Abstract
Background
Current guidelines for the management of hepatitis C virus (HCV) infections provide varying recommendations for the optimal treatment of acute HCV infections. There are limited data from small cohort studies to provide guidance on the best approach to treatment of this important patient population.
Methods
Sofosbuvir-Containing Regimens Without Interferon for Treatment of Acute HCV in HIV-1 Infected Individuals is an open-label, 2-cohort, Phase 1 clinical trial in which the second cohort assessed the safety and efficacy of 8 weeks of ledipasvir/sofosbuvir for the treatment of acute HCV infections in participants with chronic human immunodeficiency virus (HIV)-1 infections. This final analysis of the second cohort had a planned accrual of 27 participants, based on non-inferiority criteria, compared to the study-defined, historical, sustained virologic response (SVR) of 60% with pegylated-interferon/ribavirin.
Results
We enrolled 27 men (9 Hispanic; 11 White, non-Hispanic; 5 Black, non-Hispanic; 2 Asian or Pacific Islander; median age 46 years). Most (96%) had HCV genotype-1 infection and 59% had the favorable interleukin 28B CC genotype. The median baseline HCV RNA load was 6.17 log10 IU/mL (interquartile range 4.51 – 6.55). All participants (100%) achieved the primary outcome of a sustained virologic response 12 weeks after the date of the last dose of study treatment (90% confidence interval 90–100%), achieving non-inferiority versus the 60% historic benchmark. No treatment discontinuations occurred.
Conclusions
This multicenter clinical trial, investigating 8 weeks of ledipasvir/sofosbuvir for acute HCV infections in men with HIV infections, reports a 100% SVR. This study provides the rationale for larger studies of shortened courses of direct-acting antiviral therapies in persons with HIV infections, including those with high baseline HCV RNA loads.
Clinical Trials Registration
Keywords: human immunodeficiency virus, direct-acting antivirals, early infection, interferon-free, men who have sex with men
SWIFT-C assesses 8 weeks of ledipasvir/sofosbuvir treatment for acute hepatitis C virus (HCV) infections in human immunodeficiency virus–infected participants. The high treatment response (100%) suggests direct-acting antiviral therapy can be shortened in early HCV infection.
The World Health Organization recently estimated that 71 million persons are living with hepatitis C virus (HCV) infections globally and 2.3 million are estimated to be living with both HCV and human immunodeficiency virus (HIV) infections [1]. With HCV elimination as a long-term goal, incident HCV infection is identified as a threat. In 2015, 1.75 million new HCV infections were reported by the World Health Organization, an epidemic that is widespread [1–3]. Since the prevention of future infections is a mainstay of the elimination plan, the early identification and treatment of incident HCV infections also becomes critical. However, in the direct-acting antiviral (DAA) era, the best approach to the treatment of acute HCV infections remains unclear.
Currently, HCV guidelines provide different recommendations for the treatment of acute HCV infection. The European Association for the Study of Liver Disease recommends multiple 8-week DAA regimens, including ledipasvir/sofosbuvir for genotypes 1, 4, 5, and 6 [4]. The American Association for the Study of Liver Disease and Infectious Diseases Society of America Guidance Panel for the Management of HCV infection recommend that an acute infection be treated the same as a chronic infection, in which case, an 8-week regimen would only be possible with 2 DAA regimens and, in the case of ledipasvir/sofosbuvir, would require a baseline HCV RNA load <6 million IU/mL [5].
Over the past 2 years, several pilot clinical trials of all oral DAA therapies have been reported in the setting of acute or early HCV infections [6–10]. These studies show significant variability in the study populations, definitions of acute versus early infections, presence or absence of symptoms attributable to HCV infections, frequency of the interleukin 28B (IL28B) favorable genotype, DAA therapy potency, and/or length of DAA therapy. Although small and variable, these studies have identified challenges in the treatment of acute infections, including the potential impact of high baseline HCV RNA loads on treatment responses [7].
We performed an open-label, multicenter clinical trial to investigate the safety and efficacy of ledipasvir/sofosbuvir—a daily, fixed-dose combination tablet that combines an non-structural (NS) 5A inhibitor with a NS5B nucleotide inhibitor—for 8 weeks in HIV-1 co-infected individuals with acute genotype 1 or 4 HCV infections or re-infections [11, 12]. The objective of this study was to test whether this short-course treatment (8 weeks is approved only in treatment-naive patients without cirrhosis and with a baseline HCV RNA load <6 million IU/mL) was more effective than historical trials of short-course interferon and ribavirin in this patient group. In addition, we explored the safety of concomitant dosing of pharmacologically-boosted antiretroviral (ARV) regimens and tenofovir disoproxil fumarate (TDF), the combination of which were excluded in the registrational trial of this regimen in individuals with HIV infections.
METHODS
Study Design
A5327 is an open-label, 2-cohort clinical trial of the interferon-free treatment of acute HCV infections in participants with chronic HIV-1 infection. We present here Cohort 2, which assessed the safety and efficacy of 8 weeks of daily ledipasvir (90 mg)/sofosbuvir (400 mg) for the treatment of acute HCV infections in participants with chronic HIV-1 infections. The sample size of 27 participants for Cohort 2 was chosen to provide 90% power to show that the underlying, true, sustained virologic response (SVR) 12 weeks after the date of the last dose of study treatment (SVR12) rate is greater than the study-defined, historical SVR rate of 60% for pegylated-interferon/ribavirin treatment, based on a 2-sided 90% confidence interval [13]. The results of the Cohort 1 portion of the study have been previously published [8].
