Abstract
To evaluate outcomes and prognostic markers among children with relapsed Hodgkin lymphoma (HL) treated with autologous stem cell transplant (ASCT), we conducted a retrospective analysis of 36 consecutive pediatric patients treated at Memorial Sloan Kettering Cancer Center from 1989–2013. With a median follow-up of 9.6 yrs, the 10yr overall survival (OS) and event-free survival (EFS) were 74.1% and 67.1% respectively. Absence of B-symptoms, chemotherapy-sensitive disease, and transplant date after 1997 were each associated with superior EFS (HR 0.12 (p=0.0015); 0.18 (p=0.0039) and 0.17 (p=0.0208), respectively). Childhood Hodgkin International Prognostic Score at relapse (R-CHIPS) was calculated in a subset of patients (n=22) and a lower score was associated with improved OS (HR 0.29, p=0.0352) and a trend toward improved EFS (HR 0.38, p=0.0527). In summary, ASCT results in durable remission for the majority of pediatric patients with relapsed HL. R-CHIPS should be evaluated in larger cohorts as a potential predictive tool.
Introduction
Although outcomes have improved substantially for children and adolescents with Hodgkin lymphoma (HL), relapsed or refractory disease remains an issue for up to 15% of patients [1–4]. High-dose therapy followed by autologous stem cell transplantation (ASCT) has become the standard of care for relapsed/refractory disease, largely based on data from adult studies [5–16]. With no randomized studies to evaluate ASCT for children with relapsed/refractory HL, prospective and retrospective series provide insight into overall outcomes as well as potential prognostic markers. As new therapies emerge for HL, risk stratification of pediatric patients with relapsed disease will be essential to determine which patients are likely to benefit from ASCT and which patients should be selected for alternative therapy. In the current study we evaluated the long-term outcomes and potential prognostic markers among 36 pediatric patients with HL who underwent ASCT at Memorial Sloan Kettering Cancer Center (MSKCC).
Patients and Methods
Study Design:
We conducted a retrospective analysis of 36 consecutive pediatric patients age ≤21yrs with relapsed/refractory HL who underwent ASCT at MSKCC between 1989 and 2013. We collected clinical data including: age, HL histologic subtype, treatment prior to ASCT, disease status at the time of transplant, pre-transplant conditioning regimen, and outcome after ASCT. EFS was measured as the time from transplant to adverse event. Events included relapse, progression, or death from any cause. OS was measured as the time from transplant to death from any cause. Given recent data supporting the Childhood Hodgkin International Prognostic Score (CHIPS)[17] for risk stratification at the time of HL diagnosis, this score was calculated at the time of relapse to evaluate its prognostic relevance in the relapse setting. A waiver for HIPAA authorization and informed consent was obtained from the MSKCC institutional review board.
Tumor Response Evaluation:
Remission status prior to transplant was re-assesed at the time of retrospective review. Disease status was determined by physical exam, CT scan, gallium/FDG PET scans when available, and bone marrow biopsy, if indicated, and defined as follows [18]: Complete Response (CR): resolution of pathologic palpable lymphadenopathy, at least 80% reduction of the product of the perpendicular diameters (PPD) of each nodal mass, or return to normal size with no residual mass greater than 2.0 cm in transverse diameter, resolution of focal lesions in the liver or spleen, no residual disease in non-measureable assessable lesion sites, and no new lesions; Partial Response (PR): ≥50% reduction in PPD of each site of measurable disease, no new lesions; Stable Disease (SD): less than a PR but not progressive disease; Progressive Disease (PD): any of the following: a) at least 50% increase in PPD of any of the involved nodal sites or organ lesions, b) new lesions, c) progression of non-measurable assessable disease site. Residual lesions not meeting the above criteria in pateints otherwise in a CR were permitted if they were gallium or FDG negative or if a biopsy was performed and was negative for disease [19].
R-CHIPS Classification:
CHIPS classification was calculated at time of relapse (R-CHIPS). One point was awarded for each of the following: stage IV disease, bulky mediastinal adenopathy, albumin <3.5g/dL, and fever. Bulky mediastinal lymphadenopathy was defined as tumor diameter >1/3 the thoracic diameter as measured on upright PA CXR or a nodal aggregate >6cm in the longest transverse diameter. Fever was defined as temperature ≥38 degrees Celsius.
Statistical Analysis:
Kaplan-Meier survival analysis was used to estimate the probability of OS and EFS. Survival difference between groups was tested by log-rank test. Univariate Cox regression analysis was used for hazard ratio analysis. All analyses were performed using statistical software SAS Version 9.4 (SAS Institute, Cary, NC). The number of events (13 events including 10 deaths) was insufficient to perform a multivariate analysis.
Results
Patient and Treatment Characteristics:
The patient characteristics are summarized in Table 1. Thirty-five of the 36 patients were undergoing first ASCT and one patient underwent a second ASCT after first ASCT at an outside hospital with subsequent recurrence of disease. Thirty-five patients had relapsed disease; one patient had primary refractory disease. The median age at time of ASCT was 17.7 yrs (range 9.7–21.0 yrs). Histologic classification included nodular sclerosis (n=32), mixed cellularity (n=1), lymphocyte predominant (n=2), or subtype unspecified (n=1). Disease stage at initial diagnosis was advanced (stage III/IV) in 19 of 36 (52.8%) patients. Initial therapy for HL included: COPP-ABV (n=9), ABVE-PC (n=7), ABVD (n=5), MOPP-based regimens (n=4), AV-PC (n=2), BEACOPP (n=2), or other (n=7) (Table 2). Twenty of 36 (55.6%) patients received radiation therapy at initial diagnosis. The median time from initial diagnosis to relapse was 13 months (range 5 to 60 months). At the time of relapse, 22 patients (66.1%) had advanced stage disease. Second line therapies varied among patients and are summarized in Table 2. The most common regimens were ICE (n=12) and ifosfamide/vinorelbine (n=7). Four patients received brentuximab-vedotin prior to ASCT. Twenty-nine patients received radiation therapy as a part of salvage therapy or as total lymphocyte irradiation (TLI) prior to ASCT. Nineteen patients were treated on a clinical trial at the time of relapse for either salvage therapy (n=6), ASCT (n=9), or both (n=4).
