Abstract
Background:
Cytoreductive surgery for neuroendocrine tumor liver metastases improves survival and symptomatic control. However, the feasibility of adequate cytoreduction in patients with many liver metastases remains uncertain. We compared patient outcomes based on the number of lesions treated to better define the efficacy of cytoreductive surgery for numerous neuroendocrine tumor liver metastases.
Methods:
Patients undergoing hepatic cytoreductive surgery for gastroenteropancreatic neuroendocrine tumors were identified in our institutional surgical neuroendocrine tumor database. Imaging studies were reviewed to determine the liver tumor burden and percent cytoreduced. Overall survival and progressionfree survival were compared, using the number of lesions treated, percent tumor debulked, and additional clinicopathologic characteristics.
Results:
A total of 188 hepatic cytoreductive procedures were identified and stratified into groups according to the number of metastases treated: 1–5, 6–10, and >10. Median overall survival and progressionfree survival were 89.4 and 22.5 months, respectively, and did not differ significantly between groups. Greater than 70% cytoreduction was associated with significantly better overall survival than <70% cytoreduction (134 months versus 38 months).
Conclusion:
In patients with gastroenteropancreatic neuroendocrine tumors and liver metastases, >70% cytoreduction led to improved overall survival and progression-free survival and was achieved reliably in patients undergoing debulking of > 10 lesions. These data support an aggressive approach to patients with numerous neuroendocrine tumor liver metastases to achieve >70% cytoreduction.
Introduction
Neuroendocrine tumors (NETs) are a diverse group of neoplasms arising from endocrine cells in a number of different organs throughout the body. Around 60% of these neoplasms originate in the gastroenteropancreatic (GEP) axis,1 and the annual incidence of GEPNETs has increased steadily during the past 40 years to the current rate of 3.56 per 100,0 00.2 Upward of 60% of patients will present with or go on to develop metastatic disease,1,3 most commonly to the liver. Neuroendocrine tumor liver metastases (NETLMs) are associated with substantial morbidity from hormone production, as well as eventual replacement of the normal hepatic parenchyma, leading to liver failure, which is the leading cause of death in these patients.4
Despite the high frequency of metastatic disease in NETs, patient prognosis remains quite favorable, especially when compared with adenocarcinomas of the GEP axis. In contrast to many other metastatic GI malignancies, patients with NETLMs are not precluded from operative intervention. Patients may derive quite a substantial benefit from cytoreductive surgery in terms of symptomatic relief and survival.3,5–9 Recent studies have shown median overall survival of greater than 10 years in patients undergoing surgical debulking of NETLMs.7,8 As is suggested by the terms “cytoreductive” and “debulking,” operative treatment of NETLMs is rarely curative, with recurrence rates exceeding 90% even with an R0 resection.7,10 Other than major resections, a number of operative techniques, including wedge resection, enucleation, and ablation, have been employed to treat NETLMs to minimize the loss of normal liver tissue. Survival of patients treated with these parenchyma-sparing procedures has been comparable with those treated with formal hepatic resections.7,8,11
Despite the benefits of cytoreductive surgery, the optimal approach to patients with NETLMs continues to be refined. Historically, patients were considered for liver-directed surgery only when near complete12 or 90% cytoreduction10 could be achieved, but more recent series have demonstrated equivalent palliation of symptoms5 and overall survival8,9 when a lesser cytoreductive threshold of 70% is used. In the 2017 consensus guidelines from the North American Neuroendocrine Tumor Society, the use of parenchyma-sparing operative techniques along with a 70% debulking threshold was recommended for the treatment of NETLMs.3
Although there are several retrospective series supporting operative debulking when 70% cytoreduction can be achieved,8,9,11 the feasibility of reaching this goal in the face of multiple NETLMs has not been established. With delays in diagnosis of up to 7 years,1 patients are frequently not considered for debuliking because of the presence of numerous, bilobar metastases. Decreasing the threshold for cytoreduction allows for a much greater proportion of patients with NETLMs to have these procedures performed, but questions remain as to how well this can be achieved when there are many metastases present. We set out to compare patient outcomes based on the number of hepatic lesions treated to better define the efficacy of cytoreductive surgery for numerous NETLMs, the overall survival (OS) and progression-free survival (PFS), and rate of complications resulting from these procedures.
Methods
A prospectively maintained database from our single center, including patients undergoing surgery for NETs between 1999 and 2017, was reviewed. Patients undergoing hepatic cytoreductive surgery for GEPNETs, with or without concomitant resection of their primary tumor, were identified. All patients in the database provided informed consent in accordance with the protocol approved by the Institutional Review Board of the University of Iowa (Iowa City). Patient information was anonymized where possible and protected in compliance with the Health Insurance Portability and Accountability Act of 1996. Patients with primary tumors outside the gastroenteropancreatic axis (eg, bronchus, cervix) were excluded, as were patients with a different primary surgeon performing cytoreduction. We reviewed the clinicopathologic data, including patient age, sex, date of operation, operative details, nonoperative treatments, number of hepatic lesions treated, percent liver replaced, percent tumor debulked, symptomatic response, biochemical response, primary tumor site, primary tumor size, multifocality, grade, lymphovascular invasion, perineural invasion, 30-day complications, 30-day mortality, and transfusion requirements. Complications were graded using the Clavien-Dindo classification.13 The tumor grade was assigned according to the World Health Organization 2010 classification of tumors of the digestive system based on the Ki-67 index and mitotic rate.14 For patients in whom the Ki-67 index was calculated in both the primary tumor and metastases, the greater value was used to determine overall grade based on the findings of Keck et al.15
Cytoreductive procedures were performed with the aim to prolong survival or for palliation of symptoms and usually involved removal of the primary tumor and regional lymph nodes, and debulking of liver lesions. The latter consisted of formal and nonanatomic liver resections, enucleations, and ultrasonographically guided microwave and radiofrequency ablation. Patients with liver biopsies or enucleations performed for purely diagnostic purposes were excluded, and hepatic debulking was generally not attempted in patients with >50% liver replacement, patients with very numerous, small liver lesions where substantial cytoreduction was deemed improbable, or patients known to have grade 3 tumors from preoperative biopsy or earlier resection. All procedures were performed by a single surgeon, and the extent of the operative procedure was determined based on the preoperative imaging and intraoperative findings. Patients were stratified for analysis based on the number of hepatic lesions treated (1–5, 6–10, and >10 lesions).
Patients were generally seen 3 months after operation, with computed tomography/magnetic resonance imaging (CT/MRI) and serum NET markers obtained at this visit to determine the efficacy of the cytoreduction and to establish a new baseline. Subsequently, they were followed at 6-month intervals for imaging and biochemical surveillance. Patients uniformly received somatostatin analogs (SSAs) postoperatively, and disease progression was treated with either SSA dose escalation, peptide receptor radionuclide therapy (PRRT), targeted therapy, or chemotherapy. For each patient, preoperative and postoperative CTs and MRIs were reviewed by the surgeon, and these imaging studies were used to calculate the number of lesions present preoperatively, the percent of liver replaced by tumor preoperatively, and the percent of tumor successfully debulked postoperatively. The location of each NETLM on preoperative imaging was recorded on a diagram of the liver, the number of lesions totaled, and the percent hepatic replacement determined by summing the best estimates of the contribution of each metastasis. To calculate the percent tumor debulked, the preoperative and postoperative images were compared side by side, and the percent of cytoreduction was determined for each lesion. The completeness of ablation for each metastasis, weighted by its volume, was used to estimate the percent of tumor debulking achieved. Patients were stratified for analysis based on the amount of tumor cytoreduced (>90%, 70%–90%, and <70%).
