Abstract
Agrotis ipsilon (Lepidoptera: Noctuidae) is a major underground pest that damages many agricultural crops in China and other countries. A diet-incorporation-based bioassay was conducted to evaluate the sublethal effects of the novel anthranilic diamide chlorantraniliprole on the nutritional physiology, enzymatic properties and population parameters of this cutworm. Chlorantraniliprole exhibited signs of active toxicity against third instar larvae of A. ipsilon, and the LC50 was 0.187 μg.g−1 of artificial diet after treatment for 72 h. The development time of the larval, pupal and adult stages was significantly affected after chlorantraniliprole exposure, compared to the control treatment. Relative to the control treatment, chlorantraniliprole decreased pupal and adult emergence rates, fecundity and fertility and increased the proportions of developmental deformities, the adult preoviposition period (APOP) and the total preoviposition period (TPOP). Furthermore, compared to those treated with the control, A. ipsilon larvae treated with low doses of chlorantraniliprole decreased food utilization and nutrient content (protein, lipid, carbohydrate, trehalose), showed lower pupal weights and growth rates. Compared with the control treatment, chlorantraniliprole significantly reduced digestive enzyme activities and observably increased detoxifying and protective enzyme activities and hormone titers. Importantly, these chlorantraniliprole-induced changes affected life table parameters of the cutworm. These results suggest that chlorantraniliprole at low concentrations can impair A. ipsilon development duration, normal food consumption and digestion process, enzymatic properties, hormone levels, fecundity and population levels. Chlorantraniliprole exhibit the potential to be exploited as a control strategy for this cutworm.
Subject terms: Non-model organisms, Non-model organisms, Biocatalysis, Biocatalysis
Introduction
The black cutworm (BCW), Agrotis ipsilon (Lepidoptera: Noctuidae), is widely distributed in many countries and regions worldwide1,2. A. ipsilon is one of the most dangerous species of underground pests and can feed on more than 100 types of host plants (e.g., corn, wheat, cotton, soybean, vegetables and a variety of weeds3,4. One larva may bite off several plant seedlings in a night5. In particular, A. ipsilon larvae can cause serious damage at the fourth-sixth and/or higher instar stages5. BCW can cause hidden damage in fields, and it is difficult to fully expose the pests to insecticides by spraying, which results in reduced effectiveness of control strategies6. Currently, the selection of high-efficiency insecticides and suitable application methods is an important problem in the development of integrated pest management (IPM) strategies for controlling the black cutworm.
Chlorantraniliprole is a novel anthranilic diamide insecticide developed by DuPont Crop Protection that has been registered and effectively used for the control of many lepidopteran pests and non-lepidopteran species, such as Coleoptera, Diptera, Hemiptera and Isoptera species in various crops7,8. Chlorantraniliprole has a unique mode of action, activating the unregulated release of internal calcium stores, which leads to Ca2+ depletion, feeding cessation, lethargy, muscle paralysis, and insect death9. This insecticide is a useful alternative to the more toxic conventional insecticides because it is environmentally friendly and exhibits relatively low toxicity against nontarget animals in Integrated Pest Management (IPM) programs10,11.
In addition to having a direct killing effect on target pests exposed to lethal doses of insecticide12,13, also results, to a certain extent, in effects on insect physiology, biology, behavior, reproduction, longevity and so on, of survival individuals exposed to low-lethal doses of insecticide, due to the continuous degradation and variable distributions of chemicals when insecticides application in fields14,15. That is, chemicals may generate sublethal effects on target or nontarget insects when they are exposed to insecticides at low-lethal concentrations16,17, which can stimulate the rate of development and adult fecundity of targeted insects18,19, Individual differences in toxicity and sublethal effects are related to exposure time and dose of insecticide20. In addition, sublethal effects vary among different classes of insecticides and different species of targeted insects21.
The age-stage, two-sex life table, which can fully describe population dynamics in a comprehensive manner and can also explain the multiple sublethal effects on targeted insects22,23. However, these studies have only paid attention to population parameters, and other physiological and/or biochemical characteristics should also be considered after exposure to low concentrations of insecticides. Changes in bioactivity can be used to assess and predict the toxicity and potential efficacy of insecticides in the control of insect pests24,25. Hence, studying the sublethal effects of insecticides on targeted insect pests is crucial to guide the scientific application of insecticides.
The objective of this study was to obtain a comprehensive understanding of the sublethal effects of chlorantraniliprole on A. ipsilon. Based on the toxicity of chlorantraniliprole against third instar larvae, we investigated its sublethal effects on the development, population parameters and bioactivity parameters (nutritional indices; nutrient content; digestive, detoxifying and protective enzyme activities; and hormone titers) of A. ipsilon when third instar larvae were exposed to chlorantraniliprole at the LC05, LC25 and LC45 concentrations. The results may help us understand the sublethal effects of chlorantraniliprole on the population dynamics and bioactivity of A. ipsilon and provide important information about the rational application of chlorantraniliprole in IPM strategies for A. ipsilon.
Results
Toxicity of chlorantraniliprole against third instar larvae of A. ipsilon
This experiment determined the toxicity of chlorantraniliprole against third instar larvae of A. ipsilon (Table 1). The results showed that chlorantraniliprole exhibited active toxicity against A. ipsilon larvae. After 72 h of treatment, for the artificial diet, the LC50 was 0.187 μg.g−1, and the LC05, LC25, and LC45 were 0.007 μg.g−1, 0.048 μg.g−1, and 0.145 μg.g−1, respectively.
Table 1.
Toxicity of chlorantraniliprole to 3rd-instar larvae of Agrotis ipsilon.
| Insecticide | Na | Slope ± SE | LC50 (95%CL)b (μg.g−1) | LC45 (95%CL)b (μg.g−1) | LC25 (95%CL)b (μg.g−1) | LC05 (95%CL)b (μg.g−1) | χ2 (df)c | p-valuec |
|---|---|---|---|---|---|---|---|---|
| chlorantraniliprole | 840 | 2.582 ± 0.597 | 0.187 (0.153–0.229) | 0.145 (0.116–0.182) | 0.048 (0.034–0.069) | 0.007(0.004–0.013) | 1.385 (6) | 0.987 |
aNumber of larvae.
b95% confidence limits.
cChi-square value (χ2), degrees of freedom (df), and p-value as calculated by probit analysis with SPSS 23.0.
Effects of chlorantraniliprole on the growth and development of A. ipsilon
The sublethal effects of chlorantraniliprole on the growth and development parameters of A. ipsilon were determined (Table 2). Chlorantraniliprole at low-lethal concentrations did significantly shorten the development time of third and fourth instar lavae of A. ipsilon. Larval duration of fifth/sixth-instar was significantly prolonged compared to the control when third instar larvae of A. ipsilon were exposed to chlorantraniliprole. After treatment with chlorantraniliprole, the development time of pupae and adult longevity were lengthened after exposure to the LC05 concentration; however, there was no significant difference between the treatment and the control. Chlorantraniliprole significantly increased the number of deformed pupae and adults of the parental generation and offspring eggs and the percentage of seventh-instar larvae compared to the control. However, the pupation rate and adult emergence were significantly decreased when chlorantraniliprole was applied to the third instar larvae of A. ipsilon. No significant effect was observed on pupa weight and sex ratio when A. ipsilon larvae were treated with chlorantraniliprole compared to the control. The adult preoviposition period (APOP) and total preoviposition period (TPOP) of female A. ipsilon insects exposed to the low-lethal chlorantraniliprole treatments were significantly affected. In addition, the LC05 treatment did, to some extent, lengthen the oviposition period compared to that of the control group, while the LC25 and LC45 treatments shortened the oviposition period. The LC05, LC25 and LC45 treatments significantly reduced fecundity (1054.555, 838.588 and 619.843 eggs/female at LC05, LC25 and LC45, respectively) compared with the control treatment (1210.423 eggs/female) (Table 2).
Table 2.
Effects of chlorantraniliprole treatment on growth and development (mean ± SE) of A. ipsilon 3rd-instar larvae.
| Biological characteristics | Control | LC05 | LC25 | LC45 | P | ||||
|---|---|---|---|---|---|---|---|---|---|
| n | Mean ± SE | n | Mean ± SE | n | Mean ± SE | n | Mean ± SE | ||
| Third-instar period (days) | 150 | 4.121 ± 0.108 a | 189 | 3.983 ± 0.067 ab | 197 | 3.773 ± 0.061 b | 216 | 3.381 ± 0.069 c | <0.0001 |
| Fourth-instar period (days) | 149 | 4.512 ± 0.080 a | 181 | 4.308 ± 0.087 a | 189 | 4.029 ± 0.088 b | 205 | 3.817 ± 0.095 b | <0.0001 |
| Fifth-instar period (days) | 146 | 5.867 ± 0.078 c | 172 | 6.384 ± 0.073 b | 176 | 6.531 ± 0.049 ab | 197 | 6.918 ± 0.119 a | <0.0001 |
| Sixth-instar period (days) | 143 | 7.498 ± 0.084 b | 167 | 7.920 ± 0.107 a | 168 | 7.822 ± 0.056 ab | 175 | 8.026 ± 0.052 a | 0.0020 |
| Seven-instar larvae (%) | 23 | 15.920 ± 0.272 c | 39 | 23.468 ± 0.379 b | 63 | 37.735 ± 0.276 a | 61 | 34.148 ± 0.139 a | <0.0001 |
| Pupation rate (%) | 139 | 97.455 ± 0.954 a | 158 | 94.443 ± 0.748 b | 148 | 88.918 ± 0.674 c | 142 | 81.075 ± 0.531 d | <0.0001 |
| Pupae with deformities (%) | 139 | 2.718 ± 0.035 d | 158 | 3.349 ± 0.046 c | 148 | 4.642 ± 0.012 b | 142 | 5.562 ± 0.048 a | <0.0001 |
| Female pupae period (days) | 70 | 14.608 ± 0.161 ab | 78 | 15.228 ± 0.237 a | 73 | 13.823 ± 0.235 b | 70 | 12.555 ± 0.227 c | 0.0003 |
| Male pupae period (days) | 69 | 13.575 ± 0.132 a | 80 | 14.085 ± 0.355 a | 75 | 13.055 ± 0.102 ab | 72 | 12.043 ± 0.162 b | 0.0028 |
| Female pupae weight (g) | 70 | 0.339 ± 0.013 a | 78 | 0.327 ± 0.009 ab | 73 | 0.293 ± 0.019 ab | 70 | 0.275 ± 0.016 b | 0.0404 |
| Male pupae weight (g) | 69 | 0.392 ± 0.006 a | 80 | 0.354 ± 0.017 a | 75 | 0.344 ± 0.036 a | 72 | 0.311 ± 0.007 a | 0.0903 |
| Adult emergence (%) | 134 | 96.575 ± 1.032 a | 150 | 94.493 ± 0.847 ab | 135 | 91.308 ± 0.878 b | 123 | 86.253 ± 0.990 c | <0.0001 |
| Sex ratio (♀/(♀ + ♂)) (%) | 134 | 50.650 ± 0.404 a | 150 | 50.333 ± 0.599 a | 135 | 49.180 ± 0.373 a | 123 | 48.643 ± 0.468 a | 0.0470 |
| Adults with deformities (%) | 134 | 3.443 ± 0.015 c | 150 | 4.250 ± 0.017 bc | 135 | 5.465 ± 0.026 ab | 123 | 6.245 ± 0.017 a | <0.0001 |
| Female adult longevity (days) | 68 | 14.128 ± 0.182 a | 75 | 14.458 ± 0.258 a | 66 | 13.490 ± 0.159 a | 60 | 12.725 ± 0.234 a | 0.0667 |
| Male adult longevity (days) | 66 | 13.265 ± 0.263 a | 75 | 13.325 ± 0.174 a | 69 | 12.518 ± 0.219 a | 63 | 11.378 ± 0.151 a | 0.0746 |
| APOPa (days) | 68 | 3.073 ± 0.079 b | 75 | 3.285 ± 0.038 ab | 66 | 3.815 ± 0.027 ab | 60 | 4.378 ± 0.046 a | 0.0204 |
| TPOPb (days) | 68 | 53.750 ± 0.783 c | 75 | 55.060 ± 0.482 bc | 66 | 56.145 ± 0.536 ab | 60 | 57.435 ± 0.689 a | 0.0007 |
| Oviposition period (days) | 68 | 8.788 ± 0.096 ab | 75 | 9.158 ± 0.202 a | 66 | 8.673 ± 0.097 ab | 60 | 8.148 ± 0.119 b | 0.0708 |
| Fecundity (eggs/♀) | 68 | 1210.423 ± 28.090 a | 75 | 1054.555 ± 46.164 b | 66 | 838.588 ± 17.816 c | 60 | 619.843 ± 12.393 d | <0.0001 |
| Eggs with deformities (%) | 68 | 5.570 ± 0.087 c | 75 | 7.155 ±± 0.105 bc | 66 | 10.083 ± 0.107 b | 60 | 14.673 ± 0.203 a | <0.0001 |
aAdult preoviposition period.
