Summary
Genome sequencing of pathogenic fungi has revealed the presence of various effectors that aid pathogen invasion by the manipulation of plant immunity. Effectors are often individually dispensable because of duplication and functional redundancy as a result of the arms race between host plants and pathogens. To study effectors that have functional redundancy, multiple gene disruption is often required. However, the number of selection markers that can be used for gene targeting is limited. Here, we established a marker recycling system that allows the use of the same selection marker in successive transformations in the model fungal pathogen Colletotrichum orbiculare, a causal agent of anthracnose disease in plants belonging to the Cucurbitaceae. We identified two C. orbiculare homologues of yeast URA3/pyrG, designated as URA3A and URA3B, which can be used as selection markers on medium with no uridine. The gene can then be removed from the genome via homologous recombination when the fungus is grown in the presence of 5‐fluoroorotic acid (5‐FOA), a chemical that is converted into a toxin by URA3 activity. The ura3a/b double mutants showed auxotrophy for uridine and insensitivity to 5‐FOA. Using the ura3a/b mutants, transformation with the URA3B marker and its removal were successfully applied to disrupt the virulence‐related gene, PKS1. The pks1 mutants showed a reduction in virulence, demonstrating that the method can be used to study virulence‐related genes in C. orbiculare. The establishment of a URA3‐based marker recycling system in plant‐pathogenic fungi enables the genetic analysis of multiple genes that have redundant functions, including effector genes.
Keywords: Colletotrichum orbiculare, Cucurbitaceae plants, plant‐pathogenic fungus, selection marker recycling, URA3/pyrG
Introduction
Phytopathogens have evolved various strategies to overcome plant immunity, including the use of effectors that facilitate a parasitic lifestyle by regulating their host’s immune system. For example, effectors that target pattern‐triggered immunity, the first layer of plant immunity, have been reported in various pathogens. In turn, plants have developed so‐called resistance proteins to detect effectors, inducing a strong defence response against pathogens, called effector‐triggered immunity. During the process of evolution, phytopathogens and plants have developed mutual attack and defence systems that have resulted in functional redundancy and the duplication of pathogen effectors and plant immunity‐related proteins (Asai and Shirasu, 2015; Hogenhout et al., 2009; Jones and Dangl, 2006).
The Colletotrichum genus comprises over 600 species, including hemibiotrophic fungi that cause anthracnose disease in various plants, e.g. economically important crops, vegetables and fruits (Cannon et al., 2012). Therefore, the Colletotrichum genus is recognized by researchers in the plant–microbe interaction community as one of the 10 most important phytopathogenic fungi (Dean et al., 2012). Within the genus, Colletotrichum higginsianum and Colletotrichum orbiculare have been recognized as model pathosystems, as they can infect the model plants Arabidopsis thaliana and Nicotiana benthamiana, respectively (O’Connell et al., 2004; Perfect et al., 1999; Shen et al., 2001; Takano et al., 2006). The functions of several effectors have been reported in C. higginsianum and C. orbiculare. For example, the lysin motif domain (LysM) contains effectors ELP1 and ELP2 of C. higginsianum, which play dual roles in appressorial function and suppression of chitin‐triggered plant immunity (Takahara et al., 2016). In C. orbiculare, the effector NIS1 has a cell death‐inducing effect on N. benthamiana in an SGT1‐ and HSP90‐dependent manner (Yoshino et al., 2012). Furthermore, transcriptome analysis has revealed a characteristic expression pattern of effector‐encoding genes during the infection stage transition of C. higginsianum and C. orbiculare, implying that the coordinate expression of different sets of effectors is orchestrated for successful infection (Gan et al., 2013; Kleemann et al., 2012; O’Connell et al., 2012). Although the functions of several effectors have been elucidated, the vast majority of Colletotrichum effectors are still obscure. One reason for this lack of knowledge is the limited availability of selection markers for transformation. There are only four reported combinations of antibiotics (bialaphos, geneticin/G418, hygromycin and sulfonylurea) and corresponding resistance genes that can be used for the transformation of C. higginsianum and C. orbiculare, making it difficult to analyse redundant effectors (Chung et al., 2002; Dallery et al., 2017; Irieda et al., 2014).
In general, the marker recycling method is used to resolve the limitation of selection markers (Kopke et al., 2010; Zhang et al., 2017). For example, in Aspergillus fungi, the pyrG (a homologue of URA3)‐based marker recycling system has been used and developed (d’Enfert, 1996; Nielsen et al., 2006; Oakley et al., 1987). The URA3/pyrG gene encodes an orotidine‐5′‐phosphate decarboxylase involved in uridine/uracil synthesis (Weld et al., 2006). In Saccharomyces cerevisiae, mutants of URA3 show growth defects on medium lacking uridine. The uridine auxotrophy of ura3 enables URA3 expression cassettes to work as a selection marker for transformation in the ura3 mutant background. In addition, URA3/pyrG can be applied to negative selection (Boeke et al., 1984). Orotidine‐5′‐phosphate decarboxylases encoded by pyrG or URA3 orthologues convert 5‐fluoroorotic acid (5‐FOA), an analogue of the uracil precursor, to 5‐fluorouracil, a toxic compound that inhibits DNA and RNA synthesis (Flynn and Reece, 1999). Therefore, when URA3/pyrG is positioned between homologous sequences, excision of the genomic URA3/pyrG sequence by homologous recombination can be selected by 5‐FOA treatment. By utilizing this strategy, the URA3/pyrG expression cassette can be removed from the fungal genome. Indeed, the URA3/pyrG‐based marker recycling system has been utilized in S. cerevisiae (Alani et al., 1987), Aspergillus nidulans (Nielsen et al., 2006; Oakley et al., 1987), Aspergillus fumigatus (d’Enfert, 1996), Neurospora crassa (Turner et al., 1997), Candida albicans (Fonzi and Irwin, 1993) and Mucor circinelloides (Garcia et al., 2017). However, this system has never been applied to phytopathogenic fungi.
Here, we report the establishment of a URA3‐based marker recycling method in C. orbiculare 104‐T. As a proof of concept, we knocked out PKS1, a gene encoding a polyketide synthase that is required for melanin synthesis involved in virulence (Takano et al., 1995), using the URA3B (one of two pyrG homologues in C. orbiculare) expression cassette as a selection marker. The PKS1 mutants showed reduced virulence on plants, consistent with previous studies demonstrating that the URA3B selection marker can be applied to study virulence‐related genes. In the pks1 mutant background, DMAT3, a secondary metabolism key enzyme encoding gene, was disrupted using the URA3B selection marker, demonstrating that the marker recycling system can be applied to sequential transformation and gene deletion. The establishment of a URA3‐based marker recycling system enables genes that have redundant functions, such as effectors in pathogenic fungi, to be studied.
Results
The C. orbiculare genome encodes two URA3/pyrG homologues, URA3A and URA3B
To check whether URA3 homologues are present in plant‐pathogenic fungi, including C. orbiculare, BLAST (blastp; default setting) search was performed using S. cerevisiae Ura3p as a query. Figure 1a shows that each of the 10 different plant fungal pathogens, which were selected as the 10 most important fungal pathogens based on scientific/economic importance (Dean et al., 2012), has at least one URA3/pyrG homologue, except for Melampsora lini. In particular, C. orbiculare has two putative URA3/pyrG genes, named URA3A (Cob_06825) and URA3B (Cob_03887). As shown in Fig. S1 (see Supporting Information), URA3A is more similar than URA3B to S. cerevisiae Ura3p. However, reverse transcription‐quantitative polymerase chain reaction (RT‐qPCR) analysis revealed that URA3B is the major URA3 gene, because of its constitutive expression in all tested developmental stages (Fig. 1b). This notion is supported by the fact that Fusarium oxysporum URA3, which is the only predicted URA3 homologue in the genome, is more similar to URA3B than URA3A (Fig. S1).
Figure 1.
