Summary
Wheat blast was first reported in Brazil in 1985. It spread rapidly across the wheat cropping areas of Brazil to become the most important biotic constraint on wheat production in the region. The alarming appearance of wheat blast in Bangladesh in 2016 greatly increased the urgency to understand this disease, including its causes and consequences. Here, we summarize the current state of knowledge of wheat blast and aim to identify the most important gaps in our understanding of the disease. We also propose a research agenda that aims to improve the management of wheat blast and limit its threat to global wheat production.
Keywords: infection physiology, integrated disease management, international quarantine, origin and diversification, population biology and epidemiology, Pyricularia graminis‐tritici
The Emergence and Spread of Wheat Blast
Wheat blast disease was first discovered in the state of Paraná, Brazil in 1985 (Igarashi et al., 1986); it was found in the neighbouring states of São Paulo and Mato Grosso do Sul in 1986 (Goulart et al., 1990), followed by Rio Grande do Sul in 1987 (Igarashi, 1991) (Fig. 1). Since then, wheat blast has become a major disease across central and southern Brazil and is now well established in South America. The disease was not widespread in Brazil before the 1985 epidemic (Igarashi, 1991; Igarashi et al., 1986), although a previous description from 1936 (Puttemans, 1936) suggests that wheat blast may already have been present in the state of Rio de Janeiro (Fig. 1).
Figure 1.
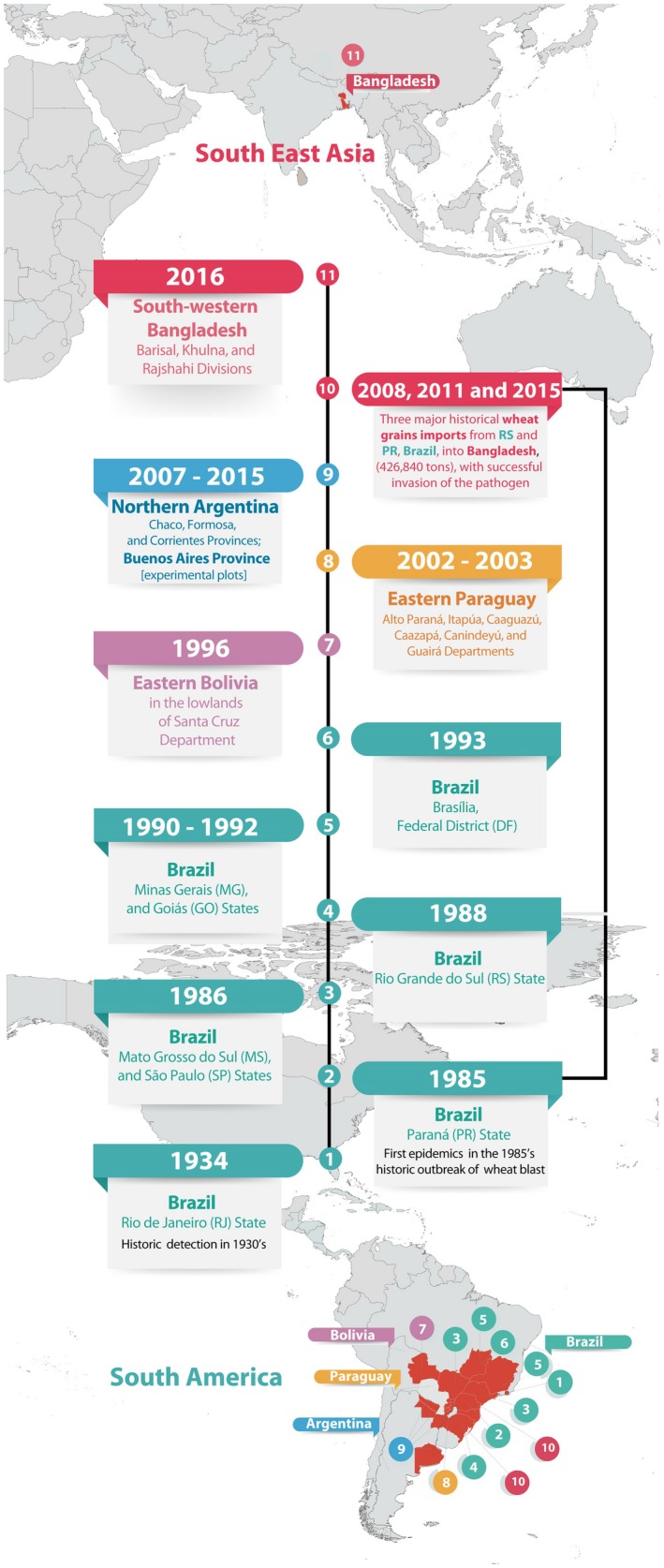
Timeline of events for the spread of wheat blast, from its emergence in South America to its invasion into South‐East Asia. Regions with confirmed wheat blast are highlighted in red. [Colour figure can be viewed at wileyonlinelibrary.com]
Wheat blast is caused by a hemibiotrophic Ascomycete in the Pyricularia species complex. The correct name for the Pyricularia lineage(s) causing wheat blast is currently under debate (Ceresini et al., 2018; Gladieux et al., 2018). In this review, we refer to the collection of lineages found to be primarily associated with wheat as Pyricularia graminis‐tritici (Pygt), following Castroagudín et al. (2016). We address the taxonomic debate about the species status of Pygt later in this review. After its origin in Paraná, the pathogen followed the agricultural expansion to the warm Cerrado areas of central‐western Brazil, arriving in Minas Gerais in 1990 (Lima, 2004), Goiás in 1992 (Prabhu et al., 1992) and Brasília in 1993 (Anjos et al., 1996), spreading about 1200 km north from its origin. Pygt also invaded new wheat agroecosystems located 1700 km to the north‐west of Paraná, arriving in Bolivia in 1996 (Barea and Toledo, 1996) and eastern Paraguay in 2002 (Viedma et al., 2010; Viedma and Morel, 2002). It also spread to cooler regions 1200 km south‐west of Paraná, reaching Chaco and Corrientes provinces in Argentina in 2007 (Alberione et al., 2008; Cabrera and Gutiérres, 2007; Perelló et al., 2015) (Fig. 1).
Seed‐borne inoculum probably facilitated the long‐distance dispersal of Pygt and allowed it to invade other agroecosystems in South America and now South‐East Asia (Gomes et al., 2017; Goulart and Paiva, 1992a; Goulart et al., 1995). Despite the risk of introducing Pygt into their local agroecosystems, 65 countries imported Brazilian wheat or mixtures of wheat and rye between January 2006 and September 2017, with quantities as high as 1.14 million tons of seeds or grain (Ceresini et al., 2018; Government of Brazil, 2017). A series of imports between 2008 and 2015, totalling more than 425 000 tons of possibly contaminated mixtures of wheat and rye grain harvested from Brazilian wheat blast epidemic areas, preceded the emergence of Pygt in Bangladesh in 2016 (Islam et al., 2016).
The invasion of wheat blast into Bangladesh in 2016 (Callaway, 2016; Islam et al., 2016) and its possible spread into India in 2017 (Government of India, 2016; Press Trust of India, 2017) brought wheat blast to the attention of Asian governments and the international community of plant pathologists, exposing an urgent need to develop strategies to contain the spread of this destructive pathogen (Islam et al., 2016; Malaker et al., 2016; McDonald and Stukenbrock, 2016; Sadat and Choi, 2017; Saharan et al., 2016; Sharma, 2017; Singh et al., 2016). A suitable climate, coupled with highly susceptible cultivars, could lead to severe outbreaks of wheat blast if it spreads further into India’s north‐eastern plains and Pakistan. This could have serious consequences for regional food security (Government of India, 2016; Saharan et al., 2016).
The Origin of the Wheat Blast Pathogen
Early studies conducted in the 1990s indicated a non‐rice origin for the wheat blast pathogen (Urashima et al., 1993). The findings of cross‐infection and inter‐fertility between fungal strains originating from different grass hosts occurring near wheat fields suggested that non‐rice hosts played a key role in the emergence of wheat blast in Brazil (Castroagudín et al., 2016; Pereira et al., 2014; Urashima et al., 1993). Isolates of Pygt found on wheat can infect a wide range of invasive, native and cultivated grass hosts from the tribes Hordeae, Festuceae, Aveneae, Chlorideae, Agrosteae and Paniceae (Urashima et al., 1993). Physical proximity between wheat and other grass species under natural field conditions was proposed to facilitate genetic exchange among the Pyricularia populations infecting different hosts, enabling host shifts (Stukenbrock and McDonald, 2008). Field experiments are needed to test these hypotheses and to provide a better understanding of the role played by non‐wheat hosts in the epidemiology of wheat blast.
Evidence supporting the hypothesis that wheat blast emerged via a host shift from a Pyricularia population infecting Lolium came from analyses of genetic variation in the avirulence genes PWT3 and PWT4 (Inoue et al., 2017). In this model, Lolium‐derived isolates carrying the Ao avirulence allele at the PWT3 locus infected a susceptible wheat cultivar carrying the rwt3 allele conditioning susceptibility, with the 1980s wheat blast outbreak in Brazil enabled by the widespread cultivation of susceptible wheat cultivars carrying rwt3. Later, selection on the less common Rwt3 wheat varieties favoured the emergence of pathogen strains with non‐functional PWT3 alleles. The model proposes that these pwt3 strains became the epidemic wheat blast population prevalent in South America (Inoue et al., 2017). Although this model made use of available data, the study did not include any Lolium‐associated populations of the pathogen from wheat blast endemic areas in Brazil, and did not consider that the association of Pyricularia with Lolium was reported 7 years after the Brazilian wheat blast outbreak (Igarashi et al., 1986; Nunes et al., 2002; Urashima et al., 1993). This model should be further tested using extensive collections of Lolium‐derived Pygt from Brazil to compare levels of gene and genotype diversity with the wheat‐infecting population and to determine whether the sequences of PWT3 and PWT4 in the Brazilian Lolium‐infecting population are consistent with this model.
Population genomic analyses, including 36 Pygt strains originating from many different hosts (including Lolium) and 59 strains of other Pyricularia species, could not determine whether the wheat blast pathogen in Brazil had a single or multiple origins. The absence of strict host specialization amongst the major Pygt subclades indicates that the capacity to infect wheat may have originated multiple times (Castroagudín et al., 2017; Fig. 2). This finding is consistent with the emergence of wheat blast in Brazil through a series of host shifts from populations of Pygt infecting native or invasive grass species growing near wheat fields (Castroagudín et al., 2015, 2017; Fig. 2). Supporting this hypothesis was the discovery of contemporary gene flow between the wheat‐infecting Pygt population and the Pygt population infecting other grass species, with the notable exception of the rice‐derived population P. oryzae (Castroagudín et al., 2017). Despite the lack of evidence for extensive contemporary gene flow, Gladieux et al. (2018) found evidence for historic introgression events amongst Pygt and P. oryzae populations. Further support came from the finding that the most frequent Pygt virulence groups, defined by glasshouse inoculations of single strains onto seedlings and detached ears of seven wheat differential lines (Maciel et al., 2014), were shared between the grass‐ and wheat‐infecting populations, suggesting movement of Pygt between nearby grasses and wheat crops (Castroagudín et al., 2017). Field experiments should be conducted to directly test this hypothesis. The Pygt populations found on wheat and other hosts have significantly higher genetic variation than the populations of P. oryzae causing rice blast in Brazil (Castroagudín et al., 2017; Saleh et al., 2012). All of the population genetic and population genomic analyses conducted thus far suggest that the rice blast pathogen may not provide an appropriate model for understanding wheat blast.
Figure 2.
