Summary
Leucine‐rich repeat‐receptor‐like proteins (LRR‐RLPs) and LRR‐receptor‐like kinases (LRR‐RLKs) trigger immune signalling to promote plant resistance against pathogens. LRR‐RLPs lack an intracellular kinase domain, and several of these receptors have been shown to constitutively interact with the LRR‐RLK Suppressor of BIR1‐1/EVERSHED (SOBIR1/EVR) to form signalling‐competent receptor complexes. Ligand perception by LRR‐RLPs initiates recruitment of the co‐receptor BRI1‐Associated Kinase 1/Somatic Embryogenesis Receptor Kinase 3 (BAK1/SERK3) to the LRR‐RLP/SOBIR1 complex, thereby activating LRR‐RLP‐mediated immunity. We employed phosphorylation analysis of in planta‐produced proteins, live cell imaging, gene silencing and co‐immunoprecipitation to investigate the roles of SOBIR1 and BAK1 in immune signalling. We show that Arabidopsis thaliana (At) SOBIR1, which constitutively activates immune responses when overexpressed in planta, is highly phosphorylated. Moreover, in addition to the kinase activity of SOBIR1 itself, kinase‐active BAK1 is essential for AtSOBIR1‐induced constitutive immunity and for the phosphorylation of AtSOBIR1. Furthermore, the defence response triggered by the tomato LRR‐RLP Cf‐4 on perception of Avr4 from the extracellular pathogenic fungus Cladosporium fulvum is dependent on kinase‐active BAK1. We argue that, in addition to the trans‐autophosphorylation of SOBIR1, it is likely that SOBIR1 and BAK1 transphosphorylate, and thereby activate the receptor complex. The signalling‐competent cell surface receptor complex subsequently activates downstream cytoplasmic signalling partners to initiate RLP‐mediated immunity.
Keywords: BAK1/SERK3, Cf‐4, immunity, phosphorylation, RLK, RLP, SOBIR1
Introduction
Plants are sessile organisms and possess several layers of defence to protect themselves against pathogens. One layer comprises transmembrane (TM) receptors which are present at the cell surface. Unlike mammals, plants have evolved highly expanded families of TM receptors, which sense non‐self or danger‐related molecules in the extracellular space and initiate downstream signalling to mount plant immunity (Zipfel, 2014). TM receptors comprise receptor‐like kinases (RLKs) and receptor‐like proteins (RLPs). Both RLKs and RLPs contain ectodomains for ligand recognition, which, in many cases, are leucine‐rich repeat (LRR) domains (Böhm et al., 2014; Couto and Zipfel, 2016; Macho and Zipfel, 2014). LRR‐RLKs (further referred to as RLKs) carry an intracellular kinase domain, whereas LRR‐RLPs (further referred to as RLPs) do not. Whilst the expansion of receptor families enables co‐evolution with infectious pathogens, it challenges plants to link newly evolved receptors with downstream immune signalling pathways. In line with this, it is now emerging that receptors form heteromeric kinase complexes to induce immune signalling (Dufayard et al., 2017; Fischer et al., 2016).
RLPs, lacking an intracellular signalling domain, constitutively interact with the RLK Suppressor Of BIR1‐1/EVERSHED (SOBIR1/EVR, hereafter referred to as SOBIR1), thereby providing a kinase domain that is thought to function in downstream signalling (Gao et al., 2009; Gust and Felix, 2014; Leslie et al., 2010; Liebrand et al., 2014). SOBIR1 was initially found to interact with Cf proteins from tomato (Solanum lycopersicum, Sl), conferring resistance to the extracellular fungal pathogen Cladosporium fulvum (Liebrand et al., 2013). Since this initial discovery, it has been shown that SOBIR1 constitutively interacts with many RLPs involved in immunity and development (Bi et al., 2014; Catanzariti et al., 2017; Domazakis et al., 2018; Hegenauer et al., 2016; Jehle et al., 2013; Liebrand et al., 2013; Ma and Borhan, 2015; Wang et al., 2018; Zhang et al., 2014; Zhang et al., 2013a). In addition to the stabilization of the associated RLP by SOBIR1, SOBIR1 is thought to be involved in downstream signalling on RLP activation by its matching ligand (Liebrand et al., 2013, 2014).
The RLK BRI1‐Associated Receptor Kinase 1/Somatic Embryogenesis Receptor Kinase 3 (BAK1/SERK3, hereafter referred to as BAK1) functions as a co‐receptor of several well‐studied RLKs from Arabidopsis, including Flagellin‐Sensing 2 (FLS2), Elongation Factor‐Tu Receptor (EFR) and the brassinosteroid (BR) receptor Brassinosteroid‐Insensitive 1 (BRI1) (Gómez‐Gómez and Boller, 2000; Li and Chory, 1997; Nam and Li, 2002; Zipfel et al., 2006). These RLKs form complexes with BAK1 on association with their ligands (Chinchilla et al., 2007; Heese et al., 2007; Santiago et al., 2013; Somssich et al., 2015; Sun et al., 2013a, 2013b). RLK/BAK1 complex formation is followed by the transphosphorylation of both kinase domains, the initiation of downstream signalling and the internalization of the activated RLK/BAK1 complex through endocytosis (Couto and Zipfel, 2016; Frescatada‐Rosa et al., 2015; Schwessinger et al., 2011; Wang et al., 2008). Differential auto‐ and transphosphorylation have been suggested to take place for BAK1 when complexed with RLKs, signalling for either defence or development, and this differential phosphorylation eventually leads to the desired output (Macho et al., 2014, 2015; Oh et al., 2011; Schwessinger et al., 2011; Wang et al., 2008).
Similar to the situation with RLKs, BAK1 has also recently been found to be recruited to two‐component RLP/SOBIR1 complexes on ligand recognition by the RLP involved (Albert et al., 2015; Domazakis et al., 2018; Postma et al., 2016; Wang et al., 2018). For example, BAK1 is specifically recruited to the Cf‐4/SOBIR1 complex on perception of Avr4 from C. fulvum by Cf‐4 (Postma et al., 2016). Similarly, Albert et al. (2015) showed that the RLP23/SOBIR1 complex recruits BAK1 to mediate immunity triggered by necrosis and ethylene‐inducing peptide‐like 1 proteins (NLPs). Reminiscent of BAK1‐mediated receptor complex activation for cell surface complexes involving the RLKs FLS2, EFR and BRI1, it is likely that BAK1, on recruitment, also forms signalling‐competent receptor complexes with RLP/SOBIR1 bipartite RLKs, thereby mediating RLP signalling. In agreement with this, SOBIR1 kinase activity is essential for its function downstream of Cf‐4, as it has been shown that a kinase‐dead mutant of SOBIR1 is unable to complement Cf‐4/Avr4 signalling and endocytosis of the Cf‐4/SOBIR1 complex (Bi et al., 2015; Liebrand et al., 2013; Postma et al., 2016). However, until now, it has remained unclear how the kinase domain of SOBIR1 contributes to RLP/SOBIR1 receptor complex signalling.
SOBIR1 was initially identified as a positive regulator of cell death. It was found that Arabidopsis BAK1‐Interacting RLK 1 (bir1‐1) loss‐of‐function mutants showed severe dwarfing as a result of constitutive immunity, which was partially suppressed by a sobir1 loss‐of‐function mutation (Gao et al., 2009). Furthermore, overexpression of SOBIR1 in Arabidopsis induced constitutive immunity, which was observed as constitutive cell death and defence gene activation (Gao et al., 2009). BIR1 is a negative regulator of defence, which sequesters BAK1 away from active signalling complexes (Gao et al., 2009; Liu et al., 2016). Liu et al. (2016) showed that, upon silencing of BIR1 in Arabidopsis, more BAK1 becomes available, and constitutively interacts with SOBIR1.
Recently, we have observed that transient heterologous overexpression of Arabidopsis thaliana (At)SOBIR1 in Nicotiana tabacum (tobacco, Nt) and N. benthamiana (Nb), in contrast with transient overexpression of SlSOBIR1 and NbSOBIR1, also results in constitutive immunity (Wu et al., 2018). This constitutive immunity is typically manifested by the induction of cell death [the hypersensitive response (HR)] and mitogen‐activated protein kinase (MAPK) activation (Wu et al., 2018). Kinase‐dead AtSOBIR1D498N does not induce constitutive immunity, indicating that SOBIR1 kinase activity is essential for this phenomenon. The lack of constitutive immune activation by SlSOBIR1 and NbSOBIR1 in tobacco and N. benthamiana might be explained by the negative regulation of these Solanaceous orthologues of SOBIR1 by endogenous phosphatases of the Solanaceous plants tobacco and N. benthamiana. Negative regulation of immune receptors via dephosphorylation is a well‐known phenomenon (Couto and Zipfel, 2016). BAK1, for example, has been shown to be negatively regulated by Protein Phosphatase type 2A (PP2A) (Segonzac et al., 2014). Possibly, endogenous Solanaceous phosphatases successfully negatively regulate the activity of tomato and N. benthamiana SOBIR1 via dephosphorylation. However, these phosphatases might not be able to keep AtSOBIR1 in check, for example, because of the lower affinity for this heterologous SOBIR1 orthologue (Wu et al., 2018).
Here, we set out to determine how SOBIR1 activates defence signalling. For this, we exploited the phenomenon of AtSOBIR1 triggering constitutive immune signalling in N. benthamiana, leading to cell death. We show that AtSOBIR1 is clearly phosphorylated when transiently expressed in N. benthamiana, whereas the kinase‐dead mutant AtSOBIR1D498N is not. We demonstrate that the overall phosphorylation status of AtSOBIR1 is positively linked with its constitutive immune activity. We found that SOBIR1 constitutively forms homodimers, as well as heterodimers, with BAK1. Interestingly, the Cf‐4/Avr4‐triggered defence response, as well as AtSOBIR1 constitutive immunity and AtSOBIR1 phosphorylation, all depend on defence signalling‐competent BAK1, in addition to SOBIR1 kinase activity. These findings are in agreement with a model in which BAK1, which is recruited to the RLP/SOBIR1 complex on ligand perception by an RLP, and SOBIR1 probably transphosphorylate each other to signal for immunity.