Participant Population
Adults (aged ≥18 years) with a chronic HIV-1 infections and documented confirmation of an acute HCV infection or HCV re-infection within 6 months prior to entry were recruited from 9 different acquired immunodeficiency syndrome (AIDS) clinical research sites in the United States. Acute HCV infection was defined, per the European AIDS Treatment Network Acute Hepatitis C Infection Consensus Panel [14], as:
The new (<24 weeks prior to study entry) elevation of alanine aminotransferase (ALT) to ≥5X the upper limit of normal OR >250 U/L in a participant with a documented, normal ALT in the preceding 12 months or to ≥10X the upper limit of normal OR >500 U/L in a participant with an abnormal or no measured ALT at baseline in the preceding 12 months and a detectable HCV RNA load, excluding those participants with any prior, positive anti-HCV; or
A detectable HCV RNA level in a participant with prior, negative anti-HCV antibodies or an undetectable HCV RNA load within the preceding 6 months.
Participants with re-infections were eligible if they had both (1) documentation of a treatment-induced or spontaneous clearance of a prior infection, as evidenced by positive anti-HCV antibodies and 2 negative HCV RNA level results a minimum of 6 months apart, and (2) subsequently met the definition of acute infection. Enrollment was limited to those infected with HCV genotypes 1 or 4.
Participants were not required to be on ARVs, but those who were on ARVs were required to have evidence of HIV RNA suppression. Excluded ARVs were didanosine, stavudine, and ritonavir-boosted tipranavir. Pharmacologically-boosted ARV regimens were allowed, including ritonavir-boosted protease inhibitors and elvitegravir/cobicistat in combination with TDF. Other inclusion criteria included creatinine clearance (CrCl) ≥60 mL/min, as calculated by the Cockcroft-Gault equation. Full eligibility criteria are provided in the study protocol, available in the Supplementary Material.
We obtained ethics committee approval at all participating centers, in accordance with the principles of the 2008 Declaration of Helsinki. All participants provided written informed consent before undergoing any protocol-specified procedures. An independent study-monitoring committee reviewed the progress of the study. The study was registered with the Clinicaltrials.gov registry (NCT02128217).
Study Endpoints and Procedures
The primary efficacy endpoint was the rate of SVR, which was defined as an undetectable HCV RNA load (less than the lower limit of quantification, target not detected [<LLOQ TND]; Roche COBAS Taqman HCV Test 2.0; LLOQ 15 IU/mL) at SVR12. If a participant had no HCV RNA load measurement at week 12, the measurement was imputed as <LLOQ TND if the immediately-preceding and immediately-succeeding measurements were both <LLOQ TND; otherwise, it was imputed as detectable. Imputation criteria were also applied at other virologic time points.
The primary safety endpoint was the occurrence, during treatment or within 28 days after treatment discontinuation, of Grade ≥ 2 adverse events (AEs) as defined by the Division of AIDS, including both clinical AEs and laboratory, serious AEs, according to International Conference on Harmonisation criteria, or treatment-limiting AEs [15]. Due to the potential for increased tenofovir exposure in participants on pharmacologically-boosted ARV regimens, renal toxicity thresholds included confirmation of any 1 of the following 3 criteria [1]: an increase in baseline creatinine >0.4 mg/dL [2], CrCl <50 mL/min [3], or incident 2+ proteinuria [12]. Secondary endpoints included viral kinetics and treatment-emergent resistance to NS5A or non-structural 5B nucleotide.
The study visits after screening occurred at baseline (entry); treatment weeks 1, 2, 4, and 8; and post-treatment weeks 2, 4, 8, 12, and 24. HCV and HIV-1 RNA testing was done centrally at Quest Diagnostics. HIV-1 RNA loads were analyzed using Abbott RealTime HIV-1. Whole blood was obtained either at the screening or baseline visit for IL28B genotyping (Roche Taqman). The study drug was dispensed once, at the entry visit. Self-reported adherence was assessed by participant self-reports of missed doses during the 4 days prior to each visit.
Statistical Analysis
The primary efficacy objective was assessed by estimating the proportion of participants who started study treatment and achieved SVR12 in an intent-to-treat analysis. A 2-sided 90% confidence interval was calculated for the proportion using the Blyth-Still-Casella method for binomial outcomes. If this confidence interval was entirely above 60%, then there would be reasonable evidence that the underlying, true SVR12 rate was greater than 60% (ie, non-inferior to 60%). The Spearman correlation was used to assess the association of the time of the first undetectable HCV RNA load measurement with the baseline log10 HCV RNA load. The primary safety objective was addressed by estimating, in the same way as the primary efficacy endpoint, the proportion of participants who had 1 of the defined AEs. Changes in serum creatinine and creatinine clearance were compared between selected HIV ARV treatment groups (TDF combined with either cobicistat or a ritonavir-boosted protease inhibitor versus other TDF-containing regimens) at each measurement week using the Wilcoxon rank sum test. The area under the curve of change in these parameters was also calculated using the trapezoidal rule and compared between the same HIV ARV treatment groups as above.
