Abstract
Background
Antidepressants are among the most commonly prescribed drugs worldwide. They are often discontinued, frequently without the knowledge of the prescribing physician. It is, therefore, important for physicians to be aware of the withdrawal and rebound phenomena that may arise, in order to prevent these phenomena, treat them when necessary, and counsel patients appropriately.
Methods
This review is based on a comprehensive, structured literature search on antidepressant withdrawal phenomena that we carried out in the CENTRAL, PubMed (Medline), and Embase databases. We classified the relevant publications and reports by their methodological quality.
Results
Out of a total of 2287 hits, there were 40 controlled trials, 38 cohort studies and retrospective analyses, and 271 case reports that met the inclusion criteria. Withdrawal manifestations are usually mild and self-limiting; common ones include dizziness, headache, sleep disturbances, and mood swings. More serious or prolonged manifestations rarely arise. There is an increased risk with MAO inhibitors, tricyclic antidepressants, venlafaxine, and paroxetine; on the other hand, for agomelatine and fluoxetine, abrupt discontinuation seems to be unproblematic. There is also some evidence of rebound phenomena, i.e., of higher relapse rates or especially severe relapses of depression after the discontinuation of an antidepressant.
Conclusion
A robust evidence base now indicates that there can be acute withdrawal phenomena when antidepressants are discontinued. Putative rebound phenomena have not been adequately studied to date. It is recommended that antidepressants should be tapered off over a period of more than four weeks.
Antidepressants are among the drugs most frequently prescribed not only in psychiatry but also other medical specialties. In 2017, 1.49 billion defined daily doses of antidepressants were prescribed in the health insurance system in Germany (not including private prescriptions and hospital treatments) (1). In addition to depression, they have also been approved for other indications such as anxiety and obsessive compulsive disorders. Sound knowledge of the side effects and risks of antidepressant medication is essential in order to inform and treat patients.
Besides adverse drug reactions during antidepressant use, adverse phenomena that occur following treatment discontinuation are increasingly becoming the focus of attention. Withdrawal phenomena of this kind were known as early on as in the early 1960s (2, e1). However, awareness of the significance of this topic remains low despite its considerable relevance. It is likely that a third of patients discontinue antidepressant medication within 1 month and 50% of patients by the end of the third month (e2), often without consulting their treating physician. A Danish study showed that the most frequent calls to a national medical advice hotline were accounted for by inquiries relating to antidepressant withdrawal phenomena (e3). It is essential, therefore, to provide patients at the start of treatment with relevant information on the risks of abrupt discontinuation, as recommended by the German clinical practice guidelines on unipolar depression (3).
If adverse symptoms occur following discontinuation (or dose reduction) of treatment, a distinction needs to be made between withdrawal syndrome, rebound phenomena, and re-emergence of the primary disorder (table 1).
Table 1. Differential diagnosis following antidepressant discontinuation or dose reduction.
| Syndrome | Characteristic |
| Withdrawal syndrome, ADS (antidepressant discontinuation syndrome), acute discontinuation syndrome |
● Rapid onset following discontinuation |
| ● Transient, self-limiting | |
| ● Rapid improvement following resumption of the medication | |
| ● Symptoms may resemble (or differ from) primary disorder (depression) | |
| ● Typically nonspecific symptoms (“FINISH,” see text), possibly specific serotonergic/ cholinergic syndromes | |
| Rebound | ● Re-emergence of symptoms of the primary disorder to a greater extent than prior to medication and/or |
| ● Higher risk for relapse compared to patients not receiving antidepressants | |
| ● Counter-regulatory mechanisms activated by treatment and excessive counter-regulation following drug discontinuations | |
| Relapse | Re-emergence of the same disease episode due to loss of pharmacological effect |
| Recurrence | New episode of a recurring primary disorder following previous recovery (remission over 6–9 months) due to loss of pharmacological effect |
An accurate differential diagnosis is important, since it has crucial clinical consequences. For example, in the case of transient withdrawal phenomena, one can usually take a wait-and-see approach or treat symptomatically. In the case of disease recurrence, on the other hand, medication may need to be resumed. If pharmaceutical drugs are actually known to be associated with a risk of rebound following discontinuation, this needs to be taken into account as early on as at the time of making the indication and providing patient information.
Methods
A comprehensive and structured database search was carried out (JH) in CENTRAL, PubMed (Medline) (up to January 2017) and Embase (up to April 2017) (ebox). Manual searches were also carried out and the references in relevant articles assessed. All controlled studies, cohort studies, observational studies, case series, and case reports on antidepressant withdrawal and rebound phenomena in subjects aged over 18 years were included. The included studies were classified according to methodological quality (JH) (etable 1).
eBOX. Explicit database search entries.
● PUBMED
(antidepress* or agomelatine* or amineptine* or amitriptyline* or amoxapine* or bupropion* or butriptyline* or chlorimipramine* or citalopram* or clomipramine* or desipramine* or desvenlafaxine* or dibenzepin* or dosulepin* or dothiepin* or doxepin* or duloxetine* or escitalopram* or fluoxetine* or fluvoxamine* or imipramine* or isocarboxazid* or lofepramine* or levomilnacipran* or MAOI* or monoamine oxidase inhibitors or maprotiline* or mianserin* or milnacipran* or mirtazapine* or moclobemide* or nefazodone* or nortriptyline* or paroxetine* or phenelzine* or protriptyline* or reboxetine* or selegiline* or sertraline* or setiptiline* or SSRI or SSNRI* or SNRI* or tca or selective serotonin reuptake inhibitors or serotonin-norepinephrine reuptake inhibitors or tetracyclic* or tianeptine* or tranylcypromine* or trazodone* or trimipramine* or tricyclic* or venlafaxine* or viloxazine* or vortioxetine*) Title/Abstract
AND
(withdraw* or discontinu*) Title OR (withdraw* or discontinu*) Other Term
● CENTRAL
(antidepress* or agomelatine* or amineptine* or amitriptyline* or amoxapine* or bupropion* or butriptyline* or chlorimipramine* or citalopram* or clomipramine* or desipramine* or desvenlafaxine* or dibenzepin* or dosulepin* or dothiepin* or doxepin* or duloxetine* or escitalopram* or fluoxetine* or fluvoxamine* or imipramine* or isocarboxazid* or lofepramine* or levomilnacipran* or MAOI* or monoamine oxidase inhibitors or maprotiline* or mianserin* or milnacipran* or mirtazapine* or moclobemide* or nefazodone* or nortriptyline* or paroxetine* or phenelzine* or protriptyline* or reboxetine* or selegiline* or sertraline* or setiptiline* or SSRI or SSNRI* or SNRI* or tca or selective serotonin reuptake inhibitors or serotonin-norepinephrine reuptake inhibitors or tetracyclic* or tianeptine* or tranylcypromine* or trazodone* or trimipramine* or tricyclic* or venlafaxine* or viloxazine* or vortioxetine*) AND (withdraw* or discontinu*) Record Title OR Keyword
● EMBASE
([antidepress* or agomelatine* or amineptine* or amitriptyline* or amoxapine* or bupropion* or butriptyline* or chlorimipramine* or citalopram* or clomipramine* or desipramine* or desvenlafaxine* or dibenzepin* or dosulepin* or dothiepin* or doxepin* or duloxetine* or escitalopram* or fluoxetine* or fluvoxamine* or imipramine* or isocarboxazid* or lofepramine* or levomilnacipran* or MAOI* or monoamine oxidase inhibitors or maprotiline* or mianserin* or milnacipran* or mirtazapine* or moclobemide* or nefazodone* or nortriptyline* or paroxetine* or phenelzine* or protriptyline* or reboxetine* or selegiline* or sertraline* or setiptiline* or SSRI or SSNRI* or SNRI* or tca or selective serotonin reuptake inhibitors or serotonin-norepinephrine reuptake inhibitors or tetracyclic* or tianeptine* or tranylcypromine* or trazodone* or trimipramine* or tricyclic* or venlafaxine* or viloxazine* or vortioxetine*].ab.)
