Abstract
Preeclampsia (PE) is characterized by poor placentation, consequent on aberrant extravillous trophoblast (EVT) cell function during placental development. The SRC family of proteins is important during pregnancy, especially SRC-3, which regulates placental morphogenesis and embryo survival. Although SRC-3 expression in mouse trophoblast giant cells has been documented, its role in the functional regulation of extravillous trophoblasts and the development of PE remains unknown. This study found that SRC-3 expression was significantly lower in placentas from PE pregnancies as compared to uncomplicated pregnancies. Additionally, both CoCl2-mimicked hypoxia and suppression of endogenous SRC-3 expression by lentivirus short hairpin RNA attenuated the migration and invasion abilities of HTR-8/SVneo cells. Moreover, we demonstrated that SRC-3 physically interacts with AKT to regulate the migration and invasion of HTR-8 cells, via the AKT/mTOR pathway. We also found that the inhibition of HTR-8 cell migration and invasion by CoCl2-mimicked hypoxia was through the SRC-3/AKT/mTOR axis. Our findings indicate that, in early gestation, accumulation of HIF-1α inhibits the expression of SRC-3, which impairs extravillous trophoblastic invasion and migration by directly interacting with AKT. This potentially leads to insufficient uterine spiral artery remodeling and placental hypoperfusion, and thus the development of PE.
Subject terms: Non-coding RNAs, Reproductive disorders
Introduction
Preeclampsia (PE) is a serious pregnancy-specific complication that affects 3–8% of all pregnancies and is the leading cause of maternal and fetal morbidity/mortality1,2. It has long been believed that the development of PE stems from defective placentation, however, the precise etiology of PE remains poorly understood3,4. Trophoblast cells are at the interface between the embryonic and maternal vascular systems. The migration and invasion capacity of these cells play a crucial role in placentation, embryo implantation, and other important functions. Abnormalities in trophoblast cell function during placental development result in poor placentation and fetal growth restriction and are associated with PE5,6. In addition, accumulating evidence suggests that hypoxia is a critical event during the development of PE, one that is associated with abnormal differentiation of trophoblasts and increases in proinflammatory cytokine expression levels and oxidative stress7–9. Previous studies have shown that hypoxia leads to defective trophoblast invasion; this has been attributed to several factors, however, the underlying mechanism has yet to be fully elucidated10–13.
The p160 steroid receptor co-activator (SRC) family member SRC-3 (also known as NCOA3, AIB1, ACTR, pCIP, RAC3, and TRAM-1) is an oncogene that has been reported to be amplified and/or overexpressed in a variety of tumors, including ovarian cancer, esophageal cancer, colorectal cancer, and breast cancer14–16. Recently, studies have reported that SRC-3 participates in tumorigenesis by regulating the proliferation and invasion of cancer cells15,17. Animal studies have revealed that overexpression of SRC-3 in transgenic mice promotes the development of breast cancer18. Trophoblasts share a number of similarities with cancer cells and trophoblast tissue has been defined as a ‘pseudo-malignant’ or ‘physiological metastasis’; trophoblasts may thus be associated with similar expression patterns of SRC-319. Additionally, the SRC family (including SRC-1, SRC-2, and SRC-3) is expressed in the human placenta; as its expression initially increases following conception and continually increases during gestation, it is considered critical for maintaining pregnancy20. A previous study of SRC-3 knockout mice indicated that the loss of SRC-3 in mice placenta led to reduced fetal capillaries and maternal blood sinusoids in the labyrinth area of these mice as compared to wild-type mice21. Our previous work suggested that SRC-3 influences the migration and tube formation of endothelial cells, which is related to vascular endothelial dysfunction and recognized features of PE22. SRC-3 was also detected in trophoblast giant cells21, which are widely accepted as mediating the invasion of the endometrium during mice placentation23,24. However, the role of SRC-3 in the regulation of trophoblastic invasion and migration remains unknown.
In the present study, SRC-3-deficient trophoblast cells and a CoCl2-mimicked hypoxia model were used to investigate whether SRC-3 impacts the proliferation, migration, and invasion of trophoblast cells, as well as to determine the relevance of SRC-3 to the subsequent development of PE.
Results
Clinical characteristics
The clinical characteristics of the study subjects are shown in Table 1. The age and parity were similar between the PE and the uncomplicated pregnancy groups. Women suffering from PE had significantly higher antenatal body mass index (BMI), systolic blood pressure, diastolic blood pressure, and proteinuria, but mean gestational age at birth, neonatal birth weight, and placental weight were lower, as compared to uncomplicated pregnant women.
Table 1.
Clinical characteristics of the human subjects.
| Category | Control (n = 25) | Preeclampsia (n = 25) |
|---|---|---|
| Age (years) | 29.1 ± 2.83 | 29.4 ± 2.59 |
| Gestational age at birth (weeks) | 40.07 ± 0.44 | 36.86 ± 1.60*** |
| Body mass index (BMI; kg/m2) | 27.98 ± 1.42 | 30.33 ± 2.31*** |
| Gravidity | 1.90 ± 0.68 | 1.95 ± 0.58 |
| Proteinuria (g/24 h) | 0.05 ± 0.01 | 2.69 ± 0.07*** |
| Systolic blood pressure (mmHg) | 110.8 ± 6.65 | 158.5 ± 8.67*** |
| Diastolic blood pressure (mmHg) | 73.7 ± 7.25 | 106.4 ± 8.24*** |
| Neonatal birth weight (g) | 3402 ± 313.53 | 2640 ± 121.52*** |
| Neonatal birth length (cm) | 50.01 ± 0.99 | 47.24 ± 0.92*** |
| Placental weight (g) | 555.5 ± 28.37 | 473.4 ± 25.44*** |
Body mass index (BMI) formula: weight (kg)/height2 (m2).
*p < 0.05, **p < 0.01, ***p < 0.001.
SRC-3/p-AKT/p-mTOR expression is downregulated in PE human placentas
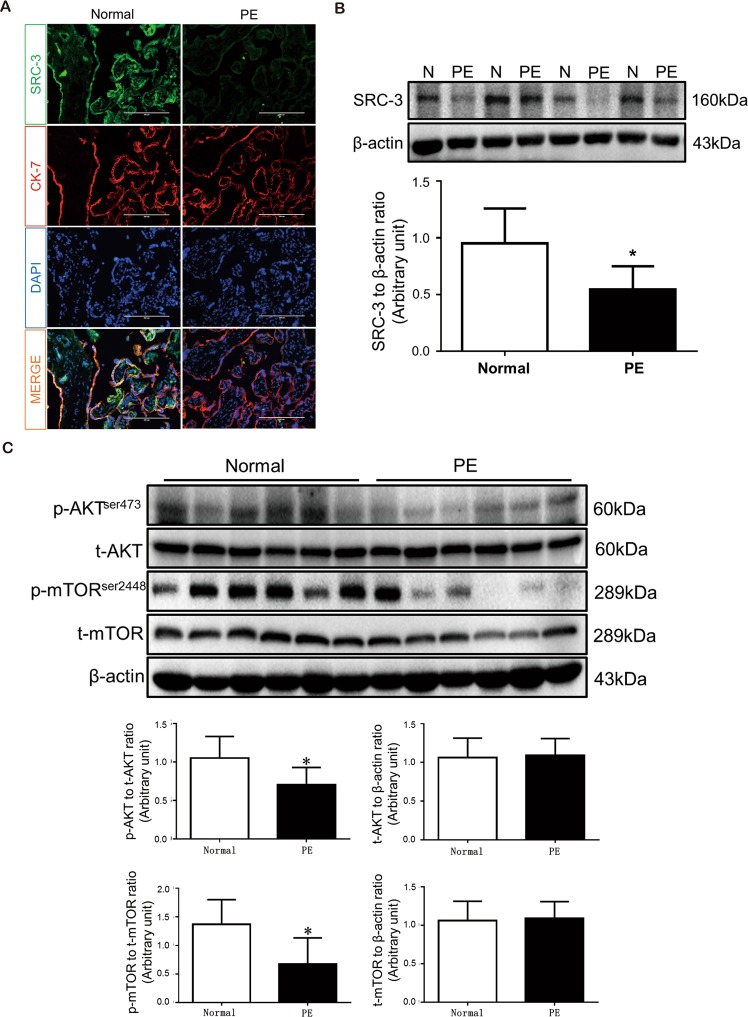
To determine the involvement of SRC-3 in PE development, we first determined the expression pattern of SRC-3 in human placentas by immunofluorescence (IF) staining. As shown in Fig. 1A, SRC-3 was ubiquitously expressed in placental tissue and was primarily found in trophoblasts. Intriguingly, the expression level of SRC-3 was significantly lower in PE placentas than in placentas from uncomplicated pregnancies. Western blotting demonstrated that SRC-3 protein levels were reduced by 43% in PE placentas (Fig. 1B), while the phosphorylation levels of AKT and mTOR were also significantly reduced in PE human placentas as compared to placentas from uncomplicated pregnancies (Fig. 1C).
