Abstract
FASL (CD178) is known for its role in triggering apoptosis, mostly in relation with immune cells but additional functions have been reported more recently, including those in bone development. Examination of postnatal FasL‐deficient mice (gld) showed an increased bone deposition in adult mice when compared with wild types. However, a different phenotype was observed prenatally, when the gld bone was underdeveloped. The aim of the following investigation was to evaluate this indication for an growth‐dependent bone phenotype of gld mice and to search for the ‘switch point’. This study focused on the mandibular/alveolar bone as an important structure for tooth anchorage. In vivo micro‐computed tomography (CT) analysis was performed at different stages during the first month (6, 12 and 24 days) of postnatal bone development. In 6‐day‐old gld mice, a decrease in bone volume/tissue volume (BV/TV), trabecular thickness and trabecular number was revealed. In contrast, the 12‐day‐old gld mice showed an increased BV/TV and trabecular thickness in the alveolar bone. The same observation applied for bone status in 24‐day‐old gld mice. Therefore, changes in the bone phenotype occurred between day 6 and 12 of the postnatal development. The switch point is likely related to the changing proportion of bone cells at these stages of development, when the number of osteocytes increases. Indeed, the immunohistochemical analysis of FASL localized this protein in osteoblasts, whereas osteocytes were mostly negative at examined stages. The impact of FASL particularly on osteoblasts would agree with an earlier in vivo observed effect of FASL deficiency on expression of Mmp2, typical for osteoblasts, in the gld mandibular/alveolar bone. Notably, an age‐dependent bone phenotype was reported in Mmp2‐deficient mice.
Keywords: alveolar bone phenotype, fasL, microCT, mouse, postnatal development
Introduction
FASL (CD178), the exclusive ligand of the FAS receptor (CD95), is an important cell regulator with a major effect on the extrinsic apoptotic pathway (Wajant, 2002). In addition to this role, novel, non‐apoptotic‐related functions of FASL have been reported (Wajant et al. 2003). Both apoptotic and non‐apoptotic effects of FASL are likely to take place during bone development, where two major ways of ossification can be distinguished: endochondral and intramembranous (Aghaloo et al. 2010). The endochondral ossification, typical for long bones, includes a cartilaginous anlage, whereas the intramembranous one, typical for the craniofacial region, does not. In both types of bone, osteoblasts, osteocytes and osteoclasts participate in bone formation, remodelling and homeostasis (Florencio‐Silva et al. 2015).
From previous reports, FASL seems to play multiple roles in osteogenesis. The FAS‐FASL system is involved in the induction of apoptosis in osteoblasts (Ozeki et al. 2002), and of pre‐osteoclasts (Nakamura et al. 2007; Krum et al. 2008). Non‐apoptotic functions of FASL were also reported during the differentiation of osteoclastic (Park et al. 2005) and osteoblastic cells in vitro (Kovacic et al. 2007). In vivo, non‐apoptotic evidence of FASL functions was first reported in endochondral bone (Katavic et al. 2003), and recently also in intramembranous bone (Svandova et al. 2018), where a decreased expression of Mmp2 was demonstrated in samples from FasL‐deficient (gld) mice. Examination of adult gld mice showed an increased bone deposition when compared with wild types (Katavic et al. 2003). However, the bone architecture appeared as underdeveloped prenatally (Svandova et al. 2018).
The following investigations therefore aimed to further determine a growth‐dependent change in the bone phenotype of gld mice. Intramembranous mandibular/alveolar bone, as a structure necessary for proper tooth anchorage, was examined in the region of the first lower molar. In vivo micro‐computed tomography (CT) analysis was performed to search for the ‘switch point’ between the early and late bone phenotypes. Additional temporo‐spatial investigation was performed using histological sections to search for possible mechanisms underlying the phenomenon.
Materials and methods
Animals
Mice (Mus musculus) of the ICR strain were studied at postnatal (P) stages P6, 12 and 24. The FasL‐deficient mice (gld) strain B6Smn.C3‐FASL gld/J, and the corresponding controls (C57BL/6J) were purchased from The Jackson Laboratory (Bar Harbor, ME, USA) and staged at P6, 12 and 24. Mice were kept in the animal facilities of the Institute of Animal Physiology and Genetics, The Czech Academy of Sciences, v.v.i., Czech Republic, and Faculté de Chirurgie Dentaire, Université Paris Descartes, Paris, France. All experiments with animals were performed using a protocol approved by the Animal Care Committee of the University Paris Descartes (Agreement APAFIS #6173 N°2015112518204933). Animals were maintained according to the guidelines for ethical conduct developed by the European Communities Council Directive (animal breeding agreement C92‐049‐01). All efforts were made to minimize animal pain or discomfort.
