Abstract
Leishmaniasis is one of eight neglected tropical diseases currently endemic in 102 countries/areas around the world. In recent years, cutaneous leishmaniasis (CL) has been increasingly observed among migrants, travelers, ecotourists, and military personnel. Because of its great capacity to mimic other dermatoses, CL is one of the great imitators and can mislead practitioners, which can result in untreated lesions that cause scars. CL is a disfiguring disease, especially for women, and often leaves scars on visible body sites, causing psychological, social, and economic problems. CS is a challenge, especially in nonendemic regions, such as Australia, because experience with diagnosis and management of the disease is limited.
Keywords: Cutaneous leishmaniasis, neglected diseases, disfiguring diseases, stigma, women's health
Introduction
Leishmaniasis is listed as one of the eight neglected tropical diseases by the World Health Organization (WHO), and the number of cases in endemic areas has significantly increased in recent years (Galgamuwa et al., 2018). The increase is caused by unicellular parasites that are injected into the skin by small, flying insects (Fig. 1; Hodiamont et al., 2014).
Fig. 1.
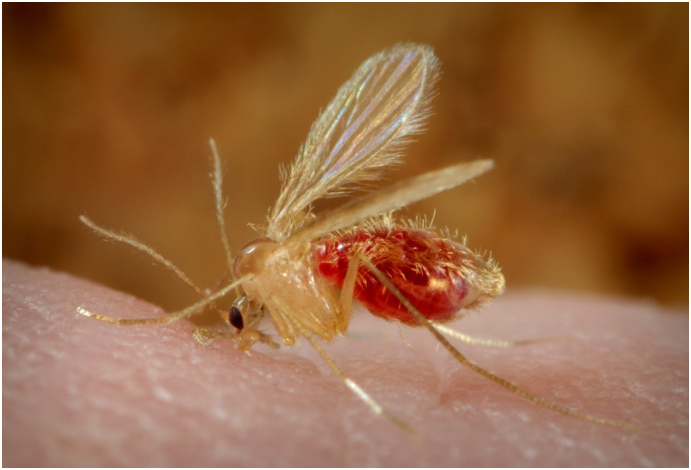
Sand fly (Phlebotomus) content provider (Source: https://de.wikipedia.org/wiki/Datei:Phlebotomus_pappatasi_bloodmeal_continue2.jpg)
The only proven vectors of human disease are sandflies, the species of genus Phlebotomus in the Old World and Lutzomyia in the New World (Sundar and Chakravarty, 2015, World Health Organization, 2010). Transmission is by the vector, which transmits the disease from infected to healthy humans (anthroponotic infection) or from an animal reservoir to humans (zoonotic infection; Sundar and Chakravarty, 2015). Sandflies mostly show maximum activity in warm weather (summertime) and clear nights with low wind speed (Salam et al., 2014).
Leishmaniasis is currently endemic to 102 countries/areas according to the WHO (Uzun et al., 2018, World Health Organization, 2016). Mortality and morbidity from leishmaniasis is estimated at 2 to 4 million disability-adjusted life years worldwide (Uzun et al., 2018, World Health Organization, 2004). Leishmaniasis causes different clinical syndromes that are determined by the balance between parasitic factors (e.g., tropisms, virulence, resistance, species) and the host–immune response (Copeland and Aronson, 2015). There are four main forms of the disease: visceral leishmaniasis (VL, also known as kala-azar); post–kala-azar dermal leishmaniasis (PKDL); cutaneous leishmaniasis (CL); and mucocutaneous leishmaniasis (MCL; WHO, 2016). Twelve countries (Afghanistan, Algeria, Brazil, Colombia, Iran, Morocco, Pakistan, Saudi Arabia, Syria, Peru, Tunisia, and Turkey) were identified as having a high burden (> 2500 cases per year) of CL by the WHO (2016). On the other hand, 13 countries were identified as high burden for VL: Bangladesh, Brazil, China, Ethiopia, India, Kenya, Nepal, Paraguay, Somalia, South Sudan, Spain, Sudan, and Uganda (WHO, 2016).
Currently, leishmaniasis is increasingly seen among migrants, travelers, ecotourists, and military personnel. Recent outbreaks of leishmaniasis could be due to increased human migration because of violent conflicts (e.g., in Syria) that cause the introduction of disease to newer surroundings (Dawit and Girma, 2013, Salam et al., 2014). Furthermore, Leishmania has increasingly been seen as an opportunistic pathogen of HIV-infected adults (Alvar et al., 2012, Galgamuwa et al., 2018). It is a challenge especially in nonendemic regions because experience with the diagnosis and management of the disease is limited.
Clinical manifestation
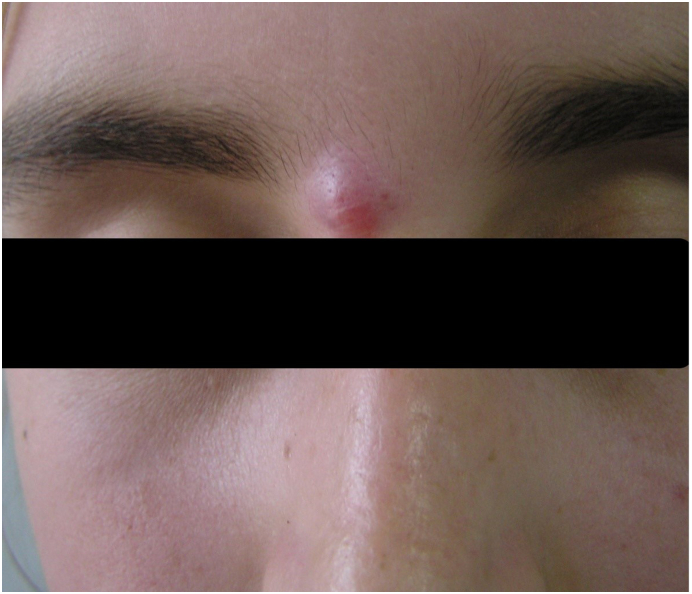
People who have not previously had the disease are susceptible to CL. Cutaneous lesions can either be a single, limited skin lesion or multiple, large, locally destructive skin lesions (Blum et al., 2014). Clinical lesions appear after an incubation period, which often can be many months. Spontaneous healing often results in lifelong immunity (Uzun et al., 2018). CL lesions usually develop on body parts that are mainly open and exposed to the environment, such as the face, forearms, and lower legs. The disease generally presents as a painless, brownish, erythematous papule after a long (2-8 months) incubation period. Infection mainly occurs during the summer months, which causes the disease to appear later during the winter months. These newly appearing papules then gradually enlarge, turning into a nodule or plaque within 6 months (Fig. 2).
