Abstract
Background:
The protection conferred by influenza vaccination is generally thought to last less than a year, necessitating annual re-vaccination. However, the speed with which influenza vaccine effectiveness (VE) might decline during a year is unknown. This is of particular importance for locations with year-round influenza activity.
Methods:
We analysed data from a test-negative study of influenza VE in hospitalized children in Hong Kong over five consecutive years. We examined how VE changed by time between vaccination and hospitalisation.
Findings:
We analysed data on 15,695 children admitted from September 2012 through August 2017. Among study participants, the majority of vaccinations occurred in the final quarter of each year. Influenza admissions occurred year-round with peaks in January through March in most years and a large summer peak in 2016. We estimated that VE against influenza A or B decreased from 79% (95% CI: 64%−88%) for children vaccinated within 0.5 to 2 months, to 60% (95% CI: 46%−71%) within 2 to 4 months, to 57% (95% CI: 39%−70%) within 4–6 months of vaccination, and to 45% (95% CI: 22%−61%) within 6–9 months of vaccination. In separate analyses by type/subtype, we estimated that VE declined by 2–5 percentage points per month.
Interpretation:
We observed clear evidence of reductions in VE during the 9 months after vaccination in children. Our findings confirm the importance of annual vaccination in children. Influenza vaccines that provide broader and longer-lasting protection are needed to provide year-round protection in regions with irregular influenza seasonality or prolonged periods of influenza activity.
INTRODUCTION
Influenza viruses cause disease burden in all age groups and severe disease most frequently in individuals at the extremes of age.1 Influenza vaccination is currently the most effective measure available for preventing influenza virus infections and associated severe outcomes. Children are thought to play a major role in influenza transmission, and vaccination of children is an important strategy to prevent influenza virus infection and transmission, offering both direct protection of individuals and the potential for indirect protection for the population through herd immunity.2,3
In Hong Kong, located on the southern coast of China, the government subsidises influenza vaccination for children aged 6 months to 12 years of age and provides free vaccination to children with chronic medical conditions.4 Access to hospital care is universal through a public health care system covering 90% of bed-days.5 The northern hemisphere formulation of the influenza vaccine is used, and the trivalent inactivated vaccine (TIV), quadrivalent inactivated vaccines (QIV) and trivalent live attenuated influenza vaccine (LAIV) (available in Hong Kong in the 2012/13 and 2013/14 season) are available from September or October until April or May each year. The climate is subtropical with prolonged periods of influenza activity, winter epidemics in most years, and unpredictable timing of epidemics at other times of the year.6
Annual revaccination is recommended for influenza because circulating influenza viruses continue to change antigenically, and usually at least one vaccine strain is changed each year.7 In addition, some studies have reported reductions in antibody titers from 1 month to 6 months after vaccination,8–10 and other studies have reported reductions in vaccination effectiveness (VE) with increasing time since vaccination.11–15
In this established study, we previously reported influenza VE against hospitalization in children in 2009–2013,16 in 2009–2014 for influenza B viruses,17 in 2015/16,18 and in 2016/17.19 Here, we analysed data from five consecutive years to assess how influenza VE changes by time intervals between vaccination and hospitalization, taking advantage of almost year-round circulation of influenza in Hong Kong.
METHODS
Study design
We analysed data from a test-negative study designed to monitor influenza VE against hospitalization in children in Hong Kong across 5 years from 1 September 2012 through 31 August 2017.16–19 Children between 6 months and 17 years of age were included in the analysis if they were admitted to general wards in four public hospitals in Hong Kong with fever ≥38°C and any respiratory symptom such as runny nose, cough or sore throat. As previously described,16–19 nasopharyngeal aspirates were collected from all eligible children and initially tested by direct immunofluorescence assay and then subsequently tested by culture and reverse transcription polymerase chain reaction (RT-PCR). Research staff obtained children’s influenza vaccination history by interviewing parents or legal guardians at the time of hospitalization. Detailed vaccination history including time of vaccination and type of vaccine given was cross-checked with vaccination records. For children vaccinated in the public sector, information was verified with a Clinical Management System, which records immunization history for public hospitals in Hong Kong. For those who received vaccination in the private sector, their vaccination cards were checked by research staff. If date of vaccination or type of vaccine was not clear on the vaccination card, information was cross-checked with private clinics for further clarification with parental consent. Prior to the 2015/16 season, children were enrolled from two public hospitals in Hong Kong island, with two additional hospitals in Kowloon from September 2015 onwards. The study protocol was approved by the Institutional Review Boards of the University of Hong Kong/Hospital Authority Hong Kong West Cluster, and the Hospital Authority Kowloon West Cluster.
Children were categorized as vaccinated if they received the influenza vaccination for the current season after 1 August and at least two weeks prior to hospitalization with appropriate dosage.20 A small number of children who had received vaccine less than 2 weeks prior to admission or who required 2 doses but had received only 1 dose were classified as unvaccinated. For children who required 2 doses and received 2 doses, time since vaccination was counted from the second dose.
Statistical analysis
First, we compared the characteristics between influenza-positive cases and negative-controls by chi-squared tests or Fisher’s exact test. The causal effect of influenza vaccination in reducing the risk of influenza hospitalisation is confounded by age and calendar time.21,22 We used regression analysis to account for this potential confounding effect, permitting a causal inference on the effect of influenza vaccination on the risk of hospitalization, i.e. the VE.23,24 We used logistic regression models adjusting for age, age-squared and cumulative calendar month (as a categorical variable) without intercept, assuming baseline risk and vaccination coverage differed by calendar month. The age-squared term was included to allow for the possibility of a nonlinear association between age and VE. VE was estimated by one minus the adjusted odds ratio comparing the vaccination odds among test-positive cases verses test-negative controls. As an initial analysis of the potential changes in VE, we estimated VE against all influenza in three defined periods which corresponded to typical influenza epidemic periods in Hong Kong: (1) September to December (low activity) (2) January to April (winter peak) and (3) May to August (summer peak).6,25 As a complementary analysis, we examined VE against all influenza by time from vaccination to hospitalization: 1) ≥0.5 months (≥14 days) and ≤2 months (shown as 0.5–2 months), 2) >2 and ≤4 months (shown as 2–4 months), 3) >4 and ≤6 months (shown as 4–6 months) and 4) >6 and ≤9 months (shown as 6–9 months). Children who had missing vaccination date were not included in this analysis. Sensitivity analysis was conducted using conditional logistic regression models, adjusting for age, age-squared and matched by cumulative calendar month or calendar week.
