Abstract
Dendrobium is known for its pharmacological actions including anti-cancer effect, anti-fatigue effect, gastric ulcer protective effect, and so on. At present, only studies on endophytic fungi of Dendrobium affecting the metabolites of host plants have been reported, very little research has been done on endophytic bacteria. In this study, we have demonstrated the great diversity of endophytic bacteria in 6 Dendrobium samples from different origins and cultivars. According to the results of the culture-independent method, the endophytic bacterial community in Dendrobium stems showed obvious different in the 6 samples and was influenced by origin and cultivar. Some bacteria including Ralstonia, Comamonas and Lelliottia were first detected in Dendrobium in this study. Based on the culture-dependent method, a total of 165 cultivable endophytic bacteria isolates were isolated from the sterilized Dendrobium stems, and were classified into 43 species according to the 16S rRNA gene sequence analysis. Moreover, 14 of the 43 strains showed antimicrobial activity against phytopathogen using the Kirby-Bauer method. Strain NA-HTong-7 (Bacillus megaterium, 99.12%) showed the highest antimicrobial activity. This study was the first comprehensive study on endophytic bacteria of Dendrobium from different origins and cultivars, which provides new insights into the endophytic bacteria from Dendrobium.
Subject terms: Applied microbiology, Bacterial host response
Introduction
Dendrobium is one of the largest genera among Orchidaceae family plants about 1500 species distributed all over the world. There are 74 species and 2 variants of genus Dendrobium Sw. in China, mainly distributed in south and southwest of China1. Dendrobium, as the Traditional Chinese Medicine (TCM), makes tremendous contributions to public healthcare and promotes the development of TCM2,3. According to the Chinese pharmacopoeia, Dendrobium has great clinical effect on human stomach and kidney4. Modern pharmacological actions of Dendrobium in hepatoprotective effect, anti-cancer effect, hypoglycemic effect, anti-fatigue effect, gastric ulcer protective effect, and so on were also reported. This may mainly be attributed to polysaccharides which are considered as the major bioactive components of Dendrobium5.
Endophytes are a class of endosymbiotic microorganisms widespread among plants that colonize intercellular and intracellular spaces of all known plant compartments but do not cause any plant diseases or significant morphological changes6. The endophyte is likely to be an important determinant of plant health and productivity, and has received substantial attention in recent years as a subject of scientific and commercial interest7. Numerous studies have highlighted the ability of plant-associated microbes to influence important traits such as growth, disease resistance, abiotic stress tolerance, water retention, and the synthesis of plant growth-promoting hormones8,9. They produce a large amount of novel and bioactive secondary metabolites that are not only beneficial to the host plants but also economically important to humans for the potential applications in pharmaceutical, agricultural, and food industries10,11. In view of the importance of endophytes to plants metabolites, recent studies have begun to probe the importance of endophytes to medicinal plants12–15. Studies have shown that the content of secondary metabolites from the same medicinal plant species can be different depending on their location of cultivation, which could in part be related to different composition in their associated microbes when grown at different sites.
At present, only studies on endophytic fungi of Dendrobium have been reported, very little research has been done on endophytic bacteria. In the published literature, most of the studies on endophytes of Dendrobium were mainly based on the culture-dependent method16–20. However, less than 1% of bacteria can be usually be cultured, which is detrimental to the study of the comprehensive roles of endophytic bacteria21. Therefore, in this study, Illumina MiSeq was used to sequence the V5–V7 regions of the bacteria 16S rRNA gene, and combined with the culture-dependent methods to identify the endophytic bacteria in the Dendrobium samples. At the same time, the Kirby-Bauer test was used to screen for antimicrobial activity of the isolated endophytic bacterial strain. The results will provide new insights into the endophytic bacteria from Dendrobium.
Results
Diversity of endophytic bacteria based on Illumina-based analysis
Quality control and analysis of sequencing data
A total of 713, 422 reads and 655 OTUs were obtained after clustering at a 97% similarity level from 6 samples (Three parallel in each sample). Each library contained 29, 709 to 54, 747 reads. The rarefaction curves showed that the sequencing work was relatively comprehensive in covering the bacterial diversity, as the curves tended to approach saturation, indicating that the selected sequence data adequately reflected the bacterial abundance of these samples. In other words, the sequencing data covered almost all the species in the community of the samples under the indication of the rarefaction curve (Supplementary Fig. S1). On the other hand, the distributions of endophytic microbial composition in different Dendrobium stems were detected on different taxonomy level and other bacterial diversity indices including Shannon, Simpson, Ace and Chao were applied for the estimating of endophytic community complexity. All library coverage is above 99.97% (Supplementary Table S1).
Composition and diversity of the endophytic bacterial community in Dendrobium samples
As many as 99.99% of the bacterial sequences were classified from phylum to genus according to the program QIIME using the default setting, while 0.01% of bacterial sequences were unclassified. The valid sequences were classified into 22 different phyla, 40 classes, 93 orders, 190 families, and 373 genera. The overall bacterial composition of each sample was similar, while the distribution was varied. Proteobacteria (55.24%) was the dominant phylum, following by Actinobacteria (25.58%), Firmicutes (12.86%) and Bacteroidetes (5.46%). While the remaining 0.86% contained eighteen very low-abundant phyla (Supplementary Fig. S2).
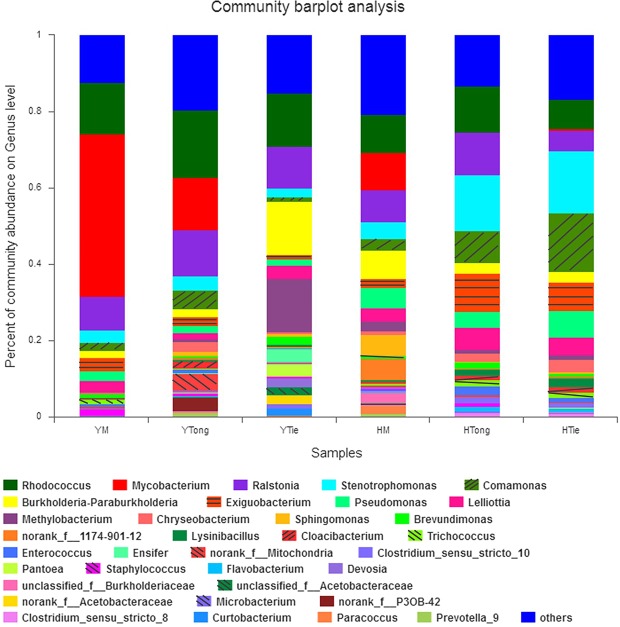
The main endophytic bacterial community for each sample was also analyzed at the genus level (Fig. 1). The results showed that the distribution of each dominant genus among the 6 Dendrobium samples was varied.
Figure 1.
Composition and relative abundance of endophytic bacterial in different samples on the genus level (The color of the column represents the different genera, and the length of the column represents the proportion size of the genus. Sequences that could not be classified into any known group were assigned as “unclassified”. Genera making up less than 1% of total composition in each sample were classified as “other”).
