Abstract
Autoimmune bullous diseases during pregnancy pose a therapeutic challenge for medical dermatologists. There are main concerns with regard to the regimen, dose, route of administration, and potential harm to the fetus. Many therapeutic options may be safe during pregnancy despite official classifications. Furthermore, there are always questions regarding management during the lactation period. Additionally, issues exist about male and female fertility and the time of discontinuation of certain medications before conception. In this article, we present an overview of the literature based on answers to these issues to solve common and uncommon management problems that arise about a spectrum of autoimmune bullous diseases before conception, as well as during pregnancy and the lactation period.
Keywords: Pemphigus, pemphigoid, bullous, pregnancy, lactation fertility
Introduction
Autoimmune bullous diseases (AIBD) comprise typical examples of autoantibody-mediated, organ-specific autoimmune disorders. They are clinically recognized by the formation of blisters on the skin and/or the mucosal membranes. Blister formation is mainly caused by circulating and tissue-bound autoantibodies against adhesion structure molecules. In the pemphigus group, cadherin family proteins partially comprise the desmosome, are responsible for maintaining cell-to-cell adhesion, and are recognized as antigens. In the pemphigoid group, target antigens derive from structural proteins of the dermal-epidermal junction. Discrete clinical forms of AIBD are routinely diagnosed by histology, immunofluorescence, and the detection of circulating autoantibodies against target autoantigens with enzyme-linked immunosorbent assay techniques (Schmidt and Zillikens 2013).
Pemphigus is most frequently diagnosed after the fifth decade of life and bullous pemphigoid after the seventh decade. Epidemiological data with regard to the incidence of AIBD around the world vary (Alpsoy et al. 2015). Literature on the epidemiology of AIBD in specific groups, such as children, adolescents, and pregnant or lactating women, is extremely limited. Accordingly, although there are consensus statements and international guidelines about the diagnosis and treatment of AIBD, there are no specific instructions about pregnancy and lactation.
AIBD during pregnancy can be challenging for clinicians (Fig. 1, Fig. 2). There are concerns with regard to the regimen, dose, route of administration, and potential harm to the fetus and questions regarding management during the lactation period. Additionally, there are issues about male and female fertility and the time of discontinuation of certain medications before conception. In this article, we present an overview of the literature based on answers to these issues to solve common and uncommon management problems that arise about a spectrum of AIBD before conception, as well as during pregnancy and the lactation period.
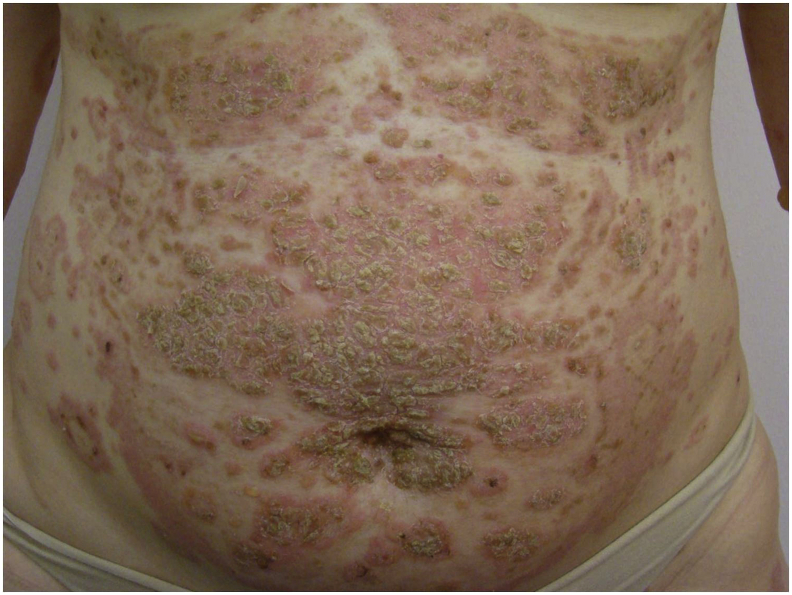
Fig. 1.
