Abstract
Introduction
Inflammation is associated with obesity condition and plays a pivotal role in the onset and progression of many chronic diseases. Among several nutraceutical foods, hazelnuts (Corylus avellana L.) are considered an excellent anti-inflammatory and hypolipidemic food being the second richest source of monounsaturated fatty acids among nuts and because they are rich in vitamins, minerals, and phenolic compounds.
Materials and Methods
A prospective pilot clinical trial on 24 healthy volunteers who consumed daily, as a snack, 40 g of hazelnuts (261.99 kcal/1096.17 kJ) for six weeks was conducted. Anthropometric measurements, body composition analysis, and nutrigenomic analysis on 12 anti-inflammatory and antioxidant genes were evaluated at baseline (T0) and after hazelnut intervention (T1).
Results
No significant changes were detected on body composition analysis after hazelnut consumption. Conversely, significant upregulation was detected for SOD1 (2−ΔΔCt = 2.42), CAT (2−ΔΔCt = 2.41), MIF (2−ΔΔCt = 4.12), PPARγ (2−ΔΔCt = 5.89), VDR (2−ΔΔCt = 3.61), MTHFR (2−ΔΔCt = 2.40), and ACE (2−ΔΔCt = 2.16) at the end of the study.
Conclusions
According to emerging evidences, hazelnut consumption does not lead to weight gain probably due to the improvement of the body's antioxidant capacity by the upregulation of genes implied in oxidant reactions and inflammation.
1. Introduction
Inflammation is a constant feature associated with the onset and progression of many chronic degenerative diseases, dramatically increasing in western countries, and one of the leading causes of its insurgence is the high adipose tissue content [1]. White adipose tissue (WAT) is a metabolic organ able to satisfy body functional demands through the storage, in case of an extra caloric intake, or the mobilization, in case of metabolic demands, of energy. WAT is composed of adipocytes, which has marked cellular heterogeneity, vascularisation, and innervation, with a complex hormonal homeostatic system [2]. The relationship between obesity and inflammation was firstly observed in the 1990s. The metabolic dysfunction of adipocytes is the main cause of chronic inflammation in WAT, through the increasing expression of many biologically functional cytokines/chemokines considering the major mediators of inflammation in particular in obesity condition, like tumor necrosis factor-α (TNF-α), interleukin-6 (IL-6), interleukin-1β (IL-1β), interleukin-8 (IL-8), and interleukin-17D (IL-17D) [3]. The release of those inflammation cytokines determined the subsequent activation of tissue-resident macrophages [4]. In obesity, the excess of lipids and the consequent surplus of WAT can cause also an increase of reactive oxygen species (ROS) levels and the reduction of the antioxidant defenses, events that determine the induction of systemic oxidative stress [5].
Among several nutraceutical foods, tree nuts are edible dry fruits that, together with cereals and legumes, have characterized the human diet since preagricultural times, representing a high-energy and nutrient-dense food, rich in minerals, vitamins, and bioactive compounds [6]. The scientific evidence arises from both epidemiological observations and clinical trials, showing beneficial effects of nut intake on the health status. The reason why a diet rich in nuts has these health benefits is due to their high content in monounsaturated fatty acids (MUFAs) and polyunsaturated fatty acids (PUFAs) [7], sterols, and fibers and to the presence of bioactive molecules like vitamin E (α-tocopherol), vitamin B, arginine, and polyphenols [8, 9]. In particular, the nut antioxidant capacity was widely discussed, highlighting the bioactivity potential of nut phenolic compounds [10]. There is also a suggestion of regulatory effects of nuts on inflammation, demonstrated by the change of circulating inflammatory markers and the expression of ligands for inflammatory molecules in circulating monocytes after two weeks of an enriched nut diet [11]. Furthermore, nuts are particularly rich in calories but their enriching in diets does not change the body mass index (BMI). Actually, a higher weight loss and a greater protection against cardiovascular risk factors in subjects who consumed nuts in their diet compared to who follow other dietary plans were shown [12–14]. These observations support the theory for which the introduction of nuts in weight loss or weight control diets determines a more favourable outcome.
For all these reasons, nuts are included in the American Heart Association dietary metrics for defining ideal cardiovascular health in their recent report on setting goals for health promotion and disease reduction for 2020 [15]. Furthermore, the World Health Organization (WHO) recommend a daily intake of nuts as a fundamental part of a cardioprotective diet [16].
Among nuts, hazelnuts (Corylus avellana L.) belong to the Betulaceae family and are considered excellent anti-inflammatory and hypolipidemic food [17], being the second richest source of MUFAs among nuts and because they are rich in vitamin E and tocopherols, phytosterols (mainly α-sitosterol), magnesium, copper and selenium, L-arginine, polyphenols, folate, and fibers. Among the phenolic compounds, gallic acid, p-hydroxybenzoic acid, epicatechin, caffeic acid, sinapic acid, and quercetin are found in hazelnuts, with the highest unsaturated/saturated fatty acid ratio among nuts [12].
To our knowledge, only one study defined the right intake of hazelnuts to consume in order to maintain body weight [18], and few works have had a nutrigenomic approach to study the effects of hazelnut consumption [19, 20]. However, nowadays, no studies have identified the right intake of hazelnuts to be able to maintain or improve body weight and/or body composition and enhance anti-inflammatory gene expression. The mechanisms behind weight and of body composition changes given by hazelnut consumption, as well as their modulatory effect on the inflammatory genomic upstream pathway, represent a new field to discover.
The aims of this pilot study were to investigate the effect of hazelnut treatment (HNT) on body composition and on genomic response of genes related to oxidative stress and inflammation.
2. Materials and Methods
2.1. Subjects and Study Design
For this prospective clinical trial, 30 healthy volunteers were recruited and analyzed from the staff of the Clinical Nutrition and Nutrigenomic Section, Department of Biomedicine and Prevention of the University of Rome Tor Vergata. Body composition data and blood samples for genomic analysis were collected at baseline (T0) and after 6 weeks of hazelnut intervention (T1) in order to evaluate nutritional, oxidative, and inflammation statuses. In this pilot study, every recruited subject was considered a control of himself. Lifestyle habits of healthy volunteers did not change during the study period. No abnormality was presented during the study period. All participants recruited in the study authorized their participation by reading and signing the informed consent, conducted in accordance with the provisions of the Ethics Committee of Medicine, University of Rome Tor Vergata, and with the Helsinki Declaration of 1975 as revised in 1983. This protocol has been registered with Trial Registration Number ClinicalTrials.gov. ID: NCT01890070.
2.2. Exclusion Criteria
In order to be included in the study, subjects had to respect the following exclusion criteria: active tobacco smoking; pregnancy; breastfeeding; type 1 and type 2 diabetes; past or active cardiovascular diseases; metabolic, endocrine, liver, kidney, and autoimmune disorders; chronic viral (hepatitis C and B and HIV) and cancer diseases; corticosteroid and chronic anti-inflammatory therapy; and participation in other dietary trials.