Symptomatic response to debulking was evaluated by reviewing the preoperative and postoperative clinic notes. Patients were assessed for a decrease in frequency of bowel movements or for subjective improvement in diarrhea, flushing, abdominal pain, wheezing, or fatigue. Patients with functional pancreatic NETs (PNETs) were additionally appraised for a decrease in symptoms for their specific hormonal syndrome. Symptomatic response was classified as improved, stable, or worse. In patients with small bowel NETs (SBNETs), serum levels of serotonin, chromogranin A, neurokinin A, and pancreastatin were routinely obtained before debulking and at the 3- to 4-months follow-up visit. For PNETs, these hormones plus pancreatic polypeptide and other relevant functional markers (eg, gastrin for gastrinoma) were collected. A biochemical response was defined as a >50% decrease in at least one of the markers that were increased preoperatively.
Overall survival (OS) and progression-free survival (PFS) were calculated using the Kaplan-Meier method. Univariate analyses of clinicopathologic variables associated with survival were performed using Cox proportional hazard regression modeling. Complication rates were compared using Fisher’s exact test. The relationship between the number of lesions treated and the percent tumor debulked was examined using the Kruskal-Wallis test, analysis of variance, and Fisher’s exact test. Clinicopathologic characteristics were compared between groups using the Kruskal-Wallis test and Fisher’s exact test. Multivariate analysis was performed using Cox regression. Variables found to be significant on univariate analysis were included in a number of multivariate models, which were compared using the Akaike information criterion (AIC) to find the best fit.16 For patients who had undergone more than one cytoreductive operation, only the first procedure was used to calculate OS, while PFS, response to debulking, and complication rates were treated separately for all subsequent surgeries. All statistical analyses were performed in R (v 3.4.1, R Core Team, 2017, Vienna, Austria), using the packages rms (v 5.1–1, Harrell, FE, 2017) and eeptools (v 1.1.0, Knowles, JE, 2017).
Results
A total of 184 patients undergoing 188 cytoreductive operations met the inclusion criteria (Table 1). Of these, the majority were SBNETs (128) and PNETs (41). Other primary sites in the series included stomach (4), rectum (3), duodenum (3), pancreas and small bowel (3), colon (1), and 5 were unknown. Other than a single patient with MEN1, none had a known hereditary cancer syndrome. There were 100 male and 84 female patients; 3 males and 1 female underwent 2 cytoreductive procedures. The median age at the time of operation was 58 years. The majority of patients (74%) had concurrent resection of their primary tumor. Most patients were treated with SSAs before cytoreduction (61%), and this number increased to 97% postoperatively. Additional nonoperative treatments received by patients in this series included hepatic artery embolization ([HAE] 26%), systemic therapy (chemotherapy and targeted therapy; 26%), and PRRT (16%). Patient characteristics were similar between groups, with the exception of the duration of median follow-up, which was significantly less in the > 10 lesions group, and the number of patients receiving preoperative SSAs, which was greater in the >10 lesions group. When median follow-up time was examined using the reverse Kaplan-Meier estimator17 (which assesses follow-up in the absence of death), the > 10 lesions group continued to have significantly lesser median follow-up.
Table 1.
Comparison of patient characteristics stratified by number of NETLMs treated
| Overall | 1–5 Treated NETLMs | 6–10 Treated NETLMs | >10 Treated NETLMs | P value | |
|---|---|---|---|---|---|
| Number of operations | 188 | 73 | 54 | 61 | – |
| Median NETLMs treated (range) | 7 (1–67) | 3 (1–5) | 7.5 (6–10) | 17 (11–67) | – |
| Sex | .34 | ||||
| Male | 103 | 42 | 25 | 36 | – |
| Female | 85 | 31 | 29 | 25 | – |
| Median age (range, y) | 58 (15–80) | 60 (22–80) | 56.5 (15–79) | 58 (33–78) | .35 |
| Median follow-up (range, months) | 29.3 (0.4 – 214.8) | 39.4 (0.4 – 214.8) | 28.4 (2.1 – 207.1) | 18.8 (0.4 – 89.4) | < .01 |
| Median follow-up (reverse KM, 95% CI, months) | 39.2 (33.3 – 45.7) | 77.1 (49.6 – 117.1) | 40.4 (24.2 – 77.8) | 27.3 (15.8 – 35.4) | < .01 |
| Primary site | .26 | ||||
| SBNET | 128 | 48 | 34 | 46 | – |
| PNET | 41 | 14 | 15 | 12 | – |
| Other primary | 19 | 11 | 5 | 3 | – |
| Grade | .35 | ||||
| G1 | 72 | 34 | 17 | 21 | – |
| G2 | 97 | 31 | 30 | 36 | – |
| G3 | 15 | 6 | 5 | 4 | – |
| Lymphovascular invasion | 128 (88%) | 48 (90%) | 38 (86%) | 42 (86%) | .74 |
| Perineural invasion | 116 (83%) | 42 (82%) | 33 (77%) | 41 (89%) | .29 |
| Nonoperative treatments | – | ||||
| Hepatic artery embolization | 49 (26%) | 19 (27%) | 13 (24%) | 17 (28%) | .91 |
| Peptide receptor Radionucleotide therapy | 30 (16%) | 10 (14%) | 10 (19%) | 10 (16%) | .80 |
| Systemic therapy | 49 (26%) | 18 (26%) | 13 (24%) | 18 (30%) | .81 |
| Somatostatin analog preop | 114 (61%) | 41 (56%) | 28 (52%) | 45 (74%) | .03 |
| Somatostatin analog Postop | 180 (97%) | 66 (93%) | 54 (100%) | 60 (98%) | .09 |
The median number of lesions seen on preoperative imaging was 10, with a median liver replacement of 10% (Table 2). Both the number of lesions seen on imaging and liver replacement were positively correlated with the number of lesions treated. The mean tumor debulking achieved was 79%, and this did not differ significantly between the different groups, based on number of lesions treated (79% in the 1–5 lesions treated group, 78.% in the 6–10 lesions group, and 78% in the >10 lesions group). In contrast, the median tumor debulked was significantly greater in the 1–5 lesions group compared with the other 2 groups (95% versus 80% and 80%, respectively; P = . 01). A total of 70% cytoreduction was achieved in 76% of patients in the 1–5 lesion group, 84% in the 6–10 group, and 78% of the >10 group (P = . 62). Biochemical response was equivalent for all 3 groups, with a mean of 69% of patients demonstrating a greater than 50% decrease in at least 1 preoperatively increased NET marker. The rate of symptomatic response was greater in the >10 lesions group (60%) compared with the 1–5 (45%) and 6–10 (33%) groups (P = . 03). The most common complications were wound infections and anemia, and the most common major complications (grade 3 or 4) were bleeding (requiring intervention) and intraabdominal infections (Table 3). The overall rates of complications (52%) and grade 3 or 4 complications (15%) did not differ significantly between these three groups. Thrombocytopenia was a common postoperative lab finding and was seen more frequently in the >10 lesions treated group (44% versus 17% versus 12%; P < .01). No patients remained thrombocytopenic on follow-up, with many showing a transient thrombocytosis at their first postoperative visit (data not shown). The median OS for the entire cohort was 89 months, and the median PFS was 23 months. Neither OS nor PFS differed significantly between groups, based on number of lesions treated (Figs 1, A and 1, B).
