Abstract
Published histological studies of the hilar plate or entire biliary remnant at the time of Kasai portoenterostomy (KHPE) have not provided deep insight into the pathogenesis of biliary atresia, relation to age at surgery, prognosis or the basis for successful drainage. We report the detailed histological findings in 172 centrally reviewed biliary remnants with an average of six sections per subject. The predominant active lesion consisted of active fibroplasia with or without a mononuclear cell infiltrate concentric to ducts or peribiliary glands. Necroinflammatory lesions were rare and clustered in a few subjects. Histological heterogeneity of lesions was common within a given remnant. However, histologic patterns were homogeneously inactive or active in most subjects; homogeneity did not correlate with age at KHPE, presence/absence of congenital anomalies at laparotomy indicative of heterotaxy, and outcome. Remnants from the youngest subjects were more likely than older subjects to be homogeneously inactive suggesting significantly earlier onset in the youngest subset. Conversely remnants from the oldest subjects were often homogeneously active suggesting later onset or slower progression. More data is needed in remnants from subjects <30 days old at KHPE and in those with visceral anomalies. Prevalence of partially preserved epithelium in active fibroplastic BA lesions at all ages suggests that epithelial regression or injury may not be a primary event or that re-epithelialization is already underway at the time of KHPE. We hypothesize that outcome after KHPE is the result of competition between active fibroplasia and re-epithelialization of retained, collapsed but not obliterated lumens. The driver of active fibroplasia is unknown.
Introduction
The etiology of biliary atresia remains an enigma. Multiple pathways and potential etiologic subsets have been proposed; the relative influence of environment and genetics continues to be debated (1, 2, 3). The histopathology of the biliary remnant in BA previously has attracted interest for potential prognostic value and as a source of insight into pathogenesis. Remnant histology has been described mainly in tissue from the most proximal portion of the hepatic ducts near the hilum (4–6). Forty-eight hilar remnants, of which 26 included the entire hepatic duct and gall bladder, were serially sectioned by Gautier who observed inflammation and fibrosis with variable retention of epithelial structures that he sorted into main ducts and peribiliary glands. Bland fibrotic remnants were mostly distal in these samples. Based on histopathology, Gautier speculated that BA is an ascending process that begins at or prior to birth and that destructive inflammation might be a secondary feature. In more extensively sampled remnants of the extrahepatic biliary tree, Tan et. al. observed that absent duct remnants and absent inflammation at the porta hepatis associated with poor prognosis but this study did not provide a detailed histopathological analysis of the entire remnants (7). Other reports suggest that histology of the fibrous plate may predict outcome (8–10), but there has been little agreement.
The success rate of the KHPE is approximately 50–60% and better outcome is generally related to younger age at surgery 11–13)). However, a survival benefit has yet to be established and may not exist in infants less than 30 days old at the time of operation (14). This subset may grow as methods for early detection improve (15, 16). Unfortunately, histologic study of biliary remnants has thus far provided little insight into conditions for success or failure of the KHPE, nor provided deeper understanding of etiopathogenesis of BA.
The CHildREN Network*, a consortium of 15 institutions in North America sponsored by the NIDDK has compiled clinical and pathological data on 500 subjects with biliary atresia accessioned between 2003 and 2015. The CHildRENS database includes centrally reviewed liver biopsies obtained at or near the time of the KHPE (17) and protocol-driven step-sectioned biliary remnants in most subjects. We report here the detailed histological findings in biliary remnants from 172 subjects based on conformity to the submission protocol with an average of six sections per subject. We correlate findings with gender, age at KHPE, presence/absence of congenital anomalies at laparotomy indicative of heterotaxy, and outcome.
Methods
Clinical data from PROBE* consisted of gender, age at KHPE, the major associated congenital anomalies known as biliary atresia-splenic malformation syndrome-heterotaxy syndrome (BASM), and other major anomalies (18).
Information collected at surgery consisted of intra-abdominal anatomic abnormalities, status of the common bile duct, findings on intra-operative cholangiogram and anatomic type (Ohi classification) of biliary atresia (19) Outcome was defined as normalization of total serum bilirubin at 3 months post KHPE or survival with the native liver at age 2 years post KHPE.
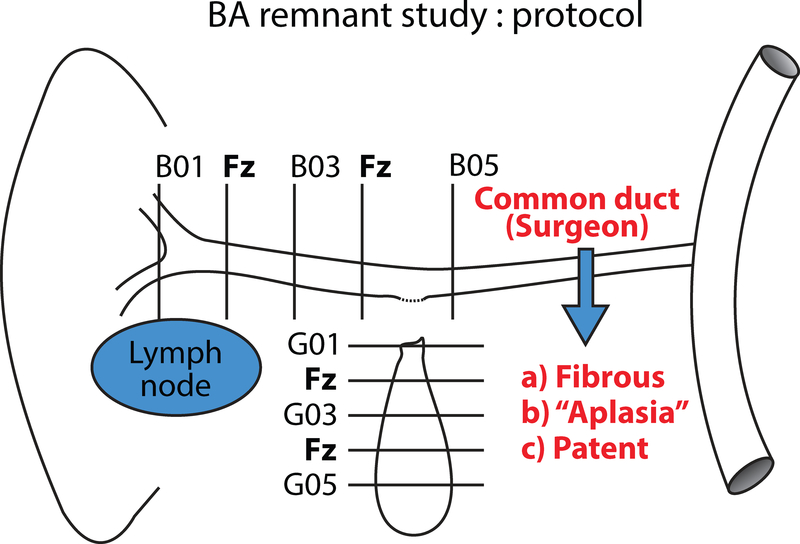
All biliary remnants were oriented by the surgeon (proximal to distal). Most were photographed at the time of gross examination. Step-sections perpendicular to the axis of the hepatic duct from the hilum to the proximal common duct were designated hepatic duct series B. A second series of axial cross sections extending from the cystic duct to the tip of the gall bladder were designated cystic duct/gall bladder series G (Figure 1). Identifiable common duct components were infrequently received and were included in continuity with the hepatic duct series.
Figure 1.
This schematic demonstrates how the biliary remnants in this study were sampled for FFPE with alternate slices frozen for research. The average number of paraffin blocks per case was 6. Additional levels in the B and G series were labelled by expanding the sequences in the schematic as needed*.
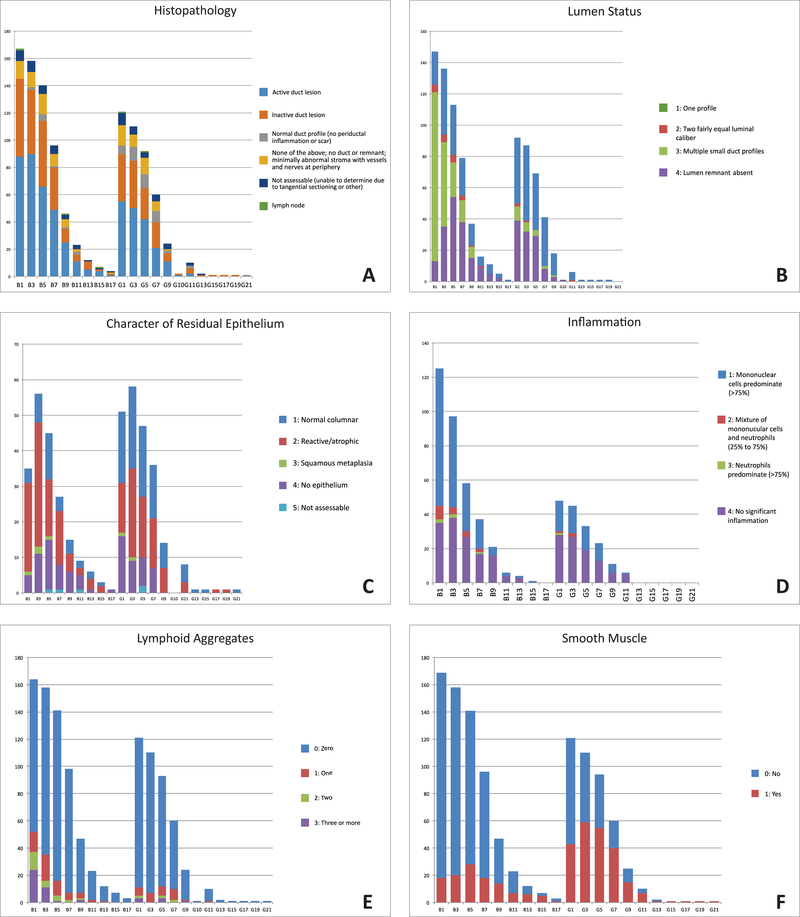
Central evaluation and classification of one H&E stained section from each level was by consensus of 4 or more experienced pediatric pathologists using a protocol designed for remnant evaluation, ChildREN Network Form 10B. This protocol interrogates for each level as follows: inclusion of liver tissue in the section; classification of the lesion based on the appearance of the duct remnant as active or inactive based on character and amount of the inflammatory infiltrate and dominance of active fibrosis vs inactive mature scar; subtypes of inflammatory cells; number of residual lumens; character and amount of residual surface epithelium in residual main channels; presence of smooth muscle bundles; contiguous lymph nodes; ectopic cartilage; and number of contiguous compact nests of tertiary lymphoid tissue. Remnant data were entered into an Excel file at the Data Coordinating Center and analyzed by the senior author. Summary of results are displayed in bar graphs (Figure 2A–I) and in Tables 1–2.
