Abstract
Background
Two randomized, double-blind, sham-controlled trials (ACT1, ACT2) evaluated non-invasive vagus nerve stimulation (nVNS) as acute treatment for cluster headache. We analyzed pooled ACT1/ACT2 data to increase statistical power and gain insight into the differential efficacy of nVNS in episodic and chronic cluster headache.
Methods
Data extracted from ACT1 and ACT2 were pooled using a fixed-effects model. Main outcome measures were the primary endpoints of each study. This was the proportion of participants whose first treated attack improved from moderate (2), severe (3), or very severe (4) pain intensity to mild (1) or nil (0) for ACT1 and the proportion of treated attacks whose pain intensity improved from 2–4 to 0 for ACT2.
Results
The pooled population included 225 participants (episodic: n = 112; chronic: n = 113) from ACT1 (n = 133) and ACT2 (n = 92) in the nVNS (n = 108) and sham (n = 117) groups. Interaction was shown between treatment group and cluster headache subtype (p < 0.05). nVNS was superior to sham in episodic but not chronic cluster headache (both endpoints p < 0.01). Only four patients discontinued the studies due to adverse events.
Conclusions
nVNS is a well-tolerated and effective acute treatment for episodic cluster headache.
Trial registration
The studies were registered at clinicaltrials.gov (ACT1: NCT01792817; ACT2: NCT01958125).
Keywords: Episodic cluster headache, chronic cluster headache, non-invasive vagus nerve stimulation, acute treatment, neuromodulation, meta-analysis
Introduction
Cluster headache is a highly disabling brain disorder typically characterized by attacks of excruciating unilateral headache associated with ipsilateral cranial autonomic features and often an urge to move (1). Attacks last from 15 minutes to 3 hours and may occur up to eight times a day, frequently at night (2). About 80% of patients have episodic cluster headache, in which attacks come in periods lasting from 1 week to several months and are separated by attack-free periods of more than 1 month. The remaining 20% experience chronic cluster headache, in which attacks continue to recur for more than 1 year without attack-free periods longer than a month (3).
Research into differential treatment effects among episodic and chronic cluster headache subtypes warrants further attention in clinical trials. Cluster headache therapy for the acute treatment of both subtypes should preferably have a low adverse event profile and be easy to use and must be fast acting because the pain builds up quickly and is extremely severe. Current acute treatments for cluster headache, although limited, have shown efficacy, but clinical disadvantages include daily dosing limitations (1), cardiovascular contraindications (4), inconvenience, and/or unavailability. Pain-free rates of up to 49% (subcutaneous sumatriptan) at 15 minutes have been reported in clinical studies of triptans for the acute treatment of cluster headache (5), and inhalation of 100% oxygen was shown to relieve attacks at 15 minutes in 78% of patients (6). Results for episodic and chronic cluster headache subtypes are not commonly evaluated separately, likely because of the much smaller chronic cluster headache subgroups typically enrolled (7–9). Studies that separately evaluated both subtypes demonstrated higher treatment effects in episodic cluster headache than in chronic cluster headache but were not powered to dissect any differential effects (7,10,11). Consistent with these findings, recent data suggested that a monoclonal antibody targeting the calcitonin gene-related peptide (CGRP) ligand was ineffective for the preventive treatment of chronic cluster headache but effective for preventing attacks in episodic cluster headache (12).
Non-invasive vagus nerve stimulation (nVNS; gammaCore; electroCore, Inc., Basking Ridge, NJ, USA) is a novel, easy-to-use, non-invasive neuromodulation treatment option for cluster headache (13). In ACT1, acute nVNS treatment showed efficacy in participants with episodic cluster headache but not in the chronic cluster headache subgroup or in the total population that included all participants of both subtypes (11). The ACT1 trial was underpowered for analysis of the differential effect size in episodic and chronic cluster headache separately. A further study using the same nVNS and sham devices (ACT2) had similar results (10). The study designs and populations of ACT1 and ACT2 were similar, particularly in terms of sham and active stimulation and data collection. This allowed for pooled data analysis and, because of greater statistical power, formal verification of possible differential effects of nVNS in episodic and chronic cluster headache. Given the rarity of the disorders, combined analysis of both studies may also provide a more complete depiction of the efficacy, tolerability, and application options of nVNS in the two different cluster headache subtypes. We present here a pooled data analysis of both studies, focusing on the possibility of differential effects in episodic and chronic cluster headache.