Table 1:
Patient Characteristics
| Variable | n (%) | |
|---|---|---|
| Median age in yrs (range) | 17.7 (9.7–21) | |
| Gender | Male Female |
16 (44.4%) 20 (55.6%) |
| Pathologic classification | Nodular Sclerosis Mixed Cellularity Lymphocyte predominant Subtype unspecified |
32 (88.9%) 1 (2.8%) 2 (5.6%) 1 (2.8%) |
| Stage at diagnosis | I II III IV |
2 (5.6%) 15 (41.7%) 8 (22.2%) 11 (30.6%) |
| Time to relapse | Median in months (range) ≤12 months* >12 months |
13 (5–60) 18 (50%) 18 (50%) |
| Stage at relapse | I II III IV |
0 14 (38.9%) 4 (11.1%) 18 (50%) |
| R-CHIPS | 0 1 2 3 data not available |
7 (19.4%) 11 (30.6%) 3 (8.3%) 1 (2.8%) 14 (38.9%) |
includes one patient with primary refractory disease
Table 2:
Treatment Characteristics
| Variable | n (%) |
|---|---|
| Upfront Therapy COPP-ABV ABVE-PC MSK-MDP ABVD MOPP-based AV-PC BEACOPP Other* |
9 (25%) 7 (19.4%) 5 (13.9%) 5 (13.9%) 4 (11.1%) 2 (5.6%) 2 (5.6%) 2 (5.6%) |
| Salvage Therapy ICE Vinorelbine and/or gemcitabine-containing regimen Ifofamide/Etoposide Brentuximab-vedotin-containing regimen Variable other regimens** Data unavailable |
12 (33.3%) 12 (33.3%) 4 (11.1%) 4 (11.1%) 3 (8.3%) 1 (2.8%) |
| Radiation therapy: At initial diagnosis At relapse (salvage therapy or ASCT preparative regimen) After ASCT |
20 (55.6%) 29 (80.6%) 2 (5.6%) |
| Preparative Regimen Cyclophosphamide Etoposide TLI Cyclophosphamide Etoposide Carmustine BEAM |
14 (38.9%) 18 (50%) 4 (11.1%) |
| Disease Status at ASCT CR PR SD PD |
22 (61.1%) 5 (13.9%) 6 (16.7%) 3 (8.3%) |
| BMT Date ≤1997 >1997 |
18 (50%) 18 (50%) |
COPP-ABV, Cyclophosphamide, Vincristine, Prednisone, Procarbazine, Doxorubicin, Bleomycin, Vinblastine; ABVE-PC, Doxorubicin, Bleomycin, Vincristine, Etoposide, Prednisone, Cyclophosphamide; MSK-MDP, Doxorubicin, Procarbazine, Prednisone, Vincristine, Cyclophosphamide; ABVD, Doxorubicin, Bleomycin, Vinblastine, Dacarbazine; MOPP, Mustargen, Vincristine, Procarbazine, Prenisone; AV-PC, Doxorubicin, Vincristine, Prednisone, Cyclophosphamide; BEACOPP, Bleomycin, Etoposide, Doxorubicin, Cyclophosphamide, Vincristine, Procarbazine, Prednisone; ICE, Ifosfamide, Carboplatin, Etoposide; Cy, Cyclophosphamide; Etop, Etoposide; BEAM, Carmustine, Etoposide, Cytarabine, Melphalan.
Other up front chemotherapy regimens include: VAMP (Vincristine, Doxorubicin, Methotrexate, Prednisone) and OPPA (Vincristine, Prednisone, Procarbazine, Doxorubicin).
Variable other regimens include: Doxorubicin, etoposide, cyclophosphamide (n=1), DECAL (n=1), BEACOPP (n=1)
Of note, two patients with lymphocyte predominant HL are included in our cohort. The first patient initially presented with stage IIIA disease, relapsed 18 months after diagnosis with IIIA disease, and proceeded to ASCT with stable disease after salvage therapy. The second patient initially presented with stage IA disease, relapsed 22 months after diagnosis with stage IIIA disease, and proceeded to transplant with a CR after salvage therapy. Both remain alive and without evidence of disease.
Disease Status at the time of ASCT:
All 36 patients underwent a disease evaluation prior to ASCT. This consisted of a CT alone in 10 patients, a CT and gallium scan in 11 patients, and a CT and FDG/PET scan in 15 patients. All 15 patients who underwent ASCT after 2002 had an FDG/PET scan to determine remission status. Among these patients, 7 had no FDG uptake and 6 had a low level of uptake that was interpreted as negative. Two patients had significant FDG/PET uptake that was considered positive. Both patients with a positive FDG/PET scan underwent a biopsy and both were negative for disease. Of note, the level of uptake in the mediastinal blood pool and liver was not routinely reported for the majority of patients and Deauville score could not be calculated. Taking into account both CT scan and metabolic imaging when available, 22 patients (61.1%) underwent ASCT in CR. The remaining patients had a PR (n=5, 13.9%), SD (n=6, 16.7%), or PD (n=3, 8.3%).
