Abstract
Sandwich complexes are an indispensable part of organometallic chemistry, which is becoming increasingly important in the field of lanthanide-based single molecule magnets. Herein, a fundamental class of pure sandwich complexes, [(η9-C9H9)Ln(η8-C8H8)] (Ln=Nd, Sm, Dy, Er), is reported. These neutral and sandwiched lanthanide compounds exclusively contain fully π-coordinated coplanar eight and nine membered CH rings. The magnetic properties of these compounds are investigated, leading to the observation of slow relaxation of the magnetization, including open hysteresis loops up to 10 K for the Er(III) analogue. Fast relaxation of the magnetization is likewise observed near zero field, a highly important characteristic for quantum information processing schemes. Our synthetic strategy is straightforward and utilizes the reaction of [(η8-C8H8)LnI(thf)n] complexes with [K(C9H9)]. Although all compounds are fully characterized, structural details of the title compounds can also be deduced by Raman spectroscopy only.
Subject terms: Organometallic chemistry, Ligands
Lanthanide sandwich complexes represent both a fundamental class of organometallic compounds and promising molecular magnets for information storage. Here the authors unveil a class of lanthanide sandwich complexes containing fully π-coordinated 8- and 9-membered rings, and show their slow relaxation of the magnetization.
Introduction
Sandwich complexes, that is, compounds bearing exclusively two planar, cyclic and π-bonded ligands, are a fundamental class of compounds in organometallic chemistry. In fact, the discovery of ferrocene [(η5-C5H5)2Fe] by Kealy and Pauson1 and the subsequent structural analysis by Fischer and Wilkinson paved the way to modern organometallic chemistry2,3. Ever since, the quest for new sandwich complexes has been a central part of modern organometallic chemistry. In the last decades, sandwich complexes, and particularly ferrocene, have become widely used compounds, which found a variety of applications, e.g. in synthesis, catalysis, electrochemistry, medicine and even as fuel additive4. In a classical homoleptic sandwich or metallocene complex, two identical aromatic ring systems equally bind with all carbon atoms to a metal center5. Well established examples of this structural motif are the above-mentioned ferrocene, bis(benzene)chromium and uranocene1,6,7. Besides these homoleptic complexes, there are also examples of heteroleptic sandwich complexes ligated by two different aromatic ring systems. As aromatic moieties, rings ranging from three to nine-membered systems have been established in organometallic chemistry. Despite the large variety of possible ligand permutations, considering these seven different ring sizes, only a limited number of structurally characterized ligand combinations has been reported in terms of homoleptic and heteroleptic complexes. These are the four non-substituted homoleptic metallocene archetypes with five to nine-membered rings: [(η5-C5H5)2M]1–3, [(η6-C6H6)2M]7, [(η8-C8H8)2M]6,8, and [(η9-C9H9)2M] (Fig. 1)9–11. Considering heteroleptic sandwich complexes, only four non-substituted types were structurally characterized, which are: [(η5-C5H5)M(η4-C4H4)]12, [(η6-C6H6)M(η5-C5H5)]13,14, [(η7-C7H7)M(η5-C5H5)]15–18, and [(η8-C8H8)M(η5-C5H5)] (Fig. 1)18–20. All of these are cyclopentadienyl derivatives combined with four to eight-membered rings.
Fig. 1.

Examples of sandwich complexes. Structurally characterized homoleptic and heteroleptic metallocenes with Cx ring systems (x = 4–9; M = metal atom)
Obviously, the vast majority of sandwich complexes is ligated by cyclopentadienyl derived moieties. In contrast, complexes ligated by larger aromatic monocycles are scarce. Therefore, we define the class of sandwich complexes having more than 16 carbon atoms coordinated to the central metal atom as super sandwich compounds, to distinguish them from classical sandwich complexes.
One of the most recent application of sandwich compounds in rare earth chemistry is their use as single molecule magnets (SMMs)21–30. Such lanthanide-based SMMs have been shown to act as quantum computing units, so-called qudits, for the implementation of Grover´s quantum search algorithm31, and more recently have displayed magnetic hysteresis at liquid nitrogen temperatures32–34.
A popular model for the SMM behavior of mononuclear lanthanide complexes focuses on the stabilization of the corresponding lanthanide ions mJ ground state by tuning the local electron density around the lanthanide ion generated by the ligand sphere27. Two prominent examples proving this concept are dysprosium and erbium. For example, the highest mJ state (±15/2) for dysprosium(III) has an oblate shape, thus an axial ligand field enhances the anisotropic properties of dysprosium containing complexes35. Recently, significant advances were reported by using homo- and heteroleptic cyclopentadienyl based dysprosium(III) metallocene cations, which exhibit a highly axial ligand field, enabling record high anisotropy barriers32–34. On the other hand the highest mJ state of erbium(III) is prolate shaped, therefore, an equatorial ligand field is beneficial in this case. This can be achieved by introducing one or two η8-C8H8 ligands, which exert a strong equatorial ligand field, into the coordination sphere of erbium ions27,28,35–37. These two examples highlight that the local symmetry generated around the central lanthanide ion, determined by the ligand field and the rigidity of the complex, plays a crucial role in the design of SMMs23,24,38–42. A review published recently pointed out that other, uncommon ligand systems, such as the cyclononatetraenyl anion may shed light on interesting properties in terms of SMM behavior and fundamental magneto-structural correlations23.
