Abstract
OBJECTIVE:
Timely treatment with neuraminidase inhibitor (NAI) drugs appears to improve survival in adults hospitalized with influenza. We analyzed California surveillance data to determine whether NAI treatment improves survival in critically ill children with influenza.
METHODS:
We analyzed data abstracted from medical records to characterize the outcomes of patients aged 0 to 17 years hospitalized in ICUs with laboratory-confirmed influenza from April 3, 2009, through September 30, 2012.
RESULTS:
Seven hundred eighty-four influenza cases aged <18 years hospitalized in ICUs had information on treatment. Ninety percent (532 of 591) of cases during the 2009 H1N1 pandemic (April 3, 2009–August 31, 2010) received NAI treatment compared with 63% (121 of 193) of cases in the postpandemic period (September 1, 2010–September 30, 2012; P < .0001). Of 653 cases NAI-treated, 38 (6%) died compared with 11 (8%) of 131 untreated cases (odds ratio = 0.67, 95% confidence interval: 0.34–1.36). In a multivariate model that included receipt of mechanical ventilation and other factors associated with disease severity, the estimated risk of death was reduced in NAI-treated cases (odds ratio 0.36, 95% confidence interval: 0.16–0.83). Treatment within 48 hours of illness onset was significantly associated with survival (P = .04). Cases with NAI treatment initiated earlier in illness were less likely to die.
CONCLUSIONS:
Prompt treatment with NAIs may improve survival of children critically ill with influenza. Recent decreased frequency of NAI treatment of influenza may be placing untreated critically ill children at an increased risk of death.
Keywords: influenza, neuraminidase inhibitor, antiviral, critically ill, mortality, children, pediatric, pediatric ICU
Influenza A(H1N1)pdm09 (pH1N1) virus was first identified in California in April 2009 and caused a global pandemic1-3 that disproportionately affected children and young adults.2 As a result, in April 2009, the California Department of Public Health (CDPH) initiated surveillance for critically ill and fatal cases of laboratory-confirmed influenza.
During the pH1N1 pandemic, the reported morbidity and mortality in California were high, with 2144 persons admitted to an ICU and 608 deaths, including 45 deaths in persons aged <18 years. In the first few months of the pandemic, national hospitalization rates for laboratory-confirmed pH1N1 were 4.5-fold higher among children aged <2 years, 2-fold higher among children aged 2 to 4 years, and 1.6-fold higher among children aged 5 to 17 years than among adults.4 In California, during April 3 through August 11, 2009, of 345 persons hospitalized aged <18 years with laboratory-confirmed influenza, more than one-quarter of these hospitalized cases required intensive care, and 9 (3%) were fatal.5 Infants aged <6 months were most likely to be hospitalized.
Since the onset of the pH1N1 pandemic, prompt initiation of antiviral treatment has been recommended for all patients with suspected or confirmed influenza (1) requiring hospitalization; (2) in a high-risk group with comorbidity associated with severe disease as defined by the Advisory Committee for Immunization Practices (ACIP), including children aged <2 years; and (3) with complicated illness regardless of previous health status.6,7 The neuraminidase inhibitors (NAI) currently available for treatment include enteral oseltamivir phosphate, inhaled zanamivir,8 and the investigational intravenous formulations of peramivir and zanamivir. During the pH1N1 pandemic, the Food and Drug Administration issued Emergency Use Authorizations to treat hospitalized children <1 year old with enteral oseltamivir and to allow intravenous peramivir for treatment of hospitalized patients. In December 2012, the Food and Drug Administration approved use of enteral oseltamivir for treatment of symptomatic infants aged ≥14 days that are suspected of having influenza and that have had symptoms for <48 hours (http://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm333205.htm). However, the ACIP and Centers for Disease Control and Prevention recommendations also include oseltamivir treatment of influenza in infants aged <14 days.8
The clinical efficacy and safety of oseltamivir for severe influenza in hospitalized patients have been questioned because data from phase 3 treatment trials remain unavailable for review.9 Several observational studies have demonstrated a reduction in outcomes such as length of hospital stay and risk of critical illness requiring ICU admission or death among hospitalized adults treated with NAIs before, during, and after the pH1N1 pandemic.10-19 However, relatively few studies have evaluated the effectiveness of antiviral treatment of influenza in hospitalized children. We previously found that in cases aged <18 years hospitalized with pH1N1, those treated with NAIs within 48 hours of symptom onset were less likely to require ICU admission or die compared with those never treated.5 In this study, we analyzed antiviral treatment and survival of children aged 0 to 17 years admitted to ICUs with laboratory-confirmed influenza reported to CDPH during the pandemic and in the 2 subsequent postpandemic influenza seasons.
