Abstract
Tissue inhibitor of metalloproteinase-3 (TIMP-3), a secreted glycoprotein, plays an important role in carcinogenesis. It can bind to many proteinases to suppress their activity and thus protect the extracellular matrix from degradation. TIMP-3 may have many anticancer properties, including apoptosis induction and antiproliferative, antiangiogenic, and antimetastatic activities. This review summarizes the structure, proteinase inhibition ability, genetic and epigenetic regulation, cancer therapy potential, and contribution to cancer development of TIMP-3. Furthermore, in this review we discuss its potential as a biomarker for predicting cancer progression and the current state of drugs that target TIMP-3, either alone or in combination with clinical treatment. In conclusion, TIMP-3 can be a biomarker of cancer and a potential target for cancer therapy. This review article can serve as a basis to understand how to modulate TIMP-3 levels as a drug target of cancers.
Keywords: cancer therapy, extracellular matrix, matrix metalloproteinase, metastasis, tissue inhibitors of metalloproteinases-3
Introduction
Despite recent improvements, cancer treatment remains associated with several challenges. Cancer development is a multifactorial and multistep process and involves several genetic and epigenetic regulations. In addition, cancer cells possess several unique characteristics, also known as the cancer hallmark, that confer the cells with resistance against the human immune system and cancer treatment. The cancer hallmark includes tumor-promoting inflammation, enabling replicative immortality, avoiding immune destruction, evading growth suppressors, sustaining proliferative signaling, deregulating cellular energetics, resisting cell death, genome instability and mutation, inducing angiogenesis, and activating invasion and metastasis.1,2 Metastasis, which is the major cause of death among cancer patients, involves multiple processes including extracellular matrix (ECM) remodeling and degradation. Degradation of the ECM is required for tumor cell metastasis; this is achieved by several proteinases such as the plasmin system and particularly, the matrix metalloproteinases (MMPs).3–6 MMPs are known to play an important role in the tissue invasion and metastasis of cancer cells. The tissue inhibitors of MMPs (TIMPs) are endogenous inhibitors of MMPs, and regulation of MMPs by TIMPs is particularly important for the maintenance of the ECM. Disruption of the balance between the activities of MMPs and TIMPs during carcinogenesis may affect invasion and metastasis7–9 and may worsen patient outcomes.10 TIMP-3, a member of the TIMP family, is a 24-kDa secreted glycoprotein, and its gene is located on chromosome 22q12.1–q13.2. Knockout of the TIMP-3 gene in mice resulted in increased MMP, a disintegrin and MMPs with thrombospondin motifs (ADAMTS) activity, and cartilage degradation, suggesting that reduced TIMP-3 levels may cause osteoarthritis.11 In addition, the absence of TIMP-3 leads to poor cardiac remodeling and has been associated with myocardial infarction or hypertension.12,13 In cancer studies, TIMP-3 plays an important role in the cancer hallmark by controlling cell death, angiogenesis, tumor inflammation, and tumor cell invasion and dissemination.14 For instance, TIMP-3 restoration in cancer cells inhibits cell growth and promotes cell apoptosis.15,16 In addition, TIMP-3 overexpression improves the sensitivity of osteosarcoma to clinical drug treatment through interleukin (IL)-6 inhibition.17 TIMP-3 also acts as a potential antiangiogenesis agent by inhibiting endothelial cell tube formation.18 Moreover, TIMP-3 can inhibit cancer cell migration, invasion, and metastasis in vitro and in vivo.19,20 Clinical studies have reported reduced TIMP-3 expression in cases of several cancer types compared with normal controls;19–22 the loss of TIMP-3 may lead to poor outcomes, including large tumor size, high tumor stage, and metastasis.23–25 Herein, we review the structure and function of TIMP-3 and discuss its contribution to carcinogenesis and its potential in cancer therapy.
TIMP-3
TIMP classification
The TIMP family contains four members: TIMP-1, TIMP-2, TIMP-3, and TIMP-4. The molecular weight of the TIMPs is approximately 21 kDa, and they contain an N-terminal domain and a C-terminal domain. In contrast to that in TIMP-1 and TIMP-3, the C-terminal domain in TIMP-2 and TIMP-4 is negatively charged. Compared with the nonglycosylation of TIMP-2 and TIMP-4, TIMP-1 contains two N-glycosylation sites at Asn26 and Asn,27 and TIMP-3 contains a single N-glycosylation site in the C-terminal domain.28 Glycosylated forms of TIMP-3 have higher affinity for glycan-bound MMPs and are protected from endocytosis and degradation.29 Unlike other TIMPs, TIMP-3 is the only TIMP that binds firmly to the ECM after its secretion.30 This binding is via the interaction of the N-terminal domain with heparan sulfate and sulfated glycosaminoglycans.31
Transcriptional regulation of TIMP-3
The expression of TIMP-3 can be regulated by transcriptional regulation. Transcriptional regulation contains two major parts: the first part involves transcription factors and the transcription apparatus and the second part involves chromatin and its regulators.26 Gene expression regulated by transcription factors is one of the most common transcriptional regulations. Transcription factors including Elf3, sp1, smad2, and smad4 have been reported to target on the promoter of TIMP-3 and regulated TIMP-3 expression.32–36 Jobling et al. discovered that ETS transcription factor Elf-3 was expressed in human retinal pigment epithelium (RPE) cell lines. Transfection of Elf3a and Elf3b overexpression vector increased promoter activity of TIMP-3.32 TIMP-3 promoter contains four sp1 binding sites in the region near the transcription start site.35 Zerrouqi et al. indicated that P14ARF increased expression of TIMP-3 in human glioblastoma cell line is sp1 dependent. Knockdown of sp1 by siRNA suppressed TIMP-3 promoter activity that is enhanced by P14ARF.34 Other studies also demonstrated that sp1 regulated TIMP-3 promoter transcription activity via the ERK pathway.33,35 Treatment of ERK inhibitor decreased binding ability of sp1 to DNA.35 TIMP-3 is also a target for Smad pathway mediated by transforming growth factor (TGF)-β. Qureshi et al. suggested that the transcription factors Smad2 and Smad4 must bind to the promoter of TIMP-3 in the presence of TGF-β.36 In addition, TIMP-3 expression can also be regulated by histone modification such as histone acetylation and histone methylation. Shinojima et al. used chromatin immunoprecipitation and showed that transcriptional repression of TIMP-3 was associated with increased H3K27me3 and decreased H3K9ac histone marks at TIMP-3 promoter.37 Many proteins have also been reported to be involved in the process of histone modification. HDAC9 is one of the histone deacetylases (HDACs) that has been indicated to suppress TIMP-3 via promoter histone hypoacetylation.38 KDM1A, also known as LSD1, caused TIMP-3 repression through H3K4me2 demethylation at TIMP-3 promoter.39 The enhancer of zeste homolog 2 (EZH2), which has histone methyltransferase activity, is known to reduced TIMP-3 expression by catalyzing H3K27me3.40
MMP inhibitory activity of TIMP-3
TIMPs are endogenous inhibitors of MMPs and exhibit marked antiproteinase activity against MMPs, ADAMs, and ADAMTSs.41 TIMPs can use the N-terminal region to bind to the catalytic domain of MMPs to inhibit their activity and form a stable bond with the C-terminal hemopexin domain of proMMPs via the C-terminal region.42 However, the extent of MMP inhibition differs between each TIMP; TIMP-1 strongly inhibits MMP-9 but poorly inhibits MT1-MMP, MT3-MMP, MT5-MMP, and MMP-19,30 and TIMP-2 strongly inhibits MMP-2 and can inhibit other MMP members. TIMP-1, TIMP-2, and TIMP-4 inhibit only a few ADAMs.43–45 In addition, TIMP-2 can form a ternary complex composed of TIMP-2-pro-MMP-2-MT1-MMP, which resulted in the activation of pro-MMP-2.30 TIMP-4 can also form a TIMP-4-pro-MMP-2-MT1-MMP complex, but unlike TIMP-2, leading to inhibit the activation of pro-MMP-2 via inhibition of MT1-MMP.46 TIMP-3 can form a similar terminal complex to inhibit pro-MMP-2 activation. Knockout of TIMP-3 in cell promoted activation of pro-MMP-2 mediated by MT1-MMP.47 In contrast to other members of the TIMP family with limited inhibitory activity for ADAMs, TIMP-3 can effectively inhibit ADAM10, ADAM12, ADAM17, ADAM28, ADAM33, ADAMTS-1, ADAMTS-2, ADAMTS-4, and ADAMTS-5.30 For instance, the ECM protein-degrading activity of ADAM12 can only be blocked by TIMP-3, but not by TIMP-1, TIMP-2, and TIMP-4.48 In addition, TIMP-2 and TIMP-3 inhibit the aggrecanase activity of ADAMTS1, but TIMP1 and TIMP4 have no significant inhibitory effect.49
TIMP-3 in cancer