Figure 2.
Topographic bar graphs demonstrating distribution in remnants of histopathologic subsets (a), lumen status (b), residual epithelium character (c), inflammatory status (d), tertiary lymphoid aggregates (e), and smooth muscle (f).
Table 1.
Biliary Remnants: histological classification of 1072 levels in 172 subjects
| B | G | |||
|---|---|---|---|---|
| Active | Necroinflammatory | 19 | 9 | 10 |
| 516/1072 | Active fibroplasia | 263 | 160 | 103 |
| Active fibroplasia/mixed inflammation | 234 | 182 | 52 | |
| Inactive | Perilumenal fibrosis | 88 | 41 | 47 |
| 342/1072 | Fibrous cord | 208 | 127 | 81 |
| Perilumenal myxoid fibrosis | 32 | 17 | 15 | |
| Paucicollagenous angiomyxoid central zone/no lumen | 101 | 57 | 44 | |
| Other | ||||
| 178/1072 | Indeterminate | 9 | 2 | 7 |
| Section not assessable | 68 | 37 | 31 | |
| Normal duct profile | 50 | 11 | 39 | |
| Totals | 1072 | 643 | 429 |
Table 2.
Biliary Remnants: Tertiary Lymphoid Aggregates vs Age at KHPE in 168 subjects
| 0–40d | 41–60d | 61–79d | 80–151d | ||
|---|---|---|---|---|---|
| Subjects | n-30 | N=48 | N=55 | N=36 | 168 |
| Lymphoid Aggregates: none n=901 levels | 19 | 39 | 40 | 27 | 125 |
| Lymphoid Aggregates: 1 n=86 | 3 | 5 | 4 | 3 | 14 |
| Lymphoid Aggregates: 2 n=32 | 3 | 0 | 3 | 4 | 10 |
| Lymphoid Aggregates: 3 or more n=44 | 8 | 2 | 5 | 4 | 19 |
| 32 | 46 | 52 | 38 | 168 |
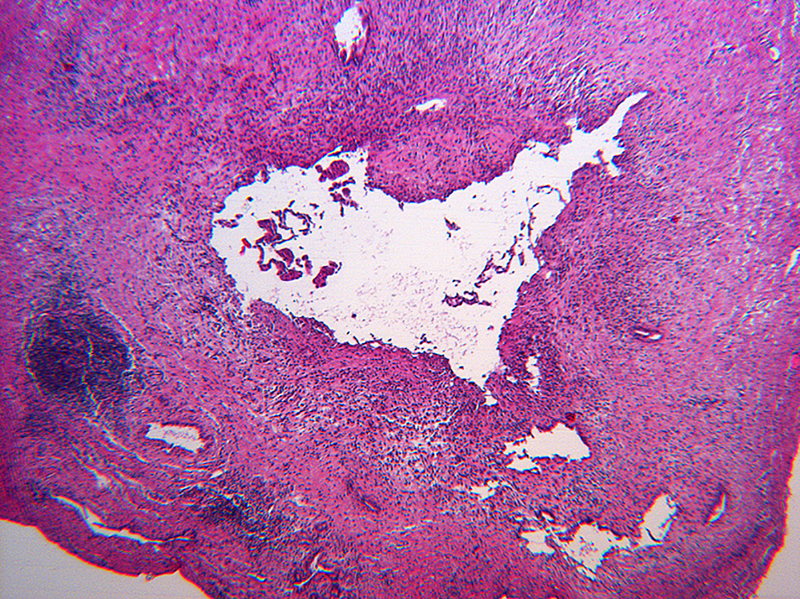
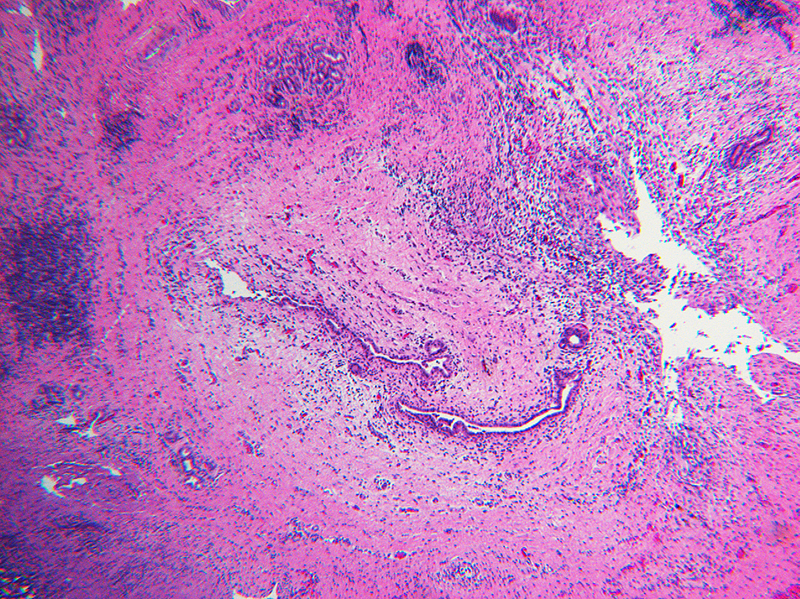
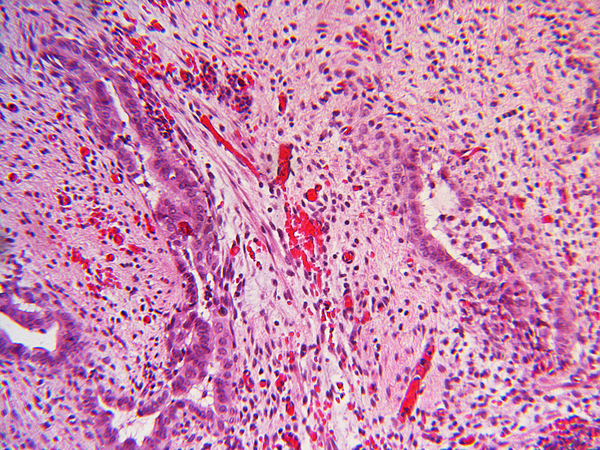
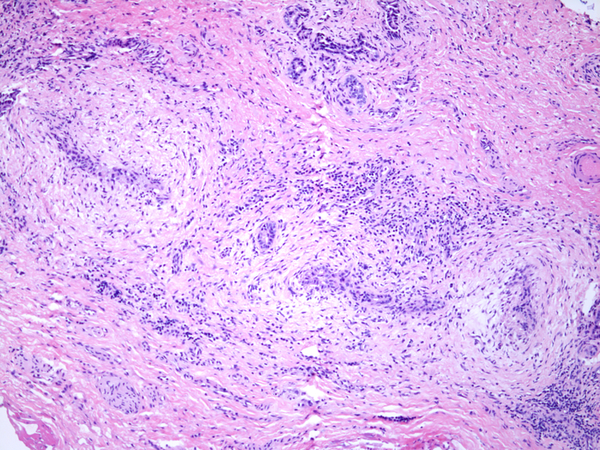
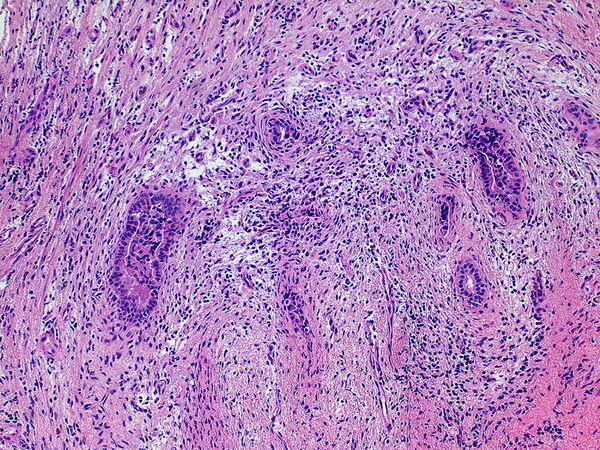
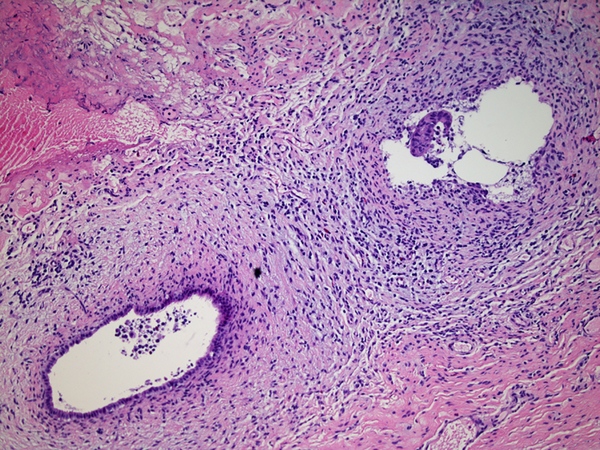
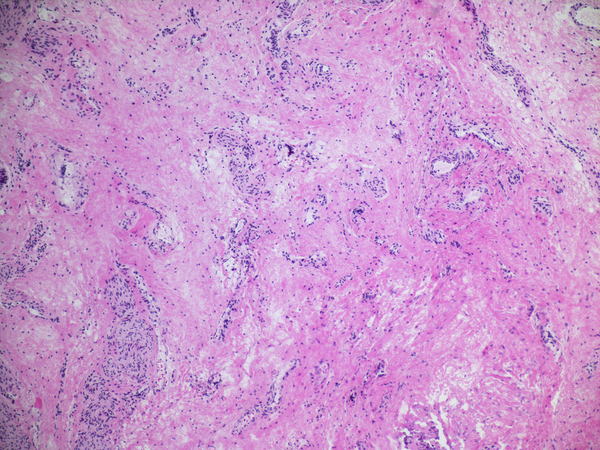
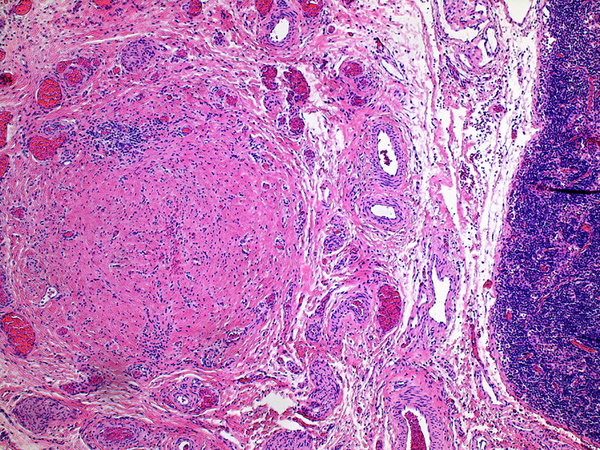
Inflammation was assessed semi-quantitatively as present or absent at low magnification (4X) and further qualified as to predominance of either mononuclear or polymorphonuclear cells. The designation active was subclassified based upon presence of concentric necrosis/exudate/granulation tissue (Figure 3), or cellular reactive fibroblastic tissue around residual lumens (Figure 4). Active concentric fibroplasia was designated mixed when a consensus was reached that the accompanying mononuclear cell inflammatory infiltrate exceeded minimal to absent (Figure 5).