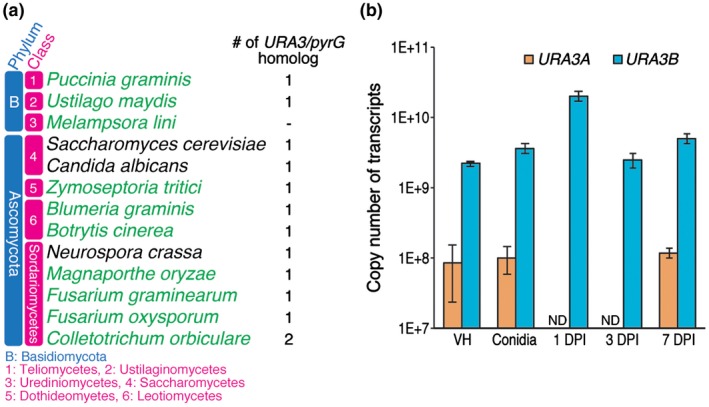
URA3/pyrG homologues are conserved in plant fungal pathogens. (a) The number of predicted URA3/pyrG homologues in selected plant fungal pathogens (green type) and model fungi (black type). Homologues were predicted by BLAST search using the Saccharomyces cerevisiae Ura3p amino acid sequence as a query. (b) URA3A and URA3B expression profiles quantified by reverse transcription‐quantitative polymerase chain reaction (RT‐qPCR) analysis. Bars represent the absolute number of transcripts. RNAs were extracted from vegetative hyphae (VH), conidia, epidermal cells at 1 day post‐inoculation (DPI), epidermal cells at 3 DPI and whole leaf tissue at 7 DPI. Total RNA levels were normalized using the RIBOSOMAL PROTEIN I5 gene (Cob_11000), as reported previously (Gan et al., 2013). Error bars represent standard errors. n = 3. ND indicates not detected. Primers used to detect URA3A, URA3B and RPI5 are listed in Table S3 (see Supporting Information). [Colour figure can be viewed at wileyonlinelibrary.com]
URA3A and URA3B double knock‐out mutants exhibit uridine auxotrophy and 5‐FOA insensitivity
Because URA3B is constitutively expressed, we first knocked out URA3B by homologous recombination using the pNK028 plasmid harbouring neomycin phosphotransferase II (NPTII), a geneticin/G418 resistance gene (Fig. 2a). At least four ura3b knock‐out strains were obtained and the gene disruption was confirmed by genomic PCR using the primer sets Po1/Po2, Po3/Po4 and Po5/Po6 (Fig. 2a,b). As predicted, all ura3b mutants showed no grow on potato dextrose agar (PDA), but were able to grow on PDA supplemented with 10 mm uridine, suggesting that URA3B is indispensable for uridine synthesis in vivo (Fig. 2c). The growth of wild‐type C. orbiculare was inhibited by the addition of 1 mg/mL 5‐FOA to PDA, implying that URA3B also synthesizes a toxic compound from 5‐FOA. However, the 5‐FOA sensitivity varied among the ura3b mutants (Fig. 2c), suggesting that residual URA3 enzyme activity was contributed by the other URA3 homologue URA3A. Therefore, we decided to knock out URA3A in ura3b and established four double knock‐out lines by homologous recombination using pNK032 (Fig. 2d). As shown in Fig. 2e, genomic PCR confirmed that the URA3A gene was disrupted in ura3a/b#1‐4. Importantly, all four strains showed uridine auxotrophy and 5‐FOA insensitivity, demonstrating that the URA3‐based enzyme activity in C. orbiculare were completely lost in the ura3a/b#1‐4 mutants (Fig. 2f).
Figure 2.
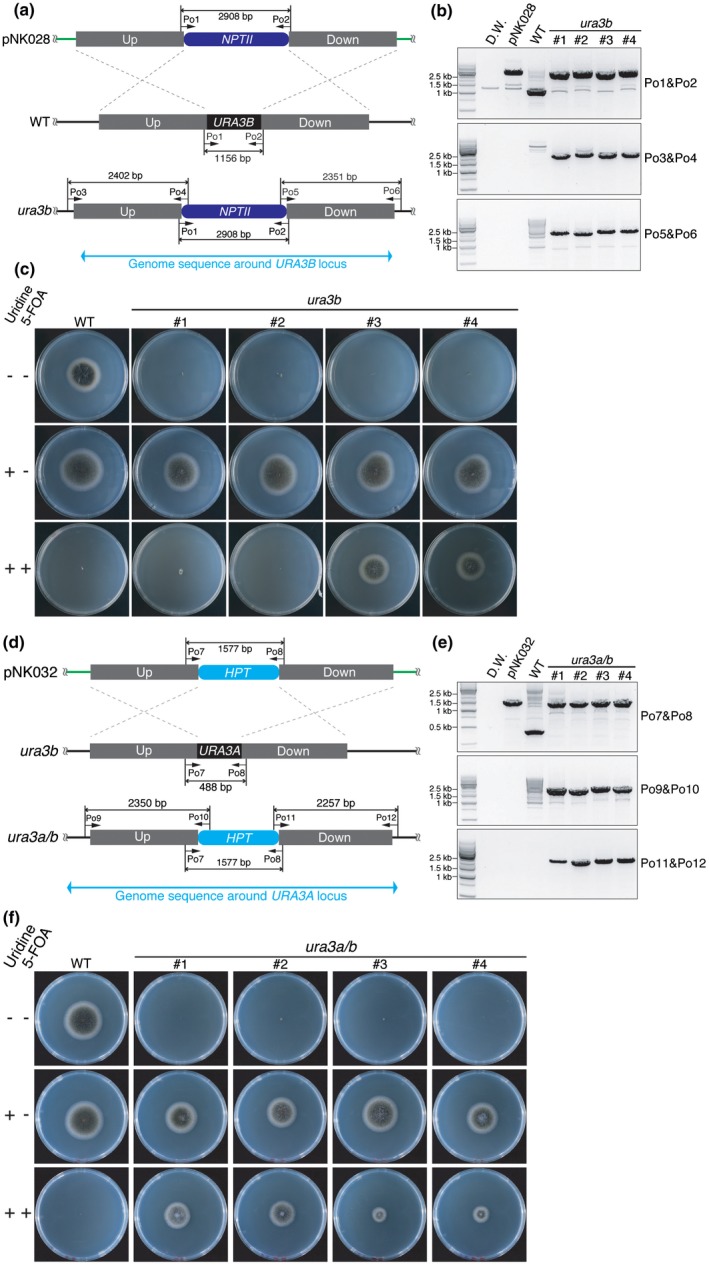
URA3A and URA3B double knockout mutants show uridine auxotrophy and 5‐fluoroorotic acid (5‐FOA) insensitivity. (a) Schematic diagrams of URA3B (Cob_03887) knockout in Colletotrichum orbiculare 104‐T wild‐type (WT) strain. The pNK028 plasmid contains 2 kb of upstream (Up) and downstream (Down) sequences of the URA3B coding sequence (CDS). The neomycin phosphotransferase II (NPTII) expression cassette is located between the Up and Down sequences as a selection marker against G418. Black arrows represent primers used for genomic DNA polymerase chain reaction (PCR). (b) Genomic DNA PCR showed that URA3B was knocked out. The primer set Po1/Po2 generates 2908‐ and 1156‐bp amplicons from the genome of WT and ura3b, respectively. The primer sets Po3/Po4 and Po5/Po6 generate 2402‐ and 2351‐bp bands from the genome of ura3b, but not from that of WT. (c) WT and four independent ura3b strains were cultured on potato dextrose agar (PDA), PDA with 10 mm uridine and PDA with 10 mm uridine and 1 mg/mL 5‐FOA for 6 days at 25 °C in the dark. (d) Schematic diagrams of URA3A (Cob_06825) knockout in ura3b. The pNK032 plasmid includes the 2‐kb upstream (Up) and downstream (Down) sequences of the URA3A locus. The Down sequence partially includes the 3′‐end of the URA3A CDS. The hygromycin phosphotransferase (HPT) expression cassette is located between the Up and Down sequences as a selection marker against hygromycin. (e) Genomic DNA PCR showed that URA3A was knocked out, resulting in ura3a/b double mutants. ura3a/b#1‐2 and ura3a/b#3‐4 are descendants of ura3b#3 and ura3b#2, respectively. The primer set Po7/Po8 generates 488‐ and 1577‐bp amplicons from the genome of WT (or ura3b) and ura3a/b, respectively. The primer sets Po9/Po10 and Po11/Po12 generate 2350‐ and 2257‐bp bands from the genome of ura3a/b, but not from that of WT (or ura3b). The primers used are listed in Table S3 (see Supporting Information). (f) The same experiment as described in (c) was performed. Details of each strain are listed in Table S1 (see Supporting Information). [Colour figure can be viewed at wileyonlinelibrary.com]
The URA3B expression cassette functions as a selection marker in ura3a/b mutants
To test whether the uridine auxotrophy selection marker is usable in C. orbiculare, PKS1, which encodes a polyketide synthase involved in melanin synthesis (Takano et al., 1995, 1997), was targeted for disruption by the pNK059 plasmid. The plasmid has the URA3B expression cassette as a selection marker, in which URA3B is driven by the Tef (TRANSLATION ELONGATION FACTOR) promoter of Aureobasidium pullulans (Wymelenberg et al., 1997) (Fig. 3a). Successful knock out of PKS1 was confirmed by genomic PCR (Fig. 3b). Consistently, pks1/ura3a/b‐Tef::URA3B#1 and pks1/ura3a/b‐Tef::URA3B#2 showed the albino phenotype, characteristic of pks1 mutants and caused by a lack of melanin (Takano et al., 1995) (Fig. 3c).
Figure 3.