RESULTS
Participants
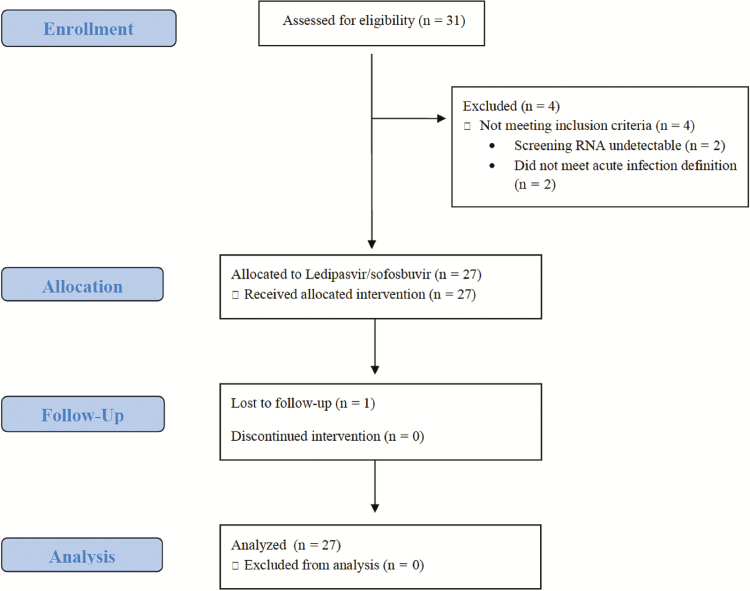
There were 27 participants enrolled into Cohort 2 between August 2015 and September 2016 (Figure 1). There were 7 participants who were initially screened and did not enroll; these included 3 participants who were re-screened and subsequently enrolled. The other 4 screen failures included 2 participants who had undetectable HCV RNA loads at the time of screening and 2 participants who failed to meet the HCV acute infection entry criteria. All participants were male; the median age was 46 years (interquartile range 38–50 years); and 9 (33%) were Hispanic; 5 (19%) were Black, non-Hispanic; and 11 (41%) were White, non-Hispanic (Table 1). There were 5 participants in Cohort 2 who met the protocol-defined criteria for HCV re-infection; the rest met the criteria for a first acute HCV infection. Most participants (89%) met the ALT elevation criteria for entry. The median time from the first laboratory evidence of an acute HCV infection until the study entry was 116 days (range 89–166 days). Most participants (96%) had HCV genotype 1 infections; 1 had a genotype 4 infection. The majority of participants (59%) had the favorable IL28B CC genotype. There was 1 participant who had an HCV RNA load <LLOQ TND at entry (the screening HCV RNA load was detectable). There were 14 (52%) participants who had baseline HCV RNA loads ≥ 1 000 000 IU/mL, including 6 (22%) with a baseline HCV RNA load ≥ 6 000 000 IU/mL and 4 (15%) with baseline HCV RNA loads ≥ 10 000 000 IU/mL.
Figure 1.
Sofosbuvir-Containing Regimens Without Interferon for Treatment of Acute HCV in HIV-1 Infected Individuals cohort 2 flow diagram.
Table 1.
Characteristics of Patients at Baseline (Entry)
| Ledipasvir/Sofosbuvir for 8 Weeks (N = 27) |
|
|---|---|
| Male sex, n (%) | 27 (100) |
| Median age, y (IQR) | 46 (38–50) |
| White, non-Hispanic, n (%) | 11 (41) |
| Black, non-Hispanic, n (%) | 5 (19) |
| Hispanic or Latino (regardless of race), n (%) | 9 (33) |
| Asian or Pacific Islander, n (%) | 2 (7) |
| Intravenous drug use, n (%) | |
| Current | 1 (4%) |
| Previous | 4 (15%) |
| Reported history of STI, n (%) | 11 (41) |
| IL28B CC favorable genotype, n (%) | 16 (59) |
| HCV genotype, n (%) | |
| 1a | 23 (85) |
| 1b | 3 (11) |
| 4 | 1 (4) |
| First HCV infection, n (%) | 22 (81) |
| Median HCV RNA load, IU/mL (IQR) | 1 490 000 (32 500-3 560 000) |
| Median HCV RNA load, log10 IU/mL (IQR) | 6.17 (4.51–6.55) |
| HCV RNA load ≥ 6 million IU/mL, n (%) | 6 (22) |
| Median time (days) from first laboratory evidence of acute infection (IQR) | 116 (98–156) |
| Median CD4, cells/µL (IQR) | 561 (441–698) |
| HIV-1 RNA <50 copies/mL, n (%) | 27 (100) |
| Median ALT, mg/dL (IQR) | 133 (47–393) |
| Median AST, mg/dL (IQR) | 83 (35–224) |
| Median total bilirubin, mg/dL (IQR) | 0.60 (0.50–1.20) |
| Median creatinine, mg/dL (IQR) | 0.96 (0.88–1.15) |
Abbreviations: ALT, alanine aminotransferase; AST, aspartate aminotransferase; HCV, hepatitis C virus; HIV, human immunodeficiency virus; IL28B, interleukin 28B; IQR, interquartile range; STI, sexually-transmitted infection.
All participants were receiving ARVs prior to entry, with HIV-1 RNA loads <50 copies/mL; the median baseline CD4+ cell count was 561 cells/mm3 (interquartile range 441–698 cells/mm3). There were 12 (44%) participants on ritonavir or cobicistat-boosted ARV regimens, 22 (81%) on a TDF-based regimen, and 1 on tenofovir alafenamide (Table 2). There were 9 (33%) participants on a pharmacologically-boosted TDF-based regimen.
Table 2.
