Abstract
Background and Purpose
Hepatic mitochondrial pyruvate carrier (MPC) transports pyruvate into mitochondria. This study investigated the involvement of MPC1 in hepatic glucagon response, in order to identify a possible pharmacological intervention.
Experimental Approach
The correlation between hepatic glucagon response and MPC1 induction was investigated in fasted mice and primary hepatocytes. The effects of ginsenoside Rb1 on MPC1 function were observed.
Key Results
Glucagon challenge raised blood glucose with hepatic MPC1 induction, and inhibition of MPC induction coincided with a reduced rise in blood glucose. cAMP‐responsive element‐binding protein (CREB) knockdown blocked glucagon‐induced MPC1 expression, while CREB overexpression increased MPC1 expression. Luciferase reporter, chromatin immunoprecipitation assay, and promoter mutation confirmed that CREB increased MPC1 transcription through gene promoter induction. CREB regulated transcription co‐activator 2 nuclear translocation was also required for CREB to promote MPC1 induction. Glucagon shifted mitochondrial pyruvate towards carboxylation for gluconeogenesis via the opposite regulation of pyruvate dehydrogenase and carboxylase with respect to MPC1 induction. MPC1 induction was necessary for glucagon to promote pyruvate‐driven hepatic glucose production (HGP), but glucagon failed to influence HGP from other gluconeogenic substrates routed into the tricarboxylic acid cycle, independent of MPC. Rb1 blocked cAMP signalling by inhibiting AC activity and deactivated CREB by dephosphorylation, possibly contributing to inhibiting MPC1 induction to reduce HGP.
Conclusions and Implications
CREB transcriptionally up‐regulates MPC1 to provide pyruvate for gluconeogenesis. Rb1 reduced cAMP formation which consequently reduced CREB‐mediated MPC1 induction and thereby might contribute to limiting pyruvate‐dependent HGP. These results suggest a therapeutic strategy to reduce hyperglycaemia in diabetes.
Abbreviations
- CREB
cAMP‐responsive element‐binding protein
- CRTC2
CREB regulated transcription co‐activator 2
- HGP
hepatic glucose production
- MPC
mitochondrial pyruvate carrier
- PC
pyruvate carboxylase
- PDH
pyruvate dehydrogenase
- PDK4
pyruvate dehydrogenase kinase 4
- PGC‐1α
PPARγ co‐activator 1α
- TCA
tricarboxylic acid
What is already known
Hepatic MPC1 is increased in diabetes.
What this study adds
CREB mediates glucagon action to up‐regulate MPC1 expression via transcriptional regulation.
Ginsenoside Rb1 reduces cAMP formation and inhibits MPC1 induction.
What is the clinical significance
Suppression of MPC1 induction could reduce hyperglycaemia in diabetes.
1. INTRODUCTION
During fasting, increase in circulating pancreatic glucagon stimulates the gluconeogenic programme for glucose output to provide fuel for glucose‐dependent tissues, especially the brain. The hepatic glucagon response up‐regulates gluconeogenic genes to promote hepatic glucose production (HGP). The supply of gluconeogenic substrates is essential for glucose production. Although free amino acids, derived from the breakdown of muscle protein, can be routed through the mitochondrial matrix to support HGP during fasting, mitochondrial pyruvate is thought to be the major gluconeogenic substrate (Katz & Tayek, 1999; Rauckhorst et al., 2017). Cytoplasmic pyruvate is derived from several sources in the cytosol including glycolysis and systemically produced lactate and alanine. The import of pyruvate into mitochondria is mediated by the mitochondrial pyruvate carrier (MPC, SLC54, Bricker et al., 2012; Halestrap, 1975). In mitochondria, pyruvate is oxidized to acetyl‐CoA by pyruvate dehydrogenase (PDH) which is localized in the mitochondrial matrix. Alternatively, mitochondrial pyruvate may be carboxylated by pyruvate carboxylase (PC), an initial step in gluconeogenesis. In spite of being required for glucose homeostasis in the fasting state, enhanced hepatic glucagon response and excessive HGP are responsible for hyperglycaemia in diabetes (Unger & Cherrington, 2012).
MPC is a complex of MPC1 and MPC2 subunits, embedded in the inner mitochondrial membrane. Because MPC1 and MPC2 associate in an oligomer, loss of either protein can impair the stability of the other, resulting in loss of MPC function (Bender, Pena, & Martinou, 2015; Bricker et al., 2012; Herzig et al., 2012). In high fat diet‐fed mice, MPC mediates increased hepatic mitochondrial pyruvate utilization, contributing to hyperglycaemia and liver fibrosis (Rauckhorst et al., 2017). In diabetic mice, MPC function is enhanced to ensure efficient gluconeogenesis while loss of MPC activity attenuates hyperglycaemia (Gray et al., 2015; McCommis et al., 2015). MPC expression and function could be regulated at different transcriptional or post‐transcriptional levels (Bender & Martinou, 2016). It is therefore worth investigating whether or not the hepatic response to glucagon regulates MPC to ensure mitochondrial pyruvate availability for gluconeogenesis.
By binding to membrane GPCRs, glucagon triggers the activation of AC to increase cAMP production. Like other second messengers, cAMP activates the cAMP‐responsive element‐binding protein (CREB) by phosphorylation through PKA. CREB is a transcription factor that stimulates hepatic gluconeogenesis directly by binding to promoters for phosphoenolpyruvate carboxykinase and glucose‐6‐phosphatase genes (Altarejos & Montminy, 2011; Herzig et al., 2001). In addition, CREB also up‐regulates PPARγ co‐activator 1α (PGC‐1α) and NF‐κB‐inducing kinase to amplify gluconeogenesis (Altarejos & Montminy, 2011; Sheng et al., 2012). In fact, CREB is a multifunctional transcription factor that regulates metabolic signals in nutrient sensing (Altarejos & Montminy, 2011), a phenomenon which suggests that CREB activation could transcriptionally up‐regulate MPC induction, enabling the supply of mitochondrial pyruvate for efficient HGP.
The thiazolidinediones, which are known to attenuate hyperglycaemia in Type 2 diabetes, were unexpectedly found to inhibit MPC in muscle cells (Divakaruni et al., 2013), suggesting a possible alternative route for pharmacological intervention. Ginsenosides, the major active constituents in ginseng, are widely used in traditional Chinese medicine for the treatment of metabolic disorders. Ginsenosides are reported to reduce gluconeogenesis, ameliorate insulin resistance, and promote glucose transport (Bai et al., 2018; Liu et al., 2017), demonstrating their potential in the regulation of glucose homeostasis. Ginsenoside Rb1 (Rb1) is the most abundant of the ginsenosides with a demonstrable biological activity against glucose and lipid metabolic disorders. Rb1 is known to exert antiobesity and antihyperglycaemic effects in obese mice (Xiong et al., 2010). Although Rb1 ameliorates metabolic disorders via diverse mechanisms, such as regulation of nervous activity in the hypothalamus and insulin sensitivity in skeletal muscle (Shen et al., 2015; Xiong et al., 2010), its regulation of hepatic glucagon response and glucose production remains unrevealed. In this study, we explored the effect of Rb1 on pyruvate‐driven HGP from the perspective of mitochondrial pyruvate transport, with the aim of finding a potential of pharmacological intervention. Although different sources can serve as gluconeogenic substrates, we have shown that glucagon promoted pyruvate‐driven glucose production in a MPC1‐dependent manner due to the transcriptional up‐regulation of MPC1 by CREB activation. Rb1 reduced cAMP generation by inhibiting AC activity with CREB inactivation. This regulation might contribute to limiting mitochondrial pyruvate transport, indicating the pharmacological potential of inhibiting MPC1, in order to reduce HGP.
2. METHODS
2.1. Animals and ethical statement
The animal care and all experimental procedures were approved by the Animal Ethics Committee of China Pharmaceutical University. All efforts were made to minimize animal suffering and to reduce the number of animals used (in line with the 3Rs). Animal studies are reported in compliance with the ARRIVE guidelines (Kilkenny et al., 2010) and with the recommendations made by the British Journal of Pharmacology (McGrath & Lilley, 2015). Because hepatic gluconeogenesis and MPC1 induction occur in C57BL/6J mice is not related to the sex of the animals (Gray et al., 2015), we chose male mice in this study throughout for experimental consistency. Six to 8‐week‐old male C57BL/6J mice (18–22 g, RRID:IMSR_JAX:000664) were provided by the Laboratory Animal Center of Nanjing Qinglongshan (Nanjing, China). All animals were housed under specific pathogen‐free conditions. Mice were housed in a temperature‐controlled facility (23–25°C) and humidity 40–60% on a 12‐hr light–dark cycle with free access to standard food and water. The numbers of mice used for each experimental group are shown in the figure legends. Animals were randomized for treatment. Data collection and evaluation of all experiments were performed without knowledge of the group identity.
2.2. Preparation of primary mouse hepatocytes and cell culture
Primary hepatocytes were isolated from overnight fasted mice according to the method previously reported (Li et al., 2018). In brief, the liver was digested with collagenase IV (3 ml·min−1), and the hepatocytes were harvested by centrifuging. After filtering, the hepatocytes were collected and resuspended for culturing in DMEM supplemented with 10% (v/v) FBS, 100 U·ml−1 penicillin, and 100 μg·ml−1 streptomycin. After 4 hr, the medium was replaced with fresh DMEM containing 10% (v/v) FBS for continuous culture. The prepared hepatocytes were identified with CK‐18 staining to ensure purity. HepG2 cell line was obtained from the American Type Culture Collection (ATCC Cat# HB‐8065, RRID:CVCL_0027), and 293T cell line was obtained from Stem Cell Bank, Chinese Academy of Sciences (Cat# SCSP‐502, RRID:CVCL_0063). HepG2 and 293T cells were both cultured in DMEM supplemented with 10% (v/v) FBS without penicillin and streptomycin for transfection. Cultured cells were starved for 4 hr in serum‐free medium before experiments.
