Abstract
Background
In resource-constrained areas, generic direct-acting antivirals (DAAs) have considerably reduced the cost of hepatitis C virus (HCV) therapy while there remain significant costs related to the baseline and follow-up virologic assays.
Aim
The aim was to assess the efficacy and safety of HCV therapy in Myanmar with pan-genotypic generic DAA sofosbuvir/velpatasvir (SOF/VEL) and with and without the baseline genotype testing, while the duration of treatment and use of ribavirin (RBV) was dictated by cirrhosis and prior treatment failure.
Methods
Between September 2016 and June 2017, data from the 359 participants who completed treatment with SOF/VEL (± RBV) for 12–24 weeks were analyzed. Two hundred one patients did not have the baseline HCV genotype tested.
Results
Regimens included SOF/VEL for 12 weeks (n = 43), SOF/VEL/RBV for 12 weeks (n = 275), or SOF/VEL/RBV for 24 weeks (n = 41). The mean age was 52 years, 44% were men (n = 159), 41 (11.4%) had a history of previous DAA therapy, 7 (1.9%) had a history of hepatocellular carcinoma, and 55 (15.3%) had cirrhosis. Overall, the sustained viral response (SVR)12 rate was 98.6% (354/359) and with a good adverse event profile. SVR rates were similar to those with and without baseline genotype testing and also across all genotypes in those who had genotype tested.
Conclusions
In Myanmar, generic and pan-genotypic SOF/VEL ± RBV is a highly effective and safe treatment for HCV, regardless of the HCV genotype, and therefore, the requirement for the baseline genotype can be eliminated. Future strategies should include elimination of treatment and end of treatment HCV RNA testing to enhance treatment uptake and further reduce cost.
Keywords: sofosbuvir/velpatasvir, hepatitis C, generic, pan-genotypic, direct-acting antiviral, Myanmar
Abbreviations: direct-acting antivirals, DAAs; hemoglobin, Hgb; hepatitis B virus, HBV; hepatitis C virus, HCV; hepatocellular carcinoma, HCC; human immunodeficiency virus, HIV; ledipasvir, LDV; line probe assay, LiPA; pegylated interferon, PEG-IFN; ribavirin, RBV; sofosbuvir/velpatasvir, SOF/VEL; sustained viral response, SVR
Chronic hepatitis C viral (HCV) infection affects an estimated 71 million people worldwide and is a leading cause of cirrhosis, liver transplantation, hepatocellular carcinoma (HCC), and liver-related death.1 While the considerable morbidity and mortality of chronic HCV is an international concern, there are notable geographic differences in the prevalence and genotype distribution of HCV. In Western countries such as the United States, an estimated 1.6% of the population is infected with HCV, while in Southeast Asia, the proportion is significantly higher at an estimated prevalence of 2–12%.2 Globally, HCV genotypes - 1, 2, and 3 are most common, with genotype - 1 accounting for 46% of cases.2, 3 However, in Southeast Asia, the most common genotypes are 1, 3, and 6.4 In Myanmar, in particular, genotype - 6 is the most prevalent genotype, accounting for almost half of the HCV infections, while genotype - 1 only accounts for 11% of the cases.5
In order for HCV to be eradicated, rates of sustained virologic response (SVR) need to be higher than those of new HCV infections.6 Since 2011, the standard of care for chronic HCV infection has shifted from pegylated interferon (PEG-IFN)–based treatment to direct-acting antiviral (DAA)–based therapy. In Asia, while PEG-IFN–based treatment is expensive and requires more intense follow-up of patients, it has resulted in SVR rates of approximately 70% for genotype - 1, approximately 80–90% for genotype - 2/3, and 80% for genotype 6, 7, 8 The introduction of pan-genotypic DAAs has led to significant increases in the tolerability and efficacy of chronic HCV treatment (>90% SVR rates for all genotypes).9 As such, the remaining challenges are identification of cases, education on transmission modes and prevention, and minimizing pretherapy assessment and on-treatment monitoring to be able to facilitate a broader implementation of an elimination strategy. Decreasing the cost of DAA-based therapy, especially in resource-constrained regions such as Myanmar, can increase rates of treatment and thus lead to lower transmission of infection rates. An example of the correlation between making DAAs more accessible and decreasing new infection rate was seen in 2015 when the Netherlands introduced unrestricted access to DAAs for all newly infected HCV patients.10 This new policy resulted in a 51% reduction in new HCV infections for men who have sex with men.10 This result is especially notable because this group had not had a reduction in new infections in the last decade.10 While DAA-based HCV treatment is expensive in the Western world, several highly effective and safe pan-genotypic generic drugs have become available in resource-constrained areas, including Myanmar; bioequivalent data are only available for sofosbuvir (SOF).11, 12, 13 To further decrease the cost of DAA-based therapy, this experience analyzes the efficacy of treatment without the baseline requirement for HCV genotype testing. The cost of genotype testing in Myanmar is around 250 USD, whereas the cost of a 12-week course of a pan-genotypic regimen is between 1050 and 1350 USD. Because these DAA drugs are pan-genotypic, it is likely that genotype testing can be eliminated without compromising efficacy of treatment.
Methods
Patients and Study Design
This open-label, real-world prospective non-randomized observational study was conducted at a single center, Yangon GI & Liver Centre, in Yangon, Myanmar, between September 2016 and June 2017. Adult patients with documented chronic HCV infection, including those with compensated or decompensated cirrhosis (ascites determined by ultrasound), were enrolled in the study, regardless of previous experience with PEG-IFN/ribavirin (RBV) or DAA-containing regimens. Patients with hepatitis B virus (HBV) coinfection, HIV coinfection, and HCC, as well as patients who could not afford genotyping, were included in this treated cohort. Patients were excluded from treatment if they were pregnant, desired to conceive in the near feature, or were lactating and if they had chronic kidney disease with creatinine clearance <30 ml/min as estimated by the Cockcroft-Gault method. Patients with significant medical comorbidities such as ischemic heart diseases, chronic pulmonary diseases, and psychiatric disorders were also excluded from treatment.
