Abstract
The burden of psoriasis is particularly high for women, who report lower levels of happiness (women: 18.5%; men: 11.3% lower vs. general population) and are more likely to experience stress (women: > 60%; men: 42%), loneliness (women: 25-28%; men: 19-24%), stigmatization (Feelings of Stigmatization Questionnaire score; women: 93.2; men: 78.0), and reduced sexual activity (women: 33%; men: 19%) compared with men. The onset of psoriasis is bimodal, with one incidence peak (15-30 years) that coincides with the prime reproductive age for women, which poses specific challenges for their treatment. However, well-established guidelines for the treatment of women of childbearing age are lacking. Many women experience stabilization (21%) or improvement (55%) of their skin during pregnancy, but up to a quarter can experience disease worsening, and postpartum flares are common (> 50%). Therefore, balancing the risk of treatment with the risk of uncontrolled disease is important. Because half of pregnancies are unplanned, the implications of therapeutic options must be considered for all women with psoriasis who are sexually active, irrespective of intentions to start a family. Timely initiation of these discussions by health care professionals is paramount to prevent unintentional toxicity to the developing fetus. For example, acitretin, methotrexate, and oral psoralen/ultraviolet A are all contraindicated in pregnancy. Reassuringly, safety data for other psoriasis treatments during pregnancy are increasingly available, particularly for anti-tumor necrosis factor therapies. Despite encouraging data from pregnancy exposure registries and clinical studies now being included in anti-tumor necrosis factor drug labels, comfort with prescribing these therapies to pregnant women remains low (U.S. dermatologists: 21%; EU-5 dermatologists: 10%). In this article, we review issues specific to treating women of childbearing age with psoriasis and highlight the need for treatment guidelines to ensure consistent care and optimal outcomes for these patients.
Keywords: psoriaris, psoriatic arthritis, women, treatment, pregnancy, lactation
Introduction
Psoriasis (PSO) is a chronic inflammatory disease with manifestations that include red, scaly, itchy and/or painful patches, plaques, or papules. These symptoms typically have a predilection for the scalp and extensor surfaces but can be widespread in severe cases. Up to 40% of patients with PSO will develop psoriatic arthritis (PsA), typically within 5 to 10 years of PSO disease onset (Mease and Armstrong, 2014). PsA is a chronic rheumatic disease characterized by synovitis, enthesitis, dactylitis, and spondylitis (Mease and Armstrong, 2014). Joint involvement can precede skin manifestations or may occur in parallel in a minority number of cases (Kumar et al., 2014, Leung et al., 2007). Beyond PsA, people with PSO are also at increased risk of developing a range of comorbidities related to the metabolic syndrome, including hypertension, hyperlipidemia, obesity, and type 2 diabetes mellitus (Augustin et al., 2010, Singh et al., 2017).
The prevalence of PSO varies worldwide (World Health Organization, 2016); in countries with a predominantly Caucasian population, it is frequently reported to be between 1% and 3% (Kurd and Gelfand, 2009, Mease and Armstrong, 2014). Disease onset is bimodal, with one incidence peak occurring at 15 to 30 years of age and the second between 50 to 60 years (Bandoli et al., 2010, Crow, 2012, Kimball et al., 2014). However, > 75% of cases present before the age of 40 years (Tauscher et al., 2002).
For women, a diagnosis of PSO (average age: 28 years; Levine and Gottlieb, 2009, Tauscher et al., 2002) and therapy initiation often overlap with peak reproductive years (18-45 years; Crow, 2012, Kimball et al., 2014), which can pose specific challenges for treatment. Formal treatment guidelines for women of childbearing age with PSO are not currently available, but U.S. and European drug labeling now summarizes safety data associated with pregnancy and lactation to help inform treatment selection for this patient population.
Women with PSO can experience disease activity fluctuations with hormonal changes and with each stage of the journey to motherhood (Ceovic et al., 2013). In a prospective study of 163,763 women (n = 1253 incident cases of PSO), irregular menstrual cycles and surgical menopause were associated with a higher risk of PSO, whereas there was a trend among younger women toward a lower PSO risk in those who had experienced multiple births and longer breastfeeding periods (Wu et al., 2016).
Burden of psoriasis for women
PSO can have a profoundly negative impact on patients’ quality of life. In a survey of patients with PSO or PsA (n = 5604) conducted by the National Psoriasis Foundation (NPF), the majority of patients felt that PSO was a problem in their daily life (94%), affected their overall emotional well-being (88%), and interfered with their enjoyment of life (82%; Armstrong et al., 2012). The emotional impact of PSO was greater in patients with severe (> 10% self-reported body surface area [BSA] involvement) compared with those with mild disease (< 3% BSA; Armstrong et al., 2012).
Women with PSO and PsA show reductions in quality of life in all domains assessed by the 36-Item Short Form Health Survey questionnaire (Salaffi et al., 2009, Wahl et al., 2000). According to the World Psoriasis Happiness Report 2017, women with severe PSO experience a greater negative impact on their happiness compared with men, with gaps of − 18.5% vs. − 11.3%, respectively, compared with women and men in the general population (LEO Innovation Lab and The Happiness Research Institute, 2017). Furthermore, women with PSO are more likely to experience stress (> 60% vs. 42%) and loneliness (25-28% vs. 19-24%) than men with the disease (LEO Innovation Lab and The Happiness Research Institute, 2017). Among patients with PsA, women also appear to have higher disability and fatigue severity scores (Generali et al., 2016).
Women report higher (worse) Dermatology Life Quality Index scores than men, despite scoring lower (better) on the Psoriasis Area Severity Index (n = 2450), which suggests a mismatch between subjective and objective disease activity measures (Lesuis et al., 2012). This finding highlights the importance of considering subjective measures in the treatment decision-making process to ensure that female patients are not undertreated (Lesuis et al., 2012).
Patients with PSO and PsA have an increased risk of depression, anxiety, and suicidality (Kurd et al., 2010, McDonough et al., 2014). In a study using prospectively collected data from the Organization of Teratology Information Specialists (OTIS) Autoimmune Diseases in Pregnancy Project, the prevalence of depression in women with PSO was approximately two-fold higher compared with women without the condition (21% vs. 12%, respectively; p = .03; Bandoli et al., 2010).
PSO can also lead to sexual impairment. In response to a questionnaire (n = 481), a quarter of Dutch Psoriasis Association patients reported a decline in sexual activity after disease onset, with women affected to a greater extent than men (33% vs. 19%, respectively; p < .0001; Meeuwis et al., 2011). Approximately half of the women with PSO who responded had sexual dysfunction (n = 88 of 181 patients; Female Sexual Function Index cutoff score: ≤ 26.55). Moreover, using the Female Sexual Distress Scale (cutoff score: ≥ 15), 38% of women with PSO showed sexual distress. Women with current genital lesions exhibited significantly more sexual distress versus women not currently affected (p = .001; Meeuwis et al., 2011). In a study of adult patients with PSO (n = 354) in the U.S. and Ireland, 63% reported experiencing genital PSO at some stage (Ryan et al., 2015). Of the patients with currently active genital PSO, 43% reported a decreased frequency of intercourse. Of note, pain, burning, and discomfort during intercourse were reported significantly more frequently by women than men (Ryan et al., 2015). PsA is also known to interfere with the physical ability to have intercourse, especially when the lower back and sacroiliac joints are involved (Gupta and Gupta, 1997, Kurizky and Mota, 2012).
Compared with men, women experience higher stigmatization as a result of PSO, as assessed by the Feelings of Stigmatization Questionnaire (mean total score of 93.2 vs. 78.0; p = .001), which includes questions on anticipation of rejection, feelings of being flawed, sensitivity to the opinions of others, and secretiveness (Hawro et al., 2017).
Health care professionals (HCPs) can support and reassure patients by initiating discussions around intimacy and dating in an open and honest manner. Guidance on the management of PSO in an intimate relationship and on using make-up and clothing to improve confidence is available via PSO patient support groups and online (National Psoriasis Foundation, 2017, PlaquePsoriasis.com, 2018). Women with PSO may also require support with ascertaining what expectations to have of their therapy; patients may not always be aware of the optimal achievable condition of their skin, particularly if they do not consult a dermatologist who prescribes the full repertoire of PSO and PsA treatments, including systemic or biologic agents.
Management of psoriasis in women of childbearing age
PSO has a unique impact on women of childbearing age. The condition is not thought to affect fertility (Rademaker et al., 2018); however, a 22% lower likelihood of pregnancy has been reported among women with PSO compared with the overall population (Cather et al., 2012). The underlying reasons are likely complex and may include voluntary childlessness, concern with regard to disease activity during pregnancy or postpartum, fear of the effect of PSO therapy on pregnancy, reduced intimacy due to embarassment, and/or physical inability to have intercourse.