Fig. 2.
Newly appeared cutaneous leishmaniasis papule
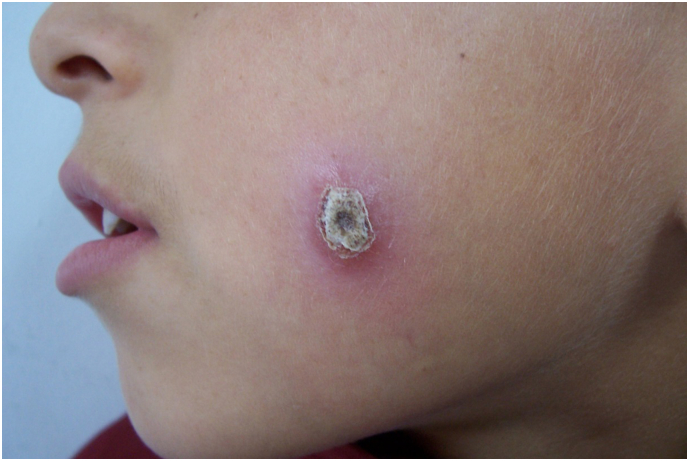
Subsequently, these indurated nodules usually ulcerate from their center, and a brownish crust (tightly adhered to the base) covers the infection area and ulcer. The most distinctive clinical picture of CL is the appearance of sloping, firm margins with a prominent central crater of the ulcer, called volcanic ulcer. This which can help differentiate CL from other causes of chronic ulcer (Fig. 3). Wet-type CL, which is caused by L. major, has a shorter incubation period (˂2 months) and a shorter time to self-cure (˂6 months) than the dry type (Uzun et al., 2018). These limited lesions usually heal spontaneously, leaving a depressed scar.
Fig. 3.
Indurated nodule with central ulcer and brownish crust (tightly adhered to the base) covering the ulcer
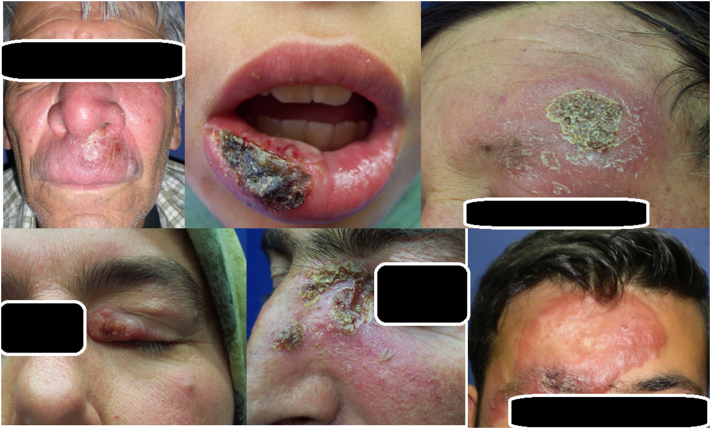
Because of its mimicking nature, CL is called the great imitator and can mislead practitioners because of clinical manifestations that mimic malignant tumors (e.g., basal or squamous cell carcinomas with nodular or nodulo-ulcerative lesions), erysipelas, eczema, sporotrichosis, lupus vulgaris, psoriasis, atypical mycobacterial infections, and other deep fungal infections (Fig. 4; Akcalı et al., 2008, Karincaoglu et al., 2004, Uzun et al., 1999a, Uzun et al., 1999b, Uzun et al., 2018).
Fig. 4.
Different clinical manifestations of cutaneous leishmaniasis, mimicking a wide range of dermatological diseases
CL should be considered in differential diagnoses of patients with a history of living in endemic areas or a history of travel to an endemic region, 1 to 6 months before the lesion appears. Although CL occurs in patients of all ages, onset is more common in children and young individuals (Uzun et al., 2018).
VL is the most severe form of leishmaniasis and is characterized by prolonged fever, splenomegaly, hepatomegaly, pancytopenia, progressive anemia, and weight loss. Fortunately, VL rarely occurs, and these patients often have underlying immunosuppression. If left untreated, VL is often fatal (Blum et al., 2014, Sundar and Chakravarty, 2015).
PKDL occurs in 5% to 15% of endemic areas of VL, such as South Asia and East Africa, as a consequence of VL (Mondal et al., 2018, Zijlstra et al., 2003). PKDL is not fatal, and patients do not feel ill; thus, patients are not usually in search of treatment. However, PKDL lesions are reservoirs for leishmaniasis transmission (Aronson, 2017). PKDL can be seen as hypopigmented macules and patches, erythematous papules and nodules, or mixed polymorphic forms of these lesions (Mondal et al., 2018). Lesions usually start as a single or few macular lesions that gradually increase in size and distribution. Differential diagnoses include pityriasis versicolor, pityriasis alba, vitiligo, leprosy, discoid lupus erythematosus, and lupus vulgaris (WHO, 2012). Treatment should be with systemic therapies.
Cutaneous leishmaniasis in women
Permanently disfiguring scars caused by CL and PKDL create a lifelong stigma for individuals (Fig. 5; Yanik et al., 2004). A recent review by Bennis et al. (2018) investigated the current knowledge of the psychological burden and stigma related to CL by evaluating several reports in electronic databases. The researchers found that quantitative assessments using standard scales and qualitative research suggest that CL is a source of psychological suffering, stigmatization, and decreased quality of life (QoL).
Fig. 5.
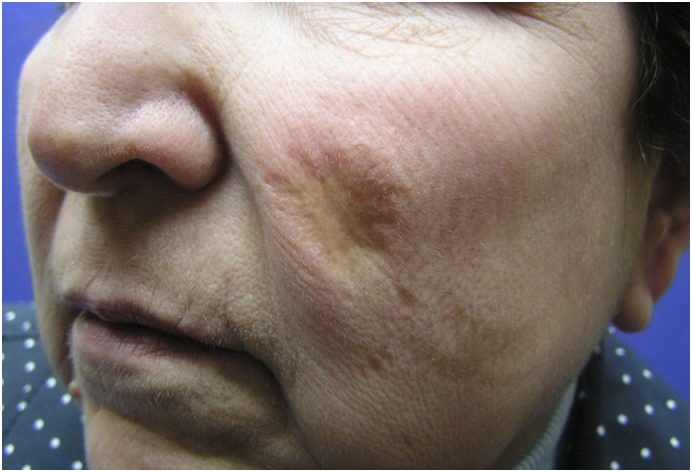
Permanent disfiguring scar on the face can create a lifelong stigma for individuals
In a study by Vares et al. (2013), CL had the maximum effect on patients’ QoL regarding symptoms and emotions, such as embarrassment or self-consciousness, according to the Dermatology Life Quality Index. In the study, patients with lesions that had an ulcerous appearance had a lower QoL. On the other hand, patients with papular lesions had a higher QoL, which was attributed to the papular lesions’ natural qualities, such as being usually smaller than other lesions and seen more frequently in early stages of the disease.