Antiretroviral Regimens
| Ledipasvir/Sofosbuvir for 8 Weeks (N = 27) |
|
|---|---|
| Receiving HIV treatment at screen, n (%) | 27 (100) |
| Protease inhibitor, n (%) | 7 (26) |
| Darunavir/ritonavir | 3 (11) |
| Atazanavir/ritonavir | 4 (15) |
| Non-nucleoside reverse transcriptase inhibitor, n (%) | 9 (30) |
| Efavirenz | 3 (11) |
| Rilpivirine | 5 (18) |
| Nevirapine | 1 (4) |
| Integrase inhibitor, n (%) | 14 (52) |
| Raltegravir | 3 (11) |
| Dolutegravir | 6 (22) |
| Elvitegravir | 5 (18) |
| Nucleoside reverse transcriptase inhibitor, n (%) | 27 (100) |
| Tenofovir/emtricitabinea | 23 (85) |
| Abacavir/lamivudine | 4 (15) |
Abbreviation: HIV, human immunodeficiency virus.
aThere was 1 participant on tenofovir alafenamide and were 22 participants on tenofovir disoproxil fumarate.
Efficacy
Among the 27 participants enrolled, all 27 (100%) achieved the primary outcome of SVR12 (90% confidence interval 90–100%; Table 3), thus achieving non-inferiority versus the historic benchmark of 60%. All participants completed the planned course of treatment; there were no premature treatment discontinuations. There were 2 participants (Supplementary Figure 1, IDs 650944 and 2705686) who had declines in HCV RNA loads from screening to entry that resulted in levels <LLOQ. There were also 2 participants (Supplementary Figure 1, IDs 428450 and 2705690) who achieved undetectable HCV RNA loads during treatment but had 1 detectable HCV RNA load each at a post-treatment week (weeks 4 and 8, respectively; 74 and 161 IU/mL, respectively). However, subsequent measurements for both participants were <LLOQ TND, and both achieved SVR12 and SVR24. There were 26 (96%) participants that achieved SVR24, with 1 participant lost to follow-up after achieving SVR12.
Table 3.
Virologic Response During and After Therapy
| Response | Ledipasvir/Sofosbuvir for 8 Weeks (N = 27) |
|---|---|
| HCV RNA load <LLOQ TND | |
| On therapy, n (%) | |
| Week 1 | 5 (19) |
| Week 2 | 12 (44) |
| Week 4 | 22 (82) |
| End of therapy (week 8), n (%) | 25 (92)a |
| After end of therapy, n (%) | |
| Week 2 (SVR2) | 27 (100) |
| Week 4 (SVR4) | 26 (96)b |
| Week 8 (SVR8) | 26 (96)b |
| Week 12 (SVR12) | 27 (100) |
| Week 24 (SVR24) | 26 (96)c |
| Virologic breakthrough during treatment | 0 |
| Relapse in patients with HCV RNA load <LLOQ TND at end of therapy | 0 |
Each instance of SVR and a number indicates the SVR that number of weeks after the date of the last dose of study treatment.
Abbreviations: <LLOQ TND, less than lower limit of quantification target not detected; HCV, hepatitis C virus; SVR, sustained virologic response.
aThere were 2 subjects that missed the week 8 visit and did not meet imputation criteria.
bThere were 2 subjects that each had a detectable HCV RNA load (1 at week 4 after therapy and 1 at week 8 after therapy; 74 and 161 IU/mL, respectively). Both achieved undetectable HCV RNA loads at week 4 on therapy and both achieved SVR12.
cThere was 1 subject that was lost to follow-up after achieving SVR12.
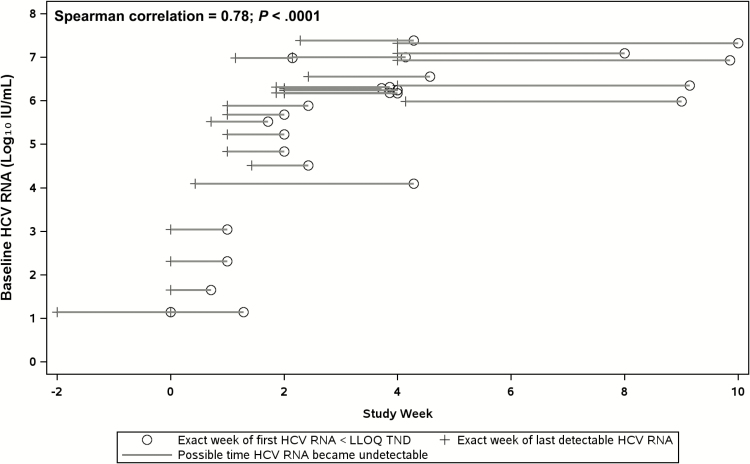
Figure 2 illustrates the time at which participants achieved an undetectable HCV RNA load, according to their baseline log10 HCV RNA load. The Spearman correlation for the association of the time of the first undetectable HCV RNA load and the baseline log10 HCV RNA load was 0.78 (P < .001), suggesting that the time to achieve an undetectable HCV RNA load was longer among the participants with higher baseline HCV RNA loads.
Figure 2.
Time to achieve an undetectable HCV RNA load, according to baseline HCV viral load. The horizontal axis represents the study week and the vertical axis represents HCV RNA load log10 IU/mL. The crosses indicate the last time each participant had a detectable HCV RNA load; the open circles show the times of the first undetectable HCV RNA loads; the lines represent the range of possible times when a participant may have become undetectable. Abbreviations: <LLOQ TND, less than lower limit of quantification target not detected; HCV, hepatitis C virus.
Safety
There were 9 participants (33%, 90% confidence interval 20–50%) who had a Grade 2 or 3 AE, either while on treatment or up to 28 days after treatment discontinuation (Table 4). There was 1 serious AE (a skull fracture), which was reported as not related to the study drug by the site investigator. There were no Grade 4 AEs reported. Grade 2 or higher AE classifications included: 2 participants with new diagnoses; 4 participants with signs or symptoms; and 6 participants with laboratory abnormalities (Supplementary Table 1).