Methods
Study design and participants
We pooled and analyzed the data of all 225 participants (age 18 years or older) with episodic (n = 112) or chronic (n = 113) cluster headache (2) from two prospective, randomized (ratio 1:1), double-blind, sham-controlled, multicenter clinical trials. Demographic data for both trials were self-reported by participants and were validated by the investigator. The trials had similar designs with a few exceptions. The main difference was the primary endpoint. For ACT1, this was “the proportion of participants whose first treated attack had improved (on a 5-point pain intensity scale) from pain intensity of moderate (2), severe (3), or very severe (4) to mild (1) or nil (0) at 15 minutes after treatment initiation”. For ACT2, the primary outcome was “the proportion of all treated attacks that had improved from pain intensity 2–4 to 0 at 15 minutes after treatment initiation for that attack”. ACT1 had a 1-month double-blind period followed by a 3-month open-label phase, whereas ACT2 had a 1-week run-in period followed by a 2-week double-blind period and then a 2-week open-label phase. In both studies, participants had to treat attacks as soon as possible after onset with three consecutive 120-second applications of nVNS. In ACT2, however, participants could apply up to three additional stimulations if not pain-free at 9 minutes after initiation of the first treatment. Finally, although in ACT1 all treatments were applied to the right cervical vagus nerve, in ACT2 the participants were encouraged to treat ipsilateral to the pain.
Both studies were conducted in accordance with the Declaration of Helsinki, Good Clinical Practice, and local laws. The protocols were reviewed by the appropriate national regulatory agency for each site, and additional reviews were completed by regional or local independent ethics committees as required. The studies were registered at ClinicalTrials.gov (ACT1 (11): NCT01792817 and ACT2 (10): NCT01958125), and all participants had signed informed consent forms prior to study inclusion.
Data sources
We pooled the final data sets from ACT1 and ACT2 and used the full analysis set to analyze all efficacy endpoints and adverse events in the total population and separately in the episodic and chronic cluster headache subgroups.
Statistical analyses
We used logistic regression models to estimate odds ratios and associated 95% confidence intervals (95% CIs) for (i) the proportion of participants whose first treated attack had improved from pain intensity 2–4 to 0–1 at 15 minutes after treatment initiation (the ACT1 primary endpoint) and (ii) the proportion of participants in whom ≥ 50% of all treated attacks had improved from pain intensity 2–4 to 0–1 at 15 minutes after treatment initiation. Analyses resulting in pooled estimates included site as a covariate in the logistic regression models.
Generalized linear mixed-effects regression models (with logit link and binomial response distribution) were used to estimate the proportion of all treated attacks that had improved from pain intensity 2–4 to 0 at 15 minutes after treatment initiation for that attack (the ACT2 primary endpoint). Population averaged/marginal models (to account for repeated headache attacks within patient) were utilized, and the structure of the covariance matrix was specified as compound symmetry. p values for comparisons between the nVNS and sham groups were determined from resulting F tests.
Individual participant data were used in the meta-analysis. Fixed effects meta-analysis models were used to estimate the pooled effects of nVNS treatment, given the small number of studies being pooled and because the ACT1 and ACT2 studies were homogeneous for participant populations and results. In addition, there was no evidence of treatment by study interactions for any of the outcomes examined.
In the meta-analysis models, interactions between treatment group and cluster headache subgroup were examined to determine whether the magnitude of treatment effect varied significantly by cluster headache subtype.
First-order interactions between treatment group and cluster headache subgroup were examined to determine whether the magnitude of treatment effect varied significantly by cluster headache subtype.
Two-sided p values < 0.05 were considered statistically significant. p values are provided for all efficacy analyses without adjustment for multiple comparisons. Data were analyzed using SAS 9.4 (SAS Institute Inc., Cary, NC, USA).
Results
In total, we included 225 participants in the pooled efficacy analysis, 133 from ACT1 and 92 from ACT2, who comprised the intent-to-treat populations and were randomly assigned to nVNS (n = 108) or sham (n = 117). Demographic and baseline characteristics were reported for the pooled safety population and did not differ between the nVNS and sham treatment groups (Table 1). Participants with chronic cluster headache were about 3 years older at disease onset than participants with episodic cluster headache (p < 0.05).
Table 1.
Demographic and baseline characteristics (pooled analysis).
| Characteristic | By treatment group |
By cluster headache subgroup |
||
|---|---|---|---|---|
| nVNS (n = 124) | Sham (n = 129) | Episodic (n = 131) | Chronic (n = 122) | |
| Age, mean (SD), years | 45.4 (12.4)a | 47.8 (11.2)b | 47.3 (12.4)c | 45.9 (11.1)d |
| Male, n (%) | 94 (76) | 105 (81) | 106 (81) | 93 (76) |
| Ethnic origin, n (%)e | ||||
| Asian | 5 (4) | 1 (1) | 4 (3) | 2 (2) |
| Black | 5 (4) | 7 (5) | 9 (7) | 3 (3) |
| White | 113 (91) | 120 (93) | 117 (89) | 116 (95) |
| Missing | 1 (1) | 1 (1) | 1 (1) | 1 (1) |
| Cluster headache type, n (%) | ||||
| Episodic cluster headache | 65 (52) | 66 (51) | 131 (100) | 0 |
| Chronic cluster headache | 59 (48) | 63 (49) | 0 | 122 (100) |
| Age at cluster headache onset, mean (SD), years | 30.8 (13.8)a | 33.5 (13.2)b | 30.7 (13.9)c | 33.9 (13.0)d |
| Treatments used to manage cluster headache, n (%) | ||||
| Acute n/N (%) | 114/123 (93) | 121/125 (97) | 121/130 (93) | 114/118 (97) |
| Prophylactic n/N (%) | 75/123 (61) | 92/125 (74) | 83/130 (64) | 84/118 (71) |
| ACT1 population, No. (%) | 73 (59) | 77 (60) | 101 (77) | 49 (40) |
| ACT2 population, No. (%) | 51 (41) | 52 (40) | 30 (23) | 73 (60) |
n/N: number of subjects using treatment/total number of subjects with data; nVNS: non-invasive vagus nerve stimulation; SD: standard deviation.
an = 108; bn = 115; cn = 120; dn = 103.
ePercentages may not add up to 100% because of rounding.
