Abstract
The study of skeletochronology and bone tissue as a record of information on ontogenetic stages and events is widely used for improving the knowledge about life histories (LHs) of extinct and extant vertebrates. Compared with dinosaurs and extant reptiles, mammalian bone histology has received little attention. Here, we calibrate for the first time bone and dental age with histological bone characteristics and LH stages in ontogenetic series of red deer. We rely on known LHs of different aged individuals of captive Cervus elaphus hippelaphus from Austria to correlate epiphyseal closure, dental eruption pattern, bone growth marks and bone tissue patterns in femora and tibiae, and of wild Cervus elaphus hispanicus from Spain. Our data show that females (of both subspecies) attain skeletal maturity earlier than males. At this moment, epiphyseal closure (in femora and tibiae) and dental eruption are complete and long bones start to deposit an external fundamental system. The results also show that the attainment of reproductive maturity in red deer occurs slightly before skeletal maturity.
Keywords: cyclical growth marks, dental eruption pattern, epiphyseal closure, external fundamental system, growth, skeletal maturity
Introduction
A significant research goal in biological disciplines is to gather information about age at death or estimate some life history (LH) patterns from mammalian remains. This approach could provide demographic data such as individual age, sexual and skeletal maturity or the age structures of extant and fossil populations, and improve our knowledge about their way of life. This information is especially useful in policies of conservation and wildlife management as well as in ecological studies of both extant and fossil organisms. However, correlated, age‐calibrated anatomical data, which are the basis for correct LH interpretations, are lacking in all mammalian groups.
The study of epiphyseal closure in limb bones is based on the degree of fusion between diaphyses and epiphyses. In juvenile specimens, epiphyses are unconnected, enabling the growth in length (O'Connor, 2000). As the specimen reaches adult size, linear growth stops and the epiphyses start to fuse with the diaphyses (Popkin et al. 2012). When completely fused, the limb bone can only increase in width. Closure sequence differs between species; it has been documented on some species of cervids such as white‐tailed deer (Odocoileus virginianus; Purdue, 1983; Flinn et al. 2013), fallow deer (Dama dama; Carden & Hayden, 2002), roe deer (Capreolus capreolus; Ruddle, 1997) and red deer (Cervus elaphus; Mariezkurenna, 1983). It is also possible to estimate the specimen's age by its dental eruption pattern (O'Connor, 2000). Azorit et al. (2002) described the sequence of eruption and replacement in Spanish red deer (Cervus elaphus hispanicus), and Brown & Chapman (1991) for English red deer (C. elaphus elaphus). Other studies focused on dental features were developed in cervids, such as the study of dental measurements (Cederlund et al. 1991; Cooper et al. 2013) or dental wear (Veiberg et al. 2007; Azorit, 2011).
The study of cyclical growth marks (CGMs) is frequently used in age estimations (skeletochronology). These marks originate from growth alterations. Cementum marks or dental increments have been widely used as an age‐estimation method in cervids due to their annual periodicity (Mitchell, 1967; Cederlund et al. 1991; Azorit et al. 2004). CGMs can also be found in mammalian limb bones (Köhler et al. 2012). This finding is supported by numerous histological studies in several mammalian groups such as primates (Castanet et al. 2004; Castanet, 2006), rodents and lagomorphs (García‐Martínez et al. 2011; Moncunill‐Solé et al. 2016; Orlandi‐Oliveras et al. 2016) and ungulates (Marín‐Moratalla et al. 2011, 2013; Köhler et al. 2012; Jordana et al. 2016; Nacarino‐Meneses et al. 2016a,b; Orlandi‐Oliveras et al. 2018). Data about growth rates of extinct deer and their relation with LH have been obtained recently by means of the analysis of bone histology (Amson et al. 2015; Kolb et al. 2015a). However, a study of bone histology in extant deer emerges as essential and has not been performed so far to provide a firm basis for the interpretation of fossil deer.
In view of this, the aim of this research is to correlate, for the first time, the data obtained from bone histology with the different techniques commonly used for ageing mammals (epiphyseal closure and dental eruption pattern) and to define a common pattern that describes the timing between these morphological traits and the LH characteristics (Table 1) of wild and captive populations of C. elaphus. Moreover, our complete sample, which includes two different subspecies of C. elaphus, provides the opportunity to make, for the first time, a horizontal comparison of the patterns of skeletal and sexual maturation in subspecies of red deer.
Table 1.
Main life history traits of Cervus elaphus by sex according to the dimorphic regime of the species (Mitchell et al. 1977; Clutton‐Brock et al. 1982; Carranza, 2017)
| Traits | Average weight | Age at sexual maturity | Age at skeletal maturity | Age at parturition | Litter size |
|---|---|---|---|---|---|
| Data | |||||
| Males | 80–160 kg | 4–5 years | 5 years | – | – |
| Females | 50–100 kg | 2.5–3 years | – | 2–3 years | 1 fawn |
It is important to remark that, although the analysis of a wild population with known individual age could provide important information about the deer LH, monitoring a wild population is a difficult and expensive task, requiring large amounts of time. Results here obtained provide the basis for growth interpretation of deer and can be applied to other groups of hoofed animals.
Materials and methods
Study sample
A total of 32 deer specimens of two subspecies (C. e. hispanicus and Cervus elaphus hippelaphus) belonging to four populations of different European geographical areas were collected (Table 2). We studied individuals of three wild populations (n = 1, 8 and 10 specimens) of C. e. hispanicus from La Rioja (northern Spain), Badajoz (southwest Spain) and Lleida (northeast Spain), respectively, and of one captive population of C. e. hippelaphus (n = 13 specimens) from the Wildlife Institute of Vienna (Austria). This latter population was kept in semi‐wild conditions in an area of 45 ha with additional fodder supply (pellets) during the winter season. Females gave birth in May–June (and only exceptionally later) and they generally had one calf, with the exception of female IPS‐74171, which had two calves at the age of 7 years. Most calves were hand‐raised by bottle until their 3rd month of life, coinciding with the time at which calves are able to feed independently in wild populations. We had very accurate information for the Austrian population regarding age, sex and some other important ecological and LH variables (e.g. castration in males, weight; Table 2). Specimens from Spanish populations were obtained from hunting practices. Sex was identified (Table 2) and age was estimated according to dental parameters (Table 3). In Spain, red deer together with wild boar (Sus scrofa) are the two most hunted wild ungulates due to their economic significance (Olea & Miguel‐Ayanz, 2006). This activity has disrupted the wild condition of deer, particularly in the southwestern region of Spain, where the Mediterranean dehesa habitat predominates. Dehesa managers usually install fences in some hunting reserves; in these places, artificial conditions are maintained and, consequently, deer density is increased (Olea & Miguel‐Ayanz, 2006). Some specimens of C. e. hispanicus here analysed belonged to this region (Badajoz), and two others lived in temperate forests. It is also important to bear in mind the loss of genetic variability caused by the trophy stalking or the introduction of European deer in some reserves with the purpose of obtaining larger trophies (Carranza, 2017), compromising the true wild status of the red deer in Spain.
Table 2.