However, CL is especially detrimental for women and young girls in poor, uneducated, and underprivileged societies because of isolation and exclusion of these women from all aspects of life, such as education, business, and social activities (Fig. 6). In some areas, women are considered most exposed to mosquito bites because they usually have more active roles in farming activities, taking care of cattle, and dealing with manure and waste (Bennis et al., 2017). Studies in Pakistan and Afghanistan suggested that the number and visibility of CL scars is the determining factor for the level of stigma and social exclusion suffered by patients with CL. Especially affected girls and women generally believe that CL considerably alters their natural beauty and reduces their prospects of employment, marriage, and being valued in society (Brooker et al., 2004, Reithinger et al., 2005).
Fig. 6.
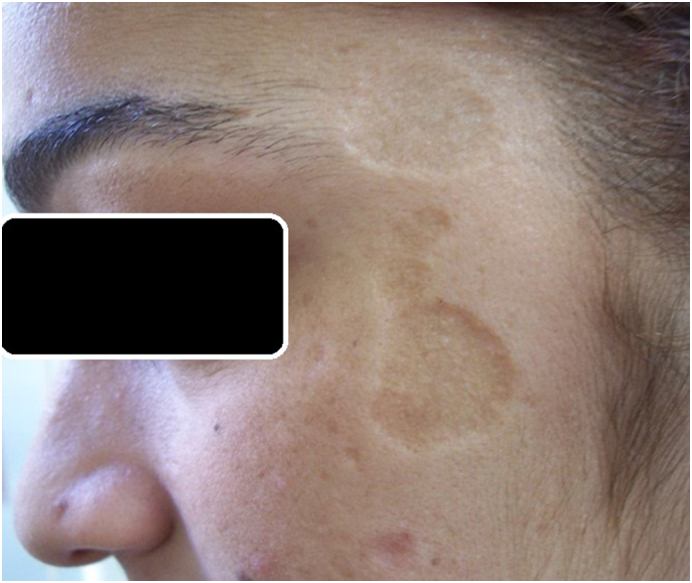
Leishmaniasis scars can cause isolation and exclusion of the individual, resulting in significant alteration in psychological, social, and economic well-being
This was more prominent in patients of a young age who tended to experience more stigma (Chahed et al., 2016) The results of the study by Chahed et al. also pointed out that the nature of the stigma experienced is associated with certain general fears and anticipation of rejection, sometimes even unrelated to the patient’s true experience from society. The researchers suggested that the disability associated with the disfigurement depends on how patients see themselves in their own environment (i.e., personal, social, and professional; Chahed et al., 2016). However, this is different in some countries, such as Pakistan or in Afghanistan, where young girls and women experience dire consequences in marriage and family life; they are particularly victimized as unacceptable for marriage, sometimes by their own families. Moreover, these women are usually separated from their children by their family during the disease and are not allowed to breastfeed or spend time with their children because they are thought to be a source of contamination (Bennis et al., 2017, Reithinger et al., 2005, Simsek et al., 2008).
In rural societies, unaffected women usually remain polite with young women who are affected by CL by sharing household chores or social activities, but even they do not agree to allow their sons to marry these affected young women. Others feel uncomfortable sharing meals with somebody with open sores, mostly for fear of contamination (Bennis et al., 2017). In their study, Bennis et al. also found that the psychosocial impact of CL was more severe than that of acne and similar to that of scar tissue from burns.
In contrast, in Suriname, where CL mainly affects men, the only factor that generated stigma was the presence of a big (or multiple) CL lesion(s), but no barriers to marriage were observed (Ramdas et al., 2016). In a cross-sectional study by Simsek et al. (2008), 270 women were selected using the probability cluster sampling method at a 95% confidence interval to investigate the prevalence and predictors of mental disorders among women in Sanliurfa, Southeastern Turkey. The study results revealed that the frequency of any mental disorder was significantly different according to the history of previous trauma; anemia, history of leishmaniasis; domestic violence including physical, verbal, and economic; economic situation; and participation in decisions at home (P = .05).
Cutaneous leishmaniasis has long been endemic to Sanliurfa and is called a beauty scar. In the study by Yanik et al., patients with CL had more psychiatric problems, such as anxiety and depression, as well as reduced self-body satisfaction (Yanik et al., 2004). The researchers found that patients with CL who have active lesions also have a lower QoL score than others. CL lesions on exposed body parts such as the face and hands, active CL for > 1 year, permanent scar formation, and social stigmatization cause anxiety, depressive symptoms, and decreased body satisfaction and QoL in patients with CL.
All these studies and reports show the high burden of CL on women and women’s health.
Diagnosis
Considering leishmaniasis in the differential diagnoses is the most important step for medical practitioners. They need to confirm the diagnosis using one of the laboratory methods (e.g., dermal scraping smear, culture, incisional biopsy, or polymerase chain reaction [PCR]; Uzun et al., 2018). The most commonly used laboratory method, especially in endemic areas, is the smear, which is a simple and inexpensive diagnostic tool (Durdu et al., 2009, Uzun et al., 2018). Performing a slit-skin smear at the margin of the CL lesion increases the sensitivity of the smear. The smear is stained with Giemsa or Wright stain and then examined with direct microscopy (Fig. 7). In slit-skin smears, although papulonodular PKDL lesions are usually positive, macular lesions often fail to show parasites (WHO, 2012).
Fig. 7.
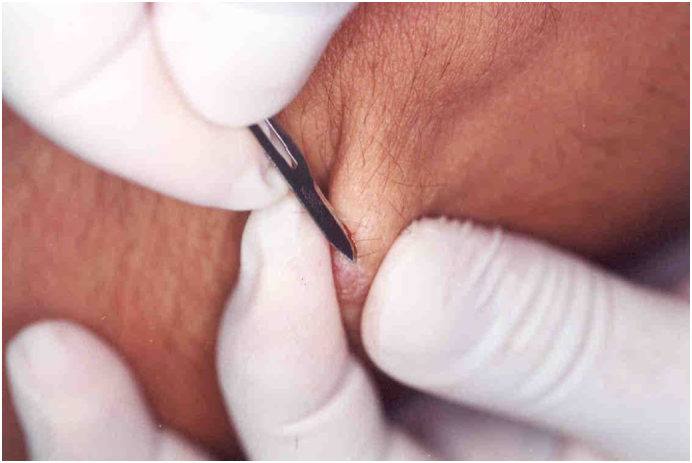
In the case of the papular or nodular cutaneous leishmaniasis lesions, a longitudinal incision should be made with a scalpel (no: 15), followed by a dermal scraping. Subsequently, the scraped dermal material is spread out on a microscope slide, and the smear slide is stained with Giemsa or Wright stain for examination with direct microscopy
Leishmania amastigotes are ellipsoid-shaped parasites that are 2 to 4 μm in length, with an eccentric nucleus and paranuclear kinetoplast in the cytoplasm of macrophages or extracellularly (Fig. 8). Many parasites within the cytoplasm of the macrophages may appear in the formation of a swarm of bees (Uzun et al., 2018).