Table 4.
Adverse Events (During Study Treatment, Through to 28 Days After End of Study Treatment) and Treatment Discontinuations
| Event | Ledipasvir/Sofosbuvir for 8 Weeks (N = 27) |
|---|---|
| ≧Grade 2 AEs, n (%) | 9 (33) |
| Grade 4 AE, n (%) | 0 |
| Serious AE, n (%) | 1 |
| Treatment D/C due to AE, n (%) | 0 |
| Death, n (%) | 0 |
| Grade 3‒4 laboratory abnormality, n (%) | 0 |
Grade 2 AEs consist of fatigue, nasal discharge, nasal congestion, vomiting, abdominal pain, or cough; Grade 3 AEs consist of traumatic skull fractures, varicella zoster, or oral pain due to dental work.
Grade 2 Lab AEs consist of elevated total bilirubin, elevated lipase, elevated potassium, elevated non-fasting glucose, elevated AST, ALT, or alkaline phosphatase; Grade 3 Lab AEs consist of elevated lipase, elevated total bilirubin, or elevated AST.
Abbreviations: AE, adverse event; ALT, alanine aminotransferase; AST, aspartate aminotransferase; D/C, discontinuation.
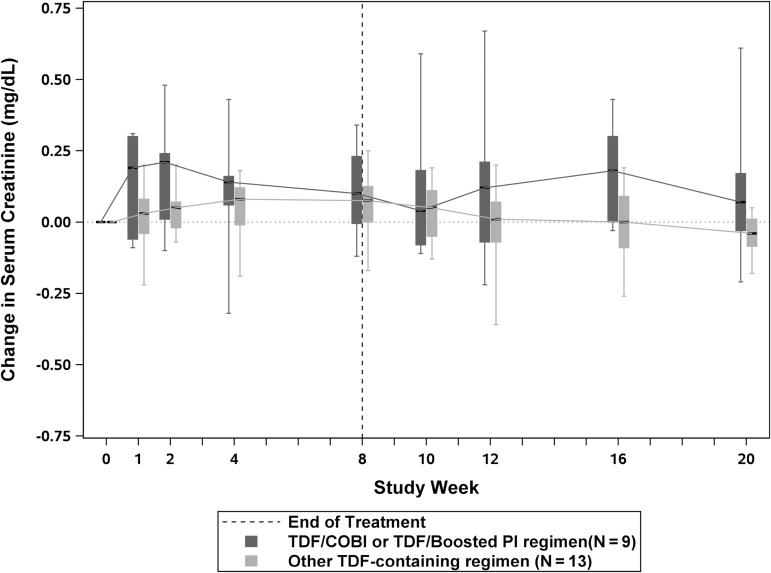
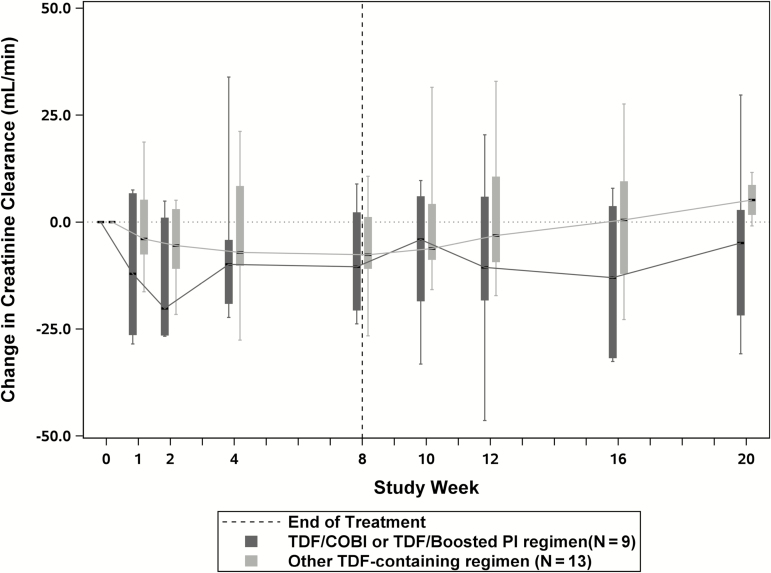
Renal toxicities were monitored for changes in serum creatinine, changes in creatinine clearance, and incident proteinuria. There were 2 participants who had increases in serum creatinine from baseline of more than 0.4 mg/dL. Both participants were on pharmacologically-boosted TDF-based regimens (atazanavir/ritonavir and elvitegravir/cobicistat). There was 1 participant who experienced creatinine increases at weeks 4 and 8 post-treatment and there was 1 participant who experienced an increase at week 2 on therapy and stabilized; no medication change was required in either case. Figure 3 displays the median change in serum creatinine values over time for participants taking pharmacologically-boosted TDF-containing ARV regimens (n = 9) compared to participants taking other TDF-containing regimens (n = 13). The area under the curve of changes in serum creatinine from baseline to the end of treatment was not different between the 2 groups (P = .18). Figure 4 displays the median change in CrCl for the same groups, also showing no difference in the area under the curve (P = .32).
Figure 3.
Serum creatinine change from baseline during study treatment and post-treatment follow-up periods among participants on TDF-containing antiretroviral regimens. The horizontal axis represents the study weeks and the vertical axis represents the changes in serum creatinine levels from baseline. The dark gray boxes represent the interquartile ranges for changes in serum creatinine for participants on pharmacologically-boosted TDF-containing antiretroviral regimens, and the light gray boxes represent the interquartile ranges for changes in serum creatinine for participants on all other TDF-containing antiretroviral regimens. The hashed vertical line represents the end of dosing and start of follow-up. Abbreviations: COBI, cobicistat; PI, protease inhibitor; TDF, tenofovir disoproxil fumarate.
