INTRODUCTION
Penicillin allergy is the most commonly reported drug allergy. While 10% of patients report a penicillin allergy, up to 15% of hospitalized patients report a penicillin allergy.1-3 Given that 90-95% of patients with a reported penicillin allergy tolerate penicillin,4 hospitalized patients with infections often unnecessarily avoid penicillins and other beta-lactams, such as the cephalosporins. Reporting a history of penicillin allergy can result in the choice of a clinically-inferior antibiotic5,6 and lead to more adverse effects.7,8 When the beta-lactam alternative antibiotic chosen is more broad-spectrum, antimicrobial resistance ensues. Indeed, patients with reported penicillin allergies have an increased incidence of methicillin-resistant Staphylococcus aureus.7
Unverified beta-lactam allergies interfere with optimal care of infections in the hospital. In methicillin-sensitive Staphylococcus aureus (MSSA) bacteremia, giving the alternative drug (vancomycin) results in more treatment failure (15% vs 9%) and death (18% vs 7%) than using the penicillin allergy history to guide beta-lactam treatment.9 Despite this, a penicillin allergy history was the strongest negative predictor of receiving optimal therapy for patients with MSSA bacteremia.6 Performing a penicillin skin test (PST) prior to MSSA bacteremia treatment was determined to be cost-saving over a 1-year time horizon when PST costs less than $959.98.10
Patients with gram negative bacteremia treated with a beta-lactam alternative because of their allergy history experienced 10% more treatment failures.11 Although aztreonam is often used for inpatient gram negative bacterial infections in patients with beta-lactam allergy histories because there is no risk of beta-lactam cross-reactivity -- except in patients with ceftazidime allergy -- it is less effective against Pseudomonas Spp.12 and is more costly (at least double the cost/day compared to the beta-lactams cefepime or ceftazidime).13 The high cost of aztreonam explains some of the higher costs observed for inpatients with reported penicillin allergy. Recently, inpatient costs for patients with reported penicillin allergy were $1,145 to $4,254 more per patient compared with patients without a penicillin allergy history.14
Inpatients who did not get preferred beta-lactam therapy due to their report of allergy were also found to be at 3-fold greater risk of adverse events (a composite outcome of readmissions for the same infection, acute kidney injury, Clostridioides difficile infection, and drug-related adverse events) compared to those without reported allergy.8 Notably, patients in that study who received beta-lactams despite reported penicillin allergy did not have an increased risk of adverse events compared to those without reported allergy.8
Confirming allergy histories prior to medication prescribing is a core aspect of patient safety.15,16 There are layers of both human and electronic (i.e., allergy alerts) support to ensure patients do not receive drugs to which they are allergic, or potentially allergic given cross-reactivity patterns.17 Although choosing alternative drugs when faced with documented drug allergies aligns with safety principles generally, evidence supports questioning the allergy history for optimal care when patients with beta-lactam allergy histories need antibiotic treatment.
Allergy specialists have performed penicillin allergy evaluations in outpatient settings for decades, but penicillin allergy evaluations have not historically been part of hospital practice. However, given the clear impact that a reported penicillin allergy has on inpatient care of infectious diseases and antibiotic stewardship, penicillin allergy assessments are now considered important to antibiotic stewardship and have multidisciplinary support.18,19 Prescribing beta-lactam antibiotics to patients with beta-lactam allergy histories occurs in approximately 5% of all United States (US) hospital patients, considering that 15% of hospital patients report a penicillin allergy, 40-50% require antibiotics, and 75% of hospital infections should be treated with a beta-lactam.8,20 In this Review, we identified a variety of acute care beta-lactam allergy pathways by intervention type, with a focus on unifying themes important to the intervention process and outcome measures considered central to antibiotic stewardship, quality improvement, and patient safety.
METHODS
To identify illustrative beta-lactam allergy pathway articles to include in this Review, we performed term-by-term and combined term PubMed searches of the following keywords: antimicrobial stewardship, outcome, de-labeling, electronic guideline, penicillin, beta-lactam, allergy, skin test, drug challenge, cephalosporin, and hypersensitivity. The last search date was October 31, 2018, but Partners HealthCare System (PHS) papers published after this date were also considered. All studies available in English and full-text were included if they described an acute care beta-lactam allergy pathway, defined as a coordinated inpatient program for beta-lactam allergy assessments as a tool of antibiotic stewardship. Pathways were grouped by their primary approach; while some pathways were based solely on the allergy history, many used the allergy history with direct drug challenges, PST, or both (i.e., comprehensive beta-lactam allergy pathways). For all identified articles, we reviewed and summarized their intervention details and reported outcomes. Although our approach was intended to comprehensively capture beta-lactam allergy pathway articles, no systematic literature review nor meta analyses was performed.
BETA-LACTAM ALLERGY PATHWAYS: INTERVENTIONS
History-based
The allergy history alone is a powerful tool that can be used to improve antibiotic prescribing. There were eight articles describing pathways exclusively reliant on the allergy history to improve antibiotic choice (Table 1).21-28 These interventions were largely designed and implemented by pharmacists. The most commonly targeted alternative antibiotic was aztreonam, but carbapenem use was a focus of one article.21-23,25,27,28 The history in these pathways was often standardized; for example, one group used a penicillin allergy screening tool25 and another created a penicillin allergy guidance card.27
Table 1.