Although TIMPs may have substantial roles in cancer, the mechanisms and outcomes for each TIMP vary. For example, high TIMP-1 expression has been reported to be associated with poor prognosis in most cancers. In an in vitro study, TIMP-1 expression was found to promote cancer cell survival in acute myeloid leukemia (AML) and melanoma.50,51 Clinical studies have shown that a high level of TIMP-1 is associated with a shortened relapse-free and cancer-specific survival in endometrial carcinoma52 and poor overall survival in laryngeal squamous cell carcinoma.53 Both increased and decreased expressions of TIMP-2 and TIMP-4 have been reported to be associated with greater cancer risk. Overexpression of TIMP-2 has been reported to increase proliferation of choriocarcinoma cells.54 By contrast, TIMP-2 inhibits angiogenesis via an MMP-independent mechanism.55 TIMP-4 is expressed in some cancers; however, its role in carcinogenesis remains unclear. In a recent study, TIMP-4 was found to regulate stemness in cervical cancer by enriching tumor progenitor cells.56 Studies in human cancer tissues have suggested that TIMP-3 may play a tumor suppressive role, and that TIMP-3 gene expression is downregulated in brain tumors, esophageal adenocarcinoma, gastric adenocarcinoma, clear cell renal cell carcinoma, meningiomas, and pancreatic endocrine tumors.57–61 In an in vitro study, TIMP-3 expression was observed to induce cancer cell apoptosis in cervical cancer, fibrosarcoma, and breast cancer cell lines.62 In an animal model, TIMP-3-deficient prostate tumors showed increased expression of inflammation markers, including monocyte chemoattractant protein (MCP) 1, COX2, tumor necrosis factor (TNF)-α, and IL-1β.63 In addition, TIMP-3 has been widely described as a potential angiogenesis inhibitor. A study reported that purified and renatured TIMP-3 inhibits angiogenesis, as revealed by the CAM assay.64 Moreover, TIMP-3 can inhibit the migration and invasiveness of cancer cells in vitro.65–67 By contrast, some reports have suggested that TIMP-3 promotes cancer. Higher TIMP-3 mRNA levels were observed in the stroma of head and neck cancer cells than in the normal epithelial cells, and high levels of TIMP-3 have been shown to be associated with a significant reduction in the overall survival rate.68 Our previous study also revealed that plasma TIMP-3 is a potential biomarker of the tumor stage in patients with oral squamous cell carcinoma.21 TIMP-3-knockout mice demonstrated resistance to developing breast and liver cancer despite the increase in their inflammatory response.69,70
Genetic and epigenetic regulation of TIMP-3 in cancer
Gene polymorphism
Gene expression may be regulated by gene polymorphisms, which are variations in the DNA sequence resulting from a >1% nucleotide change within a population.71,72 Polymorphisms of TIMP-3 have been reported in many cancers including adenocarcinoma, bladder cancer, breast cancer, hepatocellular carcinoma, oral cancer, and prostate cancer.23,73–77 The TIMP-3 polymorphic rs9862 allele is associated with increased plasma levels of TIMP-3 and higher risk of oral cancer than with the wild-type allele.23 In adenocarcinoma of the gastroesophageal junction, polymorphisms (rs130274, rs715572, rs1962223, and rs5754312) in TIMP-3 and polymorphism rs9862 in the TIMP-3 promoter are associated with survival.77 Moreover, rs8136803 (TT) is associated with decreased disease-free survival in breast cancer patients.78
DNA methylation and histone modification
Loss or downregulation of TIMP-3 expression has been linked to promoter hypermethylation of the TIMP-3 gene in several types of cancer including esophageal adenocarcinoma, head and neck squamous cell carcinoma, ovarian cancer, and pancreatic endocrine tumors.27,61,79 Hypermethylation in the promoter region usually causes transcriptional silencing because it affects the ability of the transcription factor to bind to the target gene. TIMP-3 methylation in the sp1 binding site and the TATA box of the promoter are associated with low expression of TIMP-3 protein in gastric cancer cell lines.80 DNA methyltransferases (DNMTs) are key enzymes causing gene methylation, and dysregulation of DNMTs has been reported in tumorigenesis.81 Knockout of both DNMT1 and DNMT3B in colorectal carcinoma cell lines caused gene demethylation of TIMP-3, which resulted in recovery of TIMP-3 mRNA expression.82 TIMP-3 expression is also regulated by ten–eleven translocation 1 (TET1), a dioxygenase involved in cytosine demethylation. TET1 can maintain the expression of TIMP-3 by inhibiting its methylation and, thus, suppressing cancer cell invasion.83 Loss of TIMP-3 expression can also be regulated by histone H3K27 methylation via upregulation of the EZH2 in non-small cell lung cancer (NSCLC).40 In addition, TIMP-3 expression was suppressed by KDM1A, a histone demethylase that removes H3K4me2 from TIMP-3 promoter and promotes tumor cells invasion in NSCLC.39 In prostate cancer, treatment with histone methylation inhibitor 3-deazaneplanocin A and trichostatin A restored expression of TIMP-3.37
MicroRNA and long noncoding RNAs
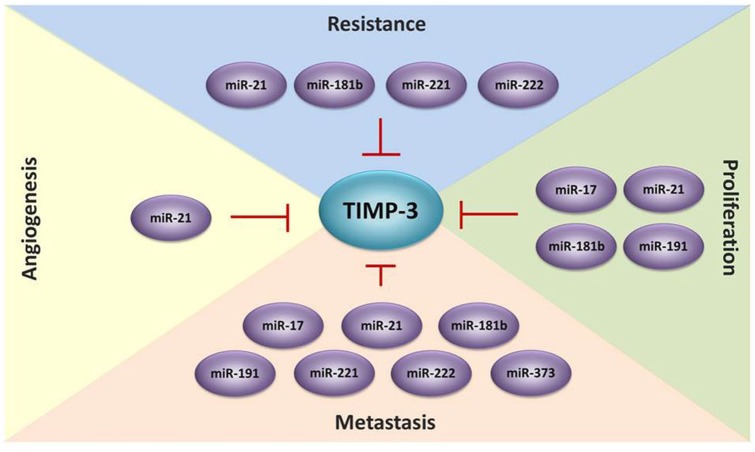
Accumulating evidence suggests that microRNAs (miRNAs) and long noncoding RNAs (lncRNAs) affect cancer development.84 MiRNAs involved in the post-transcriptional regulation of TIMP-3. The mechanism of MicroRNA to suppress gene expression is by controlling mRNA stability and translation through base pairing to the 3′ untranslated region (3′-UTR). TIMP-3 regulation by miRNAs including miR-17-3p, miR-17-5p, miR-21, miR-21-5p, miR-181b, miR-191, miR-221, miR-222, and miR-373 has been widely reported in different cancers (Table 1).85–101 For instance, mature miR-17-5p and the passenger strand miR-17-3p promoted prostate cancer growth and invasion by targeting TIMP-3.85 Upregulation of miR-21 was correlated with decreased TIMP-3 expression in patients with breast cancer, cervical cancer, cholangiocarcinoma, and pancreatic ductal adenocarcinoma.87,88,90,91 In an in vitro analysis, miR-373 could induce esophageal squamous cell carcinoma cell migration and invasion by inhibiting TIMP-3 expression.101 In an in vivo study, miR-181b upregulation by TGF-β reduced TIMP-3 expression, and transfection with anti-miR-181b in the hepatocellular carcinoma cell line SK Hep-1 suppressed tumor growth in nude mice.94 Some studies have suggested that miRNAs targeting TIMP-3 are also crucial in cancer resistance development. For instance, miR-21-5p was upregulated in patients with gastric cancer and induced drug resistance to doxorubicin by targeting TIMP-3.92 Upregulation of miR-221 in oral squamous cell carcinoma cells increases resistance to doxorubicin by silencing TIMP-3 expression.97 Moreover, miR-221 inhibition enhances the sensitivity of human oral squamous cell carcinoma cells to doxorubicin by upregulating TIMP-3 expression98 (Figure 1). lncRNAs are RNA transcripts that are longer than 200 nucleotides. Accumulating evidence indicates that lncRNAs play critical roles in tumorigenesis through various mechanisms such as transcriptional, post-transcriptional, and epigenetic regulation.102–105 The lncRNA BC032913 enhances TIMP-3 expression and inhibits nuclear β-catenin expression, thus suppressing the migration, invasion, and metastatic potential of colorectal cancer cells.106 The lncRNA DANCR suppresses TIMP-3 expression by increasing the binding ability of EZH2 and H3K27me3 to the TIMP-3 promoter in prostate cancer.107
Table 1.
MicroRNAs target TIMP-3 during cancer progression.
| MicroRNAs | Cancer type | Function | Reference |
|---|---|---|---|
| miR-17-3p and miR-17-5p | Prostate cancer | Tumor growth and invasion ↑ | Yang et al.85 |
| miR-21 | Melanoma | Tumor invasion ↑ | Martin del Campo et al.86 |
| Breast cancer | Cancer metastasis ↑ | Li et al.87 | |
| Cervical cancer ESCC PDAC |
Proliferation, migration and invasion ↑ Tumor growth and invasion ↑ Apoptosis ↓ Worse survival of patients |
Zhang et al.88
Wang et al.89 Nagao et al.91 |
|
| miR-21-5p | Gastric cancer Breast cancer |
Doxorubicin resistance Tumor growth ↑ Angiogenesis ↑ |
Chen et al.92
Dai et al.93 |
| miR-181b | HCC | Tumor growth, migration and invasion ↑ Doxorubicin resistance |
Wang et al.94 |
| Gastric cancer | Cancer metastasis ↑ | Zhou et al.95 | |
| miR-191 | EAOC | Proliferation and invasion ↑ | Dong et al.96 |
| miR-221 | OSCC | Doxorubicin resistance Adriamycin resistance |
Du et al.97
Chen et al.98 |
| miR-221 and miR-222 |
NSCLC and HCC Breast cancer |
TRAIL resistance Migration ↑ Tamoxifen resistance |
Garofalo et al.99
Gan et al.100 |
| miR-373 | ESCC | Migration and invasion ↑ | Liu et al.101 |
EAOC, endometriosis-associated ovarian cancer; ESCC, esophageal squamous cell carcinoma; HCC, hepatocellular carcinoma; NSCLC, non-small cell lung cancer; OSCC, oral squamous cell carcinoma; PDAC, pancreatic ductal adenocarcinoma; TRAIL, TNF-related apoptosis-inducing ligand.