Pemphigus foliaceus during pregnancy: Superficial erosions and crusts on the abdomen
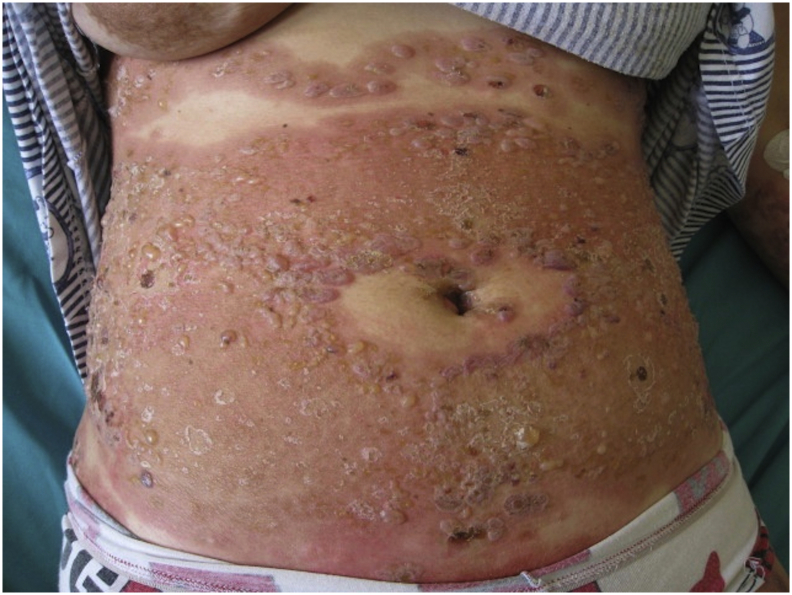
Fig. 2.
Pemphigoid gestationis (postpartum): Erythema and tense bullae, typically sparing the periumbilical area
Methods
We first defined the questions (i.e., common and less common) about disease course and treatment during pregnancy and lactation. We also added questions on contraception, fertility, and conception in patients with known disease.
Subsequently, we performed a Medline literature search using the terms “pemphigus and pregnancy”, “pemphigoid and pregnancy”, “linear IgA dermatosis and pregnancy”, “dermatitis herpetiformis and pregnancy”, and “epidermolysis bullosa acquisita and pregnancy”. We extracted data that could answer the predefined questions and combined it to write this narrative review.
Results and discussion
Answers on the course of the disease
What is the course of pemphigus during pregnancy? Is it different depending on the trimester of pregnancy? Data to support the answer to this question come from a limited number of publications (Table 1). Daneshpazhooh et al. (2011) reported on a series of 52 cases of pemphigus during pregnancy in Iran. Among these, 54% of known cases before conception were exacerbated during pregnancy. A significant number of pregnant women with a history of pemphigus relapsed during the postpartum period (47.1%;.Daneshpazhooh et al. 2011).
Table 1.
Autoimmune bullous diseases: Course during pregnancy, after delivery, or during subsequent pregnancies
| Relapse during pregnancy | Improvement during pregnancy | Relapse after delivery | Relapse in subsequent pregnancies | |
|---|---|---|---|---|
| Pemphigus vulgaris | v | N/A | v | Few cases |
| Pemphigus foliaceus | v | N/A | v | Few cases |
| Pemphigoid gestationis | v | N/A | v | V |
| Linear IgA dermatosis | N/A | v | v | N/A |
| Dermatitis herpetiformis | N/A | N/A | v | N/A |
| Epidermolysis bullosa acquisita | Few cases | Few cases | N/A | N/A |
IgA, immunoglobulin A; N/A, xxx; v, reported in several case reports and series
In a review of the literature on pemphigus cases in pregnancy published in 2015 and covering the period between 1966 and 2014, among 47 identified cases, 21 cases had pemphigus onset before pregnancy and 26 during pregnancy. Pemphigus was exacerbated in 61.9% of patients with a known history (Lin et al. 2015). Pemphigus exacerbations are more common during the first and second trimesters of pregnancy. Increased plasma concentrations of steroids during the third trimester may play a somewhat protective role (Kaplan and Callen 1983).
