Abstract
Background
Globally, most deaths due to childhood pneumonia occur at the community level. Some countries are still using oral co-trimoxazole, despite a World Health Organization recommendation of oral amoxicillin for the treatment of fast-breathing pneumonia in children at the community level.
Methods
We conducted an unblinded, cluster-randomized, controlled-equivalency trial in Haripur District, Pakistan. Children 2–59 months of age with fast-breathing pneumonia were treated with oral amoxicillin suspension (50 mg/kg/day) for 3 days in 14 intervention clusters and oral co-trimoxazole suspension (8 mg trimethoprim/kg and 40 mg sulfamethoxazole/kg/day) for 5 days in 14 control clusters by lady health workers (LHW). The primary outcome was treatment failure by day 4 for intervention clusters and by day 6 for control clusters. The analysis was per protocol.
Results
Out of the 15 749 cases enrolled in the study, 9153 cases in intervention and 6509 cases in control clusters were included in the analysis. Treatment failure rates were 3.6% (326) in intervention clusters and 9.1% (592) in control clusters. After adjusting for clustering, the risk of treatment failure was lower in intervention clusters (risk difference [RD] -5.5%, 95% confidence interval [CI] -7.4–-3.7%) than in control clusters. Children with incomplete adherence had a small increase in treatment failure versus those with complete adherence (RD 2.9%, 95% CI 1.6–4.1%). No deaths or serious adverse events occurred.
Conclusions
A 3-day course of oral amoxicillin, administered by LHWs, is an effective and safe treatment for fast-breathing pneumonia in children 2–59 months of age. A shorter course of amoxicillin improves adherence to therapy, is low in cost, and puts less pressure on antimicrobial resistance.
Clinical Trials Registration
ISRCTN10618300.
Keywords: community treatment, fast-breathing pneumonia, oral amoxicillin, short-course therapy, cluster-randomized trial
This cluster-randomized trial compared 3-day oral amoxicillin versus 5-day oral co-trimoxazole for the at-home treatment of fast-breathing pneumonia among children aged 2–59 months by community health workers in Pakistan. Oral amoxicillin treatment was significantly better than oral co-trimoxazole treatment.
Pneumonia causes over 900000 child deaths per year globally [1]. Over 120 million cases of pneumonia occur annually, of which 12% may require hospitalization [2]. Approximately 63% children under 5 years of age with pneumonia symptoms are taken to an appropriate provider [3].
Community health workers (CHWs) manage children 2–59 months of age with pneumonia using community case management (CCM), which increases access to treatment and reduces pneumonia-specific mortality [4, 5]. Current World Health Organization (WHO)/United Nations International Children’s Emergency Fund guidelines for integrated CCM recommend 5 days of treatment with oral amoxicillin for fast-breathing pneumonia (previously classified as pneumonia) [6, 7]. Despite this, countries currently using oral co-trimoxazole as the first-line pneumonia treatment are reluctant to shift to amoxicillin, due to its higher cost. Shortening the duration of amoxicillin treatment from 5 to 3 days will reduce the cost, but evidence is needed for its effectiveness at the community level.
This question was addressed by 3 studies in outpatient health facility settings by comparing 3 days of oral amoxicillin with 5 days in children 2–59 months with fast-breathing pneumonia, and the differing durations were found to be equivalent [8–10]. The WHO currently recommends that fast-breathing pneumonia be treated with 3 days of oral amoxicillin on an outpatient basis in low–human immunodeficiency virus settings [11, 12]. No published data exist on the effectiveness of 3-day oral antibiotics for fast-breathing pneumonia treatment by CHWs.
We undertook 2 parallel trials in the Haripur District of Pakistan: 1 to assess the management of chest-indrawing pneumonia (reported elsewhere) [13] and the other to assess the management of fast-breathing pneumonia in children 2–59 months of age by CHWs, called lady health workers (LHWs). We report here the results of the fast-breathing pneumonia trial.
METHODS
Study Design and Intervention
We conducted a cluster-randomized, equivalency trial among children 2 to 59 months of age with WHO-defined fast-breathing pneumonia. Children were treated by LHWs with 3 days of oral amoxicillin in intervention clusters and 5 days of oral co-trimoxazole in control clusters.
Study Setting and Population
Details of the setting, the population, and the LHW program have been previously reported [13] (see Supplementary Material Box 1).
Randomization and Masking
The severe pneumonia study [13] and this study had the same population, study period, and randomization technique. Clusters were defined as the 44 union councils within the Haripur district. We randomized at the cluster level because the intervention was focused on training lady health workers, and any training would likely have affected the provision of care to control patients had we randomized at the individual-patient level. We excluded 16 union councils that were located in an urban area [7] or were inaccessible [9], and randomized 28 clusters with 511 LHWs. A WHO expert not associated with the trial performed the stratified randomization of clusters using STATA (version 10.0). Stratification was based on child population and mortality and literacy rates of mothers [14]. This was done to attempt to minimize confounding. Caregivers and study personnel were not masked to the treatment assignment.