2.3. Dietary Intervention
During the study period, all the recruited subjects consumed daily 40 g of hazelnuts (261.99 kcal/1096.17 kJ), cultivar Tonda Gentile Romana, provided by Coopernocciola in Vico Matrino (Viterbo, Italy). A 24 h dietary recall was performed at the baseline to all participants. All subjects were administered a standard isocaloric diet according to the following Mediterranean diet criteria: 55% of carbohydrates, 20% of proteins (>50% of vegetable derivation), <30% of lipids (on total kcal: saturated fat <10%, 6–10% polyunsaturated fatty acids (PUFA), n-6/n-3 PUFA ratio of 3 : 1, 15% of monounsaturated fatty acids (MUFA), and <1% transfatty acids), and 30 g of fiber. Caloric intake calculation of all standard isocaloric diets was based on a 24 h dietary recall evaluation for each subject. Standard diets were elaborated with a proper software (Dietosystem, DS Medica, Milan, Italy). All subjects were asked to eliminate any other type of hazelnuts or nuts from their diet.
2.4. Anthropometric Measurements and Body Composition Analysis
All volunteers were subjected to an anthropometric evaluation after overnight fasting. Body weight and height were measured according to previously described methods [21]. Body weight was evaluated with balance scale to the nearest 0.1 kg (Invernizzi, Rome, Italy). Height was measured with a stadiometer to the nearest 0.1 cm (Invernizzi, Rome, Italy). BMI was calculated using the following formula: BMI = body weight (kg)/height (m)2. Body composition analysis was assessed by Dual Energy X-Ray Absorptiometry (DXA) (i-DXA, GE Medical Systems, Milwaukee, WI, USA) [22].
2.5. Biochemical Analysis
Blood tests were carried out at the “Policlinico Tor Vergata (PTV)” of Rome, Italy. Analyses were performed at baseline after a 12-hour overnight fast. Blood samples (10 mL) were collected into EDTA tubes (Vacutainer®), placed in ice, and plasma was separated by centrifugation. Laboratory analysis included complete blood count, total cholesterol (TC), high-density lipoprotein cholesterol (HDL-C), low-density lipoprotein cholesterol (LDL-C), and triglycerides (Tg). Complete blood count, except serum lipid and Tg analyses, was carried out with an ADVIA®1800 Chemistry System (Siemens Healthcare). Serum lipid profile components were determined by standard enzymatic colorimetric techniques (Roche143 Modular P800, Roche Diagnostics, Indianapolis, IN, USA). Serum Tg were measured by a coupled enzymatic method on the Beckman Synchron LX20 automated system.
2.6. Sample Collection. RNA Extraction and Analysis
Blood samples were collected and stabilized in PAX gene Blood RNA Tubes (Pre AnalytiX, Qiagen, Hombrechtikon, Switzerland) and then stored at -80°C until use. Blood samples were pooled according to intervention times (baseline and postintervention). Total RNA was purified with a PAX gene Blood miRNA Kit, following the manufacturer's instructions (Pre AnalytiX, Qiagen, Hombrechtikon, Switzerland), and quantified through spectrophotometry (Nanodrop, Wilmington, USA). Specific RT2 Profiler PCR Arrays (Qiagen, Netherlands) were used for human oxidative stress (PAHS-065ZA, Qiagen, Netherlands) and human inflammation (PAHS-097ZA, Qiagen, Netherlands) pathways. The gene expression of the following 12 genes was analyzed: superoxide dismutase 1 (SOD1) (NCBI Accession number: NM_000454.4), catalase (CAT) (NCBI Accession number: NM_001752.3), macrophage migration inhibitory factor (MIF) (NCBI Accession number: NM_002415.1), peroxisome proliferator-activated receptor gamma (PPARγ) (NCBI Accession number: NM_001354667), vitamin D receptor (VDR) (NCBI Accession number: NM_000367.2), methylenetetrahydrofolate reductase (MTHFR) (NCBI Accession number: NM_001330358.1), angiotensin I-converting enzyme (ACE) (NCBI Accession number: NM_000789.3), apolipoprotein E (APOE) (NCBI Accession number: NM_001302691.1), interleukin 6 receptor (IL6R) (NCBI Accession number: NM_000565.3), nuclear factor of kappa light polypeptide gene enhancer in B-cell 1 (NFKB1) (NCBI Accession number: NM_003998.3), insulin-like growth factor 2 receptor (IFG2R) (NCBI Accession number: NG_011785.3), and upstream transcription factor 1 (USF1) (NCBI Accession number: NM_001276373.1). Each qRT-PCR experiment was performed in triplicate and repeated at least twice, in line with the manufacturer's instructions (Qiagen, Netherlands). β-Actin (ACTB) (NM 001101) was used as a housekeeping gene. A comparative threshold (CT) cycle was used to determine the gene expression level. A CT value was normalized using the formula ΔCT = CT (gene) − CT (housekeeping gene). The relative gene expression levels were determined according to the following formula: ΔΔCT = ΔCT sample − ΔCT calibrator. The value used to plot relative gene expression was determined using the expression fold change (FC) = 2–ΔΔCT.
2.7. Statistical Analysis
Statistical analysis was carried out using IBM SPSS 21.0 for Windows (Armonk, NY: IBM Corp, USA). Power calculation was evaluated on total cholesterol, with a 2-sided test and an α = 0.05. After the Shapiro-Wilk test, a paired t-test or a nonparametric Wilcoxon test was performed to evaluate differences before and after hazelnut interventions. All tests were considered significant at p ≤ 0.05. For genomic analysis, the value used to plot relative gene expression was determined using the expression fold change (FC) = 2−ΔΔCT. Only genes with a FC ≥2 were considered significantly upregulated for differentially expressed genes. Conversely, genes with a FC≤0.5 were considered significantly downregulated for differentially expressed genes.
3. Results
3.1. Subjects Characteristics
Of the thirty subjects enrolled, one of them was excluded from the trial (subject declined to participate) (Figure 1). At the end, twenty-four subjects completed the trial. Any changes to trial outcomes after the trial commenced occurred. The power of the study was 0.61. The average age of subjects was 51.58 ± 9.37 years (58.3% male and 41.7% female) (Table 1).
Figure 1.
Flow chart study design.
Table 1.