METHODS
CDPH instituted mandatory reporting for all Californians who were hospitalized or died with influenza from April 3 through August 10, 2009. From August 11, 2009, through September 30, 2012, requirements were changed for mandatory reporting of all Californians aged 0 to 64 years with laboratory confirmed influenza who died; reporting became voluntary for Californians aged 0 to 64 years with laboratory-confirmed influenza that required care in an ICU. For the purposes of this study, a case was defined as a California resident aged 0 to 17 years that had influenza virus nucleic acid detected in a respiratory specimen of any type by reverse-transcription polymerase chain reaction assay and was hospitalized in an ICU with signs and symptoms of acute respiratory infection. Fatal cases had influenza listed as a cause of death in either the death certificate or medical record. Testing was performed at local public health laboratories, commercial laboratories or the CDPH Viral and Rickettsial Disease Laboratory. Providers and hospitals reported cases to local health jurisdictions, which then reported cases to CDPH. Using a standardized case report form local health jurisdiction and CDPH staff abstracted data from medical and autopsy records regarding demographics, clinical presentation, and hospital course, comorbid conditions, laboratory results and type, and dosing and dates of antiviral medications.
Nonfatal and fatal patients were compared with respect to demographics, clinical characteristics, and underlying risk factors. The χ2 test was used for comparisons of categorical variables with large numbers and Fisher’s exact test was used for comparisons of categorical variables with expected values <5. The Wilcoxon 2-sample test was used for comparisons of continuous variables. To better understand and visually inspect the confounding effect of clinical severity on the relationship of treatment with survival, bivariate Mantel-Haenszel adjustment was conducted and graphically assessed. Variables that may increase the severity of clinical illness that were significantly associated with fatality in univariate analysis were incorporated into a multivariable logistic regression model. Case fatality proportions were determined for cases categorized by numbers of days from onset of symptoms to initiation of antiviral therapy and were compared with those who were never treated with antiviral agents. The Cochran-Armitage test for trend was used to assess the association of survival with the time between symptom onset and initiation of antiviral treatment. All analyses were performed by using SAS 9.2 (SAS Institute, Cary, NC).
This activity was reviewed by the State of California Committee for the Protection of Human Subjects and determined to be a public health response that did not require institutional review board approval.
RESULTS
During the period of April 3, 2009, to September 30, 2012, 850 California residents aged 0 to 17 years who required intensive care or died with laboratory-confirmed influenza were reported. Of these, 827 (97%) were hospitalized in ICUs, and 23 (3%) died outside of the hospital. Overall, 784 (95%) of the hospitalized patients had information available on antiviral treatment and were analyzed.
Pandemic Versus Postpandemic
The majority of patients reported during the pandemic (April 3,2009–August 31, 2010) had test results consistent with pH1N1 virus infection (90%; 531 of 591). Influenza virus testing during the postpandemic period (September 1, 2010–September 30, 2012) was consistent with pH1N1 in 22% (42 of 192), influenza A subtype H3 in 22% (42 of 192), nonsubtyped influenza A in 28% (53 of 192), and influenza B in 28% (53 of 192). Overall, 532 (90%) of 591 patients reported during the pandemic received NAI treatment during their illness compared with 121 (63%) of the 193 cases in the postpandemic period (P < .0001). The interval from symptom onset to NAI treatment was similar for patients hospitalized in the pandemic period (median 3 days, range 0–33 days) and the postpandemic period (median 3 days, range 0–21 days; P = .2).