Figure 1.
SRC-3 expression pattern and AKT/mTOR signaling pathway component expression in normal and PE human placentas. (A) IF staining of SRC-3 (green) and CK7 (red) in frozen sections of human term-placentas; nuclei were counterstained by DAPI (blue). Scale bars: 200 μm. (B) Western blots of SRC-3 in human term-placentas, n = 5, *p < 0.05. (C) Western blots of AKT, p-AKT, mTOR, and p-mTOR protein expression in human term-placentas. β-actin served as a loading control, n = 6, *p < 0.05. All experiments were repeated at least three times.
Inhibition of SRC-3 expression does not alter trophoblast viability
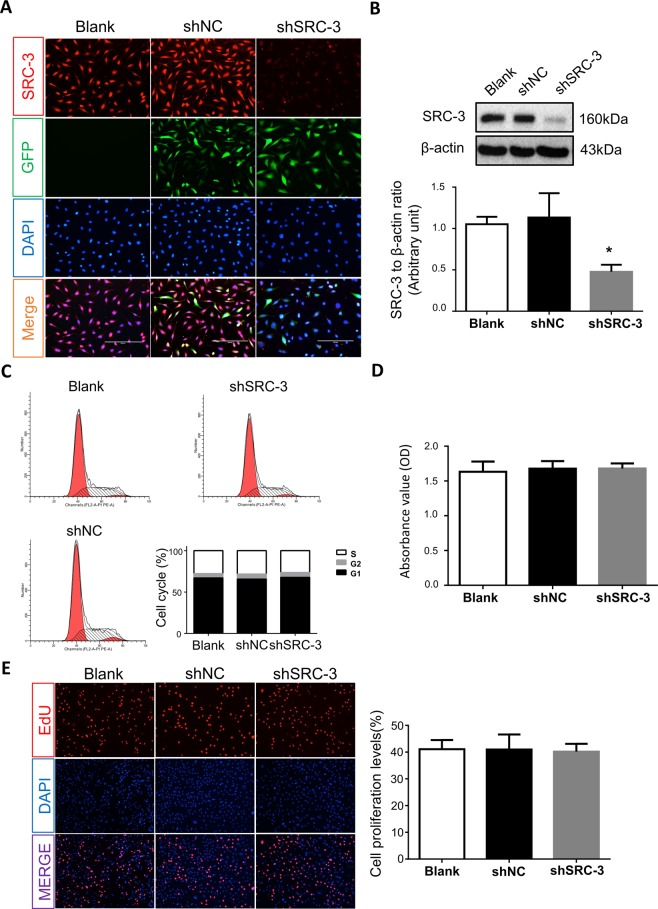
To further investigate the role of SRC-3 in trophoblast cells, SRC-3 expression levels in human HTR-8/SVneo trophoblast cells were reduced by short hairpin RNA (shRNA) transfection. IF staining demonstrated that SRC-3 levels in HTR-8/SVneo cells were markedly reduced by shSRC-3 transfection (Fig. 2A), and Western blotting confirmed that SRC-3 expression was reduced by nearly 50% (Fig. 2B).
Figure 2.
SRC-3 knockdown in HTR-8/SVneo cells. Wild-type, GFP-labeled shSRC-3, or shNC-transfected HTR-8/SVneo cells were subjected to: (A) IF staining for SRC-3 (red); transfected cells were recognized by GFP staining (green), while nuclei were counterstained with DAPI (blue). (B) SRC-3 protein expression levels were confirmed by western blot analysis; β-actin served as a loading control, n = 3, *p < 0.05; Cell proliferation was assessed by (C) flow cytometry, (D) CCK-8 staining, and (E) EdU staining. All experiments were repeated three times.
Since SRC-3 has been reported to be involved in the regulation of cell growth and proliferation in various cancer cells14,15,25, we next assessed the effects of SRC-3 on trophoblast proliferation. Flow cytometry analysis revealed that downregulation of SRC-3 by shRNA did not result in cell-cycle arrest in HTR8/SVneo cells (Fig. 2C). Consistent with this finding, neither CCK-8 nor EdU staining assays demonstrated that the proliferation of trophoblasts was influenced by SRC-3 inhibition (Fig. 2D,E), which implies that SRC-3 may not be critical for DNA replication in HTR8/SVneo cells. SRC-3-knockdown (KD) cells demonstrated comparable proliferation rates to blank control and scramble shRNA (shNC) transfected cells (Fig. 2C), further indicating that SRC-3 is not a determinant of proliferation in trophoblasts.