2.3. Pyruvate and glucagon tolerance tests
For pyruvate and glucagon tolerance tests, overnight fasted mice were treated with either Rb1 (20 or 50 mg·kg−1) or metformin (200 mg·kg−1) by gavage 2 hr before pyruvate (2 g·kg−1) or glucagon (2 mg·kg−1) administration by intraperitoneal injection. In the tolerance tests, UK5099 was administered by intraperitoneal injection (10 mg·kg−1). Blood was collected at regular intervals by tail bleeding for the assay of blood glucose or glucagon levels using commercial kits. After indicated treatment, mice were anaesthetized with inhalational isoflurane and killed by rapid cervical dislocation for the collection of the liver.
2.4. cAMP and AMP quantification and the assay of AC activity
Two hours after glucagon challenge in fasted mice, the liver was collected for the assay of intracellular cAMP and AMP using commercial kits (Cell Signaling Technology, Danvers, MA, USA), and the results were normalized to total cell protein content. Primary mouse hepatocytes were treated with glucagon (100 nM) for 2 hr and lysed in cell lysis buffer, and the supernatant fraction was harvested for the assays of cAMP and AMP. For the determination of AC activity, primary mouse hepatocytes were incubated with Rb1 for 2 hr, and the cells were harvested for the assay using the commercial kit.
2.5. Lactate accumulation and glucose production in hepatocytes
Hepatocytes were incubated in KRB solution (118‐mM NaCl, 4.7‐mM KCl, 1.2‐mM MgSO4, 1.2‐mM KH2PO4, 1.2‐mM CaCl2, 20‐mM NaHCO3, 25‐mM HEPES pH 7.4, and 0.025% BSA) containing relevant substrates or indicated agents for 6 hr, and then the medium was harvested for the assay of produced lactate or glucose using commercial kits. Glucose output was expressed as the percentage of untreated basal glucose output.
2.6. Transfection
We employed Lipofectamine 2000 reagent (Invitrogen, CA, USA) to transfect HepG2 cells. The cells were transfected with siRNA targeting CREB, MPC1, or CREB regulated transcription co‐activator 2 (CRTC2) for knockdown, while plasmid targeting CREB or MPC1 and empty vector (pCDNA and pEX‐3; GenePharma, Suzhou, China) were used for overexpression. After 24 hr of transfection, cells were cultured in a fresh medium for further experiments.
2.7. Luciferase reporter assay
The pGL3‐basic MPC1 promoter‐luciferase plasmid, wild type CREB, or mutant CREB (c.397T→G, encoding p.Ser133Ala) plasmid were co‐transfected into primary mouse hepatocytes. The transfection of the pGL3‐basic MPC1 (wild type) promoter‐luciferase plasmid, CREB plasmid, or mutant MPC1 promoter‐luciferase plasmid (the mutant sites is indicated in figure) were conducted in 293T cells using Lipofectamine® 2000. Twenty‐four hours after transfection, cells were treated as indicated in figure legends, and the cell lysates were used for luciferase assays using the dual‐luciferase substrate system (Promega, America). A Renilla luciferase reporter was used at 0.1 μg per well as an internal control.
2.8. Chromatin immunoprecipitation assay
Chromatin immunoprecipitation (ChIP) assay was performed according to the protocol from the SimpleChIP Enzymatic Chromatin IP Kit (Cell Signaling, 9002). Briefly, cells were fixed for 10 min at room temperature in 1% paraformaldehyde to cross‐link proteins to DNA. The chromatin was harvested using enzymic digestion. An aliquot of each sample was set aside as input control, while the remaining portion was immunoprecipitated with antibodies against CREB overnight at 4°C, with normal rabbit IgG as control. Immune complexes were washed in ChIP buffer, and the DNA‐protein cross‐links was reversed by addition of NaCl and kept at 65°C for 2 hr. After proteinase K digestion, DNA was purified with columns and then subjected to qRT‐PCR amplification using primers specific for the MPC1 promoter: site 1: forward primer: 5′‐CAAGCGATTCTTCTGTCTCAGCCTC‐3′; reverse primer: 5′‐AAGTCCCACCGCGGGTGCATATTGA‐3′. Site 2: forward primer: 5′‐GGGGATGTCCATTATTAACACTAAT‐3′; reverse primer: 5′‐ACGCCTGTAATCTCAGCACTTT‐3′. DNA enrichment was evaluated by average values of the eluate with immunoprecipitated DNA normalized to average values of input.
2.9. Quantitative real‐time PCR
Total mRNA was isolated, and cDNAs were synthesized using the HieffTM First Strand cDNA Synthesis Kit (Yeasen, Shanghai, China). RT‐PCR was performed on the CFX96™ Real‐Time PCR Detection System (Bio‐Rad, USA), details of which is described in Data S1.
2.10. Western blot analysis and immunoprecipitation
The antibody‐based procedures used comply with the recommendations made by the British Journal of Pharmacology. Liver tissue or cell protein was extracted with lysis buffer; the lysates were centrifuged; and the supernatants were collected. After quantification, the protein was resolved by 10–12% SDS‐PAGE, transferred onto PVDF membranes, then blocked in 5% non‐fat dry milk prepared in TBST, and incubated with primary antibodies followed by the corresponding secondary antibodies (Table S2). The relative expression level of the target protein was normalized to β‐actin or VDAC1. The antibody reactivity was then detected by ECL and quantified with ImageJ software (Version 1.48, RRID:SCR_003070).
For immunoprecipitation, lysates from treated primary mouse hepatocytes were centrifuged, and the supernatant was collected. Next, the anti‐CREB antibody was immunoprecipitated overnight at 4°C and then with protein A + G agarose beads according to the protocols. CRTC2 expressions were analysed by western blotting.
2.11. Immunofluorescence
After treatment, HepG2 cells were fixed with 4% paraformaldehyde for 20 min. The cells were permeabilized with 0.2% Triton X‐100 and incubated with 3% BSA for blocking non‐specific staining, followed by incubation with anti‐CRTC2 primary antibodies overnight at 4°C. After washing, the cells were incubated with FITC‐labelled goat anti‐mouse IgG antibody in the dark at 37°C for 1 hr and then incubated in DAPI for 15 min in the dark at 37°C. After washing, the cells were visualized under a confocal scanning microscope (Zeiss LSM 700).
2.12. Data and statistical analyses
The data and statistical analysis comply with the recommendations on experimental design and analysis in pharmacology (Curtis et al., 2018). All experiments were randomized and blinded. The results are expressed as the means ± SD of the indicated number (n) of independent experiments. The significance of differences was analysed by one‐way ANOVA followed by Tukey's test (more than two groups) or a Student's t test (two groups). The post hoc tests are performed only when F achieved P < .05, and there was no significant variance in homogeneity. All tests were two‐sided, and P < .05 was considered statistically significant. Statistical analysis was performed using GraphPad Prism 6.0 software (RRID:SCR_002798).
2.13. Materials
Ginsenoside Rb1 (purity ≥98%) was obtained from Nanjing Spring & Autumn Biological Engineering Co., Ltd. (China), while metformin was purchased from Shanghai Sangon Biological Engineering Co., Ltd. (China). UK5099 and CPI‐613 were from Apexbio Technology LLC (Houston, USA). Bt2‐cAMP was bought from Sigma (St. Louis, MO, USA). Forskolin and 666‐15 were purchased from MedChem Express (New Jersey, USA). H‐89 was from Beyotime (Shanghai, China), while reconstituted glucagon is a product of Novo Nordisk (Bagsvaerd, Denmark).
2.14. Nomenclature of targets and ligands
Key protein targets and ligands in this article are hyperlinked to corresponding entries in http://www.guidetopharmacology.org, the common portal for data from the IUPHAR/BPS Guide to PHARMACOLOGY (Harding et al., 2018), and are permanently archived in the Concise Guide to PHARMACOLOGY 2017/18 (Alexander, Fabbro, et al., 2017; Alexander, Kelly, et al., 2017).
3. RESULTS
3.13. Induction of MPC1 is associated with hyperglycaemia in response to glucagon and pyruvate load
In the fasting state, pancreatic glucagon secretion promotes hepatic gluconeogenesis. To determine if MPC1 induction is involved in hepatic gluconeogenesis, we measured hepatic MPC1 expression in fasting mice and found increased MPC1 protein expression (Figure 1a). Glucagon challenge further increased MPC1 expression (Figure 1a). Pyruvate load increased glucagon secretion (Figure 1b), and the MPC inhibitor UK5099 effectively attenuated the hyperglycaemic response with suppression of MPC1 protein expression (Figure 1c,d). Similarly, UK5099 also reduced the elevated blood glucose levels in response to glucagon challenge in the fasted mice (Figure 1e). These results indicate that MPC1 induction is involved in hepatic gluconeogenesis.
Figure 1.