X‐ray micro‐CT examination of samples
For bone evaluation, mice were anesthetized (isoflurane, induction at 3–11.4% under an airflow of 0.8–1.5 L min−1, and 1.5–2% under 400–800 mL min−1 thereafter) to perform in vivo micro‐CT. They were imaged using an X‐ray micro‐CT device (Quantum FX Caliper; Life Sciences, Perkin Elmer, and Waltham, MA, USA). The X‐ray source was set at 90 kV and 160 μA. Tridimensional images were acquired with an isotropic voxel size of 20 μm. An internal density phantom, calibrated in mg of hydroxyapatite, was used to scale bone density. Full 3D high‐resolution raw data were obtained by rotating both the X‐ray source and the flat panel detector 360° around the sample (scanning time: 3 min). First, image stacks were oriented in the same direction using dataviewer (Skyscan, release 1.5.2.4; Kontich, Belgium) to determine the region of interest (ROI). Secondly, quantification of the jaw bone microarchitecture below the first molar was performed using CT scan analyser software (Skyscan, release 1.13.5.1). ROI was drawn in the axial sections along the cortical zone between the mesial roots of the first and second molar. Batch processing was applied on our data including a median filter (with radius 1) for noise reduction, and adaptive thresholding was performed with a radius of 1, between 398 and 532 mg HA cm−3. Following segmentation, batch processing calculated 3D parameters of the binary image: bone volume fraction BV/TV (%), trabecular thickness Tb.Th (mm), trabecular number Tb.N (1/mm), trabecular separation Tb.Sp (mm), trabecular pattern factor (Tb.Pf) and structure model index. Histograms within the ROI were saved to calculate bone mineral density (BMD). Analyses were performed at postnatal days 6, 12 and 24 (P6, P12 and P24). Due to the young age of the animals, the examined mice were of mixed gender. For each parameter, gld mice (n = 6 animals) were compared with wild type controls (n = 6 animals). Statistical analyses (t‐test) were performed with graphpad prism software (v. 7.04). Bone 3D reconstructions were performed with CT scan‐analyser software (Bruker).
Histological and immunohistochemical detection
Mouse heads were fixed in 4% paraformaldehyde for 24 h. Samples were also dehydrated in alcohol series, treated with xylene and embedded in paraffin. Histological sections of mandibles (5 μm) were then prepared. Haematoxylin‐eosin staining was used to visualize the morphological appearance of tissue.
For immunohistochemical detection, antigen retrieval was carried out in citrate buffer (pH = 6.0) for 10 min at 98 °C for MMP2 and SOST antibodies. The following primary antibodies were used: FASL (FASL/N‐20, sc‐834; Santa Cruz Biotechnology, USA), MMP2 (MMP2/H‐76, sc‐10736; Santa Cruz Biotechnology) and SOST (AF1589; R&D Systems). The primary antibodies were diluted 1 : 50 (FASL) and 1 : 150 (MMP2, SOST) and applied overnight at 4 °C. Peroxidase‐conjugated streptavidin‐biotin system (Vectastain, USA) and chromogen substrate diaminobenzidine (K3466; Dako, USA) reactions were used to visualize the positive cells as brown. Tissues were counterstained with haematoxylin. The primary antibodies were omitted in the negative control, as shown in Supporting Information Fig. S1.
Tartrate‐resistant acid phosphatase (TRAP) in osteoclasts was detected by a staining solution containing: naphthol AS‐TR phosphate disodium salt (0.0023 m, N6125; Sigma‐Aldrich, Germany) dissolved in N,N‐dimethylformamide, sodium tartrate dibasic dihydrate (0.1 m, S‐8640; Sigma‐Aldrich) and Fast Red TR Salt (0.1%, 368881; Sigma‐Aldrich) dissolved in acetate buffer containing of glacial acetic acid (0.2 m) and sodium acetate (0.2 m). Samples were incubated in the solution for 1 h at 37 °C. Haematoxylin was used for counterstaining.