Demographic and Clinical Characteristics
Four hundred seventy-three patients (61%) were boys. The median age was 6 years (range 0 weeks-17 years). Five hundred twenty-one cases (68%) had a comorbid condition considered by the ACIP as increasing the risk of severe influenza complications20; nearly half of cases had chronic pulmonary disease (366; 48%). Other frequently reported chronic medical conditions included neurologic disorders such as cerebral palsy/developmental delay and seizure disorder (277; 36%), chronic cardiac disease (105; 14%), and immunosuppression (77; 10%). The median length of hospital stay was 6 days (range 1–238 days). The median timeframes from symptom onset to hospitalization and intensive care admission were 2 days (range 0–32 days) and 3 days (range 0–372 days), respectively.
Forty-nine (6%) children died. Compared with nonfatal patients, fatal patients were more likely to have an ACIP comorbid condition (P = .005), radiographic evidence of pneumonia (P = .0007), and require mechanical ventilation (P < .0001; Table 1). There was no significant difference in distribution by gender, race/ethnicity, or age in the nonfatal compared with fatal cases; younger children (either <2 or <4 years) were not at increased risk for death.
TABLE 1.
Risk Factors Associated With Fatal Outcomes in 784 Critically Ill Cases Aged 0 to 17 Years With Laboratory-Confirmed Influenza in California, April 2009 through September 2012
| Characteristic present, n (% fatal)a |
Characteristic absent, n (% fatal)a |
Univariate |
Multivariateb |
|||
|---|---|---|---|---|---|---|
| OR (CI) | χ2 P | OR (CI) | χ2 P | |||
| Neuraminidase inhibitor treatmentc | 653 (6) | 131 (8) | 0.7 (0.3–1.4) | .3 | 0.4 (0.2–0.8) | .02 |
| Characteristics of severe diseased | ||||||
| ACIP comorbid conditions for severe influenzae | 521 (8) | 249 (3) | 3.0 (1.3–6.9) | .005 | 2.4 (1.0–5.6) | .05 |
| Pneumonia | 539 (8) | 202 (2) | 6.0 (1.9–19.7) | .0007 | 3.2 (0.9–11.0) | .06 |
| Mechanical ventilation | 292 (16) | 428 (0.2) | 81.9 (11.2–597.4) | <.0001 | 70.1 (9.5–517.3) | <.0001 |
| Secondary bacterial infectionf | 74 (8) | 710 (6) | 1.4 (0.5–3.3) | .5 | n/a | n/a |
n/a, not applicable.
Data were missing for the following categories: ACIP comorbid conditions (14 cases), pneumonia (43 cases), and mechanical ventilation (64 cases).
In addition to NAI treatment, significant variables in univariate analysis that were associated with fatality were incorporated into the multivariate logistic regression model and included presence of ACIP comorbid condition, diagnosis of pneumonia, and requirement of mechanical ventilation. Secondary bacterial infection was not included.
Twenty-one cases had missing antiviral treatment information.
Includes cases with known information only.
Conditions defined in Fiore (2010)7 include chronic heart disease (n = 105), chronic lung disease (n = 366), metabolic disease (n = 73), immunosuppressive conditions (n = 77), and neurologic disease (n = 277) and are not mutually exclusive.
Secondary bacterial coinfection was defined by isolation of bacteria from either a sterile site or a lower respiratory tract specimen in conjunction with a new infiltrate on chest radiograph, and excluded bacterial or fungal infections that were likely hospital-acquired (eg, diagnosed >48 h after hospital admission; Centers for Disease Control and Prevention/National Healthcare Safety Network Surveillance Definition of Healthcare-Associated Infection and Criteria for Specific Types of Infections in the Acute Care. Available at: www.cdc.gov/nhsn/pdfs/pscmanual/17pscnosinfdef_current.pdf). Organisms identified included methicillin-resistant Staphylococcus aureus (1), methicillin-sensitive S aureus (1), Streptococcus pneumoniae (3), Streptococcus viridans (1), Legionella pneumophila (1), and Neisseria meningitidis (1).