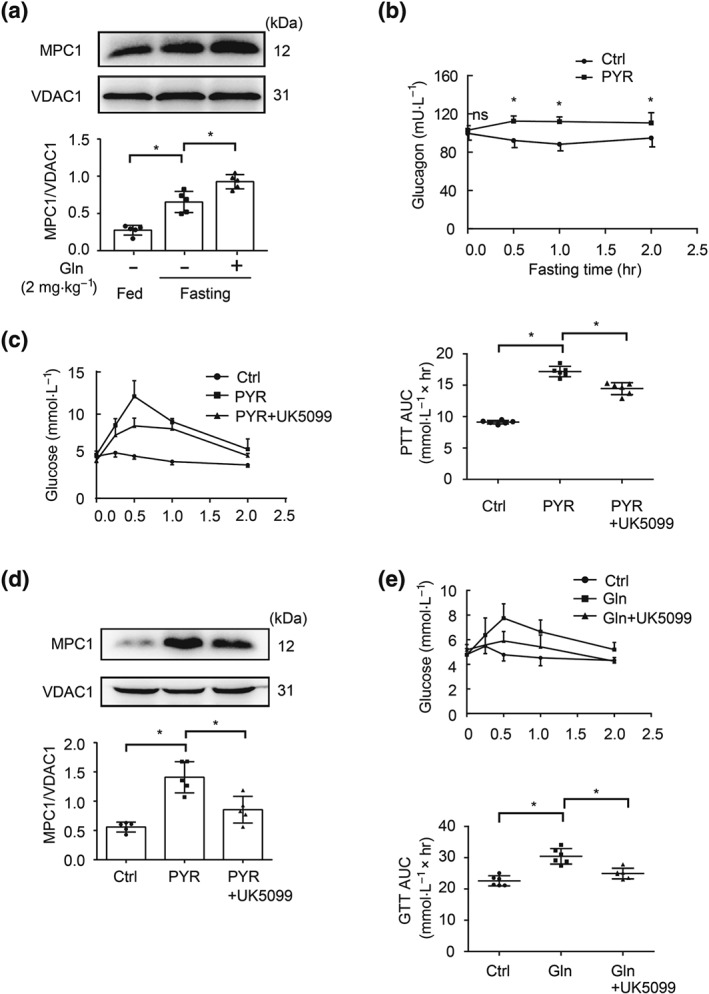
MPC1 expression in the liver of fasted mice. (a) Hepatic MPC1 protein expression during fasting (n = 5 each group); (b) glucagon secretion in response to pyruvate load (n = 6 each group); (c) blood glucagon levels in pyruvate tolerance tests (n = 6 each group); (d) protein expression of MPC1 in the liver of pyruvate‐treated mice (n = 5 each group); (e) blood glucose levels in glucagon tolerance test (n = 6 each group). Data shown are individual values with means ± SD. *P < .05, significantly different as indicated. Ctrl, control; Gln, glucagon; PYR, pyruvate
3.14. Glucagon increases MPC1 expression in hepatocytes
Glucagon activates AC to generate cAMP that initiates the hepatic glucagon response. In primary mouse hepatocytes, glucagon stimulation increased cAMP production with increased MPC1 protein expression (Figure 2a,b). Similarly, the AC activator forskolin and cAMP analogue Bt2‐cAMP also induced MPC1 gene and protein expression (Figure 2c–f), indicative of the role of cAMP in MPC1 induction. In response to cAMP formation, PKA activates CREB by phosphorylation. In line with this regulation, the PKA inhibitor H‐89 attenuated forskolin‐induced MPC1 protein expression in primary mouse hepatocytes (Figure 2g). The CREB inhibitor 666‐15 attenuated glucagon‐induced MPC1 protein expression, which suggests that glucagon increases MPC1 induction through CREB‐mediated transcriptional regulation (Figure 2h).
Figure 2.
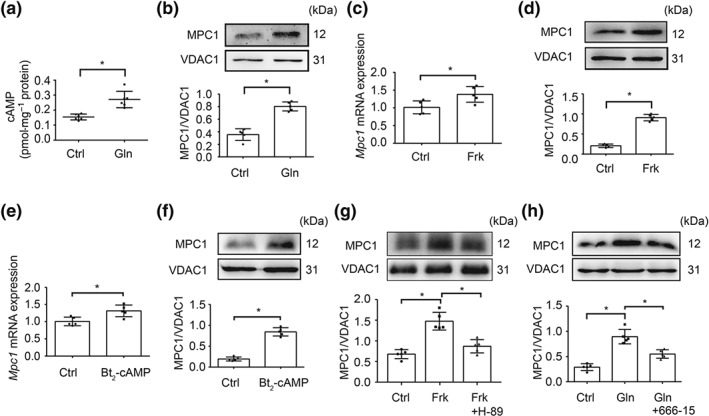
Glucagon increased MPC1 expression in hepatocytes. (a) cAMP contents in primary mouse hepatocytes treated with glucagon (100 nM) for 2 hr (n = 6 each group); (b) protein expression of MPC1 in primary mouse hepatocytes stimulated with glucagon (100 nM) for 2 hr (n = 5 each group); (c–f) MPC1 gene and protein expression in primary mouse hepatocytes exposed to forskolin (10 μM) or Bt2‐cAMP (100 μM) for 2 hr (n = 5 each group); (g) protein expression of MPC1 in primary mouse hepatocytes stimulated with forskolin (10 μM) for 2 hr in the presence of H‐89 (20 μM; n = 5 each group); (h) MPC1 protein expression in primary mouse hepatocytes incubated with glucagon (100 nM) for 2 hr (n = 5 each group). Data shown are individual values with means ± SD. *P < .05, significantly different as indicated. Ctrl, control; Frk, forskolin; Gln, glucagon
3.15. CREB transcriptionally up‐regulates MPC1
CREB is a transcriptional regulator encoding gluconeogenic genes. We transfected HepG2 cells with plasmid for CREB overexpression and found increased gene and protein expressions of MPC1 (Figure 3a,b) without affecting MPC2 gene expression (Figure S1a). In contrast, knockdown of CREB with siRNA diminished glucagon‐induced MPC1 gene and protein expressions (Figure 3c,d). Luciferase reporter assays showed that glucagon increased the activity of MPC1 in primary mouse hepatocytes (Figure 3e). In response to cAMP formation, PKA activates CREB by phosphorylation at Ser133 (Altarejos & Montminy, 2011). When primary mouse hepatocytes were transfected with wild type CREB plasmid, glucagon further increased luciferase reporter activity of MPC1, but the action was blocked by transfection of CREB (Ser133) mutant plasmid (Figure 3e). These results indicate the potential interaction between CREB and MPC1 induction. Two potential CREB‐binding sites in the MPC1 promoter region were identified in JASPAR database (Figure 3f), which display significant homology with the canonical CREB recognition motif TGACGTAA (Altarejos & Montminy, 2011). Subsequent ChIP assay showed that glucagon induced the recruitment of CREB to the MPC1 gene promoter at site 1 but not site 2 (Figure 3f). Concordantly, CREB failed to increase luciferase activity in 293T cells when co‐transfected with MPC1 mutant at site 1 vectors (Figure 3g). These results support the critical role and specific potential site for the transcriptional regulation of MPC1 gene by CREB. In this regard, forskolin‐ and Bt2‐cAMP induced glucose production were inhibited by the CREB inhibitor 666‐15 in primary mouse hepatocytes (Figure S1b,c). CRTC2 is a functional co‐activator for cAMP‐response genes, facilitating CREB for transcriptional regulation (Altarejos & Montminy, 2011). The result of immunoprecipitation showed that glucagon increased CRTC2 binding to CREB (Figure 3h). When CRTC2 was knocked down in HepG2 cells, glucagon failed to induce MPC1 gene expression (Figure 3i). These results indicate that nuclear translocation of CRTC2 with binding to CREB is required for CREB to promote MPC1 induction. In support of this, CREB overexpression failed to induce MPC1 gene expression in CRTC2 knockdown HepG2 cells (Figure 3j).
Figure 3.
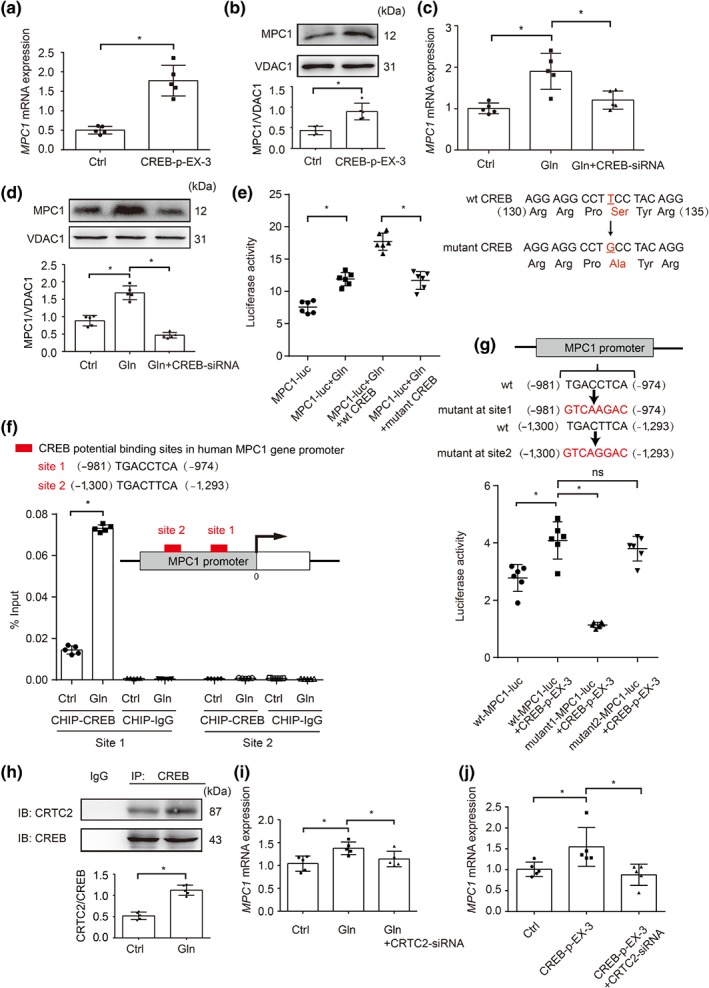
CREB transcriptionally up‐regulated MPC1 expression. (a) The mRNA levels of MPC1 in HepG2 cells transfected with CREB overexpressed plasmid (n = 5 each group); (b) protein expression of MPC1 in HepG2 cells transfected with CREB overexpressed plasmid (n = 5 each group); (c) relative mRNA abundance of MPC1 in glucagon‐stimulated HepG2 cells (100 nM, 2 hr) transfected with CREB siRNA (n = 5 each group); (d) protein expression of MPC1 in glucagon‐stimulated HepG2 cells (100 nM, 2 hr) transfected with CREB siRNA (n = 5 each group); (e) luciferase reporter assay for the promoting effect of glucagon (100 nM) on MPC1 gene promoter with wild type CREB or mutant CREB (c.397T→G, encoding p.Ser133Ala) plasmid were co‐transfected into primary mouse hepatocytes (the luciferase activity was normalized with the internal control, n = 6 each group); (f) ChIP–quantitative PCR analyses of CREB binding to MPC1 promoter in HepG2 cells stimulated by glucagon (100 nM) for 2 hr (n = 5 each group); (g) luciferase reporter assay for the promoting effect of CREB on MPC1 gene promoter or mutant MPC1 gene promoter in 293T cells (the luciferase activity was normalized with the internal control, n = 6 each group); (h) CRTC2 protein expression in precipitated CREB protein when primary mouse hepatocytes was stimulated with glucagon (100 nM) for 2 hr (n = 5 each group); (i) relative mRNA abundance of MPC1 in HepG2 cells transfected with CRTC2 siRNA when exposed to glucagon (100 nM) for 2 hr (n = 5 each group); (j) relative mRNA abundance of MPC1 in HepG2 cells transfected with CRTC2 siRNA with or without CREB overexpressed plasmid (n = 5 each group). Data shown are individual values with means ± SD. *P < .05, significantly different as indicated. Ctrl, control; Gln, glucagon; ns, not significant; wt, wild type
3.16. MPC1 is needed for glucagon to promote HGP
Logically, enhanced MPC function should reduce hepatic lactate accumulation due to the limited conversion of cytosolic pyruvate to lactate by LDH. Indeed, glucagon stimulation reduced lactate accumulation in the liver and primary mouse hepatocytes. This effect was attenuated by MPC inhibitor UK5099 in primary mouse hepatocytes (Figure 4a,b), indicating the role of MPC in glucagon action. When pyruvate was used as the only substrate in the culture medium (KRB solution containing 10 mM of pyruvate), glucagon also reduced lactate accumulation, but this effect was diminished by UK5099 and CREB inhibitor 666‐15 (Figure 4c). Concordantly, glucagon‐induced glucose production was attenuated by UK5099 in primary mouse hepatocytes (Figure 4d). Because gene expression of Pygl (glycogen phosphorylase, liver form) and Gys2 (glycogen synthase 2) were not affected (Figure S2a,b), we reasoned that the glucose produced was possibly minimally influenced by glycogenolysis. Methyl pyruvate is inner membrane‐permeable, freely entering into mitochondria without the transport by MPC. As expected, methyl pyruvate‐driven glucose production was not affected by glucagon or co‐treatment with MPC inhibitor UK5099 (Figure 4e), providing evidence to support our speculation that glucagon promotes pyruvate‐driven HGP dependent on MPC1. Aside from pyruvate, alanine and glutamine are also gluconeogenic substrates, which enter into mitochondria without MPC transport (Jeoung, Harris, & Harris, 2014). When alanine and glutamine were used as the substrates, no significant effects of glucagon on glucose production were observed (Figure 4f). These results indicate that under the chosen experimental conditions, glucagon‐induced HGP was mainly driven from pyruvate, and MPC function was needed.