Counting of osteocytes
The percentage of osteocytes within a total number of counted cells (osteoblasts and osteocytes) was calculated. The counting was based on morphological appearance of cells. Osteocytes were evaluated as cells ‘entrapped’ in bone matrix, and osteoblasts as mononuclear cuboidal cells ‘sitting’ on bone matrix. Three independent sections were used for each stage (P6, P12, P24), and the alveolar bone adjacent to the developing roots of the first molar was analysed.
Results
Formation of gld alveolar bone displays age‐specific alterations
Based on the phenotypic differences in prenatal vs. adult gld bone (Katavic et al. 2003; Svandova et al. 2018), early postnatal stages (P6, P12, P24) were investigated in the FasL‐deficient mice and compared with wild type control animals. For this purpose, micro‐CT analysis was performed to determine the critical period when the phenotype changes. This study was focused on the mandibular/alveolar bone region associated with the first lower molar.
At P6, alveolar bone of gld mice showed a statistically significant decrease in BMD (Fig. 1A; P < 0.0001), BV/TV (Fig. 1B; P < 0.0001), Tb.N (Fig. 1D; P < 0.0001) and Tb.Th (Fig. 1E; P < 0.0001). A statistically significant increase was observed in Tb.Pf (Fig. 1F; P = 0.0005), but the slight increase in Tb.Sp (Fig. 1C, P = 0.2167) was not significant. These results show a decreased formation of bone in gld mice at P6, as compared with wild type mice.
Figure 1.
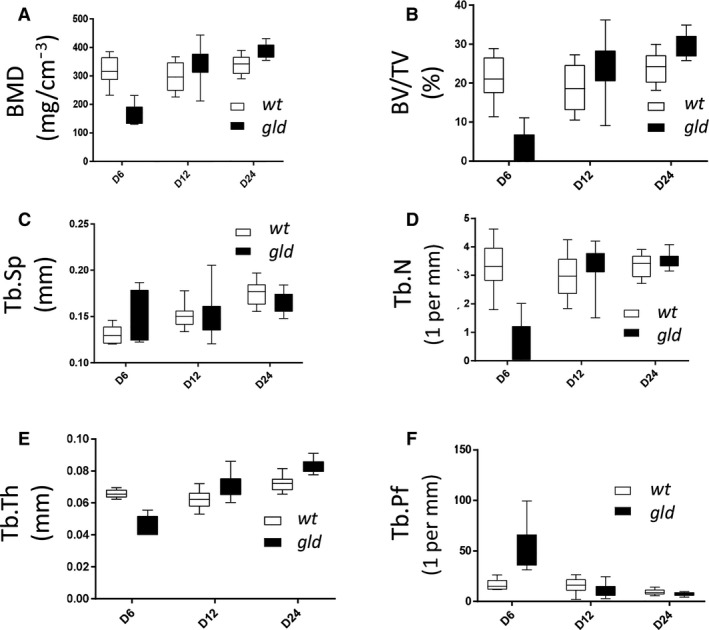
Micro‐architectural parameter analysis of gld mice as compared with wild type. Quantitative in vivo micro‐CT analysis of (A) bone mineral density (BMD), (B) bone volume fraction (BV/TV), (C) trabecular separation (Tb.Sp), (D) trabecular number (Tb.N), (E) trabecular thickness (Tb.Th) and (F) trabecular pattern factor (Tb.Pf). Analyses were performed at postnatal day 6, 12 and 24 (P6, P12 and P24). For each parameter, gld mice (n = 6 animals) were compared with wild type controls (n = 6 animals, t‐test). Statistical analyses were performed with graphpad prism software (v. 7.04).
At P12, the Tb.Th (Fig. 1E, P < 0.0001) was significantly increased in gld alveolar bone, and an increase without statistical significance was observed in BMD (Fig. 1A; P = 0.0877), Tb.N (Fig. 1D; P = 0.3556), Tb.Sp (Fig. 1C; P = 0.9761) and BV/TV (Fig. 1B; P = 0.0804) in gld when compared with wild types. Tb.Pf (Fig. 1F; P = 0.1054) was decreased but the decrease was not statistically significant when compared with wild types. These observations suggest an increased bone formation in gld mice at P12 in contrast to P6.
