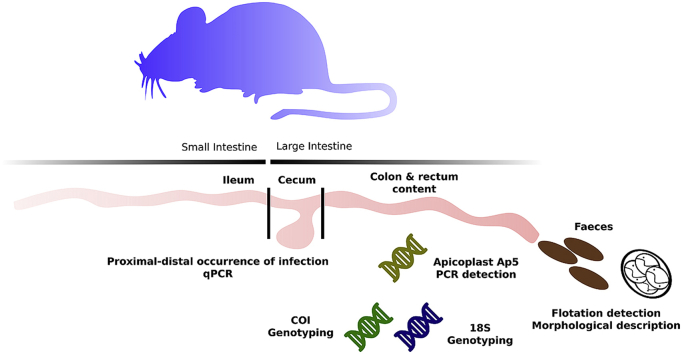
Abstract
Detection and quantification of coccidia in studies of wildlife can be challenging. Therefore, prevalence of coccidia is often not assessed at the parasite species level in non-livestock animals. Parasite species – specific prevalences are especially important when studying evolutionary questions in wild populations. We tested whether increased host population density increases prevalence of individual Eimeria species at the farm level, as predicted by epidemiological theory.
We studied free-living commensal populations of the house mouse (Mus musculus) in Germany, and established a strategy to detect and quantify Eimeria infections. We show that a novel diagnostic primer targeting the apicoplast genome (Ap5) and coprological assessment after flotation provide complementary detection results increasing sensitivity. Genotyping PCRs confirm detection in a subset of samples and cross-validation of different PCR markers does not indicate bias towards a particular parasite species in genotyping. We were able to detect double infections and to determine the preferred niche of each parasite species along the distal-proximal axis of the intestine. Parasite genotyping from tissue samples provides additional indication for the absence of species bias in genotyping amplifications. Three Eimeria species were found infecting house mice at different prevalences: Eimeria ferrisi (16.7%; 95% CI 13.2–20.7), E. falciformis (4.2%; 95% CI 2.6–6.8) and E. vermiformis (1.9%; 95% CI 0.9–3.8). We also find that mice in dense populations are more likely to be infected with E. falciformis and E. ferrisi.
We provide methods for the assessment of prevalences of coccidia at the species level in rodent systems. We show and discuss how such data can help to test hypotheses in ecology, evolution and epidemiology on a species level.
Keywords: Eimeria, Coccidia, House mice, Diagnostic PCR, Species-specific prevalence, qPCR
Graphical abstract
Highlights
-
•
Flotation and PCR provide complementary results for Eimeria detection in house mice.
-
•
Genotyping PCRs confirm detections.
-
•
E. ferrisi, E. falciformis, and E. vermiformis infect natural populations of M. musculus.
-
•
Double infections and preferentially infected tissues could be identified using qPCR.
-
•
Potential virulence prevalence trade-off for Eimeria of house mice.
1. Introduction
House mice (Mus musculus) are the most commonly used mammalian model for biomedical research worldwide (Houdebine, 2004; Vandenbergh, 2013). Laboratory mouse strains are derived mainly from the subspecies M. m. domesticus with genetic contributions from other subspecies (M.m musculus and M. m. castaneus) (Nishioka, 2011; Yang et al., 2007). Establishment of suitable mouse models to better understand infections with coccidia is an ongoing process. Wild rodents and especially wild house mice are an attractive system for first steps in this direction (Ehret et al., 2017).
Eimeria (Schneider, 1875) is, with around 1700 species, the most speciose genus in the phylum Apicomplexa (Duszynski, 2011; Perkins et al., 2000). For economical reasons, the most studied parasites in this group are those infecting livestock (Shirley et al., 2005; Su et al., 2003). At least one third of the slmdescribed species, however, infects rodents (Levine and Ivens, 1965; Zhao and Duszynski, 2001b).
The most commonly used method for detection and identification of coccidia is the flotation and microscopical observation of oocysts shed in faeces during the patent period of infection (Ryley et al., 1976). Unsporulated oocysts, however, are difficult or impossible to differentiate into species (Levine and Ivens, 1988, 1965). Thus, prior to identification the oocyst should be sporulated under specific conditions. In addition, expertise and experience is required for species identification, especially in cases (like ours) of very similar oocyst morphology in different species (Duszynski and Wilber, 1997; Long and Joyner, 1984). For that reason, tools based on DNA amplification and sequencing have been included as routine strategy not only for detection, but also for taxonomic assessment (Fernandez et al., 2003; Hnida and Duszynski, 1999; Kawahara et al., 2010; Morris et al., 2007; Schnitzler et al., 1999; Su et al., 2003; Vrba et al., 2010).
Up to 16 species of Eimeria have been described from house mice (Levine and Ivens, 1965) and some of them use different niches in the intestine. The reasons for this diversity are still elusive (Zhao and Duszynski, 2001a) and artificial splitting of morphologically plastic forms of the same species (in the same of different hosts) might contribute to this.
Eimeria species described from house mice include E. falciformis, the first coccidia described in house mice (Eimer, 1870), which has sometimes been regarded as the most prevalent species in mice (Becker, 1934; Owen, 1976). This species (and especially the BayerHaberkorn1970 isolate) are the most commonly studied coccidia model in laboratory mice. Life cycle progression (Haberkorn, 1970) and host response (Mesfin et al., 1978; Schelzke et al., 2009; Schmid et al., 2012) are relatively well studied and the whole genome of this species has been sequenced and annotated in detail (Heitlinger et al., 2014).
E. vermiformis was first described in 1971 (Ernst et al., 1971) but since then, to our knowledge, not reported in wild house mice. Similar to E. falciformis, most of the information on this species comes from laboratory infection experiments (Figueiredo-Campos et al., 2018; Rose et al., 1990; Rose and Millard, 1985; Todd Jr and Lepp, 1971), making the timing of life cycle progression and its effect on the host relatively well studied.
E. ferrisi was originally described from M. m. domesticus from North America (Ankrom et al., 1975; Levine and Ivens, 1965). Laboratory infections with this parasite have confirmed its shorter life cycle, compared to E. vermiformis or E. falciformis (Schito et al., 1996).
To the best of our knowledge, just few investigation of prevalences and intensities of coccidia has been conducted in free-living populations of M. musculus (Ball and Lewis, 1984; Ernst et al., 1971; Golemanski, 1979; Owen, 1976; Parker et al., 2009; Yakimoff and Gousseff, 1938). In the present work we studied the prevalence of Eimeria in house mice from a transect of the well-studied European house mice hybrid zone (HMHZ) (Boursot et al., 2003; Ďureje et al., 2012; Phifer-Rixey and Nachman, 2015). We established methods for detection, species identification and quantification of Eimeria in these wild commensal populations of house mice.
2. Material and methods
2.1. Collection of samples
Between 2015 and 2017, 378 house mice (Mus musculus) were captured in 96 farms and private properties in a transect 152.27 km long and 114.48 km wide, within the German federal state of Brandenburg (capture permit No. 2347/35/2014) (Fig. 1A, Supplementary data 1). On average 20 traps were set overnight per locality. Mice were house individually in cages over night and euthanised by cervical dislocation. All mice were dissected within 24 h after capture. Faeces for microscopical diagnosis of Eimeria spp. were preserved in potassium dichromate (K2Cr2O7) 2.5% (w/v) and stored at 4 °C until further processing, colon content was preserved in liquid nitrogen and stored at −80 °C. For a subset of 163 mice (from Brandenburg in 2016) tissue samples from cecum and ileum were collected for DNA extraction and molecular identification of Eimeria spp. All samples were kept in liquid nitrogen during transportation and maintained at −80 °C until processing.
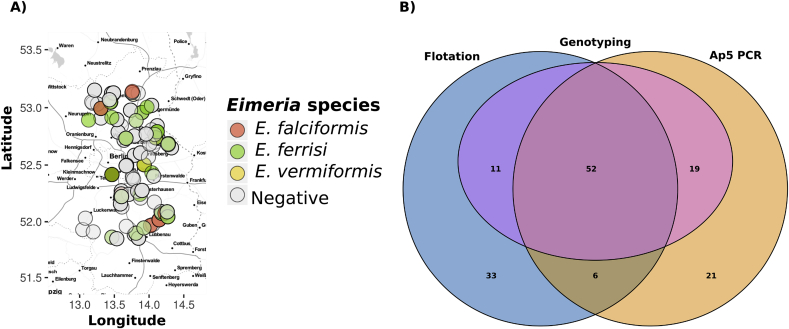
Fig. 1.
Geographical localization of house mice (Mus musculus) collected for this study and comparison of diagnostic methods for Eimeria. A) Localization from the 378 mice included in the present study, colors indicate the Eimeria species identified for each. B) Venn diagram showing the overlap between detection methods and successful genotyping identification of the isolates. (For interpretation of the references to color in this figure legend, the reader is referred to the Web version of this article.)
2.2. Flotation and microscopical analysis of oocyst
Fecal samples were washed with tap water to eliminate potassium dichromate and homogenized. After oocyst were flotated using a saturated salt solution (specific gravity = 1.18–1.20), they were collected by centrifugation (3234×g/room temperature/10 min), washed with distilled water (1800×g/room temperature/10 min). The flotations were screened for the presence of oocyst using a Leica® DM750 M light microscope under the 10X objective. To estimate the intensity of infection, flotated oocysts were counted using a Neubauer chamber and the results were expressed as oocyst per gram (OPG) of faeces. Samples were then preserved in a fresh solution of potassium dichromate 2.5% (w/v) and sporulated in a water bath at 30 °C for 10–12 days for further characterisation.