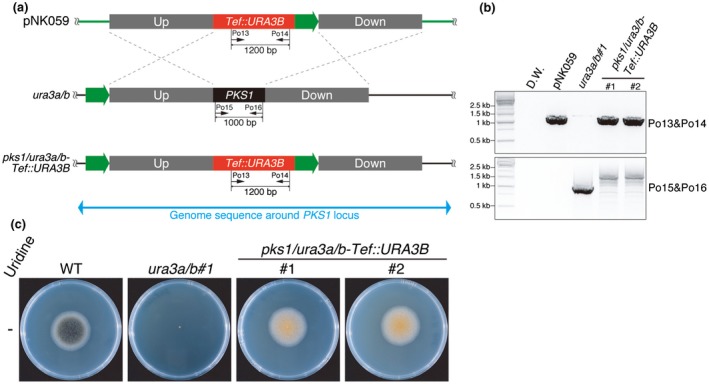
The URA3B expression cassette driven by the Tef (TRANSLATION ELONGATION FACTOR) promoter works as a uridine auxotrophy selection marker in ura3a/b. (a) Schematic diagrams of PKS1 (Cob_09513) knock out in ura3a/b. The pNK059 plasmid has 2 kb of upstream (Up) and downstream (Down) sequences of the PKS1 locus. The Down sequence includes the partial PKS1 coding sequence (CDS). The URA3B expression cassette driven by the Tef constitutive promoter (Tef::URA3B) is located between the Up and Down sequences as a uridine auxotrophy marker. Green arrows represent a 500‐bp sequence located upstream of the Up sequence on the Colletotrichum orbiculare genome. The 500‐bp sequence is designed to allow homologous recombination induced by 5‐fluoroorotic acid (5‐FOA) treatment for excision of Tef::URA3B. (b) Genomic DNA polymerase chain reaction (PCR) showed that PKS1 was knocked out. The primer set Po13/Po14 generates the 1200‐bp amplicon if the Tef::URA3B cassette is present. The primer set Po15/Po16, generates the 1000‐bp band if the PKS1 CDS is present. The primers used are listed in Table S3 (see Supporting Information). (c) Wild‐type (WT), ura3a/b#1 and two independent pks1/ura3a/b‐Tef::URA3B strains were cultured on potato dextrose agar (PDA) for 6 days at 25 °C in the dark. The details of each strain are listed in Table S1 (see Supporting Information). [Colour figure can be viewed at wileyonlinelibrary.com]
The excision of the URA3B expression cassette can be selected by 5‐FOA treatment, demonstrating establishment of the marker recycling system in C. orbiculare
In the pks1/ura3a/b‐Tef::URA3B strains, the Tef::URA3B cassette is designed to be located between 500 bp of completely homologous sequences (Fig. 4a, green boxes). If pks1/ura3a/b‐Tef::URA3B is incubated on PDA plates containing 5‐FOA, the strains that lose the Tef::URA3B cassette by homologous recombination should be selected. As predicted, pks1/ura3a/b strains without the Tef::URA3B cassette could be isolated from pks1/ura3a/b‐Tef::URA3B strains after growth on PDA containing 1 mg/mL 5‐FOA and 10 mm uridine. Then, using eight randomly selected colonies per strain, the removal of the Tef::URA3B cassette was checked by genomic DNA PCR. No bands were observed from genomic DNA of all pks1/ura3a/b strains (#1–4 are shown as representatives in Fig. 4b) using the primer set Po13/Po14, which generates 1200‐bp amplicons in the presence of the Tef::URA3B cassette. The pks1/ura3a/b strains were unable to grow on PDA, but grew on PDA supplemented with 10 mm uridine (Fig. 4c). These strains also show low sensitivity to 5‐FOA treatment, suggesting the absence of the Tef::URA3B cassette. Together, we conclude that the removal of the Tef::URA3B marker from pks1/ura3a/b‐Tef::URA3B can be selected by 5‐FOA treatment.
Figure 4.
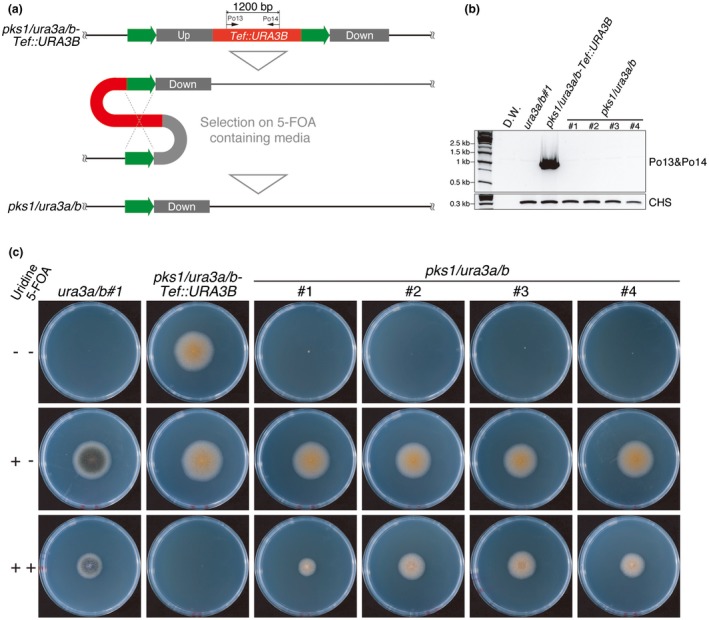
The Tef::URA3B cassette was removed by homologous recombination and selected by 5‐fluoroorotic acid (5‐FOA) treatment. (a) Schematic diagrams of the removal of the Tef::URA3B cassette (Cob_09513). Green arrows show the homologous 500‐bp sequences for recombination. (b) Genomic DNA polymerase chain reaction (PCR) showed that Tef::URA3B was removed. The primer set Po13/Po14 generates the 1200‐bp amplicon if the Tef::URA3B cassette is present. The 266‐bp bands corresponding to CHITIN SYNTHASE (CHS) were amplified using the primer set CHS_79F and CHS_345R (Carbone and Kohn, 1999) to show the presence of genomic DNA. The primers used are listed in Table S3 (see Supporting Information). (c) ura3a/b#1, pks1/ura3a/b‐Tef::URA3B#1 and pks1/ura3a/b#1‐4 strains were cultured on potato dextrose agar (PDA), PDA with 10 mm uridine and PDA with 10 mm uridine plus 1 mg/mL 5‐FOA for 6 days at 25 °C in the dark. All pks1/ura3a/b#1‐4 strains showed uridine auxotrophy and 5‐FOA insensitivity similar to ura3a/b#1, demonstrating successful removal of the Tef::URA3B cassette. The details of each strain are listed in Table S1 (see Supporting Information). [Colour figure can be viewed at wileyonlinelibrary.com]
URA3B marker knock in enables in planta virulence assay in C. orbiculare
We tested whether the marker recycling system could be applied to study virulence in planta. As shown in Fig. 5d, ura3a/b mutants did not trigger disease symptoms on cucumber cotyledons, most probably because uridine was not acquired from the host plant. To check whether externally added uridine complements the phenotype of ura3a/b, wild‐type, ura3a/b and ura3a/b supplemented with 10 mm uridine were inoculated onto cucumber cotyledons. Externally added uridine partially complemented the disease phenotype of ura3a/b (Fig. S2c,d, see Supporting Information), showing that uridine is required for the virulence of C. orbiculare. Then, to determine at which stage the pathogenicity of ura3a/b mutants was arrested, the rates of conidial penetration, an early event of infection, were assessed. Although appressoria were formed as in the wild‐type, the ura3a/b mutants could not penetrate into the cucumber cells at all (Fig. S2a,b). This deficiency in the penetration rate of ura3a/b was partially complemented by externally added uridine (Fig. S2d). These results suggest that infection of ura3a/b is arrested at the penetration stage and uridine is required for successful penetration.
Figure 5.