At P24, micro‐CT examination revealed a statistically significant increase in BMD (Fig. 1A; P = 0.0009), BV/TV (Fig. 1B; P = 0.0009) and Tb.Th (Fig. 1E; P < 0.0001) in gld mice. Tb.N (Fig. 1D; P = 0.1121) was also increased but without statistical significance. In contrast, Tb.Sp (Fig. 1C; P = 0.0949) and Tb.Pf (Fig. 1F; P = 0.0412) appeared slightly decreased in gld mice, alhough, these values were not statistically significant.
These results, together with the 3D reconstructions of the mandibular/alveolar bone (Fig. 2), suggest that gld mice presented an altered mandibular bone formation process, where, after an initial decrease in BV, density and trabecular thickness, a switch took place at around P12, when mineralization increased, as compared with wild type animals.
Figure 2.
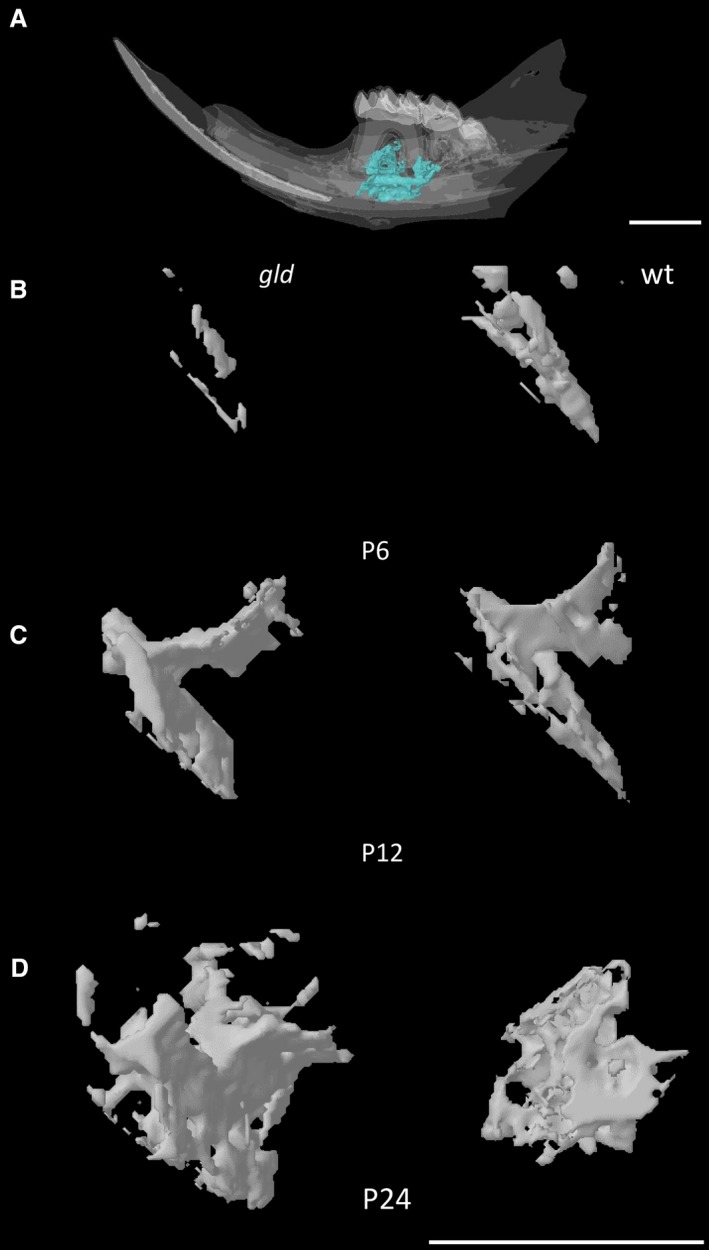
Micro‐CT reconstruction of the analysed mandibular/alveolar bone. Representative 3D reconstruction images of a P24 WT mandible showing the region of interest (A), Representative 3D reconstructions of the mandibular/alveolar bone below the first molar as observed in gld and control mice at P6 (B), 12 (C) and 24 (D). Scale bar: 1 mm.
FASL expression decreases with advanced bone development in parallel with MMP2
To search for a possible mechanism underlying these bone alterations, we examined the pattern of FASL expression in young postnatal wild type bone in serial histological sections of the mandible. The correlation of FASL expression with osteoblasts (osteocalcin) and osteoclasts (TRAP) is shown in Fig. S1. Since a previous study has shown that FASL regulates Mmp2 expression in osteoblasts (Svandova et al. 2018), expression of MMP2 was followed in parallel.