Results
Constitutive immune activity of AtSOBIR1 is positively linked with its phosphorylation status
Leslie et al. (2010) showed that the kinase domain of AtSOBIR1 trans‐autophosphorylates in vitro at serine (Ser), threonine (Thr) and tyrosine (Tyr) residues. To determine whether in planta phosphorylation of SOBIR1 plays a role in signalling for constitutive immunity induced by this RLK (Wu et al., 2018), we transiently overexpressed full‐length enhanced green fluorescent protein (eGFP)‐tagged Arabidopsis and tomato SOBIR1 proteins, and the corresponding kinase‐dead mutants in which the catalytic aspartic acid (Asp, D) is mutated to asparagine (Asn, N), in N. benthamiana in combination with P19. Subsequently, we analysed their overall phosphorylation status by Pro‐Q staining. This revealed that AtSOBIR1 is highly phosphorylated, whereas SlSOBIR1 and both kinase‐dead mutants are not (Fig. 1A). It appears that the phosphorylation status of AtSOBIR1 is positively linked with its constitutive immune activity (Fig. S1A, see Supporting Information) (Wu et al., 2018). The lack of a Pro‐Q signal for kinase‐dead AtSOBIR1 suggests that the wild‐type RD‐kinase of AtSOBIR1 trans‐autophosphorylates in planta, as it has previously been shown to do in vitro (Leslie et al., 2010). In addition, the low phosphorylation status of AtSOBIR1D489N suggests that it might be necessary for AtSOBIR1 to first activate a potential signalling partner, which then, in its turn, fully activates AtSOBIR1 by transphosphorylation. Observations by confocal microscopy showed that, similar to previous observations for SlSOBIR1 (Postma et al., 2016), both wild‐type AtSOBIR1‐eGFP and kinase‐dead AtSOBIR1R489N‐eGFP localize at the plasma membrane (PM), where such events can take place (Fig. 1B).
Figure 1.
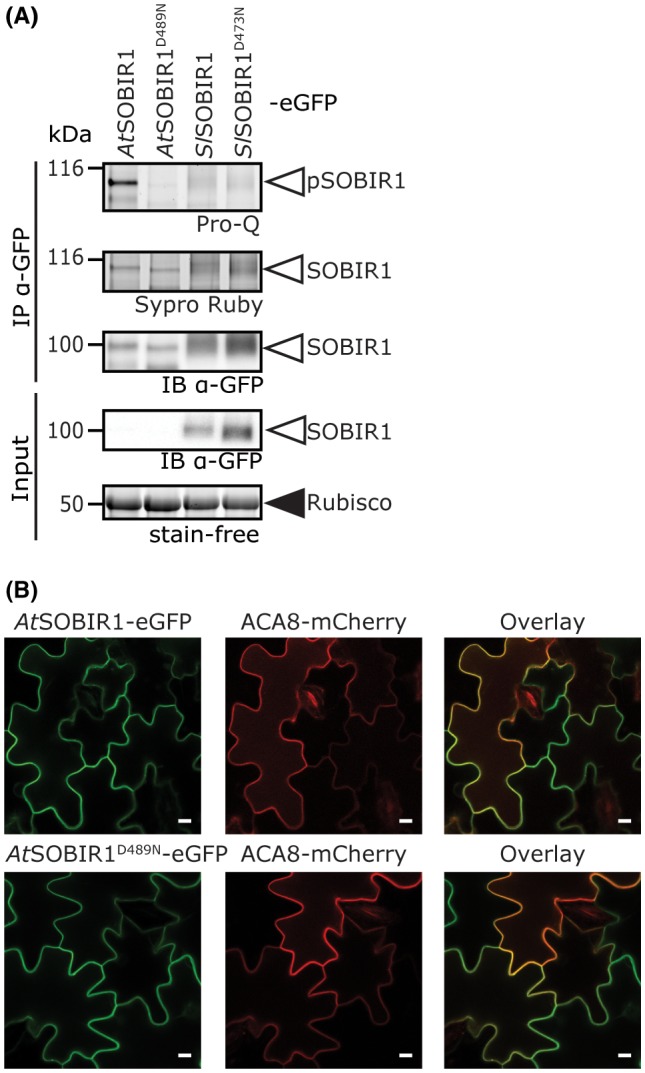
Constitutive immune activity of Arabidopsis thaliana (At)SOBIR1 positively links with its phosphorylation status. (A) Pro‐Q phosphoprotein staining of immunoprecipitated enhanced green fluorescent protein (eGFP)‐tagged SOBIR1 variants from Arabidopsis and tomato shows constitutive phosphorylation of AtSOBIR1 (pAtSOBIR1), but not of Solanum lycopersicum (Sl)SOBIR1. Furthermore, both the Arabidopsis and tomato kinase‐dead mutants show no obvious phosphorylation. Agroinfiltrations were performed on Nicotiana benthamiana leaves at an optical density at 600 nm (OD600) of 1, with co‐infiltration of P19 also at an OD600 of 1. Leaves were harvested about 40 h after agroinfiltration, before cell death became apparent, followed by immunoprecipitation (IP) using anti‐GFP affinity beads. Sypro Ruby staining and immune blotting (IB) show SOBIR1 protein levels. It should be noted that the band representing pAtSOBIR1 shows a slightly higher molecular weight than unphosphorylated AtSOBIR1D498N on the Sypro Ruby‐stained gel and αGFP IB. The ribulose‐1,5‐bisphosphate carboxylase/oxygenase (Rubisco) band of the input shows equal loading. (See also Fig. S1.) It should be noted that, because of its low level of accumulation, AtSOBIR1 is not visible in the input sample. (B) AtSOBIR1 and its kinase‐dead version localize at the plasma membrane. Leaves of tobacco were agroinfiltrated with constructs driving the expression of eGFP‐tagged wild‐type AtSOBIR1 or the kinase‐dead mutant AtSOBIR1D489N, and plasma membrane‐localized ACA8‐mCherry was co‐expressed. Overlay images indicate the co‐localization of the proteins fused to GFP and mCherry, as a yellow colour is produced (right panels). Confocal microscopy analysis was performed at 2 days post‐infiltration (dpi). White bars represent 10 µm. The experiment was performed three times and representative photographs are shown.
Interestingly, constitutive immunity and the functionality of SOBIR1 downstream of Cf‐4 are dependent on SOBIR1 kinase activity (Liebrand et al., 2013; Wu et al., 2018). The stabilization of Cf‐4 by SOBIR1 is independent of SOBIR1 kinase activity, and so this stabilization effect by itself cannot explain the role of SOBIR1 in RLP‐mediated signalling (Fig. S1B) (Liebrand et al., 2013). This again points to a signalling role by the kinase domain of SOBIR1. The lack of a Pro‐Q signal for SlSOBIR1 supports the hypothesis that negative regulation probably takes place through dephosphorylation of this solanaceous SOBIR1 by endogenous phosphatases in N. benthamiana (Fig. 1A), as suggested by Wu et al. (2018) and reviewed by Couto and Zipfel (2016).
Together, these data show that the constitutive immune activity of AtSOBIR1 is positively linked with its phosphorylation status, and that the immune activity of SlSOBIR1 is probably kept in check by maintaining low phosphorylation levels.
SOBIR1 constitutively forms homodimers
To explore whether trans‐autophosphorylation of SOBIR1 might play a role in signalling for defence by the RD‐kinase SOBIR1, we analysed whether SOBIR1 forms homodimers in planta. Transient co‐expression of eGFP‐ and Myc‐tagged SOBIR1 orthologues, followed by immunoprecipitation (IP) of eGFP‐tagged SOBIR1, resulted in the co‐purification of SOBIR1‐Myc (Fig. 2A). This indicates that both AtSOBIR1 and SlSOBIR1 form homodimers in planta. In addition, kinase‐dead variants of AtSOBIR1 and SlSOBIR1 also form homodimers (Fig. S2, see Supporting Information). This shows that the lack of phosphorylation by kinase‐dead AtSOBIR1D489N is not caused by an inability to form homodimers. Much higher amounts of SOBIR1‐Myc co‐purified with the IP of Cf‐4‐eGFP, used as a positive control, than with the IP of SOBIR1‐eGFP (Fig. 2A). This suggests that probably only a small pool of SOBIR1 protein is present in the form of homodimers in planta, and this probably explains why the homodimerization of SOBIR1 was not observed previously (Liebrand et al., 2013). Similar to the earlier observation by Liebrand et al. (2013), SOBIR1‐Myc does not co‐purify with FLS2‐eGFP, here used as a negative control (Fig. 2A).
Figure 2.
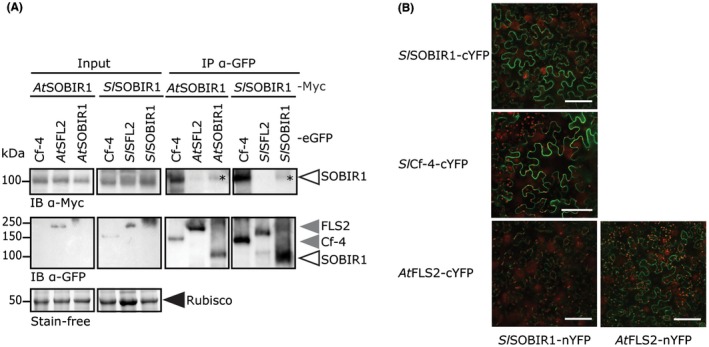
SOBIR1 constitutively forms homodimers in planta. (A) Myc‐tagged versions of AtSOBIR1 and SlSOBIR1 co‐immunoprecipitate with eGFP‐tagged versions of AtSOBIR1 and SlSOBIR1 (asterisks), respectively, and with Cf‐4‐eGFP, but not with Flagellin‐Sensing 2 (FLS2)‐eGFP. Co‐agroinfiltrations of the various affinity‐tagged proteins were performed in combination with P19 in N. benthamiana leaves at an optical density at OD600 of 0.6 for each construct. Leaves were harvested at 2 dpi, and subjected to IP using anti‐GFP beads, followed by immune blotting (IB). The Rubisco band of the input shows equal loading. The experiment was performed twice and representative results are shown. It should be noted that, because of the low accumulation levels, not all proteins are visible in the input samples. (B) A split‐YFP (yellow fluorescent protein) experiment shows the interaction between SlSOBIR1‐nYFP and SlSOBIR1‐cYFP at the plasma membrane. Leaves of N. benthamiana were agroinfiltrated with constructs driving expression of the indicated constructs, and analysed for interaction at 2 dpi using confocal microscopy. Chloroplast autofluorescence is depicted in red. White bars represent 100 µm. The experiment was performed three times and representative photographs are shown. [Colour figure can be viewed at wileyonlinelibrary.com]
A split‐YFP (yellow fluorescent protein) experiment confirmed the homodimerization of SlSOBIR1 in planta (Fig. 2B). For this, SlSOBIR1 fused to the N‐terminal half of YFP (nYFP) was co‐expressed with SlSOBIR1 fused to the C‐terminal half of YFP (cYFP). Subsequent observation by confocal microscopy revealed a clear signal of reconstituted full‐length YFP. As a positive control, co‐expression of SlSOBIR1‐nYFP with Cf‐4‐cYFP was performed, which also resulted in a clear YFP signal. The expression of SlSOBIR1‐nYFP in combination with AtFLS2‐cYFP, performed as a negative control, did not reconstitute a YFP signal (Fig. 2B). A clear YFP signal on co‐expression of AtFLS2‐cYFP and AtFLS2‐nYFP (Fig. 2B) confirmed that FLS2 also forms homodimers in planta (Sun et al., 2012).
