Summary
Botrytis cinerea is the causative agent of grey mould on over 1000 plant species and annually causes enormous economic losses worldwide. However, the fungal factors that mediate pathogenesis of the pathogen remain largely unknown. Here, we demonstrate that a novel B. cinerea‐specific pathogenicity‐associated factor BcHBF1 (hyphal branching‐related factor 1), identified from virulence‐attenuated mutant M8008 from a B. cinerea T‐DNA insertion mutant library, plays an important role in hyphal branching, infection structure formation, sclerotial formation and full virulence of the pathogen. Deletion of BcHBF1 in B. cinerea did not impair radial growth of mycelia, conidiation, conidial germination, osmotic‐ and oxidative‐stress adaptation, as well as cell wall integrity of the ∆Bchbf1 mutant strains. However, loss of BcHBF1 impaired the capability of hyphal branching, appressorium and infection cushion formation, appressorium host penetration and virulence of the pathogen. Moreover, disruption of BcHBF1 altered conidial morphology and dramatically impaired sclerotial formation of the mutant strains. Complementation of BcHBF1 completely rescued all the phenotypic defects of the ∆Bchbf1 mutants. During young hyphal branching, host penetration and early invasive growth of the pathogen, BcHBF1 expression was up‐regulated, suggesting that BcHBF1 is required for these processes. Our findings provide novel insights into the fungal factor mediating pathogenesis of the grey mould fungus via regulation of its infection structure formation, host penetration and invasive hyphal branching and growth.
Keywords: appressorium, Botrytis cinerea, host penetration, hyphal branching, infection cushions, sclerotial formation, virulence
Introduction
Botrytis cinerea is a typical necrotrophic plant fungal pathogen that inflicts grey mould on over 1000 plant species (Fillinger and Elad, 2016), including almost all vegetable and fruit crops, and annually causes US$10 billion to US$100 billion in losses worldwide (Weiberg et al., 2013). In the field, the main infection source of the pathogen is conidia that germinate on plant surfaces and form appressorium‐like structures to facilitate host penetration (Gourgues et al., 2004). Mycelia of the pathogen are able to form highly melanized ‘specialized hyphal networks’ or ‘clumps of hyphae’ called infection cushions that also promote the pathogen host invasion (Cao et al., 2018; Liu et al., 2018; Marschall and Tudzynski, 2016). Besides infection structures (appressoria and infection cushions), occasionally, host invasion by the pathogen can occur via germ tube apices (Choquer et al., 2007; Van den Heuvel and Waterreus, 1983). B. cinerea induces a very wide range of symptoms; the most typical symptoms on leaves and soft fruits are soft rots, accompanied by collapse and water soaking of parenchyma tissues, followed by a rapid appearance of grey masses of conidia that initiate the next round of infection. B. cinerea produces sclerotia that develop within dying host tissues and serve as the long‐term survival structures in the life cycle and the primary inoculum in the disease cycle. The pathogen can also survive as mycelia, surviving within crop debris and inside some seeds to serve as primary inoculum. Sclerotia germinate in early spring to produce conidiophores and multinucleate conidia, serving as a primary source of inoculum (Williamson et al., 2007).
Host infection by B. cinerea is complicated and tightly regulated by numerous pathogenicity‐associated factors including extracellular enzymes, proteins and metabolites. To secure a successful host infection, B. cinerea conidia need to germinate and form appressoria to penetrate hosts. However, B. cinerea conidia hardly ever germinates in the presence of only water. The pathogen gluconeogenesis thus play a crucial role in the initiation of conidial germination since the process allows the pathogen to cope with the limitation of glucose and/or other carbon sources in the infection niches (Liu et al., 2018). B. cinerea genes involved in autophagy, a mechanism of the cell that disassembles and recycles unnecessary or dysfunctional cellular components, also greatly influence conidial germination and virulence (Liu et al., 2019; Ren et al., 2017, 2018a, b). During infection, B. cinerea secretes a large number of extracellular virulence components including cell wall degrading enzymes, oxidoreductases, cerato‐platanin family proteins, toxic compounds such as botrydial, oxalic acid (Frías et al., 2011; van Kan, 2006), and even small RNAs (Wang et al., 2017; Weiberg et al., 2013). Cell wall degrading enzymes may facilitate host penetration and the conversion of host tissue into fungal biomass, while toxic compounds and reactive oxygen species (ROS) may contribute to killing of the host cells, other proteins or molecules may participate in pathogen adhesion, signal transduction, adaptation to infection‐associated environmental stresses and modulation of host immune systems during infection. These features are regarded to contribute to the pathogen with a very broad host range; for reviews, see (Nakajima and Akutsu, 2014; Sharma and Kapoor, 2017; Williamson et al., 2007).
B. cinerea is an aggressive necrotrophic pathogen; interestingly, the fungal pathogen can also systemically colonize host plants without causing disease symptoms (van Kan et al., 2014; Veloso and van Kan, 2018), or sometimes remain in a long period of quiescence before rotting tissues when the host physiology changes and the environment is conducive (Williamson et al., 2007). Infestation by the pathogen can occur anytime from seedling to ripe product. Serious damage can also occur in any stage of seedling, development, maturity, storage and sale (Dean et al., 2012). Currently, grey mould control mainly relies on fungicides; however, due to the pathogen’s genetic flexibility and high evolutionary potential, there is increasing concern about the development and abundance of fungicide‐resistant B. cinerea field strains and the overtake of fungicide‐efficiency by the fungicide‐resistant strains (Hahn, 2014; Leroch et al., 2013; Romanazzi et al., 2016).
B. cinerea has been regarded as the second most important phytopathogenic fungus based on its scientific and economic significance (Dean et al., 2012). The availability of the pathogen’s genomic sequence and its high amenability to genetic and molecular genetic manipulation, together with its economic relevance, have contributed to B. cinerea being one of the most extensively studied necrotrophic fungal pathogen (Dean et al., 2012; Van Kan et al., 2017). Owing to the features of significant phenotypic variations, haploid nature, small genome size and reduced repeat content and its high amenability, B. cinerea has long been served as a model aggressive necrotrophic pathogen to study molecular mechanisms underlying host‐pathogen interactions (Amselem et al., 2011; Dean et al., 2012; Van Kan et al., 2017). The knowledge about the molecular mechanisms underlying B. cinerea host interactions has been greatly expanded in the last two decades due to the development of many technologies (Fillinger and Elad, 2016; Nakajima and Akutsu, 2014; Sharma and Kapoor, 2017). However, many details of B. cinerea pathogenesis and its disarming of host resistance still remain obscure (Fillinger and Elad, 2016).
In this study, we demonstrate that B. cinerea hyphal branching‐related factor 1 (BcHbf1) is a novel pathogenicity‐associated factor that is only found in B. cinerea genome and is required for conidial morphogenesis, hyphal branching, infection structure development, sclerotial formation and full virulence of the pathogen. Mycelium development and host invasion as well as hyphal invasive growth correspond to the up‐regulation of BcHBF1 expression. BcHBF1 is dispensable for pathogen conidiation, conidial and sclerotial germination, radial growth of mycelia and osmotic‐ and oxidative‐stress adaptation as well as cell wall integrity. Our findings provide new insights into BcHBF1 mediation of the development and pathogenicity of the necrotrophic fungal pathogen.
Results
BcHBF1 is a novel B. cinerea‐specific virulence‐associated gene
To comprehensively understand molecular mechanisms underlying B. cinerea host interactions, we generated a B. cinerea T‐DNA insertion mutant library that contains about 50 000 transformants and identified a mutant strain M8008 with a significant reduction in pathogenicity on detached tomato and strawberry leaves from the mutant library. Thermal Asymmetric Interlaced‐Polymerase Chain Reaction (TAIL‐PCR) and sequencing analysis of the mutant strain M8008 indicated that a T‐DNA inserted into the position 24 bp upstream of the start coding region of an open reading frame (ORF) (BCIN_06g00240) (Fig. 1A) that has been previously annotated as a gene encoding a hypothetical protein (Van Kan et al., 2017). The deduced hypothetical protein contains 465 amino acid residues (Fig. S1A) and only a few evolutionarily conserved domains, i.e. (non)cytoplasmic and transmembrane domains, were detected in the hypothetical protein (Fig. S1B). Bioinformatics analysis suggests that the hypothetical protein may be a membrane protein that contains a non‐cytoplasmic domain, a transmembrane region/TMhelix, and a cytoplasmic domain (Fig. S1B). A BLAST search demonstrated that protein orthologs of the hypothetical protein were not detected, implying that the hypothetical protein is a B. cinerea‐specific virulence‐associated factor. Further functional analysis suggested that the protein is required for the pathogen hyphal branching (see below), the T‐DNA tagged gene was thus designated as BcHBF1.
Figure 1.