NAI Treatment
Of the 784 patients, 653 (83%) were treated with NAIs and 131 (17%) were not. The overall median duration of NAI treatment was 5 days (range 0–16 days). Of the 653 treated patients, 38 (6%) died compared with 11 (8%) of 131 untreated patients (odds ratio [OR] = 0.67, 95% confidence interval [CI]: 0.34–1.36). In bivariate analysis stratifying on mechanical ventilation, antiviral therapy was significantly associated with decreased mortality (OR = 0.38, 95% CI: 0.17–0.87) but not in similar stratification on pneumonia (OR = 0.64, 95% CI: 0.29–1.38). In a multivariate model that incorporated variables that were significant in the univariate analysis, receipt of antiviral therapy was associated with decreased mortality (OR = 0.36, 95% CI 0.16–0.84; Table 1).
Timing of NAI Treatment
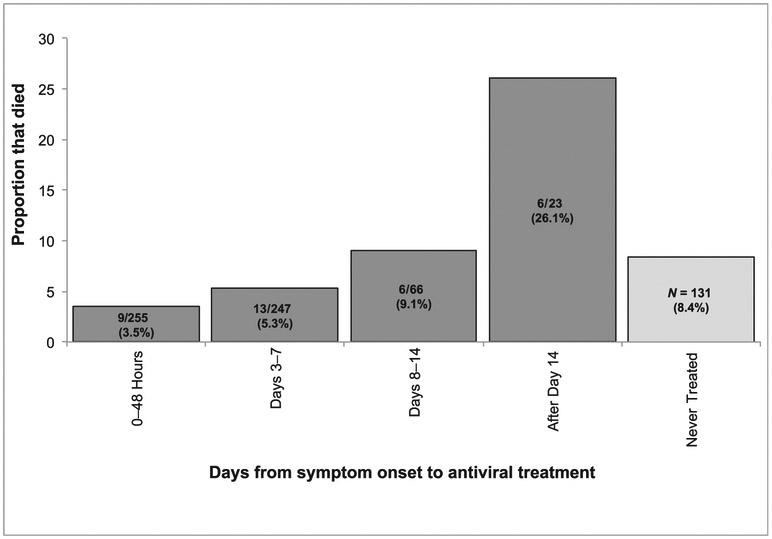
For the 591 (91%) cases with available information on timing of antiviral treatment, the median time from onset of symptoms to starting NAI treatment was 3 days (range 0–33 days). Of treated cases, 502 (84.9%) began treatment during their first week of illness, 66 (11.2%) during the second week and 23 (3.9%) were treated subsequently (Fig 1). Of 255 cases treated with an NAI within 48 hours of symptom onset, 9 (3.5%) died compared with 11 (8%) of 131 untreated cases (P = .04). There was a significant difference between the median time from onset of symptoms to treatment of nonfatal cases (median 3 days, range 0–33 days) compared with fatal cases (5 days, range 0–29 days; P = .004). Early treatment with NAIs sooner after illness onset was associated with decreased mortality (Fig 1; test for trend P = .0002).
FIGURE 1.
Mortality over time in critically ill children aged 0 to 17 years with laboratory-confirmed influenza in California, with and without NAI treatment, April 2009 through September 2012. Note that all fatal cases received mechanical ventilation before death.