As AtSOBIR1 is phosphorylated in planta (Fig. 1A), and SOBIR1 is able to constitutively form homodimers in planta, it is mechanistically possible that SOBIR1 trans‐autophosphorylates.
Constitutive immune activity of AtSOBIR1 is dependent on BAK1
To analyse whether BAK1, in addition to its role in RLP/SOBIR1‐mediated immunity, also plays a role in AtSOBIR1‐induced constitutive immunity and phosphorylation of SOBIR1, we examined whether, in addition to ligand‐induced BAK1 recruitment, a constitutive interaction between BAK1 and SOBIR1 takes place. To test this, we co‐expressed tagged BAK1 and SOBIR1 in N. benthamiana in the presence of P19. Interestingly, we found that BAK1 co‐immunoprecipitates with AtSOBIR1D489N and SlSOBIR1 (Fig. 3A). Wild‐type AtSOBIR1 often only accumulates to low levels because of its constitutive immune activity (Gao et al., 2009; Wu et al., 2018), and a complex of SOBIR1 and BAK1 is likely to be degraded by the plant to prevent the onset of an immune response and, consequently, cell death (Mbengue et al., 2016). Therefore, the presence of a possible interaction between AtBAK1 and AtSOBIR1 could not be determined (Fig. 3A). The fact that we could not determine an interaction between AtBAK1 and AtSOBIR1 is in agreement with the findings of Liu et al. (2016), who showed an interaction between AtSOBIR1 and AtBAK1 only on silencing of BIR1 in Arabidopsis. It is probable that a small pool of SOBIR1 and BAK1 constitutively interacts when overexpressed in planta, and this pool becomes larger on ligand elicitation (Albert et al., 2015; Postma et al., 2016; Wang et al., 2018) or BIR1 silencing (Liu et al., 2016). Together, these results suggest that SOBIR1 forms heterodimers with BAK1, independent of the constitutive immune activity of SOBIR1, and the strong phosphorylation status of constitutively active AtSOBIR1 cannot simply be explained by SOBIR1 interaction with BAK1, as SlSOBIR1 also constitutively interacts with BAK1 and phosphorylation of SlSOBIR1 is not apparent (Fig. 1A).
Figure 3.
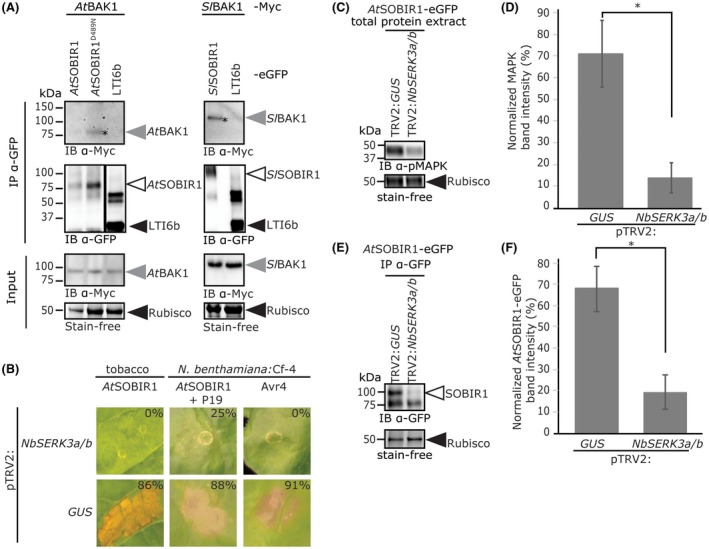
Constitutive immune activity of AtSOBIR1 is dependent on BAK1. (A) SOBIR1 constitutively interacts with BAK1. eGFP‐tagged versions of SOBIR1 and Myc‐tagged versions of BAK1 were transiently co‐expressed in N. benthamiana at an OD600 of 0.6 for each construct. Leaves were harvested at 1 dpi, as AtSOBIR1 triggers a constitutive cell death visible at 2 dpi, followed by IP and protein detection on IB. It should be noted that anti‐Myc IB, revealing the co‐IP of BAK1‐Myc (asterisks), shows relatively faint signals, which suggests that only a small pool of the total amount of SOBIR1 and BAK1 protein constitutively interacts. The plasma membrane (PM) protein LOW TEMPERATURE‐INDUCED 6b (LTI6b) (Kurup et al., 2005), included as a negative control, is visible at 30 kDa. The band at 60 kDa could be a dimer of LTI6b that is not fully denatured. The representative results of three independent experiments are shown. (B) Inoculation of tobacco and N. benthamiana:Cf‐4 with pTRV2:NbSERK3a/b leads to compromised constitutive immune activity of AtSOBIR1. AtSOBIR1‐eGFP was transiently expressed by agroinfiltration at an OD600 of 1 in pTRV2:GUS‐ or pTRV2:NbSERK3a/b‐inoculated tobacco or N. benthamiana:Cf‐4. In N. benthamiana:Cf‐4, AtSOBIR1 was co‐expressed with P19 at an OD600 of 1. Transient expression of Avr4 at OD600 = 0.03 shows a compromised hypersensitive response (HR) in pTRV2:NbSERK3a/b‐inoculated N. benthamiana:Cf‐4 plants. The percentages of cell death were scored as described in Experimental procedures. The experiments were performed at least three times, with testing of at least three individual leaves per sample. Representative photographs taken at 3 dpi are shown. (C) Inoculation with pTRV2:NbSERK3a/b leads to reduced mitogen‐activated protein kinase (MAPK) activation on transient overexpression of AtSOBIR1. AtSOBIR1‐eGFP was transiently co‐expressed with P19, both at an OD600 of 1, in N. benthamiana plants that had been previously inoculated with the indicated TRV recombinants. About 40 h after agroinfiltration, total protein was extracted and analysed for MAPK activation using anti‐p42/p44‐erk antibody. The Rubisco band shows equal loading. The experiment was performed three times, and representative results are shown. (D) Quantification of the results of the experiment shown in (C). Ratios were obtained by dividing the band intensity of phosphorylated MAPK by the intensity of the Rubisco input band. Data are presented as mean ± standard error (SE). The asterisk indicates a significant difference (P < 0.05), as determined by Student’s t‐test. (E) Inoculation with pTRV2:NbSERK3a/b leads to reduced accumulation of AtSOBIR1‐eGFP. AtSOBIR1‐eGFP was transiently co‐expressed with P19, both at an OD600 of 1, in N. benthamiana plants that had been previously inoculated with the indicated TRV recombinants. About 40 h after agroinfiltration, total protein was extracted and subjected to IP, followed by protein detection on IB. The Rubisco background band of the IP samples is depicted to show equal loading. The experiment was performed three times, and representative results are shown. (F) Quantification of the results of the experiment shown in (D). Ratios were obtained by dividing the band intensity of AtSOBIR1‐eGFP by the band intensity of the Rubisco background in the IP samples. Data are presented as the mean ± SE. The asterisk indicates a significant difference (P < 0.05), as determined by Student’s t‐test. [Colour figure can be viewed at wileyonlinelibrary.com]
To further address whether BAK1 is required for AtSOBIR1 constitutive immune activity, we employed virus‐induced gene silencing (VIGS) to knock down the expression of the AtBAK1 homologues SERK3a/b and SERK1 in tobacco and N. benthamiana (Heese et al., 2007; Postma et al., 2016). For this, the VIGS construct pTRV2:NbSERK3a/b (Heese et al., 2007) was agroinoculated into tobacco (cv. Samsun) (Zhang et al., 2013b), together with pTRV2:GUS as a negative control. Three weeks after agroinoculation, plants exhibited the characteristic stunting phenotype caused by silencing of the BAK1 homologues (Heese et al., 2007; Li et al., 2002; Nam and Li, 2002), and leaf sectors were transiently transformed to overexpress AtSOBIR1. Constitutive immune activity of AtSOBIR1 was highly compromised in plants inoculated with pTRV2:NbSERK3a/b, but not in pTRV2:GUS‐inoculated tobacco plants (Fig. 3B). A similar experiment was performed in N. benthamiana:Cf‐4 (Gabriëls et al., 2006), for which inoculation with pTRV2:NbSERK3a/b also resulted in severe stunting, confirming BAK1 silencing (Heese et al., 2007; Li et al., 2002; Nam and Li, 2002), and suppressed AtSOBIR1 constitutive immune activity (Fig. 3B). Compromised Cf‐4‐mediated HR on expression of Avr4 in these plants confirmed the successful knockdown of NbSERK3a/b, as these BAK1 homologues have been shown previously to be required for Cf‐4/Avr4‐triggered HR (Postma et al., 2016).
To confirm that the compromised cell death response, observed on overexpression of AtSOBIR1 in N. benthamiana inoculated with pTRV2:NbSERK3a/b, is a consequence of reduced constitutive immune activity, we examined MAPK activation. MAPKs are constitutively activated on overexpression of AtSOBIR1 (Wu et al., 2018), and immune blotting (IB) showed that MAPK activation by overexpression of AtSOBIR1 is reduced in plants inoculated with pTRV2:NbSERK3a/b, but not in pTRV2:GUS‐inoculated plants (Fig. 3A,C).
Previously, it has been shown that the accumulation levels of AtSOBIR1 variants inversely correlate with their constitutive immune activity (Wu et al., 2018). Interestingly, although showing less constitutive immune activity, AtSOBIR1 accumulates to lower levels in leaves of pTRV2:NbSERK3a/b‐inoculated N. benthamiana plants, when compared with pTRV2:GUS‐inoculated plants, in which AtSOBIR1 shows strong immune activity. This suggests that these BAK1 homologues are also important for the accumulation of SOBIR1 (Fig. 3E,F). Therefore, the reduced accumulation of AtSOBIR1 might also partially explain the reduced immune phenotypes.
Together, these experiments show that SOBIR1 forms heterodimers with BAK1, and that SOBIR1 constitutive immunity and accumulation are dependent on BAK1.
SOBIR1‐mediated immunity is dependent on kinase‐active BAK1
To further elucidate by which mechanism(s) BAK1 regulates the constitutive immune activity of AtSOBIR1, transient co‐transformations of AtSOBIR1 with (untagged) BAK1 variants, mutated in their kinase domain, were performed. These experiments revealed a dominant‐negative effect of the BAK1 mutant AtBAK1C408Y (also known as AtBAK1‐5) and kinase‐dead AtBAK1D416N (Schwessinger et al., 2011) in the form of suppression of AtSOBIR1 constitutive immune activity in both N. benthamiana and tobacco (Figs 4A and S3A,B, see Supporting Information). Similarly, the Cf‐4/Avr4‐induced HR was suppressed by co‐expression of AtBAK1C408Y and AtBAK1D416N with Avr4 in N. benthamiana:Cf‐4 plants (Figs 4B and S3C). AtBAK1C408Y is known to compromise immunity mediated by FLS2 and EFR, but does not affect BR signalling or cell death induction (Schwessinger et al., 2011). AtBAK1D416N exhibits a broad loss‐of‐function phenotype, as this mutant has lost its ability to signal in FLS2‐ and EFR‐mediated immune signalling, as well as in BR and cell death signalling (Schwessinger et al., 2011). Our results suggest that the transiently overexpressed AtBAK1 mutants out‐compete endogenous functional BAK1 homologues, resulting in compromised AtSOBIR1 constitutive immunity. These findings show that the constitutive immunity of AtSOBIR1 is dependent on defence signalling‐competent BAK1.