Timely initiation of discussions about family planning and ongoing dialogue with HCPs are critical for all women of childbearing age. The implications of treatment must be considered in all women with PSO who are sexually active, even if they are not actively planning to start a family. This is especially pertinent in light of research suggesting that approximately half of all pregnancies are unplanned, including among the PSO and PsA patient population (Kavanaugh et al., 2015, Murase et al., 2019). Furthermore, most women discover they are pregnant between weeks 4 and 7 (calculated from the date of the last menstrual period), which corresponds to a fetal gestational age of 2 to 5 weeks and coincides with a critical window of early fetal development (American Pregnancy Association, 2018). Therefore, women of childbearing age may benefit from counselling to avoid unintentional teratogen exposure (Rademaker et al., 2018). There are many safe treatment options for this patient group, as discussed in detail herein, but certain therapies mandate the use of birth control measures.
Women with PSO should be made aware also of the importance of notifying their treating physician of a potential pregnancy. A recent U.S. NPF survey (n = 141) revealed that of women with PSO who had experienced pregnancy (n = 66), only 41% had informed their HCP of the pregnancy immediately; while 21% did not notify their HCP at any point (Lebwohl et al., 2018).
Psoriasis and pregnancy
PSO activity often improves or stabilizes during pregnancy, and a subset of women show a marked improvement in their skin condition (Murase et al., 2005). In a study investigating the effect of hormonal fluctuations around pregnancy on PSO (n = 47), 55% of women reported improvement and 21% indicated no change (Murase et al., 2005). In patients with ≥ 10% psoriatic BSA who experienced improvement (n = 16; mean BSA: 40%), lesions decreased by > 80% between the first and third trimesters (Murase et al., 2005). However, there is evidence that women with PSO and PsA may also experience skin disease worsening (or stable high disease activity) during pregnancy (PSO: ~ 23%; PsA: ~ 9%; Murase et al., 2005, Polachek et al., 2017, Ursin et al., 2018).
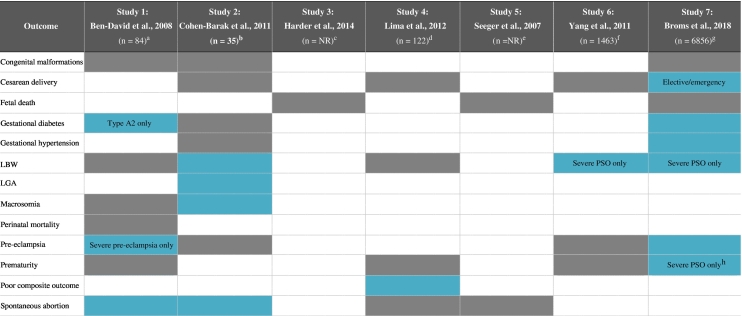
Pregnancy outcomes data for women with PSO have shown variable results (Table 1), and further studies are warranted. In a 2016 systematic review including nine observational studies (six studies rated as good quality), there was no clear evidence of a consistently increased risk of adverse pregnancy outcomes for women with PSO (Bobotsis et al., 2016). Furthermore, a study using data from a U.S. nationwide inpatient sample database (2003-2011; n = 11,204 women with PSO or PsA) revealed that, after adjusting for potential confounders, maternal PSO/PsA had no significant impact on inpatient mortality or fetal death. The prevalence of preterm delivery, premature rupture of membranes, postpartum hemorrhage, and cesarean delivery was similar between the PSO/PsA population and the control group (Boddeda et al., 2018).
Table 1.
Study data evaluating pregnancy outcomes in women with PSO and PsA (Ben-David et al., 2008, Cohen-Barak et al., 2011, Harder et al., 2014, Lima et al., 2012, Seeger et al., 2007, Yang et al., 2011, Broms et al., 2018)
 Statistically significant
Statistically significant
 Not statistically significant
Not statistically significant
BMI, body mass index; LBW, low birth weight; LGA, large for gestational age; NR, not reported; PsA, psoriatic arthritis; PSO, psoriasis
aPrevious abortion was adjusted for maternal age, gestational diabetes mellitus A2, and severe preeclampsia. Cesarean delivery was adjusted for previous cesarean delivery, malpresentation, hypertensive disorders, and labor dystocia.
bNo adjusting for confounding factors was specified.
cIncluded 2553 pregnancies; outcomes were adjusted for maternal pre-pregnancy BMI, smoking, weekly alcohol consumption before pregnancy, and maternal age.
dMultivariate outcomes analysis was adjusted for ethnicity, marital status, education level, depression, hypertension, pregnancies per woman, age, BMI, hospital of delivery, diabetes, assisted reproductive technologies, substance abuse/cigarette smoking, medication use, anemia, and infection.
eIncluded 197 pregnancies; incidence rate ratios were adjusted for age.
fOutcomes analysis was adjusted for infant sex and parity, mother’s age, education level, marital status, income, gestational hypertension, diabetes, anemia, and coronary heart disease, as well as the age and education level of the father.
gMultivariate analyses reported (Model 2) were adjusted for country, maternal age and parity, smoking status, BMI, depression, diabetes and hypertension; separate analyses were performed comparing women with severe and non-severe PSO with women without PSO.
hModerate preterm birth only (32-36 weeks).
However, certain studies have found a link between disease activity and pregnancy complications or adverse pregnancy outcomes. In a recent cohort study using prospectively collected data from Denmark and Sweden (April 2007-December 2012; n = 8097 births to PSO/PsA mothers), an increased risk of gestational diabetes, gestational hypertension, preeclampsia, and emergency cesarean delivery was reported in women with PSO (Broms et al., 2018). The lifestyle factors and comorbidities adjusted for by the authors included country, maternal age and parity, smoking status, body mass index, depression, diabetes, and hypertension (Broms et al., 2018). The risks were higher for women with severe PSO, who were also found to have an increased risk of moderate preterm birth (32-36 weeks) and low birth weight (Broms et al., 2018). The increased risk of low birth weight and preeclampsia in women with severe PSO is in accordance with findings from a previous cohort study that evaluated pregnancy outcomes in 1483 pregnant women with PSO (Table 1; Yang et al., 2011). This analysis was adjusted for characteristics of the infant (sex and parity), the mother (age, education level, marital status, income, gestational hypertension, diabetes, anemia, and coronary heart disease), and the father (age and education level).
Data on pregnancy outcomes in women with PsA are more limited (Mouyis et al., 2017). The cohort study by Broms et al. identified an increased risk of gestational hypertension and preeclampsia for women with PsA (after adjusting for confounding factors as mentioned; Broms et al., 2018). Recently presented data from a Swedish cohort study (2007-2014) also found an adjusted odds ratio (aOR) of preterm birth of 1.80 (95% confidence interval [CI], 1.22-2.64) among 397 PsA pregnancies (n = 289 mothers; Remaeus et al., 2018). However, the aOR of preeclampsia, gestational diabetes and stillbirth did not differ between the comparator groups (Remaeus et al., 2018). The authors of the study adjusted for the mother’s country of birth, education level, smoking status, body mass index, parity, age, and year of birth, but not for comorbidities such as diabetes mellitus. Data from a large prospective cohort analysis of women enrolled as part of the OTIS Autoimmune Disease in Pregnancy Project before 20 weeks gestation (2004-2018) also showed that PsA was associated with an increased risk for moderate preterm delivery (adjusted risk ratio [aRR]: 1.81; 95% CI, 1.01-3.26), preterm labor (aRR: 2.05; 95% CI, 1.21-3.48), oligohydramnios (aRR: 3.79; 95% CI, 1.34-10.74), and cesarian section (aRR: 1.63; 95% CI, 1.26-2.12) versus the healthy comparator group (Smith et al., 2018a). However, adjustment for confounding factors was not reported in this abstract.
Postpartum period
Disease activity may improve during pregnancy, but many women with PSO experience flares postpartum (Murase et al., 2005, Polachek et al., 2017). In U.S. patients with PSO, of 1239 live pregnancies identified in the MarketScan database (January 2010-September 2016), 1897 flares were recorded. The average monthly flare rates revealed an increased risk postpartum: 71.3, 77.4, and 126.1 flares per month were calculated for the pre-conception, pregnancy, and postpartum periods, respectively (Lee et al., 2019). Murase et al. (2005) demonstrated that psoriatic BSA approximately doubled between 30 weeks’ gestation and 6 weeks postpartum. However, the increased BSA values observed did not exceed those of the first trimester, suggesting a return to the patients’ baseline status (Murase et al., 2005).
Estrogen fluctuations are speculated to be associated with BSA improvement during pregnancy (elevated levels) and disease worsening postpartum (subsequent reduction; Boyd et al., 1996, Murase et al., 2005), but no definitive evidence exists in support of a causative effect (Danesh and Murase, 2015). In addition, psoriatic treatments during pregnancy are commonly discontinued (Lebwohl et al., 2018, Lee et al., 2018, Ursin et al., 2018), and the impact of therapy cessation on postpartum disease activity cannot be discounted.