Herein, we present a long sought for class of sandwich complexes [(η9-C9H9)Ln(η8-C8H8)], which exclusively contain fully π-coordinated eight and nine-membered rings. Synthesizing these compounds was already attempted by Streitwieser et al.43 in 1973, shortly after the first successful synthesis of KC9H9 was reported by Katz and coworkers43,44. Their strategy was based on a one-pot reaction between LnCl3 (Ln=Ce(III), Pr(III), Nd(III), Sm(III)), K2C8H8, and KC9H9. However, they could only isolate complexes of the type [(η8-C8H8)LnCl(thf)2] thereby highlighting, that the C9H9– anion does not form sandwich complexes analogous to C8H82–. After a 45 years quest for [(η9-C9H9)Ln(η8-C8H8)], we now report a synthetic protocol based on two distinct steps.
Results
Synthesis and crystallographic characterization of [(η9-C9H9)Ln(η8-C8H8)]
First, we synthesized the starting material KC9H9 following the procedure of Katz et al.44 The 1H-NMR spectrum shows only one sharp resonance at δ 7.05 ppm, which is attributed to the nine ring protons and consistent with the regular 1-all-cis-configuration being present in solution. Additionally, the molecular structure of the dimethoxyethane solvate [(η9-C9H9)K(DME)2] (1) was established by X-ray diffraction experiments. A flat and aromatic nine-membered carbon ring is observed with C–C bond lengths ranging from 1.389(3) Å to 1.394(3) Å, which is in the expected region for aromatic sp2-hybridized carbon atoms (Fig. 2). Only the perfectly nonagonal all cis-isomer was found in the solid state and no positional disorder, indicating the presence of the cis,cis,cis,trans-cyclononatetraenyl isomer, was observed. This is in contrast to very recent findings from Nocton et al.11, who also reported on the solid-state structure of [(η9-C9H9)K(OEt2)]. They obtained KC9H9 from diethyl ether as a mixture of cis- and trans-isomers of the C9H9– ring and discussed the influence of the isomer on its subsequent reactivity. The potassium ion is centered below the ring and shows a complete η9-coordination with K–C bond distances ranging from 3.085(2)–3.154(2) Å.
Fig. 2.
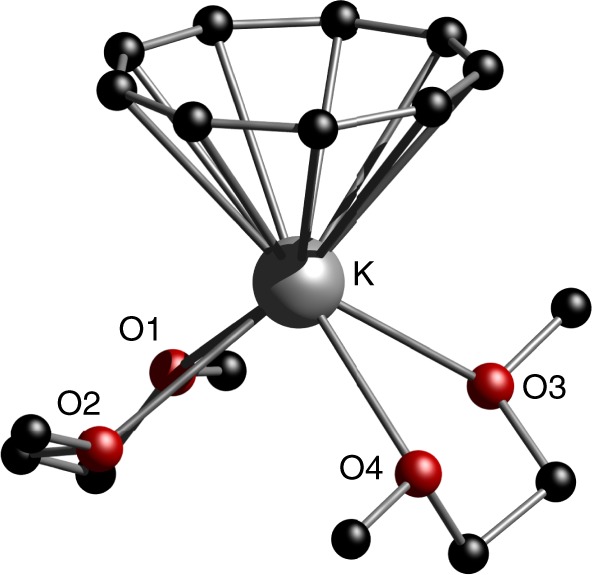
Molecular solid-state structure of compound 1. [(η9-C9H9)K(DME)2] 1. Color code: K, gray; C, black; O, red. Hydrogen atoms are omitted for clarity
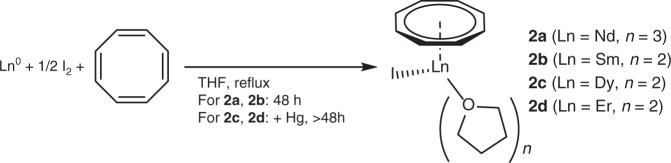
With KC9H9 in hand, we next aimed to synthesize defined [(η8-C8H8)LnI] complexes, in which the residual iodide ligand can be replaced by C9H9– in a salt metathesis approach. Therefore, we synthesized [(η8-C8H8)LnI(thf)n] (Ln=Nd(III) (2a), Sm(III) (2b), Dy(III) (2c), Er(III) (2d); n = 2 (Sm, Dy, Er), 3 (Nd)) according to a facile synthetic protocol reported by Mashima et al.45, which is based on the direct reaction of the lanthanide metal, cyclooctatetraene and iodine in hot THF (Fig. 3).
Fig. 3.
General synthetic procedure part 1. Synthesis of the cyclooctatetraene-complexes [(η8-C8H8)LnI(thf)n] (Ln = Nd (2a), Sm (2b), Dy (2c), Er (2d))
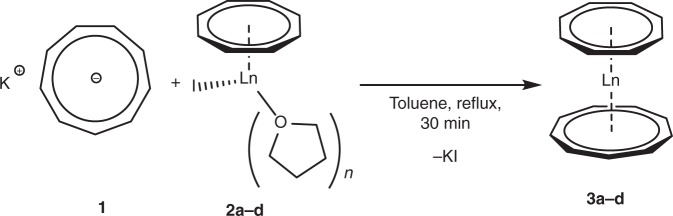
Compounds 2a and 2b, which have been reported earlier by Mashima et al.45, were obtained within two days. Complex 2c and 2d could only be obtained on this route after activation of the metal by in situ amalgamation. Nevertheless, significantly longer reactions times (3–4 weeks) were needed to obtain crystalline yields of 57% (2c) and 43% (2d). Ultimately, we were able to react the [(η8-C8H8)LnI(thf)2] complexes with 1 in refluxing toluene, which gave the title compounds [(η9-C9H9)Ln(η8-C8H8)] in moderate crystalline yields of 36% (Nd, 3a), 32% (Sm, 3b), 31% (Dy, 3c) and 32% (Er, 3d) (Fig. 4).
Fig. 4.
