Abstract
The most common neurological complication in patients receiving successful combination antiretroviral therapy (cART) is peripheral neuropathic pain. Data show that distal symmetric polyneuropathy (DSP) also develops along with murine acquired immunodeficiency syndrome (MAIDS) after infection with the LP-BM5 murine retrovirus mixture. Links between cannabinoid receptor 2 (CB2R) and peripheral neuropathy have been established in animal models using nerve transection, chemotherapy-induced pain, and various other stimuli. Diverse types of neuropathic pain respond differently to standard drug intervention, and little is currently known regarding the effects of modulation through CB2Rs. In this study, we evaluated whether treatment with the exogenous synthetic CB2R agonists JWH015, JWH133, Gp1a, and HU308 controls neuropathic pain and neuroinflammation in animals with chronic retroviral infection. Hind-paw mechanical hypersensitivity in CB2R agonist-treated versus untreated animals was assessed using the MouseMet electronic von Frey system. Multicolor flow cytometry was used to determine the effects of CB2R agonists on macrophage activation and T-lymphocyte infiltration into dorsal root ganglia (DRG) and lumbar spinal cord (LSC). Results demonstrated that, following weekly intraperitoneal injections starting at 5 wk p.i., JWH015, JWH133, and Gp1a, but not HU308 (5 mg/kg), significantly ameliorated allodynia when assessed 2 h after ligand injection. However, these same agonists (2x/wk) did not display antiallodynic effects when mechanical sensitivity was assessed 24 h after ligand injection. Infection-induced macrophage activation and T-cell infiltration into the DRG and LSC were observed at 12 wk p.i., but this neuroinflammation was not affected by treatment with any CB2R agonist. Activation of JAK/STAT3 has been shown to contribute to development of neuropathic pain in the LSC and pretreatment of primary murine microglia (2 h) with JWH015-, JWH133-, or Gp1a-blocked IFN-gamma-induced phosphorylation of STAT1 and STAT3. Taken together, these data show that CB2R agonists demonstrate acute, but not long-term, antiallodynic effects on retrovirus infection-induced neuropathic pain.
1. Introduction
Approximately 35–50% of HIV-1 patients undergoing successful combination antiretroviral therapy (cART) experience peripheral neuropathic pain, which currently makes it the most common neurological complication of infection [1–4]. Our laboratory has been investigating the role of activated, brain-infiltrating peripheral immune cells in driving chronic activation of brain-resident microglia following viral infection, particularly through production of IFN-γ [5, 6]. Studies demonstrate that this type of immune-induced activation of resident glial cells is also emerging as a common mechanism underlying various types of chronic pain, reviewed in [7]. Despite its clinical significance, neuropathic pain in the context of well-controlled HIV infection is still an understudied area, in part because of the lack of convenient animal models. Correspondingly, data from us and others show that distal symmetric polyneuropathy (DSP) develops in animals with murine acquired immunodeficiency syndrome (MAIDS) by 6–8 wk following infection with the LP-BM5 retrovirus mixture [8, 9].
Chronic viral infection and infection-induced inflammation are known to induce production of neurotoxic mediators which have been associated with DSP, reviewed in [10]. While HIV itself does not replicate in neurons, neuropathological studies have demonstrated the presence of proviral DNA, mRNA, and p24 antigen within macrophages in dorsal root ganglia (DRG) of HIV-infected patients [11]. A consistent neuropathological abnormality in DSP patients is the presence of activated macrophages in the DRG, which express MHCII antigens and proinflammatory cytokines [12]. In many forms of DSP, the disease correlates well with the degree of macrophage infiltration and microglial cell activation [13, 14]. Numerous studies have demonstrated a critical role of activated microglia [15–18], as well as astrocytes [19–21] and satellite glial cells in the DRG [22–25], in the development of neuropathic pain.
At the present time, there are no FDA-approved pharmacologic agents available which are specifically designed for treatment of chronic HIV-associated neuropathy. The analgesics currently used (i.e., opioids) are only modestly effective and possess significant CNS side effects [26, 27]. Derivatives of Cannabis sativa have frequently been used as analgesics, and links between cannabinoids and peripheral neuropathy have been established in animal models using nerve transection, chemical-induced pain, and various other stimuli [28–32]. It is well known that diverse types of peripheral neuropathic pain respond differently to standard drug intervention [33, 34], but little is currently known regarding the effects of inflammatory modulation through cannabinoid receptor 2 in the context of peripheral neuropathy due to chronic retroviral infection.
There are two distinct types of cannabinoid receptors: CB1Rs which are expressed primarily in neurons and CB2Rs which are found mainly in cells of the immune system and glia [35, 36]. So, exploring potential therapies centered on CB2R-specific agonists has the potential benefit of more specifically targeting immune cell activation while simultaneously avoiding unwanted, psychoactive side effects associated with activating CB1Rs. CB2R activation clearly results in suppressive effects such as reduced cytokine release and inhibition of immune cell chemotaxis [37]. So, CB2Rs have enormous potential for pharmacological manipulation, but because of the sheer diversity of their physiological influences, huge challenges also exist. Although an abundance of experimental data demonstrate the effectiveness of cannabinoids as potentially effective analgesics, it has been difficult to translate the use of cannabis extracts into clinically effective therapies. Overall, to date, results from reported clinical trials do not make a compelling argument for their use in various types of neuropathic pain, recently reviewed in [38]. Nevertheless, new approaches which lead to development of more specific and effective analgesics for neuropathic pain are desperately needed, and immunomodulation through the CB2R system is clearly a promising strategy. The application of more specific, synthetic CB2R agonists may be one approach to increase the clinical efficacy of cannabinoid therapy.
Although not a perfect representation of human AIDS, the MAIDS (i.e., LP-BM5 infection) model in mice does allow us to investigate mechanisms that drive neuropathic pain during chronic retroviral infection. In a previous study, we have reported neuroinflammation and nitrosative damage associated with peripheral neuropathy in this murine model, but no drugs or therapeutic treatment interventions were used [9]. In experiments presented here, we follow up to our previous study by testing the hypothesis that treatment using synthetic CB2R agonists can control the neuropathic pain and limit the neuroinflammation seen during chronic retroviral infection.
2. Materials and Methods
2.1. Ethical Approval
The animal care protocol employed was approved by our Institutional Animal Care and Use Committee (Protocol Number 1709-35110A). Animal well-being was monitored according to Research Animal Resources (RAR) procedures. All efforts were made to reduce animal suffering, and when required, mice were euthanized using isoflurane inhalation.
2.2. Reagents
Chemicals were from Sigma-Aldrich (St. Louis, MO); fetal bovine serum was from Peak Serum (Wellington, CO); DNase was from Life Technologies (Carlsbad, CA); CB2R agonists: JWH015, JWH133, Gp1a, and HU308, were from Tocris Bioscience (Minneapolis, MN).
2.3. Virus and Animals
LP-BM5 was acquired from the NIH AIDS Reagent Program (Germantown, MD, USA). Stocks of virus were prepared as cell-free supernatants of SC-1 cells, as described previously [6]. The XC plaque assay for BM5eco was used to determine viral titers. Female C57BL/6 mice were obtained from The Jackson Laboratory, Bar Harbor, ME, USA, and were housed at the RAR facility. Mice were infected via intraperitoneal (i.p.) injection (2 × 104/PFU dose/2 doses with 3 d between doses) of the LP-BM5 retrovirus mixture [6].
2.4. Assessment of Mechanical Allodynia
The MouseMet electronic von Frey system (Topcat Metrology, Cambridge, UK) was used to assess both left and right hind-paw mechanical hypersensitivity weekly between 5 and 12 wk following LP-BM5 infection. Mice were acclimated to the testing chambers for 20 min before testing. Bilateral measurements of six sets were performed for each mouse, and data were calculated as the paw withdrawal threshold in grams of force.