At P6, the immature bone mainly consisted of osteoblasts, but some osteocytes had already become apparent (Fig. 3A,A′). Immunostaining for FASL was detected in the osteoblasts and in numerous cells of the surrounding connective tissue. In contrast, osteocytes residing in the bone matrix lacunae were FASL‐negative (Fig. 3B,D). Of note, MMP2 was also detected in osteoblasts, whereas osteocytes remained negative (Fig. 3C,E).
Figure 3.
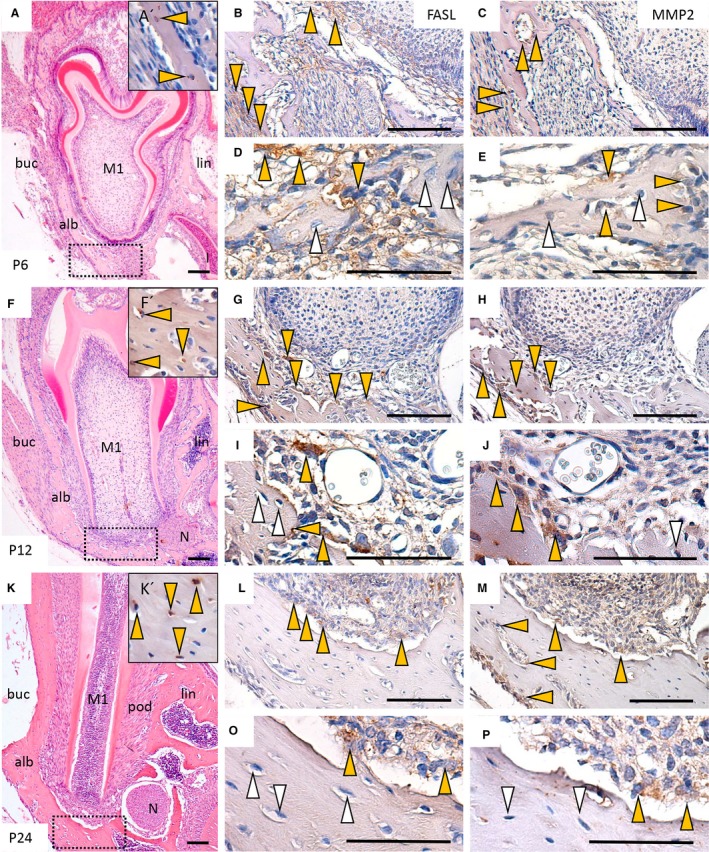
Immunohistochemical detection of FASL and MMP2 in development of alveolar bone in wild type mice. Morphological staining (haematoxylin‐eosin) of lower jaw at P6 (A), P12 (F) and P24 (K). Immunodetection of SOST‐positive osteocytes at P6 (A′), P12 (F′) and P24 (K′); immunodetection of FASL at P6 (B,D), P12 (G,I) and P24 (L,N); immunodetection of MMP2 at P6 (C, E), P12 (H, J), P22 (M, O). Alb (alveolar bone), buc (buccal), M1 (first molar), N (nerve), lin (lingual), pod (periodontium). Yellow arrows point to positive cells, white arrows point to negative cells. Scale bar: 50 μm (D,E,I,J,N,O), 100 μm (A,B,C,F,G,H,K, L,M).
At P12, as bone matured, transformation of osteoblasts into osteocytes (SOST‐positive) proceeded (Fig. 3F,F′). Again, FASL was present mostly in osteoblasts and in cells of the surrounding connective tissue. Some large osteoclastic cells sitting on the bone matrix were also FASL‐positive (Fig. 3G,I). This cell‐specific expression pattern was very similar to the one observed for MMP2 (Fig 3H, J). In the examined region, osteocytes were negative for both FASL and MMP2 (Fig. 3H,J).
At P24, osteocyte number rapidly increased (Fig. 3K,K′) in contrast to stage P6, as documented in Fig. S2. Within the alveolar bone, FASL expression was concentrated particularly in a few of the remaining osteoblasts (Fig. 3L,N). A general decrease in MMP2 expression was also apparent, labelling was essentially maintained in osteoblasts, whereas osteocytes were negative in examined alveolar bone (Fig. 3M,P).