Between–cluster headache type interactions
There was a first-order interaction between treatment group and cluster headache subtype (p < 0.05) in models estimating the ACT1 and ACT2 primary endpoints in the ACT1, ACT2, and pooled populations (Table 2). Thus, results are presented overall and by cluster headache subtype.
Table 2.
Interaction model results.
| ACT1 Primary endpoint | Estimate | SE | Wald Chi-square | Pr > ChiSq |
|---|---|---|---|---|
| ACT1 | 0.5290 | 0.2428 | 4.7468 | 0.0294 |
| ACT2 | 0.4611 | 0.2683 | 2.9534 | 0.0857 |
| Pooled | 0.4588 | 0.1642 | 7.8111 | 0.0052 |
| ACT2 Primary endpoint |
Estimate | SE | t value | Pr > |t| |
| ACT1 | 2.1267 | 0.9507 | 2.24 | 0.0270 |
| ACT2 | 2.1778 | 1.0512 | 2.07 | 0.0412 |
| Pooled | 1.9577 | 0.6403 | 3.06 | 0.0025 |
SE: standard error.
Treatment response at 15 minutes in first treated attack
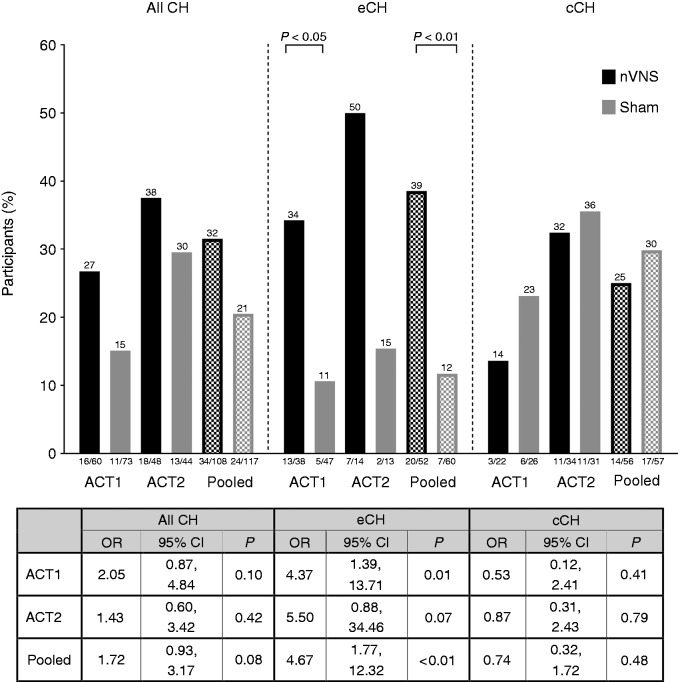
Among participants with episodic cluster headache, more participants treated with nVNS than with sham achieved the ACT1 primary endpoint: Improvement of the first treated attack at 15 minutes to pain intensity 0–1 in the ACT1 (absolute difference, 24%; p = 0.01) and pooled (absolute difference, 27%; p < 0.01) study populations, but not in the ACT2 study population (absolute difference, 35%; p = 0.07; Figure 1).
Figure 1.
Proportion of participants who responded at 15 minutes for the first treated attack.
cCH: chronic cluster headache; CH: cluster headache; CI: confidence interval; eCH: episodic cluster headache; nVNS: non-invasive vagus nerve stimulation; OR: odds ratio.
Note: p values are from logistic regression; pooled analyses included study as a covariate.
Proportion of all attacks pain-free at 15 minutes
Among participants with episodic cluster headache, the proportion of all treated attacks that had improved at 15 minutes to pain-free (the ACT2 primary endpoint) was higher in the nVNS-treated group than in the sham-treated group for ACT1 (absolute difference, 9%; p < 0.05), ACT2 (absolute difference, 41%; p < 0.05), and pooled (absolute difference, 22%; p < 0.01) study populations (Figure 2).
Figure 2.
Proportion of all treated attacks that achieved pain-free status at 15 minutes.
cCH: chronic cluster headache; CH: cluster headache; CI: confidence interval; eCH: episodic cluster headache; GEE: generalized estimating equation; nVNS: non-invasive vagus nerve stimulation.