General synthetic procedure part 2. Synthesis of the heteroleptic sandwich complexes [(η9-C9H9)Ln(η8-C8H8)] (Ln = Nd (3a), Sm (3b), Dy (3c), Er (3d); n = 2 (Sm, Dy, Er), 3 (Nd))
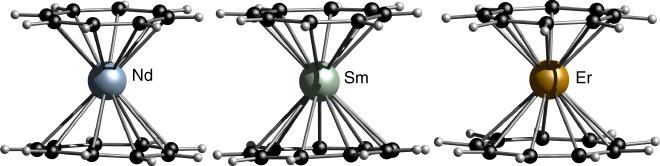
Single crystals of the heteroleptic sandwich complexes [(η8-C8H8)Ln(η9-C9H9)] (Ln=Nd (3a), Sm (3b), Dy, (3c), Er (3d)) were obtained from toluene. The solid-state structures of 3c and 3d show a disorder of the eight and the nine-membered rings (see Supplementary Information for details). Especially the molecular solid-state structure of 3d exhibits a complicated disorder with split positions of the Er(III) ion, showing slight indications of ring slipping and tilting in both ligands. The Er-C distances in the eight-membered ring vary between 2.406(11) Å and 2.670(10) Å with 5 carbon atoms closer located to the Er(III) ion than the others. Similarly, the Er-C distances in the nine-membered ring vary between 2.468(12) Å and 2.733(10) Å with four carbon atoms in closer proximity to the Er(III) ion (see Supplementary Table 10 for detailed bond lengths). However, this might also be caused by the unusual split Er(III) positions and thus, do not reflect the actual binding mode of the two aromatic moieties. We, therefore, performed a DFT geometry optimization and found the energetic minimum for 3d to be a perfect sandwich-type configured [(η9-C9H9)Er(η8-C8H8)] molecule (see Fig. 5). As a result, we propose 3d to comprise two fully π-coordinated and coplanar aromatic ligands, although the solid-state structure does not undoubtedly proof this assumption. On the other hand, 3a and 3b did not show this type of disorder. Their solid-structures exhibit perfect sandwich-type configuration with both rings bound to the central lanthanide ion in a coplanar fashion (Fig. 5).
Fig. 5.
Molecular structures of compounds 3a–d. [(η9-C9H9)Nd(η8-C8H8)] 3a (left) and [(η9-C9H9)Sm(η8-C8H8)] 3b (middle) in the solid state. Only on part of the disordered structures is depicted here. Geometry optimized structure of 3d (right). Color code: H, light; C, black; Nd, cyan; Sm, pale green; Er, orange
Only the structural parameters of 3a are discussed here in detail (Ln-Cg distances for 3a–d are given in Table 1). In compound 3a, the neodymium atom is centered between both rings with Nd-C bond lengths of 2.613(8)–2.653(8) Å to the eight-membered ring and Nd-C bond lengths of 2.845(6)–2.915(7) Å to the nine-membered ring. The distances of the Nd atom to the ring centroids (Cg) are Nd-Cg(8) 1.8925(3) Å and Nd-Cg(9) 2.0441(3) Å. The Cg(8)-Nd-Cg(9) angle accounts for 176.47(1)° and underpins an almost ideal coplanar arrangement. Interestingly, the η8-C8H8 unit is, albeit the lower ring diameter, significantly closer to the neodymium ion than the η9-C9H9 unit. This is probably caused by the higher negative charge of η8-C8H8 compared to η9-C9H9, leading to a stronger electrostatic attraction.
Table 1.
Ln-Cg distances in the solid state of compounds 3a–d
| Compound | Ln-Cg(C8H8) [Å] | Ln-Cg(C9H9) [Å] |
|---|---|---|
| 3a | 1.8925 (3) | 2.0441 (3) |
| 3b | 1.8687 (5) | 1.9908 (6) |
| 3c | 1.8869 (4) | 1.8752 (3) |
| 3d | 1.6725 (4) | 1.7248 (4) |
Raman-spectroscopic analysis
We further investigated the bonding situation and ligand aromaticity using Raman spectroscopy and vibrational analysis. Fourier transform Raman (FT-Raman) spectra of 3a–3d were recorded powdered samples (Fig. 6). The band assignments were validated by quantum chemical calculations. The Raman spectra can be divided in two sections: (i) above 300 cm–1 the spectra of all molecules are almost identical as the signals are solely attributable to both sandwich ligands. Vibrational coupling to the lanthanide cations is not expected due to the orthogonality of the in-plane vibrations of the ligands with respect to that of the lanthanide-ring centroid46. Therefore, the signals at 3042 (η8-C8H8) and 3006 cm–1 (η9-C9H9) are attributed to the fully symmetric C–H-valence motions, those at 1495 (η8-C8H8) and 1517 cm–1 (η9-C9H9) to the antisymmetric C–C-stretching vibrations of the ligands. A third group of bands belongs to the (local) symmetric ring breathing modes at 749 cm–1 (νsym(η8-C8H8)) and at 681 cm–1 (νsym(η9-C9H9)). Analyzing these modes is an unambiguous proof of the ring size and the bond strength within these aromatic ligand systems. According to normal coordinate analyses on C5H5–, C6H6 and C7H7+ 47 the stretching force constant values of the C–H and C–C bonds are each of comparable size. Assuming, this is furthermore true for larger aromatic CnHn monocyclic ligand systems, the approximate wavenumber of the fully symmetric ring breathing mode is easily calculated using a mathematical expression deduced in the Supplemental Information (see Supplementary Equation 1). This formula nicely reproduces the observed ring breathing mode energies of the two ligands (ν(C8H82–) = 754 cm–1 (calc. 761 cm–1) and ν(C9H9–) = 680 cm–1 (calc. 680 cm–1)) and therefore, confirms the comparable bonding situation in these ligand systems with those of aromatic ligands like C5H5–, C6H6 and C7H7+ (see SI for details). (ii) At vibrational energies lower than 300 cm–1 lanthanide-ring centroid stretching modes are expected in the Raman spectra of [(η9-C9H9)Ln(η8-C8H8)]. Due to the larger (η9-C9H9)-Ln bond lengths their vibrations are found between 100 and 166 cm–1, whereas those of the shorter Ln-(η8-C8H8) bonds are observed between 236 and 247 cm–1 (Nd, 3a: 137.0 (s), 242.1 (s); Sm, 3b: 126.8 (s), 236.0 (vs); Dy, 3c: 100 (s), 237.2 (s) and Er, 3d: 165.9 (m), 207.4 (m)). Both vibrations remind of the symmetric and antisymmetric modes of triatomic linear molecules like CO2. For these bands, the agreement with the results of the quantum chemical calculations is only of limited accordance due to possible coupling with lattice vibrations (see Table 4b in the SI). However, 3a–d are rare cases in modern organometallic chemistry, in which the coordination mode of the ligands can be determined by Raman spectroscopy as sole method.