The role of the T-helper (Th) cells in the development of autoimmune diseases is well known and crucial. During pregnancy there is a disruption of the Th1:Th2 balance. In fact, there is a shift toward more Th2 and fewer Th1 cells, which causes different outcomes in various autoimmune diseases. Pemphigus as a Th2-dominant autoimmune disease tends to relapse due to the shift toward Th2 cell differentiation in pregnancy. Interleukin-4, the master cytokine of Th2 response, has been recently proposed as a therapeutic target for pregnant patients with Th2 dominant diseases (Tavakolpour and Rahimzadeh 2016). The disease does not commonly relapse during every pregnancy. There are very few cases (3) with relapses during subsequent pregnancies in the literature (Kaplan and Callen 1983).
What is the course of pemphigoid diseases during pregnancy? With regard to pemphigoid diseases, data exist on pemphigoid gestationis (PG), which is associated with pregnancy. PG usually presents between the 28th and 32nd week of pregnancy and in a few cases during the postpartum period (Moore and Werth 2016). With PG, an increased fetal risk (e.g., placental insufficiency, growth restriction, prematurity, and small for gestational age) should always be considered (Cohen et al. 2018).
For other AIBD, such as linear immunoglobulin A (IgA) dermatosis, acquired epidermolysis bullosa, dermatitis herpetiformis, and mucous membrane pemphigoid, data are based on case reports and series (Matsuura et al. 2017). In 2002, Collier et al. (1994) reported on a series of 12 women with known linear IgA dermatosis that improved during pregnancy, but most cases (75%) relapsed within 4 months after delivery (Collier et al. 1994). With regard to epidermolysis bullosa acquisita, reported cases during pregnancy are even more scarce; hormonal factors seem instead to induce or exacerbate the condition during pregnancy (Kubo et al. 1997). The first case of a flare of dermatitis herpetiformis in a patient with a 16- year history of the disease was reported in 1982. The patient gave birth to a healthy full-term infant but flared after delivery. The authors discussed the possibility of hemolytic anemia of the newborn due to dapsone transmission through the breast milk (Tuffanelli 1982).
Do the aforementioned entities relapse during the next pregnancy or pregnancies? PG usually recurs in subsequent pregnancies with early onset and increased severity and in only in 5% of cases did it skip a pregnancy (Cohen et al. 2018). No data are available on relapses of linear IgA disease, epidermolysis bullosa acquisita, and dermatitis herpetiformis in subsequent pregnancies (Moore and Werth 2016).
Response to treatment
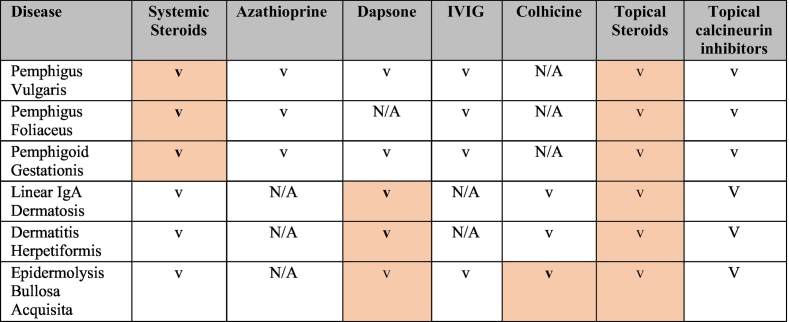
Is treatment with systemic steroid treatments safe during pregnancy and lactation? Is there an optimal regimen (Table 2)? An optimal regimen cannot be evidence-based because there is lack of controlled trials. In the most extensive series of patients with pemphigus during pregnancy (Daneshpazhooh et al. 2011), prednisone was well documented as the first-line treatment. A comprehensive review of published cases up to 2014 reported the safe use of systemic prednisone during pregnancy at the lowest effective dose (Lin et al. 2015). Overall, there is no sufficient evidence that exposure of the fetus to systemic steroids increases the risk for orofacial clefts, as indicated by a recent meta-analysis (Xiao et al. 2017). To achieve disease control in a pregnant patient with a flaring autoimmune bullous disorder, a dose of no more than 20 mg/day of prednisone is recommended and, when needed, the addition of a steroid-sparing agent (Kushner et al., 2018, Murase et al., 2014). Systemic steroids are compatible with lactation because small amounts are transferred to the milk, but mothers are recommended to breastfeed 4 hours after steroid intake so that the concentration of the steroid metabolites within the milk is minimized (Butler et al. 2014).
Table 2.