Fig. 8.
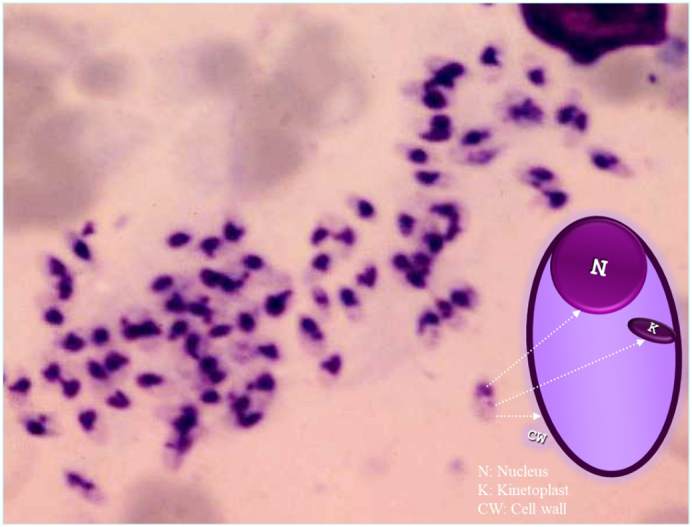
Leishmania amastigotes with eccentric nucleus and paranuclear kinetoplast in a “swarm of bees” formation.
Cultures from an exudate, fine-needle aspirate or scraping also show good results. The gold standard medium for culture is Novy–MacNeal–Nicolle testing with positive results in 1 to 3 weeks, or Schneider Drosophila medium, which gives positive results in 1 week (Hadi and Hadi, 2014). Incisional skin biopsy with staining and culture is important to rule out cutaneous tuberculosis, atypical mycobacterial infections, and deep fungal infections where the clinician is not sure about the diagnosis.
Serological testing based on the detection of anti-Leishmania antibodies in patient serum and agglutination of the parasite is also used and are particularly valuable in VL and MCL (Salam et al., 2014). Leishmania parasites that cause CL can now be genotyped with PCR techniques to detect Leishmania DNA. Immunologic tests cannot distinguish between current and past infections. However, PCR-based methods are considered more specific and can detect current infections (Chappuis et al., 2007, Goto and Lindoso, 2010, Salam et al., 2014). PCR offers a rapid, highly sensitive, and specific modality of diagnosis and provides Leishmania species–specific treatment.
Treatment
Due to the absence of a vaccine and the emergence of drug resistance, leishmaniasis continues to be a burden on endemic countries. Additionally, with increased migration, there is a significant chance that leishmaniasis could spread to other areas, introducing the pathogen to newer environments and leading to mutations and the emergence of more virulent subtypes (Salam et al., 2014). There is a need to report and document cases from endemic and nonendemic regions that can give information about disease distribution (Salam et al., 2014, Singh et al., 2010).
For treatment, one should consider the clinical type of the disease, the Leishmania species involved, and the geography of infection. The main aims of CL treatment are to accelerate the healing process and to prevent complications (e.g., ugly scars), development of dysfunction, spreading, and recurrence of lesions (Reithinger et al., 2007, Uzun et al., 2018).
Per the Turkish guidelines, confirmed CL lesions (< 1 cm in diameter) that are located on covered body areas can be left untreated, but practitioners must keep in mind that untreated patients can also be a source of the disease. The main indications for the treatment of CL are accepted as follows: cosmetically unacceptable lesions; persistent lesions; large lesions; lesions on the joints or face; multiple lesions; lesions located in the mucosa or near the lips, eyelids, or intranasal areas; lesions accompanied by nodular lymphangitis; psychologically affected patients; and patients with immunodeficiencies (Uzun et al., 2018).
First-line therapy
Pentavalent antimonials
Pentavalent antimonials (SbV) are the first-line therapy and have been widely used for > 70 years (Salam et al., 2014). SbV is available as sodium stibogluconate (Pentostam) and meglumine antimoniate (Glucantime; Sundar and Chakravarty, 2015). Glucantime can be administered intramuscularly or intralesionally (IL), and sodium stibogluconate can be administered intravenously or IL (Uzun et al., 2018). IL therapy is a suitable option for acute lesions that are noninflammatory and not very large (< 4 cm in diameter) because this administration method has limited side effects (Uzun, 2008, Uzun et al., 2018). Although systemic side effects are rarely seen in IL treatment, injections are better performed in a procedure room where practitioners can intervene against possible anaphylactic reaction (Uzun et al., 2018). IL therapy should be considered first when a patient has contraindications to systemic therapy (Blum et al., 2004, Uzun et al., 2018). The drug is administered directly into the lesion with a fine-needle (27-30 gauge) syringe, such as an insulin syringe. The entire lesion must be blanched (optimal dose) to reach an effective dose of the drug in the lesion area (1-5 mL per session every 3-7 days; Uzun et al., 2018).
SbV is administered systemically with a dose of 20 mg/kg/day for 20 days for CL and 28 to 30 days for MCL (Sundar and Chakravarty, 2015). After appropriate treatment, the recurrence rate is < 2% (Simsek et al., 2007, Uzun et al., 2018). Arthralgia, myalgia, and elevated hepatic and pancreatic enzymes are common adverse events, and major side effects are cardiac arrhythmia (prolonged QTc interval: > 0.5 seconds), ventricular premature beats, ventricular tachycardia, and torsades de pointes (Sundar and Chakravarty, 2015). Side effects appear to be dose related and more common in patients with renal and liver impairment and those with cardiac arrhythmias. On the other hand, careful follow-up is important during treatment with systemic therapy, and hospitalization of these patients is usually needed to monitor the side effects.
Failure of treatment is often associated with inappropriate doses (below the therapeutic doses, which is ˂10 mg/kg/day, or insufficient dose due to obesity) and insufficient treatment duration (˂10 days; Schubach et al., 1998, Uzun et al., 2018).