Transplant Characteristics:
Transplant characteristics are summarized in Table 2. ASCT preparative regimens included cyclophosphamide, etoposide, and TLI (n=14, 38.9%), cyclophosphamide, etoposide, and carmustine (n=18, 50%) or carmustine, etoposide, cytarabine, melphalan (BEAM) (n=4, 11.1%). The TLI-based regimen was only used in patients who had not received prior RT. Most patients treated after 1997 (17 of 18) received chemotherapy-only preparative regimens. The stem cell source consisted of bone marrow in 9 patients and peripheral blood in 27 patients. Two patients received radiation as consolidation therapy post-transplant.
Hematopoietic Recovery and Transplant-related Mortality:
Hematopoietic recovery after ASCT was defined by: (1) neutrophil engraftment: the first day of an absolute neutrophil count > 500 × 106 for 3 consecutive days and (2) platelet engraftment: the first day of unsupported platelet count of >20 × 103/ml for 3 consecutive days. Hematologic engraftment occurred in all patients. The mean time to neutrophil engraftment was 11 days (range 9–22 days) and the mean time to platelet engraftment data was 20 days (range 14–151 days). There were no cases of transplant-related mortality in this cohort.
Overall and Disease Free Survival:
With a median follow-up 9.6 years (range 1–25.5 years) the estimated 10-year OS and EFS are 74.1% and 67.1%, respectively (Figure 1). The causes of death among the 10 of 36 patients who died were: Hodgkin lymphoma (n=8), end stage renal disease (n=1), and cardiac disease (n=1). Relapse after BMT occurred in 11 of 36 patients. The median time from ASCT to relapse was 10 months (range 1.8 to 57.3 months). Among those who relapsed, two had entered ASCT in a CR, three in PR, and six with SD or PD. Of the 11 patients who relapsed after ASCT, eight subsequently died of Hodgkin lymphoma. Three patients are alive without evidence of disease: one after treatment with gemcitabine alone x 21 cycles, one after methotrexate, thioguanine and cladribine, and one after brentuximab x 9 cycles and allogeneic transplant. There were no secondary malignancies reported to date in this cohort.
Figure 1:
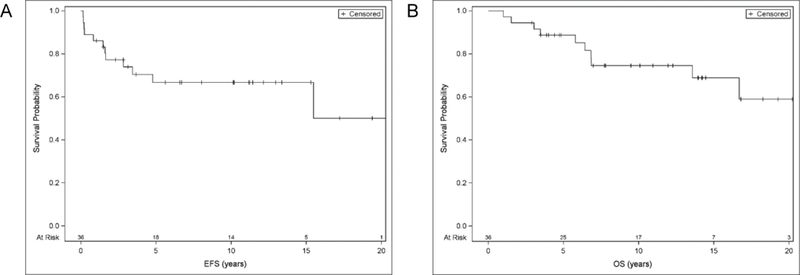
Probabilities of event free survival (A) and overall survival (B) for the entire cohort
Analysis of Risk Factors:
To evaluate potential markers predictive of outcome we evaluated patient and treatment characteristics by univariate analysis using the Cox regression model (Table 3). Patient age, stage at diagnosis, stage at relapse, time to relapse, and cytoreduction regimen were not associated with significant differences in EFS or OS. The absence of B symptoms at relapse was associated with improved EFS and OS (HR 0.12, p=0.0015 and 0.18, p=0.0170 respectively). Chemotherapy-sensitive disease (defined as CR or PR at time of ASCT) was associated with improved EFS and OS (HR 0.18, p=0.0039 and 0.22, p=0.0219 respectively). Kaplan-Meier estimates and log-rank test of EFS and OS also demonstrated superior outcome among patients with chemo-sensitive disease (10-year survival rate: EFS 78.0% vs. 33.3%, p=0.001; OS 85.8% vs. 44.4%, p=0.013) (Figure 2 A-B).