Available data of subspecies of Cervus elaphus and number of LAGs in both the fibrolamellar complex and the external fundamental system
| ID | Subspecies | Location | Sex group | Weight (kg) | Known age (years) | Femora | Tibiae | ||
|---|---|---|---|---|---|---|---|---|---|
| FLC | EFS | FLC | EFS | ||||||
| IPS‐56303 | C. e. hippelaphus | Vienna, A | Female | 104 | 5 | 3 | 4 | – | – |
| IPS‐56304 | C. e. hippelaphus | Vienna, A | Female | 94.5 | 5 | 3 | 3 | – | – |
| IPS‐74171 | C. e. hippelaphus | Vienna, A | Female | 133 | 13 | 3a | 6 | – | – |
| IPS‐56306 | C. e. hippelaphus | Vienna, A | Female | 95 | 4 | 3a | 2 | – | – |
| IPS‐56307 | C. e. hippelaphus | Vienna, A | Female | 148.5 | 13 | 3a | 6 | – | – |
| IPS‐56308 | C. e. hippelaphus | Vienna, A | Male | 56 | 0.91 | 1 | 0 | – | – |
| IPS‐106838 | C. e. hippelaphus | Vienna, A | Maleb | 225 | 5 | 5 | 0 | 5 | 0 |
| IPS‐106839 | C. e. hippelaphus | Vienna, A | Male | 116 | 1.17 | 1 | 0 | 1 | 0 |
| IPS‐106840 | C. e. hippelaphus | Vienna, A | Male | 117 | 1.08 | 1 | 0 | 1 | 0 |
| IPS‐106841 | C. e. hippelaphus | Vienna, A | Maleb | 210 | 5 | 5 | 0 | 5 | 0 |
| IPS‐106842 | C. e. hippelaphus | Vienna, A | Male | 127 | 1.25 | 1 | 0 | 1 | 0 |
| IPS‐106843 | C. e. hippelaphus | Vienna, A | Male | – | – | 5 | 5 | 7 | 0 |
| IPS‐109289 | C. e. hippelaphus | Vienna, A | Male | 79 | 0.58 | 0 | 0 | 0 | 0 |
| IPS‐60869 | C. e. hispanicus | Badajoz, SP | Female | – | – | 3 | 3 | – | – |
| IPS‐60870 | C. e. hispanicus | Badajoz, SP | Male | – | – | 2 | 0 | – | – |
| IPS‐60871 | C. e. hispanicus | Badajoz, SP | Male | – | – | 5 | 0 | – | – |
| IPS‐60872 | C. e. hispanicus | Badajoz, SP | Male | – | – | 4 | 3 | – | – |
| IPS‐60873 | C. e. hispanicus | Badajoz, SP | Male | – | – | 4 | 0 | – | – |
| IPS‐60874 | C. e. hispanicus | Badajoz, SP | Female | – | – | 3 | 2 | – | – |
| IPS‐60875 | C. e. hispanicus | Badajoz, SP | Male | – | – | 4 | 3 | – | – |
| IPS‐60876 | C. e. hispanicus | Badajoz, SP | Female | – | – | 1 | 0 | – | – |
| IPS‐60877 | C. e. hispanicus | Badajoz, SP | Male | – | – | 4 | 4 | – | – |
| IPS‐60878 | C. e. hispanicus | Badajoz, SP | Female | – | – | 1 | 0 | – | – |
| IPS‐83490 | C. e. hispanicus | Lleida, SP | Female | – | – | ‐ | ‐ | 1 | 0 |
| IPS‐83491 | C. e. hispanicus | Lleida, SP | Female | – | – | 0 | 0 | 0 | 0 |
| IPS‐83492 | C. e. hispanicus | Lleida, SP | Female | – | – | 0 | 0 | 0 | 0 |
| IPS‐83503 | C. e. hispanicus | Lleida, SP | Male | – | – | 4 | 0 | 4 | 0 |
| IPS‐83505 | C. e. hispanicus | Lleida, SP | Male | – | – | 3 | 0 | 3 | 0 |
| IPS‐83506 | C. e. hispanicus | Lleida, SP | Male | – | – | 0 | 0 | 0 | 0 |
| IPS‐83507 | C. e. hispanicus | Lleida, SP | Male | – | – | 1 | 0 | 1 | 0 |
| IPS‐83513 | C. e. hispanicus | Lleida, SP | Male | – | – | – | – | 8 | 0 |
| IPS‐62075 | C. e. hispanicus | La Rioja, SP | Female | – | – | 3 | 3 | – | – |
A, Austria; ID, specimen; EFS, external fundamental system; FLC, fibrolamellar complex; SP, Spain.
The innermost LAG has been reconstructed.
Castrated specimens.
Table 3.
Information about the stage of epiphyseal closure, stage and estimated age according dental eruption (Brown & Chapman, 1991), stage and estimated age according dental wear (Anders et al. 2011; Azorit, 2011), presence of EFS of subspecies of Cervus elaphus and estimated age group of each specimen
| ID | EFS | Epiphyseal fusion | Dental eruption | Dental wear | Age group | ||||
|---|---|---|---|---|---|---|---|---|---|
| Femora | Tibia | Femora (p/d) | Tibia (p/d) | Stage | Estimated age in months | Stage | Estimated age in years | ||
| IPS‐56303 | Yes | – | Fd/Fd | – | Complete | 38–40 | IDAS 3 (adult) | 5.5–8.5 | Adult |
| IPS‐56304 | Yes | – | Fd/Fd | – | Complete | 38–40 | IDAS 3 (adult) | 5.5–8.5 | Adult |
| IPS‐74171 | Yes | – | Fd/Fd | – | Complete | 38–40 | IDAS 4 (late ad.) | 7.5–13.5 | Adult |
| IPS‐56306 | Yes | – | Fd/Fd | – | Complete | 38–40 | IDAS 3 (adult) | 4.5–6.5 | Adult |
| IPS‐56307 | Yes | – | Fd/Fd | – | Complete | 38–40 | IDAS 4 (late ad.) | 7.5–13.5 | Adult |
| IPS‐56308 | No | – | Un/Un | – | M2 erupting | 10 | IDAS 2 (juvenile) | < 3.5 | Juvenile |
| IPS‐106838 | No | No | Fd/Fd | Fd/Fd | Complete | 38–40 | IDAS 3 (adult) | 4.5–6.5 | Juvenile‐adult |
| IPS‐106839 | No | No | Un/Un | Un/Un | M3 forming | 15 | IDAS 2 (juvenile) | < 3.5 | Juvenile |
| IPS‐106840 | No | No | Un/Un | Un/Un | M3 forming | 13 | IDAS 2 (juvenile) | < 3.5 | Juvenile |
| IPS‐106841 | No | No | Fd/Fd | Fd/Fd | Complete | 38–40 | IDAS 3 (adult) | 4.5–6.5 | Juvenile‐adult |
| IPS‐106842 | No | No | Un/Un | Un/Fg | M3 forming | 15 | IDAS 2 (juvenile) | < 3.5 | Juvenile |
| IPS‐106843 | Yes | No | Fd/Fd | Fd/Fd | Complete | 38–40 | – | – | Adult |
| IPS‐109289 | No | No | Un/Un | Un/Un | M2 erupting | 10 | IDAS 2 (juvenile) | < 3.5 | Juvenile |
| IPS‐60869 | Yes | – | Fd/Fd | – | Complete | 38–40 | IDAS 3 (adult) | 4.5–6.5 | Adult |
| IPS‐60870 | No | – | Fg/Fg | – | M3 erupting | 18 | IDAS 2 (juvenile) | < 3.5 | Juvenile |
| IPS‐60871 | No | – | Fg/Fg | – | Complete | 38–40 | IDAS 3 (adult) | 3.0–4.0 | Juvenile‐adult |
| IPS‐60872 | Yes | – | Fd/Fd | – | Complete | 38–40 | IDAS 3 (adult) | 3.0–4.0 | Adult |
| IPS‐60873 | No | – | Fg/Fg | – | M3 erupting | 27 | IDAS 2 (juvenile) | < 3.5 | Juvenile |
| IPS‐60874 | Yes | – | Fd/Fd | – | Complete | 38–40 | IDAS 3 (adult) | 3.0–4.0 | Adult |
| IPS‐60875 | Yes | – | Fd/Fd | – | Complete | 38–40 | IDAS 3 (adult) | 5.5–8.5 | Adult |
| IPS‐60876 | No | – | Un/Un | – | M2 erupting | 10 | IDAS 2 (juvenile) | < 3.5 | Juvenile |
| IPS‐60877 | Yes | – | Fd/Fd | – | Complete | 38–40 | IDAS 3 (adult) | 5.5–8.5 | Adult |
| IPS‐60878 | No | – | Un/Un | – | M2 erupting | 10 | IDAS 2 (juvenile) | < 3.5 | Juvenile |
| IPS‐83490 | – | No | – | Un/Un | M1 erupting | < 5 | IDAS 1 (infant) | < 3.5 | Juvenile |
| IPS‐83491 | No | No | Un/Un | Un/Un | M1 erupting | 5 | IDAS 1 (infant) | < 3.5 | Juvenile |
| IPS‐83492 | No | No | Un/Un | Un/Fg | M1 erupting | < 5 | IDAS 1 (infant) | < 3.5 | Juvenile |
| IPS‐83503 | No | No | Fg/Fg | Fg/Fd | – | – | – | – | Juvenile‐adult |
| IPS‐83505 | No | No | Un/Un | Un/Fg | – | – | – | – | Juvenile |
| IPS‐83506 | No | No | Fg/Fg | Fg/Fg | – | – | – | – | Juvenile |
| IPS‐83507 | No | No | Fg/Un | Un/Fg | – | – | – | – | Juvenile |
| IPS‐83513 | – | No | – | Fd/Fd | – | – | – | – | – |
| IPS‐62075 | Yes | – | Fg/Fg | – | – | – | – | – | – |
d, distal; EFS, external fundamental system; Fd, fused; Fg, fusing; M1, first lower molar; M2, second lower molar; M3 third lower molar; ID, specimen; IDAS, individual dental age stage; p, proximal; Un, unfused.