Anthropometric and clinical baseline characteristic of study subjects.
| Parameter (n = 24) | Male | Female | ||||
|---|---|---|---|---|---|---|
| Frequency (n, %) | (n = 14; 53.8%) | (n = 10; 41.7%) | ||||
| Median | P25 | P75 | Median | P25 | P75 | |
| Age (years) | 56.50 | 52.00 | 60.50 | 47.50 | 41.25 | 56.25 |
| SBP (mmHg) | 125.50 | 112.25 | 140.00 | 108.00 | 101.75 | 121.50 |
| DBP (mmHg) | 74.50 | 65.00 | 88.25 | 72.50 | 63.75 | 75.50 |
| Height (cm) | 172.00 | 165.00 | 173.25 | 161.00 | 155.75 | 162.00 |
| Weight (kg) | 75.00 | 69.50 | 82.95 | 63.95 | 58.83 | 78.68 |
| BMI (kg/m2) | 26.23 | 24.34 | 27.36 | 25.37 | 22.99 | 30.07 |
| Neck circumference (cm) | 41.00 | 39.75 | 42.63 | 37.00 | 35.38 | 38.25 |
| Waist circumference (cm) | 88.50 | 82.75 | 92.25 | 81.50 | 72.00 | 85.88 |
| Abdominal circumference (cm) | 94.00 | 87.13 | 97.00 | 92.75 | 86.75 | 99.75 |
| Hip circumference (cm) | 97.50 | 94.00 | 99.25 | 100.50 | 96.00 | 114.13 |
| Waist/hip ratio | 0.91 | 0.87 | 0.96 | 0.79 | 0.73 | 0.84 |
| TBFat (kg) | 19.30 | 16.20 | 22.60 | 22.90 | 20.51 | 37.41 |
| TBFat (%) | 25.60 | 22.28 | 28.58 | 38.45 | 33.05 | 42.28 |
| ABFat (%) | 33.15 | 25.08 | 37.53 | 43.00 | 33.98 | 51.75 |
| GBFat (%) | 25.70 | 22.98 | 27.15 | 39.35 | 36.88 | 49.63 |
| TBLean (kg) | 53.94 | 48.04 | 58.36 | 38.39 | 36.44 | 40.93 |
| BMC (g) | 2896.00 | 2631.75 | 3108.50 | 2313.00 | 2059.50 | 2634.75 |
| ASMMI | 8.74 | 8.37 | 9.29 | 6.67 | 6.42 | 7.59 |
| t-score | -0.40 | -0.93 | 0.13 | 0.25 | -1.13 | 1.60 |
| Neutrophils (K/μL) | 2850.00 | 2000.00 | 3530.00 | 3070.00 | 2190.00 | 3360.00 |
| Lymphocytes (K/μL) | 1800.00 | 1240.00 | 2000.00 | 2120.00 | 1160.00 | 2540.00 |
| Platelets (K/μL) | 191000.00 | 167000.00 | 204000.00 | 243000.00 | 173000.00 | 256000.00 |
| TC (mg/dL) | 193.00 | 149.00 | 245.00 | 214.00 | 159.00 | 270.00 |
| HDL-C (mg/dL) | 50.00 | 43.00 | 66.00 | 67.00 | 38.00 | 70.00 |
| LDL-C (mg/dL) | 131.00 | 80.00 | 159.00 | 146.00 | 75.00 | 178.00 |
| Tg (mg/dL) | 95.00 | 42.00 | 113.00 | 99.00 | 54.00 | 124.00 |
| NLR | 1.46 | 1.11 | 2.40 | 1.52 | 0.83 | 2.22 |
| PLR | 120.00 | 77.78 | 129.41 | 106.60 | 65.04 | 186.72 |
| Tg/HDL-C | 1.44 | 0.68 | 2.51 | 1.81 | 0.77 | 2.24 |
| TC/HDL-C | 3.86 | 2.40 | 4.00 | 3.87 | 2.34 | 4.07 |
| kcal/diet | 1960.00 | 1650.00 | 2050.00 | 1840.00 | 1230.00 | 2050.00 |
Anthropometric and clinical characteristic at baseline (T0) and after HNT (T1). Results are expressed as the median, minimum, and maximum for each parameter. A paired t-test (a) or a nonparametric Wilcoxon test (b) was performed to evaluate differences before and after hazelnut intervention. All tests were considered significant at p ≤ 0.05. SBP: systolic blood pressure; DBP: diastolic blood pressure; TBFat: total body fat; ABFat: android body fat; GBFat: gynoid body fat; TBLean: total body lean; BMC: bone mineral content; ASMMI: appendicular skeletal muscle mass index; TC: total cholesterol; HDL-C: high-density lipoprotein cholesterol; LDL-C: low-density lipoprotein cholesterol; Tg: triglycerides; NLR: neutrophil-lymphocyte ratio; PLR: platelet-lymphocyte ratio.
3.2. Body Composition and Bioclinical Analysis
After 6 weeks of HNT (T1), a significant reduction of the abdominal circumference (p = 0.04; Δ% = −0.53%) was observed, compared to baseline (T0) values. At the same time, in DXA measurements of bone mineral content (BMC), total body lean (TBLean), android body fat (ABFat), gynoid body fat (GBFat), and total body fat (TBFat), no significant changes were detected. The same observations were highlighted for the changes in the appendicular skeletal muscle mass index (ASMMI) and t-score parameters (Table 2). Furthermore, among the biochemical parameters, only TC (p = 0.01; Δ% = −10.48%) and LDL-C (p = 0.01; Δ% = −12.12%) had a significant reduction after 6 weeks of HNT, as well as the TC/HDL-C ratio (p = 0.03; Δ% = −5.83%). No other changes were highlighted for serum lipid profile and inflammation markers (Table 2).
Table 2.
Anthropometric and clinical characteristic at baseline (T0) and after hazelnut treatment (HNT) (T1).
| Baseline (T0) | HNT (T1) | Δ% | p | |||
|---|---|---|---|---|---|---|
| Median | Min–Max | Median | Min–Max | |||
| SBP (mmHg) | 116.50 | 96.00-169.00 | 120.00 | 100.00-150.00 | 3.00 | 0.81a |
| DBP (mmHg) | 73.00 | 57.00-93.00 | 75.00 | 60.00-86.00 | 2.74 | 0.28a |
| Weight (kg) | 71.40 | 53.50-93.00 | 71.05 | 53.50-93.00 | -0.49 | 0.46a |
| BMI (kg/m2) | 25.95 | 20.64-35.60 | 25.76 | 20.64-35.44 | -0.71 | 0.55a |
| Neck circumference (cm) | 39.50 | 33.00-44.00 | 40.00 | 34.00-43.00 | 1.27 | 0.71b |
| Waist circumference (cm) | 86.25 | 66.50-101.00 | 85.00 | 66.00-103.00 | -1.45 | 0.90a |
| Abdominal circumference (cm) | 94.00 | 73.00-110.00 | 93.50 | 81.00-110.50 | -0.53 | 0.04a |
| Hip circumference (cm) | 98.25 | 92.00-117.00 | 99.00 | 91.00-116.00 | 0.76 | 0.44b |
| Waist/hip ratio | 0.87 | 0.71-1.03 | 0.87 | 0.69-1.00 | 0.00 | 0.34a |
| TBFat (kg) | 34.75 | 14.60-53.10 | 34.95 | 13.50-52.60 | 0.58 | 0.89b |
| TBFat (%) | 29.65 | 16.3-54 | 29.05 | 18.00-53.30 | -2.02 | 0.73a |
| ABFat (%) | 28.75 | 16.10-48.30 | 28.80 | 15.40-48.00 | 0.17 | 0.15a |
| GBFat (%) | 21.08 | 12.02-38.02 | 21.34 | 12.49-38.13 | 1.21 | 0.89a |
| TBLean (kg) | 47.63 | 32.87-70.81 | 48.09 | 32.40-68.42 | 0.97 | 0.34a |
| BMC (g) | 2703.00 | 1667.00-3742.00 | 2622.00 | 1692.00-3627.00 | -3.00 | 0.29a |
| ASMMI | 8.37 | 5.98-10.28 | 8.05 | 6.06-10.57 | -3.83 | 0.11a |
| t-score | -0.1000 | -1.90-1.90 | -0.1000 | -1.90-1.90 | 0.00 | 0.87a |
| Neutrophils (K/μL) | 2970.00 | 2220-6860 | 3245 | 2170-8481 | 11.71 | 0.40a |