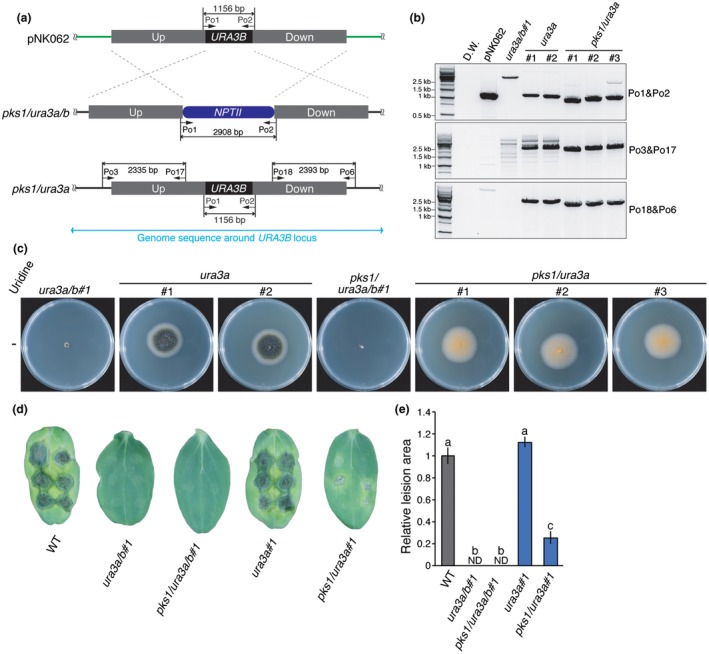
URA3B knock‐in to its original locus enabled in planta virulence assay using the ura3a/b mutant‐based marker recycling system. (a) Schematic diagrams of URA3B knock‐in experiments on the ura3a/b mutant strains. The pNK062 plasmid contains 2 kb of upstream (Up) and downstream (Down) sequences of the URA3B coding sequence (CDS) on the Colletotrichum orbiculare 104‐T genome. The complete URA3B CDS, including intron, is located between the Up and Down sequences. (b) Genomic DNA polymerase chain reaction (PCR) showed that URA3B was knocked in, resulting in ura3a and pks1/ura3a mutants. The primer set Po1/Po2 is described in Fig. 2b. If the URA3B CDS is knocked in to ura3a/b and pks1/ura3a/b mutants, primer sets Po3/Po17 and Po18/Po6 generate 2335‐ and 2393‐bp bands, respectively. The primers used are listed in Table S3 (see Supporting Information). (c) All strains were cultured on potato dextrose agar (PDA) for 6 days at 25 °C in the dark. ura3a#1, ura3a#2, pks1/ura3a#1, pks1/ura3a#2 and pks1/ura3a#3 strains can grow on PDA without uridine addition, demonstrating the knock‐in of URA3B. (d) Conidia of each strain were inoculated onto plants. At 9 days post‐germination, cotyledons of cucumber were inoculated with six drops of 10 µL conidia solution at 5 × 105 conidia/mL. Photographs were taken after 6 days of incubation. The disease symptoms of ura3a#1 were similar to those of the WT strain, showing that URA3B is able to complement the reduced virulence phenotype of the ura3a/b mutant. The reduced virulence phenotype of pks1/ura3a#1 reflects the contribution of PKS1 to the virulence of C. orbiculare. The details of each strain are listed in Table S1 (see Supporting Information). (e) The area of each lesion shown in (d) was measured using Image J software. Values were normalized to set WT as unity. n = 12. Error bars represent standard errors. ND indicates not detected. Different letters on the bars represent significant differences (Tukey’s test, P < 0.01). [Colour figure can be viewed at wileyonlinelibrary.com]
Next, we knocked in the URA3B gene in ura3a/b to its original locus using pNK062 (Fig. 5a). The successful knock in of the URA3B gene was confirmed by genomic DNA PCR (Fig. 5b) and phenotypic analysis (Fig. 5c). No significant difference in disease symptoms between wild‐type and URA3B knocked‐in strains was observed, demonstrating that URA3B alone was sufficient to complement the reduced virulence phenotype of ura3a/b (Fig. 5d,e). Then, we knocked in URA3B in pks1/ura3a/b mutants to produce pks1/ura3a mutants, and infected cucumber (Cucumis sativus) cotyledons. As shown in Fig. 5d,e, pks1/ura3a showed significantly less virulence than ura3a, indicating the involvement of PKS1 in virulence, as described previously (Takano et al., 1995). These data demonstrate that the marker recycling system can be applied to the in planta assays to test genes involved in virulence.
DMAT3 knock‐out in the pks1/ura3a/b mutant using the marker recycling system
To check whether the marker recycling system can be repeatedly used for transformation and gene targeting, we performed one round of additional gene disruption in the pks1/ura3a/b mutant. As a target, DMAT3 (Cob_04983), which encodes a predicted secondary metabolite (SM) key gene, dimethylallyl transferase, was selected for gene knock‐out by the pNK098 plasmid (Fig. 6a). First, the Tef::URA3B expression cassette was inserted into the upstream region of the DMAT3 coding sequence (CDS) in pks1/ura3a/b. Successful insertion of the Tef::URA3B cassette was confirmed by genomic DNA PCR (Fig. 6b), resulting in three independent strains, named pNK098HR pks1/uras3/b. In these strains, the Tef::URA3B cassette and the DMAT3 CDS were located between 500 bp of completely homologous sequences (Fig. 6a, green boxes) for the marker and CDS removal. Second, removal of the Tef::URA3B cassette and DMAT3 CDS was selected for by incubating pNK098HR pks1/uras3/b on PDA plates containing 5‐FOA and uridine. The successful removal of the Tef::URA3B cassette and DMAT3 CDS was confirmed by genomic DNA PCR (Fig. 6c), resulting in three independent dmat3/pks1/ura3a/b mutant strains. These results prove the concept of sequential transformation and gene targeting using the marker recycling system reported here.
Figure 6.
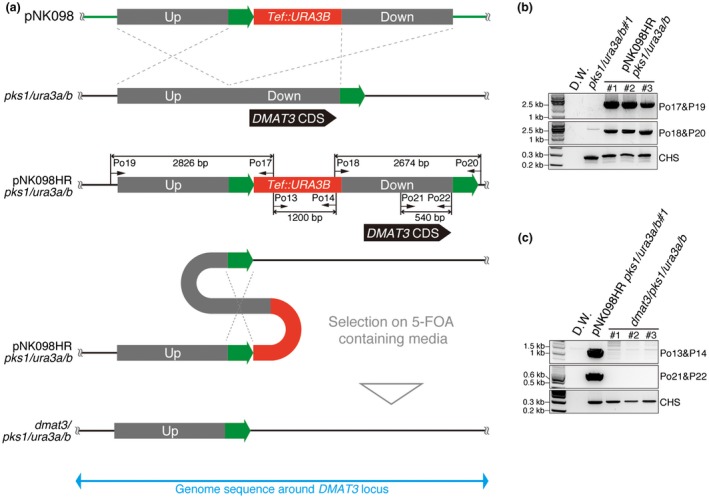
DMAT3 knock‐out in the pks1/ura3a/b mutant. (a) Schematic diagrams of the DMAT3 coding sequence (CDS) knock‐out experiments in the pks1/ura3a/b background. The pNK098 plasmid includes about 2 kb of upstream (Up) and downstream (Down) sequences around the DMAT3 locus. The Down sequence contains the complete DMAT3 CDS shown by a black box. Green arrows show homologous 500‐bp sequences for recombination originally from the downstream region of DMAT3 CDS. (b) Genomic DNA polymerase chain reaction (PCR) showed successful homologous recombination by pNK098, resulting in pNK098HR pks1/ura3a/b strains. The primer sets Po17/Po19 and Po18/20 generate 2826‐ and 2674‐bp bands, respectively, from pNK098HR pks1/ura3a/b, but not from the genome of pks1/ura3a/b. The 266‐bp bands corresponding to CHITIN SYNTHASE (CHS) were amplified to show the presence of genomic DNA. (c) Genomic DNA PCR showed the successful removal of the Tef::URA3B expression cassette and the DMAT3 CDS. The primer sets Po13/Po14 and Po21/Po22 generate 1200‐ and 540‐bp bands, respectively, from the genome of pNK098HR pks1/ura3a/b, but not from that of dmat3/pks1/ura3a/b. The primers used are listed in Table S3 (see Supporting Information). [Colour figure can be viewed at wileyonlinelibrary.com]
Discussion
The limited availability of selection markers represents a bottleneck for effector studies in many plant‐pathogenic fungi. Here, we report the establishment of a marker recycling system using URA3/pyrG homologues in the phytopathogenic filamentous fungus, C. orbiculare. The URA3B cassette was successfully used as a selection marker in C. orbiculare ura3a/b double mutants to create PKS1 knock‐out lines. Selection for loss of the URA3B cassette via homologous recombination can then easily be performed by growth on media supplemented with 5‐FOA. In addition, we successfully knocked out DMAT3 in the pks1 mutant background, proving the concept of sequential transformation by the marker recycling system. Importantly, the reintroduction of URA3B at its original locus is able to restore the growth of ura3a/b mutants to wild‐type levels in planta. Thus, using this system, we should be able to assess the function of any gene of interest in planta. The avoidance of positional effects is critical, as shown in Candida albicans, an opportunistic fungal pathogen of animals, where the virulence phenotype of the fungus in mice varied as a function of the relocation of URA3 in the genome, as the expression of URA3 during infection is affected by its genomic locus (Staab and Sundstrom, 2003; Sundstrom et al., 2002). The reintroduction of URA3B is an additional step in the protocol, but the use of the same vector and the high efficiency of homologous recombination make this process straightforward. As the URA3B cassette can be recycled, knock‐out analysis of multiple genes with functional redundancy is now possible in C. orbiculare. Similarly, the URA3‐based marker recycling system can be applied to other pathogenic fungi that are transformable with a relatively high homologous recombination rate. The genome sequences of other transformable plant pathogens, Ustilago maydis, Zymoseptoria tritici, Botrytis cinerea, Magnaporthe oryzae, Fusarium graminearum, F. oxysporum and C. higginsianum, revealed that these organisms contain only one copy of URA3 (Fig. 1a). Thus, it is possible that setting up the ura3 knock‐out system in these pathogens may be easier than that in C. orbiculare.