Fig. 6.
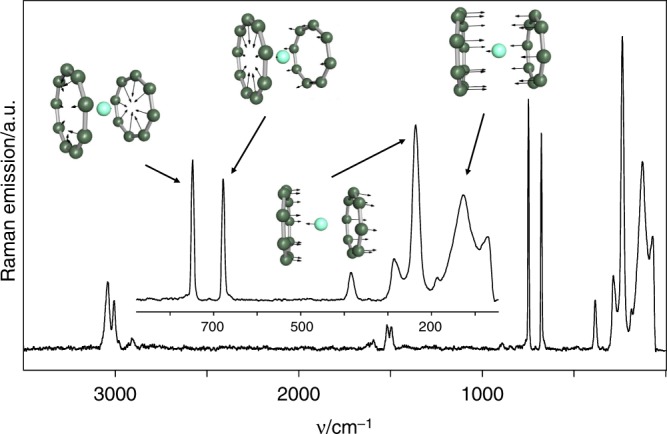
Raman-spectroscopic analysis. FT-Raman spectrum of [(η9-C9H9)Sm(η8-C8H8)] 3b. The motion vectors of four prominent bands are inserted. Hydrogen atoms and their motion vectors are omitted for clarity
Magnetic properties of [(η9-C9H9)Er(η8-C8H8)] (3d)
Although the single-crystal X-ray structures of 3c and 3d show some disorder, we conclude an almost coplanar η9-C9H9 and η8-C8H8 arrangement of the ligands as observed in 3a and 3b, based on the Raman-spectroscopic analysis. This arrangement is known to exert an equatorial ligand field, which drastically stabilizes prolate shaped mJ states of lanthanide ions. The prime example for this family of trivalent lanthanide ions in terms of single molecule magnetic behavior is without doubt erbium (vide supra), where the equatorial ligand field exerted by a η8-C8H8 ligand, can yield SMMs with not just large energy barriers to the relaxation of the magnetization, but also leading to open hysteresis loops at temperatures as high as 10 K26,27,36,48. We, therefore, carried out detailed magnetic studies on compound 3d, to test whether the asymmetric η8-C8H8/η9-C9H9 ligand field enhances the magnetic anisotropy of the sandwiched erbium ion. The rationale is two-fold: (i) as mentioned above η8-C8H8 ligands exert a strong equatorial ligand field, resulting in erbium compounds showing slow relaxation of the magnetization; and (ii) the introduction of a larger cyclic ring as η9-C9H9 could allow a closer Er-C contact, which could increase the equatorial ligand field, therefore enhancing the anisotropic characteristics of 3d. DC magnetic susceptibility studies of 3d were conducted in an applied field of Hdc = 1 kOe. At room temperature the χMT(T) (χM is the molar magnetic susceptibility) value is 11.25 cm3 K mol–1, in agreement with the expected value for an isolated Er(III) ion (c.f. 11.48 cm3 K mol–1 for J = 15/2, gJ = 6/5). The moment decreases smoothly upon cooling, until ca. 6 K, where it sharply decreases to 7.13 cm3 K mol–1 at 2 K (see Supplementary Fig. 11). The abrupt drop in χMT(T) indicates magnetic blocking, where pinning of the magnetic moment in the immobilized crystalline material occurs.
The dynamic behavior of 3d was probed via magnetic susceptibility AC studies under zero applied DC field. A single peak is observed in the temperature and frequency dependent out-of-phase magnetic susceptibility, i.e. χM”(T) and χM”(ν), respectively. This result is in agreement with the dynamic studies for [(η8-C8H8)2Er]– 28, while they differ from the asymmetric [(η5-C5H5)Er(η8-C8H8)] counterparts24, where two maxima were observed. The χM”(T, ν) reveals a temperature dependent maximum at temperatures between 16–26 K, whilst below 15 K the maximum in χM”(ν) remains practically constant (Fig. 7a). Between 18 and 26 K, the Arrhenius analysis of τ at different temperatures show a relaxation dominated thermally activated Orbach process, whereas below 9 K temperature independent processes dominate. The curvature between 10 and 15 K suggests that other relaxations pathways, such as Raman, are also active. The distribution of the relaxation parameter (α) likewise indicates a narrow distribution of relaxation times between 20 K and 26 K (α ≤ 0.18(1)), while for temperatures below 15 K the parameter is greater (α ≥ 0.18(1)). The energy barrier Ueff of 251(1) cm–1 and τ0 = 1.3(2) × 10–10 s (Fig. 7b) are very similar to the ones observed for homoleptic and heteroleptic erbium complexes24,27,28,36. The plateau at temperatures between 2 and 5 K marks the quantum tunneling of the magnetization regime, with a τQTM = 0.18(1) s. Application of an optimal field of 2 kG (at which relaxation is slower), efficiently suppressed QTM, leading to an almost purely temperature dependent relaxation (green symbols in Fig. 7b and SI (see Supplementary Fig. 14)), with a slightly enhanced Ueff = 261(1) cm–1.