| Lymphocytes (K/μL) | 1695.00 | 1160.00-2680.00 | 1660.00 | 754.00-2700.00 | -7.11 | 0.18b |
| Platelets (K/μL) | 194500.00 | 166000.00-293000.00 | 214000.00 | 148000.00-324000.00 | 6.01 | 0.14a |
| TC (mg/dL) | 181.00 | 149.00-214.00 | 167.00 | 102.00-200.00 | -10.48 | 0.01a |
| HDL-C (mg/dL) | 51.50 | 35.00-79.00 | 47.50 | 37.00-80.00 | -6.93 | 0.22a |
| LDL-C (mg/dL) | 114.00 | 75.00-146.00 | 103.00 | 47.00-125.00 | -12.12 | 0.01a |
| Tg (mg/dL) | 100.50 | 42.00-170.00 | 82.50 | 44.00-151.00 | -11.99 | 0.20a |
| NLR | 2.08 | 0.83-4.48 | 2.14 | 0.95-6.68 | 28.87 | 0.18b |
| PLR | 127.08 | 61.94-198.28 | 135.69 | 64.91-309.02 | 18.10 | 0.08a |
| Tg/HDL-C | 2.02 | 0.68-3.55 | 1.87 | 0.61-3.02 | -5.61 | 0.55b |
| TC/HDL-C | 3.71 | 2.28-5.03 | 3.27 | 2.23-4.6 | -5.83 | 0.03a |
| kcal/diet | 1965.00 | 1230.00-2780.00 | 2226.99 | 1491.99-3041.99 | 11.38 | 0.03a |
Anthropometric and clinical characteristic at baseline (T0) and after HNT (T1). Results are expressed as the median, minimum, and maximum for each parameter. A paired t-test (a) or a nonparametric Wilcoxon test (b) was performed to evaluate differences before and after hazelnut intervention. All tests were considered significant at p ≤ 0.05. SBP: systolic blood pressure; DBP: diastolic blood pressure; TBFat: total body fat; ABFat: android body fat; GBFat: gynoid body fat; TBLean: total body lean; BMC: bone mineral content; ASMMI: appendicular skeletal muscle mass index; TC: total cholesterol; HDL-C: high-density lipoprotein cholesterol; LDL-C: low-density lipoprotein cholesterol; Tg: triglycerides; NLR: neutrophil-lymphocyte ratio; PLR: platelet-lymphocyte ratio.
3.3. Gene Expression Data
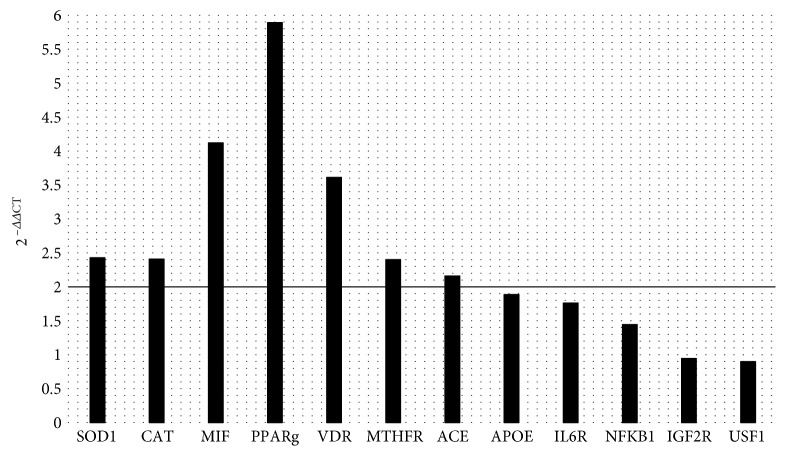
Significant upregulation with a fold change exceeding the threshold set at 2 was detected for SOD1 (2−ΔΔCt = 2.42), CAT (2−ΔΔCt = 2.41), MIF (2−ΔΔCt = 4.12), PPARγ (2−ΔΔCt = 5.89), VDR (2−ΔΔCt = 3.61), MTHFR (2−ΔΔCt = 2.40), and ACE (2−ΔΔCt = 2.16) after HNT (Figure 2). No significant gene expression changes were observed for APOE, IL6R, NFKB1, IFG2R, and USF1 (0.5 < 2−ΔΔCt < 2).
Figure 2.
Gene expression after hazelnut treatment. Different levels of the fold change of 12 genes were analyzed: superoxide dismutase 1 (SOD1), catalase (CAT), macrophage migration inhibitory factor (MIF), peroxisome proliferator-activated receptor gamma (PPARγ), vitamin D receptor (VDR), methylenetetrahydrofolate reductase (MTHFR), angiotensin I-converting enzyme (ACE), apolipoprotein E (APOE), interleukin 6 receptor (IL6R), nuclear factor of kappa light polypeptide gene enhancer in B-cell 1 (NFKB1), insulin-like growth factor 2 receptor (IFG2R), and upstream transcription factor 1 (USF1). Genes with a FC ≥ 2 were considered significantly upregulated for differentially expressed genes; genes with a FC ≤ 0.5 were considered significantly downregulated for differentially expressed genes.
4. Discussion
Hazelnuts have been demonstrated to be able to reduce atherogenic/cardiovascular risk and inflammation [12, 18, 23]. The beneficial effects of hazelnuts on inflammation are due to the content of various nutrients and bioactive substances, and their biochemical profile depends on the cultivar and the country of origin. Nowadays, Turkey is the main country producing hazelnuts in the world, followed by Italy, where Tonda Gentile Romana is found, a typical cultivar from the Latium region, which is particularly rich in stearic, oleic, and linoleic fatty acids [24]. However, the reason why a diet rich in hazelnuts has beneficial effects on health is because of their high content of MUFAs, PUFAs, fibers, α-tocopherol, phytosterols, phenolic compounds, magnesium, copper, and selenium [7, 12]. Some of these components have a potential antioxidant effect through their major nonenzymatic antioxidant proprieties [25]. Constitutive enzymes function or even through their ability to increase the expression of genes involved in anti-inflammatory and antioxidant processes and/or to reduce the expression of inflammatory genes [26–28]. It is well known that the imbalance of the ROS production and the body's antioxidant capacity determines oxidative stress, which is implicated in several pathological processes. Despite the fact that 30% of obese subjects are considered metabolically healthy, a condition where insulin sensitivity, visceral fat content, and intima media thickness of the carotid artery are similar to healthy normal weight, the majority of obese patients were metabolically unhealthy, with an increased risk of cardiovascular problems, metabolic disorders, arterial hypertension, and chronic heart disease development [3]. Adipocytes represent the main cause of chronic inflammation in WAT because of the increased expression of several cytokines/chemokines and ROS production, within a reduction of the antioxidant defenses [5, 29]. In particular, obesity-related disorders are mainly due to the increasing levels of visceral adiposity tissue (VAT), which are considered extremely dangerous because they are related to the high concentration of inflammatory cytokines and obesity-related cardiometabolic problems [3]. It is well known that nuts are particularly rich in fats and calories and usually considered a hypercaloric food, but it was observed that hazelnuts or nut-enriched diets do not change weight or BMI. In fact, several epidemiological studies detected an inverse or a null association between nut consumption and BMI [17–20]. More specifically, consumption up to 60 g of hazelnuts per day did not affect weight and improve blood cholesterol levels [18].