AND
([withdraw* or discontinu*].ti. OR .kw.)
eTable 1. Methodological quality in terms of the diagnostic question (frequency, severity, and characteristics of discontinuation phenomena with antidepressants).
| Level of methodological quality/evidence level | Specific features | Strengths/limitations |
| I | Discontinuation under placebo substitution compared to continuation of medication, randomized, blinded | Lowest risk of bias, including assessment of absolute frequencies of occurrence |
| II | Discontinuation of one drug compared to discontinuation of another drug | Comparison of various drugs with each other for the risk of ADS, no assessment of absolute incidence as there is no continuation arm |
| III | Discontinuation under placebo substitution (blinded for patients), no control group | Lack of control, but lower risk of bias due to patient blinding |
| IVa | Cohort studies, case series, unblinded | High risk of bias |
| IVb | Individual case reports | Highest risk of bias, no causal relationships can be assessed |
ADS, antidepressant discontinuation syndrome
Results
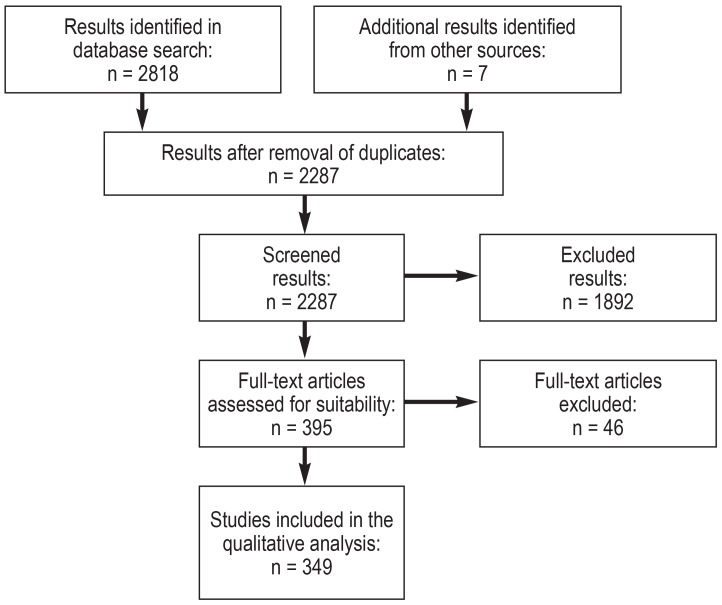
Once duplicates had been excluded, the literature search yielded 2287 hits, 349 of which met the inclusion criteria. These included 40 controlled studies, 38 cohort studies and retrospective analyses, and 271 case reports. The eFigure shows the PRISMA flow chart.
eFigure.
PRISMA flow chart
Acute discontinuation syndrome/withdrawal syndrome
A separate syndrome has now been defined in English-speaking countries in relation to antidepressants: antidepressant discontinuation syndrome (ADS). There is a standardized checklist, discontinuation-emergent signs and symptoms (DESS), which lists the symptoms described in the literature and is used as standard in numerous studies (4, e4). A number of symptoms can resemble the primary disease (depression: e.g., anxiety, suicidal thoughts), whereas others can be clearly differentiated from the disorder (e.g., electric shock-like paresthesia, diarrhea). Table 2 provides an overview of the clinical picture of ADS.
Table 2. Clinical presentations of antidepressant withdrawal symptoms*1.
|
Systemic, cardiac |
Flu-like symptoms*2, dizziness/drowsiness*2, tachycardia*2, impaired balance, fatigue, weakness, headache, dyspnea |
| Sensory | Parasthesia*2, electric shock–like sensation (“brain zapps/body zapps”)*2, sensory disorders, dysesthesia, itch, tinnitus, altered taste, blurred vision, visual changes |
| Neuromuscular | Muscle tension*2, myalgia*2, neuralgia*2, agitation*2, ataxia*2, tremor |
| Vasomotor | Perspiration*2, flushing*2, chills*2, impaired temperature regulation |
| Gastrointestinal | Diarrhea*2, abdominal pain*2, anorexia, nausea, vomiting |
| Sexual | Premature ejaculation*2, genital hypersensitivity*2 |
| Sleep | Insomnia, nightmares, vivid dreams, hypersomnia |
| Cognitive | Confusion*2, disorientation*2, amnesia*2, reduced concentration |
| Affective | Irritability, anxiety, agitation, tension, panic, depressive mood, impulsivity, sudden crying, outbursts of anger, mania, increased drive, mood swings, increased suicidal thoughts, derealization, depersonalization |
| Psychotic | Visual and auditory hallucinations |
| Delirium | (Typically only with tranylcypromine) |
The mnemonic aid used in English, FINISH, helps in its timely identification (e5):
Flu-like symptoms
Insomnia (disturbed sleep, vivid dreams/nightmares)
Nausea
Imbalance (vertigo, light-headedness)
Sensory disturbances (electric shock-like sensations, dysesthesia)
Hyperarousal (anxiety, agitation, irritability, etc.)
Box 1 summarizes the characteristics of ADS. Different specific features are seen depending on the drug.
Box 1. Characteristics of ADS.
Rapid onset mostly within 1 week following discontinuation (generally peaking after 36–96 h), after approximately 3–5 half-lives
Spontaneous resolution within 2 (–6) weeks (depending on half-life)
Predominantly mild, reversible symptoms
Rapid and generally complete resolution if medication is resumed
Nonspecific physical symptoms typically predominate
Commonest symptoms: dizziness, nausea, headache, disturbed sleep, irritability/emotional lability
ADS, antidepressant discontinuation syndrome
Selective serotonin reuptake inhibitors
A sufficiently large number of methodologically high-quality studies are available on selective serotonin reuptake inhibitors (SSRI; evidence level I and II according to eTable 1). With its especially long half-life, fluoxetine is particularly unproblematic, even in the case of abrupt discontinuation (7, 8). Sertraline and in particular citalopram and escitalopram pose low risk. Studies revealed no significant benefit for tapered withdrawal compared to continuing the medication (9), while abrupt discontinuation carries low risk (approximately 20% compared to 10% in the continuation arms) (10, 11).
Paroxetine is associated with a high risk for ADS compared to other SSRI and, in the case of abrupt discontinuation, ADS symptoms are seen in over 30% of patients (7, 12, 13). With the exception of paroxetine, which causes ADS symptoms more closely resembling those seen with tricyclic antidepressants in terms of frequency and severity (14), SSRI-related ADS is generally mild and self-limiting.
