Abstract
Given the high mortality rate and clinical impact associated with sinusoidal obstruction syndrome (SOS), many studies have attempted to better characterize the disease and potential treatment strategies. However, the unpredictability of SOS onset represents a major obstacle when developing reproducible and controlled clinical trials in humans. Similarly, although in vitro studies have elucidated many of the molecular and cellular mechanisms of SOS, they often lack clinical relevance and translatability, highlighting the importance of experimental in vivo research. Animal models have greatly varied in the approach used to induce SOS in accordance with the numerous causes of human disease. Thus far, the most common and prevalent model is the monocrotaline-induced model in rats, which has served as the basis for both new diagnostic and treatment studies and has been revised over the last 20 years to optimize its use. Furthermore, radiotherapy, oxaliplatin-based chemotherapy, and even hematopoietic stem cell transplantation have been recently used to better replicate human SOS in animals. Nevertheless, because of the novelty of such research, further studies should be conducted to better understand the reproducibility and applicability of these newer models. Thus, this review seeks to summarize the methods and results of experimental in vivo models of SOS and compare the efficacy of these various adaptations.
Keywords: blue liver disease, in vivo, monocrotaline, oxaliplatin, veno-occlusive disease
Abbreviations: BM SPC, Bone Marrow Endothelial Progenitor Cell; CRLM, Colorectal Liver Metastases; CV, Central Vein; HSCT, Hematopoietic Stem Cell Transplantation; HVOD, Hepatic Veno-Occlusive Disease; MCT, Monocrotaline; MMP-9, Matrix Metalloproteinase-9; NO, Nitric Oxide; PA, Pyrrolizidine Alkaloid; RILD, Radiation-Induced Liver Disease; SEC, Sinusoidal Endothelial Cell; SOS, Sinusoidal Obstruction Syndrome
Sinusoidal obstruction syndrome (SOS) is a common, drug-induced form of liver injury related to chemotherapy regimens used in pre-hematopoietic stem cell transplantation (HSCT) conditioning as well as in treatment for colorectal liver metastases (CRLM).1 Clinically, the disease is referred to as “blue liver disease” and can be especially dangerous in cases of CRLM because it impairs liver resectability by decreasing the regenerative capacity and functional reserve of the liver.2 When untreated, SOS has been associated with a mortality rate that can exceed 80% in situations of multiorgan failure.3, 4, 5 Even in nonfatal cases, patients can suffer from a wide array of symptoms including jaundice, tender hepatomegaly, ascites, and weight gain.3, 6 SOS is primarily a circulatory disease characterized by severe damage to the liver sinusoids. Loss of sinusoidal wall integrity caused by gaps among sinusoidal endothelial cells (SECs) allows red blood cells, leucocytes, and cellular debris to enter and embolize downstream.2, 7
Although SOS was first identified in livestock after ingestion of pyrrolizidine alkaloids (PAs), such as monocrotaline (MCT), causes of modern, human SOS can generally be divided into three categories: 1) acute high-dose chemotherapy, 2) chronic ingestion of PAs, and 3) side effects of radiation therapy.1, 8 These three causes result in versions of SOS that may differ clinically and histologically, such as in time course of development, which can vary from 25 days to 1–2 months, and in the presence or absence of coagulative necrosis.8 However, all versions exhibit the characteristic sinusoidal changes, occasionally with centrilobular hemorrhaging and occlusion of the central vein, that are unique to SOS due to SEC injury, compared with other forms of liver damage.
In vivo animal research has offered a novel way to experimentally uncover the impact of SOS with randomized, reproducible, and controlled experiments that also have clinical relevance. Animal-based studies have investigated the effects of SOS on hepatic regeneration,9 the role of specific protein markers in SOS development,10 and certain metabolic changes that can assist in SOS diagnosis.11 These models have also been used to test a variety of preventative and therapeutic treatments with varying results.12, 13, 14, 15 Nevertheless, as new research has been published, scientists have attempted to replicate both pathological and pathophysiological appearance of human SOS by adapting key features of their models, such as the type of animal studied or method of SOS induction used, as well as finer details, such as the dosage of toxin or timeline of application. However, this variability between the studies inhibits our ability to simply group them into a single category. Instead, to truly understand the progress achieved by animal models of SOS, we must analyze their results through the lens of their differences.
As such, animal-based in vivo studies have made significant contributions toward understanding and treating SOS. These studies also benefit from high levels of reproducibility and efficacy that are lacking in human research of SOS because of its reliance on patient reports and case studies. Still, not all aspects of these models are ubiquitous across all studies, and understanding the advantages (and disadvantages) of these adaptations will allow us to improve our analysis and application of animal models as a whole. In light of this situation, this article seeks to do the following:
-
1.
Review the use of animal models in the context of SOS, focusing primarily on the MCT-induced rat model.
-
2.
Compare the methodologies used in different studies and explain how these methods may relate to their respective aims and results.
-
3.
Comment on the study of SOS using animal models induced by other, non-MCT methods.
Relevant studies to this review were selected from the following databases: PubMed, Science Direct, Wiley Online Library, SpringerLink, and MEDLINE. Searches were conducted using these keywords: “sinusoidal obstruction syndrome”; “hepatic veno-occlusive disease”; “experimental model”; “animal”; “in vivo”; “rats”; “mice”; “pigs”; “monocrotaline”; “pyrrolizidine alkaloid”; “hematopoietic stem cell transplantation”; “radiation-induced liver disease”; “Gynura segetum”; “FOLFOX”; and “oxaliplatin”. Exclusion criteria included 1) studies that were not published in full or not in English and 2) studies that did not explicitly induce or study SOS or hepatic veno-occlusive disease (HVOD) in animals. For example, a study that administered MCT to animals for the sole reason of testing MCT toxicity would not have been included, even if the animals developed SOS-like characteristics, because it would not be a controlled model of SOS.