In a more detailed analysis, we then selected specific periods of time for stratified analyses by influenza type/subtype, identifying periods for each type/subtype in which there was no change in the vaccine strain and no change in circulating strains. Various combinations of measurements for intervals since vaccination were compared—linear, square, cubic and cubic spline—in logistic regression models with the best-fitting model determined by likelihood ratio test. We used a generalised additive regression model (GAM) for days since vaccination using non-restricted cubic spline (with five knots). We used this model because the change in VE estimates by time since vaccination might not be linear, and GAM could allow for a flexible measurement of the potential nonlinear trend.26 The placement of the knots was determined by GAM plots. For influenza A(H1N1)pdm09, we pooled data from five seasons because there was no change in the vaccine strain, and no significant antigenic changes in circulating viruses over that period which remained well-matched to the vaccine strain.27 To minimize potential biases that might arise from changes in vaccine match over time and between seasons, we focused on the 2016/17 season for influenza A(H3N2), and the 2015/16 season for influenza B. Sensitivity analysis for influenza B was conducted by restricting study period with influenza virus activity from January through August 2016. To assess interval validity of the GAM, additional sensitivity analysis was done by using 1000 times bootstrap resampling from data by influenza type/subtype. Analyses were conducted using R version 3.4.0 (R Foundation for Statistical Computing, Vienna, Austria).
Role of the funding source
This work was financially supported by a grant from the Health and Medical Research Fund (HKS-18-E18). This work was supported by the Research Grants Council of the Hong Kong Special Administrative Region (project no. 17105414 and T11–705/14N). BJC is supported by the Harvard Center for Communicable Disease Dynamics from the National Institute of General Medical Sciences (grant no. U54 GM088558). CW Leung is supported by a Mini Research Grant of Clinical Research Centre, Princess Margaret Hospital. The WHO Collaborating Centre for Reference and Research on Influenza is supported by the Australian Government Department of Health. The funding bodies had no role in study design, data collection and analysis, preparation of the manuscript, or the decision to publish. The corresponding author had full access to all of the data and the final responsibility to submit for publication.
RESULTS
From September 2012 through to August 2017, a total of 15,659 hospitalised children were enrolled in our study, including 2,500 (16.5%) who tested positive for influenza A or B and 13,195 (83.5%) who tested negative (Table 1). Influenza hospitalizations were identified in almost every week of the 5-year study period (Figure 1). There were large influenza epidemics in 4/5 winters, with the exception being the winter of 2016/17 when there was low to moderate activity from January through to June 2017 followed by a larger epidemic in June-July in which influenza A(H3N2) predominated (Figure 1). Receipt of influenza vaccination occurred throughout most of the year, while 70%−80% of all vaccinated children were vaccinated by the end of December (Figure 2).
Table 1.
Comparison of cases testing positive for any influenza virus and test-negative cases in Hong Kong, September 2012 to August 2017
| Characteristic | Influenza-positive (n=2500) N (%) |
Influenza-negative (n=13195) N (%) |
p-value a | ||
|---|---|---|---|---|---|
| Age group | |||||
| 6m-2y | 923 | (36.9%) | 6863 | (52.0%) | <0.001 |
| 3–5y | 834 | (33.4%) | 3637 | (27.6%) | |
| 6–17y | 743 | (29.7%) | 2695 | (20.4%) | |
| Female | 1135 | (45.4%) | 5745 | (43.5%) | 0.090 |
| Season | |||||
| 2012/13 | 142 | (5.7%) | 1610 | (12.2%) | <0.001 |
| 2013/14 | 194 | (7.8%) | 1040 | (7.9%) | |
| 2014/15 | 203 | (8.1%) | 1401 | (10.6%) | |
| 2015/16 | 1051 | (42.0%) | 4566 | (34.6%) | |
| 2016/17 | 910 | (36.4%) | 4578 | (34.7%) | |
| 3 Periods | |||||
| Sep - Dec | 283 | (11.3%) | 4526 | (34.3%) | <0.001 |
| Jan - Apr | 1521 | (60.8%) | 4649 | (35.2%) | |
| May - Aug | 696 | (27.8%) | 4020 | (30.5%) | |
| Receipt of influenza vaccination b | |||||
| Overall | 159 | (6.4%) | 1445 | (11.0%) | <0.001 |
| By age group | |||||
| 6 months – 2 years | 36 | (3.9%) | 514 | (7.5%) | <0.001 |
| 3 – 5 years | 65 | (7.8%) | 608 | (16.7%) | |
| 6 – 17 years | 58 | (7.8%) | 323 | (12.0%) | |
| By intervals since vaccination c | 154/159 | (96.9%) | 1368/1445 | (94.7%) | |
| .5 – 2 months | 16 | (10.4%) | 337 | (24.6%) | 0.001 |
| 3 – 4 months | 54 | (35.1%) | 377 | (27.6%) | |
| 5 – 6 months | 38 | (24.7%) | 336 | (24.6%) | |
| 7 – 9 months | 42 | (27.3%) | 279 | (20.4%) | |
| 10 – 12 months | 4 | (2.6%) | 39 | (2.9%) | |
p-values estimated by chi-squared tests or Fisher’s exact test whenever appropriate
Receipt of influenza vaccination defined as receipt of a quadrivalent or trivalent inactivated influenza vaccine with an age-appropriate schedule within 12 months prior admission and for current season.
A total of 82/1604 (5.1%) children were with missing vaccination time.
Figure 1.