Selective serotonin and noradrenaline reuptake inhibitors
There is robust evidence (level I and II) on selective serotonin and noradrenaline reuptake inhibitors (SNRI). Venlafaxine (and desvenlafaxine) carry a higher risk for ADS (15) compared to both the SNRI duloxetine (e6) and SSRI (escitalopram, sertraline) (13, e7, e8). Venlaflaxine also appears to more frequently cause severe forms of ADS (e8). Moreover, this appears to be the case with particularly early onset withdrawal symptoms (according to some case reports, as early on as after a delayed dose), which can be linked to the drug’s extremely short half-life (box 1). Duloxetine carries a low risk for ADS (e6) in comparison; this, however, rises in the high-dose range (120 mg/day; e9). The third SNRI, milnacripran, showed no ADS symptoms in a methodologically high-quality study (level I, in the psychosomatic indication “fibromyalgia”) even upon abrupt discontinuation (16). Similarly, an open study revealed only isolated occurrences of anxiety (e10).
Tricyclic antidepressants
The evidence on tricyclic antidepressants (TCA) is limited, and there are only a handful of methodologically high-quality studies (level I and II), some with very low case numbers. However, these point to a high risk for ADS. Even when amitriptyline was tapered out gradually, 80% of patients exhibited symptoms (N = 15), albeit primarily mild and self-limiting (17). Imipramine was comparable to the SSRI paroxetine (14). Methodologically weak studies and case series (level III and IV) yield evidence that there is a risk for severe effects following discontinuation of TCA (18). Symptoms related to cholinergic overdrive are clinically characteristic (2).
MAO inhibitors
There are only case reports and two studies of low methodological quality on MAO inhibitors (MAO-I) (19, e11, e12). Taking these methodological limitations into account, MAO-I appear to be associated with a particularly high risk for ADS; severe courses appear to be more common. Delirium was described in 50% of case reports on ADS following discontinuation of tranylcypromine (19).
Agomelatine
A number of methodologically high-quality studies (level I) demonstrate that ADS does not occur even following abrupt discontinuation of agomelatine (20– 22).
Mirtazapine and bupropion
Although studies are lacking, a handful of case reports suggest that discontinuation of mirtazapine and bupropion can also cause ADS (e13– e15) (table 3).
Table 3. ADS risk for individual drugs.
| Risk of ADS | Antidepressant |
 Very high risk Very high risk
|
Tranylcypromine, phenelzine |
 High risk High risk
|
Paroxetine, tricyclic antidepressants, venlafaxine (desvenlafaxine) |
 Moderate risk Moderate risk
|
Citalopram, escitalopram, sertraline, duloxetine, vortioxetine |
 Low risk Low risk
|
Fluoxetine, milnacipran |
 No risk No risk
|
Agomelatine |
 Unclear risk (insufficient evidence) Unclear risk (insufficient evidence) |
Mirtazapine, bupropion |
ADS, antidepressant discontinuation syndrome
Severe cases of ADS
Uncontrolled studies and (online) surveys suggest higher incidence rates of antidepressant withdrawal effects in general, as well as more severe symptoms (23). However, one needs to take into consideration the methodological limitations and the danger of incorrectly attributing causality to associations. For example, blinded randomized controlled trials revealed equally high rates of withdrawal symptoms in the control arms (>30%), i.e., in which the antidepressant was continued (9, 15). Controlled, high-quality studies point to a primarily self-limiting course involving mild symptoms. In rare cases, symptoms that were classified as more severe were seen. These were mainly sleep disorders and nervousness/anxiety (desvenlafaxine [24]). Severe courses involving extrapyramidal motor symptoms (such as parkinsonism and akathisia) or paradoxical activation/mania are known from methodologically weaker studies and case reports. These were described following discontinuation of tricyclic antidepressants (e11, e16), MAO inhibitors (19, e17), SSRI (e18– e20), venlafaxine (e21, e22), and mirtazapine (e13) in patients with uni- and bipolar disorders, as well as symptoms that are of particular clinical relevance such as suicidal thoughts (25). The sensation of electric shocks experienced by patients as particularly impairing (especially with SSRI and venlafaxine) is a specific aspect worthy of note (26, e23).
Rebound phenomena
Rebound phenomena refer to the organism’s increased susceptibility following drug discontinuation—comparable to the image of a ball which, when pushed under water and suddenly released, not only returns to the surface, but actually rises out of the water: the symptoms of the underlying disease return to a greater extent than prior to drug initiation, or there is a greater risk of relapse compared to patients that did not receive medication.
Individual case reports and case series report persistent depressive syndrome following antidepressant discontinuation—more severe in nature compared to before starting medication or with additional psychopathological symptoms, some of which are challenging to treat (6). Some authors define these as persistent post-discontinuation syndromes if symptoms persist for longer than 6 weeks (6, e24). Anxiety and panic disorders, sleep disorders, and cyclothymic/bipolar disorders have been reported following discontinuation of paroxetine, escitalopram, citalopram, and fluvoxamine, whereby paroxetine appears to harbor a particularly high risk (6, 27, e25– e27). The available evidence does not permit any statements to be made on the frequency of rebound phenomena. There is only one open and uncontrolled study in this regard, which describes persistent mood swings following discontinuation of paroxetine in three of 20 patients (27).
Our literature search does not systematically answer the question of whether an increased risk of recurrence following discontinuation can be demonstrated. However, a 2011 meta-analysis (28) showed that depressive patients who experienced remission with antidepressants relapsed more frequently following discontinuation (42.0%–55.6%) than did those that experienced remission with placebo (24.7%). The risk was higher for antidepressants that alter monoaminergic neurotransmission more strongly, i.e,. in particular MAO-I and TCA. The risk of relapse is particularly high in the first 6 months following discontinuation (e28). Evidence suggests that the risk of relapse is higher the longer the drug was previously taken (e29). However, the reliability of this evidence from secondary analyses is limited due to study design (e.g., separate observation of study arms). Given its considerable clinical relevance, the topic urgently requires further research.
Basic principles
The minimum treatment duration required for the development of withdrawal phenomena has been insufficiently demonstrated; at least 4 weeks appear to be necessary (e30). There is robust evidence for SSRI and SNRI that there is a risk of ADS from 8 weeks, and that this risk does not change to any relevant extent with longer treatment (7, e4, e9, e31– e33). ADS appears to develop irrespective of the primary disorder (e31, e34).
Pharmacodynamics
There is insufficient experimental data as yet. The anticholinergic effect of numerous TCA can cause neuroadaptive counter-regulation, as a result of which acetylcholinergic neurotransmission is increased, causing symptoms characteristic of cholinergic overdrive following discontinuation (2). From a clinical perspective, the majority of ADS symptoms correspond to the picture of serotonin syndrome (e35), particularly with SSRI (6) (table 2), which can be explained at least in part by the particular effects of antidepressants on serotonin transporters. This is due to the fact that a number of antidepressants not only block the serotonin and norepinephrine transporters, but also cause a reduction (and not a counter-regulatory increase) in these transporters when used long term (e36– e38), which may result in persistent serotonin hyperfunction following discontinuation (the transporters reduce the level of serotonin in the synaptic cleft). In animal studies, transporter density only normalized in a delayed manner (e39). A hyper-responsive serotonergic system was also observed in animal studies following SSRI discontinuation (e40). The following appears to apply as a basic principle: the stronger and more directly an antidepressant affects the balance of the neurotransmitter system, the more pronounced the withdrawal and rebound symptoms.