Figure 3.
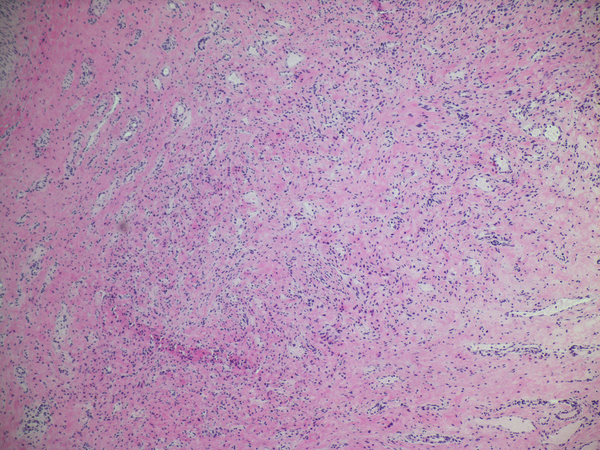
Necroinflammatory lesions in BA remnants: All panels show remnant levels B-1 (hilum). A and B: Single lumens with necrotic epithelium are accompanied by intense inflammation, granulation tissue and tertiary lymphoid aggregates (arrows). C. Variably necrotic and retained injured epithelium is accompanied by stromal edema, loose fibroblast proliferation with focal smooth muscle differentiation, minor collagen deposition, reactive epithelium and a mixed mononuclear cell-neutrophil infiltrate.
Figure 4.
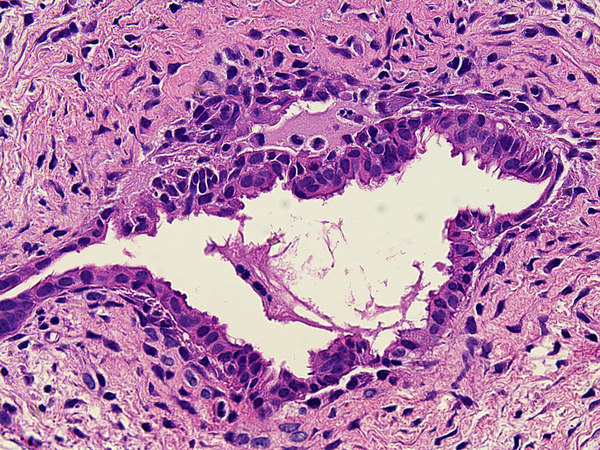
Active fibroplasia in BA remnants: A, remnant level B-1(hilum) shows active fibroplasia without inflammation related to duct remnants and peribiliary glands with intact epithelium (insert). A non-reactive hilar lymph node at lower right lacked germinal centers. B, remnant level B-1 shows reactive epithelium with focal subepithelial fluid (arrow). C, remnant level B-5 shows two lumen residues, possibly located near junction of cystic and hepatic ducts, with asynchronous incomplete fibrous obliteration. The larger lumen on the left is accompanied by prominent peribiliary glands.
Figure 5.
Active fibroplasia classified as mixed with mononuclear cell inflammation. A, remnant level B-1 shows two larger lumens, probably obliquely sectioned surrounded by slightly edematous fibroblastic collars with mild mononuclear cell infiltrates of varied density. Peribiliary glands show mild reactive change of similar nature. B, remnant level B-1 shows mononuclear cells lightly infiltrating zone of circumferential reactive myofibroblasts around epithelial channels of variable size with uncertain distinction between main duct and peribiliary glands. Nuclear debris is prominent (arrow). C, remnant level B-5 shows two lumens, one showing circumferential reactive fibroplasia with preserved epithelium and the other showing mononuclear cells intermixed with reactive fibroblasts and extensive epithelial erosion, more typical of the necroinflammatory subset.