Table 3:
Univariate Analysis of Potential Prognostic Markers
| Variable | Prognostic Marker | Reference | EFS | OS | ||
|---|---|---|---|---|---|---|
| HR (95% CI) |
p | HR (95% CI) | p | |||
| Age | ≤ 18yr | >18yr | 0.39 (0.12–1.33) | 0.1326 | 0.27 (0.07–1.06) | 0.0612 |
| Stage at Diagnosis |
I, II | III, IV | 0.50 (0.15–1.69) | 0.2656 | 0.55 (0.14–2.15) | 0.3907 |
| Stage at Relapse | I, II | III, IV | 0.31 (0.07–1.4) | 0.1275 | 0.20 (0.02–1.55) | 0.1230 |
| Time to Relapse | >12mo | ≤ 12mo | 0.67 (0.2–2.19) | 0.5076 | 0.59 (0.17–2.11) | 0.4203 |
| B Symptoms at Relapse | Absent | Present | 0.12 (0.03–0.45) | 0.0015 | 0.18 (0.04–0.74) | 0.0170 |
| Disease Status at ASCT |
CR, PR | SD, PD | 0.18 (0.05–0.57) | 0.0039 | 0.22 (0.06–0.81) | 0.0219 |
| Cytoreduction Regimen | Cy/Etop/TLI | Cy/Etop/Car | 3.39 (0.88–13.06) | 0.0756 | 1.78 (0.43–7.46) | 0.4291 |
| BMT Date | >1997 | ≤ 1997 | 0.21 (0.05–0.96) | 0.0436 | 0.12 (0.02–0.99) | 0.0489 |
| R-CHIPS |
1 unit decrease | 0.38 (0.15–1.01) | 0.0527 | 0.29 (0.09–0.92) | 0.0352 | |
Figure 2:
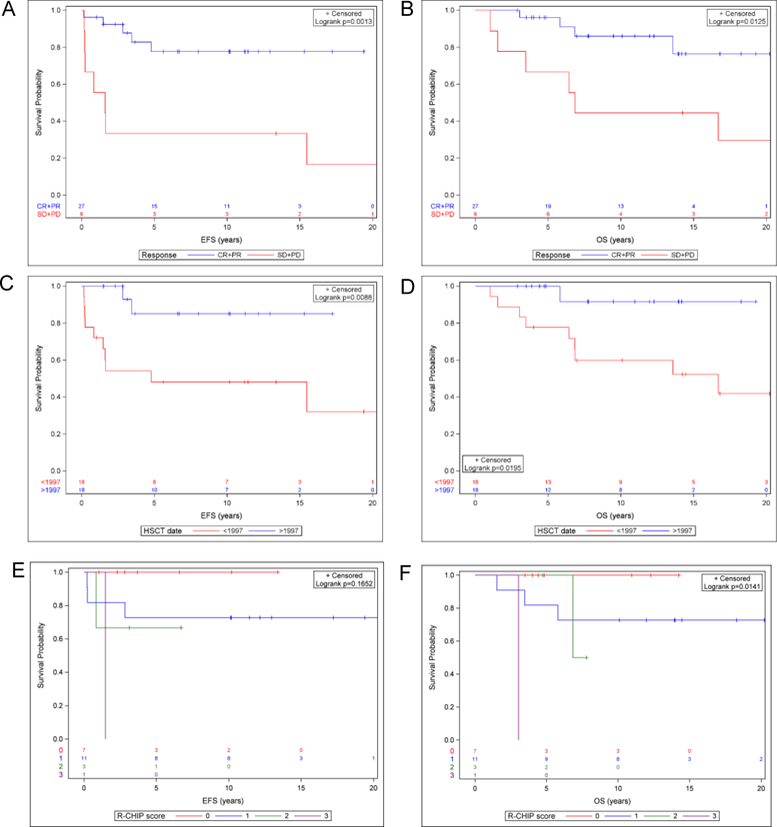
(A-B) Probabilities of event free survival (A) and overall survival (B) for patients with chemotherapy sensitive disease (defined as CR or PR at time of ASCT) compared to chemotherapy resistant disease (defined as SD or PD). (C-D) Probabilities of event free survival (C) and overall survival (D) for patients transplanted in 1989–1997 compared to 1998–2013. (E-F) Probabilities of event free survival (E) and overall survival (F) based on R-CHIPS.
Outcome by Transplant Date:
To determine the impact of ASCT date on EFS and OS, we divided the cohort into patients who underwent ASCT from 1989–1997 (n=18) and 1998–2013 (n=18). Transplant after 1997 was associated with superior outcomes for both EFS and OS (HR 0.17, p=0.0208 and 0.12, p=0.0489 respectively). Patients who underwent transplant after 1997 had a 10 year EFS and OS of 85.8% and 91.7% respectively, compared to 48.8% and 59.1% from 1989–1997 (Figure 2 C-D). Of note, after 1997, all 18 patients entered ASCT in CR as compared to 4 of 18 (22%) patients prior to 1997. Metabolic imaging (gallium or FDG/PET) was used to determine remission status for all patients (n=18) after 1997 as compared to 8 of 18 (44.4%) prior. In addition, the treatment regimens utilized at relapse varied between the two time periods. From 1989–1997 12 of 18 patients received ifosfamide/carboplatin/etoposide (ICE) or an ifosfamide/etoposide-containing regimen. Other patients received a BCNU-based regimen (n=2) or a doxorubicin/etoposide-based regimen (n=1). The salvage therapy for one patient was not available. After 1997, 9 of 18 patients received a vinorelbine and/or gemcitabine-containing regimen. Others received a brentuximab containing regimen (n=4), ICE (n=3), BEACOPP (n=1), or DECAL (dexamethasone, etoposide, cisplatin, cytarbine, and L-asparaginase, n=1).
Outcome by CHIPS:
The Childhood Hodgkin Lymphoma International Prognostic Score (CHIPS) was developed by the Children’s Oncology Group (COG) to risk stratify pediatric patients with HL at the time of diagnosis. This model was found to be predictive of EFS among pediatric patients with intermediate risk Hodgkin lymphoma enrolled on the COG AHOD0031 prospecitve trial [17,18]. To determine if this system may also predict outcome in the relapse setting, we calculated R-CHIPS at the time of relapse. Among 22 patients with data available to calculate R-CHIPS, the distribution by R-CHIPS is as follows: 0 (n=7), 1 (n=11), 2 (n=3), 3 (n=1). There were no patients with an R-CHIPS of 4. Patients a lower R-CHIPS demonstrated improved OS and a trend toward improved EFS. Specifically, by univariate hazard ratio analysis, each 1 point decrement in R-CHIPS score was associated with improved OS (HR 0.29, p=0.0352) and a trend toward improved EFS (HR 0.38, p=0.0527) (Table 3). Analysis by Kaplan-Meier estimate and log-rank test also demonstrated a significant difference in OS among patients when stratified by R-CHIPS (0 vs. 1 vs. 2 vs. 3) (p=0.014) and a trend toward significance for EFS (p=0.165) (Figure 2E-F).