Pharmacokinetics
There is a correlation within the drug classes between plasma elimination time of the drugs and severity and time of onset of ADS (7, 8). Thus, antidepressants with a short half-life pose a greater risk for the development of (more severe) withdrawal symptoms (etable 2). As such, rapid metabolizers likely also pose a greater risk for ADS (29). The onset of discontinuation symptoms appears to occur in around three to five half-lives following discontinuation (e41). An increase in the risk of ADS at higher doses appears to apply only in the high-dose range (duloxetine 120 mg/day; escitalopram 20 mg/day) (e9, e31, 10).
eTable 2. Half-lives (HL) of selected antidepressants (e60).
| Drug and active metabolite | HL |
| Selective serotonin-reuptake inhibitors | |
| Citalopram | 38–48 h |
| Escitalopram | 27–32 h |
| Fluoxetine plus N-methyl fluoxetine |
4–6 days 4–16 days |
| Fluvoxamine | 21–43 h |
| Paroxetine | 12–44 h |
| Sertraline | 22–36 h |
| Selective serotonin and noradrenaline reuptake inhibitors | |
| Desvenlafaxine | 10–17 h |
| Duloxetine | 9–19 h |
| Milnacipran | 5–8 h |
| Venlafaxine plus O-desmethylvenlafaxine |
14–18 h 10–17 h |
| Tricyclic antidepressants | |
| Amitriptyline plus nortriptyline |
10–28 h 18–44 h |
| Clomiparmin plus N-desmethyl clomipramine |
16–60 h 37–43 h |
| Doxepin plus N-desmethyldoxepin |
15–20 h |
| Imipramine plus desipramine |
11–25 h 15–18 h |
| Trimipramine | 23–24 h |
| MAO inhibitors | |
| Moclobemide | 2–7 h |
| Tranylcypromine | 1–3 h |
| Others | |
| Agomelatine | 1–2 h |
| Bupropion plus hydroxybupropion |
1–15 h 17–47 h |
| Mirtazapine | 20–40 h |
Discussion
Differential diagnosis
Differentiating between ADS and (re-)emergence of the primary psychiatric disorder is crucial. There is considerable symptom overlap between ADS and a depressive episode or anxiety disorder, as well as a (hypo-)manic episode (table 2). Misinterpretation of symptoms can result in unnecessary and potentially harmful medication (e.g., if ADS is misinterpreted as a manic episode and subsequently misdiagnosed as a bipolar affective disorder). Likewise, when changing medication, ADS due to the discontinued drug may be incorrectly identified as an adverse drug reaction to the new drug. A guiding criterion when making this differentiation can be the temporal course, which is characterized by early onset as well as fluctuations, and tends to be transient (29). The likeliest time course is onset in the first week following discontinuation and resolution in the second week (11). The fact that ADS is generally more strongly and specifically defined by somatic symptoms, with symptoms untypical for depression such as dizziness, nausea, sensory impairment, and flu-like symptoms, can be used to help in the differentiation (7). Similarly, particular sleep disturbances such as vivid dreams and nightmares point to ADS (29, e35).
Treatment and prevention
The most important treatment approach likely lies in prevention. Since symptoms are mild and self-limiting in the majority of cases, detailed patient education is often sufficient; if necessary, patients can receive symptomatic treatment in the form of hypnotic agents or anti-muscarinic substances for TCA and cholinergic rebound (30). In the case of severe symptoms, the antidepressant can be resumed, which generally leads to complete symptom remission within 24 h (e42, e43). This also applies to extrapyramidal symptoms and paradoxical activation/mania. A gradual tapering can then be undertaken. Although antidepressant tapering is not able to completely rule out the risk of ADS, it appears to reduce its severity (15). A time period of 2 weeks is too short (15, 25)—the German clinical practice guidelines recommend reducing a drug over a period of at least 4 weeks (3). Findings from narcolepsy research even suggest minimum periods of 3 months (e44). Treating physicians should make decisions depending on the particular drug and taper over longer periods in the case of high initial doses and high-risk drugs (table 3). Fluoxetine has proven itself in case reports as a “rescue” substance for withdrawal symptoms from other SSRI (e45, e46) and venlafaxine (e47). It can be used instead of the discontinued drug if ADS does emerge and then presumably abruptly discontinued after a number of weeks.
Risk of rebound
Signs of rebound phenomena following discontinuation are to be taken seriously; however, these are often challenging to clinically differentiate from a re-emergence of the primary disorder, since this, too, can change in terms of symptoms and severity over its natural course. Depressive syndromes, for example, are often combined with anxiety disorders, and manic episodes of bipolar disorders often emerge in a delayed manner following what originally appeared to be unipolar depression (31– 33).
The concern that, once started, an antidepressant can no longer be discontinued due to the risk of sudden, severe, and in some cases treatment-resistant recurrence (34– 36) should prompt caution when starting an antidepressant, all the more so in the case of moderate depression, particularly since antidepressants are scarcely superior to placebo here (37– 39).
The question of dependence is discussed in Box 2.
BOX 2. Do antidepressants cause addiction?
There has long been a debate on whether the withdrawal symptoms caused by antidepressant discontinuation indicate addiction (e48, e49). According to ICD-10, dependence on a substance, whether drugs, alcohol, or medications, is present if at least three of six criteria are met: tolerance development, withdrawal symptoms, strong desire to use the substance, difficulty controlling its use, neglecting other interests, and persistent substance use despite harmful effects (e50). Withdrawal symptoms and tolerance development are considered to be the effect of counter-regulatory neuroadaptation opposing the effects of the substance in order to preserve homeostasis (40). On the one hand, this can lead to the loss of the original drug effect. Reports have described that, following discontinuation, reinstatement of an antidepressant is no longer able to achieve the original effect, which can be interpreted as tolerance development (e51, e52). On the other hand, abrupt discontinuation of the substance causes withdrawal symptoms, since the newly established balance is disrupted by discontinuing substance use. Withdrawal symptoms are thus not a manifestation of a desire for the discontinued substance; they are much more the effect of previous adaption, as is the case of many centrally or peripherally active medications, e.g., ß-blockers or acetylsalicylic acid (e53– e55). Therefore, tolerance development and withdrawal symptoms are not specific to drug effects and are not sufficient—even in the case of a protracted course (e48)—to diagnose an addiction disorder.
Unlike addiction-forming drugs, antidepressants do not cause an uncontrolled desire to use the substance or a loss of control, and also do not confine behavior or interests to antidepressant use (40). Persistent substance use despite harmful effects has not been described. Only for the MAO inhibitor tranylcypromine have isolated cases of unauthorized dose increases been described (e56, e57), which may be due to the special pharmacological effect on dopaminergic neurotransmission (e58, e59).
Summary
A large number of studies on ADS, some of which are of very high quality, are now available. Symptoms are generally mild and self-limiting. MAO-I, tricyclic antidepressants, paroxetine, and venlafaxine carry a higher risk. With the exception of fluoxetine and agomelatine, gradual tapering of all antidepressants is recommended in order to prevent ADS. Not enough research has been carried out as yet on rebound phenomena. Patients should be educated on the risks of discontinuation and possible rebound phenomena at the start of antidepressant treatment.
Key Messages.
Antidepressants are associated with discontinuation phenomena. MAO inhibitors, tricyclic antidepressants, paroxetine, and venlafaxine carry high risk.