Medications to treat autoimmune bullous disorders during pregnancy and standard of care
IgA, immunoglobulin A; IVIG, intravenous immunoglobulin; N/A, xxx; v, considered safe
Highlighted areas are the preferred schemes.
Are there any risks from topical corticosteroid therapy during pregnancy and lactation? According to a recent Cochrane review on the use of topical steroids in pregnancy, there is no association between the mother's use of topical steroids of any potency and type of delivery, congenital malformations, premature births, or low Apgar score (Chi et al. 2015). Some evidence exists that the use of high doses of very potent steroids during pregnancy may be associated with low birth weight (Chi et al. 2015). The topical use of steroids during lactation is considered safe, but it is recommended that mothers should avoid the application of highly potent steroids directly on the nipple (Butler et al. 2014).
Is there any difference between fluorinated and nonfluorinated topical corticosteroids? The answer to this question derives from the reported efficacy and side effects of topical steroids in atopic dermatitis. Fluorinated topical steroids are effective but may cause severe skin atrophy after chronic use. Moreover, in the skin of a patient with an autoimmune blistering disorder, these treatments may induce systemic absorption due to the disrupted skin barrier. Nonfluorinated topical steroids have fewer side effects (Gregurek-Novak 2001).
The classification of topical corticosteroids in the United States ranges from Class 1 (most potent) to Class 7 (least potent). In the United Kingdom, the classification has four different categories (Carlos 2013). Potency varies depending on the fluorination of the active molecule, the area of the body on which they are used, the nature of the vehicle (i.e., cream or ointment), the integrity of the skin barrier, and the frequency of the administration (Chi et al., 2017, Ference, 2009). Fluorinated steroids, such as clobetasol propionate, may have an enhanced efficacy, but there may be a degree of percutaneous absorption and a potential for systemic exposure of the mother and fetus (Ference 2009). Despite the low level of evidence, the use of mild-to-moderate potency topical steroids (i.e., methylprednisolone aceponate) is recommended during pregnancy (Chi et al. 2017).
Is the use of topical calcineurin inhibitors permissible in pregnancy? Although there are no data from studies during pregnancy, topical calcineurin inhibitors may be applied on small areas, no more than 5 g/day for 2 to 3 weeks, or when needed (Murase et al., 2014, Rademaker et al., 2017). For breastfeeding mothers, the recommendation is to not apply topical calcineurin inhibitor directly on the nipple (Butler et al. 2014).
Which immunomodulatory agent can be used safely during pregnancy and lactation? Azathioprine (AZA) is considered safe during pregnancy, and the recommended dosage is to not exceed 2 mg/kg/day. When a pregnant patient is at 32 weeks gestation and the leukocyte count is < 1 standard deviation below the mean, half the dose of AZA is recommended (Kushner et al., 2018, Murase et al., 2014). There are only few data to the contrary, for example from the Swedish Medical Birth Register. According to this register, infants exposed to AZA during early pregnancy may be at a moderately increased risk of congenital malformations, specifically ventricular/atrial septal defects, growth restriction, and preterm delivery (Cleary and Källén 2009).
The significant part of 6-mercaptopurine, which is the metabolite of AZA, is excreted in the breast milk within 4 hours after AZA intake. Ingestion by the infant has been estimated to be extremely low. It is recommended, though, that the breastfeeding be done at least 4 hours after AZA intake (Christensen et al. 2008).
Dapsone is also considered safe during pregnancy, as long as glucose-6-phosphate dehydrogenase levels have been measured before initiation. Close blood monitoring after initiation for the risk of maternal or neonatal anemia is of utmost significance (Kushner et al. 2018). Dapsone has a long half-life, and its concentration in the breast milk is relatively high; therefore, dapsone is better avoided during lactation (Butler et al. 2014).
Colchicine is safe during pregnancy (Skorpen 2016) and lactation (Herscovici et al. 2015) based on data from the rheumatologic literature. Intravenous immunoglobulins are also considered a safe therapy during both pregnancy and lactation (Butler et al., 2014, Murase et al., 2014). Mycophenolate mofetil (MMF) is an immunosuppressant agent that is commonly used in the treatment of AIBD. However, MMF is very dangerous during pregnancy. Postmarketing studies have indicated a potential increased risk of first-trimester miscarriage; microtia; external auditory canal atresia; cleft lip/palate; and finger, cardiac, renal, ocular, and central nervous system abnormalities (Kim et al., 2013, Murase et al., 2014). MMF also compromises the efficacy of the birth control pill; thus, women should use other forms of contraception for 6 weeks after stopping therapy.