On the whole, the TiePi samples (YTie and HTie) have higher community abundance than other samples but irrelevant to the origins, followed by the TongPi (YTong and HTong) samples, and the MiHu (YM and HM) samples have lowest community abundance. For every single sample, the dominant genera were different from each other (Table 1). For YM, there were 15 genera with over 1% abundance, and the dominant genera were Mycobacterium (42.68%) and Rhodococcus (13.44%). For YTie, there were 15 genera with over 1% abundance, and the dominant genera were Burkholderia-Paraburkholderia (14.03%), Methylobacterium (13.98%), Rhodococcus (13.86%) and Ralstonia (10.95%). For YTong, there were 13 genera with over 1% abundance, and the dominant genera were Rhodococcus (17.60%), Mycobacterium (13.74%) and Ralstonia (12.04%). For HM, there were 13 genera with over 1% abundance, and the dominant genus was Rhodococcus (10.06%). For HTie, there were 17 genera with over 1% abundance, and the dominant genera were Stenotrophomonas (16.35%) and Comamonas (15.25%). For HTong, there were 15 genera with over 1% abundance, and the dominant genera were Stenotrophomonas (14.53%), Rhodococcus (12.23%) and Ralstonia (11.21%). The relative abundance of the same genus was significant difference among the samples. For example, Acetobacteraceae, Curtobacterium, Devosia, Ensifer and Pantoea were only detected in YTie sample. Staphylococcus and Sphingomonas was the specific genus for YM and HM, respectively. Although Mycobacterium was detected in all five samples except HTong, but it was only dominant in YM.
Table 1.
Comparison of percentage (%) of the metagenome sequences affiliated with the dominant bacterial genera (average abundance >1%) for the 6 Dendrobium samples.
| Genus | YM | YTie | YTong | HM | HTie | HTong |
|---|---|---|---|---|---|---|
| Acetobacteraceae | — | 4.32 | — | — | — | — |
| Burkholderia-Paraburkholderia | 1.82 | 14.03 | 2.10 | 7.23 | 2.82 | 2.72 |
| Brevundimonas | 0.90 | 2.07 | — | — | 0.91 | 1.52 |
| Comamonas | 2.23 | 1.04 | 4.80 | 3.11 | 15.25 | 8.52 |
| Curtobacterium | — | 1.79 | — | — | — | — |
| Chryseobacterium | 0.47 | — | 2.61 | 0.96 | 3.25 | 2.14 |
| Cloaciibacterium | — | — | 1.86 | — | 1.37 | 1.26 |
| Devosia | — | 2.29 | — | — | — | — |
| Exiguobacterium | 3.59 | — | 2.34 | 2.50 | 7.45 | 9.97 |
| Enterococcus | 0.64 | — | — | — | 1.11 | 2.17 |
| Ensifer | — | 3.61 | — | — | — | — |
| Flavobacterium | 0.96 | 1.33 | ||||
| Lelliottia | 2.73 | 3.46 | 1.62 | 3.38 | 4.74 | 5.81 |
| Lysinibacillus | — | — | — | — | 2.25 | 1.69 |
| Mycobacterium | 42.68 | 0.17 | 13.74 | 9.75 | 0.44 | — |
| Methylobacterium | — | 13.98 | 2.55 | 1.30 | — | |
| Mitochondria | 0.52 | — | 4.52 | — | — | — |
| Pseudomonas | 2.49 | 1.69 | 1.96 | 5.35 | 6.84 | 4.14 |
| Paracoccus | 2.18 | |||||
| Pantoea | — | 3.29 | — | — | — | — |
| Prevotella | 1.21 | |||||
| Rhodococcus | 13.44 | 13.86 | 17.60 | 10.06 | 7.66 | 12.23 |
| Ralstonia | 8.76 | 10.95 | 12.04 | 8.40 | 5.31 | 11.21 |
| Stenotrophomonas | 3.21 | 2.30 | 3.84 | 4.37 | 16.35 | 14.53 |
| Sphingomonas | — | — | — | 5.53 | — | — |
| Staphylococcus | 1.47 | |||||
| Trichococcus | 1.09 | — | — | — | 1.58 | 1.64 |
| others | 12.27 | 15.12 | 19.59 | 20.69 | 16.84 | 13.15 |
Venn diagram was used to identify the common and unique OTUs among different samples, OTU richness assessed from the 6 samples showed no obvious difference. The OTU abundance of Dendrobium stems from Huoshan was higher than Dendrobium stems from Yingshan on the whole. Samples from Huoshan and Yingshan shared 283 same OTUs, but harbored 237 and 135 unique OTUs respectively (Supplementary Fig. S3a). HM, HTie and HTong as samples from Huoshan shared 75 same OTUs, while harbored 134, 120 and 64 unique OTUs respectively (Supplementary Fig. S3b). YM, YTie and YTong as samples from Yingshan shared 73 same OTUs, while harbored 35, 88 and 130 unique OTUs respectively (Supplementary Fig. S3c). YM and HM shared 77 same OTUs, while harbored 58 and 190 unique OTUs respectively (Supplementary Fig. S3d). YTie and HTie shared 128 same OTUs, while harbored 108 and 171 unique OTUs respectively (Supplementary Fig. S3e). YTong and HTong shared 126 same OTUs, while harbored 159 and 105 unique OTUs respectively (Supplementary Fig. S3f).
In order to test the significance of inter-group differences among the samples in the grouping, Partial Least Squares Discriminant Analysis (PLS-DA) was used to conduct linear discrimination and classification modeling for grouping the samples. There were significant differences among different samples at the OTU level. The first principal coordinate separated the samples was based on the cultivars (COMP1). HM, HTie and HTong showed significant difference at level of COMP1, which showed an obvious difference about the bacteria community composition among cultivars of Dendrobium. It indicated that the cultivar was a factor to shape the community composition. While YM, YTie and YTong showed slight difference at the level of COMP1, which may require further investigation. On the level of COMP2, HM and YM, HTie and YTie showed significant difference, indicating that the origin also influences the shape of the endophytic community composition. While HTong and YTong showed significant difference at level of COMP1, which should be taken more attention (Fig. 2a).
Figure 2.