Figure 4.
Serum creatinine clearance change from baseline during study treatment and post-treatment follow-up periods among participants on TDF-containing antiretroviral regimens. The horizontal axis represents the study weeks and the vertical axis represents changes in serum creatinine clearance from baseline. The dark gray boxes represent the interquartile ranges for changes in serum creatinine clearance for participants on pharmacologically-boosted TDF-containing antiretroviral regimens, and the light gray boxes represent the interquartile ranges for changes in serum creatinine clearance for participants on all other TDF-containing antiretroviral regimens. The hashed vertical line represents the end of dosing and start of follow-up. Abbreviations: COBI, cobicistat; PI, protease inhibitor; TDF, tenofovir disoproxil fumarate.
Adherence
Self-reported adherence was assessed by a questionnaire at each on-treatment study visit. Self-reported adherence was excellent: only 1 participant reported a single missed dose of ledipasvir/sofosbuvir through week 8; and at week 8, only 5 participants reported 1 or more missed doses in the 4 days preceding the study visit.
DISCUSSION
This multicenter, clinical trial reports that a short course of 8 weeks of combination therapy with ledipasvir/sofosbuvir resulted in sustained virologic responses in all participants with HIV infections and acute HCV co-infections (predominantly genotype 1). This cohort was primarily asymptomatic and was identified due to elevations in liver transaminases. In addition, this 8-week course of ledipasvir/sofosbuvir was safe in participants with HIV infections receiving pharmacologically-boosted TDF-based ARV regimens.
Short-course therapies have historically been highly effective in the treatment of acute HCV infections due to strong innate and adaptive immune responses, but these responses decrease over time [16–20]. Variations in outcomes among the published studies of ledipasvir/sofosbuvir in acute infections and this study might be due to the timing of treatment initiation or to variations in the prevalence of the favorable IL28B CC genotype. Deterding et al allowed for enrollment immediately following the identification of an infection (mean time from diagnosis to start of therapy was 32.8 days), resulting in a largely-symptomatic cohort and a low median HCV RNA load (4.04 log10 IU/mL) at baseline [6]. Rockstroh et al used the same definition for acute infection as our study [7]. While the median time from diagnosis to treatment was not provided by Rockstroh et al, the median HCV RNA load reported at baseline was 5.6 log10 IU/mL. This suggests that as the time since a first diagnosis increases, the baseline HCV RNA load increases, which may reflect the immune transition from an early to a more chronic infection profile. This is further supported by our finding that the baseline HCV RNA load was correlated (r = 0.78) with the time to reach an undetectable HCV RNA load; thus, higher HCV RNA loads had slower viral kinetics, potentially due to the loss of immune mediate viral clearance.
Thus, 6 weeks of therapy may be sufficient when patients are treated early in their acute infection and, in particular, when they have favorable baseline predictors, including a high IL28B CC frequency, elevated total bilirubin, and low HCV RNA load at baseline. However, if patients are identified later in their acute infection course, as evidenced by a lack of symptoms and high HCV RNA load at baseline, 8 weeks appears to improve the treatment response. This is further supported by the recent report of successful treatment of acute HCV infections with an 8-week course of elbasvir/grazoprevir [21]. This Phase 3b study enrolled 80 men who have sex with men, both with (91%) and without HIV and with acute HCV infections confirmed within 6 months of the estimated date of infection. The mean time between estimated infection and HCV treatment was 4.4 months. Only 1 patient experienced a relapse. Since the current American Association for the Study of Liver Disease and Infectious Diseases Society of America HCV Guidance recommends treating an acute infection the same as a chronic infection, depending on the DAA therapy available to the patient, this may be a 12-week course of therapy. This study and others suggest that this increased length of therapy is unlikely to be necessary; a shorter course of therapy would decrease costs and drug exposure.
The 2 participants who achieved undetectable HCV RNA loads during treatment each had 1 detectable HCV RNA load value (at post-treatment weeks 4 and 8), but follow-up confirmation testing showed re-suppression of the RNA load and both achieved SVR12 and SVR24. Several possible explanations for this include false-positive results due to a high-sensitivity polymerase chain reaction assay or a transient re-infection with the innate clearance of the new infection. This underscores the importance of follow-up testing in individuals with ongoing risks of HCV infections.
As expected, the ledipasvir/sofosbuvir regimen was well tolerated, with no treatment discontinuations and a single serious AE during study treatment that was deemed not related. Of particular interest in this study was the potential for renal toxicity, due to the allowance of pharmacologically-boosted TDF-containing ARV regimens. In this study, 9 participants were on pharmacologically-boosted TDF-containing ARV regimens and 13 participants were on other TDF-containing ARV regimens. There was no difference in changes in creatinine or CrCl during the study period between these 2 groups. The 2 participants who met the renal toxicity threshold for increased creatinine were on pharmacologically-boosted TDF-containing ARV regimens. Neither required a change in their ARV regimen or discontinuation of the DAA regimen; however, monitoring is recommended and consideration of switching the ARV regimen is reasonable, particularly in patients with risks of conditions such as diabetes and hypertension. Thus, 8 weeks of ledipasvir/sofosbuvir is safe in persons with HIV infections on pharmacologically-boosted TDF-containing ARV regimens with a baseline CrCl >60 mL/min.
