Abstract
Rhinocerebral mucormycosis is a rapidly life‐threatening disease caused by a fungal infection. Every diabetic patient with sinusitis symptoms, headache, and visual changes needs radiological approach and nasal endoscopy to rule out mucormycosis. The mortality rate is 50%‐85%, despite an early diagnosis and a correct treatment.
Keywords: amphotericin B, endoscopic sinus surgery, mucor, rhinocerebral mucormycosis, zygomycetes
1. INTRODUCTION
We present a case of a patient of 59‐year‐old with an untreated diabetes who developed Rhinocerebral mucormycosis. He was treated with endoscopic sinus surgery (ESS) and medical treatment with amphotericin B intravenous, intrathecal, and endosinusal administration. Despite an early diagnosis, the patient died 2 weeks after admission.
Mucormycosis is an aggressive, angioinvasive, and often rapidly life‐threatening disease caused by fungal infection. There are seven genera of Mucorales fungi found in human infections: Rhizopus, Mucor, and Rhizomucor, Cunninghamella, Absidia (now Lichtemia), Saksenaea, and Apophysomyces. The Mucorales are ubiquitous, but their exact ecology is unknown. They are found in rotting biological material and are thermotolerant. The disease has a global distribution, and Mucorales infection is influenced by seasonal variation.1 This disease, in developed countries, is rare and mostly affects diabetic or immunosuppressed patients or subjects undergoing chemotherapy. Conversely, in developing countries, especially in the Asian subcontinent, mucormycosis affects patients suffering from massive trauma or uncontrolled diabetes.2 Mucormycosis remains a rare disease, it is most common in high‐risk patients, and it represents 8.3%‐13% of all fungal infections detected in autopsies.3 A recent study in the general population in Spain found an incidence of 0.43/1 000 000 inhabitants and 0.62/100 000 hospital admissions, while the analysis of hospital records in France showed an increasing incidence from 0.7/1 000 000 in 1997 to 1.2/1 000 000 in 2006.4, 5, 6 Rhinocerebral mucormycosis (ROCM) is the most frequent kind of mucormycosis in diabetic patients. The disease starts after inhalation of fungal spores into the paranasal sinuses. The fungal infection quickly spreads into adjacent tissues. At the time of germination, the infection spreads to the cavernous sinus, the palate, and the sphenoid sinus progressively involving the brain and the orbits. The fungus infects the central nervous system through the orbital apex or the cribriform plate of the ethmoid bone and eventually leads to the death of the patient. Hematogenous dissemination of the infection can be promoted by cerebral vascular invasion of the fungus, sometimes with the formation of fungal aneurysms. Ultimately, imaging studies are usually unsatisfactory for ROCM diagnosis, and a correct diagnosis of ROCM is usually carried out by histopathological examination of the hyphae of the Mucorales in samples.7 We present a case of ROCM in a diabetic patient, treated with endoscopic sinus surgery (ESS) and medical treatment with amphotericin B intravenous, intrathecal, and endosinusal administration.
2. CASE REPORT
We present a case of a patient of 59‐year‐old with a family history of diabetes, hypothyroidism,8, 9, 10 and adeno‐tonsillar surgery.11 He suffered from benign positional vertigo12 and vocal cord cysts.13 The patient came to General First Aid with symptoms attributable to sinusitis with bilateral periorbital cellulitis and headache (Figure 1). CT skull study documented widespread opacification and thickening of the maxillary, ethmoidal, and sphenoid sinuses. Moreover, there is a considerable presence of soft tissue swelling in the right periorbital site (Figure 2). The patient is admitted to our otolaryngologist‐operating unit. At the neurological counseling, osteotendinous reflexes appear norm vivacious and symmetrical, without any deficit of the extrinsic ocular muscles and absent meningeal signs. Diabetologic consultation is also performed, since the patient has a sugar level in the blood of more than 600 mg/dL and immediately starts insulin and moisturizing therapy. The patient had misdiagnosed diabetes, so he did not perform any hypoglycemic therapy before hospitalization. The remaining biotemporal examinations are concerned; a leukocytosis with GB over 20 000 is reported. A normal blood gas analysis was obtained.