DISCUSSION
We reviewed available epidemiologic and clinical data for >780 critically ill children with laboratory-confirmed influenza in California over a 3-year period during and after the pH1N1 pandemic. Patients treated with NAIs were less likely to die compared with untreated patients (6% compared with 8%, respectively), suggesting NAI treatment was beneficial [OR = 0.67, 95% CI: 0.34–1.36). The risk of death in patients requiring mechanical ventilation was much higher, even when treated (OR = 81.9,95% CI: 11.2–597.4). In a multivariate model that included receipt of mechanical ventilation and other factors associated with disease severity, the risk of dying was reduced for cases treated with NAIs (OR = 0.36, 95% CI: 0.16–0.84). Timing of NAI treatment was important: children treated earlier in their illness were less likely to die than those who were treated later, and cases treated within 48 hours of illness onset were significantly more likely to survive compared with those never treated.
There is strong evidence that NAI treatment of hospitalized adults is beneficial when initiated early in the clinical course of influenza, although evidence from randomized placebo-controlled trials is lacking.20 In large observational studies, hospitalized adults infected with seasonal, pH1N1 or influenza A (H5N1) viruses were less likely to die or require intensive care when NAIs were initiated no later than 4 days from onset of symptoms.10-19 Initiation of NAI treatment within 5 days of symptom onset increased the likelihood of survival in a study of >1800 adults hospitalized in ICUs in California.21 A recent meta-analysis reviewing data from 90 studies of adults and children with pH1N1 found NAI treatment within 48 hours of symptom onset reduced the likelihood of severe outcomes such as death and ICU admission.22
There are fewer and less consistent data on the effectiveness of NAI treatment of influenza in hospitalized children, with some studies finding no association with improved survival. Early NAI use in hospitalized children has been associated with a decreased likelihood of ICU admission and need for mechanical ventilation; mortality was not assessed in these studies.23,24 A study of 287 hospitalized, previously healthy children found no difference in length of stay, diagnosis of pneumonia, ICU admission or death in NAI-treated compared with untreated patients; however, the young age of patients (40% aged <6 months) may have prompted hospitalization for observation purposes rather than because of severity of illness.25 A retrospective cohort study of >500 children with severe seasonal influenza illness admitted to PICUs over 6 influenza seasons (2001–2007) found that patients treated with oseltamivir within 24 hours of hospital admission had an 18% reduction in total hospital days (P = .02) but no significant reduction in length of PICU stay, in-hospital mortality, and readmission rates.26
In contrast, a handful of small studies have suggested that early NAI treatment improves survival. During the initial phase of the 2009 H1N1 pandemic, initiation of oseltamivir within 48 hours of symptom onset was associated with a decreased likelihood of ICU admission or death (P = .02) in 345 children hospitalized in California.5 Oseltamivir treatment initiated within 24 hours of hospitalization was protective against death (P = .02) for 147 critically ill children in Argentina.27 Delayed initiation of oseltamivir increased the likelihood of death for 193 children hospitalized with highly pathogenic avian influenza A (H5N1) virus infection, with a 75% increase in the adjusted OR for death for each day of delay.28 Likewise, our review of nearly 800 critically ill children with influenza patients suggests improvement in survival with prompt NAI treatment.
Of note, frequency of NAI treatment in our ICUs was 90% during the pandemic but fell to 63% in the following 2 years. A reduction in antiviral treatment since the 2009 H1N1 pandemic has also been noted through population-based surveillance for hospitalized children with influenza in 10 US states; 84% of children admitted to an ICU with laboratory-confirmed influenza received antiviral treatment during the 2009 H1N1 pandemic compared with 73% during the 2010–2011 season.29 Among all hospitalized pediatric influenza patients, there was a 27% decline in the proportion treated with antiviral agents from 2009 to 2010–2011.29 These results and our findings suggest that further efforts are needed to educate clinicians to increase antiviral treatment in hospitalized children with seasonal influenza, including those who are critically ill.