Figure 1.
TIMP-3 is regulated by microRNAs during cancer progression. MicroRNAs play an important role to silence the expression of TIMP-3 and promote cell proliferation, migration, invasion, metastasis, angiogenesis and drug resistance in several types of cancer.
Function of TIMP-3 in cancer
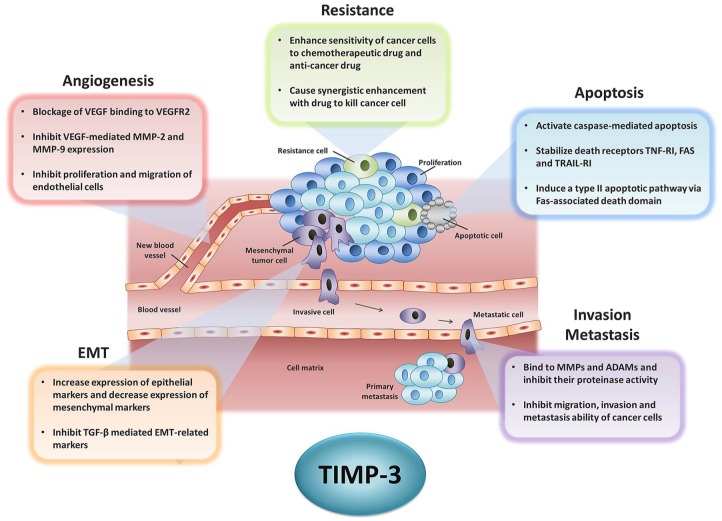
TIMP-3 functions as a tumor suppressor gene in many types of cancer. It exerts anticancer effects through mechanisms such as the inhibition of cell proliferation, the induction of apoptosis, the inhibition of drug resistance of cancer and inhibition of angiogenesis, migration and invasion, cancer metastasis, and epithelial–mesenchymal transition (Figure 2). In this section, we focus on the aforementioned anticancer abilities of TIMP-3.
Figure 2.
The role of TIMP-3 in cancer progression. Tumor development contains many complex mechanisms including avoiding from cell apoptosis, growth without limit, angiogenesis, resistance to drug treatment, changing cell morphology from epithelial type to mesenchymal type (EMT), and metastasizing to the new organ. This figure lists the anticancer capacity of TIMP-3 that has been reported in previous studies.
ADAM, a disintegrin and metalloproteinase; EMT, epithelial–mesenchymal transition; MMP, matrix metalloproteinase; TGF, transforming growth factor; TIMP, tissue inhibitors of metalloproteinase; TNF, tumor necrosis factor; VEGF, vascular endothelial growth factor.
Cell proliferation and apoptosis
Cancer cells grow in an uncontrolled fashion and evade the host immune system. Overexpression of TIMP-3 suppresses proliferation and induces apoptosis in different cancer cell lines.62 TIMP-3 has been shown to induce cell apoptosis by activating mitochondrion-mediated caspase-3 in highly metastatic prostate cancer cell lines PC-3 and DU-145.108 In addition, TIMP-3 can enhance the sensitivity of cancer cells to apoptosis by stabilizing the death receptor. Mark et al. reported that TIMP-3 induces a type II apoptotic pathway via a Fas-associated death domain-mediated mechanism.109 TIMP-3 overexpression in melanoma cells induces apoptosis by stabilizing the TNF receptor-1 (R1), FAS, and TNF-related apoptosis-inducing ligand receptor-1 (TRAIL-R1) on the cell surface; stabilization of death receptors results in the activation of apoptosis markers caspase-8 and caspase-3.110 Other studies have also suggested that TIMP-3 can block ADAM17 activity, which induces shedding of TNF-R1 and TNF-α from the cell surface.111,112 Notably, TIMP-3 can also promote death of nonadherent small-cell lung carcinoma cells even if the cells do not present cell surface death receptors or caspase-8.113 In vivo studies have shown that prostate cancer cells transfected with TIMP-3 suppress tumor growth and induce tumor apoptosis in nude mice.114 Similar results have also been shown in colon carcinoma, melanoma, and neuroblastoma; TIMP-3 overexpression suppresses tumor growth in vivo.15,115,116
Angiogenesis
Angiogenesis, which is the process of new blood vessel formation, plays a key role in tumor growth and metastasis. TIMP-3 has been shown to inhibit angiogenesis through regulating angiogenesis-related proteins or directly inducing apoptosis of endothelial cells. Vascular endothelial growth factor (VEGF) is a key mediator of blood vessel development. TIMP-3 can inhibit angiogenesis by blocking VEGF from binding to VEGF receptor-2.117 In leukemia cells, TIMP-3 can inhibit the proliferation and migration of human umbilical vein endothelial cells (HUVECs) and reduce VEGF-mediated MMP-2 and MMP-9 expression.67 Kang et al. used a yeast 2-hybrid system and found a TIMP-3-interacting partner angiotensin II type 2 receptor (AGTR2). They suggested that combination treatment with TIMP-3 and AGTR2 inhibits VEGF-mediated proliferation of HUVECs.118 TIMP-3 can also inhibit angiogenesis by promoting endothelial cell apoptosis. Qi et al. suggested that TIMP-3 may induce apoptosis of endothelial cells by triggering a FAK/Paxillin cell survival pathway but not a caspase-dependent cell death pathway.119 Upregulation of TIMP-3 by transcription factor sp1 via P14ARF inhibited endothelial cell migration and vessel formation.34 In an in vivo study, TIMP-3 knockout in nude mice but not in the tumor showed enhanced growth of tumor and increased angiogenesis.120
Migration and invasion and metastasis
Metastasis is the process of cancer cells breaking apart from the primary tumor and traveling through the blood or lymph vessels to form new tumors in other organs or tissues.2,7,121,122 Metastasizing cancer cells can secrete proteinases to degrade the ECM and further migrate and invade into the blood. Overexpression and unrestricted activation of MMPs may promote malignant conversion of cancer cells.123,124 TIMP-3 has been shown to block proteinase activity and inhibit tumor migration and invasion. Overexpression of TIMP-3 in osteosarcoma decreases MMP-1 and MMP-2 expression and suppresses cell migration and invasion ability.66 In addition, TIMP-3 can protect ECM degradation mediated by ADAM12, the expression of which is correlated with the status and stage of breast cancer.48 Inhibition of the invasive activity of melanoma cells is more pronounced in the case of TIMP-3 overexpression compared with TIMP-1 and TIMP-2 overexpression.16 Loss of TIMP-3 promotes NSCLC cell invasion via TNF-mediated IL-6 production.24 High levels of soluble CD44 are associated with malignant cancer and metastasis; however, TIMP-3 potentially inhibits CD44 shedding by targeting ADAM-like proteases and MT1-MMP.125,126 In an animal study, melanoma and lymphoma cells in TIMP-3-/- mice were observed to have higher metastatic ability, metastasizing to multiple organs, and lung tissues from TIMP-3-/- mice showed higher MMP-2 and MMP-9 enzyme activity than did those from wide-type mice.127
EMT
EMT refers to the transition of an epithelial cell into a mesenchymal cell and is considered an important indicator of cancer cell metastasis.128,129 Restoration of TIMP-3 expression in thyroid tumor cells increased their cell adhesion ability, thereby increasing the expression of the epithelial marker cytokeratin 8/18 and decreasing that of the mesenchymal marker vimentin.19 In addition, transfection of pcDNA-TIMP-3 in gastric cancer cells followed by treatment with TGF-β revealed that TIMP-3 partially rescues EMT-related marker expression induced by TGF-β.95
TIMP-3 as a target for cancer therapy
In addition to traditional surgery, chemotherapy, and radiotherapy, current cancer research is increasingly focusing on identifying biomarkers for early prediction of cancer progression, immunotherapy, and the use of targeted therapy against tumor suppressor genes or oncogenes.
TIMP-3 as a biomarker for predicting cancer progression
Cancer may rapidly progress from an early stage to an advanced stage, which hinders early diagnosis and accurate monitoring of progression. Plasma and serum levels of TIMP-3 can be easily detected using enzyme-linked immunosorbent assay. A clinical study showed that the increase in plasma levels of TIMP-3 was significantly higher in those with large tumors (>T2) than in those with small tumors among betel quid chewers with oral cancer.23 TIMP-3 protein and mRNA can be extracted from tissues of patient with cancer and detected using Western blotting, immunohistochemistry, and real-time polymerase chain reaction. TIMP-3 protein expression has been shown to be correlated with tumor stage in NSCLC.24 Low TIMP-3 mRNA expressions was observed in patients with high-grade clear cell renal cell carcinoma.59 Loss of TIMP-3 expression in gastric cancer tissues is associated with tumor size, histologic grade, lymphatic invasion, venous invasion, invasive depth, lymph node metastasis, distant metastasis, and TNM stage.25 Gene polymorphism and DNA methylation could be identified from DNA extracted from blood, body fluids, and salivary rinse and used as biomarkers to predict cancer progression in many cancers. TIMP-3 polymorphism rs9862 has been associated with an increased risk of developing a tumor of size >T2 among betel quid chewers with oral cancer.23 A study revealed that TIMP-3 hypermethylation in patients with esophageal squamous cell carcinoma is associated with poorer prognosis for both disease-free and overall survival than that of patients without TIMP-3 methylation.130 Patients with AML harboring methylation of TIMP-3 show higher frequency of adverse cytogenetic prognosis than those with a favorable or intermediate prognosis.131 TIMP-3 promoter hypermethylation detected from pretreatment salivary rinse is significantly associated with local recurrence-free survival in patients with head and neck squamous cell carcinoma.132 Methylation of the TIMP-3 promoter identified from body fluids has been reported to be a useful biomarker for predicting tumor size, differentiation, T stage, lymph node metastasis, distant metastasis, and clinical stage in patients with gastric cancer25 (Table 2).