HCV genotypes and subtypes were evaluated with line probe assay (LiPA) technology (VERSANT HCV genotype assay [LiPA] Bayer Healthcare manufactured by Innogenetics, Ghent, Belgium), and HCV RNA was quantified by Roche COBAS TaqMan HCV Test, version 2.0. The presence of cirrhosis or advanced fibrosis (F3/F4) was assessed using Fibroscan® (Echosens, France), abdominal ultrasonography, aspartate aminotransferase: platelet ration index, and clinical evaluation. The cut-off transient elastography threshold for cirrhosis was ≥12.5 kPa.
There were two cohorts in whom the therapy outcomes were assessed. As a non-randomized strategy, one cohort had baseline genotype done while the other cohort had no genotype testing done, and both these groups received the generic version of SOF/velpatasvir (SOF/VEL) (400 mg/100 mg) once daily with or without weight-based RBV (1,000 mg for patients with body weight ≤75 kg or 1,200 mg for patients with body weight ≥75 kg) in a divided dose twice daily for 12 or 24 weeks. SOF/VEL with RBV for 24 weeks was given to treatment-experienced patients. SOF/VEL with RBV for 12 weeks was given to participants with Metavir ≥ F3 and patients with HCV genotype - 3 or 6. SOF/VEL without RBV for 12 weeks was given to treatment-naive patients without cirrhosis. There were some exceptions to this pattern in that six treatment-naive patients with cirrhosis were not given RBV. In addition, 18 patients with genotype 3 or 6 were initially not given RBV. This was because the initial strategy was to not use RBV in patients with cirrhosis. However, there were a few relapsers in an earlier experience with DAAs, and therefore, RBV was added to the SOF/VEL regimen. In addition, the high relapse rate with SOF/ledipasvir (LDV) in genotype 6 prompted the change in the strategy.14 The monthly cost of generic SOF/VEL/RBV combination therapy varies between 350 and 450 USD, with different manufacturers from India, Pakistan, and Bangladesh. In Myanmar, the available generic versions of SOF/VEL (400 mg/100 mg) are Sofosvel, Velso and Velfos, which are manufactured by Beacon Pharmaceutical in Bangladesh, Genix Pharma Private Limited in Pakistan, and Getz Pharma Private Limited in Pakistan, respectively. HCV RNA was analyzed at the baseline, at treatment weeks 4, 8, 12, and 24 (if applicable) and at post-treatment week 12.
Safety Assessments
Follow-up visits for safety assessments were scheduled for all patients at screening, at the start of treatment, at treatment weeks 4, 8, 12, and/or 24, and at post-treatment week 12. Safety evaluation included monitoring for adverse events, clinical laboratory testing, physical examination, and vital sign measurement, as well as reporting of adverse events. Anemia was defined as mild (hemoglobin [Hgb] values between 10 and 12 g/dL), moderate (Hgb values between 8.5 and 10 g/dL), or severe (Hgb values less than or equal to 8.5 g/dL). Occurrence of anemia during treatment was managed by 200-mg RBV dose reduction, use of erythropoietin, and/or blood transfusions. This study received approval from the institutional review board to review and analyze the data for publication purposes.
Statistical Analysis
Two-sample t-test, χ2 contingency test, and Fisher's exact test were used where appropriate. Non-parametric methods were used to test the difference in median for variables that are not normally distributed, with the summary statistics for these variables expressed as median (interquartile range). All P-values represent the results of two-sided tests. P-values < 0.05 were considered statistically significant. All data analyses were performed using NCSS 8 software.15
Results
Demographics
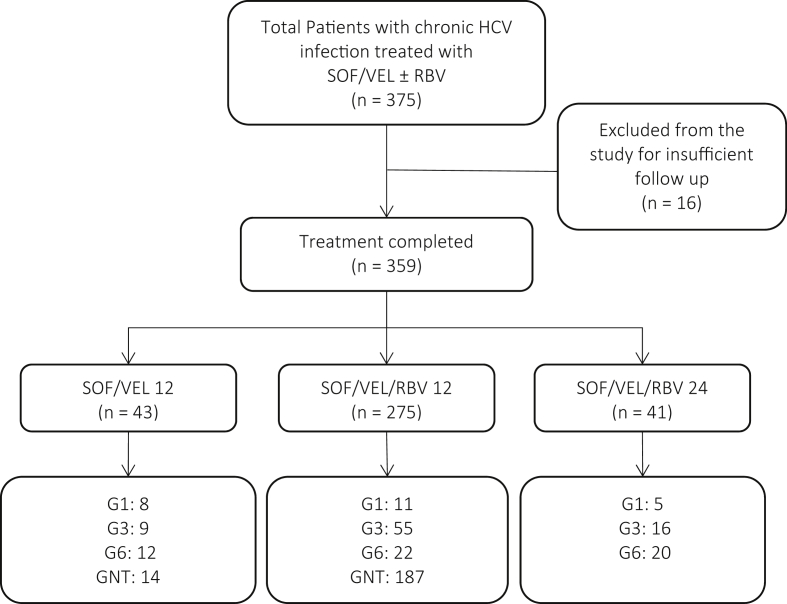
Of the 375 participants who were initially screened for this study, 16 were excluded because of insufficient follow-up. The remaining 359 participants completed treatment of either SOF/VEL for 12 weeks (n = 43), SOF/VEL and RBV for 12 weeks (n = 275), or SOF/VEL and RBV for 24 weeks (n = 41) between September of 2016 and June of 2017 (Figure 1). Table 1 shows the pretreatment baseline demographic and laboratory characteristics and characterization of fibrosis/cirrhosis stage of the study population categorized by whether the participant's genotype was tested. The mean age of the entire group was 52 years, 44% of the participants were men (n = 159), and the median body mass index was 23.0 kg/m2. A total of 27 participants (7.5%) had diabetes, six (1.7%) were coinfected with HBV, and three (0.8%) were coinfected with HIV. Of the 359 participants, 41 (11.4%) had a history of previous DAA therapy and 7 (1.9%) had a history of HCC. The previously used regimens included SOF/daclatasvir for 12 weeks (n = 7), SOF/daclatasvir for 24 weeks (n = 4), SOF/LDV for 12 weeks (n = 24), SOF/LDV for 24 weeks (n = 3), and SOF/RBV for 24 weeks (n = 3). Before treatment, 55 (15.3%) of the participants had cirrhosis, and 4 (1.9%) had decompensated cirrhosis. Two hundred twenty-one (61.6%) participants had HCV RNA >800,00 IU/μL. The pretreatment median albumin level was 3.6 g/dL, the median total bilirubin was 0.7 mg/dL, the median serum creatinine level was 0.8 mg/dL, and the mean platelet count was 210.0 k/UL. Of the 158 participants whose genotypes were tested, 24 (15.2%) had genotype - 1 (20.8% were 1a and 79.2% were 1b), 80 (50.6%) had genotype - 3 (20.0% were 3a; 57.5% were 3b; and 22.5% were unspecified), and 54 had genotype - 6 (44.4% were 6-c-l; 11.1% were 6-m; 16.7% were 6-n; and 27.8% were unspecified). Table 2 compares the baseline characteristics of the three different treatment groups: treatment-naive patients treated with SOF/VEL/RBV for 12 weeks, treatment-naive patients treated with SOF/VEL for 12 weeks, and treatment-experienced patients treated with SOF/VEL/RBV for 24 weeks. All the patients who had previously undergone DAA-based therapy were treated with SOF/VEL/RBV for 24 weeks.