With the exception of fluoxetine and agomelatine, antidepressants should be tapered over more than 4 weeks if possible.
Patients should be informed about rebound phenomena and withdrawal syndromes prior to starting treatment.
The evidence on rebound phenomena is currently insufficient, despite the fact that this risk is crucial to the indication for antidepressant medication.
Acknowledgments
Translated from the original German by Christine Rye.
Footnotes
Conflict of interest statement
The authors state that there are no conflicts of interest.
References
- 1.Schwabe U, Ludwig WD. Arzneiverordnungen 2017 im Überblick Arzneiverordnungs-Report 2018. In: Schwabe U, Paffrath D, Ludwig WD, Klauber J, editors. Heidelberg: Springer. Berlin: 2018. pp. 3–26. [Google Scholar]
- 2.Dilsaver SC, Greden JF. Antidepressant withdrawal phenomena. Biol Psychiatry. 1984;19:237–256. [PubMed] [Google Scholar]
- 3.DGPPN, BÄK, KBV, AWMF für die Leitliniengruppe Unipolare Depression. S3-Leitlinie/Nationale VersorgungsLeitlinie Unipolare Depression-Langfassung. AWMF-Register Nr nvl-005. www.awmforg/leitlinien/detail/ll/nvl-005html (last accessed on 27 December 2017) [Google Scholar]
- 4.Baldwin DS, Cooper JA, Huusom AK, Hindmarch I. A double-blind, randomized, parallel-group, flexible-dose study to evaluate the tolerability, efficacy and effects of treatment discontinuation with escitalopram and paroxetine in patients with major depressive disorder. Int Clin Psychopharmacol. 2006;21:159–169. doi: 10.1097/01.yic.0000194377.88330.1d. [DOI] [PubMed] [Google Scholar]
- 5.Fava GA, Gatti A, Belaise C, Guidi J, Offidani E. Withdrawal symptoms after selective serotonin reuptake inhibitor discontinuation: a systematic review. Psychother Psychosom. 2015;84:72–81. doi: 10.1159/000370338. [DOI] [PubMed] [Google Scholar]
- 6.Chouinard G, Chouinard VA. New classification of selective serotonin reuptake inhibitor withdrawal. Psychother Psychosom. 2015;84:63–71. doi: 10.1159/000371865. [DOI] [PubMed] [Google Scholar]
- 7.Michelson D, Amsterdam J, Apter J, et al. Hormonal markers of stress response following interruption of selective serotonin reuptake inhibitor treatment. Psychoneuroendocrinology. 2000;25:169–177. doi: 10.1016/s0306-4530(99)00046-3. [DOI] [PubMed] [Google Scholar]
- 8.Zajecka J, Fawcett J, Amsterdam J, et al. Safety of abrupt discontinuation of fluoxetine: a randomized, placebo-controlled study. J Clin Psychopharmacol. 1998;18:193–197. doi: 10.1097/00004714-199806000-00003. [DOI] [PubMed] [Google Scholar]
- 9.Allgulander C, Florea I, Huusom AK. Prevention of relapse in generalized anxiety disorder by escitalopram treatment. Int J Neuropsychopharmacol. 2006;9:495–505. doi: 10.1017/S1461145705005973. [DOI] [PubMed] [Google Scholar]
- 10.Markowitz JS, DeVane CL, Liston HL, Montgomery SA. An assessment of selective serotonin reuptake inhibitor discontinuation symptoms with citalopram. Int Clin Psychopharmacol. 2000;15:329–333. doi: 10.1097/00004850-200015060-00003. [DOI] [PubMed] [Google Scholar]
- 11.Montgomery SA, Nil R, Dürr-Pal N, Loft H, Boulenger JP. A 24-week randomized, double-blind, placebo-controlled study of escitalopram for the prevention of generalized social anxiety disorder. J Clin Psychiatry. 2005;66:1270–1278. doi: 10.4088/jcp.v66n1009. [DOI] [PubMed] [Google Scholar]
- 12.Kaufman MJ, Henry ME, Frederick B, et al. Selective serotonin reuptake inhibitor discontinuation syndrome is associated with a rostral anterior cingulate choline metabolite decrease: a proton magnetic resonance spectroscopic imaging study. Biol Psychiatry. 2003;54:534–539. doi: 10.1016/s0006-3223(02)01828-0. [DOI] [PubMed] [Google Scholar]
- 13.Montgomery SA, Huusom AK, Bothmer J. A randomised study comparing escitalopram with venlafaxine XR in primary care patients with major depressive disorder. Neuropsychobiology. 2004;50:57–64. doi: 10.1159/000078225. [DOI] [PubMed] [Google Scholar]
- 14.GlaxoSmithKline. A double-blind comparative study of withdrawal effects following abrupt discontinuation of treatment with paroxetine in low or high dose or imipramine. GSK - Clinical Study Register 1992; www.gsk-clinicalstudyregister.com (last accessed on 27 December 2017) [Google Scholar]
- 15.Ninan PT, Musgnung J, Messig M, Buckley G, Guico-Pabia CJ, Ramey TS. Incidence and timing of taper/posttherapy-emergent adverse events following discontinuation of desvenlafaxine 50 mg/d in patients with major depressive disorder. Prim Care Companion CNS Disord. 2015;17 doi: 10.4088/PCC.14m01715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Saxe PA, Arnold LM, Palmer RH, Gendreau RM, Chen W. Short-term (2-week) effects of discontinuing milnacipran in patients with fibromyalgia. Curr Med Res Opin. 2012;28:815–821. doi: 10.1185/03007995.2012.677418. [DOI] [PubMed] [Google Scholar]
- 17.Giller E, Bialos D, Harkness L, Jatlow P, Waldo M. Long-term amitriptyline in chronic depression. Hillside J Clin Psychiatry. 1985;7:16–33. [PubMed] [Google Scholar]
- 18.Charney DS, Heninger GR, Sternberg DE, Landis H. Abrupt discontinuation of tricyclic antidepressant drugs: evidence for noradrenergic hyperactivity. Br J Psychiatry. 1982;141:377–386. doi: 10.1192/bjp.141.4.377. [DOI] [PubMed] [Google Scholar]
- 19.Gahr M, Schonfeldt-Lecuona C, Kolle MA, Freudenmann RW. Withdrawal and discontinuation phenomena associated with tranylcypromine: a systematic review. Pharmacopsychiatry. 2013;46:123–129. doi: 10.1055/s-0032-1333265. [DOI] [PubMed] [Google Scholar]
- 20.Goodwin GM, Emsley R, Rembry S, Rouillon F. Agomelatine prevents relapse in patients with major depressive disorder without evidence of a discontinuation syndrome: a 24-week randomized, double-blind, placebo-controlled trial. J Clin Psychiatry. 2009;70:1128–1137. doi: 10.4088/JCP.08m04548. [DOI] [PubMed] [Google Scholar]
- 21.Stein DJ, Ahokas A, Albarran C, Olivier V, Allgulander C. Agomelatine prevents relapse in generalized anxiety disorder: a 6-month randomized, double-blind, placebo-controlled discontinuation study. J Clin Psychiatry. 2012;73:1002–1008. doi: 10.4088/JCP.11m07493. [DOI] [PubMed] [Google Scholar]