Figure 4.
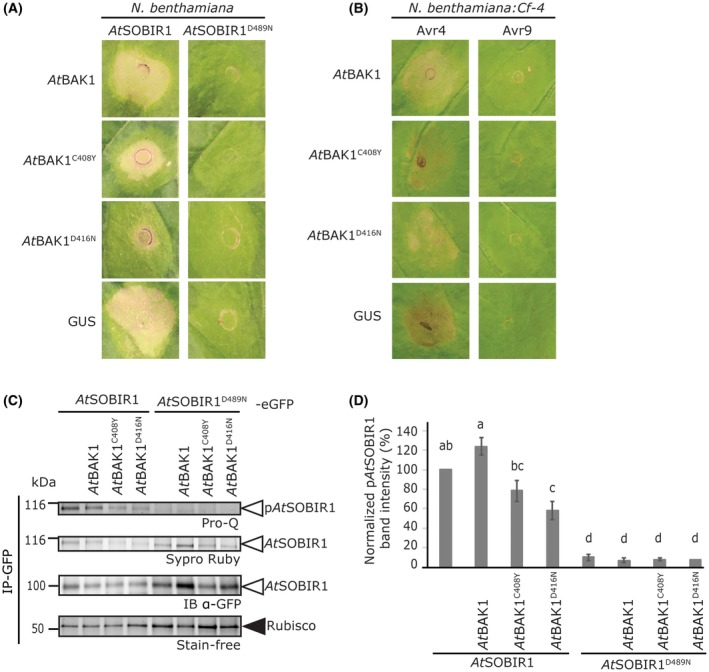
SOBIR1‐mediated immunity is dependent on kinase‐active BAK1. (A) Transient co‐expression of AtSOBIR1 with the AtBAK1C408Y or AtBAK1D416N mutant results in reduced AtSOBIR1 constitutive immune activity, when compared with co‐expression with wild‐type AtBAK1 or β‐glucuronidase (GUS). The indicated constructs were agroinfiltrated in N. benthamiana at an OD600 of 0.5, with co‐infiltration of P19 at an OD600 of 1. Photographs were taken at 3 dpi, and are representative of two repetitions, with eight agroinfiltrated leaves per sample. (See also Fig. S3A,B.) (B) Transient co‐expression of Avr4 in N. benthamiana:Cf‐4 with the mutants AtBAK1C408Y or AtBAK1D416N results in reduced Cf‐4/Avr4 HR, when compared with co‐expression with wild‐type AtBAK1 or GUS. The indicated constructs were agroinfiltrated at an OD600 of 0.5, with co‐infiltration of Avr4 or Avr9 at an OD600 of 0.03. Photographs were taken at 3 dpi, and are representative of three repetitions, with agroinfiltration of at least six leaves per sample. (See also Fig. S3C.) (C) Phosphorylation of AtSOBIR1 is dependent on kinase‐active BAK1. The indicated eGFP‐tagged AtSOBIR1 and untagged BAK1 constructs were co‐agroinfiltrated at an OD600 of 0.5, in combination with P19 at an OD600 of 1. At 2 dpi, leaves were harvested and subjected to immunoprecipitation (IP) using GFP‐affinity beads, and proteins were subsequently detected by anti‐GFP immune blotting (IB) and Pro‐Q stain. The ribulose‐1,5‐bisphosphate carboxylase/oxygenase (Rubisco) background band of the IP samples is depicted to show equal loading. The experiment was performed twice, and representative results are shown. (D) Quantification of the band intensity of phosphorylated AtSOBIR1 as shown in (C). Ratios were obtained by dividing the band intensity of pAtSOBIR1 from Pro‐Q staining by the band intensity reflecting the total amount of immunoprecipitated AtSOBIR1 in Sypro Ruby staining. Data are presented as the mean ± standard error (SE). The band intensity of the control sample, expression of AtSOBIR1 without co‐expression of BAK1, was set at 100%. The letters indicate significant differences at P < 0.05, as determined by one‐way analysis of variance (ANOVA), including a Tukey post hoc test. [Colour figure can be viewed at wileyonlinelibrary.com]
To determine whether co‐expression of the signalling‐incompetent BAK1 mutants affects the phosphorylation status of AtSOBIR1, we co‐agroinfiltrated AtSOBIR1‐eGFP with either wild‐type BAK1 or the different BAK1 mutants in N. benthamiana, in the presence of P19. Subsequent IP of AtSOBIR1, followed by Pro‐Q staining, revealed a reduction in the phosphorylation level of AtSOBIR1 when co‐expressed with AtBAK1C408Y or AtBAK1D416N, when compared with its co‐expression with wild‐type BAK1 (Fig. 4C,D). This analysis suggests that the strong phosphorylation of AtSOBIR1 is probably the result of transphosphorylation of AtSOBIR1 by BAK1.
Taken together, these data show that the elevated phosphorylation status of constitutively active SOBIR1 depends on signalling‐competent BAK1. This suggests that immune signalling by SOBIR1 involves transphosphorylation events with BAK1.
Discussion
BAK1 is a well‐known co‐receptor for ligand‐binding RLKs, such as BRI1, EFR and FLS2 (Chinchilla et al., 2009), and has been found recently to be recruited to activated RLP/SOBIR1 complexes, which have been proposed to function as two‐component RLKs (Albert et al., 2015; Domazakis et al., 2018; Liebrand et al., 2014; Postma et al., 2016; Wang et al., 2018). Here, we show that the kinase activity of AtSOBIR1 is essential for its phosphorylation. In addition, we show that SOBIR1 and BAK1 act together to signal for defence. Interestingly, in addition to kinase‐active SOBIR1, kinase‐active BAK1 is essential for AtSOBIR1 constitutive immune activity, for strong phosphorylation of AtSOBIR1 and for the Cf‐4/Avr4‐triggered HR. Based on our findings and on current data concerning RLKs, such as FLS2, which recruit and transphosphorylate BAK1 on ligand binding (Schwessinger et al., 2011; Somssich et al., 2015; Wang et al., 2008; Yan et al., 2012), we speculate that SOBIR1/RLP bimolecular RLKs function in a similar way when in a complex with BAK1. We propose a model in which AtSOBIR1 trans‐autophosphorylates and transphosphorylates BAK1, thereby activating this co‐receptor (Fig. 5). Subsequent transphosphorylation and full activation of AtSOBIR1 by activated BAK1 probably enables AtSOBIR1‐induced constitutive immunity (Fig. 5A). The model also applies to Cf‐4/Avr4‐mediated immunity, where the recognition of Avr4 by Cf‐4 possibly initiates SOBIR1 trans‐autophosphorylation, followed by transphosphorylation of BAK1 by SOBIR1, and, in turn, transphosphorylation of SOBIR1 by activated BAK1 to fully activate SOBIR1 (Fig. 5B). Fully activated SOBIR1 subsequently initiates further downstream signalling.
Figure 5.
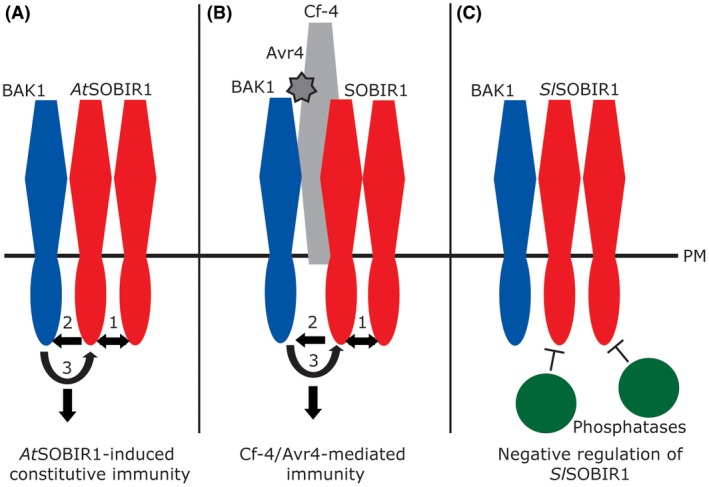
SOBIR1 and BAK1 act together to signal for defence. In this model, we propose that SOBIR1 and BAK1 act together to signal for downstream defence activation. In addition to the trans‐autophosphorylation of AtSOBIR1, it is likely that AtSOBIR1 transphosphorylates BAK1. By transphosphorylating BAK1, this co‐receptor becomes activated, followed by the transphosphorylation and activation of AtSOBIR1 by activated BAK1 to enable AtSOBIR1‐induced constitutive immunity (A) and the Cf‐4/Avr4‐mediated HR (B). The arrows represent auto‐ or transphosphorylation events, and the numbers indicate the proposed order of the various phosphorylation events. The phosphorylation and activity of Solanum lycopersicum (Sl)SOBIR1 are likely to be down‐regulated by endogenous phosphatases, when not activated by Avr4 (C). PM, plasma membrane. [Colour figure can be viewed at wileyonlinelibrary.com]
First, we found that the phosphorylation status of SOBIR1 and its constitutive immune activity are positively linked, as constitutively active AtSOBIR1 is clearly phosphorylated, and phosphorylation of kinase‐dead AtSOBIR1 is not apparent (Fig. 1). In addition, we observed suppressed phosphorylation of AtSOBIR1 when co‐expressed with signalling‐incompetent BAK1 mutants (Fig. 4). This suggests that BAK1 plays a role in the transphosphorylation and activation of AtSOBIR1. Knockdown of the expression of BAK1 homologues NbSERK1 and NbSERK3a/b in N. benthamiana causes AtSOBIR1 to accumulate to lower levels (Fig. 3E), which might also partially explain the compromised AtSOBIR1‐induced constitutive immunity in these NbSERK3a/b‐silenced plants. Stabilization of RLKs and RLPs by regulatory RLKs has been observed previously, and so it is likely that SOBIR1 and BAK1 promote each other’s accumulation (Imkampe et al., 2017; Liebrand et al., 2013; Wang et al., 2008). Because silencing of BAK1 homologues suppresses the accumulation of SOBIR1, attempts to measure changes in the phosphorylation level of SOBIR1 on silencing of BAK1 homologues were not successful.