SRC-3 regulates migration and invasion of HTR8/SVneo cells through the AKT/mTOR signaling pathway
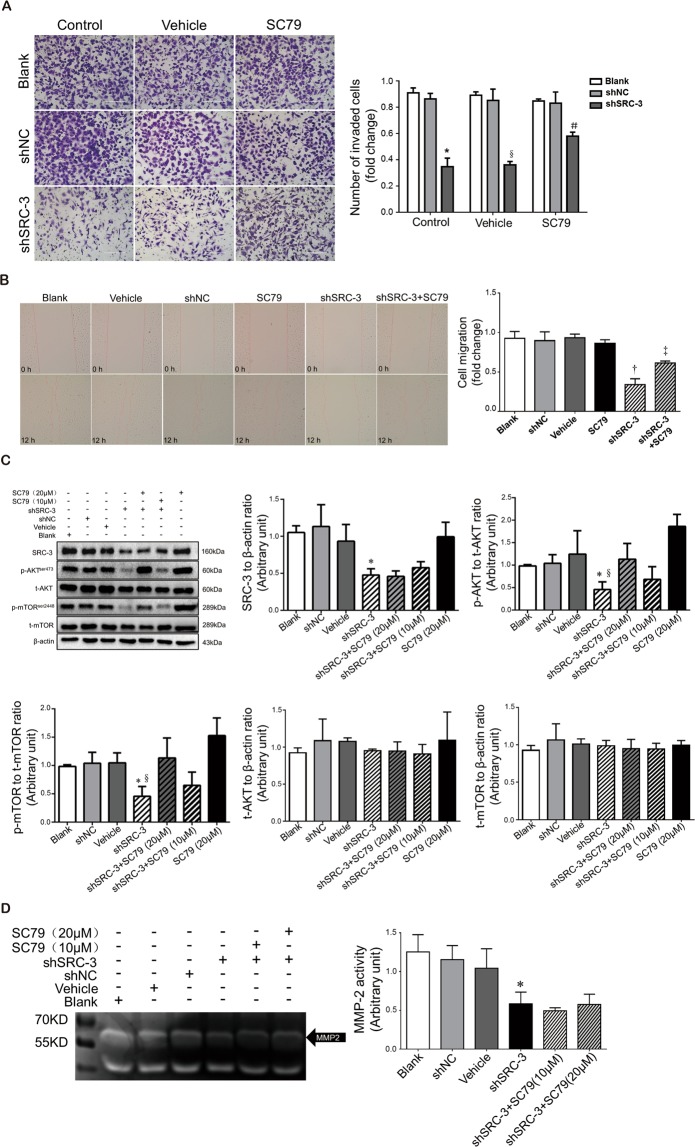
Migration and invasion of trophoblast cells play a crucial role in placentation, and downregulation of AKT/mTOR signaling has been correlated with cancer cell migration and invasion26–28. We thus evaluated the role of SRC-3 in modulating the migratory and invasive capabilities of trophoblast cells. Matrigel-based invasion assays demonstrated that inhibition of SRC-3 expression significantly diminished the invasiveness of HTR8/SVneo cells (Fig. 3A); the loss of invasiveness was largely rescued following treatment with SC79, an AKT activator. Similarly, wound-healing experiments determined that the migratory capability of trophoblast cells was suppressed in the presence of shSRC-3 (Fig. 3B) but could be enhanced by SC79 treatment. Western blotting results showed that the knockdown of SRC-3 significantly reduced p-AKT and p-mTOR levels, while the activation of AKT and mTOR was restored by SC79 treatment in a dose-dependent manner (Fig. 3C). Furthermore, gelatin zymography illustrated that SRC-3 interference significantly decreased MMP-2 activity in HTR8/SVneo cells; the loss in MMP-2 activity could not be restored by either low- or high-dose SC79 treatment (Fig. 3D). Taken together, these results indicate that SRC-3 regulates the migration and invasion of HTR8/SVneo cells via the AKT/mTOR pathway, independent of MMP-2.
Figure 3.
Downregulation of SRC-3 expression suppresses the migration and invasion of HTR8/SVneo cells through the AKT/mTOR signaling pathway. Non-transfected and shNC- or shSRC-3-transfected HTR8/SVneo cells were treated with 20 μM SC79 or 0.1% DMSO (vehicle control) and subjected to: (A) Matrigel transwell assays. Invaded cells were stained and counted after 24 h. n = 3, *p < 0.001 vs. Blank control and shNC control, §p < 0.001 vs. Blank vehicle & shNC vehicle, #p < 0.01 vs. shSRC-3 control & shSRC-3 vehicle. Scale bars: 200 μm. (B) Wound-healing assays. Images were taken at 0 h and 12 h of treatment. Quantification of the areas of migration is shown in the bar graph. n = 3, †p < 0.001 vs. Blank & shNC, ‡p < 0.001 vs. shSRC-3. (C) Western blotting of SRC-3, AKT, p-AKT, mTOR, and p-mTOR in the aforementioned groups of cells, and a lower dose of SC79 (10 μM) treatment group was added, n = 3, *p < 0.01 vs. shNC, §p < 0.01 vs. shSRC-3 + SC79 (20 μM). (D) Gelatin zymography of MMP-2 activity in the culture medium of cells in the presence of vehicle (0.1% DMSO), 10 μM SC79, or 20 μM SC79 over 24 h, n = 3, *p < 0.05 vs. shSRC-3. Scale bars: 400 μm (D). All experiments were repeated three times.
SRC-3 physically interacts with AKT in HTR-8/SVneo
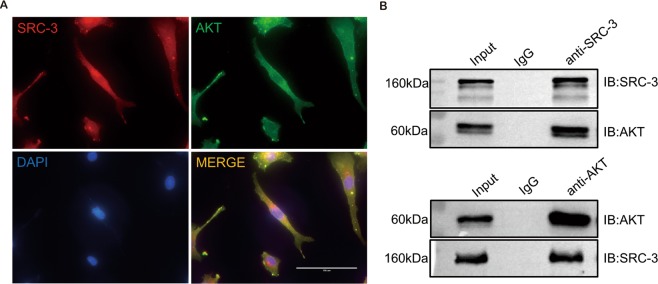
The family of SRC proteins contain a bHLH/PAS domain, which is normally involved in protein–protein interactions, indicating that SRC proteins may regulate downstream effectors through physical interactions. Indeed, it has been reported that SRC-3 binds the C-terminal activation domain of myocardin and enhances myocardin-mediated transcriptional activation of its downstream factors29,30. Therefore, we next investigated whether the regulatory effects of SRC-3 on trophoblast cell invasion are mediated by direct interactions between SRC-3 and AKT. First, IF staining showed that AKT was ubiquitously expressed in HTR8/SVneo cells, whereas SRC-3 was predominantly localized in the nucleus (Fig. 4A). The co-localization of SRC-3 and AKT in the nucleus suggests that SRC-3 might bind to AKT and thus modulate the transcriptional activity of AKT in trophoblast cells. To further validate the putative physical interaction between SRC-3 and AKT, reciprocal co-immunoprecipitation (IP) experiments were performed; the results clearly show that SRC-3 binds to AKT in trophoblasts (Fig. 4B).
Figure 4.
SRC-3 directly interacts with AKT in HTR-8/SVneo cells. (A) Representative images of IF staining of SRC-3 (red) and AKT (green) in HTR-8/SVneo cells. Nuclei were counterstained with DAPI (blue). Scale bars: 100 μm. (B) Co-IP of AKT with SRC-3 in HTR8/SVneo cells. All experiments were repeated three times.