After cleaning and selecting suitable specimens, we collected a total of 30 femora and 15 tibiae to perform the study. We chose femora and tibiae because these long bones preserve the most complete record of growth marks in tetrapods (Padian et al. 2013). This material is housed at the ICP – Institut Català de Paleontologia Miquel Crusafont, Barcelona (Spain).
Epiphyseal closure and eruption pattern
Estimations of individual age at death were made according to the degree of epiphyses fusion in long bones and the eruption pattern (in specimens whose mandible was available). Proximal and distal epiphyses of femora and tibiae were examined and classified into three different categories according to their degree of closure, i.e. unfused (Un) when the epiphyses were completely separated, fusing (Fg) when it was possible to appreciate the suture between epiphyses and diaphysis, and fused (Fd) when it was not possible to observe the suture previously mentioned. We considered the study by Mariezkurenna (1983) on red deer and Flinn et al. (2013) on white‐tailed deer to age specimens according to their epiphyseal closure. Thus, we established two age groups: < 3.5 years when epiphyses are Un or Fg, and ≥ 3.5 years when they are completely fused (Table 2). According to Brown & Chapman (1991), who defined dental age classes from radiography in red deer, we established eight age categories. We used seven stages from Brown & Chapman's (1991) original classifications and added one additional category (Table 3):
0–5 months: first lower molar erupting; 5 months: first lower molar erupted but still unworn (Brown & Chapman Fig. 1A);10 months: first lower molar erupted, wear has started; 13 months: second lower molar erupted but still unworn; 15 months: all lower premolars are mineralizing; 18 months: second lower molar erupted, wear has started; 27 months: third lower molar erupted but still unworn; lower premolars are all erupting and will replace the lost deciduous precursors; 38–40 months: all teeth are completely formed and initial wear is observable on all teeth. Beyond this age, it was possible to age individuals from their teeth using the dental wear approach. Anders et al. (2011) established a formula of six IDAS (individual dental age stage) based on the dental wear of different groups of eutherians to distinguish the categories of prenatal, infantile, juvenile, adult, late adult and senile. More specifically, Azorit (2011) recognized four age groups from 4 to 13 years after the complete dental eruption for red deer: 3.0–4.0 years, 4.5–6.5 years, 5.5–8.5 years and 8.5–13.5 years.
Figure 1.
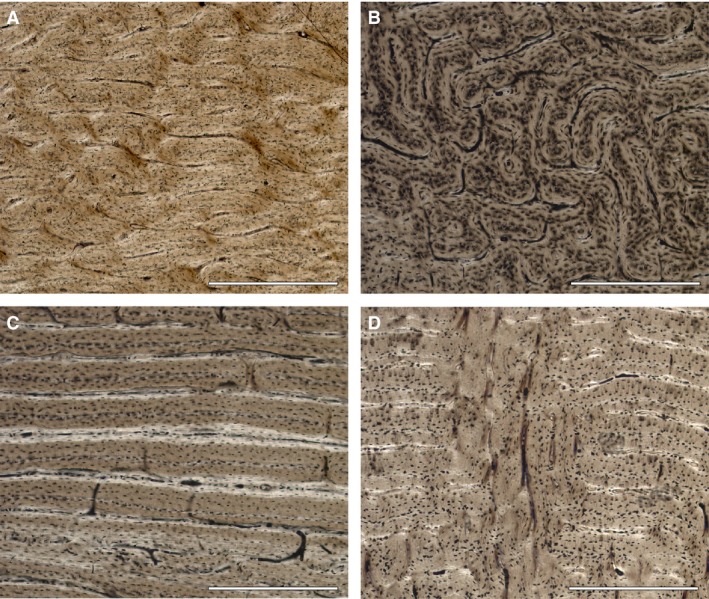
Long bones histology of Cervus elaphus. (A) Laminar bone in the femur of an adult male (IPS‐83513). (B) Plexiform bone in the femur of an adult male (IPS‐60877). (C) Reticular bone in the femur of a juvenile female (IPS‐83490). (D) Radial canals present in the tibia of a juvenile male (IPS‐83508). Scale bar: 500 μm.
With this information, we defined skeletal maturity as the moment at which all epiphyses are completely fused and all teeth have completely erupted (i.e. ca. 3.5 years). Accordingly, we established three age categories (Table 2): juvenile (< 3.5 years), adult (> 3.5 years) and juvenile–adult transition (≈ 3.5 years).
Histological analysis
The necessary metrics (i.e. maximum length, functional length, length of the diaphysis, antero‐posterior diameter, total diameter, minimum perimeter of the diaphysis and antero‐posterior and total diameter of both, distal and proximal, epiphyses) were taken for each bone. Thereafter, thin sections of 30 femora and 15 tibiae were prepared following the protocol of the ICP laboratory. A chunk of approximately 3 cm from the middle of the diaphysis was extracted from each femur (from 1.5 cm above to 1.5 cm below the mid‐shaft) and embedded in Araldite 2020 epoxy resin. This block was cut into two halves with a low‐speed diamond saw (IsoMet low speed saw, Buehler). The cut surfaces were polished with a MetaServ polishing machine and fixed to a frosted glass using ultraviolet curing epoxy resin. Once the sample was fixed, it was cut with a diamond saw (Petrothin, Buehler) up to a thickness of 100–120 μm and finely polished again to perfect the slide. Finally, sections were covered with DPX to improve the visualization under the microscope.
After preparation, thin sections were studied using a ZEISS Scope A1 microscope, with an integrated camera, under polarized light to analyse the different types of bone tissue and growth marks.