MCT-induced model of SOS
Early Models of SOS
Although different animal-based models have been proposed and tested to study SOS, the most common and notable one of the last 20 years has been the MCT-induced model of SOS, specifically in rats. MCT is part of the toxic group of PAs, which are found in many species of Crotalaria plants. The use of PAs in experimental models of SOS, formerly known as “hepatic veno-occlusive disease”, can actually be traced as far back as the 1950s and 1960s when preliminary research was conducted to understand both the pathology of the disease as well as the toxic effects of PAs. These models originated due to findings of sinusoidal lesions that developed (in both humans and animals) in the West Indies, Jamaica, and Barbados, where prevalence of PA-containing plants was high.16, 17, 18, 19 Studies by Mclean et al and Hill et al originally experimented with oral and intraperitoneal application, respectively, of MCT in rats.18, 19 Hoping to reproduce human disease in lower primates, Allen et al induced HVOD in Macaca speciosa monkeys, whereas Bras et al studied the disease in cattle because of the prevalence of PA ingestion in livestock.16, 17 However, these studies struggled with predicting and controlling the fraction of animals that would develop HVOD as well as the time course of the disease.8 Toward the later 1900s, further attempts were conducted to revise these models and even included new animals, but still to no avail.20, 21, 22
Standardization of the MCT-induced Model of SOS in Rats
Nevertheless, in 1999, DeLeve et al were able to standardize a MCT-induced model of SOS for experimental use in rats, which would go on to serve as the basis for most, if not all, future MCT-induced models of SOS.8 To track disease progression, the authors induced SOS in rats with 160 mg/kg doses of MCT. The primary outcomes that were measured and corresponding methodologies used can be found in Table 1. MCT was identified to induce similar histological changes (SOS) in rats as humans, reflecting its ability to accurately represent the disease.
Table 1.
Methods Used to Measure SOS Characteristics by DeLeve et al (1999).8
| Outcome | Histology | Morphology | Inflammation | Biochemical parameters | Physical parameters |
|---|---|---|---|---|---|
| Method | Light microscopy | Electron microscopy | Immunohistochemistry | Blood sampling | Various |
| Specific measures |
|
|
|
|
|
CV, central vein; SECs, sinusoidal endothelial cells; SOS, sinusoidal obstruction syndrome.
DeLeve et al also developed a scoring system to classify the staging and severity of SOS over a 10-day course8 (Figure 1). Stages were separated into “early” (characterized by coagulative necrosis) and “late” (when fibrosis develops), and severity was determined from the summed scores of different histological outcomes including endothelial damage, hemorrhaging, and necrosis (for early HVOD) or fibrosis (for late HVOD) (Table 1). Of note, days 8–10 are unique because the disease progressed to severe, late HVOD in some animals, whereas others experienced almost a full recovery (Figure 1). Interestingly though, this is quite similar to the disease progression in humans as studies have shown that patients with SOS may spontaneously recover during later stages of the disease.4
Figure 1.
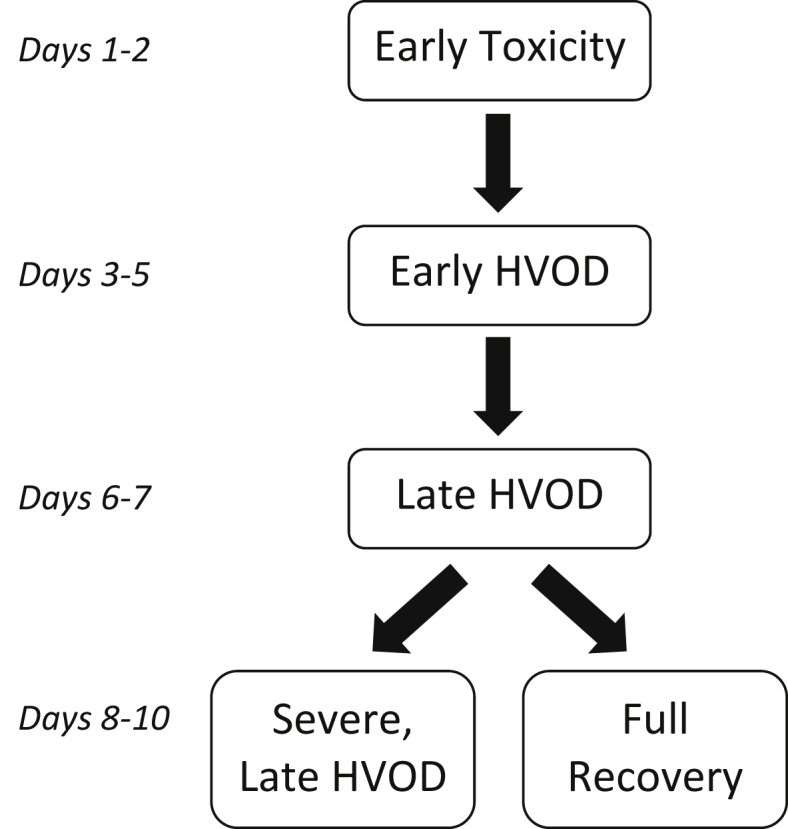
Timeline of hepatic veno-occlusive disease in rats after MCT treatment in the study by DeLeve et al (1999)8. HVOD, hepatic veno-occlusive disease; MCT, monocrotaline.
In addition to histological changes, DeLeve et al identified morphological changes in the sinusoidal lining caused by SOS. Their results showed sinusoidal wall destruction and increased lobular inflammatory infiltrate localized in the centrilobular region and central vein (CV), both of which peaked during severe, early HVOD (days 3–5). The rats also exhibited a sharp increase in body weight, accumulation of ascites, and hepatomegaly. Beyond day 5, bilirubin levels exceeded 2 mg/dl, and hematocrit levels significantly decreased.8 These findings correlate with the Seattle and Baltimore criteria for diagnosis of human SOS, which include weight gain, ascites, hepatomegaly, and hyperbilirubinemia,3 indicating that this model replicates both the morphological changes and clinical symptoms of SOS.
Use of the MCT-induced Model to Understand and Treat SOS
Since its publication in 1999, the study by DeLeve et al has served as the basis for most future animal research on SOS and its treatment strategies. Table 2, Table 3, Table 4 break down studies that used the MCT model of SOS by primary overall goal of disease characterization (pathophysiology or diagnostics), prevention, or treatment.
Table 2.
Studies on the Characterization of SOS Using the MCT-induced Model in Rats (Unless Otherwise Stated).
| Category | Primary author | Year | MCT dosage (mg/kg) | Summary of key result(s) |
|---|---|---|---|---|
| Mechanism | DeLeve, L.23 | 2003 | 160 | Decrease in hepatic nitric oxide promotes the onset of SOS by allowing an increase in MMP activity. |
| DeLeve, L.24 | 2003 | 160 | Microcirculatory obstruction is initiated by embolization of red blood cells, sinusoidal lining cells, and adherent monocytes after swelling of SECs. | |
| DeLeve, L.10 | 2003 | 160 | Early increase in MMP-9 activity results in primary morphological changes caused by SOS. Increased MMP-9 activity is likely due to F-actin depolymerization in SECs caused by MCT. | |
| Hirata, M.25 | 2017 | 90 | MCT-treated rats were positive for CD41 and P-selectin, markers of platelet aggregation, suggesting that extravasated platelet aggregation in the space of Disse is associated with SOS onset. | |
| Outcomes | Schiffer, E.9 | 2009 | 160 | SOS impairs hepatic regeneration after 70% hepatectomy resulting in hepatocellular injury. |
| Jafari, A.26 | 2017 | 90 | SOS resulted in 25% increase in mortality after 70% hepatectomy. | |
| Diagnosis | Conotte, R.11 | 2014 | 100 | Identified metabolic changes unique to SOS to aid in disease diagnosis. |
| Park, S.27,a | 2017 | 90 | Liver shear-wave velocity measured by acoustic radiation force impulse elastography increases in proportion to degree of SOS injury and can be used in potential diagnosis and severity assessments. |
MCT, monocrotaline; MMP, matrix metalloproteinase; SECs, sinusoidal endothelial cells; SOS, sinusoidal obstruction syndrome.