We note some important limitations and observations. There was likely under-reporting of pH1N1 cases ascertained from voluntary passive reporting by clinicians. In this observational study design in which antiviral treatment was not randomized, selection bias is always possible, and the treated versus untreated groups may have varied in clinical severity. Compared with untreated cases, children treated with NAIs experienced longer median length of hospital stay and higher frequency of mechanical ventilation; it is possible that clinicians might have been more likely to treat the most relatively critically ill patients with NAIs than others admitted to ICUs (eg, nonventilated patients). If the patients who received NAIs were more severely ill before treatment, our estimates of the effect of NAI treatment are biased toward lack of benefit. Delays in initiating therapy with NAIs may also have reduced their effectiveness; 15% of cases began therapy at least 1 week after onset of influenza illness. Approximately 6% of data in our multivariable model were missing; we performed a sensitivity analysis to check the impact and found little difference in the analysis results.
Additionally, we were unable to analyze other treatment modalities or clinical complications that may have affected outcomes despite treatment with NAIs; for example, systematic testing for bacterial coinfections was not performed for all cases, and we did not have available information on coadministration of corticosteroids, which have been implicated in more severe outcomes in hospitalized influenza cases.30 Finally, our results likely represent patients infected with influenza viruses susceptible to NAIs because only 3 of 423 cases tested in California during this surveillance period were infected with influenza viruses containing the H275Y mutation in neuraminidase, which confers resistance to oseltamivir (CDPH, unpublished data).
Our results suggest that prompt NAI therapy in children with influenza virus infection who are hospitalized in an ICU may improve survival, including in those most severely ill who require mechanical ventilation. These findings also emphasize the need for, and the difficulty in obtaining, better evidence of the efficacy and optimal timing of NAI therapy in children; large randomized controlled trials of NAIs could provide better evidence, but at great expense, and present ethical issues because current guidelines recommend initiation of NAI treatment as soon as possible in hospitalized children with influenza.7 Nevertheless, prompt initiation of NAIs seems prudent in a critical care setting where the likelihood of severe morbidity and mortality outweighs concern for side effects. This message needs additional emphasis given that in this study, more than one-third of critically ill children with influenza did not receive antiviral treatment in the postpandemic period.
WHAT’S KNOWN ON THIS SUBJECT: Few data on treating children hospitalized for influenza with neuraminidase inhibitors are available, contributing to uncertainty regarding the benefits of treatment.
WHAT THIS STUDY ADDS: This study of nearly 800 critically ill children suggests that treatment with neuraminidase inhibitors improves survival from influenza. This message needs additional emphasis, given that in the past 2 seasons over one-third of cases did not receive antiviral treatment.
ACKNOWLEDGMENTS
We thank James Watt for his review and helpful comments. Although we cannot name them all, we gratefully acknowledge the contributions of the clinicians throughout California and all the staff at California local health departments who diligently worked to help acquire the epidemiologic and clinical information and ensured that these cases were reported to CDPH.
FUNDING: All phases of this study were supported by the California Department of Public Health. No external funding was secured for this study.
ABBREVIATIONS
- ACIP
Advisory Committee for Immunization Practices
- CDPH
California Department of Public Health
- CI
confidence interval
- NAI
neuraminidase inhibitor
- OR
odds ratio
- pH1N1
influenza A(H1N1)pdm09
Footnotes
FINANCIAL DISCLOSURE: The authors have indicated they have no financial relationships relevant to this article to disclose.
POTENTIAL CONFLICT OF INTEREST: The authors have indicated they have no potential conflicts of interest to disclose.