Figure 1.
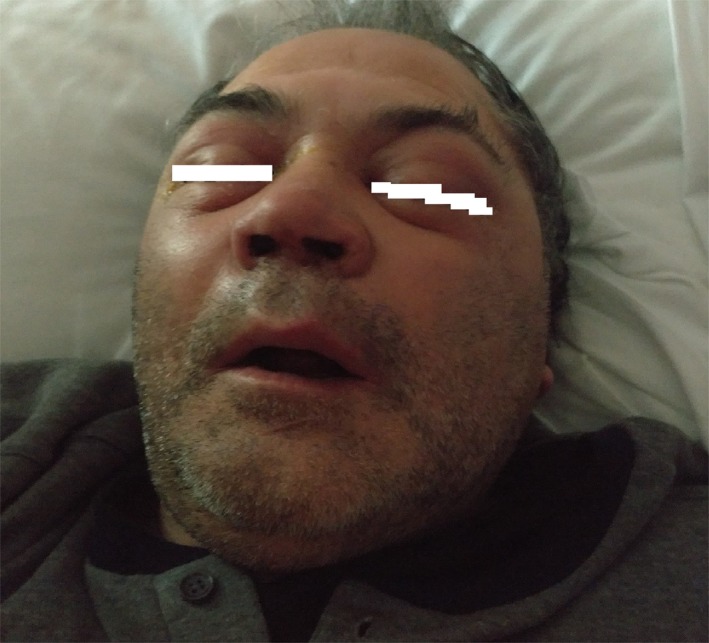
The patient comes to our observation with bilateral periorbital cellulitis
Figure 2.
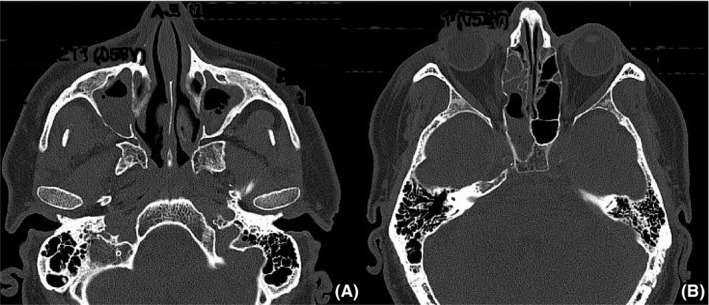
Assial CT study documented opacification of the maxillary sinuses (A), of the right sphenoid sinus and the ethmoidal cells (B)
The next day a worsening of the neurological situation has been recorded, with altered facial sensitivity and deficiency of masticatory muscles. Brain MRI is performed with contrast agent that documenting the presence of the known pathological material/tissue in the context of the sinuses. Moreover, diffuse swelling of the lower palpebral soft tissues is appreciated, bilaterally. The muscles of the right infratemporal fossa and the omolateral pterygoid muscles are also partially swollen. Normal condition of the Meckel cave and the cavernous sinuses (Figure 3A,B). Comparing the worsening of the neurological picture, the lack of response to antibiotic therapy and persistent high level of glucose in the blood, a possible fungal infection is suspected. Bilateral ESS was performed without assisted navigation,14, 15, 16 practicing uncibullectomy with a large bilateral meatotomy, bilateral ethmoidectomy, left intranasal sphenoidectomy, and transethmoidal right sphenoidectomy (Figure 4). Pathological tissue has been sent to extemporaneous histopathological examination to search fungal hyphae. The result was negative.
Figure 3.