Table 2.
TIMP-3 as biomarker in cancer progression.
| Cancer type | Sample source | Clinical significance | Reference |
|---|---|---|---|
| TIMP-3 expression | |||
| Oral cancer | Plasma protein | Tumor size | Su et al.21 |
| NSCLC | Tissue protein | Tumor stage | Wu et al.24 |
| CCRCC | Tissue mRNA | Tumor grade | Gu et al.58 |
| Gastric cancer | Tissue protein | Tumor size Histologic grade Distant metastasis TNM stage |
Yu et al.25 |
| TIMP-3 polymorphism | |||
| Oral cancer | Blood DNA | Tumor size | Su et al.23 |
| Breast cancer | Blood DNA | Survival | Bashash et al.77 |
| Adenocarcinoma | Blood DNA | Survival | Wieczorek et al.76 |
| TIMP-3 methylation | |||
| ESCC | Tissue DNA | Disease-free survival Overall survival |
Ninomiya et al.130 |
| AML | Bone marrow DNA | Cytogenetic prognosis | Raneros et al.131 |
| HNSCC | Salivary rinse DNA | Local recurrence-free survival | Sun et al.132 |
| Gastric cancer | Body fluid DNA | Disease-free survival Tumor size Differentiation T stage Lymph node metastasis Distant metastasis |
Yu et al.25 |
AML, acute myeloid leukemia; CCRCC, clear cell renal cell carcinoma; ESCC, esophageal squamous cell carcinoma; HNSCC, head and neck squamous cell carcinoma; NSCLC, non-small cell lung cancer.
Combination of TIMP-3 and clinical treatment in cancer therapy
Cancers cells can develop drug resistance, which could limit the efficacy of traditional therapies. Combination therapy, the use of more than one type of therapy in treatment, has the potential to delay or reduce drug resistance of cancer. In prostate cancer, adenovirus-mediated expression of TIMP-3 highly sensitizes prostate cancer cells to the chemotherapeutic drug paclitaxel (Taxol) and promotes synergistic enhancement in cell death.108 TIMP-3 increases cisplatin-induced apoptosis in laryngeal carcinoma by facilitating a mitochondria-dependent apoptosis mechanism such as cytochrome c release and caspase activation.133 In addition, TIMP-3 overexpression in osteosarcoma cells facilitates cisplatin-induced apoptosis, whereas TIMP-3 knockdown by siRNA has an opposite effect.66 In an animal study, combination therapy with adenovirus-TIMP-3 and the broad-spectrum antitumor agent cisplatin inhibited cervical cancer xenograft growth more effectively than cisplatin only.134
Inhibition of cancer progression through TIMP-3
Because TIMP-3 expression is downregulated or deregulated in cancer, current studies are focusing on normalizing or reactivating the expression of TIMP-3 (Table 3). In recent years, the use of natural products has gained much attention in cancer chemoprevention. For example, Andrographolide, a Chinese herbal medicine that is isolated from the stem and leaves of Andrographis paniculata, can inhibit angiogenesis by suppressing miR-21-5p expression and enhancing TIMP-3 expression.93 Diallyl disulfide, one of the organosulfur compounds derived from Allium vegetables, can inhibit migration and invasion in gastric cancer and upregulate tumor suppressor gene expression, including that of TIMP-3 and E-cadherin.135 Green tea polyphenols and their major component epigallocatechin-3-gallate restore TIMP-3 expression by attenuating epigenetic silencing of EZH2 and HDACs, thus inhibiting invasion in breast cancer.136 Arctigenin, derived from the seeds of Arctium lappa, may also increase TIMP-3 expression and inhibit tumor growth in prostate cancer.137 Mithramycin A (MMA), an antibiotic against Gram-positive soil bacteria, has antitumor and antimetastatic effects; MMA can interact with the catalytic pocket of DNMT1, which results in promoter demethylation and mRNA restoration of TIMP-3.138 Although beneficial, cancer chemoprevention is associated with many challenges, such as toxicity, side effects, efficacy only at high doses, low bioavailability, and rapid metabolism.139 In addition to the natural products, some synthetic products can also be used against cancer. NucAnt 6L (N6L), a synthetic peptide, can suppress the invasion ability of melanoma through TIMP-3 release from sulfated glycosaminoglycans.140 The synthetic peptide p700, which is derived from the N-terminal domain of TIMP-3, inhibits VEGF-family receptors, angiogenesis, and tumor growth.141 An artificially designed i-lncRNA can target multiple oncogenic miRNAs, thereby protecting the tumor suppressor gene TIMP-3 in diffuse large B-cell lymphoma.142 TAPI-0, a functional analog of TIMP-3, which inhibits AML cell-induced short-term natural killer (NK) cell abnormalities, may contribute to NK cell-based immunotherapy of AML.143 Other studies have focused on the epigenetic regulation of TIMP-3. Jharna et al. suggest that the quinoline-based compound SGI-1027 acts as a hypomethylation agent by inhibiting DNMT1 activity and inducing its degradation. In addition, they claimed that treatment with SGI-1027 facilitates re-expression of the tumor suppressor genes MLH1, P16, and TIMP-3 in cancer cell lines.144 MPT0G013, a HDAC inhibitor, has been reported to induce TIMP-3 expression and further inhibit angiogenesis and tumor growth in vivo.145 Moreover, TIMP-3 may be a target for demethylation treatments in AML patients. TIMP-3 demethylation by azacytidine (AZA) or decitabine (DAC) can inhibit the release of soluble NKG2D ligand (NKG2DL) mediated by ADAM17 and enhance the lytic activity of NK cells through immune recognition mediated by the NKG2D–NKG2DL engagement.131 Although hypomethylating agents (HMAs) such as AZA and DAC have been widely used in the clinical treatment of AML. However, there are still many limitations and side effects in the use of HMAs,146 and it is easy to cause excessive inhibition of DNA methylation. Moreover, DNA hypomethylation may cause some autoimmune diseases such as systemic lupus erythematosus (SLE),147,148 and may also activate some cancer metastasis genes such as u-PA to accelerate cancer metastasis.149 Therefore, second-generation HMAs and other combined treatments are still evolving and ultimately expected to improve efficacy and reduce side effects.150
Table 3.
Anticancer agents targeting to TIMP-3.
| Agent name | Targeting cancer | Anticancer function | Reference |
|---|---|---|---|
| Natural products | |||
| Andrographolide | Breast cancer | Antiangiogenesis | Dai et al.93 |
| Diallyl disulfide | Gastric cancer | Inhibit migration and invasion | Su et al.135 |
| EGCG | Breast cancer | Inhibit invasion | Deb et al.136 |
| Arctigenin | Prostate cancer | Inhibit tumor growth | Wang et al.137 |
| Mithramycin A | Lung cancer | Inhibit metastasis | Lin et al.138 |
| Synthetic products | |||
| NucAnt 6L | Melanoma | Inhibit invasion | Destouches et al.140 |
| p700 | Breast cancer | Inhibit tumor growth and angiogenesis | Chen et al.141 |
| lncRNA | Diffuse large B-cell lymphoma | Induce apoptosis and inhibit tumor growth | Su et al.142 |
| TAPI-0 | AML | Inhibit AML cell-induced STNK cell abnormalities | Arriga et al.143 |
| Epigenetic agents | |||
| SGI-1027 | Multiple cancers | Block DNMT1 and reactivate TIMP-3 | Datta et al.144 |
| MPT0G013 | Colon cancer | Inhibit angiogenesis, tumor growth, metastasis | Wang et al.145 |
| decitabine | AML | Enhance the lytic activity of NK cells | Raneros et al.131 |
DNMT1, DNA methyltransferase 1; EGCG, epigallocatechin-3-gallate; NK, natural killer; p700, synthetic peptide derived from N-terminal domain of TIMP-3; STNK, short-term natural killer.
Conclusion
TIMP-3 is unique among the TIMP family members because it is the only TIMP that can bind firmly to the ECM after secretion. In addition, TIMP-3 can inhibit not only MMPs but also a wide range of ADAMs and ADAMTSs. Accumulating evidence indicates that the TIMP-3 gene acts as a tumor suppressor gene by inducing apoptosis and inhibiting proliferation, angiogenesis, and metastasis. However, TIMP-3 expression is downregulated by genetic and epigenetic alternation in most cancers. In this review, we have systematically described the contribution of TIMP-3 in cancer and its potential in cancer therapy including its utility as a predictor of cancer progression, its efficacy in combination therapy for cancer treatment, and its potential as a direct target for cancer therapy. Further research is required to harness the potential of TIMP-3 in diagnosing and treating cancer.