The URA3‐based marker recycling system offers several advantages over other systems, such as Cre‐loxP, Flp‐FRT and β‐recombinase‐six, which have been used in prokaryotes and eukaryotes (Abuin and Bradley, 1996; Johansson and Hahn‐Hägerdal, 2004; Lambert et al., 2007; Szewczyk et al., 2014; Yuliya et al., 2010; Zhang et al., 2017). Cre, Flp and β‐recombinase catalyse the recombination between sites, named loxP, FRT and six, respectively. As these enzymes lead to the excision of DNA between the two recombination sites, one of the sites is left behind in the genome (Kilby et al., 1993; Wirth et al., 2007). Thus, unlike our homologous recombination‐based URA3 system, foreign sequences accumulate in the genome if sequential transformation is performed, which potentially induces genome instability. Therefore, the URA3/pyrG‐based marker recycling system should allow the creation of much more stable multiple knock‐out lines. Further, the URA3B cassette has been demonstrated to function as both a positive and negative selection marker, avoiding the need for opposite markers as used in the β‐recombinase‐based system, which utilizes the bialaphos resistance gene as a positive selection marker for transformation and the thymidine kinase gene as a negative selection marker for excision (Szewczyk et al., 2014). Thus, the URA3/pyrG‐based marker recycling system should allow the construction of smaller vectors.
In Aspergillus nidulans, a model organism for fungal research, URA3/pyrG‐based transformation and related technologies have been developed (Dohn et al., 2018; Oakley et al., 1987). For example, Szewczyk et al. (2006) were able to improve the speed of transformation experiments by utilizing PCR fragments (not plasmids) for gene targeting. Further, Nayak et al. (2006) identified and knocked out the A. nidulans homologue (nkuA) of the human KU70 gene, which is important for non‐homologous end joining of DNA in double‐strand breaks. A lack of nkuA reduces the frequency of non‐homologous integration of DNA fragments for transformation, leading to higher gene targeting efficiency. The homologue of nkuA has also been knocked out in C. higginsianum (Ushimaru et al., 2010), the causal agent of anthracnose on Brassicaceae plants, leading to improved gene targeting efficiency. Thus, it is likely that the method may be applicable to other members of the Colletotrichum fungi. The application of these advanced technologies developed in A. nidulans and other fungi to C. orbiculare is theoretically possible and could make sequential gene targeting and transformation more rapid and easier in combination with the marker recycling system.
In recent years, the genomes of many phytopathogenic fungi, such as Colletotrichum species, M. oryzae, U. maydis and Z. tritici, have been sequenced and the presence of multiple effector proteins has been predicted (Dean et al., 2005; Gan et al., 2013; Ma et al., 2010; O’Connell et al., 2012; Spanu et al., 2010). In Colletotrichum, transcriptome analysis revealed that the expression of several SM synthesis‐related genes is strongly induced during infection, especially at the biotrophic phase, in addition to effector proteins (Dallery et al., 2017; Gan et al., 2013). These findings suggest that SMs synthesized by fungi may have a virulence function. One SM synthesis‐related gene, btcAco, whose expression is induced during infection, was knocked out, and its function was assessed (Gao et al., 2018). Although btcAco is involved in SM synthesis, virulence effects in btcAco disrupted mutants were not detected (Fig. S3, see Supporting Information). One possible reason for this lack of virulence phenotype is that SMs may also have functional redundancy in virulence. We anticipate that the URA3/pyrG marker recycling system will contribute to elucidate the functions of effectors, including proteins, and SMs.
Experimental Procedures
Fungal transformation
Fungal transformation was performed using the polyethylene glycol‐mediated protoplast transformation protocol described previously (Kubo et al., 1991). Colletotrichum orbiculare 104‐T (MAFF240422) was used as the wild‐type strain (Ishida and Akai, 1969). Derivative strains from C. orbiculare 104‐T and plasmids used for transformation are listed in Tables S1 and S2 (see Supporting Information), respectively.
Selection of removal of the URA3B expression cassette after 5‐FOA treatment
The strains pks1/ura3a/b‐Tef::URA3B#1 (CoNK0031) and pks1/ura3a/b‐Tef::URA3B#3 (CoNK0033) were cultured on PDA (Nissui Pharmaceutical Co., Ltd., Taito‐ku, Tokyo, Japan) for 6 days and their conidia were collected. About 1 × 105 conidia of CoNK0031 and CoNK0033 were spread onto PDA with 1 mg/mL 5‐FOA monohydrate (Wako Pure Chemical Industries, Ltd., Chuo‐ku, Tokyo, Japan) and 10 mm uridine (Tokyo Chemical Industry Co., Ltd., Chuo‐ku, Tokyo, Japan) in sterilized no. 2 square plates (Eiken Chemical Co., Ltd., Taito‐ku, Tokyo, Japan). Then, the plates were incubated at 25 °C in the dark for 4 days. The surviving colonies were transferred to new PDA with 1 mg/mL 5‐FOA and 10 mm uridine, and incubated under the same conditions for 4 days. The surviving colonies were selected and removal of the Tef::URA3B cassette was examined by fungal colony PCR. Selected transformants were designated as pks1/ura3a/b#1 (CoNK0041), pks1/ura3a/b#2 (CoNK0042), pks1/ura3a/b#3 (CoNK0043) and pks1/ura3a/b#4 (CoNK0044).
Plasmid construction
All primers used are listed in Table S3 (see Supporting Information). The genomic DNA of C. orbiculare 104‐T used for PCR was isolated as described previously (Gan et al., 2013).
pNK028: PCR‐1 and PCR‐2 were amplified from C. orbiculare 104‐T genomic DNA using the primer sets IF‐pII99EcoRV+URA3BUP_F plus IF‐pII99EcoRV+URA3BUP_R and pNK028‐DW_F plus pNK028‐DW_R. The pII99 plasmid harbouring NPTII, a geneticin/G418 resistance gene (Namiki et al., 2001), was digested with EcoRV (TaKaRa Bio, Inc., Kusatsu, Shiga, Japan) and the larger fragment was fused to PCR‐1 using the In‐Fusion HD Cloning Kit (TaKaRa Bio, Inc.) according to the manufacturer’s instructions, resulting in pNK028P1. pNK028P1 was digested with BamHI (TaKaRa Bio, Inc.) and the larger fragment was assembled with PCR‐2 using the In‐Fusion HD Cloning Kit, resulting in the pNK028 plasmid for the URA3A knock‐out harbouring NPTII.
pNK032: PCR‐3 and PCR‐4 were amplified from C. orbiculare 104‐T genomic DNA using the primer sets URA3AUP_fwd plus URA3AUP_rev and HF‐pNK032‐DW_F plus IF‐pENTR4URA3AKO‐R. PCR‐5 was amplified from the pCB1004 plasmid (Sweigard et al., 1997) using the primer set HygR_fwd plus HF‐pNK032‐HygR_R. PCR‐6 was amplified from pENTR4 Dual Selection (Thermo Fisher Scientific. Inc., Waltham, Massachusetts, USA) using the primer set pENTR4_Dual_selection_F plus pENTR4_Dual_selection_R. PCR‐3 to PCR‐6 were assembled using NEBuilder HiFi DNA Assembly Mix (New England Biolabs, inc., Ipswich, Massachusetts, USA) according to the manufacturer’s protocol, resulting in pNK032 for URA3B knock‐out harbouring hygromycin phosphotransferase (HPT), which confers resistance to hygromycin B.
pNK059: PCR‐7, PCR‐8 and PCR‐9 were amplified from C. orbiculare 104‐T genomic DNA using the primer sets IF1‐Cob_09513_UP_F plus HFBK‐pNK059_R, HFBK‐pNK059_F plus Cob_09513_UP(‐500)_R and Cob_09513_Dw_F plus IF3‐Cob_09513KO_DW_R, respectively. PCR‐10, having the Tef promoter sequence, and PCR‐11, having the SCD1 terminator, were amplified from Tef‐GFP plasmid using the primer sets HF‐pNK059Frag1_F plus HF‐pNK059Frag1_R and HF‐pNK059Frag3_F plus HF‐pNK059Frag3_R. The sequences of PCR‐10 and PCR‐11 are given in Data S1 (see Supporting Information). PCR‐12 was amplified from C. orbiculare 104‐T cDNA using the primer set HF‐pNK059Frag2_F plus HF‐pNK059Frag2_R. PCR‐10 to PCR‐12 were assembled by NEBuilder HiFi DNA Assembly Master Mix, resulting in PCR‐13, which is the Tef promoter‐driven URA3B expression cassette. PCR‐14 was amplified from the pAGM4723 plasmid (Engler et al., 2014) using pCIH47732_IF_F plus pICH47732_IF_R2. PCR‐7, PCR‐8, PCR‐13, PCR‐9 and PCR‐14 were assembled by NEBuilder HiFi DNA Master Mix, resulting in pNK059.
pNK062: PCR‐15 was amplified from pNK028 using the primer set HF‐pNK062‐BB_F and HF‐pNK062‐BB_R. PCR‐16 was amplified from C. orbiculare 104‐T genomic DNA using the primer set HF‐pNK062‐IN_F and HF‐pNK062‐BB_R. PCR‐15 and PCR‐16 were assembled using NEBuilder HiFi DNA Assembly Master Mix, resulting in pNK062.
pNK098: PCR‐17, PCR‐18 and PCR‐19 were amplified from C. orbiculare 104‐T genomic DNA using the primer sets HF‐pNK098UP_F plus HF‐pNK098UP_R, HF‐pNK098HS_F plus HF‐pNK098HS_R and HF‐pNK098DOWN_F plus HF‐pNK098DOWN_R, respectively. PCR‐20 harbouring the URA3B expression cassette was amplified from pNK059 using the primer set HF‐URA3BCas_F plus HF‐URA3BCas_R. Then, PCR‐6, PCR‐17, PCR‐18, PCR‐19 and PCR‐20 were assembled using NEBuilder HiFi DNA Assembly Master Mix, resulting in pNK098.