Discussion:
In the absence of randomized trials in pediatrics to compare ASCT and chemotherapy alone, the data to support ASCT is extrapolated from adult randomized studies as well as and pediatric prospective and retrospective series. Two randomized studies in adults evaluated chemotherapy versus high dose chemotherapy followed by ASCT. In a randomized comparison of BEAM followed by ASCT versus the same chemotherapy agents at lower doses (mini-BEAM) in 40 adults with relapsed or refractory HL, patients randomized to BEAM + ASCT had a superior EFS (3yr EFS 53% vs. 10%, p=0.025) [20]. Similar outcomes were observed in a prospective randomized trial of dexamethasone + BEAM (dexa-BEAM) vs. dexa-BEAM followed by high dose BEAM and ASCT. Among 161 adults, freedom from treatment failure (FFTF) was superior in patients randomized to the ASCT arm (3yr FFTF 55% vs. 34%, p=0.019) [21]. Furthermore, a Cochrane review of three randomized controlled trials comparing ASCT with chemotherapy in adults with relapsed HL concluded that there is moderate evidence to suggest a progression-free survival (PFS) benefit for patients treated with ASCT and a trend toward improvement with regard to OS [22].
The outcome of pediatric patients with relapsed or refractory HL treated with ASCT has been described in one prospective trial and a series of retrospective reports. The ST-HD-86 trial prospectively studied 176 children with relapsed/refractory HL, 53 of whom underwent ASCT. The 10-year OS among patients who underwent ASCT was 51% [23]. Several groups have reported pediatric outcomes based on retrospective series with a 5-yr EFS ranging from 45 to 65% and 5-yr OS ranging from 55 to 74% (Table 4)[24–29]. A recent review of the Center for International Blood and Marrow Transplant Research (CIBMTR) registry reported longer follow-up in pediatric and young adult patients with a 10-yr PFS of 41%, highlighting the importance of long-term follow-up to capture late relapses and long-term toxicity[28]. In our cohort, we report a 10-yr EFS and OS 67.1% and 74.1% respectively, which compares favorably with prior series.
Table 4:
Summary of recent literature on ASCT for relapsed HL in pediatrics
| Reference | n | Age in yrs: median (range) | Follow-up in yrs: median (range) | EFS | OS |
|---|---|---|---|---|---|
| S Akhtar et al, BMT, 2010[33] | 58 | 17 (14–21) | 3.6 | 45% | 55% |
| Shafer et al, Leuk/Lymph, 2010[30] | 37 (27 ASCT 10 allogeneic SCT) |
21 (12–35) | 3.6 (0.2–11.4) | 60%+ | 73% |
| Metzger et al, Cancer 2010[27] | 50 | 16 (4–22) | 4.4 (1.2–16.6) | 57.8%* | 74.2% |
| Gorde-Grosjean et al, BJH 2012[25] | 70 (50 ASCT, 2 allogeneic SCT, 18 no transplant) | 14 (2–17) | 3.3 (0.2–11.7) | 55% |
69% |
| Satwani et al, BMT 2015[28] | 606 | 23 (15–29) | 5.3 (0.3–18) | 47%+ | 58% |
| Garfin et al, BBMT 2015[24] | 89 | 18 (8–21) | 3.25 (0.03–21) | 65% | 71% |
| Hazar et al, Ped Transplant 2015[26] | 66 | 15 (7–20) | 3.25 (1.2–12.7) | 54.3% | 63.1% |
| Present study | 36 | 18 (9–21) | 9.6 (1–25.5) | 67.1% | 74.1% |
freedom from second treatment failure
PFS
Prognostic markers among children with relapsed HL are of particular interest. Retrospective series have evaluated patient and disease markers associated with higher risk for relapse with some themes emerging across studies. Primary refractory disease, short remission duration, and poor response to salvage chemotherapy have been consistent markers of inferior outcome [30–33]. Other poor prognostic factors including extranodal disease, mediastinal bulk, B symptoms, anemia, elevated ESR, elevated LDH have been reported [23,25,27,30,32]. Consistent with prior studies, in our cohort absence of B symptoms and chemotherapy-sensitive disease were associated with superior EFS and OS.
When evaluating outcome by date of transplant, we found superior outcomes among patients who underwent ASCT after 1997. Other retrospective series have reported similar observations and attributed this to a variety of factors including decreased transplant-related mortality, the use of post-transplant radiation therapy, and improved patient selection[24]. In our cohort, transplant-related mortality was not a contributing factor as there were no transplant-related deaths. The superior outcome after 1997 could be due to improved patient selection with the use of metabolic imaging to determine remission status. The prognostic relevance of metabolic imaging prior to ASCT is well established among adults with HL but has previously not been studied in children[34–36]. After 1997, all 18 patients in our cohort entered ASCT in CR as compared to 4 of 18 (22%) patients prior to 1997. Of patients transplanted after 1997, 100% achieved a complete metabolic remission (15 by FDG/PET, 3 by gallium scan). This is in comparison to the earlier cohort, in which among the four patients with a complete remission, two had a metabolic CR and two had CT imaging only. In addition, the salvage treatment regimens varied between the two time periods. In the more recent era, many patients received a vinorlbine, gemcitabine, or brentuximab containing regimen.