Studied both MCT-induced and FOLFOX-induced SOS (See Table 5).
Table 3.
Studies on the Prevention of SOS Using the MCT-induced Model in Rats (Unless Otherwise Stated).
| Therapy type | Primary author | Year | Specific treatment | MCT dosage (mg/kg) | Proposed mechanism |
|---|---|---|---|---|---|
| Antibiotics | DeLeve, L.10 | 2003 | Doxycycline | 160 | MMP-9 inhibition |
| Anticoagulants | Ikezoe, T.29,a | 2017 | Fifth epidermal growth factor–like domain of thrombomodulin | 200 | Anticoagulation |
| Nakamura, K.13 | 2014 | Soluble thrombomodulin | 90 | Anticoagulation | |
| Antioxidants | Ezzat, T.30 | 2012 | Flavonoid monoHER | 160 | MMP-9 inhibition reinforcement of microvasculature |
| Periasamy, S.31 | 2013 | Sesame oil | 90 | MMP-9 inhibition | |
| Wang, X.32 | 2000 | Glutathione | 160 | Detoxification | |
| Herbal medicine | Narita, M.14 | 2009 | Dai-kenchu-to—processed ginger | 90 | Prevents neutrophil accumulation and hepatocyte coagulative necrosis |
| Chymase inhibitor | Masubuchi, S.33,b | 2013 | Chymase inhibitor TY-51469 | 120 | MMP-9 inhibition |
| Kinase inhibitors | Nakamura, K.34 | 2012 | Sorafenib | 90 | MMP-9 inhibition |
| Okuno, M.35 | 2015 | Regorafenib | 90 | MMP-9 inhibition | |
| Phosphodiesterase-III inhibitor | Miyata, T.36 | 2017 | Cilostazol | 90 | Antiplatelet aggregation/anticoagulation SEC protection |
| Narita, M.37 | 2009 | Olprinone | 90 | SEC protection |
MCT, monocrotaline; MMP-9, matrix metalloproteinase-9; monoHER, 7-mono-O-(β-hydroxyethyl)-rutoside; SECs, sinusoidal endothelial cells; SOS, sinusoidal obstruction syndrome.
Used mice model of SOS.
Used hamster model of SOS.
Table 4.
Studies on the Treatment of SOS Using the MCT-induced Model in Rats (Unless Otherwise Stated).
| Therapy type | Primary author | Year | MCT dosage (mg/kg) | Specific treatment | Time of treatment (after MCT) | Proposed mechanism |
|---|---|---|---|---|---|---|
| Antioxidants | Periasamy, S.38 | 2011 | 90 | Sesame oil | +24 h | MMP-9 inhibition; antiinflammation |
| Zhang, J.28 | 2017 | 90 | Natural flavonoids—Quercetin + Baicalein | +6 h, +30 h | MMP-9 inhibition; antiinflammation | |
| Zheng, Z.39 | 2016 | 90 | Chlorogenic acid | +6 h, +30 h | MMP-9 inhibition; antiinflammation; detoxification | |
| Stem cells | Harb, R.15 | 2009 | 160 | Bone marrow endothelial progenitor (BM CD133+) cells | +4 d | SEC repair |
MCT, monocrotaline; MMP-9, matrix metalloproteinase-9; SECs, sinusoidal endothelial cells.
Characterization of SOS Using Animal Models
Studies focused on understanding SOS progression, effects, and diagnosis are described in Table 2. The three most notable are those published by DeLeve et al in 2003, continuing their work on the original MCT model. In one study, on microcirculatory obstruction in the liver, they observed swollen SECs as early as 12 h after MCT treatment. This created gaps allowing red blood cells to enter the space of Disse and deteriorate the sinusoidal lining, all of which embolized and reduced sinusoidal blood flow.24 Embolization in the space of Disse was further confirmed in a recent study by Hirata et al (2017)25 (Table 2). In the two other studies, DeLeve et al found that the loss of sinusoidal integrity is directly related to an increase in matrix metalloproteinase-9 (MMP-9) activity, which is reinforced by a decrease in nitric oxide (NO) production.10, 23 In the liver, NO is usually produced by SECs and Kupffer cells, both of which are adversely affected by MCT treatment. Based on these results, the authors proposed a hypothesis for the onset of MCT-induced SOS starting with metabolic activation of MCT to MCT pyrrole, which binds to actin in the SECs, resulting in the disassembly of F-actin and increased MMP activity. Depolymerization of F-actin leads to rounding up of SECs, while increased MMP activity breaks down the extracellular matrix in the space of Disse, all of which results in red blood cells penetrating the endothelium and embolizing downstream.10
Experimental Treatment Strategies for SOS
The three studies by DeLeve et al discussed in the previous section are especially important because they serve as the theoretical motivation for many of the treatment strategies against SOS. As seen in Table 3, although many of the specific prophylactic treatments of SOS have differed, most of them share an underlying function of either inhibiting MMP-9 or preventing coagulation in the microvasculature, two of the preliminary changes involved in SOS onset as found by DeLeve et al.10, 23, 24 In fact, failing to target these mechanisms actually resulted in incomplete prevention of SOS. For example, studies by Ezzat et al (2012)30 and DeLeve et al (2003)10 were both able to prevent the histological changes associated with SOS due to MMP-9 inhibition. However, Narita et al (2009) were unable to completely prevent SOS onset because Dai-kenchu-to, the Japanese herbal medicine they studied, lacked a protective effect on hepatic SECs from the rounding-up effects of MMPs.14
From a therapeutic or curative standpoint, many of the strategies seen in Table 4 are similar to the preventative measures in Table 3, especially the use of sesame oil by Periasamy et al (2011) and flavonoids by Zhang et al (2017).28, 38 Even chlorogenic acid, which was tested as a treatment by Zheng et al (2016), acted through similar mechanisms as other preventative compounds, including MMP-9 inhibition and anti-inflammation39 (Table 4). This similarity between compound types and modes of action suggests that these therapeutic strategies may, in fact, be more likely to operate through preventative measures. However, the study by Harb et al (2009) stands apart because as opposed to targeting the molecular mechanism behind SOS, their method exclusively treated damaged SECs.15 The authors isolated bone marrow endothelial progenitor cells (BM SPCs), positive for stem cell (CD133 and CD45) and endothelial cell (CD31) markers, to treat MCT-administered rats. Results showed that SOS can be induced by the depletion of BM SPCs and that these cells can activate regeneration of previously damaged SECs.15
The study by Harb et al (2009) was also the only to apply treatment with enough time after MCT exposure to constitute significant progress of SOS. The studies by Periasamy et al, Zhang et al, and Zheng et al all concluded their treatment within 30 h after MCT application (Table 4). According to the original model by DeLeve et al, by this time point, the animals would only show signs of “early toxicity” (Figure 1). On the other hand, Harb et al applied stem cell treatment 4 days after MCT, by which point the animals should have faced mild-to-severe, early SOS. To be clinically relevant, an animal model should account for the appropriate amount of time for human SOS to manifest, be diagnosed, and have a specific treatment prescribed, which is not likely to be accomplished by the “early toxicity” phase. For these reasons, the study by Harb et al (2009) can be considered the only true therapeutic or “curative” measure for SOS thus far, whereas the other studies should only be considered extensions of SOS prevention.