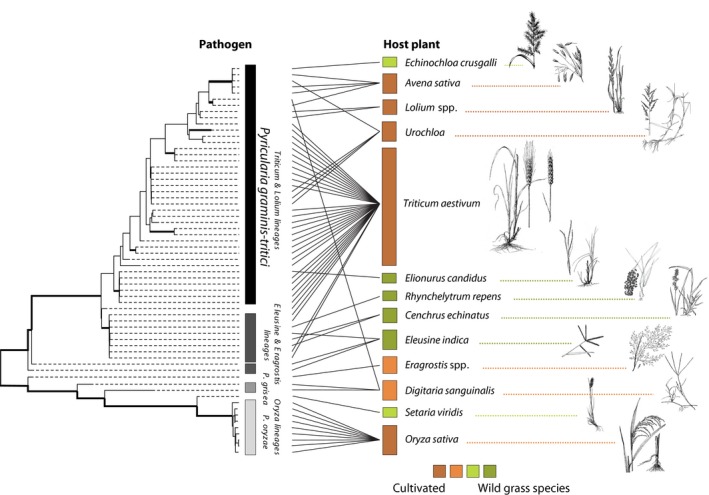
Maximum likelihood phylogenetic tree of Pyricularia lineages based on 28 427 genome‐wide single nucleotide polymorphisms. Bold lines indicate strong (100%) bootstrap support. The gradients across wild and cultivated host plant species are indicated by different colours. Plant drawings were adapted from original images obtained from the USDA Natural Resources Conservation Service ‐ NRCS (2018). [Colour figure can be viewed at wileyonlinelibrary.com]
The broader host range and apparent genetic diversification of Pygt relative to P. oryzae raise many questions about the functional role of within‐species polymorphisms. Mutations in the avirulence genes PWT3 and PWT4 are the only genetic factors that have thus far been associated with adaptation of Pygt to its host, allowing the fungus to escape recognition (Inoue et al., 2017). However, hundreds of presence/absence gene polymorphisms have been detected both within Pygt and between the wheat‐ and rice‐infecting populations (Chiapello et al., 2015; Yoshida et al., 2016). The gene categories most affected by gene deletion polymorphisms include genes encoding effectors and key secondary metabolites (Yoshida et al., 2016). Such genetic variation has been shown to fuel rapid adaptation to new host genotypes in other plant pathogens (Hartmann and Croll, 2017; Hartmann et al., 2017). Genomic analyses of the Pygt strains sampled from the recent outbreak in Bangladesh in 2016 and 2017 indicated the occurrence of a single clonal genotype, presenting a significant contrast to the high genetic diversity found at the pathogen’s centre of origin in South America (Islam et al., 2016). This suggests that Pygt experienced a significant bottleneck during its introduction into Bangladesh. Preventing the introduction of additional, sexually compatible strains of the pathogen into Bangladesh will be an important strategy to prevent the Asian Pygt population from undergoing the rapid diversification through recombination that happened in South America (Zhan et al., 2015).
The Wheat Blast Pathogen is Highly Diverse and has a Broad Host Range
From the time at which the rice blast pathogen was first named Pyricularia oryzae in 1892 (Tosa and Chuma, 2014), several Pyricularia‐like isolates associated with blast symptoms on barley (Hordeum vulgare), millet (Eleusine coracana, Pennisetum glaucum, Setaria italica), oats (Avena sativa), perennial ryegrass (Lolium perenne), wheat (Triticum aestivum) and more than 50 other grass species have been classified under the P. oryzae species complex (Couch et al., 2005; Couch and Kohn, 2002; Murakami et al., 2003; Takabayashi et al., 2002; Urashima and Kato, 1998). Based on assumptions with regard to host specificity, mating ability and genetic relatedness, P. oryzae was thereafter split into several pathotypes to reflect a limited host range: e.g. the Avena pathotype was attributed to isolates pathogenic on oats, the Eleusine pathotype to isolates pathogenic on finger millet (Eleusine coracana), the Lolium pathotype was pathogenic on perennial ryegrass and the Triticum pathotype was pathogenic on wheat (Farman, 2002; Kato et al., 2000; Oh et al., 2002; Tosa et al., 2004; Urashima et al., 1999). Although it became the status quo, this system of sorting Pyricularia isolates based on the assumption of host‐specialized populations does not reflect the current known biology of the blast pathogens, because many Pyricularia have been shown to have broader host ranges (Kato et al., 2000). For example, isolates of the Triticum pathotype can cause blast on barley, oats, rye (Secale cereale), signalgrass (Urochloa brizantha) and more than 10 other grass species (Castroagudín et al., 2016; Urashima et al., 1993), isolates of the Avena pathotype can infect wheat (Oh et al., 2002) and isolates of the Lolium pathotype can also infect wheat (Farman et al., 2017; Tosa et al., 2004). Further complicating the definition of species boundaries in this species complex is that different sets of strains, typically coming from different hosts, geographically distant locations and often collected many years apart, are mixed together into a single analysis oriented around a single set of genetic markers or phenotypes, making it difficult to compare and unify results coming from different analyses. We believe that new population genomic studies that bring together a representative set of Pyricularia strains collected from different hosts in sympatry (i.e. from the same geographical region and in the same time frame) will clarify the association between host range and the possibility of recent speciation.
Since 2010, a series of comprehensive phylogenetic studies have revisited the relationships among Pyricularia and Pyricularia‐like species, leading to substantial changes in the order Magnaporthales (Choi et al., 2013; Hirata et al., 2007; Klaubauf et al., 2014; Luo and Zhang, 2013; Murata et al., 2014). Taxonomists have proposed a definitive name change from Magnaporthe spp. to Pyricularia spp. (Klaubauf et al., 2014; Luo and Zhang, 2013; Murata et al., 2014). Older multi‐gene phylogenetic analyses of highly conserved housekeeping genes already recognized that P. oryzae and P. grisea were independent phylogenetic species (Couch and Kohn, 2002). Newer phylogenetic analyses based on 859 067 single nucleotide polymorphisms (SNPs) distributed across entire genomes have indicated that Pyricularia isolates associated with Triticum and Avena form a single monophyletic clade clearly distinct from the P. oryzae clade (Yoshida et al., 2016). These results were coherent with earlier phylogenomic analyses (Chiapello et al., 2015) and suggest that P. oryzae may not provide a good model for understanding the biology of the wheat blast pathogen.
A recent study based on a multi‐gene phylogeny for 128 isolates of Pyricularia spp. sampled from sympatric populations associated with rice, wheat and grasses growing near wheat fields distinguished the new species P. graminis‐tritici associated with wheat and several other hosts (Castroagudín et al., 2016). This study, which used the same 10 housekeeping genes that separated P. oryzae from P. grisea (Couch and Kohn, 2002), identified two Pyricularia species associated with wheat blast: Pygt and the formerly described Triticum pathotype of P. oryzae (PoT), which were both independent of the Oryza pathotype. The assignment of the new Pygt species was challenged by Gladieux et al. (2018), although it was also evident in their analysis that the rice‐ and wheat‐associated genotypes were in separate clades and that wheat‐associated genotypes were highly diverse compared with the rice‐associated genotypes. Gladieux et al. (2018) presented three arguments against the assignment of a separate species to the wheat‐associated isolates: (1) a lack of phylogenetic concordance for most of the loci analysed; (2) evidence for historic gene flow at specific loci between the genomes of rice‐ and wheat‐associated isolates and; (3) the occurrence of multiple, deeply diverging lineages within the original P. oryzae. The concordance of phylogenetic signals at multiple loci is often considered to be a prerequisite for the assignment of a new fungal species (Taylor et al., 2000). However, the phylogenetic species recognition system is very conservative and may fail to identify recent speciation events, which may be especially common in plant‐pathogenic fungi associated with agroecosystems (Stukenbrock and McDonald, 2008). Genomic analyses of the early stages of speciation in fungi, plants and animals have shown that the phylogenetic concordance of conserved loci emerges well after reproductive isolation of populations as a result of reduced hybrid fitness (Seehausen et al., 2014).
A more recent study including 95 genomic sequences of the wheat blast fungus from Brazil and other Pyricularia species supported the designation of Pygt as a distinct, highly diverse species with a broad host range (Castroagudín et al., 2017; Fig. 2), including strains from the pathotype Triticum (PoT) lineage which was placed into a separate clade when only 10 housekeeping genes were used (Castroagudín et al., 2016). Hence, the genome‐scale analysis merged the previously described PoT lineage into the Pygt species. This study also showed that Pygt is capable of causing blast on many other crop species, including barley, oats, perennial ryegrass and signalgrass, as well as native and introduced grass species frequently occurring as weeds in wheat fields (e.g. Chloris distichophylla, Cynodon spp., Digitaria insularis, Equinochloa crusgalli, Panicum maximum, Rhynchelytrum repens and Sorghum sudanense) (Castroagudín et al., 2017; Fig. 2). This new study has three important implications: (1) Pygt is not a wheat‐specialized pathogen; (2) the hypothesis of grass‐specific populations for the overall P. oryzae species complex is falsified; and (3) P. oryzae may not provide a suitable model for understanding the biology of Pygt.
The recognition of Pygt as a distinct species has important implications for quarantine regulations worldwide (Castroagudín et al., 2017). A lack of recognition that the formerly geographically restricted wheat blast pathogen Pygt was not the same species as the globally distributed rice blast pathogen P. oryzae may explain in part the lack of phytosanitation screens that led to the introduction of Pygt into Bangladesh (Government of Brazil, 2017; Ceresini et al., 2018). It is possible that local officials assumed that the wheat blast pathogen Pygt was the same species as the rice blast pathogen P. oryzae, which was already a well‐established pathogen in Bangladesh (Government of the People’s Republic of Bangladesh, 1989, 2011; Khan et al., 2016). Regardless of the root cause(s) of the introduction of wheat blast into Asia, it is clear that improved quarantines should be implemented to limit the further spread of Pygt into new regions.
Wheat Blast has Limited the Expansion of Wheat Production in Brazil
Wheat blast has limited the expansion of wheat cropping in Brazil. An analysis of the history of wheat production in the state of Mato Grosso do Sul illustrates its impact. In 1987, the wheat crop reached a peak of 428 000 hectares in Mato Grosso do Sul. Mainly as a result of wheat blast epidemics (Fig. 3A), but also in response to frequent droughts and falling commodity prices, wheat fields are now rare in this state, dropping by 95% to less than 20 000 ha in 2016 (Companhia Nacional de Abastecimento ‐ CONAB, 2017).
Figure 3.

Symptoms associated with wheat blast, including severe head infection in an irrigated wheat field in the Cerrado (A), and infections on leaves (B), spikes (C) and rachis (D, E). [Colour figure can be viewed at wileyonlinelibrary.com]
Wheat blast has also prevented the expansion of tropical wheat into the Cerrado region spanning the states of Minas Gerais, Goiás and the Brasília Federal District (Fig. 4). There would be many advantages associated with wheat cultivation in the Brazilian Cerrado. When used in a crop rotation, wheat decreases pathogen inoculum for other crops in the rotation, including the soybean crop that is vital for Brazil’s export economy. The Brazilian Cerrado is closer to the major cities located in central and northern Brazil, resulting in significantly lower transportation costs and making wheat produced in the Cerrado more economically competitive than imported wheat, especially from Argentina (Ceresini et al., 2018; Maciel, 2011). However, the fear of yield losses caused by wheat blast leads many growers to avoid cropping tropical wheat (Ceresini et al., 2018; Maciel, 2011).
Figure 4.
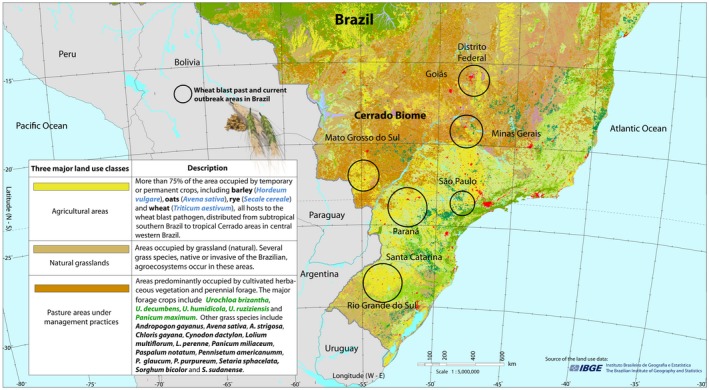
A map of Brazil showing the distribution of the three major land use categories and their overlap with areas affected by wheat blast. Land use data were provided by The Brazilian Institute of Geography and Statistics ‐ IBGE (2016). [Colour figure can be viewed at wileyonlinelibrary.com]
Biochemical and Physiological Alterations Associated with Wheat Blast
Foliar symptoms of wheat blast are rarely seen before heading and include elliptical to elongate lesions showing white to light brown centres and dark grey to reddish‐brown borders (Fig. 3B). Typical symptoms are observed on the heads when all spikelets above the point of infection in the rachis turn white (Fig. 3C). Symptoms on heads can vary from elliptical lesions with bleached centres on glumes to partial or total spike bleaching, sterility and empty grains (Goulart and Paiva, 1992b; Goulart et al., 1990; Urashima et al., 2009). Multiple points of infection in the rachis typically spread upwards and downwards from its node (Fig. 3D,E). When grains are produced, wheat blast reduces grain yield and quality, which can render the grain unfit for human consumption (Urashima et al., 2009). The shrunken and wrinkled grains commonly found in blasted wheat fields are used mainly for animal feed (Goulart and Paiva, 1992b; Goulart et al., 1990; Urashima et al., 2009).
Infection of plant leaves and spikes by Pygt causes many biochemical and physiological changes. Infected flag leaves show lower ribulose‐1,5‐bisphosphate‐carboxylase/oxygenase (RuBisCO) activity and a reduced RuBisCO activation state (Debona et al., 2013), as well as reduced CO2 assimilation (Rios et al., 2017). Infected leaves also suffer from lower CO2 influx as a result of stomatal closure (Aucique‐Pérez et al., 2014; Debona et al., 2013). Net carbon assimilation (A) correlates negatively with CO2 internal concentration (C i), and A is negatively correlated with blast severity (Aucique‐Pérez et al., 2014; Debona et al., 2013). A decrease in light‐saturated A (A max) and the light saturating point (LSP), coupled with an increase in dark respiration (R d), indicating plant photoinactivation, have been reported in infected leaves (Aucique‐Pérez et al., 2017). The photosynthetic machinery of infected leaves is impaired as indicated by the lower values of maximum photosystem II (PSII) quantum efficiency (F v/F m), capture efficiency of excitation energy by the open PSII reaction centres (F v′/F m′), photochemical quenching coefficient (q P) and electron transfer rate (ETR).