RT‐qPCR
Total RNA isolation and DNA removal were carried out using an RNeasy Plant Mini Kit and RNase‐Free DNase Set (Qiagen, Venlo, Limburg, Netherlands) following the manufacturer’s protocol. cDNAs were synthesized from isolated RNAs with the ReverTraAce qPCR RT Kit (Toyobo Co., Ltd., Kita‐ku, Osaka, Japan) using the included primer mix and following the manufacturer’s instructions. All RT‐qPCRs were performed with THUNDERBIRD SYBR qPCR Mix (Toyobo Co., Ltd., Kita‐ku, Osaka, Japan) and an MX3000P Real‐Time qPCR System (Stratagene, Santa Clara, California, USA). Primer sets qURA3A_F3 plus qURA3A_R3, qURA3B_F2 plus qURA3B_R2 and 8436qF_ref plus 8436qR_ref were used to detect transcripts of URA3A, URA3B and ribosomal protein I5, respectively. The primer sequences used in the experiments are listed in Table S3. Plasmids with the coding sequences of URA3A and URA3B were used as standards for the absolute quantification of URA3A and URA3B transcripts.
Fungal infection assay of cucumber
Fungal strains were cultured on PDA at 24 °C for 6 days in the dark. Then, their conidia were collected by centrifugation at 3000 g for 5 min and washed twice with sterilized water. Droplets (10 µL) of conidia at 5 × 105 conidia/mL in water were inoculated onto cucumber cotyledons at 10 days post‐germination. Seeds of C. sativus, cucumber Suyo strain (Sakata Seed Corp., Yokohama, Kanagawa, Japan), were planted in a mix of equal amounts of Supermix A (Sakata Seed Corp.) and vermiculite. Then, cucumbers were grown at 24 °C under a 10‐h light/14‐h dark cycle.
Alignment of URA3 proteins
The amino acid sequence of Saccharomyces cerevisiae Ura3p and C. orbiculare URA3A and URA3B were aligned using MAFFT software (Katoh et al., 2002). Aligned sequences were formatted using CLC Genomics Workbench8.0 (CLC bio, Aarhus, Midtjylland, Denmark).
Accession numbers
Ura3p (SGD:S000000747) from the Saccharomyces Genome Database (https://www.yeastgenome.org/). URA3A (ENH84876.1), URA3B (ENH87716.1), PKS1 (ENH81867.1), DMAT3 (ENH86929.1) and F. oxysporum f. sp. cubense URA3 (EMT68416.1) from the GenBank databases.
Supporting information
Fig. S1 Amino acid sequence alignment of Saccharomyces cerevisiae Ura3p with Colletotrichum orbiculare URA3A and URA3B, and Fusarium oxysporum URA3.
Fig. S2 The lesser disease symptom phenotype of ura3a/b is partially complemented by externally added uridine.
Fig. S3 Colletotrichum orbiculare btcAco knock‐out mutants do not show reduced virulence on cucumber leaves. The experiment was performed in the same conditions as in Fig. 5d.
Table S1 Fungal strain list.
Table S2 Plasmid list.
Table S3 Oligo list.
Data S1 DNA sequences of PCR‐10 and PCR‐11.
Acknowledgements
Colletotrichum orbiculare 104‐T strain and the Tef‐GFP plasmid were kindly provided by Dr Yoshitaka Takano (Kyoto University, Kyoto, Japan). We thank Dr Pamela Gan and Ms Ayako Tsushima for critical reading of the manuscript. This work was supported by RIKEN Special Postdoctoral Researcher Program (N.K.), JSPS KAKENHI 18K14440 (N.K.) and 17H06172 (K.S.). The authors state that they have no conflicts of interest to declare associated with this article.
References
- Abuin, A. and Bradley, A. (1996) Recycling selectable markers in mouse embryonic stem cells. Mol. Cell. Biol. 16, 1851–1856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alani, E. , Cao, L. and Kleckner, N. (1987) A method for gene disruption that allows repeated use of URA3 selection in the construction of multiply disrupted yeast strains. Genetics, 116, 541–545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asai, S. and Shirasu, K. (2015) Plant cells under siege: plant immune system versus pathogen effectors. Curr. Opin. Plant Biol. 28, 1–8. [DOI] [PubMed] [Google Scholar]
- Boeke, J.D. , Croute, F.L. and Fink, G.R. (1984) A positive selection for mutants lacking orotidine‐5′‐phosphate decarboxylase activity in yeast: 5‐fluoro‐orotic acid resistance. Mol. Gen. Genet. 197, 345–346. [DOI] [PubMed] [Google Scholar]
- Cannon, P.F. , Damm, U. , Johnston, P.R. and Weir, B.S. (2012) Colletotrichum – current status and future directions. Stud. Mycol. 73, 181–213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carbone, I. and Kohn, L.M. (1999) A method for designing primer sets for speciation studies in filamentous ascomycetes. Mycologia, 91, 553–556. [Google Scholar]
- Chung, K.R. , Shilts, T. , Li, W. and Timmer, L.W. (2002) Engineering a genetic transformation system for Colletotrichum acutatum, the causal fungus of lime anthracnose and postbloom fruit drop of citrus. FEMS Microbiol. Lett. 213, 33–39. [DOI] [PubMed] [Google Scholar]
- Dallery, J.‐F. , Lapalu, N. , Zampounis, A. , Pigné, S., Luyten, I., Amselem, J., Wittenberg, A.H.J., Zhou, S., de Queiroz, M.V., Robin, G.P., Auger, A., Hainaut, M., Henrissat, B., Kim, K.‐T., Lee, Y.‐H., Lespinet, O., Schwartz, D.C., Thon, M. R. and O’Connell, R.J. (2017) Gapless genome assembly of Colletotrichum higginsianum reveals chromosome structure and association of transposable elements with secondary metabolite gene clusters. BMC Genomics. 18, 667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dean, R. , Van Kan, V. , Pretorius, Z.A. , Hammond‐Kosack, K.E. , Di pietro, A. , Spanu, P.D. , Rudd, J.J. , Dickman, M. , Kahmann, R. , Ellis, J. and Foster, G.D. (2012) The Top 10 fungal pathogens in molecular plant pathology. Mol. Plant Pathol. 13, 414–430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dean, R.A. , Talbot, N.J. , Ebbole, D.J. , Farman, M.L., Mitchell, T.K., Orbach, M.J., Thon, M., Kulkarni, R., Xu, J.R., Pan, H., Read, N.D., Lee, Y.H., Carbone, I., Brown, D., Oh, Y.Y., Donofrio, N., Jeong, J.S., Soanes, D.M., Djonovic, S., Kolomiets, E., Rehmeyer, C., Li, W., Harding, M., Kim, S., Lebrun, M.H., Bohnert, H., Coughlan, S., Butler, J., Calvo, S., Ma, L.J., Nicol, R., Purcell, S., Nusbaum, C., Galagan, J.E. and Birren, B.W. (2005) The genome sequence of the rice blast fungus Magnaporthe grisea . Nature, 434, 980–986. [DOI] [PubMed] [Google Scholar]
- Dohn, J.W. , Grubbs, A.W. , Oakley, C.E. and Oakley, B.R. (2018) New multi‐marker strains and complementing genes for Aspergillus nidulans molecular biology. Fungal Genet. Biol. 111, 1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- d’Enfert, C. (1996) Selection of multiple disruption events in Aspergillus fumigatus using the orotidine‐5′‐decarboxylase gene, pyrG, as a unique transformation marker. Curr. Genet. 30, 76–82. [DOI] [PubMed] [Google Scholar]
- Engler, C. , Youles, M. , Gruetzner, R. , Ehnert, T.‐M. , Werner, S. , Jones, J.D.G. , Patron, N.J. and Marillonnet, S. (2014) A golden gate modular cloning toolbox for plants. ACS Synth. Biol. 3, 839–843. [DOI] [PubMed] [Google Scholar]
