Abstract
Background
The presence of left ventricular diastolic dysfunction (LVDD) in patients with cirrhosis leads to a restriction of activities and a poor health related quality of life (HRQoL), which should be taken into consideration when treating them for liver and cardiac complications.
Aims
The prevalence, complications, predictors of HRQoL and survival in cirrhotic patients with LVDD were studied.
Methods
We report a prospective cohort study of 145 consecutive cirrhotic patients with LVDD who were evaluated for cardiac functional status at enrollment and followed up for hepatic complications, cardiac events, outcome and HRQoL using the Minnesota Living With Heart Failure Questionnaire (MLHFQ) over a period of 2 years.
Results
In total, 145 (mean age 61 years, 59% male) patients were included. Seventeen patients died with 10.5%, 22.5% and 40% mortality rates in patients with Grades 1, 2 and 3 LVDD respectively over 24 months. The parameters that were significant for predicting mortality on bivariate analysis were MELD, MELDNa, hepatic venous pressure gradient, MLHFQ, and left ventricular (LV) diastolic function (e′ and E/e′ ratio), but only MELD, MELDNa and E/e′ remained significant on multivariate analysis. The E/e′ ratio (8.7 ± 3.3 in survivors vs. 9.1 ± 2.3 in non-survivors) predicted outcome. On univariate analysis, the predictors of poor HRQoL were the Child-Pugh score ≥9.8 (OR 2.6; 95% confidence intervals (CI) 2.3–9.1, P = 0.041), MELD score ≥ 15.7 (OR 2.48; 95% CI 1.4–3.9, P = 0.029), refractory ascites (OR 1.9; 95% CI 1.1–6.1, P = 0.050), and E/e′ ratio ≥7.6 (OR 1.9; 95% CI 1.8–7.1, P = 0.036) The presence of Class II/III (P = 0.046) symptoms of heart failure and MLHFQ≥ 45 (P = 0.042) were predictors of mortality at 24 months.
Conclusion
The grade of LVDD correlates with liver function, clinical events, risk of renal dysfunction and HRQoL. Evaluation of novel therapies which target symptomatic improvement in LVDD, should be done with suitable outcome measures, including HRQoL assessment.
Keywords: left ventricular diastolic dysfunction, cirrhotic cardiomyopathy, Health related Quality of Life, cirrhosis, Heart Failure
Abbreviations: 2D, two-dimensional; A, atrial wave-filling peak; ASE, the American Society of Echocardiography; AUC, area under the curve; BNP, brain natriuretic peptide; CI, confidence interval; CO, cardiac output; DT, deceleration time; E, E-wave transmitral peak early filling; e′, early diastolic mitral annular velocity; E/A, early diastolic mitral inflow velocity/late diastolic; E/e′ ratio, E-wave transmitral/early diastolic mitral annular velocity; FHVP, free hepatic venous pressure; GI, gastrointestinal; HE, hepatic encephalopathy; HR, heart rate; HRS, hepatorenal syndrome; HVPG, hepatic venous pressure gradient; IVRT, isovolumetric relaxation time; LT, liver transplantation; LV, left ventricular; LVDD, left ventricular diastolic dysfunction; LVEF, left ventricular ejection fraction; MAP, mean arterial pressure; MELD, Model for End-Stage Liver Disease; MLHFQ, Minnesota Living with Heart Failure questionnaire; PAP, pulmonary artery pressure; PCWP, pulmonary capillary wedged pressure; PH, portal hypertension; RAP, right atrial pressure; RR, relative risk; OR, Odds Ratio; SBP, spontaneous bacterial peritonitis; SD, standard deviation; TDI, tissue Doppler imaging; TIPS, transjugular intrahepatic portosystemic shunt; TTE, transthoracic echocardiography; USG, ultrasonography; WHVP, wedged hepatic venous pressures
Cirrhotic cardiomyopathy is a chronic cardiac dysfunction characterized by impaired contractile responsiveness to stress stimuli, and/or impaired diastolic relaxation, and electrophysiological abnormalities with prolonged QT interval, in the absence of other known cardiac disease.1, 2
The degree of severity can be graded according to average E/e′ ratio.3 The prevalence of left ventricular diastolic dysfunction (LVDD) is relatively high in patients with cirrhosis (43%–70%) despite a normal EF4 and is not related to the etiology of liver disease.5, 6, 7 LVDD is responsible for complications of cirrhosis including acute kidney injury (AKI),8 hepatic encephalopathy (HE), and sepsis induced shock,9, 10 pulmonary edema following liver transplantation,11, 12 and after transjugular intrahepatic portosystemic shunt (TIPS)13 Patients with LVDD have greater probability of mortality8 with the echocardiographic E/e′ ratio acting as an independent prognostic factor for survival.14 Health related quality of life (HRQoL) is a predictor of hospitalization and death in patients with heart failure(HF).15, 16 It is a subjective, patient-centered outcome that is recognized by researchers and clinicians as an important outcome for patients with HF.17 We therefore performed a prospective cohort study aimed at investigating the prognosis, predictors of survival and HRQoL in cirrhotic patients with LVDD followed up over a period of 2 years.
Patients and Methods
Consecutive patients with histological or clinical cirrhosis were screened for LVDD between November 2014 to November 2015 at the outpatient hepatology clinic at the Institute of Liver and Biliary Sciences (ILBS). The inclusion criteria were patients with cirrhosis aged between 18 and 65 years and having LVDD on 2D echocardiography. The exclusion criteria were; patients ≥65 years, chronic renal disease, pregnancy and peripartum cardiomyopathy, hypertension, coronary artery disease (CAD), valvular heart disease, sick sinus syndrome/pacemaker, thyroid dysfunction, or were diagnosed with severe psychiatric impairment other than depression or anxiety, severe anemia or absence of consent to participate. We excluded CAD by performing a battery of tests including electrocardiograms, dobutamine stress echocardiography, Holter examination and treadmill test for inducible ischemia. Sample size was calculated using G*Power, a statistical analysis program, with an effect size of 0.5, alpha 0.05, and power 0.85. It was estimated that a total sample size of 122 patients would be required. Assuming an attrition of 15%, 145 patients were finally recruited for the study. Patients with overt hepatic encephalopathy and concurrent malignancy were excluded. The trial was approved by the Institutional Ethics Committee and was performed in accordance with the Declaration of Helsinki. Patients were enrolled consecutively with written informed consent.