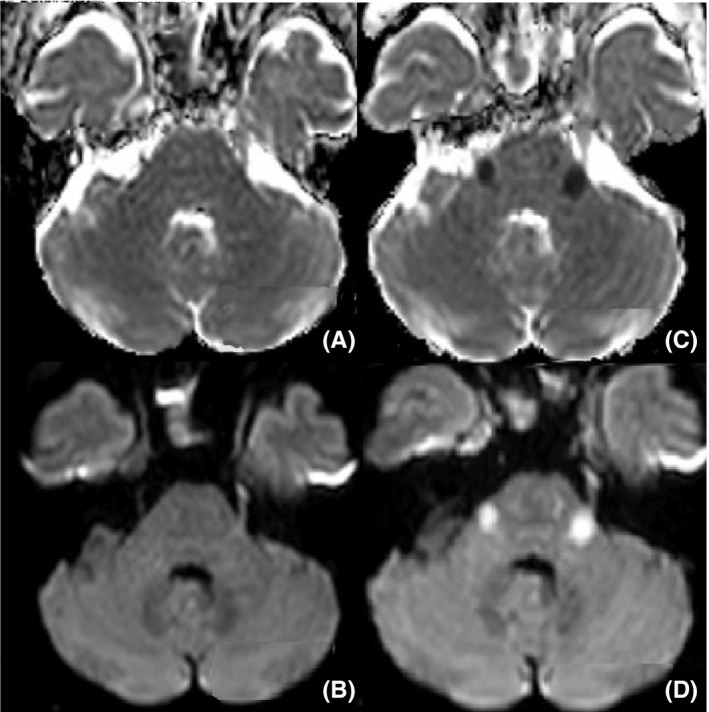
MRI sequences ADC and DWI done before (A; B) and after (C; D) 24 h that documented the appearance of two lesions in bilateral pontine sites, near the trigeminal nerve emergence
Figure 4.
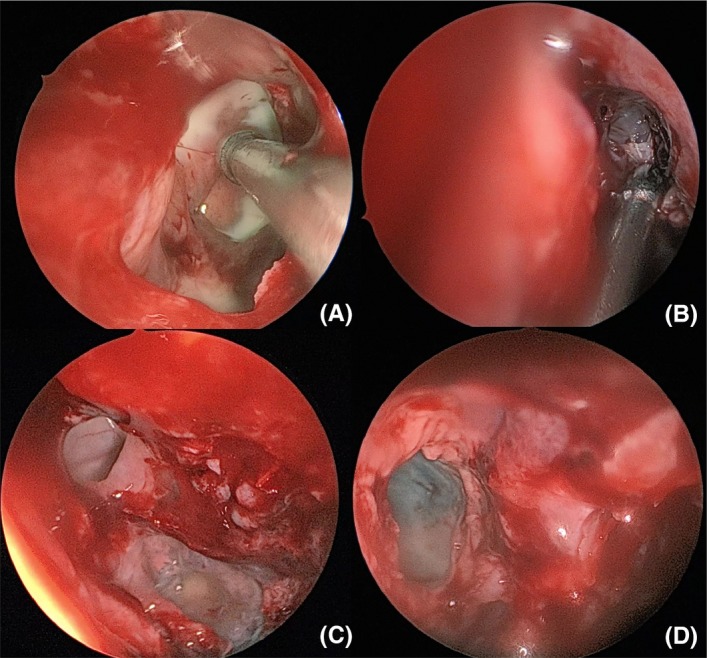
Presence of whitish spongy secretions in right (A) and left (B) maxillary sinuses, in right posterior ethmoid (C) and left sphenoid sinuses (D) during endoscopic surgery
The day after the surgical treatment, there were new worsening of clinical conditions, especially from the neurological point of view, with a paralysis of the III, IV, V, and IV bilateral cranial nerves.
A new MRI documents the appearance of two lesions in bilateral pontine sites, near the trigeminal nerve emergence (Figure 3C,D), pathological tissue in the context of both the Meckel Cave and both cavernous sinuses, increased swelling of the periorbital soft tissues bilaterally.
Whitish spongy secretions are present in the nasal cavities and in the oral cavity (Figure 5). A new biopsy sampling is performed in the paranasal sinuses, given the strong suspicion of a fungal lesion. This time the histopathological examination is positive for the presence of Mucor's fungal hyphae, order of Mucorales.