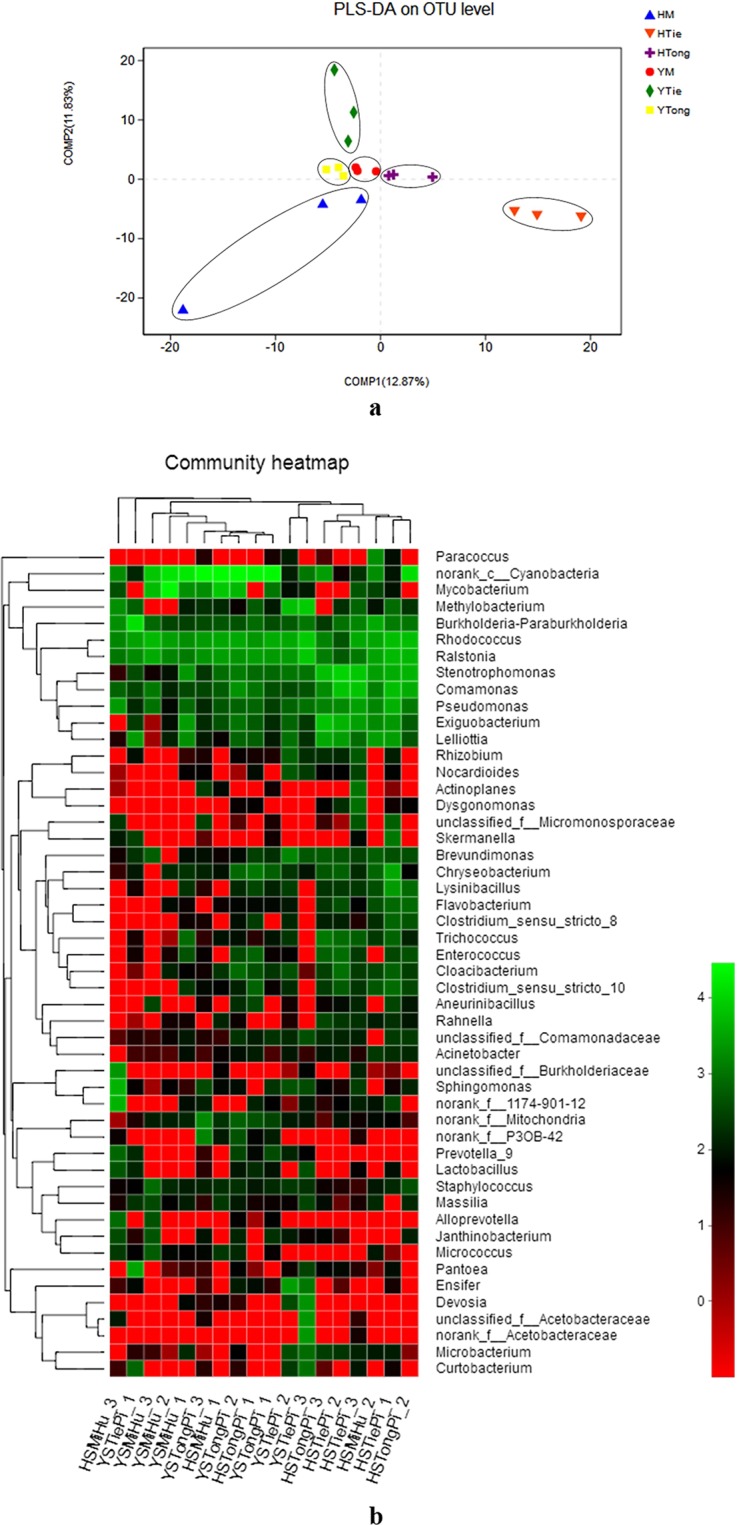
The differences among the samples in the grouping (a- Partial Least Squares Discriminant Analysis (PLS-DA) illustrates differences between bacterial communities in the 6 samples; b- Heatmap of the top 50 most abundant genera in bacterial communities detected in the 6 samples. Dendrograms for hierarchical cluster analysis grouping genera and sample locations are shown at the left and at the top, respectively).
A heatmap was drawn based on the distributions and abundances of the 6 samples on genus level (Fig. 2b). Hierarchical clustering analysis was used to detect the similarity of endophytic bacteria between samples. Based on the results, it was found that the samples from Houshan were clustered together, and other samples were located on the other branch except the HSTongPi_1, HSMiHu_1 and HSMiHu_3. So we deduced that the endophytic bacteria community was affected by their origins primarily.
Diversity of endophytic bacteria based on culture-dependent method
A total of 165 cultivable endophytic bacteria isolates were isolated from the sterilized Dendrobium stems, including 9 isolates from YM, 10 isolates from YTong, 106 isolates from YTie, 16 isolates from HM, 18 isolates from HTong and 6 isolates from HTie. All the isolated endophytic bacteria were identified by 16S rRNA gene sequence analysis, and then compared by the EzTaxon Database (https://www.ezbiocloud.net/). The endophytic bacteria were classified into 3 different phyla, 20 genera and 43 species. The 43 species were submitted to NCBI GenBank under Accession Number (MK389421- MK389463). The phylogenetic tree was shown in the Fig. 3.
Figure 3.
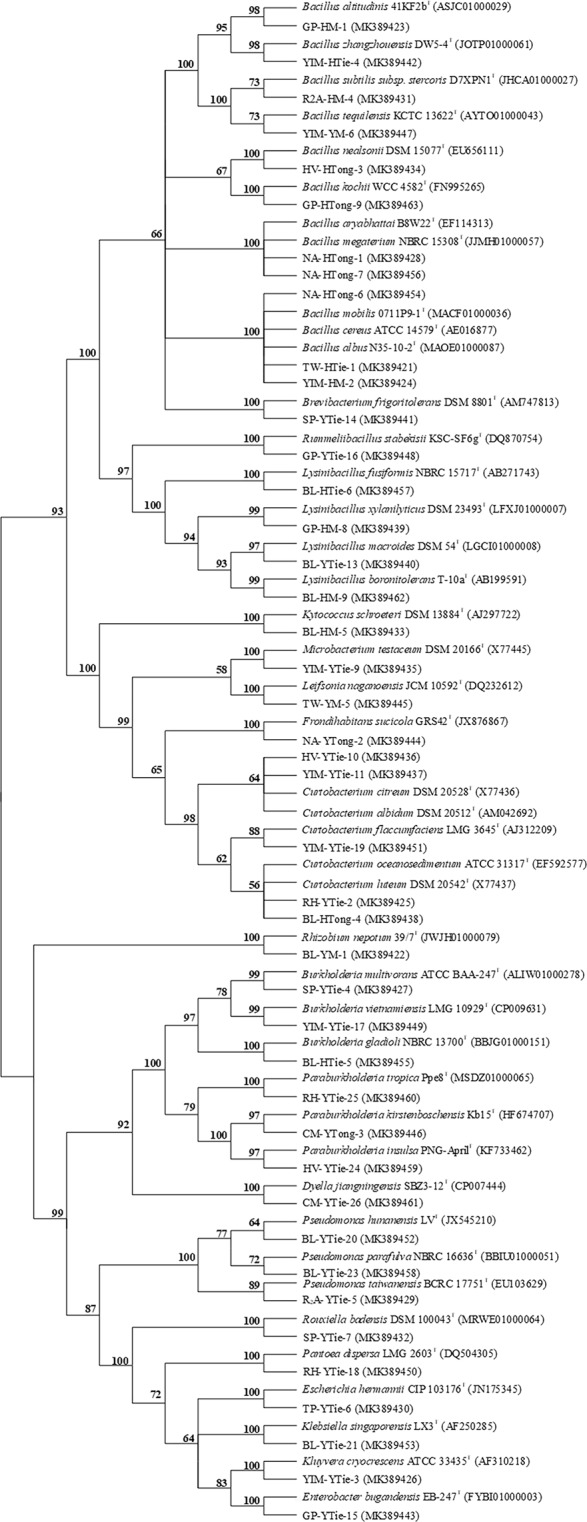
The phylogenetic tree of the 43 species identified in this study (GenBank accession numbers are given in parentheses. Strain name: medium type-sample type- strain number. For example: GP-HM-1 means that the bacterial strain No. 1 isolated from D. huoshanense collected from Huoshan using M-WA agar (GP) culture medium).