Footnotes
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
Conflict of interest statement: The authors declare that there is no conflict of interest.
ORCID iD: Shun-Fa Yang  https://orcid.org/0000-0002-0365-7927
https://orcid.org/0000-0002-0365-7927
Contributor Information
Chun-Wen Su, Institute of Medicine, Chung Shan Medical University, Taichung.
Chiao-Wen Lin, Institute of Oral Sciences, Chung Shan Medical University, Taichung; Department of Dentistry, Chung Shan Medical University Hospital, Taichung.
Wei-En Yang, Department of Medical Research, Chung Shan Medical University Hospital, Taichung.
Shun-Fa Yang, Institute of Medicine, Chung Shan Medical University, 110 Chien-Kuo N. Road, Section 1, Taichung 402; Department of Medical Research, Chung Shan Medical University Hospital, Taichung.
References
- 1. Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell 2011; 144: 646–674. [DOI] [PubMed] [Google Scholar]
- 2. Su SC, Hsieh MJ, Yang WE, et al. Cancer metastasis: mechanisms of inhibition by melatonin. J Pineal Res 2017; 62: 12370. [DOI] [PubMed] [Google Scholar]
- 3. Massague J, Obenauf AC. Metastatic colonization by circulating tumour cells. Nature 2016; 529: 298–306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Su SC, Lin CW, Yang WE, et al. The urokinase-type plasminogen activator (uPA) system as a biomarker and therapeutic target in human malignancies. Expert Opin Ther Targets 2016; 20: 551–566. [DOI] [PubMed] [Google Scholar]
- 5. Chien MH, Lin CW, Cheng CW, et al. Matrix metalloproteinase-2 as a target for head and neck cancer therapy. Expert Opin Ther Targets 2013; 17: 203–216. [DOI] [PubMed] [Google Scholar]
- 6. Yang JS, Lin CW, Su SC, et al. Pharmacodynamic considerations in the use of matrix metalloproteinase inhibitors in cancer treatment. Expert Opin Drug Metab Toxicol 2016; 12: 191–200. [DOI] [PubMed] [Google Scholar]
- 7. Ho HY, Lin CW, Chien MH, et al. Melatonin suppresses TPA-induced metastasis by downregulating matrix metalloproteinase-9 expression through JNK/SP-1 signaling in nasopharyngeal carcinoma. J Pineal Res 2016; 61: 479–492. [DOI] [PubMed] [Google Scholar]
- 8. Yang SF, Wu TF, Tsai HT, et al. New markers in pelvic inflammatory disease. Clin Chim Acta 2014; 431: 118–124. [DOI] [PubMed] [Google Scholar]
- 9. Hsieh MJ, Chen JC, Yang WE, et al. Dehydroandrographolide inhibits oral cancer cell migration and invasion through NF-kappaB-, AP-1-, and SP-1-modulated matrix metalloproteinase-2 inhibition. Biochem Pharmacol 2017; 130: 10–20. [DOI] [PubMed] [Google Scholar]
- 10. Jiang Y, Goldberg ID, Shi YE. Complex roles of tissue inhibitors of metalloproteinases in cancer. Oncogene 2002; 21: 2245–2252. [DOI] [PubMed] [Google Scholar]
- 11. Sahebjam S, Khokha R, Mort JS. Increased collagen and aggrecan degradation with age in the joints of Timp3(-/-) mice. Arthritis Rheum 2007; 56: 905–909. [DOI] [PubMed] [Google Scholar]
- 12. Tian H, Cimini M, Fedak PW, et al. TIMP-3 deficiency accelerates cardiac remodeling after myocardial infarction. J Mol Cell Cardiol 2007; 43: 733–743. [DOI] [PubMed] [Google Scholar]
- 13. Basu R, Lee J, Morton JS, et al. TIMP3 is the primary TIMP to regulate agonist-induced vascular remodelling and hypertension. Cardiovasc Res 2013; 98: 360–371. [DOI] [PubMed] [Google Scholar]
- 14. Jackson HW, Defamie V, Waterhouse P, et al. TIMPs: versatile extracellular regulators in cancer. Nat Rev Cancer 2017; 17: 38–53. [DOI] [PubMed] [Google Scholar]
- 15. Bian J, Wang Y, Smith MR, et al. Suppression of in vivo tumor growth and induction of suspension cell death by tissue inhibitor of metalloproteinases (TIMP)-3. Carcinogenesis 1996; 17: 1805–1811. [DOI] [PubMed] [Google Scholar]
- 16. Ahonen M, Baker AH, Kahari VM. Adenovirus-mediated gene delivery of tissue inhibitor of metalloproteinases-3 inhibits invasion and induces apoptosis in melanoma cells. Cancer Res 1998; 58: 2310–2315. [PubMed] [Google Scholar]
- 17. Han XG, Mo HM, Liu XQ, et al. TIMP3 overexpression improves the sensitivity of osteosarcoma to cisplatin by reducing IL-6 production. Front Genet 2018; 9: 135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Ma DH, Chen JI, Zhang F, et al. Inhibition of fibroblast-induced angiogenic phenotype of cultured endothelial cells by the overexpression of tissue inhibitor of metalloproteinase (TIMP)-3. J Biomed Sci 2003; 10: 526–534. [DOI] [PubMed] [Google Scholar]
- 19. Anania MC, Sensi M, Radaelli E, et al. TIMP3 regulates migration, invasion and in vivo tumorigenicity of thyroid tumor cells. Oncogene 2011; 30: 3011–3023. [DOI] [PubMed] [Google Scholar]
- 20. Lin H, Zhang Y, Wang H, et al. Tissue inhibitor of metalloproteinases-3 transfer suppresses malignant behaviors of colorectal cancer cells. Cancer Gene Ther 2012; 19: 845–851. [DOI] [PubMed] [Google Scholar]
- 21. Su CW, Su BF, Chiang WL, et al. Plasma levels of the tissue inhibitor matrix metalloproteinase-3 as a potential biomarker in oral cancer progression. Int J Med Sci 2017; 14: 37–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Gu X, Fu M, Ding Y, et al. TIMP-3 expression associates with malignant behaviors and predicts favorable survival in HCC. PLoS One 2014; 9: e106161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Su CW, Huang YW, Chen MK, et al. Polymorphisms and plasma levels of tissue inhibitor of metalloproteinase-3: impact on genetic susceptibility and clinical outcome of oral cancer. Medicine 2015; 94: e2092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Wu DW, Tsai LH, Chen PM, et al. Loss of TIMP-3 promotes tumor invasion via elevated IL-6 production and predicts poor survival and relapse in HPV-infected non-small cell lung cancer. Am J Pathol 2012; 181: 1796–1806. [DOI] [PubMed] [Google Scholar]
- 25. Yu JL, Lv P, Han J, et al. Methylated TIMP-3 DNA in body fluids is an independent prognostic factor for gastric cancer. Arch Pathol Lab Med 2014; 138: 1466–1473. [DOI] [PubMed] [Google Scholar]
- 26. Lee TI, Young RA. Transcriptional regulation and its misregulation in disease. Cell 2013; 152: 1237–1251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Darnton SJ, Hardie LJ, Muc RS, et al. Tissue inhibitor of metalloproteinase-3 (TIMP-3) gene is methylated in the development of esophageal adenocarcinoma: loss of expression correlates with poor prognosis. Int J Cancer 2005; 115: 351–358. [DOI] [PubMed] [Google Scholar]
- 28. Boon L, Ugarte-Berzal E, Vandooren J, et al. Glycosylation of matrix metalloproteases and tissue inhibitors: present state, challenges and opportunities. Biochem J 2016; 473: 1471–1482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Troeberg L, Lazenbatt C, Anower EKMF, et al. Sulfated glycosaminoglycans control the extracellular trafficking and the activity of the metalloprotease inhibitor TIMP-3. Chem Biol 2014; 21: 1300–1309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Brew K, Nagase H. The tissue inhibitors of metalloproteinases (TIMPs): an ancient family with structural and functional diversity. Biochim Biophys Acta 2010; 1803: 55–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Yu WH, Yu S, Meng Q, et al. TIMP-3 binds to sulfated glycosaminoglycans of the extracellular matrix. J Biol Chem 2000; 275: 31226–31232. [DOI] [PubMed] [Google Scholar]
- 32. Jobling AI, Fang Z, Koleski D, et al. Expression of the ETS transcription factor ELF3 in the retinal pigment epithelium. Invest Ophthalmol Vis Sci 2002; 43: 3530–3537. [PubMed] [Google Scholar]
- 33. Qureshi HY, Sylvester J, El Mabrouk M, et al. TGF-beta-induced expression of tissue inhibitor of metalloproteinases-3 gene in chondrocytes is mediated by extracellular signal-regulated kinase pathway and Sp1 transcription factor. J Cell Physiol 2005; 203: 345–352. [DOI] [PubMed] [Google Scholar]
- 34. Zerrouqi A, Pyrzynska B, Febbraio M, et al. P14ARF inhibits human glioblastoma-induced angiogenesis by upregulating the expression of TIMP3. J Clin Invest 2012; 122: 1283–1295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Yan D, Chen D, Hawse JR, et al. Bovine lactoferricin induces TIMP-3 via the ERK1/2-Sp1 axis in human articular chondrocytes. Gene 2013; 517: 12–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Qureshi HY, Ricci G, Zafarullah M. Smad signaling pathway is a pivotal component of tissue inhibitor of metalloproteinases-3 regulation by transforming growth factor beta in human chondrocytes. Biochim Biophys Acta 2008; 1783: 1605–1612. [DOI] [PubMed] [Google Scholar]