2.5. Isolation of Leukocytes from Lumbar Spinal Cord (L3–L5) and Dorsal Root Ganglia for Flow Cytometry
Tissue mononuclear cells were isolated from the lumbar spinal cord (LSC) of uninfected, infected, and infected, CB2R agonist-treated animals using procedures described previously [39, 40]. Mice were anesthetized with ketamine/xylazine and transcardial perfused with saline before tissue collection. The LSCs were dissected (n=4–6) and finely minced in RPMI 1640 at RT. Preparations of single cells were suspended in 30% Percoll and banded on a 70% Percoll cushion. Leukocytes obtained at the 30–70% Percoll interface were harvested and counted. A nonenzymatic dissociation method was employed to isolate mononuclear cells from DRG, as described previously [41]. Six ganglia (L3–L5) per mouse were collected (n=4–6 animals/group/experiment). DRGs were homogenized using a 1 ml syringe attached with a 21 G needle and then a 23 G needle, filtered through a cell strainer, and counted as described previously.
Isolated cells were treated with Fc Block (i.e., anti-CD32/CD16) to inhibit nonspecific Ab binding. Cells were stained with anti-immune surface markers (i.e., anti-MHC-II-PE (clone M5/114.15.2; cat# 12-5321-82; eBioscience, San Diego, CA, USA), anti-CD11b-AF700 (clone M1/70; cat# 56-0112-82; eBioscience), anti-CD45-PE-Cy5 (clone 30-F11; cat# 15-0451-81; eBioscience), anti-CD4-BV510 (clone GK1.5; cat# 100449; BioLegend, San Diego, CA, USA), and anti-CD8-PE-Cy7 (clone 53-6.7; cat# 25-0081-82; eBioscience)). Counts were acquired (105/sample) using an LSR flow cytometer (BD Biosciences, San Jose, CA), and the FACSDIVA software was used to calculate the absolute cell number. The FlowJo software (Tree Star, Ashland, OR, USA) was used to analyze the data.
2.6. Microglial Cell Cultures
Cerebral cortical cells were collected from one-day-old C57BL/6 pups followed by cell dissociation using trypsin (0.25% in HBSS) for 30 min. Cells were centrifuged and washed 3 times in HBSS, suspended in DMEM with FBS (10%), Fungizone® (250 pg/mL), gentamicin (50 μg/mL), penicillin (100 U/mL), and streptomycin (100 μg/mL), and seeded onto Falcon tissue culture flasks (75 cm2). Culture media were changed at d 1 and d 4. Microglial cells were harvested at d 12 and plated onto 12-well Falcon cell culture plates in DMEM with FBS (6%) for 60 min at 37°C. Cell culture media were replaced with DMEM without FBS overnight, followed by pretreatment with CB2R agonists for 2 h prior to IFN-γ stimulation for 20 min.
2.7. Western Blotting
Following treatment, microglial culture media were removed and cells were lysed with the 2x Laemmli sample buffer containing 5% 2-mercaptoethanol. The samples were then heated at 100°C for 5 min and vortexed before being loaded onto 4–15% precast acrylamide gels for electrophoresis. Gels were transblotted onto nitrocellulose membranes (0.45 µm), rinsed in TTBS (Tris-HCl with NaCl and Tween 20), and blocked with 5% Blotto (Santa Cruz, Dallas, TX) for 1 h at RT. Membranes were then probed with rabbit anti-β-actin (#4970) and rabbit anti-phospho-Stat3(Tyr705) (#9131) or -Stat1(Tyr701) (#9171) Abs (Cell Signaling Technology, Danvers, MA) overnight at 4°C. After washing 3x with TTBS, alkaline phosphatase (AP) conjugated secondary antibody (1 : 5,000 in 1% Blotto, Promega, Madison, WI) was added to the membranes at RT for 1 h. Following 3x washing with TTBS, membranes were rinsed in the assay buffer (1x) twice (2 min each) and then incubated in the substrate solution (CDP-Star, Applied Biosystems, Foster City, CA) for 10 min followed by imaging using an Odyssey Imaging System (LI-COR Biosciences, Lincoln, NE).
2.8. Statistical Analysis
One-way ANOVA with Fisher's LSD post hoc test was used for the analysis of behavioral testing. ANOVA with Tukey's test was employed for graphical analysis. Significant differences were obtained when p < 0.05.
3. Results
3.1. Acute Antiallodynic Effects of CB2R Agonists on Retrovirus-Infected Animals
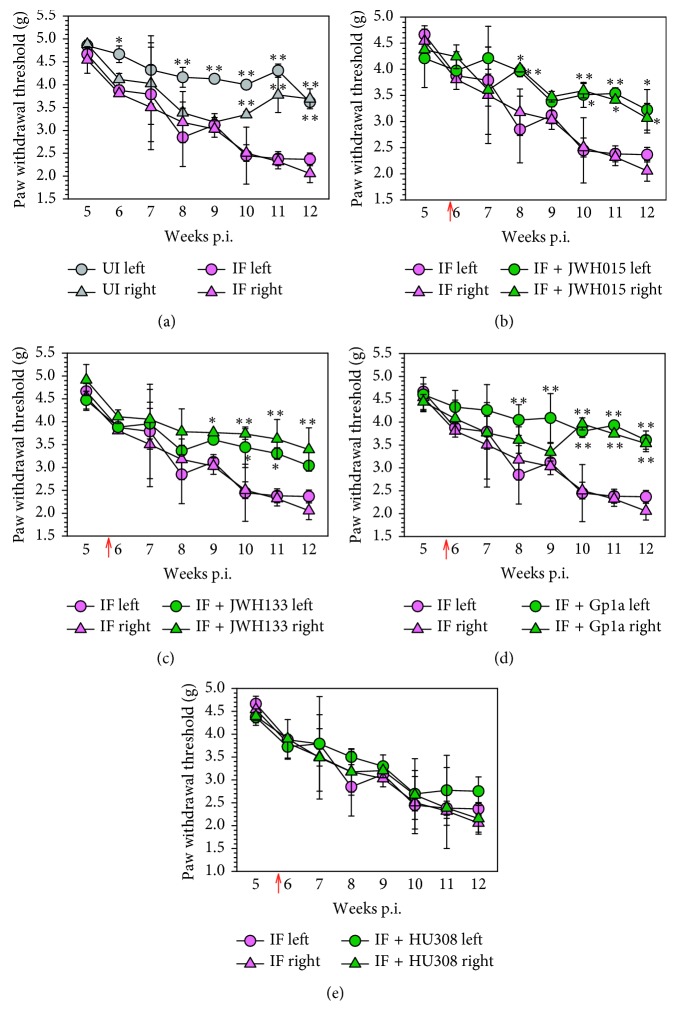
C57BL/6 mice infected with the LP-BM5 retrovirus mixture have previously been reported to show symptoms of DSP by 6 wk p.i. [8, 9]. Here, we evaluated whether treatment with the exogenous synthetic CB2R agonists: JWH015, JWH133, Gp1a, and HU308, could control neuropathic pain and neuroinflammation in animals with this chronic retroviral infection. Using the MouseMet electronic von Frey system, we assessed hind-paw mechanical hypersensitivity in CB2R agonist-treated versus untreated animals. We were first able to once again repeat findings that mice infected with LP-BM5 exhibited mechanical hind-paw hypersensitivity by 6 wk p.i., with no difference between left and right paws (Figure 1(a)). Additional results showed that weekly intraperitoneal injections (5 mg/kg) starting at 5 wk p.i. of JWH015 (Figure 1(b)), JWH133 (Figure 1(c)), and Gp1a (Figure 1(d)), but not HU308 (Figure 1(e)), significantly ameliorated allodynia when assessed 2 h after injection of the CB2R agonist. Although viral loads were found to persist within DRG and LSC tissues, no differences in either BM5def or BM5eco were detected between CB2R agonist-treated and untreated groups when assessed using real-time PCR at 12 wk p.i. (data not shown), as described in [9].
Figure 1.