Table 2.
Comparison of operative characteristics stratified by number of NETLMs treated
| Overall | 1–5 Treated NETLMs | 6–10 Treated NETLMs | >10 Treated NETLMs | P value | |
|---|---|---|---|---|---|
| Median lesions preop (range) | 10 (0–100) | 3 (0–60) | 9 (1–100) | 20 (1–100) | < .01 |
| Median % liver replacement (range) | 10 (0–70%) | 2 (0–55%) | 10 (2–60%) | 15 (2–70%) | < .01 |
| Primary resected | 139 ( 74%) | 54 (74%) | 41 (76%) | 44 (72%) | .87 |
| Median % tumor debulked (range) | 85 (0–100%) | 95 (0–100%) | 80 (20–100%) | 80 (50–100%) | .01 |
| Mean % tumor debulked (range) | 78.7 (0–100%) | 79.3 (0–100%) | 78.2 (20–100%) | 78.4 (50–100%) | .95 |
| Number reaching 70% cytoreduction | 138 (79%) | 50 (76%) | 41 (84%) | 47 (78%) | .62 |
| Biochemical response | 111 (69%) | 42 (71%) | 30 (67%) | 39 (68%) | .88 |
| Symptomatic response | 80 (45%) | 28 (42%) | 17 (33%) | 35 (60%) | .03 |
| All complications | 98 (52%) | 35 (48%) | 33 (61%) | 30 (49%) | .29 |
| Major complications (grade 3, 4) | 28 (15%) | 10 (14%) | 7 (13%) | 11 (18%) | .76 |
| Median overall survival (95% CI, months) | 89 (72.6 – NR) | 80 (65.9 – NR) | 131.5 (72.6 – NR) | 89 (67.3 – NR) | .55 |
| Median progression-free survival (95% CI, months) | 22.5 (18.8 – 31.8) | 23.3 (18.2 – 56.1) | 21.6 (18.1 – 38.4) | 21.8 (17 – 3.7) | .55 |
Table 3.
Complications stratified by number of NETLMs treated*
| 1–5 Treated NETLMs (n=73) | 6–10 Treated NETLMs (n=54) | >10 Treated NETLMs (n=61) | P value | |
|---|---|---|---|---|
| Overall Complications | 35 (48%) | 33 (61%) | 30 (49%) | .29 |
| Minor Complications | 31† (42%) | 29‡ (54%) | 27§ (44%) | .3 |
| Major Complications (Grade 3,4) | 10¶ (14%) | 7∥ (13%) | 11# (18%) | .76 |
| Thrombocytopenia | 9 (12%) | 9 (17%) | 27 (44%) | < .01 |
Complications were classified using the Clavien-Dindo system.
Wound infection or dehiscence (17), anemia (9), infectious (4), acute kidney injury (3), bile leak (2), clostridium difficile (2), seizure (2), atrial fibrillation (1), pneumothorax (1), ileus (1), urinary tract infection (1), and urinary retention (1).
Wound infection or dehiscence (10), anemia (8), infectious (7), urinary tract infection (3), pancreatic fistula (2), atrial fibrillation (2), dehydration (1), urinary retention (1), clostridium difficile (1), portal vein thrombosis (1), carcinoid crisis (1), neuropathy (1), dysphonia (1), and altered mental status (1).
Anemia (10), wound infectious or dehiscence (8), acute kidney injury (6), urinary tract infection (4), clostridium difficile (4), infectious (4), ileus (3), urinary retention (3), atrial fibrillation (2), pancreatic fistula (1), failure to thrive (1), heparin induced thrombocytopenia (1), pancreatitis (1), arrhythmia (1), and seroma (1).
Bleeding (3), bile leak (3), intraabdominal infection (3), pleural effusion (1), septic shock (1), respiratory failure (1), aki requiring stenting (1), gastric outlet obstruction (1)
bleeding (1), bile leak (1), intraabdominal infection (1), pancreatic fistula (1), septic shock (1), respiratory failure (1), and small bowel obstruction (1).
Bleeding (3), intraabdominal infection (3), renal failure (2), respiratory failure (2), choledocholithiasis (1), tumor lysis syndrome (1), failure to thrive (1), carcinoid crisis (1), and bile leak (1).
NOTE: Anemia defined as hemoglobin < 7 g/dl. Thrombocytopenia defined as a platelet count <100,000 per μl.
Fig 1.
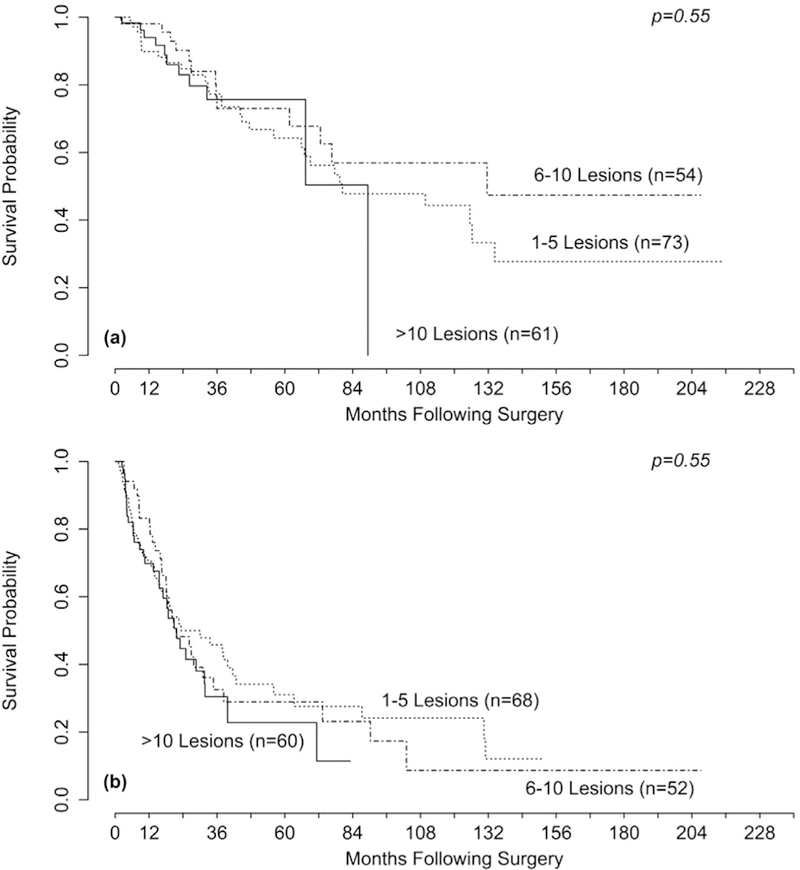
Kaplan-Meier curves for (A) overall survival (OS) and (B) progression-free survival (PFS) stratified by the number of NETLMs treated. The P value was obtained using Cox regression. There was no significant difference in OS or PFS between groups.