Since 1 kg of fat contains 9000 kcal [30], an excess of 261.99 kcal per day for 6 weeks, due to the intake of 40 g of hazelnuts, would have led to a fat tissue gain of 1 kg, while no significant difference of weight and/or fat mass emerged from the results. Interestingly, as demonstrated by previous observations [12–14], although we noticed a small but significant reduction of abdominal circumference (p < 0.05), we did not observe any changes in weight, BMI, android, gynoid body fat, and total body fat measured by DXA after 6 weeks of a reported 1096.17 kJ/d increase in energy intake with the hazelnut-enriched diets. Furthermore, in this study, we observed a significant reduction of TC, LDL-C, and TC/HDL-C ratio according to Perna et al. [14].
There are several reasons why nut consumption does not determine weight gain and improve cholesterol profile. Firstly, nuts contain a good amount of proteins and fibers, and they have a low glycemic index, contributing to enhance satiety through the reduction of calorie intake from other food sources [31]. In addition, the high content of dietary fibers could reduce the bioavailability of cholesterol from food, and the antioxidant molecules contained in hazelnuts contribute to their antiatherogenic effect [14]. Moreover, the typical crunchy texture of nuts promotes satiety through the mechanical act of chewing, which determines the secretion of food intake regulation [32]. Secondly, previous works suggested that nut consumption leads to the energy expenditure and thermogenic effect increase, probably because of the high unsaturated/saturated fat ratio characteristic of the nuts [33]. Thirdly, lipids of nuts are not easily bioavailable [34], and then, a great part of them is excreted with feces and not accessible for metabolization [35]. Fourthly, the application of Atwater factors to nuts determined an overestimation of energy contents [36].
Another possible explanation about nut consumption and the maintenance of weight or BMI could be suggested by the antioxidant potential of hazelnuts. It is well known that some foods have peculiar antioxidant activities [37], which can affect some disturbances such as obesity. In fact, the relation between obesity and inflammation could be considered bidirectional. Few studies demonstrated that a previous inflammatory status is found before the overweight and obesity onset, becoming a possible risk factor of them [38, 39]. In the SUN study, Ramallal et al. [40] observed that subjects who follow a proinflammatory diet have a clinically relevant weight gain (3-5 kg), as compared with those in the anti-inflammatory diet group. The antioxidant capacity determined by nuts was widely discussed, highlighting the bioactivity potential of nut phenolic compounds [13].
Unfortunately, only few studies observed the effect of hazelnut consumption on inflammatory and/or anti-inflammatory gene expression, and the most relevant results were related to the upregulation of some antioxidant genes [25, 26]. In this pilot study, we confirmed that after hazelnut consumption there is an upregulation of the two of the major antioxidant enzymes, SOD1 (2−ΔΔCt = 2.42) and CAT (2−ΔΔCt = 2.41), which are two of the most important genes involved in the antioxidant pathway, thanks to their ability to catalyze the reaction that leads from superoxide (O2 −) to oxygen and water production. Furthermore, during our trial, we observed an upregulation of MIF (2−ΔΔCt = 4.12). MIF, a cytokine able to regulate innate and acquired immune responses, is usually associated to atherosclerosis progression, obesity, insulin resistance, and inflammatory diseases [41]. Recently, MIF was identified as a key regulator of antioxidant response element (ARE), a DNA enhancer that controls the expression of phase II detoxifying enzymes and cytoprotective proteins for redox homeostasis [42]. In this view, in accordance with other studies [43, 44], MIF seems to be a sensor for oxidative stress, and its upregulation, as shown in our work, could represent a new way to reduce oxidative stress, because of its role as a key regulator of ARE-mediated gene expression. Furthermore, MIF regulates the antioxidant system by binding and activating transcription factors such as Nrf2, which during oxidative stress translocate into the nucleus, inducing ARE activation. In our pilot study, hazelnut consumption is associated also with the upregulation of PPARγ (2−ΔΔCt = 5.89), an enzyme that cooperates in the modulation of oxidative stress, antioxidant response, and inflammatory diseases. In fact, PPARγ has a reciprocal transcriptional regulation with Nrf2, acting synergically in the activation of antioxidant genes [45]. The upregulation of SOD1, CAT, MIF, and PPARγ observed in this study is in line with previous works, which have demonstrated the antioxidant proprieties of a diet rich in nuts, related to the regulation of cellular pathways of atherosclerosis, inflammation, and oxidative stress [46] (Figure 2). At the same time, PPARγ, which controls M2 macrophage activation, seems to be related to VDR expression. The reduction of VDR expression could determine the abolishment of PPARγ effects, establishing a possible VDR-PPARγ pathway that, along with vitamin D, regulates macrophage phenotype [47]. Furthermore, VDR inhibits the initiation of endothelial inflammatory diseases, like atherosclerosis, and reducing inflammatory cytokine/chemokine production in macrophages, when it is activated by vitamin D [48]. The upregulation of VDR (2−ΔΔCt = 3.61) observed in our study (Figure 2) amplified the antioxidant and anti-inflammatory potential of hazelnuts. In fact, the increased VDR gene expression overlaps PPARγ upregulation, suggesting a possible effect of HNT on the VDR-PPARγ pathway.
High blood concentrations of homocysteine (Hcy), an important intermediary in the metabolism of methionine and cysteine, are another potential inflammatory factor. Hcy induces the production of several proinflammatory cytokines, and at the same time, in endothelial cells, high levels of Hcy induce the oxidative inactivation of nitric oxide with the collaboration of several ROS. Furthermore, Hcy plays an important role in the conservation of intracellular glutathione pools and then in oxidative stress conditions. A full MTHFR expression and activity are strictly necessary for the proper recycle of Hcy. Its reduced gene expression could lead to mental disorders, developmental delays, cardiovascular diseases, and cancer [49]. The increased MTHFR gene expression (2−ΔΔCt = 2.40) observed in this pilot study after HNT could reinforce the antioxidant effect of hazelnut consumption (Figure 2). In fact, if MTHFR gene is upregulated, we can speculate a reduction of ROS production and inflammation.