Figure 1.
The study treatment scheme is outlined for patients who completed treatment by HCV genotype. SOF, sofosbuvir; VEL, velpatasvir; RBV, ribavirin; GNT, genotype not tested; HCV, hepatitis C virus.
Table 1.
Pretreatment Baseline Characteristics.
| Characteristics | Genotype tested | Genotype not tested | P-value | Total |
|---|---|---|---|---|
| N | 158 | 201 | 359 | |
| Mean age (range) | 51.2 (23–81) | 51.9 (19–90) | 0.6 | 51.5 |
| Gender (M/F) | 74/84 | 85/116 | 0.74 | 159/200 |
| Median BMI (IQR) | 24.0 (21–27) | 23.0 (21–25) | 0.06 a | 23.0 |
| Median ALT (IU/L) (IQR) | 29 (23–45) | 28 (23.5–32) | 0.2 a | 28.0 |
| Median AST (IU/L) (IQR) | 23.5 (21–37.5) | 24 (21–29.5) | 0.68 a | 24.0 |
| Median bilirubin (mg/dl) (IQR) | 0.7 (0.6–0.8) | 0.7 (0.6–0.8) | 0.62 a | 0.7 |
| Median albumin (g/dl) (IQR) | 3.6 (3.5–3.8) | 3.5 (3.4–3.7) | 0.0009 a | 3.6 |
| Median AFP (IU/L) (IQR) | 5.05 (3.8–7.2) | 5.3 (4.3–7) | 0.12 a | 5.3 |
| Median sCr (IQR) | 0.8 (0.8–1.0) | 0.8 (0.7–0.9) | 0.09 a | 0.8 |
| Median Hgb (IQR) | 12.0 (11.5–13.0) | 11.8 (11.4–12.8) | 0.06 a | 11.9 |
| Mean WBC count (range) | 7.0 (3.3–12.11) | 7.2 (3.4–11.4) | 0.44 | 7.12 |
| Median platelet (IQR) | 210 (188–256) | 210 (189–234) | 0.92 a | 210 |
| HCV RNA > 800,000 IU/ml | ||||
| Yes | 99 | 122 | 0.14 | 221 |
| No | 59 | 79 | 138 | |
| Genotype | ||||
| 1 | 24 | n/a | b | 24 |
| 1a | 5 | n/a | 5 | |
| 1b | 19 | n/a | 19 | |
| 3 | 80 | n/a | 80 | |
| 3a | 16 | n/a | 16 | |
| 3b | 46 | n/a | 46 | |
| Unspecified | 18 | n/a | 18 | |
| 6 | 54 | n/a | 54 | |
| 6c-l | 24 | n/a | 34 | |
| 6m | 6 | n/a | 6 | |
| 6n | 9 | n/a | 9 | |
| Unspecified | 15 | n/a | 15 | |
| Previous IFN-based therapy | ||||
| Yes | 0 | 0 | b | |
| No | 158 | 201 | ||
| Previous DAA therapy | ||||
| Yes | 41 | 0 | <1 × 10−6 | |
| No | 117 | 201 | ||
| Cirrhosis | ||||
| Yes | 35 | 30 | 0.52 | |
| No | 171 | 123 | ||
| Ribavirin | ||||
| Yes | 129 | 187 | 0.00097 | |
| No | 29 | 14 | ||
| Diabetes | ||||
| Yes | 14 | 13 | 0.39 | |
| No | 144 | 188 | ||
| HBV/HCV coinfection | 2 | 4 | 0.6 | |
| HIV/HCV coinfection | 1 | 2 | 0.71 | |
| History of HCC | 1 | 6 | 0.11 | |
| History of alcohol use | ||||
| Yes | 7 | 2 | 0.04 | |
| No | 151 | 199 | ||
| Previous therapy with PPIs | ||||
| Yes | 0 | 0 | b | |
| No | 158 | 201 | ||
When any cell is less than 5 in the contingency table, Fisher's exact test was calculated.
BMI, body mass index; IQR, interquartile range; ALT, alanine aminotransferase; AST, aspartate aminotransferase; AFP, alpha-fetoprotein; sCr, serum creatinine; Hgb, hemoglobin; WBC, white blood cell; IFN, interferon; DAA, direct-acting antiviral; HBV, hepatitis B virus; HCV, hepatitis C virus; HIV, human immunodeficiency virus; HCC, hepatocellular carcinoma; PPI, proton pump inhibitor.
*Transient Elastography (TE) for HCV 12.5 kPA (F4).
Normality rejected, Wilcoxon rank-sum test used to test the difference between two groups, with the corresponding summary statistics presented as median (interquartile range).
Statistics not possible.
Table 2.