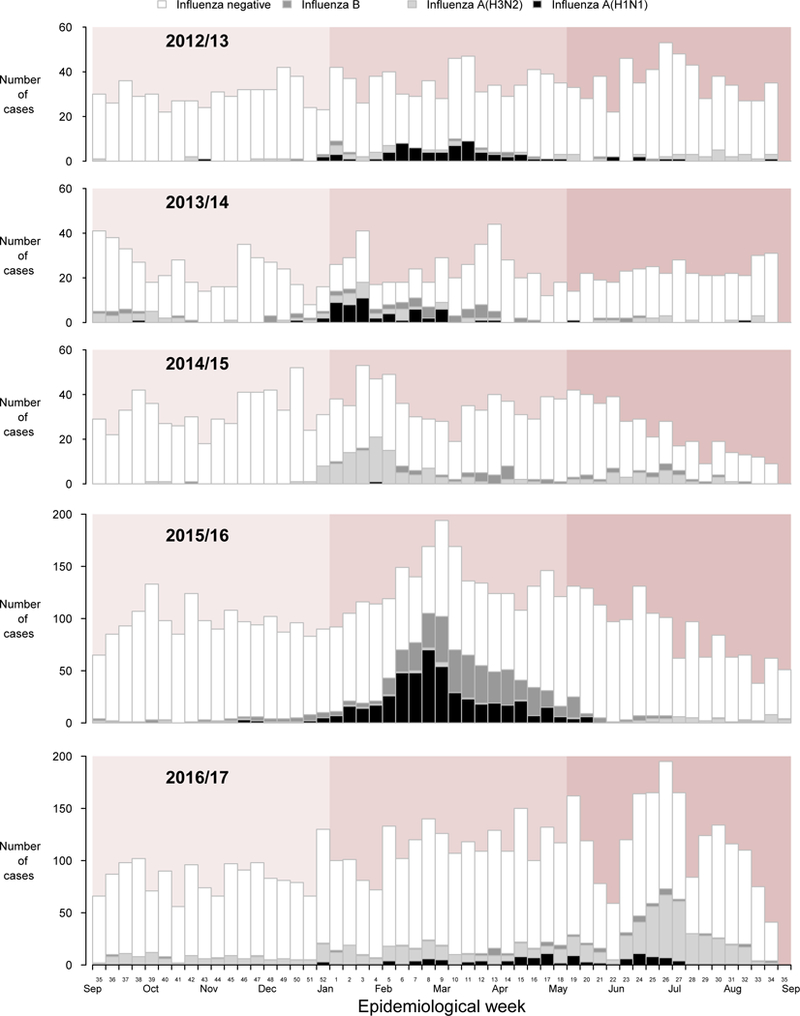
Timeline of recruitment of cases testing positive or negative for influenza virus by type/subtype in Hong Kong (background colour indicated period from September to December, January to April and May to August), September 2012 to August 2017.
Figure 2.
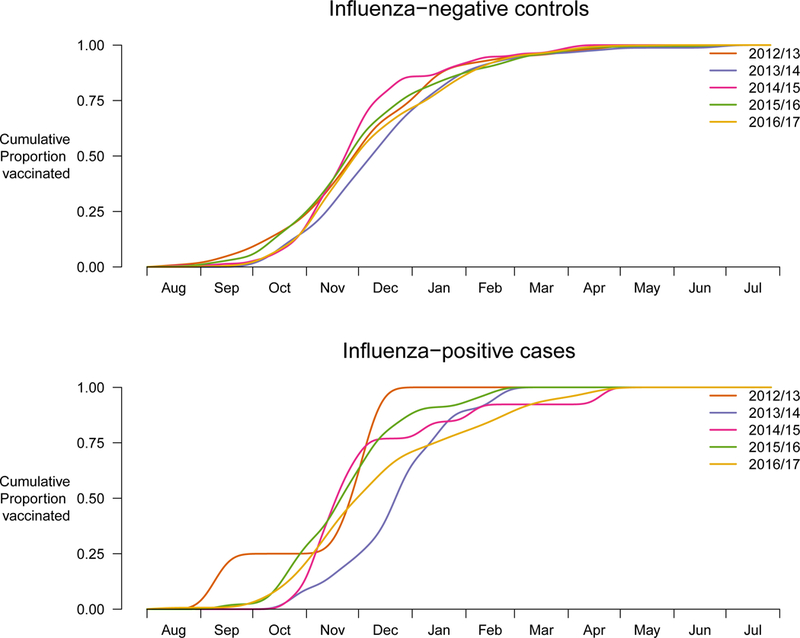
Cumulative proportion of vaccination for children hospitalized with acute respiratory infections by calendar time for influenza-negative controls and positive cases, September 2012 through August 2017.
To assess the concordance of VE estimates across calendar time, we estimated VE for 3 defined periods, both overall and for each of the 5 seasons, and by influenza type/subtype and age group (Figure S1). Pooled across 5 seasons, VE for children of all ages was 79% (95% confidence interval (CI): 42%, 92%) for the period of September to December, compared to 67% (95% CI: 57%, 74%) for January to April, and 43% (95% CI: 25%, 57%) for May to August. There was a statistically significant difference between the estimates for January-April and May-August (p=0.004) (Figure S1). Influenza type/subtype and age-specific analysis are shown in Figures S2–5.
The median days since vaccination among all vaccinated children was 119 days (IQR: 117), and mean was 127 days (SD: 75). Children aged 6 to 17 years old tended to have longer intervals since vaccination compared with children 6 months to 2 years old and children 3 to 5 years old (p<0.001). No association was observed between days since vaccination and sex. To examine whether decreasing VE across calendar time was confounded by timing of vaccination, we further compared VE estimates against influenza A or B by four defined intervals since vaccination (Table S1). A small number of vaccinated children (82/1604, 5.1%) were excluded from this analysis due to missing date of vaccination. There was clear evidence of a decrease in VE with longer intervals since vaccination, from 79% within 2 months of vaccination to 45% at 6–9 months after vaccination. Similar patterns were observed within each age strata (Figure 3). There was a statistically significant difference at 5% level between VE of within 2 months compared with VE of 2 to 4 months (p=0.037). Results by conditional logistic regression models showed comparable VE estimates (Figure S6).
Figure 3.