Flag leaves and grains infected by Pygt show lower fructose, glucose and sucrose concentrations and reduced sucrose phosphate synthase (SPS) activity (Rios et al., 2017). Infected flag leaves show a high starch concentration because of the down‐regulation of α‐ and β‐amylase genes (Rios et al., 2017). In grains obtained from infected spikelets, starch concentration is decreased in response to lower sucrose transport from photosynthetic organs. An increase in the breakdown of starch in grains from infected spikelets has been linked with the expression of α‐ and β‐amylase genes at an early stage of Pygt infection (Rios et al., 2017). Plants infected by Pygt during reproduction show alterations in the metabolism of organic acids and amino acids (Rios et al., 2017). The concentrations of amino acids derived from glycolytic intermediates (e.g. alanine, cysteine, phenylalanine and valine), as well as arginine, cysteine, histidine, methionine, proline and tryptophan, are higher in infected leaves. Fructose, glucose, sucrose and starch concentrations are dramatically reduced in grains obtained from infected spikelets (Rios et al., 2017).
High concentrations of hydrogen peroxide (H2O2) and superoxide anion radical (O2 •), and a reduction in lipid peroxidation, are essential for leaf and spike resistance to blast, especially on cultivars with a high level of partial resistance (Debona et al., 2012; Silva, 2017). Higher activities of ascorbate peroxidases (APXs), catalases (CATs), glutathione‐S‐transferases (GSTs), glutathione reductases (GRs), peroxidases (POXs) and superoxide dismutases (SODs) have been associated with the high level of basal resistance of some cultivars (Debona et al., 2012). Blast severity decreases on leaves of plants sprayed with picolinic acid because of a rapid response of antioxidant metabolism (higher APX, GST, POX and SOD activities) in the removal of reactive oxygen species (Aucique‐Pérez, 2016). Polyphenoloxidase activity on infected flag leaves is high regardless of the basal level of resistance of the cultivar. Phenylalanine ammonia‐lyase activity and the concentrations of phenolics and lignin are higher on more resistant cultivars (Silva, 2017). Chitinase and β‐1,3‐glucanase activities increase in response to Pygt infection on leaves and spikes of cultivars with higher resistance (Silva, 2017).
Population Genetics of PYGT and Epidemiology of Wheat Blast
Contemporary populations of Pygt collected from across the wheat‐growing regions of Brazil in 2012 and 2013 (including more than 500 strains taken from wheat and grass species growing in or nearby infected fields) contained high genetic and virulence diversity, including 198 multilocus microsatellite genotypes (MLMGs) and 25 virulence groups (Castroagudín et al., 2017). These populations exhibited a mixed reproductive system in which cycles of sexual reproduction generated novel genotypes that could be selected by the local environment, enabling amplification and dispersal of well‐adapted clones via asexual reproduction (Castroagudín et al., 2017). A similar genetic structure was observed in populations of Pygt previously sampled from central‐southern Brazil (Maciel et al., 2014). Morphological evidence supporting the hypothesis of sexual reproduction included the detection of proto‐perithecia and perithecia forming on senescing stems of wheat and other grasses under experimental conditions, suggesting that sexual reproduction can occur on crop residues or within senescent tissues of alternative hosts during the saprotrophic phase of the disease cycle (Castroagudín et al., 2017; Moreira et al., 2015). An important area for future research will be to identify the main hosts, tissues and environmental conditions that support the development of the sexual cycle in nature. The complete development of perithecia was observed on Phalaris canariensis (canarygrass), indicating that it is a promising candidate for the observation of the teleomorph in the field.
Given that Pygt isolates are capable of infecting wheat and other grasses, and can move back and forth between hosts, we hypothesize that sexual recombination may occur preferentially on non‐wheat hosts with the resulting ascospores and/or conidia infecting nearby wheat fields, giving rise to the highly diverse Pygt population detected on wheat. This scenario is supported by previous reports of gene flow amongst wheat‐ and other Poaceae‐adapted populations of Pygt (Castroagudín et al., 2017), the sharing of genotypes and virulence phenotypes between the host groups (Castroagudín et al., 2017), the cross‐infection and inter‐fertility between isolates from other poaceous hosts and wheat (Bruno and Urashima, 2001; Galbieri and Urashima, 2008; Urashima et al., 1993), and the occurrence of gametic equilibrium among neutral genetic markers (Castroagudín et al., 2017), consistent with sexual recombination in most populations, including those from non‐wheat hosts (Castroagudín et al., 2017).
The populations of Pygt from other grass species included strains isolated from signal grass (U. brizantha) (Castroagudín et al., 2017). As signal grass is an extensively grown pasture grass occupying more than 90 million hectares in Brazil (Jank et al., 2014), and is frequently found alongside wheat fields (The Brazilian Institute of Geography and Statistics ‐ IBGE, 2016; Fig. 4), it may play an important role as a key inoculum reservoir for wheat blast, providing a spatial and temporal link connecting wheat fields across central and western Brazil. A high degree of gene flow across a spatial scale of more than 2000 km was found in Brazil (Castroagudín et al., 2017). This high gene flow, which would maintain many alleles and reduce the impact of genetic drift, could reflect efficient wind dispersal of the pathogen’s conidia or ascospores over short distances (Urashima et al., 2007), in addition to long‐distance dispersal via infected seeds of wheat and Urochloa (Gomes et al., 2017; Goulart et al., 1995; Goulart and Paiva, 1993a, 1993b). Overall, these findings suggest that non‐wheat hosts may play an important role in the epidemiology of wheat blast, further complicating control efforts. There is an urgent need for field experiments to better understand the epidemiology of wheat blast, aiming to elucidate the relative importance of infected seeds, conidia and ascospores, coming from both wheat and non‐wheat hosts, as sources of primary and secondary inoculum.
One of the important findings on the population genetics of Pygt was the discovery of a super‐race, named virulence group A, which caused blast on the entire tested panel of wheat and barley cultivars (Castroagudín et al., 2017; Ceresini et al., 2018). Virulence group A was not a clone; this phenotype was found in many different Pygt genetic backgrounds. This suggests that the virulence group A phenotype may represent the loss of a major avirulence gene that is now segregating in many different genetic backgrounds as a result of recombination. It was detected at a higher frequency on Brazilian wheat, but was also found on several grass species invading wheat fields, including Avena sativa, Cenchrus echinatus, Chloris distichophylla, Cynodon spp., Echinochloa crusgalli, Digitaria insularis, D. sanguinalis, Eleusine indica, Eragrostis plana, Panicum maximum, Rhynchelytrum repens, Sorghum sudanense and U. brizantha (Castroagudín et al., 2017; Ceresini et al., 2018). Other common virulence groups were also shared between the grass‐ and wheat‐infecting Pygt populations, providing additional evidence for the movement of Pygt between wheat fields and nearby grasses.
Strategies for the Management of Wheat Blast
Reinforcing quarantine and bio‐safety rules in wheat blast‐free regions
Because wheat blast has now escaped from its endemic areas in South America and is causing epidemics in South‐East Asia, the primary global concern is to prevent additional spread of the pathogen to disease‐free countries and to avoid potential outbreaks in new regions. As infected seeds can spread the pathogen over long distances (Cruz and Valent, 2017; Goulart and Paiva, 1990; Silva et al., 2009; Urashima et al., 1999), the strengthening of quarantine and seed trading laws will provide the best course of action to prevent the further spread of wheat blast (Mezzalama, 2016; Sadat and Choi, 2017; Singh, 2017; Valent et al., 2013). Trade in wheat seeds from the wheat blast endemic areas in Latin America and South‐East Asia should be strictly regulated, if not totally prohibited. A globally organized awareness programme should advise quarantine regulators worldwide to implement appropriate rules to address the threat posed by the spread of wheat blast. Local country officials should more strictly enforce laws prohibiting the re‐direction of wheat grains imported to make flour into the local wheat seed industry. The introduction of wheat blast into Bangladesh appeared to follow the latter path (Ceresini et al., 2018; Islam et al., 2016; Sadat and Choi, 2017).
Management strategies in wheat blast endemic areas
Integrated disease management (IDM) strategies will be needed to minimize crop losses without impacting environmental sustainability (Mehta, 2014). The implementation of IDM tactics should be organized locally, taking into account the particular conditions of each affected region (Mehta, 2014). These tactics should be based on a knowledge of the pathogen’s population biology and epidemiology, including its disease cycle, survival strategy, means of dispersal, host range, main reproductive mode(s) and the weather conditions most conducive for disease development. Unfortunately, much of this knowledge is still missing.
Crop rotations or other cultural and sanitary practices
Weedy grass hosts appear to play a key role as bridges between wheat fields and cropping seasons and as sources of primary and/or secondary inoculum. Reducing the population of known weed hosts within and nearby wheat fields may be difficult to implement, but could reduce local Pygt inoculum (Mehta, 2014). It appears that Pygt can survive and produce sexual fruiting bodies (perithecia) on crop residues between wheat cropping seasons (Castroagudín et al., 2017; Urashima and Kato, 1998). If minimum tillage is not essential for soil conservation purposes, deep ploughing could decrease the initial Pygt inoculum on crop stubble (Igarashi, 1991; International Maize and Wheat Improvement Center ‐ CIMMYT Wheat Program, 2016). Following the introduction of wheat blast into South‐East Asia, local officials advised the elimination of crop residues from wheat, barley, millet and oats, as well as the eradication of invasive grass hosts, including Brachiaria spp., Cenchrus spp., Chloris spp., Digitaria spp., Echinochloa spp., Eleusine indica and Lolium spp. (Government of India, 2016; Plantwise, 2016; Sadat and Choi, 2017). This management strategy may not be cost‐effective for small farmers or appropriate for regions in which soil conservation agriculture is needed (International Maize and Wheat Improvement Center ‐ CIMMYT Wheat Program, 2016). Crop rotation can be a useful option for the management of wheat blast if non‐grass crops, such as soybean and common vetch (Santos et al., 2000), are available. In South‐East Asia, jute (Corchorus olitorius) cultivation could be an option for crop rotation (Government of India, 2016). Altering the sowing date to prevent an overlap between flowering or grain filling stages and blast‐conducive periods characterized by high temperatures, rain and high relative humidity is an effective cultural practice to manage wheat blast in South America (Coelho et al., 2016; Mehta, 2014; Mehta et al., 1992). Field experiments designed to dissect the epidemiology of wheat blast will be needed to determine which of these strategies will have the greatest impact.
Certified healthy seeds and improved detection methods for seed‐borne inoculum
Because wheat blast can be seed‐borne and seed‐transmitted (Goulart and Paiva, 1990; Martins et al., 2004), infected seeds are thought to be the primary source of inoculum for long‐distance dispersal (Cruz and Valent, 2017; Goulart and Paiva, 1990; Silva et al., 2009; Urashima et al., 1999, 2009). Spores of Pygt are able to survive and remain infectious for up to 2 years on both the surface and inside seeds. Even apparently healthy seeds harvested from infected fields can carry fungal spores (Reis et al., 1995). Consequently, the use of certified pathogen‐free seeds, or fungicide‐treated wheat seeds, should be mandatory for both internal seed markets and for export from countries with wheat blast (Toledo, 2015). In Brazil, wheat seed fungicide treatments were mandated by law (Mehta, 2014), but, since the 1970s, the legislation has been relaxed and seed health testing has been neglected (Mehta, 2014). This decreased vigilance facilitated the dispersal of seed‐borne Pygt inoculum across Brazil’s wheat cropping areas, as well as into Bolivia and Paraguay, through frequent imports of Brazilian wheat seed used for sowing (Ceresini et al., 2018).
Accurate methods for the detection of Pygt in asymptomatic seeds will be needed to limit or prevent the spread of the pathogen into disease‐free areas. Healthy wheat seed will also decrease the initial inoculum in wheat blast endemic areas (Akhtar et al., 2016; Mezzalama, 2016). Ideal detection methods should combine several specific molecular markers as targets and use template DNA extracted directly from potentially infested seed lots (Chen et al., 2016; Pieck et al., 2016). A recent report has illustrated the current lack of robust molecular tools that can detect infected seeds (Gupta et al., 2018), and has highlighted the need to develop improved detection methods.
Fungicide seed treatment
The only fungicide labelled for seed treatment against wheat blast in Brazil is the demethylation inhibitor (DMI) difenoconazole. The eradicant fungicide dimethyldithiocarbamate thiram, which is labelled for the control of other seed‐borne wheat pathogens, is also effective against Pygt (Bockus et al., 2015; Goulart and Paiva, 1991).