A constitutive interaction between SOBIR1 and BAK1 has been suggested previously (Liu et al., 2016; Postma et al., 2016). Although Liu et al. (2016) did not show an interaction between AtSOBIR1 and AtBAK1 in N. benthamiana, they did so in Arabidopsis on silencing of a negative regulator of defence, AtBIR1 (Gao et al., 2009; Liu et al., 2016). Silencing of BIR1 led to higher BAK1 availability, and a clearer interaction of BAK1 with SOBIR1 (Liu et al., 2016). In the experiment described here, the accumulation of AtSOBIR1 is relatively low because of its constitutive immune activity in N. benthamiana (Wu et al., 2018), and an interaction between AtBAK1 and AtSOBIR1 is not visible (Fig. 3A). For AtSOBIR1D489N and SlSOBIR1, we observed an interaction with BAK1, because these SOBIR1 variants accumulate to higher amounts as they do not trigger a constitutive cell death response. A constitutive interaction between SOBIR1 and BAK1 is anticipated to occur only at very low levels, as this interaction is specifically stimulated on ligand perception by the SOBIR1‐associated RLP, in order to activate the immune system (Albert et al., 2015; Domazakis et al., 2018; Postma et al., 2016; Wang et al., 2018). Pre‐formation of immune complexes at nanoclusters at the PM, without their activation by the matching ligand, has been shown previously (Bücherl et al., 2017; Jarsch et al., 2014; Somssich et al., 2015). It was found that various pre‐formed immune complexes are present at the PM, spatially separated into different nanoclusters. Such a separation into different nanodomains enables rapid and diverse responses. Thus, a small pool of pre‐formed RLP/SOBIR1/BAK1 complexes, probably kept in check by endogenous phosphatases as suggested previously (Wu et al., 2018), might enable rapid and specific responses to elicitation in resistant plants.
In addition to the sequestration of co‐receptors required for downstream signalling, immune receptor activity is also regulated at the level of phosphorylation (Couto and Zipfel, 2016). For instance, BAK1 and FLS2 are negatively regulated by PP2A and PP2C, respectively (Couto et al., 2016; Gómez‐Gómez et al., 2001; Segonzac et al., 2014). Here, we show that AtSOBIR1, which can constitutively activate immune responses, is clearly phosphorylated when overexpressed in N. benthamiana, whereas SlSOBIR1 is not (Fig. 1A). It is likely that endogenous phosphatases of N. benthamiana keep SlSOBIR1 in check by dephosphorylation (Fig. 5C), but do not have the correct affinity for the phosphorylated kinase domain of AtSOBIR1, and therefore cannot properly dephosphorylate this non‐solanaceous protein. This could lead to excessive phosphorylation and thereby constitutive immune activation, as suggested previously (Wu et al., 2018).
Similar to BAK1, SOBIR1 is a typical RD‐kinase as it contains an arginine (R) and aspartic acid (D) residue in its catalytic site. RD‐kinases are generally thought to require phosphorylation of their activation loop to acquire the active conformation (Johnson et al., 1996; Kornev et al., 2006; Nolen et al., 2004). Our observation that kinase‐dead AtSOBIR1D489N does not show substantial phosphorylation, when compared with wild‐type AtSOBIR1, when transiently expressed in N. benthamiana, suggests that AtSOBIR1 needs to trans‐autophosphorylate to a certain level in order to become signalling competent. As SOBIR1 forms homodimers, it is possible that such trans‐autophosphorylation takes place (Fig. 2). This trans‐autophosphorylation is probably required to keep SOBIR1 in a signalling‐active state. In addition, the lack of apparent phosphorylation of AtSOBIR1D489N suggests that AtSOBIR1 needs to transphosphorylate its signalling partner BAK1 in order for this partner to become signalling competent, which then, in turn, transphosphorylates AtSOBIR1. Indeed, on overexpression of BAK1 together with SOBIR1, we observed elevated phosphorylation levels of SOBIR1 (Fig. 4C, D). Moreover, overexpression of AtSOBIR1 with signalling‐incompetent BAK1 mutants, which are unable to signal for defence, resulted in reduced phosphorylation levels of SOBIR1. These observations suggest that, for full phosphorylation of SOBIR1, and immune signalling by SOBIR1, transphosphorylation by BAK1 is probably necessary. This is in concert with previous studies on ligand binding RLKs and BAK1, for which it was concluded that transphosphorylation of RLK and BAK1 is necessary to initiate signalling on ligand perception (Wang et al., 2008; Yan et al., 2012). It is likely that, on ligand recognition by an RLP, the RLP and associated SOBIR1 undergo conformational changes, leading to SOBIR1 trans‐autophosphorylation, thereby providing SOBIR1 with the ability to trans‐phosphorylate BAK1, which is recruited on ligand perception by the RLP. This transphosphorylation event locks BAK1 in the active conformation (Yan et al., 2012), and provides BAK1 with the capacity to transphosphorylate and fully activate SOBIR1. The resulting activated complex is subsequently able to trigger downstream defence signalling (Fig. 5).
Interestingly, we observed reduced AtSOBIR1 phosphorylation levels and cell death on overexpression of AtBAK1C408Y (also referred to as BAK1‐5) together with AtSOBIR1 (Fig. 4). AtBAK1C408Y was initially found to be impaired in complementing defence signalling by FLS2 and EFR (Schwessinger et al., 2011). Nevertheless, AtBAK1C408Y exhibits only slightly reduced kinase activity, and is not impaired in BR or cell death signalling. Therefore, this mutant is important to unlink the ability of BAK1 to signal for defence, cell death and development. We observed that AtBAK1C408Y is impaired in signalling for AtSOBIR1‐induced constitutive immunity, as well as in mediating the transphosphorylation of AtSOBIR1 (Fig. 4). Thus, AtSOBIR1 specifically signals for immunity together with BAK1. This further confirms that the constitutive immune activity induced by AtSOBIR1 follows the same immune pathway as Cf‐4/Avr4‐mediated HR.
In conclusion, we have shown that SOBIR1 and BAK1 probably act together to signal for defence. Our in planta data support a model in which SOBIR1 trans‐autophosphorylates, and on elicitation transphosphorylates BAK1, and is, in turn, transphosphorylated by activated BAK1 to signal for immunity. We envisage that this model not only describes signalling for AtSOBIR1 constitutive immunity, but also signalling on BAK1 heterodimer formation with ligand‐activated bipartite RLP/SOBIR1 complexes (Fig. 5).
Experimental Procedures
Binary vectors for Agrobacterium‐mediated transformation and VIGS
The constructs pBIN‐KS‐35S::AtSOBIR1‐eGFP, pBIN‐KS‐35S::AtSOBIR1D489N‐eGFP, pBIN‐KS‐35S::SlSOBIR1‐eGFP, pBIN‐KS‐35S::SlSOBIR1D486N‐eGFP, pBIN‐KS‐35S::SlCf‐4‐eGFP, pGWB20‐35S::SlSERK3‐myc, pGWB20‐35s::SlSOBIR1‐Myc and pGWB20‐35s::AtSOBIR1‐Myc have been described previously (Liebrand et al., 2012, 2013). AtBAK1‐Myc has been described by Halter et al. (2014b). Avr4 was expressed using the pMOG800 construct (Van der Hoorn et al., 2000). P19 (Voinnet et al., 2015), pBIN61‐GUS, pTRV1 (Liu et al., 2002,b), pTRV2:GUS (Tameling and Baulcombe, 2007) and pTRV2:NbSERK3a/b (Heese et al., 2007) have been described elsewhere. AtBAK1, AtBAK1C408Y and AtBAK1D416N originate from Schwessinger et al. (2011). GFP‐LTI6b has been described previously (Kurup et al., 2005). AtFLS2‐eGFP and SlFLS2‐eGFP were described by Robatzek et al. (2006, 2007). SlSOBIR1‐cYFP, SlSOBIR1‐nYFP and SlCf‐4‐cYFP (Postma et al., 2016), and pACA8::ACA8‐mCherry, AtFLS2‐cYFP and AtFLS2‐nYFP (Frei dit Frey et al., 2012), have been described elsewhere.
Plant growth conditions
Nicotiana tabacum (tobacco) (cv. SR1 and cv. Samsun) and N. benthamiana [wild‐type and N. benthamiana stably expressing SlCf‐4 under its native promoter (referred to as N. benthamiana:Cf‐4; Gabriëls et al., 2006] were grown under 16 h light at 25 °C and 8 h darkness at 21 °C, with ~75% relative humidity.
VIGS in tobacco and N. benthamiana
VIGS using TRV‐based vectors was performed in tobacco (cv. Samsun) and N. benthamiana:Cf‐4 as described previously (Liebrand et al., 2012; Zhang et al., 2013b).
Agrobacterium‐mediated transient transformation
Agrobacterium‐mediated transient transformations (agroinfiltrations) were performed as described previously (Van der Hoorn et al., 2000). Binary constructs expressing affinity‐tagged proteins were agroinfiltrated with Agrobacterium tumefaciens cultures at an OD600 of 1 in combination with P19 at an OD600 of 1, unless indicated otherwise. Leaves were harvested for protein isolation and IP at 2 dpi, unless indicated otherwise. Percentages of HR were quantified by visual scoring for full HR (100%), mildly reduced HR (60%), strongly reduced HR (30%) and no HR (0%).
IPs, IB, phosphorylation analysis and MAPK activation analysis
IPs and co‐IPs were performed as described previously (Liebrand et al., 2013). To detect phosphorylated proteins, a protein extraction buffer was used as described by Karlova et al. (2006, 2009), with minor modifications; instead of Tris and Triton‐X, 100 mm NaPi (pH 7.2) and 1% IGEPAL CA‐630 (NP40) were used, respectively. Pre‐cast TGX gels were used for Pro‐Q and Sypro Ruby analyses (Bio‐Rad, Veenendaal, the Netherlands, #456‐1095). Pro‐Q diamond phosphoprotein gel stains and subsequent Sypro Ruby stains were performed according to the manufacturer’s recommendations (Invitrogen, Life Technologies, Carlsbad, CA, USA; Taylor et al., 2013). TGX stain‐free gels were used for all other protein analyses (Bio‐Rad, #456‐8085), and total protein was visualized using the stain‐free method or with Coomassie Brilliant Blue (CBB). The following antibodies were used for protein detection on IB: αGFP‐HRP (130‐091‐833, MACS antibodies, Bergisch Gladbach, Germany), αMyc (cMyc9E10, sc‐40, Santa Cruz Biotechnology, Heidelberg, Germany), αMouse‐HRP (GE Healthcare, Eindhoven, The Netherlands), anti‐p42/p44‐erk (NEB: Bioké Dellaertweg 9b 2316 WZ, Leiden) and goat anti‐rabbit (Sigma Zwijndrecht, the Netherlands). Band intensities were measured using Image Lab software (Bio‐Rad), and ratios were calculated as indicated in the figures. To quantify immunoprecipitated protein bands, the ribulose‐1,5‐bisphosphate carboxylase/oxygenase (Rubisco) background band in the IP sample was taken as an internal standard for the total protein concentration of the sample.