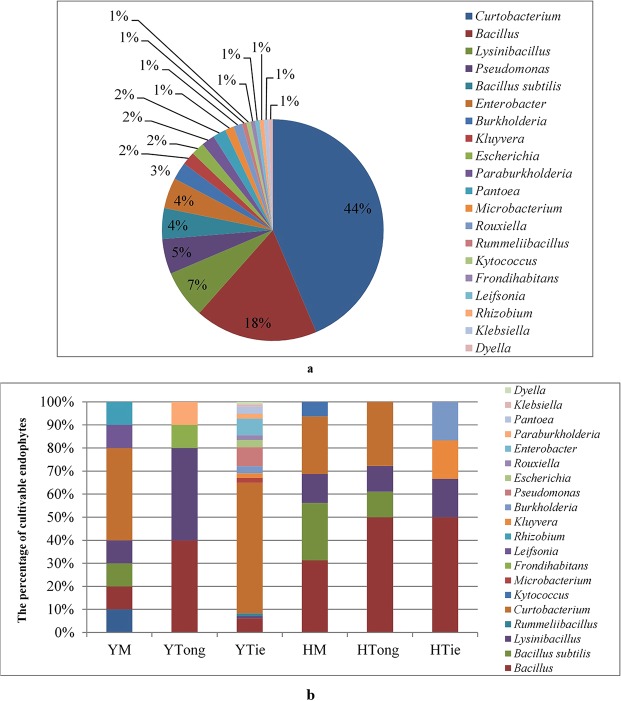
The 20 genera were dominated by Curtobacterium (44%) and Bacillus (18%). Except the predominant genera mentioned above, rare reported endophytic genera such as Rouxiella, Rummeliibacillus, Kytococcus were also appeared (Fig. 4a). Predominant endophytic bacteria for Dendrobium stems showed a slightly different result: the results showed that Bacillus (40.00%) and Lysinibacillus (40.00%) were predominant in YTong, while Bacillus (50.00%) was predominant in HTong. Curtobacterium (56.70%) was predominant in YTie, while Bacillus (50.00%) was predominant in HTie, which harbored very low-abundant endophytes compared to YTie. Interestingly, Curtobacterium (44.44%) was dominated in YM, while Bacillus (31.25%) was common in HM, this phenomenon is similar to YTie and HTie (Fig. 4b).
Figure 4.
Diversity of endophytic bacteria composition of cultivable endophytes (a -Percent of cultivable endophytes on Genus level on the whole; b - Comparison of Genus level distributions for the 6 samples).
Obviously, the distribution of the cultivable endophytic bacterial community was influenced by the difference of origin and cultivar. The bacterial diversity of YTie was greater than any other Dendrobium stems. This result was consistent with the result of Illumina-based analysis.
Antimicrobial activity of endophytic bacteria against phytopathogen
The 43 species were tested for their antimicrobial activity against Athelia rolfsii, Myrothecium roridum and Pectobacterium carotovorum subsp. actinidiae using the Kirby-Bauer method. The supernatant and sediment were extracted separately used ethyl acetate and acetone. Among them, 14 strains showed antimicrobial activity against at least one pathogen in this study. These 14 strains belonged to 9 different genera, namely Burkholderia, Bacillus, Paraburkholderia, Kluyvera, Rouxiella, Microbacterium, Brevibacterium, Pseudomonas and Dyella. The genus Bacillus showed the highest antimicrobial activity against the selected pathogenic fungi and pathogenic bacteria. Out of the 14 strains, 1 strain was from HTie, 2 strains were from HTong, 1 strain was from YTong and 10 strains were from YTie (Table 2).
Table 2.
Antimicrobial activity screening of endophytic bacteria against phytopathogen and their identification based on 16S rRNA gene sequencing analysis.
| Strain | Number | GenBank accession number | Closest species in 16S rRNA gene sequences database | samilarity% | Active part | Pathogenic bacteria | Pathogenic fungi | |
|---|---|---|---|---|---|---|---|---|
| Pectobacterium carotovorum subsp. actinidiae | Athelia rolfsii | Myrothecium roridum | ||||||
| 228 | BL-HTie-5 | MK389455 | Burkholderia gladioli | 98.93 | supernatant | − | +++ | +++ |
| sediment | − | +++ | +++ | |||||
| 214 | NA-HTong-6 | MK389454 | Bacillus albus | 98.88 | supernatant | − | + | ++ |
| sediment | − | ++ | ++ | |||||
| 207 | NA-HTong-7 | MK389456 | Bacillus megaterium | 99.12 | supernatant | ++ | +++ | +++ |
| sediment | − | +++ | +++ | |||||
| 65 | CM-YTong-3 | MK389446 | Paraburkholderia kirstenboschensis | 98.87 | supernatant | − | + | + |
| sediment | − | − | − | |||||
| 166 | YIM-YTie-3 | MK389426 | Kluyvera cryocrescens | 98.43 | supernatant | − | − | + |
| sediment | − | − | − | |||||
| 70 | SP-YTie-7 | MK389432 | Rouxiella badensis | 99.8 | supernatant | − | ++ | ++ |
| sediment | − | + | + | |||||
| 97 | YIM-YTie-9 | MK389435 | Microbacterium testaceum | 98.78 | supernatant | − | ++ | ++ |
| sediment | − | − | + | |||||
| 13 | SP-YTie-14 | MK389441 | Brevibacterium frigoritolerans | 99.93 | supernatant | − | + | + |
| sediment | − | − | − | |||||
| 124 | YIM-YTie-17 | MK389449 | Burkholderia vietnamiensis | 97.48 | supernatant | − | − | − |
| sediment | − | − | ++ | |||||
| 107 | BL-YTie-20 | MK389452 | Pseudomonas hunanensis | 99.79 | supernatant | − | − | + |
| sediment | − | − | − | |||||
| 236 | BL-YTie-23 | MK389458 | Pseudomonas parafulva | 99.29 | supernatant | − | − | + |
| sediment | − | − | − | |||||
| 194 | HV-YTie-24 | MK389459 | Paraburkholderia insulsa | 99.02 | supernatant | − | + | − |
| sediment | − | + | − | |||||
| 202 | RH-YTie-25 | MK389460 | Paraburkholderia tropica | 99.04 | supernatant | − | − | − |
| sediment | − | + | + | |||||
| 135 | CM-YTie-26 | MK389461 | Dyella jiangningensis | 99.64 | supernatant | − | ++ | + |
| sediment | − | ++ | − | |||||
− indicates no activity; +, indicates positive activity, diameter of zones of inhibition less than 10 mm; ++, indicates medium activity, diameter of zones of inhibition between 10 and 20 mm; +++, indicates strong activity, diameter of zones of inhibition greater than 20 mm.
In the assay of ethyl acetate extracts of the supernatant, only NA-HTong-7 showed antimicrobial activity against all three phytopathogens, and its inhibitory activity was better than the 70% Mancozeb (750 fold dilution) (Supplementary Fig. S4). While other strains showed inhibition activity against at least one of the pathogenic fungi, except YIM-YTie-17 and RH-YTie-25 which showed no activity to any of the phytopathogens. Especially, the extracts of NA-HTong-7 and BL-HTie-5 showed the highest antifungal activity against Athelia rolfsii and Myrothecium roridum, with the diameter of zones of inhibition greater than 20 mm.
In the assay of acetone extracts of the sediment, all the isolates showed no antimicrobial activity against the pathogenic bacteria. Interestingly, the extracts of NA-HTong-7 and BL-HTie-5 also showed the highest antifungal activity against Athelia rolfsii and Myrothecium roridum. The extracts of CM-YTong-3, YIM-YTie-3, SP-YTie-14, BL-YTie-20 and BL-YTie-23 showed no antimicrobial activity against any of the three phytopathogens, while the extracts of NA-HTong-6, SP-YTie-7, YIM-YTie-9, YIM-YTie-17, HV-YTie-24, RH-YTie-25 and CM-YTie-26 showed inhibition activity against at least one of the pathogenic fungi.