Disease forecasting based on weather conditions
The optimum weather conditions for wheat blast development include long and frequent periods of leaf wetness (24–40 h), coupled with high temperatures (25–30 °C) (Cardoso et al., 2008). In Brazil, a predictive model for wheat blast outbreaks based on daily climatic data, named Sisalert (Plant Disease Epidemic Risk Prediction System), was developed to calculate the risk of an epidemic (available at: https://dev.sisalert.com.br/monitoramento/?page_id=14; Fernandes et al., 2017; Nicolau et al., 2016). In the USA, a climatic model adapted from this Brazilian model indicated that the weather conditions were favourable to wheat blast in 25% of the winter wheat cropping regions, with suitable conditions for wheat blast outbreaks in 70% of the years for Louisiana, Mississippi and Florida (Cruz et al., 2016). Similar predictive models should be developed for other countries facing wheat blast outbreaks, including Bolivia, Paraguay, Bangladesh and India. The major advantage of using a model like Sisalert based on the automated collection of weather data is to provide a warning of an imminent risk of wheat blast infection (West et al., 2017; West and Kimber, 2015). This alert enables real‐time decision‐making for fungicide applications.
Breeding for wheat blast resistance
The breeding of wheat cultivars for improved blast resistance has not been very successful despite constant efforts over the last 30 years (Cruz et al., 2010; Maciel et al., 2008; Prestes et al., 2007; Urashima et al., 2004). With the realization that other grass species, such as Urochloa or Lolium, may play a key role in wheat blast epidemiology, it may be time to consider breeding for resistance to Pygt in these other grass hosts. Resistance to wheat blast is not stable because resistant varieties become susceptible when they are deployed across different geographical locations spanning a 2800‐km transect (Urashima et al., 2004). Initially, the unstable resistance was thought to result from cultivar‐by‐environment interactions that differed across Brazil (Castroagudín et al., 2016; Duveiller et al., 2010; Maciel et al., 2014). Today, it seems more likely that the instability of blast resistance reflects a breakdown of resistance genes underlying gene‐for‐gene (GFG) interactions, with new races of the pathogen emerging in different regions to render the deployed resistance ineffective.
Extensive phenotyping of 173 Pygt strains encompassing 80 unique MLMGs from six geographical regions in Brazil (Castroagudín et al., 2017) differentiated 25 seedling virulence groups (SVGs), named SVGs A–Y (Ceresini et al., 2018). The predominant group was SVG L, representing 47% of the isolates tested, whereas SVG A was the second most frequent group, found in 13% of the isolates tested. Many of the wheat cultivars used as differentials lost their resistance to wheat blast in the heading stage, corroborating earlier findings (Urashima and Kato, 1998). The same 173 isolates fell into nine head virulence groups (HVGs) when virulence was assessed on detached, mature wheat heads (Castroagudín et al., 2017; Ceresini et al., 2018). HVG A was the predominant virulence group, found in 78% of isolates, followed by HVG B, found in 14% of isolates. These two HVGs were found in all geographical regions, including the populations sampled from non‐wheat hosts in Mato Grosso do Sul and Paraná. HVG A caused blast on the entire set of differential cultivars, suggesting its designation as a ‘super‐race’. HVG A was also found on the non‐wheat hosts Avena sativa, Cenchrus echinatus, Chloris distichophylla, Cynodon spp., Digitaria insularis, D. sanguinalis, Echinochloa crusgalli, Eleusine indica, Eragrostis plana, Panicum maximum, Rhynchelytrum repens, Sorghum sudanense and U. brizantha.
Few sources of resistance to wheat blast have been identified in Brazil until now. Amongst these sources, the Brazilian wheat cultivars BRS 229 and BR 18‐Terena have been regularly used in breeding programmes because of their higher levels of field resistance to head blast (Brunetta et al., 2006; Goulart et al., 1991; Goulart and Paiva, 1992b; Sousa, 2002). However, these resistant cultivars have shown susceptibility in some environments, probably as a result of the emergence of new Pygt races in these areas (Urashima et al., 2001, 2005). Therefore, the identification of new sources of resistance is crucial.
A potential source of resistance was identified in Aegilops tauschii (syn. Aegilops squarrosa) (Urashima and Kato, 1994) in 1994. The bread wheat cultivar Renan (Hanzalová et al., 2007) and the barley lineage CGN02857 (a barley accession obtained in East Africa and maintained by the CGN germplasm collection at Wageningen University and Research Centre), both derived from crosses with A. tauschii, were considered to be promising sources of wheat blast resistance.
Recently, the 2NS/AS chromosomal translocation from A. ventricosa was associated with resistance to wheat blast (Cruz et al., 2016). Compared with accessions lacking 2NS/AS, near‐isogenic lines of both spring and winter wheat carrying the segment showed a 64%–81% reduction in head blast severity in field trials conducted under natural epidemics in Bolivia. Cultivars derived from the CIMMYT line Milan, which possesses the 2NS/AS translocation, also showed high levels of blast resistance under field conditions (Cruz et al., 2016). Other 2NS/AS carriers derived from Milan, such as Canindé I, CD 116 and Sausal ClAT, have been widely deployed in South America, but the stability of this source of resistance remains unclear (Kohli et al., 2011). There is evidence that the 2NS/AS resistance present in the bread wheat cultivar Renan can be overcome by three of the nine Pygt virulence groups identified in Brazil, including the super‐race HVG A (Castroagudín et al., 2017; Ceresini et al., 2018). Consequently, additional sources of resistance should be sought and developed.
Four resistance genes (Rmg2, Rmg3, Rmg7, Rmg8) have been identified in common wheat (T. aestivum) and in tetraploid emmer wheat (T. dicoccum) (Anh et al., 2015; Tagle et al., 2015; Zhan et al., 2008). These four resistance genes function against the Br48 strain of Pygt that was collected in 1991. The sources of these resistance genes include T. aestivum cultivar Thatcher (carrier of Rmg2 and Rmg3; Zhan et al., 2008), T. dicoccum lines KU112 (St17), KU120 (St24) and KU122 (St25), carriers of Rmg7 (Tagle et al., 2015), and T. aestivum line S615, carrier of Rmg8 (Anh et al., 2015). It is not yet known whether these resistance genes will be effective against contemporary populations of Pygt.
Embrapa’s new cultivar Lagoa Vermelha has one of Thatcher’s relatives (NewThatch) in its pedigree. In the 2017 screening of Embrapa wheat genotypes in a wheat blast hotspot in Uberaba, Minas Gerais (MG), Lagoa Vermelha showed high levels of blast resistance during an epidemic year. However, in Patos de Minas, MG, under more tropical conditions, cultivar Thatcher showed high susceptibility to blast (J. L. N. Maciel, personal communication). Interestingly, the Rmg2 and Rmg3 genes giving blast resistance in cultivar Thatcher are not effective at high temperatures and do not provide resistance for ear infections (Zhan et al., 2008). Consequently, they were probably not associated with the levels of resistance found in Lagoa Vermelha. Instead, it is more plausible that cultivar Lagoa Vermelha carries other wheat blast resistance genes.
Progress in breeding for wheat blast resistance will probably be facilitated through the development of uniform sets of host and pathogen differentials that can be used as controls to enable comparisons of results and better coordination of breeding efforts distributed across several locations. Screening of germplasm for wheat blast resistance should include a representative set of the contemporary virulence diversity existing in the wheat blast population across Brazil, including the HVG A super‐race. An optimal field screening for resistance would include several locations across different agroecosystems, including Mato Grosso Sul State, which is a hotspot for the pathogen’s virulence diversity, with the highest number of SVGs (11) and HVGs (6). For countries interested in pre‐emptive breeding for wheat blast resistance, germplasm should be screened for blast resistance in Brazil, where the pathogen is genetically diverse, virulent, endemic and distributed across a temperature cline ranging from the colder regions of Rio Grande do Sul to the warmer Cerrado areas of Goiás (Fig. 4). Three recent examples of this strategy are as follows: a work plan between CIMMYT and the Indian Council of Agricultural Research (ICAR) to test 40 wheat varieties and advanced lines at wheat blast hotspots in Argentina, Bolivia, Brazil and Paraguay (Government of India, 2016); the testing of spring wheat cultivars with and without the 2NS/AS translocation near Quirusillas, in Bolivia (Cruz et al., 2016); EMBRAPA Cenargen, at the request of Swiss breeders in cooperation with the Federal University of Lavras, introduced Swiss wheat landraces to test against the most common SVGs and HVGs characterized there (E. Alves, UFLA, personal communication).
Fungicide applications on leaves and ears
In South America, fungicides are often used to manage wheat blast even though their efficacy is low, with only a small reduction in blast severity observed on symptomatic spikes in treated fields (Maciel, 2011; Pagani et al., 2014). Decreases in crop losses were detected only when mixtures of strobilurin and triazole fungicides were applied early on moderately resistant wheat varieties with low or moderate disease pressure (Pagani et al., 2014; Rios et al., 2016a, 2016b; Rocha et al., 2014). In all cases, the effectiveness of fungicide sprays at early heading and early grain‐filling stages was associated with a decrease in Pygt inoculum produced on the lower wheat leaves, with a subsequent reduction in ear infections (Cruz et al., 2015).
In South‐East Asia, recommendations for the management of wheat blast include spraying strobilurins combined with triazoles (i.e. trifloxystrobin and tebuconazole) (Government of India, 2016). In Brazil, under high disease pressure, these fungicides resulted in only partial control (Maciel, 2011; Maciel et al., 2014), reducing disease severity by 50% (Maciel, 2011). The reduced efficacy of fungicides for the management of wheat blast in Brazil was attributed to many factors, including highly favourable weather conditions coupled with high levels of cultivar susceptibility (Cruz et al., 2011), the highly diverse Pygt population (Urashima et al., 2004), the difficulties of reaching the infection sites on spikelets (Panisson et al., 2004) and the inherent inefficacy of some active ingredients (Goulart et al., 1996; Maciel, 2011; Pagani et al., 2014; Urashima and Kato, 1994). Because Pygt has a broad host range, including several invasive grass species on which fungicides are not sprayed (Castroagudín et al., 2015; Reis and Casa, 2016), a continuous external source of new inoculum might compromise the efficacy of chemical management (Castroagudín et al., 2017). All of these factors should be taken into consideration when fungicides are deployed to manage wheat blast in South‐East Asia.
In Brazil, 28 fungicides were labelled for the management of wheat blast, including 11 triazoles and seven mixtures of quinone outside inhibitors (QoIs) and triazoles (Ministério da Agricultura Pecuária e Abastecimento ‐ MAPA, 2017). These two fungicide groups were used intensively over one to three decades to manage rusts and other foliar diseases of wheat (Debona et al., 2009; Navarini and Balardin, 2012; Reis et al., 1977; Tormen et al., 2013), and it is possible that their use to control other diseases inadvertently selected for resistance in the associated Pygt populations, explaining their low efficacy against wheat blast. In fact, resistance to both QoI (azoxystrobin and pyraclostrobin) and triazole (tebuconazole and epoxiconazole) fungicides was found to be pervasive in populations of the pathogen across the major wheat cropping areas from central western to southern Brazil (Castroagudín et al., 2015; Ceresini et al., 2018; Oliveira et al., 2015; Poloni, 2016). All contemporary populations of Pygt sampled in 2012 and 2013 showed high resistance to azoxystrobin, pyraclostrobin and to both epoxiconazole and tebuconazole, with half maximal effective concentration (EC50) values at least 30–200 times higher than the wild‐type isolates (Ceresini et al., 2018). Mutations in the cytb and Cyp51A genes were associated with higher levels of resistance to strobilurins and triazoles in these populations (Castroagudín et al., 2015; Ceresini et al., 2018; Oliveira et al., 2015; Poloni, 2016). These observations suggest that these strobilurin and triazole fungicides are not likely to provide long‐term solutions to the management of wheat blast in South‐East Asia.
Recently, five new fungicide formulations were labelled for wheat diseases in Brazil, each containing the second‐generation carboxamide fluxapiroxade (a succinate dehydrogenase inhibitor, SDHI) combined with the QoI pyraclostrobin and/or the DMI epoxiconazole (Ministério da Agricultura Pecuária e Abastecimento ‐ MAPA, 2017), the two fungicide classes to which the Brazilian populations of Pygt were already resistant (Poloni, 2016). Because none of these five formulations were labelled specifically for wheat blast, their effectiveness has yet to be determined. An important factor to consider is that the second‐generation SDHIs are also high‐risk fungicides (Ishii and Hollomon, 2015) and resistance could emerge in Pygt populations if point mutations occur in any of the three genes encoding the targeted components of the SDH complex (SDH B, C and D) (Amiri et al., 2013). When 170 Pygt isolates sampled from different locations in 2013 were tested for sensitivity to fluxapyroxad, the majority of the isolates were insensitive, with moderately resistant isolates found in five of the six tested field populations (Casado, 2017). This was surprising because these Pygt populations had not yet been exposed to second‐generation SDHIs. No data are yet available regarding mutations in the sdh subunits B, C and D that could explain the observed levels of resistance. To reduce the risk that Pygt will become resistant to SDHI fungicides, the recommendation is to deploy SDHIs only in mixtures with low‐risk fungicides, such as mancozeb and chlorothalonil (van den Bosch et al., 2014), but it is clear that a monitoring programme should be implemented to detect the emergence of known resistance mutations in these genes.