Baseline Characteristics of Treatment-Naive Patients Treated With SOF/VEL/RBV 12 Weeks and SOF/VEL 12 Weeks and Treatment-Experienced Patients Treated With SOF/VEL/RBV 24 Weeks.
| Characteristics | SOF/VEL 12 weeks | SOF/VEL/RBV 12 weeks | SOF/VEL/RBV 24 weeks | P-value |
|---|---|---|---|---|
| N | 43 | 275 | 41 | |
| Mean age (range) | 51.6 (24–78) | 51.2 (19–90) | 53.8 (34–71) | 0.77 |
| Gender (M/F) | 21/22 | 116/159 | 22/19 | 0.31 |
| Median BMI (IQR) | 24 (21–27) | 23 (21–25) | 24 (22–27) | 0.02a |
| Median ALT (IU/L) (IQR) | 26 (21–33) | 28 (23–36) | 27 (23–40) | 0.15a |
| Median AST (IU/L) (IQR) | 21 (19–25) | 24 (21–33) | 23 (21–34) | 0.003a |
| Median bilirubin (mg/dl) (IQR) | 0.7 (0.6–0.8) | 0.7 (0.6–0.8) | 0.8 (0.65–0.9) | 0.27a |
| Median albumin (g/dl) (IQR) | 3.6 (2.4–4.1) | 3.6 (2.2–5.2) | 3.5 (2.7–3.9) | 0.08a |
| Median AFP (IU/L) (IQR) | 4.9 (3.9–6.8) | 5.4 (3.9–7.3) | 4.5 (3.6–6.5) | 0.15a |
| Median sCr (IQR) | 0.8 (0.8–0.9) | 0.8 (0.7–0.9) | 0.8 (0.8–1.0) | 0.68a |
| Median Hgb (IQR) | 11.7 (11.4–12.8) | 11.9 (11.5–12.8) | 11.9 (11.3–12.8) | 0.21a |
| Mean WBC (range) | 7.0 (3.3–12.11) | 7.1 (3.4–11.4) | 7.1 (3.4–10.31) | 0.07 |
| Median platelet (IQ R) | 210 (186–245) | 210 (191–242) | 198 (175–231) | 0.43a |
| HCV RNA > 800,000 IU/ml, n (%) | ||||
| Yes | 28 (65.1) | 166 (60.4) | 27 (65.9) | 0.72 |
| No | 15 (34.9) | 109 (39.6) | 14 (34.1) | |
| Genotype, n (%) | ||||
| 1 | 8 (18.6) | 11 (4.0) | 5 (12.2) | 0.006 |
| A | 1 | 3 | 1 | |
| B | 7 | 8 | 4 | |
| 3 | 9 (20.9) | 55 (20.0) | 16 (39.0) | |
| A | 0 | 15 | 1 | |
| B | 7 | 32 | 7 | |
| Unspecified | 2 | 8 | 8 | |
| 6 | 12 (27.9) | 22 (8.0) | 20 (48.8) | |
| C-L | 7 | 5 | 12 | |
| M | 1 | 4 | 1 | |
| N | 1 | 5 | 3 | |
| Unspecified | 3 | 8 | 4 | |
| Not tested | 14 (32.6) | 187 (68.0) | 0 (0.0) | |
| Previous IFN-based therapy, n (%) | ||||
| Yes | 0 (0.0) | 0 (0.0) | 0 (0.0) | b |
| No | 43 (100.0) | 275 (100.0) | 41 (100.0) | |
| Previous DAA therapy, n (%) | ||||
| Yes | 0 (0.0) | 0 (0.0) | 41 (100.0) | |
| No | 41 (100.0) | 275 (100.0) | 0 (0.0) | |
| Cirrhosis, n (%) | ||||
| Yes | 6 (14.0) | 46 (16.7) | 13 (31.7) | 0.051 |
| No | 37 (86.0) | 229 (83.3) | 28 (68.3) | |
| Ribavirin, n (%) | ||||
| Yes | 0 (0.0) | 275 (100.0) | 41 (100.0) | |
| No | 43 (100.0) | 0 (0.0) | 0 (0.0) | |
| Diabetes, n (%) | ||||
| Yes | 1 (2.3) | 22 (8.0) | 4 (9.8) | 0.37 |
| No | 42 (97.7) | 253 (92.0) | 37 (90.2) | |
| HBV/HCV coinfection, n (%) | 2 (4.7) | 3 (1.1) | 1 (2.4) | 0.14 |
| HIV/HCV coinfection, n (%) | 0 (0.0) | 3 (1.1) | 0 (0.0) | 1 |
| History of HCC, n (%) | 0 (0.0) | 6 (2.2) | 1 (2.4) | 0.83 |
| History of alcohol use, n (%) | ||||
| Yes | 0 (0.0) | 8 (2.9) | 1 (2.4) | 0.72 |
| No | 43 (100.0) | 267 (97.1) | 40 (97.6) | |
| Previous therapy with PPIs, n (%) | ||||
| Yes | 0 (0.0) | 0 (0.0) | 0 (0.0) | b |
| No | 43 (100.0) | 275 (100.0) | 41 (100.0) | |
SOF, sofosbuvir; VEL, velpatasvir; RBV, ribavirin; BMI, body mass index; IQR, interquartile range; ALT, alanine aminotransferase; AST, aspartate aminotransferase; AFP, alpha-fetoprotein; sCr, serum creatinine; Hgb, hemoglobin; WBC, white blood cell; IFN, interferon; DAA, direct-acting antiviral; HBV, hepatitis B virus; HCV, hepatitis C virus; HIV, human immunodeficiency virus; HCC, hepatocellular carcinoma; PPI, proton pump inhibitor.
Normality of residuals rejected, Kruskal–Wallis one-way ANOVA on rank test was used to test the difference between two groups, with the corresponding summary statistics presented as median (interquartile range).
Statistics not possible.