Eimeria isolates, corresponding to different phylogenetic groups (see below), were selected to take photomicrographs of sporulated oocysts using a Carl-Zeiss microscope at 100x magnification. Measurements were made on ~30 oocysts and ~30 sporocysts, using Adobe Photoshop CC v14.2.1 (3778 pixels = 100, 0000 μm). Length and width were measured and reported in micrometers. The Length/Width (L/W) ratio was calculated for both oocysts and sporocysts including means, standard deviation and variation coefficients. Additionally, main morphological traits (oocyst wall, oocyst residuum, micropyle, polar granule, sporocyst residuum, refractile bodies and Stieda body) were described, according to the protocol of Duszynski and Wilber (1997).
2.3. DNA extraction
DNA from colon content was extracted using the NucleoSpin® Soil kit (MACHEREY-NAGEL GmbH & Co. KG, Düren, Germany) according to the manufacturer's recommendations, adding a mechanical lysis process in a Mill Benchtop Mixer MM 2000 (Retsch GmbH, Haan, Germany). DNA from cecum and ileum tissues was isolated using the innuPREP DNA Mini Kit (Analytik Jena AG, Jena, Germany) following the instructions of the manufacturer after disruption of the tissue with liquid nitrogen in a mortar. Quality and quantity of isolated DNA was measured spectrophotometrically in a NanoDrop 2000c (Thermo Scientific, Waltham, USA).
2.4. PCR amplification for detection (ap tRNA) and identification (nu 18S rDNA and mt COI)
For detection of Eimeria, amplification of a conserved tRNA region of the apicoplast genome (Ap5) was used. Primers Ap5_Fwd (YAAAGGAATTTGAATCCTCGTTT) and Ap5_Rev (YAGAATTGATGCCTGAGYGGTC) were designed based on the complete apicoplast genomes sequences available in the GenBank from Eimeria tenella (NC_004823.1), E. falciformis (CM008276.1) and E. nieschulzi (JRZD00000000.1).
For all samples with oocysts detected during flotation or successful amplification of Ap5, genotyping PCRs were performed to confirmation of detection and further identification of parasite species. A fragment of nuclear small subunit ribosomal RNA (~1500 bp) and mitochondrial cytochrome C oxidase subunit I (~800 bp) were amplified using primers 18S_EF and 18S_ER (Kvičerová et al., 2008) and Cocci_COI_For/Rev (Ogedengbe et al., 2011), respectively. An alternative pair of primers was used in case of failure to amplify COI: Eim_COI_M_F (ATGTCACTNTCTCCAACCTCAGT) and Eim_COI_M_R (GAGCAACATCAANAGCAGTGT). These primers were designed based on the mitochondrial genome of E. falciformis (CM008276.1) (Heitlinger et al., 2014) and amplify a ~700 bp fragment of COI gene.
PCR reactions were carried out in a Labcycler (SensoQuest GmbH, Göttingen, Germany) using 0.025 U/μL of DreamTaqTM DNA Polymerase (Thermo Scientific, Waltham, USA), 1X DreamTaq Buffer, 0.5 mM dNTP Mix, 0.25 μM from each primer and 1–20 ng/μL of DNA template in 25 μL reaction. A concentration of 0.25 mM dNTP Mix and a supplementation with 2 mM MgCl2 was used for the amplification of Ap5. The thermocycling protocol consist of 95 °C initial denaturation (4 min) followed by 35 cycles of 92 °C denaturation (45 s), annealing at 52 °C (30 s/Eim_COI); 53 °C (45 s/18S_E); 55 °C (30 s/Cocci_COI); 56 °C (30 s/Ap5); 72 °C extension 90 s (18S_E), 20 s (Cocci_COI/Eim_COI) or 45s (Ap5) and a final extension at 72 °C (10 min). DNA from oocyst of E. falciformis BayerHaberkorn1970 strain and DNA from colon content of a non-infected NMRI mouse were used as positive and negative controls, respectively.
All PCR products with the expected size were purified using the SAP-Exo Kit (Jena Bioscience GmbH, Jena, Germany) and Sanger sequenced from both directions by LGC Genomics (Berlin, Germany). Quality assessment and assembly of forward and reverse sequence was performed in Geneious v6.1.8. All sequences were submitted to NCBI GenBank (Accession numbers: nu 18S rDNA [MH751925—MH752036] and mt COI [MH777467—MH777593 and MH755302—MH755324] (Supplementary data S2).
2.5. Eimeria detection in tissue by qPCR
For mice collected in 2016 (n = 163) cecum and ileum tissue was screened using qPCR. Primers targeting a short fragment of mt COI were used to amplify DNA from intracellular stages of Eimeria (Eim_COI_qX-F, TGTCTATTCACTTGGGCTATTGT; Eim_COI_qX-R GGATCACCGTTAAATGAGGCA). Amplification reactions with a final volume of 20 μL contained 1X iTaqTM Universal SYBR® Green Supermix (Bio-Rad Laboratories GmbH, München, Germany), 400 nM of each primer and 50 ng of DNA template. Cycling in a Mastercycler® RealPlex 2 (Eppendorf, Hamburg, Germany) was performed with the following program: 95 °C initial denaturation for 2 min, followed by 40 cycles of denaturation at 95 °C for 15 s, annealing at 55 °C for 15 s and extension 68 °C for 20 s. Melting curves were analysed to detect eventual primer dimer formation or non-specific amplification. As internal reference for relative quantification the CDC42 gene from the nuclear genome of the house mouse was amplified (Ms_gDNA_CDC42_F CTCTCCTCCCCTCTGTCTTG; Ms_gDNA_CDC42_R TCCTTTTGGGTTGAGTTTCC). Infection intensity was estimated as the ΔCt between mouse and Eimeria amplification (CtMouse- CtEimeria). To correct for background noise a detection threshold was estimated at ΔCt = −5 and only results above this value were considered infected. ΔCtIleum and ΔCtCecum were compared for samples above the threshold in both tissues to assess primary tissue occurrence (Ahmed et al., 2019). In samples positive for qPCR, Eimeria genotyping was performed based on DNA extracted from tissue, as described above (see 2.4).
2.6. Molecular identification of Eimeria spp. isolates: 18S and COI phylogenetic analysis
As strategy for molecular identification, datasets of nu 18S and mt COI sequences were compiled. Sequences generated for the present work were compared to databases sequences using NCBI BLAST and most similar sequences were selected. Based on this, sequences for E. falciformis, E. vermiformis and E. ferrisi were downloaded from GenBank as a reference. COI sequences were aligned by translation using the Multiple Align algorithm and translation frame 1 with the genetic code for “mold protozoan mitochondrial”, 18S sequences were aligned using MUSCLE (Edgar, 2004), both through Geneious v6.1.8.
Phylogenetic trees for all datasets were constructed using Maximum Likelihood (ML) and Bayesian inference (BI) methods, implemented in PhyML v3.0 (Guindon et al., 2010) and MrBayes v3.2.6 (Huelsenbeck and Ronquist, 2001; Ronquist et al., 2012), respectively. The most appropriate evolutive models for each datasets were determined in JModelTest v2.1.10 (Posada, 2008). For ML trees, a bootstrap analysis with 1000 replicates was performed, whereas MCMC for BI was run with two cold and two hot chains for 1,000,000 generations or until the average split frequency was below 0.05. The concatenated dataset was analysed using partitions and locus-specific models. Visualization of the trees was done with FigTree v1.4.2 (Rambaut, 2012).
2.7. Statistical analysis
All statistical analyses were performed in R (R Development Core Team, 2008). Prevalence of Eimeria was calculated as the proportion of positive samples in the total number of analysed samples. The 95% confidence interval [CI 95%] was calculated using the Sterne's exact method (Sterne, 1954) implemented in the package “epiR” v0.9-99 (Nunes et al., 2018). Prevalences were tested for statistical differences with the Fisher's exact test (Fisher, 1922).
To assess the significance in primer bias, logistic regression models were used to estimate the probability to successfully amplify and sequence a specific genetic marker for each Eimeria species. The response variable in these models was the amplification and sequencing success with a particular primer pair (COCCI_COI_F/R, Eim_COI_M_F/R or 18S_E F/R), and the predictors were the species identity (as determined with the other markers only, to make response and predictors independent) and additionally the detection of an infection with Ap5 and Flotation. These models were fitted first for COCCI_COI_F/R as response, then for the combined probability of successful COI genotyping and finally for 18S genotyping as response. Tables were produced for the summary of models using the package “jtools” v2.0.0 (Long, 2019).
For each Eimeria species logistic regression models were used to test whether the infection is influenced by host density or by the presence of the other two Eimeria. We use as response variable the infection status by E. ferrisi, E. falciformis or E. vermiformis, independently, and the total number of mice cough per locality per year and the infection status by a different Eimeria species as predictors.
Differences on oocyst and sporocyst L/W ratios between Eimeria species were tested for significance with an analysis of variance fitting a linear model using the species assignment as predictor with a Tukey HSD post hoc test adjusting for multiple comparisons.
3. Results
3.1. Sampling and Eimeria spp. detection
We used flotation of oocyst from faeces and PCR amplification of a novel diagnostic maker (Ap5) from colon content DNA to detect Eimeria parasites in a total of 378 house. Overall prevalence was 25.9% [95% CI = 21.7–30.7] (98/378) for PCR and 27.0% [95% CI = 22.7–31.7%] (102/378) for flotation. These estimates are not significantly different (Fisher exact test, p > 0.05). However, both techniques considered together estimate a higher prevalence of 37.6% [95% CI = 32.8–42.6] (142/378), meaning that 44 and 40 positive results were detected only by flotation or PCR, respectively (Fig. 1B). We further aimed to provide species specific identification and to consolidate results from the two different detection methods.