Acute antiallodynic effects of CB2R agonists on retrovirus-infected animals. (a) C57BL/6 mice were randomly assigned into uninfected (UI) or LP-BM5-infected (IF) groups (n=8/group), and hind-paw mechanical hypersensitivity was assessed weekly via the MouseMet electronic von Frey system between 5 and 12 wk p.i. Following LP-BM5 infection, synthetic CB2R ligands were injected (5 mg/kg, i.p.) once/wk starting at 5 wk·p.i. (red arrow). Assessment of allodynia was performed weekly 2 h after injection of the indicated ligand: (b) JWH015; (c) JWH133; (d) Gp1a; (e) HU308. Data are presented as the mean ± SE paw withdrawal threshold with n=8/group. ∗p < 0.05 and ∗∗p < 0.01 vs. corresponding paw/wk p.i. (ANOVA with Fisher's LSD post hoc analysis).
3.2. Lack of Long-Term Antiallodynic Effects of CB2R Agonists on Hind-Paw Mechanical Hypersensitivity
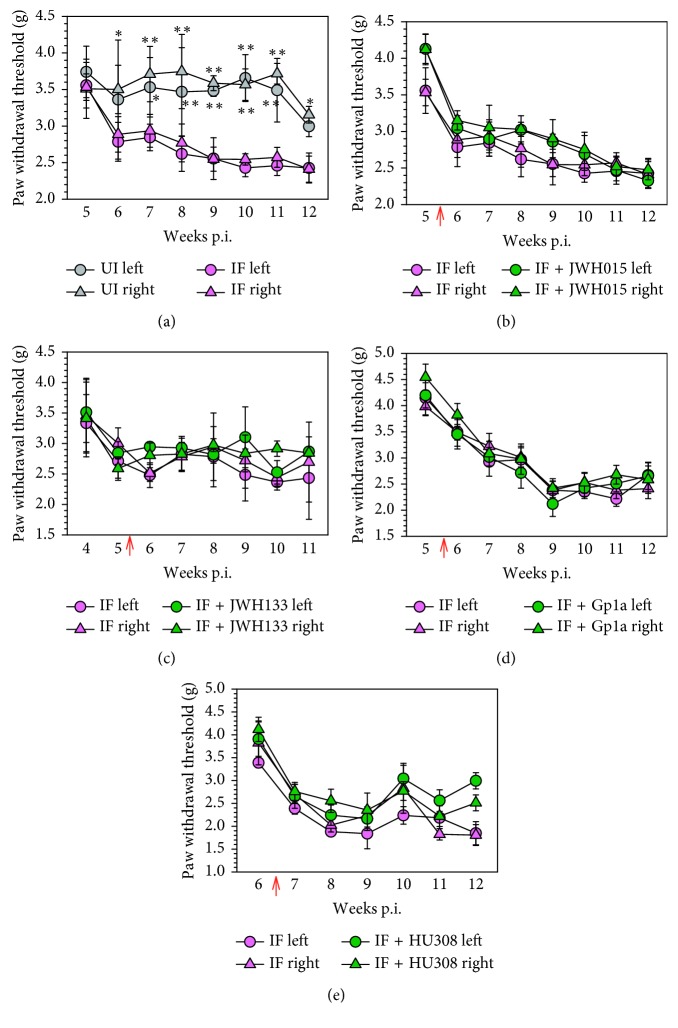
Our next experiments examined the duration of the antiallodynic effect following CB2R agonist treatment. In a parallel experiment, we again established that mice infected with LP-BM5 exhibited mechanical hind-paw hypersensitivity (Figure 2(a)). Interestingly, when the same CB2R agonists were injected twice per week starting at 5 wk p.i. (6 wk for HU308) and mechanical sensitivity was assessed 24 h after ligand injection, treatment with JWH015 (Figure 2(b)), JWH133 (Figure 2(c)), Gp1a (Figure 2(d)), or HU308 (Figure 2(e)) was not found to display significant antiallodynic effects. Likewise, animals treated with vehicle alone (i.e., 10% DMSO/10% TWEEN 80 in saline) did not display analgesic effects.
Figure 2.
Lack of long-term antiallodynic effects of CB2R agonists on hind-paw mechanical hypersensitivity. (a) LP-BM5-infected mice were randomly assigned into uninfected (UI) or infected (IF) groups (n=8/group), and hind-paw mechanical hypersensitivity was assessed weekly via the MouseMet electronic von Frey system between 5 and 12 wk p.i. After LP-BM5 infection, synthetic CB2R ligands were injected (5 mg/kg, i.p.) twice/wk starting at 5 or 6 wk (HU308) p.i. (red arrow). Assessment of hind-paw mechanical hypersensitivity was performed weekly 24 h after first injection of the indicated CB2R ligand: (b) JWH015; (c) JWH133; (d) Gp1a; (e) HU308. Data are presented as the mean ± SE paw withdrawal threshold with n=8/group. ∗p < 0.05 and ∗∗p < 0.01 vs. corresponding paw/wk p.i. (ANOVA with Fisher's LSD post hoc analysis).
3.3. Effects of CB2R Agonist Treatment on Macrophage and T-Cell Infiltration into the DRG of LP-BM5-Infected Animals