Figure 5.
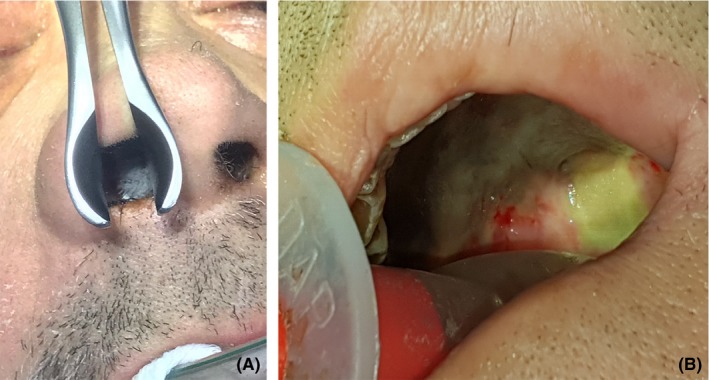
Whitish spongy secretions are present in the nasal cavities (A) and in the oral cavity (B)
The patient is transferred in the intensive care unit and started therapy with Amphotericin B (from 5 to 10 mg/kg/d) intravenously and with paranasal sinuses wash. The patient during the recovery was intubated and stabilized from the hemodynamic and respiratory point of view.
After 2 days, a new MRI is performed, which highlight the presence of parietal, occipital, and frontal focal lesions and thrombosis of the left internal carotid artery (Figure 6). Given the poor response of the therapy, Amphotericin B is administered intrathecally 1.5 mg three times a week. In the following days, the clinical conditions worsened, with a negative response to therapy and poor glycemic compensation. The patient died 2 weeks after admission.
Figure 6.
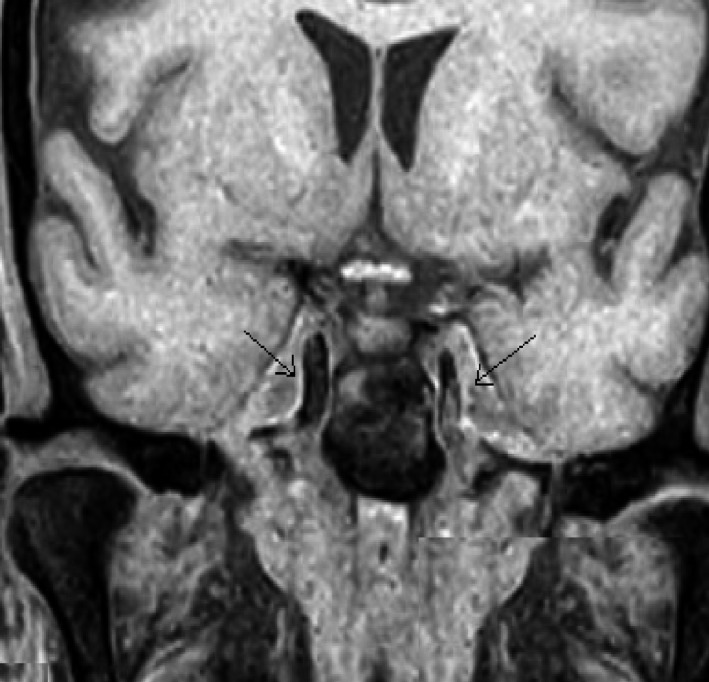
T1 SPIR sequence in MRI with contrast enhancement, showing the presence of internal carotid thrombosis, indicated by the arrow
3. DISCUSSION
The starter symptoms of ROCM are compatible with those of periorbital cellulitis and sinusitis. They are characterized by facial and eye pain or blurry vision with facial numbness. The typical clinical symptoms of mucormycosis in predisposed subjects combine orbital inflammation, multiple cranial nerve palsies, eyelid edema, blepharoptosis, proptosis, unilateral periorbital facial pain, headache, acute ocular motility alteration, external or internal ophthalmoplegia, and acute vision loss. The pathognomonic sign of mucormycosis is a black necrotic eschar.