Definitions
The diagnosis of cirrhosis was based on clinical criteria (splenomegaly, ascites, hepatic encephalopathy or variceal bleed, etc.), imaging (irregular surface, altered echotexture, presence of collaterals or dilated portal vein) and endoscopic presence of varices. The American Society of Echocardiography defines LVDD as the presence of septal e′ < 8 cm/s, lateral e′ < 10 cm/s, mitral inflow patterns and LA volume index ≥ 34 mL/m2.3 The presence of CAD was defined as a prior myocardial infarction or any degree of obstruction on coronary imaging. The diagnosis of HF was made according to published criteria.18 The classification of the etiologies of HF followed previous guidelines, and the diagnosis of HF was made through both clinical and imaging modalities.19 Standard Medical Treatment: All patients were given standard medical treatment including dietary advice, diuretics if indicated in the form of spironolactone with frusemide, lactulose and/or rifaximin in patients with prior encephalopathy. Indications for beta blocker therapy were hepatic venous pressure gradient (HVPG) ≥12 mmHg, presence of large varices, or prior variceal bleeding. None of the patients received any other antihypertensive drugs like calcium channel blockers, angiotensin receptor blocker (ARB) or inhibitor (ACE) therapy.
At inclusion, demographic data, the patient history, and medication were recorded. A full physical examination and a broad screening panel of tests (including liver function tests, hematology, lipid profile, HbA1c, creatinine, and electrolytes) were performed. All patients underwent hepatic venous and cardiac catheterization from the femoral route at the time of enrollment with calculation of HVPG, right atrial pressure (RAP), pulmonary capillary wedge pressure (PCWP) and pulmonary artery pressure (PAP).
Health Related Quality of Life (HRQoL) assessment
The Minnesota Living with Heart Failure questionnaire (MLHFQ) is a measure of HRQoL that is used to assess the patient's perceptions of the influence of HF on physical and emotional aspects of life. The 21 items are summed and ranged from 0 to 105 with higher scores indicating worse HRQoL. The MLHFQ is a self-administered disease-specific questionnaire for patients with HF,20 comprising 21 items rated on six-point Likert scales, representing different degrees of impact of HF on HRQoL, from 0 (none) to 5 (very much). It provides a total score (range 0–105, from best to worst HRQoL), as well as scores for two dimensions, physical (8 items, range 0–40) and emotional (5 items, range 0–25). The other eight items (of the total of 21) are only considered for the calculation of the total score.21 This HRQoL scale has been validated in several studies in patients with heart failure22 and is available in multiple Indian vernacular languages including Hindi, Punjabi, Telugu and Marathi, making it a useful tool for estimating HRQoL in India.23, 24 Prior studies have classified a score of <24 on the MLHFQ represents a good QoL, a score ≥24 and < 45 represents a moderate QoL, and a score ≥ 45 represents a poor QoL.21
Echocardiographic Assessment
Echocardiography was performed by an experienced operator in accordance with the recommendations of the American Society of Echocardiography. From a long axis parasternal view, the left ventricular (LV) systolic and diastolic septal wall thickness and the LV diameter [LV end systolic diameter (LVESD) and LV end diastolic diameter (LVEDD)] were measured in M-mode. LV volumes and LV Ejection Fraction (LVEF) were estimated using Simpson's modified biplane method. An LVEF above 50% was considered normal. The following parameters were recorded and measured: peak early (E wave) and atrial (A wave) flow velocities, their ratio E/A, and the E wave deceleration time. Tissue Doppler Imaging (TDI) was obtained from the four-chamber apical view and tissue velocity was calculated. The myocardial peak systolic velocity (S′) was measured in lateral mitral annulus to define systolic function. Tissue velocities were also measured in the lateral mitral annulus during the diastole to obtain peak myocardial velocities during early (E′) and atrial filling (A′) phases. Isovolumetric Ventricular Relaxation Time (IVRT) was measured by TDI. The intra observer variation for echocardiographic measurements was <10%. LVEDD and LVESD are the left ventricular dimensions at the end of diastole and systole respectively. Early (E) and late (A) peak velocity was measured across the mitral valve. The mitral deceleration time (DT) is the interval from the peak of the early velocity until the end of early diastolic filling. The IVRT is the interval from the closure of the aortic valve until mitral valve opening. It was measured by continuous wave imaging from the apical window, displaying aortic and mitral flow together. The echocardiographic evaluation was repeated in patients annually in Grade 1 LVDD and semiannually in Grades 2/3 LVDD. Beta blockers were withheld two days prior to baseline echocardiographic evaluation and were restarted after the procedure on the same day. However, on follow up visits, betablockers were not withheld. LVDD was defined and classified according to American Society of Echocardiography (ASE) guidelines.3, 8 as below:
Grade 1: e′ <8 cm/sec, E/e′ ratio <8, E/A ratio <0.8, and deceleration time (DT) >200 ms;
Grade 2: e′ <8 cm/sec, E/e′ ratio 9–15, E/A ratio 0.8–1.5, and DT 160–200 ms; and
Grade 3: e′ <8 cm/sec, E/e′ ratio >15, E/A ratio >2, and DT <160 ms
Statistical Analysis
Patient characteristics were summarized as proportions with means and standard deviations/range. The measurements obtained by the above-mentioned studies were used to calculate mean values for each patient and the group. Categorical variables were compared using the chi-square test or Fisher's exact test. Normal continuous variables were compared using the Student's t-test. P value< 0.05 was considered significant. Qualitative variables were compared with Chi square test. Repeated measure analysis/Friedman test was applied followed by post hoc comparison by Bonferroni method. Logistic regressions, t-tests, Pearson correlation, Kaplan–Meier plots with log-rank test, and Cox regressions were used to explore the relationships among HRQoL, functional status, and survival. Predictors of MLHFQ scores and mortality were analyzed using logistic regression. With bivariate Cox proportional hazards models, we first estimated the unadjusted relationship between each co variate and mortality. Then the independent relationship between LVDD, HRQoL and mortality, while adjusting for predictors of mortality was evaluated. We then computed the adjusted hazard ratios (HRs) and 95% confidence intervals (CI) to estimate the strength of association of each predictor with time to the clinical event or death. With logistic regression analysis, we also calculated the HR for mortality with varying HRQoL while adjusting for liver disease severity. Data were analyzed with SPSS software version 22 (IBM SPSS Statistics, Armonk, USA).