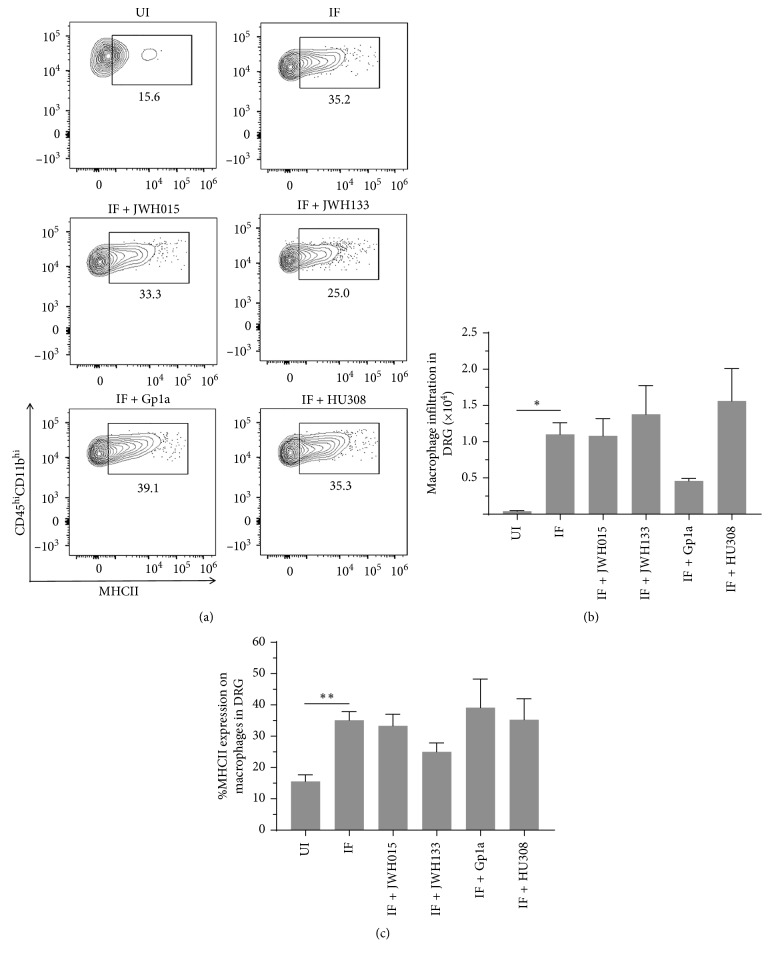
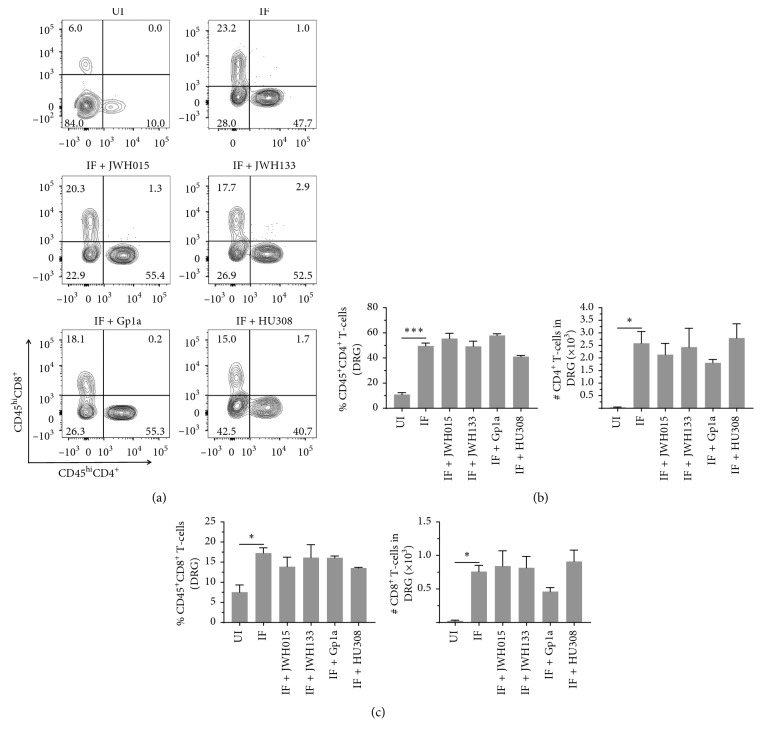
We have recently reported inflammation of the DRG in LP-BM5-infected mice, as assessed by increased infiltration of CD45hiCD11b+ macrophages, as well as CD4+ and CD8+ T-cells, concomitant with development of DSP [9]. Here, we went on to use multicolor flow cytometry to determine the effects of CB2R agonist treatment on macrophage infiltration into DRG and cellular activation, as assessed by upregulation of MHCII (Figure 3(a)). We observed significant differences in macrophage numbers (Figure 3(b)) and MHCII expression (Figure 3(c)) between uninfected (UI) and infected (IF) animals when assessed at 12 wk p.i. However, this macrophage-induced neuroinflammation was not found to be affected by treatment with any CB2R agonist (Figure 3). An apparent decrease in macrophage infiltration was observed following treatment with Gp1a, but this difference was not statistically significant. Likewise, significant CD4+ and CD8+ T-lymphocyte infiltration into the DRG was observed in infected mice at 12 wk p.i., when compared to uninfected animals (Figure 4(a)). Similar to the situation with macrophages, this T-cell neuroinflammation was not affected by treatment with any CB2R agonist. Neither the percentage nor the number of CD4+ (Figure 4(b)) or CD8+ (Figure 4(c)) T-cells within the DRG-infiltrating CD45hi leukocyte population was affected by treatment with CB2R agonists.
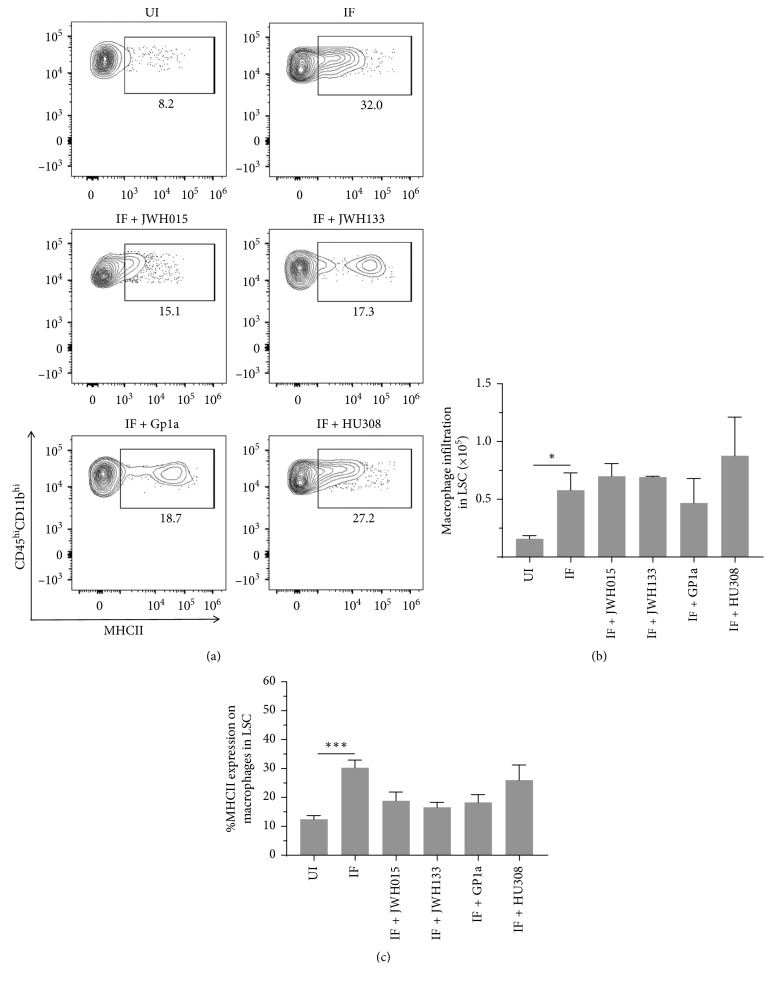
Figure 3.
Effects of CB2R agonist treatment on macrophage infiltration into the DRG of LP-BM5-infected animals. After LP-BM5 infection, synthetic CB2R ligands were injected (5 mg/kg, i.p.) twice/wk starting at 5 or 6 wk (HU308) p.i. At 12 wk p.i., DRG were dissected and mononuclear cells from six ganglia (L3–L5) per mouse were isolated using nonenzymatic dissociation. Isolated cells were then stained using Abs specific for CD45, CD11b, and MHCII for analysis by flow cytometry. (a) Representative contour plots show percentages of macrophages (i.e., CD45hiCD11bhi) expressing MHCII within uninfected (UI), infected (IF), and infected (IF + indicated CB2R ligand), agonist-treated DRG. (b) Numbers of macrophages infiltrating DRG of animals belonging to the indicated treatment groups. (c) Frequency of macrophages expressing MHCII within DRG of animals belonging to the indicated groups. Pooled data present absolute numbers (mean ± SE) from two independent experiments using 4–6 animals/group. ∗p < 0.05; ∗∗p < 0.01.
Figure 4.