Treatment Efficacy
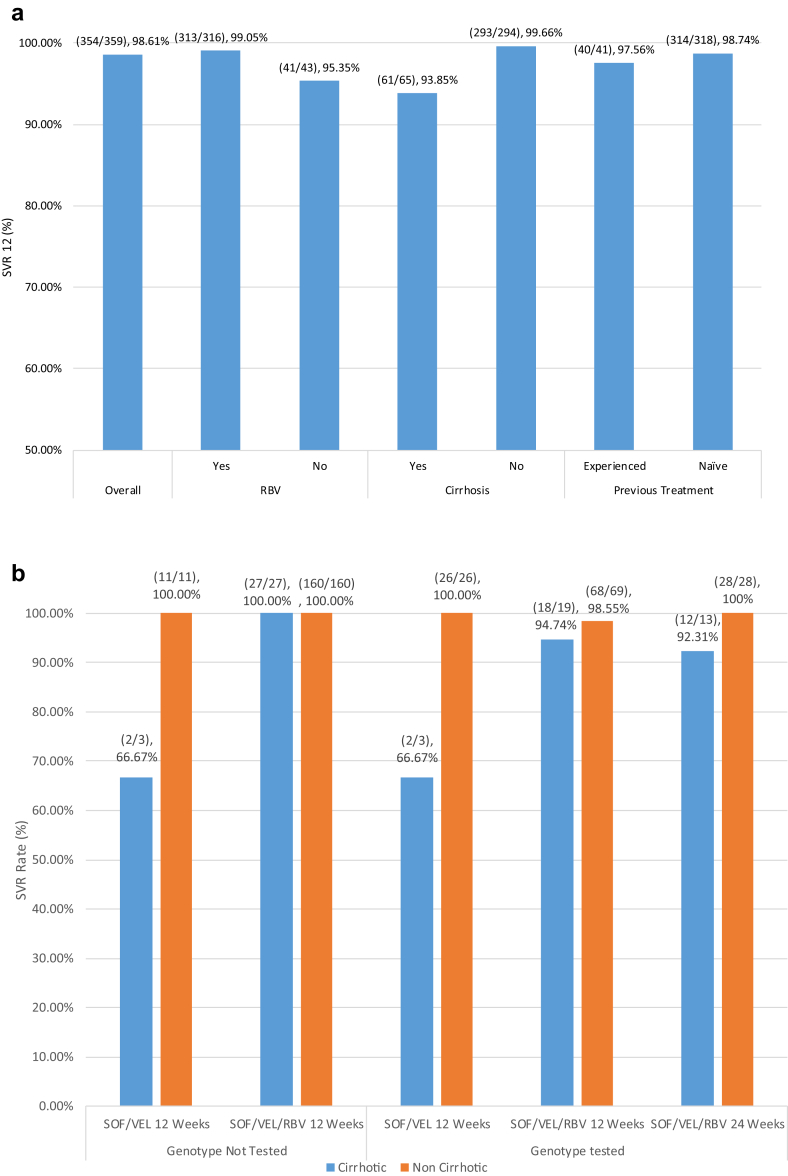
The overall rate of SVR12 in this study was 98.6% (354/359) as per the protocol analysis (Figure 2a). Virologic relapses occurred in five patients (see Supplemental Table). One of these patients had a history of previous DAA therapy failure and was, thus, treated with 24 weeks of SOF/VEL/RBV, while the rest were treatment naive. Two of the treatment-naive patients were treated with only SOF/VEL for 12 weeks, while the other two were treated with SOF/VEL/RBV for 12 weeks. All but one of the five had a Fibroscan score of F4 indicating cirrhosis. Finally, the HCV genotypes of these patients varied, one did not undergo the baseline genotype testing, two were genotype 3b, one was 3a, and one was 6n. There was no significant difference in virologic response between patients who had or did not have their genotype tested. There were also no significant differences in virologic response between patients with HCV genotype - 1, 3, and 6. Figure 2a shows SVR rates categorized by whether the participant's treatment regimen included RBV, had cirrhosis, or they had previously failed HCV therapy. While patients who were treated with RBV had a slightly higher rate of SVR (n = 313/316; 99.05%) than without RBV (n = 41/43; 95.35%), these results were not statistically significant (P = 0.11). On the other hand, cirrhosis was a statistically significant (P = 0.004) predictor of SVR. Patients without cirrhosis (293/294; 99.66%) had a slightly higher rate of SVR than those with (n = 61/65; 93.85%). In addition, as seen in Figure 2b, directing the use of RBV and extending the duration of therapy to 24 weeks based on cirrhosis and prior treatment failure did not impact SVR. The only two groups of patients who had SVR rates below 90% were1 patients without the baseline genotype testing who were treated with SOF/VEL for 12 weeks and had cirrhosis (n = 2/3, 66.67%) and2 patients with the baseline genotype testing who were treated with SOF/VEL for 12 weeks and had cirrhosis (n = 2/3, 66.67%), while the sample size was small in both these groups.
Figure 2.
(a) Overall rate of sustained virologic response 12 weeks after the end of therapy (SVR12) to SOF/VEL ± RBV and rates of SVR12 based on the use of RBV, presence of cirrhosis before treatment, and previous treatment status. The Fisher's exact test P-value for RBV use is 0.11, for presence of cirrhosis is 0.004, and for previous treatment is 0.46. (b) Percentage of patients who achieved sustained virologic response 12 weeks after the end of therapy (SVR12) to SOF/VEL ± RBV. Patients are categorized based on whether their genotype was tested, what treatment regimen they received, and presence of cirrhosis before treatment. SVR, sustained viral response; SOF, sofosbuvir; VEL, velpatasvir; RBV, ribavirin.
Safety and Adverse Events
There were no incidents of serious adverse events and no discontinuation of treatment due to adverse events during the course of this study (Table 3). The most frequently reported side effects were fatigue (21.2%) and anemia (11.1%). A small proportion of patients also reported decreased appetite (1.1%) and headaches (0.6%). Only one patient reported experiencing insomnia. Most of these side effects were reported by patients treated with RBV. As a result, 39 patients (12.3%) had RBV dose reductions. No blood transfusions were necessary, and erythropoietin-stimulating agent was used for only two patients. Finally, Hgb reductions ≥1.5 g/dL (11.4%), platelet reductions <150 (9.2%), and white blood cell reductions <4.0 (2.8%) were observed in a small proportion of patients.
Table 3.