Fever is fluctuating and may be absent in more than half of the reported cases. The number of white blood cells is often high if the patient is immunocompetent. The radiological study before the operation is essential to define the extent of the disease. The CT study highlights the edematous mucosa and the possible destruction of the periorbital tissues, the inflammatory processes of the paranasal sinuses, and the bone margins. The CT study remains the best radiological method to study the phlogistic invasion of the disease. The rupture of bone margins occurs in the late stages of the disease after soft tissue necrosis. MRI is essential to evaluate the intracranial extension and involvement of the meninges of the pathology, the thrombosis of the internal carotid artery of cavernous portions, and the cavernous sinus thrombosis. Contrast‐enhanced MRI imaging may also show perineural invasion of infection. MRI imaging is more specific for the study of periorbital soft tissue infection than CT. In the early stages of the disease, patients may have a normal radiological examination with either CT or MRI. In the case of high suspicion of ROCM, endoscopic paranasal sinus surgery and biopsy sampling must always be performed.17 Radiological studies are important for assessing the extent of the disease, but the final diagnosis of mucormycosis occurs through the histopathological study that reveals the fungal invasion of the tissues.
The histological examination of Mucorales fungus in biopsy and their interpretation may be very difficult. These organisms are usually difficult to recognize on hematoxylin‐eosin stains. Gomori methenamine silver stains and Periodic acid‐Schiff are the best methods for a completely characterized appearance of the fungus. Despite this, it is possible to highlight only fragments, even with the use of cell wall coloring.5 The hyphae of the Mucorales are typical and consent for a presumptive diagnosis from clinical specimens. The hyphae are broad (5‐15 micron), irregularly branched, and have rare septations (Figure 7). This is in contrast with the hyphae of ascomycetous molds, such as Aspergillus, which are narrower (2‐5 micron), exhibit regular branching, and have many septations. The lack of regular settings can contribute to the fragile nature of hyphae and to the difficulty of growing mucormycosis agents from clinical samples, which is translated into a correct diagnosis of an untimely laboratory, like in our case at the first extemporaneous biopsy. Excessive movement of clinical samples can cause irreversible damage to hyphae.18
Figure 7.
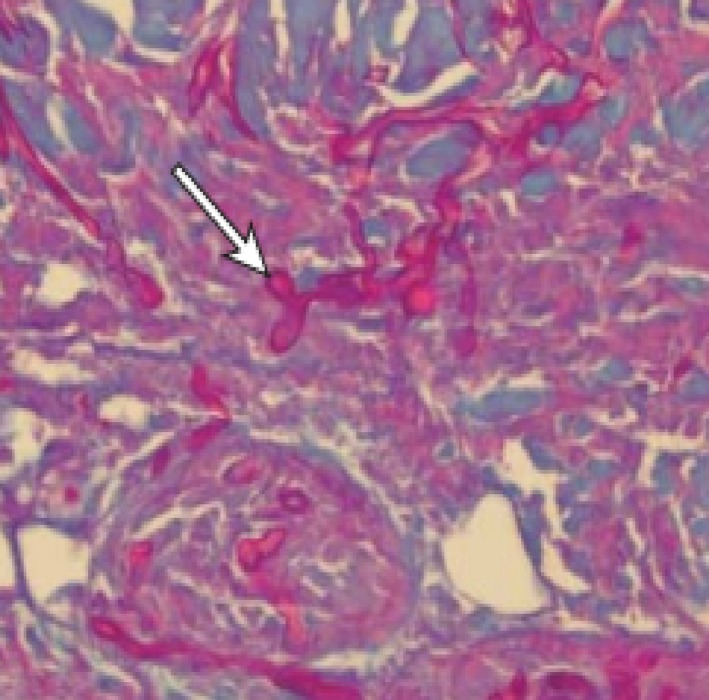
Periodic acid‐Schiff stain showed the elongated, pink fungal hyphae, irregularly branched and with rare septations (arrow)