Results
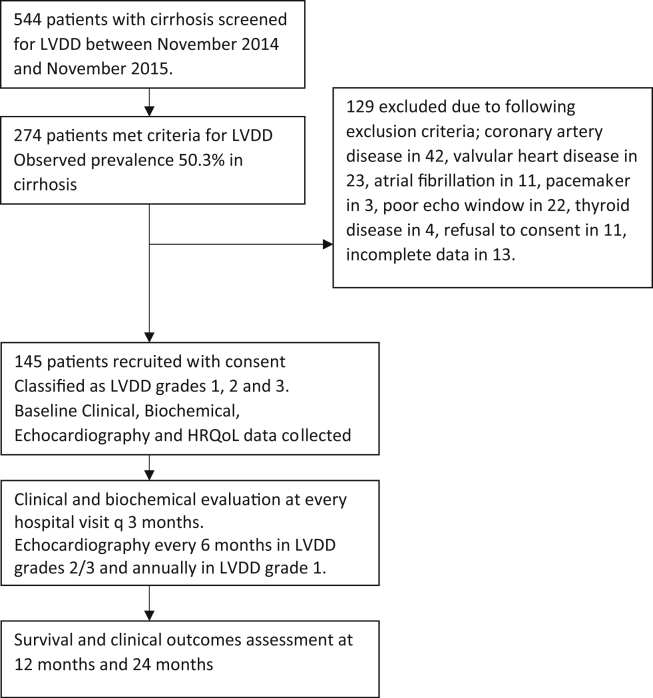
A total of 544 patients with cirrhosis were screened for LVDD between November 2014 to November 2015, of which 274 met criteria for LVDD, with an observed prevalence of 50.3% in cirrhosis. Of these, 129 were excluded because of associated CAD in 42, significant valvular heart disease in 23, atrial fibrillation in 11, pacemaker in situ in 3, poor echo window in 22, thyroid disease in 4, refusal to consent in the protocol in 11 and incomplete HRQoL data in 13 (Figure 1). Finally, 145 patients were included and followed for a median duration of 20 months (range 3–44 months). The mean age of included patients was 61 years (range 41–65 years) and 54 (59%) were men. The mean body mass index was 24 ± 4.0 kg/m2 (range 16–31). The underlying etiology for cirrhosis was ethanol (55.8%), nonalcoholic steatohepatitis (33.7%), chronic viral hepatitis (5.5%), autoimmune hepatitis (1.3%), or cryptogenic cirrhosis (3.4%). The mean CTP, MELD and MELDNa scores were 9.3 ± 1.6, 14.4 ± 4.0 and 17.2 ± 4.4 respectively. Seventy-seven (53.1%) patients had clinical signs of decompensation prior to inclusion. At baseline, patients (54%) had ascites, 17.9% had diabetes, 11% were either current or past smokers (11%) and 22.1% had dyslipidemia. Sixty-one (42%) patients were treated with lactulose, 61 (42%) with beta-blockers, 57 (39.3%) with loop diuretics, and 49 (33.7%) with spironolactone. No other antihypertensive drugs were used in this cohort. Only 16 (%) of patients smoked; i.e. 11.7%, 7.5% and 15% in grades 1, 2 and 3 LVDD respectively.
Figure 1.
Patient enrollment flowchart.
Association of Severity of LVDD and Circulatory Dysfunction
We found that 58.6%, 27.5% and 13.7% of the 145 subjects had grades 1, 2 and 3 LVDD respectively. LVDD was characterized by a reduced e, and an increased E/e′ ratio. Patients with grades 2 and grade 3 LVDD had higher E/e′, IVRT, LA volume, RAP and PAP, and lower MAP, than in patients with grade 1 LVDD. E/e′ ratio and left atrial (LA) volume increased with higher grades of LVDD. All patients had preserved EF (50–55%). Left ventricular systolic function, estimated by CO, left ventricular stroke work and cardiac chronotropic function were reduced in patients with grade 2 as compared to grade 1 LVDD. Patients with grade 2 LVDD showed lower MAP, as compared to patients with grade 1 LVDD. HVPG was higher in the patients with grades 3 vs 1 LVDD (17.9 ± 4.0 vs 15.7 ± 3.4; P = 0.034) (Table 1).
Table 1.
Demographic, Clinical, Biochemical, Echocardiographic, Hemodynamic, Functional Status and HRQoL Data of Patients with Cirrhosis at Time of Inclusion Classified According to Grade of LVDD.
| Characteristics | Grade 1 LVDD N = 85 (1) |
Grade 2 LVDD N = 40 (2) |
Grade 3 LVDD N = 20 (3) |
P Value (1 vs 2) |
P Value (1 vs 3) |
|---|---|---|---|---|---|
| Mean ± standard deviation | |||||
| Age (years) | 52.3 ± 7.8 | 54.8 ± 8.2 | 51.8 ± 7.2 | 0.411 | 0.544 |
| Body Mass Index (kg/m2) | 22.4 ± 3.8 | 19.4 ± 2.8 | 20.3 ± 2.1 | 0.032 | 0.421 |
| Child-Pugh score | 9.0 ± 1.6 | 9.0 ± 1.8 | 10.1 ± 1.2 | 0.538 | 0.065 |
| MELD score | 13.1 ± 4.0 | 13.6 ± 4.1 | 16.6 ± 4.8 | 0.943 | 0.014 |
| MELD Na score | 15.9 ± 5.4 | 16.9 ± 5.9 | 19.4 ± 4.3 | 0.027 | 0.041 |
| Diabetes mellitus n (%) | 18 (21.1%) | 4 (10%) | 4 (20%) | 0.058 | 0.155 |
| Dyslipidemia n (%) | 23 (27%) | 5 (12.5%) | 4 (20%) | 0.047 | 0.344 |
| Smoking n (%) | 10 (11.7%) | 3 (7.5%) | 3 (15%) | 0.452 | 0.092 |
| Echocardiographic data | |||||
| Diastolic function | |||||
| e′, cm/sec | 8.3 ± 1.6 | 6.8 ± 1.6 | 6.8 ± 1.6 | 0.000 | 0.000 |