History-based beta-lactam allergy pathways in acute care
| Study | Location | Setting | Patient Selection | Study Design | Intervention Description | Outcome(s) measured | |
|---|---|---|---|---|---|---|---|
| Swearingen (2016)21 | Knoxville, TN, US | Academic medical center | Patients with mild penicillin allergy histories treated with aztreonam | Pre/Post (117 orders pre-period and 63 orders in post-period) | Restriction of aztreonam to patients with penicillin anaphylaxis, accomplished by education of pharmacy and patient-specific multidisciplinary communication | Alternative ABX use | A significant decrease in median aztreonam DOT (4.0 vs 2.0) post-period and persisted 1-year post-intervention |
| BL use | 36 ABX changes to BLs: 9 to ceftazidime, 9 to meropenem, 7 to cefepime, 6 to ceftriaxone, 5 to non-BL agents | ||||||
| Hospital length of stay and mortality | No change | ||||||
| Caplinger (2016)22 | Boise, ID, USA | Veterans affairs medical center | Patients receiving an anti-pseudomonal carbapenem | Pre/Post considering all orders for carbapenems (not just in BL allergy) | Risk stratification related to BL cross-reactivity inserted into the computerized decision support system | Alternative ABX use | Reduced anti-pseudomonal carbapenem use, considering initiations per 1000 patient-days Carbapenem use in patients with documented No significant change in carbapenem use in BL allergy (60% vs 54%, p=0.59) |
| Safety | No adverse events | ||||||
| Estep (2016)23 | Jacksonville, FL, US | Academic medical center | Patients ordered for aztreonam without clinician-witnessed anaphylaxis, pregnancy, nursing, incarcerated, or enrolled in a clinical trial | Pre/Post (79 patients pre-period and 107 patients post-period) | Antimicrobial stewardship quality initiative “ASQI” that involved EHR review and patient/family interview if feasible regarding allergy, and pharmacy evaluation based on collected information | Alternative ABX use | Reduced median time to aztreonam discontinuation in hours (30.7 vs12.7 |
| BL use | 35% switched to BL post-implementation versus 23% pre-implementation | ||||||
| Safety | No adverse effects associated with BL | ||||||
| Cost | Annual savings of $28,134 considering acquisition cost | ||||||
| Sigona (2016)24 | Syracuse, NY, US | Academic medical center | Adult hospitalized patients with documented BL allergy and receiving non-penicillin ABX | Prospective interview of 32 patients, 24 candidates for a BL ABX recommendation | Pharmacist history of BL allergy and recommendations to change ABX | BL use | 21 patients (66%) were switched to a BL |
| Staicu (2016)25 | Rochester, NY, US | Community teaching hospital | Adult patients ordered for aztreonam | Pre/post (303 orders for 281 patients pre-period and 193 orders for 178 patients in post-period) | ASP team (ID physicians, ID pharmacists) developed history tool (“Penicillin Allergy Screening Tool,” PAST); disseminated electronically on ASP websites) and hard copy (posters). Education of pharmacist and providers, pharmacists mandatory pass-rate >80%. Modification of sepsis order set | Alternative ABX use | Reduced aztreonam DOT per 1000 patient-days (9.5 to 4.4); reduced mean rate of inappropriate aztreonam usage from 4.0 DOT to 0.8 DOT per 1000 patient-days |
| BL use | 84% switched to another ABX: 15% to penicillin, 47% to a cephalosporin, 1% to a carbapenem, and 28% to a fluoroquinolone | ||||||
| Safety | No serious HSRs; one patient grade 2 rash to cefepime | ||||||
| Cost | Estimated annual direct cost avoidance of $60,000-$100,000 | ||||||
| Krey (2017)26 | Green Bay, WI, US | Community teaching hospital | Inpatients with reported BL allergy who received ABX for more than 24 hours (excluding surgical prophylaxis) | Pre/Post (91 pre-period and 88 post-period) | Implementation of local practice guidelines reliant on a scripted BL allergy history tool for pharmacy technician use. Pharmacist documented allergies, screened patients for prior BL use, and recommended ABX to providers following guideline informed by AI andID physicians. Education of pharmacists and prescribers. | Alternative ABX use | No difference in use of non-BL ABX (87% vs 84%, p=0.61) |
| BL use | More transitions to BL ABX (26% vs 11%,p=0.02) Among patients discharged on ABX, BL ABX were more frequently prescribed (57% vs 36%, p=0.006) |
||||||
| Phan (2018)27 | Jacksonville, FL, US | Community teaching hospital | Adult patients with self-reported penicillin allergy on aztreonam | Pre/Post (140 patients in each period) | Education of pharmacy, pharmacy interception of orders for aztreonam, penicillin allergy guidance pocket card. | Alternative ABX use | Rate of aztreonam and fluoroquinolone use decreased |