Hypoxia suppresses the migration and invasion of HTR8/SVneo cells through inhibition of the SRC-3/AKT/mTOR signaling axis
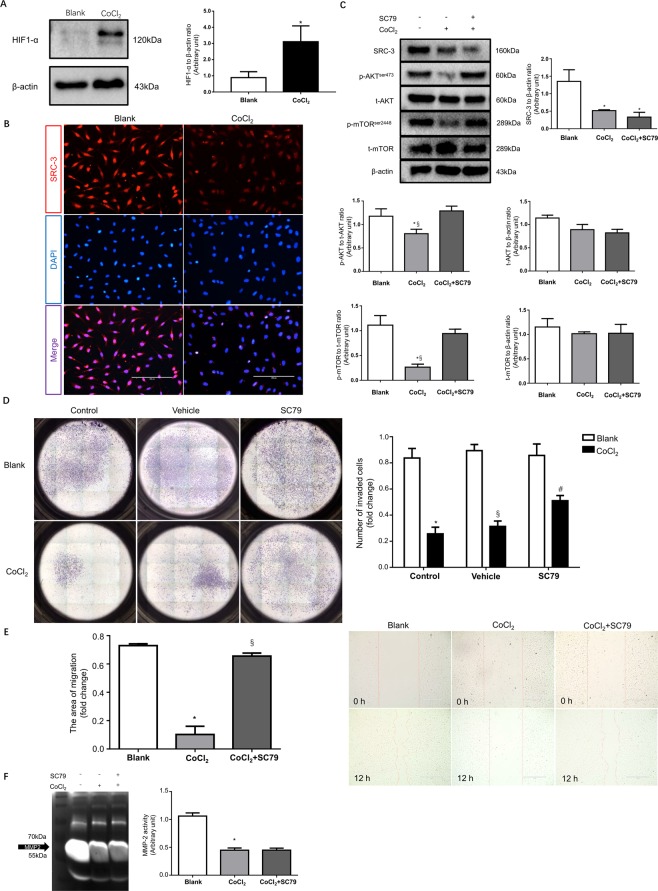
To explore the mechanism underlying SRC-3 downregulation in PE placenta, HTR8/SVneo cells were subjected to cobalt chloride (CoCl2) treatment to establish an in vitro trophoblast hypoxia model. The expression of HIF-1 was significantly elevated by CoCl2, confirming that a hypoxia response had been induced in HTR8/SVneo cells (Fig. 5A). Subsequently, IF staining showed that CoCl2-mimicked hypoxia dramatically attenuated the expression of SRC-3 in HTR8/SVneo cells (Fig. 5B); this was further validated by Western blotting (Fig. 5C). In addition, CoCl2 treatment significantly decreased the phosphorylation of AKT and mTOR in trophoblasts, but it did not change the total protein expression levels of AKT and mTOR (Fig. 5C). Although SC79 treatment almost fully alleviated the dephosphorylation of AKT and mTOR induced by CoCl2, it failed to rescue SRC-3 expression in trophoblasts. These results indicate that hypoxia impairs SRC-3 expression in trophoblasts, which subsequently results in the inhibition of the AKT/mTOR pathway. Furthermore, CoCl2 treatment significantly suppressed the invasion of HTR8/SVneo cells; this suppression was blunted in the presence of SC79 (Fig. 5D). Similarly, trophoblasts treated with CoCl2 were associated with a reduction in migration, which could be blocked by SC79 treatment (Fig. 5E). However, SC79 treatment failed to rescue MMP-2 inhibition in HTR-8 cells resulting from CoCl2 treatment (Fig. 5F). These findings are consistent with our observations in PE placentas, and suggest that CoCl2-induced downregulation of SRC-3 compromises trophoblastic invasion and migration through the AKT/mTOR signaling pathway independent of MMP-2.
Figure 5.
Hypoxia impairs the migration and invasion of HTR8/SVneo cells through inhibition of the SRC-3/AKT/mTOR signaling axis. After 48 h of 250 μM CoCl2 treatment, HTR8/SVneo cells were subjected to: (A) Western blot analysis of HIF1-α. (B) IF staining of SRC-3, scale bar: 200 μm. (C) Western blot analysis of SRC-3, AKT, p-AKT, mTOR, and p-mTOR levels in HTR8/SVneo cells in the presence of 250 μM CoCl2 and/or 20 μM SC79. The blank control was also included, n = 3, *p < 0.05 vs. Blank, §p < 0.05 vs. CoCl2 + SC79. (D) HTR8/SVneo cells subjected to Matrigel transwell assays in the presence of vehicle (0.1% DMSO), 250 μM CoCl2, and/or 20 μM SC79. Invaded cells were counted after 24 h. n = 3, *p < 0.001 vs. Blank control, §p < 0.001 vs. Blank vehicle, #p < 0.01 vs. CoCl2 control. (E) Wound-healing assays for HTR8/SVneo cells in the presence of 250 μM CoCl2 and/or 20 μM SC79. Images were taken at 0 h and after 12 h of treatment. The areas of migration were quantified in the bar graph. n = 3, *p < 0.001 vs. Blank, §p < 0.001 vs. CoCl2. (F) Gelatin zymography of MMP-2 activity in culture medium of HTR8/SVneo cells in the presence of 250 μM CoCl2 and/or 20 μM SC79, *p < 0.001 vs. Blank. All experiments were repeated three times.
Discussion
SRC-3 has been intensively studied in several malignant tumors and is thought to be an oncogene that promotes carcinogenesis15,31. Since first trimester trophoblasts show considerable similarities to malignant cells, they have been suggested to utilize similar mechanisms for the regulation of proliferation, migration, and invasion32. Accumulating evidence has suggested that SRC-3 might play a pivotal role in maintaining pregnancy20,21. In this study, we demonstrated that PE placentas express lower levels of SRC-3 than placentas from uncomplicated pregnancies, which is consistent with a prior report22 and potentially implicates a loss of placental SRC-3 expression in the development of PE. It has been shown that SRC-3-knockout mice have smaller sinusoids in the labyrinth area21. In contrast, upregulation of SRC-3 promotes the proliferation and growth of tumor tissues in a broad spectrum of cancers15,29.
We utilized multiple approaches to evaluate the effects of SRC-3 on trophoblast proliferation. No marked differences were found between SRC-3-knockdown and wild-type HTR8/SVneo cells, despite AKT/mTOR being downregulated by knockdown of SRC-3. There are extensive cross-talk pathways between the PI3K/AKT/mTOR and the ERK-MAPK signaling pathways33,34. When any of these pathways are blocked, cross-inhibition and activation of compensatory signaling often occurs35–37. For example, it has been shown that blocking each pathway alone is less efficient in suppressing cancer cell proliferation than the combined inhibition of PI3K and MAPK33,35,38. Further investigation into the effects of SRC-3 on ERK signaling will be helpful to fully elucidate the role of SRC-3 in modulating the proliferation and viability of trophoblast cells.
SRC-3 has been reported to regulate cell invasion15,16, and as expected, repression of SRC-3 expression coincided with reduced invasion and migration of trophoblast cells. Migration- and invasion-related extracellular matrix (ECM) degradation requires the action of MMP-239,40, and MMP-2 is regulated by PI3K/AKT signaling26,27. Therefore, we further studied whether the SRC-3-AKT signaling axis regulates trophoblast migration by modulating MMP-2 activity. MMP-2 activity was reduced in SRC-3-knockdown cells. Previous studies have further suggested that SRC-3 could be an upstream regulator of the PI3K signaling pathway18,41,42. Our data confirmed that SRC-3 expression is positively correlated with AKT and mTOR phosphorylation. Intriguingly, although SC79 treatment restored the p-mTOR levels, as well as the invasion and migration capabilities of SRC-3-KD trophoblast cells, it did not rescue MMP-2 activity, suggesting that the regulation of trophoblastic invasion and migration by the SRC-3-AKT-mTOR signaling axis is not mediated by MMP-2. However, SRC-3 is a polyomavirus enhancer activator 3 (PEA3) coactivator and in breast cancer cells SRC-3 forms complexes with PEA3 on MMP-2 promoters to enhance its expression43. Further studies in trophoblasts are required.
Since the SRC family contains a bHLH/PAS domain that mediates protein–protein interactions29,30, we speculated that SRC-3 might directly interact with AKT and regulate its activation in trophoblast cells. Indeed, our results are the first to confirm that SRC-3 binds to AKT; this may be critical for SRC-3/AKT signal transduction and the subsequent regulation of trophoblastic function.