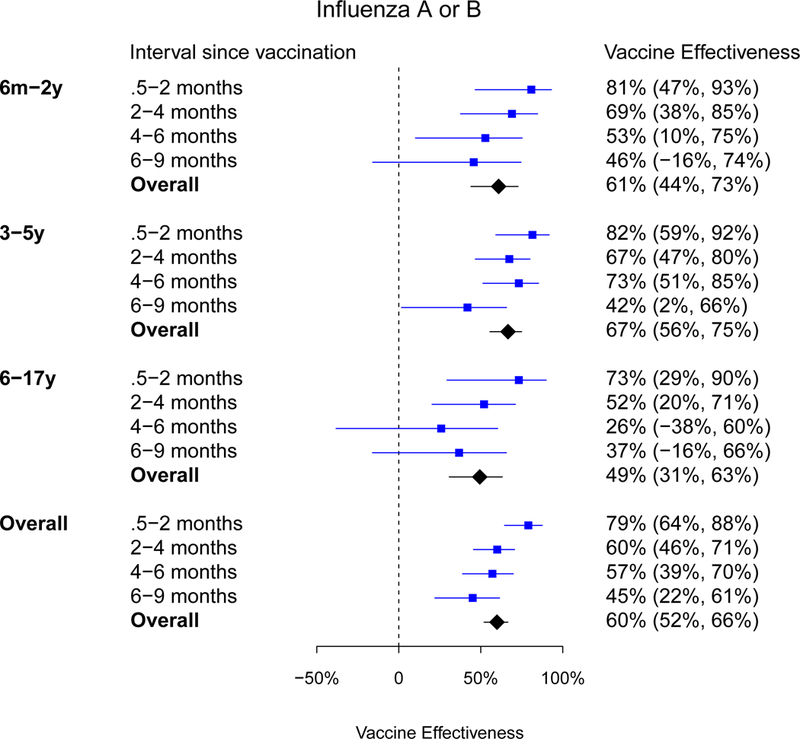
Estimated influenza vaccine effectiveness against influenza A or B among children in Hong Kong by age group and intervals since vaccination to hospitalization, September 2012 to August 2017.
VE was estimated using logistic regression models adjusted for age, age-squared and calendar month.
A GAM model incorporating time since vaccination as a cubic spline was identified, by likelihood ratio test, to provide a better fit compared with linear/square/cubic models for influenza A(H1N1)pdm09 and B (all p-values <0.05). No difference was observed for influenza A(H3N2). To examine VE for influenza A(H1N1)pdm09, we pooled data from all 5 years, and found that VE declined with time since vaccination with the mean rate of approximately 5 percentage points per month, with an increase in the waning rate over time observed from the point estimates, although the confidence intervals were wide (Figure 4A).
Figure 4.

Estimated influenza vaccine effectiveness against (A) influenza A(H1N1), September 2012 to August 2017, and (B) influenza A(H3N2), September 2016 to August 2017 among children in Hong Kong by intervals and days since vaccination to hospitalization.
VE was estimated using generalised additive logistic regression models adjusted for age, age-squared and calendar month.
To examine VE for influenza A(H3N2) further, we focused on the 2016/17 season. We found that VE declined by around 2 percentage points per month (Figure 4B). The point estimate for VE against influenza A(H3N2) was highest (55%; 95% CI: 12%, 77%) 14 days post-vaccination, and declined to 39% (95% CI: 10%, 59%) by day 250.
We focused on the 2015/16 season to analyse VE against influenza B further. In that season, B/Yamagata and B/Victoria co-circulated, with B/Yamagata predominating later in the year (Figure 5A). Most vaccinated children in Hong Kong had received QIV (339/510, 66.5%), while a smaller fraction (76/510, 14.9%) had received the TIV which included a B/Yamagata strain only, and 18.6% (95/510) could not recall the type of vaccine. When using intervals since vaccination as a categorical variable, we did not find evidence of a decline in VE estimates over the first three time periods (Figure 5). For VE estimate of vaccination from >6 to 9 months, we observed a decline from shorter intervals, though the confidence interval was wide. When we analysed the VE of all vaccines (i.e. TIV and QIV) using splines, we found a decline of around 3 percentage points per month (Figure 5B), and the pattern for VE of QIV was very similar (Figure 5C). We found similar waning pattern in sensitivity analyses (Figures S7, S8).
Figure 5.
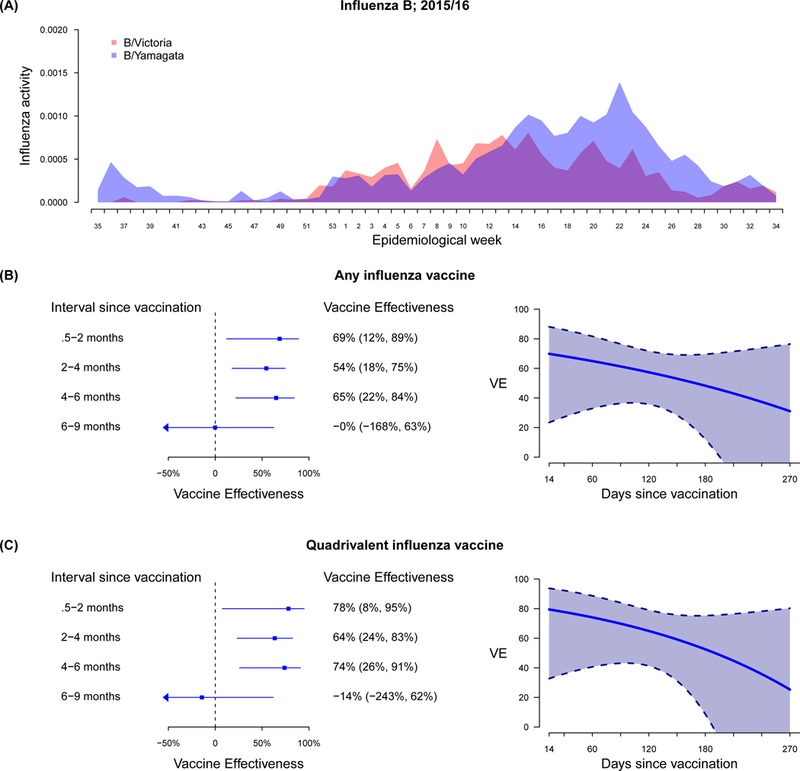
(A) Influenza activity by influenza B/Victoria and B/Yamagata lineage, and laboratory confirmed influenza B/Yamagata lineage among total influenza B positive specimens; (B) estimated effectiveness of any influenza vaccine, and (C) estimated effectiveness of quadrivalent influenza vaccine against influenza B among children in Hong Kong by intervals and days since vaccination to hospitalization, September 2015 to August 2016.