A recent retrospective study found no significant change in PsA disease activity during pregnancy, but a statistically significant aggravation was observed during the postpartum period compared with the third trimester (n = 33 live births; p = .01; Berman et al., 2018). Similarly, in a prospective multicenter study including 103 women with PsA (n = 108 pregnancies) from a Norwegian nationwide register, PsA disease activity decreased during pregnancy and increased within 6 months postpartum (Ursin et al., 2018). However, disease activity from planning to 12 months after delivery was stable, with 75% of women with PsA shown to be in remission (Disease Activity Score-28 [DAS28] ≤ 2.6) or to have low disease activity (2.6 < DAS28 ≤ 3.2) at these time points (Ursin et al., 2018). Overall disease stability was observed despite disease-modifying antirheumatic drug use (synthetic, biologic, or both) decreasing from 58% prior to pregnancy to 17% in late pregnancy (Ursin et al., 2018).
On the other hand, discontinuation of biologic treatment before pregnancy or during the first trimester (n = 15 of 21 pregnancies) was associated with significant disease worsening during pregnancy and in the postpartum period (odds ratio: 7; 95% CI, 0.5-98.6), but no significant changes were reported in the small number of patients who continued on biologic therapy beyond the first trimester (n = 6; anti-tumor necrosis factor [anti-TNF]: n = 5; ustekinumab: n = 1; Berman et al., 2018). In line with these findings, although only a small number of women with PsA used anti-TNFs during pregnancy (n = 7) in the Norwegian study, disease activity 6 months postpartum was significantly lower in this group (mean DAS28-C-reactive protein with anti-TNF: 2.22 vs. without anti-TNF: 2.72; p = .043; Ursin et al., 2018).
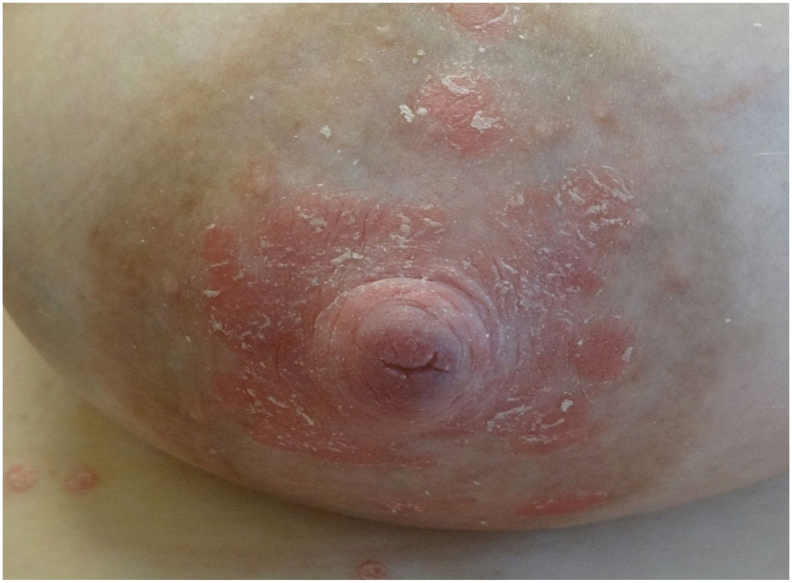
If a mother can and chooses to breastfeed, it can benefit both the mother and the child (Jain and Gordon, 2011). However, nursing mothers are prone to nipple pain due to dermatitis and the development of new psoriasis plaques (koebnerization; Fig. 1; Heller et al., 2012). PSO and PsA management before, during, and after pregnancy is highly important to achieve optimal outcomes for both mothers and infants (Porter et al., 2017).
Fig. 1.
Koebnerization: Development of new psoriasis plaques on the nipple due to infant suckling (image courtesy of Jenny Murase, MD, Department of Dermatology, University of California San Francisco and Palo Alto Medical Foundation Medical Group).
Unmet needs of women with psoriasis and psoriatic arthritis on their journey to motherhood
Pre-conception
Women with PSO planning a pregnancy require support from their HCPs with initiating discussions about family planning. The Patient Journey survey of women of childbearing age (18-45 years) with psoriatic diseases (PSO: n = 367; PsA: n = 142) from the U.S., Japan, and the European Union 5 (EU5; Germany, France, Italy, Spain, and the United Kingdom) revealed that before pregnancy, more than three-quarters of women with PSO and PsA had to initiate these discussions with HCPs themselves (Murase et al., 2019). This finding was corroborated by the NPF survey, which found that only 7% of patients who discussed family planning with their treating physician stated that this discussion was initiated by their HCP (Lebwohl et al., 2018).
Inadequate information and guidance from HCPs may contribute to women delaying pregnancy or seeking information elsewhere. More than half of women with PsA and a quarter of women with PSO admitted delaying their decision to become a mother; their main fear was passing on health issues to their child (Murase et al., 2019). According to the NPF survey, most patients with PSO planning to become pregnant sought advice from the internet (88%); only 21% had consulted their treating physician (Lebwohl et al., 2018).
HCPs should also be able to confidently address patients’ questions on the heritability of PSO and PsA. If only one parent has PSO, a child will have a 28% lifetime risk of developing the condition; if both parents have PSO, the risk increases to 65% (Swanbeck et al., 1997).
Pregnancy
Recent surveys have highlighted several unmet needs of women with PSO and PsA around pregnancy. The Patient Journey survey indicated that women lack information on the impact of treatment decisions on pregnancy (PSO: 31%; PsA: 40%; Murase et al., 2019). Furthermore, only 28% of women with PSO had a treatment plan aligned between different HCPs (only 18% for women with PSO on biologics) compared with 64% for women with PsA (Murase et al., 2019).
The U.S. NPF survey (n = 141) revealed that during pregnancy, 65% of patients who had been pregnant stopped treatment (of any type), 79% of whom did so out of fear of harming their baby (Lebwohl et al., 2018). In 40% of cases where patients had stopped all treatment for PSO, the decision was initiated by the patient themselves; in 47% of cases, treatment was stopped by their treatment provider (Lebwohl et al., 2018). Of the patients who stopped treatment, 44% experienced a worsening in the severity of their disease, but most patients did not have a plan for flare management during pregnancy (≥ 65%; Lebwohl et al., 2018).
Postpartum
A disease management plan for new mothers is particularly important, owing to the increased risk of postpartum flares. However, the NPF survey showed that of patients who had experienced a pregnancy and had stopped all treatment, almost a quarter had not been advised on restarting treatment postpartum (Murase et al., 2005). Misconceptions during this phase are also common. The Patient Journey survey revealed that 44% of women with PSO and 78% of women with PsA felt they had to choose between treatment or breastfeeding (Murase et al., 2019. Furthermore, up to a quarter of women felt they lacked information on the impact of treatment decisions on breastfeeding (PSO: 16%; PsA: 24%; Murase et al., 2019). According to the survey, fewer than half of patients with PSO (46%) consulted a dermatologist during pregnancy (first and/or second and/or third trimester), compared with 86% who visited an obstetrician/gynecologist (Murase et al., 2019.
Alignment with a multidisciplinary team for patient-centric care can improve the patient experience and prevent conflicting recommendations (Guise and Segel, 2008). Patients and family members should be encouraged to play an active role in the development of their plan of care and coordination of their care team. This strategy helps patients to better understand their options, empowers them to make their values and preferences known, and ensures that they have understood the consequences of any decisions made (Barry and Edgman-Levitan, 2012).
Treatment of psoriasis/psoriatic arthritis and options compatible with pregnancy
Many treatment options are available for PSO and PsA, including topical agents, phototherapy, oral systemic agents and biologics. Of note, women of childbearing age (18-44 years) with PSO were reported to undergo more treatment changes than any other cohort assessed (i.e., men aged 18-44 years, men aged 45-65 years, and women aged 45-65 years), which can be an indicator of poor disease control (Anderson and Feldman, 2015, Smith et al., 2018) or increased focus on appearance. This group of patients was also the most likely to discontinue treatment (Lee et al., 2018).
Balancing the risk of PSO/PsA treatment with the risk of uncontrolled disease is critically important in women of reproductive age, but it can be challenging for physicians (Porter et al., 2017, Rademaker et al., 2018). New U.S. Food and Drug Administration labeling now summarizes data associated with fertility, pregnancy, and lactation to facilitate effective discussions around the risks and benefits of drugs with patients, supporting informed decisions. European Medicines Agency guidelines also allow the recommendation of medication use during pregnancy and breastfeeding. If no increased incidence of malformations is observed within at least 300 or 1000 first trimester–exposed, prospectively collected pregnancies with known pregnancy outcomes, the conclusion might be reached that a drug is not responsible for a 10-fold, 2-fold, or more increase of the overall incidence of malformations (European Medicines Agency, 2008).
Pregnancy outcomes data are increasingly available, and ongoing studies, such as the MotherToBaby study using the regulatory body-recognized OTIS registry, provide further valuable information (MotherToBaby, 2018). HCPs have a responsibility to interpret emerging safety data in a timely manner and work with their patients to create a customized disease management plan for all women of reproductive age, irrespective of family planning intentions. A number of articles provide guidance on the treatment of women of childbearing age with PSO or PsA (Johansen et al., 2018, Murase et al., 2014, Pottinger et al., 2018, Rademaker et al., 2018, Smith et al., 2017). However, treatment guidelines for PSO, similar to those developed for the management of rheumatic diseases (Flint et al., 2016a, Flint et al., 2016b, Gotestam Skorpen et al., 2016), are still urgently needed.