Predictive models may be able to better risk stratify pediatric patients with relapsed HL, although no single model has been universally adopted. Bröckelmann et al (2017) reported a multivariate analysis of risk factors for survival among 656 adult patients treated for relapsed/refractory HL between 1993 and 2013, with validation in an additional 389 patients [37]. In this analysis, stage IV disease, time to relapse ≤ 3 months, ECOG performance status ≥ 1, bulk ≥ 5cm and inadequate response to salvage chemotherapy were equally weighted risk factors for both progression-free survival (HR 1.70, p<0.001) and overall surivival (HR 1.63, p <0.0001). In a recent retrospective study of 606 patients age 15–29 with relapsed/refractory HL registered with the CIBMTR, Satwani et al (2015) developed a prognostic scoring system using 4 factors associated with inferior PFS: chemo-resistance, Karnofsky/Lansky score <90%, extranodal disease, and time to first relapse <1 year [28]. The study categorized patients as low risk (score=0), intermediate risk (score 1 or 2) or high risk (score 3 or 4) and found a statistically significant difference in PFS among the groups (PFS of 75%, 56% and 23% for low, intermediate, and high risk respectively, p<0.001). We were unable to apply these criteria to our cohort as ECOG performance status and/or Karnofsky/Lansky scores were recorded in an insufficient number of patients. The Children’s Oncology Group recently developed the Childhood Hodgkin International Prognostic Score (CHIPS) to predict outcome among children with Hodgkin lymphoma at the time of initial diagnosis. The CHIPS was generated through a multivariate analysis of 1,103 pediatric patients with intermediate risk disease enrolled on COG AHOD0031. Among patients treated on AHOD0031, the 4-year EFS was 93.1%, 88.5%, 77.6%, and 69.2% for CHIPS scores of 0, 1, 2, and 3, respectively (p=<0.001). Since CHIPS has not been studied in the relapse setting, we calculated a “relapse CHIPS” (R-CHIPS) for patients with available data (n=22). Decreased R-CHIPS was associated with superior OS and a trend toward improved EFS. This suggests that R-CHIPS might be predictive in the relapsed setting and supports the study of R-CHIPS in larger cohorts.
This retrospective study has several limitations that must be considered when interpreting data. This study captures patients who received an ASCT and does not report on patients with relapsed/refractory HL who did not undergo ASCT. As selection criteria for ASCT may have become more stringent with the use of metabolic imaging, this introduces a potential bias. Since the definitions for disease response evaluation changed over the reported time period, we re-assesed disease status prior to ASCT retrospectively for this study based on anatomic response criteria [18] and metabolic imaging, if available [19]. We do not have the data available in regards to the total number of patients with relapsed HL during each era, as compared to the number of patients who ultimately underwent ASCT. It is possible that a subset of patients who did not achieve a CR with salvage therapy were referred for allogeneic transplant and were not captured in this cohort.
Patients in this study also received different chemotherapy regimens, different total radiation therapy dose and field exposure, and variable SCT conditioning regimens, reflecting changes in standard of care over the 27-year period of data collection. Lastly the sample size and small number of events limit our ability to conduct a multivariate analysis of potential prognostic markers. Despite this, our cohort represents a large series of pediatric patients from a single-center with the benefit of long-term follow-up.
As novel agents emerge for Hodgkin lymphoma, the role for ASCT will likely be evolving. Brentuximab vedotin has demonstrated clinical activity in adult and pediatric patients who have relapsed after autologous stem-cell transplant [38]. In pediatrics, brentuximab vedotin is currently being evaluated in both the upfront and relapse settings relapse (NCT02166463, NCT01780662). The checkpoint inhibitors nivolumab and pembrolizumab have both shown activity in adults with refractory Hodgkin lymphoma, and studies are ongoing in pediatrics (NCT02332668, NCT02927769, NCT02304458) [39–41]. As clinical trials are being designed for these agents in pediatric Hodgkin lymphoma, it is essentaial to understand predictive markers to determine which patients to select for alternative therapies. For example, members of the Children’s Oncology Group recently proposed a risk-adapted, response-based approach to determine therapy for pediatric patients with relapsed HL, including specifications for which patients to recommend for ASCT [42]. This proposal is the basis of an ongoing phase II trial evaluating the combination of nivolumab and brentuximab in pediatric patients with relapsed HL (NCT02927769).
In summary, we report the 27-year experience of autologous SCT for the treatment of relapsed/refractory Hodgkin lymphoma in children at MSKCC. This study demonstrates that ASCT offers the prospect of durable, disease-free survival for a significant proportion of pediatric patients with relapsed HL. Specifically, the outcome among patients who received an ASCT in recent years (1998–2013) was favorable (EFS/OS: 85.8%/91.7%), likely in part due to the increased use of functional imaging to determine disease status prior to ASCT and improved therapies prior to transplant. To our knowledge this is the first report evaluating the potential utility of CHIPS in the relapse setting. In our cohort a lower R-CHIPS was associated with superior OS, and a trend toward improved EFS, suggesting that R-CHIPS should be studied further as a potential prognostic marker in relapsed HL.
Supplementary Material
Footnotes
Acknowledgements and Declaration of Interests: The authors have no conflicts of interest to disclose.