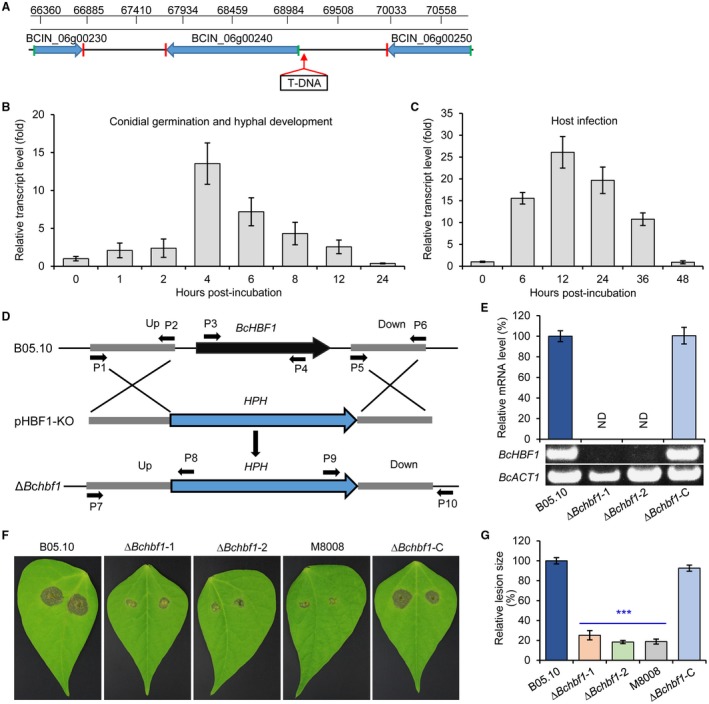
BcHBF1 is a pathogenicity‐associated gene. (A) Schematic diagram indicates the position of T‐DNA insertion in B. cinerea genome of the mutant M8008. (B) Expression of BcHBF1 in B. cinerea during conidial germination and the subsequent hyphal development. (C) Expression profile of B. cinerea BcHBF1 during host infection by the pathogen. (D) Strategy for generation of BcHBF1 gene disruption (∆Bchbf1) mutant strains. (E) Detection of BcHBF1 expression in the wild type (WT) (B05.10), ∆Bchbf1 and complemented (∆Bchbf1‐C) strains via real‐time quantitative Reverse Transcription‐Polymerase Chain Reaction (qRT‐PCR. (F) Pathogenicity assay for the WT, T‐DNA insertional mutant M8008, ∆Bchbf1 mutant and complemented strains. Droplets of conidial suspension (mixture of conidial suspension [1 × 106 conidia/mL] and PDB, vol: vol = 1: 1, 5 µL) of each strain were inoculated. Diseased leaves were photographically documented at 72 h post‐inoculation/incubation (hpi) at 20 °C in dark. (G) Quantification of lesion size caused by the indicated strains at 72 hpi. Data represent means ± standard deviations (SDs) from at least three independent experiments with triplicate samples examined for each treatment. ***: significant at P < 0.001.
To evaluate the roles of BcHBF1 in B. cinerea development and pathogenesis, we profiled BcHBF1 expression during the pathogen conidial germination, mycelial development and host infection via real‐time quantitative Reverse Transcription (qRT‐PCR) approach. Our data demonstrated that the expression of BcHBF1 was at a relatively low level at 1 h–2 h after the initiation of conidial germination and maintained an up‐regulated level to 4 h post‐inoculation/incubation (hpi) and reached a peak (13.54‐fold) at 4 hpi (Fig. 1B). However, after 4 hpi, the expression of BcHBF1 gradually decreased. During a time course of 48 h of host infection, BcHBF1 expression was up‐regulated after 6 hpi, reached a peak at 12 hpi and then back to normal level at 48 hpi (Fig. 1C). The results imply that BcHBF1 plays an important role in the pathogen’s hyphal development and host infection.
To analyse the roles of BcHBF1 in the pathogen growth and virulence, we first generated B. cinerea HBF1 gene knockout (KO) mutant ∆Bchbf1 using the illustrated strategy (Fig. 1D) and its complemented strain ∆Bchbf1‐C as previously described (Liu et al., 2018). We then performed pathogenicity assays for the wild type (WT) (B05.10), T‐DNA mutant M8008, ∆Bchbf1, and complemented ∆Bchbf1‐C strains of the pathogen (conidial suspension in ½ potato dextrose broth [PDB]) after confirmation of the absence of gene BcHBF1 in the mutant strains via PCR detection and qRT‐PCR analysis (Fig. 1E). Our result indicated that loss of BcHBF1 significantly reduced virulence of the ∆Bchbf1 mutants, which corresponded to pathogenicity reduction in the mutant M8008. Complementation of the mutant strain ∆Bchbf1‐1 with the B. cinerea WT HBF1 allele rescued the pathogenicity defect of the mutants (Fig. 1F and G). These results demonstrate that B. cinerea HBF1 is a novel virulence‐associated factor.
BcHBF1 is required for B. cinerea conidial morphogenesis but dispensable for radial growth of mycelia and conidiation
To test a role of BcHBF1 in mediation of B. cinerea growth, we determined mycelial growth of the WT, ΔBchbf1, and complemented strains on complete medium (CM) plates. Our results demonstrated that when mycelial plugs (Fig. S2A) or conidia (Fig. S2B) of the tested strains were inoculated on CM plates, mycelial growth (rate) of the tested strains was similar during a time course of 3 days (for mycelial plugs) or 4 days (for conidia) of incubation (Fig. S2). These results indicated that loss of BcHBF1 in B. cinerea does not affect radial growth of the fungal mycelia.
To investigate a role of BcHBF1 in the pathogen conidiation and conidial morphogenesis, we inoculated mycelial plugs or conidia suspension of the tested strains on CM plates and determined conidiation ability and conidial morphogenesis of these strains at 10 days to 15 days post‐incubation (dpi). Our quantitative data demonstrated that loss of BcHBF1 did not impair conidiation of the ΔBchbf1 mutant strains (Fig. 2A and B), indicating that BcHBF1 is dispensable for the pathogen conidiation.
Figure 2.
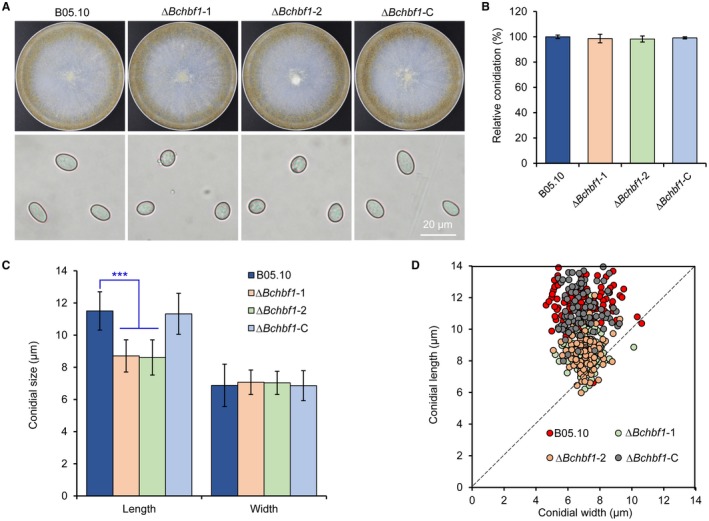
BcHBF1 is required for B. cinerea conidial morphogenesis but dispensable for conidiation. (A) BcHBF1 mediates B. cinerea conidiation (upper panel) and conidial morphogenesis (lower panel). The indicated B. cinerea WT, ∆Bchbf1 and complemented strains were incubated on CM plates at 20 °C for 10 days and photographically documented. (B) Quantification of relative conidiation of the indicated strains at 10 days post‐incubation/inoculation (dpi) on CM plates. (C) Comparison of conidial size of the indicated strains. (D) Loss of BcHBF1 increases the number of globose and less elliptical conidia (closer to the dash line) in the ∆Bchbf1 mutant strains. More than 100 10‐day‐old conidia of each strain were measured under a microscope in each experiment. Data represent means ± standard deviations (SDs) from three independent experiments with triplicate colonies/slides were analyzed for each strain. *** indicates significant at P < 0.001.
Further analysis of conidial morphology revealed that the length of ΔBchbf1 mutant conidia was significantly shorter than those of the WT and complemented strains (11.5 ± 1.2 μm, 8.7 ± 1.0 μm, and 11.3 ± 1.3 μm for conidia produced by the WT, ΔBchbf1, and ΔBchbf1‐C strains, respectively). However, conidial width of all the tested strains did not display any significant difference (6.9 ± 1.3 μm, 7.1 ± 0.8 μm, and 6.9 ± 0.9 μm for the WT, ΔBchbf1, and ΔBchbf1‐C strains, respectively) (Fig. 2C and D). The ΔBchbf1 mutant conidia are more globose and less elliptical than those of controls as demonstrated by the length‐to‐width ratio of the mutant conidia, which is closer to 1 (1.67, 1.23 and 1.65 for the WT, ΔBchbf1 and ΔBchbf1‐C strains, respectively) (Fig. 2D). These data demonstrated that loss of BcHBF1 in B. cinerea reduced conidial size of the pathogen, although the conidial widths of the mutants were similar to those of the control and complemented strains (Fig. 2C and D). Taken together, our data demonstrate that BcHBF1 controls B. cinerea conidial morphogenesis and is dispensable for the pathogen radial growth of mycelia and conidiation.
BcHBF1 mediates B. cinerea hyphal branching but is dispensable for its conidial germination
To test whether BcHBF1 mediates B. cinerea conidial germination and the subsequent hyphal/mycelial development, we determine conidial germination and hyphal development on solid CM plates (Fig. 3) or on glass slides with liquid CM (Fig. S3) using the conidia harvested from CM plates at 10 dpi to 15 dpi. Our findings demonstrated that during a time course of 6 h incubation, conidial germination and germling development of the tested strains did not display significant difference (Fig. 3A and C; Fig. S3A and C). However, statistical analysis of the branched hyphae and the total hyphal length of the tested strains revealed that the difference in hyphal development between the WT and mutant strains gradually increased from 8 hpi (Fig. 3 and S3). At 14 hpi, the number of branched hyphae of the ΔBchbf1 mutants was only about half of those of the WT and complemented strains (each conidium containing 2–3 and 5–6 branched hyphae in the mutant and WT strains, respectively) (Fig. S3A and B); and at 32 hpi, a similar length (~600 µm) of branched hypha contained 2–3 and 7–8 branched hyphae in the mutant and WT strains, respectively (Fig. S3A). Similar result of hyphal branching was observed when conidia of the strains cultured on solid CM at 24 hpi (Fig. 3A and B). At 8 hpi to 24 hpi, compared to the WT strain, the total hyphal length of the ΔBchbf1 mutant significantly reduced (Fig. 3A and C; and Fig. S3A and C). These data demonstrate that loss of BcHBF1 did not impair conidial germination and the germ tube development of the mutant strains, but BcHBF1 played an important role in mycelial development via mediating hyphal branching.