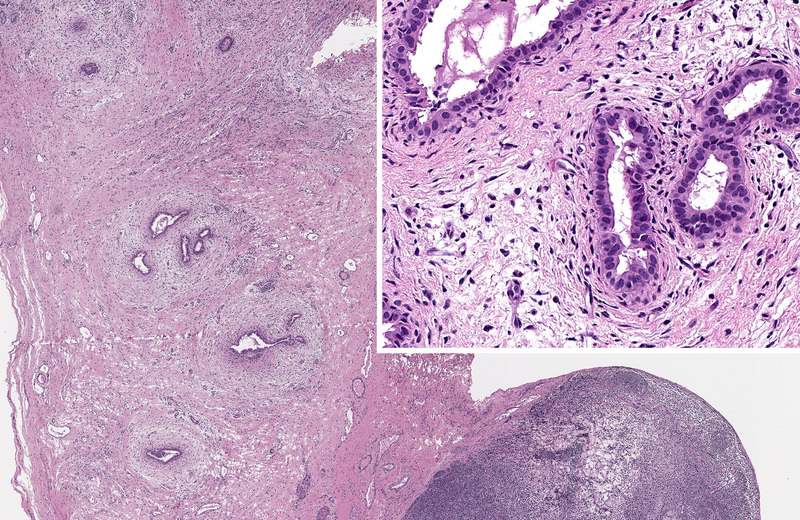
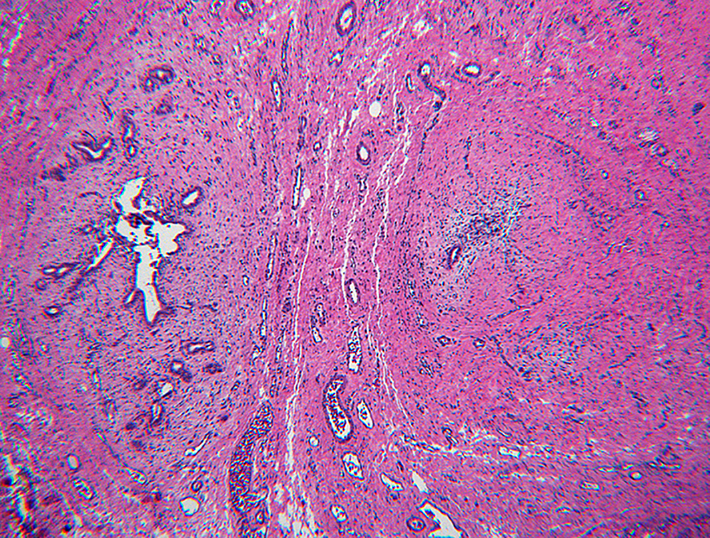
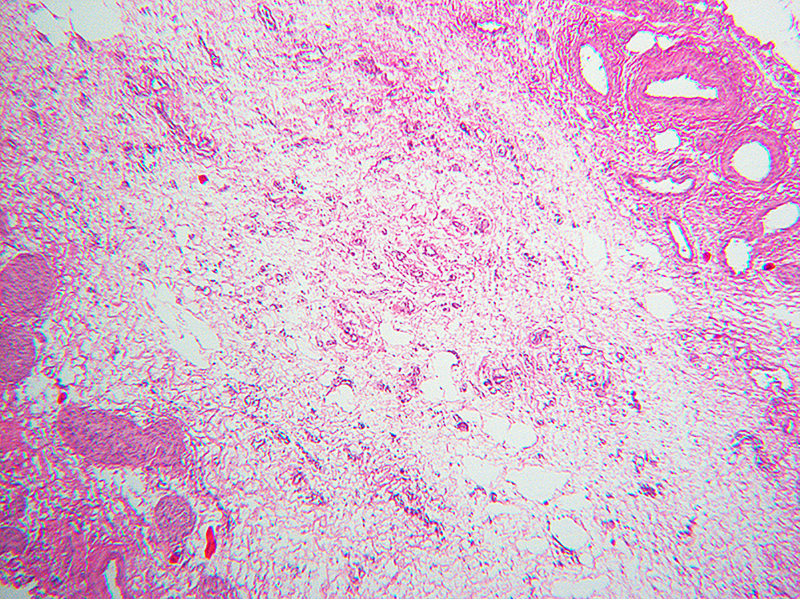
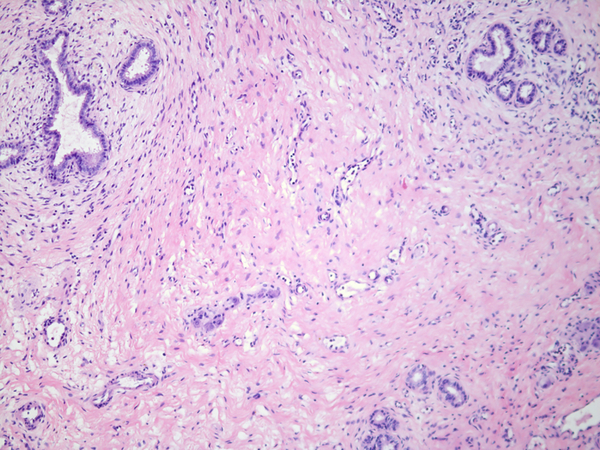
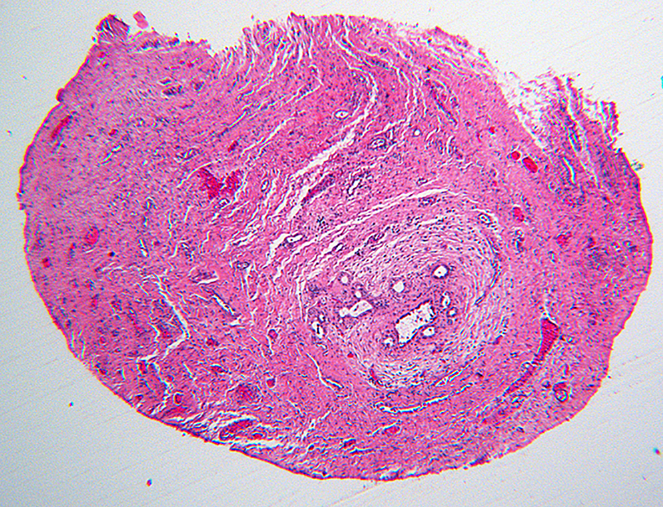
Inactive lesions were defined by paucicellular dense fibrous scar tissue with little or no central inflammation. Inactive lesions included discrete dense fibrous cords and less well defined but recognizable localized scar tissue. Inflammation was usually absent; “minor” mononuclear infiltrates occasionally remained, though overshadowed by the extent or completeness of duct replacement by mature fibrous tissue (Figure 6). Occasionally inactive lesions contained tiny epithelial cell nests consistent with peribiliary glands with or without a larger tubular structure that may have been a stenotic residual lumen embedded in scar. One-hundred-one levels included in the inactive category were judged to be well-oriented based on centrifugal/peripheral distribution of normal vascular structures and nerves along the tissue section margin. In these, an identifiable central duct remnant or discrete scar was absent, but in many such sections, the central zone was occupied by pauci-collagenous variable neovascularized loose fibromyxoid tissue (Figure 7). These remnants consistently lacked inflammation.
Figure 6.
Paucicollagenous obliteration, a subset of inactive remnants. A, remnant level B-3 shows subtle central obliterative lesion with normal vessels and nerves displaced to periphery. B, remnant level B-7 shows ill-defined central vascularized scar. C, remnant level B-5 shows ill-defined central vascularized scar. Notably absent are peribiliary glands.
Figure 7.
Inactive fibrous cords. A, remnant level B-5 is the seat of a non-discrete central scar without inflammation. Cicatricial collagen surrounds surviving peribiliary glands at the periphery, one of which communicates with a compressed lumen lined by tall cholangiocytes. B, remnant level B-1 shows discrete fibrous cord with a few retained minute epithelial nests. C, remnant G-1 shows a discrete fibrous cord. An inactive lymph node is adjacent.
Tertiary lymphoid aggregates, defined as condensed aggregates of small lymphoid cells located at the periphery of a duct remnant (Figure 3) were distinguished from more diffuse mononuclear cell-rich infiltrates or inconspicuous loose aggregates and were tabulated as a variable independent of active or inactive status of the inflammatory infiltrate.
Alternate categories were as follows: ‘normal duct/gall bladder profile’ or ‘not assessable’ because of insufficient tissue or oblique orientation.