Protein localization studies
Confocal laser scanning microscopy (CLSM) was performed using a Leica SP5 laser point scanning microscope (Leica Camera AG, Wetzlar, Germany), mounted with hybrid detectors (HyD), as described previously (Beck et al., 2012). For CLSM analysis of eGFP, constructs were transiently expressed in adult tobacco plants by infiltration with Agrobacterium tumefaciens suspensions of OD600 = 0.3. At 2 dpi, GFP fluorophores were excited using a 488‐nm argon laser and fluorescence emission was captured between 495 and 540 nm. mCherry fluorophores were excited using a 561‐nm argon laser and fluorescence emission was captured between 580 and 620 nm. For GFP‐only images, chloroplast autofluorescence was captured between 700 and 800 nm. For CLSM analysis of bimolecular fluorescence complementation (BiFC, split‐YFP) experiments, cYFP and nYFP constructs were transiently co‐expressed by co‐infiltration of adult N. benthamiana plants using Agrobacterium tumefaciens suspensions, each at OD600 = 0.3. Reconstituted YFP molecules were excited using a 514‐nm argon laser, and fluorescence emission was captured between 520 and 550 nm. Chloroplast autofluorescence was captured between 700 and 800 nm. Images were taken using a 20× objective (for eGFP) or 40× objective (for YFP), and processed using Leica LAS‐AF and FIJI (ImageJ) software packages.
Supporting information
Fig. S1 Kinase activity of SOBIR1 is not required for Cf‐4 stabilization. (A) Transient overexpression of Arabidopsis thaliana (At)SOBIR1 induces cell death in tobacco and in Nicotiana benthamiana when co‐expressed with P19. Agroinfiltrations were performed at an optical density at 600 nm (OD600) of 1. Where indicated, P19 was also co‐infiltrated at an OD600 of 1. Photographs were taken at 3 days post‐infiltration (dpi). It should be noted that constitutive immune activity of AtSOBIR1 requires its kinase activity. Furthermore, overexpression of Solanum lycopersicum (Sl)SOBIR1 from the Solanaceous plant tomato does not result in cell death. [See also Wu et al. (2018)]. (B) Co‐expression of wild‐type SlSOBIR1 as well as kinase‐dead SlSOBIR1D473N stabilizes Cf‐4 when co‐expressed in N. benthamiana. It should be noted that the signal of Cf‐4 is increased when overexpressed with both wild‐type and kinase‐dead SlSOBIR1, and highly increased on co‐expression with P19. Co‐agroinfiltrations of the affinity‐tagged proteins were performed in N. benthamiana leaves at an OD600 of 1 for each construct. Leaves were harvested at 2 dpi, and subjected to immunoprecipitation (IP) using anti‐green fluorescent protein (anti‐GFP) beads, followed by immune blotting (IB). The ribulose‐1,5‐bisphosphate carboxylase/oxygenase (Rubisco) band of the input shows equal loading. It should be noted that Cf‐4 is only visible in the input when co‐infiltrated with P19. CBB, Coomassie Brilliant Blue.
Fig. S2 Kinase‐dead SOBIR1 constitutively forms homodimers in planta. (A) Myc‐tagged versions of AtSOBIR1D489N and SlSOBIR1D473N co‐immunoprecipitate with eGFP‐tagged versions of AtSOBIR1D489N and SlSOBIR1D473N (asterisks), respectively, and with Cf‐4‐eGFP, but not with Flagellin‐Sensing 2 (FLS2)‐eGFP. Co‐agroinfiltrations of the various affinity‐tagged proteins were performed in combination with P19 in leaves of N. benthamiana at an OD600 of 0.6 for each construct. Leaves were harvested at 2 dpi, and subjected to IP using anti‐GFP beads, followed by IB. The Rubisco band of the input shows equal loading. It should be noted that, because of the low accumulation levels, not all proteins are visible in the input samples.
Fig. S3 AtSOBIR1‐mediated immunity is dependent on kinase‐active BAK1. (A) Transient co‐expression of AtSOBIR1 in tobacco with AtBAK1C408Y or AtBAK1D416N results in reduced AtSOBIR1 constitutive immune activity, when compared with co‐expression of AtSOBIR1 with wild‐type AtBAK1 or GUS. The indicated constructs were agroinfiltrated at an OD600 of 0.7. Photographs were taken at 2 dpi, and are representative of the agroinfiltration of eight leaves per sample. (B) Quantification of the percentage of cell death as shown in Fig. 4A. Percentages of constitutive cell death are presented as the mean ± standard error (SE). The letters indicate significant differences at P < 0.05, as determined by one‐way analysis of variance (ANOVA), including a Tukey post hoc test. (C) Quantification of the percentage of hypersensitive response (HR) as shown in Fig. 4B. Percentages of Avr4‐induced HR are presented as mean ± SE. The letters indicate significant differences at P < 0.05, as determined by one‐way ANOVA, including a Tukey post hoc test.
Acknowledgements
We thank Unifarm personnel for excellent plant care. We acknowledge Maria Font Farré for initiating experiments on the dominant‐negative effect of BAK1, and Cris Wijnen for help with statistics. A.M.v.d.B. is supported by the Netherlands Organization for Scientific Research (NWO), Earth and Life Sciences (ALW). Research by J.P. and S.R. is supported by the Gatsby Charitable Foundation.
References
- Albert, I. , Böhm, H. , Albert, M. , Feiler, C.E. , Imkampe, J. , Wallmeroth, N. , Brancato, C. , Raaymakers, T.M. , Oome, S. , Zhang, H. , Krol, E. , Grefen, C. , Gust, A.A. , Chai, J. , Hedrich, R. , Van den Ackerveken, G. and Nürnberger, T. (2015) An RLP23–SOBIR1–BAK1 complex mediates NLP‐triggered immunity. Nat. Plants, 1, 15 140. [DOI] [PubMed] [Google Scholar]
- Beck, M. , Zhou, J. , Faulkner, C. , MacLean, D. and Robatzek, S. (2012) Spatio‐temporal cellular dynamics of the Arabidopsis flagellin receptor reveal activation status‐dependent endosomal sorting. Plant Cell, 24, 4205–4219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bi, G. , Liebrand, T.W. , Cordewener, J.H. , America, A.H. , Xu, X. and Joosten, M.H.A.J. (2014) Arabidopsis thaliana receptor‐like protein AtRLP23 associates with the receptor‐like kinase AtSOBIR1. Plant Signal. Behav. 9, e27937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bi, G. , Liebrand, T.W.H. , Bye, R.R. , Postma, J. , van der Burgh, A.M. , Robatzek, S. , Xu, X. and Joosten, M.H.A.J. (2015) SOBIR1 requires the GxxxG dimerization motif in its transmembrane domain to form constitutive complexes with receptor‐like proteins. Mol. Plant Pathol. 17, 96–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Böhm, H. , Albert, I. , Fan, L. , Reinhard, A. and Nürnberger, T. (2014) Immune receptor complexes at the plant cell surface. Curr. Opin. Plant Biol. 20, 47–54. [DOI] [PubMed] [Google Scholar]
- Bücherl, C.A. , Jarsch, I.K. , Schudoma, C. , Segonzac, C. , Mbengue, M. , Robatzek, S. , MacLean, D. , Ott, T. and Zipfel, C. (2017) Plant immune and growth receptors share common signalling components but localise to distinct plasma membrane nanodomains. eLife, 6, e25114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Catanzariti, A.M. , Do, H.T. , Bru, P. , Sain, M. , Thatcher, L.F. , Rep, M. and Jones, D.A. (2017) The tomato I gene for Fusarium wilt resistance encodes an atypical leucine‐rich repeat receptor‐like protein whose function is nevertheless dependent on SOBIR1 and SERK3/BAK1. Plant J. 89, 1195–1209. [DOI] [PubMed] [Google Scholar]
- Chinchilla, D. , Shan, L. , He, P. , de Vries, S. and Kemmerling, B. (2009) One for all: the receptor‐associated kinase BAK1. Trends Plant Sci. 14, 535–541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chinchilla, D. , Zipfel, C. , Robatzek, S. , Kemmerling, B. , Nürnberger, T. , Jones, J.D. , Felix, G. and Boller, T. (2007) A flagellin‐induced complex of the receptor FLS2 and BAK1 initiates plant defence. Nature, 448, 497–500. [DOI] [PubMed] [Google Scholar]
- Couto, D. , Niebergall, R. , Liang, X. , Bücherl, C.A. , Sklenar, J. , Macho, A.P. , Ntoukakis, V. , Derbyshire, P. , Altenbach, D. , Maclean, D. , Robatzek, S. , Uhrig, J. , Menke, F. , Zhou, J.‐M. and Zipfel, C. (2016) The Arabidopsis protein phosphatase PP2C38 negatively regulates the central immune kinase BIK1. PLoS Pathog., 12, e1005811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Couto, D. and Zipfel, C. (2016) Regulation of pattern recognition receptor signalling in plants. Nat. Rev. Immunol. 16, 537–552. [DOI] [PubMed] [Google Scholar]
- Domazakis, E. , Wouters, D. , Visser, R. , Kamoun, S. , Joosten, M.H.A.J. and Vleeshouwers, V.G.A.A. (2018) The ELR‐SOBIR1 complex functions as a two‐component RLK to mount defense against Phytophthora infestans . Mol. Plant–Microbe Interact. 31, 795–802. 10.1094/mpmi-09-17-0217-r. [DOI] [PubMed] [Google Scholar]
- Dufayard, J.‐F. , Bettembourg, M. , Fischer, I. , Droc, G. , Guiderdoni, E. , Périn, C. , Chantret, N. and Diévart, A. (2017) New insights on leucine‐rich repeats receptor‐like kinase orthologous relationships in angiosperms. Front. Plant Sci. 8, 381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer, I. , Diévart, A. , Droc, G. , Dufayard, J.‐F. and Chantret, N. (2016) Evolutionary dynamics of the Leucine‐Rich Repeats Receptor‐Like Kinase (LRR‐RLK) subfamily in angiosperms. Plant Physiol. 170, 1595–1610. Doi 10.1104/pp.15.01470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frei dit Frey, N. , Mbengue, M. , Kwaaitaal, M. , Nitsch, L. , Altenbach, D. , Haweker, H. , Lonzano‐Duran, R. , Njo, M.F. , Beeckman, T. and Huettel, B. (2012) Plasma membrane calcium ATPases are important components of receptor‐mediated signalling in plant immune responses and development. Plant Physiol. 159, 798–809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frescatada‐Rosa, M. , Robatzek, S. and Kuhn, H. (2015) Should I stay or should I go? Traffic control for plant pattern recognition receptors. Curr. Opin. Plant Biol. 28, 23–29. [DOI] [PubMed] [Google Scholar]