Figure 3.
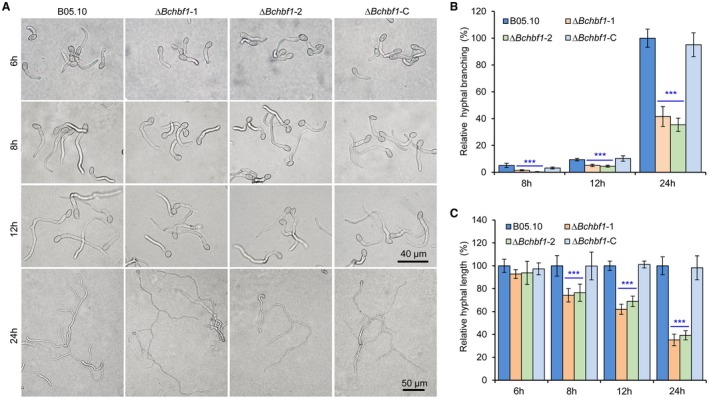
BcHBF1 is dispensable for B. cinerea conidial germination but required for hyphal branching. (A) Droplets of conidial suspension (5 × 105 conidia/mL, 10 µL) of each strain were inoculated on solid CM plates and incubated at 20 °C. Germinated conidia were photographically documented at the indicated hpi. (B and C) Quantification of hyphal branching (B) and total hyphal length (C) of the indicated strains at the indicated hpi. Data represent means ± standard deviations (SDs) from three independent experiments with triplicate plates examined for each treatment. ***: significant at P < 0.001.
Disruption of BcHBF1 impairs B. cinerea sclerotium formation
Sclerotia play important roles in the pathogen survival in hostile environments, sexual reproduction and primary inoculum in the disease cycle (Veloso and van Kan, 2018; Williamson et al., 2007). To test a role of BcHBF1 in sclerotium production, we inoculated conidia or mycelial plugs of the WT, ΔBchbf1, and ΔBchbf1‐C strains on CM plates, incubated these strains at 20 °C in dark condition, and observed and photographically documented sclerotial formation by these strains in a period of 30 days. Our results indicated that sclerotial production by the ΔBchbf1 strains was dramatically impaired when compared to the control and complemented strains (Fig. 4A, B and C). Disruption of BcHBF1 delayed sclerotial formation in the mutant strains about 2 weeks (Fig. 4A and B) and sclerotia produced by the mutants reduced (P < 0.001) (Fig. 4A and B) and were much smaller in size (~12% of the WT control) (Fig. 4A and C). However, sclerotial germination assays indicated that these smaller sclerotia produced by the mutants did not display significant difference in germination and the subsequent mycelial growth (Fig. 4D). These findings indicate that BcHBF1 plays an important role in B. cinerea sclerotial formation, but has little effect on sclerotial germination.
Figure 4.
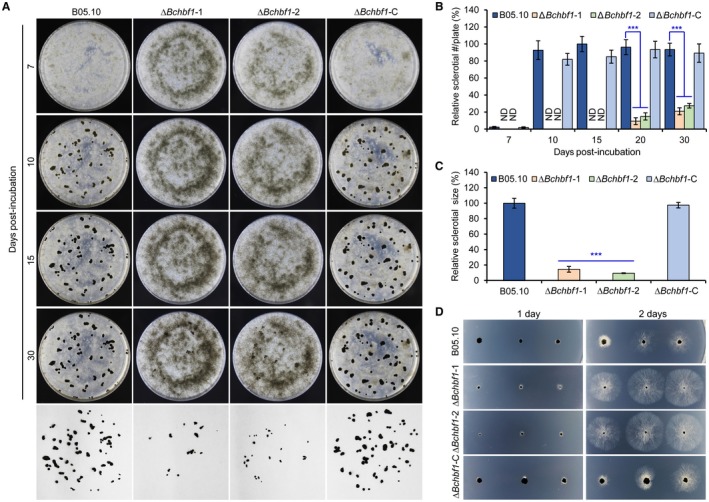
Disruption of BcHBF1 in B. cinerea impairs sclerotial production. (A) Conidia of the wild type (WT), ∆Bchbf1, and ∆Bchbf1‐C strains were inoculated on CM plates at 20 °C in darkness. Sclerotial production by each strain was observed at the indicated dpi. (B) Quantification of sclerotial formation by the indicated strains during a time course of 30 days of incubation. ND: Not detected. (C) Quantification of the sizes of sclerotia produced by the indicated strains via ImageJ (https://imagej.nih.gov/ij/). (D) Germination of sclerotia produced by the indicated strains. The representative images are from one of the experiments, at least three independent experiments were performed, and all the experiments resulted in similar results. Data represent means ± standard deviations (SDs) from three independent experiments in which triplicate plates were analyzed for each strain in each experiment. ***: significant at P < 0.001.
BcHBF1 is dispensable for B. cinerea osmotic‐ and oxidative‐stress adaptation as well as cell wall integrity
To test whether loss of BcHBF1 may affect B. cinerea adaptation to infection‐related stresses, we compared the radial growth rates of the WT, ΔBchbf1 and complemented strains on CM containing the osmotic stress agents NaCl and KCl, the oxidative‐stress agent H2O2, and the cell wall disturbing agents sodium dodecyl sulfate (SDS) and Congo Red (CR) (Cao et al., 2018; Feng et al., 2017; Liu et al., 2018). Our results demonstrated that after 4 days of incubation of conidia (Fig. 5A) or mycelial plugs (Fig. 5B) on CM containing the indicated stress‐mimetic agents, mycelial growth of all the tested strains did not display any significant difference (Fig. 5A, B, D and E), which was consistent with microscopic observation of conidial germination and germling development of these tested strains on CM containing the stress‐mimic agents (Fig. 5C, F and G). These data suggest that BcHBF1 is dispensable for the pathogen osmotic‐ and oxidative‐stress adaptation as well as cell wall integrity.
Figure 5.
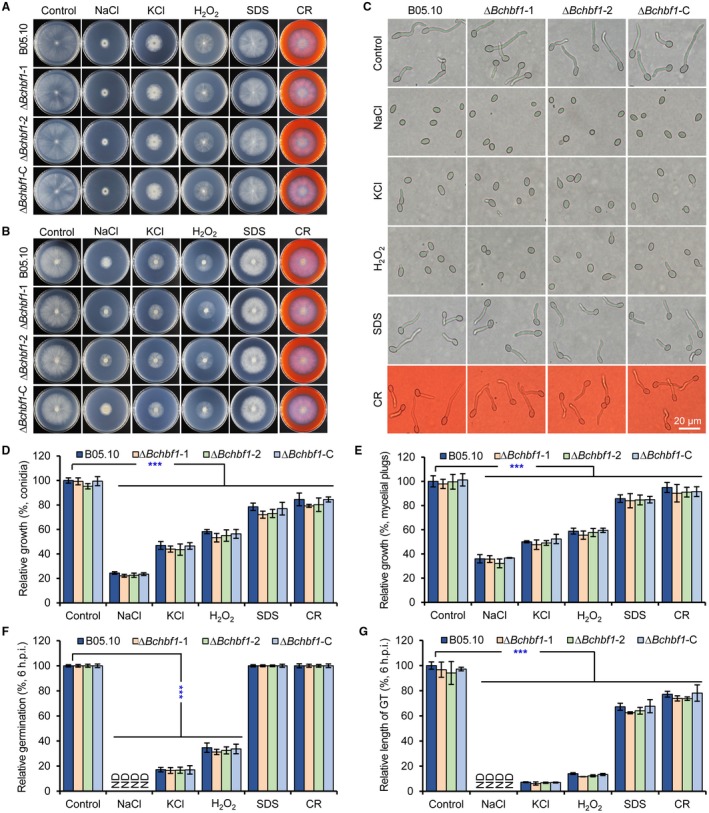
BcHBF1 is dispensable for osmotic‐ and oxidative‐stress adaptation as well as cell wall integrity of B. cinerea. (A) Conidial germination (1 × 106 conidia/mL, 1 µL) and hyphal development of the indicated wild type (WT), ∆Bchbf1, and ∆Bchbf1‐C strains of B. cinerea on CM plates supplemented with the osmotic stress agents NaCl (1 M) and KCl (1 M), the oxidative‐stress agent H2O2 (5 mM), and the cell wall disturbing agents sodium dodecyl sulfate (SDS, 0.005%) and Congo Red (CR, 300 μg/mL). (B) Mycelial radial growth (inoculated with fresh mycelial plugs, 5 mm in diameter) of the indicated strains on CM plates containing the indicated stress‐mimetic agents as presented in (A). Representative photographs were taken at 4 dpi. (C) Conidial germination (5 × 105 conidia/mL, 2 µL) of the indicated strains incubated on CM plates containing the assorted stress agents as presented in (A) for 6 h. (D and E) Quantification of the relative mycelial growth of the indicated strains growing from inoculated conidia (D) or mycelial plugs (5 mm in diameter) (E) on CM plates supplemented with the indicated stress‐mimetic agents as presented in (A). (F and G) Quantification of the relative conidial germination rates (F) and germ tube development (G) of the indicated strains incubated for 6 h on CM plates containing the indicated stress agents as presented in (A). Representative images are from one experiment, at least three independent experiments were performed and all the experiments resulted in similar results. Data represent means ± standard deviations (SDs) from three independent experiments in which triplicate plates were examined for each strain in each experiment. ***: significant at P < 0.001.