The primary limitations of this study are due to its relatively small study size, allowing us to power the study only to show non-inferiority of the primary outcome to the study-defined historical SVR rate, assuming a high underlying, true response rate, and we did not have the statistical power to assess the absolute effectiveness of this 8-week regimen at high (eg, >6 log10 IU/mL) viral loads. In addition, although the study allowed the enrollment of both men and women with HIV-1 infections, no women were enrolled, and only 5 subjects reported active (n = 1) or previous intravenous drug use. Thus, whether the results of this study apply more broadly to people who are not HIV-infected men is unclear.
In conclusion, treatment with 8 weeks of ledipasvir/sofosbuvir in subjects with HIV infections and HCV infections, predominantly acute genotype 1 infections, was well tolerated and highly efficacious, achieving non-inferiority compared to historical responses to interferon and ribavirin during acute HCV infections. Larger studies are needed, therefore, to better define the characteristics of HIV-infected patients with acute HCV infections who would require a longer (eg, 8-week) course and of those who can be treated with a shorter (eg, 6-week) course of ledipasvir/sofosbuvir, with the goal of curing all. These data add to the growing body of literature that supports an 8-week recommendation for the treatment of acute HCV infections.
Supplementary Data
Supplementary materials are available at Clinical Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Notes
The A5327 study team members are Beverly Alston-Smith, Laura Weichmann, Thucuma Sise, Emily Cosimano, Cheryl Jennings, Sikhulile Moyo, and Oswald Dadson. The A5327 site investigators are Annie Luetkemeyer and Jay Dwyer, at University of California, San Francisco, Acquired Immunodeficiency Syndrome Clinical Research Site (site 801; grant number 5UM1AI069496); Valery Hughes and Joanne Grenade, at Weill Cornell Uptown CRS (site 7803; grant numbers 5UM1 AI069419 and UL1 TR000457); Todd Stroberg and Tiina Ilmet, at Weill Cornell Chelsea CRS (site 7804; grant numbers 5UM1 AI069419 and UL1 TR000457); Sarah Henn and Kristi Kiger, at Whitman-Walker Health (site 31791; grant number UM1AI069465); Teri Flynn and Amy Sbrolla, at Massachusetts General Hospital (site 101; grant number 2UM1AI069412-09); Kathleen Nuffer and David Wyles, at University of California, San Diego Antiviral Research Center (site 701; grant AI069432); Donna McGregor and Claudia Hawkins, at Northwestern University CRS (site 2701; grant AI 069471); Brett Williams and Tondria Green, at Rush University Medical Center CRS (site 2702; grant number U01 AI069471); Pablo Tebas and Deborah Kim, at Penn Therapeutics CRS (site 6201; grant numbers Acquired Immunodeficiency Syndrome Clinical Trials Group UM-AI069534-09 and Center for AIDS Research P30-AI045008-17); Roger Bedimo and Holly Wise, at Trinity Health and Wellness Center CRS (site 31443; grant number U01 AI069471); Roberto C. Arduino and Aristoteles Villamil, at Houston AIDS Research Team (site 31473; grant number 2 UM1 AI069503).
Acknowledgments. The authors and study team thank all participants, the Acquired Immunodeficiency Syndrome Clinical Trials Group, SDMC, participating CRSs, and Specialty Laboratories. They also thank Gilead Sciences for providing sofosbuvir and ribavirin and funding hepatitis C virus RNA testing.
Disclaimer. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Financial support. This work was supported by the National Institute of Allergy and Infectious Diseases of the National Institutes of Health (grant numbers UM1 AI068634, UM1 AI068636, and UM1 AI106701). The research has also been supported in part by Gilead Sciences.
Potential conflicts of interest. S. N.’s institution has received grants from AbbVie, Bristol Meyers Squibb, Gilead, Janssen, Merck, Tacere, and Vertex. D. S. F. has common stock ownership in Gilead Sciences and his institution has received grants from Gilead Sciences. M. D. H. has received research grants from the National Institutes of Health to support the statistical and data management center for this study. A. Y. K.’s institution has received research grants from Gilead Sciences. A. L.’s institution has received research grants from AbbVie, Merck, Gilead, and Proteus. J. G. M. is an employee and stockholder of Gilead. D. M. B. is an employee of Gilead Sciences. M. G. P. served on an advisory board for Abbott and received honoraria from Abbott, Genentech, and Merck, outside the submitted work. J. J. K.’s institution has received research grants from Gilead Sciences. K. M. M.’s institution has received research grants from Gilead, Merck, and BMS. R. T. C.’s institution has received research grants from Gilead, AbbVie, Merck, Bristol Meyers Squibb, Boehringer Ingelheim, and Mass Biologics. All other authors report no potential conflicts. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
Contributor Information
Acquired Immunodeficiency Syndrome Clinical Trials Group (ACTG) A5327 Study Team:
Beverly Alston-Smith, Laura Weichmann, Thucuma Sise, Emily Cosimano, Cheryl Jennings, Sikhulile Moyo, Oswald Dadson, Annie Luetkemeyer, Jay Dwyer, Valery Hughes, Joanne Grenade, Todd Stroberg, Tiina Ilmet, Sarah Henn, Kristi Kiger, Teri Flynn, Amy Sbrolla, Kathleen Nuffer, David Wyles, Donna McGregor, Claudia Hawkins, Brett Williams, Tondria Green, Pablo Tebas, Deborah Kim, Roger Bedimo, Holly Wise, Roberto C Arduino, and Aristoteles Villamil