Comparison of Methodologies
Comparing the results of different studies using the MCT model has elucidated various SOS treatments. However, many of these studies differ on certain methodologies, which could have affected their results, the most notable and disputed of which is the dosage of MCT used to induce SOS, as seen in the inconsistencies throughout Table 2, Table 3, Table 4.
Based on the original model proposed by DeLeve et al (1999), future studies of SOS should have used 160 mg/kg of MCT. Moreover, in preliminary tests of their model, DeLeve et al discovered a narrow “window” of MCT dosage in rats, specifically from 100 to 200 mg/kg, below which did not induce SOS and above which rapidly killed all animals.8 Yet, as seen in Table 2, Table 3, Table 4, not all studies adhered to these guidelines. Even those that used other small rodents varied the dosage of MCT.29, 33 Moreover, after 2009, many studies using rats switched to 90 mg/kg doses, although still citing the original model by DeLeve et al.13, 14, 25, 26, 27, 28, 34, 35, 36, 37, 38, 39 However, as the first study to use this dosage, Narita et al (2009) briefly summarize SOS development in their model in four phases over seven days—early toxicity on day 1, severe sinusoidal changes and coagulative necrosis on days 2–3, development of fibrosis on days 4–6, and almost complete recovery on day 7.37 This four-phase course of SOS produced similar histopathological changes to human SOS (day 2) and matches the ten-day staging of the 160 mg/kg model proposed by DeLeve et al (Figure 1), a result that directly opposes the “dosage window” of 100–200 mg/kg.
Periasamy and Liu recently commented on an article published by Ezzat et al (2012)30 and argued, based on a pilot study they conducted, that 160 mg/kg doses of MCT cause a high mortality rate (37.5%), whereas a 90 mg/kg dosage was much safer and more realistic.38, 51 In response, however, Ezzat et al supported their original 160 mg/kg dosage because it had been used in many previous studies without such mortality risk. They even argued that any animal model that produced this mortality rate would not be accurately representing the subacute, chronic, and potentially reversible nature of SOS, thus showing that the correct dosage of MCT to induce SOS in rats is still up for debate.52
Other non-MCT models of SOS
The aforementioned studies have shown that MCT can reproduce SOS in small animals in an effective and controllable way. Still, scientists have developed other methods of inducing SOS to refine their models with varying results.
Gynura segetum
For example, one such compound studied is G. segetum (Tusanqi), a plant traditionally used in Chinese medicine which contains other PAs similar to MCT.53 Although pre-HSCT conditioning therapy is the most common reason for developing SOS in the Western Hemisphere, in China, ingestion of PA-containing plants, such as G. segetum, frequently causes SOS in humans as well.54 As a PA, G. segetum acts similarly to MCT, specifically by increasing MMP-9 levels, as shown in a study by Yu et al43 (Table 5). More recently, a study by Qiu et al (2018) identified 18 metabolites, commonly found in urine and plasma, that can serve as potential diagnostic markers for G. segetum–induced hepatotoxicity.42 Further research should be conducted to determine whether the same markers are present in the MCT model.
Table 5.
Main Features of Non-MCT-induced Animal Models of SOS.
| Induction method | Animal | Primary author | Year | Dosage | Timing and periodicity | Primary goal |
|---|---|---|---|---|---|---|
| Gynura segetum | Mice | Chen, Z.40 | 2011 | 30 g/kg | Daily for 30 d | Prevention of SOS with ligustrazine |
| Zhu, H.41 | 2011 | 30 g/kg | Daily for 30 d | Prevention of SOS with prednisone | ||
| Rats | Qiu, S.42 | 2018 | 3.75, 7.5, and 15 g/kg (3 groups) | Daily for 15 d | Identification of biomarkers and metabolic mechanisms in G. segetum hepatotoxicity | |
| Yu, X-z.43 | 2013 | 600 mg/kg | Daily for 3 w | MMP-9 expression in SOS | ||
| Radiotherapy | Cynomolgus monkeys | Yannam, G.44 | 2014 | 30, 36, 40, and 50 Gy hypofractionated (4 groups) | Daily for 5 d | Model characterization |
| Dogs | Shulman, H.45,a | 1987 | 9.2–16 Gy (TBI) ± 90–180 mg/m2 (L-PAM) ± 30, 60, 125, and 250 mg/kg (MCT) | Various | Model characterization | |
| FOLFOX | Mice | Robinson, S.46 | 2013 | 6 mg/kg (OX) + 50 mg/kg (5-FU) + 90 mg/kg (folinic acid) | Weekly for 5 w 5-FU and folinic acid 2 h after OX |
SOS pathogenesis and model characterization |
| Robinson, S.47 | 2013 | 6 mg/kg (OX) + 50 mg/kg (5-FU) + 90 mg/kg (folinic acid) | Weekly for 5 w 5-FU and folinic acid 2 h after OX |
Impact of CRLM on SOS severity after FOLFOX therapy | ||
| Rats | Park, S.27 | 2017 | 5 mg/kg (OX) + 20 mg/kg (5-FU) + 90 mg/kg (folinic acid) | Weekly for 7 w | Diagnosis and severity assessments of SOS using liver shear-wave velocity measured by ARFI elastography | |
| HSCT | Mice | Qiao, J.48 | 2015 | 7.5 Gy (TBI) + 5 × 106 (BM MNC) | Single-dose HSCT | Prevention of SOS using infusion of endothelial progenitor cells |
| Yeom, M.49 | 2015 | 10 mg iron dextran + 75 cGy/min (TBI) + 1 × 107 (BM MNC) + 5 × 106 (splenocytes) | Iron 5 d/w (consecutively) HSCT 4 h after TBI Single-dose HSCT |
Impact of secondary iron overload on post-HSCT SOS | ||
| Zeng, L.50 | 2013 | 7.5 Gy (TBI) + 5 × 106 (BM MNC) | HSCT 4 h after TBI Single-dose HSCT |
Model characterization |
5-FU, 5-fluorouracil; ARFI, acoustic radiation force impulse; BM MNC, bone marrow mononuclear cell; CRLM, colorectal liver metastases; FOLFOX, Folinic acid, Fluorouracil, Oxaliplatin; HSCT, hematopoietic stem cell transplantation; L-PAM, l-phenylalanine mustard; MCT, monocrotaline; MMP-9, matrix metalloproteinase-9; OX, oxaliplatin; SOS, sinusoidal obstruction syndrome; TBI, total-body irradiation.