Note: p values are from F tests; pooled analyses included study as a fixed effect. Graphed data are GEE model adjusted percentages with 95% CIs. Data for number of subjects using treatment/total number of subjects with data (n/N) attacks are unadjusted numbers.
Key secondary endpoints
The proportion of participants who were pain-free at 15 minutes in ≥ 50% of their treated attacks was higher for nVNS than for sham (absolute difference, 18%; p = 0.01) in participants with episodic cluster headache in the pooled study population (Figure 3(a)). The absolute difference for this endpoint in episodic cluster headache was 14% (p = 0.05) in the ACT1 study population and 28% (p = 0.11) in the ACT2 population. There were no other treatment differences for this endpoint in any of the other study populations. The proportion of participants who were pain-free or with mild pain at 15 minutes in ≥ 50% of their treated attacks was higher for nVNS than for sham in participants with episodic cluster headache in the ACT1 (absolute difference, 19%; p = 0.04), ACT2 (absolute difference, 49%; p = 0.02), and pooled (absolute difference, 27%; p < 0.01) study populations. The ≥ 50% response rates for pain freedom or mild pain at 15 minutes also favored nVNS in the total population of the ACT2 (absolute difference, 26%; p < 0.01) and pooled (absolute difference, 14%; p = 0.01) study populations (Figure 3(b)).
Figure 3.
Pain-free (a) or responder (b) status at 15 minutes for ≥ 50% of attacks.
cCH: chronic cluster headache; CH: cluster headache; CI: confidence interval; eCH: episodic cluster headache; nVNS: non-invasive vagus nerve stimulation; OR: odds ratio.
Note: OR > 1 favors nVNS. p values are from logistic regression; pooled analyses included study as a covariate.
Adverse events
Thirty-eight participants in the nVNS group and 45 participants in the sham group experienced at least one adverse event (Table 3). Two participants in the nVNS group had at least one serious adverse event (SAE): One participant in ACT1 reported exacerbations of cluster headache and one in ACT2 reported lower abdominal and back pain. In the sham group of ACT2, one participant reported anxiety and depression as an SAE. Among the pooled adverse event data, the most common adverse device effect of nVNS was perioral muscle contraction during treatment, with all other adverse device effects that were reported by ≥ 5% of participants (i.e. most common) occurring in the sham group. In ACT1, the most common adverse device effects of nVNS were lip or facial drooping, pulling, and twitching (i.e. occurring in ≥ 5% of participants). In ACT2, application site irritation, application site paresthesia, and skin irritation were the most common adverse device effects of nVNS (i.e. occurring in > 1 participant). There were no serious adverse device effects.
Table 3.
Incidence of adverse events, serious adverse events, and adverse device effects (pooled data).
| AEs and ADEs | nVNS (n = 123)a | Sham (n = 129) |
|---|---|---|
| Participants with ≥ 1 AE, n (%) | 38 (31) | 45 (35) |
| Participants with ≥ 1 SAE, n (%) | 2 (2)b,c | 1 (1)c,d |
| Participants with ≥ 1 ADE, n (%) | 20 (16) | 34 (26) |
| ADEs occurring in ≥ 5% of participants in either treatment group, n (%) | ||
| Dysgeusia | 0 | 8 (6) |
| Erythema at treatment site | 0 | 9 (7) |
| Perioral myokymia during treatment | 8 (7) | 0 |
ADE: adverse device effect; AE: adverse event; CH: cluster headache; nVNS: non-invasive vagus nerve stimulation; SAE: serious adverse event.
One patient from the safety population assigned to the nVNS group (n = 124) provided only baseline data and was therefore excluded from the safety analysis.
Included one participant with an SAE of CH (two occurrences) in ACT1 and one participant with SAEs of lower abdominal pain and back pain in ACT2.
SAEs were not considered related to the study device.
Included one participant with SAEs of anxiety and depression in ACT2.
Discussion
We pooled the data of two large clinical trials, ACT1 and ACT2, to enable both a formal analysis of acute efficacy separately for episodic and chronic cluster headache and a larger pooled analysis of tolerability. Meta-analysis of the entire population of 225 participants demonstrated significant efficacy of nVNS as an acute treatment for episodic cluster headache but not for the acute treatment of chronic cluster headache. The data show that repeated use of nVNS was well tolerated and that there were no obvious short-term safety concerns.
A recent mechanistic model of autonomic function provides further insights into the possible differential efficacy of nVNS observed between the two cluster headache subtypes. Reductions in kinetic oscillation stimulation–induced lacrimation in healthy subjects suggested that the symptom relief seen with nVNS in ACT1 and ACT2 was likely attributable to a bilateral inhibitory effect on parasympathetic output of the trigeminal autonomic reflex (14,15). The possibility of a differential parasympathetic response among patients with episodic and chronic cluster headache could play a role in the differential acute efficacy of nVNS between the episodic and chronic cluster headache subgroups in the ACT1 and ACT2 studies. Several other mechanistic studies were previously reported, suggesting that multiple distinct mechanisms may contribute to the efficacy of nVNS as an acute treatment. In animal models, two doses of vagus nerve stimulation (VNS) inhibited acute nociceptive activation of trigeminocervical neurons for up to 3 hours (16), and VNS attenuated pain responses and Fos protein expression (17). In healthy volunteers, nVNS caused activation of the nucleus tractus solitarius as measured with functional magnetic resonance imaging (18). Taken together, the beneficial acute effects of nVNS in episodic cluster headache seem biologically plausible.