Screening and Enrolment
LHWs screened all children 2–59 months of age presenting with a cough and fast or difficult breathing (see Table 1 for eligibility criteria). A single, respiratory-rate cut-off of ≥50 breaths per minute was used in the trial, in accordance with the National LHW program guideline for the classification of fast-breathing pneumonia [15].
Table 1.
Inclusion and Exclusion Criteria
| Inclusion criteria |
| • Child aged 2 to 59 months, presenting to lady health workers with fast-breathing pneumonia (cough and/or difficulty breathing, with a respiratory rate of 50 breaths or more); and |
| • Residing in the study area (ie, intervention or control clusters). |
| Exclusion criteria |
| • Chest-indrawing pneumonia (lower chest indrawing, regardless of respiratory rate, in children with a history of cough and/or difficulty breathing); |
| • Very severe disease (presence of any danger sign in a child with history of cough and/or difficult breathing: unable to drink/breastfeed; convulsions; vomits everything; abnormally sleepy/difficult to wake); |
| • Diarrhea, with severe dehydration; |
| • Severe clinical malnutrition; |
| • Previously enrolled in the study in the prior 2 weeks; |
| • Caretaker refusal to participate in the study; or |
| • Already on antibiotic treatment. |
Ethical Issues
Ethical approval was provided by the WHO Ethical Review Committee and the Technical Committee on Innovations of the National LHW program. Boston University’s Institutional Review Board gave approval for analysis of anonymized data. Children’s legal guardians provided written informed consent.
Case Management Training
From each of the 28 public health facilities, 2 health care providers (doctors, lady health visitors, or medical technicians), all previously trained in Integrated Management of Childhood Illness (IMCI), were provided with additional development as trainers in pneumonia case management by Master Trainers at the District Headquarter Hospital in Haripur.
The LHWs in the control (245) and intervention (266) clusters received training in the national program protocol for the management of fast-breathing pneumonia [15]. Intervention-cluster LHWs received additional training for the treatment of fast-breathing pneumonia with oral amoxicillin. Both groups also received standard training in the study procedures. More details have been reported elsewhere [13].
Treatment and Follow-up
In the control clusters, LHWs treated fast-breathing pneumonia as per the LHW program standard of care [15], using oral co-trimoxazole at 40 mg sulphamethoxazole/8mg trimethoprim/kg/day (200mg sulphamethoxazole/40mg trimethoprim/5 ml), given twice daily for 5 days. In the intervention clusters, LHWs treated fast-breathing pneumonia for 3 days with oral amoxicillin at 50mg/kg/day (250mg/5ml), given twice daily. LHWs followed up on all children, either in their homes or at their health house, on days 2 and 14 after enrollment for the assessment and recording of clinical outcomes on standardized forms. The LHWs also visited children in the intervention clusters on day 4 and in the control clusters on day 6 upon completion of therapy to assess their outcomes.
Adherence was defined as the receipt of all 6 doses of oral amoxicillin for the intervention-cluster patients and 10 doses of oral co-trimoxazole for the control-cluster patients. It was assessed during each follow-up visit by asking caregivers to confirm the quantity of medicine given for each dose and whether any dose was missed, and by checking the remaining antibiotic liquid in the bottle.
Supervision and Quality Assurance
Data collection assistants independently verified both the diagnoses of pneumonia among all cases (within 24 hours of enrollment by LHWs) and all treatment failures (Table 2). An independent assessor (study physician) not involved with the treatment of the child and not blinded to the cluster assignment verified 5% of all treatment-failure cases. Other details of data collection assistants’ training, supervision, follow-up, and efforts to maintain the skills of LHWs have been previously reported [13] (see Supplementary Material Box 1).
Table 2.