Ordinary nut consumption, maybe due to the polyphenol content, is associated with beneficial effects on blood pressure [47]. High blood pressure is one of the risk factors associated with oxidative stress and inflammation. Usually, increased levels of vascular ACE, the key regulator of the renin-angiotensin system and kallikrein-kinin system, are associated with increased blood pressure [50]; however, evidences have been reported between ACE gene expression and hypertensive condition [51]. In our study, hazelnut consumption leads to an increase of ACE gene expression (2−ΔΔCt = 2.16) (Figure 2), but not to a significant change in SBP or DBP (p > 0.05), strengthening the observation of Mohammadifard N. et al. that maybe only mixed nut and pistachio consumption could be associated with better blood pressure outcomes [52].
5. Conclusions
In conclusion, the reasons why nuts do not determine weight gain are various, like satiety induction, energy expenditure, thermogenesis increase, and the low nutrient bioavailability. According to emerging evidences, hazelnut antioxidant capacity could be another reason. Although this pilot study was not without limitations, as the lack of biochemical analysis, serum protein levels of investigated genes, antioxidant markers, and the limited sample size, our results suggest that an ordinary consumption of small amount of hazelnuts could be a nutritional treatment for all chronic degenerative diseases underlying oxidative stress and inflammation reinforcing the WHO recommendation. Furthermore, the lack of weight gain and, more importantly, the lack of fat mass gain with the hazelnut consumption, which is still considered a high-calorie food, could represent an encouragement for the inclusion of this food not only in the anti-inflammatory dietary patterns but also in all weight loss diets. Our data should be confirmed on a larger number of subjects, with a prospective long-term controlled trial.
Acknowledgments
We are indebted to all the subjects who volunteered in the study. We also thank the entire medical team from the Section of Clinical Nutrition and Nutrigenomic, University of Rome Tor Vergata, for their technical assistance in conducting the clinical aspects of this study.
Abbreviations
- ABFat:
Android body fat
- ACE:
Angiotensin I-converting enzyme
- ARE:
Antioxidant response element
- APOE:
Apolipoprotein E
- ASMMI:
Appendicular skeletal muscle mass index
- BMI:
Body mass index
- BMC:
Bone mineral content
- CAT:
Catalase
- CCL5:
Copper chaperone for superoxide dismutase
- DBP:
Diastolic blood pressure
- DXA:
Dual energy X-ray absorptiometry
- FC:
Fold change
- GBFat:
Gynoid body fat
- GPX1, GPX2:
Glutathione peroxidases 1 and 2
- GSR:
Glutathione reductase
- HNT:
Hazelnut treatment
- HSPA1A:
Heat shock protein 1A
- Hcy:
Homocysteine
- IFG2R:
Insulin-like growth factor 2 receptor
- IL-17D:
Interleukin-17D
- IL-1β:
Interleukin-1β
- IL-6:
Interleukin-6
- IL-8:
Interleukin-8
- IL6R:
Interleukin 6 receptor
- KRT1:
Keratin
- MIF:
Macrophage migration inhibitory factor
- MBL:
Mannose-binding lectin (protein C) 2 soluble
- MTHFR:
Methylenetetrahydrofolate reductase
- MUFAs:
Monounsaturated fatty acids
- NFKB1:
Nuclear factor of kappa light polypeptide gene enhancer in B-cell 1
- PPARγ:
Peroxisome proliferator-activated receptor gamma
- PUFAs:
Polyunsaturated fatty acids
- ROS:
Reactive oxygen species
- SOD1:
Superoxide dismutase 1
- SBP:
Systolic blood pressure
- TXNRD1:
Thioredoxin reductase 1
- TBFat:
Total body fat
- TBLean:
Total body lean
- TNF-α:
Tumor necrosis factor-α
- USF1:
Upstream transcription factor 1
- VAT:
Visceral adiposity tissue
- VDR:
Vitamin D receptor
- WAT:
White adipose tissue
- WHO:
World Health Organization.
Data Availability
The data used to support the findings of this study are available from the corresponding author upon request.
Disclosure
The funding sponsors had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; and in the decision to publish the results.
Conflicts of Interest
The authors declare no conflict of interest.
Authors' Contributions
LDR designed the study. SG, SB, LA, VA, MM, AC, IJ, and IC acquired the data. LDR and GC analyzed and interpreted the data. LDR and GC drafted the work and substantively revised it. SG had the primary responsibility of the study. All authors read and approved the submitted and final version of the manuscript. All authors agree to be personally accountable for the author's own contributions and for ensuring that questions related to the accuracy or integrity of any part of the work, even ones in which the author was not personally involved, are appropriately investigated, resolved, and documented in the literature. Laura Di Renzo and Giorgia Cioccoloni contributed equally to this work.
Supplementary Materials
Graphical Abstract: effects of 6 weeks of hazelnut administration on body composition and gene expression. Effects of hazelnut administration on body composition and gene expression of 7 genes belonging to the oxidative stress and the related involved pathway. Superoxide dismutase 1 (SOD1), catalase (CAT), macrophage migration inhibitory factor (MIF), peroxisome proliferator-activated receptor gamma (PPARγ), vitamin D receptor (VDR), methylenetetrahydrofolate reductase (MTHFR), angiotensin I-converting enzyme (ACE), interleukin-10 (IL10), RAS, M1 and M2 macrophages, nuclear factor- (erythroid-derived 2) like 2 (Nrf2), and antioxidant response element (ARE).