Univariate analysis of clinicopathologic factors associated with OS revealed that age, grade, Ki-67 index, percent liver replacement, number of lesions seen preoperatively, and percent tumor debulked were all significant (Table 4). Multivariate analysis was performed incorporating age, Ki-67 index or grade, percent liver replacement, number of lesions seen preoperatively, and percent tumor debulked. The A1C was used to compare several models, using 4 to 6 variables, and ultimately the model found to be most predictive included age, whether or not 70% debulking was achieved, grade, and percent liver replacement, all of which were significantly associated with OS.
Table 4.
Univariate and multivariate analysis of clinicopathologic variables associated with overall survival.
| Hazard ratio (univariate) | P value (univariate) | P value (multivariate) | |
|---|---|---|---|
| Sex (male versus female) | 1.05 | .87 | – |
| Age | 1.41 per 10 y | < .01 | < .01 |
| Grade | < .01 | – | |
| Grade 1 versus grade 2 | 2.12 | .02 | .02 |
| Grade 1 versus grade 3 | 11.69 | < .01 | < .01 |
| Primary Ki-67 | 1.23 per 5% | < .01 | – |
| Met Ki-67 | 1.63 per 5% | < .01 | – |
| Ki-67 Overall | 1.27 per 5% | < .01 | – |
| Mitotic rate | 1.07 | .11 | – |
| Lymphovascular invasion | 2.19 | .14 | – |
| Perineural invasion | 1.06 | .82 | – |
| Percent liver replacement | 1.37 per 10% | < .01 | .01 |
| NETLMs preop | 1.22 per 10 lesions | < .01 | – |
| Multifocality | 0.73 | 0.31 | – |
| Primary site (SB versus pancreas) | 0.82 | 0.53 | – |
| Number of lesions treated | 1.02 per 10 lesions | 0.94 | – |
| Percent tumor debulked | 0.81 per 10% | <0.01 | – |
| ≥70% versus <70% debulking | 0.34 | <0.01 | 0.01 |
| >90% versus ≤90% debulking | 0.53 | 0.055 | – |
| >90% versus 70%–90% Debulking | 0.82 | 0.61 | – |
| Surgery before 2010 | 0.98 | .95 | – |
| Primary resected | 1.09 | .81 | – |
| Primary size (largest dimension) | 1.07 per 1 cm | .24 | – |
| Nonsurgical treatments | – | ||
| Somatostatin analog preop | 1.83 | .03 | – |
| Somatostatin analog postop | 0.62 | .45 | – |
| Systemic therapy | 2.16 | < .01 | – |
| Hepatic artery embolization | 1.76 | .05 | – |
| Peptide receptor radionucleotide therapy | 0.69 | .26 | – |
On univariate analysis of factors associated with PFS, grade, Ki-67 index, percent liver replacement, number of lesions seen preoperatively, primary site (small bowel versus pancreas), percent tumor debulked, and concurrent primary resection were all found to be significant (Table 5). Again, several models incorporating combinations of Ki-67 index or grade, percent liver replacement, lesions seen preoperatively, percent tumor debulked, primary site, and concurrent primary resection were compared. The model found to be most predictive of PFS included Ki-67 index, percent liver replacement, percent tumor debulked, and concurrent primary resection, all of which were found to be significantly associated with PFS.
Table 5.
Univariate and multivariate analysis of clinicopathologic variables associated with progression-free survival
| Hazard ratio (univariate) | P value (univariate) | P value (multivariate) | |
|---|---|---|---|
| Sex (male versus female) | 0.93 | .70 | – |
| Age | 1.08 per 10 years | .36 | – |
| Grade | < .01 | – | |
| Grade 1 versus grade 2 | 2.25 | < .01 | – |
| Grade 1 versus grade 3 | 5.78 | < .01 | – |
| Primary Ki-67 | 1.58 per 5% | < .01 | – |
| Met Ki-67 | 1.35 per 5% | < .01 | – |
| Overall Ki-67 | 1.41 per 5% | < .01 | < .01 |
| Mitotic rate | 1.04 | .11 | – |
| Lymphovascular nvasion | 1.80 | .11 | – |
| Perineural nvasion | 1.19 | .57 | – |
| Percent liver replacement | 1.27 per 10% | < .01 | .04 |
| NETLMs preop | 1.18 per 10 lesions | < .01 | – |
| Multifocality | 0.77 | .30 | – |
| Primary site (SB versus pancreas) | 0.62 | .03 | – |
| Number of lesions treated | 1.06 per 10 lesions | .66 | – |
| Percent tumor debulked | 0.79 per 10% | < .01 | < .01 |
| ≥70% versus <70% debulking | 0.31 | < .01 | – |
| >90% versus ≤90% debulking | 0.36 | < .01 | – |
| >90% versus 70–90% debulking | 0.48 | < .01 | – |
| Surgery before 2010 | 0.76 | .23 | – |
| Primary resected | 0.61 | .02 | < .01 |
| Primary size (largest dimension) | 1.09 per 1 cm | .07 | – |
| Nonsurgical treatments | – | ||
| Somatostatin analog preop | 1.82 | < .01 | – |
| Somatostatin analog postop | 1.00 | 1.0 | – |
| Systemic therapy | 3.08 | < .01 | – |
| Hepatic artery embolization | 1.71 | < .01 | – |
| Peptide receptor radionucleotide therapy | 1.56 | .06 | – |
When survival was examined relative to specific debulking thresholds, 70% cytoreduction was associated with better OS compared with <70% (median 134.3 months vs 37.6 months, P < .01), and 90% cytoreduction was not associated with improved OS when compared with 70%–90% (median not reached versus 134 months, P = .6) or to <90% (median not reached versus 76.6 months, P = . 06; Table 4, Fig 2, A). In contrast, improved PFS was seen when comparing 70%–90% cytoreduction to <70% (20.6 vs 10.8 months), and when comparing >90% cytoreduction to 70–90% (56.1 vs 20.6 months, P < .01 each; Table 5, Fig 2, B). Patients in whom >90% cytoreduction was achieved had significantly fewer lesions preoperatively, fewer lesions treated, and lesser percent liver replacement (Table 6). Conversely, patients who had <70% cytoreduction had significantly more lesions seen preoperatively and greater percent liver replacement.
Fig 2.