- Flynn, P.J. and Reece, R.J. (1999) Activation of transcription by metabolic intermediates of the pyrimidine biosynthetic pathway. Mol. Cell. Biol. 19, 882–888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fonzi, W.A. and Irwin, M.Y. (1993) Isogenic strain construction and gene mapping in Candida albicans . Genetics, 134, 717–728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gan, P. , Ikeda, K. , Irieda, H. , Narusaka, M. , O’Connell, R.J. , Narusaka, Y. , Takano, Y. , Kubo, Y. and Shirasu, K. (2013) Comparative genomic and transcriptomic analyses reveal the hemibiotrophic stage shift of Colletotrichum fungi. New Phytol. 197, 1236–1249. [DOI] [PubMed] [Google Scholar]
- Gao, L. , Narita, K. , Ozaki, T. , Kumakura, N. , Gan, P. , Minami, A. , Liu, C. , Lei, X. , Shirasu, K. and Oikawa, H . (2018) Identification of novel sesterterpenes by genome mining of phytopathogenic fungi Phoma and Colletotrichum sp. Tetrahedron Lett. 59, 1136–1139. [Google Scholar]
- Garcia, A. , Adedoyin, G. , Heitman, J. and Lee, S.C. (2017) Construction of a recyclable genetic marker and serial gene deletions in the human pathogenic Mucorales Mucor circinelloides. G3 Genes Genom. Genet. 7, 2047–2054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hogenhout, S.A. , Van der Hoorn, R.A.L. , Terauchi, R. and Kamoun, S. (2009) Emerging concepts in effector biology of plant‐associated organisms. Mol. Plant–Microbe Interact. 22, 115–122. [DOI] [PubMed] [Google Scholar]
- Irieda, H. , Maeda, H. , Akiyama, K. , Hagiwara, A. , Saitoh, H. , Uemura, A. , Terauchi, R. and Takano, Y. (2014) Colletotrichum orbiculare secretes virulence effectors to a biotrophic interface at the primary hyphal neck via exocytosis coupled with SEC22‐mediated traffic. Plant Cell, 26(5), 2265–2281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishida, N. and Akai, S. (1969) Relation of temperature to germination of conidia and appressorium formation in Colletotrichum lagenarium . Mycologia, 61, 382–386. [Google Scholar]
- Johansson, B. and Hahn‐Hägerdal, B. (2004) Multiple gene expression by chromosomal integration and CRE‐loxP‐mediated marker recycling in Saccharomyces cerevisiae In: Recombinant Gene Expression. Methods in Molecular Biology, pp. 287–296. Humana Press; Available at https://link.springer.com/protocol/10.1385%2F1-59259-774-2%3A287. [DOI] [PubMed] [Google Scholar]
- Jones, J.D.G. and Dangl, J.L. (2006) The plant immune system. Nature, 444, 323–329. [DOI] [PubMed] [Google Scholar]
- Katoh, K. , Misawa, K. , Kuma, K. and Miyata, T. (2002) MAFFT: a novel method for rapid multiple sequence alignment based on fast Fourier transform. Nucleic Acids Res. 30, 3059–3066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kilby, N.J. , Snaith, M.R. and Murray, J.A.H. (1993) Site‐specific recombinases: tools for genome engineering. Trends Genet. 9, 413–421. [DOI] [PubMed] [Google Scholar]
- Kleemann, J. , Rincon‐Rivera, L.J. , Takahara, H. , Neumann, U., Ver Loren van Themaat, E., van der Does, H.C., Hacquard, S., Stüber, K., Will, I., Schmalenbach, W., Schmelzer, E. and O’Connell, R.J. (2012) Sequential delivery of host‐induced virulence effectors by appressoria and intracellular hyphae of the phytopathogen Colletotrichum higginsianum . PLOS Pathog. 8, e1002643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kopke, K. , Hoff, B. and Kück, U. (2010) Application of the Saccharomyces cerevisiae FLP/FRT recombination system in filamentous fungi for marker recycling and construction of knockout strains devoid of heterologous genes. Appl. Environ. Microbiol. 76, 4664–4674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kubo, Y. , Nakamura, H. , Kobayashi, K. , Okuno, T. and Furusawa, I. (1991) Cloning of a melanin biosynthetic gene essential for appressorial penetration of Colletotrichum lagenarium . Mol. Plant–Microbe Interact. 4, 440–445. [DOI] [PubMed] [Google Scholar]
- Lambert, J.M. , Bongers, R.S. and Kleerebezem, M. (2007) Cre‐lox‐based system for multiple gene deletions and selectable‐marker removal in Lactobacillus plantarum . Appl. Environ. Microbiol. 73, 1126–1135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma, L.‐J. , van der Does, H.C. , Borkovich, K.A. , Coleman, J.J., Daboussi, M.J., Di Pietro, A., Dufresne, M., Freitag, M., Grabherr, M., Henrissat, B., Houterman, P.M., Kang, S., Shim, W.B., Woloshuk, C., Xie, X., Xu, J.R., Antoniw, J., Baker, S.E., Bluhm, B.H., Breakspear, A., Brown, D.W., Butchko, R.A., Chapman, S., Coulson, R., Coutinho, P.M., Danchin, E.G., Diener, A., Gale, L.R., Gardiner, D.M., Goff, S., Hammond‐Kosack, K.E., Hilburn, K., Hua‐Van, A., Jonkers, W., Kazan, K., Kodira, C.D., Koehrsen, M., Kumar, L., Lee, Y.H., Li, L., Manners, J.M., Miranda‐Saavedra, D., Mukherjee, M., Park, G., Park, J., Park, S.Y., Proctor, R.H., Regev, A., Ruiz‐Roldan, M.C., Sain, D., Sakthikumar, S., Sykes, S., Schwartz, D.C., Turgeon, B.G., Wapinski, I., Yoder, O., Young, S., Zeng, Q., Zhou, S., Galagan, J., Cuomo, C.A., Kistler, H.C. and Rep, M. (2010) Comparative genomics reveals mobile pathogenicity chromosomes in Fusarium . Nature, 464, 367–373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Namiki, F. , Matsunaga, M. , Okuda, M. , Inoue, I. , Nishi, K. , Fujita, Y. and Tsuge, T. (2001) Mutation of an arginine biosynthesis gene causes reduced pathogenicity in Fusarium oxysporum f. sp. melonis . Mol. Plant–Microbe Interact. 14, 580–584. [DOI] [PubMed] [Google Scholar]
- Nayak, T. , Szewczyk, E. , Oakley, C.E. , Osmani, A. , Ukil, L. , Murray, S.L. , Hynes, M.J. , Osmani, S.A. and Oakley, B.R. (2006) A versatile and efficient gene‐targeting system for Aspergillus nidulans . Genetics, 172, 1557–1566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nielsen, M.L. , Albertsen, L. , Lettier, G. , Nielsen, J.B. and Mortensen, U.H. (2006) Efficient PCR‐based gene targeting with a recyclable marker for Aspergillus nidulans . Fungal Genet. Biol. 43, 54–64. [DOI] [PubMed] [Google Scholar]
- O’Connell, R. , Herbert, C. , Sreenivasaprasad, S. , Khatib, M. , Esquerré‐Tugayé, M.‐T. and Dumas, B. (2004) A novel Arabidopsis–Colletotrichum pathosystem for the molecular dissection of plant–fungal interactions. Mol. Plant–Microbe Interact. 17, 272–282. [DOI] [PubMed] [Google Scholar]
- O’Connell, R.J. , Thon, M.R. , Hacquard, S. , Amyotte, S.G. , Kleemann, J. , Torres, M.F. , Damm, U. , Buiate, E.A. , Epstein, L. , Alkan, N. , Altmüller, J. , Alvarado‐Balderrama, L. , Bauser, C.A. , Becker, C. , Birren, B.W. , Chen, Z. , Choi, J. , Crouch, J.A. , Duvick, J.P. , Farman, M.A. , Gan, P. , Heiman, D. , Henrissat, B. , Howard, R.J. , Kabbage, M. , Koch, C. , Kracher, B. , Kubo, Y. , Law, A.D. , Lebrun, M.‐H. , Lee, Y.‐H. , Miyara, I. , Moore, N. , Neumann, U. , Nordström, K. , Panaccione, D.G. , Panstruga, R. , Place, M. , Proctor, R.H. , Prusky, D. , Rech, G. , Reinhardt, R. , Rollins, J.A. , Rounsley, S. , Schardl, C.L. , Schwartz, D.C. , Shenoy, N. , Shirasu, K. , Sikhakolli, U.R. , Stüber, K. , Sukno, S.A. , Sweigard, J.A. , Takano, Y. , Takahara, H. , Trail, F. , van der Does, H.C. , Voll, L.M. , Will, I. , Young, S. , Zeng, Q. , Zhang, J. , Zhou, S. , Dickman, M.B. , Schulze‐Lefert, P. , Ver Loren van Themaat, E. , Ma, L.‐J. and Vaillancourt, L.J. (2012) Lifestyle transitions in plant pathogenic Colletotrichum fungi deciphered by genome and transcriptome analyses. Nat. Genet. 44, 1060–1065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oakley, B.R. , Rinehart, J.E. , Mitchell, B.L. , Oakley, C.E. , Cannona, C. , Gray, G.L. and May, G.S. (1987) Cloning, mapping and molecular analysis of the pyrG (orotidine‐5′‐phosphate decarboxylase) gene of Aspergillus nidulans . Gene, 61, 385–399. [DOI] [PubMed] [Google Scholar]