VE was estimated using generalised additive logistic regression models adjusted for age, age-squared and calendar month.
DISCUSSION
We analysed data from 15,695 hospitalized children across 5 calendar years in a location with almost year-round influenza activity. We observed decreased VE estimates against influenza A(H1N1), A(H3N2) and B by time since vaccination (Figure 4, Figure 5). Point estimates dropped by 2–5 percentage points per month. However, the confidence intervals became wider for later periods since vaccination. Given that most children in Hong Kong are vaccinated in the last quarter of each year (Figure 2), vaccination would have provided greatest protection for the winter months, but appears to have conferred reduced protection in the spring and summer.
Our findings are consistent with previous studies.11–13,19,28–42 Evidence of waning VE against influenza (H3N2) has been reported by Europe,11,30,32,34,36 Australia40 and the US,12,29 either for a single season24,30,32,34,36,40 or a combination of seasons.11,8 For instance, in 2011/12, Europe experienced a late season predominated by influenza A(H3N2), and studies found intra-seasonal waning VE against influenza A(H3N2).30,32,34,36 Nevertheless, findings on the rate of waning by influenza type/subtype have been inconsistent among studies. Ferdinands et al. combined data from 4 seasons and estimated that VE waned by 7% per month for influenza A(H3N2) and influenza B, and 6% to 11% per month for influenza A(H1N1) for populations of all ages.12 A European VE study pooling 5 seasons’ data identified modest waning against influenza B and rapid waning against influenza A(H3N2) to 0% effectiveness at around 3 to 4 months.11 Waning against influenza A(H1N1) was not identified.11 This study also reported differences in waning against influenza B by age, i.e., faster waning among older adults.11 A meta-analysis compared pooled VE estimates for receipt of vaccination 15–90 days to 91–180 days based on available estimates, and the authors found statistically significant waning in pooled VE estimates against influenza A(H3N2) and influenza B but not influenza A(H1N1).14
There are a number of potential mechanisms which could explain a decline in VE over time.43 One established mechanism is that vaccine-induced antibody titers also decline over time.9,10,44 This has been observed in children in Hong Kong and the United States, where vaccine-induced antibody titers measured by the hemagglutination inhibition assay waned by around half between 30 days and 6 months after vaccination.9,10 However, antibody titers measured by the hemagglutination inhibition assay may not be perfect correlates of vaccine protection, and it is unclear whether a decline in antibody titers of this magnitude would lead to substantial differences in VE. A second potential mechanism is the antigenic drift in circulating viruses, discussed in the following paragraphs. A final potential mechanism is heterogeneity in susceptibility between vaccinated and unvaccinated people, where unvaccinated people might be more likely to be infected earlier in the season.43,45,46 However, a multi-season study in the US observed increased risk of infection later in the season irrespective of whether they included all individuals or restricted their analyses to vaccinees only.12
Waning effectiveness may be caused by antigenic drift in circulating viruses. For example, in the 2011/12 season in Europe, A(H3N2) viruses exhibited evidence of antigenic drift within the season,47 such that lower VE later in the season was consistent with reduced vaccine match to the A/Perth/16/2009-like component, as seen in antigenic assays.34 More generally, pooling data across years including some early well-matched seasons with better VE together with other later seasons with drifted viruses and poorer VE would provide evidence of waning VE through the year. To avoid pooling data across years with different degrees of match and different timing of seasons, we restricted analysis to the 2016/17 season for influenza A(H3N2) when it dominated (Figure 4). During that season, genetic variability was seen among A(H3N2) viruses, and the degree to which this affected antigenic match remains unclear because of ongoing problems with using standard antigenic assays for these viruses.48 The net decrease in VE seen in that season was 2 percentage points (absolute) and 4 percentage points (relative) per month compared with VE at 14 days.
In contrast, the A(H1N1) component, of the vaccine remained A/California/7/2009 (H1N1)-like virus from 2012/13 through 2016/17, suggesting limited evidence of antigenic drift during this period.27 Thus, we pooled our data for these years, but observed limited waning of around 5 percentage points (absolute), and 5 percentage points per month relative compared with VE at 14 days on average. The confidence intervals of VE estimates were wide after 6 months since vaccination (Figure 4 A). The steepness of the decay in VE was greater for A(H1N1)pdm09 than A(H3N2) and monotonic (Figure 4), suggesting the limited role of match in waning or that the waning effect may differ by subtype.
For influenza B, we restricted to the 2015/16 season, when there was substantial circulation of influenza B. While there was co-circulation of both lineages, the majority of vaccinated children in Hong Kong had received QIV, and an analysis of VE restricted to QIV provided very similar estimates of VE to the overall analysis (Figure 5, Figure S7). VE was relatively stable for 6 months, and then dropped substantially at >6 to 9 months after vaccination (Figure 5, Figure S7). We do not have any evidence of sudden antigenic changes in circulating viruses that would exain such a drop.