There are a number of possible explanations for why patients with chronic cluster headache have a poorer response to acute treatment. Inter-paroxysmal pain is considerably more common in chronic than in episodic cluster headache (19), complicating achievement of pain-free status in the chronic subgroup. The presence of inter-paroxysmal pain was not measured in ACT1 or ACT2, representing a limitation of this pooled analysis. This information may have provided further insight into the observed differences among the two cluster headache subtypes. Spontaneous transitions from episodic to chronic cluster headache, and vice versa, do occur and are unpredictable (20,21). Given the cohort size, such transitions may have occurred in at least some participants, potentially influencing treatment response. A possible pathophysiological reason for a differential treatment response in episodic and chronic cluster headache involves differences in gray matter volume in pain processing areas (22). Such differences likely reflect the brain's adaptive capacity to different stimuli (23). There may also be differences in brain pharmacology between chronic and episodic cluster headache (24). Although establishing causal links to chronicity is challenging, such differences may contribute to disparity in treatment response. Central sensitization is considered essential for the pathologic mechanisms underpinning the chronification of primary headache disorders (25). The changes underlying treatment refractoriness in some patients with chronic cluster headache may involve consolidation of neuronal connections and networks in the disease mechanism.
Several similarities of ACT1 and ACT2, including participant characteristics, overlapping outcome measures, and use of the same nVNS and sham devices, allowed for pooled data evaluation of differential effects in episodic and chronic subtypes, but the trials also have differences. The double-blind period in ACT1 was 4 weeks, whereas in ACT2 it was 2 weeks to minimize exposure to sham. Because both studies were acute attack treatment trials and cluster headache attacks are clinically stable, this should not have been a major issue. The allowance of extra pulses in ACT2 may have contributed to the greater efficacy seen in ACT2. Application of three consecutive stimulations takes 6–8 minutes; given the 15-minute endpoint, the paradigm may have biased against a response in the total population. We also do not know whether the move from right-sided stimulation to stimulation ipsilateral to the pain was important. The anatomy suggests that the vagal afferents are bilateral. This may have increased the placebo rate, which would have rendered a positive outcome more challenging. Taken together, the similarities seem more relevant than the only minor dissimilarities, thus allowing for meta-analysis of both studies.
The results from this pooled analysis offer additional evidence for the clinical utility and advantages of nVNS in patients with cluster headache. Adverse events were mild, and there were no safety concerns during the trial. Long-term safety can be judged only after monitoring repeated use of nVNS over longer periods. The portability of nVNS and the fact that, in contrast to triptans, nVNS may be used more than three times per day and without major restrictions to co-medication highlight its practical utility. The ability to use the device in patients with cardiovascular contraindications to triptans is an important advantage of nVNS (4). In previous trials, nVNS therapy was associated with adherence rates ≥ 90% and treatment satisfaction rates ≥ 50% and was generally regarded as easy to use (13).
The apparent lack of acute nVNS efficacy in subjects with chronic cluster headache attacks is somewhat surprising, considering that clinical efficacy was shown when nVNS was used preventively in patients with chronic cluster headache (13). However, the combined ACT1 and ACT2 data, as well as recent data for a CGRP-targeted monoclonal antibody (12), reinforce previous data that suggest that acute (13,26) and preventive (27) treatments are more challenging in chronic than in episodic cluster headache. Consistent with this, a consensus statement from the European Headache Federation recommended amending the International Classification of Headache Disorders (2) to include a sub-classification for treatment of refractory chronic cluster headache (28).
The differential results in chronic versus episodic cluster headache have important implications for participant selection and minimum size of future therapeutic studies in cluster headache. Clinical trial design requirements, the challenges encountered selecting eligible participants, and patient motivation to enroll have generally shifted the selection of study participants toward an episodic subtype dominance (6). Attack numbers in patients with cluster headache often exceed limits for triptan therapy, making these patients more likely to overuse opioids and other analgesic medications (29), generally precluding inclusion in controlled trials. Combining participants with episodic and chronic cluster headache into one analysis may distort outcomes and, driven by the dominance of participants with the episodic subtype, may result in a potentially false conclusion that both groups are responders. Our analysis represents the first adequately powered analysis to assess the differential effect of a specific treatment between the two forms of cluster headache. The results underline the importance of redoubling efforts at developing further suitable acute treatments for patients with chronic cluster headache.