Fig. 7.
Magnetic properties of 3d. a Experimental out-of-phase magnetic susceptibility (χM”(ν)) for 3d in the temperature range of 16 to 26 K (filled circles). Solid lines are the fittings to a generalized Debye model. b Arrhenius analysis of the relaxation times (τ) for 3d with HDC = 0 (red) and with HDC = 2 kG (green). The black line corresponds to the thermally active regime, whilst the blue dashed line represents the QTM regime. c Hysteresis measurements for 3d between ±2 T and from 1.8 K to 10 K employing a field sweep rate of 700 G/s; d optimized structure of 3d and direction of the principal magnetic anisotropy axis obtained from CASSCF calculations (green arrow). e ab initio calculated electronic states of the J = 15/2 manifold of the 4I term of 3d and the most probable relaxation pathway for the magnetization represented by the red arrows, involving spin phonon excitation to the first and second doublets. The thick black lines represent the Kramers doublet states as function of their magnetic moments
An open magnetic hysteresis is the ultimate proof of the strong anisotropic behavior in SMMs and their bistable magnetic behavior. Extrapolation of the Arrhenius data to low temperature indicates that the observation of magnetic hysteresis below 12 K is feasible, where the relaxation time is 100 s. To confirm the SMM behavior and the slow relaxation observed through AC studies, magnetization hysteresis loops were collected between 1.8–10 K. Figure 7c shows butterfly-like hysteresis loops between 2 and 10 K and a field ranging from ±2 T, leading to a blocking temperature (TB) of 10 K. Note that, albeit the energy barrier being rather large, the hysteresis loops are practically close at zero field, strongly indicating that QTM is rather efficient, as commonly observed in lanthanide-based SMMs.
To gain deeper insight into the relaxation mechanism and the anisotropic magnetic properties of 3d, CASSCF/SO-RASSI/SINGLE_ANISO calculations were performed49–54. Due to the highly disordered character of the crystal structure of 3d, CASSCF calculation were carried out employing an optimized crystal structure (see SI for details). The electronic calculation predicts a highly axial ground state with gz = 17.95 and gx,y ≈ 10–5. As observed in Fig. 7d, the anisotropy axis is perpendicular to the η8-C8H8 and η9-C9H9 planes. Employing the ligand field parameters from electronic calculations, we find that the ground, first and second excited states are almost colinear and highly pure mJ = ±15/2 and ±13/2 and ±1/2 states, respectively. The relative energies for the first and second excited state are 140 and 268 cm–1. The succeeding excited states are highly pure and huddled over 330–490 cm–1. As it can be observed in Fig. 7e, ab-initio results reproduce rather well the χMT(T) and M(H) (see Supplementary Fig. 11) with only small differences. The discrepancies might arise by structural distortions not reflected in the geometry optimization. Using the average matrix elements of magnetic moment between the electronic states, it is predicted that the most efficient magnetic relaxation pathway is to occur via thermally assisted QTM through the second excited state at 268 cm–1. As observed, this state is very close to the Ueff obtained from dynamic studies (cf. ~260 cm−1 (Fig. 7e)). Interestingly, the energy barrier is also very similar to the antisymmetric vibration of the C8/C9 rings observed in the Raman spectrum (240 cm–1). As molecular vibrations have been predicted to facilitate spin-phonon coupling, these could be relevant for the relaxation in 3d33.
Note that the strongly equatorial ligand field exerted by the η8-C8H8 and η9-C9H9 ligands is optimal at stabilizing the largest mJ state for Er(III), characterized by a prolate electron density, as demonstrated by the magnetic studies and supported by CASSCF calculations. In contrast, for the Dy(III) ions an axial ligand field is more suitable to stabilize the largest mJ = 15/2 state, thus the anisotropic magnetic properties in [(η9-C9H9)Dy(η8-C8H8)] are expectedly worse, as confirmed by AC tests and other reports27,55.
Discussion
By synthesizing [(η9-C9H9)Ln(η8-C8H8)] (Ln=Nd, Sm, Dy, Er), we unveiled a fundamental class of pure sandwich complexes. The title compounds represent a long sought asymmetric organometallic motif, leading to the observation of hysteresis loops up to 10 K. In addition, we observe fast quantum tunneling of the magnetization near zero field, which opens the possibility of nuclear spin read-out with the 167Er(III) analog of [(η9-C9H9)Er(η8-C8H8)]56. Our results clearly highlight the significance of not just a long desired and extremely elusive organometallic complex class, but are also of relevance to future quantum spintronic applications.
Methods
Synthetic methods
Experiments were carried out under a dry, oxygen-free argon atmosphere using Schlenk-line and glovebox techniques. All solvents and reagents were rigorously dried and deoxygenated before use. All compounds were characterized by single-crystal X-ray diffraction studies. Further details are available in the Supplementary Information (see section Supplementary Methods).
Supplementary information
Acknowledgements
K.I.T. is acknowledged for financial support. The authors acknowledge computational support by the state of Baden-Württemberg through bwHPC and the Deutsche Forschungsgemeinschaft (DFG) through grant No INST 40/467-1 FUGG.
Author contributions
L.M. synthesized and analyzed all compounds with support from C.S. and S.B. L.M. and C.S. conducted X-ray experiments. E.M.P. and M.R. conducted and interpreted magnetic measurements and carried out the ab-initio CASSCF calculations and interpreted the results. R.K. performed and analyzed quantum chemical calculations. PWR originated the idea, supervised the work, and interpreted the results. All authors contributed to the preparation of the manuscript.