| BL use | Increased frequency of cephalosporin use from 56 of 140 patients (40%) on cephalosporins pre-intervention and 106/140 (76%) post-intervention | ||||||
| Safety | 4.3% of patients discontinued ABXs due to ADR in pre-implementation group compared to 0.0% discontinuation in post-implementation group | ||||||
| Clark (2018)28 | Jacksonville, FL, US | Community teaching hospital | Inpatients with self-reported BL allergy | Pre/Post (95 patients pre, 65 patients post) | Standardized BL allergy questionnaire implemented by pharmacist trainees. Electronically identified eligible patients (BL allergy, aztreonam orders) | Alternative ABX use | Reduced aztreonam: doses per 1000 patient-days (21.2 vs 9.1, p=0.003), DOT per 1000 patient-days (8.8 vs 4.2, p-0.016) |
| BL use | Of 24 patients on aztreonam in post-period, 22 had therapy changed, most commonly to BL (59%) | ||||||
| Safety | No reported adverse events | ||||||
| Cost | $12,889 saved in post-implementation period, estimated annual cost saving of $37,857 | ||||||
Abbreviations: ABX, antibiotics; DOT, days of therapy; BL, beta-lactam; EHR, electronic health record; ASP, antibiotic stewardship program; ID, infectious diseases; HSR, hypersensitivity reaction; ADR, adverse drug reaction
Drug challenge-based
Administration of beta-lactam direct drug challenges to inpatients requires more resources than the allergy history alone. Direct drug challenges (also known as test dose challenges) are commonly one to three doses in the US. To perform drug challenges, hospitals needed infrastructure for ordering and preparing the drug challenge, and protocols for observing patients during their challenge. Acute care settings benefit from having many of the resources required for drug challenges readily available, including nursing observation, monitoring equipment, and access to anti-allergic medications or anaphylaxis kits. Drug challenges in acute care settings were performed to penicillin or amoxicillin, with the aim of disproving the penicillin allergy in low risk patients.29 Alternatively, drug challenges were performed to the indicated therapeutic beta-lactam.30 While the former facilitated penicillin allergy de-labeling, the latter facilitated the timely, appropriate antibiotic treatment for acutely infected individuals.
There were two pathways that were exclusively direct drug challenge-based beta-lactam allergy pathways (Table 2).29,31 One proposed challenge-based pathway in the United Kingdom (UK) was an e-questionnaire that used the allergy history and host factors in a computerized decision support system that determined patient risk (high vs low). Low risk patients would then undergo a direct 250 mg amoxicillin challenge.31 A program active at two tertiary care referral centers in Australia also used allergy history-based guidance to direct oral penicillin challenges.29
Table 2.
Challenge-based beta-lactam allergy pathways in acute care
| Study | Location | Setting | Patient Selection | Study Design | Intervention Description | Outcome(s) measured | |
|---|---|---|---|---|---|---|---|
| Krishna (2017)31 | Birmingham, United Kingdom | Large teaching hospital | Adult patients that are not unconscious and without altered mental status | Development and conceptual framework | Electronic guideline including an e-questionnaire about the allergy history and host factors and answers that interfaces with computerized decision support system (“CDSS,” electronic software that guides the risk stratification into high or low risk). Low risk patients undergo a direct 250 mg amoxicillin challenge | Not applicable | |
| Trubiano (2018)29 | Melbourne, Australia | 1 referral cancer hospital and 1 academic medical center | Adult patients with penicillin allergy labels including a cohort with cancer | Pre/Post of 46 patients including 10 inpatients with cancer and 19 inpatients without cancer, all with low-risk penicillin allergy | Allergy history-based guidance for oral penicillin re-challenge program | Alternative ABX use | Restricted ABX 90 days pre/post decreased from 55% to 23% |
| BL use | Penicillin use 90 days pre/post was increased from 23% to 77% | ||||||
| Safety | There were no ADRs | ||||||
Abbreviations: ABX, antibiotic; BL, beta-lactam; ADR, adverse drug reaction
While PHS pathways used direct full dose and test dose challenges as the major component of their intervention, PST was also available at three sites, and therefore PHS pathways are considered comprehensive pathways (Table 4).6,30,32-35
Table 4.