3.2. Molecular identification of Eimeria isolates - (phylogenetic analysis nu 18S and mt COI)
Eimeria species were identified by phylogenetic analysis of nu 18S and mt COI sequences, the most commonly used molecular markers of apicomplexan parasites. To identify our isolates, sequences were compared with references from the NCBI database. Sequences from three previously described Eimeria species infecting M. musculus showed highest BLAST similarities and phylogenetic clustering. This approach ignores the problem whether isolates from different hosts would be assigned to the same phylogenetic clusters while they are regarded different species by taxonomists.
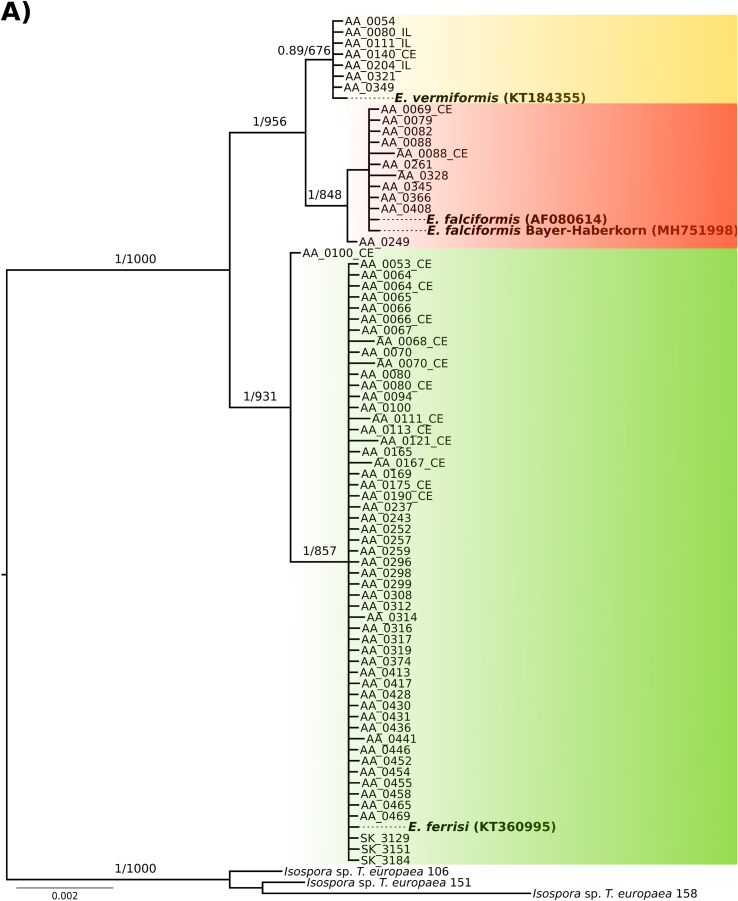
The nu 18S phylogenetic tree was inferred based on 80 sequences (540–1795 bp), 73 of them from wild house mice generated in our study (3 from ileum tissue, 16 from cecum tissue and 54 from colon content, see below). Eimeria species previously described in house mouse were represented by E. falciformis (AF080614), E. vermiformis (KT184355) and E. ferrisi (KT360995). In addition, one newly generated sequence from E. falciformis strain BayerHaberkorn1970 (MH751998) was also included. Sequence identity of our isolates to this references sequences was above 98% and even 100% in most of the cases for this marker. Isospora sp. sequences identified in Talpa europaea moles were used as outgroup. Both ML and BI rooted trees shared a topology placing our sequences at the same positions in relation to reference sequences with high support (bootstrap values and posterior probabilities are shown in Fig. 2A). The sequences clustered in three well supported monophyletic groups (Fig. 2A).
Fig. 2.
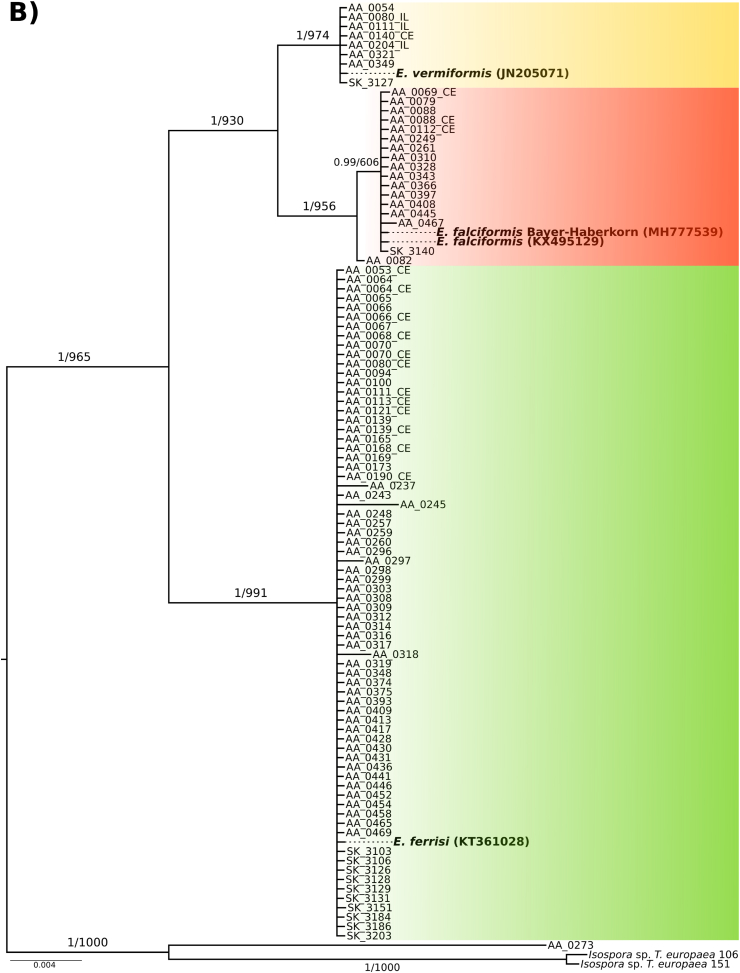
Phylogenetic trees based on 18S rRNA and COI sequences. Sequences of 18S A) and COI B) were used to infer the molecular identification of wild-derived isolates of Eimeria. In both phylogenies, our isolates clustered in three groups one close to E. falciformis (red), other close to E. ferrisi (green) and finally one to E. vermiformis (yellow). Numbers in the branches represent the Bayesian posterior probability and the non-parametric bootstrap value. In bold are indicate the reference sequences for each species. CE and IL make reference to sequence derived from cecum or ileum tissue DNA, respectively. (For interpretation of the references to color in this figure legend, the reader is referred to the Web version of this article.)
The phylogenetic tree for mt COI was based on 103 sequences (519–804 bp), 97 of which were obtained from Eimeria infecting wild house mice (3 from ileum, 16 from cecum tissue and 78 from colon content). Reference sequences from house mouse Eimeria (E. ferrisi, KT361028; E. falciformis, KX495129 and MH777539; E. vermiformis JN205071) identified by BLAST searches showed an identity of above 98% to respective groups of our isolates. We defined Isospora sp. from Talpa europaea as outgroup for rooting. ML and BI rooted trees based on alignments of these COI sequences shared a general topology with respect to the placement of our isolates in relation to reference sequences. Bootstrap values and posterior probabilities for support of these placements are shown in Fig. 2B. The sequences derived from house mice cluster in three monophyletic groups including reference sequences for E. falciformis (n = 17, sequences from our study), E. ferrisi (n = 72) and E. vermiformis (n = 8) (Fig. 2B). Phylogenies based on concatenated supermatrices for the two markers show the same topology concerning placement of isolates from the present study (Supplementary data S3 and S4).