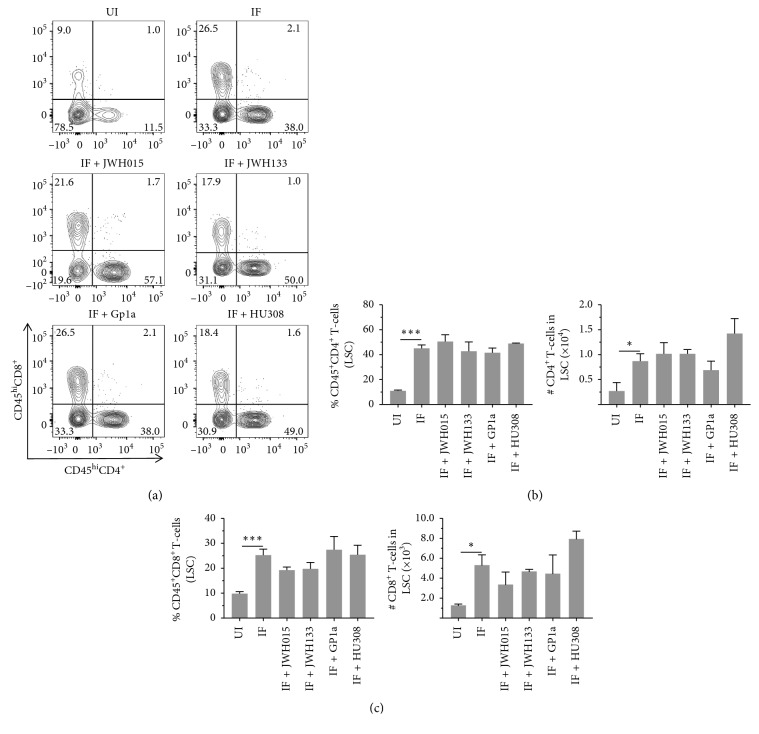
Effects of CB2R agonists on lymphocyte infiltration into the DRG. After LP-BM5 infection, synthetic CB2R ligands were injected (5 mg/kg, i.p.) twice/wk starting at 5 or 6 wk (HU308) p.i. At 12 wk p.i., DRG were dissected and mononuclear cells from six ganglia (L3–L5) per mouse were isolated using nonenzymatic dissociation. Mononuclear cells were then labeled with Abs specific for CD45, CD4, and CD8 for analysis by flow cytometry. (a) Representative contour plots show percentages of CD4+ and CD8+ T-lymphocytes within uninfected (UI), infected (IF), and infected (IF + indicated CB2R ligand), agonist-treated DRG. (b) Frequency and number of CD4+ T-cells infiltrating into DRG of animals belonging to the indicated treatment groups. (c) Frequency and number of CD8+ T-cells infiltrating into DRG of animals belonging to the indicated treatment groups. Pooled data present absolute numbers (mean ± SE) of CD4+ and CD8+ T-cells from two independent experiments using 4–6 animals per group. ∗p < 0.05; ∗∗∗p < 0.001.
3.4. Effects of CB2R Agonist Treatment on Macrophage and T-Cell Infiltration into the LSC of LP-BM5-Infected Animals
Multicolor flow cytometry was also used to determine the effects of CB2R agonists on macrophage and T-lymphocyte infiltration into the LSC. Here, infection-induced infiltration of CD45hiCD11b+ macrophages was observed at 12 wk p.i. (Figure 5(a)). Significant differences in macrophage numbers (Figure 5(b)) and MHCII expression (Figure 5(c)) were detected between uninfected (UI) and infected (IF) animals when assessed at 12 wk p.i., but again this macrophage inflammation was found not to be affected by treatment with any CB2R agonist (IF + indicated CB2R agonist). Likewise, significant CD4+ and CD8+ T-lymphocyte infiltration into the LSC was observed within infected mice at 12 wk p.i., when compared to uninfected animals (Figure 6(a)). Similar to the situation with macrophages, this T-cell neuroinflammation was not affected by treatment with any CB2R agonist. The percentage and number of CD4+ (Figure 6(b)) or CD8+ (Figure 6(c)) T-cells within the LSC-infiltrating CD45hi leukocyte population were not found to be affected by treatment with CB2R agonists.
Figure 5.
Effects of CB2R agonist treatment on macrophage infiltration into the LSC of LP-BM5-infected animals. After LP-BM5 infection, synthetic CB2R ligands were injected (5 mg/kg, i.p.) twice/wk starting at 5 or 6 wk (HU308) p.i. At 12 wk p.i., LSCs were dissected and infiltrating leukocytes were banded on a 30–70% Percoll cushion, collected, and labeled with Abs specific for CD45, CD11b, and MHCII for analysis by flow cytometry. (a) Representative contour plots show the percentages of macrophages (i.e., CD45hiCD11bhi) expressing MHCII within uninfected (UI), infected (IF), and infected (IF + indicated CB2R ligand), agonist-treated LSC. (b) Numbers of macrophages infiltrating into the LSC of animals belonging to the indicated treatment groups. (c) Frequency of macrophages expressing MHCII within the LSC of animals belonging to the indicated groups. Pooled data present absolute numbers (mean ± SE) from two independent experiments using 4–6 animals/group. ∗p < 0.05; ∗∗∗p < 0.001.
Figure 6.
Effects of CB2R agonist treatment on T-lymphocyte infiltration into the LSC of LP-BM5-infected animals. After LP-BM5 infection, synthetic CB2R ligands were injected (5 mg/kg, i.p.) twice/wk starting at 5 or 6 wk (HU308) p.i. At 12 wk p.i., LSCs were dissected and infiltrating leukocytes were banded on a 30–70% Percoll cushion, collected, and labeled with Abs specific for CD45, CD4, and CD8 for analysis by flow cytometry. (a) Representative contour plots show percentages of CD4+ and CD8+ T-lymphocytes within uninfected (UI), infected (IF), and infected (IF + indicated CB2R ligand), agonist-treated LSC. (b) Frequency and number of CD4+ T-cells infiltrating into the LSC of animals belonging to the indicated treatment groups. (c) Frequency and number of CD8+ T-cells infiltrating into the LSC of animals belonging to the indicated treatment groups. Pooled data present absolute numbers (mean ± SE) of CD4+ and CD8+ T-cells from two independent experiments using 4–6 animals per group. ∗p < 0.05; ∗∗∗p < 0.001.
3.5. CB2R Agonists Dampen STAT3/STAT1 Activation in Microglia
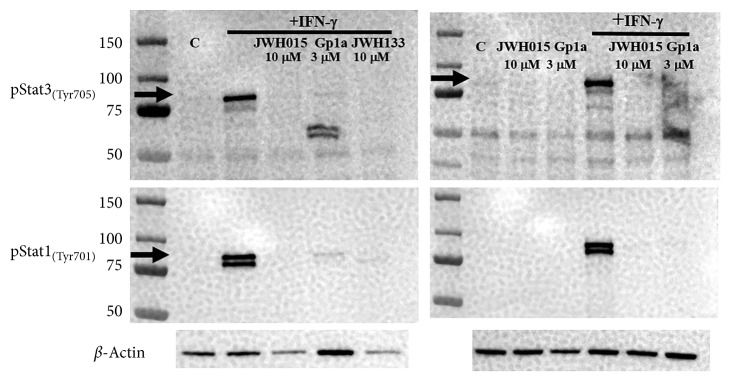
It has been reported that STAT3 contributes to chronic pain following nerve injury [42]. The activity of STAT3 is significantly enhanced following peripheral nerve damage [43], and blocking its activation ameliorates mechanical allodynia [42]. For this reason, we went on to examine the effect of pretreatment with CB2R agonists on IFN-γ-induced STAT3/STAT1 phosphorylation in primary murine microglia. We examined whether pretreatment of primary murine microglial cells (2 h) with JWH015, JWH133, or Gp1a could block IFN-γ-induced phosphorylation of STAT3/STAT1. In these studies, we found that treatment of murine microglia with IFN-γ (20 min) clearly induced STAT3/STAT1 phosphorylation, and pretreatment of the cultures with JWH015 (10 μM), JWH133 (10 µM), or Gp1a (3 µM) for 2 h prior to IFN-γ stimulation dampened activation of this pathway (Figure 7).
Figure 7.
CB2R agonists dampen STAT3 and STAT1 activation in primary microglial cells. Primary murine microglial cultures were left untreated or treated with the indicated CB2R ligands: JWH015 (10 μM), Gp1a (3 µM), or JWH133 (10 µM), for 2 h followed by IFN-γ stimulation for 20 min. Cell lysates were collected in the 2x sample buffer, electrophoresed, transblotted, and probed for β-actin and phosphorylated STAT3(Tyr705) or STAT1(Tyr701).
4. Discussion
CB2Rs have been shown to be expressed at the leading edge of activated microglia [44], and their specific engagement is well known to suppress multiple cellular responses [45]. In addition to effects on macrophages and microglia, CB2R agonists have also been shown to inhibit T-cell function including cytokine production, cellular migration, proliferation, and, more recently, mixed lymphocyte reactions (MLRs) [46–50]. In previous studies, we have found inhibition of HIV-1 p24 expression in primary human microglia and CD4+ T-cells by the synthetic, nonselective CB1R and CB2R agonists WIN55,212-2 and CP55,940 was mediated primarily through CB2Rs [51, 52]. These same agents also suppressed production of proinflammatory mediators such as nitric oxide (NO) by human astrocytes [53]. We have also reported that WIN55,212-2 and JWH015 blunted HIV-1 gp120-induced damage to dopaminergic neurons, as measured by dopamine transporter (DAT) function (viz. 3H-DA uptake), apoptosis (viz. DNA fragmentation), and lipid peroxidation (viz. 8-isoprostane), and that these effects were mediated through actions by CB2Rs on microglial cells [54].