Topical treatments
Topical therapies used in the management of PSO include anthralin, calcipotriol, coal tar, corticosteroids, salicylic acid, tacrolimus, and tazarotene. Many topical drugs can be used during pregnancy, but others are unsuitable. Topical agents that have suspected or known mutagenic or teratogenic properties include anthralin and tazarotene. Coal tar has been shown to have mutagenic potential in animal models when applied to extensive surface areas (Table 2). Topical corticosteroid use has also been implicated in the formation of striae on the breasts during breastfeeding, when the skin is rapidly expanding and contracting (Johansen et al., 2018, Menter et al., 2009).
Table 2.
Topical treatment options and compatibility during pregnancy and breastfeedinga
| Topical treatments | Evidence during pregnancy | Key advice during pregnancy | Key advice during breastfeeding |
|---|---|---|---|
| Anthralin (Summer Laboratories, 2006) | No data in humans | Avoid or only use if clearly needed | Only use if potential benefit justifies potential risk to infant |
| Calcipotriol (LEO Laboratories, 2015) | No data in humans | Use only on small surfaces when no alternatives exist (Murase et al., 2014) | Compatible; limit to < 20% surface area and doses < 10,000 IU/day (Murase et al., 2014, Butler et al., 2014) |
| Coal tar (Murase et al., 2014, Rademaker et al., 2018) | Minimal data in humans | Avoid; potentially mutagenic/carcinogenic (animal study data; Murase et al., 2014) | Avoid or use minimally (Butler et al., 2014) |
| Corticosteroids (G&W Laboratories, 2016) | Potent corticosteroids may be associated with increased risk of low birth weight at doses > 300 g (Chi et al., 2013, Chi et al., 2015) | Use of low- to moderate-potency topical corticosteroids in short durations is acceptable (Bae et al., 2012, Chi et al., 2011) | Compatible, except for Class I (avoid; Smith et al., 2017); avoid use of high-potency corticosteroids on the nipple (Butler et al., 2014) |
| Salicylic acid (Exeltis USA Dermatology, 2016) | Limited data in humans | Restrict use to limited local application for limited time (Murase et al., 2014) | Compatible for local, topical use; avoid use on breasts |
| Tacrolimus (Astellas Pharma, 2011) | Minimal data in humans | Use only on small surfaces when no alternatives exist (Murase et al., 2014) | Only use sparingly and not directly on the nipple (Butler et al., 2014) |
| Tazarotene (Allergan, 2017) | Teratogen | Avoid | Avoid (Murase et al., 2014) |
This information is not intended to provide treatment recommendations. All disease management decisions must be based on discussion and agreement between the patient and treating physician.
The use of class 1 corticosteroids on the nipples should be avoided. A 2015 Cochrane systematic review of 14 randomized controlled trials and cohort studies on the use of topical corticosteroids in pregnant women found no association between maternal exposure and any of the pregnancy outcomes assessed (Chi et al., 2015). However, the risk of low birth weight significantly increased when the dose of potent (to very potent) topical corticosteroids exceeded 300 g (aRR: 7.74; 95% CI, 1.49-40.11; p = .02; Chi et al., 2013).
Phototherapy
Phototherapy with broadband and narrowband ultraviolet (UV)-B is considered safe during pregnancy and breastfeeding (Rademaker et al., 2018). Owing to the potential for maternal folate photodegradation with this treatment, and the association of folate deficiency with neural tube defects, ensuring that women of childbearing age receive adequate folic acid supplementation is an important consideration (El-Saie et al., 2011, Murase et al., 2014, Rademaker et al., 2018). Furthermore, due to an increased risk of melasma, the use of facial shielding is recommended to prevent exacerbation (Murase et al., 2014). Conversely, psoralen and UV-A therapy is contraindicated during pregnancy due to the mutagenic and teratogenic properties of psoralen (Murase et al., 2014, Rademaker et al., 2018).
Systemic agents
The use of certain oral systemic PSO treatments is also incompatible with pregnancy, including oral psoralen, methotrexate, and acitretin, which have been linked to teratogenic effects and should be avoided in women of childbearing age (Murase et al., 2014). Cyclosporin is generally considered compatible with pregnancy, although the drug may be associated with an increased risk of low birth weight and prematurity (Murase et al., 2014). Cyclosporin can cause maternal hypertension; thus, its reservation as a rescue therapy for severe PSO has been recommended (Murase et al., 2014). Several societies have developed treatment recommendations around the use of systemic agents during pregnancy and breastfeeding (Bae et al., 2012, Menter et al., 2009, Porter et al., 2017).
Biologic therapies
Anti-TNF treatments are commonly used to treat PSO, and data are increasingly emerging on their safety around pregnancy (Johansen et al., 2018). The Fc-free anti-TNF certolizumab pegol (CZP) is the only biologic agent with clinical trial data in its label supporting potential use in both pregnancy and breastfeeding for chronic inflammatory diseases (European Medicines Agency, 2018, U.S. Food and Drug Administration, 2018). Published in 2017, two prospective studies showed a lack of placental transfer of CZP from mothers to infants (CRIB) (Mariette et al., 2018), and no to minimal transfer from plasma into breast milk (CRADLE) (Clowse et al., 2017). Furthermore, a recent pharmacovigilance safety database analysis (n = 528 prospective pregnancies with known outcomes with maternal exposure to CZP, including 10 twins) demonstrated no teratogenic effect of CZP when compared with the general population in the U.S. and European Union, nor an increased risk of fetal death, providing further reassurance for women of childbearing age considering treatment with CZP (Clowse et al., 2018). More recently, the adalimumab and etanercept labels were also updated to allow potential use during pregnancy, while acknowledging that they may cross the placenta (European Medicines Agency, 2007, European Medicines Agency, 2019, U.S. Food and Drug Administration, 2008). Adalimumab can also be used during breastfeeding, based on limited evidence in the published literature.
Two MothertoBaby/OTIS studies investigating pregnancy outcomes in women with chronic inflammatory diseases after exposure to anti-TNFs (etanercept and adalimumab) have so far been completed. The final publications for these studies are expected imminently (Chambers et al., 2018). An increase in major birth defects with etanercept compared with disease-matched groups was indicated (2005-2012; n = 370 exposed; aOR: 2.77; 95% CI, 1.04-7.35; Chambers et al., 2015). However, a drug-related effect was not supported due to the lack of a specific pattern of defects and the minimal placental transfer of etanercept anticipated in early pregnancy (Chambers et al., 2015).
In another recent observational multi-country registry study (Sweden, Denmark, and Finland), no increased risk of birth defects, preterm birth, or infections was observed in the offspring of mothers with chronic inflammatory diseases who were treated with etanercept (n = 425 pregnancies exposed during the first trimester) compared with mothers who were treated with nonbiologic systemic treatment (n = 3508) during pregnancy. The odds ratio for major birth defects in women exposed to etanercept during the first trimester compared with nonbiologic treatment during pregnancy was 0.96 (95% CI, 0.58-1.60) when adjusted for country, maternal disease, parity, maternal age, and smoking status in early pregnancy (Kieler et al., 2018). The adalimumab MothertoBaby/OTIS study (2004-2014; n = 257 exposed; aOR for major birth defects: 0.91; 95% CI, 0.37-2.23]) also found no evidence of a specific pattern of major birth defects in the exposed cohort (Chambers et al., 2017). Furthermore, the results of this study were reassuring for every outcome examined, including spontaneous abortion and preterm delivery (Chambers et al., 2017).
A recent update article on biologic safety for patients with PSO during pregnancy recommends that infants born to mothers treated with anti-TNF therapies during pregnancy should not be administered live vaccinations (e.g., Bacillus Calmette-Guérin (BCG) or rotavirus vaccines) for at least 6 months after birth due to an increased risk of infection (Porter et al., 2017),3 advice that is also included in the drug package inserts. The only anti-TNF label that differs with respect to vaccination recommendations is that of CZP, based on data demonstrating that CZP levels are undetectable at weeks 4 and 8 after delivery in the plasma of infants exposed in utero (Mariette et al., 2018). Accordingly, the U.S. and EU CZP labels state that the theoretical risk of live or live-attenuated vaccine administration to infants exposed to CZP in utero should be weighed against the benefits of these vaccinations (European Medicines Agency, 2018, U.S. Food and Drug Administration, 2018). Inactive vaccinations can be administered per routine practice (Porter et al., 2017).
Despite the increasing availability of reassuring data on the use of biologics during pregnancy and breastfeeding, rates of biologic use among women of childbearing age have been reported to be lower compared with those of women of all ages (Lee et al., 2018).
A recent article on biologic safety states that, although relatively limited data are available on biologic use during pregnancy and lactation, these drugs may be considered appropriate for patients with PSO (Porter et al., 2017). Moreover, anti-TNF agents should be considered over monoclonal antibodies directed against interleukin (IL) 12/23 or IL-17, owing to the greater availability of safety data for anti-TNF therapies (Porter et al., 2017). In a systematic meta-analysis of 13 studies in patients with chronic inflammatory diseases, women exposed to anti-TNFs during pregnancy had outcomes comparable to those of women who did not receive anti-TNFs (Komaki et al., 2017).