Comprehensive beta-lactam allergy pathways in acute care
| Study | Location | Setting | Patient Selection | Study Design | Type of Intervention | Outcome(s) measured | |
|---|---|---|---|---|---|---|---|
| Blumenthal (2015)30 | Boston, MA, US | Academic medical center | All patients with penicillin or cephalosporin allergy histories needing ABX were eligible | Pre/Post (49 pre patients/183 post patients) | Comprehensive guideline (Figure 1): History, skin testing, test doses. Guideline uses challenge doses (“test doses”) some with prior PST and guides AI and ID consultations. PST by AI consultation Guideline distributed as pocket cards/posters/and accessed with hospital guidelines |
Number of allergy evaluations | Increase in number of BL test doses in postperiod (183 post-period vs 49 pre-period) |
| Alternative ABX use | Guideline-driven test doses significantly decreased vancomycin (68% vs 37%), aztreonam (12% vs <1%) aminoglycosides (6% vs 1%) and fluoroquinolones (15% vs 3%) in test dose patients | ||||||
| Safety | ADRs frequency/severity in post-period were no different from pre-period (where allergy consulted on all patients getting test doses) Pre-period 6% vs post-period 4%, p=0.44 |
||||||
| Blumenthal (2016)6 | Boston, MA, US | Academic medical center | All patients with methicillin-sensitive Staphylococcus aureus bacteremia | Pre/Post (464 patients) | Comprehensive guideline Blumenthal 201528 | ABX appropriateness | Methicillin-sensitive Staphylococcus aureus bacteremia patients reporting PCN allergy increased first-line ABX from 41% preperiod to 88% post-period (p = 0.02) |
| Blumenthal and Wickner(2017)34 | Boston, MA, US | Academic medical center | Internal medicine patients with penicillin allergy histories ordered for therapeutic ABX | Pre/Post adjusting for period differences (148 patients in pre-period and 199 patients in post-period) | Comprehensive guideline from Blumenthal 201528 supported electronically with mobile-friendly website (“app”) | BL use | Increased adjusted odds of penicillin or cephalosporin use overall in eligible patients overall 1.8 [95 % CI 1.1 to 2.9] |
| Safety | No serious ADRs; one patient developed erythema and pruritus | ||||||
| Blumenthal (2017)33 | Boston area, MA, US | Large healthcare system comprised of 2 academic and 3 community sites | All patients with penicillin or cephalosporin allergy histories on ABX were eligible | Implementation methods study | Comprehensive guideline from Blumenthal 201528 supported electronically with mobile-friendly website (“app”) and computerized support including best practice alert and order set | Cost | Total projected annual cost savings with complete implementation across 5 hospitals: $8.9 million-13.7 million |
| Lambl (2018, abstract only)35 | Salem, MA, US | Community teaching hospital | Medical or surgical patients with penicillin or cephalosporin allergy histories Excluded anaphylaxis and type II-HSR |
Retrospective cohort (336 patients who received BL test doses) | Comprehensive guideline from Blumenthal 201528 supported electronically with mobile-friendly website (“app”) and computerized support including best practice alert and order set | Alternative ABX use | Decreased aztreonam use by85% and vancomycin use by 77% |
| BL use | Penicillins increased 42% and cephalosporins increased by 79% | ||||||
| ABX appropriateness | 308/321 (96%) had narrowing of ABX | ||||||
| Safety | No major ADRs/HSRs, but 7 reactions: rash (4), throat irritation (1), urticaria (1), wheezing (1) | ||||||
| Cost | Pharmacy cost savings of $38,281 ($630 per patient) | ||||||
| Ramsey (2018)49 | Rochester , NY, US | Community hospital |
|
Prospective PST evaluation | Penicillin Allergy History Algorithm (PAHA, Figure 2) on selected patients for history taking, then AI consultation and PST based on this result | PST outcomes | 64 approached: 50 skin tested Not tested included 9 for possible SCAR and 5 declined 50 skin tested: 47 (94%) negative; 2 (4%) positive; 1 (2%) not interpretable |
| BL use | 28 (56%) of skin test negative patients transitioned to PCN ABXs, 13 (26%) to first- or second-generation cephalosporins, 12 (24%) to a third- or fourth-generation, 2 (4%) to carbapenem, and 7 (14%) received a combination of these ABXs | ||||||
| Alternative ABX use | Prior to PST, ABXs were: vancomycin 41 (82%), aztreonam 11 (22%), moxifloxacin 3 (6%), daptomycin 2 (4%), linezolid 1 (2%) 863 days of vancomycin were avoided | ||||||
| ABX appropriateness | 982 days of second-line ABX exposure averted | ||||||
| Safety | No immediate ADRs or HSRs; 1 patient delayed rash; 1 patient neutropenia | ||||||
| Cost | $70 per patient saved considering cost of ABXs | ||||||
| Sacco (2019)50 | Jacksonville, FL, US | Community teaching hospital | General internal medicine patients with reported penicillin allergy | Pre/Post (42 pre-period patients and 57 post-period patients, with 8 patients receiving BL test doses in the post period) | Educational initiative and algorithm modified from Blumenthal 201528 | Alternative ABX use | Vancomycin use decreased 14% |
| BL use | Penicillin use increased 250% and cephalosporin use increased 120% | ||||||
| Safety | One patient transient rash to ceftriaxone | ||||||
| Hospital length of stay | Unchanged | ||||||
| Allergy documentation | EHR penicillin allergy documentation improved from 5% to 65% | ||||||
| Blumenthal (2019)32 | Boston area, MA, US | Large healthcare system comprised of 2 academic and 3 community sites | All patients with penicillin or cephalosporin allergy histories on ABX were eligible | Retrospective cohort (1,046 patients who received BL test doses) | Comprehensive guideline from Blumenthal 201528 supported electronically with mobile-friendly website (“app”) and computerized support including best practice alert and order set | BL use | 1,046 BL test doses were performed: 809 (77%) to cephalosporins, 148 (14%) to penicillins, and 89 (9%) to carbapenems |