- Gabriëls, S.H.E.J. , Takken, F.L.W. , Vossen, J.H. , De Jong, C.F. , Liu, Q. , Turk, S.C.H.J. , Wachowski, L.K. , Peters, J. , Witsenboer, H.M.A. , De Wit, P.J.G.M. and Joosten, M.H.A.J. (2006) cDNA‐AFLP combined with functional analysis reveals novel genes involved in the hypersensitive response. Mol. Plant–Microbe Interact. 19, 567–576. [DOI] [PubMed] [Google Scholar]
- Gao, M. , Wang, X. , Wang, D. , Xu, F. , Ding, X. , Zhang, Z. , Bi, D. , Cheng, Y.T. , Chen, S. , Li, X. and Zhang, Y. (2009) Regulation of cell death and innate immunity by two receptor‐like kinases in Arabidopsis . Cell Host Microbe, 6, 34–44. [DOI] [PubMed] [Google Scholar]
- Gómez‐Gómez, L. , Bauer, Z. and Boller, T. (2001) Both the extracellular leucine‐rich repeat domain and the kinase activity of FLS2 are required for flagellin binding and signaling in Arabidopsis . Plant Cell, 13, 1155–1163. [PMC free article] [PubMed] [Google Scholar]
- Gómez‐Gómez, L. and Boller, T. (2000) FLS2: an LRR receptor‐like kinase involved in the perception of the bacterial elicitor flagellin in Arabidopsis . Mol. Cell, 5, 1003–1011. [DOI] [PubMed] [Google Scholar]
- Gust, A.A. and Felix, G. (2014) Receptor like proteins associate with SOBIR1‐type of adaptors to form bimolecular receptor kinases. Curr. Opin. Plant Biol. 21, 104–111. [DOI] [PubMed] [Google Scholar]
- Halter, T. , Imkampe, J. , Mazzotta, S. , Wierzba, M. , Postel, S. , Bücherl, C. , Kiefer, C. , Stahl, M. , Chinchilla, D. , Wang, X. , Nürnberger, T. , Zipfel, C. , Clouse, S. , Borst Jan, W. , Boeren, S. , de Vries, Sacco C. , Tax, F. and Kemmerling, B. (2014b) The Leucine‐Rich Repeat Receptor Kinase BIR2 Is a Negative Regulator of BAK1 in Plant Immunity. Curr. Biol., 24, 134–143. [DOI] [PubMed] [Google Scholar]
- Heese, A. , Hann, D.R. , Gimenez‐Ibanez, S. , Jones, A.M.E. , He, K. , Li, J. , Schroeder, J.I. , Peck, S.C. and Rathjen, J.P. (2007) The receptor‐like kinase SERK3/BAK1 is a central regulator of innate immunity in plants. Proc. Natl. Acad. Sci. USA, 104, 12 217–12 222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hegenauer, V. , Fürst, U. , Kaiser, B. , Smoker, M. , Zipfel, C. , Felix, G. , Stahl, M. and Albert, M. (2016) Detection of the plant parasite Cuscuta reflexa by a tomato cell surface receptor. Science, 353, 478–481. [DOI] [PubMed] [Google Scholar]
- Imkampe, J. , Halter, T. , Huang, S. , Schulze, S. , Mazzotta, S. , Schmidt, N. , Manstretta, R. , Postel, S. , Wierzba, M. , Yang, Y. , vanDongen, W.M. , Stahl, M. , Zipfel, C. , Goshe, M.B. , Clouse, S. , de Vries, S.C. , Tax, F. , Wang, X. and Kemmerling, B. (2017) The Arabidopsis leucine‐rich repeat receptor kinase BIR3 negatively regulates BAK1 receptor complex formation and stabilizes BAK1. Plant Cell, 29, 2285–2303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jarsch, I.K. , Konrad, S.S.A. , Stratil, T.F. , Urbanus, S.L. , Szymanski, W. , Braun, P. , Braun, K.‐H. and Ott, T. (2014) Plasma membranes are subcompartmentalized into a plethora of coexisting and diverse microdomains in Arabidopsis and Nicotiana benthamiana . Plant Cell Online, 26, 1698–1711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jehle, A.K. , Fürst, U. , Lipschis, M. , Albert, M. and Felix, G. (2013) Perception of the novel MAMP eMax from different Xanthomonas species requires the Arabidopsis receptor‐like protein ReMAX and the receptor kinase SOBIR. Plant Signal. Behav. 8, e27408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson, L.N. , Noble, M.E.M. and Owen, D.J. (1996) Active and inactive protein kinases: structural basis for regulation. Cell, 85, 149–158. [DOI] [PubMed] [Google Scholar]
- Karlova, R. , Boeren, S. , van Dongen, W. , Kwaaitaal, M. , Aker, J. , Vervoort, J. and de Vries, S. (2009) Identification of in vitro phosphorylation sites in the Arabidopsis thaliana somatic embryogenesis receptor‐like kinases. Proteomics, 9, 368–379. [DOI] [PubMed] [Google Scholar]
- Karlova, R. , Boeren, S. , Russinova, E. , Aker, J. , Vervoort, J. and de Vries, S. (2006) The Arabidopsis somatic embryogenesis receptor‐like kinase1 protein complex includes brassinosteroid‐insensitive1. Plant Cell, 18, 626–638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kornev, A.P. , Haste, N.M. , Taylor, S.S. and Ten Eyck, L.F. (2006) Surface comparison of active and inactive protein kinases identifies a conserved activation mechanism. Proc. Natl. Acad. Sci. USA, 103, 17 783–17 788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurup, S. , Runions, J. , Köhler, U. , Laplaze, L. , Hodge, S. and Haseloff, J. (2005) Marking cell lineages in living tissues. Plant J. 42, 444–453. [DOI] [PubMed] [Google Scholar]
- Leslie, M.E. , Lewis, M.W. , Youn, J.Y. , Daniels, M.J. and Liljegren, S.J. (2010) The EVERSHED receptor‐like kinase modulates floral organ shedding in Arabidopsis . Development, 137, 467–476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li, J. and Chory, J. (1997) A putative leucine‐rich repeat receptor kinase involved in brassinosteroid signal transduction. Cell, 90, 929–938. [DOI] [PubMed] [Google Scholar]
- Li, J. , Wen, J. , Lease, K.A. , Doke, J.T. , Tax, F.E. and Walker, J.C. (2002) BAK1, an Arabidopsis LRR receptor‐like protein kinase, interacts with BRI1 and modulates brassinosteroid signaling. Cell, 110, 213–222. [DOI] [PubMed] [Google Scholar]
- Liebrand, T.W.H. , van den Burg, H.A. and Joosten, M.H.A.J. (2014) Two for all: receptor‐associated kinases SOBIR1 and BAK1. Trends Plant Sci. 19, 123–132. [DOI] [PubMed] [Google Scholar]
- Liebrand, T.W.H. , van den Berg, G.C.M. , Zhang, Z. , Smit, P. , Cordewener, J.H.G. , America, A.H.P. , Sklenar, J. , Jones, A.M.E. , Tameling, W.I.L. , Robatzek, S. , Thomma, B.P.H.J. and Joosten, M.H.A.J. (2013) Receptor‐like kinase SOBIR1/EVR interacts with receptor‐like proteins in plant immunity against fungal infection. Proc. Natl. Acad. Sci. USA, 110, 10 010–10 015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liebrand, T.W.H. , Smit, P. , Abd‐El‐Haliem, A. , de Jonge, R. , Cordewener, J.H.G. , America, A.H.P. , Sklenar, J. , Jones, A.M.E. , Robatzek, S. , Thomma, B.P.H.J. , Tameling, W.I.L. and Joosten, M.H.A.J. (2012) Endoplasmic reticulum‐quality control chaperones facilitate the biogenesis of Cf receptor‐like proteins involved in pathogen resistance of tomato. Plant Physiol. 159, 1819–1833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu, Y. , Huang, X. , Li, M. , He, P. and Zhang, Y.C. (2016) Loss‐of‐function of Arabidopsis receptor‐like kinase BIR1 activates cell death and defense responses mediated by BAK1 and SOBIR1. New Phytol. 212, 637–645. [DOI] [PubMed] [Google Scholar]
- Liu, Y. , Schiff, M. and Dinesh‐Kumar, S. (2002. a) Virus‐induced gene silencing in tomato. Plant J. 31, 777–786. [DOI] [PubMed] [Google Scholar]
- Liu, Y. , Schiff, M. , Marathe, R. and Dinesh‐Kumar, S. (2002. b) Tobacco Rar1, EDS1 and NPR1/NIM1 like genes are required for N‐mediated resistance to tobacco mosaic virus. Plant J. 30, 415–429. [DOI] [PubMed] [Google Scholar]
- Ma, L. and Borhan, M.H. (2015) The receptor‐like kinase SOBIR1 interacts with Brassica napus LepR3 and is required for Leptosphaeria maculans AvrLm1‐triggered immunity. Front. Plant Sci. 6, 933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macho, A.P. , Lozano‐Durán, R. and Zipfel, C. (2015) Importance of tyrosine phosphorylation in receptor kinase complexes. Trends Plant Sci. 20, 269–272. [DOI] [PubMed] [Google Scholar]
- Macho, A.P. , Schwessinger, B. , Ntoukakis, V. , Brutus, A. , Segonzac, C. , Roy, S. , Kadota, Y. , Oh, M.H. , Sklenar, J. , Derbyshire, P. , Lozano‐Durán, R. , Gro Malinovsky, F. , Monaghan, J. , Menke, F.L. , Huber, S.C. , Yang He, S. and Zipfel, C. (2014) A bacterial tyrosine phosphatase inhibits plant pattern recognition receptor activation. Science, 343, 1509–1512. [DOI] [PubMed] [Google Scholar]
- Macho, A.P. and Zipfel, C. (2014) Plant PRRs and the activation of innate immune signaling. Mol. Cell, 54, 263–272. [DOI] [PubMed] [Google Scholar]
- Mbengue, M. , Bourdais, G. , Gervasi, F. , Beck, M. , Zhou, J. , Spallek, T. , Bartels, S. , Boller, T. , Ueda, T. , Kuhn, H. and Robatzek, S. (2016) Clathrin‐dependent endocytosis is required for immunity mediated by pattern recognition receptor kinases. Proc. Natl. Acad. Sci. USA, 113, 11 034–11 039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nam, K.H. and Li, J. (2002) BRI1/BAK1, a receptor kinase pair mediating brassinosteroid signaling. Cell, 110, 203–212. [DOI] [PubMed] [Google Scholar]
- Nolen, B. , Taylor, S. and Ghosh, G. (2004) Regulation of protein kinases: controlling activity through activation segment conformation. Mol. Cell, 15, 661–675. [DOI] [PubMed] [Google Scholar]
- Oh, M.H. , Wu, X. , Clouse, S.D. and Huber, S.C. (2011) Functional importance of BAK1 tyrosine phosphorylation in vivo. Plant Signal. Behav. 6, 400–405. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Postma, J. , Liebrand, T.W.H. , Bi, G. , Evrard, A. , Bye, R.R. , Mbengue, M. , Kuhn, H. , Joosten, M.H.A.J. and Robatzek, S.C. (2016) Avr4 promotes Cf‐4 receptor‐like protein association with the BAK1/SERK3 receptor‐like kinase to initiate receptor endocytosis and plant immunity. New Phytol. 210, 627–642. [DOI] [PubMed] [Google Scholar]