bTotal preoviposition period.
Means marked with different lowercase letters in the same row are significantly different (P < 0.05).
Effects of chlorantraniliprole on population parameters of A. ipsilon
The effects of low-lethal chlorantraniliprole concentrations on the population parameters of A. ipsilon were calculated using age-stage, two-sex life tables (Table 3). The intrinsic rate of increase (r) and the finite rate of increase (λ) decreased significantly in insects exposed to three low-lethal chlorantraniliprole treatments. The net reproductive rate (R0) was significantly reduced by the chlorantraniliprole treatments. At LC25 and LC45, chlorantraniliprole significantly prolonged the mean generation time (T), while the LC05 treatment resulted in no significant difference compared with the control. The gross reproductive rate (GRR) under the LC05 and LC45 treatments (682.39 and 347.89 offspring/individual at LC05 and LC45, respectively) was lower than the GRR of the control treatment (863.24 offspring/individual); however, the LC25 treatment did significantly increase the GRR (968.64 offspring/individual) compared with the control (Table 3).
Table 3.
Estimates of life table parameters of A. ipsilon when 3rd-instar larvae were exposed to low-lethal concentrations of chlorantraniliprole.
| Treatments | Population parameters | ||||
|---|---|---|---|---|---|
| r (d−1) | λ (d−1) | R0 (offspring/individual) | T (d) | GRR (offspring/individual) | |
| Control | 0.1211 ± 0.0016 a | 1.1287 ± 0.0029 a | 544.66 ± 35.6515 a | 52.05 ± 0.2641 b | 863.24 ± 28.4125 b |
| LC05 | 0.1110 ± 0.0027 b | 1.1174 ± 0.0037 b | 395.42 ± 28.4212 b | 53.86 ± 0.1987 b | 682.39 ± 35.6974 c |
| LC25 | 0.0981 ± 0.0019 c | 1.1031 ± 0.0034 c | 223.47 ± 31.0527 c | 55.14 ± 0.3656 a | 968.64 ± 41.7569 a |
| LC45 | 0.0859 ± 0.0021 d | 1.0897 ± 0.0023 d | 124.68 ± 22.3698 d | 56.12 ± 0.2543 a | 347.89 ± 32.4106 d |
SEs were estimated using 200,000 bootstraps and compared with paired bootstrap tests based on the 5% significance level.
Means followed by different lowercase letters in the same column are significantly different (P < 0.05).
r = the intrinsic rate of increase; λ = the finite rate of increase; R0 = the net reproduction rate; T = the mean generation time; GRR = the gross reproduction rate.
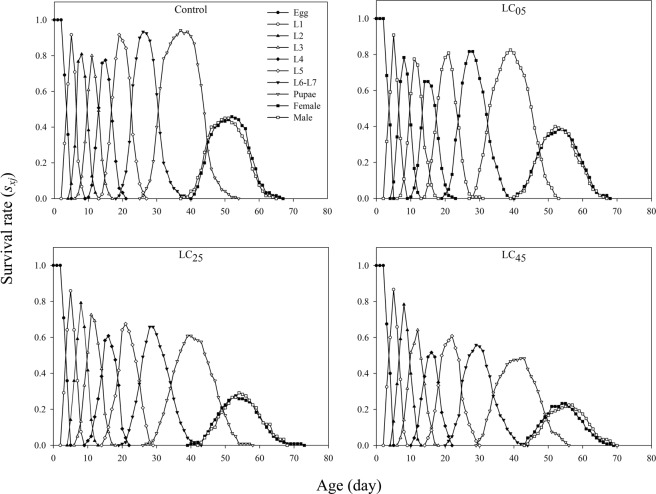
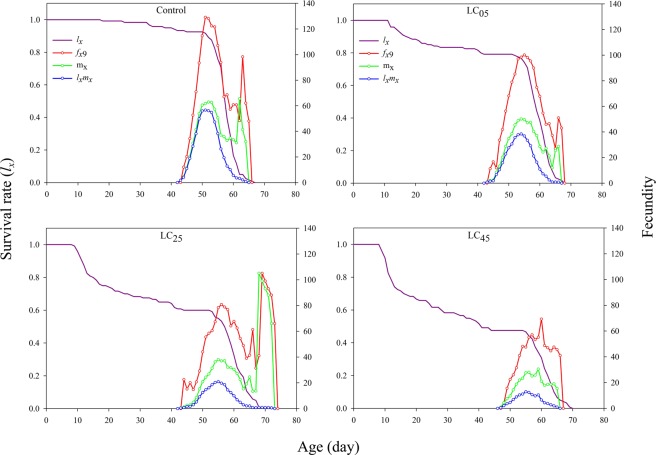
The age-stage-specific survival rate (sxj) curve is shown in Fig. 1. Because the development rate varied among individuals, a distinct overlap phenomenon was observed between the chlorantraniliprole-treated groups and the control (Fig. 1). With increasing concentration, the probability of survival from larvae to adults significantly decreased after exposure to the LC05, LC25 and LC45 treatments of chlorantraniliprole (Fig. 1). The age-specific survival rate (lx) is the probability of newborn eggs surviving to age x, regardless of stage differentiation (Fig. 2). The lx significantly decreased when the third instar larvae were exposed to the insecticide. The peak female age-specific fecundity (fx9) values under the LC05, LC25 and LC45 treatments were lower than that of the control treatment (Fig. 2). The age-specific fecundity of the total population (mx) under the LC25 treatment was higher than that of the control, while under the LC05 and LC45 treatments, the fecundity was lower than that of the control after exposure to chlorantraniliprole (Fig. 2). The lxmx value is mainly dependent on lx and mx, and the maximum lxmx values were 51, 54, 55 and 56 days for the control, LC05, LC25 and LC45 treatments, respectively, while the maximum values were 56.58, 38.38, 20.87, and 12.86 for the control, LC05, LC25 and LC45 treatments, respectively (Fig. 2).
Figure 1.
Age-stage specific survival rate (sxj) of A. ipsilon after exposure to low-lethal concentrations of chlorantraniliprole.
Figure 2.
Age-specific survival rate (lx), female age-specific fecundity (fx9), age-specific fecundity of the total population (mx), and age-specific maternity (lxmx) of A. ipsilon after exposure to low-lethal concentrations of chlorantraniliprole.
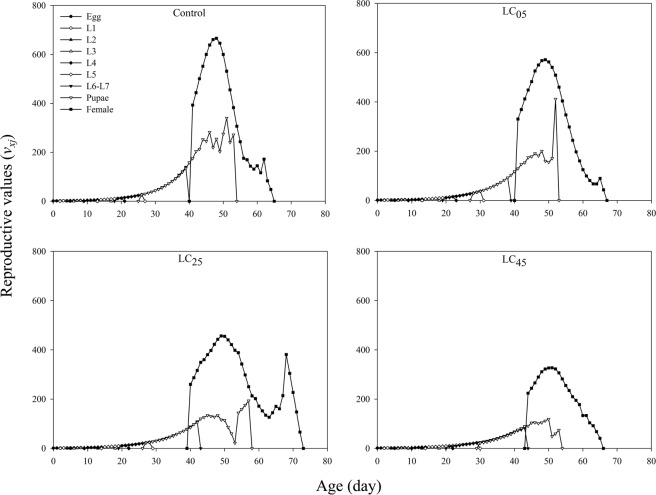
The age-stage-specific reproductive value (vxj) curves are shown in Fig. 3. However, the vxj curves for males were not produced, mainly because the contribution of males to offspring cannot be defined. There were no significant effects on the reproductive values of eggs and larvae when A. ipsilon larvae were treated with the insecticide. The peak reproductive values of the pupae under LC05 treatment were higher than that of the control, while under the LC25 and LC45 treatments, the peak was lower than that of the control. At low-lethal concentrations, chlorantraniliprole significantly decreased the female reproductive values, with peak vxj values of 666.09, 571.68, 456.63, and 327.36 for the control, LC05, LC25 and LC45, respectively (Fig. 3).
Figure 3.
Age-stage specific reproductive value (vxj) of A. ipsilon after exposure to low-lethal concentrations of chlorantraniliprole.