The organization of bone matrix can be classified as primary and secondary bone (primary lamellar/parallel‐fibered and primary woven bone/fibrolamellar complex with different types of vascularization; secondary Haversian and secondary endosteal lamellar bone) with different deposition rates and functions (Francillon‐Vieillot et al. 1990; De Margerie et al. 2002; Huttenlocker et al. 2013). According to the osteonal arrangement, various types of vascular canal orientations can be distinguished: longitudinal, laminar, plexiform, reticular and radial (Huttenlocker et al. 2013). In most mammals, the main tissue type found in long bones is a fast‐growing tissue known as fibrolamellar complex (FLC). This complex is composed of woven‐fibred bone with diverse vascular organizations (Huttenlocker et al. 2013). Sometimes, it is also possible to recognize a somewhat broad band in the periosteal zone, the external fundamental system (EFS). The EFS consists of an edge of slow‐growing tissue without vascularization formed by parallel‐fibred bone after maturity has been achieved (Chinsamy‐Turan, 2005; Woodward et al. 2013; de Andrade et al. 2015). Bone tissue is sometimes interrupted by growth marks. Here, we employ the general term BGM (bone growth mark) to describe a growth line regardless of whether its nature is cyclic, or CGM (cyclical growth mark), to describe those that follow a clearly cyclical pattern (Woodward et al. 2013). We considered a single BGM as the circumferential structure formed by one or several well‐defined lines which are situated very close together and which can be followed over the surface of the cortical slide (Huttenlocker et al. 2013).
Section images were combined to reconstruct the mid‐shaft surface using photoshop CS4. We obtained area and perimeter measurements of each growth mark using specific software (imagej). These data allowed us to calculate the growth rate for each growth zone. Although the annual period of growth arrest is still unknown, here we supposed 3 months of growth arrest (i.e. ca. 270 days of formation of growth zones) to calculate an approximation to the daily bone growth rates for each growth zone. In the case of specimens that showed a neonatal line (NL), it was also possible to calculate the growth rate of the first growth zone. In this case, we calculated the rate considering 135 days of formation (ca. half a year).
Growth areas and rates of the different groups of data were compared using the Kruskal–Wallis test. Fisher's exact test was used to find a relationship between histological data obtained from bone histology with the degree of epiphyseal fusion and dental eruption pattern.
Results
Epiphyseal closure, eruption pattern and dental wear
The stage of epiphyseal closure (unfused, fusing and fused), dental eruption and wear were examined to determine the specimen's age (Table 3). Our results revealed that the distal epiphysis of the tibia shows the earliest fusion (Table 3). These findings agree with previous studies focused on epiphyseal closure of C. elaphus (Mariezkurenna, 1983). We observed different stages of dental eruption in the mandibles. Three females from Lleida showed the earliest eruption stage. The mandible still had the deciduous premolars and the M1 was erupting. On the other hand, 14 specimens possessed their complete dentition, showing the third cusp of the M3 completely erupted and in some cases even worn. We observed the dental wear stages from infant to late adult, with no senile deer in our sample.
We also found a relation between the stage of fused epiphyses and complete dentition (P‐value = 6.73e‐05). All fused specimens had completely erupted dentition, and those with unfused or fusing stages of their epiphyses showed an incomplete dentition, with the exception of the male IPS‐60871 from Lleida. In this male, all teeth had erupted but his epiphyses were still fusing.
Histological analysis
Fast‐growing tissue interrupted by BGMs or annuli was found in every sampled specimen, except for three females (IPS‐83490, IPS‐83491, IPS‐83492), one male (IPS‐83506) from Lleida, and one male (IPS‐109289) from Vienna, which did not show any growth interruption (Table 2). The tissue consists of FLC with different vascular orientations depending on the bone and the location within it. BGMs were often surrounded by slow‐growing tissue, indicating the decline and increase in growth rate before and after the growth hiatus, respectively. The specimen from Vienna previously mentioned (IPS‐109289) showed this tissue change in the anterior part of its periosteum both in femur and tibia (Supporting Information Fig. S2). In this zone, the disorganized woven‐fibred bone shifts to a more organized tissue (parallel‐fibred bone), indicating the slowdown in growth. The full dataset about this specimen (Table 2) allows us to establish that the decrease in growth rate occurred shortly before its death. This coincides with the beginning of the winter season and hence represents the first CGM of the specimen.
In the cortex of the 29 femora, we found plexiform orientation (Fig. 1A) with small areas of reticular vascularity mainly in the medial part near the medullar cavity and in disperse zones (Fig. 1B). However, a laminar orientation prevailed in the cortex of nine of the 15 tibiae (Fig. 1C). Some specimens also showed the radial orientation of vascular canals in the inner part of the tibial cortex (Fig. 1D), which indicates very fast deposition of bone (Francillon‐Vieillot et al. 1990). Secondary bone (high density of secondary osteons) was present in the region of the linea aspera of each femur due to remodelling processes. As age increases, secondary bone expands radially into the cortex from the inner region to the external cortex of femora and tibiae. Two 13‐year‐old specimens (IPS‐74171 and IPS‐56307) also showed secondary osteons in the outermost cortex. Moreover, in 12 of them, we found the EFS. In contrast, none of the tibiae showed an EFS in its outermost cortex (Table 3).
We found a relationship between presence/absence of EFS, fused/unfused epiphyses and complete/incomplete dentition (P = 0.00024). All specimens with EFS shared a complete dentition and fused epiphyses (Fig. 2). However, in the female IPS‐62075 and the males IPS‐60871, IPS‐106838, IPS‐106841, these features were not shown together (Table 3).
Figure 2.
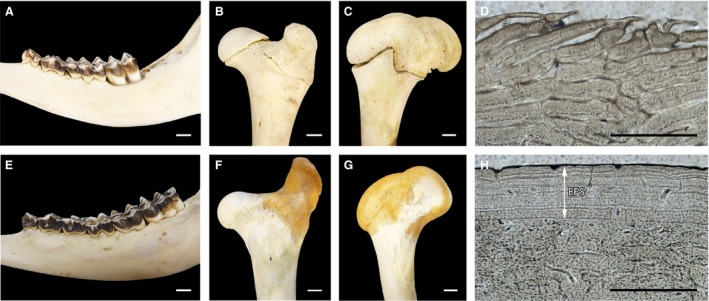
Dental eruption (A,E), stage of epiphyseal closure at the proximal (B,F) and distal region (C,G) and anterior cortex of femora (D,H) of a juvenile male (IPS‐56308) (A–D) and an adult female (IPS‐56304) (E–H). White arrow indicates the presence of EFS. White scale bar: 1 cm. Black scale bar: 500 μm.
Our results demonstrate that BGMs are recorded in both periosteal bone types, the FLC and the EFS. The bone cortex of specimens with an EFS shows a constant of four CGMs within the FLC in the case of the females and five in the case of the males of both subspecies, with the last BGM forming the border between the FLC and the EFS. Ontogenetic series of femora were reconstructed for each population by superimposition of histological slides to account for lost growth marks (Woodward et al. 2013). This allowed us to find CGMs that are partially erased by the expansion of the medullary cavity in three females of C. e. hippelaphus (IPS‐74171, IPS‐56306, IPS‐56307; Fig. S1; Table 1). In most of the analysed bones, the first BGM was either discontinuous and interrupted by growing tissue or it changed into an annulus instead of a well‐defined BGM. Moreover, we found a sudden change of tissue in the inner region of most of the tibiae analysed (Fig. 3) and in the femora of IPS‐56308, IPS‐106840, IPS‐83491, IPS‐83505, IPS‐83506 and IPS‐83507.
Figure 3.