Used combined treatment of radiotherapy, chemotherapy, and MCT.
Because they produce similar outcomes, one would expect G. segetum and MCT to be interchangeable. Unfortunately, however, G. segetum arguably impairs the study of SOS because of the extended period of exposure to high dosage toxin required to successfully induce SOS in any animal. In the studies of SOS prevention using ligustrazine40 (an alkaloid extracted from a Chinese herbal medicine) and prednisone41 (a corticosteroid), the prophylaxis was provided for one month in each case, concurrent with G. segetum exposure (Table 5). From a clinical standpoint in preventing SOS before HSCT or treatment for CRLM, undergoing prevention for one month does not seem efficient or plausible. Nonetheless, given the prevalence of SOS caused by PA ingestion in humans in China, it is important to study the G. segetum model of SOS to understand its regional impact.
The Relationship Between Radiation-induced Liver Disease and SOS
Beyond using PAs, scientists have also attempted to create experimental models of SOS which better reflect clinical onset of the disease in humans, such as the model using radiation-induced liver disease (RILD). From a pathological perspective, RILD, a side effect of radiotherapy, most commonly manifests itself as HVOD, with lesions especially prevalent around the CV.55 However, throughout the late 1900s, radiation failed to yield any promising models of SOS in a variety of animals, including rats, dogs, and even rhesus monkeys.56, 57, 58 Although these models were able to induce hepatic injury, they were unable to reproduce distinct HVOD. One study by Shulman et al (1987) showed limited potential by inducing acute HVOD in dogs but needed to combine either radiotherapy with chemotherapy or chemotherapy with MCT treatment to do so.45
More recently, scientists have discovered new methods to administer radiation therapy which circumvent previous problems. Specifically, Yannam et al (2014) used hypofractionated hepatic radiation at dosages of over 40 Gy in cynomolgus monkeys to induce noticeable changes in the CV as well as characteristic lesions of SOS44 (Table 5). Nevertheless, there is a high cost and relatively low accessibility to using cynomolgus monkeys.55 Therefore, further understanding the differences in radiosensitivity between monkeys and rodents may allow us to better develop this model in smaller animals that are easier to use.
Oxaliplatin-based Chemotherapy
Recent studies have also attempted to use oxaliplatin-based chemotherapy to induce SOS in animals with more promising results than radiotherapy. Specifically, Robinson et al (2013) used a murine chemotherapy model for five weeks and found sinusoidal dilation and hepatocyte atrophy as well as a prothrombotic state in the liver, thus confirming development of SOS46 (Table 5). Furthermore, using this model in a follow-up study, the authors found that Folinic acid, Fluorouracil, Oxaliplatin (FOLFOX)-induced SOS was exacerbated in mice in the presence of CRLM and associated tumor-related factors.47 Similarly, a study by Park et al (2017) induced SOS in rats through two separate models using both MCT- and FOLFOX-based treatment weekly for seven weeks27 (Table 5). Although this model also suffers from a longer time to induce SOS, it may aid in clinical relevance and translatability of results from animal models to humans.
Unfortunately, however, the FOLFOX-based model has faced limited reproducibility. In a study by Hubert et al (2015), which used oxaliplatin therapy in rats, the authors failed to induce sinusoidal damage after rats underwent 70% partial hepatectomy.59 Similarly, in a comment published by Lentschener et al, the authors stated that they were unable to reproduce SOS in mice, even after nearly mirroring the methodology of Robinson et al (2013).60 In a response, Robinson et al attributed these findings to potential differences in diet, substrain of mice used, or even the drug source.61 Nevertheless, these inconsistencies highlight the subtleties of this model that should be further studied.
New Efforts Using HSCT
Finally, there have also been successful attempts to create a murine model of SOS induced after total-body irradiation with HSCT, which better replicates SOS in humans (Table 5). The model was first proposed in a study by Zeng et al (2013), which used allogeneic stem cell transplantation from male C57BL/6 mice to female BALB/c mice.50 The authors used liver histological, morphological, biochemical, and physiological parameters to develop an SOS scoring system modified from DeLeve et al, classifying disease as mild, moderate, or severe.8, 50 Histological and morphological changes in mice after HSCT indicated development of SOS based on sinusoidal damage, coagulative necrosis, and CV fibrosis.
A similar model was also used in studies by Yeom et al (2015) and Qiao et al (2015) (Table 5). Yeom et al found that liver iron content increases the severity of SOS after HSCT in mice due to an increase in reactive oxygen species.49 Meanwhile, Qiao et al also used endothelial progenitor cells concurrently with their HSCT regimen to replace damaged SECs.48 Endothelial progenitor infusion reduced liver damage by inhibiting platelet activation and decreasing secretion of cytokines Interleukin-6 (IL-6) and Tumor Necrosis Factor-α (TNF-α). The successful application of HSCT-induced animal SOS in both of these studies suggests that this model may serve as a budding avenue for continued exploration.