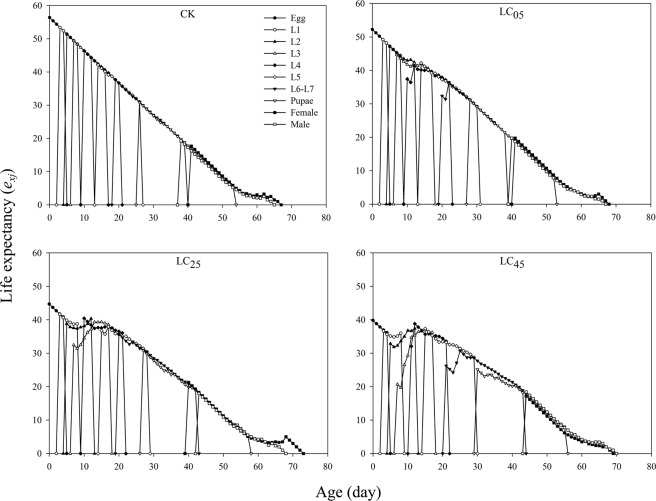
The life expectancy (exj) curves for each age-stage group of A. ipsilon are shown in Fig. 4. The exj value decreased gradually with age x because this study was carried out in the laboratory and was not adversely affected by other field conditions. At LC05, LC25, and LC45, chlorantraniliprole significantly decreased the exj of the egg and larval stages compared to the control group. The pupal exj dramatically decreased under the LC45 treatment compared to the LC05 and LC25 treatments and the control, while the exj of females decreased under the LC25 treatment compared to the LC05 and LC45 treatments (Fig. 4).
Figure 4.
Life expectancy (exj) of A. ipsilon after exposure to low-lethal concentrations of chlorantraniliprole.
Effects of chlorantraniliprole on nutritional indices
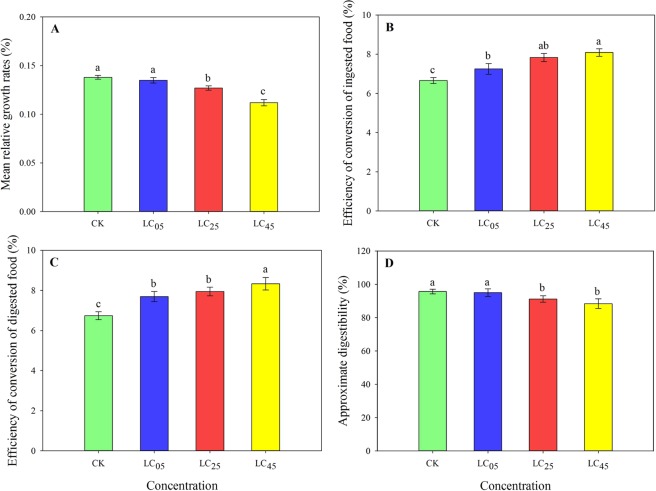
At low-lethal concentrations, chlorantraniliprole, to a certain extent, did affect the nutritional indices of A. ipsilon larvae (Fig. 5). The mean relative growth rates (MRGRs) and the approximate digestibility (AD) of the third instar larvae were significantly lower after the LC25 and LC45 treatments than after the LC05 treatment and the control treatment (Fig. 5A,D). Nevertheless, the efficiency of conversion of ingested food (ECI) and the efficiency of conversion of digested food (ECD) were much higher after exposure to the three low-lethal concentrations of chlorantraniliprole than after exposure to the control (Fig. 5B,C).
Figure 5.
Nutritional indices (A) mean relative growth rates, (B) efficiency of conversion of ingested food, (C) efficiency of conversion of digested food, (D) approximate digestibility) of third-instar larvae of A. ipsilon (Mean ± SE) after exposure to low-lethal concentrations of chlorantraniliprole. Bar with the same lowercase letters show no significant differences (Student-Newman-Keuls test, P < 0.05).
Effects of chlorantraniliprole on nutrient content
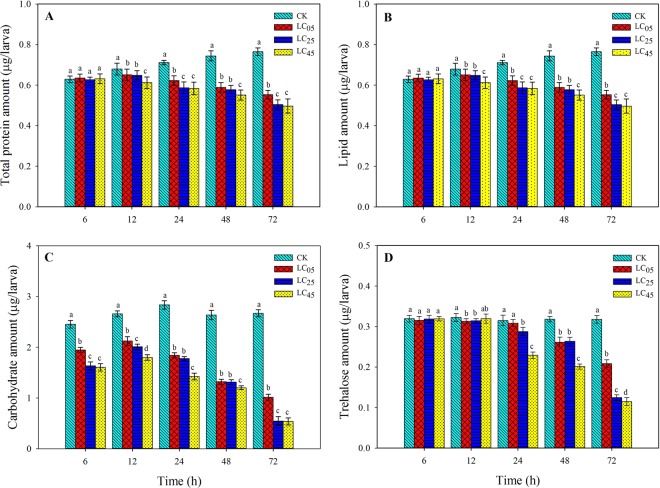
The effects of chlorantraniliprole on the nutrient content in the third instar larvae of A. ipsilon are shown in Fig. 6. At low-lethal concentrations, chlorantraniliprole significantly decreased the protein and lipid content after treatment for 12, 24, 48, and 72 h compared with the control treatment (Fig. 6A,B). However, no significant effect was observed between the LC05, LC25 and LC45 treatments and the control after 1 h of treatment. The carbohydrate content was significantly decreased under the LC05, LC25 and LC45 treatments compared with the control (Fig. 6C). In addition, chlorantraniliprole did, to some extent, reduce the trehalose content when the third instar A. ipsilon larvae were treated with the three low-lethal concentrations of the insecticide (Fig. 6D).
Figure 6.
Nutrient content (A) total protein amount, (B) lipid amount, (C) carbohydrate amount, (D) trehalose amount) in A. ipsilon (mean ± SE) after after third-instar larvae exposure to low-lethal concentrations of chlorantraniliprole. Bar with the same lowercase letters indicate no significant differences (Student-Newman-Keuls test, P < 0.05).
Effects of chlorantraniliprole on digestive enzyme activities
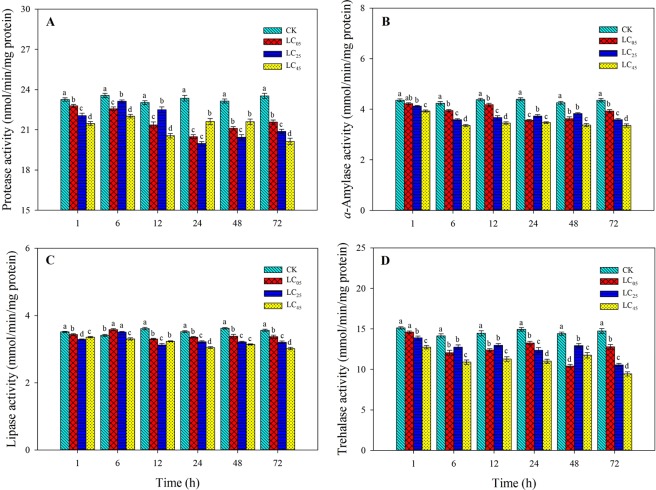
The effects of chlorantraniliprole on digestive enzyme activities in third instar A. ipsilon larvae are shown in Fig. 7. At low-lethal concentrations, chlorantraniliprole observably affected protease activity, α-amylase activity, or trehalase activity in A. ipsilon larvae compared to the effects of control treatment at 1, 6, 12, 24, 48, and 72 h (Fig. 7A,B and D). Lipase activity was significantly enhanced at 24 h after treatment with chlorantraniliprole at LC05 and LC25 compared to the activity with control treatment. However, only LC45 treatment significantly decreased lipase activity after treatment for 24 h compared to that of the control (Fig. 7C). Overall, the digestive enzyme activities decreased in a concentration-dependent manner.
Figure 7.
Digestive enzyme activities (A) protease, (B) α-amylase, (C) lipase, (D) trehalose) in A. ipsilon larvae (mean ± SE) after exposure to low-lethal doses of chlorantraniliprole. Bar with the same lowercase letters show no significant differences (Student-Newman-Keuls test, P < 0.05).
Effects of chlorantraniliprole on detoxifying enzyme activities
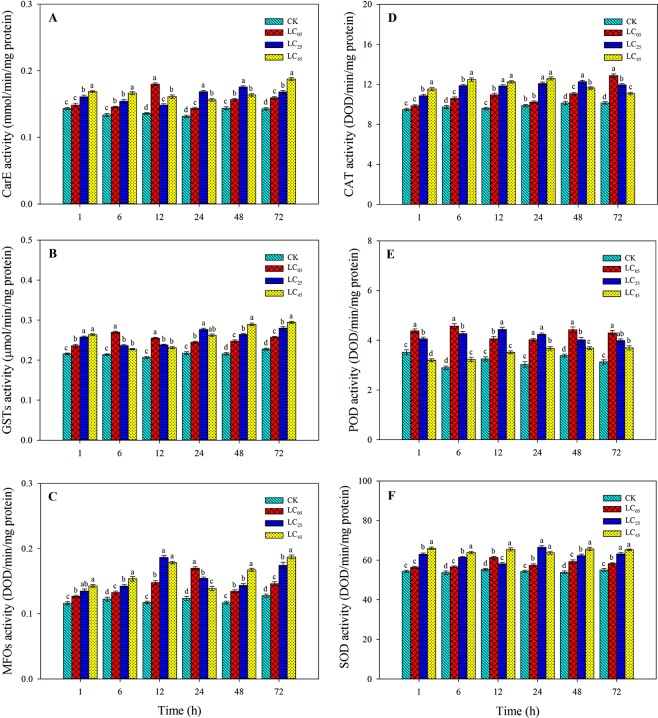
The activities of detoxifying and protective enzymes were determined after third instar A. ipsilon larvae were treated with the three low-lethal concentrations of chlorantraniliprole (Fig. 8). After only 1 h, the carboxyl esterase activity (CarE) of the LC05 treatment exhibited no significant difference compared to the control; however, under the LC25 and LC45 treatments, the CarE activity increased significantly. Chlorantraniliprole significantly enhanced the activity of CarE after treatment for 6, 12, 24, 48, and 72 h (Fig. 8A). Glutathione S-transferase activity (GSTs) and multifunctional oxidase activity (MFOs) increased significantly under LC05, LC25 and LC45 chlorantraniliprole treatments after 1, 6, 12, 24, 48, and 72 h (Fig. 8B,C).
Figure 8.
Detoxification enzyme activities (A) carboxyl esterase (CarE), (B) glutathione S-transferase (GSTs), (C) O-demethylation multi-function oxidase (MFOs)) and protective enzyme activities (D) catalase (CAT), (E) peroxidase (POD), (F) superoxide dismutase(SOD)) in third-instar larvae of A. ipsilon (mean ± SE) after exposure to low-lethal concentrations of chlorantraniliprole. Bar with the same lowercase letters indicate no significant differences (Student-Newman-Keuls test, P < 0.05).
Effects of chlorantraniliprole on protective enzyme activities
Catalase activity (CAT) and superoxide dismutase activity (SOD) significantly increased under all chlorantraniliprole treatments compared with those of the control group (Fig. 8D,F). After 1 h, the LC45 treatment significantly decreased the activity of peroxidase (POD), while POD activity increased dramatically after treatment for 6, 12, 24, 48, and 72 h compared with that of the control. At LC05 and LC25, chlorantraniliprole significantly enhanced the activity of POD compared to that of the LC45 treatment and control groups (Fig. 8E).