In our study, 28% of the influenza-positive cases were detected between May and August (Figure 1, Table 1). Influenza viruses circulate throughout the year in Hong Kong, with notable summer epidemics in the 2014/15 and 2016/17 seasons (Figure 1). Our results highlight the need for optimal vaccination strategies, for example by using enhanced vaccines that might provide greater or longer-lasting protection.7,43 Nevertheless, the current timing of vaccination around the end of each calendar year will likely provide the best chance of protection during January through April when the majority of influenza hospitalisations in children occur in Hong Kong in most years (Figure 1).42
We observed reduced VE in the 2016/17 season (Figure S1). Our VE estimates for 2016/17 season are comparable with those from the UK in 2016/17 and Australia in 2017.49,50 2016/17 and 2014/15 were seasons predominated by influenza A(H3N2). In general, VE against influenza A(H3N2) was lower compared with influenza A(H1N1) or B even for antigenically matched viruses.51 Moreover, waning in VE may have played a role during 2016/17 season whereas a late peak occurred during May to August. In terms of vaccine policy, VE estimates were estimated to continue to provide some degree of protection even during the later period of a season (Figure S1). In Hong Kong, the vaccination coverage among children was around 11% (Table 1), and further efforts are needed in increasing vaccination coverage.
One strength of this study was the near year-round influenza activity in our location, enabling us to examine the effect of vaccine protection for up to 6–9 months after vaccination. With a larger sample size, particularly since 2015, we were able to analyse individual seasons rather than pooling across seasons with the potential for different degrees of antigenic match between the vaccine strains and circulating strains. However, our findings are subject to several limitations. First, we did not incorporate vaccination and infection history for previous seasons, and thus were not able to examine the effect of repeated vaccination.52 Second, information on virus characterisation during the focus years was not available, which would provide more insight into the degree of match and the potential of changes in the degree of match throughout a season. Third, our study was restricted to children, so these results may not be generalizable to other age groups for whom the strength and duration of immune responses to vaccination might differ. That said, age was not identified as an important predictor of waning in a recent meta-analysis.14
In conclusion, we observed waning in VE by time since vaccination among children in Hong Kong, the rate of which varied by influenza type/subtype and season. It is likely that a decline in immune responses (including antibody titers) over time since vaccination contribute to this effect, while antigenic drift in circulating viruses may also play a role. It may be the case that the relative importance of antigenic match versus antibody decay differ by type and subtype. Further studies in which sera and viruses can be collected to assess antibody responses as well as antigenic drift in circulating viruses are needed to understand this issue better. Nevertheless, our results confirm that annual vaccination of children is effective, and the optimal timing of vaccination in Hong Kong is in October-December to provide the highest level of protection during winter epidemics. Improved influenza vaccines are needed to provide year-round protection for children, particularly in subtropical and tropical locations.
Supplementary Material
RESEARCH IN CONTEXT.
Evidence before this study
Influenza vaccination is the best means available for preventing seasonal influenza virus infection and associated severe outcomes. It is well understood that the immune response to vaccination includes a rise in antibody titers to a peak within approximately 4–6 weeks after vaccination, followed by a decline over the following months/years. Annual revaccination is required because (1) vaccine induced antibody titers decline substantially by 12 months, and may therefore offer little ongoing protection, and (2) circulating influenza strains continuously evolve and the influenza strains included in the vaccine need to be regularly updated to keep pace with this drift.
We searched PubMed for evidence evaluating intra-seasonal trends in immune responses to influenza vaccination, and intra-season trends in influenza vaccine effectiveness (VE). We searched for articles published by 2 April 2018, using key words “influenza/flu”, “vaccine”, “efficacy/effectiveness/antibody”, “declin*/wan*/decreas*” without language restriction. Evidence on time-varying VE was scant for locations with year-round influenza circulation. We identified 2 key studies measuring antibody responses for healthy children, which reported a decline in antibody titers within a single season. Nineteen studies, including one systematic review, estimated VE by time since vaccination. Among these, three studies from the US, Europe and Spain used data for a combination of seasons and reported intra-season waning of VE. The multicenter European study observed a rapid decline in protection against influenza A(H3N2) but no waning of VE for influenza A(H1N1). The US study estimated a similar rate of waning for influenza A(H3N2) and B.
Added value of this study
In this study, we analysed data on hospitalized children in Hong Kong over five consecutive years from 2012/13 through 2016/17. We investigated how VE changed by time intervals since vaccination and over calendar time. We found declines in influenza VE against any influenza viruses, and a different rate of waning by influenza type/subtype. In Hong Kong, a subtropical location in the northern hemisphere where vaccines are mostly administered in the final quarter of each calendar year, protection was lowest against influenza in the summer, indicating a potential need for additional public health measures during summer epidemics such as the large epidemic of influenza A(H3N2) in Hong Kong in June-July 2015 and June-July 2017. Our results have broader implications for vaccination timing in tropical and subtropical locations.
Implications of all the available evidence
In tropical and subtropical locations such as Hong Kong, it may be advantageous for vaccines to be available year-round. While most temperate locations promote influenza vaccination during a limited time period that usually precedes annual epidemics, extending vaccination campaigns over a longer period might provide better community protection against large epidemics at any time of the year. Locations with predictable influenza seasons should consider more carefully the optimal timing for vaccination campaigns to provide maximum protection during anticipated influenza seasons. Further research is warranted on alternative strategies to provide year-round protection from influenza, including universal vaccines.
ACKNOWLEDGMENTS
We thank the doctors and nurses of Princess Margaret Hospital, Queen Mary Hospital and Princess Margaret Hospital for research support. The virological data were provided by the Public Health Laboratory Services Branch, Centre for Health Protection, Department of Health, Hong Kong Special Administrative Region, China as part of the routine diagnostic service.
Funding: Health and Medical Research Fund, Hong Kong
JSMP has received research funding from Crucell NV and serves as an ad hoc consultant for GlaxoSmithKline and Sanofi. BJC has received honoraria from Sanofi Pasteur and Roche.
Footnotes
POTENTIAL CONFLICTS OF INTEREST
The authors report no other potential conflicts of interest.