Data availability
All data is available from the authors on reasonable request. The X-ray crystallographic coordinates for structures reported in this study have been deposited at the Cambridge Crystallographic Data Centre (CCDC), under deposition numbers 1894445-1894450. These data can be obtained free of charge from The Cambridge Crystallographic Data Centre via www.ccdc.cam.ac.uk/data_request/cif.
Competing interests
The authors declare no competing interests.
Footnotes
Peer review information: Nature Communications thanks Alessandro Prescimone, Alexey Popov, David Mills and an anonymous reviewer for their contribution to the peer review of this work. Peer reviewer reports are available.
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
E. Moreno-Pineda, Email: eufemio.pineda@kit.edu
M. Ruben, Email: mario.ruben@kit.edu
P. W. Roesky, Email: roesky@kit.edu
Supplementary information
Supplementary Information accompanies this paper at 10.1038/s41467-019-10976-6.
References
- 1.Kealy TJ, Pauson PL. A New Type of Organo-Iron Compound. Nature. 1951;168:1039. doi: 10.1038/1681039b0. [DOI] [Google Scholar]
- 2.Wilkinson G, Rosenblum M, Whiting MC, Woodward RB. The Stucture of Iron Bis-Cyclopentadienyl. J. Am. Chem. Soc. 1952;74:2125–2126. doi: 10.1021/ja01128a527. [DOI] [Google Scholar]
- 3.Fischer, E. O. & Pfab, W. Cyclopentadien-metallkomplexe, ein neuer Typ metallorganischer verbindungen. Z. Naturforsch., B: Chem. Sci. 7, 377–379 (1952).
- 4.Štěpnička, P. Ferrocenes: Ligands, Materials and Biomolecules (John Wiley & Sons, Ltd, Chichester, 2008).
- 5.Elschenbroich, C. Organometallics, Edn. 3. (Wiley-VCH, Weinheim, 2006).
- 6.Streitwieser A, Müller-Westerhoff U. Bis(cyclooctatetraenyl)uranium (uranocene). A new class of sandwich complexes that utilize atomic f orbitals. J. Am. Chem. Soc. 1968;90:7364–7364. doi: 10.1021/ja01028a044. [DOI] [Google Scholar]
- 7.Fischer EO, Hafner W. Di-benzol-chrom. Z. Naturforsch., B: Chem. Sci. 1955;10:665–668. doi: 10.1515/znb-1955-1201. [DOI] [Google Scholar]
- 8.Mares F, Hodgson K, Streitwieser A. Lanthanide complexes with cyclooctatetraene di-anion. J. Organomet. Chem. 1970;24:C68–C70. doi: 10.1016/S0022-328X(00)84477-0. [DOI] [Google Scholar]
- 9.Walter MD, Wolmershäuser G, Sitzmann H. Calcium, strontium, barium, and ytterbium complexes with cyclooctatetraenyl or cyclononatetraenyl ligands1. J. Am. Chem. Soc. 2005;127:17494–17503. doi: 10.1021/ja0550071. [DOI] [PubMed] [Google Scholar]
- 10.Kawasaki K, et al. A designer ligand field for blue-green luminescence of organoeuropium(II) sandwich complexes with cyclononatetraenyl ligands. Chem. Commun. 2017;53:6557–6560. doi: 10.1039/C7CC03045B. [DOI] [PubMed] [Google Scholar]
- 11.Xémard Mathieu, Zimmer Sébastien, Cordier Marie, Goudy Violaine, Ricard Louis, Clavaguéra Carine, Nocton Grégory. Lanthanidocenes: Synthesis, Structure, and Bonding of Linear Sandwich Complexes of Lanthanides. Journal of the American Chemical Society. 2018;140(43):14433–14439. doi: 10.1021/jacs.8b09081. [DOI] [PubMed] [Google Scholar]
- 12.Riley PE, Davis RE. Crystal and molecular structure of (π-cyclopentadienyl)-(π-cyclobutadiene)cobalt. J. Organomet. Chem. 1976;113:157–166. doi: 10.1016/S0022-328X(00)96130-8. [DOI] [Google Scholar]
- 13.Braunschweig H, et al. Heteroleptic [n]chromoarenophanes: ansa complexes derived from [Cr(η5-C5H5)(η6-C6H6)] Chem. Eur. J. 2013;19:270–281. doi: 10.1002/chem.201203288. [DOI] [PubMed] [Google Scholar]
- 14.Beck V, Cowley AR, O’Hare D. Magnetic and structural studies of vanadium arene complexes. Organometallics. 2004;23:4265–4270. doi: 10.1021/om0497442. [DOI] [Google Scholar]
- 15.Zeinstra JD, De Boer JL. Structure of cyclopentadienylcycloheptatrienyl-titanium. J. Organomet. Chem. 1973;54:207–211. doi: 10.1016/S0022-328X(00)85010-X. [DOI] [Google Scholar]
- 16.Engebretson G, Rundle RE. The molecular and crystal structures of π-cyclopentadienyl π-cycloheptatrienyl vanadium. J. Am. Chem. Soc. 1963;85:481–482. doi: 10.1021/ja00887a030. [DOI] [Google Scholar]
- 17.Tamm M, Kunst A, Bannenberg T, Herdtweck E, Schmid R. Cycloheptatrienyl−cyclopentadienyl−zirconium sandwich complexes: structure and bonding. Organometallics. 2005;24:3163–3171. doi: 10.1021/om050192c. [DOI] [Google Scholar]
- 18.Lyssenko KA, Antipin MY, Ketkov SY. Electron density distribution in vanadocene (η5-C5H5)2V and mixed metallocenes (η5-C5H5)M(η5-C7H7) (M=Ti, V, or Cr) and (η5-C5H5)Ti(η8-C8H8). Effect of the nature of the cyclic ligand on the character of the M–(π-ligand) bond. Russ. Chem. Bull. 2001;50:130–141. doi: 10.1023/A:1009597723152. [DOI] [Google Scholar]