| Safety | 78 patients (7.5%; 95%CI 5.9% to 9.2%) had signs or symptoms of an ADR, with 40 (3.8%; 95% CI 2.8% to 5.2%) confirmed HSRs HSRs were rash (n=19), itching (n=6), hives (n=2), tingling (n=1), bronchospasm/wheezing (n=5), angioedema/swelling (n=4), hypotension/dizziness (n=3), anaphylaxis (n=1), severe cutaneous adverse reactions (n=1) and acute interstitial nephritis (n=1) 3 HSR patients (8%) were treated with intramuscular epinephrine for anaphylaxis |
||||||
| Staicu (2018)51 | Rochester, NY, US | Community medical center | Penicillin-allergic adult inpatients on antibiotics; patients on high risk ABX (fluoroquinolones, clindamycin, vancomycin) and those on second-line therapy were prioritized. | Prospective observational study of 338 ST eligible inpatients | Pharmacist used Penicillin Allergy History Algorithm (PAHA, Figure 2). If PST was indicated, allergy/immunology physician assistant performed testing followed by synchronous allergy/immunology telemedicine consultation | Number of allergy evaluations | Of 338 screened, 50 were tested (15%) |
| Alternative ABX use | Reduction in use of vancomycin, metronidazole, aztreonam, aminoglycosides, and clindamycin | ||||||
| BL use | Of 46 PST negative patients, 33 (72%) were transitioned to a BL | ||||||
| Safety | No adverse reactions immediately or reported through follow up phone calls | ||||||
| Cost | About $350 per patient saved considering ABX cost PST performance by physician assistant rather than allergist saved about $7,600 |
||||||
Abbreviations: ABX, ABXs; PST = Penicillin Skin Test; AI., allergy/immunology; BL, BL ABXs; ABX=ABX(s); DOT=days of therapy; ADR=adverse drug reaction, AAL=ABX allergy label, HSR= hypersensitivity reaction
PST-based
Performing PST on hospitalized patients required training of skin testers when the tester was not an allergist, allergy trainee, or other allergy practitioner. PST as part of acute care beta-lactam allergy pathways was performed by allergy-trained registered nurses,36 pharmacists,37 and infectious diseases trainees (in addition to allergists).38,39 Clear PST inclusion and exclusion criteria were needed. PST-based pathways also required compounding time for reagents when Penicillin G, minor determinants, and/or ampicillin were used, since the only commercially available reagent was major determinant, or penicilloyl-polylysine (Pre-Pen). PST-based pathways additionally required patient and provider time for the performance and interpretation of the test. With shortening length of stay a hospital priority, and competing tests and procedures planned for hospitalized patients, all hospitalized patients eligible for PST are unlikely to get PST. PST-based pathways captured up to 20% of patients with a penicillin allergy on antibiotics.34,40
PST was the primary allergy intervention in the majority of beta-lactam allergy pathway studies (Table 3) 20,34,36-48 PST patients were often selected from the inpatient population with reported beta-lactam allergy on antibiotics. This selection process was potentially done because of volume considerations. For example, we estimated that Massachusetts General Hospital (MGH) would need to perform over 60 PSTs per week to capture all inpatients with penicillin allergy histories on antibiotics. Patient selection might have also been performed to optimize skin testing resources and/or improve antibiotic stewardship outcomes (i.e., capture patients most likely to benefit).41 Patient selection, for example, relied on antibiotic data, microbiology culture-data, or specialist expertise.37,43 Few PST pathways did not select based on patient need, but evaluated PST in a specific inpatient area (intensive care unit)42,44 or specific service (internal medicine).34
Table 3.
Penicillin skin testing-based beta-lactam allergy pathways in acute care settings
| Study | Location | Setting | Patient Selection | Study Design | Intervention Description | Outcome(s) measured | |
|---|---|---|---|---|---|---|---|
| Harris (1999)41 | Boston, MA, US | Academic medical center | Penicillin allergy patients identified from pharmacy searching
|
Prospective PST study |
|
PST outcome | 100 reviewed: 56 excluded 44 skin tested: 38 (86%) negative; 3 (7%) positive; 3(7%) indeterminate |
| BL use | BL used in 36% of screened and 82% of enrolled patients Cephalosporins (15), penicillin (7), penicillin and cephalosporin (1) | ||||||
| Alternative ABX use | 46 days of vancomycin use avoided; Vancomycin (n=11), fluoroquinolone (n=12), clindamycin (n=10) were stopped | ||||||
| Cost | Cost savings of ABX regimens was $103 per patient | ||||||
| Arroliga(2000)42 | Cleveland, OH, US | Academic medical center | Intensive care unit adult patients with penicillin allergy | Prospective PST study | PST performed by AI consultation | PST outcome | 24 enrolled: 3 not tested because of IgE histories 21 skin tested: 20(95%) negative; 1 (5%) indeterminate |
| BL use | 10 of 21 (48%) were treated with a penicillin ABX | ||||||
| Alternative ABX use | Prior ABXs: Ciprofloxacin (n=5), clindamycin (n=2), imipenem (n=2), and erythromycin (n=1) | ||||||
| Safety | No BL ADRs | ||||||
| Forrest (2001)43 | Vancouver, Canada | Academic medical center | Infection with positive culture susceptible to penicillin, or cellulitis, in patients with penicillin allergy history | Pre/Post (64 pre patients/95 post patients) | Skin testing guideline PST by AI consultation Guideline disseminated as posters and pocket cards to housestaff | Number of allergy evaluations | Increase in skin testing from 17% pre to 64% post in eligible patients (p<0.001) |