Conclusion
Animal models of SOS have greatly varied throughout the past 50 years in both their goals and methodologies. Of these models, the MCT-induced model of SOS has been most commonly used, especially in rats, and has aided in our research on treatments for the disease. Still, this model is not without its limitations and has been the center of debate on different aspects (sometimes without resolution), most notably the proper dosage of MCT to use. Meanwhile, newer studies have shown the potential of more novel techniques including radiotherapy, oxaliplatin-based chemotherapy, and HSCT, which may offer better insight through their additional translatability. However, as research has shown, not all of these models have been optimized yet and may face barriers in reproducibility. Furthermore, human SOS can also vary, both clinically and histologically, so it is important to determine which form is represented by each animal model.8 Finally, it is important to note that most of the models discussed have used small animals, often rodents, as subjects. Although such studies can serve as a foundation for in vivo research, experimenting on larger animals may provide us with a better picture of SOS as it develops in humans. Thus, although in vivo research has continued to provide us with valuable insight into human SOS and a better understanding of how to treat the disease, the lingering questions that exist surrounding new models suggest that further research should continue to refine these models for better clinical applicability.
Conflicts of interest
The authors have none to declare.
Acknowledgments
This work has been supported by Charles University Research Centre program UNCE/MED/006 “University Center of Clinical and Experimental Liver Surgery”.
References
- 1.Valla D.C., Cazals-Hatem D. Sinusoidal obstruction syndrome. Clin Res Hepatol Gastroenterol. 2016;40(4):378–385. doi: 10.1016/j.clinre.2016.01.006. [DOI] [PubMed] [Google Scholar]
- 2.Rubbia-Brandt L. Sinusoidal obstruction syndrome. Clin Liver Dis. 2010;14(4):651–668. doi: 10.1016/j.cld.2010.07.009. [DOI] [PubMed] [Google Scholar]
- 3.Carreras E. How I manage sinusoidal obstruction syndrome after haematopoietic cell transplantation. Br J Haematol. 2015;168(4):481–491. doi: 10.1111/bjh.13215. [DOI] [PubMed] [Google Scholar]
- 4.Coppell J.A., Richardson P.G., Soiffer R. Hepatic veno-occlusive disease following stem cell transplantation: incidence, clinical course, and outcome. Biol Blood Marrow Transplant. 2010;16(2):157–168. doi: 10.1016/j.bbmt.2009.08.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.McDonald G.B., Hinds M.S., Fisher L.D. Veno-occlusive disease of the liver and multiorgan failure after bone marrow transplantation: a cohort study of 355 patients. Ann Intern Med. 1993;118(4):255–267. doi: 10.7326/0003-4819-118-4-199302150-00003. [DOI] [PubMed] [Google Scholar]
- 6.Ito Y. A novel therapeutic strategy for liver sinusoidal obstruction syndrome. J Gastroenterol Hepatol. 2009;24(6):933–934. doi: 10.1111/j.1440-1746.2009.05839.x. [DOI] [PubMed] [Google Scholar]
- 7.Mohty M., Malard F., Abecassis M. Sinusoidal obstruction syndrome/veno-occlusive disease: current situation and perspectives-a position statement from the European Society for Blood and Marrow Transplantation (EBMT) Bone Marrow Transplant. 2015;50(6):781–789. doi: 10.1038/bmt.2015.52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.DeLeve L.D., McCuskey R.S., Wang X. Characterization of a reproducible rat model of hepatic veno-occlusive disease. Hepatology. 1999;29(6):1779–1791. doi: 10.1002/hep.510290615. [DOI] [PubMed] [Google Scholar]
- 9.Schiffer E., Frossard J.L., Rubbia-Brandt L., Mentha G., Pastor C.M. Hepatic regeneration is decreased in a rat model of sinusoidal obstruction syndrome. J Surg Oncol. 2009;99(7):439–446. doi: 10.1002/jso.21276. [DOI] [PubMed] [Google Scholar]
- 10.Deleve L.D., Wang X., Tsai J., Kanel G., Strasberg S., Tokes Z.A. Sinusoidal obstruction syndrome (veno-occlusive disease) in the rat is prevented by matrix metalloproteinase inhibition. Gastroenterology. 2003;125(3):882–890. doi: 10.1016/s0016-5085(03)01056-4. [DOI] [PubMed] [Google Scholar]
- 11.Conotte R., Colet J.M. A metabonomic evaluation of the monocrotaline-induced sinusoidal obstruction syndrome (SOS) in rats. Toxicol Appl Pharmacol. 2014;276(2):147–156. doi: 10.1016/j.taap.2014.02.008. [DOI] [PubMed] [Google Scholar]
- 12.Copple B.L., Woolley B., Banes A., Ganey P.E., Roth R.A. Anticoagulants prevent monocrotaline-induced hepatic parenchymal cell injury but not endothelial cell injury in the rat. Toxicol Appl Pharmacol. 2002;180(3):186–196. doi: 10.1006/taap.2002.9394. [DOI] [PubMed] [Google Scholar]
- 13.Nakamura K., Hatano E., Miyagawa-Hayashino A. Soluble thrombomodulin attenuates sinusoidal obstruction syndrome in rat through suppression of high mobility group box 1. Liver Int. 2014;34(10):1473–1487. doi: 10.1111/liv.12420. [DOI] [PubMed] [Google Scholar]
- 14.Narita M., Hatano E., Tamaki N. Dai-kenchu-to attenuates rat sinusoidal obstruction syndrome by inhibiting the accumulation of neutrophils in the liver. J Gastroenterol Hepatol. 2009;24(6):1051–1057. doi: 10.1111/j.1440-1746.2009.05795.x. [DOI] [PubMed] [Google Scholar]
- 15.Harb R., Xie G., Lutzko C. Bone marrow progenitor cells repair rat hepatic sinusoidal endothelial cells after liver injury. Gastroenterology. 2009;137(2):704–712. doi: 10.1053/j.gastro.2009.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Allen J.R., Carstens L.A., Olson B.E. Veno-occlusive disease in Macaca speciosa monkeys. Am J Pathol. 1967;50(4):653–667. [PMC free article] [PubMed] [Google Scholar]