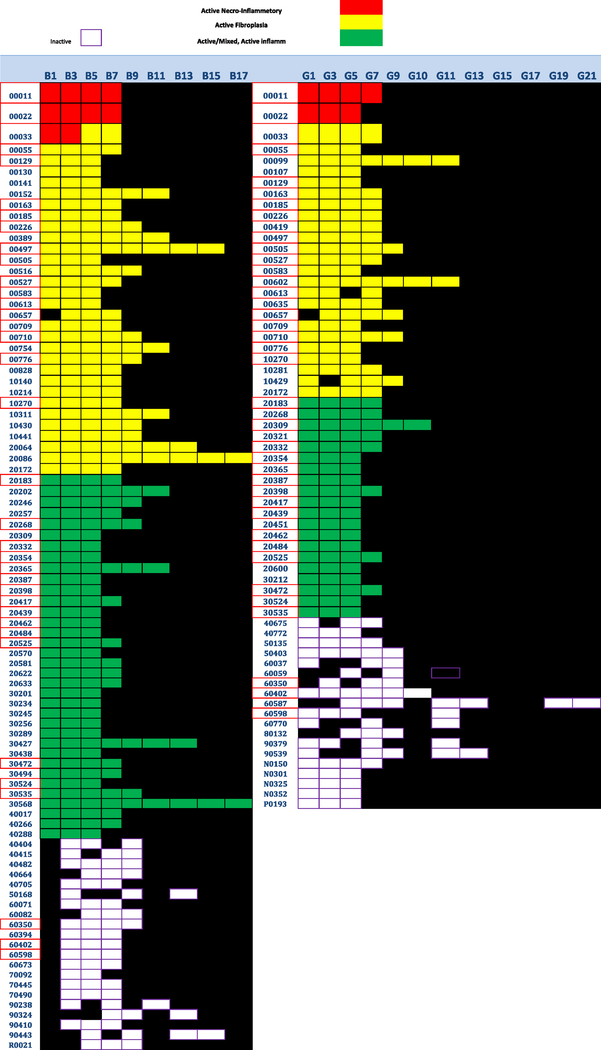
Homogeneity within a remnant was initially a suspicion based on superficial scrutiny of the data. An arbitrary definition of homogeneity was adopted based on three or more levels designated active or inactive within any B or G series. Tabulation of the data from subjects meeting this definition are presented in Table 3 and in a box plot, Figure 8.
Table 3.
Biliary Remnants: 134 Subjects with Homogeneous Histology in 3 or more levels of Hepatic Duct (B-series) vs Age at KHPE
| 0–40d | 41–60d | 61–79 | 80–151 | ||
|---|---|---|---|---|---|
| Histological class of homogeneous hepatic duct atresia specimens (B-series) | n-30 subjects | N=48 subjects | N=55 subjects | N=36 subjects | N=169 subjects* |
| Necroinflammatory. | 0 | 2 | 0 | 0 | 2 |
| Active fibroplasia. | 4 | 13 | 12 | 10 | 39 |
| Active Fibroplasia/mixed inflammation. | 5 | 10 | 15 | 9 | 39 |
| Inactive. | 14 | 13 | 16 | 11 | 57 |
| Subjects with homogeneous active or inactive histology | 23 | 38 | 43 | 30 | 134 |
| Subjects with heterogeneous histology | 7 | 10 | 12 | 6 | 35 |
Age at KHPE was not available in 3 subjects
Figure 8.
Color-coded box plot illustrates the number and location of levels in homogeneous remnants. Left, B series; right, G series. Red, necroinflammatory; Yellow, active fibroplasia; Green, active fibroplasia with mixed inflammation; White, Inactive; Black, indeterminate or non-assessable. Inclusion criteria were at least three levels in the B or G series that were either active or inactive. The only exception in the boxplot is one remnant that had only two levels classed as necroinflammatory, included here to emphasize the clustering of necroinflammatory lesions in 4 subjects accounted for 17 of 19 levels so classified (Table 1). Twenty-two of 23 remnants shown as black boxes were in the homogeneously inactive hepatic duct sets.
Results
Using a step-section approach to sampling, with alternate remnant sections frozen and retained for future research, the average number of hematoxylin and eosin stained sections per remnant was 6.2 including both hepatic duct (B) and cystic duct/gall bladder (G) series. The average number of alternate levels frozen fresh and retained for research is similar.
Overall survival with the native liver 2 years post KHPE in the BA patients with followup data was 94/165 (58%), similar to the much larger group of patients with BA who had been accessioned as ChildREN network subjects during the period of observation and to the best results reported by other groups.
Liver tissue in the most proximal hilar sample was assessed as a guide to the depth of the surgical dissection during excision of the biliary remnant. Liver tissue was present in only 30/169 subjects(18%). Total bilirubin less than 2.0 mg/dl at age 3 months post KHPE (p=0.84) and overall survival with the native liver at 2 years post KHPE (p=1.0) were unrelated to presence or absence of liver tissue in the most proximal remnant sample.
Activity and character of lesions in biliary remnant
Table 1 lists the detailed histopathological classification of all remnant levels in the B series and G series.
Histological features did not relate to position within the remnants in either series. This is dramatically demonstrated when histological diversity in the series is plotted by remnant level (Figure 2). Examples of the various classes of histological change encountered are illustrated in Figures 3–7. The range of histopathological changes was similar in the hepatic duct and gall bladder, the B and G series respectively. Necroinflammatory lesions (Figure 3a, b, c) were found in only 19/1072 levels (.02%); these occurred at all levels of remnants but with a minor predilection for the hilar region (hepatic duct levels 1 and 3). It is noteworthy that 17 of 19 examples of necroinflammatory lesions were found in remnants from just 4 subjects.
The stage in the proximal hepatic duct remnant was most commonly classified as active fibroplasia with no inflammation (Figure 4a–c) or active fibroplasia with intermixed mononuclear cell predominant inflammation (Figure 5a–c). Levels classified as inactive obliterated duct were observed at all levels of the hepatic duct and gall bladder. Well oriented levels with evidence suggesting a minimal paucicollagenous central scar without inflammation were surprisingly common (Figure 6a–c). One, or less commonly two, patent “main” lumens of diminished caliber were identified in 329 levels. In the latter scenario, both lumens were usually accompanied by a collar of active circumferential fibroplasia with or without a mononuclear inflammatory cell infiltrate but differing histologies were occasionally observed in pairs of residual ducts with inactive lesions (Figure 7a). Fibrous cords occasionally contained tiny residual lumens (Figure 7b). Gall bladder remnants displayed both active and inactive lesions and extreme variability in thickness of the smooth muscle component that often appeared to be independent of lesion activity. In 39 BA remnants, the gall bladder was histologically normal (Table 1).
Measurements of lumens were not made but estimates of lumen diameter classed as a “main lumen” were typically in the range of one-third to two-thirds of a normal infant extrahepatic bile duct (0.1 cm). Using two-tailed contingency table analysis, presence of a “main” but damaged duct with circumferential active fibroplasia, variable inflammation and a diminished main lumen (estimated at 0.3–0.5 mm) anywhere in the B series or within the first two most proximal levels did not predict favorable outcome, defined as early successful drainage or survival with native liver 2 years post KHPE.
Lumen status in the B and G-series was recorded during central review as one identifiable main lumen (n=329), 2 more or less equal lumens (n=24) or multiple (3 or more tiny lumens (n=227) or no lumen(s) identified (n=283). Multiple small ducts possibly representing peribiliary glands (3 or more) were most common in the B series; a single profile was most common in the G series (Figure 2b).
Fifty remnant levels displayed a histologically normal hepatic duct of normal diameter (n=11) or gall bladder (n=39) profile. In hepatic duct remnants from five subjects, we observed one or more levels with a duct profile of normal-size; several of these had intact epithelium underlain by mild subepithelial fibrosis without active inflammation. These rare findings were interpreted as bona fide “skip” lesions”, levels where the extrahepatic biliary tree was spared or the process of biliary atresia may have been arrested at a stage when the lumen/epithelium was not fully compromised. The majority of the histologically normal gall bladder sections were concentrated in a small number of subjects (n=7) in whom all samples of the gall bladder, though of small diameter, lacked evidence of an active or inactive atresia process.
For the largest duct profile in every sample, we estimated the amount of preserved attached epithelium as < 10%, 10–50% or > 50% of the lumenal surface (Figure 2c). Reactive/atrophic epithelium was most common in the hepatic duct series and normal columnar epithelium was most common in the cystic duct/gall bladder series. A contingency table analysis compared outcome in subjects with a main lumen lined by > 50% intact epithelium at the two most proximal levels and also at the most proximal single level of the hepatic duct to duct remnants in those same locations with less than 10% preserved epithelium. No significant difference in outcome was observed.
Inflammatory infitrates in necroinflammatory lesions were often neutrophil-rich, and occasionally lymphocyte-rich. In lesions classed as active fibroplasia with mixed inflammation, mononuclear cells always predominated (Figure 2d). Tertiary lymphoid aggregates (TLA) located eccentric to a damaged, active or inactive fibrotic duct remnant were identified somewhere in the remnant in 162 of 1063 levels from 43 subjects (Figure 2e). In 125 remnants, no TLA were observed at any level. Fourteen subjects had 1, 10 had 2 and 19 had 3 or more TLA.