In early gestation, trophoblast cells have to be resistant to a low oxygen intrauterine environment44–46. However, in placentas of pregnancies complicated by preeclampsia, low oxygen triggers a hypoxic response, which leads to an elevation of HIF-1α, a consequent reduction in trophoblast invasion, and placental hypoperfusion. The accumulation of HIF-1α in PE trophoblasts may be caused by various possible mechanisms, including an insufficient increase in placental oxygen tension after the 12th week of gestation, catechol-O-methyl transferase (COMT) deficiency47,48, decreased expression of prolyl hydroxylase 2 (PHD-2), and/or other inhibitory regulators of HIF-149–51.
We established a CoCl2-mimicked hypoxic trophoblast model and found that hypoxia reduced SRC-3 expression. Interestingly, although both hypoxia and SRC-3 knockdown lead to MMP-2 inhibition, the impaired trophoblastic invasion and migration due to hypoxia-induced SRC-3 downregulation is mediated by the AKT-mTOR pathway independent of MMP-2 activity. SRC-3 was initially discovered as a steroid receptor co-activator that could mediate chromatin remodeling and enhance receptor-dependent transcription by recruiting additional factors, such as acetyltransferases (e.g., CBP and p300) and methyltransferases (e.g., CARM1 and PRMT1)52. Thus, the involvement of these molecules in SRC-3-regulated trophoblastic function is worthy of further investigation.
Chen et al. demonstrated that in addition to trophoblast giant cells, SRC-3 is also highly expressed in layer III syncytiotrophoblasts, which are responsible for fetal-maternal nutrient exchange in the labyrinth21. As a result, SRC-3-KO mice are associated with a small labyrinth, reduced fetal vascular density, and dilated maternal blood sinuses21. In human placentas, villous trophoblast malfunction and deficient fetoplacental angiogenesis are associated with PE53–55. In addition to regulating extravillous trophoblast (EVT) invasion, SRC-3 may contribute to the development of PE by interfering with villi branching. Further investigation into both mechanisms could aid understanding of the role of SRC-3 in PE.
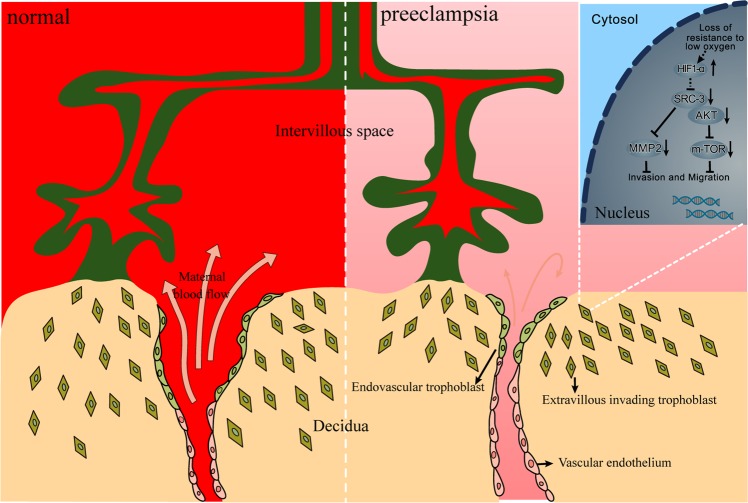
In conclusion, SRC-3 binds to AKT to activate the AKT/mTOR signaling pathway in EVT, which regulates cell migration and invasion independent of MMP-2 activity. Hypoxic response-induced SRC-3 downregulation may be an important pathologic mechanism underlying PE (Fig. 6). Our findings provide new insights into the etiology of PE and suggest potential targets for therapeutic intervention.
Figure 6.
A working model of SRC-3/AKT/mTOR signaling in the regulation of trophoblastic invasion and PE development.
Materials and Methods
Patients and tissue sample collection
25 women whose pregnancies were complicated by PE and 25 normal pregnant women admitted to the Department of Obstetrics at The First Affiliated Hospital of Chongqing Medical University were recruited. PE was diagnosed according to the criteria of the American College of Obstetrics and Gynecology. Exclusion criteria included the presence of chronic hypertension, diabetes mellitus, renal diseases, or other metabolic diseases. This study was in accordance with the principles set out in the Declaration of Helsinki and was approved by the Ethics Committee of the First Affiliated Hospital of Chongqing Medical University. All participants provided informed consent. Placental tissues were collected immediately after cesarean section, washed in ice-cold 0.9% saline, and stored at −80 °C until further processing. Tissue collection processes and preparation for immunofluorescence were performed as described previously22.
Cell culture and hypoxia treatment
HTR8/SVneo cells were kindly provided by Dr. Charles H. Graham (Kingston, ON, Canada). The HTR8/SVneo cells were maintained in RPMI-1640 (Gibco, Carlsbad, California, USA) supplemented with 10% fetal bovine serum (FBS; Gibco), at 37 °C in a humidified atmosphere with 5% CO2. To mimic hypoxia in trophoblasts, cells were treated with CoCl2 at a concentration of 250 µM for 48 h56,57. Cells were harvested using a commercial lysis buffer (Beyotime, Jiangsu, China), while culture medium was collected for zymography assays.
SRC-3 knockdown by lentiviral shRNA
The target sequence of SRC-3 for constructing lentiviral shRNA was: shSRC-3: 5′-AGACTCCTTAGGACCGCTT-3′, shNC: 5′-TTCTCCGAACGTGTCACGT-3′. HTR8/SVneo cells were transfected with shRNA and shNC (GenePharma, Shanghai, China), according to the manufacturer’s protocol. At 24 h after transfection, 5 µg/ml puromycin was added for three days to screen SRC-3-KD cells.
Immunofluorescence
Immunostaining was performed on frozen sections of human term placentas and HTR8/SVneo cells as described previously22. Briefly, the placental frozen sections and trophoblast cells were permeabilized with 0.2% Triton X-100 (Beyotime) and blocked with 1% bovine serum albumin (BSA) (Beyotime). The tissues and cells were then incubated with a primary antibody against SRC-3 (1:100, #5765), cytokeratin-7 (CK-7) (1:100, #4465) (Cell Signaling Technology, Danvers, MA, USA) at 4 °C overnight followed by incubation with the appropriate secondary antibody for 2 h (Santa Cruz Biotechnology, Dallas, TX, USA). The nuclei were counterstained by 4′, 6-diamidino-2-phenylindole (DAPI; Beyotime). Images were captured using a fluorescence microscope (Life Technologies, Waltham, MA, USA).
Western blotting
Total protein in placental tissues or HTR8/SVneo cells were extracted with RIPA lysis buffer (Beyotime) and measured by bicinchoninic acid (BCA) protein assays (Beyotime). Twenty micrograms of total protein extracted from placental tissue or cell lysates were resolved by sodium dodecyl sulfate (SDS)-polyacrylamide gel electrophoresis followed by transfer to polyvinylidene difluoride (PVDF) membranes (Roche, Germany). These were further incubated with primary antibodies against SRC-3 (1:1000, #5765), p-AKT (Ser473) (1:1000, #9271), AKT (1:1000, #9272), mTOR (1:1000, #2983), or β-actin (1:1000, #3700), purchased from Cell Signaling Technology), or a primary antibody against p-mTOR (Ser2448) (1:1000; ab109268) purchased from Abcam (Cambridge, MA, USA). Subsequently, the membranes were incubated with a secondary antibody (1:5000) linked to horseradish peroxidase (Santa Cruz Biotechnology) and then enhanced chemiluminescence detection reagents (Millipore, Danvers, MA, USA) to detect the protein of interest. Identified bands were analyzed with a ChemiDoc image analyzer (Bio-Rad, Hercules, CA, USA).