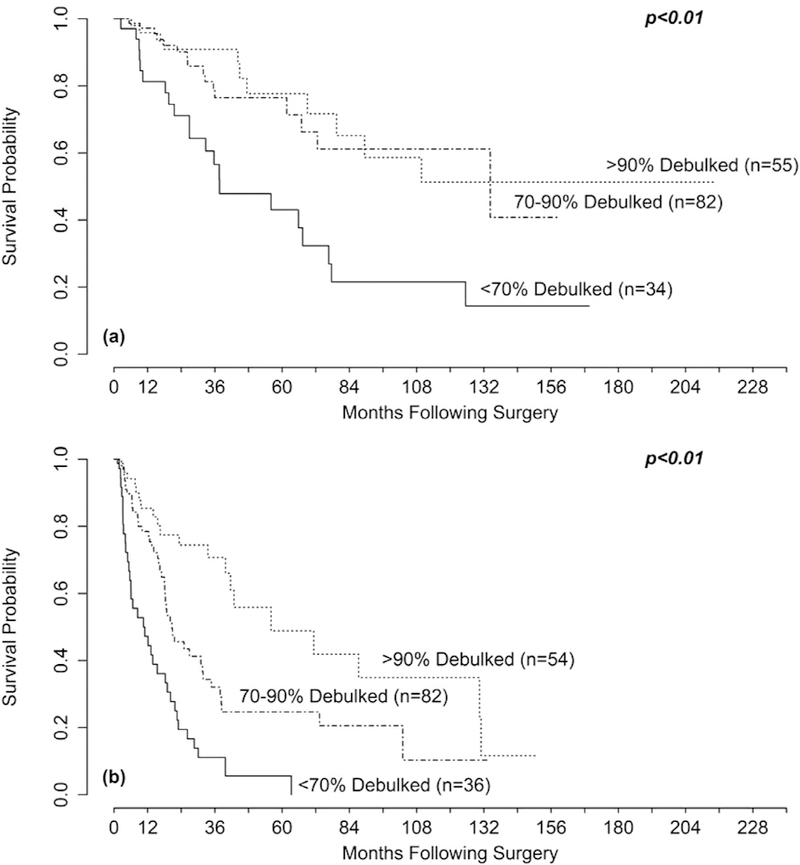
Kaplan-Meier curves for (A) overall survival (OS) and (B) progression-free survival (PFS) stratified by the amount of hepatic tumor debulked. The P values were obtained using Cox regression. There was a difference in (A) OS comparing <70% to 70%–90% (P < .01) but not 70%–90% to >90% (P=. 6). There was a significant difference in (B) PFS comparing < 70% to 70%–90% (P < .01) and 70%–90% to >90% (P < .01).
Table 6.
Comparison of operative characteristics stratified by the amount of hepatic tumor debulked
| Overall | <70% debulking | 70–90% debulking | >90% debulking | P value | |
|---|---|---|---|---|---|
| Number of operations | 175 | 37 | 83 | 55 | – |
| Median lesions preop (range) | 10 (0–100) | 22 (1–100) | 11 (1–64) | 2 (0–31) | < .01 |
| Median lesions treated (range) | 7 (1–67) | 7 (1–36) | 9 (1–67) | 3 (1–23) | < .01 |
| Median liver replacement (range) | 10% (0–70%) | 30% | 12% | 2% | < .01 |
| Primary resected | 139 (74%) | 31 (84%) | 60 (72%) | 38 (69%) | .28 |
| Biochemical response | 111 (69%) | 21 (66%) | 52 (68%) | 34 (71%) | .90 |
| Symptomatic response | 80 (45.2%) | 15 (44%) | 37 (46%) | 26 (47%) | .97 |
| Complications | 98 (52%) | 18 (49%) | 46 (55%) | 25 (45%) | .50 |
| Major complications | 28 (15%) | 6 (16%) | 12 (14%) | 8 (15%) | .96 |
| Median overall survival (95% CI, months) | 89 (72.6 – NR) | 38 (26.9 – 78) | 134.3 ( 73 – NR) | NR (79 – NR) | < .01 |
| Median progression-free survival (95% CI, months) | 22.5 (19 – 32) | 10.8 (6 – 19) | 20.6 (18 – 35) | 56 (42 – NR) | < .01 |
The percent of patients in whom 70% cytoreduction was achieved was evaluated relative to the percent liver replacement and the number of NETLMs seen preoperatively to determine whether there was a point at which this threshold was no longer reliably reached (Figs 3, A, and 3, B). A 70% cytoreduction was achieved 83% of the time with ≤45% liver replacement and 17% of the time with >45% replacement (P < .01). When ≤30 lesions were seen preoperatively, the rate of achieving 70% debulking was 85%, and when >30 lesions were seen, the rate was 46% (P < .01). Only 12 patients had >45% liver replacement, and 28 patients had more than 30 lesions.
Fig 3.
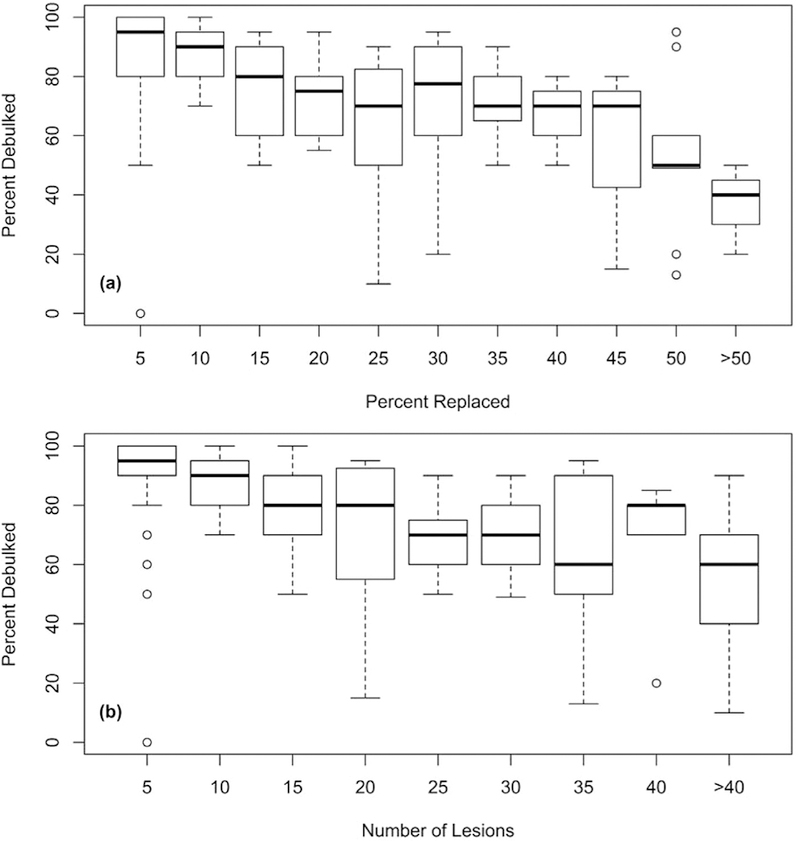
Box plots showing (A) amount of tumor debulked against the percent liver replacement and (B) number of lesions seen preoperatively. Percent liver replacement has been divided into increments of 5%, number of liver lesions has been divided into increments of 5 lesions. Groups are labeled corresponding to the top of their range (eg, the group labeled 10 in [A] contains all patients with >5% and ≤10% liver replacement).