Figure 4.
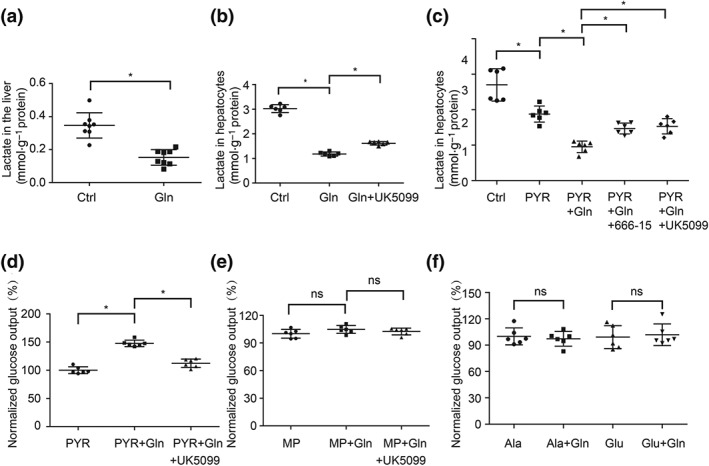
MPC1 is needed for glucagon to promote HGP. (a) Lactate accumulation in the liver of mice injected with 2 mg·kg−1 glucagon (n = 8 each group); (b) lactate contents in primary mouse hepatocytes treated with glucagon (100 nM, 6 hr) in the presence or absence of UK5099 (300 nM; n = 6 each group); (c, d) lactate and glucose production in primary mouse hepatocytes when pyruvate (10 mM) was used as the only substrate (n = 6 each group); (e, f) glucose production in primary mouse hepatocytes when methyl pyruvate (10 mM), alanine (10 mM), or glutamine (10 mM) were used as the only substrate, individually (n = 6 each group). The glucose production was expressed as the percentage of the basal level in PYR, MP, or Ala group. Data shown are individual values with means ± SD. *P < .05, significantly different as indicated. Ala, alanine; Ctrl, control; Gln, glucagon; Glu, glutamine; MP, methyl pyruvate; ns, not significant; PYR, pyruvate
3.17. CREB regulates PC and PDK4 in the context of MPC1
Upon entry into mitochondria, pyruvate is metabolized through competing metabolic pathways, oxidation by PDH, or carboxylation by PC for gluconeogenesis, depending on metabolic demands. To investigate the influence of glucagon on the fate of mitochondrial pyruvate, we examined PC and PDK4 expressions. The results show that glucagon increased Pc gene expression with impaired PDH activity, indicated by the increased Pdk4 gene expression (Figure 5a,b). Consistently, CREB overexpression in HepG2 cells increased PC gene and protein expression (Figure 5c,d). Meanwhile, PDK4 gene expression was also increased by CREB overexpression (Figure 5e). These results suggest that the hepatic glucagon response limits pyruvate oxidation to facilitate gluconeogenesis through carboxylation pathway in mitochondria. Concordantly, increased glucose output by glucagon was reduced but increased by PC inhibitor PAA and PDH inhibitor CPI‐613 (Figure 5f). Moreover, CREB overexpression‐induced gene expressions of PC and PDK4 were blocked by MPC1 knockdown in HepG2 cells (Figure 5g,h), indicating that the glucagon response regulates mitochondrial pyruvate metabolism in respect of MPC1 induction. As expected, CREB overexpression‐induced HGP was abrogated by MPC1 knockdown in HepG2 cells (Figure S3).
Figure 5.
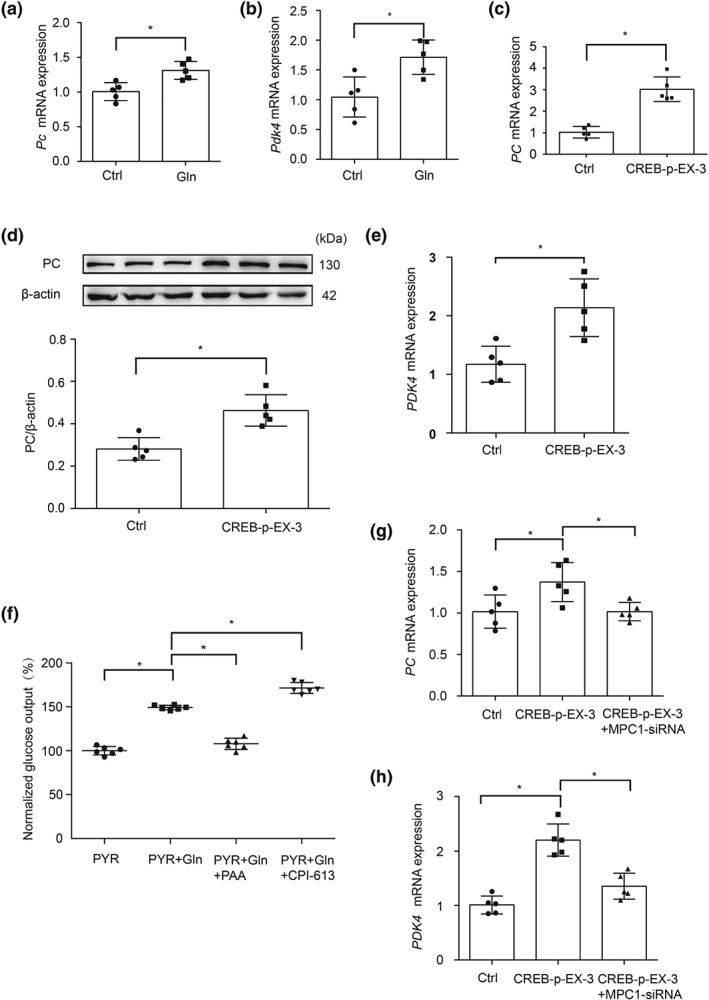
CREB regulates PC and PDK expression. (a, b) The mRNA levels of Pc and Pdk4 in primary mouse hepatocytes incubated with glucagon (100 nM) for 2 hr (n = 5 each group); (c, d) the mRNA expression and protein level of PC in HepG2 cells transfected with CREB overexpressed plasmid (n = 5 each group); (e) the mRNA expression of PDK4 in HepG2 cells transfected with CREB overexpressed plasmid (n = 5 each group); (f) glucose output in primary mouse hepatocytes incubated with glucagon (100 nM, 6 hr) in the presence or absence of phenylacetic acid (10 mM) or CPI‐613 (100 nM; n = 6 each group), the glucose production was expressed as the percentage of the basal level in PYR group; (g, h) the mRNA levels of PC and PDK4 in HepG2 cells co‐transfected with MPC1 siRNA and CREB plasmid (n = 5 each group). Data shown are individual values with means ± SD. *P < .05, significantly different as indicated. Ctrl, control; Gln, glucagon; PYR, pyruvate; PAA, phenylacetic acid
3.18. Rb1 attenuates hepatic glucagon response
Oral administration of Rb1 (20 or 50 mg·kg−1) attenuated hyperglycaemic response in glucagon tolerance test in fasted mice (Figure 6a). Meanwhile, Rb1 reduced cAMP accumulation with an increase in AMP contents in the liver (Figure 6b,c), indicating its ability to inhibit AC. Also, Rb1 inhibited AC activity in primary mouse hepatocytes (Figure 6d), and the IC50 of which was 0.88 μM (Figure S4). As a downstream regulation, Rb1 inactivated CREB by dephosphorylation in the liver of fasted mice challenged with glucagon (Figure 6e). The confocal microscopic image showed that Rb1 effectively prevented nuclear translocation of CRTC2 in HepG2 cells when exposed to forskolin (Figure 6f). As a consequence, Rb1 attenuated MPC1 protein expression (Figure 6g). Similarly, Rb1 administration also reduced hepatic MPC1 protein expression in fasted mice without glucagon stimulation (Figure 6h). Metformin lowers blood glucose by inhibiting hepatic gluconeogenesis (Miller et al., 2013). Using metformin as a positive control in vivo, we found that it exerted a similar regulation as Rb1 in mice. These results suggest the possibility that Rb1 attenuated hepatic glucagon response with the implication of MPC1 inhibition.