- 17.Bras G., Berry D.M., Gyorgy P. Plants as aetiological factor in veno-occlusive disease of the liver. Lancet. 1957, May 11;1:960–962. doi: 10.1016/s0140-6736(57)91283-7. [DOI] [PubMed] [Google Scholar]
- 18.Hill K.R., Stephenson C.F., Filshie I. Hepatic veno-occlusive disease produced experimentally in rats by the injection of monocrotaline. Lancet. 1958, Mar 22;1 doi: 10.1016/s0140-6736(58)90874-2. 623–623. [DOI] [PubMed] [Google Scholar]
- 19.Mclean E., Gyorgy P., Bras G. Veno-occlusive lesions in livers of rats fed Crotalaria fulva. Br J Exp Pathol. 1964;45(3):242. [PMC free article] [PubMed] [Google Scholar]
- 20.Epstein R.B., Lerner M.R., Min K.W., Pennington L.R., Brackett D.J. Production and therapy of veno-occlusive disease in rats following administration of monocrotaline. Blood. 1997;90(10) 1620–1620. [Google Scholar]
- 21.Min K.W., Epstein R.B. Monocrotaline induced hepatic venoocclusive disease in dogs - a histochemical and electron-microscopic study. Lab Investig. 1992;66(1) A98–A98. [Google Scholar]
- 22.Teicher B.A., Crawford J.M., Holden S.A. Glutathione monoethyl ester can selectively protect liver from high-dose BCNU or cyclophosphamide. Cancer. 1988;62(7):1275–1281. doi: 10.1002/1097-0142(19881001)62:7<1275::aid-cncr2820620705>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]
- 23.DeLeve L.D., Wang X., Kanel G.C. Decreased hepatic nitric oxide production contributes to the development of rat sinusoidal obstruction syndrome. Hepatology. 2003;38(4):900–908. doi: 10.1053/jhep.2003.50383. [DOI] [PubMed] [Google Scholar]
- 24.DeLeve L.D., Ito Y., Bethea N.W., McCuskey M.K., Wang X., McCuskey R.S. Embolization by sinusoidal lining cells obstructs the microcirculation in rat sinusoidal obstruction syndrome. Am J Physiol Gastrointest Liver Physiol. 2003;284(6):G1045–G1052. doi: 10.1152/ajpgi.00526.2002. [DOI] [PubMed] [Google Scholar]
- 25.Hirata M., Tajima H., Miyashita T. Extravasated platelet aggregation in the livers of rats with drug induced hepatic sinusoidal obstruction syndrome. Mol Med Rep. 2017;15(5):3147–3152. doi: 10.3892/mmr.2017.6407. [DOI] [PubMed] [Google Scholar]
- 26.Jafari A., Wehner S., Kalff J.C., Manekeller S. Sinusoidal obstruction syndrome in the animal model: influence on liver surgery. Langenbecks Arch Surg. 2017;402(1):115–122. doi: 10.1007/s00423-016-1506-0. [DOI] [PubMed] [Google Scholar]
- 27.Park S.H., Lee S.S., Sung J.Y. Noninvasive assessment of hepatic sinusoidal obstructive syndrome using acoustic radiation force impulse elastography imaging: a proof-of-concept study in rat models. Eur Radiol. 2018;28(5):2096–2106. doi: 10.1007/s00330-017-5179-z. [DOI] [PubMed] [Google Scholar]
- 28.Zhang J., Sheng Y., Shi L. Quercetin and baicalein suppress monocrotaline-induced hepatic sinusoidal obstruction syndrome in rats. Eur J Pharmacol. 2017;795:160–168. doi: 10.1016/j.ejphar.2016.12.015. [DOI] [PubMed] [Google Scholar]
- 29.Ikezoe T., Yang J., Nishioka C. The fifth epidermal growth factor-like region of thrombomodulin exerts cytoprotective function and prevents SOS in a murine model. Bone Marrow Transplant. 2017;52(1):73–79. doi: 10.1038/bmt.2016.195. [DOI] [PubMed] [Google Scholar]
- 30.Ezzat T., van den Broek M.A., Davies N. The flavonoid monoHER prevents monocrotaline-induced hepatic sinusoidal injury in rats. J Surg Oncol. 2012;106(1):72–78. doi: 10.1002/jso.23046. [DOI] [PubMed] [Google Scholar]
- 31.Periasamy S., Yang S.S., Chen S.Y., Chang C.C., Liu M.Y. Prophylactic sesame oil attenuates sinusoidal obstruction syndrome by inhibiting matrix metalloproteinase-9 and oxidative stress. JPEN J Parenter Enteral Nutr. 2013;37(4):529–537. doi: 10.1177/0148607112454299. [DOI] [PubMed] [Google Scholar]
- 32.Wang X., Kanel G.C., DeLeve L.D. Support of sinusoidal endothelial cell glutathione prevents hepatic veno-occlusive disease in the rat. Hepatology. 2000;31(2):428–434. doi: 10.1002/hep.510310224. [DOI] [PubMed] [Google Scholar]
- 33.Masubuchi S., Komeda K., Takai S. Chymase inhibition attenuates monocrotaline-induced sinusoidal obstruction syndrome in hamsters. Curr Med Chem. 2013;20(21):2723–2729. doi: 10.2174/0929867311320210008. [DOI] [PubMed] [Google Scholar]
- 34.Nakamura K., Hatano E., Narita M. Sorafenib attenuates monocrotaline-induced sinusoidal obstruction syndrome in rats through suppression of JNK and MMP-9. J Hepatol. 2012;57(5):1037–1043. doi: 10.1016/j.jhep.2012.07.004. [DOI] [PubMed] [Google Scholar]
- 35.Okuno M., Hatano E., Nakamura K. Regorafenib suppresses sinusoidal obstruction syndrome in rats. J Surg Res. 2015;193(2):693–703. doi: 10.1016/j.jss.2014.08.052. [DOI] [PubMed] [Google Scholar]
- 36.Miyata T., Tajima H., Hirata M. Phosphodiesterase III inhibitor attenuates rat sinusoidal obstruction syndrome through inhibition of platelet aggregation in Disse's space. J Gastroenterol Hepatol. 2018;33(4):950–957. doi: 10.1111/jgh.14004. [DOI] [PubMed] [Google Scholar]
- 37.Narita M., Hatano E., Ikai I. A phosphodiesterase III inhibitor protects rat liver from sinusoidal obstruction syndrome through heme oxygenase-1 induction. Ann Surg. 2009;249(5):806–813. doi: 10.1097/SLA.0b013e3181a38ed5. [DOI] [PubMed] [Google Scholar]
- 38.Periasamy S., Hsu D.Z., Chen S.Y., Yang S.S., Chandrasekaran V.R., Liu M.Y. Therapeutic sesamol attenuates monocrotaline-induced sinusoidal obstruction syndrome in rats by inhibiting matrix metalloproteinase-9. Cell Biochem Biophys. 2011;61(2):327–336. doi: 10.1007/s12013-011-9215-3. [DOI] [PubMed] [Google Scholar]
- 39.Zheng Z., Shi L., Sheng Y., Zhang J., Lu B., Ji L. Chlorogenic acid suppresses monocrotaline-induced sinusoidal obstruction syndrome: the potential contribution of NFkappaB, Egr1, Nrf2, MAPKs and PI3K signals. Environ Toxicol Pharmacol. 2016;46:80–89. doi: 10.1016/j.etap.2016.07.002. [DOI] [PubMed] [Google Scholar]