- Perfect, S.E. , Hughes, H.B. , O’Connell, R.J. and Green, J.R. (1999) Colletotrichum: a model genus for studies on pathology and fungal–plant interactions. Fungal Genet. Biol. 27, 186–198. [DOI] [PubMed] [Google Scholar]
- Shen, S. , Goodwin, P.H. and Hsiang, T. (2001) Infection of Nicotiana species by the anthracnose fungus, Colletotrichum orbiculare . Eur. J. Plant Pathol. 107, 767–773. [Google Scholar]
- Spanu, P.D. , Abbott, J.C. , Amselem, J. , Burgis, T.A., Soanes, D.M., Stüber, K., Ver Loren van Themaat, E., Brown, J.K., Butcher, S.A., Gurr, S.J., Lebrun, M.H., Ridout, C.J., Schulze‐Lefert, P., Talbot, N.J., Ahmadinejad, N., Ametz, C., Barton, G.R., Benjdia, M., Bidzinski, P., Bindschedler, L.V., Both, M., Brewer, M.T., Cadle‐Davidson, L., Cadle‐Davidson, M.M., Collemare, J., Cramer, R., Frenkel, O., Godfrey, D., Harriman, J., Hoede, C., King, B.C., Klages, S., Kleemann, J., Knoll, D., Koti, P.S., Kreplak, J., López‐Ruiz, F.J., Lu, X., Maekawa, T., Mahanil, S., Micali, C., Milgroom, M.G., Montana, G., Noir, S., O'Connell, R.J., Oberhaensli, S., Parlange, F., Pedersen, C., Quesneville, H., Reinhardt, R., Rott, M., Sacristán, S., Schmidt, S.M., Schön, M., Skamnioti, P., Sommer, H., Stephens, A., Takahara, H., Thordal‐Christensen, H., Vigouroux, M., Wessling, R., Wicker, T. and Panstruga, R. (2010) Genome expansion and gene loss in powdery mildew fungi reveal tradeoffs in extreme parasitism. Science, 330, 1543–1546. [DOI] [PubMed] [Google Scholar]
- Staab, J.F. and Sundstrom, P. (2003) URA3 as a selectable marker for disruption and virulence assessment of Candida albicans genes. Trends Microbiol. 11, 69–73. [DOI] [PubMed] [Google Scholar]
- Sundstrom, P. , Cutler, J.E. and Staab, J.F. (2002) Reevaluation of the role of HWP1 in systemic Candidiasis by use of Candida albicans strains with selectable marker URA3 Targeted to the ENO1 Locus. Infect. Immun. 70, 3281–3283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sweigard, J. , Chumley, F. , Carroll, A. , Farrall, L. and Valent, B. (1997) A series of vectors for fungal transformation. Fungal Genet. Rep. 44, 52–53. [Google Scholar]
- Szewczyk, E. , Kasuga, T. and Fan, Z. (2014) A new variant of self‐excising β‐recombinase/six cassette for repetitive gene deletion and homokaryon purification in Neurospora crassa . J. Microbiol. Methods. 100, 17–23. [DOI] [PubMed] [Google Scholar]
- Szewczyk, E. , Nayak, T. , Oakley, C.E. , Edgerton, H. , Xiong, Y. , Taheri‐Talesh, N. , Osmani, S.A. and Oakley, B.R. (2006) Fusion PCR and gene targeting in Aspergillus nidulans . Nat. Protoc. 1, 3111–3120. [DOI] [PubMed] [Google Scholar]
- Takahara, H. , Hacquard, S. , Kombrink, A. , Hughes, H.B., Halder, V., Robin, G.P., Hiruma, K., Neumann, U., Shinya, T., Kombrink, E., Shibuya, N., Thomma, B.P. and O'Connell, R.J. (2016) Colletotrichum higginsianum extracellular LysM proteins play dual roles in appressorial function and suppression of chitin‐triggered plant immunity. New Phytol. 211, 1323–1337. [DOI] [PubMed] [Google Scholar]
- Takano, Y. , Kubo, Y. , Kuroda, I. and Furusawa, I. (1997) Temporal transcriptional pattern of three melanin biosynthesis genes, PKS1, SCD1, and THR1, in appressorium‐differentiating and nondifferentiating conidia of Colletotrichum lagenarium . Appl. Environ. Microbiol. 63, 351–354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takano, Y. , Kubo, Y. , Shimizu, K. , Mise, K. , Okuno, T. and Furusawa, I. (1995) Structural analysis of PKS1, a polyketide synthase gene involved in melanin biosynthesis in Colletotrichum lagenarium . Mol. Gen. Genet. 249, 162–167. [DOI] [PubMed] [Google Scholar]
- Takano, Y. , Takayanagi, N. , Hori, H. , Ikeuchi, Y. , Suzuki, T. , Kimura, A. and Okuno, T. (2006) A gene involved in modifying transfer RNA is required for fungal pathogenicity and stress tolerance of Colletotrichum lagenarium . Mol. Microbiol. 60, 81–92. [DOI] [PubMed] [Google Scholar]
- Turner, G. , Jimenez, T. , Chae, S. , Baasiri, R. and Borkovich, K. (1997) Utilization of the Aspergillus nidulans pyrG gene as a selectable marker for transformation and electroporation of Neurospora crassa . Fungal Genet. Rep. 44, 57–59. [Google Scholar]
- Ushimaru, T. , Terada, H. , Tsuboi, K. , Kogou, Y. , Sakaguchi, A. , Tsuji, G. and Kubo, Y. (2010) Development of an efficient gene targeting system in Colletotrichum higginsianum using a non‐homologous end‐joining mutant and Agrobacterium tumefaciens‐mediated gene transfer. Mol. Genet. Genomics, 284, 357–371. [DOI] [PubMed] [Google Scholar]
- Weld, R.J. , Plummer, K.M. , Carpenter, M.A. and Ridgway, H.J. (2006) Approaches to functional genomics in filamentous fungi. Cell Res. 16, 31–44. [DOI] [PubMed] [Google Scholar]
- Wirth, D. , Gama‐Norton, L. , Riemer, P. , Sandhu, U. , Schucht, R. and Hauser, H. (2007) Road to precision: recombinase‐based targeting technologies for genome engineering. Curr. Opin. Biotechnol. 18, 411–419. [DOI] [PubMed] [Google Scholar]
- vanden Wymelenberg, A.J. , Cullen, D., Spear, R.N., Schoenike, B. andAndrews, J.H. (1997) Expression of green fluorescent protein in Aureobasidium pullulans and quantification of the fungus on leaf surfaces. Biotechniques, 23, 686–690. [DOI] [PubMed] [Google Scholar]
- Yoshino, K. , Irieda, H. , Sugimoto, F. , Yoshioka, H. , Okuno, T. and Takano, Y. (2012) Cell death of Nicotiana benthamiana is induced by secreted protein NIS1 of Colletotrichum orbiculare and is suppressed by a homologue of CgDN3. Mol. Plant–Microbe Interact. 25, 625–636. [DOI] [PubMed] [Google Scholar]
- Yuliya, K. , Karin, M. , Kerstin, S. , Lupas, A.N. and Regine, K. (2010) The use of FLP‐mediated recombination for the functional analysis of an effector gene family in the biotrophic smut fungus Ustilago maydis . New Phytol. 187, 957–968. [DOI] [PubMed] [Google Scholar]
- Zhang, S. , Ban, A. , Ebara, N. , Mizutani, O. , Tanaka, M. , Shintani, T. and Gomi, K. (2017) Self‐excising Cre/mutant lox marker recycling system for multiple gene integrations and consecutive gene deletions in Aspergillus oryzae . J. Biosci. Bioeng. 123, 403–411. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig. S1 Amino acid sequence alignment of Saccharomyces cerevisiae Ura3p with Colletotrichum orbiculare URA3A and URA3B, and Fusarium oxysporum URA3.
Fig. S2 The lesser disease symptom phenotype of ura3a/b is partially complemented by externally added uridine.
Fig. S3 Colletotrichum orbiculare btcAco knock‐out mutants do not show reduced virulence on cucumber leaves. The experiment was performed in the same conditions as in Fig. 5d.
Table S1 Fungal strain list.
Table S2 Plasmid list.
Table S3 Oligo list.
Data S1 DNA sequences of PCR‐10 and PCR‐11.