Discussion
In this study, the great diversity of endophytic bacteria with a considerable number of antimicrobial strains in the 6 Dendrobium stems was demonstrated, and the results showed that the distribution of the endophytic bacterial community was influenced by the difference of origins and cultivars. Meanwhile, the culture-dependent and culture-independent methods of the endophytic bacteria diversity of Dendrobium stems were evaluated. The results will provide new insights into the endophytic bacteria from Dendrobium.
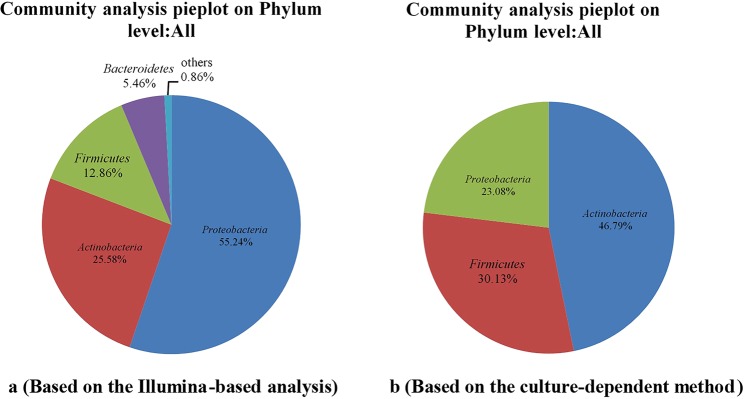
According to the results, differences were found between the two methods, especially on the genus level. It is easy to detect redundant more genera using Illumina-based analysis method and thus it is a more reliable method for investigating the endogenous microorganisms communities of plants. Twenty-two different phyla were detected based on the Illumina-based analysis. Proteobacteria (55.24%), Actinobacteria (25.58%), Firmicutes (12.86%) and Bacteroidetes (5.46%) were the four most dominant phyla, accounting for 99.14% of the reads (Fig. 5a). While the cultivable endophytic bacteria were classified into 3 different phyla, including Actinobacteria (46.79%), Firmicutes (30.13%), and Proteobacteria (23.08%) (Fig. 5b). Compared with the results of Illumina-based analysis, Bacteroidetes was not isolated by the culture-dependent method. It’s worth noting that Proteobacteria was predominant in the 6 Dendrobium stems for the Illumina-based analysis, while occupied a smaller proportion based on the culture-dependent method.
Figure 5.
Composition and relative abundance of endophytic bacterial phyla in different samples (a -Percent of the culture-independent endophytic bacteria on phylum level on the whole; b - Percent of the cultivable endophytic bacteria on phylum level on the whole).
On the genus level, there were 23 genera identified through culture-dependent methods, while 373 genera were sequenced from the 6 Dendrobium stems based on Illumina-based analysis, with 15 of the genera having an average abundance of over 1% (Supplementary Fig. S5). The significantly higher proportion of Curtobacterium (44%) and Bacillis (18%) were observed in the 6 Dendrobium stems by the culture-dependent method. However, Rhodococcus (11.84%) was occupied high abundance as revealed through the Illumina-based analysis. By the culture-dependent method, the isolates from Dendrobium stems included Bacillus, Enterobacter, Klebsiella, Pantoea, Pseudomonas, Curtobacterium, Burkholderia, Microbacterium and Lysinibacillus, which have been reported in previous studies22–25. While some bacterial genera that have not been reported from Dendrobium were detected by Illumina-based analysis, including Ralstonia, Comamonas and Lelliottia, which indicated that there is still a lot of research space for endophytes of Dendrobium26,27. It is evident that the culture-dependent method tended to produce biased results for microbes. The culturable bacteria were probably the largest, most active bacteria in Dendrobium samples28–30. A combination of culture-dependent and culture-independent methods might provide a powerful strategy to investigate microbes, which will help us identify and obtain some novel or difficult-to-cultivate endophytes.
In total, 165 cultivable endophytic bacteria were isolated and assigned into 20 genera dominanted by Curtobacterium (44%) and Bacillis (18%). Eleven different types of media were used to isolate endophytic bacteria from sterilized Dendrobium stems, and all media were supplemented with 1% plant extracts of Dendrobium stems to offer natural growth conditions for the potential endophytes in plant tissues to obtain more culturable single colonies. In fact, the genera preference of cultivable endophytic bacteria was influenced by the isolation media. According to the results, differences were found among the 11 different media (Supplementary Fig. S6). Among the media used in the experiment, YIM38 was proven to be most favorable to the growth of cultivable endophytic bacteria, with 31 strains were isolated from it. On the other hand, the endophytic strains isolated from BL medium showed higher diversity compared to other 10 media, which demonstrated that BL medium possessed more extensive adaptability for bacterial growth. It’s worth noting that Curtobacterium was the only genus isolated from all the media. The influence of different media components on endophytes diversity was showed in this study, especially the factors such as Carbon source and Nitrogen source. It suggested that environmental factors such as oxygen content should be taken more attention in investigations of plant endophytes.
Combining with culture-dependent and culture-independent methods, the endophytic bacterial community in Dendrobium stems was different in the 6 samples and the distribution of the endophytic bacterial community was influenced by the difference of origins and cultivars, but mostly the origins. This result agreed with research that endophytic bacterium strains are obtained from different Dendrobium collected from different locations showed specificity distribution characteristics, and the specific endophytic bacteria promote the formation of geo-authentic crude drug31,32.
Endophytes associated with medicinal plants may produce the same metabolites in vitro and within host plant tissue33,34, which also are the rich sources of secondary metabolites with antimicrobial activity. In this study, the antimicrobial activity of a diverse collection of endophytes isolated from Dendrobium stems, consisting of 20 genera and 43 species was analyzed. Athelia rolfsii, Myrothecium roridum as pathogenic fungi of Dendrobium, and Pectobacterium carotovorum subsp. actinidiae as pathogenic bacteria of Dendrobium, were used to determine the antimicrobial activities. In these assays, a significant fraction of the endophytic bacteria (32.6%) displayed antagonistic effects. Among the 43 species, 14 strains harbored at least one positive antimicrobial activity in this study. These 14 strains belonged to 9 different genera, but only genus Bacillus showed the highest antimicrobial activity against all three indicator pathogens. Previous studies have demonstrated that Bacillus strains associated with other medicinal plants exhibited antibacterial activity not only against phytopathogens such as Fusarium moniliforme, Fusarium oxysporumf.sp.vasinfectum, Verticillium dahlia and Burkholderia solanacearum35–38, but also the 3 pathogens used in this study39–42. The genus Bacillus is well known for the natural production of secondary metabolites with antibacterial and antifungal activities and has a strong potential to control plant diseases43–45. Our study further illustrates its potential role as a biological agent for controlling phytopathogens.
At present, the research on endophytes of Dendrobium mainly focuses on the isolation of endophytes of a single Dendrobium sample. However, to realize the potential of beneficial microbes for medicinal herb applications, research should be carried out to study variations in the effective components of the same cultivar from different origins or the same origin from different cultivars. In our study, we have combined the culture-dependent and culture-independent methods to explore the difference of endophytic bacteria of the 6 Dendrobium samples. The result demonstrated that there was a great diversity of endophytic bacteria in the 6 Dendrobium stems, with a considerable number of antimicrobial strains, and showed that the distribution of the endophytic bacterial community was influenced by the difference of origins and cultivars, especially the origins. However, we have not studied the relationship between the medicinal components of Dendrobium and the endophytes, which may be the further research plan in our laboratory.