Figure 6.
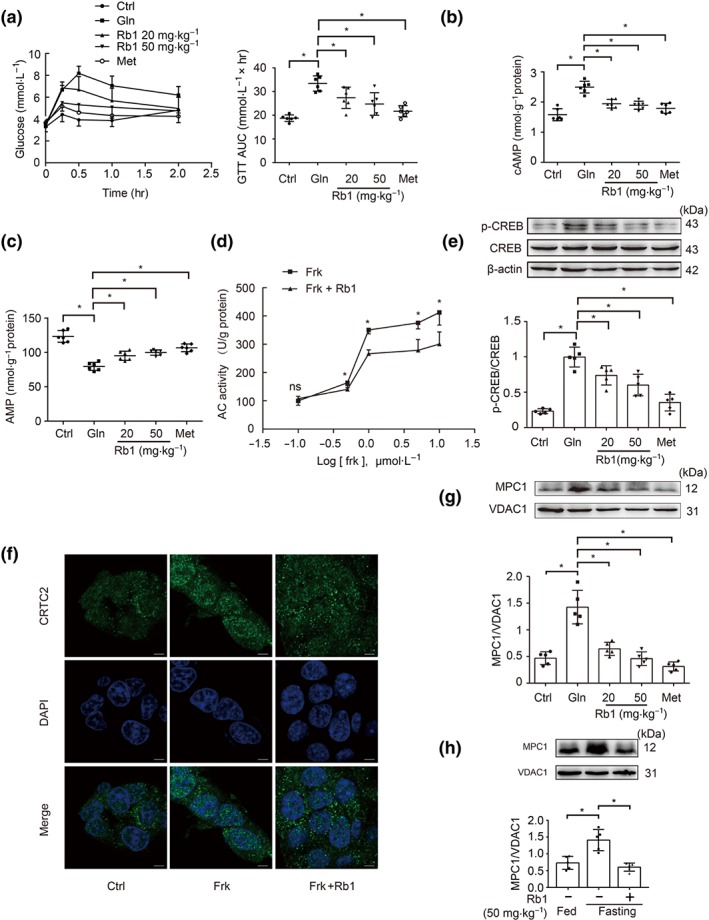
Rb1 attenuated the hepatic glucagon response. (a) Blood glucose levels in glucagon tolerance tests (2 mg·kg−1) in mice (n = 6 each group); (b, c) cAMP contents and AMP contents in glucagon‐treated (2 mg·kg−1) mice (n = 6 each group); (d) AC activity in primary mouse hepatocytes stimulated with forskolin for 2 hr in the presence of Rb1 (10 μM; n = 5 each group); (e) CREB phosphorylation in the liver of mice 2 hr after glucagon treatment (2 mg·kg−1; n = 5 each group); (f) CRTC2 nuclear translocation in HepG2 cells stimulated with forskolin (10 μM) for 2 hr. Scale bars: 5 μm (n = 5 each group); (g) protein expression of MPC1 in the liver of mice 2 hr after glucagon treatment (2 mg·kg−1; n = 5 each group); (h) MPC1 protein expression during fasting in the liver of mice 2 hr after Rb1 treatment (50 mg·kg−1; n = 5 each group). Data shown are individual values with means ± SD. *P < .05, significantly different as indicated. Ctrl, control; Frk, forskolin; Gln, glucagon; Met, metformin; ns, not significant
3.19. Rb1 restrains pyruvate‐driven glucose production with MPC1 inhibition
In HepG2 cells, Rb1 reduced forskolin‐induced MPC1 gene expression, but this effect was blocked by CREB overexpression (Figure 7a). This indicates that Rb1 inhibited MPC1 induction via CREB deactivation. Rb1 reduced glucose production and increased lactate accumulation, but these actions were blocked by MPC1 overexpression (Figure 7b,c). Rb1 reduced PC protein expression and improved PDH by dephosphorylation in the liver of fasted mice (Figure 7d,e). Similarly, forskolin‐induced PC and PDK4 gene expressions were also attenuated by Rb1 in primary mouse hepatocytes (Figure S5a,b). This regulation should reduce HGP via shifting mitochondrial pyruvate away from the gluconeogenic pathway. When methyl pyruvate, alanine, and glutamine were used as the gluconeogenic substrates, no significant effects of Rb1 on glucose production were observed (Figure 7f), indicating that MPC1 inhibition was needed for Rb1 to reduce pyruvate‐derived HGP.
Figure 7.
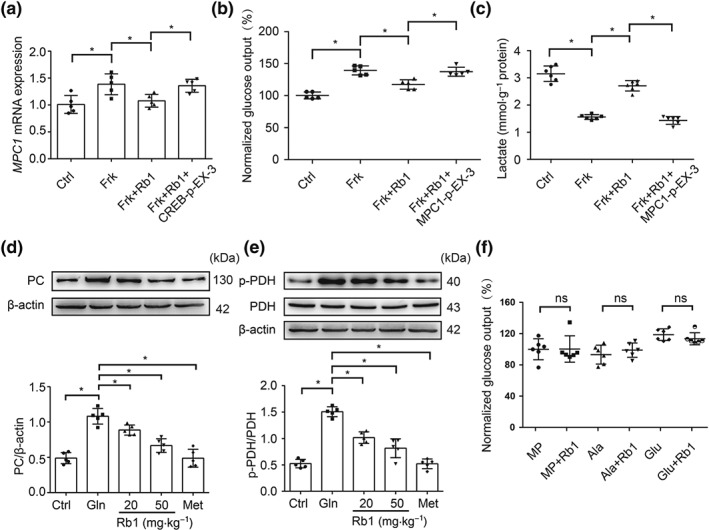
Rb1 reduced pyruvate‐driven glucose production. (a) The mRNA expression of MPC1 in HepG2 cells transfected with CREB overexpressed plasmid when co‐treated with forskolin (10 μM) and Rb1 (10 μM) for 2 hr (n = 5 each group); (b) glucose production in HepG2 cells transfected with MPC1 overexpressed plasmid when co‐treated with forskolin (10 μM) and Rb1 (10 μM) for 6 hr (n = 5 each group); the glucose production was expressed as the percentage of the basal level in Ctrl group; (c) lactate contents in HepG2 cells transfected with MPC1 overexpressed plasmid when co‐treated with forskolin (10 μM) and Rb1 (10 μM) for 6 hr (n = 6 each group); (d, e) PC protein and PDH phosphorylation in the liver of mice 2 hr after Rb1 treatment (20 or 50 mg·kg−1; n = 5 each group); (f) glucose production in primary mouse hepatocytes incubated with Rb1 (10 μM) for 6 hr when methyl pyruvate (10 mM), alanine (10 mM), or glutamine (10 mM) were used as the only substrate, individually (n = 6 each group); the glucose production was expressed as the percentage of the basal level in MP group. Data shown are individual values with means ± SD. *P < .05, significantly different as indicated. Ala, alanine; Ctrl, control; Frk, forskolin; Gln, glucagon; Glu, glutamine; MP, methyl pyruvate; Met, metformin; ns, not significant
4. DISCUSSION
Hyperglucagonaemia is often observed in diabetic patients (Unger, 1978). Enhanced hepatic glucagon response promotes excessive HGP, resulting in hyperglycaemia (Unger & Cherrington, 2012). Although most gluconeogenic substrates are routed through mitochondria, we showed that mitochondrial pyruvate transport is the initial step in glucagon‐mediated HGP, and CREB up‐regulates MPC1 to ensure mitochondrial pyruvate availability for gluconeogenesis.
Because glycolysis is inhibited when hepatic gluconeogenesis is increased, cytosolic pyruvate in the liver is predominantly derived from systemic lactate accumulation to support gluconeogenic fuel (Rognstad, 1983). MPC induction is up‐regulated in diabetic or high fat diet‐fed mice, contributing to excessive HGP (Gray et al., 2015; Li et al., 2018). Because several pathological factors can influence hepatic glucagon response in high fat diet models or obesity models, we investigated the direct impact of glucagon on MPC1 induction in normal mice fasted with glucagon load. In tolerance tests, we found that glucagon and pyruvate load‐induced hyperglycaemic responses were accompanied by induction of hepatic MPC1 and showed that AC activation‐induced cAMP generation was the initial cause of MPC1 induction. Although it is well established that glucagon promotes HGP through pyruvate‐driven endogenous glucose production, our results indicate that mitochondrial pyruvate transport by MPC1 is the initial step in the gluconeogenic pathway in the context of the hepatic response to glucagon.