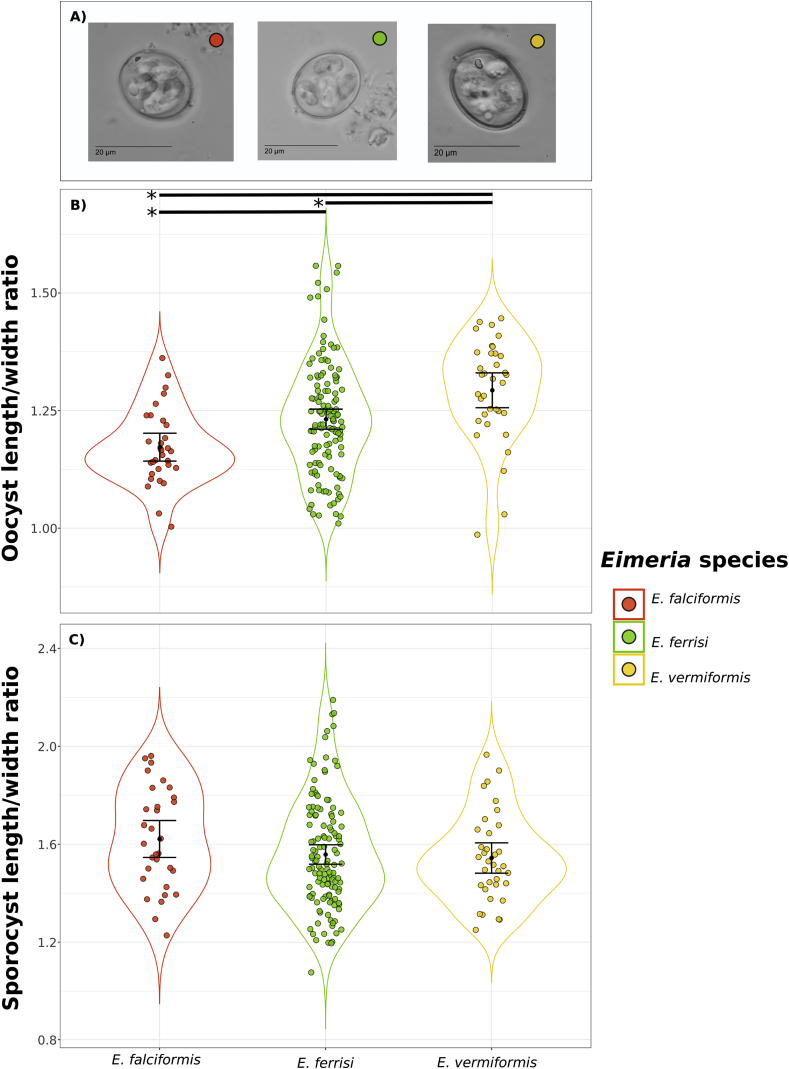
3.3. Morphometrical and morphological comparison of oocysts
For further support assignment of the three phylogenetic groups of Eimeria from house mouse, we characterized sporulated oocysts morphologically (Table 1). E. falciformis, E. ferrisi and E. vermiformis oocyst shared most of the traits we evaluated and showed overlapping morphometry (Fig. 3A). The length/width ratio of E. vermiformis oocysts, however, was significantly higher (1.29; 95%CI = 1.26–1.33; n = 35) than that of E. falciformis (1.17; 95%CI = 1.14–1.20; n = 31) and E. ferrisi oocysts (1.23; 95%CI = 1.21–1.25; n = 127) (Tukey HSD, p < 0.05) (Fig. 3B; Supplementary data S5). This means that E. vermiformis has more ellipsoidal oocysts than the other two species. Other morphological characteristics of oocysts (smooth wall, absence of micropyle, presence of polar granule and absence of oocyst residuum) are very similar or identical between the three species (Table 1).
Table 1.
Morphological and morphometrical characteristics from Eimeria wild-derived isolates and reference morphotypesa.
| Species | Oocysts |
|||||||
|---|---|---|---|---|---|---|---|---|
| Shape | Wall | Micropyle | Polar granule | Residuum | Lenght (μm) | Width (μm) | L/W ratio | |
| E. falciformis | Spherical/Ellipsoidal | Smooth | Absent | Present | Absent | 18.62 (15.29–20.76) | 15.92 (13.89–17.6) | 1.17 (1.00–1.36) |
| E. falciformis | Ovoid/Spherical | Smooth | Absent | Present | Absent/Present | 21 (15–26) | 18 (13–24) | 1.17 (1.1–1.2) |
| E. ferrisi | Spherical/Ellipsoidal | Smooth | Absent | Presentb | Absent | 17.32 (13.59–21.47) | 14 (10.97–17.44) | 1.23 (1.02–1.56) |
| E. ferrisi | Ellipsoidal/Subspherical | Smooth | Absent | Present | Absent | 17 (12–22) | 14 (11–18) | 1.22 (1.0–1.6) |
| E. vermiformis | Spherical/Ellipsoidal | Smooth | Absent | Presentb | Absent | 20.02 (16.22–22.8) | 15.57 (12.37–18.44) | 1.29 (0.99–1.45) |
| E. vermiformis | Spherical/Ellipsoidal | Lightly pitted | Absent | Present | Absent | 23.1 (18–26) | 18.4 (15–21) | 1.25 (1.1–1.4) |
|
| ||||||||
| Species | Sporocysts | Tissue localization | Reference | |||||
| Residuum |
Refractile body |
Stieda body |
Lenght (μm) |
Width (μm) |
||||
| E. falciformis | Present | Present | Presentc | 8.88 (7.03–10.30) | 5.52 (4.16–6.93) | Cecum | This work | |
| E. falciformis | Present | Present | Present | 11 (10–12) | 7 (6–8) | Ileum, cecum and colon | Eimer (1870); Haberkorn (1970) | |
| E. ferrisi | Present | Present | Present | 7.75 (5.17–11.47) | 5.03 (2.88–7.73) | Cecum | This work | |
| E. ferrisi | Absent/Present | Present | Present | 10.5 (10–11) | 5.5 (5–6) | Cecum and colon | Levine and Ivens (1965); Ankrom et al. (1975) | |
| E. vermiformis | Present | Present | Present | 8.35 (6.18–11.29) | 5.43 (4.19–7.00) | Cecum and ileum | This work | |
| E. vermiformis | Present | Present | Present | 12.8 (11–14) | 7.9 (6–10) | Jejunum, ileum and cecum | Ernst et al. (1971) | |
Measurements are means in micrometers with ranges in parenthesis.
Observed in more than 80% of the oocysts.
Present but not evident.
Fig. 3.
Morphological and morphometrical characteristics of Eimeria oocyst isolated from Mus musculus. a) Photomicrographs at 1000x amplification of Eimeria oocyst from the three species isolated from Mus musculus (red = E. falciformis; green = E. ferrisi and yellow = E. vermiformis). Length/Width ratio from b) oocyst and c) sporocysts corresponding to each species (E. falciformis n = 31; E. ferrisi n = 127 and E. vermiformis n = 35). Mean±95% Confidence Interval is plotted. * Represent significant difference (Tukey HSD, p < 0.05). (For interpretation of the references to color in this figure legend, the reader is referred to the Web version of this article.)
Morphological measurements of sporocysts are not significantly different between the three species found in house mice (Fig. 3C). We also observed the presence of a sporocyst residuum, refractile bodies and Stieda bodies in all species uniformly, in agreement with previous descriptions (Ankrom et al., 1975; Eimer, 1870; Ernst et al., 1971; Haberkorn, 1970).
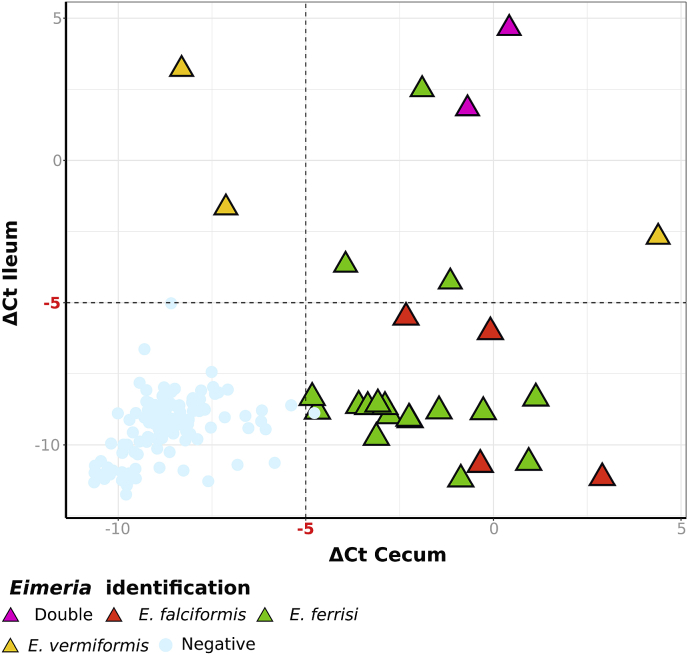
3.4. Proximal-distal occurrence of infection and double infections
We detected DNA from endogenous stages by qPCR in 27 of 163 samples analysed (Supplementary data S6). We differentiate detection between small and large intestine, analysing ileum as the most distal tissue of the small intestine and cecum as the most proximal tissue of the large intestine. Detection was either limited just to cecum (n = 19), to ileum (n = 2) or possible in both tissues (n = 6). Infections in cecum were identified as E. falciformis (n = 4), E. ferrisi (n = 17) and E. vermiformis (n = 1) Detections in ileum (n = 2) were identified as E. vermiformis. In two mice positive in both tissues, it was possible to identify E. ferrisi in cecum and E. vermiformis in ileum, providing evidence that these animals presented a double infection (that is, simultaneous infections with different isolates; Fig. 4).
Fig. 4.
qPCR detection of intracellular stages of Eimeria in cecum and ileum from Mus musculus. -Delta Ct value (CtEimeria - CtMouse) from each tissue for 164 mice are plotted on the graph. The dotted line indicate the threshold of −5, values above the line are considered positive for the corresponding tissue. Circles represent negative samples, triangles indicate samples with Eimeria species identification and colors correspond to the Eimeria species identified in those samples. (For interpretation of the references to color in this figure legend, the reader is referred to the Web version of this article.)
3.5. Amplification efficiency of different markers
As Eimeria detection by PCR and the determination of species identity could be biased especially in cases of double infections, we analysed differences in amplification efficiency of the three primer pairs used for the molecular identification of Eimeria species (Table 2). In a cross-validation approach we compare the likelihood to amplify a maker given the species identification with the other marker. The amplification and sequencing efficiency of Cocci_COI primer pair was significantly higher for E. ferrisi isolates (logistic regression, p < 0.001) than for E. falciformis isolates (the letter determined by the 18S marker). Using the novel primer pair Eim_COI_M_F/R the sequencing results were complemented and we detected no significant differences in the combined amplification efficiency for both primers (logistic regression; p = 0.62).