Materials and Methods
Plant sampling and surface sterilization
Dendrobium samples were collected from Yingshan, Hubei Province, China and Huoshan, Anhui Province, China. The plant samples included 6 cultivars originated from the two different places (Table 3). Stems were collected from 6 cultivars of Dendrobium samples randomly. The plant samples were randomly divided into three groups placed in three different plastic bags. All 18 plant samples were transported to the laboratory at 4 °C. Dendrobium samples were labeled according to the difference of origins and cultivars as YM, YTong, YTie, HM, HTong, HTie.
Table 3.
Dendrobium samples codes and their origin.
| Samples | Species | Tissue | Latitude | Longitude | Location |
|---|---|---|---|---|---|
| YM | D. huoshanense | stem | 115°31′~116°04′ | 30°00′~31°08′ | Yingshan, Hubei Province, China |
| YTong | D. moniliforme | stem | |||
| YTie | D. officinale | stem | |||
| HM | D. huoshanense | stem | 115°52′~116°32′ | 31°03′~31°33′ | Huoshan, Anhui Province, China |
| HTong | D. moniliforme | stem | |||
| Htie | D. officinale | stem |
In order to avoid the influence of microorganisms on the surface of Dendrobium, the stem samples of Dendrobium were surface-sterilized as follows: Dendrobium stems were washed by tap water and underwent ultrasonic treatment (40 kHz) for 15 min. Then surface sterilized with 1% (v/v) Tween 20 for 1 min; 2.5% (w/v) NaS2O3 for 10 min; 75% ethanol for 5 min. Next, stems were rinsed with distilled water three times. To confirm the sterilization process was successful, 200 μL of the final water rinse was plated on the eleven different media along with 1% plant extracts of Dendrobium stems (Supplementary Table S2), which consistently yielded no bacterial colonies incubated at 28 °C for two weeks.
Illumina-based analysis of endophytic bacterial
DNA extraction and PCR amplification
Microbial DNA was extracted from the samples using the E.Z.N.A.® Bacterial DNA Kit (Omega Bio-tek, Norcross, GA, U.S.) according to the manufacturer’s protocols. The final DNA concentration and purification were determined by NanoDrop 2000 UV-vis spectrophotometer (Thermo Scientific, Wilmington, USA), and DNA quality was checked by 1% agarose gel electrophoresis.
The V5–V7 regions of the bacteria 16S rRNA gene of each sample were amplified for twice. The PCR reactions were first conducted with primers 799 f (5′-AACMGGATTAGATACCCKG-3′) and 1392r (5′-ACGGGCGGTGTGTRC-3′) by thermocycler PCR system (GeneAmp 9700, ABI, USA). Subsequently, the initial PCR products were used as templates for the second amplification with primers 799 f (5′-AACMGGATTAGATACCCKG-3′) and 1193r (5′-ACGTCATCCCCACC TTCC-3′). The PCR reactions were conducted using the standard procedure46, with slight modifications. PCR reactions were performed in 20 μL mixture containing 4 μL of 5 × FastPfu Buffer, 2 μL of 2.5 mM dNTPs, 0.8 μL of each primer (5 μM), 0.2 μL of BSA, 0.4 μL of FastPfu Polymerase and 10 ng of template DNA, and then, adding up to 20 μL with ddH2O.
Illumina MiSeq sequencing
The resulted PCR products were extracted from a 2% agarose gel and further purified using the AxyPrep DNA Gel Extraction Kit (Axygen Biosciences, Union City, CA, USA) and quantified using QuantiFluor™-ST (Promega, USA) according to the manufacturer’s protocol. Purified amplicons were pooled in equimolar and paired-end sequenced (2 × 250 bp) on an Illumina MiSeq platform (Illumina, San Diego,USA) according to the standard protocols by Majorbio Bio-Pharm Technology Co. Ltd. (Shanghai, China). Sequencing was by using Illumina’s Miseq PE250 platform.
Processing of sequencing data
Raw FASTQ files were quality-filtered by Trimmomatic and merged by FLASH by the standard procedure47. Operational taxonomic units (OTUs) were clustered with 97% similarity cutoff using UPARSE (version 7.1 http://drive5.com/uparse/) with a novel ‘greedy’ algorithm that performs chimera filtering and OTU clustering simultaneously. The taxonomy of each 16S rRNA gene sequence was analyzed by RDP Classifier algorithm (http://rdp.cme.msu.edu/) against the Silva (SSU123) 16SrRNA database using confidence threshold of 70%.
Data analysis
Rarefaction curves were plotted for each sample to determine the abundance of communities and sequencing data of each sample. Alpha-diversity analyses, including community richness parameters (Chao, Ace, Sobs), community diversity parameters (Shannon, Simpson) and Community coverage index (Coverage), were calculated using the mothur software. Bacterial taxonomic distributions of sample communities were visualized using the R package software. Venn diagram was implemented using the R package to show unique and shared OTUs. Partial Least Squares Discriminant Analysis (PLS-DA) based on OTU compositions were determined. Student’s t-test was used to detect whether there was a significant difference in The Observed Richness Value (Sobs) between the samples.
Isolation and identification of endophytic bacteria
Isolation of Endophytic bacteria from Dendrobium stems
The sterilized Dendrobium stems were cut by sterile surgical scissors into 0.5~1.0 cm segments, and placed onto different types of media plates to obtain more culturable single colonies. All media were supplemented with 1% plant extracts of Dendrobium stems to offer natural growth conditions for the potential endophytes in plant tissues. The plates were incubated at 28 °C until emergence of bacterial colonies from inside the samples.
Single-colony isolation was repeated at least three times for purification of each of the endophytic bacteria isolates by use of the YIM38 agar medium. Finally, the selected endophytic bacteria isolates were stored in 50% (v/v) glycerol (Bacterial fluid: 50% glycerol = 1:1, v/v) at −80 °C for further study.
Identification of Endophytic bacteria
The endophytic microbial DNA was extracted following the standard procedure46, with slight modifications. Bacterial 16S rRNA genes were amplified using the genomic DNA as template and bacterial universal primers of 27 f and 1492r by thermocycler PCR system (Applied Biosystems, Singapore). The PCR reaction were performed in 50 μL mixture containing 25 μL of 1 × EasyTap PCR SuperMix (TransGen Biotech), 1.5 μL of universal primers 27 f, 1.5 μL of universal primers 1492r, 20 μL of sterilized water, 2 μL of genomic DNA. The PCR amplification procedure was shown as follow (Supplementary Table S3). The PCR products were checked for the expected size on 1.2% agarose gel and were sequenced at Sangon Lab (Shanghai, China) with the two primers.
The EzTaxon database (https://www.ezbiocloud.net/) was used to compare the 16S rRNA gene sequences of the endophytic bacterial strains, and the sequences were submitted to NCBI Genbank. The phylogenetic tree was constructed using the Neighbour-Joining method through the Mega7 software.