- 37. Shinojima T, Yu Q, Huang SK, et al. Heterogeneous epigenetic regulation of TIMP3 in prostate cancer. Epigenetics 2012; 7: 1279–1289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Xie D, Zhu J, Liu Q, et al. Dysregulation of HDAC9 represses trophoblast cell migration and invasion through TIMP3 activation in preeclampsia. Am J Hypertens 2019; 32: 515–523. [DOI] [PubMed] [Google Scholar]
- 39. Kong L, Zhang P, Li W, et al. KDM1A promotes tumor cell invasion by silencing TIMP3 in non-small cell lung cancer cells. Oncotarget 2016; 7: 27959–27974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Xu C, Hou Z, Zhan P, et al. EZH2 regulates cancer cell migration through repressing TIMP-3 in non-small cell lung cancer. Med Oncol 2013; 30: 713. [DOI] [PubMed] [Google Scholar]
- 41. Arpino V, Brock M, Gill SE. The role of TIMPs in regulation of extracellular matrix proteolysis. Matrix Biol 2015; 44–46: 247–254. [DOI] [PubMed] [Google Scholar]
- 42. Bourboulia D, Stetler-Stevenson WG. Matrix metalloproteinases (MMPs) and tissue inhibitors of metalloproteinases (TIMPs): positive and negative regulators in tumor cell adhesion. Semin Cancer Biol 2010; 20: 161–168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Amour A, Knight CG, Webster A, et al. The in vitro activity of ADAM-10 is inhibited by TIMP-1 and TIMP-3. FEBS Lett 2000; 473: 275–279. [DOI] [PubMed] [Google Scholar]
- 44. Jacobsen J, Visse R, Sorensen HP, et al. Catalytic properties of ADAM12 and its domain deletion mutants. Biochemistry 2008; 47: 537–547. [DOI] [PubMed] [Google Scholar]
- 45. Mochizuki S, Shimoda M, Shiomi T, et al. ADAM28 is activated by MMP-7 (matrilysin-1) and cleaves insulin-like growth factor binding protein-3. Biochem Biophys Res Commun 2004; 315: 79–84. [DOI] [PubMed] [Google Scholar]
- 46. Bigg HF, Morrison CJ, Butler GS, et al. Tissue inhibitor of metalloproteinases-4 inhibits but does not support the activation of gelatinase A via efficient inhibition of membrane type 1-matrix metalloproteinase. Cancer Res 2001; 61: 3610–3618. [PubMed] [Google Scholar]
- 47. English JL, Kassiri Z, Koskivirta I, et al. Individual Timp deficiencies differentially impact pro-MMP-2 activation. J Biol Chem 2006; 281: 10337–10346. [DOI] [PubMed] [Google Scholar]
- 48. Roy R, Wewer UM, Zurakowski D, et al. ADAM 12 cleaves extracellular matrix proteins and correlates with cancer status and stage. J Biol Chem 2004; 279: 51323–51330. [DOI] [PubMed] [Google Scholar]
- 49. Rodriguez-Manzaneque JC, Westling J, Thai SN, et al. ADAMTS1 cleaves aggrecan at multiple sites and is differentially inhibited by metalloproteinase inhibitors. Biochem Biophys Res Commun 2002; 293: 501–508. [DOI] [PubMed] [Google Scholar]
- 50. Forte D, Salvestrini V, Corradi G, et al. The tissue inhibitor of metalloproteinases-1 (TIMP-1) promotes survival and migration of acute myeloid leukemia cells through CD63/PI3K/Akt/p21 signaling. Oncotarget 2017; 8: 2261–2274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Toricelli M, Melo FHM, Hunger A, et al. Timp1 promotes cell survival by activating the PDK1 signaling pathway in melanoma. Cancers 2017; 9: E37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Honkavuori M, Talvensaari-Mattila A, Puistola U, et al. High serum TIMP-1 is associated with adverse prognosis in endometrial carcinoma. Anticancer Res 2008; 28: 2715–2719. [PubMed] [Google Scholar]
- 53. Ma J, Wang J, Fan W, et al. Upregulated TIMP-1 correlates with poor prognosis of laryngeal squamous cell carcinoma. Int J Clin Exp Pathol 2014; 7: 246–254. [PMC free article] [PubMed] [Google Scholar]
- 54. Thang NM, Kumasawa K, Tsutsui T, et al. Overexpression of endogenous TIMP-2 increases the proliferation of BeWo choriocarcinoma cells through the MAPK-signaling pathway. Reprod Sci 2013; 20: 1184–1192. [DOI] [PubMed] [Google Scholar]
- 55. Seo DW, Li H, Guedez L, et al. TIMP-2 mediated inhibition of angiogenesis: an MMP-independent mechanism. Cell 2003; 114: 171–180. [DOI] [PubMed] [Google Scholar]
- 56. Lizarraga F, Espinosa M, Ceballos-Cancino G, et al. Tissue inhibitor of metalloproteinases-4 (TIMP-4) regulates stemness in cervical cancer cells. Mol Carcinog 2016; 55: 1952–1961. [DOI] [PubMed] [Google Scholar]
- 57. Bachman KE, Herman JG, Corn PG, et al. Methylation-associated silencing of the tissue inhibitor of metalloproteinase-3 gene suggest a suppressor role in kidney, brain, and other human cancers. Cancer Res 1999; 59: 798–802. [PubMed] [Google Scholar]
- 58. Gu P, Xing X, Tanzer M, et al. Frequent loss of TIMP-3 expression in progression of esophageal and gastric adenocarcinomas. Neoplasia 2008; 10: 563–572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Masson D, Rioux-Leclercq N, Fergelot P, et al. Loss of expression of TIMP3 in clear cell renal cell carcinoma. Eur J Cancer 2010; 46: 1430–1437. [DOI] [PubMed] [Google Scholar]
- 60. Barski D, Wolter M, Reifenberger G, et al. Hypermethylation and transcriptional downregulation of the TIMP3 gene is associated with allelic loss on 22q12.3 and malignancy in meningiomas. Brain Pathol 2010; 20: 623–631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Wild A, Ramaswamy A, Langer P, et al. Frequent methylation-associated silencing of the tissue inhibitor of metalloproteinase-3 gene in pancreatic endocrine tumors. J Clin Endocrinol Metab 2003; 88: 1367–1373. [DOI] [PubMed] [Google Scholar]
- 62. Baker AH, George SJ, Zaltsman AB, et al. Inhibition of invasion and induction of apoptotic cell death of cancer cell lines by overexpression of TIMP-3. Br J Cancer 1999; 79: 1347–1355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Adissu HA, McKerlie C, Di Grappa M, et al. Timp3 loss accelerates tumour invasion and increases prostate inflammation in a mouse model of prostate cancer. Prostate 2015; 75: 1831–1843. [DOI] [PubMed] [Google Scholar]
- 64. Sun JX, Zhang CX, Chen ZZ, et al. Cloning and expression of the cDNA encoding human tissue inhibitor of metalloproteinase-3 and its inhibition on angiogenesis. Sheng Wu Hua Xue Yu Sheng Wu Wu Li Xue Bao (Shanghai) 1998; 30: 220–224. [PubMed] [Google Scholar]
- 65. Zhang H, Wang YS, Han G, et al. TIMP-3 gene transfection suppresses invasive and metastatic capacity of human hepatocarcinoma cell line HCC-7721. Hepatobiliary Pancreat Dis Int 2007; 6: 487–491. [PubMed] [Google Scholar]
- 66. Han XG, Li Y, Mo HM, et al. TIMP3 regulates osteosarcoma cell migration, invasion, and chemotherapeutic resistances. Tumour Biol 2016; 37: 8857–8867. [DOI] [PubMed] [Google Scholar]
- 67. Yu XF, Yang C, Liang LH, et al. Inhibition of human leukemia xenograft in nude mice by adenovirus-mediated tissue inhibitor of metalloproteinase-3. Leukemia 2006; 20: 1–8. [DOI] [PubMed] [Google Scholar]
- 68. Kornfeld JW, Meder S, Wohlberg M, et al. Overexpression of TACE and TIMP3 mRNA in head and neck cancer: association with tumour development and progression. Br J Cancer 2011; 104: 138–145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Defamie V, Sanchez O, Murthy A, et al. TIMP3 controls cell fate to confer hepatocellular carcinoma resistance. Oncogene 2015; 34: 4098–4108. [DOI] [PubMed] [Google Scholar]
- 70. Jackson HW, Hojilla CV, Weiss A, et al. Timp3 deficient mice show resistance to developing breast cancer. PLoS One 2015; 10: e0120107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Hua KT, Liu YF, Hsu CL, et al. 3′UTR polymorphisms of carbonic anhydrase IX determine the miR-34a targeting efficiency and prognosis of hepatocellular carcinoma. Sci Rep 2017; 7: 4466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Su SC, Hsieh MJ, Lin CW, et al. Impact of HOTAIR gene polymorphism and environmental risk on oral cancer. J Dent Res 2018; 97: 717–724. [DOI] [PubMed] [Google Scholar]
- 73. Tsai HT, Hsieh MJ, Chiou HL, et al. TIMP-3–1296 T>C and TIMP-4–55 T>C gene polymorphisms play a role in the susceptibility of hepatocellular carcinoma among women. Tumour Biol 2014; 35: 8999–9007. [DOI] [PubMed] [Google Scholar]
- 74. Srivastava P, Kapoor R, Mittal RD. Impact of MMP-3 and TIMP-3 gene polymorphisms on prostate cancer susceptibility in North Indian cohort. Gene 2013; 530: 273–277. [DOI] [PubMed] [Google Scholar]
- 75. Lei H, Hemminki K, Altieri A, et al. Promoter polymorphisms in matrix metalloproteinases and their inhibitors: few associations with breast cancer susceptibility and progression. Breast Cancer Res Treat 2007; 103: 61–69. [DOI] [PubMed] [Google Scholar]