Other therapies
Cryotherapy
Cryotherapy with liquid nitrogen (–195°C) is used once or twice weekly up to 6 weeks and has been found 95% effective in Israel, Greece, and Jordan but less effective (77%) in Turkey (Blum et al., 2004, Khatami et al., 2007, Schubach et al., 1998, Simsek et al., 2007, Sundar and Chakravarty, 2015, Uzun et al., 2004, Uzun et al., 2018). The most effective application method is a double freeze-thaw cycle of 10 to 25 seconds, including a 2-mm healthy area from the lesion border (Khatami et al., 2007, Uzun et al., 2018). The main disadvantage of this therapy is the high relapse rate (12%; Simsek et al., 2007, Uzun et al., 2004, Uzun et al., 2018). Also, permanent hypopigmentation can be seen in patients with darker skin.
Thermotherapy
Leishmania parasites do not multiply at temperatures > 39°C in vitro. Thermotherapy (i.e., burn the boil at 50°C for 30 seconds) with radiofrequency waves has been most commonly administered both for Old and New World CL with azoles (Sundar and Chakravarty, 2015).
Azoles
Azoles (e.g., ketoconazole, fluconazole, and itraconazole) act as inhibitors for ergosterol synthesis of Leishmania parasites (Sundar and Chakravarty, 2015) and have been used in the treatment of CL and MCL (al-Fouzan et al., 1991, Alrajhi et al., 2002, Alsaleh et al., 1995, Dogra and Saxena, 1996, Hodiamont et al., 2014, Momeni et al., 1996, Morizot et al., 2007, Nassiri-Kashani et al., 2005, Salmanpour et al., 2001, Weinrauch et al., 1983). However, their efficacy is still unclear (Hodiamont et al., 2014, Morizot et al., 2007). Azole benefits are their oral administration and fewer side effects. According to Turkish experience, ketoconazole at a dosage of 600 mg/day for 6 weeks can be used as an option in patients who are resistant or have severe side effects and risk to SbV therapy (Uzun et al., 2018).
(Liposomal) Amphotericin B
Amphotericin B has been used in antimony-resistant cases but is mostly used for refractory VL in India. Amphotericin B is the only anti-Leishmania drug without drug-resistance (Korzeniewski and Olszanski, 2004, Uzun et al., 2018), but it has serious adverse effects, including infusion reactions, nephrotoxicity, hypokalemia, and myocarditis. However, the liposomal form is considered safer (Sundar and Chakravarty, 2015), and treatment with 3 mg/kg/day for 5 consecutive days and a sixth dose on day 10 (cumulative dose not exceeding 18 mg/kg) has been suggested for CL (Uzun et al., 2018, Solomon et al., 2011). The drug should be administered as a slow infusion that lasts 30 to 60 minutes (Uzun et al., 2018).
Paromomycin
Paromomycin is an aminoglycoside antibacterial drug that also has therapeutic effects against leishmania parasites. There are two available forms: parenteral and 15% topical formulations (Leishcutan; David and Craft, 2009, Neuber, 2008, Uzun et al., 2018). Paromomycin can be used twice a day for 10 to 20 days as an alternative to IL SbV therapy (Blum et al., 2004, Uzun et al., 2018).
Miltefosine
Miltefosine (Impavido), a phosphocholine analogue, is a new drug for VL (Blum and Hatz, 2009, Uzun et al., 2018). Miltefosine is used 150 mg/day for 28 days (Blum et al., 2004). Its main advantages are the oral administration of the drug and the absence of serious side effects (Blum and Hatz, 2009, Uzun et al., 2018). The main disadvantages are the expense of the therapy and its availability in a small number of countries.
Pentamidine
Pentamidine (Pentacarinat) is the first-line treatment for L. guyanensis infections (Blum et al., 2004, David and Craft, 2009, Uzun et al., 2018), and is commonly used in New World CL and in MCL. Pentamidine is administered 2 to 4 mg/kg/day, for a total of 4 to 7 doses (IM or IV; maximum 300 mg/day; Uzun et al., 2018). It should be administered by IV or injected slowly (IM) with a long needle (50 mm; Blum et al., 2004). Fasting glycemia, creatinine kinase, urine for proteinuria and glycosuria, blood pressure, and heart rate must be checked before every injection (Blum et al., 2004, Hellier et al., 2000, Lightburn et al., 2003).
The efficacy and required dosage of anti-leishmanial agents vary in different areas; therefore, the WHO report with regard to treatment recommendations based on regional differences should be consulted (Sundar and Chakravarty, 2015, World Health Organization, 2010).
Follow-up
The decrease in elevated lesions to the skin level and the healing of ulcerated lesions are considered complete healing (Uzun et al., 2018). In CL, lesions should show signs of clinical improvement after 4 to 6 weeks. Complete healing with or without scar formation must be achieved within 3 to 6 months of treatment (Hodiamont et al., 2014). Patients should be evaluated for a second cure treatment if there is no complete healing during the follow-up period (Uzun et al., 2018).
In MCL, complete healing is expected during a 12-month follow-up period (Hodiamont et al., 2014). Relapses can occur after treatment, and patients should be checked every 3 months over a 1-year period (Uzun, 2008, Uzun et al., 2018).
Treatment during pregnancy and lactation
During pregnancy, larger CL lesions with different and/or exophytic clinical presentations are characteristic. There is no therapy to cure VL or PKDL during pregnancy, but a postpartum cure has been found to be complete with treatment (Morgan et al., 2007). Unfortunately, an infection of VL might result in vertical transmission and fetal loss when treatment failure occurs (Morgan et al., 2007, Pagliano et al., 2005). Physical treatment methods, such as cryotherapy or thermotherapy, can be performed if necessary for CL during pregnancy and lactation (Lupi et al., 2009, Uzun et al., 2018).
Co-infection with HIV
HIV infection is most commonly seen with VL. However, some case reports also describe CL and HIV co-infections, which are seen increasingly (Humaira et al., 2014, Kanika et al., 2011, Ndiaye et al., 1996, Tangie et al., 2017). CL lesions in HIV-co-infected individuals are usually atypical, and multiple or widespread lesions are due to immunodeficiency. However, the main risk is the development of VL in endemic areas in HIV-infected individuals. Furthermore, HIV co-infection prohibits an adequate therapeutic response and increases the risk of relapse (Akuffo et al., 2018). For treatment, HIV-infected patients also have a good response to antimonial therapies and liposomal amphotericine B (Rosatelli et al., 1998, Zijlstra et al., 2003).
Prevention
The use of long-lasting insecticide-treated nets (there are two products, PermaNet and Olyset generic, that are approved by the WHO) are the most suggested method of personal protection. Furthermore, insecticide spraying should be applied regularly indoor and outdoor (Croft and Coombs, 2003, Schubach et al., 1998, Uzun et al., 2018).