| E/e′ ratio | 9.1 ± 1.2 | 11.1 ± 2.1 | 12.1 ± 2.5 | 0.010 | 0.020 |
| LA size cm | 4.0 ± 2.2 | 4.4 ± 2.8 | 4.8 ± 2.3 | 0.157 | 0.054 |
| DT ms | 228.1 ± 26.2 | 186.7 ± 32.1 | 179.7 ± 22.1 | 0.012 | 0.032 |
| RA size cm | 3.5 ± 0.35 | 3.6 ± 0.44 | 3.4 ± 0.74 | 0.074 | 0.031 |
| E/A ratio | 1.1 ± 0.36 | 0.9 ± 0.43 | 0.7 ± 0.43 | 0.000 | 0.000 |
| Systolic function | |||||
| LVEF, % | 55–60% | 55–60% | 55–60% | NS | NS |
| LVEDV, mL | 45.3 ± 6.3 | 45.9 ± 7.4 | 48.9 ± 7.4 | 0.274 | 0.042 |
| LVESV, mL | 23.9 ± 5.2 | 24.4 ± 5.5 | 26.4 ± 4.5 | 0.619 | 0.033 |
| Hemodynamic assessment and portal hepatic measurements | |||||
| MAP, mmHg | 89.6 ± 5.8 | 86.6 ± 6.2 | 82 ± 5.2 | 0.290 | 0.032 |
| HR, bpm | 85.3 ± 10.6 | 83.9 ± 12.4 | 92 ± 8.2 | 0.236 | 0.051 |
| RAP, mmHg | 4.0 ± 1.1 | 4.4 ± 1.2 | 4.8 ± 1.4 | 0.074 | 0.033 |
| PAP, mmHg | 15.7 ± 2.3 | 15.8 ± 3.2 | 13.8 ± 3.2 | 0.457 | 0.544 |
| PCWP, mmHg | 11.9 ± 2.1 | 11.9 ± 1.5 | 13.9 ± 2.5 | 0.962 | 0.352 |
| HVPG, mmHg | 15.7 ± 3.4 | 17.5 ± 4.0 | 17.9 ± 4.0 | 0.079 | 0.034 |
| CO, L/min | 6.3 ± 1.2 | 5.9 ± 1.1 | 5.5 ± 1.4 | 0.405 | 0.027 |
| HRQoLParameters | |||||
| NYHA Classification | |||||
| I | 55 (64.7%) | 24 (60.0%) | 12 (60.0%) | 0.433 | 0.346 |
| II | 27 (31.7%) | 16 (40.0%) | 8 (40.0%) | 0.344 | 0.452 |
| III | 3 (3.5%) | – | – | ||
| IV | – | – | – | ||
| MLHFQ score, (mean ± SD) | |||||
| Physical subscale | 22.40 ± 9.5 | 22.40 ± 9.5 | 32.40 ± 9.5 | 0.394 | 0.023 |
| Emotional subscale | 12.40 ± 8.8 | 15.40 ± 4.8 | 18.4 ± 5.8 | 0.037 | 0.044 |
| Total scale | 39.4 ± 12.2 | 51.8 ± 19.9 | 64.8 ± 21.9 | 0.012 | 0.002 |
Abbreviations for the table: CO, cardiac output; e′, early diastolic mitral annular velocity; E/A, early diastolic mitral inflow velocity/late diastolic; E/e′ ratio, E-wave transmitral/early diastolic mitral annular velocity; EF, ejection fraction; IVRT, isovolumic ventricular relaxation time; HR, heart Rate; HVPG, hepatic venous pressure gradient; ejection fraction; LA, left atrium; LV, left ventricle; LVEDV, LV end diastolic volume; LVESV, LV end systolic volume; MAP, mean arterial pressure; MELD, Model for End-Stage Liver Disease; MLHFQ, Minnesota Living with Heart Failure questionnaire; NYHA New York Heart Association classification of heart failure symptoms. PAP, Pulmonary arterial pressure; RAP, right atrial pressure; PCWP, Pulmonary capillary wedge pressure.
Association of LVDD with Severity and Complications of Cirrhosis
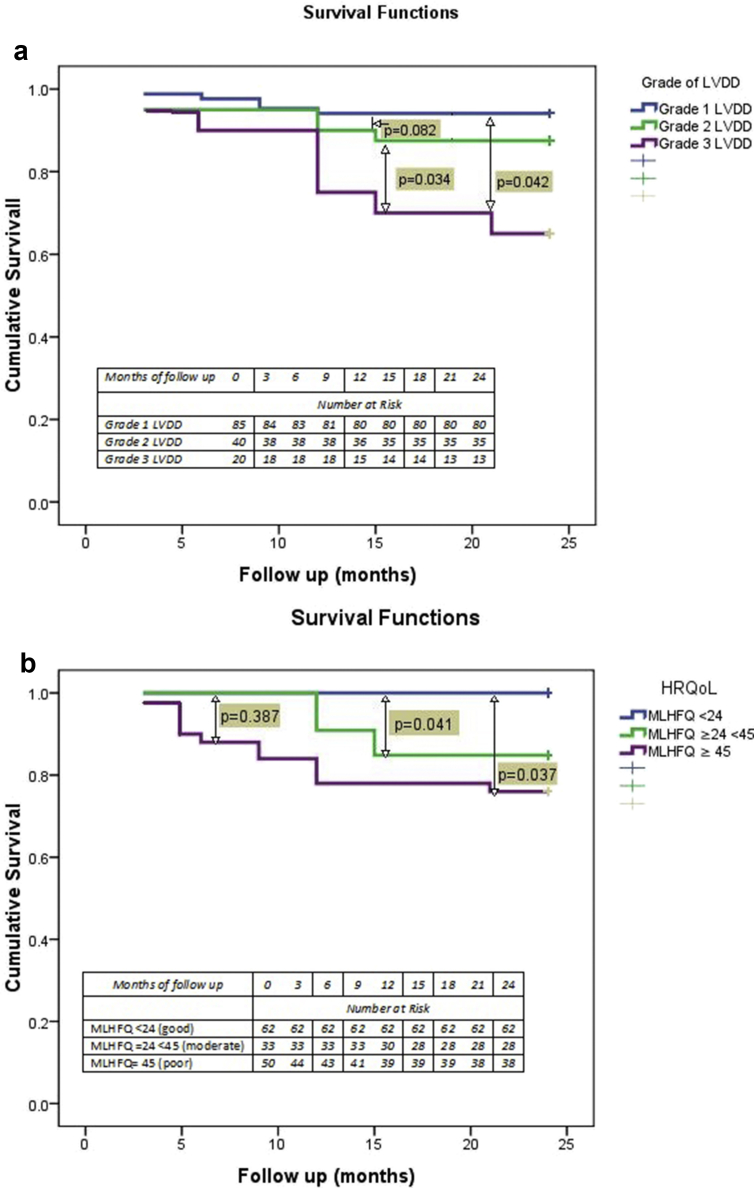
We also studied the incidence of cirrhosis related complications over the two-year period of follow up. The episodes AKI were more likely to occur in grades 2/3 vs grade 1 LVDD (OR 6.273; CI-2.89–35.4, P = 0.027). The episodes of HE were more likely to occur in the grade 2/3 LVDD as compared with grade 1 LVDD. (OR 5.6; CI-2.9–8.7, P = 0.024). Seventeen patients were admitted with variceal bleeding. Three patients underwent successful liver transplantation and two patients received a TIPS insertion. Spontaneous bacterial peritonitis was diagnosed in 8 (5.5%) patients and 29 (20%) developed other bacterial infections like pneumonia (8.2%) and urinary tract infection (2.7%) that required hospitalization. Twenty-five (17.2%) patients developed overt HE, and 18 (12.4%) developed hepatorenal syndrome. Kaplan Meier curves showed a trend of reduced survival with increasing grades of LVDD (Figure 2a).
Figure 2.
a: Kaplan Meir Curve showing survival over a period of 2 years according to grade of LVDD. b: Kaplan Meir Curve showing survival of over a period of two years according to MLHFQ Score.