Treatment Failure Criteria
| Treatment failure |
| • Death; |
| • Appearance of any danger sign (unable to drink/breastfeed, convulsions, vomits everything, abnormally sleepy/difficult to wake) up to day 4 in intervention-cluster patients or day 6 in control-cluster patients; |
| • Appearance of lower chest indrawing anytime up to day 4 or 6; |
| • Change of antibiotic (through self-referral or by caregivers) anytime up to day 4 in intervention-cluster patients or day 6 in control-cluster patients; or |
| • Fast breathing (respiratory rate ≥50 breaths per minute) on day 4 in intervention clusters or day 6 in control clusters. |
| Relapse |
| After a child’s signs disappeared on day 4 (intervention) or 6 (control), relapse was defined as the reappearance, through day 14, of any of the following: |
| • Fast breathing (respiratory rate ≥ 50 breaths per minute); |
| • Lower chest indrawing; or |
| • Appearance of any danger sign (unable to drink/breastfeed, convulsions, vomits everything, abnormally sleepy/difficult to wake). |
Statistical Analysis
The primary outcome was treatment failure (Table 2) at the end of the course of treatment (day 4 in the amoxicillin group and day 6 for the co-trimoxazole group). This study was powered as an equivalency trial, assuming 90% power, an alpha of 0.05, a failure rate in the control clusters of 9% [10], and a design effect of 2 to account for the clustering [14]. We defined equivalence as an upper and lower 95% confidence interval (CI) between +/- 5% on the risk difference (RD). This implies that our risk difference would need to be close to 0% for the confidence limits to be within this range. The sample size for the chest-indrawing trial was 2772 cases [13]. As fast-breathing pneumonia is more common than chest-indrawing pneumonia, all eligible cases of fast-breathing pneumonia presenting to the health facilities during the chest-indrawing pneumonia trial period were enrolled.
The primary analysis was per protocol, as the study was designed as an equivalence trial, but we also conducted an intention-to-treat analysis. We compared the proportion of children with treatment failures by study arms, using RDs and 95% CIs. We accounted for clustering through the use of generalized estimating equations in a linear risk model, with each union council included as a cluster, and adjusted for confounding of covariates in our model.
Role of the Funding Source
The World Health Organization was funded by the United States Agency for International Development to support the study. The United States Agency for International Development had no role in the design, conduct, or analysis of this study. The corresponding author had full access to all study data and had final responsibility for the decision to submit for publication.
RESULTS
We assigned 14 union councils to the intervention arm (266 LHWs) and 14 to the control arm (245 LHWs; Figure 1). We had a similar mean number of LHWs per cluster (19 [range 9–30] vs 17 [range 8–24], respectively) and population served per cluster (18 146 [range 12 216–24 066] vs 18 359 [range 9930–28 000], respectively) in the intervention and control arms.
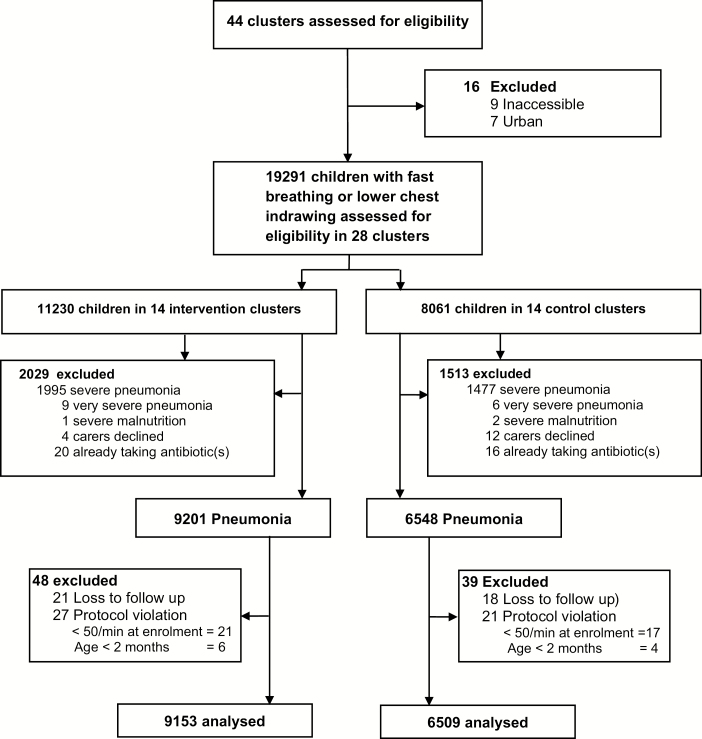
Figure 1.
Trial profile.
From April 2008 to December 2009, LHWs assessed children under 5 years of age who presented with fast breathing or chest indrawing, with 11 230 children in intervention clusters and 8061 children in control clusters (Figure 1). After exclusions, 9201 and 6548 cases of fast-breathing pneumonia were enrolled in the intervention and control clusters, respectively, and met the intention-to-treat analysis criteria. The median enrollment per cluster was higher in the intervention group (550 vs 380). After exclusion of those children lost to follow-up (21 intervention, 18 control) or protocol violations (27 intervention, 21 control), 9153 cases in the intervention clusters and 6509 cases in the control clusters were included in the per-protocol analysis. The loss to follow-ups and protocol violations were all included as negative outcomes for the intention-to-treat analysis.
The study arms were similar with respect to sex and other baseline characteristics, with minor differences in the age of children, the presence of very fast breathing, and temperatures in the 2 arms (Table 3). We found strong concordance (97%) between LHWs and the independent assessors for the respiratory rate on the day of enrollment.