In the hepatic duct remnant only, 63 subjects had no TLA, forty-four subjects had one TLA, and 57 subjects had two or more TLA. There was no significant relationship between either absence or presence of any lymphoid aggregates anywhere in the remnant, or when evaluated only for the hilar-most samples, to age at KHPE (Table 2) or to either of the two outcome measures. Lymphoid aggregates occurred in subjects with remnants that were homogeneously either active or inactive but differences in age at KHPE or outcome between these subgroups were not statistically significant.
Bundles of smooth muscle were found in 738 sections and were absent in 334 sections of remnants. Inconspicuous, partly circumferential bundles were identified in 29% of sections of the hepatic duct remnant (B series) and 53 % of sections of the gall bladder remnant (G series). Circumferential completeness and muscle quality and quantity in the gallbladder were not assessed.
A single microscopic nodule of cartilage was identified in non-inflamed stroma adjacent to one level of one gall bladder remnant.
Histological homogeneity, defined as active or inactive at all levels in remnant from a given subject was a common feature in the B series (n=89 or 53% of 168 subjects), and less common in the G series (n=65 or 38% of subjects). Remnant homogeneity throughout both B and G series was less common (n=36 or 21% of subjects).
Remnants from subjects with histological homogeneity in either the B or the G series are illustrated in a box plot that conveys the number of levels so classified in each of these remnants (Figure 8).
The age at the time of KHPE in those subjects with histological homogeneous hepatic duct remnants appear in Table 3. To investigate a possible relationship between age at KHPE and homogeneously active or inactive hepatic duct profiles we used contingency table analysis (Fisher exact test, 2-tailed) to compare the activity or lack thereof in remnants from subjects < 40 days old with those 41–151 days old (p=0.0198). No statistically significant differences were observed between the three older cohorts. This data suggests that inactive end-stage histology was relatively more common in the youngest cohort and progressive active fibroplasia was more common by far and to an equal degree in the three older cohorts. However, neither active nor inactive homogeneous subsets of the hepatic duct remnant at KHPE predicted outcome defined either as normalization of total bilirubin at 3 months post KHPE or as survival with the native liver 2 years post KHPE. It is notable that almost all of the B-1 levels that are represented as black boxes in the box plot occurred in remnants that were deemed homogeneously inactive; these most proximal B levels had been classified as not assessable or indeterminate, as might be expected if the remnant was indeed homogeneously inactive.
Visceral heterotaxy
Visceral heterotaxy was defined at laparotomy and judged by the surgeon to be present in 8 subjects who had asplenia/polysplenia, situs inversus, and/or a combination of malrotation, midline liver and preduodenal portal vein. Four additional subjects had malrotation only. The mean age of these 12 subjects at KHPE was 59 days (range 26–106 days). These remnants had an average of 6 levels examined which were classified as active (n=38), inactive(n=32), inactive with minimal scar (n=2), normal (n=1), and non-assessable (n=1). Four of 12 remnants were homogeneous: 3 with active fibroplasia and 1 with necroinflammatory lesions at multiple levels. The overall pattern of homogeneous versus heterogeneous histology did not differ from that observed in remnants from subjects without visceral anomalies.
Discussion
The success of the KHPE over the last 4 decades has provided opportunity to examine the remnants of the biliary tree in infants with biliary atresia within the first few months of life. Compared to previous studies, our prospective multi-institutional study of 172 centrally reviewed step-sectioned biliary remnants provides important insights into the histopathology of BA as it exists at the time of KHPE, but raises as many questions as it resolves.
We have confirmed previous histological studies that emphasized histological heterogeneity (4, 7, 8). We demonstrate that necroinflammatory lesions are rare (19/ 1072 = .02%) and are not related to young age at KHPE, nor do they increase or decrease in frequency with age at KHPE; most examples were present at multiple levels in a small subgroup of patients, including one with malrotation. These findings cast serious doubt on the proposition that biliary atresia is initiated by a flagrant acute destructive process. Florid inflammatory cholangitis was so uncommon that it is unlikely to play a primary role in pathogenesis of BA unless very rapid progression to fibrosis occurs in most patients by the time remnants are excised. Moreover, finding such lesions at multiple levels of the hepatic duct in only a few subjects is more consistent with the hypothesis that these overtly destructive inflammatory lesions are secondary either to acquired infection or to bile-induced chemical irritation. We favor use of the umbrella term fibrous obliteration to describe the lesion of BA until the initial events that lead to BA are unraveled.
Composition of reactive tissue in proximal hepatic duct remnant
The most prevalent lesion at any age is active fibroplasia with or without an accompaniment of mononuclear inflammatory cells. Active cellular circumferential fibroplasia, was common at the liver hilum, where it was observed in 60% of the two most proximal remnant sections. Homogeneous active fibroplasia throughout the remnant was common in all age groups except subjects 40 days old or less (n=30) who had a significantly higher ratio of end stage lesions to active fibroplasia than the older cohorts. Because only 11 samples were from infants 30 days old or less at the time of KHPE, many more evaluations are needed from the youngest infants to verify this. The number of remnants from infants with visceral anomalies including heterotaxy was also small (n=12) but it is notable that patterns of active vs inactive histology did not differ from those with no associated visceral anomalies. More samples are needed in this subgroup to confirm the apparent lack of relationship of remnant histology to anomaly status.
Our findings have implications for any discussion regarding the time of onset of the BA process, and the rate of progression to complete histological obstruction. The histology at the earliest stages of BA is unknown but clinical evidence of elevated conjugated bilirubin in the earliest post-natal blood sample (15, 16), suggests frequent onset at or before birth. KHPE for BA in infants 30 days old is uncommon, probably less than 10% of all cases to date (14). Remnants from these very young infants are not well characterized as yet. However, we found that homogeneous inactive end-stage atresia in the remnant is more likely in the youngest than in the older cohorts at the time of KHPE. This suggests the possible existence of two populations in BA differing in the time of onset and/or in the rate of progression to complete obstruction, both of which are important variables that are not understood.
Substantial level to level variability was observed in both the hepatic duct and gall bladder in about 20% of the samples. Equally common in heterogeneous hepatic duct remnants were active lesions that consisted of partly epithelial-lined ducts or clusters of periductal glands accompanied by active fibroplasia with or without an associated inflammatory infiltrate, and inactive lesions consisting of an irregular ill-defined scar, a discrete fibrous cord, or a mere hint of a central pauci-collagenous scar in an otherwise well-oriented section lacking a more obvious remnant. In remnants with heterogeneous histology, it may be inferred that lumenal compromise often advances unpredictably or may even spontaneously arrest. This would help explain why successful drainage may be achieved even when KHPE is performed in infants who are 3–4 months old (20–22).
Based upon overall prevalence and the plausible presumption of evolution from fibroplasia to end stage obliteration, active circumferential fibroplasia is the most likely determinant of the fate of the biliary tree. The active-appearing fibroblasts in BA remnants react as myofibroblasts with antibodies to smooth muscle actin and also have been referred to as “juvenile” type compared to “mature” fibroblasts (8, 22, 23). Active fibroplasia is commonly accompanied by a variable infiltrate of mononuclear cells that defines a large subset. Future studies using immunomarkers to characterize the infiltrate would be important because fibroplasia may be driven by cytokines produced by the infiltrate, however inconspicuous it might be.
In the hepatic duct series, partial obliquity of the samples undoubtedly contributed to reviewers’ difficulty in distinguishing residual main channel from peribiliary glands (see Figures 5B and 7B). A higher proportion of normal profiles was observed in the gall bladder compared to the hepatic duct. In 7 subjects, all sections of the gall bladder, though smaller than normal, were judged to be histologically normal and unaffected by the atresia process.
Smooth muscle is well developed in a normal infant gall bladder but inconstant and scant around the hepatic duct. Our topographic assessment of smooth muscle in the entire remnant disclosed that small clusters of smooth muscle fibers occur within the zones of active fibroplasia. Whether these are reactive or pre-existing is unclear. We speculate that a substantial number of the gall bladders in BA may be deficient in smooth muscle to a degree that may be independent of obliterating histology. Possibly disuse of the GB due to proximal obstruction is a factor. If it can be substantiated by further study that reduced smooth muscle in BA gall bladders is developmental rather than destructive, the possibility of a defect in mesenchymal differentiation merits consideration. One remnant had a nodule of ectopic cartilage adjacent to the gall bladder, a finding previously reported in BA remnants near the liver hilum (24, 25) and possibly a manifestation of mesenchymal instability in BA. The frequency of this finding in normal infants is unknown.