Predictors of Mortality in Patients with LVDD
There were 17 deaths in our study cohort with 10.5%, 22.5% and 40% mortality rates in patients with Grades 1, 2 and 3 LVDD respectively over a period of 24 months (See Figure 2b). Nine patients were lost to follow up. Causes of death included severe sepsis (58%), refractory gastrointestinal bleed (17%) and hepatocellular carcinoma (11.7%). Table 2 shows the baseline characteristics that predicted survival at 12 months. On bivariate analysis, the parameters that were significant for predicting mortality were CTP, MELD, and MELDNa, HVPG, MLHFQ and LV diastolic function (e′ and E/e′ ratio). In multivariate analysis, MELD, MELD Na and E/e′ score were significant for predicting 2-year mortality. The E/e′ ratio (8.7 ± 3.3 in survivors vs. 9.1 ± 2.3 in non-survivors) and the IVRT (86.5 ± 15.6 ms in survivors and 98.2 ± 16.3 in non-survivors) also predicted outcomes (P = 0.043) (Table 3) Cox proportional hazards models were used to find a direct association between degree of LVDD and mortality across three models with different levels of adjustment. In both the partially adjusted models i.e. Model 1 with adjustment for age and gender, and Model 2 with adjustment as Model 1 and presence of diabetes and dyslipidemia, we found a direct association of grades 2 and 3 LVDD with mortality. In case of Model 3 where adjustment was done as for Model 2 and baseline MELD, the strength of association remained significant for Grade 3 LVDD alone, not Grade 2 (Table 4).
Table 2.
Comparison of Baseline Clinical, Biochemical, Echocardiographic and Hemodynamic Characteristics of Patients at Baseline as per Survival at 24 Months.
| Characteristics | Survivor (n = 128) | Non survivor (n = 17) | P Value |
|---|---|---|---|
| BMI (kg/m2) | 21.4 ± 3.6 | 20.4 ± 2.2 | 0.364 |
| Diabetes mellitus (n = 26) | 22 (84.6%) | 4 (15.3%) | 0.570 |
| Dyslipidemia (n = 32) | 23 (71.8%) | 9 (28.1%) | 0.470 |
| Etiology of Cirrhosis | |||
| Alcohol | 72 (56.2%) | 8 (47%) | 0.206 |
| NASH | 44 (34.3%) | 5 (29.4%) | 0.107 |
| Other | 12 (9.3%) | 4 (23.5%) | 0.241 |
| Child-Pugh score | 9.0 ± 0.9 | 10.0 ± 0.62 | 0.000 |
| MELD score | 13.0 ± 3.7 | 17.5 ± 4.2 | 0.000 |
| MELD Na | 16.9 ± 4.9 | 22.07 ± 4.2 | 0.000 |
| MLHFQ Score | 42.4 ± 16.9 | 59.5 ± 22.2 | 0.044 |
| Echocardiographic data | |||
| Diastolic function | |||
| e′, cm/sec | 10.6 ± 2.3 | 11.8 ± 4.4 | 0.332 |
| E/e′ ratio | 8.7 ± 3.3 | 9.1 ± 2.3 | 0.058 |
| IVRT, ms | 86.5 ± 15.6 | 98.2 ± 16.3 | 0.043 |
| E/A ratio | 1.1 ± 0.2 | 1.1 ± 0.3 | 0.643 |
| DT, ms | 217.8 ± 33 | 224.7 ± 48 | 0.467 |
| Systolic function | |||
| LVEF, % | 55–60% | 55–60% | |
| LVEDV, mL | 45.4 ± 6.7 | 45.9 ± 9.4 | 0.854 |
| LVESV, mL | 24.1 ± 5.3 | 24.1 ± 6.2 | 0.860 |
| Hemodynamic assessment and portal hepatic measurements at baseline | |||
| MAP, mmHg | 89.9 ± 6.1 | 89.6 ± 4.6 | 0.809 |
| HR, bpm | 84.2 ± 11.5 | 87.7 ± 7.3 | 0.100 |
| RAP, mmHg | 4.24 ± 1.24 | 4.57 ± 1.3 | 0.395 |
| PAP, mmHg | 15.6 ± 3.1 | 16.5 ± 3.8 | |
| PCWP, mmHg | 12 ± 1.8 | 12.9 ± 2.03 | 0.103 |
| CO, L/min | 6.4 ± 1.4 | 6.0 ± 0.9 | |
| HVPG, mmHg | 16.7 ± 3.8 | 19.3 ± 4.9 | 0.023 |
Abbreviations for the table: BMI, body mass index; CO, cardiac output; e′, early diastolic mitral annular velocity; E/A, early diastolic mitral inflow velocity/late diastolic; E/e′ ratio, E-wave transmitral/early diastolic mitral annular velocity; EF, ejection fraction; IVRT, isovolumic ventricular relaxation time; HR, heart Rate; HVPG, hepatic venous pressure gradient; MLHFQ, Minnesota Living with Heart Failure questionnaire;; LV, left ventricle; LVEDV, LV end diastolic volume; LVESV, LV end systolic volume; MAP, mean arterial pressure; MELD, Model for End-Stage Liver Disease; PAP, Pulmonary arterial pressure; RAP, right atrial pressure; PCWP, Pulmonary capillary wedge pressure.
Table 3.
Results of Cox Proportional Hazards Regression Analysis to Assess Predictors of 24- Month Mortality on Bivariate and Multivariate Analysis.
| Characteristic | Bivariate analysis |
Multivariate analysis |
||||
|---|---|---|---|---|---|---|
| HR | 95% CI | P Value | HR | 95% CI | P Value | |
| MELD | 1.63 | 1.21–1.44 | 0.029 | 1.42 | 1.22–1.75 | 0.030 |
| MELD Na | 2.48 | 1.4–2.3.9 | 0.031 | 1.35 | 1.18–1.77 | 0.043 |
| E/e′ | 1.42 | 1.22–1.62 | 0.023 | 1.19 | 1.13–1.5 | 0.045 |
| e′ | 1.15 | 0.98–1.56 | 0.057 | |||
| HVPG | 1.28 | 1.11–1.55 | 0.044 | |||
| MLHFQ | 1.18 | 1.12–1.45 | 0.056 | |||
| CTP | 1.15 | 1.02–1.77 | 0.046 | |||
Abbreviations: CTP, Child Turcotte Pugh Score; HR, hazard ratio; 95% CI, 95% confidence interval; e′, early diastolic mitral annular velocity; E/e′ ratio, E-wave transmitral/early diastolic mitral annular velocity; HVPG, hepatic venous pressure gradient; INR, international normalized ratio; MLHFQ, Minnesota Living with Heart Failure questionnaire; MELD, Model for end stage liver disease; MELD Na, Model for end stage liver disease Na.
Table 4.