- 22.Montgomery SA, Kennedy SH, Burrows GD, Lejoyeux M, Hindmarch I. Absence of discontinuation symptoms with agomelatine and occurrence of discontinuation symptoms with paroxetine: a randomized, double-blind, placebo-controlled discontinuation study. Int Clin Psychopharmacol. 2004;19:271–280. doi: 10.1097/01.yic.0000137184.64610.c8. [DOI] [PubMed] [Google Scholar]
- 23.Davies J, Read J. A systematic review into the incidence, severity and duration of antidepressant withdrawal effects: Are guidelines evidence-based? Addict Behav. 2018 doi: 10.1016/j.addbeh.2018.08.027. pii: S0306-4603(18)30834-7. [DOI] [PubMed] [Google Scholar]
- 24.Khan A, Musgnung J, Ramey T, Messig M, Buckley G, Ninan PT. Abrupt discontinuation compared with a 1-week taper regimen in depressed outpatients treated for 24 weeks with desvenlafaxine 50 mg/d. J Clin Psychopharmacol. 2014;34:365–368. doi: 10.1097/JCP.0000000000000100. [DOI] [PubMed] [Google Scholar]
- 25.Tint A, Haddad PM, Anderson IM. The effect of rate of antidepressant tapering on the incidence of discontinuation symptoms: a randomised study. J Psychopharmacol. 2008;22:330–332. doi: 10.1177/0269881107081550. [DOI] [PubMed] [Google Scholar]
- 26.Ellison JM. SSRI withdrawal buzz. J Clin Psychiatry. 1994;55:544–545. [PubMed] [Google Scholar]
- 27.Fava GA, Bernardi M, Tomba E, Rafanelli C. Effects of gradual discontinuation of selective serotonin reuptake inhibitors in panic disorder with agoraphobia. Int J Neuropsychopharmacol. 2007;10:835–838. doi: 10.1017/S1461145706007462. [DOI] [PubMed] [Google Scholar]
- 28.Andrews PW, Kornstein SG, Halberstadt LJ, Gardner CO, Neale MC. Blue again: perturbational effects of antidepressants suggest monoaminergic homeostasis in major depression. Front Psychol. 2011;2 doi: 10.3389/fpsyg.2011.00159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Harvey BH, Slabbert FN. New insights on the antidepressant discontinuation syndrome. Hum Psychopharmacol. 2014;29:503–516. doi: 10.1002/hup.2429. [DOI] [PubMed] [Google Scholar]
- 30.Dilsaver SC, Feinberg M, Greden JF. Antidepressant withdrawal symptoms treated with anticholinergic agents. Am J Psychiatry. 1983;140:249–251. doi: 10.1176/ajp.140.2.249. [DOI] [PubMed] [Google Scholar]
- 31.Beesdo K, Bittner A, Pine DS, et al. Incidence of social anxiety disorder and the consistent risk for secondary depression in the first three decades of life. Arch Gen Psychiatry. 2007;64:903–912. doi: 10.1001/archpsyc.64.8.903. [DOI] [PubMed] [Google Scholar]
- 32.Wittchen HU, Beesdo K, Bittner A, Goodwin RD. Depressive episodes-evidence for a causal role of primary anxiety disorders? Eur Psychiatry. 2003;18:384–393. doi: 10.1016/j.eurpsy.2003.10.001. [DOI] [PubMed] [Google Scholar]
- 33.Boschloo L, Spijker AT, Hoencamp E, et al. Predictors of the onset of manic symptoms and a (hypo) manic episode in patients with major depressive disorder. PLoS One. 2014;9 doi: 10.1371/journal.pone.0106871. e106871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Fava M, Schmidt ME, Zhang S, Gonzales J, Raute NJ, Judge R. Treatment approaches to major depressive disorder relapse Part 2: reinitiation of antidepressant treatment. Psychother Psychosom. 2002;71:195–199. doi: 10.1159/000063644. [DOI] [PubMed] [Google Scholar]
- 35.Fava GA, Rafanelli C, Grandi S, Canestrari R, Morphy MA. Six-year outcome for cognitive behavioral treatment of residual symptoms in major depression. Am J Psychiatry. 1998;155:1443–1445. doi: 10.1176/ajp.155.10.1443. [DOI] [PubMed] [Google Scholar]
- 36.Fava M, Detke MJ, Balestrieri M, Wang F, Raskin J, Perahia D. Management of depression relapse: re-initiation of duloxetine treatment or dose increase. J Psychiatr Res. 2006;40:328–336. doi: 10.1016/j.jpsychires.2005.06.005. [DOI] [PubMed] [Google Scholar]
- 37.Fournier JC, DeRubeis RJ, Hollon SD, et al. Antidepressant drug effects and depression severity: a patient-level meta-analysis. JAMA. 2010;303:47–53. doi: 10.1001/jama.2009.1943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kirsch I, Deacon BJ, Huedo-Medina TB, Scoboria A, Moore TJ, Johnson BT. Initial severity and antidepressant benefits: a meta-analysis of data submitted to the Food and Drug Administration. PLoS Med. 2008;5 doi: 10.1371/journal.pmed.0050045. e45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Cipriani A, Furukawa TA, Salanti G, et al. Comparative efficacy and acceptability of 21 antidepressant drugs for the acute treatment of adults with major depressive disorder: a systematic review and network meta-analysis. Lancet. 2018;391:1357–1366. doi: 10.1016/S0140-6736(17)32802-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Heinz A, Daedelow L, Wackerhagen C, Di Chiara G. Addiction theory matters—why there is no dependence on caffeine or antidepressant medication. Addict Biol. 2019 doi: 10.1111/adb.12735. [DOI] [PubMed] [Google Scholar]
- E1.Kramer JC, Klein DF, Fink M. Withdrawal symptoms following dicontinuation of imipramine therapy. Am J Psychiatry. 1961;118:549–550. doi: 10.1176/ajp.118.6.549. [DOI] [PubMed] [Google Scholar]
- E2.Hotopf M, Hardy R, Lewis G. Discontinuation rates of SSRIs and tricyclic antidepressants: a meta-analysis and investigation of heterogeneity. Br J Psychiatry. 1997;170:120–127. doi: 10.1192/bjp.170.2.120. [DOI] [PubMed] [Google Scholar]
- E3.Van Geffen EC, Brugman M, Van Hulten R, Bouvy ML, Egberts AC, Heerdink ER. Patients‘ concerns about and problems experienced with discontinuation of antidepressants. Int J Pharm Pract. 2007;15:291–293. [Google Scholar]
- E4.Rosenbaum JF, Fava M, Hoog SL, Ascroft RC, Krebs WB. Selective serotonin reuptake inhibitor discontinuation syndrome: a randomized clinical trial. Biol Psychiatry. 1998;44:77–87. doi: 10.1016/s0006-3223(98)00126-7. [DOI] [PubMed] [Google Scholar]
- E5.Berber MJ. FINISH: remembering the discontinuation syndrome Flu-like symptoms, Insomnia, Nausea, Imbalance, Sensory disturbances, and Hyperarousal (anxiety/agitation) J Clin Psychiatry. 1998;59 [PubMed] [Google Scholar]