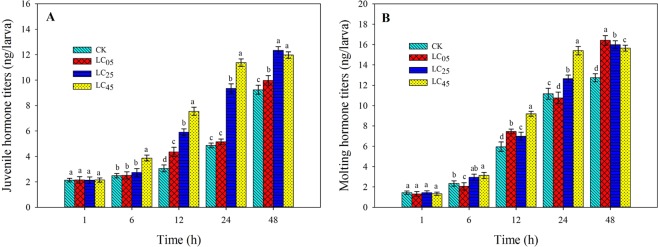
Effects of chlorantraniliprole on hormone titers
The effects of low-lethal concentrations of chlorantraniliprole on hormone titers in A. ipsilon larvae were determined (Fig. 9). The juvenile hormones (JH) and molting hormones (MH) titers clearly increased with growth and development of the third instar larvae of A. ipsilon. As the concentration increased, the JH and MH titers of the A. ipsilon larvae increased to some extent after exposure to the three treatment concentrations of chlorantraniliprole. However, there was no significant difference between the treatments and the control after chlorantraniliprole treatment for 1 h. The LC05 treatment significantly reduced the hormone titers after treatment for 6 h and 24 h compared to the control.
Figure 9.
Hormone titers (A) juvenile hormone titers, (B) molting hormone titers) in A. ipsilon larvae after treatment with chlorantraniliprole. Bar with the same lowercase letters show no significant differences (Student-Newman-Keuls test, P < 0.05).
Discussion
When selecting insecticides, in addition to acute toxicity, chronic toxicity against target pests should also be closely considered, as chronic toxicity could result in reduced survival, lifespan, growth and development, fecundity, and population growth after exposure to low doses of the toxic substance26,27. Insecticides with different modes and mechanisms of action can be applied to generate various effects against targeted insects at low-lethal concentrations28,29. Moreover, sublethal effects of the same insecticide on different types of target pests can also be significantly different21,30. In this study, we confirmed that chlorantraniliprole exhibits active toxicity against third instar larvae. Moreover, chlorantraniliprole at low-lethal doses considerably influenced the development, reproduction and demographic parameters of this cutworm. Many lepidopteran pests are also highly sensitive to this insecticide7–9, which has been registered for controlling many lepidopteran and non-lepidopteran pests in China31. Previous studies have shown that chlorantraniliprole can be used not only for foliar applications but also for seed treatment because it has excellent systemic properties and persistence32,33. Chlorantraniliprole could be applied for efficiency controlling the cutworm by seed treatment, which is an important strategy for insect pest management.
The larval stages of target insects can be significantly prolonged after exposure to low-lethal doses of insecticides11. We observed that chlorantraniliprole at low-lethal concentrations could also prolong A. ipsilon larval development (fifth and sixth stages). Previous studies have demonstrated that low doses of chlorantraniliprole could prolong the development time of many lepidopteran insect pests10,34–36. These results are consistent with those observed with the anthranilic diamide insecticide cyantraniliprole, which also prolonged the larval development of some lepidopteran pests29,37. We also found that the LC05 treatment of this insecticide could lengthen the durations of the pupal and adult stages. This effect was mainly due to the starvation caused by cessation of feeding, which led to the energy of the larvae being diverted to detoxification and metabolic processes rather than being used for individual growth and development9,27. The proportion of seventh-instar larvae attained increased when the A. ipsilon larvae were treated with this insecticide. The time needed to accumulate sufficient nutrients to maintain normal growth and development of A. ipsilon was longer when the third instar larvae were exposed to chlorantraniliprole than that needed with the control treatment.
Life table analysis is an effective tool for assessing the sublethal effects of insecticides on target insects at the population level, rather than at the individual level38,39. We found that low-lethal concentrations of chlorantraniliprole significantly impared the population parameters, including R0, λ, r and T, which are the most useful indicators of reproductive expectations for insect populations40. Chlorantraniliprole significantly extended the APOP and TPOP. The oviposition period was significantly prolonged under the LC05 treatment, while the LC25 and LC45 treatments significantly shortened the A. ipsilon female oviposition period. All the treatments resulted in a significant decrease in the total number of eggs laid by the female adults. Thus, these results indicated that female fecundity was significantly decreased after chlorantraniliprole treatment, largely because the mating times and oviposition period were greatly reduced. Similar studies have shown that chemicals can have adverse effects on female mating behavior and fecundity of target insects41,42. These results showed that low-lethal concentrations of chlorantraniliprole markedly inhibited A. ipsilon population growth.
Sublethal effects, such as hormesis the for F0 generation and selection for the F1 generation, of insecticides are considered to be common toxicological phenomena and could result in pest resurgence and secondary outbreaks43,44. The hormetic effects of insecticides have been described by Calabrese and Baldwin45 and stimulatory effects (low-dose stimulation and high-dose inhibition) have been observed in various insects46,47. Fecundity in adult females of target insect pests has been seen to significantly increase upon treatment with low-lethal concentrations of pesticides46,47. However, many studies have also shown that low doses of insecticides have adverse effects on pest reproduction37. The effects of hormesis are closely associated with insect species, pesticide mode of action and physiological states.
Demographic parameters, including the age-stage-specific survival rate (sxj), age-specific survival rate (lx), female age-specific fecundity (fx9), age-specific maternity (lxmx), female age-stage-specific reproductive value (vxj) and life expectancy (exj) of A. ipsilon were reduced dramatically after exposure to chlorantraniliprole, which had a negative effect at the population levels. Nevertheless, the LC25 chlorantraniliprole treatment did, to some extent, increase the age-specific fecundity of the total population (mx), which was the same stimulatory effect as that observed for the GRR. This result proved that LC25 treatment may have had a certain stimulatory effect on the fecundity of the A. ipsilon population. However, no studies have shown that the mx and GRR can be used to determine whether the target insects produce hormesis after exposure to pesticides. Therefore, chlorantraniliprole at low-lethal concentrations does not stimulate adult fecundity of A. ipsilon. In this study, we examined the sublethal effects of this chemical on only the parental generation of A. ipsilon under laboratory conditions. Therefore, field trials and long-term studies on offspring should be conducted in the future.
Feeding efficiency is an important aspect of feeding ecology, describing the ability of insects to convert ingested food to biomass and the effects of external and internal factors48. In our study, the AD was significantly reduced, largely due to feeding inhibition after the A. ipsilon larvae were treated with chlorantraniliprole. Nathan et al. showed that retarded movement of food in the digestive tract and prolonged larval development time resulted in increased AD values49. This finding is similar to our results, in which the development time of A. ipsilon larvae was lengthened after exposure to low-lethal doses of chlorantraniliprole. Stoyenoff et al. found that the high AD was mainly due to the passage of food through the digestive tract at a decreased rate when the target insect had consumed a low amount of food50. With larval growth, treatment with this chemical also led to significant reduction in the MRGR, which is an index that is closely associated with insect weight, with increasing concentration of insecticide29. Chlorantraniliprole reduced the pupal weight; however, no significant difference was observed in pupal weight after exposure to low-lethal doses of the insecticide. Similarly, the MRGRs of Cnaphalocrocis medinalis were dramatically reduced after fourth-instar larvae were treated as described by Nathan49. Liu et al. also found that fraxinellone greatly decreased the MRGRs of Ostrinia furnacalis51. In contrast, the ECI and ECD significantly increased with increasing concentrations of this chemical. Previous work has demonstrated that insecticides and plant extracts have negative effects on the feeding efficiency of targeted insects48,52. These findings suggest that artificial diets treated with insecticides may act as inhibitors by denying the sufficient nutrients for normal growth and metabolic processes. This phenomenon may be directly associated with the low survival rate, developmental delay and reproduction of the A. ipsilon population after exposure to chlorantraniliprole.
Proteins, carbohydrates and lipids are important chemical constituents of insect bodies that provide the necessary energy to maintain bodily functions and participate in the metabolic processes of many physiological and biochemical reactions29,38. In this study, chlorantraniliprole at low doses significantly reduced the protein content. When protein levels are low, metabolic processes may be disrupted by the degradation of proteins to amino acids and by the TCA cycle compensating for the decrease in energy caused by pesticide stress53. Protease activity was significantly reduced after exposure to the chemical. This result was consistent with another report, which showed that clitocypin inhibits protease activity in Leptinotarsa decemlineata (Say)54. Protease activity in Bradysia odoriphaga was decreased upon treatment with benzothiazole21 and chlorfenapyr38. The carbohydrate content was markedly decreased when A. ipsilon was treated with chlorantraniliprole, which may be due to reduction of food consumption. Simultaneously, α-amylase activity was also significantly reduced after exposure to the insecticide, resulting in reduced conversion of glycogen to glucose and hydrolysis of starch to maltose after chlorantraniliprole treatment21. These results were consistent with the reports of Etebari et al.55 and Yazdani et al.48 and may also explain the decrease in carbohydrate content. In addition, chlorantraniliprole significantly affected the lipid content and lipase activity, which may be associated with the disruption of lipid metabolism and hormone secretion after treatment with the chemical56. Zibaee et al. found that the activity of lipase in Eurygaster integriceps was reduced after exposure to Artemisia annua extract57. Many studies have also demonstrated that chemical insecticide compounds affect lipid content and lipase activity29,38. The levels of these macronutrients were reduced in the body when the feeding efficiency of the target insects was affected by insecticides, and this effect varied among feeding conditions and different growth stages38. Upon insecticide treatment, the normal consumption and digestion processes are affected, mainly due to imbalance of the secretion and function of digestive enzymes in target insects57.
Trehalose is the main blood sugar in the hemolymph of insects and is an important energy source in many physiological activities, such as metamorphosis, flight and reproduction, regulating trehalose metabolism and controlling the utilization of glucose29,58. Trehalose can effectively prevent protein denaturation and loss of function under adverse conditions38. In this study, trehalose content and trehalase activity decreased significantly after exposure to chlorantraniliprole. Previous studies have demonstrated an increase in the proportion of trehalose among total carbohydrates to enhance the ability to adapt to insecticidal stress58. This effect is similar to those of benzothiazole and chlorfenapyr on B. odoriphaga29,38 and that of hexaflumuron on Apolygus lucorum59. In addition, the trehalose content can also affect the food selection and feeding behavior of insects60. Therefore, trehalose plays a very important role in the regulation of the growth and development of target insects.
Insecticides not only affect the growth and development, fecundity and population parameters of target insects but also disrupt the activities of protective enzymes and detoxifying enzymes38,61. In the present study, chlorantraniliprole at low-lethal concentrations significantly increased the activities of CAT and SOD in A. ipsilon larvae at all the studied time points compared with the control groups, which was consistent with the results of Zhang et al.62 POD activity was initially inhibited and subsequently activated after exposure to the LC05 treatment of chlorantraniliprole. The LC25 and LC45 treatments significantly induced SOD activity. CAT, POD and SOD are three major protective enzymes that maintain a state of dynamic equilibrium, protecting target insects from free-radical attacks62,63. Therefore, activation or inhibition of SOD, CAT and POD activities by insecticide treatment could disrupt the physiological metabolic homeostasis and ultimately lead to growth inhibition and insect death.