BcHBF1 plays a crucial role in infection structure development
Infection structures play a critical role in B. cinerea host penetration and virulence (Cao et al., 2018; Feng et al., 2017; Liu et al., 2018). To evaluate the effect of BcHBF1 on infection structure formation, we determined appressorium and infection cushion formation of the WT, ∆Bchbf1 and ∆Bchbf1‐C strains. Our data demonstrated that when the strains were incubated in ½ PDB on glass slides for 8 h, formation of appressoria by the mutant strains was significantly impaired; the relative appressorium production of the ΔBchbf1 mutant strains was about 35% of the WT control and complemented strains (Fig. 6A and B). Appressorium formation in B. cinerea can be induced by fructose (Doehlemann et al., 2006; Liu et al., 2018), thus, we observed appressorium formation by conidia of the tested strains in the presence of different concentrations of fructose for 8 h. Our findings indicated that addition of fructose significantly (P < 0.001) facilitated appressorium formation by the ΔBchbf1 mutant strains. In absence of fructose, only 35% of the mutant conidia formed the appressorium structures, whereas, in the presence of 1 mM fructose, about 60% of the mutant conidia formed the structure (Fig. 6A and B). Addition of fructose only slightly promoted appressorium formation by the WT and complemented strains; about 88% and 89%, 81% and 85% of the WT and complemented conidia formed appressoria in the absence and presence of fructose (1 mM) in ½ PDB, respectively (Fig. 6A and B). In the presence of 10 mM fructose, appressorium production by all the tested strains did not display significant difference (almost all the conidia produced appressorium structure) (Fig. 6A and C). When the tested strains were cultured with liquid CM on glass slides, the WT and complemented ∆Bchbf1‐C strains formed infection cushions at about 18 hpi and some of them were well developed at 24 hpi (Fig. 7A); formation of infection cushions by the ∆Bchbf1 mutants was not observed until 30 hpi. At 36 hpi, infection cushions formed by the mutants were much smaller; the relative size of infection cushions produced by the mutants were only 42% of those of the WT and complemented strains (Fig. 7A and B). The numbers of infection cushions produced by the ΔBchbf1 mutants significantly reduced at 48 hpi and the numbers of infection cushions formed by the all the tested strains were gradually closer after 60 hpi (Fig. 7C). Taken together, these data demonstrated that BcHBF1 plays a role in mediating infection structure formation, which may partially account for the virulence‐attenuation of the pathogen losing BcHBF1.
Figure 6.
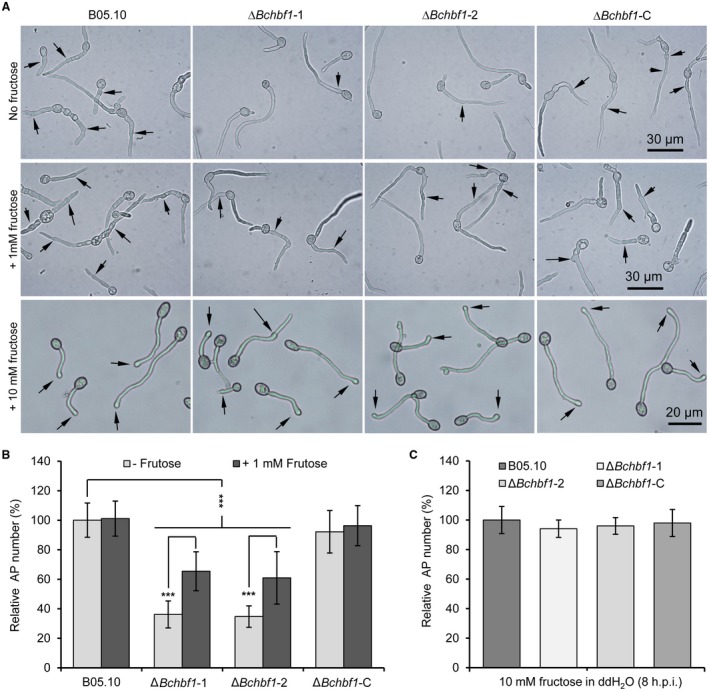
Loss of BcHBF1 reduces appressorium formation in B. cinerea. (A) BcHBF1 is required for B. cinerea appressorium formation and addition of fructose promotes the structure formation in the ∆Bchbf1 mutant strains. (B and C) Quantification of appressorium formation in the absence or presence of low concentration (1 mM) of fructose (B) and of higher concentration (10 mM) of fructose (C). For each strain, more than 100 conidia were examined in each experiment. Data represent means ± standard deviations (SDs) from three independent experiments. ***: significant at P < 0.001.
Figure 7.
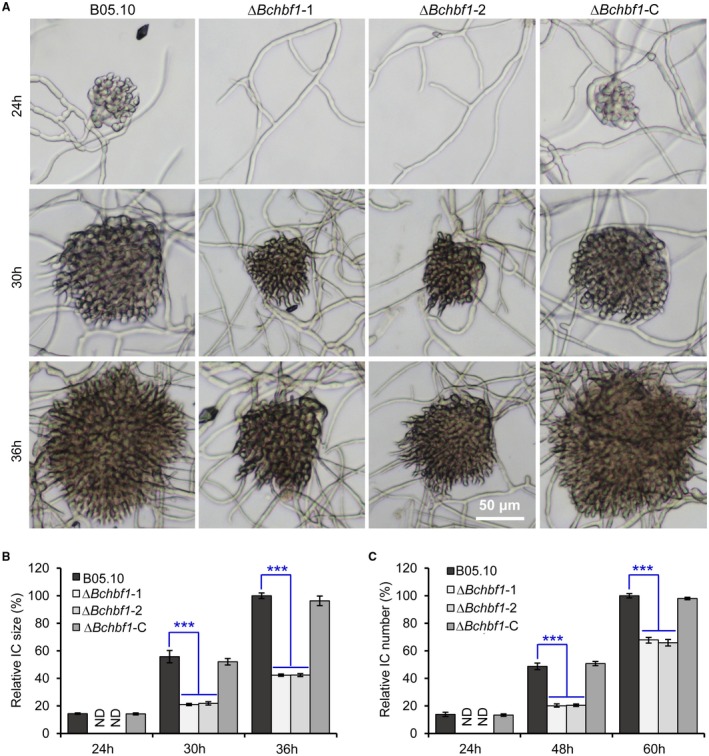
Loss of BcHBF1 impairs infection cushion development in B. cinerea. (A) Infection cushion formation of the indicated B. cinerea WT, ∆Bchbf1 and ∆Bchbf1‐C strains during a time course (36 h) of incubation at 20 ℃. Representative images are from one of three independent experiments; all the experiments resulted in similar results. (B and C) Quantification of the sizes (B) and numbers (C) of infection cushions produced by the indicated strains at the indicated hpi. Data represent means ± standard deviations (SDs) from three independent experiments in which triplicate slides were analyzed for each strain in each experiment. ***: significant at P < 0.001.
BcHBF1 is required for full virulence in B. cinerea
To comprehensively evaluate the role of BcHBF1 in B. cinerea virulence, we inoculated detached host leaves (green bean) with conidial suspension droplets (5 µL, 5 × 105 conidia/mL) (Fig. 8A) and mycelial plugs (Fig. 8B). Our data demonstrated that in conidial suspension inoculation, lesions caused by the WT and complemented strains were observed at 48 hpi; the ΔBchbf1 mutant strains did not produce obvious lesions on green bean leaves until 72 hpi (Fig. 8A and C). In mycelial plug inoculation, lesions caused by all the tested strains were observed at 24 hpi; the ΔBchbf1 mutant strains also induced smaller lesions (P < 0.05) on green bean leaves at the indicated time points post‐inoculation (Fig. 8B and D). The results demonstrate that compared to the WT and ΔBchbf1‐C strains, the grey mould disease caused by the mutants was less severe and delayed.
Figure 8.
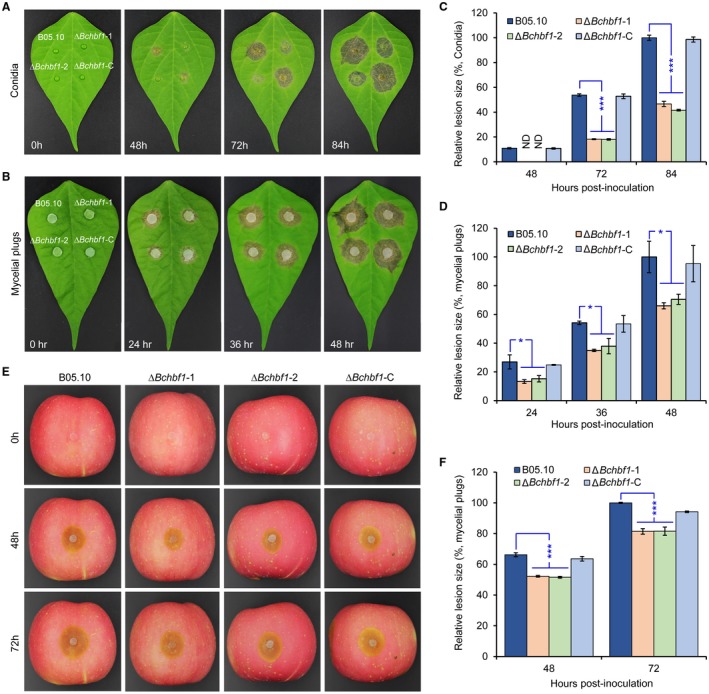
BcHBF1 is required for full virulence of B. cinerea. (A, B) Diseased green bean leaves caused by the indicated B. cinerea WT, ∆Bchbf1 and complemented strains during a time course (84 h) of infection. Droplets of conidial suspension (mixture of conidial suspension [1 × 106 conidia/mL] and PDB, vol: vol = 1: 1, 5 µL) (A) and mycelial plugs (5 mm in diameter) (B) of each strain were inoculated. The inoculated leaves were incubated at 20 °C in dark and diseased leaves were photographically documented at the indicated hpi. (C and D) Quantification of the lesion sizes caused by the indicated strains shown in (A) and (B), respectively. (E) Loss of BcHBF1 in B. cinerea impairs virulence of the pathogen on apple fruit via wound‐inoculation approach. (F) Quantification of the lesion sizes caused by the indicated strains shown in (E). Representative images are from one experiment. Data represent means ± standard deviations (SDs) from at least four independent experiments. *, ***: significant at P < 0.05, 0.001, respectively.