Previous studies demonstrate that animals begin to display hind-paw mechanical hypersensitivity approximately 5 to 6 wk following infection with the LP-BM5 retrovirus mixture [8, 9] and that viral infection is well established at this time point. For this reason, we initiated weekly i.p. injections of the selective, synthetic CB2R agonists: JWH015, JWH133, Gp1a, and HU308, at this time point p.i. As in a number of other preclinical studies where cannabinoids were found to be effective in models of neuropathic pain, reviewed in [55], we found that 3 of the ligands (JWH015, JWH133, and Gp1a), but not HU308, were effective in reducing mechanical allodynia induced by chronic retroviral infection when assessed 2 h following their injection (Figure 1). While many earlier cannabinoid studies implicated a CB1R-mediated analgesic action, the effectiveness of selective CB2R agonists has also been reported in neuropathic pain models [56, 57]. Specifically, CB2R is recognized as key for paclitaxel chemotherapy-induced neuropathic pain [58, 59]. At this time, it is unknown why HU308 displayed a different effect when compared with the other three CB2R agonists, but functional selectivity by which different cannabinoid agonists possess differential effects (e.g., agonist, inverse agonist, or antagonist), mode of binding (e.g., orthosteric or allosteric), and activation of signal transduction pathways (e.g., adenylate cyclase or MAP kinase) has been described for CB2R ligands [60]. Factors such as these may play a role in the differential effect exhibited by HU308. Finally, no direct effect on viral load was detected following treatment with any of the CB2R agonists, as assessed using real-time PCR at 12 wk p.i. performed as previously described [9].
Neuropathic pain is a response to damage, and we have previously detected elevated levels of 3-nitrotyrosine within the LSC and DRG of LP-BM5-infected animals, an indicator of nitric oxide- (NO-) induced protein damage [9]. 3-Nitrotyrosine was observed in both IB4+ small and NF200+ large DRG sensory neurons. Increasing attention is being focused on nonneuronal mechanisms involving immune cells that may amplify or resolve chronic pain. Because of the well-characterized anti-inflammatory and neuroprotective effects of synthetic CB2R agonists, in another set of experiments, we hypothesized that these agonists would control chronic, damage-induced pain. However, this hypothesis was not supported. When the same CB2R agonists were injected twice per week starting at 5 or 6 wk p.i. and antiallodynic responses were assessed 24 h after ligand injection, neither JWH015, JWH133, Gp1a, nor HU308 displayed significant long-term antiallodynic effects (Figure 2).
We have previously reported that LP-BM5 infection drives CD45hiCD11b+ macrophages as well as CD4+ and CD8+ T-cell neuroinflammation in the DRG of infected animals [9]. These findings were confirmed here, where significantly more leukocytes were found within ganglia of infected animals. Interestingly, treatment with any of the CB2R agonists did not inhibit leukocyte migration in vivo, when tissues were harvested at 12 wk p.i. (Figures 3 and 4). A similar situation was observed when leukocyte infiltration into the spinal cord was examined. When LSCs were examined using multicolor flow cytometry at 12 wk p.i., macrophage and T-cell infiltration into tissues of infected animals was observed, but CB2R agonist treatment had no inhibitory effects on chemotaxis (Figures 5 and 6). Given the well-recognized effects of CB2R agonists on cellular migration [48, 49, 61], our lack of chemotaxis inhibition was surprising.
Although mechanisms responsible for the acute effects of CB2R agonist treatment remain to be determined, because they did not produce long-lasting antiallodynic effects or affect infection-induced immune cell infiltration into the DRG or LSC, it appears unlikely that the short-term effects we observed 2 h after injection were due to modulation of immune-mediated tissue damage. More likely, the observed short-term antiallodynia resulted from CB2R-mediated interference with signal transduction pathways and the blockade of cytokine release associated with the response. Proinflammatory cytokines produced by CNS-infiltrating T-lymphocytes are well known to activate resident glial cells. In the feline immunodeficiency virus (FIV) model of DSP, STAT1 and iNOS have been demonstrated to be responsible for neuroinflammation-induced damage to DRG neurons [52]. Because TNF-α- and IL-1β-induced STAT3 activation in the DRG has also been reported to mediate mechanical allodynia following oxaliplatin treatment [62], we investigated the effects of the synthetic CB2R agonists on STAT3. These studies used cultures of primary murine microglia along with a 2 h pretreatment with JWH015, JWH133, or Gp1a (i.e., the agonists which displayed short-term antiallodynic effects). Results obtained from these studies showed that CB2R agonist treatment potently blocked IFN-γ-induced STAT1 and STAT3 phosphorylation (Figure 7).
5. Conclusions
Data presented here show that synthetic CB2R agonists demonstrate acute, but not long-term, antiallodynic effects on retrovirus infection-induced neuropathic pain.
Acknowledgments
This work was supported by award DA-039464 from the National Institute on Drug Abuse.
Data Availability
The data used to support the findings of this study are included within the article.
Disclosure
The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Conflicts of Interest
All authors declare that there are no conflicts of interest.
References
- 1.Morgello S., Estanislao L., Simpson D., et al. HIV-associated distal sensory polyneuropathy in the era of highly active antiretroviral therapy. Archives of Neurology. 2004;61(4):546–551. doi: 10.1001/archneur.61.4.546. [DOI] [PubMed] [Google Scholar]
- 2.Ellis R. J., Rosario D., Clifford D. B., et al. Continued high prevalence and adverse clinical impact of human immunodeficiency virus-associated sensory neuropathy in the era of combination antiretroviral therapy. Archives of Neurology. 2010;67(5):552–558. doi: 10.1001/archneurol.2010.76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Evans S. R., Ellis R. J., Chen H., et al. Peripheral neuropathy in HIV: prevalence and risk factors. AIDS. 2011;25(7):919–928. doi: 10.1097/qad.0b013e328345889d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Phillips T. J., Cherry C. L., Cox S., Marshall S. J., Rice A. S. Pharmacological treatment of painful HIV-associated sensory neuropathy: a systematic review and meta-analysis of randomised controlled trials. PLoS One. 2010;5 doi: 10.1371/journal.pone.0014433.e14433 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mutnal M. B., Hu S., Little M. R., Lokensgard J. R. Memory T cells persisting in the brain following MCMV infection induce long-term microglial activation via interferon-γ. Journal of NeuroVirology. 2011;17(5):424–437. doi: 10.1007/s13365-011-0042-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mutnal M. B., Schachtele S. J., Hu S., Lokensgard J. R. T-cell reconstitution during murine acquired immunodeficiency syndrome (MAIDS) produces neuroinflammation and mortality in animals harboring opportunistic viral brain infection. Journal of Neuroinflammation. 2013;10(1):p. 98. doi: 10.1186/1742-2094-10-98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ji R.-R., Berta T., Nedergaard M. Glia and pain: is chronic pain a gliopathy? Pain. 2013;154(1):10–28. doi: 10.1016/j.pain.2013.06.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cao L., Butler M. B., Tan L., Draleau K. S., Koh W. Y. Murine immunodeficiency virus-induced peripheral neuropathy and the associated cytokine responses. Journal of Immunology. 2012;189(7):3724–3733. doi: 10.4049/jimmunol.1201313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chauhan P., Sheng W. S., Hu S., Prasad S., Lokensgard J. R. Nitrosative damage during retrovirus infection-induced neuropathic pain. Journal of Neuroinflammation. 2018;15(1):p. 66. doi: 10.1186/s12974-018-1107-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kamerman P. R., Moss P. J., Weber J., Wallace V. C. J., Rice A. S. C., Huang W. Pathogenesis of HIV-associated sensory neuropathy: evidence from in vivo and in vitro experimental models. Journal of Peripheral Nervous System. 2012;17(1):19–31. doi: 10.1111/j.1529-8027.2012.00373.x. [DOI] [PubMed] [Google Scholar]