In vitro cell migration and invasion assays
Invasion assays were performed in 24-well plates with Matrigel (BD Biosciences, USA)-coated Trans well inserts (8.0 μm, Merck Millipore, Darmstadt, Germany) as described previously58, with the exception that 8 × 104 cells were plated in the upper chamber of the membrane in these experiments.
The migratory ability of the HTR8/SVneo cells was determined by wound healing assay. In brief, cells were seeded in a 6-well plate and cultured to 80% confluence, and then a scratch on the cell monolayer was made using a pipette tip. The cells were then rinsed twice with fresh culture medium and allowed to stay in culture for another 24 h. Five fields were randomly selected and imaged at the beginning (0 h) and the end of the culture period (24 h). The number of invaded and migrated cells was counted under a microscope. All experiments were performed in triplicate.
CCK-8 proliferation assay
Proliferation of HTR8/SVneo cells was measured with the use of a CCK-8 (Biotool, Houston, TX, USA) according to the manufacturer’s instructions. Briefly, 5 × 103 cells were seeded in 96-well plates that contained RPMI-1640 and 10% FBS. Cell proliferation was examined 3 h after standard procedures. The absorbance at 450 nm was measured by a microplate reader (Thermo Fisher Scientific, Waltham, MA, USA). All experiments were repeated three times.
Cell-cycle analysis
Several pretreated cells were seeded in a 6-well plate at a density of 8 × 105 HTR8/SVneo cells per well. These cells were subsequently detached using trypsin and fixed with ice-cold 70% ethanol. The cells were then stained for cell-cycle analysis using a Coulter DNA-Prep Reagents Kit (Beckman Coulter, UK). Cellular DNA levels from each sample were determined with a FACScan flow cytometer (CytoFLEX, Beckman, China). All experiments were performed in triplicate.
EdU staining
A total of 5 × 103 HTR8/SVneo cells were seeded in 96-well plates and cultured to 80% confluence. Cells were treated with 25 µM of 5-ethynyl-2′-deoxyuridine (Cell-Light™ EdU Apollo®567 In Vitro Imaging Kit; Ribobio, Guangzhou, China) for 2 h at 37 °C, and then cells were fixed in 4% paraformaldehyde (PFA). Subsequently, the cells were permeabilized in 0.5% Triton-X for 10 min and exposed to 1X Apollo reaction cocktail (Cell-Light™ EdU Apollo®567 In Vitro Imaging Kit; Ribobio) for 30 min. Finally, cell nuclei were counterstained with Hoechst 33342 for 30 min and visualized with a fluorescence microscope (Life Technologies).
Gelatin zymography
The culture medium from various pre-treated HTR8/SVneo cells was diluted with 4X sample buffer (8% SDS (w/v), 0.04% bromophenol blue (w/v), 0.25 M Tris), and incubated at 37 °C for 30 min. Equal amounts of protein were subjected to electrophoresis. Subsequently, renaturation buffer (2.5% Triton X-100 and 50 mM Tris–HCl (pH 7.5)) was used to wash the gels for 30 min at room temperature twice, followed by incubating the gels in a calcium assay buffer (50 mM Tris, 10 mM CaCl2, 1 mM ZnCl2, 1% Triton X-100, pH 7.5) at 37 °C overnight. Next, the gels were stained with Coomassie Brilliant Blue R250 at room temperature for 1 h and de-stained in 10% acetic acid (v/v). Finally, the gels were scanned by an image analyzer, the Quantity One System (Bio-Rad).
Co-immunoprecipitation (Co-IP)
Co-IPs were performed according to a standard protocol using a Pierce co-IP Kit (ThermoFisher Scientific). Briefly, differently pre-treated cells were harvested in ice-cold IP Lysis/Wash Buffer, before centrifugation at 13 000 × g for 10 min to pellet the cell debris. Then, the IP was performed by the addition of antibodies to cell lysates: rabbit anti-AKT antibody (Cell Signaling Technology) to IP the SRC-3 protein, and rabbit anti-SRC-3 antibody (Cell Signaling Technology) to IP the AKT protein. All co-IP steps were performed at 4 °C unless otherwise indicated. Subsequently, protein A/G beads (Thermo Fisher Scientific) were added for an additional 2 h. The immunoprecipitated proteins were washed five times with IP Lysis/Wash Buffer. Finally, proteins were resolved by SDS/PAGE and immunoblotted with antibodies as indicated.
Statistical analysis
All values are expressed as means ± standard deviation (SD). The data were analyzed using GraphPad Prism software (version 6.0, California, USA). Independent t-tests were used for intergroup comparisons of continuous variables. Statistical differences among multiple groups were evaluated by one-way analysis of variance (ANOVA), followed by the least significant difference multiple-comparisons test, as appropriate. P-values < 0.05 were considered to be statistically significant.
Supplementary information
Acknowledgements
This work was supported by grants from Chinese Ministry of Science and Technology (2018YFC1004103; 2016YFC1000407), National Natural Sciences Foundation of China (81671488, 81871189; 81771613, 81520108013; 81601304), Chongqing Municipal Education Commission (CXTDX201601014), Science and Technology Commission of Chongqing (cstc2017jcyjBX0045), and Chongqing Entrepreneurship and Innovation Supporting Program for Returned Overseas Students (cx2017104). We would like to acknowledge the support from “111 program” of Ministry of Education P.R.C and State Administration of Foreign Experts Affairs P.R.C.
Author Contributions
C.T., P.B., H.Q., and N.S. conceived and designed the experiments; C.H., P.X., H.G., Y.Y., Y.L., P.Z., L.W., F.Z., and L.X. performed the experiments and collected data; C.H., C.T., and C.P. analyzed the data and prepared the figures, C.T., H.Q., and N.S. provided funding resource; C.H. and C.T. wrote the paper.
Data Availability
Supporting data and essential materials for reproducibility of this study are available upon request made to the corresponding authors.
Competing Interests
The authors declare no competing interests.
Footnotes
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Chengjin He and Nan Shan contributed equally.
Contributor Information
Hongbo Qi, Email: qihongbo728@163.com.
Chao Tong, Email: chaotongcqmu@163.com.
Supplementary information
Supplementary information accompanies this paper at 10.1038/s41598-019-46699-3.