Detoxifying enzymes are the main enzymes involved in the metabolism of toxic substances in insects38,61. In this study, the activities of CarE and GSTs increased significantly in the chlorantraniliprole treatments compared to the control groups. Previous studies have demonstrated that CarE and GSTs are the primary enzymes involved in the metabolism of xenobiotics (e.g., insecticides) in target insects29,38,64. In addition, chlorantraniliprole at low-lethal doses greatly increased the MFO activity compared to the control. A significant difference was observed between the treatments and the control, and this difference increased as the concentration of the chemical increased. These results were consistent with those of Zhang et al.62. and Devorshak et al.65. Furthermore, the detoxification process requires a large amount of energy, which may lead to reduced nutrient content in insects, as observed in this study. This finding has also been confirmed in reports by Zhao et al.29, Devorshak et al.65 and Yazdani et al.48. In addition, Zhao et al. found that these enzymes allow the treated insects to survive, and the activities of these enzymes may lead to an increase in total protein content after insecticide treatment29. However, this result was different from the results of this study, which may be partly due to the unique mechanism of action of chlorantraniliprole. These results indicated that these enzymes could play a role in chlorantraniliprole metabolism and detoxification.
MH and JH regulate many physiological processes (e.g., molting, ecdysis, reproduction and behavior) during the growth and development of target insects52. In the present study, no significant difference was observed between the chlorantraniliprole treatments and the control after treatment for 1 h; however, the MH and JH titers significantly increased after treatment with the chemical with increasing treatment time. Previous research has suggested that JH and MH titers vary among insect stages and host plant species66. The development time could be delayed by alteration of the hormone titers in insects after insecticide treatment52. Chlorantraniliprole at low doses prolonged not only larval development but also pupal development. However, previous findings have shown that MFOs metabolize and decrease the levels of JHs and JH analogs, which may result in shortening of the development time of target insects by insecticide treatment62,67. This study shows that the mechanism of action of chlorantraniliprole on A. ipsilon is complex, and further genomic and proteomics studies are required.
Materials and Methods
Insect rearing and insecticide
A. ipsilon adults were originally collected from a cornfield in Ningyang City (35.76N, 116.80E) in the Shandong Province of China during June 2012. After collection, adult moths were transferred to a spawning container (30 cm L × 15 cm W × 15 cm H) and provided with a 20% honey solution as food. The eggs were collected daily and moved to an incubation container (15 cm L × 15 cm W × 10 cm H) to hatch.
Newly hatched larvae were transferred into 24-well cell culture plates (Jet, Biofil, TCP010024) and reared with fresh leaves of pesticide-free cabbage (Brassica oleracea L.) before third instar larvae developed. Then, third instar and/or higher instar larvae were reared individually in 12-well cell culture plates (Jet, Biofil, TCP010012) to prevent cannibalism and were given an artificial diet68. Larvae were transferred into finger-shaped glass tubes (2.2-cm diam., 8 cm H) at the last larval instar so that larvae could molt normally into the pupal stage. All pupae were collected and then shifted into a spawning container after emergence. To maintain genetic diversity of the colony, feral A. ipsilon adults were collected in local cornfields annually. Rearing was maintained at 28 ± 1 °C, with 75 ± 5% relative humidity (RH) and a photoperiod of 16:8 h (L: D) in a climate-controlled room.
The chlorantraniliprole (95.3% technical grade) in this study was supplied by Shanghai DuPont Agrochemical Co., Ltd., China. Molting hormone (95%, MH) and juvenile hormone (93%, JH) were purchased from Sigma Chemical Co., USA.
Lethal effects of chlorantraniliprole on third instar larvae of A. ipsilon
The bioassay method to assess the effects of chlorantraniliprole on newly molted third instar larvae (<12 h) of A. ipsilon was determined by mixing an artificial diet with insecticide. The active ingredient was dissolved with analytical grade acetone at a concentration of 100 mg/L. In a preparatory test to find the effective dose range, a series of desired chlorantraniliprole concentrations were prepared by diluting with distilled water. Chlorantraniliprole was added to the diet and mixed thoroughly to obtain concentrations of 4.0, 2.0, 1.0, 0.5, 0.25, and 0.125 μg.g−1; the temperature of the artificial diet was dropped below 50 °C during preparation processes. The control treatment was mixed with distilled water. The diet was cut into 1-cm3 cubes.
The third instar larvae (<12 h) of the same batch were transferred into 12-well cell culture plates and starved 3–4 h before toxicity tests. Larvae were fed with the artificial diet treated with different concentrations of chlorantraniliprole. For each treatment, 120 third instar larvae were used, and the bioassay employed a completely randomized design with seven treatments and five replicates. Larvae that showed extreme shrinkage compared with that of the control animals or larvae that were unable to move normally when stimulated with a writing brush after 72 h of treatment were considered dead.
Sublethal effects of chlorantraniliprole on the growth and population parameters of A. ipsilon
To assess the sublethal effects of chlorantraniliprole on A. ipsilon, artificial diets containing chlorantraniliprole at LC05, LC25 and LC45 and distilled water for the control were prepared. Newly molted third instar larvae were selected and each individual larva was considered one replicate. The tested larvae were fed an insecticide-treated diet (at LC05, LC25, LC45) or an untreated diet (as a control) prepared in advance as described above.
The surviving A. ipsilon larvae from all the treatments were selected and individually transferred into clean 12-well cell culture plates after 72 h of treatment. The larvae were reared with an artificial diet without chlorantraniliprole treatment provided ad libitum and monitored daily until they reached the pupal stage. Pupae that did shrink, soft and rigidity of body were considered deformity. All pupae were individually shifted into finger-shaped glass tubes until adult insects emerged. The pupae (two days old) were weighed using an electronic analytical balance (Sartorius, BT125D, Germany). Adults that did deficiency and asymmetry of wings were considered deformity. Newly emerged A. ipsilon adults were collected from the glass tubes, sexed and paired (one female with one male) in a spawning container (10 cm diam. × 10 cm height), and 20% honey solution was provided as food to the adult moths. The oviposition container containing adults was checked daily for oviposition after eggs were first observed. Eggs were collected daily on Kraft paper, which served as an oviposition substrate and was replaced with new Kraft paper daily. All eggs were counted on the next day with a magnifying glass. Eggs that did dehydrated and absence of pedicel were considered deformity. During the experiment, all artificial diets and the 20% honey solution were kept fresh to ensure normal growth of larvae and adults.
Effects of chlorantraniliprole on nutritional indices of A. ipsilon larvae
To evaluate the effects of chlorantraniliprole on food consumption and utilization by A. ipsilon larvae, artificial diets containing chlorantraniliprole at the LC05, LC25, and LC45 concentrations were prepared in advance. An artificial diet treated with distilled water was used as the control. The weights of all tested insects and food provided in this experiment were determined using an electronic analytical balance. Then, the third instar larvae were reared as described above. After treatment for 72 h, the surviving larvae and any remaining artificial diet and fecal matter produced were separated and weighed. To compensate for the decrease in weight of the food provided to the larvae because of evaporation, we simultaneously conducted a control experiment by maintaining weighed diets in 12-well cell culture plates and reweighed these diets after 72 h. Each of 20 larvae were tested per replicate, and six replicates we examined for each treatment. This experiment was conducted for 72 h. The food utilization rates were estimated according to the following formulas69:
where W1 = the weight of larvae before treatment (g); W2 = the weight of larvae after treatment (g); T = the duration of the experimental period (days); Q = the fresh weight gain of larvae during the experimental period (g); and F = the mass of fecal matter produced by larvae during the experimental period (g).
Effects of chlorantraniliprole on the nutrient content of A. ipsilon
For preparation of samples for physiological and biochemical assays, newly molted third instar larvae (<12 h) were fed artificial diets containing chlorantraniliprole at the LC05, LC25 and LC45 concentrations (as described above). The surviving larvae were selected and weighed after treatment. Larvae were placed in individual 2.0-mL centrifuge tubes and treated with liquid nitrogen, and then the samples were stored at −80 °C for preservation. One larva was tested per replicate, and 20 replicates were examined for each treatment. All procedures were carried out in an ice bath when the enzyme sources were prepared.
Carbohydrate content
Individual larvae were homogenized in 500 μL of 10% trichloroacetic acid (TCA) on ice and centrifuged at 20,000 g force for 10 min at 4 °C. For each sample, 30 μL of the supernatant was mixed with 70 μL of 10% TCA. Then, 600 μL of 0.2% anthrone (200 mg of anthrone dissolved in 100 mL of 98% H2SO4) was added to each sample tube, which was then heated in a thermostat-controlled water bath at 90 °C for 10 min. The absorbance was measured at 630 nm using the BioTek SynergyTM 2 Multi-Mode Reader (BioTek Instruments, Inc. Winooski, Vermont, USA). The carbohydrate content of all the samples was quantified based on the standard curve for glucose.
Lipid content
Individual larvae were homogenized in 200 μL of 2% Na2SO4. Lipids were extracted with 750 μL of methanol:chloroform (1:2) for 4 h. Then, the mixture was centrifuged at 20,000 g force for 10 min at 4 °C. Five hundred microliters of the supernatant of each sample was transferred into a new tube and dried at 40 °C for 12 h. All samples were treated with 500 μL 98% H2SO4 individually and incubated in a water bath at 90 °C for 10 min. Finally, 30 μL of each sample was mixed with 270 μL of vanillin reagent (6 mg of vanillin dissolved in 1 mL of distilled water and 4 mL of 85% H3PO4). The absorbance was measured at 530 nm after 30 min, and the standard curve was prepared using cholesterol.
Trehalose content
Individual larvae were homogenized with 100 μL of 20 mM phosphate buffer (pH 5.8) on ice, followed by centrifugation at 20,000 g force at 4 °C for 10 min. Then, 30 μL of the supernatant was mixed with 30 μL of 1% H2SO4 and incubated in a thermostat-controlled water bath at 90 °C for 10 min. After cooling on ice for 3 min, 30 μL of 30% KOH was added to each sample, and the mixture was incubated again at 90 °C for 10 min. Next, 600 μL of 0.2% anthrone was added after cooling for 3 min on ice, and the mixtures were incubated again at 90 °C for 10 min. After cooling on ice, the absorbance was measured at 630 nm, and trehalose was used to establish the standard curve, which was used for determining the trehalose content.
Total protein content
The total protein content was determined according to the method developed by Bradford (1976)70. Individual larvae were homogenized in 600 μL of 50 mM Tris-HCl (pH 7.1; containing 0.5% Triton X-100 and 20% sucrose) on ice, and then the samples were centrifuged at 20,000 g force at 4 °C for 10 min. Then, 30 μL of the supernatant was subsequently mixed with 150 μL of 0.01% Coomassie Brilliant Blue G-250 as a dye. Then, the absorbance was measured at 595 nm using a BioTek SynergyTM 2 multimode reader. The total protein content was calculated using a standard curve based on bovine serum albumin.