Biological control and biofortification
Several biocontrol agents which have been shown to reduce rice blast symptoms under field conditions should be tested for their efficacy against wheat blast. Microbes that control rice blast include Bacillus methylotrophicus, Chaetomium globosum and Trichoderma harzianum (Oliveira et al., 2015; Park et al., 2005; Singh et al., 2012). Some non‐fungicidal chemicals have already been evaluated for their ability to control wheat blast. For example, potassium silicate inhibited fungal growth in vitro and potassium phosphate reduced blast severity on three wheat cultivars (Cruz et al., 2011). Applications of silicon restricted Pygt colonization of wheat leaves by triggering the flavonoid biosynthetic pathway and the deposition of phenolic compounds. Silicon applications also intensified the expression of defence‐related genes (Cruz et al., 2011, 2015b, 2015; Silva et al., 2015). Treatments with silicon in field experiments increased yields by 26%–92%, whereas phosphite treatments increased yields by 9%–80% (Pagani et al., 2014), suggesting promising lines of research for the management of wheat blast with non‐fungicidal chemicals.
A Forward‐Looking Research Agenda for Wheat Blast
There are many important gaps in our knowledge of wheat blast that will need to be filled in order to develop long‐term management strategies. Although population genetic and genomic studies have provided important insights into the possible origins of Pygt and its likely mechanisms of long‐distance dispersal, surprisingly little is understood about wheat blast epidemiology at the field scale. Replicated field experiments are urgently needed to determine the main sources of primary and secondary inoculum and to evaluate the relative contributions of infected seeds, conidia and ascospores to wheat ear infections. Although the population genetic studies suggest that Urochloa and other non‐wheat grasses could be important sources of inoculum to fuel wheat blast epidemics, field experiments must be conducted to test this hypothesis. Additional field experiments will be needed to determine the relative contributions of crop residues, infected seeds and non‐wheat grasses to the persistence of Pygt between growing seasons and to identify the most important environments for sexual reproduction. The discovery that the Pygt population in Bangladesh appears to be a single clone (Islam et al., 2016) is inconsistent with the hypothesis that the pathogen was introduced on infected wheat grain colonized by the highly diverse Pygt population of Brazil. Although this lack of diversity in the Asian population is good news from the perspective of disease management (less genetic diversity suggests lower evolutionary potential and slower adaptation to fungicide treatments and resistant cultivars), it suggests that much remains to be learned about wheat seed infection.
Equally important will be to identify new sources of wheat blast resistance that are effective and stable. These efforts could be made more efficient by agreeing on a uniform set of host and pathogen differentials to include in screening programmes. Although it seems obvious that the screening for resistance should take place under selection by the highly diverse Pygt population in Brazil, it is not obvious what sources of germplasm should be used in the case of a newly emergent disease that did not co‐evolve with its main economic host (wheat), especially given the broad host range of Pygt. If non‐wheat hosts, such as Urochloa, are shown to be the primary host for the pathogen, with blast disease on wheat mainly collateral damage occurring as a result of inoculum spillover from an epidemic on the non‐wheat host under particular environmental conditions, it may be more appropriate to breed for resistance in the non‐wheat host.
Similarly, if a non‐wheat grass is the primary host of Pygt, management strategies may need to focus on the control of the infection in the non‐wheat host in order to prevent spillover into nearby wheat fields. For example, biological control agents or fungicides may be more effective if applied to the primary, non‐wheat host instead of the wheat field. A crop rotation designed to remove Pygt inoculum from wheat stubble of previous crops is unlikely to be effective if the primary source of inoculum is coming from the perennial Urochloa pastures growing near the wheat field. It is becoming clear that we need to know much more about the biology of Pygt on its non‐wheat hosts.
Acknowledgements
We thank the following funding agencies that supported much of the research described in this review: FAPESP (São Paulo Research Foundation, Brazil) research grants (2013/10655‐4, 2015/10453‐8 and 2017/50456‐1) to P.C.C., EMBRAPA‐Monsanto research grant (Macroprogram II‐02.11.04.006.00.00) to J.L.N.M., and research grants from FINEP (Funding Authority for Studies and Projects, Brazil) and FAPEMIG (Minas Gerais Research Foundation, Brazil) to E.A. (CAG‐APQ‐01975‐15). E.A., F.A.R. and P.C.C. were supported by research fellowships from the Brazilian National Council for Scientific and Technological Development ‐ CNPq (Pq‐2 307361/2012‐8 and 307295/2015‐0). V.L.C. was supported by a Post‐Doctorate Research Fellowship FAPESP (PDJ 2014/25904‐2, from 2015 to 2017). S.I.M. was supported by a Postdoctoral Research Fellowship PNPD from CAPES (Higher Education Personnel Improvement Coordination, Brazil). D.C. is supported by the Swiss National Science Foundation (grant 31003A_173265).
References
- Akhtar, J. , Kandan, A. , Singh, B. , Kumar, P. , Khan, Z. , Gawade, B.H. , Kumar, S. and Dubey, S.C. (2016) Diagnosis of seed‐borne plant pathogens for safe introduction and healthy conservation of plant genetic resources In: Current Trends in Plant Disease Diagnostics and Management Practices (Kumar P., Gupta V.K., Tiwari A.K. and Kamle M., eds), pp. 429–440. Cham: Springer International Publishing. [Google Scholar]
- Alberione, E. , Bainotti, C. , Cettour, I. and Salines, J. (2008) Evaluación de enfermedades en trigos en siembra de verano en el NEA argentino‐Campaña 2007/2008 In: 7mo. Congreso Nacional de Trigo (Univ. Nacional La Pampa, eds), pp. 2 . Santa Rosa, La Pampa, Argentina. [Google Scholar]
- Amiri, A. , Heath, S.M. and Peres, N.A. (2013) Resistance to fluopyram, fluxapyroxad, and penthiopyrad in Botrytis cinerea from strawberry. Plant Dis. 98, 532–539. [DOI] [PubMed] [Google Scholar]
- Anh, V.L. , Anh, N.T. , Tagle, A.G. , Vy, T.T.P. , Inoue, Y. , Takumi, S. , Chuma, I. and Tosa, Y. (2015) Rmg8, a new gene for resistance to Triticum isolates of Pyricularia oryzae in hexaploid wheat. Phytopathology, 105, 1568–1572. [DOI] [PubMed] [Google Scholar]
- Anjos, J.R.N. , Silva, D.B. , Charchar, M.J.D. and Rodrigues, G.C. (1996) Occurrence of blast fungus (Pyricularia grisea) on wheat and rye in the savanna region of Central Brazil. Pesq. Agropec. Trop. 31, 79–82. [Google Scholar]
- Aucique‐Pérez, C.E. (2016) Wheat resistance to blast using a non‐host selective toxin and host metabolic reprogramming through a successful infection by Pyricularia oryzae. PhD Dissertation. Federal University of Viçosa, Viçosa, Minas Gerais, Brazil, pp. 89. [Google Scholar]
- Aucique‐Pérez, C.E. , de Menezes Silva, P.E. , Moreira, W.R. , DaMatta, F.M. and Rodrigues, F.Á. (2017) Photosynthesis impairments and excitation energy dissipation on wheat plants supplied with silicon and infected with Pyricularia oryzae . Plant Physiol. Biochem. 121, 196–205. [DOI] [PubMed] [Google Scholar]
- Aucique‐Pérez, C.E. , Rodrigues, F.Á. , Moreira, W.R. and DaMatta, F.M. (2014) Leaf gas exchange and chlorophyll a fluorescence in wheat plants supplied with silicon and infected with Pyricularia oryzae . Phytopathology, 104, 143–149. [DOI] [PubMed] [Google Scholar]
- Barea, G. and Toledo, J. (1996) Identificación y zonificación de piricularia o bruzone (Pyricularia oryzae) en el cultivo del trigo en el Dpto. de Santa Cruz In: CIAT. Informe Técnico. Proyecto de Investigación Trigo, Santa Cruz, pp. 76–86.
- Bockus, W.W. , Cruz, C.D. , Stack, J.P. and Valent, B. (2015) Effect of seed‐treatment fungicides on sporulation of Magnaporthe oryzae from wheat seed, 2014. Plant Dis. Manag. Rep. 9, ST004. [Google Scholar]
- van den Bosch, F. , Paveley, N. , van den Berg, F. , Hobbelen, P. and Oliver, R. (2014) Mixtures as a fungicide resistance management tactic. Phytopathology, 104, 1264–1273. [DOI] [PubMed] [Google Scholar]
- Brunetta, D. , Bassoi, M.C. , Dotto, S.R. , Scheeren, P.L. , Miranda, M.Z.D. , Tavares, L.C.V. and Miranda, L.C. (2006) Characteristics and agronomic performance of wheat cultivar BRS 229 in Paraná State, Brazil. Pesq. Agropec. Bras. 41, 889–992. [Google Scholar]
- Bruno, A.C. and Urashima, A.S. (2001) Sexual relationship between Magnaporthe grisea from wheat and from other hosts. Fitopatol. Bras. 26, 21–26. [Google Scholar]
- Cabrera, M.G. and Gutiérres, S.A. (2007) Primer registro de Pyricularia grisea en cultivos de trigo del NE de Argentina In: Actas de la Reunión de Comunicaciones Científicas y Tecnológicas, SGCYT (Universidade Nacional de Nordeste /Secretaría Gral. de Ciencia y Técnica (SGCYT), eds). Corrientes: Nacional University of Nordeste. [Google Scholar]
- Callaway, E. (2016) Devastating wheat fungus appears in Asia for first time. Nature, 532, 421–422. [DOI] [PubMed] [Google Scholar]
- Cardoso, C.A.A. , Reis, E.M. and Moreira, E.N. (2008) Development of a warning system for wheat blast caused by Pyricularia grisea . Summa Phytopathol. 34, 216–221. [Google Scholar]
- Casado, P.S. (2017) Validation of the microplate method and studies on resistance to the fungicide fluxapyroxad in populations of Pyricularia graminis‐tritici in Brazil. MSc Dissertation, University of São Paulo State, Ilha Solteira, São Paulo, pp. 53. [Google Scholar]
- Castroagudín, V.L. , Ceresini, P.C. , de Oliveira, S.C. , Reges, J.T.A. , Maciel, J.L.N. , Bonato, A.L.V. , Dorigan, A.F. and McDonald, B.A. (2015) Resistance to QoI fungicides is widespread in Brazilian populations of the wheat blast pathogen Magnaporthe oryzae . Phytopathology, 105, 284–294. [DOI] [PubMed] [Google Scholar]
- Castroagudín, V.L. , Danelli, A. , Moreira, S.I. , Reges, J.T.A. , Carvalho, G. , Maciel, J.L.N. , Bonato, A.L.V. , Forcelini, C.A. , Alves, E. , McDonald, B. and Croll, D. (2017) The wheat blast pathogen Pyricularia graminis‐tritici has complex origins and a disease cycle spanning multiple grass hosts. bioRxiv, 203455 Available at: 10.1101/203455 [accessed on 05 July 2018]. [DOI] [Google Scholar]