| PST outcome | 61 skin tested in post-period: 58 (95%) negative; 3 (5%) positive | ||||||
| BL use | 54 of 58 (93%) skin test negative patients switched to treatment with penicillin | ||||||
| Cost | No significant difference between actual costs and projected cost of alternative ABX | ||||||
| Arroliga (2003)44 | Cleveland, OH, US | Academic medical center | Intensive care unit adult patients with penicillin allergy | Prospective PST study | PST performed by AI consultation | PST outcome | 100 enrolled: 4 not tested (serum sickness (n=2), patient refusal (n=2)) 96 skin tested: 85 (89%) negative; 1 (1%) positive; 10 (10%) indeterminate |
| BL use | 31 of 38 (82%) received a BL (38 of the 96 patients were initially receiving therapeutic ABXs) | ||||||
| Alternative ABX use | Prior ABXs: 73 patients (73%) received vancomycin, 27 patients (27%) received quinolones, 11 patients (11%) received a third-generation cephalosporin, and 6 patients (6%) received ABXs such as clindamycin or metronidazole | ||||||
| Safety | No adverse events related to either PST or BL administration | ||||||
| Macy (2004)36 | San Diego, CA, US | Community hospital | General medical and surgical patients hospitalized with a penicillin allergy history | Nested case-cohort (compares convenience sample of PST inpatients to age and sex-matched controls) | PST performed by allergy registered nurse | PST outcome | 8.7% of penicillin-allergic inpatients tested 141 skin tested: 133 (94%) negative; 8 (6%) positive |
| BL use | PST patients more frequently had more penicillins (17% vs 7%) and cephalosporins (59% vs 48%) | ||||||
| Alternative ABX use | Vancomycin use low overall and nonsignificantly lower in cases than controls (19 cases/24 courses vs 29 controls/39 courses) | ||||||
| Safety | No adverse reactions associated with skin testing ADRs: 0.7% tested vs 1.4% controls Tested patient ADR was to macrolide (not BL) |
||||||
| Wall (2004)37 | West Des Moines, IA, US | Academic medical center |
|
Prospective PST evaluation | ID physician ordered PST, which was performed by allergist-trained pharmacists. Challenge performed for indeterminate tests BL use at discretion of physician with full dose. |
PST outcome | 26 enrolled: 3 patients did not receive PST due to mild initial ADR but were switched to a penicillin or BL 23 skin tested: 22 (96%) negative; 1 (4%) indeterminate 23 of 23 not allergic after challenge |
| BL use | All 26 patients enrolled in the skin testing service received a penicillin or BL | ||||||
| Alternative ABX use | An average of 48 doses of vancomycin and 12 doses of levofloxacin per patient were avoided | ||||||
| Safety | No reactions occurred | ||||||
| Rimawi (2013)39 | Greenville, NC, US | Community teaching hospital |
|
Prospective PST evaluation | PST by ID trainee | PST outcome | 482 considered and 146 skin tested 145 (99%) negative; 1(1%) positive |
| BL use | 145 of 146 (99%) switched to BL treatment without an adverse reaction | ||||||
| Alternative ABX use | Prior to intervention: 31 patients received aztreonam, 26 received ciprofloxacin, 14 received vancomycin, 14 received clindamycin, 12 received a combination of ciprofloxacin and clindamycin, 12 received linezolid, 12 received moxifloxacin, 6 received tobramycin, 4 received doxycycline, 3 received daptomycin, 3 received trimethoprim/sulfamethoxazole, 1 received gentamycin, and 1 received a combination of ciprofloxacin and tobramycin | ||||||
| Safety | No adverse reactions to BLs | ||||||
| Cost | Cost savings of $225/patient. Projected annual savings of $82,000 | ||||||
| Ward (2015)45 | London, United Kingdom | Academic medical center | Patients with infective endocarditis and penicillin allergy history | PST outcome | 16 patients skin tested: 16 (100%) negative | ||
| BL use | 14 of 16 patients (88%) had first-line BL use: flucloxacillin (7), amoxicillin (3), benzyl penicillin (3) and ceftriaxone (1) | ||||||
| Alternative ABX use | Prior to intervention: vancomycin (13), gentamycin (5), meropenem (2), fusidic acid (1), rifampicin (1) | ||||||
| Safety | 1 patient (7%) developed BL rash | ||||||
| King (2016)46 | Summit, NJ, US | Community teaching hospital |
|
Retrospective analysis of PST patients | PST by hospital-affiliated allergist by AI consultation. If negative, multi-step graded amoxicillin challenge. | PST outcome | 50 PST: 50 (100%) negative 49 (98%) patients tolerated amoxicillin challenge |
| BL use | 37 patients (76%) switched to a BL | ||||||
| Alternative ABX use | Reduction in the number of patients on a non-BL due to historical BL allergy | ||||||
| Safety | 1 patient has anaphylactic reaction | ||||||
| Cost | Patients switched to a BL after PST led to savings of $11,005 or $297 per patient | ||||||
| Heil (2016)38 | Baltimore, MD, US | Academic medical center | Patients selected from:
|
Prospective observational study | PST by ID trainee | Number of allergy evaluations | Year prior PST service, 21 patients received PST compared to 76 PST in 11 months after established PST service |
| PST outcome | 90 assessed, 76 skin tested 64 (84% ) negative; 3(4%) positive; 9 (12%) indeterminate |
||||||
| BL use | 54 of 64 (84%) negative patients had ABX changes 55% changed to penicillin, 40% to cephalosporin, and 5% to carbapenem |
||||||
| ABX appropriateness | 63% narrower spectrum ABXs; 80% more effective therapy | ||||||
| Safety | No serious adverse effects; 3 patients had a benign delayed rash to BLs after tolerating challenge | ||||||
| Cost | 61% less costly therapy. Cost savings of $26,000 per year | ||||||