The more recent anti-IL agents (immunoglobulin [Ig] G monoclonal antibodies) are thought to be actively transported across the placenta (Johansen et al., 2018) in a manner similar to IgG anti-TNFs (e.g., adalimumab) but unlike certolizumab pegol, as previously discussed. Pregnancy outcomes data in women with PSO, PsA, or Crohn’s disease from the Janssen Biotech safety database (through 2017; prospective: n = 130; retrospective: n = 76) identified no specific risks with exposure to the anti-IL 12/23 ustekinumab during pregnancy or within 2 months prior to conception (Mahadevan et al., 2018).
Furthermore, a recent analysis of safety outcomes with maternal or paternal exposure to the anti-IL 17A secukinumab from the Novartis global safety database (n = 153 pregnancies with known outcomes) found no safety signals with regard to spontaneous abortions or congenital malformations (Warren et al., 2018). These results are reassuring, but the majority of patients discontinued treatment during the first trimester, which is prior to the anticipated placental transfer of secukinumab. Overall, safety data on anti-IL agents are currently scarce, and because their mechanism of action is distinct from that of anti-TNFs, large pharmacovigilance studies on the safety of these agents during pregnancy are needed. At present, the ustekinumab and secukinumab labels suggest avoidance of their use during pregnancy as a precautionary measure, due to the lack of adequate data on use in pregnant women. No clinical data on the use of the newer, small molecule apremilast during pregnancy are available at present; however, a pregnancy registry has been established (NCT02775500; MotherToBaby, 2019).
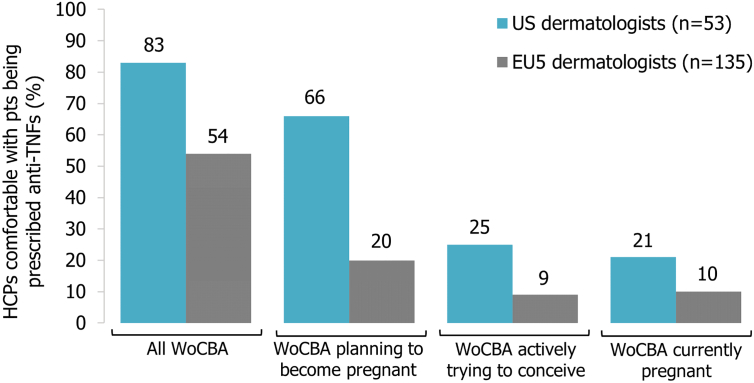
Despite the importance of HCPs being adequately informed about treating women of childbearing age with anti-TNFs, a recent physicians’ survey conducted in the EU5 and U.S. suggested that dermatologists (EU5: n = 135 [November-December 2017]; U.S.: n = 53 [July 2017]) were the least likely to prescribe anti-TNFs for women with chronic inflammatory diseases compared with rheumatologists (EU5/U.S.) and gastroenterologists (U.S. only; Ryan et al., 2018). Although more than half of participating dermatologists across both regions agreed that controlled disease reduces the risk of pregnancy complications, their comfort with prescribing anti-TNF therapies consistently declined with the onset of family planning, particularly in Europe (Fig. 2; Ryan et al., 2018). EU5 dermatologists (83%) were also more likely to recommend discontinuation of anti-TNFs before conception than U.S. dermatologists (57%) and to agree that women should stop anti-TNF therapy once pregnant (EU5: 72%; U.S.: 45%; Ryan et al., 2018). Possible explanations for this difference may be the fact that fewer EU5 dermatologists agreed on making disease control during pregnancy their priority, coupled with more EU5 than U.S. dermatologists being very concerned about adverse events in pregnant women taking anti-TNFs.
Fig. 2.
Dermatologists’ level of comfort with women of childbearing age being prescribed anti-tumor necrosis factor therapy (adapted from Ryan et al., 2018) HCP = health care professional; pts = patients; TNF = tumor necrosis factor; WoCBA = women of childbearing age.
Biologics are considered compatible for use while breastfeeding (Butler et al., 2014, Rademaker et al., 2018) due to their large molecular size and the proteolytic environment in the neonatal gastrointestinal tract (Zelikin et al., 2016). Accordingly, clinical evidence is now available to support minimal to no transfer of CZP into breast milk (Clowse et al., 2017). A recent study investigating the detection of biologics in breast milk from women treated for inflammatory bowel diseases (PIANO registry) also concluded that concentrations were low (maximum: 0.29-1.57 μg/mL) or not detectable (n = 43 of 72 women) in breast milk, with CZP demonstrating the lowest peak (infliximab [n = 29], adalimumab [n = 21], CZP [n = 13], ustekinumab [n = 6], natalizumab [n = 2], and golimumab [n = 1]; Matro et al., 2018). Nevertheless, a large proportion of dermatologists felt that breastfeeding women should either not be on anti-TNF therapy (EU5: 58%; U.S.: 43%) or indicated uncertainty (EU5: 31%; U.S.: 28%), highlighting the need for education in this area (Ryan et al., 2018).
Conclusions
PSO can profoundly affect people’s quality of life and mental health; with the burden for women being particularly high. Compared with men, women experience lower levels of happiness and greater levels of stress, loneliness, stigmatization, and sexual impairment. The onset of PSO also often coincides with peak reproductive years for women, posing specific challenges for their treatment. Half of all pregnancies are unplanned; thus, it is critically important that, unless on adequate contraception, all women of childbearing age have a treatment plan that is compatible with pregnancy, irrespective of family planning intentions.
There is also a need for better information of HCPs with respect to therapeutic options for women of reproductive age, particularly in light of the increasing safety data available for biologic therapies, and for the establishment of formal treatment guidelines. Timely initiation of discussions by treating HCPs, and ongoing dialogue, with women of childbearing age with regard to compatible treatment options are required to optimize the care and outcomes for this patient population.
This article is accompanied by a Patient Page, a short summary to guide patients who may wish to access credible information with regard to PSO disease burden among women and their options with regard to their pregnancy journey.
Footnotes
Conflicts of interest: A.B. Gottlieb is a consultant, advisory board member, and/or speaker for Janssen, Celgene, Bristol-Myers Squibb, Beiersdorf, AbbVie, UCB Pharma, Novartis, Incyte, Eli-Lilly, Reddy Labs, Valeant, Dermira, Allergan, Sun Pharmaceutical Industries, Xbiotech, Leo, and Avotres Therapeutics and has received research/educational grants from Janssen, Incyte, Novartis, Xbiotech, UCB Pharma, and Boeringer Ingelheim. C. Ryan is a consultant, advisor, and/or speaker for AbbVie, Boehringer Ingelheim, Dermira, Dr. Reddy’s Laboratories, Janssen, Leo, Lilly, Medimetriks, Novartis, Regeneron/Sanofi, and UCB Pharma. J. E. Murase is a consultant for UpToDate, Dermira, UCB Pharma, Sanofi, Ferndale, and Regeneron.
Acknowledgments: The authors acknowledge Cécile Ecoffet, PharmD, UCB Pharma, Brussels, Belgium, for courtesy critical review of the manuscript, and Arianna Psichas, PhD, Costello Medical, United Kingdom, for writing and editorial assistance in the preparation of this manuscript for publication based on the authors’ input and direction, with funding from UCB Pharma.
For patient information on skin cancer in women, please click on Supplemental Material to bring you to the Patient Page. Supplementary data to this article can be found online at https://doi.org/10.1016/j.ijwd.2019.04.021.