- Castroagudín, V.L. , Moreira, S.I. , Pereira, D.A.S. , Moreira, S.S. , Brunner, P.C. , Maciel, J.L.N. , Crous, P.W. , McDonald, B.A. , Alves, E. and Ceresini, P.C. (2016) Pyricularia graminis‐tritici, a new Pyricularia species causing wheat blast. Persoonia, 37, 199–216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ceresini, P.C. , Castroagudín, V.L. , Rodrigues, F.A. , Rios, J.A. , Aucique‐Pérez, C.E. , Moreira, S.I. , Alves, E. , Croll, D. and Maciel, J.L.N. (2018) Wheat blast: past, present, and future. Annu. Rev. Phytopathol. 56, 427–456, Available at: 10.1146/annurev-phyto-080417-050036 [accessed on 05 July 2018]. [DOI] [PubMed] [Google Scholar]
- Chen, X. , Ma, L. , Qiang, S. and Ma, D. (2016) Development of a loop‐mediated isothermal amplification method for the rapid diagnosis of Ascochyta rabiei L. in chickpeas. Sci. Rep. 6, 25 688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiapello, H. , Mallet, L. , Guérin, C. , Aguileta, G. , Amselem, J. and Kroj, T. (2015) Deciphering genome content and evolutionary relationships of isolates from the fungus Magnaporthe oryzae attacking different host plants. Genome Biol. Evol. 7, 2896–2912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi, J. , Park, S.‐Y. , Kim, B.‐R. , Roh, J.‐H. , Oh, I.‐S. , Han, S.‐S. and Lee, Y.H. (2013) Comparative analysis of pathogenicity and phylogenetic relationship in Magnaporthe grisea species complex. PLoS One, 8, e57196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coelho, M.D.O. , Torres, G.M. , Cecon, P.R. and Santana, F.M. (2016) Sowing date reduces the incidence of wheat blast disease. Pesq. Agropec. Bras. 51, 631–637. [Google Scholar]
- Companhia Nacional de Abastecimento ‐ CONAB (2017) Acompanhamento da safra brasileira: grãos: vol 4, Safra 2016/2017—Oitavo levantamento. Brasília, DF: CONAB, pp. 144. [Google Scholar]
- Couch, B.C. , Fudal, I. , Lebrun, M.‐H. , Tharreau, D. , Valent, B. , van Kim, P. , Nottéghem, J.L. and Kohn, L. (2005) Origins of host‐specific populations of the blast pathogen Magnaporthe oryzae in crop domestication with subsequent expansion of pandemic clones on rice and weeds of rice. Genetics, 170, 613–630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Couch, B.C. and Kohn, L.M. (2002) A multilocus gene genealogy concordant with host preference indicates segregation of a new species, Magnaporthe oryzae, from M. grisea . Mycologia, 94, 683–693. [DOI] [PubMed] [Google Scholar]
- Cruz, C.D. , Kiyuna, J. , Bockus, W.W. , Todd, T.C. , Stack, J.P. and Valent, B. (2015. a) Magnaporthe oryzae conidia on basal wheat leaves as a potential source of wheat blast inoculum. Plant Pathol. 64, 1491–1498. [Google Scholar]
- Cruz, C.D. , Magarey, R.D. , Christie, D.N. , Fowler, G.A. , Fernandez, J.M. , Bockus, W.W. , Valent, B. and Stack, J.P. (2016. a) Climate suitability for Magnaporthe oryzae Triticum pathotype in the United States. Plant Dis. 100, 1979–1987. [DOI] [PubMed] [Google Scholar]
- Cruz, C.D. , Peterson, G.L. , Bockus, W.W. , Kankanala, P. , Dubcovsky, J. , Jordan, K.W. , Akhunov, E. , Chumley, F. , Baldelomar, F.D. and Valent, B. (2016. b) The 2NS translocation from Aegilops ventricosa confers resistance to the Triticum pathotype of Magnaporthe oryzae . Crop Sci. 56, 990–1000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cruz, C.D. and Valent, B. (2017) Wheat blast disease: danger on the move. Trop. Plant Pathol. 42, 210–222. [Google Scholar]
- Cruz, M.F.A. , Debona, D. , Rios, J.A. , Barros, E.G. and Rodrigues, F.A. (2015b) Potentiation of defense‐related gene expression by silicon increases wheat resistance to leaf blast. Trop. Plant Pathol. 40, 394–400. [Google Scholar]
- Cruz, M.F.A. , Diniz, A.P.C. , Rodrigues, F.A. and Barros, E.G. (2011) Foliar application of products on the reduction of blast severity on wheat. Trop. Plant Pathol. 36, 424–428. [Google Scholar]
- Cruz, M.F.A. , Prestes, A.M. , Maciel, J.L.N. and Scheeren, P.L. (2010) Partial resistance to blast on common and synthetic wheat genotypes in seedling and in adult plant growth stages. Trop. Plant Pathol. 35, 24–31. [Google Scholar]
- Cruz, M.F.A. , Silva, L.A.F. , Rios, J.A. , Debona, D. and Rodrigues, F.Á. (2015. c) Microscopic aspects of the colonization of Pyricularia oryzae on the rachis of wheat plants supplied with silicon. Bragantia, 74, 207–214. [Google Scholar]
- Debona, D. , Figueiró, G.G. , Corte, G.D. , Navarini, L. , Domingues, L.S. and Balardin, R. (2009) Effect of seed treatment with fungicides and acibenzolar‐S‐methyl in soybean cultivars on Asian rust control and seedlings growth. Summa Phytopathol. 35, 26–31. [Google Scholar]
- Debona, D. , Rodrigues, F.Á. , Rios, J.A. , Martins, S.C.V. , Pereira, L.F. and DaMatta, F.M. (2013) Limitations to photosynthesis in leaves of wheat plants infected by Pyricularia oryzae . Phytopathology, 104, 34–39. [DOI] [PubMed] [Google Scholar]
- Debona, D. , Rodrigues, F.Á. , Rios, J.A. and Nascimento, K.J.T. (2012) Biochemical changes in the leaves of wheat plants infected by Pyricularia oryzae . Phytopathology, 102, 1121–1129. [DOI] [PubMed] [Google Scholar]
- Duveiller, E. , Hodson, D. and Tiedmann, A. (2010) Wheat blast caused by Magnaporthe grisea: a reality and new challenge for wheat research. In: 8th International Wheat Conference (Braun, H.J., Snape, J.W., Šíp, V. , eds), pp. 247–248. St. Petersburg: Vavilov Research Institute of Plant Industry. [Google Scholar]
- Farman, M. , Peterson, G. , Chen, L. , Starnes, J. , Valent, B. , Bachi, P. , Murdock, L. , Hershman, D. , Pedley, K. , Fernandes, J.M. and Bavaresco, J. (2017) The Lolium pathotype of Magnaporthe oryzae recovered from a single blasted wheat plant in the United States. Plant Dis. 101, 684–692. [DOI] [PubMed] [Google Scholar]
- Farman, M.L. (2002) Pyricularia grisea isolates causing gray leaf spot on perennial ryegrass (Lolium perenne) in the United States: relationship to P. grisea isolates from other host plants. Phytopathology, 92, 245–254. [DOI] [PubMed] [Google Scholar]
- Fernandes, J.M.C. , Pavan, W. , Hölbig, C.A. , Karrei, M. , de Vargas, F. , Bavaresco, J.L.B. , Lazzaretti, A.T. and Tsukahara, R.Y. (2017) A weather‐based model for predicting early season inoculum build‐up and spike infection by the wheat blast pathogen. Trop. Plant Pathol. 42, 230–237. [Google Scholar]
- Galbieri, R. and Urashima, A.S. (2008) Sexual characterization, compatibility and occurrence of sexual reproduction among isolates of Pyricularia grisea from different hosts. Summa Phytopathol. 34, 22–28. [Google Scholar]
- Gladieux, P. , Condon, B. , Ravel, S. , Soanes, D. , Nunes Maciel, J.L. , Nhani, A. , Chen, L. , Terauchi, R. , Lebrun, M.H. , Tharreau, D. and Mitchell, T. (2018) Gene flow between divergent cereal‐ and grass‐specific lineages of the rice blast fungus Magnaporthe oryzae. mBio, 9, e01219–01217. Available at: 10.01210.01128/mBio [accessed on 05 July 2018]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomes, D.P. , Rocha, V.S. , Pereira, O.L. and Souza, M.A.d. (2017) Damage of wheat blast on the productivity and quality of seeds as a function of the initial inoculum in the field. J. Seed Sci. 39, 66–74. [Google Scholar]
- Goulart, A.C.P. and Paiva, F.A. (1990) Transmission of Pyricularia oryzae by wheat (Triticum aestivum) seeds. Fitopatol. Bras. 15, 359–362. [Google Scholar]
- Goulart, A.C.P. and Paiva, F.A. (1991) Control of Pyricularia oryzae and Helminthosporium sativum through wheat seeds treatment with fungicides. Pesq. Agropec. Bras. 26, 1983–1988. [Google Scholar]
- Goulart, A.C.P. and Paiva, F.A. (1992a) Associated fungi in wheat (Triticum aestivum L.) seeds produced in Mato Grosso do Sul, in 1990 and 1991. Rev. Bras. Sementes, 14, 221–225. [Google Scholar]
- Goulart, A.C.P. and Paiva, F.A. (1992b) Incidence of (Pyricularia oryzae) in different wheat cultivars under field conditions. Fitopatol. Bras. 17, 321–325. [Google Scholar]
- Goulart, A.C.P. and Paiva, F.A. (1993a) Fungi incidence in wheat (Triticum aestivum) seeds produced in Mato Grosso do Sul. Fitopatol. Bras. 18, 107–109. [Google Scholar]
- Goulart, A.C.P. and Paiva, F.A. (1993b) Survival of Pyricularia orizae Cav on wheat seeds under different storing conditions. Rev. Bras. Sementes, 15, 153–156. [Google Scholar]
- Goulart, A.C.P. , Paiva, F.A. and Andrade, P.J.M. (1995) Relationship between incidence of blast in wheat seeds and the presence of Pyricularia grisea in the harvested seeds. Fitopatol. Bras. 20, 184–189. [Google Scholar]
- Goulart, A.C.P. , Paiva, F.A. and Colman, O.P. (1991) Reação de cultivares de trigo (Triticum aestivum L.) à brusone (Pyricularia oryzae Cav) em condições de campo In: Reunião da Comissão Centro‐Sul Brasileira de Pesquisa de Trigo (Embrapa‐Uepae, eds), Vol. 7, pp. 118–120. Curitiba: Embrapa—Uepae. [Google Scholar]
- Goulart, A.C.P. , Paiva, F.A. , Filho, G.A.M. and Richetti, A. (1996) Effect of the time and number of applications of tebuconazole and mancozeb fungicides on wheat blast control (Pyricularia grisea); technical and economic feasibility. Fitopatol. Bras. 21, 381–387. [Google Scholar]
- Goulart, A.C.P. , Paiva, F.A. and Mesquita, A.N. (1990) Occurrence of wheat blast (Pyricularia oryzae) in the state of Mato Grosso do SuI. Fitopatol. Bras. 15, 112–114. [Google Scholar]
- Government of Brazil (2017) System of Analysis of Foreign Trade Information. Available at: https://aliceweb.mdic.gov.br [accessed on 30 October 2017]. [Google Scholar]
- Government of India (2016) Minutes of the meeting on “Occurrence of blast disease on wheat” held under the Chairmanship of Agriculture Commissioner on 28th September, 2016 at Kolkata. File no. 4–2/20 13‐NFSM (Ministry of Agriculture & Farmers Welfare Department of Agriculture Cooperation & Farmers Welfare Crops Division NFSM Cell, ed.). Krishi Bhawan, New Delhi, pp. 14. [Google Scholar]
- Government of the People’s Republic of Bangladesh (1989) The Destructive Insects and Pests Rules, 1966 (Plant Quarantine) Amendment, 1989 (Department of Agricultural Extension—National Plant Quarantine Authority, ed.). Dhaka, pp. 48. [Google Scholar]
- Government of the People’s Republic of Bangladesh (2011) Plant Quarantine Act, 2011 (Department of Agricultural Extension—National Plant Quarantine Authority, ed.). Dhaka‐1215, pp. 16. [Google Scholar]
- Gupta, D.R. , Reyes Avila, C.S. , Win, J. , Soanes, D.M. , Ryder, L.S. , Croll, D. , Bhattacharjee, P. , Hossain, M.S. , Mahmud, N.U. , Mehbub, M.S. and Surovy, M.Z. (2018) The MoT3 assay does not distinguish between Magnaporthe oryzae wheat and rice blast isolates from Bangladesh. bioRxiv, 345215 Available at: 10.1101/345215 [accessed on 05 July 2018]. [DOI] [Google Scholar]
- Hanzalová, A. , Dumalasová, V. , Sumíková, T. and Bartoš, P. (2007) Rust resistance of the French wheat cultivar Renan. Czech J. Genet. Plant Breed. 43, 53–60. [Google Scholar]
- Hartmann, F.E. and Croll, D. (2017) Distinct trajectories of massive recent gene gains and losses in populations of a microbial eukaryotic pathogen. Mol. Biol. Evol. 34, 2808–2822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartmann, F.E. , Sanchez‐Vallet, A. , McDonald, B.A. and Croll, D. (2017) A fungal wheat pathogen evolved host specialization by extensive chromosomal rearrangements. ISME J. 11, 1189–1204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirata, K. , Kusba, M. , Chuma, I. , Osue, J. , Nakayashiki, H. , Mayama, S. and Tosa, Y. (2007) Speciation in Pyricularia inferred from multilocus phylogenetic analysis. Mycol. Res. 111, 799–808. [DOI] [PubMed] [Google Scholar]