Table 3.
Baseline Characteristics of Children Aged 2–59 Months With Fast-breathing Pneumonia in Intervention and Control Clusters
| Intervention n = 9153 |
Control n = 6509 |
|
|---|---|---|
| Boys | 4851 (53.0%) | 3444 (52.9%) |
| Age | ||
| Median (IQR) in months | 21 (12–36) | 19 (10–34) |
| <6 months | 799 (8.7%) | 749 (11.5%) |
| 6–11 months | 1682 (18.4%) | 1316 (20.2%) |
| 12–59 months | 6672 (72.9%) | 4444 (68.3%) |
| History of current illness | ||
| Cough | 8982 (98.1%) | 6414 (98.5%) |
| Difficult breathing | 7394 (80.8%) | 5623 (86.4%) |
| Fast breathing | 8817 (96.3%) | 6309 (96.9%) |
| Fever | 7211 (78.8%) | 5412 (83.1%) |
| Assessment on day 1 | ||
| Respiratory rate, as breaths per min, median (IQR) | 55 (53–58) | 55 (52–57) |
| Very fast breathinga | 53 (0.6%) | 55 (0.8%) |
| Temperature, in °F, median (IQR) | 100 (98–100) | 100 (98–101) |
| Enrollment per cluster, median (IQR) | 550 (471–746) | 380 (299–649) |
The intervention cluster received community treatment with 3 days of oral amoxicillin; the control cluster received community treatment with 5 days of oral co-trimoxazole. Data are given as n (%), unless otherwise indicated.
Abbreviation: IQR, interquartile range.
aRespiratory rate of at least 70 breaths per minute for children aged 2–11 months and at least 60 breaths per minute for children aged 12–59 months.
Study Outcomes
Overall, rates of treatment failure were low. By day 4, treatment failure among children in the intervention clusters (ie, receiving 3 days of amoxicillin treatment) was 3.6% (326/9153), versus 9.1% (592/6509) for the children in control clusters (receiving 5 days co-trimoxazole treatment; cluster-adjusted RD -5.5%, 95% CI -7.4–-3.7%; Table 4). Even though this was an equivalency trial, we found 3 days of oral amoxicillin treatment to be superior to 5 days of oral co-trimoxazole treatment. The results differed little in the intention-to-treat analysis (RD -5.6, 95% CI -7.6–-3.6%). The major reasons for treatment failure were the persistence of fast breathing, fever, or lower chest indrawing on days 4 and 6 in both groups and self-referral or a change of antibiotics by caregivers. After adjusting for baseline imbalances in age, very fast breathing, and fever, the risk difference increased slightly (RD -7.1, 95% CI -9.8–-4.4%).
Table 4.
Cluster-adjusted Treatment Failure by Day 4 (Primary Outcome Intervention) and Day 6 (Primary Outcome Control) in Children with Fast-breathing Pneumonia in the Intervention and Control Clusters
| Reason for Failure | Intervention (Amoxicillin Arm) | Control (Co-Trimoxazole Arm) | RD (95% CI)a |
|---|---|---|---|
| Treatment failure by day 4–6b | 326/9153 (3.6%) | 592/6509 (9.1%) | -5.5% (-7.3 to -3.7%) |
| Intention to treat failure by day 4–6 | 374/9201 (4.1%) | 631/6548 (9.6%) | -5.6% (-7.6 to -3.6%) |
| Reasons for failurec | |||
| Danger signs by day 4–6b | 2/9153 (0.0%) | 7/6509 (0.1%) | -0.09% (-0.16 to 0.00%) |
| Inability to drink | 0/9055 (0.0%) | 2/6459 (0.0%) | -0.03% (-0.07 to 0.01%) |
| Abnormally sleepy | 1/9055 (0.0%) | 2/6455 (0.0%) | -0.02% (-0.07 to 0.03%) |
| Vomits everything | 1/9058 (0.0%) | 5/6457 (0.1%) | -0.07% (-0.14 to 0.01%) |
| Convulsions | 0/9057 (0.0%) | 1/6457 (0.0%) | -0.02% (-0.05 to 0.02%) |
| Fever on day 4–6b | 87/8972 (1.0%) | 125/6471 (1.9%) | -0.96% (-1.68 to -0.00%) |
| Lower chest indrawing by day 4–6b |
66/9093 (0.7%) |
72/6489 (1.1%) |
-0.38% (-0.77 to -0.00%) |
| Fast breathing on day 4–6b,d | 187/9055 (2.1%) | 356/6487 (5.5%) | -3.42% (-4.83 to -2.01%) |
| Change of antibiotic by day 4–6b,e | 47/9153 (0.5%) | 188/6509 (2.9%) | -2.38% (-3.32 to -1. 43%) |
Abbreviations: CI, confidence interval; RD, risk difference.
aAdjusted for clustering using generalized estimating equations.
bDay 4 for amoxicillin group and day 6 for co-trimoxazole group.
cTotal failures is not equal to the sum of the individual failure reasons, as treatment could fail for more than 1 reason.
dRespiratory Rate = > 50 breaths per minute.
eSelf-referral or medication (antibiotic) by caregivers.