Screening for antimicrobial activity of endophytic bacteria against phytopathogen
Collection of the solvent extract of endophytic bacteria
The single colony of selected endophytic bacteria were picked and inoculated into 150 mL of the YIM38 liquid medium in 500 mL Erlenmeyer flasks followed by incubation with continuous shaking at 28 °C for 2 days at 200 rpm, while the single colony of selected endophytic actinomycetes were shaking for 7 days under the same conditions. The supernatant and sediment were collected separately after the culture broth was centrifuged at 9500 rpm for 20 min at 4 °C. The supernatant was mixed with an equal volume of ethyl acetate, then the ethyl acetate layer was collected by means of a separating funnel and dried using a rotary evaporator (RE-52AA; YARONG, China) at 38 °C. The sediment mixed with 15 mL of acetone for 12 h was filtered through a 0.22 μm nylon membrane filter and dried by using a rotary evaporator (RE-52AA; YARONG, China) at 38 °C. The extracts were then redissolved in 1 mL of methanol and stored at −20 °C for antimicrobial activity screening.
Screening for antimicrobial activity
Athelia rolfsii, and Myrothecium roridum as pathogenic fungi of Dendrobium, and Pectobacterium carotovorum subsp. actinidiae as pathogenic bacteria of Dendrobium, were used in the present study to determine the antimicrobial activities of the solvent extract of endophytic bacteria (Supplementary Table S4). These phytopathogens were obtained from the Sanming Academy Of Agricultural Sciences, Fujian Province, China.
The Kirby-Bauer test was used to screen for antimicrobial activity. Respectively, 10 mL cultures of the pathogenic fungi grown 2 days in the Potato Dextrose (PD) liquid medium at 28 °C was added to the 100 ml of the PD agar medium, while 10 mL cultures of the pathogenic bacteria grown 12 h in the Luria – Bertani (LB) liquid medium at 28 °C was added to the 100 ml of the LB agar medium, mixed gently, and then poured slowly on the petri dish used as the test plate. Sterilized paper discs (diameter, 6 mm) were saturated with the solvent extract (20 μL) and placed onto the test plates inoculated with the test organism. An equivalent volume of methanol instead of the endophytic bacteria was used as a negative control, and an equivalent volume of 70% Mancozeb (750 fold dilution) was used as a positive control. Plates were then incubated 16 h under aerobic conditions at 28 °C. The effect of antimicrobial activity was determined by measuring the diameter of the inhibition zones (using electronic digital caliper, 0–150 mm).
Supplementary information
Acknowledgements
This research was supported by China Agriculture Research System (CARS-21) and the National Natural Science Foundation of China (31800005).
Author Contributions
S.-S.W., J.-M.L. and B.F. conceived and designed the experiments. S.-S.W., J.-M.L., J.-N.L. and N.J. performed the experiments. S.-S.W., J.-M.L., J.S., Y.-F.S. and X.-F.D. analyzed the data. S.-S.W. and J.-M.L. wrote the manuscript. All authors reviewed the manuscript.
Data Availability
All data underlying this publication are available in the manuscript.
Competing Interests
The authors declare no competing interests.
Footnotes
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Shan-shan Wang and Jia-meng Liu contributed equally.
Contributor Information
Jia-Meng Liu, Email: ljiam88@sina.com.
Bei Fan, Email: caasfanbei@163.com.
Xiao-Feng Dai, Email: CAASXiaofengDai@163.com.
Supplementary information
Supplementary information accompanies this paper at 10.1038/s41598-019-46863-9.
References
- 1.Zeng, S. J. Species introduction of Dendrobium officinale in China. J. Flowers. 19–26 (2015).
- 2.Wu YQ, Si JP. Industrial status and sustainable development of Dendrobium officinale. J. China J Chin Mater Med. 2010;35(15):2033–2037. doi: 10.4268/cjcmm20101525. [DOI] [PubMed] [Google Scholar]
- 3.Zhang M, Liu HY. Development status and prospect of Dendrobium officinale industry. J. Modern Chinese Medicine. 2010;12(10):8–11. [Google Scholar]
- 4.Chinese Pharmacopoeia Commission. Chinese Pharmacopoeia, vol. 1, Chinese Medical Science and Technology Press (2015).
- 5.Tang Hanxiao, Zhao Tianwen, Sheng Yunjie, Zheng Ting, Fu Lingzhu, Zhang Yongsheng. Dendrobium officinale Kimura et Migo: A Review on Its Ethnopharmacology, Phytochemistry, Pharmacology, and Industrialization. Evidence-Based Complementary and Alternative Medicine. 2017;2017:1–19. doi: 10.1155/2017/7436259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Miliute I, Buzaite O, Baniulis D, Stanys V. Bacterial endophytes in agricultural crops and their role in stress tolerance: a review. J. Zemdirbyste-agriculture. 2015;102(4):465–478. doi: 10.13080/z-a.2015.102.060. [DOI] [Google Scholar]
- 7.Turner TR, James EK, Poole PS. The plant microbiome. J. Genome Biology. 2013;14(6):209. doi: 10.1186/gb-2013-14-6-209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Martina K, Schmidt R, Ramadan E, Bauer R, Berg G. The microbiome of medicinal plants: diversity and importance for plant growth, quality, and health. J. Frontiers in Microbiology. 2013;4:400. doi: 10.3389/fmicb.2013.00400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Huang W, Long C, Lam E. Roles of Plant-Associated Microbiota in Traditional Herbal Medicine. J. Trends in Plant Science. 2018;23(7):559–562. doi: 10.1016/j.tplants.2018.05.003. [DOI] [PubMed] [Google Scholar]
- 10.Strobel GA. Endophytes as sources of bioactive products. J. Microbes and Infection. 2003;5(6):535–544. doi: 10.1016/S1286-4579(03)00073-X. [DOI] [PubMed] [Google Scholar]
- 11.Gutierrez RM, Gonzalez AM, Ramirez AM. Compounds Derived from Endophytes: A Review of Phytochemistry and Pharmacology. J. Current Medicinal Chemistry. 2012;19(18):2992–3030. doi: 10.2174/092986712800672111. [DOI] [PubMed] [Google Scholar]
- 12.Strobel G, Daisy B. Bioprospecting for microbial endophytes and their natural products. J. Microbiology & Molecular Biology Reviews Mmbr. 2003;67(4):491–502. doi: 10.1128/MMBR.67.4.491-502.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Vieira MLA, et al. Diversity and antimicrobial activities of the fungal endophyte community associated with the traditional Brazilian medicinal plant Solanum cernuum Vell. (Solanaceae). (Report) J. Canadian Journal of Microbiology. 2012;58(1):54–66. doi: 10.1139/w11-105. [DOI] [PubMed] [Google Scholar]
- 14.Dilfuza E, et al. Endophytic Bacteria Improve Plant Growth, Symbiotic Performance of Chickpea (Cicer arietinum L.) and Induce Suppression of Root Rot Caused by Fusarium solani under Salt Stress. J. Frontiers in Microbiology. 2017;8:1887. doi: 10.3389/fmicb.2017.01887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mohamad OAA, et al. Evaluation of the Antimicrobial Activity of Endophytic Bacterial Populations From Chinese Traditional Medicinal Plant Licorice and Characterization of the Bioactive Secondary Metabolites Produced by Bacillus atrophaeus Against Verticillium dahlia. J. Frontiers in Microbiology. 2018;9:924. doi: 10.3389/fmicb.2018.00924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hu KX, Hou XQ, Guo SX. Distribution of endophytic fungi in Dendrobium officinale. J. Chinese journal of microbiology. 2010;37(1):0037–0042. [Google Scholar]
- 17.Parthibhan S, Rao MV, Senthil Kumar T. Culturable fungal endophytes in shoots of Dendrobium aqueum Lindley – An imperiled orchid. J. Ecological Genetics and Genomics. 2017;3–5:18–24. doi: 10.1016/j.egg.2017.06.004. [DOI] [Google Scholar]
- 18.Zhang LC, Guo SX. Isolation and antimicrobial activity of 5 endophytic fungi from Dendrobium officinale. J. Chin J pharmacol. 2009;V44(20):1540–1542. [Google Scholar]
- 19.Zhang YW, Kang JC. Research on endophytic fungi of Dendrobium officinale. J. Acta agricultura agriculturae Montenegro. 2005;24(5):438–441. [Google Scholar]
- 20.Zhang HW, Zhong CQ. Isolation and preliminary identification of endophytic bacteria from Dendrobium candidum. J. Hubei agricultural science. 2013;52(8):1811–1813. [Google Scholar]
- 21.Pei C, et al. Diversity of endophytic bacteria of Dendrobium officinale based on culture-dependent and culture-independent methods. J. Biotechnology & Biotechnological Equipment. 2016;31(1):1–8. [Google Scholar]
- 22.Yang SZ, Wu YX, D.L.Zhu LH, He YQ. Isolation, identification and functional analysis of endophytic bacteria from Dendrobium drumma. J. Chinese agricultural science bulletin. 2014;30(25):171–176. [Google Scholar]
- 23.Wang, M. Y. et al. Bacterial community structure analysis of endophytic of Dendrobium officinale. J. Western forestry science. (5), 106–111 (2014).