To investigate a role of BcHBF1 on B. cinerea expansion in planta, we inoculated apple fruits with mycelial plugs via wound‐inoculation approach, which provides entrances for mycelia or germ tubes, thereby enabling pathogen entry into host cells via an infection structure‐independent mechanism. Our finding indicated that the ΔBchbf1 mutants could cause necrotic lesions. However, the relative lesion size induced by the mutants on wounded apple fruits was still significantly smaller than those induced by the WT and complemented strains (Fig. 8E and F). At 48 hpi and 72 hpi, the relative lesion size caused by the mutants reached 79% and 82%, respectively, of that caused by the WT control (Fig. 8E and F). These data suggest that BcHBF1 plays an important role in B. cinerea invasive growth in planta.
To test the effect of BcHBF1 on host invasion, we performed onion epidermal cell infection assay to analyse host penetration by the WT, ΔBchbf1 and complemented strains and found that at 12 hpi, the WT and complemented strains penetrated into onion epidermal cells and such penetration by the mutants was not observed until 18 hpi. However, the length of primary invasive hyphae of the WT and complemented strains was longer than that of the mutants (Fig. 9A). At 24 hpi to 36 hpi, compared to the WT and complemented strains, the mutants displayed fewer branch numbers (P < 0.001) of invasive hyphae (Fig. 9B and D) and less degree of invasive hyphal elongation (Fig. 9B). These findings suggest that it takes a longer time for the mutants to establish a successful host invasion and infection. Taken together, our data demonstrate that BcHBF1 plays an important role in B. cinerea host penetration, invasive hyphal development in planta, and is required for the full virulence of the pathogens.
Figure 9.
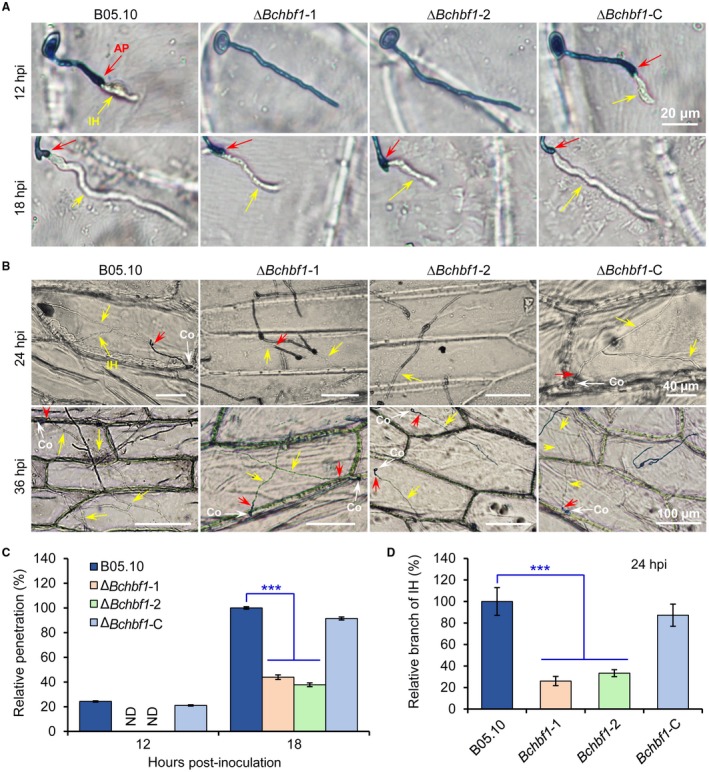
Loss of BcHBF1 impairs B. cinerea host penetration and invasive hyphal development. (A) Loss of BcHBF1 delays plant‐tissue penetration by the ∆Bchbf1 mutants. Droplets (10 µL) of conidial suspension (mixture of conidial suspension [1 × 105 conidia/mL] and ddH2O, vol: vol = 1: 10) of each strain were inoculated on onion epidermis (with extensive wash). The inoculated epidermis were incubated at 20 °C in dark; and at the indicated time points post‐inoculation, the inoculated epidermis were performed lactophenol blue staining and then photographically documented. (B) Disruption of BcHBF1 impairs B. cinerea invasive hyphal in planta branching and growth during infection. Bars in upper and lower panels are 40 µm and 100 µm, respectively. (C) Quantification of host penetration by the indicated strains at 12 hpi and 18 hpi. (D) Quantification of branch number of invasive hyphae of the indicated strains at 24 hpi. Representative images are from one of three independent experiments. Red arrows: appressoria and/or penetration points. Yellow arrows: branched or unbranched invasive hyphae. Co: conidium. IH: invasive hyphae. Data represent means ± standard deviations (SDs) from at least three independent experiments. ***: significant at P < 0.001.
Discussion
Understanding the molecular mechanisms of B. cinerea pathogenesis and host responses is critical for control of plant grey mould; and virulence‐associated factors that facilitate host infection by the aggressive pathogen play important roles in these processes. Insertional mutagenesis via the Agrobacterium tumefaciens‐mediated transformation (ATMT) method is a powerful approach to identify factors with novel functions in the fungal pathogen (Giesbert et al., 2012; Schumacher et al., 2014, 2018; Sharma et al., 2018). Using the ATMT approach, we previously identified novel pathogenesis‐associated factors, including the pre‐rRNA processing factor Nop53 (Cao et al., 2018) and phosphoenolpyruvate carboxykinase gene BcPCK1 in gluconeogenesis (Liu et al., 2018) in B. cinerea, and dissected the mechanisms of how those factors mediate the vegetative and pathogenic development as well as virulence of the pathogen (Cao et al., 2018; Liu et al., 2018). In this study, we identified a virulence‐attenuated T‐DNA tagged mutant M8008 from the same B. cinerea T‐DNA mutant library. Further sequence, genetics and functional analyses demonstrate that BcHBF1 is responsible for pathogenicity reduction in the mutant. BcHBF1 is a novel B. cinerea‐specific factor whose homologs have not been identified in genomes of any other known organisms. BcHBF1 mediates conidial morphogenesis, hyphal branching (both in vitro and in planta), infection structure development and virulence of the pathogen, which corresponds to the up‐regulation of BcHBF1 expression during these processes. However, BcHBF1 plays a limited role in B. cinerea conidiation, conidial germination, radial growth of mycelia, osmotic‐ and oxidative‐stress adaptation as well as cell wall integrity.
Sclerotia serve as specific structures that survive in hostile environments including winters and grow out into hyphae under suitable conditions; they therefore act as the primary inoculum in the disease cycle. Moreover, sclerotia play an important role in sexual reproduction, in which B. cinerea sclerotia may serve as female (sclerotial) parental tissue, which can be fertilized by microconidia from an isolate of opposite mating type (the spermatial parent) to produce fruiting bodies that contain sexual ascospores (Faretra et al., 1988; Veloso and van Kan, 2018; Williamson et al., 2007). Our findings demonstrate that BcHBF1 plays a crucial role in sclerotial formation in B. cinerea. Disruption of BcHBF1 dramatically delayed and reduced sclerotial formation as well as altered sclerotial morphology of the mutants (Fig. 4), implying that BcHBF1 may also regulate sexual reproduction of the pathogen via mediating sclerotial formation. In B. cinerea sexual production, the cellular details of plasmogamy and initiation of apothecia remain largely unknown. Moreover, the apothecia are undocumented or rare in most crops attacked by the pathogen (Williamson et al., 2007). Although our data indicated that loss of BcHBF1 did not impair germination of the mutant sclerotia, the effect of BcHBF1 on sexual production via sclerotial spermatization remains to be clarified. Many B. cinerea factors that are critical for B. cinerea sclerotial formation have been recently characterized, including diacylglycerol O‐acyl transferase 2 (DGAT2) (Sharma et al., 2018), autophagy‐related proteins (Liu et al., 2019; Ren et al., 2017, 2018a), the kynurenine 3‐monooxygenase (Kang et al., 2018), the essential ER protein BcPdi1 (Marschall and Tudzynski, 2017), and the MADS‐Box transcription factor Bcmads1 (Zhang et al., 2016). BcHbf1, together with these above‐mentioned factors, share a common in controlling the pathogen sclerotial formation; however, the association of these factors that are critical for the production of sclerotia remains obscure, and the clarification of the connection of these crucial factors for sclerotial production may constitute an intriguing research direction in the pathogen development.
Hyphal branching is frequently associated with pathogenicity of fungal pathogens. A successful host infection by fungal plant pathogens requires differential hyphal branching in the time course of infection (Liu et al., 2017; Mohammadi et al., 2017; Rolke and Tudzynski, 2008). The rice blast fungus Magnaporthe oryzae forms hyphae that do not branch prior to the penetration, and upon reaching epidermal cells, the hyphae of the pathogen start to branch, which enables extensive cell colonization (Caracuel‐Rios and Talbot, 2007). During infection, the plant pathogen Claviceps purpurea, which causes ergot of cereals and grasses, forms hyphae with extreme apical dominance; however, after tapping the vascular bundles, the fungus hyphae undergo frequent branching required for colonization of the whole ovary (Rolke and Tudzynski, 2008). The ZtVf1 transcription factor in wheat pathogen Zymoseptoria tritici is required for the full virulence and involved in hyphal branching of the pathogen. Loss of ZtVf1 reduces hyphal branching and biomass production of the ZtVf1 mutant. The reduced virulence and tissue colonization of the ZtVf1 mutant might be partly attributed to the lower hyphal branching and less fungal biomass production (Mohammadi et al., 2017). In this study, we demonstrate that loss of BcHBF1 resulted in a lower degree of hyphal branching in both vegetative growth and host infection, suggesting that the reduced virulence of the ∆Bchbf1 mutant might be partly attributed to a lower degree of invasive hyphal branching and elongation or development.