In the amoxicillin group, 9.8% (900/9153) of children did not complete the full course of treatment, vs 21.8% (1418/6509) of children in the co-trimoxazole group. In both the intervention and control clusters, treatment failure was 1.5 times higher among non-adherent patients, compared to adherent patients (Table 5).
Table 5.
Clinical Treatment Failure Rates Among Children With Fast-breathing Pneumonia Completing Full Treatment of Antibiotics and Those Not Completing Full Treatment, in Intervention and Control Clusters
| Treatment Compliance | Clinical Cure/Treatment Failure | Amoxicillin n (%) |
Co-Trimoxazole n (%) |
|---|---|---|---|
| Children completing full treatment |
Cured | 7974 (96.6) | 4679 (91.9) |
| Failure | 279 (3.4) | 412 (8.1) | |
| Total | 8253 (90.2) | 5091 (78.2) | |
| Children not completing full treatment |
Cured | 853 (94.8) | 1238 (87.3) |
| Failure | 47 (5.2) | 180 (12.7) | |
| Total | 900 (9.8) | 1418 (21.8) | |
| Overall | Cured | 8827 (96.4) | 5917 (90.9) |
| Failure | 326 (3.6) | 592 (9.1) | |
| Total | 9153 (100.0) | 6509 (100.0) |
We identified several predictors of treatment failure in a multivariable analysis (data not shown). In addition to failure to complete the full course of treatment (RD 2.9%, 95% CI 1.6–4.1%), children 2–5 months of age (RD 5.4%, 95% CI 3.9–6.9%) and 6–11 months of age (RD 3.2%, 95% CI 2.2–4.2%) had substantially more treatment failures than children 12–59 months of age.
There were no deaths among study patients. Of the 8827 children in the amoxicillin group who were well on day 4, 47 (0.5%) relapsed between days 4 and 14. In the co-trimoxazole group, of the 5917 children who were well on day 6, 52 (0.8%) relapsed between day 6 and 14.
Adverse Events
Overall adverse events were 2.5% (386/15 662). In the 234 (2.6%) children in the intervention group, 152 (1.7%) developed diarrhea, 77 (0.8%) developed a skin rash, and 5 (0.05%) began vomiting. In the 86 children in the control group, 49 (0.8%) developed diarrhea and 37 (0.6%) developed skin rashes. Among those with adverse events, therapy was changed in only 15 (0.2%) children in the intervention group and 13 (0.2%) in the control group.
DISCUSSION
Our study shows that the treatment of fast-breathing pneumonia by LHWs with 3 days of oral amoxicillin resulted in a significantly lower treatment failure rate than the standard of care of 5 days of oral co-trimoxazole. Adherence to treatment was also much higher in the 3-day amoxicillin-treatment group. In both the intervention and control groups, treatment failure was 1.5 times higher among those children who did not complete antibiotic treatment. Treatment with 3 days of amoxicillin was safe, as there were no deaths or adverse events needing therapy changes, and the relapse rate was very low.
The treatment failure rate in the amoxicillin group in our study was similar to the failure rate of 2.8% in the amoxicillin group of the placebo-controlled trial conducted in the squatter settlements of Karachi in infants less than 2 months of age with fast breathing [16]. The lower treatment rates in both studies probably are due to the availability of CHWs round the clock in the community, facilitating early care-seeking and the prompt initiation of treatment. The treatment failure rate in the co-trimoxazole group (9.1%) in our study was similar (9.5%) to the trial comparing 3 days of oral amoxicillin with 5 days of co-trimoxazole that was conducted in rural India [10]. Our findings are supportive of the previous outpatient trials that showed the equivalent effectiveness of 3 days and 5 days of oral amoxicillin treatment for fast-breathing pneumonia [8, 9]. Our treatment failure rate for the 3-day amoxicillin group was lower than that reported by the INDIACLEN Short Course Amoxicillin Pneumonia (ISCAP) [8] trial. Greenberg et al compared 3 days of amoxicillin treatment vs 5 days and 10 days of treatment in Israel for radiologically-proven pneumonia [17]. They found higher treatment failure rates in the 3-day group, as compared to the 5-day and 10-day groups. However, our study population differed from their study population, as our patients were enrolled in the community at an earlier stage of disease, whereas radiological changes appear later in the disease. A study that enrolled 2000 cases of fast-breathing pneumonia in 6 tertiary-care hospitals across Pakistan found radiological evidence of pneumonia in only 14% of cases [18].