Summary of Treatment Related Adverse Events and Laboratory Abnormalities During Treatment and Follow-up in Patients With SOF/VEL/RBV 12 Weeks and SOF/VEL 12 Weeks.
| Adverse Events/Laboratory Abnormalities | SOF/VEL 12 weeks | SOF/VEL/RBV 12 weeks | SOF/VEL/RBV 24 weeks | P-value |
|---|---|---|---|---|
| N | 43 | 275 | 41 | |
| Any adverse events, n (%) | ||||
| Yes | 14 (32.6) | 76 (27.6) | 18 (43.9) | 0.1 |
| No | 29 (67.4) | 199 (72.4) | 23 (56.1) | |
| Any serious adverse events, n (%) | ||||
| Yes | 0 (0.0) | 0 (0.0) | 0 (0.0) | a |
| No | 43 (100.0) | 275 (100.0) | 41 (100.0) | |
| Discontinuation of treatment due to adverse events, n (%) | 0 (0.0) | 0 (0.0) | 0 (0.0) | a |
| Common adverse events, n (%) | ||||
| Fatigue | 14 (32.6) | 52 (18.9) | 10 (24.4) | 0.06 |
| Anemia | 0 (0.0) | 32 (11.6) | 8 (19.5) | 0.005 |
| Headache | 0 (0.0) | 2 (0.7) | 0 (0.0) | 1 |
| Diarrhea | 0 (0.0) | 0 (0.0) | 0 (0.0) | a |
| Nausea | 0 (0.0) | 0 (0.0) | 0 (0.0) | a |
| Vomiting | 0 (0.0) | 0 (0.0) | 0 (0.0) | a |
| Decreased appetite | 0 (0.0) | 2 (0.7) | 2 (4.9) | 0.086 |
| Insomnia | 0 (0.0) | 0 (0.0) | 1 (2.4) | 1 |
| Pruritus | 0 (0.0) | 0 (0.0) | 0 (0.0) | a |
| Ribavirin dose reduction, n (%) | ||||
| Yes | n/a | 32 (11.6) | 7 (17.1) | 0.32 |
| No | n/a | 243 (88.4) | 34 (82.9) | |
| Injection of erythropoietin, n (%) | ||||
| Yes | 0 (0.0) | 1 (0.4) | 1 (2.4) | 1 |
| No | 43 (100.0) | 274 (99.6) | 40 (97.6) | |
| Blood transfusion, n (%) | ||||
| Yes | 0 (0.0) | 0 (0.0) | 0 (0.0) | a |
| No | 43 (100.0) | 275 (100.0) | 41 (100.0) | |
| Hemoglobin reduction ≥1.5 g/dl, n (%) | ||||
| Yes | 1 (2.3) | 32 (11.6) | 8 (19.5) | 0.039 |
| No | 42 (97.7) | 243 (88.4) | 33 (80.5) | |
| WBC reduction <4.0, n (%) | ||||
| Yes | 1 (2.3) | 7 (2.5) | 2 (4.9) | 0.55 |
| No | 42 (97.7) | 268 (97.5) | 39 (95.1) | |
| Platelet reduction <150, n (%) | ||||
| Yes | 5 (11.6) | 24 (8.7) | 4 (9.8) | 0.77 |
| No | 38 (88.4) | 251 (91.3) | 37 (90.2) | |
SOF, sofosbuvir; VEL, velpatasvir; RBV, ribavirin; n/a, not applicable; WBC, white blood cell.
Statistics not possible.
Discussion
Because HCV genotypes and treatment outcomes vary significantly based on the region, it is essential to study treatment strategies in context. This experience of pan-genotypic generic DAA SOF/VEL with or without RBV reveals excellent rates of SVR (SVR 98.6%; n = 354/359) for HCV-infected patients in Myanmar, regardless of the genotype.
Multiple phase three studies have evaluated the efficacy of SOF/VEL with and without RBV for 12 or 24 weeks as a treatment for chronic HCV.16, 17, 18 These studies have shown that regardless of the genotype, degree of fibrosis, or prior HCV treatment failure, a regimen of SOF/VEL resulted in high rates of SVR.16, 17, 18 One study, in particular, compared SVR rates of SOF/VEL with a standard treatment of SOF/RBV and found that the SVR rates were higher for patients in the SOF/VEL group.18 It is important to note that a majority of the patients in these studies were infected with HCV genotype - 1, whereas relatively small proportions were infected with HCV genotype - 3 and - 6, the two most common HCV genotypes in Myanmar.16, 17, 18 Regardless, however, the studies did report high rates of SVR from treatment with SOF/VEL for all HCV genotypes.16, 17, 18
While these results are promising for the efficacy of SOF/VEL in registration trials, such studies have excluded certain regions such as Myanmar. Previous experience with HCV treatment in Myanmar has shown that treatment with the fixed-dose combination of generic DAA SOF and LDV resulted in lower rates than the expected SVR rates, in patients with genotype 6.14 These results were attributed both to the presence of cirrhosis and to, perhaps, a difficulty in treating a specific subtype of genotype - 6 (genotype 6e, within subtypable 6c-l).14 It was hypothesized that this subtype was uniquely difficult to treat because LDV has an EC50 of 1.1 nM for genotype 6a but an EC50 of 264 nM for genotype 6e.14, 19 The regimen of SOF/VEL with or without RBV used in this study, however, does appear to have overcome the suboptimal efficacy with SOF and LDV in patients with genotype - 6 (SVR = 98.1, n = 53/54).