Answers on family planning
Contraception issues
The chronicity of ΑΙΒD has an impact on young couples’ sexual life and family planning. With regard to contraception and the standard-of-care regimens, there is some diversity of options about the safety of contraception with intrauterine devices (IUDs) in women taking azathioprine (Murase et al. 2014). On the other hand, apart from three case reports of women taking AZA and becoming pregnant with an IUD in place, there is no evidence with regard to the concern of decreased effectiveness of IUDs in the literature, which covers transplanted patients taking AZA and other immunocompromised populations, such as HIV-infected women (Paulen et al. 2010).
Which drugs have no impact on male and female fertility? Systemic steroids, azathioprine, and methotrexate have no negative impact on male and female fertility (Dejaco et al., 2001, Øtensen et al., 2006). Despite the lack of data, intravenous immunoglobulins seem not to cause any harmful effects to fertility (Øtensen et al. 2006).
How long should a woman discontinue treatment with specific immunomodulatory agents before conception (Table 3)? MMF should be discontinued at least 6 weeks before a planned pregnancy (Leroy et al. 2015), and methotrexate must be stopped at least 3 months prior (Øtensen et al. 2006). Female patients treated with cyclophosphamide are advised to wait for one ovulation cycle after discontinuation before conception (Leroy et al. 2015). Rituximab has a long half-life, and women are advised to avoid pregnancy < 12 months after the last infusion (Leroy et al. 2015).
Table 3.
Medications to be discontinued before pregnancy
| Medication | Time |
|---|---|
| Cyclophosphamide | One ovulation cycle |
| Methotrexate | 3 months |
| Mycophenolate mofetil | 6 weeks |
| Rituximab | 12 months |
How long should a man discontinue treatment with specific immunomodulatory agents for family planning? There are no available data, and many researchers suggest that the recommendation is the same for both parents. There is a lack of evidence with regard to the effects of methotrexate on male fertility. However, there is a recommendation to stop methotrexate 3 months prior to conception based on the timeframe of spermatogenesis. In terms of safety and unclear evidence, male patients treated with methotrexate should also be informed of the role of sperm cryopreservation (Gutierrez and Hwang 2017).
Conclusions
Among AIBD, only pemphigus typically relapses during pregnancy, and pemphigoid gestations typically present during this period. Limited data are available for other AIBD. Treatment of AIBD during pregnancy and lactation does not differ significantly because systemic steroids (considered the standard of care in most cases) can be administered with some dose restrictions. MMF, which is commonly used as an additional immunosuppressant agent for AIBD, must be discontinued at least 6 weeks before pregnancy. When a woman has a known history of an autoimmune blistering disorder, pregnancy is better planned during a remission state and after at least 3 to 6 months of disease quiescence (Wan et al. 2016). A change in contraindicated medications during the preconception phase is also recommended, with a wait of at least 2 to 3 months to reach a therapeutic effect with the alternative treatment and ensure disease stability first. When conception is achieved and the AIBD is still active, closer monitoring is required.