In cardiomyocytes and cancer cells, deacetylation modification increases MPC protein induction by improving its stability (Liang, Li, Huang, Li, & Li, 2015; Vadvalkar et al., 2017). Pyruvate load also increases MPC expression by preventing degradation, a process which occurs at 8 hr after MPC1 induction (Li et al., 2018). However, we found that glucagon‐induced MPC1 induction is unlikely to be due to post‐translational regulation, because both glucagon and forskolin increased MPC1 protein induction with up‐regulation of gene expression in less than 8 hr. In response to cAMP generation and PKA activation, CREB phosphorylation increases its activity to transcriptionally regulate lipid and glucose genes by binding to the promoters (Altarejos & Montminy, 2011). We therefore speculate that CREB transcriptionally up‐regulates MPC1 to ensure efficient HGP. CREB knockdown blocked glucagon‐induced MPC1 gene and protein expression, indicative of the possibility. PKA activates CREB by phosphorylation at Ser133 to enhance binding to most promoters with CREB‐binding sites, in cells exposed to cAMP (Altarejos & Montminy, 2011). Glucagon increased luciferase reporter activity of MPC1, but mutation of CREB at Ser133 blocked the response, indicating the need for CREB activation in the transcriptional regulation of MPC1. Increased CREB occupancy at the site of MPC1 promoter and loss of function by promoter mutation provided solid evidence in support of transcriptional regulation. PGC‐1α is a co‐activator involved in transcriptional regulation of gluconeogenic genes (Altarejos & Montminy, 2011). PGC‐1α is known to induce MPC1 expression in tumour cells (Koh, Kim, Shin, & Kim, 2018). Hepatic gluconeogenesis is an anabolic process, and glycolysis is maintained at a low level (Postic, Dentin, & Girard, 2004), while the energy metabolism in tumour cells is characterized by aerobic glycolysis. Therefore, it is possible that the different metabolic settings may be the reason for the differential regulations of MPC1.
As a co‐activator, CRTC2 also senses hormonal and metabolic signals. CRTC2 is sequestered in the cytoplasm through phosphorylation‐dependent interactions with 14‐3‐3 protein (Altarejos & Montminy, 2011; Liu et al., 2008). Exposure to cAMP triggers dephosphorylation and nuclear translocation of CRTCs, which then bind to CREB on relevant promoters to augment gluconeogenic gene expression (Altarejos & Montminy, 2011). Consistent with the regulation, we demonstrated that CRTC2 is required for CREB‐mediated MPC1 induction. Because the phosphorylation and nuclear translocation of CRTC2 can be regulated by AMPK and insulin (Altarejos & Montminy, 2011; Koo et al., 2005; Liu et al., 2008), this finding points to the possible therapeutic gains of restraining CRTC2 in the cytoplasm by pharmacological intervention. This could block MPC1 induction, thus limiting excessive HGP in the context of CREB activation.
Pyruvate plays a pivotal role in connecting glucose, amino acid, and fatty acid metabolism, and therefore, the regulation of pyruvate flux into mitochondria should influence mitochondrial metabolism. However, despite suppression of glucose and pyruvate oxidation, mitochondrial function and tricarboxylic acid (TCA) cycle metabolism remain unaffected when MPC is pharmacologically inhibited (Vacanti et al., 2014). Consistent with this, in mice with liver‐specific MPC2 deficiency, euglycemia is maintained by altered gluconeogenic substrates (McCommis et al., 2015). These events indicate that suppression of mitochondrial pyruvate transport induces a form of metabolic flexibility in hepatocytes. Mitochondrial pyruvate is either oxidized in the TCA cycle by PDH or carboxylated by PC for gluconeogenic pathway. However, we found that CREB overexpression transcriptionally up‐regulated PC with impaired PDH activity, and the opposite regulation shifted mitochondrial pyruvate towards hepatic gluconeogenesis. PC‐mediated carboxylation is increased in the liver of high fat diet‐fed mice (Lee et al., 2013), while glucagon favours the partitioning of pyruvate towards carboxylation regulated by PC (Agius & Alberti, 1985). CREB is also shown to up‐regulate PC gene (Agius & Alberti, 1985). Interestingly, we found that the opposite regulation of PC and PDH activity by CREB was in respect of MPC1 induction. Although the distinct mechanism is unknown, we reason that the opposing actions of CREB in the regulation of PC and PDH should be an adaptive response to MPC1 induction, ensuring mitochondrial pyruvate viability for glucose production. Fasting triggers protein breakdown in skeletal muscle, and the released free amino acids are the major precursors of hepatic gluconeogenesis, entering mitochondria independent of MPC. Glucagon failed to influence glucose production when alanine and glutamine were used as gluconeogenic substrates, further indicating that MPC‐mediated mitochondrial pyruvate transport is the main route for HGP promotion. Additionally, the role of alanine–pyruvate interconversion in gluconeogenesis is worth considering. Alanine–pyruvate interconversion is catalysed by two alanine transaminase (ALT) enzymes: ALT1 is localized in the cytosol, while alanine transaminase 2 (ALT2) is localized within the mitochondrial matrix (Yang et al., 2009). Because alanine can enter mitochondria through an unidentified mitochondrial alanine transporter, the alanine–pyruvate cycling provides an alternative route for pyruvate carbon entry into mitochondrial pathways, especially when MPC function is impaired. The alanine–pyruvate cycling is indicative of the adaptive regulation in gluconeogenesis (McCommis et al., 2015).
Ginsenoside Rg5 reduces cAMP accumulation to ameliorate insulin resistance in muscle and to restrain hepatic glucagon response (Xiao, Lou, et al., 2017; Xiao, Yang, et al., 2017). In the present study, we found that another ginsenoside Rb1 inhibited AC activity to prevent glucagon‐induced cAMP formation with an increase in AMP accumulation. This action is probably due to reduced cellular energy charge, as the generated AMP allosterically inhibits AC activity (Fain, Pointer, & Ward, 1972). Metformin reduced hepatic cAMP accumulation by reducing cellular energy charge, contributing to the suppression of the hepatic glucagon response (Miller et al., 2013). Rb1 increases cellular AMP/ATP ratio to activate hepatic AMPK (Shen et al., 2013) and AMP generation partly explains AC inhibition by Rb1. As a consequence, Rb1 inactivated CREB by dephosphorylation, probably contributing to decresing MPC1. In response to cAMP signalling, the nuclear translocation of CRTC2 ensures the transcriptional regulation by CREB, while AMPK phosphorylates CRTC2 at Ser171 to sequester CRTC2 in the cytoplasm (Altarejos & Montminy, 2011). Insulin, by Akt activation, inhibits gluconeogenic gene expression by promoting the phosphorylation and ubiquitin‐dependent degradation of CRTC2 (Dentin et al., 2007). Rb1 is reported to activate hepatic AMPK to reduce fatty liver and increases insulin sensitivity to suppress expression of gluconeogenic genes (Shen et al., 2015), suggesting another possible way to inhibit MPC1 induction.
We have demonstrated that Rb1 reduced cAMP generation and suppressed MPC1 induction in the liver of fasted mice. It is however worthy of note that Rb1 is a multifunctional compound and ameliorates diverse metabolic disorders via different mechanisms. Several pathological factors, such as inflammation, insulin resistance, and lipid deposition, are involved in hyperglycaemia in diabetes. Therefore, a comprehensive study with an emphasis on mitochondrial pyruvate import in a diabetic animal model is necessary for the full evaluation of the antidiabetic potential of Rb1.
In summary, CREB mediates glucagon action to transcriptionally up‐regulate MPC1 induction, ensuring efficient glucose production. Rb1 blocked cAMP signalling by AC deactivation and thus inactivated CREB. This resulted in a reduced pyruvate‐driven glucose production to which the inhibition of the MPC1 induction may have contributed. The proposed mechanism is shown in Figure 8. This finding not only reveals a previously unrecognized role of ginsenosides in antidiabetic action but also suggests that inhibition of mitochondrial pyruvate import might be a potential therapeutic strategy to reduce fasting hyperglycaemia in diabetes.
Figure 8.
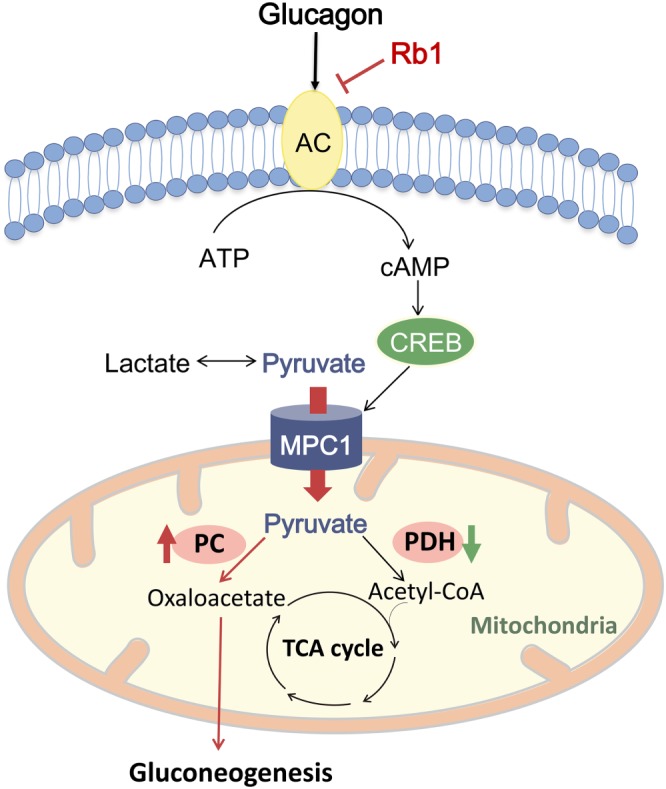
The proposed mechanism for ginsenoside Rb1 (Rb1) to restrain pyruvate‐driven hepatic glucose production. Glucagon activates AC to generate cAMP and then activates cAMP‐responsive element‐binding protein (CREB). CREB transcriptionally up‐regulates the mitochondrial pyruvate carrier 1 (MPC1) to promote mitochondrial pyruvate transport and shifts mitochondrial pyruvate metabolism towards carboxylation for gluconeogenesis by opposing regulation of pyruvate carboxylase (PC) and pyruvate dehydrogenase (PDH). Rb1 blocked cAMP signalling by AC deactivation and thus inactivated CREB to reduce pyruvate‐driven glucose production via inhibition of mitochondrial pyruvate transport through MPC1
CONFLICT OF INTEREST
All authors declare no conflicts of interest.