Despite the slight difference in SVR rates due to cirrhosis, overall generic DAA SOF/VEL with and without RBV appears to be an effective treatment for patients with HCV in Myanmar, regardless of whether their genotype is known. This result is significant because for many patients, especially in resource-constrained regions such as Myanmar, the baseline genotype testing is prohibitively expensive. The elimination of the baseline testing, which costs around 250 USD combined with the lower cost of using a generic, which, for a 12-week course, costs around 1050–1350 USD, meaning that more patients will have access to effective HCV treatment. Another challenge has been with the use of RBV in those with cirrhosis, in difficult-to-treat genotypes of 3 and 6, and for those who have experienced previous DAA therapy failure. While RBV is inexpensive, its use requires frequent on-treatment monitoring and has certain restrictions that pertain to women of child-bearing age. At the time of this study, RBV as part of the DAA regimen was recommended in European Association for the Study of the Liver (EASL) treatment guidelines; however, as per more recent American Association for the Study of Liver Diseases (AASLD) and EASL guidelines, RBV is felt not to be a requirement with the use of SOF/VEL.20, 21, 22 Furthermore, EASL guidelines recommend SOF/VEL/VOX for patients who have previously failed DAA therapy and for genotype 3 patients with compensated cirrhosis and SOF/VEL alone for treatment-naive genotype 1, 2, 4, 5, or 6 patients with compensated cirrhosis.21 AASLD guidelines similarly recommend SOF/VEL alone or SOF/VEL/VOX, depending on the genotype for the treatment of patients with compensated cirrhosis or previous history of failure to DAA therapy21, 22, 23. However, it would seem reasonable that in resource-constrained areas with limited options for difficult-to-treat patients, such as non-availability of SOF/VEL/VOX, RBV should continue to be used, in those with characteristics of cirrhosis and/or previous DAA failure, to potentially limit the number of failures. Therefore, although this was not a prospective and randomized study evaluating various regimens, based on the high rates of SVR seen in this study with the regimens used and the limited resources available in this region, it is reasonable to recommend treatment of HCV with 12 weeks of SOF/VEL for treatment-naive patients without cirrhosis, 12 weeks of SOF/VEL/RBV for treatment-naive patients with Metavir ≥3, and 24 weeks of SOF/VEL/RBV for patients with previous DAA failure, regardless of the HCV genotype and stage of fibrosis. Hopefully, as we move toward large-scale implementation of therapy with fewer drugs and expense, an RBV-free regimen can be implemented more frequently.
Moving forward, it is important to minimize the number of steps it takes to diagnose HCV and implement treatment. A significant barrier to this goal is the lack of availability of rapid point-of-care HCV RNA testing. Without this technology, it can take multiple visits from the initial screening to implementation of HCV therapy.24 New screening tools that can potentially overcome these barriers include a dried blood spot test, a saliva or rapid blood antibody test, and a point-of-care PCR test.25, 26, 27, 28 While dried blood spot testing via the finger stick can provide effective HCV testing, it still requires specialized testing at a centralized diagnostic laboratory and an additional visit for discussing the diagnosis.26, 29 These steps can serve as a barrier to efforts to bring HCV testing and treatment to rural communities in Myanmar. While finger stick or saliva rapid diagnostic HCV tests exist, they are often limited to measuring HCV antibodies, indicating mostly previous exposure rather than active infection.28 Xpert HCV Viral Load testing, called GeneXpert in many low- and middle-income countries, is a promising solution but has a better sensitivity and specificity with venipuncture sample relative to a finger stick.29 Real-world performance of the Xpert HCV Viral Load testing has reported a 100% sensitivity and 99.1% specificity.29 Yet, the test takes about 108 min to obtain a result,29 which is, again, a challenge if therapy is to be instituted at point of care in rural areas. A more recent advancement of this assay using the finger stick as opposed to a venipuncture has demonstrated 100% sensitivity and specificity, with time to result being decreased to 60 min.30
In Myanmar, an estimated 65% of the population lives in rural regions.31 Rapid diagnosis of HCV and subsequent treatment in combination with effective pan-genotypic drugs will bring physicians a step closer to HCV elimination in resource-constrained regions of the world. One could envision a strategy of large-scale point-of-care testing for HCV RNA with immediate results, followed by treatment with a pan-genotype regimen without RBV for 12 weeks, and only one time HCV RNA testing at 12 weeks of follow-up.
Conflicts of Interest
K.R.R. is a member of the scientific advisory board for Abbvie, Gilead, Merck, Shionogi, and Dova, received Support (paid to the University of Pennsylvania) from Abbvie, Merck, Gilead, Conatus, Intercept, and Mallinckrodt. Other authors do not report any conflict of interest.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jceh.2018.12.001.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- 1.Polaris Observatory H.C.V.C. Global prevalence and genotype distribution of hepatitis C virus infection in 2015: a modelling study. Lancet Gastroenterol Hepatol. 2017;2:161–176. doi: 10.1016/S2468-1253(16)30181-9. [DOI] [PubMed] [Google Scholar]
- 2.Bunchorntavakul C., Chavalitdhamrong D., Tanwandee T. Hepatitis C genotype 6: a concise review and response-guided therapy proposal. World J Hepatol. 2013;5:496–504. doi: 10.4254/wjh.v5.i9.496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gower E., Estes C., Blach S., Razavi-Shearer K., Razavi H. Global epidemiology and genotype distribution of the hepatitis C virus infection. J Hepatol. 2014;61:S45–S57. doi: 10.1016/j.jhep.2014.07.027. [DOI] [PubMed] [Google Scholar]