Conclusions
With the increase of human migration, every practitioner should be aware of this disease and keep this diagnosis in mind during their daily practice. This approach might help, especially with female patients. Leishmaniasis has detrimental effects on every aspect of women’s lives, and early diagnosis and treatment can prevent dire consequences for women.
Footnotes
Sources of support: This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors for publication.
Conflicts of interest: None.
Acknowledgments: Dr. Asli Bilgic Temel is working as a visiting fellow at St. George Hospital, University of New South Wales, and is the recipient of the Turkish Dermatology Society – Prof. Dr. Hulusi Behcet (long-term research) Scholarship in 2018.
References
- Akcalı C., Baba M., Inaloz S., Seckin D., Uzun S. Cutaneous leishmaniasis mimicking squamous cell carcinoma. Ann Acad Med Singap. 2008;37:435–436. [PubMed] [Google Scholar]
- Akuffo H., Costa C., van Griensven J., Burza S., Moreno J., Herrero M. New insights into leishmaniasis in the immunosuppressed. PLoS Negl Trop Dis. 2018;12(5) doi: 10.1371/journal.pntd.0006375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- al-Fouzan A.S., al Saleh Q.A., Najem N.M., Rostom A.I. Cutaneous leishmaniasis in Kuwait. Clinical experience with itraconazole. Int J Dermatol. 1991;30:519–521. doi: 10.1111/j.1365-4362.1991.tb04878.x. [DOI] [PubMed] [Google Scholar]
- Alrajhi A.A., Ibrahim E.A., De Vol E.B., Khairat M., Faris R.M., Maguire J.H. Fluconazole for the treatment of cutaneous leishmaniasis caused by Leishmania major. N Engl J Med. 2002;346:891–895. doi: 10.1056/NEJMoa011882. [DOI] [PubMed] [Google Scholar]
- Alsaleh Q.A., Dvorak R., Nanda A. Ketoconazole in the treatment of cutaneous leishmaniasis in Kuwait. Int J Dermatol. 1995;34:495–497. doi: 10.1111/j.1365-4362.1995.tb00622.x. [DOI] [PubMed] [Google Scholar]
- Alvar J., Velez I.D., Bern C., Herrero M., Desjeux P., Cano J. Leishmaniasis worldwide and global estimates of its incidence. PLoS One. 2012;7 doi: 10.1371/journal.pone.0035671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aronson N.E. Addressing a clinical challenge: Guidelines for the diagnosis and treatment of leishmaniasis. BMC Med. 2017;15(1):76. doi: 10.1186/s12916-017-0843-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennis I., Belaid L., De Brouwere V., Filali H., Sahibi H., Boelaert M. The mosquitoes that destroy your face. Social impact of cutaneous leishmaniasis in south-eastern Morocco, a qualitative study. PLoS One. 2017;12(12) doi: 10.1371/journal.pone.0189906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennis I., De Brouwere V., Belrhiti Z., Sahibi H., Boelaer M. Psychosocial burden of localised cutaneous leishmaniasis: A scoping review. BMC Public Health. 2018;18:358. doi: 10.1186/s12889-018-5260-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blum J.A., Hatz C.F. Treatment of cutaneous leishmaniasis in travelers 2009. J Travel Med. 2009;16:123–131. doi: 10.1111/j.1708-8305.2008.00286.x. [DOI] [PubMed] [Google Scholar]
- Blum J., Desjeux P., Schwartz E., Beck B., Hatz C. Treatment of cutaneous leishmaniasis among travellers. J Antimicrob Chemother. 2004;53:1581-66. doi: 10.1093/jac/dkh058. [DOI] [PubMed] [Google Scholar]
- Blum J., Buffet P., Visser L., Harms G., Bailey M.S., Caumes E. LeishMan recommendations for treatment of cutaneous and mucosal leishmaniasis in travelers. J Travel Med. 2014;21(2):116–129. doi: 10.1111/jtm.12089. [DOI] [PubMed] [Google Scholar]
- Brooker S., Mohammed N., Adil K., Agha S., Reithinger R., Rowland M. Leishmaniasis in refugee and local Pakistani populations. Emerg Infect Dis. 2004;10:1681–1684. doi: 10.3201/eid1009.040179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chahed M.K., Bellali H., Ben Jemaa S., Bellaj T. Psychological and psychosocial consequences of zoonotic cutaneous leishmaniasis among women in Tunisia: Preliminary findings from an exploratory study. PLoS Negl Trop Dis. 2016;10 doi: 10.1371/journal.pntd.0005090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chappuis F., Sundar S., Hailu A., Ghalib H., Rijal S., Peeling R.W. Visceral leishmaniasis: What are the needs for diagnosis, treatment and control? Nat Rev Microbiol. 2007;5:873–882. doi: 10.1038/nrmicro1748. [DOI] [PubMed] [Google Scholar]
- Copeland N.K., Aronson N.E. Leishmaniasis: Treatment updates and clinical practice guidelines review. Curr Opin Infect Dis. 2015;28(5):426–437. doi: 10.1097/QCO.0000000000000194. [DOI] [PubMed] [Google Scholar]
- Croft S.L., Coombs G.H. Leishmaniasis--current chemotherapy and recent advances in the search for novel drugs. Trends Parasitol. 2003;19(11):502–508. doi: 10.1016/j.pt.2003.09.008. [DOI] [PubMed] [Google Scholar]
- David C.V., Craft N. Cutaneous and mucocutaneous leishmaniasis. Dermatol Ther. 2009;22:491–502. doi: 10.1111/j.1529-8019.2009.01272.x. [DOI] [PubMed] [Google Scholar]
- Dawit G., Girma Z. Simenew K. A review on biology, epidemiology and public health significance of leishmaniasis. J Bacteriol Parasitol. 2013;4:166. [Google Scholar]
- Dogra J., Saxena V.N. Itraconazole and leishmaniasis: A randomised double-blind trial in cutaneous disease. Int J Parasitol. 1996;26:1413–1415. doi: 10.1016/s0020-7519(96)00128-2. [DOI] [PubMed] [Google Scholar]
- Durdu M., Baba M., Seckin D. More experiences with the Tzanck smear test: Cytologic findings in cutaneous granulomatous disorders. J Am Acad Dermatol. 2009;61:441–450. doi: 10.1016/j.jaad.2009.02.050. [DOI] [PubMed] [Google Scholar]