- 19.Kroon PA, Helmholdt RB. Structure of cyclopentadienylcyclooctateraene-titanium. J. Organomet. Chem. 1970;25:451–454. doi: 10.1016/S0022-328X(00)87847-X. [DOI] [Google Scholar]
- 20.Braunschweig H, et al. Boryl- and silyl-substituted mixed sandwich compounds of scandium. Chem. Eur. J. 2018;24:2403–2409. doi: 10.1002/chem.201704908. [DOI] [PubMed] [Google Scholar]
- 21.Zhang P, Zhang L, Tang J. Lanthanide single molecule magnets: progress and perspective. Dalton Trans. 2015;44:3923–3929. doi: 10.1039/C4DT03329A. [DOI] [PubMed] [Google Scholar]
- 22.Layfield RA. Organometallic single-molecule magnets. Organometallics. 2014;33:1084–1099. doi: 10.1021/om401107f. [DOI] [Google Scholar]
- 23.Day BM, Guo F-S, Layfield RA. Cyclopentadienyl ligands in lanthanide single-molecule magnets: one ring to rule them all? Acc. Chem. Res. 2018;51:1880–1889. doi: 10.1021/acs.accounts.8b00270. [DOI] [PubMed] [Google Scholar]
- 24.Jiang S-D, Wang B-W, Sun H-L, Wang Z-M, Gao S. An organometallic single-ion magnet. J. Am. Chem. Soc. 2011;133:4730–4733. doi: 10.1021/ja200198v. [DOI] [PubMed] [Google Scholar]
- 25.Jeletic M, et al. An organometallic sandwich lanthanide single-ion magnet with an unusual multiple relaxation mechanism. J. Am. Chem. Soc. 2011;133:19286–19289. doi: 10.1021/ja207891y. [DOI] [PubMed] [Google Scholar]
- 26.Boulon M-E, et al. Angular-resolved magnetometry beyond triclinic crystals: out-of-equilibrium studies of Cp*ErCOT single-molecule magnet. Chem. Eur. J. 2013;19:13726–13731. doi: 10.1002/chem.201302600. [DOI] [PubMed] [Google Scholar]
- 27.Ungur L, Le Roy JJ, Korobkov I, Murugesu M, Chibotaru LF. Fine-tuning the local symmetry to attain record blocking temperature and magnetic remanence in a single-ion magnet. Angew. Chem. Int. Ed. 2014;53:4413–4417. doi: 10.1002/anie.201310451. [DOI] [PubMed] [Google Scholar]
- 28.Le Roy JJ, Ungur L, Korobkov I, Chibotaru LF, Murugesu M. Coupling strategies to enhance single-molecule magnet properties of erbium–cyclooctatetraenyl complexes. J. Am. Chem. Soc. 2014;136:8003–8010. doi: 10.1021/ja5022552. [DOI] [PubMed] [Google Scholar]
- 29.Le Roy JJ, Korobkov I, Kim JE, Schelter EJ, Murugesu M. Structural and magnetic conformation of a cerocene [Ce(COT”)2]− exhibiting a uniconfigurational f1 ground state and slow-magnetic relaxation. Dalton Trans. 2014;43:2737–2740. doi: 10.1039/C3DT53280A. [DOI] [PubMed] [Google Scholar]
- 30.Le Roy JJ, Gorelsky SI, Korobkov I, Murugesu M. Slow magnetic relaxation in uranium(III) and neodymium(iii) cyclooctatetraenyl complexes. Organometallics. 2015;34:1415–1418. doi: 10.1021/om501214c. [DOI] [Google Scholar]
- 31.Moreno-Pineda E, Godfrin C, Balestro F, Wernsdorfer W, Ruben M. Molecular spin qudits for quantum algorithms. Chem. Soc. Rev. 2018;47:501–513. doi: 10.1039/C5CS00933B. [DOI] [PubMed] [Google Scholar]
- 32.Guo FS, et al. A dysprosium metallocene single‐molecule magnet functioning at the axial limit. Angew. Chem. Int. Ed. 2017;56:11445–11449. doi: 10.1002/anie.201705426. [DOI] [PubMed] [Google Scholar]
- 33.Goodwin CAP, Ortu F, Reta D, Chilton NF, Mills DP. Molecular magnetic hysteresis at 60 kelvin in dysprosocenium. Nature. 2017;548:439. doi: 10.1038/nature23447. [DOI] [PubMed] [Google Scholar]
- 34.Guo F-S, et al. Magnetic hysteresis up to 80 kelvin in a dysprosium metallocene single-molecule magnet. Science. 2018;362:1400–1403. doi: 10.1126/science.aav0652. [DOI] [PubMed] [Google Scholar]
- 35.Ungur L, Chibotaru LF. Strategies toward high-temperature lanthanide-based single-molecule magnets. Inorg. Chem. 2016;55:10043–10056. doi: 10.1021/acs.inorgchem.6b01353. [DOI] [PubMed] [Google Scholar]
- 36.Meihaus KR, Long JR. Magnetic blocking at 10 k and a dipolar-mediated avalanche in salts of the bis(η8-cyclooctatetraenide) complex [Er(COT)2]−. J. Am. Chem. Soc. 2013;135:17952–17957. doi: 10.1021/ja4094814. [DOI] [PubMed] [Google Scholar]
- 37.Hilgar JD, Bernbeck MG, Flores BS, Rinehart JD. Metal–ligand pair anisotropy in a series of mononuclear Er–COT complexes. Chem. Sci. 2018;9:7204–7209. doi: 10.1039/C8SC01361F. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Jiang S-D, Wang B-W, Su G, Wang Z-M, Gao S. A mononuclear dysprosium complex featuring single-molecule-magnet behavior. Angew. Chem. Int. Ed. 2010;49:7448–7451. doi: 10.1002/anie.201004027. [DOI] [PubMed] [Google Scholar]
