcinoma: a novel approach to subclassification and treatment. Medicine (Baltimore) 2017;96:e67451–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Ho MC, Hasegawa K, Chen XP, et al. Surgery for intermediate and advanced hepatocellular carcinoma: a consensus report from the 5th Asia-Pacific primary liver cancer expert meeting (APPLE 2014). Liver Cancer 2016;5:245–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Zhang XP, Wang K, Li N, et al. Survival benefit of hepatic resection versus transarterial chemoembolization for hepatocellular carcinoma with portal vein tumor thrombus: a systematic review and meta-analysis. BMC Cancer 2017;17:902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Guo H, Wu T, Lu Q, et al. Surgical resection improves long-term survival of patients with hepatocellular carcinoma across different Barcelona Clinic Liver Cancer stages. Cancer Manag Res 2018;10:361–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Moriya K, Namisaki T, Sato S, et al. Efficacy of bi-monthly hepatic arterial infusion chemotherapy for advanced hepatocellular carcinoma. J Gastrointest Oncol 2018;9:741–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Yeh SA, Chen YS, Perng DS. The role of radiotherapy in the treatment of hepatocellular carcinoma with portal vein tumor thrombus. J Radiat Res 2015;56:325–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Lin Q, Huang X, Zhong C, et al. Improved survival with radiotherapy in hepatocellular carcinoma with major vascular invasion: a propensity-matched analysis of surveillance, epidemiology, and end results database. Cancer Med 2019;8:515–26. [DOI] [PMC free article] [PubMed] [Google Scholar]