Table 2.
Statistical models used to analyse primer preference to different Eimeria species.
| Predictors | Model 1 (Cocci_COI) | Model 2 (Cocci_COI + Eim_COI) | Model 3 (18S_EF/R) |
|---|---|---|---|
| Intercept | −5.80 *** | −1.55 | −1.40 |
| (1.11) | (1.27) | (0.87) | |
| Ap5 | 3.50 *** | 3.99 *** | 0.78 |
| (0.59) | (0.60) | (0.62) | |
| Flotation | 1.64 ** | 2.15 *** | 1.59 ** |
| (0.52) | (0.54) | (0.51) | |
| Identification E. ferrisi (other marker) | 5.10 *** | 0.28 | 0.32 |
| (1.14) | (1.35) | (0.63) | |
| Identification E. vermiformis (other marker) | 17.23 | 11.98 | 1.71 |
| (1385.38) | (1385.38) | (1.33) | |
| No identification (other marker) | 0.97 | −3.57 ** | −3.44 *** |
| (0.92) | (1.23) | (0.87) | |
| Identification Eimeria spp. (COI) | −14.80 | ||
| (1455.40) | |||
| N | 378 | 378 | 378 |
| AIC | 128.09 | 119.18 | 143.39 |
| BIC | 151.70 | 142.79 | 170.93 |
| Pseudo R2 | 0.77 | 0.82 | 0.70 |
The upper number represents the estimate and numbers in brackets represent standard error for each predictor.
Intercept is Eimeria falciformis identification with other marker.
Other marker refers to 18S based identification for COI models or vice versa.
N (Total number of samples), AIC (Akaike information criterion), BIC (Bayesian information criterion).
***p < 0.001; **p < 0.01; *p < 0.05.
Similarly, we did not detect significant differences in the probability to obtain an 18S sequence (using the 18S_EF/R primer pair) between Eimeria species as determined by (combined) COI assessment (logistic regression; p = 0.25). Differences in PCR efficiency for Cocci_COI make it likely that markers amplify different species in case of double infections in a single isolate. Both detection by flotation and diagnostic (Ap5) PCR significantly increase the likelihood amplification for all primers (COI or 18S). The statistical models for biased amplification hence also confirm that both detection methods provide complementary results, even while controlling for detection with the other method (Table 2).
3.6. Prevalence of the different Eimeria species
Genotyping amplification and sequencing with either 18S or COI primers (or both) was possible for samples in which infections had been detected (n = 82) (Fig. 1B). Both COI and 18S genotyping PCRs hence support detection by flotation and diagnostic PCRs in this subset of samples. Furthermore amplification of both or either one maker was fully sufficient to assign the isolate to an Eimeria species (Fig. 2), allowing us to resolve prevalence on the species level.
These corrections and controls allow us to determine prevalence at the species level: E. ferrisi was identified at a higher prevalence of 16.7% (63/378, 95%CI = 13.2–20.7) in comparison to E. falciformis (16/378, 4.2% [ 95%CI = 2.6–6.8]) and E. vermiformis (7/378, 1.9% [0.9–3.8]).
Considering prevalence at the level of farms, E. ferrisi was detected in 29.2% (28/96, 95%CI = 20.7–39.0), E. falciformis in 12.5% (12/96, 95%CI = 7.1–20.7) and E. vermiformis in 7.3% (7/96, 95%CI = 3.5–14.4) of sampled localities. 25 (of in total 96) farms, had mice with single Eimeria species detected, and 10 had more than one species detected. In all cases E. ferrisi was detected (5 farms with E. ferrisi – E. falciformis, 3 farms with E. ferrisi – E. vermiformis combination, and 2 farms with the three species). Mice presenting double infections were caught at farms at which infections with the both Eimeria species was found in other mice independently.
We used the number of mice caught per farm as a proxy for population density, assuming roughly equal trapping effort at all localities. We then question whether population density affects prevalence by testing differences in the likelihood of a mouse individual to be infected dependent on that population density. We detect that the likelihood of infection is significantly increased for both E. ferrisi and for E. falciformis (logistic regression, p < 0.05; Table 3). Infection with E. falciformis got more likely by 19%, infection with E. ferrisi by 14% with each mouse caught at the same locality. We also included the detection of other Eimeria species in the model for each species and did not find a significant influence (p > 0.05) on likelihood of infection.
Table 3.
Statistical models used to analyse factors influencing infection to different Eimeria species.
| Predictors | Model 1 (E. ferrisi infection) | Model 2 (E. falciformis infection) | Model 1 (E. vermiformis infection) |
|---|---|---|---|
| Intercept | −1.62 *** | −3.32 *** | −3.99 *** |
| (0.35) | (0.61) | (0.83) | |
| Total caught mice | 0.13 * | 0.18 * | 0.11 |
| (0.06) | (0.08) | (0.10) | |
| E. ferrisi infection | 0.92 | 0.29 | |
| (0.70) | (1.06) | ||
| E. falciformis infection | 0.92 | 1.60 | |
| (0.69) | (0.92) | ||
| E. vermiformis infection | 1.61 | 0.41 | |
| (0.91) | (1.03) | ||
| N | 104 | 104 | 104 |
| AIC | 120.99 | 71.61 | 52.03 |
| BIC | 131.57 | 82.18 | 62.61 |
| Pseudo R2 | 0.18 | 0.19 | 0.17 |
The upper number represents the estimate and numbers in brackets represent standard error for each predictor.
N (Total number of samples), AIC (Akaike information criterion), BIC (Bayesian information criterion).
***p < 0.001; **p < 0.01; *p < 0.05.
4. Discussion
In this study we identify Eimeria species in wild commensal populations of house mice (Mus musculus). We show that detection and identification of this group of rodent coccidia can be challenging and propose to complement classical coprological assessment with molecular tools: a highly sensitive detection PCR, genotyping PCRs for species identification and qPCR for localization and detection of double infections. Based on this we identified three different Eimeria species in the house mouse: E. ferrisi, E. falciformis and E. vermiformis. Morphological characteristics and preferential occurrence were congruent with the assignment of isolates to the above species. We use our results to show a positive effect on host density on prevalence of E. ferrisi and E. falciformis.
Few studies report prevalences of Eimeria in wild populations of Mus musculus. Prevalences range from 3% to 40% for isolates classified either as E. falciformis, E. ferrisi or E. vermiformis (Ball and Lewis, 1984; Ernst et al., 1971; Golemanski, 1979; Levine and Ivens, 1965; Tattersall et al., 1994). Other studies make no assessment at the species level (detection as Eimeria spp) (Moro et al., 2003; Owen, 1976; Parker et al., 2009; Yakimoff and Gousseff, 1938).
A recent study in rodents (other then Mus musculus) in central Europe reported an Eimeria spp. prevalence of 32.7% based on coprological observations (Mácová et al., 2018). At 37.6% the overall prevalence for all Eimeria species in our study in house could be considered high in comparison to all these studies.
While flotation is the most commonly used for detection and quantification of coccidia (Hobbs et al., 1999; Hu et al., 2016; Rinaldi et al., 2011), we here used a complementary approach of flotations and diagnostic PCR. We observed relatively large discrepancies between both methods. Flotation and counting of oocysts has a relatively high limit of detection (Ballweber et al., 2014; Webster et al., 1996), explaining negative findings in oocyst flotations positive for PCR. Negative PCR results for samples with visible oocyst in flotations could be a result of a failure to break oocyst walls during DNA extractions and/or faecal PCR inhibition (Raj et al., 2013). Importantly, tested but couldn't find any species-specific bias in both methods making e.g. relative species prevalences reliable.
Traditional identification of Eimeria, depends on the expertise to recognise the morphology of sporulated oocyst (Levine and Ivens, 1965). We show that interpretation of morphometrical data is complex due overlap between species while measurement means agree with literature (Table 1). Considering the challenges of identification and characterisation of Eimeria isolates from field samples, we conclude that characterisation of Eimeria species requires molecular markers and phylogenetic analysis.
Sequence identity of our isolates to reference sequences from Eimeria species previously described in M. musculus was above 98% for COI, which is sometimes assumed sufficient correspond differences within species of Eimeria (Yang et al., 2015). We confirm taxonomic assignment based on highly supported maximum likelihood and Bayesian phylogenetic clustering of 18S and COI sequences. Moreover, the three identity of the three Eimeria species was supported by phenotypic characteristics: morphometry of oocysts and tissue occurrence of the infection.
For some Eimeria species precise tissue localization is described based on histology or electron microscopy (Ankrom et al., 1975; Šlapeta et al., 2001). Both methods provide detailed information on developmental stages, but are also time consuming and require a high level of expertise. As an alternative to determine (only) the rough occurrence of the infection along the proximal-distal axis of the intestine, a DNA based qPCR method allowed us not only to detect the presence of Eimeria, but also to estimate tissue specific intensity of infection. The qPCR targets a single-copy nuclear gene from the host and a mitochondrial gene of the parasite present in multiple copies (up to 180; Heitlinger et al., 2014) to increase sensitivity for Eimeria detection.