AUTHOR CONTRIBUTIONS
L.‐W.Q., Q.L., and B.‐L.L. made substantial contributions to the conception and design, acquisition, analysis and interpretation of the data, as well as in drafting the article. M.‐D.L. and J.L. performed the experiments, analysed the data, and drafted the manuscript. Y.C. and N.X. collected the data and reviewed the manuscript. G.‐X.M. designed the primers specific for the MPC1 promoter in ChIP assay. P.L. contributed to the discussion and review of the manuscript. All authors approved the final version of the paper. L.‐W.Q. and Q.L. are responsible for the integrity of this work.
DECLARATION OF TRANSPARENCY AND SCIENTIFIC RIGOUR
This Declaration acknowledges that this paper adheres to the principles for transparent reporting and scientific rigour of preclinical research as stated in the BJP guidelines for Design & Analysis, Immunoblotting and Immunochemistry, and Animal Experimentation, and as recommended by funding agencies, publishers and other organisations engaged with supporting research.
Supporting information
Data S1.
Supplementary Materials Methods
Table S1. Primer sequences used for RT‐PCR
Table S2 Primary antibodies used in western blot
Figure S1. (a) Relative mRNA abundance of MPC2 in HepG2 cells transfected with CREB overexpression plasmid (n=5 each group); (b, c) Forskolin (10 μM) and Bt2‐cAMP (100 μM) induced glucose production in primary mouse hepatocytes with or without 666‐15 (100 nM) in the presence of pyruvate (10 mM) for 6 h (n=6 each group), the glucose production was expressed as the percentage of the basal level in PYR group. Data were showed as mean ± SD. Ctrl, control; Frk, forskolin; PYR, pyruvate. *p < 0.05 and ns = not significant.
Figure S2. (a, b) Relative mRNA abundance of Pygl and Gys2 in primary mouse hepatocytes treated with pyruvate (10 mM) and glucagon (100 nM) for 6 h (n=5 each group). Data were showed as mean ± SD. Ctrl, control; Gln, glucagon; PYR, pyruvate. ns = not significant.
Figure S3. CRBE overexpression induced glucose production in HepG2 cells with or without MPC1 siRNA in the presence of pyruvate (10 mM) for 6 h (n=6 each group), the glucose production was expressed as the percentage of the basal level in PYR group. Data were showed as mean ± SD. PYR, pyruvate. *p < 0.05.
Figure S4. IC50 of Rb1 for adenyl cyclase (AC) activity stimulated with forskolin (10 μM) for 2 h (n=3 each group).
Figure S5. (a, b) Rb1 reduced Pc and Pdk4 gene expression in primary mouse hepatocytes incubated with forskolin (10 μM) for 2 h (Rb1, 10 μM; UK5099, 300 nM; n=5 each group). Data were showed as mean ± SD. Ctrl, control; Frk, forskolin. *p < 0.05.
ACKNOWLEDGEMENTS
This study was supported by the National Science Fund of China for Distinguished Young Scholars (81825023), the Natural Science Foundation of Jiangsu Province (BK20160762), and the China Postdoctoral Science Foundation (Grant 2018M640541). We thank Xiao‐Nan Ma, Yingjian Hou, and Minhui Sun from the Cellular and Molecular Biology Center of China Pharmaceutical University for their technical help. We also thank Dr. Raphael N. Alolga (China Pharmaceutical University) for editing the manuscript.
Lou M‐D, Li J, Cheng Y, et al. Glucagon up‐regulates hepatic mitochondrial pyruvate carrier 1 through cAMP‐responsive element‐binding protein; inhibition of hepatic gluconeogenesis by ginsenoside Rb1 . Br J Pharmacol. 2019;176:2962–2976. 10.1111/bph.14758
Meng‐Die Lou and Jia Li contributed equally to this work.
Contributor Information
Qun Liu, Email: liuquncpu@126.com.
Lian‐Wen Qi, Email: qilw@cpu.edu.cn.
REFERENCES
- Agius, L. , & Alberti, K. G. (1985). Regulation of flux through pyruvate dehydrogenase and pyruvate carboxylase in rat hepatocytes. Effects of fatty acids and glucagon. European Journal of Biochemistry, 152, 699–707. 10.1111/j.1432-1033.1985.tb09250.x [DOI] [PubMed] [Google Scholar]
- Alexander, S. P. H. , Fabbro, D. , Kelly, E. , Marrion, N. V. , Peters, J. A. , Faccenda, E. , … CGTP Collaborators . (2017). The Concise Guide to PHARMACOLOGY 2017/18: Enzymes. British Journal of Pharmacology, 174, S272–S359. 10.1111/bph.13877 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander, S. P. H. , Kelly, E. , Marrion, N. V. , Peters, J. A. , Faccenda, E. , Harding, S. D. , … CGTP Collaborators . (2017). The Concise Guide to PHARMACOLOGY 2017/18: Transporters. British Journal of Pharmacology, 174(Suppl 1), S360–S446. 10.1111/bph.13883 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Altarejos, J. Y. , & Montminy, M. (2011). CREB and the CRTC co‐activators: Sensors for hormonal and metabolic signals. Nature Reviews. Molecular Cell Biology, 12, 141–151. 10.1038/nrm3072 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bai, L. , Gao, J. , Wei, F. , Zhao, J. , Wang, D. , & Wei, J. (2018). Therapeutic potential of ginsenosides as an adjuvant treatment for diabetes. Frontiers in Pharmacology, 9, 423 10.3389/fphar.2018.00423 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bender, T. , & Martinou, J. C. (2016). The mitochondrial pyruvate carrier in health and disease: To carry or not to carry? Biochimica et Biophysica Acta, 1863, 2436–2442. 10.1016/j.bbamcr.2016.01.017 [DOI] [PubMed] [Google Scholar]
- Bender, T. , Pena, G. , & Martinou, J. C. (2015). Regulation of mitochondrial pyruvate uptake by alternative pyruvate carrier complexes. The EMBO Journal, 34, 911–924. 10.15252/embj.201490197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bricker, D. K. , Taylor, E. B. , Schell, J. C. , Orsak, T. , Boutron, A. , Chen, Y. C. , … Rutter, J. (2012). A mitochondrial pyruvate carrier required for pyruvate uptake in yeast, Drosophila, and humans. Science, 337, 96–100. 10.1126/science.1218099 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curtis, M. J. , Alexander, S. , Cirino, G. , Docherty, J. R. , George, C. H. , Giembycz, M. A. , … Ahluwalia, A. (2018). Experimental design and analysis and their reporting II: Updated and simplified guidance for authors and peer reviewers. British Journal of Pharmacology, 175, 987–993. 10.1111/bph.14153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dentin, R. , Liu, Y. , Koo, S. H. , Hedrick, S. , Vargas, T. , Heredia, J. , … Montminy, M. (2007). Insulin modulates gluconeogenesis by inhibition of the coactivator TORC2. Nature, 449, 366–369. 10.1038/nature06128 [DOI] [PubMed] [Google Scholar]
- Divakaruni, A. S. , Wiley, S. E. , Rogers, G. W. , Andreyev, A. Y. , Petrosyan, S. , Loviscach, M. , … Murphy, A. N. (2013). Thiazolidinediones are acute, specific inhibitors of the mitochondrial pyruvate carrier. Proceedings of the National Academy of Sciences of the United States of America, 110, 5422–5427. 10.1073/pnas.1303360110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fain, J. N. , Pointer, R. H. , & Ward, W. F. (1972). Effects of adenosine nucleosides on adenylate cyclase, phosphodiesterase, cyclic adenosine monophosphate accumulation, and lipolysis in fat cells. The Journal of Biological Chemistry, 247, 6866–6872. [PubMed] [Google Scholar]
- Gray, L. R. , Sultana, M. R. , Rauckhorst, A. J. , Oonthonpan, L. , Tompkins, S. C. , Sharma, A. , … Taylor, E. B. (2015). Hepatic mitochondrial pyruvate carrier 1 is required for efficient regulation of gluconeogenesis and whole‐body glucose homeostasis. Cell Metabolism, 22, 669–681. 10.1016/j.cmet.2015.07.027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halestrap, A. P. (1975). The mitochondrial pyruvate carrier. Kinetics and specificity for substrates and inhibitors. The Biochemical Journal, 148, 85–96. 10.1042/bj1480085 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harding, S. D. , Sharman, J. L. , Faccenda, E. , Southan, C. , Pawson, A. J. , Ireland, S. , … NC‐IUPHAR . (2018). The IUPHAR/BPS guide to pharmacology in 2018: Updates and expansion to encompass the new guide to immunopharmacology. Nucleic Acids Research, 46, D1091–D1106. 10.1093/nar/gkx1121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herzig, S. , Long, F. , Jhala, U. S. , Hedrick, S. , Quinn, R. , Bauer, A. , … Montminy, M. (2001). CREB regulates hepatic gluconeogenesis through the coactivator PGC‐1. Nature, 413, 179–183. 10.1038/35093131 [DOI] [PubMed] [Google Scholar]
- Herzig, S. , Raemy, E. , Montessuit, S. , Veuthey, J. L. , Zamboni, N. , Westermann, B. , … Martinou, J. C. (2012). Identification and functional expression of the mitochondrial pyruvate carrier. Science, 337, 93–96. 10.1126/science.1218530 [DOI] [PubMed] [Google Scholar]
- Jeoung, N. H. , Harris, C. R. , & Harris, R. A. (2014). Regulation of pyruvate metabolism in metabolic‐related diseases. Reviews in Endocrine & Metabolic Disorders, 15, 99–110. 10.1007/s11154-013-9284-2 [DOI] [PubMed] [Google Scholar]