References
- 1.Abalos E, Cuesta C, Grosso AL, Chou D, Say L. Global and regional estimates of preeclampsia and eclampsia: a systematic review. European journal of obstetrics, gynecology, and reproductive biology. 2013;170:1–7. doi: 10.1016/j.ejogrb.2013.05.005. [DOI] [PubMed] [Google Scholar]
- 2.Steegers EAP, von Dadelszen P, Duvekot JJ, Pijnenborg R. Pre-eclampsia. The Lancet. 2010;376:631–644. doi: 10.1016/s0140-6736(10)60279-6. [DOI] [PubMed] [Google Scholar]
- 3.McMaster MT, Zhou Y, Fisher SJ. Abnormal placentation and the syndrome of preeclampsia. Seminars in Nephrology. 2004;24:540–547. doi: 10.1016/j.semnephrol.2004.07.002. [DOI] [PubMed] [Google Scholar]
- 4.Cheng MH, Wang PH. Placentation abnormalities in the pathophysiology of preeclampsia. Expert review of molecular diagnostics. 2009;9:37–49. doi: 10.1586/14737159.9.1.37. [DOI] [PubMed] [Google Scholar]
- 5.Hamilton WJ, Boyd JD. Development of the human placenta in the first three months of gestation. Journal of anatomy. 1960;94:297–328. [PMC free article] [PubMed] [Google Scholar]
- 6.Hamilton WJ, Boyd JD. Trophoblast in human utero-placental arteries. Nature. 1966;212:906–908. doi: 10.1038/212906a0. [DOI] [PubMed] [Google Scholar]
- 7.Tache V, et al. Hypoxia and trophoblast differentiation: a key role for PPARgamma. Stem cells and development. 2013;22:2815–2824. doi: 10.1089/scd.2012.0596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Many A, Hubel CA, Fisher SJ, Roberts JM, Zhou Y. Invasive Cytotrophoblasts Manifest Evidence of Oxidative Stress in Preeclampsia. The American Journal of Pathology. 2000;156:321–331. doi: 10.1016/s0002-9440(10)64733-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jauniaux E, Hempstock J, Greenwold N, Burton GJ. Trophoblastic Oxidative Stress in Relation to Temporal and Regional Differences in Maternal Placental Blood Flow in Normal and Abnormal Early Pregnancies. The American Journal of Pathology. 2003;162:115–125. doi: 10.1016/s0002-9440(10)63803-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhu J, et al. Hypoxia-induced TET1 facilitates trophoblast cell migration and invasion through HIF1alpha signaling pathway. Scientific reports. 2017;7:8077. doi: 10.1038/s41598-017-07560-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fang M, et al. Hypoxia-inducible microRNA-218 inhibits trophoblast invasion by targeting LASP1: Implications for preeclampsia development. The international journal of biochemistry & cell biology. 2017;87:95–103. doi: 10.1016/j.biocel.2017.04.005. [DOI] [PubMed] [Google Scholar]
- 12.Nakashima A, et al. Impaired autophagy by soluble endoglin, under physiological hypoxia in early pregnant period, is involved in poor placentation in preeclampsia. Autophagy. 2013;9:303–316. doi: 10.4161/auto.22927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Soleymanlou N, et al. Molecular evidence of placental hypoxia in preeclampsia. The Journal of clinical endocrinology and metabolism. 2005;90:4299–4308. doi: 10.1210/jc.2005-0078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tong ZT, et al. AIB1 predicts bladder cancer outcome and promotes bladder cancer cell proliferation through AKT and E2F1. British journal of cancer. 2013;108:1470–1479. doi: 10.1038/bjc.2013.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Xu FP, et al. Overexpression of SRC-3 promotes esophageal squamous cell carcinoma aggressiveness by enhancing cell growth and invasiveness. Cancer medicine. 2016;5:3500–3511. doi: 10.1002/cam4.884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yoshida H, et al. Steroid receptor coactivator-3, a homolog of Taiman that controls cell migration in the Drosophila ovary, regulates migration of human ovarian cancer cells. Molecular and cellular endocrinology. 2005;245:77–85. doi: 10.1016/j.mce.2005.10.008. [DOI] [PubMed] [Google Scholar]
- 17.Horiguchi K, Arai S, Nishihara T, Nishikawa J. AIB1 promotes DNA replication by JNK repression and AKT activation during cellular stress. Journal of biochemistry. 2006;140:409–419. doi: 10.1093/jb/mvj167. [DOI] [PubMed] [Google Scholar]
- 18.Torres-Arzayus MI, et al. High tumor incidence and activation of the PI3K/AKT pathway in transgenic mice define AIB1 as an oncogene. Cancer cell. 2004;6:263–274. doi: 10.1016/j.ccr.2004.06.027. [DOI] [PubMed] [Google Scholar]
- 19.Zhou Y, et al. Human cytotrophoblasts adopt a vascular phenotype as they differentiate. A strategy for successful endovascular invasion? The Journal of clinical investigation. 1997;99:2139–2151. doi: 10.1172/JCI119387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kim SC, Park MN, Lee YJ, Joo JK, An BS. Interaction of steroid receptor coactivators and estrogen receptors in the human placenta. Journal of molecular endocrinology. 2016;56:239–247. doi: 10.1530/JME-15-0248. [DOI] [PubMed] [Google Scholar]
- 21.Chen X, Liu Z, Xu J. The cooperative function of nuclear receptor coactivator 1 (NCOA1) and NCOA3 in placental development and embryo survival. Molecular endocrinology. 2010;24:1917–1934. doi: 10.1210/me.2010-0201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yuan Y, et al. SRC-3 Plays a Critical Role in Human Umbilical Vein Endothelial Cells by Regulating the PI3K/Akt/mTOR Pathway in Preeclampsia. Reproductive sciences. 2018;25:748–758. doi: 10.1177/1933719117725818. [DOI] [PubMed] [Google Scholar]
- 23.Watson ED, Cross JC. Development of structures and transport functions in the mouse placenta. Physiology. 2005;20:180–193. doi: 10.1152/physiol.00001.2005. [DOI] [PubMed] [Google Scholar]
- 24.Cross JC, et al. Trophoblast functions, angiogenesis and remodeling of the maternal vasculature in the placenta. Molecular and cellular endocrinology. 2002;187:207–212. doi: 10.1016/S0303-7207(01)00703-1. [DOI] [PubMed] [Google Scholar]
- 25.Zou Z, et al. Inhibition of SRC-3 enhances sensitivity of human cancer cells to histone deacetylase inhibitors. Biochemical and biophysical research communications. 2016;478:227–233. doi: 10.1016/j.bbrc.2016.07.063. [DOI] [PubMed] [Google Scholar]
- 26.Wang L, et al. Matrix metalloproteinase 2 (MMP2) and MMP9 secreted by erythropoietin-activated endothelial cells promote neural progenitor cell migration. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2006;26:5996–6003. doi: 10.1523/JNEUROSCI.5380-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chetty C, Lakka SS, Bhoopathi P, Rao JS. MMP-2 alters VEGF expression via alphaVbeta3 integrin-mediated PI3K/AKT signaling in A549 lung cancer cells. International journal of cancer. 2010;127:1081–1095. doi: 10.1002/ijc.25134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Busch S, Renaud SJ, Schleussner E, Graham CH, Markert UR. mTOR mediates human trophoblast invasion through regulation of matrix-remodeling enzymes and is associated with serine phosphorylation of STAT3. Experimental cell research. 2009;315:1724–1733. doi: 10.1016/j.yexcr.2009.01.026. [DOI] [PubMed] [Google Scholar]
- 29.Louie MC, Zou JX, Rabinovich A, Chen HW. ACTR/AIB1 functions as an E2F1 coactivator to promote breast cancer cell proliferation and antiestrogen resistance. Molecular and cellular biology. 2004;24:5157–5171. doi: 10.1128/MCB.24.12.5157-5171.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Li HJ, et al. Steroid receptor coactivator 3 is a coactivator for myocardin, the regulator of smooth muscle transcription and differentiation. Proceedings of the National Academy of Sciences of the United States of America. 2007;104:4065–4070. doi: 10.1073/pnas.0611639104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhou HJ, et al. SRC-3 is required for prostate cancer cell proliferation and survival. Cancer research. 2005;65:7976–7983. doi: 10.1158/0008-5472.CAN-04-4076. [DOI] [PubMed] [Google Scholar]