The reason why KHPE is a successful palliative procedure for BA has never been established. Normal or minimally involved duct remnants with patent lumens are often postulated to be the basis for successful drainage, but in practice have not consistently predicted success. Uncommonly we observed one or two residual sizeable lumens surrounded by active fibroplasia confined to one or two contiguous levels. Rarely a normal or near-normal hepatic duct profile was located near the hepatic hilum. Outcomes when these two types of residual lumens were located at the most proximal levels of the hepatic duct remnant did not differ significantly from the outcomes for the entire set. Thus, no particular histology of the hepatic duct remnant near the plane of surgical resection at the hilum predicted outcome. We hypothesize that establishment of bile flow after KHPE may be more dependent upon the ability to re-epithelialize the damaged channel at the point of transection then on its existence. Regrettably there was no consistency to the depth of hilar resection in this study; only a small fraction of the hilar samples contained liver tissue permitting only a tentative judgement about the status of the most proximal hepatic duct. Epithelialization, in turn may depend on presence of functional peribiliary glands (26). Multiplicity of tiny epithelialized lumina is the term we used as a proxy for peribiliary glands that were typically concentrated near the hepatic hilum where the Kasai incision is made. Whether or not affected by active fibroplasia, these structures may be capable of restoring a functional bile duct.
Hepatic duct regression without conventional scar
101 well-oriented levels in the sequence had no duct remnant or clearly defined scar and no inflammation. In many such samples, subtle evidence for regression of a pre-existing duct segment consisted of centrally located loose myxoid fibrovascular tissue with scant collagen deposition and normal peribiliary nerves and vessels located undisturbed at the periphery of the sample. We propose that this observation defines a previously unrecognized lesion in BA remnants that highlights the variability in fibrous scar production and provides a plausable explanation of surgical observations of “aplasia” or absence of an observable remnant/cord in any part of the extrahepatic biliary tree including the common bile duct.
Tertiary lymphoid aggregates (TLA) are a form of reactive extranodal lymphoid tissue that may be a response to bacteria, viruses, self-antigens or cancer-related neoantigens. In experimental settings, TLA appear approximately 14 days after exposure to bacteria (27). Experimental studies have failed to identify CMV in TLA during active CMV infection suggesting that TLA are cytokine driven (28). TLA were present in 16% of 1072 levels examined with statistically significant predilection for the B series over the G series. With time, TLA may develop reactive centers, although none were observed in our samples.
TLA were independent of whether the entire remnant was homogeneously active or inactive. Thus, it may be concluded that aggregates, once formed tend to persist as biliary atresia evolves to complete fibrous obliteration. We observed no significant relationship of either absence or presence of 1, 2 or 3 or more lymphoid aggregates to age at KHPE (Table 2) or to the two outcome measures. This contrasts with our previous observation that presence of lymphoid follicles with reactive centers in hilar lymph nodes strongly associates with poor outcome defined as failure of the native liver to survive 2 years after KHPE (29).
If one assumes progression from initiation to active fibroplasia with or without mixed inflammatory cell infiltrate to end stage fibrosis, our findings indicate that the process of atresia, regardless of when it begins, evolves at a highly variable rate through phases of incomplete to complete obstruction. If so, further reducing the age of diagnosis and KHPE may have little or no effect on survival. On the other hand, interrupting the sequence to obliteration might be achieved by inhibiting fibroplasia at any time that restorable lumens remain.
We have documented intra-remnant variability in greater detail than in any previous reported study. This variability tends to obscure the relentlessly progressive histology of the process best appreciated in the absence of variability in the large group of remnants with homogeneous histology. Homogeneous remnants are synchronized in a pattern of either uniformly active progressive injury or inactive end stage atresia. Importantly, the youngest subjects with homogeneous remnants, <40 days old at KHPE have a higher proportion of inactive end-stage lesions than infants who are older at KHPE, but we found no relationship between outcome and age in the youngest cohort that included a small subgroup of 11 subjects who were < 30 days old at KHPE.
Contemporary reviews of BA postulate the existence of an embryonic form of biliary atresia based on very early conjugated hyperbilirubinemia and circumstantial evidence such as association with congenital anomalies not limited to visceral heterotaxy, a possible relationship to congenital cytomegalovirus infection and a morphologically distinct variant, cystic biliary atresia, that may be recognized by ultrasound in utero (30–32). Compared to sporadic BA, cystic BA has distinctive laminar sclerosis with extensive epithelial loss (24). Remnants from infants that meet the suggested criteria for embryonic BA have not been systematically evaluated and compared to those from infants with sporadic BA. In our small cohort of subjects with visceral heterotaxy we observed a pattern of active and inactive obliterative lesions similar to that seen in our larger group of subjects with sporadic BA and not differing from histology recently reported in remnants proximal to the cyst of cystic biliary atresia (24). In sporadic BA and especially in cystic BA, the importance of active fibroplasia at any point in the biliary tree to outcome is underscored by the florid myofibroplasia with total loss of surface epithelium that characterizes cysts (24). Thus, the evidence to date suggests that the morphology of atresia at the time of excision does not differ in the putative embryonic and sporadic forms. We interpret this to mean that histology in biliary remnants is not linked either to the time in early gestation when developmental anomalies are established or to the time in late gestation when cystic BA may be discovered by ultrasound. However, the tendency for homogeneous remnants from the youngest infants to have more inactive lesions would be consistent with existence of two forms of BA with either early (perinatal) or later onset, or possibly a variable rate of progression in BA that is independent of age.
We conclude from our detailed study of 172 remnants that extrahepatic BA is driven by active fibroplasia leading to fibrous or fibromyxoid lumen obliteration. Prevalence of partially preserved epithelium in active fibroplastic BA lesions at all ages suggests that epithelial regression or injury may not be a primary event or that re-epithelialization is already underway at the time of KHPE. We hypothesize that outcome after KHPE is the result of competition between active fibroplasia and re-epithelialization of retained, collapsed but not obliterated lumens
Associated mononuclear cell inflammatory infiltrate is inconstant and disproportionately minor compared to active fibroplasia. This study did not assess the nature of and potential fibrogenic role for mononuclear inflammatory cells that commonly lurk in the background in small numbers. Accordingly, in the future, either inflammation itself, or active fibroplasia may be suitable targets for early intervention. Antifibrotic agents such as those now in trials in adults with pulmonary fibrosis (32) may have a therapeutic role if safety in infants is established and if and when it is determined that early diagnosis with early KHPE (<30 days) does not improve outcomes.
Acknowledgments
We thank the Childhood Liver Research and Education Network (ChiLDREN*) investigators, coordinators, and families who participated and made the present work possible.
FUNDING: This work was supported by U01 grants from the National Institute of Diabetes, Digestive and Kidney Diseases (DK 62445 [Mount Sinai School of Medicine], DK 62497[Cincinnati Children’s Hospital Medical Center], DK 62470 [Children’s Healthcare of Atlanta], DK62481 [The Children’s Hospital of Philadelphia], DK 62456 [The University of Michigan], DK 84536 [Riley Hospital for Children], DK 84575 [Seattle Children’s Hospital], DK 62500 [UCSF Children’s Hospital], DK 62503 [Johns Hopkins School of Medicine], DK 62466 [Children’s Hospital of Pittsburgh of UPMC], DK 62453 [Children’s Hospital Colorado], DK 62452 [Washington University School of Medicine], DK 84538 [Children’s Hospital Los Angeles], DK 62436 [Ann & Robert H Lurie Children’s Hospital of Chicago]).