Appendix A. Supplementary data
Supplementary figures
References
- American Pregnancy Association Fetal development: First trimester [Internet] 2018. http://americanpregnancy.org/while-pregnant/first-trimester/ [cited 2018 May 12]. Available from:
- Anderson K.L., Feldman S.R. Reasons for treatment changes in patients with moderate to severe psoriasis. J Cutan Med Surg. 2015;19:361–366. doi: 10.1177/1203475415572797. [DOI] [PubMed] [Google Scholar]
- Armstrong A.W., Schupp C., Wu J., Bebo B. Quality of life and work productivity impairment among psoriasis patients: Findings from the National Psoriasis Foundation survey data 2003-2011. PLoS One. 2012;7 doi: 10.1371/journal.pone.0052935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Astellas Pharma . Astellas Pharma US, Inc.; Deerfield, IL: 2011. Protopic [prescribing information] [Google Scholar]
- Augustin M., Reich K., Glaeske G., Schaefer I., Radtke M. Co-morbidity and age-related prevalence of psoriasis: Analysis of health insurance data in Germany. Acta Derm Venereol. 2010;90:147–151. doi: 10.2340/00015555-0770. [DOI] [PubMed] [Google Scholar]
- Bae Y.S., Van Voorhees A.S., Hsu S., Korman N.J., Lebwohl M.G., Young M. Review of treatment options for psoriasis in pregnant or lactating women: From the Medical Board of the National Psoriasis Foundation. J Am Acad Dermatol. 2012;67:459–477. doi: 10.1016/j.jaad.2011.07.039. [DOI] [PubMed] [Google Scholar]
- Bandoli G., Johnson D.L., Jones K.L., Lopez Jiminez J., Salas E., Mirrasoul N. Potentially modifiable risk factors for adverse pregnancy outcomes in women with psoriasis. Br J Dermatol. 2010;163:334–339. doi: 10.1111/j.1365-2133.2010.09899.x. [DOI] [PubMed] [Google Scholar]
- Barry M.J., Edgman-Levitan S. Shared decision making--pinnacle of patient-centered care. N Engl J Med. 2012;366:780–781. doi: 10.1056/NEJMp1109283. [DOI] [PubMed] [Google Scholar]
- Ben-David G., Sheiner E., Hallak M., Levy A. Pregnancy outcome in women with psoriasis. J Reprod Med. 2008;53:183–187. [PubMed] [Google Scholar]
- Berman M., Zisman D., Wollman J., Levartovsky D., Rimon E., Elkayam O. The effect of pregnancy on disease activity in patients with psoriatic arthritis. J Rheumatol. 2018;45:1651–1655. doi: 10.3899/jrheum.171218. [DOI] [PubMed] [Google Scholar]
- Bobotsis R., Gulliver W.P., Monaghan K., Lynde C., Fleming P. Psoriasis and adverse pregnancy outcomes: A systematic review of observational studies. Br J Dermatol. 2016;175:464–472. doi: 10.1111/bjd.14547. [DOI] [PubMed] [Google Scholar]
- Boddeda S., Harrison N., Kishore S., Majithia V. ACR/ARHP Annual Meeting. 2018. Obstetric outcomes in women with psoriatic arthritis: Results from nationwide inpatient sample database 2003-2011; p. 969. [Google Scholar]
- Boyd A.S., Morris L.F., Phillips C.M., Menter M.A. Psoriasis and pregnancy: Hormone and immune system interaction. Int J Dermatol. 1996;35:169–172. doi: 10.1111/j.1365-4362.1996.tb01632.x. [DOI] [PubMed] [Google Scholar]
- Broms G., Haerskjold A., Granath F., Kieler H., Pedersen L., Berglind I.A. Effect of maternal psoriasis on pregnancy and birth outcomes: A population-based cohort study from Denmark and Sweden. Acta Derm Venereol. 2018;98:728–734. doi: 10.2340/00015555-2923. [DOI] [PubMed] [Google Scholar]
- Butler D.C., Heller M.M., Murase J.E. Safety of dermatologic medications in pregnancy and lactation: Part II. Lactation. J Am Acad Dermatol. 2014;70:417.e1–417.e10. doi: 10.1016/j.jaad.2013.09.009. quiz 427. [DOI] [PubMed] [Google Scholar]
- Cather J.C., Latremouille-Viau D., Horn E.J., Bao Y. Psoriasis is significantly associated with lower rates of pregnancy and live births. J Am Acad Dermatol. 2012;66 [Google Scholar]
- Ceovic R., Mance M., Bukvic Mokos Z., Svetec M., Kostovic K., Stulhofer Buzina D. Psoriasis: Female skin changes in various hormonal stages throughout life--puberty, pregnancy, and menopause. Biomed Res Int. 2013;2013:571912. doi: 10.1155/2013/571912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chambers C.D., Johnson D.L., Jones K.L. Pregnancy outcomes in women exposed to etanercept: The OTIS Autoimmune Diseases in Pregnancy Project. Birth Defects Res A. 2015;103:375–401. [Google Scholar]
- Chambers C., Johnson D.L., Kiernan E. Approach to evaluating pregnancy safety of anti-rheumatic medications in the OTIS MotherToBaby pregnancy studies: What have we learned? Rheumatology (Oxford) 2018;57:v34–v39. doi: 10.1093/rheumatology/key081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chambers C.D., Johnson D.L., Xu R. Birth outcomes following pregnancy exposure to adalimumab: The OTIS Autoimmune Diseases in Pregnancy Project. Pharmacoepidemiol Drug Saf. 2017;26:3636. [Google Scholar]
- Chi C.C., Kirtschig G., Aberer W., Gabbud J.P., Lipozenčić J., Kárpáti S. Evidence-based (S3) guideline on topical corticosteroids in pregnancy. Br J Dermatol. 2011;165:943–952. doi: 10.1111/j.1365-2133.2011.10513.x. [DOI] [PubMed] [Google Scholar]
- Chi C.C., Wang S.H., Mayon-White R., Wojnarowska F. Pregnancy outcomes after maternal exposure to topical corticosteroids: A UK population-based cohort study. JAMA Dermatol. 2013;149:1274–1280. doi: 10.1001/jamadermatol.2013.5768. [DOI] [PubMed] [Google Scholar]
- Chi C.C., Wang S.H., Wojnarowska F., Kirtschig G., Davies E., Bennett C. Safety of topical corticosteroids in pregnancy. Cochrane Database Syst Rev. 2015 doi: 10.1002/14651858.CD007346.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clowse M.E., Forger F., Hwang C., Thorp J., Dolhain R.J., van Tubergen A. Minimal to no transfer of certolizumab pegol into breast milk: Results from CRADLE, a prospective, postmarketing, multicentre, pharmacokinetic study. Ann Rheum Dis. 2017;76:1890–1896. doi: 10.1136/annrheumdis-2017-211384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clowse M.E.B., Scheuerle A.E., Chambers C., Afzali A., Kimball A.B., Cush J.J. Pregnancy outcomes after exposure to certolizumab pegol: Updated results from a pharmacovigilance safety database. Arthritis Rheumatol. 2018;70:1399–1407. doi: 10.1002/art.40508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen-Barak E., Nachum Z., Rozenman D., Ziv M. Pregnancy outcomes in women with moderate-to-severe psoriasis. J Eur Acad Dermatol Venereol. 2011;25:1041–1047. doi: 10.1111/j.1468-3083.2010.03917.x. [DOI] [PubMed] [Google Scholar]
- Crow J.M. Psoriasis uncovered. Nature. 2012;492:S50–S51. doi: 10.1038/492S50a. [DOI] [PubMed] [Google Scholar]
- Danesh M., Murase J.E. The immunologic effects of estrogen on psoriasis: A comprehensive review. Int J Womens Dermatol. 2015;1:104–107. doi: 10.1016/j.ijwd.2015.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Saie L.T., Rabie A.R., Kamel M.I., Seddeik A.K., Elsaie M.L. Effect of narrowband ultraviolet B phototherapy on serum folic acid levels in patients with psoriasis. Lasers Med Sci. 2011;26:481–485. doi: 10.1007/s10103-011-0895-0. [DOI] [PubMed] [Google Scholar]
- European Medicines Agency Adalimumab (Humira) summary of product characteristics [Internet] 2007. https://www.ema.europa.eu/en/documents/product-information/humira-epar-product-information_en.pdf [cited 2018 September 19]. Available from:
- European Medicines Agency Guideline on risk assessment of medicinal products on human reproduction and lactation: From data to labelling [Internet] 2008. https://www.ema.europa.eu/en/documents/scientific-guideline/guideline-risk-assessment-medicinal-products-human-reproduction-lactation-data-labelling_en.pdf [cited 2018 September 19]. Available from:
- European Medicines Agency Certolizumab pegol (Cimzia) summary of product characteristics [Internet] 2018. https://www.ema.europa.eu/en/documents/product-information/cimzia-epar-product-information_en.pdf [cited 2018 September 19]. Available from:
- European Medicines Agency Etanercept (Enbrel) summary of product characteristics [Internet] 2019. https://www.ema.europa.eu/en/documents/product-information/enbrel-epar-product-information_en.pdf [cited 2019 March 1]. Available from:
- Exeltis USA Dermatology . Exeltis USA Dermatology, LLC; Florham Park, NJ: 2016. Salvax [prescribing information] [Google Scholar]