The main purpose of the therapy is to correct any predisposing risk factors, an aggressive surgery to eliminate the disease and a rapid antifungal treatment. Surgical debulking is carried out mainly by the ENT specialist, sometimes assisted by the neurosurgeon or ophthalmologist if there is intracranial or orbital delivery. Surgical debridement must be as aggressive as possible. Treatment with antifungal with intravenous amphotericin B should be started as quickly as possible. Liposomal formulations of amphotericin B are preferred to classic preparations of the antifungal agent because they are more tolerable in these already systemically compromised patients and have less side effect profiles. Liposomal amphotericin B also has fewer adverse reactions than the amphotericin B lipid complex, although the latter is much less expensive and, therefore, more readily available. No significant difference has been shown in efficacy between the three formulations of the drug, except from interruption of therapy from unbearable side effects. While amphotericin is typically administered intravenously, less is known about the use of intrathecal amphotericin B. We found in literature eight published cases of intrathecal amphotericin for treatment of mucormycosis, we found that seven of the eight previously published patients reported good long‐term outcome, suggesting that early intrathecal therapy warrants further consideration for difficult‐to‐treat intracranial fungal infections. In our case, we started intrathecal therapy when the patient's conditions were extremely compromised. We did not benefit from the therapy. In any case, more studies are needed about intrathecal therapy.19, 20, 21, 22, 23, 24, 25, 26 Other studies showed that posaconazole and isavuconazole as a second line agent could be used as salvage therapy in no response cases.27, 28
In a recent review about 929 cases of zygomycosis, survival was 3% (8 of 241 patients) for cases that were not treated, 61% (324 of 532) for cases treated with amphotericin B deoxycholate, 57% (51 of 90) for cases treated with surgery alone, and 70% (328 of 470) for cases treated with antifungal therapy and surgery.29, 30, 31
Rhinocerebral mucormycosis is rapidly letal, with a mortality rate of 50%‐85% even with an aggressive surgical debridement, an intravenous antifungal therapy, and a correction of predisposing risk factors.32, 33, 34
4. CONCLUSION
As a result of this, the ROCM is a rapidly progressive disease with an increase in the mortality rate if the fungus enters the cranial cavity. In all diabetic patients with sinus symptoms, headache, alterations of vision it is necessary to suspect a mucormycosis and carry out a careful radiological evaluation and a nasal endoscopy. Despite an early diagnosis, treatment for ROCM does not guarantee a cure from the disease that often ends with the patient's death.
CONFLICT OF INTEREST
None declared.
AUTHOR CONTRIBUTIONS
BG: was treating the patient. FGazia: is corresponding author, was preparing the manuscript. CG: Bibliography research and English translation. FP: performed follow‐up examinations. FC: developed the concept and design of the study. FF: The otorhin who first saw the patient and suspected mucormycosis. FGalletti: critically revised the manuscript and gave the approval of the final version.