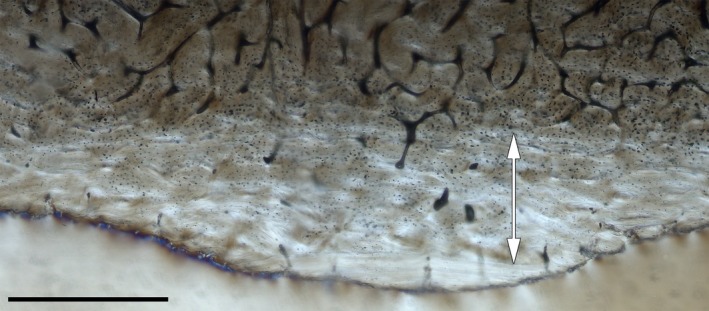
Neonatal line in the inner cortex of the femur of a young captive male (IPS‐56308). Double‐head white arrow indicates the pre‐natal tissue. Scale bar: 500 μm.
Growth curves
We plotted the growth curves using accumulative growth zone areas of femur (Fig. 4A) and tibia (Fig. 4B; i.e. active bone deposition between two BGMs). We used area instead of perimeter as a variable because it is more related to body mass and hence better reflects the ontogenetic stages. Growth follows a sigmoidal curve, showing a section of linear fast growth and another of asymptotic or slow growth. In both subspecies, females reached the inflection point at an earlier age than males (third and fourth BGM, respectively).
Figure 4.
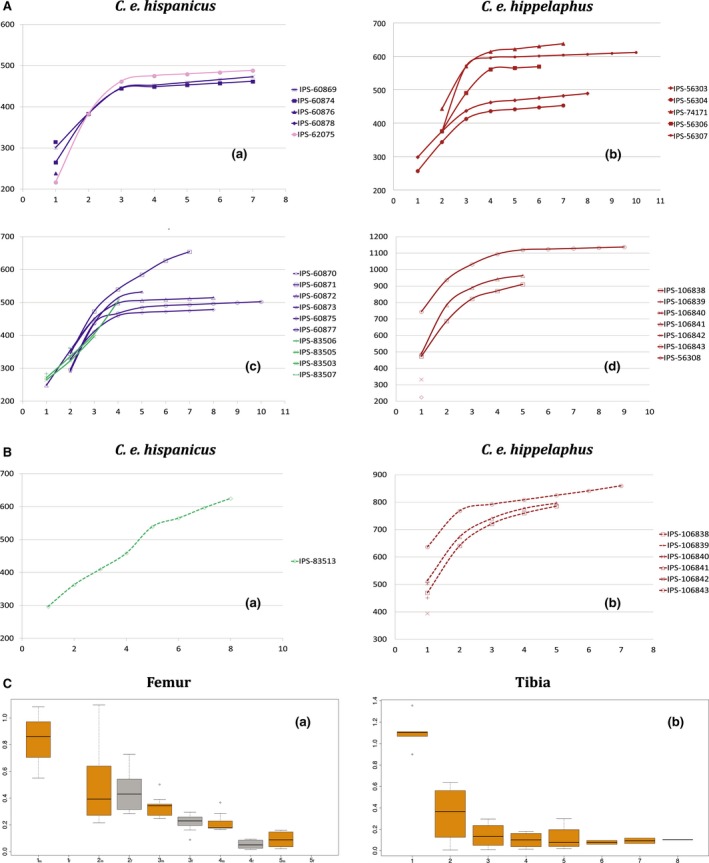
Representation of growth curves and growth rates of subspecies of Cervus elaphus. (A) Growth curves based on growth zone areas represented in mm2 of femora for Cervus elaphus hispanicus and Cervus elaphus hippelaphus. (B) Growth curves based on growth zone areas of tibia for C. elaphus. Filled symbols represent female specimens; unfilled symbols represent males. Purple lines: Spanish deer population from Badajoz. Green lines: Spanish deer population from Lleida. Pink line: Spanish deer population from La Rioja. Maroon lines: Austrian deer population. (C) Growth rates represented in mm2 day−1 for each growth zone of sampled femora (a) and tibia (b). Orange boxes: males sample. Grey boxes: Female sample. m: growth zone of males. f: growth zone of females.
Mean growth areas of femora for C. e. hispanicus and C. e. hippelaphus were 270.71 and 421 mm2, respectively, during the first year of growth, and 58.38 and 81.90 mm2 in the last year before the formation of EFS (Fig. 4C). Regarding total growth zone areas of femora, significant differences (P = 0.013) were found between subspecies but not between sexes (P = 0.0924). In the case of the tibiae, significant differences were not found between subspecies, either in total growth areas (P = 0.617) or in single areas. We calculated the growth rates for each growth zone both for tibia and femur using bone growth areas, instead of distance between BGMs (Marín‐Moratalla et al. 2013, 2014; Kolb et al. 2015b; Jordana et al. 2016). It is important to highlight that specimens from Lleida exhibited a different growth pattern than the other two populations, both in femora (Fig. 4A‐b) and tibiae (Fig. 4B‐b). With increasing age, growth rates in the femora suffered an abrupt decrease, being very low the year before the formation of the EFS (Fig. 4C‐a). By contrast, growth rates in the tibiae suffered a strong decline during the first year of growth (from 2.216 to 0.344 mm2 day−1); after that, bone growth occurred slowly and continuously (Fig. 4C‐b).
Discussion
Data obtained here allow for the first time the calibration of eruption pattern and epiphyseal closure with bone histology and key LH traits (age at skeletal and reproductive maturity) in two subspecies of extant C. elaphus. Summarizing the results, femora and tibiae of the individuals within our red deer populations showed the same number of annual marks in their FLC (before deposition of the EFS), with the exception of one adult male of C. e. hippelaphus, where the femora had fewer BGMs within the FLC than the tibia. Hence, both long bones appear to be equally useful for skeletochronology. The analysis of dental eruption pattern and epiphyseal fusion allowed us to establish different age categories for both deer subspecies.
Ageing
Deposition of zonal bone is triggered by photoperiod (Castanet et al. 2004) and seasonal environmental cycles of resource availability (Köhler et al. 2012), recording seasonal physiological cycles of hypometabolism during the unfavourable season (i.e. winter; Köhler et al. 2012). The total number of BGMs within the FLC and the EFS is expected to provide the age at death of the individual. This has been claimed to be true for ruminants in a study based on femora of wild individuals of unknown age (Jordana et al. 2016) but has been shown not to be fully reliable in equids of a known age (Nacarino‐Meneses et al. 2016a). Here, we discuss the results of our approach for the red deer.
Material of known age
Our aged material from the Vienna enclosure belongs to some but not all possible age categories, lacking animals of ages between 1 and 3 years, and 7 and 13 years. The analysis of the C. e. hippelaphus population confirms that the total number of BGMs is not as reliable for deer as expected from previous publications (Jordana et al. 2016). The data provided about the ages of these deer show that the number of BGMs present in tibiae corresponds to the known age. In the case of the femora, however, the number of BGMs only corresponds to the age before the deposition of EFS. When EFS is present, some individuals show more BGMs than expected from their age, indicating that after attainment of skeletal maturity, growth not only slows down but also ceases more frequently than before. On the other hand, there appears to be an age at which no further BGMs are deposited in the femur, leading to a lower final number of BGMs, as occurs in the oldest individuals. These outcomes agree with previous findings by Castanet et al. (2004) for the small primate Microcebus, and suggests that growth definitely ceases a few years after attainment of reproductive maturity.