- 76. Wieczorek E, Reszka E, Jablonowski Z, et al. Genetic polymorphisms in matrix metalloproteinases (MMPs) and tissue inhibitors of MPs (TIMPs), and bladder cancer susceptibility. BJU Int 2013; 112: 1207–1214. [DOI] [PubMed] [Google Scholar]
- 77. Bashash M, Shah A, Hislop G, et al. Genetic polymorphisms at TIMP3 are associated with survival of adenocarcinoma of the gastroesophageal junction. PLoS One 2013; 8: e59157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Peterson NB, Beeghly-Fadiel A, Gao YT, et al. Polymorphisms in tissue inhibitors of metalloproteinases-2 and -3 and breast cancer susceptibility and survival. Int J Cancer 2009; 125: 844–850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Liu MC, Choong DY, Hooi CS, et al. Genetic and epigenetic analysis of the TIMP-3 gene in ovarian cancer. Cancer Lett 2007; 247: 91–97. [DOI] [PubMed] [Google Scholar]
- 80. Kang SH, Choi HH, Kim SG, et al. Transcriptional inactivation of the tissue inhibitor of metalloproteinase-3 gene by DNA hypermethylation of the 5′-CpG island in human gastric cancer cell lines. Int J Cancer 2000; 86: 632–635. [DOI] [PubMed] [Google Scholar]
- 81. Zhang W, Xu J. DNA methyltransferases and their roles in tumorigenesis. Biomark Res 2017; 5: 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Rhee I, Bachman KE, Park BH, et al. DNMT1 and DNMT3b cooperate to silence genes in human cancer cells. Nature 2002; 416: 552–556. [DOI] [PubMed] [Google Scholar]
- 83. Hsu CH, Peng KL, Kang ML, et al. TET1 suppresses cancer invasion by activating the tissue inhibitors of metalloproteinases. Cell Rep 2012; 2: 568–579. [DOI] [PubMed] [Google Scholar]
- 84. Su SC, Reiter RJ, Hsiao HY, et al. Functional interaction between melatonin signaling and noncoding RNAs. Trends Endocrinol Metab 2018; 29: 435–445. [DOI] [PubMed] [Google Scholar]
- 85. Yang X, Du WW, Li H, et al. Both mature miR-17–5p and passenger strand miR-17–3p target TIMP3 and induce prostate tumor growth and invasion. Nucleic Acids Res 2013; 41: 9688–9704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Martin del Campo SE, Latchana N, Levine KM, et al. MiR-21 enhances melanoma invasiveness via inhibition of tissue inhibitor of metalloproteinases 3 expression: in vivo effects of MiR-21 inhibitor. PLoS One 2015; 10: e0115919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Li J, Zhang Y, Zhang W, et al. Genetic heterogeneity of breast cancer metastasis may be related to miR-21 regulation of TIMP-3 in translation. Int J Surg Oncol 2013; 2013: 875078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Zhang Z, Wang J, Wang X, et al. MicroRNA-21 promotes proliferation, migration, and invasion of cervical cancer through targeting TIMP3. Arch Gynecol Obstet 2018; 297: 433–442. [DOI] [PubMed] [Google Scholar]
- 89. Wang N, Zhang CQ, He JH, et al. MiR-21 down-regulation suppresses cell growth, invasion and induces cell apoptosis by targeting FASL, TIMP3, and RECK genes in esophageal carcinoma. Dig Dis Sci 2013; 58: 1863–1870. [DOI] [PubMed] [Google Scholar]
- 90. Selaru FM, Olaru AV, Kan T, et al. MicroRNA-21 is overexpressed in human cholangiocarcinoma and regulates programmed cell death 4 and tissue inhibitor of metalloproteinase 3. Hepatology 2009; 49: 1595–1601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Nagao Y, Hisaoka M, Matsuyama A, et al. Association of microRNA-21 expression with its targets, PDCD4 and TIMP3, in pancreatic ductal adenocarcinoma. Mod Pathol 2012; 25: 112–121. [DOI] [PubMed] [Google Scholar]
- 92. Chen J, Zhou C, Li J, et al. miR215p confers doxorubicin resistance in gastric cancer cells by targeting PTEN and TIMP3. Int J Mol Med 2018; 41: 1855–1866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Dai J, Lin Y, Duan Y, et al. Andrographolide inhibits angiogenesis by inhibiting the Mir-21–5p/TIMP3 signaling pathway. Int J Biol Sci 2017; 13: 660–668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Wang B, Hsu SH, Majumder S, et al. TGFbeta-mediated upregulation of hepatic miR-181b promotes hepatocarcinogenesis by targeting TIMP3. Oncogene 2010; 29: 1787–1797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Zhou Q, Zheng X, Chen L, et al. Smad2/3/4 pathway contributes to TGF-beta-induced MiRNA-181b expression to promote gastric cancer metastasis by targeting Timp3. Cell Physiol Biochem 2016; 39: 453–466. [DOI] [PubMed] [Google Scholar]
- 96. Dong M, Yang P, Hua F. MiR-191 modulates malignant transformation of endometriosis through regulating TIMP3. Med Sci Monit 2015; 21: 915–920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Du L, Ma S, Wen X, et al. Oral squamous cell carcinoma cells are resistant to doxorubicin through upregulation of miR221. Mol Med Rep 2017; 16: 2659–2667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Chen D, Yan W, Liu Z, et al. Downregulation of miR-221 enhances the sensitivity of human oral squamous cell carcinoma cells to Adriamycin through upregulation of TIMP3 expression. Biomed Pharmacother 2016; 77: 72–78. [DOI] [PubMed] [Google Scholar]
- 99. Garofalo M, Di Leva G, Romano G, et al. miR-221&222 regulate TRAIL resistance and enhance tumorigenicity through PTEN and TIMP3 downregulation. Cancer Cell 2009; 16: 498–509. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 100. Gan R, Yang Y, Yang X, et al. Downregulation of miR-221/222 enhances sensitivity of breast cancer cells to tamoxifen through upregulation of TIMP3. Cancer Gene Ther 2014; 21: 290–296. [DOI] [PubMed] [Google Scholar]
- 101. Liu W, Li M, Chen X, et al. MicroRNA-373 promotes migration and invasion in human esophageal squamous cell carcinoma by inhibiting TIMP3 expression. Am J Cancer Res 2016; 6: 1–14. [PMC free article] [PubMed] [Google Scholar]
- 102. Ayers D, Vandesompele J. Influence of microRNAs and long non-coding RNAs in cancer chemoresistance. Genes 2017; 8: E95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Rinn JL, Chang HY. Genome regulation by long noncoding RNAs. Annu Rev Biochem 2012; 81: 145–166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Wilusz JE, Sunwoo H, Spector DL. Long noncoding RNAs: functional surprises from the RNA world. Genes Dev 2009; 23: 1494–1504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Lee JT. Epigenetic regulation by long noncoding RNAs. Science 2012; 338: 1435–1439. [DOI] [PubMed] [Google Scholar]
- 106. Lin J, Tan X, Qiu L, et al. Long noncoding RNA BC032913 as a novel therapeutic target for colorectal cancer that suppresses metastasis by upregulating TIMP3. Mol Ther Nucleic Acids 2017; 8: 469–481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Jia J, Li F, Tang XS, et al. Long noncoding RNA DANCR promotes invasion of prostate cancer through epigenetically silencing expression of TIMP2/3. Oncotarget 2016; 7: 37868–37881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Deng X, Bhagat S, Dong Z, et al. Tissue inhibitor of metalloproteinase-3 induces apoptosis in prostate cancer cells and confers increased sensitivity to paclitaxel. Eur J Cancer 2006; 42: 3267–3273. [DOI] [PubMed] [Google Scholar]
- 109. Bond M, Murphy G, Bennett MR, et al. Tissue inhibitor of metalloproteinase-3 induces a Fas-associated death domain-dependent type II apoptotic pathway. J Biol Chem 2002; 277: 13787–13795. [DOI] [PubMed] [Google Scholar]
- 110. Ahonen M, Poukkula M, Baker AH, et al. Tissue inhibitor of metalloproteinases-3 induces apoptosis in melanoma cells by stabilization of death receptors. Oncogene 2003; 22: 2121–2134. [DOI] [PubMed] [Google Scholar]
- 111. Yang Y, Jalal FY, Thompson JF, et al. Tissue inhibitor of metalloproteinases-3 mediates the death of immature oligodendrocytes via TNF-alpha/TACE in focal cerebral ischemia in mice. J Neuroinflammation 2011; 8: 108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Cook EB, Stahl JL, Graziano FM, et al. Regulation of the receptor for TNFalpha, TNFR1, in human conjunctival epithelial cells. Invest Ophthalmol Vis Sci 2008; 49: 3992–3998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Kallio JP, Hopkins-Donaldson S, Baker AH, et al. TIMP-3 promotes apoptosis in nonadherent small cell lung carcinoma cells lacking functional death receptor pathway. Int J Cancer 2011; 128: 991–996. [DOI] [PubMed] [Google Scholar]
- 114. Zhang L, Zhao L, Zhao D, et al. Inhibition of tumor growth and induction of apoptosis in prostate cancer cell lines by overexpression of tissue inhibitor of matrix metalloproteinase-3. Cancer Gene Ther 2010; 17: 171–179. [DOI] [PubMed] [Google Scholar]