Conclusions
nVNS is effective in aborting attacks in episodic cluster headache. It does not show the same acute efficacy in patients with chronic cluster headache. The clearance of nVNS for the acute treatment of episodic cluster headache by the US Food and Drug Administration was, in part, based on findings from this analysis of the ACT1 and ACT2 studies. In all patients, nVNS is well tolerated. nVNS offers several advantages over existing treatment options, including its ease and flexibility of use and its ability to be used for as many attacks as the patient experiences per day, without restrictions to daily number of treatments and co-medications. nVNS is also not contraindicated in cardiovascular disease. Additional studies are needed to further elucidate the mechanism of action and possibly related reasons for failure in the acute treatment of chronic cluster headache, including potential studies of the effects of nVNS on parasympathetic output from the trigeminal autonomic reflex for patients with episodic and chronic cluster headache. This could lead to better understanding of the pathogenesis of cluster headache.
Clinical implications
This meta-analysis of data pooled from two randomized sham-controlled studies (N = 225) demonstrated significant benefits of acute non-invasive vagus nerve stimulation versus sham for subjects with episodic (n = 112) but not chronic cluster headache (n = 113).
Non-invasive vagus nerve stimulation has differential acute effects among the two cluster headache subtypes, with greater efficacy seen in episodic than in chronic cluster headache, although the mechanisms underlying these differential effects are currently unclear.
Acknowledgements
We thank the many patients with cluster headache who participated and have provided these data for their fellow sufferers. Annelie Andersson of electroCore, Inc., was responsible for coordination and execution of the ACT2 study. Medical writing support was provided by MedLogix Communications, LLC, in cooperation with the authors.
Author contributions
MDF and PJG had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis. Study concept and design: SDS, EL, SJT, MDF, PJG. Acquisition, analysis, or interpretation of data: All authors. Drafting of the manuscript: All authors. Approval of version to be published: All authors. Critical revision of the manuscript for important intellectual content: All authors. Statistical analysis: CKM. Obtained funding: EL. Administrative, technical, or material support: EL, PJG. Study supervision: PJG, SDS, EL.
Declaration of conflicting interests
The authors declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: SDS has received consultancy and advisory board fees from Abide Therapeutics, Alder Biopharmaceuticals, Allergan, Inc., Amgen, Avanir, Biohaven Pharmaceuticals, Cefaly, Curelator, Inc., Dr. Reddy’s Laboratories, Egalet Corporation, GlaxoSmithKline Consumer Health Holdings, LLC, eNeura, Impel NeuroPharma, Inc., Lilly USA, LLC, Medscape, LLC, Novartis, Inc., Satsuma Pharmaceuticals, Supernus, Teva Pharmaceuticals, Theranica, and Trigemina, Inc. DIF reports grants from Autonomic Technologies, Inc, other from Allergan, other from Avanir, grants and other from Eli Lilly, other from Zosano, other from Alder BioPharmaceuticals, other from Amgen, and grants from Merck outside the submitted work. CG has received honoraria from Allergan, Grünenthal, Desitin, Bayer, Boehringer Ingelheim, Teva GmbH, Reckitt Benckiser, Ratiopharm GmbH, Novartis, Lilly Deutschland, and Hormosan. CG has no ownership interests and does not own any pharmaceutical company stocks. CKM is an employee of North American Science Associates Inc. AT has received honoraria from Allergan, Inc. EL is an employee of electroCore, Inc., and receives stock ownership. SJT has received consultancy fees from Acorda, Alder BioPharmaceuticals, Alexza, Allergan, AlphaSights, Amgen, ATI, Avanir Pharma, Axsome Therapeutics, Charleston Laboratories, DeepBench, Dr. Reddy’s Laboratories, Eli Lilly, eNeura, Kimberly-Clark, Gerson Lehman Group, Guidepoint Global, Magellan Rx Management, Neurolief, Nordic BioTech, Novartis, Pernix, Pfizer, Satsuma, Scion NeuroStim, Slingshot Insights, Sorrento, Supernus, Teva Pharmaceuticals, WebMD, and Zosano. He receives royalties from Springer. He owns ATI stock options. He receives a salary from the American Headache Society. MDF has received consultancy fees from Medtronic and independent research support from the Netherlands Organization for Scientific Research (NWO), the European Community, ZonMw, and the Dutch Heart Foundation. PJG reports grants and personal fees from Allergan, Amgen, Eli-Lilly and Company, and eNeura, and personal fees from Alder BioPharmaceuticals, Avanir Pharma, Cipla Ltd, Dr Reddy’s Laboratories, Novartis, Pfizer Inc, Quest Diagnostics, Scion, Teva Pharmaceuticals, and Trigemina, Inc.; personal fees from MedicoLegal work, Journal Watch, Up-to-Date, Oxford University Press, Massachusetts Medical Society, and Wolters Kluwer; and a patent for magnetic stimulation for headache issued to eNeura without fee.
Funding
The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: The ACT1 and ACT2 studies were sponsored by electroCore, Inc. IFdC has received travel grants from electroCore, Inc. JCAM has received honoraria and travel grants from electroCore, Inc. SDS has been an investigator and has received consultancy and speaking fees from electroCore related to this work. DIF reports grants from electroCore, Inc. CG has received honoraria from electroCore, Inc. AT has received honoraria from electroCore, Inc. SJT has received consultancy fees from electroCore, Inc. PJG reports personal fees from electroCore, Inc., during the conduct of the study.