- 11.Hahn K., Robinson B., Anderson C., et al. Differential effects of HIV infected macrophages on dorsal root ganglia neurons and axons. Experimental Neurology. 2008;210(1):30–40. doi: 10.1016/j.expneurol.2007.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pardo C. A., McArthur J. C., Griffin J. W. HIV neuropathy: insights in the pathology of HIV peripheral nerve disease. Journal of Peripheral Nervous System. 2001;6(1):21–27. doi: 10.1046/j.1529-8027.2001.006001021.x. [DOI] [PubMed] [Google Scholar]
- 13.Jones G., Zhu Y., Silva C., et al. Peripheral nerve-derived HIV-1 is predominantly CCR5-dependent and causes neuronal degeneration and neuroinflammation. Virology. 2005;334(2):178–193. doi: 10.1016/j.virol.2005.01.027. [DOI] [PubMed] [Google Scholar]
- 14.Ristoiu V. Contribution of macrophages to peripheral neuropathic pain pathogenesis. Life Sciences. 2013;93(23):870–881. doi: 10.1016/j.lfs.2013.10.005. [DOI] [PubMed] [Google Scholar]
- 15.Coull J. A. M., Beggs S., Boudreau D., et al. BDNF from microglia causes the shift in neuronal anion gradient underlying neuropathic pain. Nature. 2005;438(7070):1017–1021. doi: 10.1038/nature04223. [DOI] [PubMed] [Google Scholar]
- 16.Ji R. R., Suter M. R. p38 MAPK, microglial signaling, and neuropathic pain. Molecular Pain. 2007;3:1744–8069. doi: 10.1186/1744-8069-3-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Raghavendra V., Tanga F., DeLeo J. A. Inhibition of microglial activation attenuates the development but not existing hypersensitivity in a rat model of neuropathy. Journal of Pharmacology and Experimental Therapeutics. 2003;306(2):624–630. doi: 10.1124/jpet.103.052407. [DOI] [PubMed] [Google Scholar]
- 18.Tsuda M., Shigemoto-Mogami Y., Koizumi S., et al. P2X4 receptors induced in spinal microglia gate tactile allodynia after nerve injury. Nature. 2003;424(6950):778–783. doi: 10.1038/nature01786. [DOI] [PubMed] [Google Scholar]
- 19.Chiang C.-Y., Sessle B. J., Dostrovsky J. O. Role of astrocytes in pain. Neurochemical Research. 2012;37(11):2419–2431. doi: 10.1007/s11064-012-0801-6. [DOI] [PubMed] [Google Scholar]
- 20.Gao Y.-J., Ji R.-R. Targeting astrocyte signaling for chronic pain. Neurotherapeutics. 2010;7(4):482–493. doi: 10.1016/j.nurt.2010.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ren K., Dubner R. Interactions between the immune and nervous systems in pain. Nature Medicine. 2010;16(11):1267–1276. doi: 10.1038/nm.2234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chen Y., Zhang X., Wang C., Li G., Gu Y., Huang L.-Y. M. Activation of P2X7 receptors in glial satellite cells reduces pain through downregulation of P2X3 receptors in nociceptive neurons. Proceedings of the National Academy of Sciences. 2008;105(43):16773–16778. doi: 10.1073/pnas.0801793105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dublin P., Hanani M. Satellite glial cells in sensory ganglia: their possible contribution to inflammatory pain. Brain, Behavior, and Immunity. 2007;21(5):592–598. doi: 10.1016/j.bbi.2006.11.011. [DOI] [PubMed] [Google Scholar]
- 24.Jasmin L., Vit J.-P., Bhargava A., Ohara P. T. Can satellite glial cells be therapeutic targets for pain control? Neuron Glia Biology. 2010;6(1):63–71. doi: 10.1017/s1740925x10000098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Liu F.-Y., Sun Y.-N., Wang F.-T., et al. Activation of satellite glial cells in lumbar dorsal root ganglia contributes to neuropathic pain after spinal nerve ligation. Brain Research. 2012;1427:65–77. doi: 10.1016/j.brainres.2011.10.016. [DOI] [PubMed] [Google Scholar]
- 26.Smith H. S. Treatment considerations in painful HIV-related neuropathy. Pain Physician. 2011;14:E505–E524. [PubMed] [Google Scholar]
- 27.Verma S., Estanislao L., Simpson D. HIV-associated neuropathic pain. CNS Drugs. 2005;19(4):325–334. doi: 10.2165/00023210-200519040-00005. [DOI] [PubMed] [Google Scholar]
- 28.Bridges D., Ahmad K., Rice A. S. C. The synthetic cannabinoid WIN55,212-2 attenuates hyperalgesia and allodynia in a rat model of neuropathic pain. British Journal of Pharmacology. 2001;133(4):586–594. doi: 10.1038/sj.bjp.0704110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fox A., Kesingland A., Gentry C., et al. The role of central and peripheral cannabinoid1 receptors in the antihyperalgesic activity of cannabinoids in a model of neuropathic pain. Pain. 2001;92(1):91–100. doi: 10.1016/s0304-3959(00)00474-7. [DOI] [PubMed] [Google Scholar]
- 30.LaBuda C. J., Koblish M., Little P. J. Cannabinoid CB2 receptor agonist activity in the hindpaw incision. European Journal of Pharmacology. 2005;527(1–3):172–174. doi: 10.1016/j.ejphar.2005.10.020. [DOI] [PubMed] [Google Scholar]
- 31.Sagar D. R., Kelly S., Millns P. J., O’Shaughnessey C. T., Kendall D. A., Chapman V. Inhibitory effects of CB1 and CB2 receptor agonists on responses of DRG neurons and dorsal horn neurons in neuropathic rats. European Journal of Neuroscience. 2005;22(2):371–379. doi: 10.1111/j.1460-9568.2005.04206.x. [DOI] [PubMed] [Google Scholar]
- 32.Kohli D. R., Li Y., Khasabov S. G., et al. Pain-related behaviors and neurochemical alterations in mice expressing sickle hemoglobin: modulation by cannabinoids. Blood. 2010;116(3):456–465. doi: 10.1182/blood-2010-01-260372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gutierrez T., Crystal J. D., Zvonok A. M., Makriyannis A., Hohmann A. G. Self-medication of a cannabinoid CB2 agonist in an animal model of neuropathic pain. Pain. 2011;152(9):1976–1987. doi: 10.1016/j.pain.2011.03.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Finnerup N. B., Sindrup S. H., Jensen T. S. The evidence for pharmacological treatment of neuropathic pain. Pain. 2010;150(3):573–581. doi: 10.1016/j.pain.2010.06.019. [DOI] [PubMed] [Google Scholar]
- 35.Cabral G. A., Marciano-Cabral F. Cannabinoid receptors in microglia of the central nervous system: immune functional relevance. Journal of Leukocyte Biology. 2005;78(6):1192–1197. doi: 10.1189/jlb.0405216. [DOI] [PubMed] [Google Scholar]
- 36.Klein T. W., Cabral G. A. Cannabinoid-induced immune suppression and modulation of antigen-presenting cells. Journal of Neuroimmune Pharmacology. 2006;1(1):50–64. doi: 10.1007/s11481-005-9007-x. [DOI] [PubMed] [Google Scholar]