References
- 1.Calder P. C., Ahluwalia N., Brouns F., et al. Dietary factors and low-grade inflammation in relation to overweight and obesity. British Journal of Nutrition. 2011;106(S3) Supplement 3:S5–78. doi: 10.1017/S0007114511005460. [DOI] [PubMed] [Google Scholar]
- 2.Wronska A., Kmiec Z. Structural and biochemical characteristics of various white adipose tissue depots. Acta Physiologica. 2012;205(2):194–208. doi: 10.1111/j.1748-1716.2012.02409.x. [DOI] [PubMed] [Google Scholar]
- 3.Engin A. B., Engin A. Obesity and Lipotoxicity. Springer International Publishing; 2017. [DOI] [Google Scholar]
- 4.Meijer K., de Vries M., al-Lahham S., et al. Human primary adipocytes exhibit immune cell function: adipocytes prime inflammation independent of macrophages. PLoS One. 2011;6(3, article e17154) doi: 10.1371/journal.pone.0017154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Manna P., Jain S. K. Obesity, oxidative stress, adipose tissue dysfunction, and the associated health risks: causes and therapeutic strategies. Metabolic Syndrome and Related Disorders. 2015;13(10):423–444. doi: 10.1089/met.2015.0095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Eaton S. B., Konner M. Paleolithic nutrition. A consideration of its nature and current implications. The New England Journal of Medicine. 1985;312(5):283–289. doi: 10.1056/NEJM198501313120505. [DOI] [PubMed] [Google Scholar]
- 7.Ros E., Mataix J. Fatty acid composition of nuts – implications for cardiovascular health. British Journal of Nutrition. 2006;96(Supplement 2):S29–S35. doi: 10.1017/bjn20061861. [DOI] [PubMed] [Google Scholar]
- 8.Savage G. P., McNeil D. L. Chemical composition of hazelnuts (Corylus avellana L.) grown in New Zealand. International Journal of Food Sciences and Nutrition. 1998;49(3):199–203. doi: 10.3109/09637489809086412. [DOI] [PubMed] [Google Scholar]
- 9.Ros E. Nuts and novel biomarkers of cardiovascular disease. The American Journal of Clinical Nutrition. 2009;89(5):1649S–1656S. doi: 10.3945/ajcn.2009.26736R. [DOI] [PubMed] [Google Scholar]
- 10.Blomhoff R., Carlsen M. H., Andersen L. F., Jacobs D. R., Jr Health benefits of nuts: potential role of antioxidants. British Journal of Nutrition. 2006;96(Supplement 2):S52–S60. doi: 10.1017/bjn20061864. [DOI] [PubMed] [Google Scholar]
- 11.Jiménez-Gómez Y., López-Miranda J., Blanco-Colio L. M., et al. Olive oil and walnut breakfasts reduce the postprandial inflammatory response in mononuclear cells compared with a butter breakfast in healthy men. Atherosclerosis. 2009;204(2):e70–e76. doi: 10.1016/j.atherosclerosis.2008.09.011. [DOI] [PubMed] [Google Scholar]
- 12.Perna S., Giacosa A., Bonitta G., et al. Effects of hazelnut consumption on blood lipids and body weight: a systematic review and Bayesian meta-analysis. Nutrients. 2016;8(12):p. 747. doi: 10.3390/nu8120747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Casas-Agustench P., Bulló M., Ros E., Basora J., Salas-Salvadó J., on behalf of the Nureta-PREDIMED Investigators Cross-sectional association of nut intake with adiposity in a Mediterranean population. Nutrition, Metabolism, & Cardiovascular Diseases. 2011;21(7):518–525. doi: 10.1016/j.numecd.2009.11.010. [DOI] [PubMed] [Google Scholar]
- 14.Wall C., Stewart A., Hancox R., et al. Association between frequency of consumption of fruit, vegetables, nuts and pulses and BMI: analyses of the International Study of Asthma and Allergies in Childhood (ISAAC) Nutrients. 2018;10(3):p. 316. doi: 10.3390/nu10030316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lloyd-Jones D. M., Hong Y., Labarthe D., et al. Defining and setting national goals for cardiovascular health promotion and disease reduction: the American Heart Association’s strategic impact goal through 2020 and beyond. Circulation. 2010;121(4):586–613. doi: 10.1161/CIRCULATIONAHA.109.192703. [DOI] [PubMed] [Google Scholar]
- 16.Hyseni L., Bromley H., Kypridemos C., et al. Systematic review of dietary trans-fat reduction interventions. Bulletin of the World Health Organization. 2017;95(12):821–830G. doi: 10.2471/BLT.16.189795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tey S. L., Brown R. C., Chisholm A. W., Delahunty C. M., Gray A. R., Williams S. M. Effects of different forms of hazelnuts on blood lipids and α-tocopherol concentrations in mildly hypercholesterolemic individuals. European Journal of Clinical Nutrition. 2011;65(1):117–124. doi: 10.1038/ejcn.2010.200. [DOI] [PubMed] [Google Scholar]
- 18.Tey S. L., Gray A. R., Chisholm A. W., Delahunty C. M., Brown R. C. The dose of hazelnuts influences acceptance and diet quality but not inflammatory markers and body composition in overweight and obese individuals. The Journal of Nutrition. 2013;143(8):1254–1262. doi: 10.3945/jn.113.174714. [DOI] [PubMed] [Google Scholar]
- 19.Di Renzo L., Carraro A., Minella D., et al. Nutrient Analysis Critical Control Point (NACCP): hazelnut as a prototype of nutrigenomic study. Food and Nutrition Sciences. 2014;5(1):79–88. doi: 10.4236/fns.2014.51011. [DOI] [Google Scholar]
- 20.Di Renzo L., Merra G., Botta R., et al. Post-prandial effects of hazelnut-enriched high fat meal on LDL oxidative status, oxidative and inflammatory gene expression of healthy subjects: a randomized trial. European Review for Medical and Pharmacological Sciences. 2017;21(7):1610–1626. [PubMed] [Google Scholar]
- 21.Di Renzo L., Bianchi A., Saraceno R., et al. 174G/C IL-6 gene promoter polymorphism predicts therapeutic response to TNF-α blockers. Pharmacogenetics and Genomics. 2012;22(2):134–142. doi: 10.1097/FPC.0b013e32834e5e7b. [DOI] [PubMed] [Google Scholar]
- 22.Colica C., Merra G., Gasbarrini A., et al. Efficacy and safety of very-low-calorie ketogenic diet: a double blind randomized crossover study. European Review for Medical and Pharmacological Sciences. 2017;21(9):2274–2289. [PubMed] [Google Scholar]
- 23.Ros E. Health benefits of nut consumption. Nutrients. 2010;2(7):652–682. doi: 10.3390/nu2070652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sciubba F., Di Cocco M. E., Gianferri R., et al. Metabolic profile of different Italian cultivars of hazelnut (Corylus avellana) by nuclear magnetic resonance spectroscopy. Natural Product Research. 2014;28(14):1075–1081. doi: 10.1080/14786419.2014.905936. [DOI] [PubMed] [Google Scholar]
- 25.Farbstein D., Kozak-Blickstein A., Levy A. P. Antioxidant vitamins and their use in preventing cardiovascular disease. Molecules. 2010;15(11):8098–8110. doi: 10.3390/molecules15118098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rocha D. M., Bressan J., Hermsdorff H. H. The role of dietary fatty acid intake in inflammatory gene expression: a critical review. São Paulo Medical Journal. 2017;135(2):157–168. doi: 10.1590/1516-3180.2016.008607072016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mocchegiani E., Costarelli L., Giacconi R., et al. Vitamin E-gene interactions in aging and inflammatory age-related diseases: implications for treatment. A systematic review. Ageing Research Reviews. 2014;14:81–101. doi: 10.1016/j.arr.2014.01.001. [DOI] [PubMed] [Google Scholar]
- 28.Gormaz J. G., Valls N., Sotomayor C., Turner T., Rodrigo R. Potential role of polyphenols in the prevention of cardiovascular diseases: molecular bases. Current Medicinal Chemistry. 2016;23(2):115–128. doi: 10.2174/0929867323666151127201732. [DOI] [PubMed] [Google Scholar]