Effects of chlorantraniliprole on A. ipsilon digestive enzyme activities protease activity
Individual larvae were homogenized with 100 μL of 20 mM phosphate buffer (pH 7.0) on ice, followed by centrifugation at 20,000 g force at 4 °C for 10 min. Casein was used as the substrate. Then, 30 μL of the supernatant was mixed with 100 μL of 1% casein and incubated in a water bath at 37 °C for 15 min. Next, this reaction was stopped via the addition of 100 μL of 0.4 M TCA, followed by centrifugation at 20,000 g force at 4 °C for 10 min. A 100 μL aliquot of the supernatant was subsequently transferred to a new tube and mixed with 500 μL of 0.4 M Na2CO3 and 100 μL of Folin-Ciocalteu reagent, after which, the sample was incubated at 37 °C for 30 min, and the absorbance was measured at 660 nm. Tyrosine was used to generate the standard curve.
Lipase activity
The crude enzyme was extracted as described in protease activity. Then, 10 μL of the enzyme solution was mixed with 18 μL of 50 mM 4-nitrophenyl butyrate and 172 μL of phosphate buffer (pH 7.0) and incubated in a water bath at 37 °C for 15 min. Next, the mixture was transferred to −20 °C for 5 min to stop the reaction. The absorbance was measured at 405 nm, and p-nitrophenol was used to establish the standard curve.
α-Amylase activity
First, the crude enzyme was extracted with 20 mM sodium phosphate buffer (pH 6.9) on ice, followed by centrifugation at 20,000 g force at 4 °C for 10 min. Next, 10 μL of the supernatant was mixed with 40 μL of 1% soluble starch and 50 μL of phosphate buffer, and the reaction was maintained in a water bath at 35 °C for 30 min. Then, the reaction was stopped by adding 100 μL of dinitrosalicylic acid (DNS) and heating in boiling water for 10 min. After cooling on ice, the absorbance was measured at 540 nm, and the standard curve was established using maltose.
Trehalase activity
First, the crude enzyme was extracted with 20 mM phosphate buffer (pH 5.8). In each tube, 30 μL of the supernatant was mixed with 60 μL of 40 mM trehalose, followed by incubation at 37 °C for 30 min. Then, the reaction was arrested by heating in boiling water for 3 min and cooling on ice. Next, 90 μL of an indicator dye (5 g of 3,5-dinitrosalicylic acid, 5 g of NaOH, 1 g of phenol and 0.25 g of anhydrous sodium sulfite dissolved in 500 mL of distilled water) was added to each tube, and the mixture was incubated in a water bath at 90 °C for 5 min. After cooling on ice, 30 μL of potassium sodium tartrate was added, and the absorbance was measured at 550 nm. The standard curve was established using glucose.
Effects of chlorantraniliprole on A. ipsilon detoxifying enzyme activities carboxyl esterase activity (CarE)
Individual larvae were homogenized in 100 μL of 40 mM phosphate buffer (pH 7.0) on ice, followed by centrifugation at 20,000 g force at 4 °C for 10 min. Then, 25 μL of the supernatant was mixed with 100 μL of 0.03 M α-naphthyl acetate, which was used as a substrate for the enzymatic reaction, and 1 mL of 1 × 10−4 M eserine, which was diluted with 40 mM phosphate buffer (pH 7.0) to 100 mL. The mixtures were incubated in a 37 °C water bath for 10 min. Next, the reactions were stopped by the addition of 75 μL of stopping solution (1% Fast Blue B salt solution and 5% sodium dodecyl sulfate solution mixed at a ratio of 2:5). The absorbance was measured at 600 nm after 30 min, and α-naphthol was used to generate the standard curve.
Glutathione S-transferase activity (GSTs)
Individual larvae were homogenized in 100 μL of 66 mM buffer (pH 7.0; 58 mg of EDTA in 66 mL of 0.1 M PBS (pH 7.0) and 34 mL of double-distilled water) on ice, followed by centrifugation at 20,000 g force at 4 °C for 10 min. Then, 10 μL of the supernatant was mixed with 5 μL of 30 mM 2,4-dinitrochlorobenzene (DNCB) and 15 μL of 50 mM glutathione. The control group was not treated with the enzyme extract. The absorbance was measured at 340 nm for 15 min. Enzyme activity was expressed in μmol/min/mg protein.
Multifunctional oxidase activity (MFOs)
Individual larvae were homogenized in 200 μL of 0.1 M phosphate buffer (pH 7.0; containing 1 mM dithiothreitol, 1 mM ethylene diamine tetraacetic acid, 0.4 mM phenylmethanesulfonyl fluoride, and 1 mM propylthiouracil on ice, followed by centrifugation at 20,000 g force at 4 °C for 10 min. Then, 90 μL of the supernatant was mixed with 100 μL of 2 mM p-nitroanisole (substrate) for 2 min. Next, 10 μL of 9.6 mM triphosphopyridine nucleotide was added, and the resulting mixture was immediately placed in a microplate reader. The absorbance was measured at 405 nm once per minute for 30 min. Enzyme activity was expressed in ΔOD/min/mg protein.
Effects of chlorantraniliprole on A. ipsilon protective enzyme activities peroxidase activity (POD)
Individual larvae were homogenized in 200 μL of precooled 1% polyvinylpyrrolidone solution on ice, followed by centrifugation at 20,000 g force at 4 °C for 10 min, and the supernatant was used as the enzyme source. Next, a 4-mL reaction system was prepared, containing 200 μL of enzyme source, 1.8 mL of acetate buffer (pH 5.0), 1 mL of 0.1% guaiacol and 1 mL of 0.08% H2O2. The absorbance was measured at 470 nm at 30 °C for 30 min using a BioTek SynergyTM 2 multimode reader. Enzyme activity was expressed in ΔOD/min/mg protein.
Catalase activity (CAT)
The protocol for enzyme source extraction was consistent with those of POD activity. The reaction system contained 2 mL of pH 7.0 phosphate buffer, 1 mL of 0.08% H2O2 (the blank instead used 1 mL of distilled water) and 100 μL of enzyme source. Absorbance was read at 240 nm at 30 °C for 30 s using the BioTek SynergyTM 2 Multi-Mode Reader. Enzyme activity was expressed in ΔOD/min/mg protein.
Superoxide dismutase activity (SOD)
Extraction of enzyme source was performed as described in POD activity. A 3-mL reaction system was prepared, containing 20 μL of enzyme source, 80 μM riboflavin, 77 μM nitro-blue tetrazolium (NBT), 13 mM methionine and 0.1 mM EDTA. The reaction was performed by illuminating at 4000 l for 5 min and stopped by placing in the dark. Then, the absorbance was measured at 560 nm at 25 °C using a BioTek Synergy 2 multimode reader. Suppression of NBT to 50% was considered to be one unit of enzyme activity (U). Enzyme activity was expressed in ΔOD/min/mg protein.
Effects of chlorantraniliprole on hormone titers of A. ipsilon Molting hormone (MH)
First, MH was accurately weighed and dissolved in methanol solution (1:1) as a stock solution. Then, standard solutions with concentrations of 55.5 ng·L−1, 27.75 ng·L−1, 13.875 ng·L−1, 6.9375 ng·L−1, 3.4688 ng·L−1, and 1.7344 ng·L−1 were prepared to obtain a standard curve. The standard solutions were used as the abscissa, and the peak areas were used as the ordinates. Individual larvae were weighed and homogenized with 2 mL of methanol (75%) in a glass homogenizer, followed by centrifugation at 20,000 g force for 10 min. Samples were extracted three times with methanol (75%) and dried completely using a pressure blowing concentrator. Next, the MH samples were dissolved in 2 mL of double-distilled water and subsequently transferred into 5 mL of chloroform. The water and chloroform (1:1) were thoroughly mixed and allowed to separate for 5 min under static conditions after the chloroform was fully mixed with the water. The upper layer was concentrated by placing the solution in a water bath at 60 °C. Then, 150 μL of standard solution was added to 150 μL of mobile phase, and the obtained solution was subsequently filtered using an organic phase membrane before being subjected to chromatographic analysis. Chromatographic conditions were described as Supplementary Information.
Juvenile hormone (JH)
First, JH was accurately weighed and dissolved in absolute ethanol (≥99.70%) as a stock solution. Then, a standard curve was prepared by serially diluting the stock solution (62.5 ng·L−1, 31.25 ng·L−1, 15.625 ng·L−1, 7.8125 ng·L−1, 3.9061 ng·L−1, and 1.9531 ng·L−1) to prepare standard solutions. Individual larvae were weighed and homogenized with 2 mL of methanol and ether (1:1) in a glass homogenizer, followed by centrifugation at 20,000 g force for 10 min. Samples were extracted three times with n-hexane. The upper phase was collected and dried completely at 40 °C under nitrogen gas. Next, 150 μL of standard solution was added to 150 μL of mobile phase, and the obtained solution was subsequently filtered using an organic phase membrane before being subjected to chromatographic analysis. Chromatographic conditions were described as Supplementary Information.
Data analysis
The data obtained from the toxicity test were corrected using Abbott’s formula71 before analysis, and data were subjected to probit analysis using SPSS (version 19.0, SPSS Inc., Chicago, IL, USA). Statistically significant mean values were determined using ANOVA, and the significant differences among the treatments were determined using the Student-Newman-Keuls test (P < 0.05).
Raw population parameter data for all A. ipsilon individuals were analyzed based on the age-stage, two-sex life table theory50,72. The age-stage-specific survival rate (sxj) represents the probability that each egg can survive to age x and stage j; the female age-specific fecundity (fx9) means the number of eggs produced by a female adult at age x; the curve for age-stage-specific reproductive values (vxj) represents the contribution of each individual to future offspring from age x to stage j. The age-specific survival rate (lx) is a simplified form of sxj, and age-specific fecundity (mx) is calculated as follows:
and
The net reproductive rate (R0) was calculated as follows:
The intrinsic rate of increase (r) was calculated as follows:
The mean generation time (T) was calculated as follows:
The finite rate of increase (λ) was calculated as follows:
Developmental time, fecundity and longevity and their mean values, standard error (SE) and differences among treatments were estimated by the bootstrap test method (B = 200,000)73,74 included in the computer program TWOSEX-MSChart10,31. Population parameters were also analyzed, and their mean values, SE values and significant differences were determined using the bootstrap method included in the computer program TWOSEX-MSChart73,74. All curves for survival rates, fecundity, reproductive value, and life expectancy were constructed using SigmaPlot 12.5 (Systat, Erkrath, Germany).
Supplementary information
Acknowledgements
This study was supported by the National Key Research Development Program of China (2018YFD0200604) and the Funds of Shandong “Double Tops” Program (SYL2017-YSTD02).
Author Contributions
F.H., S.S. and X.J. designed the study. F.H., S.S., H.T., X.S., C.Q. and S.J. performed the experiments. F.H., S.S., J.Z., X.L. and X.J. analyzed the data. F.H., S.S. and X.J. wrote the manuscript. All authors read and approved the fnal manuscript.
Competing Interests
The authors declare no competing interests.