- Flint J., Panchal S., Hurrell A., van de Venne M., Gayed M., Schreiber K. BSR and BHPR guideline on prescribing drugs in pregnancy and breastfeeding-Part I: Standard and biologic disease modifying anti-rheumatic drugs and corticosteroids. Rheumatology (Oxford) 2016;55:1693–1697. doi: 10.1093/rheumatology/kev404. [DOI] [PubMed] [Google Scholar]
- Flint J., Panchal S., Hurrell A., van de Venne M., Gayed M., Schreiber K. BSR and BHPR guideline on prescribing drugs in pregnancy and breastfeeding-Part II: Analgesics and other drugs used in rheumatology practice. Rheumatology (Oxford) 2016;55:1698–1702. doi: 10.1093/rheumatology/kev405. [DOI] [PubMed] [Google Scholar]
- G&W Laboratories South Plainfield, NJ: G&W Laboratories, Inc.; 2016. Triamcinolone ointment [prescribing information]
- Generali E., Scire C.A., Cantarini L., Selmi C. Sex differences in the treatment of psoriatic arthritis: A systematic literature review. Isr Med Assoc J. 2016;18:203–208. [PubMed] [Google Scholar]
- Gotestam Skorpen C., Hoeltzenbein M., Tincani A., Fischer-Betz R., Elefant E., Chambers C. The EULAR points to consider for use of antirheumatic drugs before pregnancy, and during pregnancy and lactation. Ann Rheum Dis. 2016;75:795–810. doi: 10.1136/annrheumdis-2015-208840. [DOI] [PubMed] [Google Scholar]
- Guise J.M., Segel S. Teamwork in obstetric critical care. Best Pract Res Clin Obstet Gynaecol. 2008;22:937–951. doi: 10.1016/j.bpobgyn.2008.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta M.A., Gupta A.K. Psoriasis and sex: a study of moderately to severely affected patients. Int J Dermatol. 1997;36:259–262. doi: 10.1046/j.1365-4362.1997.00032.x. [DOI] [PubMed] [Google Scholar]
- Harder E., Andersen A.N., Kamper-Jorgensen M., Skov L. No increased risk of fetal death or prolonged time to pregnancy in women with psoriasis. J Invest Dermatol. 2014;134:1747–1749. doi: 10.1038/jid.2014.35. [DOI] [PubMed] [Google Scholar]
- Hawro M., Maurer M., Weller K., Maleszka R., Zalewska-Janowska A., Kaszuba A. Lesions on the back of hands and female gender predispose to stigmatization in patients with psoriasis. J Am Acad Dermatol. 2017;76:648–654.e2. doi: 10.1016/j.jaad.2016.10.040. [DOI] [PubMed] [Google Scholar]
- Heller M.M., Fullerton-Stone H., Murase J.E. Caring for new mothers: diagnosis, management and treatment of nipple dermatitis in breastfeeding mothers. Int J Dermatol. 2012;51:1149–1161. doi: 10.1111/j.1365-4632.2011.05445.x. [DOI] [PubMed] [Google Scholar]
- Jain V., Gordon C. Managing pregnancy in inflammatory rheumatological diseases. Arthritis Res Ther. 2011;13:206. doi: 10.1186/ar3227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johansen C.B., Jimenez-Solem E., Haerskjold A., Sand F.L., Thomsen S.F. The use and safety of TNF inhibitors during pregnancy in women with psoriasis: A review. Int J Mol Sci. 2018;19 doi: 10.3390/ijms19051349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kavanaugh A., Cush J.J., Ahmed M.S., Bermas B.L., Chakravarty E., Chambers C. Proceedings from the American College of Rheumatology Reproductive Health Summit: The management of fertility, pregnancy, and lactation in women with autoimmune and systemic inflammatory diseases. Arthritis Care Res (Hoboken) 2015;67:313–325. doi: 10.1002/acr.22516. [DOI] [PubMed] [Google Scholar]
- Kieler H., Broms G., Gu Y. Exposure to ENBREL (etanercept) during pregnancy - Non-interventional study report [Internet] 2018. http://www.encepp.eu/encepp/openAttachment/studyResult/25594;jsessionid=Bz_xQikx7No7imWL4c-2wBmg1nBhMfY95UQS0KMMCRdKXdAFe54B!750940702 [cited 2018 March 11]. Available from:
- Kimball A.B., Leonardi C., Stahle M., Gulliver W., Chevrier M., Fakharzadeh S. Demography, baseline disease characteristics and treatment history of patients with psoriasis enrolled in a multicentre, prospective, disease-based registry (PSOLAR) Br J Dermatol. 2014;171:137–147. doi: 10.1111/bjd.13013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Komaki F., Komaki Y., Micic D., Ido A., Sakuraba A. Outcome of pregnancy and neonatal complications with anti-tumor necrosis factor-alpha use in females with immune mediated diseases; a systematic review and meta-analysis. J Autoimmun. 2017;76:38–52. doi: 10.1016/j.jaut.2016.11.004. [DOI] [PubMed] [Google Scholar]
- Kumar R., Sharma A., Dogra S. Prevalence and clinical patterns of psoriatic arthritis in Indian patients with psoriasis. Indian J Dermatol Venereol Leprol. 2014;80:15–23. doi: 10.4103/0378-6323.125472. [DOI] [PubMed] [Google Scholar]
- Kurd S.K., Gelfand J.M. The prevalence of previously diagnosed and undiagnosed psoriasis in U.S. adults: Results from NHANES 2003-2004. J Am Acad Dermatol. 2009;60:218–224. doi: 10.1016/j.jaad.2008.09.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurd S.K., Troxel A.B., Crits-Christoph P., Gelfand J.M. The risk of depression, anxiety, and suicidality in patients with psoriasis: A population-based cohort study. Arch Dermatol. 2010;146:891–895. doi: 10.1001/archdermatol.2010.186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurizky P.S., Mota L.M. Sexual dysfunction in patients with psoriasis and psoriatic arthritis--a systematic review. Rev Bras Reumatol. 2012;52:943–948. [PubMed] [Google Scholar]
- Lebwohl M., Van Voorhees A.S., Siegel M. Intl Fed Psoriasis Assoc. 2018. A comprehensive survey assessing the family planning needs of women with psoriasis; p. 34369. [Google Scholar]
- Lee E., Suruki R., Carpenter B., Harkness T., Luk D., Yassine M. The prevalence and treatment patterns of women of child bearing age (WoCBA) with psoriasis (PSO) Value Health. 2018;21:PSS34. [Google Scholar]
- Lee E., Brady B., Suruki R., Fowler R., Kim G., Stark J.L. Incidence of peri-pregnancy flares among psoriasis patients. Am Acad Dermatol. 2019:10546. [Google Scholar]
- Lesuis N., Befrits R., Nyberg F., van Vollenhoven R.F. Gender and the treatment of immune-mediated chronic inflammatory diseases: rheumatoid arthritis, inflammatory bowel disease and psoriasis: An observational study. BMC Med. 2012;10:82. doi: 10.1186/1741-7015-10-82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LEO Innovation Lab and The Happiness Research Institute World Psoriasis Happiness Report 2017 [Internet] 2017. https://docs.wixstatic.com/ugd/928487_d405a5c58b8e42ae8e2a84112fada89a.pdf?index=true [cited 2018 October 26]. Available from:
- LEO Laboratories Dublin, Ireland: LEO Laboratories Ltd.; 2015. Dovonex [prescribing information]
- Leung Y.Y., Tam L.S., Kun E.W., Li E.K. Psoriatic arthritis as a distinct disease entity. J Postgrad Med. 2007;53:63–71. doi: 10.4103/0022-3859.30334. [DOI] [PubMed] [Google Scholar]
- Levine D., Gottlieb A. Evaluation and management of psoriasis: An internist's guide. Med Clin North Am. 2009;93:1291–1303. doi: 10.1016/j.mcna.2009.08.003. [DOI] [PubMed] [Google Scholar]
- Lima X.T., Janakiraman V., Hughes M.D., Kimball A.B. The impact of psoriasis on pregnancy outcomes. J Invest Dermatol. 2012;132:85–91. doi: 10.1038/jid.2011.271. [DOI] [PubMed] [Google Scholar]
- Mahadevan U., Naureckas S., Sharma B., Tikhonov I., Szapary P., Busse C. Su1799 –Pregnancy outcomes in women exposed to ustekinumab. Gastroenterology. 2018;154:S588–S589. [Google Scholar]
- Mariette X., Forger F., Abraham B., Flynn A.D., Moltó A., Flipo R.M. Lack of placental transfer of certolizumab pegol during pregnancy: Results from CRIB, a prospective, postmarketing, pharmacokinetic study. Ann Rheum Dis. 2018;77:228–233. doi: 10.1136/annrheumdis-2017-212196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matro R., Martin C.F., Wolf D., Shah S.A., Mahadevan U. Exposure concentrations of infants breastfed by women receiving biologic therapies for inflammatory bowel diseases and effects of breastfeeding on infections and development. Gastroenterology. 2018;155:696–704. doi: 10.1053/j.gastro.2018.05.040. [DOI] [PubMed] [Google Scholar]
- McDonough E., Ayearst R., Eder L., Chandran V., Rosen C.F., Thavaneswaran A. Depression and anxiety in psoriatic disease: Prevalence and associated factors. J Rheumatol. 2014;41:887–896. doi: 10.3899/jrheum.130797. [DOI] [PubMed] [Google Scholar]
- Mease P.J., Armstrong A.W. Managing patients with psoriatic disease: The diagnosis and pharmacologic treatment of psoriatic arthritis in patients with psoriasis. Drugs. 2014;74:423–441. doi: 10.1007/s40265-014-0191-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meeuwis K.A., de Hullu J.A., van de Nieuwenhof H.P., Evers A.W., Massuger L.F., van de Kerkhof P.C. Quality of life and sexual health in patients with genital psoriasis. Br J Dermatol. 2011;164:1247–1255. doi: 10.1111/j.1365-2133.2011.10249.x. [DOI] [PubMed] [Google Scholar]
