Abstract
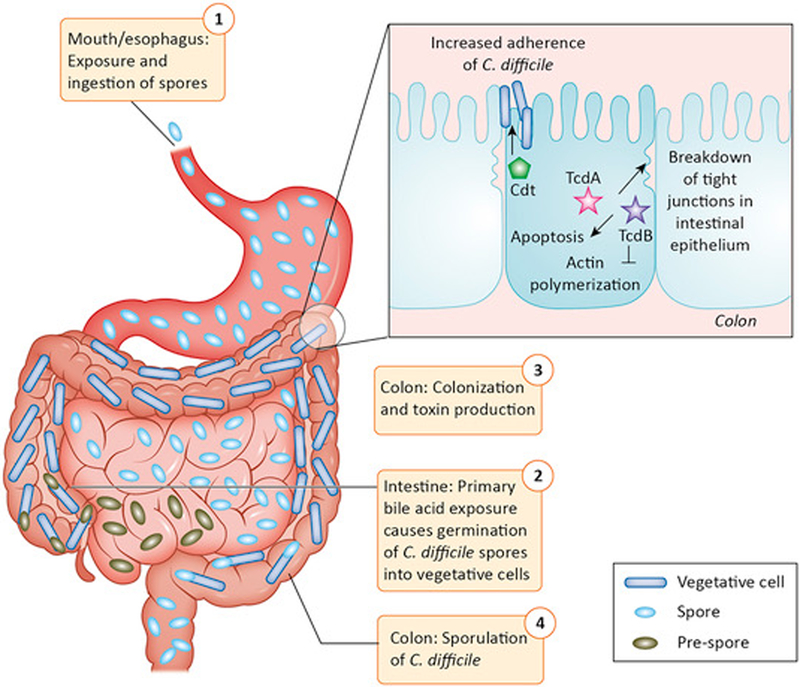
Clostridioides difficile is a spore-forming, anaerobic, intestinal pathogen that causes severe diarrhea that can lead to death. In 2011, C. difficile infected ~500,000 people in the US and killed ~29,000 people. C. difficile infection (CDI) is the most common health care related infection in the US, leading to increased health care costs of $4.8 billion dollars. This pathogen transmits via the oral-fecal route as a highly contagious and resilient spore. Upon exposure to primary bile acids in the intestine, C. difficile germinates, and in the absence of colonization resistance from the normal microbiota, the bacterium colonizes the colon and produces toxins. These toxins inhibit actin polymerization in host cells, leading to cell death. C. difficile cells can then sporulate in the intestine and exit the body via diarrheal shedding. In culture, sporulation is induced at stationary phase in a nutrient-limiting environment, but the intestinal triggers of sporulation are still unknown.
Key Facts:
C. difficile was first described in 1935, but wasn’t linked to antibiotic-associated diarrhea and pseudomembranous colitis until 1978.
C. difficile has a single, circular chromosome comprised of approximately 4.3 Mb.
C. difficile spores are resistant to heat, oxygen, and ethanol-based disinfectants, but can be killed by a 1:10 dilution of sodium hypochlorite (bleach).
CDI animal models include the hamster, mouse, young hare, infant pig, prairie dog, foal, quail, and, recently, the rat.
C. difficile toxin action has been characterized in the rat, mouse, hamster, rabbit, guinea pig, infant rhesus monkey, and the zebrafish embryo.
The two major toxins produced by C. difficile are TcdA and TcdB, which are glucosyltransferases that inhibit Rho, Rac, and Cdc42. A third toxin, Cdt, is found in the ribotype 027 strain.
Disease Facts:
People most at risk for CDI are the elderly, people in healthcare settings, and those who have received antibiotic treatment.
The antibiotics that provide the highest risk of CDI are clindamycin, floroquinolones, cephalosporins, monobactams, and carbapenems, with increased risk correlated with greater cumulative dose, number of antibiotics, and duration of exposure.
The estimated rate of CDI recurrence is 13.5% and death is 1.3%, both within the first 30 days of initial infection.
Taxonomy and classification:
Kingdom: Bacteria
Phylum: Firmicutes
Class: Clostridia
Order: Clostridiales
Family: Clostridiaceae
Genus: Clostridioides (formerly Peptoclostridium and Clostridium)
Species: difficile
Gram-positive
ACKNOWLEDGEMENTS
Details of toxin effects are adapted from Abt et al. (2016). Work in the McBride lab is supported by the U.S. National Institutes of Health through research grants A1121684 and A1116933 to S.M.M. The content of this manuscript is solely the responsibility of the authors and does not necessarily reflect the official views of the National Institutes of Health.
literature
- 1.Lessa FC, et al. , Burden of Clostridium difficile Infection in the United States. New England Journal of Medicine, 2015. 372(9): p. 825–834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dubberke ER and Olsen MA, Burden of Clostridium difficile on the Healthcare System. Clinical Infectious Diseases, 2012. 55: p. S88–S92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Raibaud P, et al. , Sodium Taurocholate, a Germination Factor for Anaerobic Bacterial-Spores Invitro and Invivo. Annales De Microbiologie, 1974. B125(3): p. 381–391. [PubMed] [Google Scholar]
- 4.Voth DE and Ballard JD, Clostridium difficile toxins: Mechanism of action and role in disease. Clinical Microbiology Reviews, 2005. 18(2): p. 247–+. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hall IC and O’Toole E, Intestinal flora in new-borin infants - With a description of a new pathogenic anaerobe, Bacillus difficilis. American Journal of Diseases of Children, 1935. 49(2): p. 390–402. [Google Scholar]
- 6.Bartlett JG, et al. , Role of Clostridium-Difficile in Antibiotic-Associated Pseudomembranous Colitis. Gastroenterology, 1978. 75(5): p. 778–782. [PubMed] [Google Scholar]
- 7.Best EL, Freeman J, and Wilcox MH, Models for the study of Clostridium difficile infection. Gut Microbes, 2012. 3(2): p. 145–167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Oka K, et al. , Establishment of an Endogenous Clostridium difficile Rat Infection Model and Evaluation of the Effects of Clostridium butyricum MIYAIRI 588 Probiotic Strain. Front Microbiol, 2018. 9: p. 1264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hutton ML, et al. , Small animal models for the study of Clostridium difficile disease pathogenesis. Fems Microbiology Letters, 2014. 352(2): p. 140–149. [DOI] [PubMed] [Google Scholar]
- 10.Abt MC, McKenney PT, and Pamer EG, Clostridium difficile colitis: pathogenesis and host defence. Nature Reviews Microbiology, 2016. 14(10): p. 609–620. [DOI] [PMC free article] [PubMed] [Google Scholar]