- 39.Gregson M, et al. A monometallic lanthanide bis(methanediide) single molecule magnet with a large energy barrier and complex spin relaxation behaviour. Chem. Sci. 2016;7:155–165. doi: 10.1039/C5SC03111G. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chilton NF, Goodwin CAP, Mills DP, Winpenny REP. The first near-linear bis(amide) f-block complex: a blueprint for a high temperature single molecule magnet. Chem. Commun. 2015;51:101–103. doi: 10.1039/C4CC08312A. [DOI] [PubMed] [Google Scholar]
- 41.Chilton NF. Design criteria for high-temperature single-molecule magnets. Inorg. Chem. 2015;54:2097–2099. doi: 10.1021/acs.inorgchem.5b00089. [DOI] [PubMed] [Google Scholar]
- 42.Liu J-L, Chen Y-C, Tong M-L. Symmetry strategies for high performance lanthanide-based single-molecule magnets. Chem. Soc. Rev. 2018;47:2431–2453. doi: 10.1039/C7CS00266A. [DOI] [PubMed] [Google Scholar]
- 43.Mares F, Hodgson KO, Streitwieser A. Monocyclooctatetraenelanthanide chlorides, a new class of cyclooctatetraene complexes. J. Organomet. Chem. 1971;28:C24–C26. doi: 10.1016/S0022-328X(00)84559-3. [DOI] [Google Scholar]
- 44.Katz TJ, Garratt PJ. Reactions of the cyclooctatetraenyl dianion with gem-dihalides. the preparation of derivatives of bicyclo [6.1.0]nonatriene. Synthesis of the cyclononatetraenyl anion. J. Am. Chem. Soc. 1964;86:5194–5202. doi: 10.1021/ja01077a032. [DOI] [Google Scholar]
- 45.Mashima K, et al. A new convenient preparation of monocyclooctatetraenyl-lanthanide complexes from metallic lanthanides and oxidants. J. Organomet. Chem. 1994;473:85–91. doi: 10.1016/0022-328X(94)80108-8. [DOI] [Google Scholar]
- 46.Köppe R, Schnöckel H, Jouany C, Gadea FX, Barthelat JC. Interaction of molecular SiS with silver atoms in an argon matrix: IR spectrum and ab initio rationalization. Heteroat. Chem. 1992;3:333–335. doi: 10.1002/hc.520030405. [DOI] [Google Scholar]
- 47.Nelson RD, Fateley WG, Lippincott ER. Normal coördinate analysis and aromatic character of five- and seven-membered aromatic (CH)n rings. J. Am. Chem. Soc. 1956;78:4870–4872. doi: 10.1021/ja01600a016. [DOI] [Google Scholar]
- 48.Rinehart JD, Long JR. Exploiting single-ion anisotropy in the design of f-element single-molecule magnets. Chem. Sci. 2011;2:2078–2085. doi: 10.1039/c1sc00513h. [DOI] [Google Scholar]
- 49.Aquilante F, et al. Molcas 8: New capabilities for multiconfigurational quantum chemical calculations across the periodic table. J. Comput. Chem. 2016;37:506–541. doi: 10.1002/jcc.24221. [DOI] [PubMed] [Google Scholar]
- 50.Siegbahn PEM, Almlöf J, Heiberg A, Roos BO. The complete active space SCF (CASSCF) method in a Newton–Raphson formulation with application to the HNO molecule. J. Chem. Phys. 1981;74:2384–2396. doi: 10.1063/1.441359. [DOI] [Google Scholar]
- 51.Olsen J, Roos BO, Jorgensen P, Jensen HJA. Determinant based configuration interaction algorithms for complete and restricted configuration interaction spaces. J. Chem. Phys. 1988;89:2185–2192. doi: 10.1063/1.455063. [DOI] [Google Scholar]
- 52.Malmqvist PÅ, Roos BO, Schimmelpfennig B. The restricted active space (RAS) state interaction approach with spin–orbit coupling. Chem. Phys. Lett. 2002;357:230–240. doi: 10.1016/S0009-2614(02)00498-0. [DOI] [Google Scholar]
- 53.Ungur L, Chibotaru LF. Ab initio crystal field for lanthanides. Chem. Eur. J. 2017;23:3708–3718. doi: 10.1002/chem.201605102. [DOI] [PubMed] [Google Scholar]
- 54.Chibotaru LF, Ungur L. Ab initio calculation of anisotropic magnetic properties of complexes. I. Unique definition of pseudospin Hamiltonians and their derivation. J. Chem. Phys. 2012;137:064112. doi: 10.1063/1.4739763. [DOI] [PubMed] [Google Scholar]
- 55.Jiang S-D, et al. Series of Lanthanide Organometallic Single-Ion Magnets. Inorg. Chem. 2012;51:3079–3087. doi: 10.1021/ic202511n. [DOI] [PubMed] [Google Scholar]
- 56.Moreno-Pineda E, Damjanović M, Fuhr O, Wernsdorfer W, Ruben M. Nuclear Spin Isomers: engineering a Et4N[DyPc2] spin qudit. Angew. Chem. Int. Ed. 2017;56:9915–9919. doi: 10.1002/anie.201706181. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data is available from the authors on reasonable request. The X-ray crystallographic coordinates for structures reported in this study have been deposited at the Cambridge Crystallographic Data Centre (CCDC), under deposition numbers 1894445-1894450. These data can be obtained free of charge from The Cambridge Crystallographic Data Centre via www.ccdc.cam.ac.uk/data_request/cif.