Cox Proportional Hazards Models With Adjustment; Hazard Ratios (HR) for Mortality Over 24 Months by Grade of LVDD Across Three Models With Different Levels of Adjustment.
| Category of LVDD | Multivariate HR (95% CI) Model 1b |
P Value | Multivariate HR (95% CI) Model 2c |
P Value | Multivariate HR (95% CI) Model 3d |
P Value |
|---|---|---|---|---|---|---|
| Grade 1a | 1.0 (reference) | 1.0 (reference) | 1.0 (reference) | |||
| Grade 2 | 1.64 (1.41–1.98) | 0.046 | 1.56 (1.38–1.77) | 0.052 | 1.16 (1.04–1.49) | 0.098 |
| Grade 3 | 1.76 (1.54–2.3) | 0.033 | 1.72 (1.32–1.92) | 0.046 | 1.67 (1.34–1.88) | 0.043 |
Left ventricular diastolic dysfunction (LVDD) grade 1 was used as reference for analysis.
Model 1: Adjusted for age and gender.
Model 2: Adjusted as Model 1, and presence of diabetes and dyslipidemia.
Model 3: Adjusted as Model 2, and baseline MELD.(Model for end stage liver disease).
Association of HRQoL with Event Free Survival
On univariate analysis, the predictors of poor HRQoL were the Child-Pugh score ≥ 9.8 (OR 2.6; 95% CI 2.3–9.1, P = 0.041), MELD score ≥ 15.7 (OR 2.48; 95% CI 1.4–3.9, P = 0.029), refractory ascites (OR 1.9; 95% CI 1.1–6.1, P = 0.050), and E/e′ ratio ≥7.6 (OR 1.9; 95% CI 1.8–7.1, P = 0.036) On multivariate analysis, only MELD, refractory ascites and E/e′ remained significant. Kaplan–Meier plots with log-rank tests demonstrated that survival was significantly worse in patients with poor MLHFQ (P = 0.023) (Figure 2b). After controlling for age, gender and MELD, patients who had MLHFQ ≥53 had increased risk of developing AKI (OR 1.4, CI 1.1–2.3; P = 0.050) and HE (OR 1.5, CI 1.1–2.9; P = 0.048) compared to patients who had better HRQoL (<53). For every two-point increase in MLHFQ score, the risk of a decompensation event during follow-up increased 1.5–1.6%. The mean HRQoL score as measured by MLHFQ was 38.3 ± 20.6 (median: 42). Table 5 shows the results of Cox proportional hazards regression model analysis for mortality as per degree of diastolic heart failure and health related quality of life adjusted for baseline MELD. The presence of Class II/III (P = 0.042) symptoms of heart failure and MLHFQ≥ 45 (P = 0.042) were predictors of mortality at 24 months.
Table 5.
Multivariate Cox Proportional Hazards Regression Model Analysis for Mortality as per Degree of Diastolic Heart Failure and Health Related Quality of Life Adjusted for Baseline MELD.
| Category | Multivariate HR (95% CI) Model 1a |
P Value |
|---|---|---|
| Degree of Heart Failure | ||
| NYHA Class I* | 1.0 (reference) | |
| NYHA Class II/IIIb | 1.44 (1.31–1.98) | 0.042 |
| HRQoL assessment by MLHFQc | ||
| MLHFQ <24 (good) | 1.0 (reference) | |
| MLHFQ ≥24 < 45 (moderate) | 1.09 (0.85–1.34) | 0.189 |
| MLHFQ ≥45 (poor) | 1.76 (1.3–3.45) | 0.042 |
Abbreviations: HR, hazard ratios; NYHA, New York Heart Association, HRQoL, health related quality of life; MLHFQ, Minnesota Living with Heart Failure questionnaire; MELD, Model for end stage liver disease.
Model 1: Adjusted for baseline MELD.
NYHA Class II and III were clubbed together due to low numbers.
< 24 on the MLHFQ represents a good QoL, a score ≥24 < 45 represents a moderate QoL, and a score ≥ 45 represents a poor QoL.
Discussion
Left ventricular diastolic dysfunction is commonly associated with advanced hepatic dysfunction, even though systolic function remains preserved.25 Patients with decompensated cirrhosis show impairment in effective arterial blood volume and arterial hypotension, lower CO and LV stroke work when compared to those without ascites.26 Our study shows a prevalence of LVDD in 50.3% of cirrhosis and describes the clinical features, degree of cardiac dysfunction, disease progression and HRQoL and mortality in a prospective cohort of cirrhotics followed up for a period of 2 years.
Association of LVDD with Outcomes
Most studies have diagnosed LVDD based on the E/A ratio <1 using 2D echocardiography. E/e′ ratio being the single best screening tool for diagnosis of LVDD using pulsed TDI, as opposed to conventional echocardiography Valeriano et al demonstrated a lower mean E/A ratio in patients with ascites.27 Ruiz del Arbol et al showed LVDD, present in 46% of patients, was a sensitive marker of advanced cirrhosis, type 1 hepatorenal syndrome development, and mortality8 Finucci et al found impaired LV relaxation, decreased E/A ratio, and delayed early diastolic transmittal filling in patients with cirrhosis compared with controls.28 E/e′ ratio as an independent prognostic factor for survival.8 The inability to sustain an increase in cardiac output efficiently to cope with the hemodynamic requirements is of crucial importance specially under stress.29 So, the increased mortality risk associated with LVDD could be related to deterioration in cardiocirculatory functions with progressive liver failure.12 We found a direct association of grades 2 and 3 LVDD with mortality in adjusted models independent of confounding variables like age, gender, diabetes, dyslipidemia. However, the strength of association with mortality remained significant for grade 3 LVDD alone when adjusted for baseline MELD. Our study suggests that degree of LVDD correlates with liver function, and the clinical events including renal dysfunction, sepsis and encephalopathy are related to worsening of circulatory function, consistent with prior studies.7, 8, 10, 29 The grade of LVDD and poor HRQoL may be a sensitive and independent marker for mortality even after adjustment for the severity of the underlying liver disease, age or gender.30
The severity of cardiac dysfunction is a marker of advanced cirrhosis and mortality, and hence future research should be aimed at improving LVDD and outcomes in cirrhosis.31, 32
Association of Other Factors with HRQoL
Patients with a low BMI were more likely to have a worse HRQoL. Our results concur with studies showing that the Child-Pugh, MELD score and low BMI are associated with poor quality of life.