Discussion
FAS‐FASL signalling is well known to activate the extrinsic apoptotic machinery, but there is an apparent pleiotropic effect that stems from multiple cellular targets and is further enhanced by apoptotic vs. non‐apoptotic activation of downstream pathways (Wajant, 2002).
In the bone, FAS‐FASL mediates osteoblastic and osteoclastic apoptotic removal (e.g. Kawakami et al. 1997; Ozeki et al. 2002; Nakamura et al. 2007; Wang et al. 2015), but there is also increasing evidence for other, non‐apoptotic roles of FASL (Wajant et al. 2003; Park et al. 2005; Kovacic et al. 2007). Analysis of the gld bone phenotype revealed increased bone formation in adult gld mice (Katavic et al. 2003), together with an underdeveloped architecture of prenatal mandibular bone (Svandova et al. 2018).
The present research therefore focused on this putative growth‐dependent gld bone phenotype. First, we searched for the period when the phenotypic transformation becomes detectable. The in vivo micro‐CT data clearly pointed to the existence of rapid postnatal changes in gld mandibular bone parameters between days 6 and 12 of mouse development, with a decreased bone formation clearly detected at day 6, followed by increased parameters of bone formation by day 12 (all compared with wild type mice).
To get a better understanding of possible mechanisms underlying this switch in phenotype, an immunohistochemical evaluation of FASL expression during postnatal development was performed. The fact that FASL expression was observed in osteoblasts, whereas osteocytes were mostly negative, suggested that the growth‐dependent phenotype could be related to the differentiation of osteoblasts into osteocytes. The hypothesis would fit with the identification of a ‘switch point’ between day 6 and 12. Indeed, during postnatal bone development, the proportion of osteocytes to osteoblasts rapidly increased, as observed from histological sections.
FASL is known to regulate osteoblast differentiation in vitro (Kovacic et al. 2007) and to impact osteogenic genes expression in mandibular/alveolar bone (Svandova et al. 2018). Mmp2 was one of the affected molecules detected as decreased in prenatal gld mandibular/alveolar bone (Svandova et al. 2018), suggesting the possibility of a connection between these two molecules. Notably, Mmp2‐deficient intramembranous bone (Inoue et al. 2006; Mosig et al. 2007) also showed growth‐dependent changes in BMD as in the gld mandible. Carrying this further, the pattern of expression MMP2 was investigated by immunohistochemistry at the same stages. As in the case of FASL, MMP2 was associated mostly to osteoblastic cells.
MMP2 was reported to be involved in several different events in bone development (Blavier & Delaisse, 1995; Gack et al. 1995; Mosig & Martignetti, 2013), including the formation of the osteocytic canalicular system (Inoue et al. 2006). This system is crucial for communication among bone cells and may be involved in the transduction of molecular signals such as SOST (Inoue et al. 2006), known to play a role in bone maintenance (Sasaki et al. 2012). Notably, in gld mandibular/alveolar bone development, the expression of Sost, a marker of osteocytes, was also decreased (Svandova et al. 2018). As osteocytes were mostly FASL‐negative at the examined stages, our data would suggest that FasL‐deficiency in osteoblasts has consequences for osteocytes during osteoblast–osteocyte differentiation.
In conclusion, stage‐specific alterations of the mandibular bone formation in gld mice were analysed and a critical period between postnatal day 6 and 12 was identified. This phenotypic transition time zone corresponds to a period of gradual increase in the proportion of osteocytes within the mandibular/alveolar bone. One factor in the mechanism underlying this turnover is likely to be related to Mmp2 being expressed in osteoblasts and being regulated in the case of FASL modulation in osteoblastic cells, which is supported by the fact that both FasL‐ and Mmp2‐deficient bone display growth‐dependent phenotypes. Nevertheless, further investigations will be necessary to search for more details on the molecular mechanisms involved at this specific time‐period.
Supporting information
Fig. S1. Correlation of FASL expression with osteoblasts (osteocalcin) and osteoclasts (TRAP) in postnatal mandible.
Fig. S2. Increasing portion of osteocytes in developing mandibular/alveolar bone.
Acknowledgements
This work was supported by the Czech Science Foundation (project GA CR 16‐18430S).