Role of the funder/sponsor
The funding source was involved in the design and conduct of the ACT1 and ACT2 studies; analysis and interpretation of the data; preparation, review, and approval of this and the ACT1 and ACT2 manuscripts; and the decision to submit the manuscripts for publication.
Co-investigators
ACT1 Study Group
Investigators are listed by study site at the time of the trial. Associated Neurologists of Southern Connecticut, Fairfield, CT – Peter J McAllister; California Medical Clinic for Headache, Santa Monica, CA – David B Kudrow; Carolina Headache Institute, Durham, NC – Anne Calhoun; Cleveland Clinic Foundation, Cleveland, OH – Stewart J Tepper; Clinvest Headache Care Center, Springfield, MO – Roger Cady; Colorado Neurological Institute, Englewood, CO – Cori Millen-Schnurr; Dent Neurologic Institute, Amherst, NY – Laszlo Mechtler; Diamond Headache Clinic, Chicago, IL – Alexander Feoktistov; Jefferson Headache Center, Philadelphia, PA – Stephen D Silberstein; Michigan Head Pain & Neurological Institute, Ann Arbor, MI – Joel Saper; Mid-Atlantic Headache Institute, Pikesville, MD – Marcia C Ribeiro; Montefiore Headache Center, Bronx, NY – Brian Grosberg; New England Regional Headache Center, Worchester, MA – Herbert G Markley; Norton Neuroscience Institute Headache and Concussion Center, Louisville, KY – Brian M Plato; Stanford University Medical Center, Department of Neurology, Stanford, CA – Sheena K Aurora; Tampa General Hospital Headache and Pain Center, Tampa, FL – Maria-Carmen Wilson; The Center for Headache Care and Research/Island Neurological Associates, PC, Plainview, NY – Ira Turner; University of Iowa Hospital and Clinics, Iowa City, IA – Connie Pieper; UT Southwestern Medical Center, Department of Neurology and Neurotherapeutics, Dallas, TX – Deborah L Friedman; West Virginia University Hospitals, Department of Neurology, Morgantown, WV – David B Watson.
ACT2 Study Group
Investigators are listed by study site at the time of the trial. Royal Free Hospital, London NHS Foundation Trust, London, UK – Peter J Goadsby, Juana CA Marin; Migraine and Headache Clinic, Königstein, Germany – Charly Gaul; Leiden University Medical Centre, Leiden, The Netherlands – Michel D Ferrari, Ilse F de Coo; The Walton Centre, Liverpool, UK – Nicolas Silver; The Southern Hospital, Glasgow, UK – Alok Tyagi; Hull Royal Infirmary, Hull, UK – Fayyaz Ahmed; Glostrup Hospital, Glostrup, Denmark – Rigmor Højland Jensen; West German Headache Center, Essen, Germany – Hans Diener, Kasja Rabe; University of Munich, Munich, Germany – Andreas Straube.
Data sharing statement
Data from the ACT1 and ACT2 studies will be publicly available at ClinicalTrials.gov with the identifiers NCT01792817 and NCT01958125, respectively. Individual participant data will not be shared.
References
- 1.May A. Cluster headache: Pathogenesis, diagnosis, and management. Lancet 2005; 366: 843–855. [DOI] [PubMed] [Google Scholar]
- 2.Headache Classification Committee of the International Headache Society (IHS). The International Classification of Headache Disorders, 3rd edition (beta version). Cephalalgia 2013; 33: 629–808. [DOI] [PubMed] [Google Scholar]
- 3.Nesbitt AD, Goadsby PJ. Cluster headache. BMJ 2012; 344: e2407. [DOI] [PubMed] [Google Scholar]
- 4.Dodick D, Lipton RB, Martin V, et al. Consensus statement: Cardiovascular safety profile of triptans (5-HT agonists) in the acute treatment of migraine. Headache 2004; 44: 414–425. [DOI] [PubMed] [Google Scholar]
- 5.Ekbom K, Monstad I, Prusinski A, et al. Subcutaneous sumatriptan in the acute treatment of cluster headache: A dose comparison study. The Sumatriptan Cluster Headache Study Group. Acta Neurol Scand 1993; 88: 63–69. [DOI] [PubMed] [Google Scholar]
- 6.Cohen AS, Burns B, Goadsby PJ. High-flow oxygen for treatment of cluster headache: A randomized trial. JAMA 2009; 302: 2451–2457. [DOI] [PubMed] [Google Scholar]
- 7.Cittadini E, May A, Straube A, et al. Effectiveness of intranasal zolmitriptan in acute cluster headache: A randomized, placebo-controlled, double-blind crossover study. Arch Neurol 2006; 63: 1537–1542. [DOI] [PubMed] [Google Scholar]
- 8.Rapoport AM, Mathew NT, Silberstein SD, et al. Zolmitriptan nasal spray in the acute treatment of cluster headache: A double-blind study. Neurology 2007; 69: 821–826. [DOI] [PubMed] [Google Scholar]