Marchesini et al showed significant differences in most HRQoL domains when comparing patients without ascites to those with either mild-to-moderate or severe ascites.32 A Dutch study also showed that patients resolution of ascites is associated with an improvement in HRQoL to levels seen in compensated disease, implying that there is potential to vastly improve patient HRQoL through effective treatment of decompensation.33 Our study showed that LVDD was associated with poorer HRQoL, affecting predominantly physical components of the MLHFQ. The assessment of HRQoL in LVDD is now being incorporated in heart failure clinical trials. Patients with better HRQoL scores at baseline have longer survival in various complications of cirrhosis such as HCC.34 Thus an analogy can be drawn between the development of LVDD and impairment of QoL.35, 36
Seven of the 17 deaths occurred in patients who were classed as Child-Pugh group B. Most of these patients had previous signs of decompensated liver disease with ascites, variceal bleeding, spontaneous bacterial peritonitis, or overt HE. Our results support the theory that decompensating events as well as Child-Pugh and MELD scores predict long-term prognosis in LVDD. The increased risk of infections concurs with previous evidence.31
Limitations
Several limitations of the study merit further discussion. Firstly, although the study was designed prospectively, it is subject to the limitations inherent in single centre observational studies. The clinical visit may be insufficient to detect compliance with therapy, and the use of the MLHFQ as a measure of HRQoL is subject to self-reporting errors. More than 42% of the patients were treated with beta-blockers and 39.3% with diuretics, the maximal tolerated doses and the duration of these treatments was not recorded, which is relevant to outcomes. Also, despite multivariate analyses, we cannot exclude that residual measured and unmeasured confounding accounts for some of these observations. Nonetheless, given the overall large number of patients observed, and long follow up period of 24 months, our results are clinically relevant, subject to a validation study with a control group of cirrhotics without LVDD. Despite these limitations, this analysis provides new insights into the factors contributing to LVDD related complications and impaired HRQoL in cirrhosis.
Conclusion
This study shows that nearly 50% of cirrhotic patients have LVDD and the of grade diastolic dysfunction correlates with liver function, clinical events, risk of renal dysfunction and HRQoL. Pulsed tissue doppler imaging is useful for diagnosing LVDD, with the E/e′ ratio being the best screening criterion. LVDD is a predictor of mortality independent of the severity of underlying liver disease. Because patients with LVDD and HF tend to experience poorer HRQoL, hence clinicians should assess HRQoL and functional status in these patients, and provide tailored, evidence-based interventions to improve outcomes in cirrhosis. Additional studies are needed to identify the most efficient management strategies to improve the prognosis as well as the quality of life. Interventions directed against LVDD may help achieve this goal.
Patient consent statement
Written informed consent has been taken from all the patients and all images have been modified to protect their identity.
Ethical clearance
Ethical clearance was obtained from the Institutional Review Board; reference number F25/5/64/ILBS/AC2013/909.
Author contributions
MP, DD, TS and SM were involved in the diagnostic procedures and data collection. JSK performed all the cardiac assessments including echocardiography. RG and SST performed all the biochemical tests. GK and MP performed the statistical analysis. All the authors have read and approved the manuscript.
Source of funding
MP was the recipient of the Young Scientist Grant, a research grant (SB/YS/LS-189/2014) provided by the Department of Science and Technology, Govt. of India.
Conflicts of interest
The authors have none to declare.
Acknowledgement
The study was supported by a working grant from the Science and Engineering Research Board, Department of Science and Technology, Government of India. SB/YS/LS-189/2014.
References
- 1.Møller S., Henriksen J.H. Cirrhotic cardiomyopathy. J Hepatol. 2010;53(1):179–190. doi: 10.1016/j.jhep.2010.02.023. [DOI] [PubMed] [Google Scholar]
- 2.Yang Y.Y., Lin H.C. The heart: pathophysiology and clinical implications of cirrhotic cardiomyopathy. J Chin Med Assoc. 2012 Dec;75(12):619–623. doi: 10.1016/j.jcma.2012.08.015. [DOI] [PubMed] [Google Scholar]
- 3.Nagueh S.F., Appleton C.P., Gillebert T.C. Recommendations for the evaluation of left ventricular diastolic function by echocardiography. Eur J Echocardiogr. 2009;10:165–193. doi: 10.1093/ejechocard/jep007. [DOI] [PubMed] [Google Scholar]
- 4.Ruiz-del-Árbol L., Serradilla R. Cirrhotic cardiomyopathy. World J Gastroenterol. 2015;21(41):11502–11521. doi: 10.3748/wjg.v21.i41.11502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Raevens S., De Pauw M., Geerts A. Prevalence and outcome of diastolic dysfunction in liver transplantation recipients. Acta Cardiol. 2014;69:273–280. doi: 10.1080/ac.69.3.3027830. [DOI] [PubMed] [Google Scholar]
- 6.Dadhich S., Goswami A., Jain V.K., Gahlot A., Kulamarva G., Bhargava N. Cardiac dysfunction in cirrhotic portal hypertension with or without ascites. Ann Gastroenterol. 2014;27:244–249. [PMC free article] [PubMed] [Google Scholar]
- 7.Karagiannakis D.S., Papatheodoridis G., Vlachogiannakos J. Recent advances in cirrhotic cardiomyopathy. Dig Dis Sci. 2015;60:1141–1151. doi: 10.1007/s10620-014-3432-8. [DOI] [PubMed] [Google Scholar]
- 8.Ruíz-del-Árbol L., Achécar L., Serradilla R. Diastolic dysfunction is a predictor of poor outcomes in patients with cirrhosis, portal hypertension, and a normal creatinine. Hepatology. 2013;58:1732–1741. doi: 10.1002/hep.26509. [DOI] [PubMed] [Google Scholar]
- 9.Pozzi M., Carugo S., Boari G. Functional and structural cardiac abnormalities in cirrhotic patients with and without ascites. Hepatology. 1997;26:1131–1137. doi: 10.1002/hep.510260507. [DOI] [PubMed] [Google Scholar]
- 10.Karagiannakis D.S., Vlachogiannakos J., Anastasiadis G., Vafiadis-Zouboulis I., Ladas S.D. Frequency and severity of cirrhotic cardiomyopathy and its possible relationship with bacterial endotoxemia. Dig Dis Sci. 2013;58:3029–3036. doi: 10.1007/s10620-013-2693-y. [DOI] [PubMed] [Google Scholar]
- 11.Mittal C., Qureshi W., Singla S., Ahmad U., Huang M.A. Pre-transplant left ventricular diastolic dysfunction is associated with post transplant acute graft rejection and graft failure. Dig Dis Sci. 2014;59:674–680. doi: 10.1007/s10620-013-2955-8. [DOI] [PubMed] [Google Scholar]