REFERENCES
- 1.Louie JK, Acosta M, Winter K, et al. ; California Pandemic (H1N1) Working Group. Factors associated with death or hospitalization due to pandemic 2009 influenza A(H1N1) infection in California. JAMA. 2009;302(17):1896–1902 [DOI] [PubMed] [Google Scholar]
- 2.Writing Committee of the WHO Consultation on Clinical Aspects of Pandemic (H1N1) 2009 Influenza. Clinical aspects of pandemic 2009 influenza A (H1N1) virus infection. N Engl J Med. 2010;362(18):1708–1719 [DOI] [PubMed] [Google Scholar]
- 3.Centers for Disease Control and Prevention (CDC). Swine influenza A (H1N1) infection in two children—Southern California, March–April 2009. MMWR Morb Mortal Wkly Rep. 2009;58(15):400–402 [PubMed] [Google Scholar]
- 4.Garg S, Fry AM, Patton M, Fiore AE, Finelli L. Antiviral treatment of influenza in children. Pediatr Infect Dis J. 2012;31(2):e43–e51 [DOI] [PubMed] [Google Scholar]
- 5.Louie JK, Gavali S, Acosta M, et al. ; California Pandemic (H1N1) Working Group. Children hospitalized with 2009 novel influenza A (H1N1) in California. Arch Pediatr Adolesc Med. 2010;164(11):1023–1031 [DOI] [PubMed] [Google Scholar]
- 6.Centers for Disease Control and Prevention. Updated interim recommendations for the use of antiviral medications in the treatment and prevention of influenza for the 2009–2010 season. Available at: www.cdc.gov/H1N1flu/recommendations.htm. Accessed October 27, 2011
- 7.Fiore AE, Uyeki TM, Broder K, et al. ; Centers for Disease Control and Prevention (CDC). Prevention and control of influenza with vaccines: recommendations of the Advisory Committee on Immunization Practices (ACIP), 2010. MMWR Recomm Rep. 2010;59(RR-8):1–62 [PubMed] [Google Scholar]
- 8.Centers for Disease Control and Prevention. Antiviral Agents for the Treatment and Chemoprophylaxis of Influenza. Recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recommendations and Reports. January 21, 2011;60(RR01):1–24. Available at: www.cdc.gov/mmwr/preview/mmwrhtml/rr6001a1.htm. Accessed October 13, 2013 [PubMed] [Google Scholar]
- 9.Jefferson T, Jones MA, Doshi P, et al. Neuraminidase inhibitors for preventing and treating influenza in healthy adults and children. Cochrane Database Syst Rev. 2012;1:CD008965. [DOI] [PubMed] [Google Scholar]
- 10.Kaiser L, Wat C, Mills T, Mahoney P, Ward P, Hayden F. Impact of oseltamivir treatment on influenza-related lower respiratory tract complications and hospitalizations. Arch Intern Med. 2003;163(14): 1667–1672 [DOI] [PubMed] [Google Scholar]
- 11.Jain S, Kamimoto L, Bramley AM, et al. ; 2009 Pandemic Influenza A (H1N1) Virus Hospitalizations Investigation Team. Hospitalized patients with 2009 H1N1 influenza in the United States, April-June 2009. N Engl J Med. 2009;361 (20):1935–1944 [DOI] [PubMed] [Google Scholar]
- 12.Rodríguez A, Díaz E, Martín-Loeches I, et al. ; H1N1 SEMICYUC Working Group. Impact of early oseltamivir treatment on outcome in critically ill patients with 2009 pandemic influenza A. J Antimicrob Chemother 2011;66(5):1140–1149 [DOI] [PubMed] [Google Scholar]
- 13.Kandun IN, Tresnaningsih E, Purba WH, et al. Factors associated with case fatality of human H5N1 virus infections in Indonesia: a case series. Lancet. 2008;372(9640):744–749 [DOI] [PubMed] [Google Scholar]
- 14.Viasus D, Paño-Pardo JR, Pachón J, et al. ; Novel Influenza A(H1N1) Study Group of the Spanish Network for Research in Infectious Diseases (REIPI). Timing of oseltamivir administration and outcomes in hospitalized adults with pandemic 2009 influenza A (H1N1) virus infection. Chest. 2011;140(4): 1025–1032 [DOI] [PubMed] [Google Scholar]