Epiphyseal closure stages match the age, females being as reliable as males. Dental eruption stages also correspond precisely to the age of up to 3.5 years (indicating the age of skeletal maturity). After this age, we can only age individuals by means of the analysis of their dental wear. Age classes and dental wear stages correspond to the age from the youngest (0–6 months) to the oldest specimen (13 years). Our findings determined that the specimens with all epiphyses unfused are younger than 1.5 years. Though a delayed epiphyseal fusion has been described in castrated specimens of sheep (Davis, 2000), we did not find variation in the maturation time of the skeletal elements of the castrated specimens.
Material of unknown age
We estimated the age for the specimens of C. e. hispanicus considering CGMs only within the FLC (Table 2). The Spanish sample is broader than that from Austria and contains a wide variety of age categories between 0.5 and 8 (estimated) years. As with the C. e. hippelaphus sample, the dental eruption and epiphyseal closure stages agree, with a few exceptions, with the age estimated from histological analyses in both males and females.
Although most of the data from our sample support that femur and tibia are equally useful for skeletochronology, there are some important remarks to make here. The tibiae of two adult males of the C. e. hispanicus (IPS‐83513) and C. e. hippelaphus (IPS‐106843) populations, showed eight and seven CGMs within the FLC and were still lacking the EFS, whereas the maximum number of CGMs within the FLC of the femora was five. This variation between the number of CGMs found in long bones, as well as the absence of EFS in the tibia, agrees with findings in equids (Nacarino‐Meneses et al. 2016b). This can be explained by the low and fairly continuous growth rate of bone deposition in tibiae. This bone takes more years than the femur to reach a time when the radial growth rate decreases, leading to more CGMs than expected in the FLC.
Because births in red deer take place in summer (May–July in C. e. hippelaphus from the Wildlife Institute, Vienna), fawns show their first CGM at ca. 6 months, coinciding with their first winter season. In our specimens, this first winter CGM appears either faint or as an annulus as a consequence of the rapid growth of deer calves (Woodward et al. 2013). Considering this, the change of tissue detected in the innermost cortex of some specimens could represent the moment of birth (i.e. the NL), as Nacarino‐Meneses & Köhler (2018) describe in their study of Equus. Specimens younger than 6 months showed this shift in the central part of the cortex, which supports its non‐cyclical origin. The change of tissue, both in femora and tibiae, often accompanied by radial canals, could be produced by the beginning of locomotion and weight bearing in newborns (Nacarino‐Meneses & Köhler, 2018).
Attainment of skeletal and reproductive maturity
The effects of the attainment of skeletal or reproductive maturity on the bone cortex are still controversial. Some researchers assume that the formation of the EFS is correlated with the attainment of sexual maturity or with the first reproduction in ungulates (Marín‐Moratalla et al. 2014; Jordana et al. 2016; Nacarino‐Meneses et al. 2016b). On the other hand, some authors circumvent this problem, relating the EFS to the attainment of skeletal maturity both in extant and extinct groups (Marín‐Moratalla et al. 2014; Amson et al. 2015; Kolb et al. 2015a). Here, we demonstrate for the first time a correlation between the presence of EFS, complete dental eruption and epiphyseal fusion of long bones of known age specimens and corroborate the presence of an EFS in femora as an indicator of skeletal maturity in red deer.
The moment of deposition of the EFS is reflected in the growth curves as an asymptotic slowdown of the growth (Fig. 4A). We calculated the age at which specimens reach skeletal maturity by counting the CGMs within the FLC before EFS formation (Chinsamy‐Turan, 2005); females attain skeletal maturity prior to males (shortly after 3.5 vs. 4.5 years, respectively), which is more than 1 year earlier than estimations by Jordana et al. (2016), who estimated ages of 5 and 6 years, respectively. The difference between females and males reveals how important the sex factor is for this LH trait. An earlier cessation of growth in females has also been shown in other deer species such as white‐tailed deer (Flinn et al. 2013) and fallow deer (Carden & Hayden, 2002). The different investment in body size between sexes is attributable to the extended period of growth of males to attain dominance in intraspecific competition, whereas females invest in reproduction (Flinn et al. 2013).
The absence of EFS in the castrated specimens could be conditioned by the fact that their age (5 years) is within the threshold of EFS formation in males. Further studies are needed to establish the effects of castration on the formation of EFSs.
According to data gathered from the captive population, we affirm that reproductive maturity in females of C. e. hippelaphus is reached at the age of 3 years because most of the females gave birth to their first calf at this age. There are no data about birth dates in Iberian deer but, according to the literature, C. e. hispanicus females can give birth as early as the age of 2 years (Carranza, 2017). First reproduction in males occurs later than in females, at the age of 5–7 years (Clutton‐Brock et al. 1983; Ibler & Fischer, 2016). This phenomenon of bimaturism, the different onset of maturity between sexes, is typically found in polygynous species such as various species of hoofed mammals and some primates (Stearns, 1992). With these results, we conclude that, in deer, reproductive maturity precedes the attainment of skeletal maturity, as previously suggested by some authors (Lee et al. 2013).
How fast do deer bones grow?
Dissimilarities found in the growth of the two subspecies might be due to (1) genetics, as C. e. hispanicus is one of the smallest subspecies of C. elaphus (Garde et al. 2010); (2) life conditions, because specimens of C. e. hippelaphus were bred in captivity while C. e. hispanicus were born in the wild; and (3) environmental conditions, as the populations belonged to different geographical regions with variable climatic conditions.
Biological differences between subspecies are based on the genetic differentiation of C. e. hispanicus due to the isolation of the territory by the Pyrenees (Garde et al. 2010). Genetic studies (Skog et al. 2009; Zachos & Hartl, 2011) support the existence of different lineages (Iberian for C. e. hispanicus and Balkan/Carpathian for C. e. hippelaphus) belonging to different glacial refuges.
Regarding the lifestyle, the captive population receives proper veterinary care and has better food availability and quality than the wild populations of Spain, which could lead to differences in growth rates (Dmitriew, 2011). Notwithstanding, it is difficult to attribute these variations to the captive conditions due to the absence of samples of wild specimens of the same location with which these could be compared, and to the non‐constant variability of other attributes such as genetics or environmental conditions (Turner et al. 2016). Moreover, the possible semi‐wild conditions of some C. e. hispanicus populations make it difficult to establish clear differences. Both subspecies belong to different geographical regions with different climatic conditions and resource availability, which implies body size variations according to Bergmann's Rule (McNab, 2010; Rosvold et al. 2014). Accordingly, the environmental factor could also be an element that affects body size.
Growth variations between C. e. hispanicus populations of Badajoz and Lleida could be attributed to their belonging to different lineages (Carranza et al. 2016); however, we should bear in mind that the existence of different genetic lineages results from natural selection acting on body mass and/or LH traits. Thus, it is also possible that their different habitat and management cause variations in growth. Faster growth rates of specimens from Badajoz could be triggered by their development in dehesas or Mediterranean forests and its possible food support due to the hunting management to ensure persistence of the deer population (Olea & San Miguel‐Ayanz, 2006). On the other hand, specimens from Lleida lived in a temperate forest of a rather native environment where low nutrition conditions, common in hard seasons, could cause slower growth rates (Popkin et al. 2012). Finally, it could also be possible that increased hunting pressure on the Lleida population, especially on fawns (see the relatively high number of fawns in our Lleida sample), might be a selective force favouring an advancement of reproductive maturity through an earlier cessation of growth associated with a decrease in body size, as proposed by LH theory (Stearns, 1992; Palkovacs, 2003).
Growth caveats
The wide variety of growth patterns within the C. e. hippelaphus population could be attributed to the captivity regime maintained in the Wildlife Research Institute of Vienna for several generations. This is supported, for instance, by studies of captive populations (Trut, 1999) in which morphological and body size variations are described to evolve within a few generations of foxes.