Discussion
The primary aim of this study was to investigate the safety and efficacy of cytoreductive surgery in patients having liver-directed procedures for large numbers of NETLMs. Surgeons who treat NETs are frequently confronted with numerous bilobar NETLMs, which are not amenable to formal resection. The 70%-debulking threshold, which has been supported by previous studies,8,9,11 was again found to be associated with significantly improved OS and PFS (Figs 2, A and 2, B). Studies from groups using the objective of achieving 90% cytoreduction have found that less than 25% of patients with NETLMs are offered debulking,6,11,18 in contrast to as many as 76% being offered debulking surgery when a threshold of 70% cytoreduction is used.8 The feasibility of reaching this 70% threshold in patients with numerous metastases, however, is not clear. We explored this question by grouping patients according to the number of hepatic lesions treated. Our data showed no significant difference in the rate at which 70% debulking was achieved in patients with 1–5, 6–10, or >10 lesions treated, no difference in biochemical response, and no significant differences in OS or PFS among these groups. Moreover, these results were obtained with similar complication rates among groups, showing that extensive hepatic cytoreduction can be performed with acceptable safety. Symptomatic response rates were greater in patients with > 10 lesions treated, and the median tumor-debulking percentage was greater when 1–5 lesions were treated, but otherwise there were no significant differences in surgical outcomes among groups, based on the number of lesions treated (Table 2).
The retrospective nature of our study and lack of nonsurgical control patients makes the comparison with nonoperative treatment difficult, but population-based retrospective studies can be used as a framework for comparison. Jiao et al19 reviewed a large community oncology network for patients diagnosed with metastatic GEPNETs between 2008 and 2012 (after the introduction of SSAs). In this study, 16% of patients were observed only, and 84% of patients received treatment with some combination of SSAs (73%), chemotherapy (18%), or sunitinib/everolimus (14%). The median OS in this group was 68 months, with age, body mass index, and primary site significantly associated with survival.19 A review of the surveillance, epidemiology, and end result database by Dasari et al evaluated the OS for patients with well to moderately differentiated metastatic NETs diagnosed between 2000 and 2012 and found a median OS of 103 months for SBNETs and 60 months for PNETs.2 It is important to note that these studies define OS from the time of diagnosis, and in our study it was defined from the time of the debulking operation. For comparison, when calculating OS in our cohort from the time of diagnosis, patients with low-grade (G1/G2) SBNETs survived a median of 163 months, and those with low-grade PNETs survived a median of 154 months. A recent multi-institutional retrospective study of 339 undergoing operative management of NETLMs by Mayo et al7 reported a 5 year OS of 74% and median OS of 125 months. Another recent study by Morgan et al11 reported on 44 patients with PNETs undergoing cytoreductive surgery, finding a 5-year OS of 81% (median not reached) and median PFS of 11 months. A number of retrospective studies have examined survival in patients undergoing debulking surgery for NETLMs, with reported 5-year OS, ranging from 41% to 87%, as summarized by Maxwell et al.8 Direct comparisons between studies are difficult because of the heterogeneous patient populations, treatments received (medical, embolic, PRRT versus operative), differet operative techniques used (major resections versus parenchymal sparing), and the cytoreduction thresholds employed (70% vs 90%). The 5-year OS for this study was 68%, and the median OS was 89 months, which included 15 patients with high-grade tumors. Of these patients, 4 were known to have high-grade tumors before resection (with Ki-67 indices ranging from 20%–28% on final pathology), 10 had biopsies showing low-grade NETs or not specifying grade, and 1 patient did not have a preoperative biopsy. The primary sites for high-grade NETS were the small bowel (7), pancreas (5), and duodenum (1), with 2 remaining unknown after exploration. When these high-grade patients were excluded, the median OS was 110 months, which is much greater than seen in national databases of patients with metastatic disease and comparable with other large cytoreduction series.
A number of clinicopathologic variables were found to be associated with OS and PFS on univariate and multivariate analysis (Tables 4 and 5). Of interest, although grade and Ki-67 index were associated with OS, mitotic rate was not, nor was the presence of lymphovascular or perineural invasion. Multivariate analysis was performed using several different models, incorporating variables found to be statistically significant on univariate analysis, which were then compared using the A1C. A few general trends were noted during model selection: Grade and Ki-67 index were strongly associated with both OS and PFS regardless of which other variables were included (Figs 4, A and 4, B). This observation is consistent with previous studies that have found that grade is a strong prognostic factor for both OS15,20 and PFS.15 Furthermore, comparing the 23-month median OS and no 5-year OS for highgrade patients in our series to the estimated 25% 5-year OS described by Richards-Taylor et al20 in a large meta-analysis suggests minimal to no benefit from debulking surgery in patients with grade 3 primaries or metastases. 1ncorporating the number of lesions seen preoperatively or the primary site tended to decrease the quality of the model, and, in models that used these variables, they were not found to significantly correlate with OS or PFS (data not shown). For OS, models incorporating percent tumor debulked as a continuous variable had similar AIC values as those which used a categoric cutoff at 70%, lending further support for the use of this threshold. Likewise, models using grade, or the log of Ki-67 index, rather than Ki-67 index as a continuous variable were found to be more predictive of OS, suggesting that, at least beyond a certain point, risk of death does not scale linearly with Ki-67 index. This finding is contrasted by models for PFS which were found to be more predictive when Ki-67 index and percent tumor debulked were treated as continuous variables rather than using grade categories or a debulking threshold of 70%.
Fig 4.
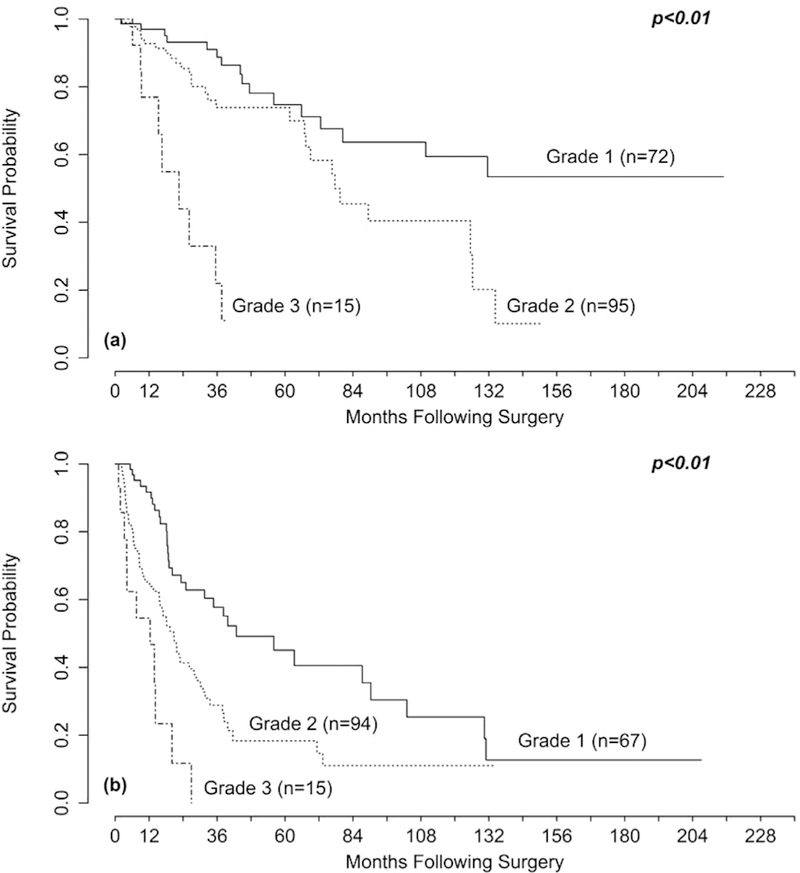
Kaplan-Meier curves for (A) overall survival (OS) and (B) progression-free survival (PFS) tratifled by tumor grade. The P values were obtained using Cox regression. There was a difference in (A) OS comparing grade 1 to grade 2 (P=.02) and grade 2 to grade 3 (P < .01) and in (B) PFS comparing grade 1 to grade 2 (P < .01) and grade 2 to grade 3 (P=.02).