B. cinerea penetrates into host plant cells or tissues mainly via its infection structures that include appressoria and infection cushions (Choquer et al., 2007; Van den Heuvel and Waterreus, 1983). Disruption of BcHBF1 reduced appressorium formation by the mutant strains (Fig. 6A and B) and delayed host penetration by the mutant appressoria (Fig. 9A and C). Loss of BcHBF1 greatly delays and reduces infection structure formation by the mutants (Figs. 6 and 7). Pathogenicity assays for the ∆Bchbf1 mutants using both the mutant conidia and mycelial plugs demonstrate that loss of BcHBF1 in the pathogen significantly impaired virulence of the mutants (Fig. 8), which corresponds to the reduction in appressorium formation, the delay of infection cushion formation and appressorium host penetration. The mutant strains displayed similar osmotic‐ and oxidative‐stress adaptation as well as cell wall integrity, implying that the adaptation of the ∆Bchbf1 mutants to the in planta stresses, if any, imposed by hosts upon penetration into host cells, is not impaired. These findings suggest that the reduced mutant appressoria as well as the delay of appressorium host penetration and infection cushion formation by the ∆Bchbf1 mutants may mainly account for their virulence reduction.
The mechanism of BcHBF1 mediating B. cinerea development and virulence remains to be characterized. Although the ∆Bchbf1 mutant strains could form a small number of appressoria, the delay of host penetration by the mutant appressoria was observed (Fig. 9A); this may partly account for the attenuated pathogenicity of the mutant strains, and raises an intriguing question about how BcHBF1 influences the capability of appressorium penetration. Analysis of BcHBF1 expression profiles demonstrates that BcHBF1 expression was up‐regulated during the development of young mycelia and host infection, suggesting that BcHBF1 is required for the pathogen germ tube and hyphal development as well as host infection. The expression profiles of BcHBF1 also correspond to the phenotypic defects including hyphal development and branching, appressorium and infection cushion formation, host penetration and invasive hyphal development of the BcHBF1 deletion mutants in these processes. However, the signal that stimulates the up‐regulation of the gene remains unknown. Subcellular localization is a key functional characteristic of a protein; therefore, subcellular localization of BcHbf1 needs to be determined, although bioinformatics predicts that BcHbf1 may be a transmembrane protein (Fig. S1B). Bioinformatics analysis suggests that the known functional domains of the deduced BcHbf1 are not detected, and functional characteristics of the protein also need to be further investigated. All the above‐mentioned issues may constitute a research direction to reveal the mysterious veil of this B. cinerea‐specific factor.
In summary, we identified a novel B. cinerea‐specific factor BcHBF1 from a B. cinerea T‐DNA mutant library and demonstrated that the B. cinerea‐specific factor plays important roles in the fungus pathogenic development and virulence. Our work provides new insights into fungal factors that enhance virulence of the grey mould fungus via promoting its infection structure formation, host penetration and invasive hyphal branching and growth.
Experimental Procedures
Fungal strains and culture conditions
B. cinerea WT (B05.10) and its derived strains including BcHBF1 gene deletion mutants ∆Bchbf1‐1, ∆Bchbf1‐2, and the ∆Bchbf1‐1 complemented (∆Bchbf1‐C) strains were cultivated and maintained on potato dextrose agar (PDA) or CM as previously described (Liu et al., 2018).
Identification of pathogenicity‐associated gene BcHBF1
A B. cinerea T‐DNA tagged transformant library (containing ~50 000 transformants) in the WT strain B05.10 was generated in our laboratory by using the ATMT approach as previously described (Giesbert et al., 2012; Rolland et al., 2003). A pathogenicity‐attenuated mutant strain M8008 was identified from the library via screening for virulence‐attenuated mutants by using both detached tomato and strawberry leaves as hosts. T‐DNA insertion regions were analysed by using TAIL‐PCR analysis method (Liu and Whittier, 1995; Terauchi and Kahl, 2000). Analyses of the T‐DNA flanking sequences and the deduced amino acid sequences of homologous genes were performed as previously described (Cao et al., 2018; Liu et al., 2018).
Generation of gene deletion mutants and complemented strains
Generation of BcHBF1 deletion mutant (∆Bchbf1) and the genetic complemented strain ∆Bchbf1‐C were performed with previously described approaches (Cao et al., 2018; Feng et al., 2017; Liu et al., 2018). Briefly, vector pXEH containing hygromycin phosphotransferase gene (HPH) cassette was used for targeted gene replacement. The 5’‐ and 3’‐homologous flanks of the targeted gene were amplified and cloned into pXEH vector in the upstream and downstream of HPH, respectively. The gene KO vector was transformed into A. tumefaciens strain AGL‐1 as previously described (Feng et al., 2017). The resultant deletion transformants were screened on PDA with 100 μg/mL hygromycin.
Vector pXEBA conferring glufosinate‐ammonium resistance was used for complementation of the ∆Bchbf1 mutants. To generate complemented vector, a fragment containing 1591 bp upstream and 577 bp downstream of the coding region of BcHBF1 was amplified by PCR and cloned into vector pXEBA. The complementary vector was transformed into A. tumefaciens strain AGL‐1 and the resultant transformants generated by the ATMT method were screened on glufosinate‐ammonium containing DCM medium (Liu et al., 2018). Diagnostic PCR was performed to verify the integration events of the selected transformants. The gene deletion and complemented strains were further confirmed by qRT‐PCR (Liu et al., 2018; Weiberg et al., 2013). Primers used in those experiments are listed in Table S1.
Fungal developmental assays
Fungal growth of the tested B. cinerea strains was determined by measuring the radial diameter of colonies on solid CM (1% glucose, 0.2% peptone, 0.1% yeast extract, 0.1% casamino acids, nitrate salts, trace elements, 0.01% vitamins, 1.2% agar, pH 6.5). Other media used in the assays included liquid CM (CM without agar), PDA (200 g potato, 20 g glucose, 20 g agar and 1 L water), and PDB (200 g potato, 20 g glucose and 1 L water).
Fungal conidiation, conidial morphology, conidium germination, infection structure formation and sclerotial formation were determined as previously described (Feng et al., 2017). Briefly, for conidium germination assays, fresh conidia of the WT, ∆Bchbf1 and ∆Bchbf1‐C strains were harvested from PDA or CM plates with ddH2O and the conidial suspension was adjusted to the concentration of 5 × 105 conidia/mL in ½ PDB. For infection cushion formation assays, droplets (20 μL) of liquid CM were placed on the surfaces of the glass slides, then conidial suspension droplets (5 × 105 conidia/mL, 2 μL) were added to the liquid media and quickly mixed, the inoculated slides were incubated in a moistened chamber at 20 °C. At the indicated time points post‐incubation, formation of infection cushions was observed and photographically documented. The abilities of infection cushion formations were determined by microscopic examination of the total number of infection cushions in five randomly selected view areas per replicate. Observation of conidial germination, infection structure formation, etc. was performed with a Nikon Eclipse 80i fluorescence microscope system. For sclerotial formation assays, strains were cultivated on CM plates at 20 °C in darkness; production of sclerotia by the test strains was observed and photographically documented during a time course of 30 days of incubation. At least three independent experiments with triplicated replicates per experiment were performed. Sclerotial germination assays were performed as previously described (Liu et al., 2018).
Plant infection experiments
Conidia of B. cinerea WT, ∆Bchbf1 and complemented strains cultivated on PDA at 20°C for 10 days to 15 days were collected, washed, and prepared to spore suspension with a concentration of 1 × 106 conidia/mL. For conidial suspension inoculation, the conidia were suspended in liquid CM. Droplets of 5 μL of spore suspension with 5 × 105 conidia/mL were inoculated on 3‐ to 6‐week‐old detached green bean leaves. For mycelial plug inoculation, green bean leaves and/or apple fruits were inoculated with actively growing B. cinerea mycelial plugs (5 mm in diameter) taken from a 3‐ to 4‐day‐old culture of the tested strains. Mycelial plug and conidial inoculated materials were incubated in containers with a sheet of plastic film sealed on the top of each container to maintain a high humidity of infection condition. The inoculated materials were photographically documented at 0, 24, 36, 48, 72, 84 and 96 hpi.
Stress adaptation assays
The adaptation of the tested strains to different stresses was analysed as previously described (Cao et al., 2018; Feng et al., 2017; Liu et al., 2018). Briefly, conidial suspension (1 μL, 1 × 106 conidia/mL) or mycelial plugs (5 mm in diameter) of the B. cinerea WT, ∆Bchbf1 and complemented strains were inoculated onto CM supplemented with different concentrations of stress agents, including osmotic stress agents NaCl and KCl, oxidative‐stress reagent H2O2 and cell wall disturbing agents SDS and CR. The inoculated plates were incubated in incubators at 20 °C. The colony diameters of the strains were measured at the indicated hpi and stress adaptation of the strains was determined by their mycelial growth and growth inhibition (Jiang et al., 2012). Triplicate colonies for each strain were analysed in each experiment and at least three independent experiments were performed.
Cytological assay
The preparation of conidia and onion epidermal cells and sample lactophenol blue staining were performed as previously described (Liu et al., 2018). The infected samples were microscopically observed, photographically documented and analysed at 12, 18, 24 and 36 hpi.
Quantitative Reverse Transcription‐Polymerase Chain Reaction (qRT‐PCR)
For conidial germination, spore suspension (100 μL) of each tested strain was plated on cellophane placed on the top of PDA plates, at 0, 1, 2, 4, 6, 8, 12 and 24 hpi; germinating conidia were collected for RNA extraction. For host infection, spore suspension (2 × 106 conidia/mL) of each strain was first diluted with liquid CM (1: 1, vol: vol) and then droplets (50 μL) of the diluted conidial suspension of each strain were inoculated on the intact leaves of green bean and incubated at room temperature. At 0, 6, 12, 24, 36 and 48 hpi, inoculated leaf samples were collected for RNA extraction. Total RNA was extracted and purified using RNase‐free DNase I to remove genomic DNA (TaKaRa, Dalian, China), and 1 μg of RNA sample of each strain was used for the first strand cDNA synthesis with the PrimeScript® RT Reagent Kit (TaKaRa, Dalian, China). Real‐time PCR was conducted using SYBR® Green I fluorescent dye detection (TaKaRa, Dalian, China). The pathogen actin gene BcACT1 was used as endogenous reference to normalize the expression levels of the measured genes, and the relative expression levels of the target genes were analysed using the relative 2–ΔΔCt method (Livak and Schmittgen, 2001).