Footnotes
Conflict of interest: The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
- Alpsoy E., Akman-Karakas A., Uzun S. Geographic variations in epidemiology of two autoimmune bullous diseases: pemphigus and bullous pemphigoid. Arch Dermatol Res. 2015;307(4):291–298. doi: 10.1007/s00403-014-1531-1. [DOI] [PubMed] [Google Scholar]
- Butler D.C., Heller M.M., Murase J.E. Safety of dermatologic medications in pregnancy and lactation Part II. Lactation. J Am Acad Dermatol. 2014;70(3):1–10. doi: 10.1016/j.jaad.2013.09.009. [DOI] [PubMed] [Google Scholar]
- Carlos G. Rational use of topical corticosteroids in dermatology. Aust Prescr. 2013;36(5):158–161. [Google Scholar]
- Chi C., Wang S., Wojnarowska F., Kirtschig G., Davies E., Bennett C. Safety of topical corticosteroids in pregnancy. Cochrane Database Syst Rev. 2015;10 doi: 10.1002/14651858.CD007346.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chi C.C., Kirtschig G., Aberer W., Gabbud J.P., Lipozenčić J., Kárpáti S. Updated evidence-based (S2e) European Dermatology Forum guideline on topical corticosteroids in pregnancy. J Eur Acad Dermatol Venereol. 2017;31(5):761–773. doi: 10.1111/jdv.14101. [DOI] [PubMed] [Google Scholar]
- Christensen L.A., Dahlerup J.F., Nielsen M.J., Fallingborg J.F., Schmiegelow K. Azathioprine treatment during lactation. Aliment Pharmacol Ther. 2008;28(10):1209–1213. doi: 10.1111/j.1365-2036.2008.03843.x. [DOI] [PubMed] [Google Scholar]
- Cleary B.J., Källén B. Early pregnancy azathioprine use and pregnancy outcomes. Birth Defects Res A Clin Mol Teratol. 2009;85(7):647–654. doi: 10.1002/bdra.20583. [DOI] [PubMed] [Google Scholar]
- Cohen S., Strowd L.C., Pichardo R.O. Pemphigoid gestationis: A case series and review of the literature. J Dermatolog Treat. 2018;29(8):815–818. doi: 10.1080/09546634.2018.1459034. [DOI] [PubMed] [Google Scholar]
- Collier P.M., Kelly S.E., Wojnarowska F. Linear IgA disease and pregnancy. J Am Acad Dermatol. 1994;30(3):407–411. doi: 10.1016/s0190-9622(94)70047-8. [DOI] [PubMed] [Google Scholar]
- Daneshpazhooh M., Chams-Davatchi C., Valikhani M., Aghabagheri A., Mortazavizadeh S.A., Barzegari M. Pemphigus and pregnancy: A 23-year experience. Indian J Dermatol Venereol Leprol. 2011;77(4):534. doi: 10.4103/0378-6323.82404. [DOI] [PubMed] [Google Scholar]
- Dejaco C., Mittermaier C., Reinisch W., Gasche C., Waldhoer T., Strohmer H. Azathioprine treatment and male fertility in inflammatory bowel disease. Gastroenterology. 2001;121(5):1048–1053. doi: 10.1053/gast.2001.28692. [DOI] [PubMed] [Google Scholar]
- Ference J.D. Choosing topical corticosteroids. Am Fam Physician. 2009;79(2):135–140. [PubMed] [Google Scholar]
- Gregurek-Novak T. Topical therapy with fluorinated and non-fluorinated corticosteroids in patients with atopic dermatitis. J Eur Acad Dermatol Venereol. 2001;15(1):81–82. doi: 10.1046/j.1468-3083.2001.00176-5.x. [DOI] [PubMed] [Google Scholar]
- Gutierrez J.C., Hwang K. The toxicity of methotrexate in male fertility and paternal teratogenicity. Expert Opin Drug Metab Toxicol. 2017;13(1):51–58. doi: 10.1080/17425255.2017.1230198. [DOI] [PubMed] [Google Scholar]
- Herscovici T., Merlob P., Stahl B., Laron-Kenet T., Klinger G. Colchicine use during breastfeeding. Breastfeed Med. 2015;10(2):92–95. doi: 10.1089/bfm.2014.0086. [DOI] [PubMed] [Google Scholar]
- Kaplan R.P., Callen J.P. Pemphigus associated diseases and induced pemphigus. Clin Dermatol. 1983;1(2):42–71. doi: 10.1016/0738-081x(83)90022-6. [DOI] [PubMed] [Google Scholar]
- Kim M., Rostas S., Gabardi S. Mycophenolate fetal toxicity and risk evaluation and mitigation strategies. Am J Transplant. 2013;13(6):1383–1389. doi: 10.1111/ajt.12238. [DOI] [PubMed] [Google Scholar]