References
- Aghaloo TL, Chaichanasakul T, Bezouglaia O, et al. (2010) Osteogenic potential of mandibular vs. long‐bone marrow stromal cells. J Dent Res 89, 1293–1298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blavier L, Delaisse JM (1995) Matrix metalloproteinases are obligatory for the migration of preosteoclasts to the developing marrow cavity of primitive long bones. J Cell Sci 108, 3649–3659. [DOI] [PubMed] [Google Scholar]
- Florencio‐Silva R, Sasso GR, Sasso‐Cerri E, et al. (2015) Biology of bone tissue: structure, function, and factors that influence bone cells. Biomed Res Int 2015, 421746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gack S, Vallon R, Schmidt J, et al. (1995) Expression of interstitial collagenase during skeletal development of the mouse is restricted to osteoblast‐like cells and hypertrophic chondrocytes. Cell Growth Differ 6, 759–767. [PubMed] [Google Scholar]
- Inoue K, Mikuni‐Takagaki Y, Oikawa K, et al. (2006) Crucial role for matrix metalloproteinase 2 in osteocytic canalicular formation and bone metabolism. J Biol Chem 281, 33814–33824. [DOI] [PubMed] [Google Scholar]
- Katavic V, Lukic IK, Kovacic N, et al. (2003) Increased bone mass is a part of the generalized lymphoproliferative disorder phenotype in the mouse. J Immunol 170, 1540–1547. [DOI] [PubMed] [Google Scholar]
- Kawakami A, Eguchi K, Matsuoka N, et al. (1997) Fas and Fas ligand interaction is necessary for human osteoblast apoptosis. J Bone Miner Res 12, 1637–1646. [DOI] [PubMed] [Google Scholar]
- Kovacic N, Lukic IK, Grcevic D, et al. (2007) The Fas/Fas ligand system inhibits differentiation of murine osteoblasts but has a limited role in osteoblast and osteoclast apoptosis. J Immunol 178, 3379–3389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krum SA, Miranda‐Carboni GA, Hauschka PV, et al. (2008) Estrogen protects bone by inducing Fas ligand in osteoblasts to regulate osteoclast survival. EMBO J 27, 535–545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mosig RA, Martignetti JA (2013) Loss of MMP‐2 in murine osteoblasts upregulates osteopontin and bone sialoprotein expression in a circuit regulating bone homeostasis. Dis Model Mech 6, 397–403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mosig RA, Dowling O, DiFeo A, et al. (2007) Loss of MMP‐2 disrupts skeletal and craniofacial development and results in decreased bone mineralization, joint erosion and defects in osteoblast and osteoclast growth. Hum Mol Genet 16, 1113–1123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura T, Imai Y, Matsumoto T, et al. (2007) Estrogen prevents bone loss via estrogen receptor alpha and induction of Fas ligand in osteoclasts. Cell 130, 811–823. [DOI] [PubMed] [Google Scholar]
- Ozeki N, Mogi M, Nakamura H, et al. (2002) Differential expression of the Fas‐Fas ligand system on cytokine‐induced apoptotic cell death in mouse osteoblastic cells. Arch Oral Biol 47, 511–517. [DOI] [PubMed] [Google Scholar]
- Park H, Jung YK, Park OJ, et al. (2005) Interaction of Fas ligand and Fas expressed on osteoclast precursors increases osteoclastogenesis. J Immunol 175, 7193–7201. [DOI] [PubMed] [Google Scholar]
- Sasaki M, Hongo H, Hasegawa T, et al. (2012) Morphological aspects of the biological function of the osteocytic lacunar canalicular system and of osteocyte‐derived factors. Oral Sci Int 9, 1–8. [Google Scholar]
- Svandova E, Vesela B, Lesot H, et al. (2018) FasL modulates expression of Mmp2 in osteoblasts. Front Physiol 9, 1314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wajant H (2002) The Fas signaling pathway: more than a paradigm. Science 296, 1635–1636. [DOI] [PubMed] [Google Scholar]
- Wajant H, Pfizenmaier K, Scheurich P (2003) Non‐apoptotic Fas signaling. Cytokine Growth Factor Rev 14, 53–66. [DOI] [PubMed] [Google Scholar]
- Wang L, Liu S, Zhao Y, et al. (2015) Osteoblast‐induced osteoclast apoptosis by FAS ligand/FAS pathway is required for maintenance of bone mass. Cell Death Differ 22, 1654–1664. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig. S1. Correlation of FASL expression with osteoblasts (osteocalcin) and osteoclasts (TRAP) in postnatal mandible.
Fig. S2. Increasing portion of osteocytes in developing mandibular/alveolar bone.