- 9.van Vliet JA, Bahra A, Martin V, et al. Intranasal sumatriptan in cluster headache: Randomized placebo-controlled double-blind study. Neurology 2003; 60: 630–633. [DOI] [PubMed] [Google Scholar]
- 10.Goadsby PJ, de Coo IF, Silver N, et al. Non-invasive vagus nerve stimulation for the acute treatment of episodic and chronic cluster headache: A randomized, double-blind, sham-controlled ACT2 study. Cephalalgia 2018; 38: 959–969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Silberstein SD, Mechtler LL, Kudrow DB, et al. Non-invasive vagus nerve stimulation for the acute treatment of cluster headache: Findings from the randomized, double-blind, sham-controlled ACT1 study. Headache 2016; 56: 1317–1332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Martinez J, Goadsby P, Dodick D, et al. Study CGAL: A placebo-controlled study of galcanezumab in patients with episodic cluster headache: Results from the 8-week double-blind treatment phase [MTIS2018-176]. Cephalalgia 2018; 38: S145–S146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gaul C, Diener HC, Silver N, et al. Non-invasive vagus nerve stimulation for PREVention and Acute treatment of chronic cluster headache (PREVA): A randomised controlled study. Cephalalgia 2016; 36: 534–546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Möller M, Schroeder CF, May A. Vagus nerve stimulation modulates the cranial trigeminal autonomic reflex. Ann Neurol 2018; 84: 886–892. [DOI] [PubMed] [Google Scholar]
- 15.Schroeder CF, Möller M, May A. nVNS sham stimulation significantly affects the trigeminal-autonomic reflex: A single-blind, randomized, controlled study. Neurology. In press. [DOI] [PubMed] [Google Scholar]
- 16.Akerman S, Simon B, Romero-Reyes M. Vagus nerve stimulation suppresses acute noxious activation of trigeminocervical neurons in animal models of primary headache. Neurobiol Dis 2017; 102: 96–104. [DOI] [PubMed] [Google Scholar]
- 17.Bohotin C, Scholsem M, Bohotin V, et al. Vagus nerve stimulation attenuates heat- and formalin-induced pain in rats. Neurosci Lett 2003; 351: 79–82. [DOI] [PubMed] [Google Scholar]
- 18.Frangos E, Komisaruk BR. Access to vagal projections via cutaneous electrical stimulation of the neck: fMRI evidence in healthy humans. Brain Stimul 2017; 10: 19–27. [DOI] [PubMed] [Google Scholar]
- 19.Marmura MJ, Pello SJ, Young WB. Interictal pain in cluster headache. Cephalalgia 2010; 30: 1531–1534. [DOI] [PubMed] [Google Scholar]
- 20.Manzoni GC, Taga A, Russo M, et al. Age of onset of episodic and chronic cluster headache – a review of a large case series from a single headache centre. J Headache Pain 2016; 17: 44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Torelli P, Cologno D, Cademartiri C, et al. Primary and secondary chronic cluster headache: Two separate entities?. Cephalalgia 2000; 20: 826–829. [DOI] [PubMed] [Google Scholar]
- 22.Naegel S, Holle D, Obermann M. Structural imaging in cluster headache. Curr Pain Headache Rep 2014; 18: 415. [DOI] [PubMed] [Google Scholar]
- 23.Naegel S, Holle D, Desmarattes N, et al. Cortical plasticity in episodic and chronic cluster headache. Neuroimage Clin 2014; 6: 415–423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.D'Andrea G, Leone M, Bussone G, et al. Abnormal tyrosine metabolism in chronic cluster headache. Cephalalgia 2017; 37: 148–153. [DOI] [PubMed] [Google Scholar]
- 25.de Tommaso M, Ambrosini A, Brighina F, et al. Altered processing of sensory stimuli in patients with migraine. Nat Rev Neurol 2014; 10: 144–155. [DOI] [PubMed] [Google Scholar]
- 26.Bahra A, Gawel MJ, Hardebo JE, et al. Oral zolmitriptan is effective in the acute treatment of cluster headache. Neurology 2000; 54: 1832–1839. [DOI] [PubMed] [Google Scholar]
- 27.Gaul C, Roguski J, Dresler T, et al. Efficacy and safety of a single occipital nerve blockade in episodic and chronic cluster headache: A prospective observational study. Cephalalgia 2017; 37: 873–880. [DOI] [PubMed] [Google Scholar]
- 28.Mitsikostas DD, Edvinsson L, Jensen RH, et al. Refractory chronic cluster headache: A consensus statement on clinical definition from the European Headache Federation. J Headache Pain 2014; 15: 79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Paemeleire K, Bahra A, Evers S, et al. Medication-overuse headache in patients with cluster headache. Neurology 2006; 67: 109–113. [DOI] [PubMed] [Google Scholar]