- 4.Hlaing N.K., Banerjee D., Mitrani R. Hepatitis C virus therapy with peg-interferon and ribavirin in Myanmar: a resource-constrained country. World J Gastroenterol. 2016;22:9613–9622. doi: 10.3748/wjg.v22.i43.9613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lwin A.A., Shinji T., Khin M. Hepatitis C virus genotype distribution in Myanmar: predominance of genotype 6 and existence of new genotype 6 subtype. Hepatol Res. 2007;37:337–345. doi: 10.1111/j.1872-034X.2007.00053.x. [DOI] [PubMed] [Google Scholar]
- 6.Hill A.M., Nath S., Simmons B. The road to elimination of hepatitis C: analysis of cures versus new infections in 91 countries. J Virus Erad. 2017;3:117–123. doi: 10.1016/S2055-6640(20)30329-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yu M.L., Chuang W.L. Treatment of chronic hepatitis C in Asia: when east meets west. J Gastroenterol Hepatol. 2009;24:336–345. doi: 10.1111/j.1440-1746.2009.05789.x. [DOI] [PubMed] [Google Scholar]
- 8.Pham T.T.T., Bunchorntavakul C., Dat H.T., Reddy K.R. A randomized trial of 48 versus 24 weeks of combination pegylated interferon and ribavirin therapy in genotype 6 chronic hepatitis C. J Hepatol. 2012;56:1012–1018. doi: 10.1016/j.jhep.2011.12.020. [DOI] [PubMed] [Google Scholar]
- 9.Lok A.S., Chung R.T., Vargas H.E., Kim A.Y., Naggie S., Powderly W.G. Benefits of direct-acting antivirals for hepatitis C. Ann Intern Med. 2017;167:812–813. doi: 10.7326/M17-1876. [DOI] [PubMed] [Google Scholar]
- 10.New hepatitis C infections among HIV-positive gay men drop by half after direct-acting antiviral roll-out in Netherlands. 2017. [Google Scholar]
- 11.Jensen D.M., Sebhatu P., Reau N.S. Generic medications for hepatitis C. Liver Int. 2016;36:925–928. doi: 10.1111/liv.13120. [DOI] [PubMed] [Google Scholar]
- 12.Hill A., Tahat L., Mohammed M.K. Bioequivalent pharmacokinetics for generic and originator hepatitis C direct-acting antivirals. J Virus Erad. 2018;4:128–131. doi: 10.1016/S2055-6640(20)30257-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Organization W.H. 2018. Progress Report on Access to Hepatitis C Treatment: Focus on Overcoming Barriers in Low-and Middle-income Countries. [Google Scholar]
- 14.Hlaing N.K.T., Mitrani R.A., Aung S.T. Safety and efficacy of sofosbuvir-based direct-acting antiviral regimens for hepatitis C virus genotypes 1-4 and 6 in Myanmar: real-world experience. J Viral Hepat. 2017;24:927–935. doi: 10.1111/jvh.12721. [DOI] [PubMed] [Google Scholar]
- 15.Hintze J. NCSS. LLC. Kaysville; Utah, USA: 2012. NCSS 8. [Google Scholar]
- 16.Curry M.P., O'Leary J.G., Bzowej N. Sofosbuvir and velpatasvir for HCV in patients with decompensated cirrhosis. N Engl J Med. 2015;373:2618–2628. doi: 10.1056/NEJMoa1512614. [DOI] [PubMed] [Google Scholar]
- 17.Feld J.J., Jacobson I.M., Hezode C. Sofosbuvir and velpatasvir for HCV genotype 1, 2, 4, 5, and 6 infection. N Engl J Med. 2015;373:2599–2607. doi: 10.1056/NEJMoa1512610. [DOI] [PubMed] [Google Scholar]
- 18.Foster G.R., Afdhal N., Roberts S.K. Sofosbuvir and velpatasvir for HCV genotype 2 and 3 infection. N Engl J Med. 2015;373:2608–2617. doi: 10.1056/NEJMoa1512612. [DOI] [PubMed] [Google Scholar]
- 19.Cheng G., Tian Y., Doehle B. In vitro antiviral activity and resistance profile characterization of the hepatitis C virus NS5A inhibitor ledipasvir. Antimicrob Agents Chemother. 2016;60:1847–1853. doi: 10.1128/AAC.02524-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Liver EAfTSoT EASL recommendations on treatment of hepatitis C 2016. J Hepatol. 2017;66:153. doi: 10.1016/j.jhep.2016.09.001. [DOI] [PubMed] [Google Scholar]
- 21.European Association for the Study of the Liver Electronic address eee, european association for the study of the L. EASL recommendations on treatment of hepatitis C 2018. J Hepatol. 2018 doi: 10.1016/j.jhep.2022.10.006. [DOI] [PubMed] [Google Scholar]
- 22.Diseases TAAftSoL, Present TIDSoA . 2018. HCV Guidance: Recommendations for Testing, Managing, and Treating Hepatitis C. [Google Scholar]
- 23.AASLD- I.D.S.A. Recommendations for Testing, Managing, and Treating Hepatitis C. 2017. Treatment-naive genotype 3 with compensated cirrhosis. [Google Scholar]
- 24.Grebely J., Applegate T.L., Cunningham P., Feld J.J. Hepatitis C point-of-care diagnostics: in search of a single visit diagnosis. Expert Rev Mol Diagn. 2017;17:1109–1115. doi: 10.1080/14737159.2017.1400385. [DOI] [PubMed] [Google Scholar]
- 25.Zhou K., Fitzpatrick T., Walsh N. Interventions to optimise the care continuum for chronic viral hepatitis: a systematic review and meta-analyses. Lancet Infect Dis. 2016;16:1409–1422. doi: 10.1016/S1473-3099(16)30208-0. [DOI] [PubMed] [Google Scholar]
- 26.Coats J.T., Dillon J.F. The effect of introducing point-of-care or dried blood spot analysis on the uptake of hepatitis C virus testing in high-risk populations: a systematic review of the literature. Int J Drug Pol. 2015;26:1050–1055. doi: 10.1016/j.drugpo.2015.05.001. [DOI] [PubMed] [Google Scholar]
- 27.Jewett A., Smith B.D., Garfein R.S., Cuevas-Mota J., Teshale E.H., Weinbaum C.M. Field-based performance of three pre-market rapid hepatitis C virus antibody assays in STAHR (Study to Assess Hepatitis C Risk) among young adults who inject drugs in San Diego, CA. J Clin Virol. 2012;54:213–217. doi: 10.1016/j.jcv.2012.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Smith B.D., Drobeniuc J., Jewett A. Evaluation of three rapid screening assays for detection of antibodies to hepatitis C virus. J Infect Dis. 2011;204:825–831. doi: 10.1093/infdis/jir422. [DOI] [PubMed] [Google Scholar]
- 29.Grebely J., Lamoury F.M.J., Hajarizadeh B. Evaluation of the Xpert HCV Viral Load point-of-care assay from venepuncture-collected and finger-stick capillary whole-blood samples: a cohort study. Lancet Gastroenterol Hepatol. 2017;2:514–520. doi: 10.1016/S2468-1253(17)30075-4. [DOI] [PubMed] [Google Scholar]
- 30.Lamoury F.M.J., Bajis S., Hajarizadeh B. Evaluation of the Xpert HCV viral Load finger-stick point-of-care assay. J Infect Dis. 2018;217:1889–1896. doi: 10.1093/infdis/jiy114. [DOI] [PubMed] [Google Scholar]
- 31.The World Bank. 2014. Rural population (% of total population) [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.