- Galgamuwa L.S., Dharmaratne S.D., Iddawela D. Leishmaniasis in Sri Lanka: Spatial distribution and seasonal variations from 2009 to 2016. Parasit Vectors. 2018;11(1):60. doi: 10.1186/s13071-018-2647-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goto H., Lindoso J.A. Current diagnosis and treatment of cutaneous and mucocutaneous leishmaniasis. Expert Rev Anti-Infect Ther. 2010;8:419–433. doi: 10.1586/eri.10.19. [DOI] [PubMed] [Google Scholar]
- Hadi S.M., Hadi A.S. Treatment of skin disease. In: Lebwohl M.G., Berth-Jones J., Heymann W.R., Coulson I., editors. Leishmaniasis. 4th ed. Elsevier; Amsterdam: 2014. pp. 373–376. [Google Scholar]
- Hellier I., Dereure O., Tournillac I., Pratlong F., Guillot B., Dedet J.P. Treatment of Old World cutaneous leishmaniasis by pentamidine isethionate. An open study of 11 patients. Dermatology. 2000;200(2):120–123. doi: 10.1159/000018343. [DOI] [PubMed] [Google Scholar]
- Hodiamont C.J., Kager P.A., Bart A., de Vries H.J., van Thiel P.P., Leenstra T. Species-directed therapy for leishmaniasis in returning travellers: A comprehensive guide. PLoS Negl Trop Dis. 2014;8(5):2832. doi: 10.1371/journal.pntd.0002832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Humaira T., Sharmeen A., Mubasshir S. Cutaneous leishmaniasis with HIV. J Coll Phys Surg Pakistan. 2014;24(Special Supplement 2):S93–S95. [PubMed] [Google Scholar]
- Kanika K., Ram A., Rajesh D., Himanshu K. A patient presenting with diffuse cutaneous leishmaniasis (DCL) as a first indicator of HIV infection in India. Am J Trop Med Hyg. 2011;85(1):64–65. doi: 10.4269/ajtmh.2011.10-0649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karincaoglu Y., Esrefoglu M., Ozcan H. Atypical clinical form of cutaneous leishmaniasis: Erysipeloid form. Int J Dermatol. 2004;43:827–829. doi: 10.1111/j.1365-4632.2004.02324.x. [DOI] [PubMed] [Google Scholar]
- Khatami A., Firooz A., Gorouhi F., Dowlati Y. Treatment of acute Old World cutaneous leishmaniasis: A systematic review of the randomized controlled trials. J Am Acad Dermatol. 2007;57:335.1–29. doi: 10.1016/j.jaad.2007.01.016. [DOI] [PubMed] [Google Scholar]
- Korzeniewski K., Olszanski R. Leishmaniasis among soldiers of stabilization forces in Iraq. Int Marit Health. 2004;55:155–163. [PubMed] [Google Scholar]
- Lightburn E., Morand J.J., Meynard J.B., Kraemer P., Chaudier B., Pages F. Management of American cutaneous leishmaniasis. Outcome apropos of 326 cases treated with high-dose pentamidine isethionate. Med Trop (Mars) 2003;63(1):35–44. [PubMed] [Google Scholar]
- Lupi O., Bartlett B.L., Haugen R.N., Dy L.C., Sethi A., Klaus S.N. Tropical dermatology: Tropical diseases caused by protozoa. J Am Acad Dermatol. 2009;60(6):897–925. doi: 10.1016/j.jaad.2009.03.004. quiz 926–8. Erratum in: J Am Acad Dermatol 2009;61(6):1059. [DOI] [PubMed] [Google Scholar]
- Momeni A.Z., Jalayer T., Emamjomeh M., Bashardost N., Ghassemi R.L., Meghdadi M. Treatment of cutaneous leishmaniasis with itraconazole. Randomized double-blind study. Arch Dermatol. 1996;132:784–786. [PubMed] [Google Scholar]
- Mondal D., Bern C., Ghosh D., Rashid M., Molina R., Chowdhury R. Quantifying the infectiousness of post-kala-azar dermal leishmaniasis towards sandflies. Clin Infect Dis. 2018 doi: 10.1093/cid/ciy891. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan D.J., Guimaraes L.H., Machado P.R., D'Oliveira A., Jr., Almeida R.P., Lago E.L. Cutaneous leishmaniasis during pregnancy: exuberant lesions and potential fetal complications. Clin Infect Dis. 2007;45(4):478–482. doi: 10.1086/520017. [DOI] [PubMed] [Google Scholar]
- Morizot G., Delgiudice P., Caumes E., Laffitte E., Marty P., Dupuy A. Healing of Old World cutaneous leishmaniasis in travelers treated with fluconazole: Drug effect or spontaneous evolution? Am J Trop Med Hyg. 2007;6:48–52. [PubMed] [Google Scholar]
- Nassiri-Kashani M., Firooz A., Khamesipour A., Mojtahed F., Nilforoushzadeh M., Hejazi H. A randomized, double-blind, placebo-controlled clinical trial of itraconazole in the treatment of cutaneous leishmaniasis. J Eur Acad Dermatol Venereol. 2005;19:80–83. doi: 10.1111/j.1468-3083.2004.01133.x. [DOI] [PubMed] [Google Scholar]
- Ndiaye P., Develoux M., Dieng M., Huerre M. Diffuse cutaneous leishmaniasis and acquired immunodeficiency syndrome in a Senegalese patient. Bull Soc Pathol Exot. 1996;89:282–286. [PubMed] [Google Scholar]
- Neuber H. Leishmaniasis. J Dtsch Dermatol Ges. 2008;6:754–765. doi: 10.1111/j.1610-0387.2008.06809.x. [DOI] [PubMed] [Google Scholar]
- Pagliano P., Carannante N., Rossi M., Gramiccia M., Gradoni L., Faella F.S. Visceral leishmaniasis in pregnancy: A case series and a systematic review of the literature. J Antimicrob Chemother. 2005;55:229–233. doi: 10.1093/jac/dkh538. [DOI] [PubMed] [Google Scholar]
- Ramdas S., van der Geest S., Schallig H.D. Nuancing stigma through ethnography: The case of cutaneous leishmaniasis in Suriname. Social Sci Med (1982) 2016;151:139–146. doi: 10.1016/j.socscimed.2015.12.044. [DOI] [PubMed] [Google Scholar]
- Reithinger R., Aadil K., Kolaczinski J., Mohsen M., Hami S. Social impact of leishmaniasis, Afghanistan. Emerg Infect Dis. 2005;11:634–636. doi: 10.3201/eid1104.040945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reithinger R., Dujardin J.C., Louzir H., Pirmez C., Alexander B., Brooker S. Cutaneous leishmaniasis. Lancet Infect Dis. 2007;7:581–596. doi: 10.1016/S1473-3099(07)70209-8. [DOI] [PubMed] [Google Scholar]