While infection with rodent Eimeria in general can be limited to the duodenum and jejunum (Kvičerová et al., 2007), house mouse Eimeria have been described to be mostly found in either the small or the large intestine (Ankrom et al., 1975; Ernst et al., 1971; Haberkorn, 1970; Levine and Ivens, 1965). Using ileum, as the most distal part of the small intestine, and cecum, as the most proximal of the large intestine, we aimed to provide the most stringent test for differences in the site of infection possible: strong infections can be expected to spread in the neighboring tissue, but one could still expect the primary tissue to be more strongly infected. Additionally, genotyping DNA derived from these tissue allowed to detect double infections with E. vermiformis in the small intestine and E. ferrisi in the large intestine. Localization generally agrees with previous descriptions for the isolates we identified as E. ferrisi, E. falciformis and E. vermiformis by phylogenetic clustering. Reports of co-infections has been done previously in A. sylvaticus from the same colony (Higgs and Nowell, 2000) or in large populations of grey and red squirrels (Hofmannová et al., 2016). To our knowledge we provide the first report of double infections in wild populations of Mus musculus.
Double infections can be problematic for identification of species by genotyping. Simultaneous infection of the cecum with E. ferrisi and E. falciformis would not be recognized with our qPCR method. We shown that amplification of COI with the commonly used primer pair Cocci_COI (Ogedengbe et al., 2011) is differentially efficient for different Eimeria isolates. This primer preference can lead to a misidentification in double infections due to the generation of “chimeric” isolates that present different and contradictory information for different markers. Attention to such discrepancies is needed when collating database sequences and when developing multi-marker approaches in general. For rodent Eimeria systems, we develop an alternative COI primer pair and find no evidence for differential amplification bias in our cross-validation of the different primer pairs.
E. ferrisi is by far the most prevalent species in our study area infecting M. musculus. Concerning the house mouse hybrid zone we find infections in M. m. domesticus, M. m. musculus and hybrids, suggesting that there are no strict geographical or host subspecies constraints for this species. Population structure for E. ferrisi (which could in turn correspond to host subspecies (Goüy de Bellocq et al., 2018; Kváč et al., 2013) can not be found at the resolution the analysed markers provide.
We found that prevalences of E. ferrisi and E. falciformis increase with increasing host density at the level of farms. This is in agreement with predictions from epidemiology that in large and dense populations contact rates increase (Tompkins et al., 2011) and microoganisms with direct transmission become more prevalent. Such prevalence – host density relationships have been well documented for Hantavirus infections in Bank vole (My. glareolus) (Adler et al., 2008; Khalil et al., 2014; Sauvage et al., 2003). In free-living populations of house mice increased host density has been observed to result in higher prevalences of Murine Cytomegalovirus (MCMV) (Singleton et al., 2000). For eukaryotic parasite the prevalence of cestodes and nematodes has been described to be host density dependent in wild and laboratory rodents (Haukisalmi and Henttonen, 1990; Scott and Lewis, 1987). We here document such host density – prevalence relationship for the first time at a species level in Eimeria of house mice.
We suggest that species level identification of parasites in wildlife systems will help to assess such questions in more detail and is absolutely required for other questions. For example virulence-prevalence trade-off (Anderson and May 1982; Frank, 1996) can only be assessed at the species level. In our system one would predict a lower virulence for the prevalent E. ferrisi compared to E. falciformis and E. vermiformis. We have indications from laboratory experiments that such a lower virulence of E. ferrisi might be observed compared to E. falciformis (Al-khlifeh et al., 2019), while contrary results have been reported before (Tilahun and Stockdale, 1981). We consider this an observation warranting further research.
In conclusion we argue that Eimeria in wildlife populations should be identified more frequently at the level of species previously described by taxonomists. We propose to integrate a set of simple methods into a reproducible procedure to achieve this aim. For Coccidians, as important parasites of vertebrates, only species specific assessment will allow to test hypotheses in evolution, ecology and epidemiology.
Note
Supplementary data associated with this article.
Conflicts of interest
The author(s) declare(s) that there is no conflict of interest.
Acknowledgements
The authors acknowledge our collaboration partners Prof. Dr. Jaroslav Piálek and his team from the Institute of Vertebrate Biology, AS CR, Brno, Department of population Biology in Studenec, Czech republic for their valuable help during the collection of samples. To Deborah Dymke, Thi Phuong Le and Julia Murata for their assistance during the processing within Heitlinger Group.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.ijppaw.2019.07.004.
Funding
This work was supported by the German Foundation of Scientific Research (DFG) [grant number: 285969495/HE 7320/2-1] and the German Academic Exchange Service (DAAD) [VHJD scholarship holder] and the Research Training Group 2046 ″Parasite Infections: From Experimental Models to Natural Systems” [VHJD associated student].
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- Adler F.R., Pearce-Duvet J.M.C., Dearing M.D. How host population dynamics translate into time-lagged prevalence: an Investigation of Sin Nombre virus in deer mice. Bull. Math. Biol. 2008;70:236–252. doi: 10.1007/s11538-007-9251-8. [DOI] [PubMed] [Google Scholar]
- Ahmed N., Heitlinger E., Affinass N., Kühl A.A., Xenophontos N., Jarquin V.H., Jost J., Steinfelder S., Hartmann S. A novel non-invasive method to detect RELM beta transcript in gut barrier related changes during a gastrointestinal nematode infection. Front. Immunol. 2019;10:1–11. doi: 10.3389/fimmu.2019.00445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al-khlifeh E., Balard A., Jarquín-Díaz V.H., Weyrich A., Wibbelt G., Heitlinger E. Eimeria falciformis BayerHaberkorn1970 and novel wild derived isolates from house mice : differences in parasite lifecycle , pathogenicity and host immune reactions. bioRxiv. 2019:1–33. doi: 10.1101/611277. [DOI] [Google Scholar]
- Anderson R.M., May R.M. Coevolution of hosts and parasites. Parasitology. 1982;85:411–426. doi: 10.1017/S0031182000055360. [DOI] [PubMed] [Google Scholar]
- Ankrom S.L., Chobotar B., Ernest J.V. Life cycle of Eimeria ferrisi levine & Ivens, 1965 in the mouse, Mus musculus. J. Protozool. 1975;22:317–323. doi: 10.1111/j.1550-7408.1975.tb05177.x. [DOI] [Google Scholar]
- Ball S.J., Lewis D.C. Eimeria (Protozoa: coccidia) in wild populations of some British rodents S. J. Zool. 1984;202:373–381. doi: 10.1111/j.1469-7998.1984.tb05089.x. [DOI] [Google Scholar]
- Ballweber L.R., Beugnet F., Marchiondo A.A., Payne P.A. American Association of Veterinary Parasitologists' review of veterinary fecal flotation methods and factors influencing their accuracy and use—is there really one best technique? Vet. Parasitol. 2014;204:73–80. doi: 10.1016/j.vetpar.2014.05.009. [DOI] [PubMed] [Google Scholar]
- Becker E.R. JAMA: the Journal of the American Medical Association. Akademiai Kiado. Verlag Paul Parey; Berlin: 1934. Coccidia and coccidiosis of domesticated, game and laboratory animals and of man. Budapest, Hungary. [DOI] [Google Scholar]
- Boursot P., Auffray J.-C., Britton-Davidian J., Bonhomme F. The evolution of house mice. Annu. Rev. Ecol. Systemat. 2003;24:119–152. doi: 10.1146/annurev.es.24.110193.001003. [DOI] [Google Scholar]
- Ďureje L., Macholán M., Baird S.J.E., Piálek J. The mouse hybrid zone in Central Europe: from morphology to molecules. Folia Zool. 2012;61:308–318. doi: 10.25225/fozo.v61.i3.a13.2012. [DOI] [Google Scholar]
- Duszynski D.W. ELS. John Wiley & Sons, Ltd; Chichester, UK: 2011. Eimeria; pp. 1192–1196. [DOI] [Google Scholar]
- Duszynski D.W., Wilber P.G. A guidelines for the preparation of species description in the Eimeriidae. J. Parasitol. 1997;83:333–336. doi: 10.2307/3284470. [DOI] [PubMed] [Google Scholar]