Statistical analysis
The quantitative data presented in this study were derived from at least three independent experiments with triplicate treatments examined. To make the results from different independent experiments comparable, the data of controls including mycelial growth, lesion size, conidial germination, etc. in each independent experiment were normalized as 100%. The significance of the data was assessed using the Student’s t‐test. A P‐value of < 0.05 was considered as a significant difference.
Author contributions
Q‐MQ, J‐KL and YL conceived the experiments; YL, J‐KL, JH, JS, M‐ZZ, Y‐YZ and Y‐YW, performed the experiments; Q‐MQ, YL and J‐KL analysed and interpreted the data; Q‐MQ and G‐HL provided reagents, Q‐MQ supervised the work; Q‐MQ and J‐KL wrote the paper.
Competing interests
The authors declare that no competing interests exist.
Supporting information
Fig. S1 Sequence analysis of BcHBF1 in B. cinerea. (A) cDNA and deduced amino acid sequence of BcHBF1. (B) The deduced protein domains and functional sites of BcHbf1 based on InterProScan (http://www.ebi.ac.uk/interpro/scan.html) analyses.
Fig. S2 BcHBF1 is dispensable for B. cinerea mycelial growth. (A and B) BcHBF1 mediation of radial growth of mycelia (A) and conidial development (B) of the indicated B. cinerea strains on complete medium (CM) plates at 20 °C. (C and D) Quantification of radical growth of mycelia (C) and conidial development (D) of the indicated strains cultured on CM for 3 days and 4 days, respectively. Data represent means ± SD from three independent experiments with triplicate plates examined for each treatment. *, **, ***: significance at P < 0.05, P < 0.01 and P < 0.001, respectively.
Fig. S3 BcHBF1 is required for hyphal branching. (A) Droplets of conidial suspension (mixture of conidial suspension [1 × 105 conidia mL for the time point 32 hpi, 1 × 106 conidia mL for other time points] and PDB, vol: vol 1:1, 10 µl) of each strain were inoculated on glass slides and incubated at 20 °C. Germinated conidia were photographically documented at the indicated hpi. (B and C) Quantification of hyphal branching (B) and length (C) of the indicated strains at the indicated hpi. Data represent means ± SD from at least three independent experiments with triplicate slides examined for each treatment. *, **, ***: significance at P < 0.05, P < 0.01 and P < 0.001, respectively.
Table S1 Primers used in this study.
Acknowledgements
This work was supported by the Chinese Special Fund for Agro‐Scientific Research in the Public Interest (201303025) (Subproject: The Molecular Bases of Host‐Botrytis cinerea Interactions), the Start‐up Funding from Jilin University (4305050102), the National Natural Science Foundation of China (Grant number: 31871913) to co‐author Q‐MQ and the National Undergraduate Innovative and Entrepreneurship Training Program (201582234, 2017A82369) to co‐authors J‐KL and YL, respectively.
References
- Amselem, J. , Cuomo, C.A. , van Kan, J.A.L. , Viaud, M. , Benito, E.P. , Couloux, A. , Coutinho, P.M. , de Vries, R.P. , Dyer, P.S. , Fillinger, S. , Fournier, E. , Gout, L. , Hahn, M. , Kohn, L. , Lapalu, N. , Plummer, K.M. , Pradier, J.‐M. , Quévillon, E. , Sharon, A. , Simon, A. , ten Have, A. , Tudzynski, B. , Tudzynski, P. , Wincker, P. , Andrew, M. , Anthouard, V. , Beever, R.E. , Beffa, R. , Benoit, I. , Bouzid, O. , Brault, B. , Chen, Z. , Choquer, M. , Collémare, J. , Cotton, P. , Danchin, E.G. , Da Silva, C. , Gautier, A. , Giraud, C. , Giraud, T. , Gonzalez, C. , Grossetete, S. , Güldener, U. , Henrissat, B. , Howlett, B.J. , Kodira, C. , Kretschmer, M. , Lappartient, A. , Leroch, M. , Levis, C. , Mauceli, E. , Neuvéglise, C. , Oeser, B. , Pearson, M. , Poulain, J. , Poussereau, N. , Quesneville, H. , Rascle, C. , Schumacher, J. , Ségurens, B. , Sexton, A. , Silva, E. , Sirven, C. , Soanes, D.M. , Talbot, N.J. , Templeton, M. , Yandava, C. , Yarden, O. , Zeng, Q. , Rollins, J.A. , Lebrun, M.‐H. and Dickman, M . (2011) Genomic analysis of the necrotrophic fungal pathogens Sclerotinia sclerotiorum and Botrytis cinerea . PLoS Genet. 7, e1002230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao, S.N. , Yuan, Y. , Qin, Y.H. , Zhang, M.Z. , de Figueiredo, P. , Li, G.H. and Qin, Q.M. (2018) The pre‐rRNA processing factor Nop53 regulates fungal development and pathogenesis via mediating production of reactive oxygen species. Environ. Microbiol. 20, 1531–1549. [DOI] [PubMed] [Google Scholar]
- Caracuel‐Rios, Z. and Talbot, N.J. (2007) Cellular differentiation and host invasion by the rice blast fungus Magnaporthe grisea . Curr. Opin. Microbiol. 10, 339–345. [DOI] [PubMed] [Google Scholar]
- Choquer, M. , Fournier, E. , Kunz, C. , Levis, C. , Pradier, J.‐M. , Simon, A. and Viaud, M. (2007) Botrytis cinerea virulence factors: new insights into a necrotrophic and polyphageous pathogen. FEMS Microbiol. Lett. 277, 1–10. [DOI] [PubMed] [Google Scholar]
- Dean, R. , Van Kan, J.A. , Pretorius, Z.A. , Hammond‐Kosack, K.E. , Di Pietro, A. , Spanu, P.D. , Rudd, J.J. , Dickman, M. , Kahmann, R. and Ellis, J. (2012) The Top 10 fungal pathogens in molecular plant pathology. Mol. Plant Pathol. 13, 414–430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doehlemann, G. , Berndt, P. and Hahn, M. (2006) Different signalling pathways involving a Gα protein, cAMP and a MAP kinase control germination of Botrytis cinerea conidia. Mol. Microbiol. 59, 821–835. [DOI] [PubMed] [Google Scholar]
- Faretra, F. , Antonacci, E. and Pollastro, S. (1988) Sexual behaviour and mating system of Botryotinia fuckeliana, teleomorph of Botrytis cinerea . Microbiol. 134, 2543–2550. [Google Scholar]
- Feng, H.Q. , Li, G.H. , Du, S.W. , Yang, S. , Li, X.Q. , de Figueiredo, P. and Qin, Q.M. (2017) The septin protein Sep4 facilitates host infection by plant fungal pathogens via mediating initiation of infection structure formation. Environ. Microbiol. 19, 1730–1749. [DOI] [PubMed] [Google Scholar]
- Fillinger, S. and Elad, Y. (2016) Botrytis – the Fungus, the Pathogen and its Management in Agricultural Systems. Switzerland: Springer International Publishing. [Google Scholar]
- Frias, M. , Gonzalez, C. and Brito, N. (2011) BcSpl1, a cerato‐platanin family protein, contributes to Botrytis cinerea virulence and elicits the hypersensitive response in the host. New Phytol. 192, 483–495. [DOI] [PubMed] [Google Scholar]
- Giesbert, S. , Schumacher, J. , Kupas, V. , Espino, J. , Segmüller, N. , Haeuser‐Hahn, I. , Schreier, P. and Tudzynski, P. (2012) Identification of Pathogenesis‐Associated Genes by T‐DNA–Mediated Insertional Mutagenesis in Botrytis cinerea: A Type 2A Phosphoprotein Phosphatase and an SPT3 Transcription Factor Have Significant Impact on Virulence. Mol. Plant–Microbe Interact. 25, 481–495. [DOI] [PubMed] [Google Scholar]
- Gourgues, M. , Brunet‐Simon, A. , Lebrun, M.H. and Levis, C. (2004) The tetraspanin BcPls1 is required for appressorium‐mediated penetration of Botrytis cinerea into host plant leaves. Mol. Microbiol. 51, 619–629. [DOI] [PubMed] [Google Scholar]
- Hahn, M. (2014) The rising threat of fungicide resistance in plant pathogenic fungi: Botrytis as a case study. J. Chem. Biol. 7, 133–141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang, J. , Yun, Y. , Liu, Y. and Ma, Z. (2012) FgVELB is associated with vegetative differentiation, secondary metabolism and virulence in Fusarium graminearum . Fungal Genet. Biol. 49, 653–662. [DOI] [PubMed] [Google Scholar]
- van Kan, J.A. (2006) Licensed to kill: the lifestyle of a necrotrophic plant pathogen. Trends Plant Sci. 11, 247–253. [DOI] [PubMed] [Google Scholar]
- van Kan, J.A. , Shaw, M.W. and Grant‐Downton, R.T. (2014) Botrytis species: relentless necrotrophic thugs or endophytes gone rogue? Mol. Plant Pathol. 15, 957–961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang, Z. , Yan, X. , Zang, J. , Wang, M. , Zhao, F. , Li, P. , Cao, H. , Han, J. , Xing, J. and Dong, J. (2018) The kynurenine 3‐monooxygenase encoding gene, BcKMO, is involved in the growth, development, and pathogenicity of Botrytis cinerea . Front. Microbiol. 9, 1039. doi: 10.3389/fmicb.2018.01039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leroch, M. , Plesken, C. , Weber, R.W. , Kauff, F. , Scalliet, G. and Hahn, M. (2013) Gray mold populations in German strawberry fields are resistant to multiple fungicides and dominated by a novel clade closely related to Botrytis cinerea . Appl. Environ. Microbiol. 79, 159–167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu, Y.G. and Whittier, R.F. (1995) Thermal asymmetric interlaced PCR: automatable amplification and sequencing of insert end fragments from P1 and YAC clones for chromosome walking. Genomics 25, 674–681. [DOI] [PubMed] [Google Scholar]