- 37.Eisenstein T. K., Meissler J. J. Effects of cannabinoids on T-cell function and resistance to infection. Journal of Neuroimmune Pharmacology. 2015;10(2):204–216. doi: 10.1007/s11481-015-9603-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Brown M. R. D., Farquhar-Smith W. P. Cannabinoids and cancer pain: a new hope or a false dawn? European Journal of Internal Medicine. 2018;49:30–36. doi: 10.1016/j.ejim.2018.01.020. [DOI] [PubMed] [Google Scholar]
- 39.Cheeran M. C.-J., Hu S., Palmquist J. M., Bakken T., Gekker G., Lokensgard J. R. Dysregulated interferon-gamma responses during lethal cytomegalovirus brain infection of IL-10-deficient mice. Virus Research. 2007;130(1-2):96–102. doi: 10.1016/j.virusres.2007.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Marten N. W., Stohlman S. A., Zhou J., Bergmann C. C. Kinetics of virus-specific CD8+-T-cell expansion and trafficking following central nervous system infection. Journal of Virology. 2003;77(4):2775–2778. doi: 10.1128/jvi.77.4.2775-2778.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Liu L., Yin Y., Li F., Malhotra C., Cheng J. Flow cytometry analysis of inflammatory cells isolated from the sciatic nerve and DRG after chronic constriction injury in mice. Journal of Neuroscience Methods. 2017;284:47–56. doi: 10.1016/j.jneumeth.2017.04.012. [DOI] [PubMed] [Google Scholar]
- 42.Liu X., Tian Y., Lu N., Gin T., Cheng C. H., Chan M. T. Stat3 inhibition attenuates mechanical allodynia through transcriptional regulation of chemokine expression in spinal astrocytes. PLoS One. 2013;8 doi: 10.1371/journal.pone.0075804.e75804 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Tsuda M., Kohro Y., Yano T., et al. JAK-STAT3 pathway regulates spinal astrocyte proliferation and neuropathic pain maintenance in rats. Brain. 2011;134(4):1127–1139. doi: 10.1093/brain/awr025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Becher B., Prat A., Antel J. P. Brain-immune connection: immuno-regulatory properties of CNS-resident cells. Glia. 2000;29(4):293–304. doi: 10.1002/(sici)1098-1136(20000215)29:4<293::aid-glia1>3.3.co;2-1. [DOI] [PubMed] [Google Scholar]
- 45.Ehrhart J., Obregon D., Mori T., et al. Stimulation of cannabinoid receptor 2 (CB2) suppresses microglial activation. Journal of Neuroinflammation. 2005;2(1):p. 29. doi: 10.1186/1742-2094-2-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Cencioni M. T., Chiurchiù V., Catanzaro G., et al. Anandamide suppresses proliferation and cytokine release from primary human T-lymphocytes mainly via CB2 receptors. PLoS One. 2010;5(1) doi: 10.1371/journal.pone.0008688.e8688 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Maresz K., Pryce G., Ponomarev E. D., et al. Direct suppression of CNS autoimmune inflammation via the cannabinoid receptor CB1 on neurons and CB2 on autoreactive T cells. Nature Medicine. 2007;13(4):492–497. doi: 10.1038/nm1561. [DOI] [PubMed] [Google Scholar]
- 48.Coopman K., Smith L. D., Wright K. L., Ward S. G. Temporal variation in CB2R levels following T lymphocyte activation: evidence that cannabinoids modulate CXCL12-induced chemotaxis. International Immunopharmacology. 2007;7(3):360–371. doi: 10.1016/j.intimp.2006.11.008. [DOI] [PubMed] [Google Scholar]
- 49.Ghosh S., Preet A., Groopman J., Ganju R. Cannabinoid receptor CB2 modulates the CXCL12/CXCR4-mediated chemotaxis of T lymphocytes. Molecular Immunology. 2006;43(14):2169–2179. doi: 10.1016/j.molimm.2006.01.005. [DOI] [PubMed] [Google Scholar]
- 50.Robinson R. H., Meissler J. J., Breslow-Deckman J. M., Gaughan J., Adler M. W., Eisenstein T. K. Cannabinoids inhibit T-cells via cannabinoid receptor 2 in an in vitro assay for graft rejection, the mixed lymphocyte reaction. Journal of Neuroimmune Pharmacology. 2013;8(5):1239–1250. doi: 10.1007/s11481-013-9485-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Peterson P. K., Gekker G., Hu S., Cabral G., Lokensgard J. R. Cannabinoids and morphine differentially affect HIV-1 expression in CD4+ lymphocyte and microglial cell cultures. Journal of Neuroimmunology. 2004;147(1-2):123–126. doi: 10.1016/j.jneuroim.2003.10.026. [DOI] [PubMed] [Google Scholar]
- 52.Rock R. B., Gekker G., Hu S., et al. WIN55,212-2-mediated inhibition of HIV-1 expression in microglial cells: involvement of cannabinoid receptors. Journal of Neuroimmune Pharmacology. 2007;2(2):178–183. doi: 10.1007/s11481-006-9040-4. [DOI] [PubMed] [Google Scholar]
- 53.Sheng W. S., Hu S., Min X., Cabral G. A., Lokensgard J. R., Peterson P. K. Synthetic cannabinoid WIN55,212-2 inhibits generation of inflammatory mediators by IL-1β-stimulated human astrocytes. Glia. 2005;49(2):211–219. doi: 10.1002/glia.20108. [DOI] [PubMed] [Google Scholar]
- 54.Hu S., Sheng W. S., Rock R. B. CB2 receptor agonists protect human dopaminergic neurons against damage from HIV-1 gp120. PLoS One. 2013;8 doi: 10.1371/journal.pone.0077577.e77577 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Fine P. G., Rosenfeld M. J. Cannabinoids for neuropathic pain. Curr Pain Headache Rep. 2014;18:p. 451. doi: 10.1007/s11916-014-0451-2. [DOI] [PubMed] [Google Scholar]
- 56.Yao B. B., Hsieh G. C., Frost J. M., et al. In vitro and in vivo characterization of A-796260: a selective cannabinoid CB2 receptor agonist exhibiting analgesic activity in rodent pain models. British Journal of Pharmacology. 2008;153(2):390–401. doi: 10.1038/sj.bjp.0707568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kinsey S. G., Mahadevan A., Zhao B., et al. The CB2 cannabinoid receptor-selective agonist O-3223 reduces pain and inflammation without apparent cannabinoid behavioral effects. Neuropharmacology. 2011;60(2-3):244–251. doi: 10.1016/j.neuropharm.2010.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Deng L., Guindon J., Cornett B. L., Makriyannis A., Mackie K., Hohmann A. G. Chronic cannabinoid receptor 2 activation reverses paclitaxel neuropathy without tolerance or cannabinoid receptor 1-dependent withdrawal. Biological Psychiatry. 2015;77(5):475–487. doi: 10.1016/j.biopsych.2014.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Rahn E. J., Zvonok A. M., Thakur G. A., Khanolkar A. D., Makriyannis A., Hohmann A. G. Selective activation of cannabinoid CB2 receptors suppresses neuropathic nociception induced by treatment with the chemotherapeutic agent paclitaxel in rats. Journal of Pharmacology and Experimental Therapeutics. 2008;327(2):584–591. doi: 10.1124/jpet.108.141994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Bow E. W., Rimoldi J. M. The structure-function relationships of classical cannabinoids: CB1/CB2 modulation. Perspectives in Medicinal Chemistry. 2016;8:17–39. doi: 10.4137/pmc.s32171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Walter L., Franklin A., Witting A., et al. Nonpsychotropic cannabinoid receptors regulate microglial cell migration. Journal of Neuroscience. 2003;23(4):1398–1405. doi: 10.1523/jneurosci.23-04-01398.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Li Y. Y., Li H., Liu Z. L., et al. Activation of STAT3-mediated CXCL12 up-regulation in the dorsal root ganglion contributes to oxaliplatin-induced chronic pain. Molecular Pain. 2017;13 doi: 10.1177/1744806917747425.1744806917747425 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data used to support the findings of this study are included within the article.