Footnotes
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Falin He and Shiang Sun contributed equally.
Supplementary information
Supplementary information accompanies this paper at 10.1038/s41598-019-46915-0.
References
- 1.Harrison RL, Lynn DE. New cell lines derived from the black cutworm, Agrotis ipsilon, that support replication of the A. ipsilon multiple nucleopolyhedrovirus and several group I nucleopolyhedroviruses. J Invertebr Pathol. 2008;99:28–34. doi: 10.1016/j.jip.2008.02.015. [DOI] [PubMed] [Google Scholar]
- 2.Binning RR, Coats J, Kong X, Hellmich RL. Susceptibility to Bt proteins is not required for Agrotis ipsilon aversion to Bt maize. Pest Manag Sci. 2015;71:601–606. doi: 10.1002/ps.3901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Liu YQ, Fu XW, Feng HQ, Liu ZF, Wu KM. Trans-regional migration of Agrotis ipsilon (Lepidoptera: Noctuidae) in North-East Asia. Ann. Entomol. Soc. Am. 2015;108:519–527. doi: 10.1093/aesa/sav050. [DOI] [Google Scholar]
- 4.El-Aziz, A., Omer, E. A. & Sabra, A. S. Chemical composition of ocimum americanum essential oil and its biological effects against Agrotis ipsilon, (Lepidoptera: Noctuidae). Research Journal of Agriculture & Biological Sciences 740–747 (2007).
- 5.Showers WB. Migratory ecology of the black cutworm. Annu Rev Entomol. 1997;42:393–425. doi: 10.1146/annurev.ento.42.1.393. [DOI] [PubMed] [Google Scholar]
- 6.Zaki FN. Field application of Steinernema feltiae, in the form of baits against the greesy cutworm Agrotis ipsilon, in an okra field in Egypt. Anzeiger Für Schädlingskunde Pflanzenschutz Umweltschutz. 1996;69:79–80. doi: 10.1007/BF01908434. [DOI] [Google Scholar]
- 7.Cao GC, et al. Toxicity of chlorantraniliprole to Cry1Ac-susceptible and resistant strains of Helicoverpa armigera. Pestic Biochem Phys. 2010;98:99–103. doi: 10.1016/j.pestbp.2010.05.006. [DOI] [Google Scholar]
- 8.Pereira NC. Evaluation of the toxic effect of insecticide chlorantraniliprole on the silkworm (Lepidoptera: Bombycidae) Open Journal of Animal Sciences. 2013;3:343–353. doi: 10.4236/ojas.2013.34051. [DOI] [Google Scholar]
- 9.Lai T, Su JY. Effects of chlorantraniliprole on development and reproduction of beet armyworm, Spodoptera exigua (Hübner) J Pest Sci. 2011;84:381–386. doi: 10.1007/s10340-011-0366-1. [DOI] [Google Scholar]
- 10.Han WS, et al. Residual toxicity and sublethal effects of chlorantraniliprole on Plutella xylostella (Lepidoptera: Plutellidae) Pest Manag Sci. 2012;68:1184–1190. doi: 10.1002/ps.3282. [DOI] [PubMed] [Google Scholar]
- 11.Nawaz M, et al. Toxicity and sublethal effects of chlorantraniliprole on the development and fecundity of a non-specific predator, the multicolored Asian lady beetle, Harmonia axyridis (Pallas) Chemosphere. 2017;178:496–503. doi: 10.1016/j.chemosphere.2017.03.082. [DOI] [PubMed] [Google Scholar]
- 12.Desneux N, Rafalimanana H, Kaiser L. Dose-response relationship in lethal and behavioural effects of different insecticides on the parasitic wasp Aphidius ervi. Chemosphere. 2004;54:619–627. doi: 10.1016/j.chemosphere.2003.09.007. [DOI] [PubMed] [Google Scholar]
- 13.Cutler GC, Scott-Dupree CD, Tolman JH, Harris CR. Toxicity of the insect growth regulator novaluron to the non-target predatory bug Podisus maculiventris (Heteroptera: Pentatomidae) Biol Control. 2006;38:196–204. doi: 10.1016/j.biocontrol.2005.12.016. [DOI] [Google Scholar]
- 14.Haynes KF. Sublethal effects of neurotoxic insecticides on insect behavior. Annu Rev Entomol. 1988;33:149–168. doi: 10.1146/annurev.en.33.010188.001053. [DOI] [PubMed] [Google Scholar]
- 15.Rehan A, Freed S. Fitness cost of methoxyfenozide and the effects of its low-lethal doses on development, reproduction, and survival of Spodoptera litura (Fabricius) (Lepidoptera: Noctuidae) Neotrop Entomol. 2015;44:513–520. doi: 10.1007/s13744-015-0306-5. [DOI] [PubMed] [Google Scholar]
- 16.Boina DR, Onagbola EO, Salyani M, Stelinski LL. Antifeedant and sublethal effects of imidacloprid on Asian citrus psyllid, Diaphorina citri. Pest Manag Sci. 2010;65:870–877. doi: 10.1002/ps.1767. [DOI] [PubMed] [Google Scholar]
- 17.Cutler GC, Ramanaidu K, Astatkie T, Isman MB. Green peach aphid, Myzus persicae (Hemiptera: Aphididae), reproduction during exposure to low-lethal concentrations of imidacloprid and azadirachtin. Pest Manag Sci. 2010;65:205–209. doi: 10.1002/ps.1669. [DOI] [PubMed] [Google Scholar]
- 18.Cutler GC. Insects, insecticides and hormesis: evidence and considerations for study. Dose response. 2013;11:154–177. doi: 10.2203/dose-response.12-008.Cutler. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Guedes RN, Cutler GC. Insecticide-induced hormesis and arthropod pest management. Pest Manag Sci. 2014;70:690–697. doi: 10.1002/ps.3669. [DOI] [PubMed] [Google Scholar]
- 20.Singh JP, Marwaha KK. Effect of low-lethal concentrations of some insecticides on growth and development of maize stalk borer, Chilo partellus (Swinhoe) larvae. Shashpa. 2000;7:181–186. [Google Scholar]
- 21.Zhao YH, et al. Effects of the microbial secondary metabolite benzothiazole on the nutritional physiology and enzyme activities of Bradysia odoriphaga (Diptera: Sciaridae) Pestic Biochem Phys. 2016;129:49–55. doi: 10.1016/j.pestbp.2015.10.017. [DOI] [PubMed] [Google Scholar]
- 22.Chi H, Getz WM. Mass rearing and harvesting based on an age-stage, two-sex life table: a potato tuberworm (Lepidoptera: Gelechiidae) case study. Environ Entomol. 1988;17:18–25. doi: 10.1093/ee/17.1.18. [DOI] [Google Scholar]
- 23.Zheng XM, Tao YL, Chi H, Wan FH, Chu D. Adaptability of small brown planthopper to four rice cultivars using life table and population projection method. Sci Rep-uk. 2017;7:42399. doi: 10.1038/srep42399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sharma P, Mohan L, Dua KK, Srivastava CN. Status of carbohydrate, protein and lipid profile in the mosquito larvae treated with certain phytoextracts. Asian Pac Tropical Med. 2011;4:301–304. doi: 10.1016/S1995-7645(11)60090-4. [DOI] [PubMed] [Google Scholar]
- 25.Silva CT, et al. Biochemical parameters of Spodoptera frugiperda (J.E. Smith) treated with citronella oil (Cymbopogon winterianus Jowitt ex Bor) and its influence on reproduction. Acta Histochem. 2016;118:347–352. doi: 10.1016/j.acthis.2016.03.004. [DOI] [PubMed] [Google Scholar]
- 26.Linde B, Veerle M, Gamal A, Guy S. Lethal and sublethal side-effect assessment supports a more benign profile of spinetoram compared with spinosad in the bumblebee Bombus terrestris. Pest Manag Sci. 2011;67:541–547. doi: 10.1002/ps.2093. [DOI] [PubMed] [Google Scholar]
- 27.Stark JD, Banks JE. Population-level effects of pesticides and other toxicants on arthropods. Annu Rev Entomol. 2003;48:505–519. doi: 10.1146/annurev.ento.48.091801.112621. [DOI] [PubMed] [Google Scholar]
- 28.Bozsik A. Susceptibility of adult Coccinella septempunctata (Coleoptera: Coccinellidae) to insecticides with different modes of action. Pest Manag Sci. 2010;62:651–654. doi: 10.1002/ps.1221. [DOI] [PubMed] [Google Scholar]
- 29.Xu CM, et al. Cyantraniliprole at sublethal dosages negatively affects the development, reproduction, and nutrient utilization of Ostrinia furnacalis (Lepidoptera: Crambidae) J Econ Entomol. 2017;110:230–238. doi: 10.1093/jee/tox269. [DOI] [PubMed] [Google Scholar]
- 30.Tang QL, Xiang M, Hu HM, An CJ, Gao XW. Evaluation of sublethal effects of sulfoxaflor on the green peach aphid (Hemiptera: Aphididae) using life table parameters. J Econ Entomol. 2015;108:2720–2728. doi: 10.1093/jee/tov221. [DOI] [PubMed] [Google Scholar]
- 31.China Pesticide Information Network, Institute Control of Agrochemicals Ministry of Agriculture, P. R. China, http://www.icama.org.cn/hysj/index.jhtml (2018).