In our study, in both the treatment groups, children under 12 months of age had a higher risk of treatment failure. In addition, children who were non-adherent to treatment were more likely to fail treatment than adherent children. The Multi-Centre Amoxicillin Short-Course Therapy (MASCOT) [8] and ISCAP trials [9] also found higher risks of treatment failure in non-adherent cases, while MASCOT also found an increased risk among children under 12 months of age.
Previously-reported data from Pakistan have shown high levels of in vitro antimicrobial resistance to co-trimoxazole [19, 20]. Additionally, treatment failure rates with co-trimoxazole in Streptococcus pneumoniae bacteraemic children with pneumonia were 28%, compared to 0% for amoxicillin [20]. As our study did not collect any blood samples or nasopharyngeal swabs for bacterial culture, we could not tell whether the difference in treatment failure rates between co-trimoxazole and amoxicillin was due to high antimicrobial resistance to co-trimoxazole or due to a difference in the efficacy of these 2 drugs for pneumonia.
There is concern that, because specificity of the WHO diagnostic criteria for pneumonia is sub-optimal [21], many children presenting with fast-breathing pneumonia may not have bacterial disease and, thus, may not need antibiotics [22], thereby increasing pressure on antimicrobial resistance [23]. We agree that some children with fast breathing may have viral pneumonia, while some may have a combination of viral and bacterial pneumonias. Awasthi et al reported a higher treatment failure rate with placebo compared to 3 days of amoxicillin for fast-breathing pneumonia in children 2–59 months of age [24]; similarly, Tikmani et al reported 2 times higher treatment failure for placebo, compared to amoxicillin, among infants under 2 months of age with fast breathing [16]. Data show that the WHO’s standard case management of pneumonia reduces antibiotic use through rational use [25, 26].
Shorter courses of amoxicillin therapy have substantial benefits for patients, families, and the health system. First, they could result in less pressure on antimicrobial resistance, as reported in Schrag et al [27]. Patients who received 5 days of treatment of amoxicillin had a lower risk of penicillin non-susceptible pneumococcal carriage, compared to those who received 10 days of therapy. ISCAP reported patients in the 5-day amoxicillin-treatment group had significantly higher rates of resistance to S. pneumoniae 15 days after enrollment, compared to the 3-day amoxicillin group [9]. Second, shorter courses of antibiotics could improve both treatment adherence and treatment outcomes, as reported by MASCOT [8], ISCAP [9], and our study. Finally, 3 days of amoxicillin treatment costs less, either to families if paid out of pocket or to the health system if provided free of charge to patients. ISCAP estimated that the average, direct medical cost for 3 days of therapy was 40% less than 5 days of therapy [9].
The main strengths of our study were that it was a cluster-randomized trial in a public-sector, community-based program in a rural setting, covering 2 pneumonia seasons. Second, we had a very large sample size, with minimal loss to follow-up. Third, we included independent confirmation of treatment failure and an assessment of adherence. The limitations included greater enrollment of cases in the intervention clusters, compared to control clusters, likely due to the communities’ greater familiarity and knowledge about the program in intervention clusters, where LHWs were also treating chest- indrawing pneumonia in the community [13]. Second, the study was not blinded, as it was not practical in a community trial. Third, there was higher treatment failure in the control group, due to change in antibiotic criterion, which could be due to the longer duration of treatment, which could motivate caregivers to seek other sources of treatment in case of persistence of symptoms. However, this demonstrates the benefit of short-course therapy. Finally, our study did not employ any microbiology tests or radiology to assist in the diagnoses of pneumonia, as it was not feasible to do so at the community level.
As our study was integrated into the existing, public-sector, community health delivery program, the results can be easily translated into policy. Pakistan has 1 of the highest under-5 child death rates globally, with pneumonia as the leading cause of death [1]. Immediate and effective measures are required to address it. Along with other preventive interventions highlighted in the Global Action Plan for Pneumonia and Diarrhea [28], making treatment available with low-cost, short-course antibiotics at the community level could improve pneumonia outcomes, prevent unnecessary deaths, and accelerate the pace of reduction in mortality among children under 5.
The WHO IMCI treatment for fast-breathing pneumonia in health facilities is oral amoxicillin for 3 days in low–human immunodeficiency virus settings [11], which has not yet been extended to CCM [29]. The inconsistency between IMCI and CCM for fast-breathing pneumonia may create confusion among caregivers and trainers of CHWs. Harmonization of treatment recommendations will reduce this confusion. LHWs were able to identify cases in the community early, leading to the prompt management and resolution of pneumonia cases and resulting in fewer treatment failures and no deaths. Extending the WHO recommendation of 3 days of amoxicillin therapy for fast-breathing pneumonia to CCM could improve access to effective, safe, and low-cost treatment for children.