Conclusions
Bone growth in extant deer is tightly related to the schedule of LH events. The formation of the EFS in femora occurs at the same time as the epiphyseal fusion of proximal and distal femur and tibia and the complete eruption of the dentition, denoting the attainment of generalized skeletal maturity. Tibiae, however, continue growing beyond this age. We found differences at the histological level between sexes, with females attaining skeletal maturity earlier than males. Finally, our study provides evidence that reproductive maturity in deer occurs shortly before skeletal maturity.
Author contributions
T.C. planned and designed the study, obtained and analysed the data, wrote the manuscript, and prepared figures and tables. D.D.M. reviewed early drafts of the manuscript and discussed the results. W.A. and G.S. raised the animals and provided veterinary care in accordance with the Ethics and Animal Welfare Committee of the University of Veterinary Medicine Vienna, and provided the skeletal material, as well as the individual data for C. e. hippelaphus. M.K. planned and designed the study, supervised the histological study, discussed the results and reviewed drafts of the article.
Supporting information
Fig. S1. Superimposition of femora of 13‐year‐old (IPS‐56307) and 5‐year‐old females (IPS‐56304). Red line indicates the first CGM lost by the expansion of the medullar cavity. Blue lines are two CGM partially eroded within FLC. Scale bar: 2 mm.
Fig. S2. Histological detail of the anterior part of the tibia of the young male IPS‐109289. The change of colour indicates the shift from woven‐fibred to parallel‐fibred bone caused by the slowdown in growth at the beginning of the winter season. Scale bar: 200 μm. Image obtained under polarized light with a λ filter.
Acknowledgements
The material of C. e. hispanicus from Boumort (Lleida), deposited at Institut Català de Paleontologia, has been collected by A. Gort. We thank M. Fernández for preparing the carcasses and for making the histological sections of this study. Our research was supported by a fellowship from the Spanish Ministry of Education (T. Calderón BES‐2016‐078938) and by the Spanish Ministry of Economy and Competitiveness (M. Köhler CGL‐2015‐63777 and D. DeMiguel CGL2016‐76431‐P), and CERCA Program. The research is recognized without financial support by the Government of Catalonia (2017 SGR 960).
References
- Amson E, Kolb C, Scheyer TM, et al. (2015) Growth and life history of Middle Miocene deer (Mammalia, Cervidae) based on bone histology. C R Palevol 14, 637–645. [Google Scholar]
- Anders U, von Koenigswald W, Ruf I, et al. (2011) Generalized individual dental age stages for fossil and extant placental mammals. Palaontol Z 85, 321–339. [Google Scholar]
- de Andrade RCP, Bantim RAM, de Lima FH, et al. (2015) New data about the presence and absence of the external. Cad Cult Ciência 14, 200–211. [Google Scholar]
- Azorit C (2011) Guía para la determinación de la edad del ciervo ibérico (Cervus elaphus hispanicus) a través de su dentición: revisión metodológica y técnicas de elección. Anales 24, 235–264. [Google Scholar]
- Azorit C, Analla M, Carrasco R, et al. (2002) Teeth eruption pattern in red deer (Cervus elaphus hispanicus) in southern Spain. An Biol 24, 107–114. [Google Scholar]
- Azorit C, Muñoz‐Cobo J, Hervás J, et al. (2004) Aging through growth marks in teeth of Spanish red deer. Wildl Soc Bull 32, 702–710. [Google Scholar]
- Brown WAB, Chapman NG (1991) Age assessment of red deer (Cervus elaphus): from a scoring scheme based on radiographs of developing molariform teeth. J Zool 224, 367–379. [Google Scholar]
- Carden RF, Hayden TJ (2002) Epiphyseal fusion in the postcranial skeleton as an indicator of age at death of European Fallow Deer (Dama dama dama, Linnaeus, 1758) In: Recent Advances in Ageing and Sexing Animal Bones. (ed. Ruscillo D.), pp. 227–236. Oxford: Oxbow Books, Park End Place; Retrieved from http://www.librarything.fr/work.php?book=8793418 [Google Scholar]
- Carranza J (2017) Ciervo – Cervus elaphus Linnaeus, 1758 In: Enciclopedia Virtual de los Vertebrados Españoles (eds Salvador A, Barja I.), p. 34 Madrid: Museo Nacional de Ciencias Naturales. [Google Scholar]
- Carranza J, Salinas M, de Andrés D, et al. (2016) Iberian red deer: paraphyletic nature at mtDNA but nuclear markers support its genetic identity. Ecol Evol 6, 905–922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castanet J (2006) Time recording in bone microstructures of endothermic animals; functional relationships. CR Palevol 5, 629–636. [Google Scholar]
- Castanet J, Croci S, Aujard F, et al. (2004) Lines of arrested growth in bone and age estimation in a small primate: Microcebus murinus . J Zool 263, 31–39. [Google Scholar]
- Cederlund G, Kjellander P, Stålfelt F (1991) Age determination of roe deer by tooth wear and cementum layers‐tests with known age material In 20th Congress of the International Union of Game Biologists, pp. 540–545. Gödöllö, Hungary. [Google Scholar]
- Chinsamy‐Turan A (2005) The Microstructure of Dinosaur Bone. Baltimore: The Johns Hopkins University Press. [Google Scholar]
- Clutton‐Brock TH, Guiness FE, Albon SD (1982) Red Deer: Behaviour and Ecology of Two Sexes. Chicago: The University of Chicago Press. [Google Scholar]
- Clutton‐Brock T, Guinness FE, Albon SD (1983) The costs of reproduction to red deer hinds. J Anim Ecol 52, 367–383. [Google Scholar]
- Cooper SM, Sieckenius SS, Silva AL (2013) Dentine method: aging white‐tailed deer by tooth measurements. Wildl Soc Bull 37, 451–457. [Google Scholar]
- Davis SJM (2000) The effect of castration and age on the development of the Shetland sheep skeleton and a metric comparison between bones of males, females and castrates. J Archaeol Sci 27, 373–390. [Google Scholar]
- De Margerie E, Cubo J, Castanet J (2002) Bone typology and growth rate: testing and quantifying ‘Amprino's rule’ in the mallard (Anas platyrhynchos). C R Biol 325, 221–230. [DOI] [PubMed] [Google Scholar]
- Dmitriew CM (2011) The evolution of growth trajectories: what limits growth rate? Biol Rev 86, 97–116. [DOI] [PubMed] [Google Scholar]
- Flinn EB, Strickland BK, Demarais S, et al. (2013) Age and gender affect epiphyseal closure in white‐tailed deer. Southeast Nat 12, 297–306. [Google Scholar]
- Francillon‐Vieillot H, de Buffrénil V, Castanet J, et al. (1990) Microstructural and mineralization of vertebral skeletal tissues In: Skeletal Biommineralization: Patterns, Processes and Evolutionary Trends (ed. Carter JG.), pp. 473–530. New York: Van Nostrand Reinhold. [Google Scholar]
- García‐Martínez R, Marín‐Moratalla N, Jordana X, et al. (2011) The ontogeny of bone growth in two species of dormice: reconstructing life history traits. C R Palevol 10, 489–498. [Google Scholar]
- Garde J, Fernández‐Santos MR, Soler AJ, et al. (2010) Ciervo ibérico (Cervus elaphus hispanicus, Hilzheimer, 1909) In: Ungulados silvestres de España : biología y tecnologías reproductivas para su conservación y aprovechamiento cinegético (ed. INIA ), pp. 157–178. Madrid: Ministerio de Ciencia e Innovación. [Google Scholar]