- Katz, J. , & Tayek, J. A. (1999). Recycling of glucose and determination of the Cori cycle and gluconeogenesis. The American Journal of Physiology, 277, E401–E407. 10.1152/ajpendo.1999.277.3.E401 [DOI] [PubMed] [Google Scholar]
- Kilkenny, C. , Browne, W. , Cuthill, I. C. , Emerson, M. , & Altman, D. G. (2010). Animal research: Reporting in vivo experiments: The ARRIVE guidelines. British Journal of Pharmacology, 160, 1577–1579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koh, E. , Kim, Y. K. , Shin, D. , & Kim, K. S. (2018). MPC1 is essential for PGC‐1α‐induced mitochondrial respiration and biogenesis. The Biochemical Journal, 475, 1687–1699. 10.1042/BCJ20170967 [DOI] [PubMed] [Google Scholar]
- Koo, S. H. , Flechner, L. , Qi, L. , Zhang, X. , Screaton, R. A. , Jeffries, S. , … Montminy, M. (2005). The CREB coactivator TORC2 is a key regulator of fasting glucose metabolism. Nature, 437, 1109–1111. 10.1038/nature03967 [DOI] [PubMed] [Google Scholar]
- Lee, P. , Leong, W. , Tan, T. , Lim, M. , Han, W. , & Radda, G. K. (2013). In vivo hyperpolarized carbon‐13 magnetic resonance spectroscopy reveals increased pyruvate carboxylase flux in an insulin‐resistant mouse model. Hepatology, 57, 515–524. 10.1002/hep.26028 [DOI] [PubMed] [Google Scholar]
- Li, A. , Liu, Q. , Li, Q. , Liu, B. , Yang, Y. , & Zhang, N. (2018). Berberine reduces pyruvate‐driven hepatic glucose production by limiting mitochondrial import of pyruvate through mitochondrial pyruvate carrier 1. eBioMedicine, 34, 243–255. 10.1016/j.ebiom.2018.07.039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang, L. , Li, Q. , Huang, L. , Li, D. , & Li, X. (2015). Sirt3 binds to and deacetylates mitochondrial pyruvate carrier 1 to enhance its activity. Biochemical and Biophysical Research Communications, 468, 807–812. 10.1016/j.bbrc.2015.11.036 [DOI] [PubMed] [Google Scholar]
- Liu, Q. , Zhang, F. G. , Zhang, W. S. , Pan, A. , Yang, Y. L. , Liu, J. F. , … Qi, L. W. (2017). Ginsenoside Rg1 inhibits glucagon‐induced hepatic gluconeogenesis through Akt‐FoxO1 interaction. Theranostics, 7, 4001–4012. 10.7150/thno.18788 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu, Y. , Dentin, R. , Chen, D. , Hedrick, S. , Ravnskjaer, K. , Schenk, S. , … Montminy, M. (2008). A fasting inducible switch modulates gluconeogenesis via activator/coactivator exchange. Nature, 456, 269–273. 10.1038/nature07349 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCommis, K. S. , Chen, Z. , Fu, X. , McDonald, W. G. , Colca, J. R. , Kletzien, R. F. , … Finck, B. N. (2015). Loss of mitochondrial pyruvate carrier 2 in the liver leads to defects in gluconeogenesis and compensation via pyruvate‐alanine cycling. Cell Metabolism, 22, 682–694. 10.1016/j.cmet.2015.07.028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGrath, J. C. , & Lilley, E. (2015). Implementing guidelines on reporting research using animals (ARRIVE etc.): New requirements for publication in BJP. British Journal of Pharmacology, 172(13), 3189–3193. 10.1111/bph.12955 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller, R. A. , Chu, Q. , Xie, J. , Foretz, M. , Viollet, B. , & Birnbaum, M. J. (2013). Biguanides suppress hepatic glucagon signalling by decreasing production of cyclic AMP. Nature, 494, 256–260. 10.1038/nature11808 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Postic, C. , Dentin, R. , & Girard, J. (2004). Role of the liver in the control of carbohydrate and lipid homeostasis. Diabetes & Metabolism, 30, 398–408. 10.1016/S1262-3636(07)70133-7 [DOI] [PubMed] [Google Scholar]
- Rauckhorst, A. J. , Gray, L. R. , Sheldon, R. D. , Fu, X. , Pewa, A. D. , Feddersen, C. R. , … Taylor, E. B. (2017). The mitochondrial pyruvate carrier mediates high fat diet‐induced increases in hepatic TCA cycle capacity. Mol Metab, 6, 1468–1479. 10.1016/j.molmet.2017.09.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rognstad, R. (1983). The role of mitochondrial pyruvate transport in the control of lactate gluconeogenesis. The International Journal of Biochemistry, 15, 1417–1421. 10.1016/0020-711X(83)90073-3 [DOI] [PubMed] [Google Scholar]
- Shen, L. , Haas, M. , Wang, D. Q. , May, A. , Lo, C. C. , Obici, S. , … Liu, M. (2015). Ginsenoside Rb1 increases insulin sensitivity by activating AMP‐activated protein kinase in male rats. Physiological Reports, 3, e12543 10.14814/phy2.12543 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen, L. , Xiong, Y. , Wang, D. Q. , Howles, P. , Basford, J. E. , Wang, J. , … Liu, M. (2013). Ginsenoside Rb1 reduces fatty liver by activating AMP‐activated protein kinase in obese rats. Journal of Lipid Research, 54, 1430–1438. 10.1194/jlr.M035907 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheng, L. , Zhou, Y. , Chen, Z. , Ren, D. , Cho, K. W. , Jiang, L. , … Rui, L. (2012). NF‐κB‐inducing kinase (NIK) promotes hyperglycemia and glucose intolerance in obesity by augmenting glucagon action. Nature Medicine, 18, 943–949. 10.1038/nm.2756 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Unger, R. H. (1978). Role of glucagon in the pathogenesis of diabetes: The status of the controversy. Metabolism, 27, 1691–1709. 10.1016/0026-0495(78)90291-3 [DOI] [PubMed] [Google Scholar]
- Unger, R. H. , & Cherrington, A. D. (2012). Glucagonocentric restructuring of diabetes: A pathophysiologic and therapeutic makeover. The Journal of Clinical Investigation, 122, 4–12. 10.1172/JCI60016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vacanti, N. M. , Divakaruni, A. S. , Green, C. R. , Parker, S. J. , Henry, R. R. , Ciaraldi, T. P. , … Metallo, C. M. (2014). Regulation of substrate utilization by the mitochondrial pyruvate carrier. Molecular Cell, 56, 425–435. 10.1016/j.molcel.2014.09.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vadvalkar, S. S. , Matsuzaki, S. , Eyster, C. A. , Giorgione, J. R. , Bockus, L. B. , Kinter, C. S. , … Humphries, K. M. (2017). Decreased mitochondrial pyruvate transport activity in the diabetic heart: Role of mitochondrial pyruvate carrier 2 (MPC2) acetylation. The Journal of Biological Chemistry, 292, 4423–4433. 10.1074/jbc.M116.753509 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao, N. , Lou, M. D. , Lu, Y. T. , Yang, L. L. , Liu, Q. , Liu, B. , … Li, P. (2017). Ginsenoside Rg5 attenuates hepatic glucagon response via suppression of succinate‐associated HIF‐1α induction in HFD‐fed mice. Diabetologia, 60, 1084–1093. 10.1007/s00125-017-4238-y [DOI] [PubMed] [Google Scholar]
- Xiao, N. , Yang, L. L. , Yang, Y. L. , Liu, L. W. , Li, J. , Liu, B. , … Li, P. (2017). Ginsenoside Rg5 inhibits succinate‐associated lipolysis in adipose tissue and prevents muscle insulin resistance. Frontiers in Pharmacology, 8, 43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiong, Y. , Shen, L. , Liu, K. J. , Tso, P. , Xiong, Y. , Wang, G. , … Liu, M. (2010). Antiobesity and antihyperglycemic effects of ginsenoside Rb1 in rats. Diabetes, 59, 2505–2512. 10.2337/db10-0315 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang, R. Z. , Park, S. , Reagan, W. J. , Goldstein, R. , Zhong, S. , Lawton, M. , … Gong, D. W. (2009). Alanine aminotransferase isoenzymes: Molecular cloning and quantitative analysis of tissue expression in rats and serum elevation in liver toxicity. Hepatology, 49, 598–607. 10.1002/hep.22657 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data S1.
Supplementary Materials Methods
Table S1. Primer sequences used for RT‐PCR
Table S2 Primary antibodies used in western blot
Figure S1. (a) Relative mRNA abundance of MPC2 in HepG2 cells transfected with CREB overexpression plasmid (n=5 each group); (b, c) Forskolin (10 μM) and Bt2‐cAMP (100 μM) induced glucose production in primary mouse hepatocytes with or without 666‐15 (100 nM) in the presence of pyruvate (10 mM) for 6 h (n=6 each group), the glucose production was expressed as the percentage of the basal level in PYR group. Data were showed as mean ± SD. Ctrl, control; Frk, forskolin; PYR, pyruvate. *p < 0.05 and ns = not significant.
Figure S2. (a, b) Relative mRNA abundance of Pygl and Gys2 in primary mouse hepatocytes treated with pyruvate (10 mM) and glucagon (100 nM) for 6 h (n=5 each group). Data were showed as mean ± SD. Ctrl, control; Gln, glucagon; PYR, pyruvate. ns = not significant.
Figure S3. CRBE overexpression induced glucose production in HepG2 cells with or without MPC1 siRNA in the presence of pyruvate (10 mM) for 6 h (n=6 each group), the glucose production was expressed as the percentage of the basal level in PYR group. Data were showed as mean ± SD. PYR, pyruvate. *p < 0.05.
Figure S4. IC50 of Rb1 for adenyl cyclase (AC) activity stimulated with forskolin (10 μM) for 2 h (n=3 each group).
Figure S5. (a, b) Rb1 reduced Pc and Pdk4 gene expression in primary mouse hepatocytes incubated with forskolin (10 μM) for 2 h (Rb1, 10 μM; UK5099, 300 nM; n=5 each group). Data were showed as mean ± SD. Ctrl, control; Frk, forskolin. *p < 0.05.