- Liu, L. , Yan, Y. , Huang, J. , Hsiang, T. , Wei, Y. , Li, Y. , Gao, J. and Zheng, L. (2017) A Novel MFS Transporter Gene ChMfs1 Is Important for Hyphal Morphology, Conidiation, and Pathogenicity in Colletotrichum higginsianum . Front. Microbiol. 8, 1953. doi: 10.3389/fmicb.2017.01953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu, J.K. , Chang, H.W. , Liu, Y. , Qin, Y.H. , Ding, Y.H. , Wang, L. , Zhao, Y. , Zhang, M.Z. , Cao, S.N. , Li, L.T. and Liu, W. (2018) The key gluconeogenic gene PCK1 is crucial for virulence of Botrytis cinerea via initiating its conidial germination and host penetration. Environ. Microbiol. 20, 1794–1814. [DOI] [PubMed] [Google Scholar]
- Liu, N. , Ren, W. , Li, F. , Chen, C. and Ma, Z. (2019). Involvement of the cysteine protease bcatg4 in development and virulence of botrytis cinerea. Curr. Genet. 65, 293–300. [DOI] [PubMed] [Google Scholar]
- Livak, K.J. and Schmittgen, T.D. (2001) Analysis of relative gene expression data using real‐time quantitative PCR and the 2(‐Delta Delta C(T)) Method. Methods 25, 402–408. [DOI] [PubMed] [Google Scholar]
- Marschall, R. and Tudzynski, P. (2016) Reactive oxygen species in development and infection processes. Semin. Cell Dev. Biol. 57, 138–146. [DOI] [PubMed] [Google Scholar]
- Marschall, R. and Tudzynski, P. (2017) The Protein Disulfide Isomerase of Botrytis cinerea: An ER Protein Involved in Protein Folding and Redox Homeostasis Influences NADPH Oxidase Signaling Processes. Front. Microbiol. 8, 960. doi: 10.3389/fmicb.2017.00960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohammadi, N. , Mehrabi, R. , Mirzadi Gohari, A. , Mohammadi Goltapeh, E. , Safaie, N. and Kema, G.H.J. (2017) The ZtVf1 transcription factor regulates development and virulence in the foliar wheat pathogen Zymoseptoria tritici . Fungal Genet. Biol. 109, 26–35. [DOI] [PubMed] [Google Scholar]
- Nakajima, M. and Akutsu, K. (2014) Virulence factors of Botrytis cinerea . J. Gen. Plant Pathol. 80, 15–23. [Google Scholar]
- Ren, W. , Zhang, Z. , Shao, W. , Yang, Y. , Zhou, M. and Chen, C. (2017) The autophagy‐related gene BcATG1 is involved in fungal development and pathogenesis in Botrytis cinerea . Mol. Plant Pathol. 18, 238–248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ren, W. , Liu, N. , Sang, C. , Shi, D. , Zhou, M. , Chen, C. , Qin, Q. and Chen, W. (2018a) The autophagy gene BcATG8 regulates vegetative differentiation and plant infection of Botrytis cinerea . Appl. Environ. Micobiol. doi: 10.1128/AEM.02455-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ren, W. , Sang, C. , Shi, D. , Song, X. , Zhou, M. and Chen, C. (2018b) Ubiquitin‐like activating enzymes BcAtg3 and BcAtg7 participate in development and pathogenesis of Botrytis cinerea . Curr. Genet. 64, 919–930. [DOI] [PubMed] [Google Scholar]
- Rolke, Y. and Tudzynski, P. (2008) The small GTPase Rac and the p21‐activated kinase Cla4 in Claviceps purpurea: interaction and impact on polarity, development and pathogenicity. Mol. Microbiol. 68, 405–423. [DOI] [PubMed] [Google Scholar]
- Rolland, S. , Jobic, C. , Fevre, M. and Bruel, C. (2003) Agrobacterium‐mediated transformation of Botrytis cinerea, simple purification of monokaryotic transformants and rapid conidia‐based identification of the transfer‐DNA host genomic DNA flanking sequences. Curr. Genet. 44, 164–171. [DOI] [PubMed] [Google Scholar]
- Romanazzi, G. , Smilanick, J.L. , Feliziani, E. and Droby, S. (2016) Integrated management of postharvest gray mold on fruit crops. Postharvest Biol. Technol. 113, 69–76. [Google Scholar]
- Schumacher, J. , Simon, A. , Cohrs, K.C. , Viaud, M. and Tudzynski, P. (2014) The transcription factor BcLTF1 regulates virulence and light responses in the necrotrophic plant pathogen Botrytis cinerea . PLoS Genet. 10, e1004040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schumacher, J. , Studt, L. and Tudzynski, P. (2018) The putative H3K36 demethylase BcKDM1 affects virulence, stress responses and photomorphogenesis in Botrytis cinerea . Fungal Genet. Biol. doi: 10.1016/j.fgb.2018.11.003. [DOI] [PubMed] [Google Scholar]
- Sharma, E. and Kapoor, R. (2017) Insights into the molecular interplay of virulence factors in Botrytis cinerea . Australas. Plant Pathol. 46, 551–561. [Google Scholar]
- Sharma, E. , Tayal, P. , Anand, G. , Mathur, P. and Kapoor, R. (2018) Functional analysis of diacylglycerol O‐acyl transferase 2 gene to decipher its role in virulence of Botrytis cinerea . Curr. Genet. 64, 443–457. [DOI] [PubMed] [Google Scholar]
- Terauchi, R. and Kahl, G. (2000) Rapid isolation of promoter sequences by TAIL‐PCR: the 5'‐flanking regions of Pal and Pgi genes from yams (Dioscorea). Mol. Gen. Genet. 263, 554–560. [DOI] [PubMed] [Google Scholar]
- Van den Heuvel, J. and Waterreus, L.P. (1983) Conidial concentration as an important factor determining the type of prepenetration structures formed by Botrytis cinerea on leaves of French bean (Phaseolus vulgaris). Plant Pathol. 32, 263–272. [Google Scholar]
- Van Kan, J.A. , Stassen, J.H. , Mosbach, A. , Van Der Lee, T.A. , Faino, L. , Farmer, A.D. , Papasotiriou, D.G. , Zhou, S. , Seidl, M.F. and Cottam, E. (2017) A gapless genome sequence of the fungus Botrytis cinerea . Mol. Plant Pathol. 18, 75–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veloso, J. and van Kan, J.A.L. (2018) Many Shades of Grey in Botrytis‐Host Plant Interactions. Trends Plant Sci. 23, 613–622. [DOI] [PubMed] [Google Scholar]
- Wang, M. , Weiberg, A. , Dellota, E. Jr , Yamane, D. and Jin, H. (2017) Botrytis small RNA Bc‐siR37 suppresses plant defense genes by cross‐kingdom RNAi. RNA Biol. 14, 421–428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiberg, A. , Wang, M. , Lin, F.M. , Zhao, H. , Zhang, Z. , Kaloshian, I. , Huang, H.D. and Jin, H. (2013) Fungal small RNAs suppress plant immunity by hijacking host RNA interference pathways. Science 342, 118–123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williamson, B. , Tudzynski, B. , Tudzynski, P. and van Kan, J.A. (2007) Botrytis cinerea: the cause of grey mould disease. Mol. Plant Pathol. 8, 561–580. [DOI] [PubMed] [Google Scholar]
- Zhang, Z. , Li, H. , Qin, G. , He, C. , Li, B. and Tian, S. (2016) The MADS‐Box transcription factor Bcmads1 is required for growth, sclerotia production and pathogenicity of Botrytis cinerea . Sci. Rep. 6, 33901. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig. S1 Sequence analysis of BcHBF1 in B. cinerea. (A) cDNA and deduced amino acid sequence of BcHBF1. (B) The deduced protein domains and functional sites of BcHbf1 based on InterProScan (http://www.ebi.ac.uk/interpro/scan.html) analyses.
Fig. S2 BcHBF1 is dispensable for B. cinerea mycelial growth. (A and B) BcHBF1 mediation of radial growth of mycelia (A) and conidial development (B) of the indicated B. cinerea strains on complete medium (CM) plates at 20 °C. (C and D) Quantification of radical growth of mycelia (C) and conidial development (D) of the indicated strains cultured on CM for 3 days and 4 days, respectively. Data represent means ± SD from three independent experiments with triplicate plates examined for each treatment. *, **, ***: significance at P < 0.05, P < 0.01 and P < 0.001, respectively.
Fig. S3 BcHBF1 is required for hyphal branching. (A) Droplets of conidial suspension (mixture of conidial suspension [1 × 105 conidia mL for the time point 32 hpi, 1 × 106 conidia mL for other time points] and PDB, vol: vol 1:1, 10 µl) of each strain were inoculated on glass slides and incubated at 20 °C. Germinated conidia were photographically documented at the indicated hpi. (B and C) Quantification of hyphal branching (B) and length (C) of the indicated strains at the indicated hpi. Data represent means ± SD from at least three independent experiments with triplicate slides examined for each treatment. *, **, ***: significance at P < 0.05, P < 0.01 and P < 0.001, respectively.
Table S1 Primers used in this study.