- Menter A., Korman N.J., Elmets C.A., Feldman S.R., Gelfand J.M., Gordon K.B. Guidelines of care for the management of psoriasis and psoriatic arthritis: section 4. Guidelines of care for the management and treatment of psoriasis with traditional systemic agents. J Am Acad Dermatol. 2009;61:451–485. doi: 10.1016/j.jaad.2009.03.027. [DOI] [PubMed] [Google Scholar]
- MotherToBaby Medications & more during pregnancy & breastfeeding [Internet] 2019. https://mothertobaby.org/ongoing-study/otezla/ [cited 2019 February 22]. Available from:
- MotherToBaby Medications & more during pregnancy & breastfeeding [Internet] 2018. https://mothertobaby.org/ [cited 2018 September 19]. Available from:
- Mouyis M.A., Thornton C.C., Williams D., Giles I.P. Pregnancy outcomes in patients with psoriatic arthritis. J Rheumatol. 2017;44:128–129. doi: 10.3899/jrheum.160929. [DOI] [PubMed] [Google Scholar]
- Murase J.E., Chan K.K., Garite T.J., Cooper D.M., Weinstein G.D. Hormonal effect on psoriasis in pregnancy and post partum. Arch Dermatol. 2005;141:601–606. doi: 10.1001/archderm.141.5.601. [DOI] [PubMed] [Google Scholar]
- Murase J.E., De Simone C., Fischer-Betz R., Ecoffet C., Tincani A. Fears and misconceptions of women with chronic inflammatory diseases on their journey to motherhood. Am Acad Dermatol. 2019;8060 [Google Scholar]
- Murase J.E., Heller M.M., Butler D.C. Safety of dermatologic medications in pregnancy and lactation: Part I. Pregnancy. J Am Acad Dermatol. 2014;70:401.e1–401.e14. doi: 10.1016/j.jaad.2013.09.010. quiz 415. [DOI] [PubMed] [Google Scholar]
- PlaquePsoriasis.com Clothing & makeup choices [Internet] 2018. https://plaquepsoriasis.com/living-with-psoriasis/how-to-cover-plaques/ [cited 2018 May 10]. Available from:
- National Psoriasis Foundation . National Psoriasis Foundation; Hightstown, NJ: 2017. The skin you're in. [Google Scholar]
- Polachek A., Li S., Polachek I.S., Chandran V., Gladman D. Psoriatic arthritis disease activity during pregnancy and the first-year postpartum. Semin Arthritis Rheum. 2017;46:740–745. doi: 10.1016/j.semarthrit.2017.01.002. [DOI] [PubMed] [Google Scholar]
- Pottinger E., Woolf R.T., Exton L.S., Burden A.D., Nelson-Piercy C., Smith C.H. Exposure to biological therapies during conception and pregnancy: A systematic review. Br J Dermatol. 2018;178:95–102. doi: 10.1111/bjd.15802. [DOI] [PubMed] [Google Scholar]
- Porter M.L., Lockwood S.J., Kimball A.B. Update on biologic safety for patients with psoriasis during pregnancy. Int J Womens Dermatol. 2017;3:21–25. doi: 10.1016/j.ijwd.2016.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rademaker M., Agnew K., Andrews M., Armour K., Baker C., Foley P. Psoriasis in those planning a family, pregnant or breast-feeding. The Australasian Psoriasis Collaboration. Australas J Dermatol. 2018;59:86–100. doi: 10.1111/ajd.12641. [DOI] [PubMed] [Google Scholar]
- Remaeus K., Johansson K., Stephansson O. Presented at RheumaPreg 2018 [OP07] 2018. Pregnancy and birth outcomes in women with psoriatic arthritis, a nation-wide Swedish cohort study, 2007–2014. [Google Scholar]
- Ryan C., Murase J.E., Ecoffet C. Anti-TNF treatments for women with chronic inflammatory diseases: Comparing attitudes and perceptions of physicians in Europe and the US. J Eur Acad Dermatol Venereol. 2018;845 [Google Scholar]
- Ryan C., Sadlier M., De Vol E., Patel M., Lloyd A.A., Day A. Genital psoriasis is associated with significant impairment in quality of life and sexual functioning. J Am Acad Dermatol. 2015;72:97883. doi: 10.1016/j.jaad.2015.02.1127. [DOI] [PubMed] [Google Scholar]
- Salaffi F., Carotti M., Gasparini S., Intorcia M., Grassi W. The health-related quality of life in rheumatoid arthritis, ankylosing spondylitis, and psoriatic arthritis: A comparison with a selected sample of healthy people. Health Qual Life Outcomes. 2009;7:25. doi: 10.1186/1477-7525-7-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seeger J.D., Lanza L.L., West W.A., Fernandez C., Rivero E. Pregnancy and pregnancy outcome among women with inflammatory skin diseases. Dermatology. 2007;214:32–39. doi: 10.1159/000096910. [DOI] [PubMed] [Google Scholar]
- Singh S., Young P., Armstrong A.W. An update on psoriasis and metabolic syndrome: A meta-analysis of observational studies. PLoS One. 2017;12 doi: 10.1371/journal.pone.0181039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith C.H., Jabbar-Lopez Z.K., Yiu Z.Z., Bale T., Burden A.D., Coates L.C. British Association of Dermatologists guidelines for biologic therapy for psoriasis 2017. Br J Dermatol. 2017;177:628–636. doi: 10.1111/bjd.15665. [DOI] [PubMed] [Google Scholar]
- Smith C.J.F., Kavanaugh A., Chambers C.D. ACR/ARHP Annual Meeting. 2018. Birth outcomes and disease activity during pregnancy in a prospective cohort of women with psoriatic arthritis and ankylosing spondylitis; p. 2431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith J.A., Wehausen B., Richardson I., Zhao Y., Li Y., Herrera V. Treatment changes in patients with moderate to severe psoriasis: A retrospective chart review. J Cutan Med Surg. 2018;22:25–30. doi: 10.1177/1203475417724438. [DOI] [PubMed] [Google Scholar]
- Summer Laboratories . Summers Laboratories, Inc.; Collegeville, PA: 2006. Drithocreme [prescribing information] [Google Scholar]
- Swanbeck G., Inerot A., Martinsson T., Enerbäck C., Enlund F., Samuelsson L. Genetic counselling in psoriasis: Empirical data on psoriasis among first-degree relatives of 3095 psoriatic probands. Br J Dermatol. 1997;137:939–942. [PubMed] [Google Scholar]
- Tauscher A.E., Fleischer A.B., Jr., Phelps K.C., Feldman S.R. Psoriasis and pregnancy. J Cutan Med Surg. 2002;6:561–570. doi: 10.1007/s10227-001-0147-1. [DOI] [PubMed] [Google Scholar]
- Allergan . Irvine, CA; 2017. Tazorac. [prescribing information] [Google Scholar]
- U.S. Food and Drug Administration . 2008. Adalimumab (Humira) prescribing information [Internet] [cited 2018 September 19]. Available from. [Google Scholar]
- U.S. Food and Drug Administration, Administration Drug. 2018. Certolizumab pegol (Cimzia) prescribing information [Internet]https://www.accessdata.fda.gov/drugsatfda_docs/label/2017/125160s270lbl.pdf [cited 2018 September 19] Available from. [Google Scholar]
- Ursin K., Lydersen S., Skomsvoll J.F., Wallenius M. Disease activity of psoriatic arthritis during and after pregnancy: A prospective multicenter study. Arthritis Care Res (Hoboken) 2018 doi: 10.1002/acr.23747. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- Wahl A., Loge J.H., Wiklund I., Hanestad B.R. The burden of psoriasis: A study concerning health-related quality of life among Norwegian adult patients with psoriasis compared with general population norms. J Am Acad Dermatol. 2000;43:803–808. doi: 10.1067/mjd.2000.107501. [DOI] [PubMed] [Google Scholar]
- Warren R.B., Reich K., Langley R.G., Strober B., Gladman D., Deodhar A. Secukinumab in pregnancy: outcomes in psoriasis, psoriatic arthritis and ankylosing spondylitis from the global safety database. Br J Dermatol. 2018;179:1205–1207. doi: 10.1111/bjd.16901. [DOI] [PubMed] [Google Scholar]
- World Health Organization Global report on psoriasis 2016 [Internet] 2016. http://apps.who.int/iris/bitstream/handle/10665/204417/9789241565189_eng.pdf;jsessionid=A6EEEE46D210EC79E4534EE8261ECB1F?sequence=1 [cited 2018 October 17]. Available from:
- Wu S., Cho E., Li W., Grodstein F., Qureshi A.A. Hormonal factors and risk of psoriasis in women: A cohort study. Acta Derm Venereol. 2016;96:927–931. doi: 10.2340/00015555-2312. [DOI] [PubMed] [Google Scholar]
- Yang Y.W., Chen C.S., Chen Y.H., Lin H.C. Psoriasis and pregnancy outcomes: a nationwide population-based study. J Am Acad Dermatol. 2011;64:71–77. doi: 10.1016/j.jaad.2010.02.005. [DOI] [PubMed] [Google Scholar]
- Zelikin A.N., Ehrhardt C., Healy A.M. Materials and methods for delivery of biological drugs. Nat Chem. 2016;8:997–1007. doi: 10.1038/nchem.2629. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary figures