- Edgar R.C. MUSCLE: multiple sequence alignment with high accuracy and high throughput. Nucleic Acids Res. 2004;32:1792–1797. doi: 10.1093/nar/gkh340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehret T., Torelli F., Klotz C., Pedersen A.B., Seeber F. Translational rodent models for research on parasitic Protozoa—a review of confounders and possibilities. Front. Cell. Infect. Microbiol. 2017;7 doi: 10.3389/fcimb.2017.00238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eimer T. Ueber die ei-oder kugelförmigen sogenannten Psorospermien der Wirbelthiere: ein Beitrag Zur Entwicklungsgeschichte Der Gregarinen Und Zur Kenntniss Dieser Parasiten Als Krankheitsursache. A. Stuber. 1870 [Google Scholar]
- Ernst J.V., Chobotar B., Hammond D.M. The Oocysts of Eimeria vermiformis sp. n. and E. papillata sp. n. (Protozoa: eimeriidae) from the Mouse Mus musculus. J. Protozool. 1971;18:221–223. doi: 10.1111/j.1550-7408.1971.tb03311.x. [DOI] [PubMed] [Google Scholar]
- Fernandez S., Pagotto A.H., Furtado M.M., Katsuyama Â.M., Madeira A.M.B.N., Gruber A. A multiplex PCR assay for the simultaneous detection and discrimination of the seven Eimeria species that infect domestic fowl. Parasitology. 2003;127:317–325. doi: 10.1017/S0031182003003883. [DOI] [PubMed] [Google Scholar]
- Figueiredo-Campos P., Ferreira C., Blankenhaus B., Veldhoen M. Eimeria vermiformis infection model of murine small intestine. BIO-PROTOCOL. 2018;8 doi: 10.21769/BioProtoc.3122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher R.A. On the interpretation of χ 2 from contingency tables, and the calculation of P. J. R. Stat. Soc. 1922;85:87. doi: 10.2307/2340521. [DOI] [Google Scholar]
- Frank S.A. Models of parasite virulence. Q. Rev. Biol. 1996;71:37–78. doi: 10.1086/419267. [DOI] [PubMed] [Google Scholar]
- Golemanski V.G. The coccidia (coccidia, eimeriidae) of small mammals from the parangalitsa, ropotamo and srebirna reserves in Bulgaria. Acta Zool. Bulg. 1979;12:12–26. [Google Scholar]
- Goüy de Bellocq J., Ribas A., Bryja J., Piálek J., Baird S.J.E. Holobiont suture zones: parasite evidence across the European house mouse hybrid zone. Mol. Ecol. mec. 2018:14938. doi: 10.1111/mec.14938. [DOI] [PubMed] [Google Scholar]
- Guindon S., Dufayard J.-F., Lefort V., Anisimova M., Hordijk W., Gascuel O. New algorithms and mehtods to estimate maximum-likelihood phylogenies: asessing the performance of PhyML 2.0. Syst. Biol. 2010;59:307–321. doi: 10.1093/sysbio/syq010. [DOI] [PubMed] [Google Scholar]
- Haberkorn A. Die Entwicklung von Eimeria falciformis (Eimer 1870) in der weissen Maus (Mus musculus) Z. für Parasitenkd. 1970;34:49–67. doi: 10.1007/BF00629179. [DOI] [Google Scholar]
- Haukisalmi V., Henttonen H. The impact of climatic factors and host density on the long-term population dynamics of vole helminths. Oecologia. 1990;83:309–315. doi: 10.1007/BF00317553. [DOI] [PubMed] [Google Scholar]
- Heitlinger E., Spork S., Lucius R., Dieterich C. The genome of Eimeria falciformis - reduction and specialization in a single host apicomplexan parasite. BMC Genomics. 2014;15:696. doi: 10.1186/1471-2164-15-696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higgs S., Nowell F. Population biology of Eimeria (Protozoa: Apicomplexa) in Apodemus sylvaticus: a capture/recapture study. Parasitology. 2000;120:355–363. doi: 10.1017/S0031182099005545. [DOI] [PubMed] [Google Scholar]
- Hnida J.A., Duszynski D.W. Taxonomy and systematics of some Eimeria species of murid rodents as determined by the ITS1 region of the ribosomal gene complex. Parasitology. 1999;119:349–357. doi: 10.1017/S0031182099004849. [DOI] [PubMed] [Google Scholar]
- Hobbs R.P., Twigg L.E., Elliot A.D., Wheeler A.G. Factors influencing the fecal egg and oocyst counts of parasites of wild european rabbits Oryctolagus cuniculus (L.) in southern western Australia. J. Parasitol. 1999;85:796–802. [PubMed] [Google Scholar]
- Hofmannová L., Romeo C., Štohanzlová L., Jirsová D., Mazzamuto M.V., Wauters L.A., Ferrari N., Modrý D. Diversity and host specificity of coccidia (Apicomplexa: eimeriidae) in native and introduced squirrel species. Eur. J. Protistol. 2016;56:1–14. doi: 10.1016/j.ejop.2016.04.008. [DOI] [PubMed] [Google Scholar]
- Houdebine L.-M. Elsevier Ltd; 2004. Chapter 6 - the Mouse as an Animal Model for Human Diseases BT - the Laboratory Mouse, the Laboratory Mouse. [DOI] [Google Scholar]
- Hu X.L., Liu G., Wang W.X., Zhou R., Liu S.Q., Li L.H., Hu D.F. Methods of preservation and flotation for the detection of nematode eggs and coccidian oocysts in faeces of the forest musk deer. J. Helminthol. 2016;90:680–684. doi: 10.1017/S0022149X15000942. [DOI] [PubMed] [Google Scholar]
- Huelsenbeck J.P., Ronquist F. MrBayes: bayesian inference of phylogenetic trees. Bioinformatics. 2001;17:754–755. doi: 10.1093/bioinformatics/17.8.754. [DOI] [PubMed] [Google Scholar]
- Kawahara F., Zhang G., Mingala C.N., Tamura Y., Koiwa M., Onuma M., Nunoya T. Genetic analysis and development of species-specific PCR assays based on ITS-1 region of rRNA in bovine Eimeria parasites. Vet. Parasitol. 2010;174:49–57. doi: 10.1016/j.vetpar.2010.08.001. [DOI] [PubMed] [Google Scholar]
- Khalil H., Hörnfeldt B., Evander M., Magnusson M., Olsson G., Ecke F. Dynamics and drivers of hantavirus prevalence in rodent populations. Vector Borne Zoonotic Dis. 2014;14:537–551. doi: 10.1089/vbz.2013.1562. [DOI] [PubMed] [Google Scholar]
- Kváč M., McEvoy J., Loudová M., Stenger B., Sak B., Květoňová D., Ditrich O., Rašková V., Moriarty E., Rost M., Macholán M., Piálek J. Coevolution of Cryptosporidium tyzzeri and the house mouse (Mus musculus) Int. J. Parasitol. 2013;43:805–817. doi: 10.1016/j.ijpara.2013.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kvičerová J., Pakandl M., Hypša V. Phylogenetic relationships among Eimeria spp. (Apicomplexa, Eimeriidae) infecting rabbits: evolutionary significance of biological and morphological features. Parasitology. 2008;135:443–452. doi: 10.1017/S0031182007004106. [DOI] [PubMed] [Google Scholar]
- Kvičerová J., Ptáčková P., Modrý D. Endogenous development, pathogenicity and host specificity of Eimeria cahirinensis couch, blaustein, Duszynski, shenbrot and Nevo, 1997 (Apicomplexa: eimeriidae) from Acomys dimidiatus (cretzschmar 1826) (rodentia: muridae) from the near east. Parasitol. Res. 2007;100:219–226. doi: 10.1007/s00436-006-0251-7. [DOI] [PubMed] [Google Scholar]
- Levine N.D., Ivens V. Cross-transmission of Eimeria spp. (Protozoa, Apicomplexa) of rodents -a review. J. Protozool. 1988;35:434–437. doi: 10.1111/j.1550-7408.1988.tb04124.x. [DOI] [PubMed] [Google Scholar]
- Levine N.D., Ivens V. The University of Ilinois Press; Urbana, Ill: 1965. The Coccidian Parasites (Protozoa, Sporozoa) of Rodents. [Google Scholar]
- Long J. 2019. Jtools: Analysis and Presentation of Social Scientific Data. [Google Scholar]
- Long P.L., Joyner L.P. Problems in the identification of species of Eimeria. J. Protozool. 1984;31:535–541. doi: 10.1111/j.1550-7408.1984.tb05498.x. [DOI] [PubMed] [Google Scholar]
- Mácová A., Hoblíková A., Hypša V., Stanko M., Martinů J., Kvičerová J. Mysteries of host switching: diversification and host specificity in rodent-coccidia associations. Mol. Phylogenetics Evol. 2018;127:179–189. doi: 10.1016/j.ympev.2018.05.009. [DOI] [PubMed] [Google Scholar]
- Mesfin G.M., Bellamy J.E., Stockdale P.H. The pathological changes caused by Eimeria falciformis var pragensis in mice. Can. J. Comp. Med. Rev. Can. Med. Comp. 1978;42:496–510. [PMC free article] [PubMed] [Google Scholar]
- Moro D., Lawson M.A., Hobbs R.P., Thompson R.C.A. Pathogens of house mice on arid boullanger island and subantartic macquarie island, Australia. J. Wildl. Dis. 2003;39:762–771. doi: 10.7589/0090-3558-39.4.762. [DOI] [PubMed] [Google Scholar]
- Morris G.M., Woods W.G., Grant Richards D., Gasser R.B. The application of a polymerase chain reaction (PCR)-based capillary electrophoretic technique provides detailed insights into Eimeria populations in intensive poultry establishments. Mol. Cell. Probes. 2007;21:288–294. doi: 10.1016/j.mcp.2007.03.001. [DOI] [PubMed] [Google Scholar]
- Nishioka Y. The origin of common laboratory mice. Genome. 2011;38:1–7. doi: 10.1139/g95-001. [DOI] [PubMed] [Google Scholar]
- Nunes M.S., Heuer T.C., Marshall J., Sanchez J., Thornton R., Reiczigel J., Robison-Cox J., Sebastiani P., Solymos P., Yoshida K., Jones G., Pirikahu S., Firestone S., Kyle R., Popp J., Jay M. 2018. epiR: Tools for the Analysis of Epidemiological Data. [Google Scholar]