References
- 1.Mack CL, Feldman AG, Sokol RJ. Clues to the etiology of bile duct injury in biliary atresia. Semin Liver Dis. 2012;32:307–16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Feldman AG, Mack CL. Biliary atresia: cellular dynamics and immune dysregulation. Semin Pediatr Surg. 2012: 21;192–200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Petersen C, Davenport M. Aetiology of biliary atresia: what is actually known? Orphanet J Rare Dis. 2013: 8; 128- [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gautier M, Jehan P, Odievre M. Histologic Study of biliary fibrous remnants in 48 cases of extrahepatic biliary atresia: correlation with postoperative bile flow restoration. J Pediatr. 1976;89:704–9. [DOI] [PubMed] [Google Scholar]
- 5.Gautier M Atresia of the extrahepatic bile ducts. Etiologic hypothesis founded on a histological study of 130 fibrous remnants. Arch Fr Pediatr. 1979;36(9 suppl):III–XII. [PubMed] [Google Scholar]
- 6.Gautier M, Eliot N. Extrahepatic biliary atresia: Morphological study of 98 biliary remnants. Arch Pathol Lab Med. 1981;105:397–402. [PubMed] [Google Scholar]
- 7.Tan CE, Davenport M, Driver M, Howard ER. Does the morphology of the extrahepatic biliary remnants in biliary atresia influence survival? A review of 205 cases. J Peditr Surg. 1994;29:459–64. [DOI] [PubMed] [Google Scholar]
- 8.Zheng S, Luo Y, Wang W, Xaio X. Analysis of the pathomorphology of the intra- and extrahepatic biliary system in biliary atresia. Eur J Pediatr Surg. 2008;18:98–102. [DOI] [PubMed] [Google Scholar]
- 9.Arva NC, Russo PA, Erlichman J, Hancock WW, Haber BA, Bhatti TR. The inflammatory phenotype of the fibrous plate is distinct from liver inflammation and can predict clinical outcome in biliary atresia. Pathol Res Pract. 2015;211:252–60. [DOI] [PubMed] [Google Scholar]
- 10.Nakamura H, Koga H, Miyano G, Okawada M, Doi T, Yamataka A. Does the level of transaction of the biliary remnant affect outcome after laparoscopic Kasai portoenterostoy for biliary atresia// J Laparoendosc Adv Surg Tech A. 2017;27:744–47. [DOI] [PubMed] [Google Scholar]
- 11.Davenport M, Caponcelli E, Livesey E, Hadzic N, Howard E. Surgical outcome in biliary atresia: etiology affects the influence of age at surgery. Ann Surg. 2008;247:694–8. [DOI] [PubMed] [Google Scholar]
- 12.Sarinet M-O, Wildhaber BE, Broue P, Lachaux A, Sarles J, Jacquemin E, Gauthier F, Chardot C. Impact of age at Kasai operation on its results in late childhood and adolescence: a rational basis for biliary atresia screening. Pediatr. 2009;123:1280–6 [DOI] [PubMed] [Google Scholar]
- 13.Song Z, Dong R, Shen Z, Chen G, Yang Y, Zheng S. Surgical outcome and etiologic heterogeneity of infants with biliary atresia who received Kasai operation less than 60 days after birth: a retrospective study. Medicine (Baltimore). 2017;96:e7267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Volpert D, White F, Finegold MF, Molleston J, Debaun M, Perlmutter DH. Outcome of early hepatic portoenterostomy for biliary atresia. Pediatr Gastroenterol Nutr. 2001; 32:265–9 [DOI] [PubMed] [Google Scholar]
- 15.Harpavat S, Ramraj R, Finegold MJ, Btandt ML, Hertel PM, Fallon SC, Shepherd RW, Shneider BL. Newborn direct or conjugated bilirubin measurements as a potential screen for biliary atresia. J Pediatr Gastrenterol Nutr 2016;62:799–803 [DOI] [PubMed] [Google Scholar]
- 16.Harpavat S, Garcia-Prats JA, Shneider BL. Newborn bilirubin screening for biliary atresia. N Engl J Med 2016;375:605–6 [DOI] [PubMed] [Google Scholar]
- 17.Russo P, McGee JC, Anders RA, Bove KE, Chen Z, Finegold MJ, Chung C, Cummings OW, Finn LS, Jaffe R, Kim GE, Lovell MA. Magid MS, Melin-Aldana H, Sheta BM, Wang, White FV, Spino C, Needle liver biopsies in cholestatic infants: correlation with clinical diagnosis of biliary atresia in a multicenter study. Am J Surg Path 2016;40;1601–15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Schwarz KB, Haber BH, Philip R, Mack CL, Jeffrey M, Bove KE, Bezerra JA, Karpen SJ, Nanda K, Shneider BL, Turmelle YP, Whitington PF, Molleston JP, Murray KF, Ng VL, René R, Wang KS, Sokol RJ, Magee JC; for the Childhood Liver Disease Research and Education Network. Extra-hepatic anomalies in infants with biliary atresia: results of a large prospective North American multi-center study. Hepatology. 2013;58:1724–31 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Superina R, Magee JC, Brandt ML, Healey PJ, Tiao G, Ryckman F, Karrer FM, Iyer K, Fecteau A, West K, Burns RC, Flake A, Lee H, Lowell JA, Dillon P, Colombani P, Ricketts R, Li Y, Moore J, Wang KS. The anatomic pattern of biliary atresia identified at time of Kasai hepatoportoenterostomy and early postoperative clearance of jaundice are significant predictors of transplant-free survival. Ann Surg. 2011; 254:577–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Davenport M, Puricelli V, Farrant P, Hadzic N, Mieli-Vergani G, Portmann B, Howard ER. The outcome of the older (> or = 100 days) infant with biliary atresia. J Pediatr Surg. 2004;39:575–81 [DOI] [PubMed] [Google Scholar]
- 21.Wong KK, Chung PH, Chan IH, Lan LC, Tam PK. Performing Kasai portoenterostomy beyond 60 days of life is not necessarily associated with a worse outcome. J Pediatr Gastroentero Nutr. 2010;51:631–4 [DOI] [PubMed] [Google Scholar]
- 22.Chen G, Zheng S, Sun S, Xiao X, Ma Y, Shen W, Chen L, Song Z. Early surgical outcomes and pathological scoring values of older infants (> 90 d old) with biliary atresia. J Pediatr Surg 2012;47:2184–8 [DOI] [PubMed] [Google Scholar]
- 23.Lobeck I, Dupre P, Sheridan R, Tiao G, Lovell M, Bove KE. Cystic biliary atresia and choledochal cysts are distinct histopathological entities. Am J Surg Pathol. 2016;41:354–64 [DOI] [PubMed] [Google Scholar]
- 24.Mirkin LD, Knisely AS. Hyaline cartilage at porta hepatis in extrahepatic biliary atresia. Pediatr Pathol Lab Med 1997;17:587–91 [PubMed] [Google Scholar]
- 25.Stahlschmidt J, Stringer MD, Wyatt J, Davison S, Rajwal S, McClean P. Histologic oddities at the porta hepatis in biliary atresia. J Pediatr Surg 2008;43:1328–32 [DOI] [PubMed] [Google Scholar]
- 26.De Jong IEM, van Leeuwen OB, Lisman T, Gouw ASH, Porte RJ. Repopulating the biliary tree from the peribiliary glands. Biochim Biophys Acta 2017;17:30272–7 Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 27.Yam-Puc JC, GarciaCordero J, Calderon-Amador J, Donis-Maturano L, Cedillo-Barron L, Flores-Romo L. Germinal center reaction following cutaneous dengue virus infection in immune-competent mice. Front Immunol. 2015;6:188–94 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Magalhaes SM, Duarte FB, Vassallo J, Costa SC, Lorand-Metze I. Multiple lymphoid nodules in bone marrow biopsy in immunocompetent patient with cytomegalovirus infection: an immunohistochemical analysis. Revista da Sciedade Brasiliera de Medicina Tropical. 2001;34:365–68 [DOI] [PubMed] [Google Scholar]
- 29.Bove KE, Sheridan R, Fei L, Anders R, Chung CT, Cummings OW, Finegold MJ, Finn L, Ranganathan S, Kim G, Lovell M, Magid MS, Melin-Aldana H, Russo P, Shehata B, Wang L, White F Chen Z, Spino C, Magee JC. Hepatic hilar lymph node reactivity at Kasai portoenterostomy for biliary atresia: correlations with age, outcome, and histology of proximal biliary remnant. Pediatr and Devel Pathol. 2017. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Humphrey TM, Stringer MD. Biliary Atresia: US diagnosis. Radiology. 2007;244:845–851. [DOI] [PubMed] [Google Scholar]
- 31.Tanaka H, Sasaki H, Wada M, Sato T, Kazama T, Nishi K, Kudo H, Nakamura M, Nio M. Postnatal management of prenatally diagnosed biliary cystic malformation. J Pediatr Surg. 2015;50:507–10. [DOI] [PubMed] [Google Scholar]
- 32.Shen O, Sela HY, Nagar H, Rabinowitz R, Jacobovich E, Chen D, Granot E. Prenatal diagnosis of biliary atresia: a case series. Early Hum Dev. 2017;111:16–19. [DOI] [PubMed] [Google Scholar]
- 33.Kolb M, Richeldi L, Behr J., et al. Nintedanib in patients with idiopathic pulmonary fibrosis and preserved lung volume. Thorax 2017;72:340–46 [DOI] [PMC free article] [PubMed] [Google Scholar]