References
- 1.Bradley MB, Cairo MS. Stem cell transplantation for pediatric lymphoma: past, present and future. Bone Marrow Transplant 2008;41:149–158. [DOI] [PubMed] [Google Scholar]
- 2.Horning SJ, Adhikari A, Rizk N, Hoppe RT, Olshen RA. Effect of treatment for Hodgkin’s disease on pulmonary function: results of a prospective study. J Clin Oncol 1994;12:297–305. [DOI] [PubMed] [Google Scholar]
- 3.Hudson MM, Donaldson SS. Treatment of pediatric Hodgkin’s lymphoma. Semin Hematol 1999;36:313–323. [PubMed] [Google Scholar]
- 4.Kelly KM, Hodgson D, Appel B, et al. Children’s Oncology Group’s 2013 blueprint for research: Hodgkin lymphoma. Pediatr Blood Cancer 2013;60:972–978. [DOI] [PubMed] [Google Scholar]
- 5.Brice P, Bouabdallah R, Moreau P, et al. Prognostic factors for survival after high-dose therapy and autologous stem cell transplantation for patients with relapsing Hodgkin’s disease: analysis of 280 patients from the French registry. Societe Francaise de Greffe de Moelle. Bone Marrow Transplant 1997;20:21–26. [DOI] [PubMed] [Google Scholar]
- 6.Czyz J, Dziadziuszko R, Knopinska-Postuszuy W, et al. Outcome and prognostic factors in advanced Hodgkin’s disease treated with high-dose chemotherapy and autologous stem cell transplantation: a study of 341 patients. Ann Oncol 2004;15:1222–1230. [DOI] [PubMed] [Google Scholar]
- 7.Gribben JG, Linch DC, Singer CR, McMillan AK, Jarrett M, Goldstone AH. Successful treatment of refractory Hodgkin’s disease by high-dose combination chemotherapy and autologous bone marrow transplantation. Blood 1989;73:340–344. [PubMed] [Google Scholar]
- 8.Horning SJ, Chao NJ, Negrin RS, et al. High-dose therapy and autologous hematopoietic progenitor cell transplantation for recurrent or refractory Hodgkin’s disease: analysis of the Stanford University results and prognostic indices. Blood 1997;89:801–813. [PubMed] [Google Scholar]
- 9.Josting A, Franklin J, May M, et al. New prognostic score based on treatment outcome of patients with relapsed Hodgkin’s lymphoma registered in the database of the German Hodgkin’s lymphoma study group. J Clin Oncol 2002;20:221–230. [DOI] [PubMed] [Google Scholar]
- 10.Lazarus HM, Rowlings PA, Zhang MJ, et al. Autotransplants for Hodgkin’s disease in patients never achieving remission: a report from the Autologous Blood and Marrow Transplant Registry. J Clin Oncol 1999;17:534–545. [DOI] [PubMed] [Google Scholar]
- 11.Martinez C, Salamero O, Arenillas L, et al. Autologous stem cell transplantation for patients with active Hodgkin’s lymphoma: long-term outcome of 61 patients from a single institution. Leuk Lymphoma 2007;48:1968–1975. [DOI] [PubMed] [Google Scholar]
- 12.Moskowitz CH, Nimer SD, Zelenetz AD, et al. A 2-step comprehensive high-dose chemoradiotherapy second-line program for relapsed and refractory Hodgkin disease: analysis by intent to treat and development of a prognostic model. Blood 2001;97:616–623. [DOI] [PubMed] [Google Scholar]
- 13.Schmitz N, Pfistner B, Sextro M, et al. Aggressive conventional chemotherapy compared with high-dose chemotherapy with autologous haemopoietic stem-cell transplantation for relapsed chemosensitive Hodgkin’s disease: a randomised trial. Lancet 2002;359:2065–2071. [DOI] [PubMed] [Google Scholar]
- 14.Sureda A, Constans M, Iriondo A, et al. Prognostic factors affecting long-term outcome after stem cell transplantation in Hodgkin’s lymphoma autografted after a first relapse. Ann Oncol 2005;16:625–633. [DOI] [PubMed] [Google Scholar]
- 15.Wadehra N, Farag S, Bolwell B, et al. Long-term outcome of Hodgkin disease patients following high-dose busulfan, etoposide, cyclophosphamide, and autologous stem cell transplantation. Biol Blood Marrow Transplant 2006;12:1343–1349. [DOI] [PubMed] [Google Scholar]
- 16.Sureda A, Arranz R, Iriondo A, et al. Autologous stem-cell transplantation for Hodgkin’s disease: results and prognostic factors in 494 patients from the Grupo Espanol de Linfomas/Transplante Autologo de Medula Osea Spanish Cooperative Group J Clin Oncol 2001;19:1395–1404. [DOI] [PubMed] [Google Scholar]
- 17.Schwartz CL, Chen L, McCarten K, et al. Childhood Hodgkin International Prognostic Score (CHIPS) Predicts event-free survival in Hodgkin Lymphoma: A Report from the Children’s Oncology Group. Pediatr Blood Cancer 2017;64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Friedman DL, Chen L, Wolden S, et al. Dose-intensive response-based chemotherapy and radiation therapy for children and adolescents with newly diagnosed intermediate-risk hodgkin lymphoma: a report from the Children’s Oncology Group Study AHOD0031. J Clin Oncol 2014;32:3651–3658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cheson BD, Pfistner B, Juweid ME, et al. Revised response criteria for malignant lymphoma. J Clin Oncol 2007;25:579–586. [DOI] [PubMed] [Google Scholar]
- 20.Linch DC, Winfield D, Goldstone AH, et al. Dose intensification with autologous bone-marrow transplantation in relapsed and resistant Hodgkin’s disease: results of a BNLI randomised trial. Lancet 1993;341:1051–1054. [DOI] [PubMed] [Google Scholar]
- 21.Schmitz N, Pfistner B, Sextro M, et al. Aggressive conventional chemotherapy compared with high-dose chemotherapy with autologous haemopoietic stem-cell transplantation for relapsed chemosensitive Hodgkin’s disease: a randomised trial. Lancet 2002;359:2065–2071. [DOI] [PubMed] [Google Scholar]