- 32.Gontijo PC, Abbade Neto DO, Oliveira RL, Michaud JP, Carvalho GA. Non-target impacts of soybean insecticidal seed treatments on the life history and behavior of Podisus nigrispinus, a predator of fall armyworm. Chemosphere. 2017;191:342–349. doi: 10.1016/j.chemosphere.2017.10.062. [DOI] [PubMed] [Google Scholar]
- 33.Hummel NA, Mészáros A, Ring DR, Beuzelin JM, Stout MJ. Evaluation of seed treatment insecticides for management of the rice water weevil, Lissorhoptrus oryzophilus Kuschel (Coleoptera: Curculionidae), in commercial rice fields in louisiana. Crop Prot. 2014;65:37–42. doi: 10.1016/j.cropro.2014.06.025. [DOI] [Google Scholar]
- 34.Lutz AL, et al. Lethal and sublethal effects of chlorantraniliprole on Spodoptera cosmioides (Lepidoptera: Noctuidae) Pest Manag Sci. 2018;74:2817–2821. doi: 10.1002/ps.5070. [DOI] [PubMed] [Google Scholar]
- 35.Hong Y, Zhao W, Jin DC. Sublethal effects of chlorantraniliprole on the experimental populations of the white-backed planthopper, Sogatella furcifera (Hemiptera: Delphacidae) Acta Entomologica Sinica. 2012;55:1161–1167. [Google Scholar]
- 36.Lanka SK, Ottea JA, Davis JA, Hernandez AB, Stout MJ. Systemic effects of thiamethoxam and chlorantraniliprole seed treatments on adult Lissorhoptrus oryzophilus (Coleoptera: Curculionidae) in rice. Pest Manag Sci. 2013;69:250–256. doi: 10.1002/ps.3382. [DOI] [PubMed] [Google Scholar]
- 37.Dong JF, Wang K, Li Y, Wang SL. Lethal and sublethal effects of cyantraniliprole on Helicoverpa assulta (Lepidoptera: Noctuidae) Pestic Biochem Phys. 2016;136:58–63. doi: 10.1016/j.pestbp.2016.08.003. [DOI] [PubMed] [Google Scholar]
- 38.Zhao YH, et al. Sublethal effects of chlorfenapyr on the life table parameters, nutritional physiology and enzymatic properties of Bradysia odoriphaga (Diptera: Sciaridae) Pestic Biochem Phys. 2018;148:93–102. doi: 10.1016/j.pestbp.2018.04.003. [DOI] [PubMed] [Google Scholar]
- 39.Zhang P, et al. Life table study of the effects of low-lethal concentrations of thiamethoxam on Bradysia odoriphaga Yang and Zhang. Pestic Biochem Phys. 2014;111:31–37. doi: 10.1016/j.pestbp.2014.04.003. [DOI] [PubMed] [Google Scholar]
- 40.Papachristos DP, Milonas PG. Adverse effects of soil applied insecticides on the predatory coccinellid Hippodamia undecimnotata (Coleoptera: Coccinellidae) Biol Control. 2008;47:77–81. doi: 10.1016/j.biocontrol.2008.06.009. [DOI] [Google Scholar]
- 41.Ioriatti C, Anfora G, Angeli G, Mazzoni V, Trona F. Effects of chlorantraniliprole on eggs and larvae of Lobesia botrana (Denis & Schiffermüller) (Lepidoptera: Tortricidae) Pest Manag Sci. 2009;65:717–722. doi: 10.1002/ps.1744. [DOI] [PubMed] [Google Scholar]
- 42.Zhang R, Jang EB, He S, Chen J. Lethal and sublethal effects of cyantraniliprole on Bactrocera dorsalis (Hendel) (Diptera: Tephritidae) Pest Manag Sci. 2015;71:250–256. doi: 10.1002/ps.3791. [DOI] [PubMed] [Google Scholar]
- 43.Qu YY, et al. Sublethal and hormesis effects of imidacloprid on the soybean aphid Aphis glycines. Ecotoxicology. 2016;24:479–487. doi: 10.1007/s10646-014-1396-2. [DOI] [PubMed] [Google Scholar]
- 44.Wang P, et al. Sublethal effects of thiamethoxam on the demographic parameters of Myzus persicae (Hemiptera: Aphididae) J Econ Entomol. 2017;110:1750–1754. doi: 10.1093/jee/tox112. [DOI] [PubMed] [Google Scholar]
- 45.Calabrese EJ, Baldwin L. The dose determines the stimulation (and poison): development of a chemical hormesis database. Int J Toxicol. 1997;16:545–559. doi: 10.1080/109158197226874. [DOI] [Google Scholar]
- 46.Zhang P, Zhao YH, Wang QH, Mu W, Liu F. Lethal and sublethal effects of the chitin synthesis inhibitor chlorfluazuron on Bradysia odoriphaga Yang and Zhang (Diptera: Sciaridae) Pestic Biochem Phys. 2017;136:80–88. doi: 10.1016/j.pestbp.2016.07.007. [DOI] [PubMed] [Google Scholar]
- 47.Ge LQ, Wu JC, Zhao KF, Chen Y, Yang GQ. Induction of Nlvg and suppression of Nljhe gene expression in Nilaparvata lugens (Stål) (Hemiptera: Delphacidae) adult females and males exposed to two insecticides. Pestic Biochem Phys. 2010;98:269–278. doi: 10.1016/j.pestbp.2010.06.018. [DOI] [Google Scholar]
- 48.Yazdani E, Sendi JJ, Aliakbar A, Nathan-Nathan SS. Effect of Lavandula angustifolia essential oil against lesser mulberry pyralid Glyphodes pyloalis Walker (Lep: Pyralidae) and identification of its major derivatives. Pestic Biochem Phys. 2013;107:250–257. doi: 10.1016/j.pestbp.2013.08.002. [DOI] [Google Scholar]
- 49.Nathan SS. Effects of Melia azedarach on nutritional physiology and enzyme activities of the rice leaffolder Cnaphalocrocis medinalis (Guenée) (Lepidoptera: Pyralidae) Pestic Biochem Phys. 2006;84:98–108. doi: 10.1016/j.pestbp.2005.05.006. [DOI] [Google Scholar]
- 50.Chi H. Life-table analysis incorporating both sexes and variable development rate among individuals. Environ Entomol. 1988;17:26–34. doi: 10.1093/ee/17.1.26. [DOI] [Google Scholar]
- 51.Liu ZL, Ho SH, Goh SH. Effect of fraxinellone on growth and digestive physiology of Asian corn borer, Ostrinia furnacalis Guenee. Pestic Biochem Phys. 2008;91:122–127. doi: 10.1016/j.pestbp.2008.03.003. [DOI] [Google Scholar]
- 52.Cai HJ, et al. Effects of tea saponin on growth and development, nutritional indicators, and hormone titers in diamondback moths feeding on different host plant species. Pestic Biochem Phys. 2016;131:53–59. doi: 10.1016/j.pestbp.2015.12.010. [DOI] [PubMed] [Google Scholar]
- 53.Schoonhoven LM. Biological aspects of antifeedants. Entomol Exp Appl. 1982;31:57–69. doi: 10.1111/j.1570-7458.1982.tb03119.x. [DOI] [Google Scholar]
- 54.Šmid I, et al. Clitocypin, a fungal cysteine protease inhibitor, exerts its insecticidal effect on Colorado potato beetle larvae by inhibiting their digestive cysteine proteases. Pestic Biochem Phys. 2015;122:59–66. doi: 10.1016/j.pestbp.2014.12.022. [DOI] [PubMed] [Google Scholar]
- 55.Etebari K, Bizhannia AR, Sorati R, Matindoost L. Biochemical changes in haemolymph of silkworm larvae due to pyriproxyphen residue. Pestic Biochem Phys. 2006;88:14–19. doi: 10.1016/j.pestbp.2006.08.005. [DOI] [Google Scholar]
- 56.Steel, J. E. In: Locke M and Smith DS (Eds.), Insect Biology in Future, Academic Press, New York p. 1006 (1985).
- 57.Zibaee A, Bandani AR. Effects of Artemisia annua L. (AsterAcea) on the digestive enzymatic profiles and the cellular immune reactions of the sun pest, Eurygaster integriceps (Heteroptera: Scutellaridae) against Beauvaria bassiana. Bull Entomol Res. 2010;100:185–196. doi: 10.1017/S0007485309990149. [DOI] [PubMed] [Google Scholar]
- 58.Haque MA, Chen J, Aldred P, Adhikari B. Drying and denaturation characteristics of whey protein isolate in the presence of lactose and trehalose. Food Chem. 2015;177:8–16. doi: 10.1016/j.foodchem.2014.12.064. [DOI] [PubMed] [Google Scholar]
- 59.Tan YA, Xiao LB, Sun Y, Zhao J, Bai LX. Sublethal effects of the chitin synthesis inhibitor, hexaflumuron, in the cotton mirid bug, Apolygus lucorum (Meyer-Dür) Pestic Biochem Phys. 2014;111:43–50. doi: 10.1016/j.pestbp.2014.04.001. [DOI] [PubMed] [Google Scholar]
- 60.Thompson SN, Dahlman DL. Blood sugar formation due to abnormally elevated gluconeogenesis: aberrant regulation in a parasitized insect, Manduca sexta Linnaeus. BBA-biomembranes. 1999;1454:133–142. doi: 10.1016/s0925-4439(99)00031-9. [DOI] [PubMed] [Google Scholar]
- 61.Ramsey JS, et al. Comparative analysis of detoxification enzymes in Acyrthosiphon pisum and Myzus persicae. Insect Mol Biol. 2010;19:155–164. doi: 10.1111/j.1365-2583.2009.00973.x. [DOI] [PubMed] [Google Scholar]
- 62.Zhang YN, et al. Insecticidal activities and biochemical properties of Pinellia ternata, extracts against the beet armyworm Spodoptera exigua. J Asia-Pac Entomol. 2017;20:469–476. doi: 10.1016/j.aspen.2017.03.003. [DOI] [Google Scholar]
- 63.Huang QC, Liu MH, Feng J, Liu Y. Effect of dietary benzoxadiazole on larval development, cuticle enzyme and antioxidant defense system in housefly (Musca domestica L.) Pestic Biochem Phys. 2008;90:119–125. doi: 10.1016/j.pestbp.2007.11.001. [DOI] [Google Scholar]
- 64.Prapanthadara L, Promter N, Koottathep S, Somboon P, Ketterman AJ. Isoenzymes of glutathione S-transferase from the mosquito Anopheles dirus species B: the purification, partial characterization and interaction with various insecticides. Insect Biochem Molec. 2000;30:395–403. doi: 10.1016/S0965-1748(00)00013-8. [DOI] [PubMed] [Google Scholar]
- 65.Devorshak C, Roe RM. The role of esterases in insecticide resistance. Rev Toxicol. 1998;2:501. [Google Scholar]
- 66.Steiner B, et al. Titers of juvenile hormone I, II and III in Spodoptera littoralis (Noctuidae) from the egg to the pupa moult and their modification by the egg-larval parasitoid Chelonus inanitus (Braconidae) J Insect Physiol. 1999;45:401–413. doi: 10.1016/S0022-1910(98)00139-5. [DOI] [PubMed] [Google Scholar]
- 67.Yu SJ, Terriere LC. Ecdysone metabolism by soluble enzymes from three species of diptera and its inhibition by the insect growth regulator TH-6040. Pestic Biochem Phys. 1977;7:48–55. doi: 10.1016/0048-3575(77)90065-7. [DOI] [Google Scholar]
- 68.Zhang, Y. J., Lu, Q., Gu, S. H., Lu, Y. H. & Wu, K. M. A kind of artificial diet preparation method for black cutworm larvae and application. China patent No. 200810112399 (2008).
- 69.Waldbauer GP. The consumption and utilization of food by insects. Adv Insect Physiol. 1968;5:229–288. doi: 10.1016/S0065-2806(08)60230-1. [DOI] [Google Scholar]
- 70.Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein dye binding. Anal. Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- 71.Abbott W. A method of computing the effectiveness of an insecticide. J econ Entomol. 1925;18:265–267. doi: 10.1093/jee/18.2.265a. [DOI] [Google Scholar]
- 72.Chi H, Liu H. Two new methods for the study of insect population ecology. Bull Inst Zool Acad Sin. 1985;24:225–240. [Google Scholar]
- 73.Chi, H. TWOSEX-MSChart: a computer program for the age-stage, two-sex life table analysis (http://140.120.197.173/Ecology/) (2018).
- 74.Efron, B. & Tibshirani, R. J. An Introduction to the Bootstrap. Chapman and Hall Press, New York (1993).
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.