- 29.Goh J., Goh K. P., Abbasi A. Exercise and adipose tissue macrophages: new frontiers in obesity research? Frontiers in Endocrinology. 2016;7 doi: 10.3389/fendo.2016.00065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Buchholz A. C., Schoeller D. A. Is a calorie a calorie? The American Journal of Clinical Nutrition. 2004;79(5):899S–906S. doi: 10.1093/ajcn/79.5.899S. [DOI] [PubMed] [Google Scholar]
- 31.Jaceldo-Siegl K., Sabaté J., Rajaram S., Fraser G. E. Long-term almond supplementation without advice on food replacement induces favourable nutrient modifications to the habitual diets of free-living individuals. The British Journal of Nutrition. 2004;92(3):533–540. doi: 10.1079/BJN20041223. [DOI] [PubMed] [Google Scholar]
- 32.McKiernan F., Mattes R. D. Effects of peanut processing on masticatory performance during variable appetitive states. Journal of Nutrition and Metabolism. 2010;2010:6. doi: 10.1155/2010/487301.487301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mattes R. D., Dreher M. L. Nuts and healthy body weight maintenance mechanisms. Asia Pacific Journal of Clinical Nutrition. 2010;19(1):137–141. [PubMed] [Google Scholar]
- 34.Mandalari G., Faulks R. M., Rich G. T., et al. Release of protein, lipid, and vitamin E from almond seeds during digestion. Journal of Agricultural and Food Chemistry. 2008;56(9):3409–3416. doi: 10.1021/jf073393v. [DOI] [PubMed] [Google Scholar]
- 35.Traoret C. J., Lokko P., Cruz A. C. R. F., et al. Peanut digestion and energy balance. International Journal of Obesity. 2008;32(2):322–328. doi: 10.1038/sj.ijo.0803735. [DOI] [PubMed] [Google Scholar]
- 36.Flores-Mateo G., Rojas-Rueda D., Basora J., Ros E., Salas-Salvadó J. Nut intake and adiposity: meta-analysis of clinical trials. The American Journal of Clinical Nutrition. 2013;97(6):1346–1355. doi: 10.3945/ajcn.111.031484. [DOI] [PubMed] [Google Scholar]
- 37.Di Renzo L., Di Pierro D., Bigioni M., et al. Is antioxidant plasma status in humans a consequence of the antioxidant food content influence? European Review for Medical and Pharmacological Sciences. 2007;11(3):185–192. [PubMed] [Google Scholar]
- 38.Barzilay J. I., Forsberg C., Heckbert S. R., Cushman M., Newman A. B. The association of markers of inflammation with weight change in older adults: the Cardiovascular Health Study. International Journal of Obesity. 2006;30(9):1362–1367. doi: 10.1038/sj.ijo.0803306. [DOI] [PubMed] [Google Scholar]
- 39.Holz T., Thorand B., Döring A., Schneider A., Meisinger C., Koenig W. Markers of inflammation and weight change in middle-aged adults: results from the prospective MONICA/KORA S3/F3 study. Obesity. 2010;18(12):2347–2353. doi: 10.1038/oby.2010.73. [DOI] [PubMed] [Google Scholar]
- 40.Ramallal R., Toledo E., Martínez J. A., et al. Inflammatory potential of diet, weight gain, and incidence of overweight/obesity: the SUN cohort. Obesity. 2017;25(6):997–1005. doi: 10.1002/oby.21833. [DOI] [PubMed] [Google Scholar]
- 41.Xu L., Li Y., Sun H., et al. Current developments of macrophage migration inhibitory factor (MIF) inhibitors. Drug Discovery Today. 2013;18(11-12):592–600. doi: 10.1016/j.drudis.2012.12.013. [DOI] [PubMed] [Google Scholar]
- 42.Yukitake H., Takizawa M., Kimura H. Macrophage migration inhibitory factor as an emerging drug target to regulate antioxidant response element system. Oxidative Medicine and Cellular Longevity. 2017;2017:6. doi: 10.1155/2017/8584930.8584930 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mathew B., Jacobson J. R., Siegler J. H., et al. Role of migratory inhibition factor in age-related susceptibility to radiation lung injury via NF-E2-related factor-2 and antioxidant regulation. American Journal of Respiratory Cell and Molecular Biology. 2013;49(2):269–278. doi: 10.1165/rcmb.2012-0291OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Koga K., Kenessey A., Powell S. R., Sison C. P., Miller E. J., Ojamaa K. Macrophage migration inhibitory factor provides cardioprotection during ischemia/reperfusion by reducing oxidative stress. Antioxidants & Redox Signaling. 2011;14(7):1191–1202. doi: 10.1089/ars.2010.3163. [DOI] [PubMed] [Google Scholar]
- 45.Das S. K., Chakrabarti R. Role of PPAR in cardiovascular diseases. Recent Patents on Cardiovascular Drug Discovery. 2006;1(2):193–209. doi: 10.2174/157489006777442441. [DOI] [PubMed] [Google Scholar]
- 46.Martínez-González M. A., Salas-Salvadó J., Estruch R., et al. Benefits of the Mediterranean diet: insights from the PREDIMED Study. Progress in Cardiovascular Diseases. 2015;58(1):50–60. doi: 10.1016/j.pcad.2015.04.003. [DOI] [PubMed] [Google Scholar]
- 47.Zhang X., Zhou M., Guo Y., Song Z., Liu B. 1,25-Dihydroxyvitamin D3 Promotes High Glucose-Induced M1 Macrophage Switching to M2 via the VDR-PPARγ signaling pathway. BioMed Research International. 2015;2015:14. doi: 10.1155/2015/157834.157834 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Slusher A. L., McAllister M. J., Huang C. J. A therapeutic role for vitamin D on obesity-associated inflammation and weight-loss intervention. Inflammation Research. 2015;64(8):565–575. doi: 10.1007/s00011-015-0847-4. [DOI] [PubMed] [Google Scholar]
- 49.Austin R. C., Lentz S. R., Werstuck G. H. Role of hyperhomocysteinemia in endothelial dysfunction and atherothrombotic disease. Cell Death and Differentiation. 2004;11(Supplement 1):S56–S64. doi: 10.1038/sj.cdd.4401451. [DOI] [PubMed] [Google Scholar]
- 50.Majumder K., Wu J. Molecular targets of antihypertensive peptides: understanding the mechanisms of action based on the pathophysiology of hypertension. International Journal of Molecular Sciences. 2015;16(1):256–283. doi: 10.3390/ijms16010256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Chandra S., Narang R., Saluja D., Bhatia J., Srivastava K. Expression of angiotensin-converting enzyme gene in whole blood in patients with essential hypertension. Biomarkers. 2014;19(4):314–318. doi: 10.3109/1354750X.2014.910550. [DOI] [PubMed] [Google Scholar]
- 52.Mohammadifard N., Salehi-Abargouei A., Salas-Salvadó J., Guasch-Ferré M., Humphries K., Sarrafzadegan N. The effect of tree nut, peanut, and soy nut consumption on blood pressure: a systematic review and meta-analysis of randomized controlled clinical trials. The American Journal of Clinical Nutrition. 2015;101(5):966–982. doi: 10.3945/ajcn.114.091595. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Graphical Abstract: effects of 6 weeks of hazelnut administration on body composition and gene expression. Effects of hazelnut administration on body composition and gene expression of 7 genes belonging to the oxidative stress and the related involved pathway. Superoxide dismutase 1 (SOD1), catalase (CAT), macrophage migration inhibitory factor (MIF), peroxisome proliferator-activated receptor gamma (PPARγ), vitamin D receptor (VDR), methylenetetrahydrofolate reductase (MTHFR), angiotensin I-converting enzyme (ACE), interleukin-10 (IL10), RAS, M1 and M2 macrophages, nuclear factor- (erythroid-derived 2) like 2 (Nrf2), and antioxidant response element (ARE).
Data Availability Statement
The data used to support the findings of this study are available from the corresponding author upon request.