Supplementary Data
Supplementary materials are available at Clinical Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Notes
S. S. and S. A. Q. conducted the literature review. S. S., A. B., D. M. T., Amanullah K., I. K., and S. A. Q. designed the study. S. S., I. uH. K., A. B., and Attaullah K. implemented the study. S. S., I. uH. K., A. B., Attaullah K., I. K., and I. A. supervised the study. S. S., I. uH. K., M. P. F., Attaullah K., and S. A. Q. analyzed the data. S. S., M. P. F., D. M. T., and S. A. Q. interpreted the data. S. S., M. P. F., D. M. T., Amanullah K., and S. A. Q. wrote the manuscript. I. uH. K. and Attaullah K. managed the data. D. M. T., I. K., I. A., and S. A. Q. monitored the study. S. S. had full access to all the data in the study and bears final responsibility for the decision to submit for publication.
Acknowledgments. The authors thank Habib-ur-Rehaman, Head of Pediatric Unit, District Head Quarter Hospital, Haripur; Farhat Yasmeen, District Coordinator, National Program of Family Planning & Primary Health Care, Haripur; Tabish Hazir, Retired Head of Pediatrics Department, Children Hospital, Pakistan Institute of Medical Sciences, Islamabad; and Saddiq ur-Rehman, Executive District Officer Health, Abbottabad.
Disclaimer. The content of this publication is solely the responsibility of the authors and does not necessarily reflect the views or policies of the National Institute of Allergy and Infectious Diseases, World Health Organization, or United States Agency for International Development, nor does the mention of trade names, commercial projects, or organizations imply an endorsement by the US Government.
Financial support. This work was supported by the Department of Child and Adolescent Health and Development of the World Health Organization (United States Agency for International Development). Save the Children team, which conducted the study, received funding from WHO under Technical Services Agreement C6-181-509. M. F. received an award from the National Institute of Allergy and Infectious Diseases (Award Number K01AI083097).
Potential conflicts of interest. All other authors report no potential conflicts. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. Liu L, Oza S, Hogan D, et al. Global, regional, and national causes of child mortality in 2000-13, with projections to inform post-2015 priorities: an updated systematic analysis. Lancet 2015; 385:430–40. [DOI] [PubMed] [Google Scholar]
- 2. Walker CLF, Rudan I, Liu L, et al. Global burden of childhood pneumonia and diarrhoea. Lancet 2013; 381:1405–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. United Nations International Children’s Emergency Fund. One is too many: Ending child deaths from pneumonia and diarrhoea. New York, New York: United Nations International Children’s Emergency Fund, 2016. [Google Scholar]
- 4. Sazawal S, Black RE; Pneumonia Case Management Trials Group Effect of pneumonia case management on mortality in neonates, infants, and preschool children: a meta-analysis of community-based trials. Lancet Infect Dis 2003; 3:547–56. [DOI] [PubMed] [Google Scholar]
- 5. Theodoratou E, Al-Jilaihawi S, Woodward F, et al. The effect of case management on childhood pneumonia mortality in developing countries. Int J Epidemiol 2010; 39(Suppl 1):i155–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. World Health Organization/United Nations International Children’s Emergency Fund. Manual for community health worker: Caring for sick child in the community. Geneva, Switzerland: World Health Organization, 2011. [Google Scholar]
- 7. World Health Organization. Integrated management of childhood illnesses: chart booklet. Geneva, Switzerland: World Health Organization, 2008. [Google Scholar]
- 8. Pakistan Multi-Centre Amoxicillin Short-Course Therapy (MASCOT) Pneumonia Study Group. Clinical efficacy of 3 days versus 5 days of oral amoxicillin for treatment of childhood pneumonia: a multicentre double blind trial. Lancet 2002; 360:835–41. [DOI] [PubMed] [Google Scholar]
- 9. ISCAP Study Group. Three days versus five day treatment with amoxicillin for non-severe pneumonia in young children: a multi-centre randomised controlled trial. BMJ 2004; 328:791–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Awasthi S, Agarwal G, Singh JV, et al. ; ICMR-IndiaClen Pneumonia Project Group Effectiveness of 3-day amoxycillin vs. 5-day co-trimoxazole in the treatment of non-severe pneumonia in children aged 2-59 months of age: a multi-centric open labeled trial. J Trop Pediatr 2008; 54:382–9. [DOI] [PubMed] [Google Scholar]
- 11. World Health Organization. Integrated management of childhood illnesses: chart booklet. Geneva, Switzerland: World Health Organization, 2014. [Google Scholar]