| Chen (2017)40 | Dallas, TX, US | Academic medical center | Adult inpatients with penicillin allergy screened by pharmacist with a prioritization schema. Patients excluded if on antihistamines and then prioritized by: no discharge order, on ABX, on carbapenem or monobactam, comorbidities of HIV/malignancy/diabetes mellitus | Prospective identification through Epic Hyperspace daily report of patients with active penicillin allergy | In person evaluation by pharmacist who did history-appropriate PST | PST outcome | 252 evaluated, 247 skin tested performed, 228 completed testing: 223 negative |
| BL use | 77 of the 223 patients with negative tests (34%) switched to a penicillin or cephalosporin, 8 patients (3.6%) were discharged with BL treatment | ||||||
| Alternative ABX use | After testing, decrease in active orders for vancomycin, clindamycin, fluoroquinolones, carbapenems, and aztreonam | ||||||
| Allergy documentation | 228 of 252 (91%) had their allergy removed. | ||||||
| Blumenthal and Wickner(2017)34 | Boston, MA, US | Academic medical center | In period with PST, PST offered to all adult medicine inpatients on therapeutic ABX with a penicillin allergy history | Pre/post analysis adjusted for period differences | PST by moonlighting allergy trainees | PST outcome | Of 278, 179 eligible and 43 tested Testing not completed for reasons including: coordination (58), patient refusal (28), team refusal (21), other (29)43 of 43 (100%) negative |
| BL use | Unadjusted BL use increased from 50% to 60% in skin tested patients (0.30) Adjusted inpatient BL use in skin tested patients increased 570% (adjusted OR 5.7 [95% CI 2.6 to 12.5) Adjusted discharge BL use in skin tested patients increased 250% (adjusted OR 2.5 (95% CI 1.04 to 6.2]) | ||||||
| Safety | There were no adverse events | ||||||
| Leis (2017)20 | Toronto, Canada | 3 community hospitals | In period with PST, PST offered when BL allergy history interfered with prescribing of the preferred BL. PST exclusions also included: Severe non-IgE reaction, IgE reaction in last 3 months, discharge anticipated within 24 hours, or patient declined. |
Pre/post analysis | PST offered M-F by ASP pharmacist at the instruction of an ID physician Negative tests included BL challenge with 4-hour observation. Intervention included EHR update and letter to patients. |
PST outcome | 386 patients in intervention periods: 232 (60%) received preferred BL therapy and 154 were PST eligible. Of 154 PST eligible, 64 (42%) were excluded and 90 (58%) had PST 90 PST: 85 (94%) negative; 1 (1%) positive; 4 (4%) nondiagnostic |
| BL use | 92% with negative PST were switched to BL The number of days of penicillin use tripled (11% to 32%, p<0.0002). |
||||||
| Alternative ABX use | Carbapenem and fluoroquinolone use decreased more than half (28% vs 13%, p<0.0002) | ||||||
| ABX appropriateness | Overall BL 50% pre-period vs 81% PST post-period | ||||||
| Safety | Frequency of ADRs: 4% pre-period and 3% post-period (p=0.40) | ||||||
| Jones (2017)47 | Savannah, GA, US | Community health system | Stewardship pharmacist evaluates patients for PST considering allergy history and medication exclusions | Pharmacy screened patients with self-reported PCN allergy. | PST initiative promoted by pharmacists with allergist oversight. PST performed by nurses and ASP pharmacist | PST outcome | 36 skin tested: 36 (100%) negative |
| BL use | 27 of 36 patients (75%) changed to a penicillin or cephalosporin, the most common change was a switch from carbapenem (with or without vancomycin) to a penicillin or cephalosporin (n=12). | ||||||
| Alternative ABX use | Carbapenem to BL change occurred in 12 patients | ||||||
| Safety | No reactions to BL | ||||||
| Cost | Average cost savings per patient was approximately $314, summing to $7554. | ||||||
| Chen (2018)48 | Dallas, TX, US | Academic medical center | Inpatients with penicillin allergy on aztreonam received clinical decision support tool Patients with histories of severe cutaneous adverse reactions, recent anaphylaxis within 4 weeks, and severe cardiac or pulmonary comorbidities excluded |
Pre/Post (250 pre-period and 91 pos-period) | A clinical decision support tool that encourages providers to order PST Orders reviewed by allergy-trained pharmacist who performed history-appropriate PST and oral challenge Pre-period was active surveillance |
Number of allergy evaluations | Penicillin allergy patients receiving aztreonam who received PST increased (24% to 85%, p<0.001) Patients not tested for reasons including: antihistamine use (19), discharge before evaluation (14), altered mental status (11), cardiopulmonary instability (4), provider cancellation (5), and patient refusal (3) |
| PST outcome | Of 77 consults placed, 58 were seen and 21 PST performed 21 skin tested: 21 (100%) negative |
||||||
| BL use | Penicillins increased from 0.32 to 0.71 (p=0.046) administrations per patient-day 58% increased penicillin exposure (p=.046) | ||||||
| Alternative ABX use | Aztreonam use declined 2.54 to 1.47 administrations per 1000 patient-days (p=0.016) | ||||||
| Cost | 53% savings post-period compared to pre-period ($1266 pre and $592 post) | ||||||
| Allergy documentation | 21 of 21 negative tests had allergy removed | ||||||
Abbreviations: ABX, antibiotics; PST, penicillin skin test; BL, beta-lactam; AI, allergy/immunology; ADR, adverse drug reaction; ID, infectious diseases; HSR, hypersensitivity reaction; ASP, antibiotic stewardship program
Comprehensive
Comprehensive beta-lactam allergy pathway approaches included those primarily implemented by generalists (Table 4)6,30,32-35,49-51 and those which placed the specialist at the center of the evaluations (Table 5).52-54
Table 5.