- E6.Hartford J, Kornstein S, Liebowitz M, et al. Duloxetine as an SNRI treatment for generalized anxiety disorder: results from a placebo and active-controlled trial. Int Clin Psychopharmacol. 2007;22:167–174. doi: 10.1097/YIC.0b013e32807fb1b2. [DOI] [PubMed] [Google Scholar]
- E7.Montgomery SA, Andersen HF. Escitalopram versus venlafaxine XR in the treatment of depression. Int Clin Psychopharmacol. 2006;21:297–309. doi: 10.1097/00004850-200609000-00008. [DOI] [PubMed] [Google Scholar]
- E8.Sir A, D‘Souza RF, Uguz S, et al. Randomized trial of sertraline versus venlafaxine XR in major depression: efficacy and discontinuation symptoms. J Clin Psychiatry. 2005;66:1312–1320. doi: 10.4088/jcp.v66n1015. [DOI] [PubMed] [Google Scholar]
- E9.Perahia DG, Kajdasz DK, Desaiah D, Haddad PM. Symptoms following abrupt discontinuation of duloxetine treatment in patients with major depressive disorder. J Affect Disord. 2005;89:207–212. doi: 10.1016/j.jad.2005.09.003. [DOI] [PubMed] [Google Scholar]
- E10.Vandel P, Sechter D, Weiller E, et al. Post-treatment emergent adverse events in depressed patients following treatment with milnacipran and paroxetine. Hum Psychopharmacol. 2004;19:585–586. doi: 10.1002/hup.644. [DOI] [PubMed] [Google Scholar]
- E11.McGrath PJ, Stewart JW, Tricamo E, Nunes EN, Quitkin FM. Paradoxical mood shifts to euthymia or hypomania upon withdrawal of antidepressant agents. J Clin Psychopharmacol. 1993;13:224–225. [PubMed] [Google Scholar]
- E12.Tyrer P. Clinical effects of abrupt withdrawal from tri-cyclic antidepressants and monoamine oxidase inhibitors after long-term treatment. J Affect Disord. 1984;6:1–7. doi: 10.1016/0165-0327(84)90002-8. [DOI] [PubMed] [Google Scholar]
- E13.MacCall C, Callender J. Mirtazapine withdrawal causing hypomania. Br J Psychiatry. 1999;175 doi: 10.1192/bjp.175.4.390a. [DOI] [PubMed] [Google Scholar]
- E14.Fauchere PA. Recurrent, persisting panic attacks after sudden discontinuation of mirtazapine treatment: a case report. Int J Psychiatry Clin Pract. 2004;8:127–129. doi: 10.1080/13651500410006134. [DOI] [PubMed] [Google Scholar]
- E15.Berigan TR. Bupropion-associated withdrawal symptoms revisited: a case report. Prim Care Companion J Clin Psychiatry. 2002;4 doi: 10.4088/pcc.v04n0208a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- E16.Mirin SM, Schatzberg AF, Creasey DE. Hypomania and mania after withdrawal of tricyclic antidepressants. Am J Psychiatry. 1981;138:87–89. doi: 10.1176/ajp.138.1.87. [DOI] [PubMed] [Google Scholar]
- E17.Rothschild AJ. Mania after withdrawal of isocarboxazid. J Clin Psychopharmacol. 1985;5:340–342. [PubMed] [Google Scholar]
- E18.Bloch M, Stager SV, Braun AR, Rubinow DR. Severe psychiatric symptoms associated with paroxetine withdrawal. Lancet. 1995;346 doi: 10.1016/s0140-6736(95)92691-7. [DOI] [PubMed] [Google Scholar]
- E19.Stoukides JA, Stoukides CA. Extrapyramidal symptoms upon discontinuation of fluoxetine. Am J Psychiatry. 1991;148 doi: 10.1176/ajp.148.9.1263a. [DOI] [PubMed] [Google Scholar]
- E20.Sathananthan GL, Gershon S. Imipramine withdrawal: an Akathisia like syndrome. Am J Psychiatry. 1973;130:1286–1287. doi: 10.1176/ajp.130.11.1286. [DOI] [PubMed] [Google Scholar]
- E21.Goldstein TR, Frye MA, Denicoff KD, et al. Antidepressant discontinuation-related mania: critical prospective observation and theoretical implications in bipolar disorder. J Clin Psychiatry. 1999;60:563–567. [PubMed] [Google Scholar]
- E22.Wolfe RM. Antidepressant withdrawal reactions. Am Fam Physician. 1997;56:455–462. [PubMed] [Google Scholar]
- E23.Reeves RR, Mack JE, Beddingfield JJ. Shock-like sensations during venlafaxine withdrawal. Pharmacotherapy. 2003;23:678–681. doi: 10.1592/phco.23.5.678.32198. [DOI] [PubMed] [Google Scholar]
- E24.Cosci F, Chouinard G, Chouinard V-A, Fava GA. The diagnostic clinical interview for drug withdrawal 1 (DID-W1)-new symptoms of selective serotonin reuptake inhibitors (SSRI) or serotonin Norepinephrine reuptake inhibitors (SNRI): inter-rater reliability. Riv Psichiatr. 2018;53:95–99. doi: 10.1708/2891.29158. [DOI] [PubMed] [Google Scholar]
- E25.Bhanji NH, Chouinard G, Kolivakis T, Margolese HC. Persistent tardive rebound panic disorder, rebound anxiety and insomnia following paroxetine withdrawal: a review of rebound-withdrawal phenomena. Can J Clin Pharmacol. 2006;13:e69–e74. [PubMed] [Google Scholar]
- E26.Belaise C, Gatti A, Chouinard VA, Chouinard G. Persistent postwithdrawal disorders induced by paroxetine, a selective serotonin reuptake inhibitor, and treated with specific cognitive behavioral therapy. Psychother Psychosom. 2014;83:247–248. doi: 10.1159/000362317. [DOI] [PubMed] [Google Scholar]
- E27.Belaise C, Gatti A, Chouinard VA, Chouinard G. Patient online report of selective serotonin reuptake inhibitor-induced persistent postwithdrawal anxiety and mood disorders. Psychother Psychosom. 2012;81:386–388. doi: 10.1159/000341178. [DOI] [PubMed] [Google Scholar]
- E28.El-Mallakh RS, Briscoe B. Studies of long-term use of antidepressants. CNS Drugs. 2012;26:97–109. doi: 10.2165/11599450-000000000-00000. [DOI] [PubMed] [Google Scholar]
- E29.Viguera AC, Baldessarini RJ, Friedberg J. Discontinuing antidepressant treatment in major depression. Harv Rev Psychiatry. 1998;5:293–306. doi: 10.3109/10673229809003578. [DOI] [PubMed] [Google Scholar]
- E30.Hohagen F, Montero RF, Weiss E, et al. Treatment of primary insomnia with trimipramine: an alternative to benzodiazepine hypnotics? Eur Arch Psychiatry Clin Neurosci. 1994;244:65–72. doi: 10.1007/BF02193521. [DOI] [PubMed] [Google Scholar]
- E31.Baldwin DS, Montgomery SA, Nil R, Lader M. Discontinuation symptoms in depression and anxiety disorders. Int J Neuropsychopharmacol. 2007;10:73–84. doi: 10.1017/S1461145705006358. [DOI] [PubMed] [Google Scholar]
- E32.Hindmarch I, Kimber S, Cockle SM. Abrupt and brief discontinuation of antidepressant treatment: effects on cognitive function and psychomotor performance. Int Clin Psychopharmacol. 2000;15:305–318. doi: 10.1097/00004850-200015060-00001. [DOI] [PubMed] [Google Scholar]