- 15.Higuera Iglesias AL, Kudo K, Manabe T, et al. Reducing occurrence and severity of pneumonia due to pandemic H1N1 2009 by early oseltamivir administration: a retrospective study in Mexico. PLoS One. 2011;6(7):e21838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lee N, Choi KW, Chan PK, et al. Outcomes of adults hospitalised with severe influenza. Thorax. 2010;65(6):510–515 [DOI] [PubMed] [Google Scholar]
- 17.Adisasmito W, Chan PK, Lee N, et al. Effectiveness of antiviral treatment in human influenza A(H5N1) infections: analysis of a Global Patient Registry. J Infect Dis. 2010;202(8)1154–1160 [DOI] [PubMed] [Google Scholar]
- 18.Hanshaoworakul W, Simmerman JM, Narueponjirakul U, et al. Severe human influenza infections in Thailand: oseltamivir treatment and risk factors for fatal outcome. PLoS ONE. 2009;4(6):e6051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.McGeer A, Green KA, Plevneshi A, et al. ; Toronto Invasive Bacterial Diseases Network. Antiviral therapy and outcomes of influenza requiring hospitalization in Ontario, Canada. Clin Infect Dis. 2007;45(12):1568–1575 [DOI] [PubMed] [Google Scholar]
- 20.Treanor JJ, Hayden FG, Vrooman PS, et al. ; US Oral Neuraminidase Study Group. Efficacy and safety of the oral neuraminidase inhibitor oseltamivir in treating acute influenza: a randomized controlled trial. JAMA. 2000;283(8):1016–1024 [DOI] [PubMed] [Google Scholar]
- 21.Louie JK, Yang S, Acosta M, et al. Treatment with neuraminidase inhibitors for critically ill patients with influenza A (H1N1)pdm09. Clin Infect Dis. 2012;55(9):1198–1204 doi: 10.1093/cid/cis636 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Muthuri SG, Myles PR, Venkatesan S, Leonardi-Bee J, Nguyen-Van-Tam JS. Impact of neuraminidase inhibitor treatment on outcomes of public health importance during the 2009–2010 influenza A(H1N1) pandemic: a systematic review and meta-analysis in hospitalized patients. J Infect Dis. 2013;207(4):553–563 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Launes C, García-García JJ, Jordán I, Martínez-Planas A, Selva L, Muñoz-Almagro C. 2009 Influenza A H1N1 infections: delays in starting treatment with oseltamivir were associated with a more severe disease. Pediatr Infect Dis J. 2011;30(7):622–625 [DOI] [PubMed] [Google Scholar]
- 24.Eriksson CO, Graham DA, Uyeki TM, Randolph AG. Risk factors for mechanical ventilation in U.S. children hospitalized with seasonal influenza and 2009 pandemic influenza A. Pediatr Crit Care Med. 2012;13(6):625–631 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bueno M, Calvo C, Méndez-Echevarría A. Oseltamivir Treatment for Influenza in Hospitalized Children without Underlying Diseases. Pediatr Infect Dis J. 2013;32(10):1066–1069 [DOI] [PubMed] [Google Scholar]
- 26.Coffin SE, Leckerman K, Keren R, Hall M, Localio R, Zaoutis TE. Oseltamivir shortens hospital stays of critically ill children hospitalized with seasonal influenza: a retrospective cohort study. Pediatr Infect Dis J. 2011;30(11):962–966 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Farias JA, Fernández A, Monteverde E, et al. Critically ill infants and children with influenza A (H1N1) in pediatric intensive care units in Argentina. Intensive Care Med. 2010;36(6):1015–1022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Oner AF, Dogan N, Gasimov V, et al. H5N1 avian influenza in children. Clin Infect Dis. 2012;55(1):26–32 [DOI] [PubMed] [Google Scholar]
- 29.Garg S, Chaves SS, Pérez A, et al. Reduced influenza antiviral treatment among children and adults hospitalized with laboratory-confirmed influenza infection in the year after the 2009 pandemic. Clin Infect Dis. 2012;55(3):e18–e21 [DOI] [PubMed] [Google Scholar]
- 30.Han K, Ma H, An X, et al. Early use of glucocorticoids was a risk factor for critical disease and death from pH1N1 infection. Clin Infect Dis. 2011;53(4):326–333 [DOI] [PubMed] [Google Scholar]