- 115. Mahller YY, Vaikunth SS, Ripberger MC, et al. Tissue inhibitor of metalloproteinase-3 via oncolytic herpesvirus inhibits tumor growth and vascular progenitors. Cancer Res 2008; 68: 1170–1179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Ahonen M, Ala-Aho R, Baker AH, et al. Antitumor activity and bystander effect of adenovirally delivered tissue inhibitor of metalloproteinases-3. Mol Ther 2002; 5: 705–715. [DOI] [PubMed] [Google Scholar]
- 117. Qi JH, Ebrahem Q, Moore N, et al. A novel function for tissue inhibitor of metalloproteinases-3 (TIMP3): inhibition of angiogenesis by blockage of VEGF binding to VEGF receptor-2. Nat Med 2003; 9: 407–415. [DOI] [PubMed] [Google Scholar]
- 118. Kang KH, Park SY, Rho SB, et al. Tissue inhibitor of metalloproteinases-3 interacts with angiotensin II type 2 receptor and additively inhibits angiogenesis. Cardiovasc Res 2008; 79: 150–160. [DOI] [PubMed] [Google Scholar]
- 119. Qi JH, Anand-Apte B. Tissue inhibitor of metalloproteinase-3 (TIMP3) promotes endothelial apoptosis via a caspase-independent mechanism. Apoptosis 2015; 20: 523–534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. Cruz-Munoz W, Kim I, Khokha R. TIMP-3 deficiency in the host, but not in the tumor, enhances tumor growth and angiogenesis. Oncogene 2006; 25: 650–655. [DOI] [PubMed] [Google Scholar]
- 121. Lin CW, Yang WE, Lee WJ, et al. Lipocalin 2 prevents oral cancer metastasis through carbonic anhydrase IX inhibition and is associated with favourable prognosis. Carcinogenesis 2016; 37: 712–722. [DOI] [PubMed] [Google Scholar]
- 122. Reiter RJ, Rosales-Corral SA, Tan DX, et al. Melatonin, a full service anti-cancer agent: inhibition of initiation, progression and metastasis. Int J Mol Sci 2017; 18: E843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123. Bernhard EJ, Gruber SB, Muschel RJ. Direct evidence linking expression of matrix metalloproteinase 9 (92-kDa gelatinase/collagenase) to the metastatic phenotype in transformed rat embryo cells. Proc Natl Acad Sci U S A 1994; 91: 4293–4297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124. Sternlicht MD, Lochter A, Sympson CJ, et al. The stromal proteinase MMP3/stromelysin-1 promotes mammary carcinogenesis. Cell 1999; 98: 137–146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125. Guo YJ, Liu G, Wang X, et al. Potential use of soluble CD44 in serum as indicator of tumor burden and metastasis in patients with gastric or colon cancer. Cancer Res 1994; 54: 422–426. [PubMed] [Google Scholar]
- 126. Nakamura H, Suenaga N, Taniwaki K, et al. Constitutive and induced CD44 shedding by ADAM-like proteases and membrane-type 1 matrix metalloproteinase. Cancer Res 2004; 64: 876–882. [DOI] [PubMed] [Google Scholar]
- 127. Cruz-Munoz W, Sanchez OH, Di Grappa M, et al. Enhanced metastatic dissemination to multiple organs by melanoma and lymphoma cells in timp-3-/- mice. Oncogene 2006; 25: 6489–6496. [DOI] [PubMed] [Google Scholar]
- 128. Cheng HL, Lin CW, Yang JS, et al. Zoledronate blocks geranylgeranylation not farnesylation to suppress human osteosarcoma U2OS cells metastasis by EMT via Rho A activation and FAK-inhibited JNK and p38 pathways. Oncotarget 2016; 7: 9742–9758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129. Wen YC, Lee WJ, Tan P, et al. By inhibiting snail signaling and miR-23a-3p, osthole suppresses the EMT-mediated metastatic ability in prostate cancer. Oncotarget 2015; 6: 21120–21136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130. Ninomiya I, Kawakami K, Fushida S, et al. Quantitative detection of TIMP-3 promoter hypermethylation and its prognostic significance in esophageal squamous cell carcinoma. Oncol Rep 2008; 20: 1489–1495. [PubMed] [Google Scholar]
- 131. Raneros AB, Puras AM, Rodriguez RM, et al. Increasing TIMP3 expression by hypomethylating agents diminishes soluble MICA, MICB and ULBP2 shedding in acute myeloid leukemia, facilitating NK cell-mediated immune recognition. Oncotarget 2017; 8: 31959–31976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132. Sun W, Zaboli D, Wang H, et al. Detection of TIMP3 promoter hypermethylation in salivary rinse as an independent predictor of local recurrence-free survival in head and neck cancer. Clin Cancer Res 2012;18(4):1082–1091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133. Shen X, Gao X, Li H, et al. TIMP-3 increases the chemosensitivity of laryngeal carcinoma to cisplatin via facilitating mitochondria-dependent apoptosis. Oncol Res 2018; 27: 73–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134. Zhang Y, Qian H, Lin C, et al. Adenovirus carrying TIMP-3: a potential tool for cervical cancer treatment. Gynecol Oncol 2008; 108: 234–240. [DOI] [PubMed] [Google Scholar]
- 135. Su B, Su J, He H, et al. Identification of potential targets for diallyl disulfide in human gastric cancer MGC-803 cells using proteomics approaches. Oncol Rep 2015; 33: 2484–2494. [DOI] [PubMed] [Google Scholar]
- 136. Deb G, Thakur VS, Limaye AM, et al. Epigenetic induction of tissue inhibitor of matrix metalloproteinase-3 by green tea polyphenols in breast cancer cells. Mol Carcinog 2015; 54: 485–499. [DOI] [PubMed] [Google Scholar]
- 137. Wang P, Solorzano W, Diaz T, et al. Arctigenin inhibits prostate tumor cell growth in vitro and in vivo. Clin Nutr Exp 2017; 13: 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138. Lin RK, Hsu CH, Wang YC. Mithramycin A inhibits DNA methyltransferase and metastasis potential of lung cancer cells. Anticancer Drugs 2007; 18: 1157–1164. [DOI] [PubMed] [Google Scholar]
- 139. Penny LK, Wallace HM. The challenges for cancer chemoprevention. Chem Soc Rev 2015; 44: 8836–8847. [DOI] [PubMed] [Google Scholar]
- 140. Destouches D, Huet E, Sader M, et al. Multivalent pseudopeptides targeting cell surface nucleoproteins inhibit cancer cell invasion through tissue inhibitor of metalloproteinases 3 (TIMP-3) release. J Biol Chem 2012; 287: 43685–43693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141. Chen YY, Brown NJ, Jones R, et al. A peptide derived from TIMP-3 inhibits multiple angiogenic growth factor receptors and tumour growth and inflammatory arthritis in mice. Angiogenesis 2014; 17: 207–219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142. Su Y, Sun B, Lin X, et al. Therapeutic strategy with artificially-designed i-lncRNA targeting multiple oncogenic microRNAs exhibits effective antitumor activity in diffuse large B-cell lymphoma. Oncotarget 2016; 7: 49143–49155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143. Arriga R, Caratelli S, Coppola A, et al. Enhancement of anti-leukemia activity of NK cells in vitro and in vivo by inhibition of leukemia cell-induced NK cell damage. Oncotarget 2016; 7: 2070–2079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144. Datta J, Ghoshal K, Denny WA, et al. A new class of quinoline-based DNA hypomethylating agents reactivates tumor suppressor genes by blocking DNA methyltransferase 1 activity and inducing its degradation. Cancer Res 2009; 69: 4277–4285. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 145. Wang CY, Liou JP, Tsai AC, et al. A novel action mechanism for MPT0G013, a derivative of arylsulfonamide, inhibits tumor angiogenesis through up-regulation of TIMP3 expression. Oncotarget 2014; 5: 9838–9850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146. Howell PM, Liu Z, Khong HT. Demethylating agents in the treatment of cancer. Pharmaceuticals 2010; 3: 2022–2044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147. Wen ZK, Xu W, Xu L, et al. DNA hypomethylation is crucial for apoptotic DNA to induce systemic lupus erythematosus-like autoimmune disease in SLE-non-susceptible mice. Rheumatology 2007; 46: 1796–1803. [DOI] [PubMed] [Google Scholar]
- 148. Zhao M, Tang J, Gao F, et al. Hypomethylation of IL10 and IL13 promoters in CD4+ T cells of patients with systemic lupus erythematosus. J Biomed Biotechnol 2010; 2010: 931018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149. Pakneshan P, Szyf M, Rabbani SA. Methylation and inhibition of expression of uPA by the RAS oncogene: divergence of growth control and invasion in breast cancer cells. Carcinogenesis 2005; 26: 557–564. [DOI] [PubMed] [Google Scholar]
- 150. Duchmann M, Itzykson R. Clinical update on hypomethylating agents. Int J Hematol. Epub ahead of print 24 April 2019. DOI: 10.1007/s12185-019-02651-9. [DOI] [PubMed] [Google Scholar]