- 40.Chen Z., Huo J.R., Yang L., Zhu H.Y. Effect of ligustrazine on mice model of hepatic veno-occlusive disease induced by Gynura segetum. J Gastroenterol Hepatol. 2011;26(6):1016–1021. doi: 10.1111/j.1440-1746.2011.06661.x. [DOI] [PubMed] [Google Scholar]
- 41.Zhu H., Chu Y., Huo J., Chen Z., Yang L. Effect of prednisone on transforming growth factor-beta1, connective tissue growth factor, nuclear factor-kappaBp65 and tumor necrosis factor-alpha expression in a murine model of hepatic sinusoidal obstruction syndrome induced by Gynura segetum. Hepatol Res. 2011;41(8):795–803. doi: 10.1111/j.1872-034X.2011.00830.x. [DOI] [PubMed] [Google Scholar]
- 42.Qiu S., Zhang H., Fei Q. Urine and plasma metabolomics study on potential hepatoxic biomarkers identification in rats induced by Gynura segetum. J Ethnopharmacol. 2018;216:37–46. doi: 10.1016/j.jep.2018.01.017. [DOI] [PubMed] [Google Scholar]
- 43.Yu X.Z., Ji T., Bai X.L. Expression of MMP-9 in hepatic sinusoidal obstruction syndrome induced by Gynura segetum. J Zhejiang Univ Sci B. 2013;14(1):68–75. doi: 10.1631/jzus.B1200112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Yannam G.R., Han B., Setoyama K. A nonhuman primate model of human radiation-induced venocclusive liver disease and hepatocyte injury. Int J Radiat Oncol Biol Phys. 2014;88(2):404–411. doi: 10.1016/j.ijrobp.2013.10.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Shulman H.M., Luk K., Deeg H.J., Shuman W.B., Storb R. Induction of hepatic veno-occlusive disease in dogs. Am J Pathol. 1987;126(1):114–125. [PMC free article] [PubMed] [Google Scholar]
- 46.Robinson S.M., Mann J., Vasilaki A. Pathogenesis of FOLFOX induced sinusoidal obstruction syndrome in a murine chemotherapy model. J Hepatol. 2013;59(2):318–326. doi: 10.1016/j.jhep.2013.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Robinson S.M., Mann D.A., Manas D.M., Oakley F., Mann J., White S.A. The potential contribution of tumour-related factors to the development of FOLFOX-induced sinusoidal obstruction syndrome. Br J Cancer. 2013;109(9):2396–2403. doi: 10.1038/bjc.2013.604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Qiao J., Qi K., Chu P. Infusion of endothelial progenitor cells ameliorates liver injury in mice after haematopoietic stem cell transplantation. Liver Int. 2015;35(12):2611–2620. doi: 10.1111/liv.12849. [DOI] [PubMed] [Google Scholar]
- 49.Yeom M.Y., Kim Y.J., Chung N.G. Hepatic veno-occlusive disease may develop in secondary iron overloaded mice after allogeneic hematopoietic stem cell transplantation with total body irradiation. Blood Res. 2015;50(3):140–146. doi: 10.5045/br.2015.50.3.140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zeng L., An L., Fang T. A murine model of hepatic veno-occlusive disease induced by allogeneic hematopoietic stem cell transplantation. Cell Biochem Biophys. 2013;67(3):939–948. doi: 10.1007/s12013-013-9587-7. [DOI] [PubMed] [Google Scholar]
- 51.Periasamy S., Liu M.Y. Sinusoidal injury induction: monocrotaline dose and hepatic sinusoidal injury in rats not correlated. J Surg Oncol. 2013;107(4):447. doi: 10.1002/jso.23251. [DOI] [PubMed] [Google Scholar]
- 52.Ezzat T., Dhar D.K., Olde Damink S.W. Sinusoidal obstruction syndrome: correct dosing of monocrotaline and the validity of the rat model. J Surg Oncol. 2013;107(4):448–449. doi: 10.1002/jso.23265. [DOI] [PubMed] [Google Scholar]
- 53.Chen M.Y., Cai J.T., Du Q. Hepatic veno-occlusive disease associated with the use of Gynura segetum. Eur J Intern Med. 2007;18(8):609. doi: 10.1016/j.ejim.2007.03.006. [DOI] [PubMed] [Google Scholar]
- 54.Wang X., Qi X., Guo X. Tusanqi-related sinusoidal obstruction syndrome in China: a systematic review of the literatures. Medicine (Baltimore) 2015;94(23):e942. doi: 10.1097/MD.0000000000000942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kim J., Jung Y. Radiation-induced liver disease: current understanding and future perspectives. Exp Mol Med. 2017;49(7):e359. doi: 10.1038/emm.2017.85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Allen J.R., Carstens L.A., Katagiri G.J. Hepatic veins of monkeys with veno-occlusive disease. Sequential ultrastructural changes. Arch Pathol. 1969;87(3):279–289. [PubMed] [Google Scholar]
- 57.Epstein R.B., Min K.W., Anderson S.L., Syzek L. A canine model for hepatic venoocclusive disease. Transplantation. 1992;54(1):12–16. doi: 10.1097/00007890-199207000-00002. [DOI] [PubMed] [Google Scholar]
- 58.Guha C., Sharma A., Gupta S. Amelioration of radiation-induced liver damage in partially hepatectomized rats by hepatocyte transplantation. Cancer Res. 1999;59(23):5871–5874. [PubMed] [Google Scholar]
- 59.Hubert C., Dahrenmoller C., Marique L., Jabbour N., Gianello P., Leclercq I. Hepatic regeneration in a rat model is impaired by chemotherapy agents used in metastatic colorectal cancer. Eur J Surg Oncol. 2015;41(11):1471–1478. doi: 10.1016/j.ejso.2015.08.152. [DOI] [PubMed] [Google Scholar]
- 60.Lentschener C., Nicco C., Terris B., Samama C.M., Coriat R. Comment on ‘The potential contribution of tumour-related factors to the development of FOLFOX-induced sinusoidal obstruction syndrome’. Br J Cancer. 2016;115(8):e7. doi: 10.1038/bjc.2016.242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Robinson S.M., Mann D.A., Manas D.M., Oakley F., Mann J., White S.A. Response to 'Comment on 'The potential contribution of tumour-related factors to the development of FOLFOX-induced sinusoidal obstruction syndrome'. Br J Cancer. 2016;115(8):e8. doi: 10.1038/bjc.2016.268. [DOI] [PMC free article] [PubMed] [Google Scholar]