- Ogedengbe J.D., Hanner R.H., Barta J.R. DNA barcoding identifies Eimeria species and contributes to the phylogenetics of coccidian parasites (Eimeriorina, Apicomplexa, Alveolata) Int. J. Parasitol. 2011;41:843–850. doi: 10.1016/j.ijpara.2011.03.007. [DOI] [PubMed] [Google Scholar]
- Owen D. Some parasites and other organisms of wild rodents in the vicinity of an SPF unit. Lab. Anim. 1976;10:271–278. doi: 10.1258/002367776781035314. [DOI] [PubMed] [Google Scholar]
- Parker S.E., Malone S., Bunte R.M., Smith A.L. Infectious diseases in wild mice (Mus musculus) collected on and around the University of Pennsylvania (Philadelphia) Campus. Comp. Med. 2009;59:424–430. [PMC free article] [PubMed] [Google Scholar]
- Perkins F.O., Barta J.R., Clopton R.E., Peirce M.A., Upton S.J. Apicomplexa. In: Lee J., Leedale G., Bradbury P., editors. The Illustrated Guide to the Protozoa. Allen Press; 2000. pp. 190–304. [Google Scholar]
- Phifer-Rixey M., Nachman M.W. Insights into mammalian biology from the wild house mouse Mus musculus. Elife. 2015;2015:1–13. doi: 10.7554/eLife.05959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Posada D. jModelTest: phylogenetic model averaging. Mol. Biol. Evol. 2008;25:1253–1256. doi: 10.1093/molbev/msn083. [DOI] [PubMed] [Google Scholar]
- R Development Core Team . R Foundation for Statistical Computing; Vienna, Austria: 2008. R: A language and environment for statistical computing.http://www.R-project.org ISBN 3-900051-07-0. [Google Scholar]
- Raj G.D., Aarthi S., Selvabharathi R., Raman M., Blake D.P., Tomley F.M. Real-time PCR-based quantification of Eimeria genomes: a method to outweigh underestimation of genome numbers due to PCR inhibition. Avian Pathol. 2013;42:304–308. doi: 10.1080/03079457.2013.790531. [DOI] [PubMed] [Google Scholar]
- Rambaut A. 2012. FigTree. [Google Scholar]
- Rinaldi L., Coles G.C., Maurelli M.P., Musella V., Cringoli G. Calibration and diagnostic accuracy of simple flotation, McMaster and FLOTAC for parasite egg counts in sheep. Vet. Parasitol. 2011;177:345–352. doi: 10.1016/j.vetpar.2010.12.010. [DOI] [PubMed] [Google Scholar]
- Ronquist F., Teslenko M., Van Der Mark P., Ayres D.L., Darling A., Höhna S., Larget B., Liu L., Suchard M. a., Huelsenbeck J.P. Mrbayes 3.2: efficient bayesian phylogenetic inference and model choice across a large model space. Syst. Biol. 2012;61:539–542. doi: 10.1093/sysbio/sys029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rose M.E., Millard B.J. Eimeria vermiformis: host strains and the developmental cycle. Exp. Parasitol. 1985;60:285–293. doi: 10.1016/0014-4894(85)90033-5. [DOI] [PubMed] [Google Scholar]
- Rose M.E., Wakelin D., Hesketh P. Eimeria vermiformis: differences in the course of primary infection can be correlated with lymphocyte responsiveness in the BALB/c and C57BL/6 mouse, Mus musculus. Exp. Parasitol. 1990;71:276–283. doi: 10.1016/0014-4894(90)90032-8. [DOI] [PubMed] [Google Scholar]
- Ryley J.F., Meade R., Hazelhurst J., Robinson T.E. Methods in coccidiosis research: separation of oocysts from faeces. Parasitology. 1976;73:311–326. doi: 10.1017/S0031182000046990. [DOI] [PubMed] [Google Scholar]
- Sauvage F., Langlais M., Yoccoz N.G., Pontier D. Modelling hantavirus on virus persistence voles : the role of indirect transmission. J. Anim. Ecol. 2003;72:1–13. [Google Scholar]
- Schelzke K., Lucius R., Liesenfeld O., Steinfelder S., Pogonka T., Stange J., Papadakis K. CD8+ cells protect mice against reinfection with the intestinal parasite Eimeria falciformis. Microb. Infect. 2009;12:218–226. doi: 10.1016/j.micinf.2009.12.005. [DOI] [PubMed] [Google Scholar]
- Schito M.L., Barta J.R., Chobotar B. Comparison of four murine Eimeria species in immunocompetent and immunodeficient mice. J. Parasitol. 1996;82:255–262. doi: 10.2307/3284157. [DOI] [PubMed] [Google Scholar]
- Schmid M., Lehmann M.J., Lucius R., Gupta N. Apicomplexan parasite, Eimeria falciformis, co-opts host tryptophan catabolism for life cycle progression in mouse. J. Biol. Chem. 2012;287:20197–20207. doi: 10.1074/jbc.M112.351999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider A. Note sur la psorospermie oviforme du poulpe. Arch. Zool. Exp. Gen. 1875;4:4045. [Google Scholar]
- Schnitzler B.E., Thebo P.L., Tomley F.M., Uggla A., Shirley M.W. PCR identification of chicken Eimeria: a simplified read-out. Avian Pathol. 1999;28:89–93. doi: 10.1080/03079459995091. [DOI] [PubMed] [Google Scholar]
- Scott M.E., Lewis J.W. Population dynamics of helminth parasites in wild and laboratory rodents. Mamm Rev. 1987;17:95–103. doi: 10.1111/j.1365-2907.1987.tb00054.x. [DOI] [Google Scholar]
- Shirley M.W., Smith A.L., Tomley F.M. The biology of avian Eimeria with an emphasis on their control by vaccination. Adv. Parasitol. 2005;60:285–330. doi: 10.1016/S0065-308X(05)60005-X. [DOI] [PubMed] [Google Scholar]
- Singleton G.R., Smith A.L., Krebs C.J. The prevalence of viral antibodies during a large population fluctuation of house mice in Australia. Epidemiol. Infect. 2000;125:719–727. doi: 10.1017/S0950268800004945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Šlapeta J.R., Modrý D., Votýpka J., Jirku M., Oborník M., Lukeš J., Koudela B. Eimeria telekii n.sp. (Apicomplexa: coccidia) from lemniscomys striatus (rodentia: muridae): morphology, pathology and phylogeny. Parasitology. 2001;122:133–143. doi: 10.1017/S0031182001007259. [DOI] [PubMed] [Google Scholar]
- Sterne T.E. Some remarks on confidence or fiducial limits. Biometrika. 1954;41:275–278. doi: 10.1093/biomet/41.1-2.275. [DOI] [Google Scholar]
- Su Y.C., Fei A.C.Y., Tsai F.M. Differential diagnosis of five avian Eimeria species by polymerase chain reaction using primers derived from the internal transcribed spacer 1 (ITS-1) sequence. Vet. Parasitol. 2003;117:221–227. doi: 10.1016/j.vetpar.2003.07.028. [DOI] [PubMed] [Google Scholar]
- Tattersall F.H., Nowell F., Smith R.H. A review of the endoparasites of wild House Mice Mus domesticus. Mamm Rev. 1994;24:61–71. doi: 10.1111/j.1365-2907.1994.tb00135.x. [DOI] [Google Scholar]
- Tilahun G., Stockdale P.H.G. Oocyst production of four species of murine coccidia. Can. J. Zool. 1981;59:1796–1800. doi: 10.1139/z81-246. [DOI] [Google Scholar]
- Todd K.T., Jr., Lepp D.L. The life cycle of Eimeria vermiformis Ernst, chobotar and hammond, 1971 in the mouse Mus musculus. J. Protozool. 1971;18:332–337. doi: 10.1111/j.1550-7408.1971.tb03327.x. [DOI] [PubMed] [Google Scholar]
- Tompkins D.M., Dunn A.M., Smith M.J., Telfer S. Wildlife diseases: from individuals to ecosystems. J. Anim. Ecol. 2011;80:19–38. doi: 10.1111/j.1365-2656.2010.01742.x. [DOI] [PubMed] [Google Scholar]
- Vandenbergh J.G. Use of house mice in biomedical research. ILAR J. 2013;41:133–135. doi: 10.1093/ilar.41.3.133. [DOI] [Google Scholar]
- Vrba V., Blake D.P., Poplstein M. Quantitative real-time PCR assays for detection and quantification of all seven Eimeria species that infect the chicken. Vet. Parasitol. 2010;174:183–190. doi: 10.1016/j.vetpar.2010.09.006. [DOI] [PubMed] [Google Scholar]
- Webster K.A., Smith H.V., Giles M., Dawson L., Robertson L.J. Detection of Cryptosporidium parvum oocysts in faeces: comparison of conventional coproscopical methods and the polymerase chain reaction. Vet. Parasitol. 1996;61:5–13. doi: 10.1016/0304-4017(95)00811-X. [DOI] [PubMed] [Google Scholar]
- Yakimoff W.L., Gousseff W.F. The coccidia of mice Mus musculus. Parasitology. 1938;30:26–28. [Google Scholar]
- Yang H., Bell T.A., Churchill G.A., Pardo-Manuel De Villena F. On the subspecific origin of the laboratory mouse. Nat. Genet. 2007;39:1100–1107. doi: 10.1038/ng2087. [DOI] [PubMed] [Google Scholar]
- Yang R., Brice B., Ryan U. Morphological and molecular characterization of Eimeria haematodi, coccidian parasite (Apicomplexa: eimeriidae) in a rainbow lorikeet (Trichoglossus haematodus) Exp. Parasitol. 2015;153:123–128. doi: 10.1016/j.exppara.2015.03.005. [DOI] [PubMed] [Google Scholar]
- Zhao X., Duszynski D. Molecular phylogenies suggest the oocyst residuum can be used to distinguish two independent lineages of Eimeria spp in rodents. Parasitol. Res. 2001;87:638–643. doi: 10.1007/s004360100393. [DOI] [PubMed] [Google Scholar]
- Zhao X., Duszynski D.W. Phylogenetic relationships among rodent Eimeria species determined by plastid ORF470 and nuclear 18S rDNA sequences. Int. J. Parasitol. 2001;31:715–719. doi: 10.1016/S0020-7519(01)00136-9. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.