One limitation of these data is that the follow-up time in each group, either analyzed as median follow-up time or with a reverse Kaplan-Meier predictor (censoring death), was significantly less in the >10 lesions treated group. This trend likely reflects a broadening of operative inclusion criteria over the years that has accompanied increased experience with parenchyma-sparing techniques and extensive hepatic debulking.
The rapid evolution of nonsurgical treatments for NETs during the past several years introduces the possibility for further bias. During the period of our study, the PROMID and CLARINET trials demonstrated the antiproliferative, benefits of SSAs,21,22 the RADIANT-4 trial showed improved PFS for patients with advanced lung or GI NETs treated with everolimus,23 treatment of patients with advanced PNETs with sunitinib revealed improved PFS,24 and the NETTER-1 trial reported improved PFS in patients with advanced midgut NETs treated with 177lu-dotatate compared with high-dose octreotide.25 Despite this changing landscape, we found similar rates of treatment with postoperative SSAs, systemic therapy, PRRT, and HAE between groups (Table 2). In addition, the date of operative debulking was not correlated with OS or PFS on univariate analysis when assessed either as a continuous variable or as a categorical variable, using 2010 (the approximate midpoint of our series) as a cutoff. Treatment with preoperative SSAs, systemic therapy, or HAE was associated with worse OS and PFS in our study, likely reflecting their use in patients with more aggressive disease (Tables 4 and 5).
In conclusion, this study validates the use of a 70% threshold for attempting cytoreduction in patients with NETLMs to improve OS and demonstrates that this target can be achieved reliably even in patients undergoing treatment of more than 10 hepatic lesions. Furthermore, complication rates, biochemical response, OS, and PFS were similar regardless of whether 1–5, 6–10, or > 10 lesions were treated. These results should not be misconstrued to suggest that there is no upper limit to the number of lesions or amount of liver replacement which can be treated effectively with cytoreductive surgery because the degree of difficulty increases with the number of lesions and percent of liver replacement. Based on this series, 70% cytoreduction is less commonly achieved in patients with >45% liver replacement or >30 NETLMs seen preoperatively, suggesting a limited role for debulking surgery in these patients, and strong consideration of nonopertive options, such as chemotherapy, targeted therapy, HAE, or PRRT. Similarly, patients with high-grade NETs did not benefit from cytoreduction, emphasizing the importance of preoperative biopsy with evaluation for Ki-67, and ultimately medical rather than operative management.
☆.
Supported by T32 grant CA148062–0 (Aaron T. Scott) and by SPORE grant P50 CA174521-01 (James R. Howe, Thomas M. O’Dorisio, Andres M. Bellizzi, Joseph S. Dillon, and Patrick Breheny).
Discussion
Dr Xavier Keutgen (Chicago, IL): Great talk. I love it. It shows that liver debulking is feasible.
There are a few points: First of all, I was pretty interested to see that you debulked 15 patients with grade 3 tumors. Can you tell us more about this? Because this is unusual.
Dr Aaron T Scott: It is. And it certainly is not our routine practice to debulk patients with known high-grade disease. Our data demonstrated significantly worse outcomes in those patients. Of these 15 patients that were in this series, 10 had biopsies before surgery, which showed either low-grade neuroendocrine tumors or showed neuroendocrine tumor without specifying the grade. One did not undergo preoperative biopsy. Of the 4 that were known to have high-grade disease before surgery, they had limited disease and the Ki-67 index on the final pathology was 20%–28%. Therefore we felt that they might benefit from cytoreductive surgery.
Dr Xavier Keutgen (Chicago, IL): Regarding your last point about the greater-than-30 lesions, I think that’s a little dangerous to put out there. At least in my experience, it’s common that you think you are dealing with maybe 15 or 20 lesions. Once you get in, suddenly you see all these tiny superficial lesions that you cannot detect preoperatively. So, should you just bail out?
Dr Aaron T Scott : No, and I think that’s an excellent point. We offer both of those cutoffs (number of lesions and percent replacement) as factors to consider rather than as hard and fast rules. And I would also emphasize the point that that the threshold of 30 liver metastases was based on what you could see preoperatively rather than what you would discover intraoperatively, which is often much more.
Dr Xavier Keutgen (Chicago, IL): As a follow-up, do you think that looking at tumor volume would be a better predictor?
Dr Aaron T Scott: I think they are both reasonable factors to consider and both the number of metastases and the tumor volume increase the difficulty of the surgery. Our data also show that the cutoff for tumor volume was more strongly associated with not being likely to achieve 70% debulking than the number of lesions.
Dr Janice Pasieka (Calgary, AB): How do you estimate this percentage? I find that very difficult. Are you doing this on preoperative imaging and then postoperative imaging? Or is this just a gestalt?
Dr Aaron T Scott: It is, as you highlighted, a very difficult job sometimes to estimate accurately.
The way that we did this in our study was to carefully review the preoperative and postoperative imaging. In our case, preoperative films were usually CT and an MRI, and postoperatively patients had a CT obtained at 3 months.
On review of the preoperative imaging, the locations and sizes of all the visible metastases were carefully noted on a diagram of the liver. The number of lesions and the sum of their volumes were estimated from this diagram to come up with the percent replacement. In order to determine the percent of cytoreduction, we compared the preoperative and postoperative films side by side. For every lesion that was noted on our diagram, we estimated the completeness of treatment for that lesion and then, by aggregating the contribution of each, came up with the final value for cytoreduction.
Dr John Chabot (New York, NY): Thank you for continuing to guide us with these very difficult decisions. I am going to push you a little bit on your last point, which was that 70% and 90% are similar.
Does that mean when you hit 70% debulking, you stop if you think you can get to 90% or 100%?
Dr Aaron T Scott: No, I think certainly we would not advocate for stopping at 70%. Although we did not demonstrate a difference in overall survival, we did show that 90% cytoreduction was associated with significantly improved progression-free survival. There may be a smaller difference in overall survival associated with 90% cytoreduction, which we were unable to detect. Get as much as you can, I think, is the take-home point.