- 12.Aggarwal S., Kang Y., Freeman J.A., Fortunato F.L., Pinsky M.R. Postreperfusion syndrome: cardiovascular collapse following hepatic reperfusion during liver transplantation. Transplant Proc. 1987;19:54–55. [PubMed] [Google Scholar]
- 13.Cazzaniga M., Salerno F., Pagnozzi G., Dionigi E., Visentin S., Cirello I. Diastolic dysfunction is associated with poor survival in cirrhotic patients with transjugular intrahepatic portosystemic shunt. Gut. 2007;56:869–875. doi: 10.1136/gut.2006.102467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nazar A., Guevara M., Sitges M. LEFT ventricular function assessed by echocardiography in cirrhosis: relationship to systemic hemodynamics and renal dysfunction. J Hepatol. 2013 Jan;58(1):51–57. doi: 10.1016/j.jhep.2012.08.027. [DOI] [PubMed] [Google Scholar]
- 15.Parissis J.T., Nikolaou M., Farmakis D. Self-assessment of health status is associated with inflammatory activation and predicts long-term outcomes in chronic heart failure. Eur J Heart Fail. 2009;11:163–169. doi: 10.1093/eurjhf/hfn032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Moser D.K., Yamokoski L., Sun J.L. Improvement in health-related quality of life after hospitalization predicts event-free survival in patients with advanced heart failure. J Card Fail. 2009;15:763–769. doi: 10.1016/j.cardfail.2009.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wu J.-R., Lennie T.A., Frazier S.K., Moser D.K. Health-related quality of life, functional status and cardiac event-free survival in patients with heart failure. J Cardiovasc Nurs. 2016 May-Jun;31(3):236–244. doi: 10.1097/JCN.0000000000000248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.McKee P.A., Castelli W.P., McNamara P.M., Kannel W.B. The natural history of congestive heart failure: the Framingham study. N Engl J Med. 1971 Dec 23;285(26):1441–1446. doi: 10.1056/NEJM197112232852601. [DOI] [PubMed] [Google Scholar]
- 19.Yancy C.W., Jessup M., Bozkurt B. 2017 ACC/AHA/HFSA focused update of the 2013 ACCF/AHA guideline for the management of heart failure: a report of the American College of Cardiology/American Heart Association Task Force on clinical practice guidelines and the Heart Failure Society of America. J Am Coll Cardiol. 2017 Aug 8;70(6):776–803. doi: 10.1016/j.jacc.2017.04.025. [DOI] [PubMed] [Google Scholar]
- 20.Bilbao A., Escobar A., García-Perez L., Navarro G., Quirós R. The Minnesota living with heart failure questionnaire: comparison of different factor structures. Health Qual Life Outcome. 2016 Feb 17;14:23. doi: 10.1186/s12955-016-0425-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Behlouli H., Feldman D.E., Ducharme A. Identifying relative cut-off scores with neural networks for interpretation of the Minnesota Living with Heart Failure Questionnaire. Conf Proc IEEE Eng Med Biol Soc. 2009;2009:6242–6246. doi: 10.1109/IEMBS.2009.5334659. [DOI] [PubMed] [Google Scholar]
- 22.Rector T.S., Kubo S.H., Cohn J.N. Validity of the Minnesota living with heart failure questionnaire as a measure of therapeutic response to enalapril or placebo. Am J Cardiol. 1993;71:1106–1107. doi: 10.1016/0002-9149(93)90582-w. [DOI] [PubMed] [Google Scholar]
- 23.Krishna B.H., Pal P., Pal P.G., Balachander J., Jayasettiaseelon E., Sreekanth Y. Yoga improves quality of life and functional capacity in heart failure Patients. Biomed Res. 2014;25(2):178–182. [Google Scholar]
- 24.Raja D.C., Kapoor A., Sinha A. Heart rate manipulation in dilated cardiomyopathy: assessing the role of ivabradine. Indian Heart J. 2018;70(2):246–251. doi: 10.1016/j.ihj.2017.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zambruni A., Trevisani F., Caraceni P., Bernardi M. Cardiac electrophysiological abnormalities in patients with cirrhosis. J Hepatol. 2006;44:994–1002. doi: 10.1016/j.jhep.2005.10.034. [DOI] [PubMed] [Google Scholar]
- 26.Sampaio F., Pimenta J., Bettencourt N. Systolic and diastolic dysfunction in cirrhosis: a tissue-Doppler and speckle tracking echocardiography study. Liver Int. 2013;33:1158–1165. doi: 10.1111/liv.12187. [DOI] [PubMed] [Google Scholar]
- 27.Valeriano V., Funaro S., Lionetti R., Riggio O., Pulcinelli G., Fiore P. Modification of cardiac function in cirrhotic patients with and without ascites. Am J Gastroenterol. 2000;95:3200–3205. doi: 10.1111/j.1572-0241.2000.03252.x. [DOI] [PubMed] [Google Scholar]
- 28.Finucci G., Desideri A., Sacerdoti D. Left ventricular diastolic function in liver cirrhosis. Scand J Gastroenterol. 1996 Mar;31(3):279–284. doi: 10.3109/00365529609004879. [DOI] [PubMed] [Google Scholar]
- 29.Wong F., Girgrah N., Graba J., Allidina Y., Liu P., Blendis L. The cardiac response to exercise in cirrhosis. Gut. 2001 Aug;49(2):268–275. doi: 10.1136/gut.49.2.268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Li X., Yu S., Li L., Han D., Dai S., Gao Y. Cirrhosis-related changes in left ventricular function and correlation with the model for end-stage liver disease score. Int J Clin Exp Med. 2014 Dec 15;7(12):5751–5757. [PMC free article] [PubMed] [Google Scholar]
- 31.Krag A., Bendtsen F., Henriksen J.H., Møller S. Low cardiac output predicts development of hepatorenal syndrome and survival in patients with cirrhosis and ascites. Gut. 2010;59(1):105–110. doi: 10.1136/gut.2009.180570. [DOI] [PubMed] [Google Scholar]
- 32.Marchesini G., Bianchi G., Amodio P., Salerno F., Merli M., Panella C. Factors associated with poor health-related quality of life of patients with cirrhosis. Gastroenterology. 2001;120(1):170–178. doi: 10.1053/gast.2001.21193. [DOI] [PubMed] [Google Scholar]
- 33.Sørensen H.T., Thulstrup A.M., Mellemkjar M. Long-term survival and cause-specific mortality in patients with cirrhosis of the liver: a nationwide cohort study in Denmark. J Clin Epidemiol. 2003;56(1):88–93. doi: 10.1016/s0895-4356(02)00531-0. [DOI] [PubMed] [Google Scholar]
- 34.Diouf M., Filleron T., Barbare J.C. The added value of quality of life (QoL) for prognosis of overall survival in patients with palliative hepatocellular carcinoma. J Hepatol. 2013 Mar;58(3):509–521. doi: 10.1016/j.jhep.2012.11.019. [DOI] [PubMed] [Google Scholar]
- 35.Garin O., Herdman M., Vilagut G. Assessing health-related quality of life in patients with heart failure: a systematic, standardized comparison of available measures. Heart Fail Rev. 2014;19(3):359–367. doi: 10.1007/s10741-013-9394-7. [DOI] [PubMed] [Google Scholar]
- 36.Arvaniti V., D'Amico G., Fede G. Infections in patients with cirrhosis increase mortality four-fold and should be used in determining prognosis. Gastroenterology. 2010;139(4) doi: 10.1053/j.gastro.2010.06.019. 1246.e5–1256.e5. [DOI] [PubMed] [Google Scholar]