REFERENCES
- 1.Iuliano AD, Roguski KM, Chang HH, et al. Estimates of global seasonal influenza-associated respiratory mortality: a modelling study. The Lancet 2017; 391(10127): 1285–300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Loeb M, Russell ML, Moss L, et al. Effect of influenza vaccination of children on infection rates in Hutterite communities: a randomized trial. JAMA 2010; 303(10): 943–50. [DOI] [PubMed] [Google Scholar]
- 3.Baguelin M, Flasche S, Camacho A, Demiris N, Miller E, Edmunds WJ. Assessing optimal target populations for influenza vaccination programmes: an evidence synthesis and modelling study. PLoS Med 2013; 10(10): e1001527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Recommendations on Seasonal Influenza Vaccination for the 2017/18 Season. Centre for Health Protection. Department of Health of Hong Kong Special Administrative Region; 2017. [Google Scholar]
- 5.Lu JF, Leung GM, Kwon S, Tin KY, Van Doorslaer E, O’Donnell O. Horizontal equity in health care utilization evidence from three high-income Asian economies. Soc Sci Med 2007; 64(1): 199–212. [DOI] [PubMed] [Google Scholar]
- 6.Wu P, Presanis AM, Bond HS, Lau EH, Fang VJ, Cowling BJ. A joint analysis of influenza-associated hospitalizations and mortality in Hong Kong, 1998–2013. Sci Rep 2017; 7(1): 929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Xu C, Thompson MG, Cowling BJ. Influenza vaccination in tropical and subtropical areas. Lancet Respir Med 2017; 5(12): 920–2. [DOI] [PubMed] [Google Scholar]
- 8.MacKenzie JS. Influenza subunit vaccine: antibody responses to one and two doses of vaccine and length of response, with particular reference to the elderly. Br Med J 1977; 1(6055): 200–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ng S, Fang VJ, Ip DK, et al. Estimation of the association between antibody titers and protection against confirmed influenza virus infection in children. J Infect Dis 2013; 208(8): 1320–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Reber AJ, Kim JH, Coleman LA, et al. Seasonal Influenza Vaccination of Children Induces Humoral and Cell-Mediated Immunity Beyond the Current Season: Cross-reactivity With Past and Future Strains. J Infect Dis 2016; 214(10): 1477–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kissling E, Nunes B, Robertson C, et al. I-MOVE multicentre case–control study 2010/11 to 2014/15: Is there within-season waning of influenza type/subtype vaccine effectiveness with increasing time since vaccination? Eurosurveillance 2016; 21(16). [DOI] [PubMed] [Google Scholar]
- 12.Ferdinands JM, Fry AM, Reynolds S, et al. Intraseason Waning of Influenza Vaccine Protection: Evidence From the US Influenza Vaccine Effectiveness Network, 2011–2012 Through 2014–2015. Clin Infect Dis 2017; 64(5): 544–50. [DOI] [PubMed] [Google Scholar]
- 13.Puig-Barberà J, Mira-Iglesias A, Tortajada-Girbés M, et al. Waning protection of influenza vaccination during four influenza seasons, 2011/2012 to 2014/2015. Vaccine 2017; 35(43): 5799–807. [DOI] [PubMed] [Google Scholar]
- 14.Young B, Sadarangani S, Jiang L, Wilder-Smith A, Chen MI. The Duration of Influenza Vaccine Effectiveness: A Systematic Review, Meta-analysis and Meta-regression of Test-Negative Design Case-control Studies. J Infect Dis 2017. [DOI] [PubMed] [Google Scholar]
- 15.Belongia EA, Skowronski DM, McLean HQ, Chambers C, Sundaram ME, De Serres G. Repeated annual influenza vaccination and vaccine effectiveness: review of evidence. Expert Rev Vaccines 2017; 16(7): 723–36. [DOI] [PubMed] [Google Scholar]
- 16.Cowling BJ, Chan K-H, Feng S, et al. The effectiveness of influenza vaccination in preventing hospitalizations in children in Hong Kong, 2009–2013. Vaccine 2014; 32(41): 5278–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chiu SS, Feng S, Chan KH, et al. Hospital-based vaccine effectiveness against influenza B lineages, Hong Kong, 2009–14. Vaccine 2016; 34(19): 2164–9. [DOI] [PubMed] [Google Scholar]
- 18.Cowling BJ, Kwan MY, Wong JS, et al. Interim estimates of the effectiveness of influenza vaccination against influenza-associated hospitalization in children in Hong Kong, 2015–16. Influenza Other Respi Viruses 2016; 11(1): 61–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chiu SS, Kwan MYW, Feng S, et al. Influenza vaccine effectiveness against influenza A(H3N2) hospitalizations in children in Hong Kong in a prolonged season, 2016/17. J Infect Dis 2018: jiy027-jiy. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Grohskopf LA, Sokolow LZ, Olsen SJ, Bresee JS, Broder KR, Karron RA. Prevention and Control of Influenza with Vaccines: Recommendations of the Advisory Committee on Immunization Practices, United States, 2015–16 Influenza Season. MMWR Morb Mortal Wkly Rep 2015; 64(30): 818–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sullivan SG, Tchetgen EJT, Cowling BJ. Theoretical basis of the test-negative study design for assessment of influenza vaccine effectiveness. Am J Epidemiol 2016; 184(5): 345–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sullivan SG, Feng S, Cowling BJ. Potential of the test-negative design for measuring influenza vaccine effectiveness: a systematic review. Expert Rev Vaccines 2014; 13(12): 1571–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sullivan SG, Cowling BJ. “Crude vaccine effectiveness” is a misleading term in test-negative studies of influenza vaccine effectiveness. Epidemiology 2015; 26(5): e60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cowling BJ, Sullivan SG. A concern over terminology in vaccine effectiveness studies. Eurosurveillance 2018; 23(10). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wu P, Goldstein E, Ho LM, et al. Excess mortality associated with influenza A and B virus in Hong Kong, 1998–2009. J Infect Dis 2012; 206(12): 1862–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hastie T, Tibshirani R. Generalized additive models for medical research. Stat Methods Med Res 1995; 4(3): 187–96. [DOI] [PubMed] [Google Scholar]