- 22.Rancea M, Monsef I, von Tresckow B, Engert A, Skoetz N. High-dose chemotherapy followed by autologous stem cell transplantation for patients with relapsed/refractory Hodgkin lymphoma. Cochrane Database Syst Rev 2013:CD009411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Schellong G, Dorffel W, Claviez A, et al. Salvage therapy of progressive and recurrent Hodgkin’s disease: results from a multicenter study of the pediatric DAL/GPOH-HD study group. J Clin Oncol 2005;23:6181–6189. [DOI] [PubMed] [Google Scholar]
- 24.Garfin PM, Link MP, Donaldson SS, et al. Improved outcomes after autologous bone marrow transplantation for children with relapsed or refractory Hodgkin lymphoma: twenty years experience at a single institution. Biol Blood Marrow Transplant 2015;21:326–334. [DOI] [PubMed] [Google Scholar]
- 25.Gorde-Grosjean S, Oberlin O, Leblanc T, et al. Outcome of children and adolescents with recurrent/refractory classical Hodgkin lymphoma, a study from the Societe Francaise de Lutte contre le Cancer des Enfants et des Adolescents (SFCE). Br J Haematol 2012;158:649–656. [DOI] [PubMed] [Google Scholar]
- 26.Hazar V, Kesik V, Aksoylar S, et al. Outcome of autologous hematopoietic stem cell transplantation in children and adolescents with relapsed or refractory Hodgkin’s lymphoma. Pediatr Transplant 2015;19:745–752. [DOI] [PubMed] [Google Scholar]
- 27.Metzger ML, Hudson MM, Krasin MJ, et al. Initial response to salvage therapy determines prognosis in relapsed pediatric Hodgkin lymphoma patients. Cancer 2010;116:4376–4384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Satwani P, Ahn KW, Carreras J, et al. A prognostic model predicting autologous transplantation outcomes in children, adolescents and young adults with Hodgkin lymphoma. Bone Marrow Transplant 2015;50:1416–1423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sumaili H, Al-Kofide A, Al-Seraihi A, et al. Outcome of pediatric patients with lymphoma following stem cell transplant: a single institution report. Leuk Lymphoma 2015;56:1327–1334. [DOI] [PubMed] [Google Scholar]
- 30.Shafer JA, Heslop HE, Brenner MK, et al. Outcome of hematopoietic stem cell transplant as salvage therapy for Hodgkin’s lymphoma in adolescents and young adults at a single institution. Leuk Lymphoma 2010;51:664–670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Baker KS, Gordon BG, Gross TG, et al. Autologous hematopoietic stem-cell transplantation for relapsed or refractory Hodgkin’s disease in children and adolescents. J Clin Oncol 1999;17:825–831. [DOI] [PubMed] [Google Scholar]
- 32.Lieskovsky YE, Donaldson SS, Torres MA, et al. High-dose therapy and autologous hematopoietic stem-cell transplantation for recurrent or refractory pediatric Hodgkin’s disease: results and prognostic indices. J Clin Oncol 2004;22:4532–4540. [DOI] [PubMed] [Google Scholar]
- 33.Akhtar S, El Weshi A, Rahal M, Abdelsalam M, Al Husseini H, Maghfoor I. High-dose chemotherapy and autologous stem cell transplant in adolescent patients with relapsed or refractory Hodgkin’s lymphoma. Bone Marrow Transplant 2010;45:476–482. [DOI] [PubMed] [Google Scholar]
- 34.Moskowitz AJ, Yahalom J, Kewalramani T, et al. Pretransplantation functional imaging predicts outcome following autologous stem cell transplantation for relapsed and refractory Hodgkin lymphoma. Blood 2010;116:4934–4937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Shah GL, Yahalom J, Matasar MJ, et al. Risk factors predicting outcomes for primary refractory hodgkin lymphoma patients treated with salvage chemotherapy and autologous stem cell transplantation. Br J Haematol 2016;175:440–447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ulaner GA, Goldman DA, Sauter CS, et al. Prognostic Value of FDG PET/CT before Allogeneic and Autologous Stem Cell Transplantation for Aggressive Lymphoma. Radiology 2015;277:518–526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Brockelmann PJ, Muller H, Casasnovas O, et al. Risk factors and a prognostic score for survival after autologous stem-cell transplantation for relapsed or refractory Hodgkin lymphoma. Ann Oncol 2017;28:1352–1358. [DOI] [PubMed] [Google Scholar]
- 38.Younes A, Bartlett NL, Leonard JP, et al. Brentuximab vedotin (SGN-35) for relapsed CD30-positive lymphomas. N Engl J Med 2010;363:1812–1821. [DOI] [PubMed] [Google Scholar]
- 39.Ansell SM, Lesokhin AM, Borrello I, et al. PD-1 blockade with nivolumab in relapsed or refractory Hodgkin’s lymphoma. N Engl J Med 2015;372:311–319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Armand P, Shipp MA, Ribrag V, et al. Programmed Death-1 Blockade With Pembrolizumab in Patients With Classical Hodgkin Lymphoma After Brentuximab Vedotin Failure. J Clin Oncol 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Younes A, Santoro A, Shipp M, et al. Nivolumab for classical Hodgkin’s lymphoma after failure of both autologous stem-cell transplantation and brentuximab vedotin: a multicentre, multicohort, single-arm phase 2 trial. Lancet Oncol 2016;17:1283–1294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Harker-Murray PD, Drachtman RA, Hodgson DC, Chauvenet AR, Kelly KM, Cole PD. Stratification of treatment intensity in relapsed pediatric Hodgkin lymphoma. Pediatr Blood Cancer 2014;61:579–586. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