- Rosatelli J., Souza C., Soares F., Foss N., Roselino A. Generalized cutaneous leishmaniasis in acquired immunodeficiency syndrome. J Eur Acad Dermatol Venerol. 1998;10:229–232. [PubMed] [Google Scholar]
- Salam N., Al-Shaqha W.M., Azzi A. Leishmaniasis in the middle East: Incidence and epidemiology. PLoS Negl Trop Dis. 2014;8(10) doi: 10.1371/journal.pntd.0003208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salmanpour R., Handjani F., Nouhpisheh M.K. Comparative study of the efficacy of oral ketoconazole with intra-lesional meglumine antimoniate (Glucantime) for the treatment of cutaneous leishmaniasis. J Dermatolog Treat. 2001;12:159–162. doi: 10.1080/09546630152607899. [DOI] [PubMed] [Google Scholar]
- Schubach A., Haddad F., Oliveira-Neto M.P., Degrave W., Pirmez C., Grimaldi G., Jr. Detection of leishmania DNA by polymerase chain reaction in scars of treated human patients. J Infect Dis. 1998;178:911–914. doi: 10.1086/515355. [DOI] [PubMed] [Google Scholar]
- Simsek F.M., Alten B., Caglar S.S., Ozbel Y., Aytekin A.M., Kaynas S. Distribution and altitudinal structuring of phlebotomine sand flies (Diptera: psychodidae) in southern Anatolia, Turkey: Their relation to human cutaneous leishmaniasis. J Vector Ecol. 2007;32:269–279. doi: 10.3376/1081-1710(2007)32[269:daasop]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- Simsek Z., Ak D., Altindag A., Günes M. Prevalence and predictors of mental disorders among women in Sanliurfa, Southeastern Turkey. J Public Health (Oxf) 2008;30(4):487–493. doi: 10.1093/pubmed/fdn025. [DOI] [PubMed] [Google Scholar]
- Singh V.P., Ranjan A., Topno R.K., Verma R.B., Siddique N.A., Ravidas V.N. Estimation of under-reporting of visceral leishmaniasis cases in Bihar, India. Am J Trop Med Hyg. 2010;82:9–11. doi: 10.4269/ajtmh.2010.09-0235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solomon M., Pavlotsky F., Leshem E., Ephros M., Trau H., Schwartz E. Liposomal amphotericin B treatment of cutaneous leishmaniasis due to Leishmania tropica. J Eur Acad Dermatol Venereol. 2011;25:973–977. doi: 10.1111/j.1468-3083.2010.03908.x. [DOI] [PubMed] [Google Scholar]
- Sundar S., Chakravarty J. An update on pharmacotherapy for leishmaniasis. Expert Opin Pharmacother. 2015;16(2):237–252. doi: 10.1517/14656566.2015.973850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tangie L.N., Desmond A., Aminde L.N., Ako A.M., Halle P.M. Cutaneous leishmaniasis in a severely immunocompromised HIV patient in Kumbo, northwest region of Cameroon: Case report. BMC Res Notes. 2017;10(1):425. doi: 10.1186/s13104-017-2751-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uzun S. Leishmaniasis. In: Tuzun Y., Gurer M.A., Serdaroglu S., Oguz O., Aksungur V.L., editors. Dermatoloji. Nobel Kitabevi; Istanbul: 2008. pp. 659–677. [Google Scholar]
- Uzun S., Uslular C., Yucel A., Acar M.A., Ozpoyraz M., Memişoğlu H.R. Cutaneous lesihmaniaisis: Evaluation of 3074 cases in the Cukurova region of Turkey. Br J Dermatol. 1999;140:347–350. doi: 10.1046/j.1365-2133.1999.02673.x. [DOI] [PubMed] [Google Scholar]
- Uzun S., Acar M.A., Uslular C., Kavukçu H., Aksungur V.L., Culha G. Uncommon presentation of cutaneous leishmaniasis as eczema-like eruption. J Eur Acad Dermatol Venereol. 1999;12:266–268. doi: 10.1111/j.1468-3083.1999.tb01044.x. [DOI] [PubMed] [Google Scholar]
- Uzun S., Durdu M., Culha G., Allahverdiyev A.M., Memisoglu H.R. Clinical features, epidemiology, and efficacy and safety of intralesional antimony treatment of cutaneous leishmaniasis: Recent experience in Turkey. J Parasitol. 2004;90:853–859. doi: 10.1645/GE-185R. [DOI] [PubMed] [Google Scholar]
- Uzun S., Gürel M.S., Durdu M., Akyol M., Fettahlıoğlu Karaman B., Aksoy M. Clinical practice guidelines for the diagnosis and treatment of cutaneous leishmaniasis in Turkey. Int J Dermatol. 2018;57(8):973–982. doi: 10.1111/ijd.14002. [DOI] [PubMed] [Google Scholar]
- Vares B., Mohseni M., Heshmatkhah A., Farjzadeh S., Safizadeh H., Shamsi-Meymandi S. Quality of life in patients with cutaneous leishmaniasis. Arch Iran Med. 2013;16(8):474–477. [PubMed] [Google Scholar]
- Weinrauch L., Livshin R., Even-Paz Z., El-On J. Efficacy of ketoconazole in cutaneous leishmaniasis. Arch Dermatol Res. 1983;275:353–354. doi: 10.1007/BF00417211. [DOI] [PubMed] [Google Scholar]
- World Health Organization The World Health Report 2004. Changing history [Internet] 2004. http://www.who.int/whr/2004/en/index.html (cited 2008). Available from:
- World Health Organization Control of the leishmaniases: Report of a meeting of the WHO Expert Committee on the Control of Leishmaniases [Internet] 2010. http://www.who.int/iris/handle/10665/44412 (cited 2018). Available from:
- World Health Organization Post-Kala-Azar dermal leishmaniasis: A manual for case management and control [Internet] 2012. http://apps.who.int/iris/bitstream/handle/10665/78608/9789241505215_eng.pdf (cited 2018). Available from:
- World Health Organization Leishmaniasis in high-burden countries: An epidemiological update based on data reported in 2014. Wkly Epidemiol Rec. 2016;91:287–296. [PubMed] [Google Scholar]
- Yanik M., Gurel M.S., Simsek Z., Kati M. The psychological impact of cutaneous leishmaniasis. Clin Exp Dermatol. 2004;29:464–467. doi: 10.1111/j.1365-2230.2004.01605.x. [DOI] [PubMed] [Google Scholar]
- Zijlstra E.E., Musa A.M., Khalil E.A., el-Hassan I.M., el-Hassan A.M. Post-kala-azar dermal leishmaniasis. Lancet Infect Dis. 2003;3(2):87–98. doi: 10.1016/s1473-3099(03)00517-6. [DOI] [PubMed] [Google Scholar]