- Robatzek, S. , Bittel, P. , Chinchilla, D. , Köchner, P. , Felix, G. , Shiu, S.‐H. and Boller, T. (2007) Molecular identification and characterization of the tomato flagellin receptor LeFLS2, an orthologue of Arabidopsis FLS2 exhibiting characteristically different perception specificities. Plant Mol. Biol. 64, 539–547. [DOI] [PubMed] [Google Scholar]
- Robatzek, S. , Chinchilla, D. and Boller, T. (2006) Ligand‐induced endocytosis of the pattern recognition receptor FLS2 in Arabidopsis . Genes Dev. 20, 537–542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santiago, J. , Henzler, C. and Hothorn, M. (2013) Molecular mechanism for plant steroid receptor activation by somatic embryogenesis co‐receptor kinases. Science, 341, 889–892. [DOI] [PubMed] [Google Scholar]
- Schwessinger, B. , Roux, M. , Kadota, Y. , Ntoukakis, V. , Sklenar, J. , Jones, A. and Zipfel, C. (2011) Phosphorylation‐dependent differential regulation of plant growth, cell death, and innate immunity by the regulatory receptor‐like kinase BAK1. PLoS Genet. 7, e1002046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Segonzac, C. , Macho, A.P. , Sanmartín, M. , Ntoukakis, V. , Sánchez‐Serrano, J.J. and Zipfel, C. (2014) Negative control of BAK1 by protein phosphatase 2A during plant innate immunity. EMBO J. 33, 2069–2079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Somssich, M. , Ma, Q. , Weidtkamp‐Peters, S. , Stahl, Y. , Felekyan, S. , Bleckmann, A. , Seidel, C.A. and Simon, R. (2015) Real‐time dynamics of peptide ligand‐dependent receptor complex formation in planta . Sci. Signal. 8, ra76 10.1126/scisignal.aab0598. [DOI] [PubMed] [Google Scholar]
- Sun, W. , Cao, Y. , Labby, K.J. , Bittel, P. , Boller, T. and Bent, A.F. (2012) Probing the Arabidopsis flagellin receptor: FLS2‐FLS2 association and the contributions of specific domains to signaling function. Plant Cell, 24, 1096–1113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun, Y. , Han, Z. , Tang, J. , Hu, Z. , Chai, C. , Zhou, B. and Chai, J. (2013a) Structure reveals that BAK1 as a co‐receptor recognizes the BRI1‐bound brassinolide. Cell Res. 23, 1326–1329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun, Y. , Li, L. , Macho, A.P. , Han, Z. , Hu, Z. , Zipfel, C. , Zhou, J.M. and Chai, J. (2013b) Structural basis for flg22‐induced activation of the Arabidopsis FLS2‐BAK1 immune complex. Science, 342, 624–628. [DOI] [PubMed] [Google Scholar]
- Tameling, W.I.L. and Baulcombe, D.C. (2007) Physical association of the NB‐LRR resistance protein Rx with a Ran GTPase‐activating protein is required for extreme resistance to Potato virus X. Plant Cell, 19, 1682–1694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor, I. , Seitz, K. , Bennewitz, S. and Walker, J.C. (2013) A simple in vitro method to measure autophosphorylation of protein kinases. Plant Methods, 9, 22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van der Hoorn, R.A.L. , Laurent, F. , Roth, R. and de Wit, P.J.G.M. (2000) Agroinfiltration is a versatile tool that facilitates comparative analyses of Avr9/Cf‐9‐induced and Avr4/Cf‐4‐induced necrosis. Mol. Plant–Microbe Interact. 13, 439–446. [DOI] [PubMed] [Google Scholar]
- Voinnet, O. , Rivas, S. , Mestre, P. and Baulcombe, D.C. (2015) Retraction: an enhanced transient expression system in plants based on suppression of gene silencing by the p19 protein of tomato bushy stunt virus (retraction of The Plant Journal 84, 846. [DOI] [PubMed] [Google Scholar]
- Wang, X. , Kota, U. , He, K. , Blackburn, K. , Li, J. , Goshe, M.B. , Huber, S.C. and Clouse, S.D. (2008) Sequential transphosphorylation of the BRI1/BAK1 receptor kinase complex impacts early events in brassinosteroid signaling. Dev. Cell, 15, 220–235. [DOI] [PubMed] [Google Scholar]
- Wang, Y. , Xu, Y. , Sun, Y. , Wang, H. , Qi, J. , Wan, B. , Ye, W. , Lin, Y. , Shao, Y. , Dong, S. , Tyler, B.M. and Wang, Y. (2018) Leucine‐rich repeat receptor‐like gene screen reveals that Nicotiana RXEG1 regulates glycoside hydrolase 12 MAMP detection. Nat. Commun. 9, 594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu, J. , van der Burgh, A. , Bi, G. , Zhang, L. , Alfano, J.R. , Martin, G. and Joosten, M.H.A.J. (2018) The bacterial effector AvrPto targets the regulatory co‐receptor SOBIR1 and suppresses defence signalling mediated by the receptor‐like protein Cf‐4. Mol. Plant–Microbe Interact. 31, 75–85. [DOI] [PubMed] [Google Scholar]
- Yan, L. , Ma, Y. , Liu, D. , Wei, X. , Sun, Y. , Chen, X. , Zhao, H. , Zhou, J. , Wang, Z. , Shui, W. and Lou, Z. (2012) Structural basis for the impact of phosphorylation on the activation of plant receptor‐like kinase BAK1. Cell Res. 22, 1304–1308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang, L. , Kars, I. , Essenstam, B. , Liebrand, T.W. , Wagemakers, L. , Elberse, J. , Tagkalaki, P. , Tjoitang, D. , van den Ackerveken, G. and van Kan, J.A. (2014) Fungal endopolygalacturonases are recognized as microbe‐associated molecular patterns by the Arabidopsis receptor‐like protein RESPONSIVENESS TO BOTRYTIS POLYGALACTURONASES1. Plant Physiol. 164, 352–364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang, W. , Fraiture, M. , Kolb, D. , Löffelhardt, B. , Desaki, Y. , Boutrot, F.F.G. , Tör, M. , Zipfel, C. , Gust, A.A. and Brunner, F. (2013a) Arabidopsis receptor‐like protein30 and receptor‐like kinase Suppressor Of BIR1‐1/EVERSHED mediate innate immunity to necrotrophic fungi. Plant Cell, 25, 4227–4241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang, Z. , Fradin, E. , de Jonge, R. , van Esse, H.P. , Smit, P. , Liu, C.‐M. and Thomma, B.P.H.J. (2013b) Optimized agroinfiltration and virus‐induced gene silencing to study Ve1‐mediated Verticillium resistance in tobacco. Mol. Plant–Microbe Interact. 26, 182–190 [DOI] [PubMed] [Google Scholar]
- Zipfel, C. (2014) Plant pattern‐recognition receptors. Trends Immunol. 35, 345–351. [DOI] [PubMed] [Google Scholar]
- Zipfel, C. , Kunze, G. , Chinchilla, D. , Caniard, A. , Jones, J.D. , Boller, T. and Felix, G. (2006) Perception of the bacterial PAMP EF‐Tu by the receptor EFR restricts Agrobacterium‐mediated transformation. Cell, 125, 749–760. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig. S1 Kinase activity of SOBIR1 is not required for Cf‐4 stabilization. (A) Transient overexpression of Arabidopsis thaliana (At)SOBIR1 induces cell death in tobacco and in Nicotiana benthamiana when co‐expressed with P19. Agroinfiltrations were performed at an optical density at 600 nm (OD600) of 1. Where indicated, P19 was also co‐infiltrated at an OD600 of 1. Photographs were taken at 3 days post‐infiltration (dpi). It should be noted that constitutive immune activity of AtSOBIR1 requires its kinase activity. Furthermore, overexpression of Solanum lycopersicum (Sl)SOBIR1 from the Solanaceous plant tomato does not result in cell death. [See also Wu et al. (2018)]. (B) Co‐expression of wild‐type SlSOBIR1 as well as kinase‐dead SlSOBIR1D473N stabilizes Cf‐4 when co‐expressed in N. benthamiana. It should be noted that the signal of Cf‐4 is increased when overexpressed with both wild‐type and kinase‐dead SlSOBIR1, and highly increased on co‐expression with P19. Co‐agroinfiltrations of the affinity‐tagged proteins were performed in N. benthamiana leaves at an OD600 of 1 for each construct. Leaves were harvested at 2 dpi, and subjected to immunoprecipitation (IP) using anti‐green fluorescent protein (anti‐GFP) beads, followed by immune blotting (IB). The ribulose‐1,5‐bisphosphate carboxylase/oxygenase (Rubisco) band of the input shows equal loading. It should be noted that Cf‐4 is only visible in the input when co‐infiltrated with P19. CBB, Coomassie Brilliant Blue.
Fig. S2 Kinase‐dead SOBIR1 constitutively forms homodimers in planta. (A) Myc‐tagged versions of AtSOBIR1D489N and SlSOBIR1D473N co‐immunoprecipitate with eGFP‐tagged versions of AtSOBIR1D489N and SlSOBIR1D473N (asterisks), respectively, and with Cf‐4‐eGFP, but not with Flagellin‐Sensing 2 (FLS2)‐eGFP. Co‐agroinfiltrations of the various affinity‐tagged proteins were performed in combination with P19 in leaves of N. benthamiana at an OD600 of 0.6 for each construct. Leaves were harvested at 2 dpi, and subjected to IP using anti‐GFP beads, followed by IB. The Rubisco band of the input shows equal loading. It should be noted that, because of the low accumulation levels, not all proteins are visible in the input samples.
Fig. S3 AtSOBIR1‐mediated immunity is dependent on kinase‐active BAK1. (A) Transient co‐expression of AtSOBIR1 in tobacco with AtBAK1C408Y or AtBAK1D416N results in reduced AtSOBIR1 constitutive immune activity, when compared with co‐expression of AtSOBIR1 with wild‐type AtBAK1 or GUS. The indicated constructs were agroinfiltrated at an OD600 of 0.7. Photographs were taken at 2 dpi, and are representative of the agroinfiltration of eight leaves per sample. (B) Quantification of the percentage of cell death as shown in Fig. 4A. Percentages of constitutive cell death are presented as the mean ± standard error (SE). The letters indicate significant differences at P < 0.05, as determined by one‐way analysis of variance (ANOVA), including a Tukey post hoc test. (C) Quantification of the percentage of hypersensitive response (HR) as shown in Fig. 4B. Percentages of Avr4‐induced HR are presented as mean ± SE. The letters indicate significant differences at P < 0.05, as determined by one‐way ANOVA, including a Tukey post hoc test.