Galletti B, Gazia F, Galletti C, et al. Rhinocerebral mucormycosis with dissemination to pontine area in a diabetic patient: Treatment and management. Clin Case Rep. 2019;7:1382–1387. 10.1002/ccr3.2255
REFERENCES
- 1. Palejwala SK, Zangeneh TT, Goldstein SA, Lemole GM. An aggressive multidisciplinary approach reduces mortality in rhinocerebral mucormycosis. Surg Neurol Int. 2016;7:61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Kolekar JS. Rhinocerebral mucormycosis: a retrospective study. Indian J Otolaryngol Head Neck Surg. 2015;67(1):93‐96. Epub 2014 Dec 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Galletti B, Mannella VK, Santoro R, et al. Ear, nose and throat (ENT) involvement in zoonotic diseases: a systematic review. J Infect Dev Ctries. 2014;8(1):17‐23. [DOI] [PubMed] [Google Scholar]
- 4. Petrikkos G, Skiada A, Lortholary O, Roilides E, Walsh TJ, Kontoyiannis DP. Epidemiology and clinical manifestations of mucormycosis. Clin Infect Dis. 2012;54(Suppl 1):S23‐S34. [DOI] [PubMed] [Google Scholar]
- 5. Torres‐Narbona M, Guinea J, Martinez‐Alarcon J, et al. Impact of mucormycosis on microbiology overload: a survey study in Spain. J Clin Microbiol. 2007;45:2051‐2053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Bitar D, Van Cauteren D, Lanternier F, et al. Increasing incidence of zygomycosis (mucormycosis), France, 1997–2006. Emerg Infect Dis. 2009;15(9):1395‐1401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Walsh TJ, Gamaletsou MN, McGinnis MR, Hayden RT, Kontoyiannis DP. Early clinical and laboratory diagnosis of invasive pulmonary, extrapulmonary, and disseminated mucormycosis (zygomycosis). Clin Infect Dis. 2012;54(Suppl 1):S55‐S60. [DOI] [PubMed] [Google Scholar]
- 8. Bruno R, Aversa T, Catena M, et al. Even in the era of congenital hypothyroidism screening mild and subclinical sensorineural hearing loss remains a relatively common complication of severe congenital hypothyroidism. Hear Res. 2015;327:43‐47. [DOI] [PubMed] [Google Scholar]
- 9. Galletti B, Bruno R, Catalano N, Cammaroto G, Freni F. Follicular carcinoma on a radio‐treated ectopic lingual thyroid. Chirurgia (Turin). 2016;29(3):88‐91. [Google Scholar]
- 10. Freni F, Galletti B, Galletti F, Dionigi G. Improved outcomes for papillary thyroid microcarcinoma care: active surveillance and case volume. Ther Adv Endocrinol Metab. 2018;9(7):185‐186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Motta G, Motta S, Cassano P, et al. Effects of guidelines on adeno‐tonsillar surgery on the clinical behaviour of otorhinolaryngologists in Italy. BMC Ear Nose Throat Disord. 2013;13:1382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Ciodaro F, Mannella VK, Nicita RA, et al. Therapeutic efficacy of the Galletti‐Contrino manoeuvre for benign paroxysmal positional vertigo of vertical semicircular canals in overweight subjects. Eur Arch Otorhinolaryngol. 2018;275(10):2449‐2455. [DOI] [PubMed] [Google Scholar]
- 13. Marseglia L, D'Angelo G, Impellizzeri P, et al. Neonatal stridor and laryngeal cyst: which comes first? Pediatr Int. 2017;59(1):115‐117. [DOI] [PubMed] [Google Scholar]
- 14. Galletti B, Gazia F, Freni F, Sireci F, Galletti F. Endoscopic sinus surgery with and without computer assisted navigation: a retrospective study. Auris Nasus Larynx. 2018; 10.1016/j.anl.2018.11.004. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 15. Freni F, Galletti B, Bruno R, et al. Multidisciplinary approach in the removal of post‐trauma foreign bodies in the head and neck district: cases report and review of literature. Acta Med Mediterr. 2019;35:405. [Google Scholar]