- Kubo A., Hashimoto K., Inoue C., Hashimoto T., Yoshikawa K. Epidermolysis bullosa acquisita exacerbated by systemic estrogen and progesterone treatment and pregnancy. J Am Acad Dermatol. 1997;36(5 I):792–794. doi: 10.1016/s0190-9622(97)80352-0. [DOI] [PubMed] [Google Scholar]
- Kushner C.J., Concha J.S.S., Werth V.P. Treatment of autoimmune bullous disorders in pregnancy. Am J Clin Dermatol. 2018:1–13. doi: 10.1007/s40257-018-0342-0. [DOI] [PubMed] [Google Scholar]
- Leroy C., Rigot J.M., Leroy M., Decanter C., Le Mapihan K., Parent A.S. Immunosuppressive drugs and fertility. Orphanet J Rare Dis. 2015;10(1):136. doi: 10.1186/s13023-015-0332-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin L., Zeng X., Chen Q. Pemphigus and pregnancy: Analysis and summary of case reports over 49 years. Saudi Med J. 2015;36(9):1033–1038. doi: 10.15537/smj.2015.9.12270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuura K., Ujiie H., Hayashi M., Muramatsu K., Yoshizawa J., Ito T. Linear IgA bullous dermatosis in a pregnant woman with autoantibodies to the non-collagenous 16A domain of type XVII collagen. Acta Derm Venereol. 2017;97(3):404–405. doi: 10.2340/00015555-2557. [DOI] [PubMed] [Google Scholar]
- Moore E.M., Werth V.P. Pemphigoid gestationis. In: Sami N., editor. Autoimmune bullous diseases: Approach and management. Springer International Publishing; New York City, NY: 2016. pp. 149–162. [Google Scholar]
- Murase J.E., Heller M.M., Butler D.C., Francisco S., View M. Continuing safety of dermatologic medications in pregnancy and lactation, Part I. Pregnancy. J Am Dermatol. 2014;70(3):401.e1–401.e14. doi: 10.1016/j.jaad.2013.09.010. [DOI] [PubMed] [Google Scholar]
- Øtensen M., Khamashta M., Lockshin M., Parke A., Brucato A., Carp H. Anti-inflammatory and immunosuppressive drugs and reproduction. Arthritis Res Ther. 2006;8(3):1–19. doi: 10.1186/ar1957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paulen M.E., Folger S.G., Curtis K.M., Jamieson D.J. Contraceptive use among solid organ transplant patients: a systematic review. Contraception. 2010;82(1):102–112. doi: 10.1016/j.contraception.2010.02.007. [DOI] [PubMed] [Google Scholar]
- Rademaker M., Agnew K., Andrews M., Armour K., Baker C., Foley P. Psoriasis in those planning a family, pregnant or breast-feeding. The Australasian Psoriasis Collaboration. Australas J Dermatol. 2017:86–100. doi: 10.1111/ajd.12641. [DOI] [PubMed] [Google Scholar]
- Schmidt E., Zillikens D. Pemphigoid diseases. Lancet. 2013;381(9863):320–332. doi: 10.1016/S0140-6736(12)61140-4. [DOI] [PubMed] [Google Scholar]
- Skorpen G.C. The EULAR points to consider for use of antirheumatic drugs before pregnancy, and during pregnancy and lactation. Ann Rheum Dis. 2016;75(5):795–810. doi: 10.1136/annrheumdis-2015-208840. [DOI] [PubMed] [Google Scholar]
- Tavakolpour S., Rahimzadeh G. New insights into the management of patients with autoimmune diseases or iflammatory disorders during pregnancy. Scand J Immunol. 2016;84(3):146–149. doi: 10.1111/sji.12453. [DOI] [PubMed] [Google Scholar]
- Tuffanelli D. 6-phosphate dehydrogenase deficiency. Arch Dermatol. 1982;118:876. [PubMed] [Google Scholar]
- Wan J., Imadojemu S., Werth V.P. Management of rheumatic and autoimmune blistering disease in pregnancy and postpartum. Clin Dermatol. 2016;34(3):344–352. doi: 10.1016/j.clindermatol.2016.02.006. [DOI] [PubMed] [Google Scholar]
- Xiao W.L., Liu X.Y., Liu Y.S., Zhang D.Z., Xue L.F. The relationship between maternal corticosteroid use and orofacial clefts-a meta-analysis. Reprod Toxicol. 2017;69:99–105. doi: 10.1016/j.reprotox.2017.02.006. [DOI] [PubMed] [Google Scholar]