- 27.Neher RA, Bedford T. nextflu: real-time tracking of seasonal influenza virus evolution in humans. Bioinformatics 2015; 31(21): 3546–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Andrews N, McMenamin J, Durnall H, et al. Effectiveness of trivalent seasonal influenza vaccine in preventing laboratory-confirmed influenza in primary care in the United Kingdom: 2012/13 end of season results. Eurosurveillance 2014; 19(27): 20851. [PubMed] [Google Scholar]
- 29.Belongia EA, Sundaram ME, McClure DL, Meece JK, Ferdinands J, VanWormer JJ. Waning vaccine protection against influenza A (H3N2) illness in children and older adults during a single season. Vaccine 2015; 33(1): 246–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Castilla J, Martinez-Baz I, Martinez-Artola V, et al. Decline in influenza vaccine effectiveness with time after vaccination, Navarre, Spain, season 2011/12. Eurosurveillance 2013; 18(5): 20388. [DOI] [PubMed] [Google Scholar]
- 31.Gherasim A, Pozo F, de Mateo S, et al. Waning protection of influenza vaccine against mild laboratory confirmed influenza A (H3N2) and B in Spain, season 2014–15. Vaccine 2016; 34(20): 2371–7. [DOI] [PubMed] [Google Scholar]
- 32.Jiménez-Jorge S, de Mateo S, Delgado-Sanz C, et al. Effectiveness of influenza vaccine against laboratory-confirmed influenza, in the late 2011–2012 season in Spain, among population targeted for vaccination. BMC Infect Dis 2013; 13(1): 441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Katz MA, Lebo E, Emukule GO, et al. Uptake and effectiveness of a trivalent inactivated influenza vaccine in children in urban and rural Kenya, 2010 to 2012. Pediatr Infect Dis J 2016; 35(3): 322–9. [DOI] [PubMed] [Google Scholar]
- 34.Kissling E, Valenciano M, Larrauri A, et al. Low and decreasing vaccine effectiveness against influenza A(H3) in 2011/12 among vaccination target groups in Europe: results from the I-MOVE multicentre case-control study. Eurosurveillance 2013; 18(5): 20390. [DOI] [PubMed] [Google Scholar]
- 35.Levy JW, Simasathien S, Watanaveeradej V, et al. Influenza vaccine effectiveness in the tropics: moderate protection in a case test-negative analysis of a hospital-based surveillance population in Bangkok between August 2009 and January 2013. PloS one 2015; 10(8): e0134318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pebody R, Andrews N, McMenamin J, et al. Vaccine effectiveness of 2011/12 trivalent seasonal influenza vaccine in preventing laboratory-confirmed influenza in primary care in the United Kingdom: evidence of waning intra-seasonal protection. Eurosurveillance 2013; 18(5): 20389. [DOI] [PubMed] [Google Scholar]
- 37.Pebody R, Warburton F, Andrews N, et al. Effectiveness of seasonal influenza vaccine in preventing laboratory-confirmed influenza in primary care in the United Kingdom: 2014/15 end of season results. Eurosurveillance 2015; 20(5). [PubMed] [Google Scholar]
- 38.Pebody R, Warburton F, Ellis J, et al. Effectiveness of seasonal influenza vaccine for adults and children in preventing laboratory-confirmed influenza in primary care in the United Kingdom: 2015/16 end-of-season results. Eurosurveillance 2016; 21(38). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Radin JM, Hawksworth AW, Myers CA, Ricketts MN, Hansen EA, Brice GT. Influenza vaccine effectiveness: Maintained protection throughout the duration of influenza seasons 2010–2011 through 2013–2014. Vaccine 2016; 34(33): 3907–12. [DOI] [PubMed] [Google Scholar]
- 40.Sullivan SG, Komadina N, Grant K, Jelley L, Papadakis G, Kelly H. Influenza vaccine effectiveness during the 2012 influenza season in Victoria, Australia: influences of waning immunity and vaccine match. J Med Virol 2014; 86(6): 1017–25. [DOI] [PubMed] [Google Scholar]
- 41.Fu C, He Q, Li Z, et al. Seasonal influenza vaccine effectiveness among children, 2010–2012. Influenza Other Respi Viruses 2013; 7(6): 1168–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rambhiaa KJ, Rambhia MT. Early bird gets the flu. What should be done about waning intraseasonal immunity against seasonal influenza? Clin Infect Dis 2018: ciy748. [DOI] [PubMed] [Google Scholar]
- 43.Lipsitch M Challenges of vaccine effectiveness and waning studies. Clin Infect Dis 2018; In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Petrie JG, Ohmit SE, Johnson E, Truscon R, Monto AS. Persistence of antibodies to influenza hemagglutinin and neuraminidase following one or two years of influenza vaccination. J Infect Dis 2015; 212(12): 1914–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lipsitch M, Jha A, Simonsen L. Observational studies and the difficult quest for causality: Lessons from vaccine effectiveness and impact studies. Int J Epidemiol 2016; 45(6): 2060–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.O’Hagan JJ, Hernán MA, Walensky RP, Lipsitch M. Apparent Declining Efficacy in Randomized Trials: Examples oftheRV144 HIV Vaccine and CAPRISA 004 Microbicide Trials. AIDS (London, England) 2012; 26(2): 123–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Summary of the 2011–2012 influenza season in the WHO European Region. World Health Organization; 2012. [Google Scholar]
- 48.Review of global influenza activity, October 2016–October 2017 Wkly Epidemiol Rec: l’Organisation mondiale de la Santé, World Health Organization; 2017. p. 761–79. [Google Scholar]
- 49.Pebody R, Warburton F, Ellis J, et al. End-of-season influenza vaccine effectiveness in adults and children, United Kingdom, 2016/17. Eurosurveillance 2017; 22(44). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sullivan SG, Chilver MB, Carville KS, et al. Low interim influenza vaccine effectiveness, Australia, 1 May to 24 September 2017. Eurosurveillance 2017; 22(43). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Belongia EA, Simpson MD, King JP, et al. Variable influenza vaccine effectiveness by subtype: a systematic review and meta-analysis of test-negative design studies. The Lancet Infectious Diseases 2016. [DOI] [PubMed] [Google Scholar]
- 52.McLean HQ, Thompson MG, Sundaram ME, et al. Impact of repeated vaccination on vaccine effectiveness against influenza A (H3N2) and B during 8 seasons. Clin Infect Dis 2014; 59(10): 1375–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