- 16. Galletti B, Gazia F, Galletti C, Galletti F. Endoscopic treatment of a periorbital fat herniation caused by spontaneous solution of continuity of the papyracea lamina. BMJ Case Rep. 2019;12(4):e229376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Chaganti J, Marriott D, Steel T, Donovan J, Biggs N. Perineural trigeminal nerve abscess secondary to mucor sinusitis: serial diffusion‐weighted MRI and literature review. Clin Radiol. 2011;66(11):1106‐1109. [DOI] [PubMed] [Google Scholar]
- 18. Skiada A, Lass‐Floerl C, Klimko N, Ibrahim A, Roilides E, Petrikkos G. Challenges in the diagnosis and treatment of mucormycosis. Med Mycol. 2018;56(suppl_1):93‐101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Grannan BL, Yanamadala V, Venteicher AS, Walcott BP, Barr JC. Use of external ventriculostomy and intrathecal anti‐fungal treatment in cerebral mucormycotic abscess. J Clin Neurosci. 2014;21(10):1819‐1821. [DOI] [PubMed] [Google Scholar]
- 20. Whalen MJ, Beyt BE Jr. Cryptic cerebral phycomycosis. Ann Intern Med. 1979;91:655. [DOI] [PubMed] [Google Scholar]
- 21. Hamill R, Oney LA, Crane LR. Successful therapy for rhinocerebral mucormycosis with associated bilateral brain abscesses. Arch Intern Med. 1983;143:581‐583. [PubMed] [Google Scholar]
- 22. Fong KM, Seneviratne EM, McCormack JG. Mucor cerebral abscess associated with intravenous drug abuse. Aust NZ J Med. 1990;20:74‐77. [DOI] [PubMed] [Google Scholar]
- 23. Hamilton JF, Bartkowski HB, Rock JP. Management of CNS mucormycosis in the pediatric patient. Pediatr Neurosurg. 2003;38:212‐215. [DOI] [PubMed] [Google Scholar]
- 24. Adler DE, Milhorat TH, Miller JI. Treatment of rhinocerebral mucormycosis with intravenous interstitial, and cerebrospinal fluid administration of amphotericin B: case report. Neurosurgery. 1998;42:644‐648. [DOI] [PubMed] [Google Scholar]
- 25. Ibrahim M, Chitnis S, Fallon K, Roberts T. Rhinocerebral mucormycosis in a 12‐year‐old girl. Arch Neurol. 2009;66:272‐273. [DOI] [PubMed] [Google Scholar]
- 26. Tsung LL, Zhu XL, Chu WC, Sun DT, Cheung KL, Leung TF. Intraventricular amphotericin for absidiomycosis in an immunocompetent child. Hong Kong Med J. 2010;16:137‐140. [PubMed] [Google Scholar]
- 27. Hibi A, Amakusa Y. Intracranial subdural abscess with polymicrobial infections due to frontal sinusitis in an adolescent: life‐threatening complication of a common disease. Clin Case Rep. 2018;6(3):516‐521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Kämppi A, Kämppi L, Kemppainen P, Kanerva M, Toppila J, Auranen M. Focal atrophy of the unilateral masticatory muscles caused by pure trigeminal motor neuropathy: case report. Clin Case Rep. 2018;6(5):939‐943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Roden MM, Zaoutis TE, Buchanan WL, et al. Epidemiology and outcome of zygomycosis: a review of 929 reported cases. Clin Infect Dis. 2005;41(5):634‐653. [DOI] [PubMed] [Google Scholar]
- 30. Pagliuca G, Rosato C, Martellucci S, et al. Cytologic and functional alterations of nasal mucosa in smokers: temporary or permanent damage? Otolaryngol Head Neck Surg. 2015;152(4):740‐745. [DOI] [PubMed] [Google Scholar]
- 31. Milardi D, Cacciola A, Calamuneri A, et al. The olfactory system revealed: non‐invasive mapping by using constrained spherical deconvolution tractography in healthy humans. Front Neuroanat. 2017;11:32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Rapidis AD. Orbitomaxillary mucormycosis (zygomycosis) and the surgical approach to treatment: perspectives from a maxillofacial surgeon. Clin Microbiol Infect. 2009;15(Suppl. 5):98‐102. [DOI] [PubMed] [Google Scholar]
- 33. Nikolaos P, Eleni P, Vassilios P, Christina V. A case of successfully treated rhinocerebral mucormycosis: dental implications. Int J Dent. 2010;2010:273127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Prabhu S, Alqahtani M, Al SM. A fatal case of rhinocerebral mucormycosis of the jaw after dental extractions and review of literature. J Infect Public Health. 2018;11(3):301‐303. [DOI] [PubMed] [Google Scholar]