- Huttenlocker AK, Woodward H, Hall BK (2013) The biology of bone In: Bone Histology Offossil Tetrapods (eds Padian K, Lamm E‐T.), pp. 13–34. Berkeley: University of California Press. [Google Scholar]
- Ibler B, Fischer K (2016) Costs of reproduction. A demographical approach to examine life‐history trade‐offs in two old‐world deer species. Mamm Biol 81, 455–463. [Google Scholar]
- Jordana X, Marín‐Moratalla N, Moncunill‐Solè B, et al. (2016) Ontogenetic changes in the histological features of zonal bone tissue of ruminants: a quantitative approach. C R Palevol 15, 265–276. [Google Scholar]
- Köhler M, Marin‐Moratalla N, Jordana X, et al. (2012) Seasonal bone growth and physiology in endotherms shed light on dinosaur physiology. Nature 487, 358–361. [DOI] [PubMed] [Google Scholar]
- Kolb C, Scheyer TM, Lister AM, et al. (2015a) Growth in fossil and extant deer and implications for body size and life history evolution. BMC Evol Biol 15, 19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolb C, Scheyer TM, Veitschegger K, et al. (2015b) Mammalian bone palaeohistology: a survey and new data with emphasis on island forms. PeerJ 3, e1358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee AH, Huttenlocker AK, Padian K, et al. (2013) Analysis of growth rates In: Bone Histology of Fossil Tetrapods (eds Padian K, Lamm E‐T.), pp. 217–264. Berkeley: University of California Press. [Google Scholar]
- Mariezkurenna K (1983) Contribución al conocimiento del desarrollo de dentición y el esqueleto postcraneal de Cervus elaphus . Munibe 35, 149–202. [Google Scholar]
- Marín‐Moratalla N, Jordana X, García‐Martínez R, et al. (2011) Tracing the evolution of fitness components in fossil bovids under different selective regimes. C R Palevol 10, 469–478. [Google Scholar]
- Marín‐Moratalla N, Jordana X, Köhler M (2013) Bone histology as an approach to providing data on certain key life history traits in mammals: implications for conservation biology. Mamm Biol 78, 422–429. [Google Scholar]
- Marín‐Moratalla N, Cubo J, Jordana X, et al. (2014) Correlation of quantitative bone histology data with life history and climate: a phylogenetic approach. Biol J Linn Soc 112, 678–687. [Google Scholar]
- McNab BK (2010) Geographic and temporal correlations of mammalian size reconsidered: a resource rule. Oecologia 164, 13–23. [DOI] [PubMed] [Google Scholar]
- Mitchell B (1967) Growth layers in dental cement for determining the age of red deer (Cervus elaphus L.). J Anim Ecol 36, 279–293. [Google Scholar]
- Mitchell B, Staines CW, Welch D (1977) Ecology of Red Deer. A Research Review Relevant to Their Management in Scotland. Cambridge: Institute of Terrestrial Ecology. [Google Scholar]
- Moncunill‐Solé B, Orlandi‐Oliveras G, Jordana X, et al. (2016) First approach of the life history of Prolagus apricenicus (Ochotonidae, Lagomorpha) from Terre Rosse sites (Gargano, Italy) using body mass estimation and paleohistological analysis. C R Palevol 15, 235–245. [Google Scholar]
- Nacarino‐Meneses C, Köhler M (2018) Limb bone histology records birth in mammals. PLoS One 13, e0198511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nacarino‐Meneses C, Jordana X, Köhler M (2016a) First approach to bone histology and skeletochronology of Equus hemionus . C R Palevol 15, 277–287. [Google Scholar]
- Nacarino‐Meneses C, Jordana X, Köhler M (2016b) Histological variability in the limb bones of the Asiatic wild ass and its significance for life history inferences. PeerJ 4, e2580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Connor T (2000) The Archaeology of Animal Bones. Stroud: Sutton Publishing. [Google Scholar]
- Olea L, San Miguel‐Ayanz A (2006) The Spanish dehesa. A traditional Mediterranean silvopastoral system linking production and nature conservation. 21st Gen Meet Eur Grassl Fed 11, 1–13. [Google Scholar]
- Orlandi‐Oliveras G, Jordana X, Moncunill‐Solé B, et al. (2016) Bone histology of the giant fossil dormouse Hypnomys onicensis (Gliridae, Rodentia) from Balearic Islands. C R Palevol 15, 247–253. [Google Scholar]
- Orlandi‐Oliveras G, Nacarino‐Meneses C, Koufos GD, et al. (2018) Bone histology provides insights into the life history mechanisms underlying dwarfing in hipparionins. Sci Rep 8, 1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Padian K, Lamm E‐T, Werning S (2013) Selection of specimens In: Bone Histology Offossil Tetrapods (eds Padian K, Lamm E‐T.), pp. 35–54. Berkeley: University of California Press. [Google Scholar]
- Palkovacs EP (2003) Explaining adaptive shifts in body size on islands: a life history approach. Oikos 103, 37–44. [Google Scholar]
- Popkin PRW, Baker P, Worley F, et al. (2012) The Sheep Project (1): determining skeletal growth, timing of epiphyseal fusion and morphometric variation in unimproved Shetland sheep of known age, sex, castration status and nutrition. J Archaeol Sci 39, 1775–1792. [Google Scholar]
- Purdue JR (1983) Epiphyseal closure in white‐tailed deer. J Wildl Manage 47, 1207–1213. [Google Scholar]
- Rosvold J, Herfindal I, Andersen R, et al. (2014) Long‐term morphological changes in the skeleton of red deer (Artiodactyla, Cervidae) at its northern periphery. J Mammal 95, 626–637. [Google Scholar]
- Ruddle JL (1997) An Investigation of Bone Histology as a Potential Age Indicator in Roe Deer. London: University College London; http://discovery.ucl.ac.uk/1317650/ [Google Scholar]
- Skog A, Zachos FE, Rueness EK, et al. (2009) Phylogeography of red deer (Cervus elaphus) in Europe. J Biogeogr 36, 66–77. [Google Scholar]
- Stearns SC (1992) The Evolution of Life Histories. New York: Oxford University Press. [Google Scholar]
- Trut L (1999) Early canid domestication: the farm‐fox experiment. Am Sci 87, 160. [Google Scholar]
- Turner TR, Cramer JD, Nisbett A, et al. (2016) A comparison of adult body size between captive and wild vervet monkeys (Chlorocebus aethiops sabaeus) on the island of St. Kitts. Primates 57, 211–220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veiberg V, Loe LE, Mysterud A, et al. (2007) The ecology and evolution of tooth wear in red deer and moose. Oikos 116, 1805–1818. [Google Scholar]
- Woodward HN, Padian K, Lee AH (2013) Skeletochronology In: Bone Histology Offossil Tetrapods (eds Padian K, Lamm E‐T.), pp. 195–216. Berkeley: University of California Press. [Google Scholar]
- Zachos FE, Hartl GB (2011) Phylogeography, population genetics and conservation of the European red deer Cervus elaphus . Mamm Rev 41, 138–150. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig. S1. Superimposition of femora of 13‐year‐old (IPS‐56307) and 5‐year‐old females (IPS‐56304). Red line indicates the first CGM lost by the expansion of the medullar cavity. Blue lines are two CGM partially eroded within FLC. Scale bar: 2 mm.
Fig. S2. Histological detail of the anterior part of the tibia of the young male IPS‐109289. The change of colour indicates the shift from woven‐fibred to parallel‐fibred bone caused by the slowdown in growth at the beginning of the winter season. Scale bar: 200 μm. Image obtained under polarized light with a λ filter.