- 12. World Health Organization. Recommendations for management of common childhood conditions: evidence for technical update of pocket book recommendations: newborn conditions, dysentery, pneumonia, oxygen use and delivery, common causes of fever, severe acute malnutrition and supportive care. Geneva, Switzerland: World Health Organization, 2012. [PubMed] [Google Scholar]
- 13. Bari A, Sadruddin S, Khan A, et al. Community case management of severe pneumonia with oral amoxicillin in children aged 2-59 months in Haripur district, Pakistan: a cluster randomised trial. Lancet 2011; 378:1796–803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Hayes R, Moulton L.. Cluster randomized trials. Boca Raton, Florida: CRC Press, 2009. [Google Scholar]
- 15. Ministry of Health, Government of Pakistan. National program for family planning and primary health care. curriculum for lady health workers (Urdu). Islamabad, Pakistan: Ministry of Health, Government of Pakistan, 2008. [Google Scholar]
- 16. Tikmani SS, Muhammad AA, Shafiq Y, et al. Ambulatory treatment of fast breathing in young infants aged <60 days: a double-blind, randomized, placebo-controlled equivalence trial in low-income settlements of Karachi. Clin Infect Dis 2017; 64:184–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Greenberg D, Givon-Lavi N, Sadaka Y, Ben-Shimol S, Bar-Ziv J, Dagan R. Short-course antibiotic treatment for community-acquired alveolar pneumonia in ambulatory children: a double-blind, randomized, placebo-controlled trial. Pediatr Infect Dis J 2014; 33:136–42. [DOI] [PubMed] [Google Scholar]
- 18. Hazir T, Nisar YB, Qazi SA, et al. Chest radiography in children aged 2-59 months diagnosed with non-severe pneumonia as defined by World Health Organization: descriptive multicentre study in Pakistan. BMJ 2006; 333:629–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Mastro TD, Ghafoor A, Nomani NK, et al. Antimicrobial resistance of pneumococci in children with acute lower respiratory tract infection in Pakistan. Lancet 1991; 337:156–9. [DOI] [PubMed] [Google Scholar]
- 20. Straus WL, Qazi SA, Kundi Z, Nomani NK, Schwartz B. Antimicrobial resistance and clinical effectiveness of co-trimoxazole versus amoxycillin for pneumonia among children in Pakistan: randomised controlled trial. Pakistan Co-trimoxazole Study Group. Lancet 1998; 352:270–4. [DOI] [PubMed] [Google Scholar]
- 21. Rambaud-Althaus C, Althaus F, Genton B, D’Acremont V. Clinical features for diagnosis of pneumonia in children younger than 5 years: a systematic review and meta-analysis. Lancet Infect Dis 2015; 15:439–50. [DOI] [PubMed] [Google Scholar]
- 22. Hazir T, Nisar YB, Abbasi S, et al. Comparison of oral amoxicillin with placebo for the treatment of world health organization-defined nonsevere pneumonia in children aged 2-59 months: a multicenter, double-blind, randomized, placebo-controlled trial in pakistan. Clin Infect Dis 2011; 52:293–300. [DOI] [PubMed] [Google Scholar]
- 23. Klugman KP, Lonks JR. Hidden epidemic of macrolide-resistant pneumococci. Emerging Infectious Diseases 2005; 11:802–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Awasthi S, Agarwal G, Kabra SK, et al. Does 3-day course of oral amoxycillin benefit children of non-severe pneumonia with wheeze: a multicentric randomised controlled trial. PLoS One 2008; 3:e1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Qazi SA, Rehman GN, Khan MA. Standard management of acute respiratory infections in a children’s hospital in Pakistan: impact on antibiotic use and case fatality. Bull World Health Organ 1996; 74:501–7. [PMC free article] [PubMed] [Google Scholar]
- 26. Gouws E, Bryce J, Habicht JP, et al. Improving antimicrobial use among health workers in first-level facilities: results from the multi-country evaluation of the Integrated Management of Childhood Illness strategy. Bull World Health Organ 2004; 82:509–15. [PMC free article] [PubMed] [Google Scholar]
- 27. Schrag SJ, Peña C, Fernández J, et al. Effect of short-course, high-dose amoxicillin therapy on resistant pneumococcal carriage: a randomized trial. JAMA 2001; 286:49–56. [DOI] [PubMed] [Google Scholar]
- 28. World Health Organization. End preventable deaths: Global action plan for prevention and control of pneumonia and diarrhea. Geneva, Switzerland: World Health Organization, 2013. [Google Scholar]
- 29. Young M, Wolfheim C, Marsh DR, Hammamy D. World Health Organization/United Nations Children’s Fund joint statement on integrated community case management: an equity-focused strategy to improve access to essential treatment services for children. Am J Trop Med Hyg 2012; 87:6–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.