- Igarashi, S. (1991) Update on wheat blast (Pyricularia oryzae) in Brazil In: Proceedings of the international conference on wheat for the nontraditional warm areas (Saunders D. ed.), pp. 3 Foz do Iguaçu; Mexico DF: CIMMYT. [Google Scholar]
- Igarashi, S. , Utiamada, C.M. , Igarashi, L.C. , Kazuma, A.H. and Lopes, R.S. (1986) Occurrence of Pyrcularia sp. in wheat (Triticum aestivum L.) in the State of Paraná, Brazil. Fitopatol. Bras. 11, 351–352. [Google Scholar]
- Inoue, Y. , Vy, T.T.P. , Yoshida, K. , Asano, H. , Mitsuoka, C. , Asuke, S. , Anh, V.L. , Cumagun, C.J. , Chuma, I. , Terauchi, R. and Kato, K. (2017) Evolution of the wheat blast fungus through functional losses in a host specificity determinant. Science, 357, 80–83. [DOI] [PubMed] [Google Scholar]
- International Maize and Wheat Improvement Center ‐ CIMMYT Wheat Program (2016) Understanding and Managing the Threat of Wheat Blast in South Asia, South America, and Beyond (pp. 4). Mexico: CIMMYT; Available at: https://hdl.handle.net/10883/16947 [accessed on 7 November 2017]. [Google Scholar]
- Ishii, H. and Hollomon, D.W. (2015) Fungicide Resistance in Plant Pathogens: Principles and a Guide to Practical Management. Tokyo: Springer Japan. [Google Scholar]
- Islam, M.T. , Croll, D. , Gladieux, P. , Soanes, D.M. , Persoons, A. , Bhattacharjee, P. , Hossain, M.S. , Gupta, D.R. , Rahman, M.M. , Mahboob, M.G. and Cook, N. (2016) Emergence of wheat blast in Bangladesh was caused by a South American lineage of Magnaporthe oryzae . BMC Biol. 14, 84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jank, L. , Barrios, S. C. , do Valle, C. B. , Simeão, R. M. and Alves, G. F. (2014) The value of improved pastures to Brazilian beef production. Crop Pasture Sci. 65, 1132–1137. [Google Scholar]
- Kato, H. , Yamamoto, M. , Yamaguchi‐Ozaki, T. , Kadouchi, H. , Iwamoto, Y. , Nakayashiki, H. , Yukio, T.O.S.A. , Mayama, S. and Naoki, M.O.R.I. (2000) Pathogenicity, mating ability and DNA restriction fragment length polymorphisms of Pyricularia populations isolated from Gramineae, Bambusideae and Zingiberaceae plants. J. Gen. Plant Pathol. 66, 30–47. [Google Scholar]
- Khan, M.A.I. , Ali, M.A. , Monsur, M.A. , Kawasaki‐Tanaka, A. , Hayashi, N. , Yanagihara, S. , Obara, M. , Mia, M.A.T. , Latif, M.A. and Fukuta, Y. (2016) Diversity and distribution of rice blast (Pyricularia oryzae Cavara) races in Bangladesh. Plant Dis. 100, 2025–2033. [DOI] [PubMed] [Google Scholar]
- Klaubauf, S. , Tharreau, D. , Fournier, E. , Groenewald, J.Z. , Crous, P.W. , de Vries, R.P. and Lebrun, M.H. (2014) Resolving the polyphyletic nature of Pyricularia (Pyriculariaceae). Stud. Mycol. 79, 85–120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohli, M.M. , Mehta, Y.R. , Guzman, E. , De Viedma, L. and Cubilla, L.E. (2011) Pyricularia blast—a threat to wheat cultivation. Czech J. Genet. Plant Breed. 47, S130–S134. [Google Scholar]
- Lima, M.I.P.M. (2004) Giberela ou brusone? Orientações para a identificação correta dessas enfermidades em trigo e em cevada. Passo Fundo, RS: EMBRAPA Trigo. [Google Scholar]
- Luo, J. and Zhang, N. (2013) Magnaporthiopsis, a new genus in Magnaporthaceae (Ascomycota). Mycologia, 105, 1019–1029. [DOI] [PubMed] [Google Scholar]
- Maciel, J.L.N. (2011) Magnaporthe oryzae, the blast pathogen: current status and options for its control. Plant Sci. Rev. 2011, 233–240. [Google Scholar]
- Maciel, J.L.N. , Ceresini, P.C. , Castroagudín, V.L. , Kema, G.H.J. and McDonald, B.A. (2014) Population structure and pathotype diversity of the wheat blast pathogen Magnaporthe oryzae 25 years after its emergence in Brazil. Phytopathology, 104, 95–107. [DOI] [PubMed] [Google Scholar]
- Maciel, J. L. N., Paludo, E. A. , Só e Silva, M. , Scheeren, P. L. and Caierão, E. (2008) Reação à brusone de genótipos de trigo do programa de melhoramento da Embrapa Trigo no estádio de planta adulta (pp. 14). Passo Fundo: Embrapa Trigo. [Google Scholar]
- Malaker, P.K. , Barma, N.C.D. , Tiwari, T.P. , Collis, W.J. , Duveiller, E. , Singh, P.K. , Joshi, A.K. , Singh, R.P. , Braun, H.J. , Peterson, G.L. , Pedley, K.F ., Farman, M.L and Valent, B . (2016) First report of wheat blast caused by Magnaporthe oryzae pathotype Triticum in Bangladesh. Plant Dis. 100, 2330. [Google Scholar]
- Martins, T.D. , Lavorenti, N.A. and Urashima, A.S. (2004) Methods to examine transmission of Pyricularia grisea from seeds to seedlings of Triticale. Fitopatol. Bras. 29, 425–428. [Google Scholar]
- McDonald, B.A. and Stukenbrock, E.H. (2016) Rapid emergence of pathogens in agro‐ecosystems: global threats to agricultural sustainability and food security. Philos. Trans. R. Soc. B, 371, 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehta, Y.R. (2014) Pillars of integrated disease management In: Wheat Diseases and their Management (Y. R. Mehta, ed), pp. 17–63. New York: Springer International Publishing Switzerland. [Google Scholar]
- Mehta, Y.R. , Riede, C.R. , Campos, L.A.C. and Kohli, M.M. (1992) Integrated management of major wheat diseases in Brazil: an example for the Southern Cone region of Latin America. Crop Prot. 11, 517–524. [Google Scholar]
- Mezzalama, M. (2016) Seed Health: Fostering the Safe Distribution of Maize and Wheat Seed—General Guidelines, 4th edn. Ciudad de Mexico: CIMMYT. [Google Scholar]
- Ministério da Agricultura Pecuária e Abastecimento ‐ MAPA (2017) Agrofit—Sistemas de Agrotóxicos Fitossanitários, Coordenação Geral de Agrotóxicos e Afins. Available at: https://agrofit.agricultura.gov.br/agrofit_cons/principal_agrofit_cons [accessed on 20 November 2017].
- Moreira, S.I. , Ceresini, P.C. and Alves, E. (2015) Sexual reproduction in Pyricularia oryzae . Summa Phytopathol. 41, 175–182. [Google Scholar]
- Murakami, J. , Tomita, R. , Kataoka, T. , Nakayashiki, H. , Tosa, Y. and Mayama, S. (2003) Analysis of host species specificity of Magnaporthe grisea toward foxtail millet using a genetic cross between isolates from wheat and foxtail millet. Phytopathology, 93, 42–45. [DOI] [PubMed] [Google Scholar]
- Murata, N. , Aoki, T. , Kusaba, M. , Tosa, Y. and Chuma, I. (2014) Various species of Pyricularia constitute a robust clade distinct from Magnaporthe salvinii and its relatives in Magnaporthaceae . J. Gen. Plant Pathol. 80, 66–72. [Google Scholar]
- Navarini, L. and Balardin, R.S. (2012) Foliar diseases and control by fungicides on yield and quality of wheat grains. Summa Phytopathol. 38, 294–299. [Google Scholar]
- Nicolau, M. , Danelli, A.L.D. , Pavan, W. and Fernandes, J.M.C. (2016)Using a Bayesian model for estimating air borne infection risks: wheat blast. In: V International Symposium on Fusarium Head Blight and II International Workshop on Wheat Blast (DelPonte E.M., Bergstrom G.C., Pavan W., Lazzaretti A. and Fernandes J.M.C., eds), pp. 159 Florianópolis, SC; Passo Fundo, RS: Universidade de Passo Fundo. [Google Scholar]
- Nunes, C.D.M. , Bração, N. , Rodrigues, R.C. and Reis, J.C. (2002) Occurrence of blight on ryegrass. Fitopatol. Bras. 27(Supplement), 803. [Google Scholar]
- Oh, H.S. , Tosa, Y. , Takabayashi, N. , Nakagawa, S. , Tomita, R. , Don, L.D. , Kusaba, M. , Nakayashiki, H. and Mayama, S. (2002) Characterization of an Avena isolate of Magnaporthe grisea and identification of a locus conditioning its specificity on oat. Can. J. Bot. 80, 1088–1095. [Google Scholar]
- Oliveira, S.C.D. , Castroagudín, V.L. , Maciel, J.L.N. , Pereira, D.A.D.S. and Ceresini, P.C. (2015) Cross‐resistance to QoI fungicides azoxystrobin and pyraclostrobin in the wheat blast pathogen Pyricularia oryzae in Brazil. Summa Phytopathol. 41, 298–304. [Google Scholar]
- Pagani, A.P. , Dianese, A.C. and Café Filho, A.C. (2014) Management of wheat blast with synthetic fungicides, partial resistance and silicate and phosphite minerals. Phytoparasitica, 42, 609–617. [Google Scholar]
- Panisson, E. , Boller, W. and Reis, E.M. (2004) Spray deposition evaluation on wheat anthers to study the head blight (Gibberella zeae) chemical control. Eng. Agric. 24, 111–120. [Google Scholar]
- Park, J.H. , Choi, G.J. , Jang, K.S. , Lim, H.K. , Kim, H.T. , Cho, K.Y. and Kim, J.C. (2005) Antifungal activity against plant pathogenic fungi of chaetoviridins isolated from Chaetomium globosum . FEMS Microbiol. Lett. 252, 309–313. [DOI] [PubMed] [Google Scholar]
- Pereira, J.F. , Consoli, L. , Bombonatto, E.A.S. , Bonato, A.L.V. and Maciel, J.L.N. (2014) Development of genomic SSR markers and molecular characterizaton of Magnaporthe oryzae isolates from wheat in Brazil. Biochem. Genet. 52, 52–70. [DOI] [PubMed] [Google Scholar]
- Perelló, A. , Martinez, I. and Molina, M. (2015) First report of virulence and effects of Magnaporthe oryzae isolates causing wheat blast in Argentina. Plant Dis. 99, 1177. [Google Scholar]
- Pieck, M.L. , Ruck, A. , Farman, M.L. , Peterson, G.L. , Stack, J.P. , Valent, B. and Pedley, K.F. (2016) Genomics‐based marker discovery and diagnostic assay development for wheat blast. Plant Dis. 101, 103–109. [DOI] [PubMed] [Google Scholar]
- Plantwise (2016) Wheat Blast Pest Management Decision Guide: Green List, pp. 1 Guildford, Surrey: CABI; Available at: https://www.plantwise.org/FullTextPDF/2016/20167800929.pdf [accessed on 8 November 2017]. [Google Scholar]
- Poloni, N.M. (2016) Widespread distribution of triazole fungicide resistance and evolution of the cyp51A gene in populations of the wheat blast pathogen Pyricularia oryzae in Brazil MSc Dissertation, pp. 44 Ilha Solteira, University of São Paulo State, UNESP. [Google Scholar]
- Prabhu, A.S. , Filippi, M.C. and Castro, N. (1992) Pathogenic variation among isolates of Pyricularia oryzae infecting rice, wheat and grasses in Brazil. Trop. Pest Manag. 38, 367–371. [Google Scholar]
- Press Trust of India (2017) Deadly wheat blast disease spreads to Bengal districts In: The New Indian Express (Vijay Joshi, ed). Chennai: Express Publications (Madurai) Limited. [Google Scholar]
- Prestes, A. , Arendt, P. , Fernandes, J. and Scheeren, P. (2007) Resistance to Magnaporthe grisea among Brazilian wheat genotypes In: Wheat Production in Stressed Environments (H. T. Buck, J. E. Nisi, and N. Salomón, eds), pp. 119–123. Amsterdam: Springer, Netherlands. [Google Scholar]
- Puttemans, A. (1936) Alguns dados para servir à historia da phytopathologia no Brasil e às primeiras notificações de doenças de vegetaes neste paiz. Rodriguesia, 2, 17–36. [Google Scholar]
- Reis, E. and Casa, R. (2016) Doenças do trigo In: Manual de fitopatologia: doenças das plantas cultivadas, Vol. 2 (Amorin L., Rezende J., Bergamim Filho A. and Camargo J., eds), pp. 737–744. São Paulo: Editora Agronomica Ceres Ltda. [Google Scholar]