References
- 1. World Health Organization. Global Hepatitis Report 2017 [License: CC BY-NC-SA 3.0IGO]. Geneva, Switzerland: World Health Organization; 2017. [Google Scholar]
- 2. van de Laar T , Pybus O , Bruisten S , et al. . Evidence of a large, international network of HCV transmission in HIV-positive men who have sex with men. Gastroenterology 2009; 136:1609–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Zibbell JE , Iqbal K , Patel RC , et al. ; Centers for Disease Control and Prevention Increases in hepatitis C virus infection related to injection drug use among persons aged ≤30 years - Kentucky, Tennessee, Virginia, and West Virginia, 2006-2012. MMWR Morb Mortal Wkly Rep 2015; 64:453–8. [PMC free article] [PubMed] [Google Scholar]
- 4. EASL Recommendations on Treatment of Hepatitis C 2016. J Hepatol 2016; h.d.d.o.j. [DOI] [PubMed] [Google Scholar]
- 5. American Association for the Study of Liver Disease, Infectious Diseases Society of America . Recommendations for testing, managing, and treating hepatitis C Available at: http://www.hcvguidelines.org/ [DOI] [PMC free article] [PubMed]
- 6. Deterding K , Spinner CD , Schott E , et al. ; HepNet Acute HCV IV Study Group Ledipasvir plus sofosbuvir fixed-dose combination for 6 weeks in patients with acute hepatitis C virus genotype 1 monoinfection (HepNet Acute HCV IV): an open-label, single-arm, phase 2 study. Lancet Infect Dis 2017; 17:215–22. [DOI] [PubMed] [Google Scholar]
- 7. Rockstroh JK , Bhagani S , Hyland RH , et al. . Ledipasvir-sofosbuvir for 6 weeks to treat acute hepatitis C virus genotype 1 or 4 infection in patients with HIV coinfection: an open-label, single-arm trial. Lancet Gastroenterol Hepatol 2017; 2:347–53. [DOI] [PubMed] [Google Scholar]
- 8. Naggie S , Marks KM , Hughes M , et al. ; AIDS Clinical Trials Group (ACTG) A5327 Study Team Sofosbuvir plus ribavirin without interferon for treatment of acute hepatitis C virus infection in HIV-1-infected individuals: SWIFT-C. Clin Infect Dis 2017; 64:1035–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. El Sayed A , Barbati ZR , Turner SS , et al. ; New York Acute Hepatitis C Surveillance Network Sofosbuvir in the treatment of early HCV infection in HIV-infected men. HIV Clin Trials 2017; 18:60–6. [DOI] [PubMed] [Google Scholar]
- 10. Martinello M , Gane E , Hellard M , et al. . Sofosbuvir and ribavirin for 6 weeks is not effective among people with recent hepatitis C virus infection: The DARE-C II study. Hepatology 2016; 64:1911–21. [DOI] [PubMed] [Google Scholar]
- 11. Gilead Sciences. Harvoni U.S. prescribing information. Foster City, California: Gilead Sciences; 2015. [Google Scholar]
- 12. Naggie S , Cooper C , Saag M , et al. ; ION-4 Investigators Ledipasvir and sofosbuvir for HCV in patients coinfected with HIV-1. N Engl J Med 2015; 373:705–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Vogel M , Nattermann J , Baumgarten A , et al. . Pegylated interferon-alpha for the treatment of sexually transmitted acute hepatitis C in HIV-infected individuals. Antivir Ther 2006; 11:1097–101. [PubMed] [Google Scholar]
- 14. Acute hepatitis C in HIV-infected individuals: recommendations from the European AIDS Treatment Network (NEAT) consensus conference. Aids 2011; 25:399–409. [DOI] [PubMed] [Google Scholar]
- 15. US Department of Health and Human Services, N.I.o.H., National Institute of Allergy and Infectious Diseases, Division of AIDS. Division of AIDS (DAIDS) table for grading the severity of adult and pediatric adverse dvents, Version 2.0. Available at: http://rsc.tech-res.com/Document/safetyandpharmacovigilance/DAIDS_AE_GRADING_TABLE_v2_NOV2014.pdf
- 16. Matthews GV , Hellard M , Haber P , et al. ; Australian Trial in Acute Hepatitis C Study Group Characteristics and treatment outcomes among HIV-infected individuals in the Australian trial in acute hepatitis C. Clin Infect Dis 2009; 48:650–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Corey KE , Mendez-Navarro J , Gorospe EC , Zheng H , Chung RT. Early treatment improves outcomes in acute hepatitis C virus infection: a meta-analysis. J Viral Hepat 2010; 17:201–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Deterding K , Grüner N , Buggisch P , et al. ; Hep-Net Acute HCV-III Study Group Delayed versus immediate treatment for patients with acute hepatitis C: a randomised controlled non-inferiority trial. Lancet Infect Dis 2013; 13:497–506. [DOI] [PubMed] [Google Scholar]
- 19. Kahan SM , Wherry EJ , Zajac AJ. T cell exhaustion during persistent viral infections. Virology 2015; 479-480:180–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Wolski D , Foote PK , Chen DY , et al. . Early transcriptional divergence marks virus-specific primary human CD8+ T cells in chronic versus acute infection. Immunity 2017; 47:648–663.e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Boerekamps A , Weggheleire AD , van den Berk GE , et al. . The DAHHS 2 Study: 8 weeks of grazoprevir plus elbasvir for acute hepatitis C virus genotype 1 or 4 infection. In: Program and abstracts of the 25th Conference on Retroviruses and Opportunistic Infections (Boston, MA). 2018: Abstract 128. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.