Footnotes
Presented at the 2018 annual meeting of the American Association of Endocrine Surgeons.
References
- 1.Hallet J, Law CH, Cukier M, Saskin R, Liu N, Singh S. Exploring the rising incidence of neuroendocrine tumors: A population-based analysis of epidemiology, metastatic presentation, and outcomes. Cancer. 2015;121:589–2597. [DOI] [PubMed] [Google Scholar]
- 2.Dasari A, Shen C, Halperin D, Zhao B, Zhou S, Xu Y, et al. Trends in the incidence, prevalence, and survival outcomes in patients with neuroendocrine tumors in the United States. JAMA Oncol, 2017;3:1335–1342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Howe JR, Cardona K, Fraker DL, Kebebew E, Untch BR, Wang YZ, et al. The surgical management of small bowel neuroendocrine tumors: Consensus guidelines of the North American Neuroendocrine Tumor Society. Pancreas, 2017;46: 715–731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Givi B, Pommier SJ, Thompson AK, Diggs BS, Pommier RF. Operative resection of primary carcinoid neoplasms in patients with liver metastases yields significantly better survival. Surgery. 2006;140:891–897. [DOI] [PubMed] [Google Scholar]
- 5.Chambers AJ, Pasieka JL, Dixon E, Rorstad O. The palliative benefit of aggressive surgical intervention for both hepatic and mesenteric metastases from neuroendocrine tumors. Surgery. 2008;144:645–651. [DOI] [PubMed] [Google Scholar]
- 6.Chamberlain RS, Canes D, Brown KT, Saltz L, Jarnagin W, Fong Y, et al. Hepatic neuroendocrine metastases: Does intervention alter outcomes? J Am Coll Surg, 2000;190:432–445. [DOI] [PubMed] [Google Scholar]
- 7.Mayo SC, de Jong MC, Pulitano C, Clary BM, Reddy SK, Gamblin TC, et al. Surgical management of hepatic neuroendocrine tumor metastasis: Results from an international multiinstitutional analysis. Ann Surg Oncol. 2010;17: 3129–3136. [DOI] [PubMed] [Google Scholar]
- 8.Maxwell JE, Sherman SK, O’Dorisio TM, Bellizzi AM, Howe JR. Liver-directed surgery of neuroendocrine metastases: What is the optimal strategy? Surgery. 2016;159:320–333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Graff-Baker AN, Sauer DA, Pommier SJ, Pommier RF. Expanded criteria for carcinoid liver debulking: Maintaining survival and increasing the number of eligible patients. Surgery. 2014;156:1369–1376. [DOI] [PubMed] [Google Scholar]
- 10.Sarmiento JM, Heywood G, Rubin J, Ilstrup DM, Nagorney DM, Que FG, Surgical treatment of neuroendocrine metastases to the liver: A plea for resection to increase survival. J Am Coll Surg. 2003;197:29–37. [DOI] [PubMed] [Google Scholar]
- 11.Morgan RE, Pommier SJ, Pommier RF. Expanded criteria for debulking of liver metastasis also apply to pancreatic neuroendocrine tumors. Surgery. 2018;163:218–225. [DOI] [PubMed] [Google Scholar]
- 12.Elias D, Lasser P, Ducreux M, Duvillard P, Ouellet JF, Dromain C, et al. Liver resection (and associated extrahepatic resections) for metastatic well-differentiated endocrine tumors: A 15-year single center prospective study. Surgery. 2003;133:375–382. [DOI] [PubMed] [Google Scholar]
- 13.Dindo D, Demartines N, Clavien PA. Classification of surgical complications: A new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg. 2004;240:205–213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bosman FT, Carneiro F, Hruban RH, Theise ND, WHO classification of tumours of the digestive system, 4th ed. Lyon, France: IARC Press; 2010. [Google Scholar]
- 15.Keck KJ, Choi A, Maxwell JE, Li G, O’Dorisio TM, Breheny P, et al. Increased grade in neuroendocrine tumor metastases negatively impacts survival. Ann Surg Oncol, 2017;24:2206–2212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Akaike H Information theory and an extension of the maximum likelihood principle. In: Petrov BN, Csaki F, eds. Second international symposium on information theory Akadémiai Kiado; 1973:267–281 [Google Scholar]
- 17.Schemper M, Smith TL. A note on quantifying follow-up in studies of failure time. Control Clin Trials, 1996;17:343–346. [DOI] [PubMed] [Google Scholar]
- 18.Glazer ES, Tseng JF, Al-Refaie W, Solorzano CC, Liu P, Willborn KA, et al. Long-term survival after surgical management of neuroendocrine hepatic metastases. HPB (Oxford). 2010;12:427–433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jiao X, Pulgar S, Boyd M, Braiteh F, Mirakhur B, Pitman Lowenthal S, et al. Treatment Patterns and Clinical Outcomes in Patients With Metastatic Gastroen-teropancreatic Neuroendocrine Tumors Treated in the Community Practice Setting in the United States. Pancreas. 2018;47:173–182. [DOI] [PubMed] [Google Scholar]
- 20.Richards-Taylor S, Ewings SM, Jaynes E, Tilley C, Ellis SG, Armstrong T, et al. The assessment of Ki-67 as a prognostic marker in neuroendocrine tumours: A systematic review and meta-analysis. J Clin Pathol. 2016;69:612–618. [DOI] [PubMed] [Google Scholar]
- 21.Rinke A, Muller HH, Schade-Brittinger C, Klose KJ, Barth P, Wied M, et al. Placebocontrolled, double-blind, prospective, randomized study on the effect of oc-treotide LAR in the control of tumor growth in patients with metastatic neuroendocrine midgut tumors: A report from the PROMID Study Group. J Clin Oncol. 2009;27:4656–4663. [DOI] [PubMed] [Google Scholar]
- 22.Caplin ME, Pavel M, Cwikla JB, Phan AT, Raderer M, Sedlackova E, et al. Lanreotide in metastatic enteropancreatic neuroendocrine tumors. N Engl J Med, 2014;371:224–233. [DOI] [PubMed] [Google Scholar]
- 23.Yao JC, Fazio N, Singh S, Buzzoni R, Carnaghi C, Wolin E, et al. Everolimus for the treatment of advanced, non-functional neuroendocrine tumours of the lung or gastrointestinal tract (RADIANT-4): A randomised, placebo-controlled, phase 3 study. Lancet, 2016;387:968–977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Raymond E, Dahan L, Raoul JL, Bang YJ, Borbath I, Lombard-Bohas C, et al. Sunitinib malate for the treatment of pancreatic neuroendocrine tumors. N Engl J Med, 2011;364:501–513. [DOI] [PubMed] [Google Scholar]
- 25.Strosberg J, El-Haddad G, Wolin E, Hendifar A, Yao J, Chasen B, et al. Phase 3 trial of 177 lu-dotatate for midgut neuroendocrine tumors. N Engl J Med, 2017;376:125–135 [DOI] [PMC free article] [PubMed] [Google Scholar]