- 24.Yan L, Yang RJ, Wang QM. Isolation and identification of endophyte of Dendrobium candidum from Yunnan province. J. Journal of yunnan agricultural university (natural science). 2015;30(5):760–765. [Google Scholar]
- 25.Zhang L, Tong WJ, Xue QY, Ding XY. Diversity and growth promoting ability of endophytic and root-surrounding bacteria of Dendrobium moniliforme. J. Journal of plant resources and environment. 2015;24(3):32–40. [Google Scholar]
- 26.Tong WJ, Zhang L, Xue QY, Hou BW, Ding XY. Bacterial isolation and growth potential of endophytic of Dendrobium from different habitats. J. Journal of plant resources and environment. 2014;23(1):16–23. [Google Scholar]
- 27.Chen ZB, et al. High-throughput analysis of endophytic bacterial communities in different parts of Dendrobium candidum. J. Henan agricultural science. 2017;46(11):98–102. [Google Scholar]
- 28.Ellis RJ, et al. Cultivation-Dependent and Cultivation-Independent Approaches for Determining Bacterial Diversity in Heavy-Metal-Contaminated Soil. J. Applied and Environmental Microbiology. 2003;69(6):3223–3230. doi: 10.1128/AEM.69.6.3223-3230.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bakken, L. R. et al. Culturable and nonculturable bacteria in soil. J. Modern Soil Microbiology Marcel Dekker. 59, 47–61 (1997).
- 30.Amann RI, Ludwig W, Schleifer KH. Phylogenetic identification and in situ detection of individual microbial cells without cultivation. J. Microbiol Rev. 1995;59(1):143–169. doi: 10.1128/mr.59.1.143-169.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chen ML, et al. Effects of endophytic bacteria on the formation of geo-authentic crude drug. J. Chin J traditional Chinese medicine informatics. 2006;13(9):40–42. [Google Scholar]
- 32.Gong Y, He BW, Wang SZ, Mao BZ. Research progress of endophyte of Dendrobium officinale. J. Zhejiang agricultural science. 2017;58(8):1372–1375. [Google Scholar]
- 33.Kusari S, Pandey SP, Spiteller M. Untapped mutualistic paradigms linking host plant and endophytic fungal production of similar bioactive secondary metabolites. J. Phytochemistry. 2013;91:81–87. doi: 10.1016/j.phytochem.2012.07.021. [DOI] [PubMed] [Google Scholar]
- 34.Santosa PJCD, et al. Diaporthe endophytica and D. terebinthifolii from medicinal plants for biological control of Phyllosticta citricarpa. J. Microbiological Research. 2016;186–187:153–160. doi: 10.1016/j.micres.2016.04.002. [DOI] [PubMed] [Google Scholar]
- 35.Yang HT, Chen K, Li JS, Gou Y, Xu YK. Colonization of Bacillus megaterium in the rhizosphere of wheat and its control effect on plant fungal diseases. J. Shandong sci. 2003;16(3):12–17. [Google Scholar]
- 36.Slepecky RA, Hemphill HE. The Genus Bacillus—Nonmedical. J. Prokaryotes. 2006;4:530–562. doi: 10.1007/0-387-30744-3_16. [DOI] [Google Scholar]
- 37.Dilfuza E, Stephan W, Behrendt U, Ahmad P, Berg G. Antimicrobial Activity of Medicinal Plants Correlates with the Proportion of Antagonistic Endophytes. J. Frontiers in Microbiology. 2017;8:199. doi: 10.3389/fmicb.2017.00199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yang HT, Tang WH, Chi JG, Xu YK, Wang JN. Identification of phytogenic control strain B1301 and its mechanism of action and control effect on ginger bacterial wilt. J. Chinese journal of biological control. 2002;18(1):21–23. [Google Scholar]
- 39.Hu ZH, et al. Prevent and Control Southern Blight of Atractylides macrocephala by Adding Probiotic such as Bacillus subtilis. J. Chinese journal of traditional Chinese medicine. 2017;35(10):159–162. [Google Scholar]
- 40.Wang, Y. Purification and characterization of extracellular antimicrobial substances of Bacillus subtilis strain Bv10. D. Guangxi university, 10.7666/d.Y2407965 (2013).
- 41.Xu, L. N. et al. Biodiversity of Endophytic Bacteria in Wild Mulberry Tree and Inhibition Effect of ME0717 Strain on Pathogens. J. Silkworm science. 34(02) (2016).
- 42.Zhang J, et al. Isolation and identification of antagonistic bacteria against pectobacterium carotovorum in Pinellia ternate. J. Guizhou agricultural science. 2015;43(9):150–152. [Google Scholar]
- 43.Stein T. Bacillus subtilis antibiotics: structures, syntheses and specific functions. J. Mol. Microbiol. 2005;56(4):845–857. doi: 10.1111/j.1365-2958.2005.04587.x. [DOI] [PubMed] [Google Scholar]
- 44.Cavaglieri L, Orlando J, Rodríguez MI, Chulze S, Etcheverry M. Biocontrol of Bacillus subtilis against Fusarium verticillioides in vitro and at the maize root level. J. Research in Microbiology. 2005;156(5):748–754. doi: 10.1016/j.resmic.2005.03.001. [DOI] [PubMed] [Google Scholar]
- 45.Ramalingam R, Abeer H, Abd_Allah EF. Bacillus: A Biological Tool for Crop Improvement through Bio-Molecular Changes in Adverse Environments. J. Frontiers in Physiology. 2017;8:667. doi: 10.3389/fphys.2017.00667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zhou, S. Q., Huang, X. L., Huang, D. Y., Hu, X. W. & Chen, J. L. A rapid method for extracting DNA from Actinomycetes by Chelex-100. J. Biotechnology bulletin. 2, 123–125 (2010).
- 47.Wang YY, et al. 16S rRNA gene high-throughput sequencing reveals shift in nitrogen conversion related microorganisms in a CANON system in response to salt stress. J. Chemical Engineering Journal. 2017;317:512–521. doi: 10.1016/j.cej.2017.02.096. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data underlying this publication are available in the manuscript.