- Reis, E.M. , Blum, M.C. and Forcelini, C.A. (1995) Survival of Pyricularia oryzae associated with wheat seeds. Summa Phytopathol. 21, 43–44. [Google Scholar]
- Reis, E.M. , Eichler, M.R. and Anardi, C. (1977) Efeito da acidificação, dosagem e número de aplicações de triadimefom, no controle de doenças fúngicas do trigo In: Reunião Anual Conjunta de Pesquisa de Trigo, Vol. 9 Londrina, PR: Embrapa, CNPT. [Google Scholar]
- Rios, J.A. , Rios, V.S. , Aucique‐Pérez, C.E. , Cruz, M.F.A. , Morais, L.E. , DaMatta, F.M. and Rodrigues, F.A. (2017) Alteration of photosynthetic performance and source–sink relationships in wheat plants infected by Pyricularia oryzae . Plant Pathol. 66, 1496–1507. [Google Scholar]
- Rios, J.A. , Rios, V.S. , Paul, P.A. , Souza, M.A. , Araujo, L. and Rodrigues, F.A. (2016a) Fungicide and cultivar effects on the development and temporal progress of wheat blast under field conditions. Crop Prot. 89, 152–160. [Google Scholar]
- Rios, J.A. , Rios, V.S. , Paul, P.A. , Souza, M.A. , Neto, L.B.M.C. and Rodrigues, F.A. (2016b) Effects of blast on components of wheat physiology and grain yield as influenced by fungicide treatment and host resistance. Plant Pathol. 66, 877–889. [Google Scholar]
- Rocha, J.R.A.S.C. , Pimentel, A.J.B. , Ribeiro, G. and Souza, M.A. (2014) Efficiency of fungicides in wheat blast control. Summa Phytopathol. 40, 347–352. [Google Scholar]
- Sadat, M.D. and Choi, J. (2017) Wheat blast: a new fungal inhabitant to Bangladesh threatening world wheat production. Plant Pathol. J. 33, 103–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saharan, M.S. , Bhardwaj, S.C. , Chatrath, R. , Sharma, P. , Choudhary, A.K. and Gupta, R.K. (2016) Wheat blast disease—an overview. J. Wheat Res. 8, 1–5. [Google Scholar]
- Saleh, D. , Xu, P. , Shen, Y. , Li, C. , Adreit, H. , Milazzo, J. , Ravigné, V. , Bazin, E. , Nottéghem, J.L. , Fournier, E. and Tharreau, D. (2012) Sex at the origin: an Asian population of the rice blast fungus Magnaporthe oryzae reproduces sexually. Mol. Ecol. 21, 1330–1344. [DOI] [PubMed] [Google Scholar]
- Santos, H.P. , Lhamby, J.C.B. , Prestes, A.M. and Lima, M.R. (2000) Effect of soil management and of crop rotation systems on wheat yield and diseases. Pesq. Agropec. Bras. 35, 2355–2361. [Google Scholar]
- Seehausen, O. , Butlin, R.K. , Keller, I. , Wagner, C.E. , Boughman, J.W. , Hohenlohe, P.A. , Peichel, C.L. , Saetre, G.P. , Bank, C. , Brännström, Å. and Brelsford, A. (2014) Genomics and the origin of species. Nat. Rev. Genet. 15, 176–192. [DOI] [PubMed] [Google Scholar]
- Sharma, R. (2017) Wheat blast research: status and imperatives. Afr. J. Agric. Res. 12, 377–381. [Google Scholar]
- Silva, C.P. , Nomura, E. , Freitas, E.G. , Brugnaro, C. and Urashima, A.S. (2009) Efficiency of alternative treatments in the control of Pyricularia grisea in wheat seeds. Trop. Plant Pathol. 34, 127–131. [Google Scholar]
- Silva, E.T. (2017) Defense responses in flag leaves and spikes of two wheat cultivars differing in their basal level of resistance to blast. MSc Dissertation. Viçosa, Minas Gerais: Federal University of Viçosa, UFV, pp. 56. [Google Scholar]
- Silva, W.L.D. , Cruz, M.F.A. , Fortunato, A.A. and Rodrigues, F.Á. (2015) Histochemical aspects of wheat resistance to leaf blast mediated by silicon. Sci. Agric. 72, 322–327. [Google Scholar]
- Singh, D.P. (2017)Wheat blast caused by Magnaporthe oryzae pathotype Triticum: present status, variability, and strategies for management In: Management of Wheat and Barley Diseases. (Singh D.P., ed.), pp. 635–643. Waretown, NJ: Apple Academic Press. [Google Scholar]
- Singh, P.K. , Singh, A.K. , Singh, H.B. and Dhakad, B.K. (2012) Biological control of rice blast disease with Trichoderma harzianum in direct seeded rice under medium low land rainfed conditions. Environ. Ecol. 30, 834–837. [Google Scholar]
- Singh, R.P. , Singh, P.K. , Rutkoski, J. , Hodson, D.P. , He, X. , Jørgensen, L.N. , Hovmøller, M.S. and Huerta‐Espino, J. (2016) Disease impact on wheat yield potential and prospects of genetic control. Annu. Rev. Phytopathol. 54, 303–322. [DOI] [PubMed] [Google Scholar]
- Sousa, P.G. (2002) BR 18‐Terena: wheat cultivar for Brazil. Pesq. Agropec. Bras. 37, 1039–1043. [Google Scholar]
- Stukenbrock, E.H. and McDonald, B.A. (2008) The origins of plant pathologens in agro‐ecosystems. Annu. Rev. Phytopathol. 46, 75–100. [DOI] [PubMed] [Google Scholar]
- Tagle, A.G. , Chuma, I. and Tosa, Y. (2015) Rmg7, a new gene for resistance to Triticum isolates of Pyricularia oryzae identified in tetraploid wheat. Phytopathology, 105, 495–499. [DOI] [PubMed] [Google Scholar]
- Takabayashi, N. , Tosa, Y. , Oh, H.S. and Mayama, S. (2002) A gene‐for‐gene relationship underlying the species‐specific parasitism of Avena/Triticum isolates of Magnaporthe grisea on wheat cultivars. Phytopathology, 92, 1182–1188. [DOI] [PubMed] [Google Scholar]
- Taylor, J.W. , Jacobson, D.J. , Kroken, S. , Kasuga, T. , Geiser, D.M. , Hibett, D.S. and Fisher, M.C. (2000) Phylogenetic species recognition and species concepts in fungi. Fungal Genet. Biol. 31, 21–32. [DOI] [PubMed] [Google Scholar]
- The Brazilian Institute of Geography and Statistics ‐ IBGE (2016) Changes in Land Cover and Use 2000–2010—2010–2014, pp. 33 Rio de Janeiro: IBGE; Available at: https://geoftp.ibge.gov.br/informacoes_ambientais/cobertura_e_uso_da_terra/mudancas/documentos/mudancas_de_cobertura_e_uso_da_terra_2000_2010_2012_2014.pdf [accessed on 3 July 2018]. [Google Scholar]
- Toledo, J. (2015) Tratamiento de Semillas In: Manual de recomendaciones técnicas para el cultivo de trigo (Camacho J.A. and Villaroel‐Morales R., eds), pp. 47–55. Santa Cruz: Asociación de Productores de Oleaginosas y Trigo. [Google Scholar]
- Tormen, N.R. , Lenz, G. , Minuzzi, S.G. , Uebel, J.D. , Cezr, H.S. and Balardin, R.S.C.R. (2013) Reaction of wheat cultivars to leaf rust and yellow spot and responsiveness to fungicides. Cienc. Rural, 43, 239–246. [Google Scholar]
- Tosa, Y. and Chuma, I. (2014) Classification and parasitic specialization of blast fungi. J. Gen. Plant Pathol. 80, 202–209. [Google Scholar]
- Tosa, Y. , Hirata, K. , Tamba, H. , Nakagawa, S. , Chuma, I. , Isobe, C. , Osue, J. , Urashima, A.S. , Don, L.D. , Kusaba, M. and Nakayashiki, H. (2004) Genetic constitution and pathogenicity of Lolium isolates of Magnaporthe oryzae in comparison with host species‐specific pathotypes of the blast fungus. Phytopathology, 94, 454–462. [DOI] [PubMed] [Google Scholar]
- Urashima, A.S. , Bruno, A.C. and Lavorenti, N.A. (2001) Segregation analysis of avirulence of Magnaporthe grisea of wheat. Fitopatol. Bras. 26, 644–648. [Google Scholar]
- Urashima, A.S. , Galbieri, R. and Stabili, A. (2005) DNA fingerprinting and sexual characterization revealed two distinct populations of Magnaporthe grisea in wheat blast from Brazil. Czech J. Genet. Plant Breed. 41, 238–245. [Google Scholar]
- Urashima, A.S. , Grosso, C.R.F. , Stabili, A. , Freitas, E.G. , Silva, C.P. , Netto, D.C.S. , Franco, I. and Bottan, J.M. (2009) Effect of Magnaporthe grisea on seed germination, yield and quality of wheat In: Advances in Genetics, Genomics and Control of Rice Blast Disease. (Wang G.‐L. and Valent B., eds), pp. 267–277. Dordrecht: Springer, Netherlands. [Google Scholar]
- Urashima, A.S. , Hashimoto, Y. , Don, L.D. , Kusaba, M. , Tosa, Y. , Nakayashiki, H. and Mayama, S. (1999) Molecular analysis of the wheat blast population in Brazil with a homolog of retrotransposon MGR583. Ann. Phytopathol. Soc. Jpn. 65, 429–436. [Google Scholar]
- Urashima, A.S. , Igarashi, S. and Kato, H. (1993) Host range, mating type, and fertility of Pyricularia grisea from wheat in Brazil. Plant Dis. 77, 1211–1216. [Google Scholar]
- Urashima, A.S. and Kato, H. (1994) Varietal resistance and chemical control of wheat blast fungus. Summa Phytopathol. 20, 107–112. [Google Scholar]
- Urashima, A.S. and Kato, H. (1998) Pathogenic relationship between isolates of Pyricularia grisea of wheat and other hosts at different host developmental stages. Fitopatol. Bras. 23, 30–35. [Google Scholar]
- Urashima, A.S. , Lavorent, N.A. , Goulart, A.C.P. and Mehta, Y.R. (2004) Resistance spectra of wheat cultivars and virulence diversity of Magnaporthe grisea isolates in Brazil. Fitopatol. Bras. 29, 511–518. [Google Scholar]
- Urashima, A.S. , Leite, S.F. and Galbieri, R. (2007) Efficiency of aerial dissemination of Pyricularia grisea . Summa Phytopathol. 33, 275–279. [Google Scholar]
- USDA Natural Resources Conservation Service ‐ NRCS (2018) The PLANTS Database. Greensboro, NC: National Plant Data Team; Available at: https://plants.usda.gov [accessed on 5 July 2018]. [Google Scholar]
- Valent, B. , Bockus, W. , Cruz, C. , Stack, J. , Farman, M. , Hershman, D. , Paul, P. , Peterson, G. , Pedley, K. and Magarey, R. (2013) Recovery plan for wheat blast caused by Magnaporthe oryzae Triticum pathotype In: USDA National Plant Disease Recovery System (USA United States Department of Agriculture, eds), pp. 33 Available at: https://www.ars.usda.gov/SP2UserFiles/Place/00000000/opmp/Wheat%20Blast%20Recovery%20Plan%20Final.pdf [accessed on 1 October 2017].
- Viedma, L.Q. , Kohli, M. , Cubilla, L. and Cabrera, G. (2010) Manejo integrado de mancha amarilla y la Piricularia en el cultivo de trigo en Paraguay In: Tercer Seminario Nacional Detrigo del Grano al Pan (Kohli M., Cubilla L.E. and Cabrera G., eds), pp. 2 Asunción: PY Capeco, Inbio. [Google Scholar]
- Viedma, L.Q. and Morel, W. (2002) Añublo o Piricularia del Trigo (Wheat Blast). Capitán Miranda, Itapúa: MAG/DIA/CRIA. Programa de Investigación de Trigo, CRIA. [Google Scholar]
- West, J.S. , Canning, G.G.M. , Perryman, S.A. and King, K. (2017) Novel technologies for the detection of Fusarium head blight disease and airborne inoculum. Trop. Plant Pathol. 42, 203–209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- West, J.S. and Kimber, R.B.E. (2015) Innovations in air sampling to detect plant pathogens. Ann. Appl. Biol. 166, 4–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshida, K. , Saunders, D.G. , Mitsuoka, C. , Natsume, S. , Kosugi, S. , Saitoh, H. , Inoue, Y. , Chuma, I. , Tosa, Y. , Cano, L.M. and Kamoun, S. (2016) Host specialization of the blast fungus Magnaporthe oryzae is associated with dynamic gain and loss of genes linked to transposable elements. BMC Genomics, 18, 370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhan, J. , Thrall, P.H. , Papaïx, J. , Xie, L. and Burdon, J.J. (2015) Playing on a pathogen's weakness: using evolution to guide sustainable plant disease control strategies. Annu. Rev. Phytopathol. 53, 19–43. [DOI] [PubMed] [Google Scholar]
- Zhan, S.W. , Mayama, S. and Tosa, Y. (2008) Identification of two genes for resistance to Triticum isolates of Magnaporthe oryzae in wheat. Genome, 51, 216–221. [DOI] [PubMed] [Google Scholar]