- 32.Ferretti C, Bruni L, Dangles-Marie V, Pecking AP, Bellet D. Molecular circuits shared by placental and cancer cells, and their implications in the proliferative, invasive and migratory capacities of trophoblasts. Human reproduction update. 2007;13:121–141. doi: 10.1093/humupd/dml048. [DOI] [PubMed] [Google Scholar]
- 33.Aksamitiene E, Kiyatkin A, Kholodenko BN. Cross-talk between mitogenic Ras/MAPK and survival PI3K/Akt pathways: a fine balance. Biochemical Society transactions. 2012;40:139–146. doi: 10.1042/BST20110609. [DOI] [PubMed] [Google Scholar]
- 34.Mendoza MC, Er EE, Blenis J. The Ras-ERK and PI3K-mTOR pathways: cross-talk and compensation. Trends in biochemical sciences. 2011;36:320–328. doi: 10.1016/j.tibs.2011.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Moench R, et al. Exclusive inhibition of PI3K/Akt/mTOR signaling is not sufficient to prevent PDGF-mediated effects on glycolysis and proliferation in colorectal cancer. Oncotarget. 2016;7:68749–68767. doi: 10.18632/oncotarget.11899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Serra V, et al. PI3K inhibition results in enhanced HER signaling and acquired ERK dependency in HER2-overexpressing breast cancer. Oncogene. 2011;30:2547–2557. doi: 10.1038/onc.2010.626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Aksamitiene E, Kholodenko BN, Kolch W, Hoek JB, Kiyatkin A. PI3K/Akt-sensitive MEK-independent compensatory circuit of ERK activation in ER-positive PI3K-mutant T47D breast cancer cells. Cellular signalling. 2010;22:1369–1378. doi: 10.1016/j.cellsig.2010.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chang F, et al. Involvement of PI3K/Akt pathway in cell cycle progression, apoptosis, and neoplastic transformation: a target for cancer chemotherapy. Leukemia. 2003;17:590–603. doi: 10.1038/sj.leu.2402824. [DOI] [PubMed] [Google Scholar]
- 39.Shimonovitz S, et al. Developmental regulation of the expression of 72 and 92 kd type IV collagenases in human trophoblasts: A possible mechanism for control of trophoblast invasion. American Journal of Obstetrics and Gynecology. 1994;171:832–838. doi: 10.1016/0002-9378(94)90107-4. [DOI] [PubMed] [Google Scholar]
- 40.Staun-Ram E, Goldman S, Gabarin D, Shalev E. Expression and importance of matrix metalloproteinase 2 and 9 (MMP-2 and -9) in human trophoblast invasion. Reproductive biology and endocrinology: RB&E. 2004;2:59. doi: 10.1186/1477-7827-2-59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ying H, et al. Dual functions of the steroid hormone receptor coactivator 3 in modulating resistance to thyroid hormone. Molecular and cellular biology. 2005;25:7687–7695. doi: 10.1128/MCB.25.17.7687-7695.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zhou G, Hashimoto Y, Kwak I, Tsai SY, Tsai MJ. Role of the Steroid Receptor Coactivator SRC-3 in Cell Growth. Molecular and cellular biology. 2003;23:7742–7755. doi: 10.1128/mcb.23.21.7742-7755.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Qin L, et al. The AIB1 oncogene promotes breast cancer metastasis by activation of PEA3-mediated matrix metalloproteinase 2 (MMP2) and MMP9 expression. Molecular and cellular biology. 2008;28:5937–5950. doi: 10.1128/MCB.00579-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Red-Horse K, et al. Trophoblast differentiation during embryo implantation and formation of the maternal-fetal interface. Journal of Clinical Investigation. 2004;114:744–754. doi: 10.1172/jci200422991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Genbacev O, Zhou Y, Ludlow JW, Fisher SJ. Regulation of human placental development by oxygen tension. Science (New York, N.Y.) 1997;277:1669–1672. doi: 10.1126/science.277.5332.1669. [DOI] [PubMed] [Google Scholar]
- 46.Yang X, et al. AMPK Hyper-Activation Alters Fatty Acids Metabolism and Impairs Invasiveness of Trophoblasts in Preeclampsia. Cellular physiology and biochemistry: international journal of experimental cellular physiology, biochemistry, and pharmacology. 2018;49:578–594. doi: 10.1159/000492995. [DOI] [PubMed] [Google Scholar]
- 47.Kanasaki K, et al. Deficiency in catechol-O-methyltransferase and 2-methoxyoestradiol is associated with pre-eclampsia. Nature. 2008;453:1117–1121. doi: 10.1038/nature06951. [DOI] [PubMed] [Google Scholar]
- 48.Wong W, et al. Preeclampsia: 2-methoxyestradiol induces cytotrophoblast invasion and vascular development specifically under hypoxic conditions. The American journal of pathology. 2010;176:710–720. doi: 10.2353/ajpath.2010.090513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hirota K, Semenza GL. FIH-1: a novel protein that interacts with HIF-1alpha and VHL to mediate repression of HIF-1 transcriptional activity. Genes & development. 2001;15:2675–2686. doi: 10.1101/gad.924501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Mole DR, et al. Targeting of HIF-alpha to the von Hippel-Lindau ubiquitylation complex by O2-regulated prolyl hydroxylation. Science (New York, N.Y.) 2001;292:468–472. doi: 10.1126/science.1059796. [DOI] [PubMed] [Google Scholar]
- 51.Rolfo A, et al. Abnormalities in oxygen sensing define early and late onset preeclampsia as distinct pathologies. PloS one. 2010;5:e13288. doi: 10.1371/journal.pone.0013288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.McKenna NJ, O’Malley BW. Combinatorial control of gene expression by nuclear receptors and coregulators. Cell. 2002;108:465–474. doi: 10.1016/S0092-8674(02)00641-4. [DOI] [PubMed] [Google Scholar]
- 53.Todros T, et al. Umbilical Doppler waveforms and placental villous angiogenesis in pregnancies complicated by fetal growth restriction. Obstetrics and gynecology. 1999;93:499–503. doi: 10.1016/s0029-7844(98)00440-2. [DOI] [PubMed] [Google Scholar]
- 54.Huppertz B. Trophoblast differentiation, fetal growth restriction and preeclampsia. Pregnancy hypertension. 2011;1:79–86. doi: 10.1016/j.preghy.2010.10.003. [DOI] [PubMed] [Google Scholar]
- 55.Ji L, et al. Placental trophoblast cell differentiation: physiological regulation and pathological relevance to preeclampsia. Molecular aspects of medicine. 2013;34:981–1023. doi: 10.1016/j.mam.2012.12.008. [DOI] [PubMed] [Google Scholar]
- 56.Yamanaka-Tatematsu M, et al. Autophagy induced by HIF1alpha overexpression supports trophoblast invasion by supplying cellular energy. PloS one. 2013;8:e76605. doi: 10.1371/journal.pone.0076605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ma R, et al. Expressions of vitamin D metabolic components VDBP, CYP2R1, CYP27B1, CYP24A1, and VDR in placentas from normal and preeclamptic pregnancies. American journal of physiology. Endocrinology and metabolism. 2012;303:E928–935. doi: 10.1152/ajpendo.00279.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Yang Z, et al. Downregulated Kruppel-like factor 8 is involved in decreased trophoblast invasion under hypoxia-reoxygenation conditions. Reproductive sciences. 2014;21:72–81. doi: 10.1177/1933719113488448. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Supporting data and essential materials for reproducibility of this study are available upon request made to the corresponding authors.