- E33.Judge R, Parry MG, Quail D, Jacobson JG. Discontinuation symptoms: comparison of brief interruption in fluoxetine and paroxetine treatment. Int Clin Psychopharmacol. 2002;17:217–225. doi: 10.1097/00004850-200209000-00002. [DOI] [PubMed] [Google Scholar]
- E34.Bogetto F, Bellino S, Revello RB, Patria L. Discontinuation syndrome in dysthymic patients treated with selective serotonin reuptake inhibitors: a clinical investigation. CNS Drugs. 2002;16:273–283. doi: 10.2165/00023210-200216040-00006. [DOI] [PubMed] [Google Scholar]
- E35.Boyer EW, Shannon M. The serotonin syndrome. N Engl J Med. 2005;352:1112–1120. doi: 10.1056/NEJMra041867. [DOI] [PubMed] [Google Scholar]
- E36.Benmansour S, Altamirano AV, Jones DJ, et al. Regulation of the norepinephrine transporter by chronic administration of antidepressants. Biol Psychiatry. 2004;55:313–316. doi: 10.1016/s0006-3223(03)00676-0. [DOI] [PubMed] [Google Scholar]
- E37.Kittler K, Lau T, Schloss P. Antagonists and substrates differentially regulate serotonin transporter cell surface expression in serotonergic neurons. Eur J Pharmacol. 2010;629:63–67. doi: 10.1016/j.ejphar.2009.12.010. [DOI] [PubMed] [Google Scholar]
- E38.Mirza NR, Nielsen EØ, Troelsen KB. Serotonin transporter density and anxiolytic-like effects of antidepressants in mice. Prog Neuropsychopharmacol Biol Psychiatry. 2007;31:858–866. doi: 10.1016/j.pnpbp.2007.01.020. [DOI] [PubMed] [Google Scholar]
- E39.Benmansour S, Owens WA, Cecchi M, Morilak DA, Frazer A. Serotonin clearance in vivo is altered to a greater extent by antidepressant-induced downregulation of the serotonin transporter than by acute blockade of this transporter. J Neurosci. 2002;22:6766–6772. doi: 10.1523/JNEUROSCI.22-15-06766.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- E40.Klomp A, Hamelink R, Feenstra M, Denys D, Reneman L. Increased response to a 5-HT challenge after discontinuation of chronic serotonin uptake inhibition in the adult and adolescent rat brain. PLoS One. 2014;9 doi: 10.1371/journal.pone.0099873. e99873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- E41.Montgomery D. ECNP consensus meeting March 2000 Guidelines for investigating efficacy in GAD. Eur Neuropsychopharmacol. 2002;12:81–87. doi: 10.1016/s0924-977x(01)00147-x. [DOI] [PubMed] [Google Scholar]
- E42.Coupland NJ, Bell CJ, Potokar JP. Serotonin reuptake inhibitor withdrawal. J Clin Psychopharmacol. 1996;16:356–362. doi: 10.1097/00004714-199610000-00003. [DOI] [PubMed] [Google Scholar]
- E43.Amsden GW, Georgian F. Orthostatic hypotension induced by sertraline withdrawal. Pharmacotherapy. 1996;16:684–686. [PubMed] [Google Scholar]
- E44.Phelps J. Tapering antidepressants: is 3 months slow enough? Med Hypotheses. 2011;77:1006–1008. doi: 10.1016/j.mehy.2011.08.035. [DOI] [PubMed] [Google Scholar]
- E45.Keuthen NJ, Cyr P, Ricciardi JA, Minichiello WE, Buttolph ML, Jenike MA. Medication withdrawal symptoms in obsessive-compulsive disorder patients treated with paroxetine. J Clin Psychopharmacol. 1994;14:206–207. doi: 10.1097/00004714-199406000-00010. [DOI] [PubMed] [Google Scholar]
- E46.Benazzi F. Fluoxetine for clomipramine withdrawal symptoms. Am J Psychiatry. 1999;156:661–662. doi: 10.1176/ajp.156.4.661a. [DOI] [PubMed] [Google Scholar]
- E47.Giakas WJ, Davis JM. Intractable withdrawal from venlafaxine treated with fluoxetine. Psychiatr Ann. 1997;27:85–92. [Google Scholar]
- E48.Voderholzer U. Machen Antidepressiva abhängig? Pro Psychiatr Prax. 2018;45:344–345. [Google Scholar]
- E49.Lichtigfeld FJ, Gillman MA. The possible abuse of and dependence on major tranquillisers and tricyclic antidepressants. S Afr Med J. 1994;84:5–6. [PubMed] [Google Scholar]
- E50.WHO. International Statistical Classification of Diseases and Related health problems, 10th revision, 5th edition. World Health Organization. 2015 [Google Scholar]
- E51.Fava GA, Offidani E. The mechanisms of tolerance in antidepressant action. Prog Neuropsychopharmacol Biol Psychiatry. 2011;35:1593–1602. doi: 10.1016/j.pnpbp.2010.07.026. [DOI] [PubMed] [Google Scholar]
- E52.Bosman RC, Waumans RC, Jacobs GE, et al. Failure to respond after reinstatement of antidepressant medication: a systematic review. Psychother Psychosom. 2018;87:268–275. doi: 10.1159/000491550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- E53.Reidenberg MM. Drug discontinuation effects are part of the pharmacology of a drug. J Pharmacol Exp Ther. 2011;339:324–328. doi: 10.1124/jpet.111.183285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- E54.Reid JL, Campbell BC, Hamilton CA. Withdrawal reactions following cessation of central alpha-adrenergic receptor agonists. Hypertension. 1984;6:II71–II75. doi: 10.1161/01.hyp.6.5_pt_2.ii71. [DOI] [PubMed] [Google Scholar]
- E55.O‘Brien ET, MacKinnon J. Propranolol and polythiazide in treatment of hypertension. Br Heart J. 1972;34:1042–1044. doi: 10.1136/hrt.34.10.1042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- E56.Westermeyer J. Addiction to tranylcypromine (Parnate): a case report. Am J Drug Alcohol Abuse. 1989;15:345–350. doi: 10.3109/00952998908993414. [DOI] [PubMed] [Google Scholar]
- E57.Griffin N, Draper RJ, Webb MG. Addiction to tranylcypromine. Br Med J (Clin Res Ed) 1981;283 doi: 10.1136/bmj.283.6287.346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- E58.Ainsworth K, Smith SE, Zetterström TS, Pei Q, Franklin M, Sharp T. Effect of antidepressant drugs on dopamine D1 and D2 receptor expression and dopamine release in the nucleus accumbens of the rat. Psychopharmacology (Berl) 1998;140:470–477. doi: 10.1007/s002130050791. [DOI] [PubMed] [Google Scholar]
- E59.Heinz AJ, Beck A, Meyer-Lindenberg A, Sterzer P, Heinz A. Cognitive and neurobiological mechanisms of alcohol-related aggression. Nat Rev Neurosci. 2011;12:400–413. doi: 10.1038/nrn3042. [DOI] [PubMed] [Google Scholar]
- E60.Hiemke C, Bergemann N, Clement HW, et al. Consensus guidelines for therapeutic drug monitoring in neuropsychopharmacology: update 2017. Pharmacopsychiatry. 2018;51:9–62. doi: 10.1055/s-0043-116492. [DOI] [PubMed] [Google Scholar]