Specialist-based comprehensive BL allergy pathways in acute care
| Study | Location | Setting | Patient Selection | Study Design | Type of Intervention | Outcome(s) measured | |
|---|---|---|---|---|---|---|---|
| Banks (2015)52 and Ressner (2016)53 | Bethesda, MD, US | Military Medical Center | General medicine or intensive care unit patients with penicillin allergy | Conceptual framework | Automatic AI consultation for triage of evaluations that should be inpatient vs outpatient (Figure 3) | Not applicable | |
| Trubiano (2017)54 | Melbourne, Australia | 2 tertiary care referral centers | Referrals of patients with ABX allergies from clinical specialists and adverse drug reaction committees. All ABX allergies considered but most patients had penicillin (54%) or cephalosporin (18%) allergy | Prospective observational study of 141 patients referred and 118 patients completing testing. | Comprehensive ABX allergy assessments carried out by dedicated pharmacist and physician: ABX skin testing, patch testing, challenge doses, consultation guidance, allergy label removals | Alternative ABX use | Decrease usage of glycopeptide, carbapenem, lincosamide, and fluoroquinolone |
| BL use | Increase in penicillin (7% to 23%) and 1st/2nd generation cephalosporins (11% vs 18%) | ||||||
| ABX appropriateness | Guideline-preferred therapy increased from 12-18% to 83% | ||||||
| Allergy documentation | Revised allergy labels (94%), removed allergy labels (83%), including patients with more than 1 ABX allergy label removed (27%) | ||||||
Abbreviations: AI, allergy/immunology; ABX, antibiotics; BL, beta-lactam
The PHS pathway approach was studied in seven total articles, and was implemented by generalists, and described and evaluated in two academic and three community hospitals in the greater Boston area.6,30,32-35 The PHS approach includes penicillin and cephalosporin hypersensitivity algorithms reliant on the allergy history to guide challenge doses that are largely performed without preceding PST (Figure 1).30 The pathways direct PST when needed (i.e., patients reporting IgE-mediated allergy symptoms to a penicillin who required a penicillin or potentially cross-reactive cephalosporin), and institutionally available, but encourage full dose and test dose (i.e., standardized 2-step graded) drug challenges. 30,32-35 This approach was initially implemented at MGH; when PST was indicated based on the allergy history and desired therapeutic antibiotic, PST was performed by Allergy/Immunology consultation.6,30 This intervention was modified to include optional decision support on a mobile-friendly website (functionally, an “app”), and studied at the Brigham and Women’s Hospital (BWH).34 After pathway modification to facilitate adoption at PHS community hospitals without access to Allergy/Immunology/PST, the guideline was implemented for all inpatients and units across five hospital sites.33,35 The PHS approach has been used for teaching articles,55 implemented at non-PHS academic hospitals, such as Dartmouth-Hitchcock Medical Center (Erin L. Reigh, MD, Personal Communication, March 2, 2019), and studied at one non-PHS community hospital on the general medicine service.50
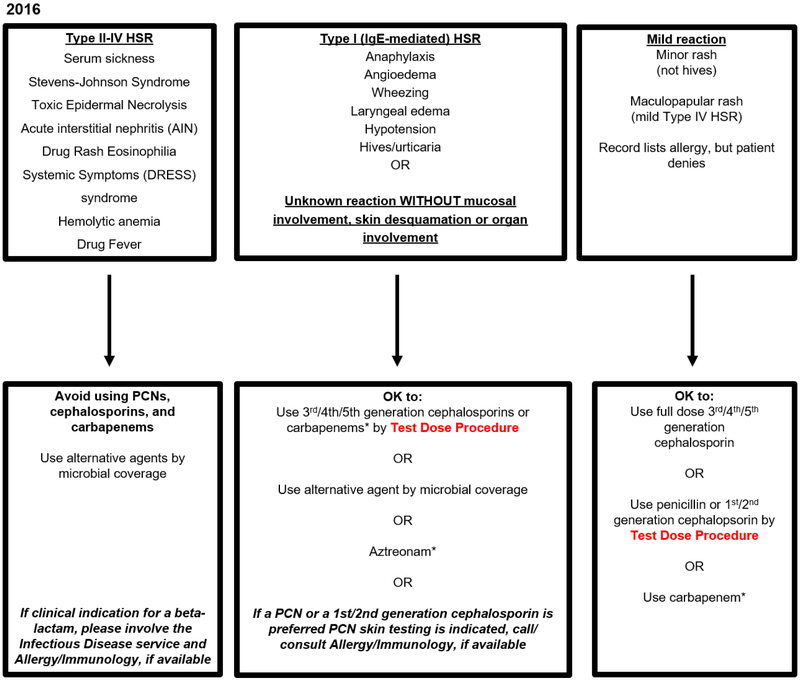
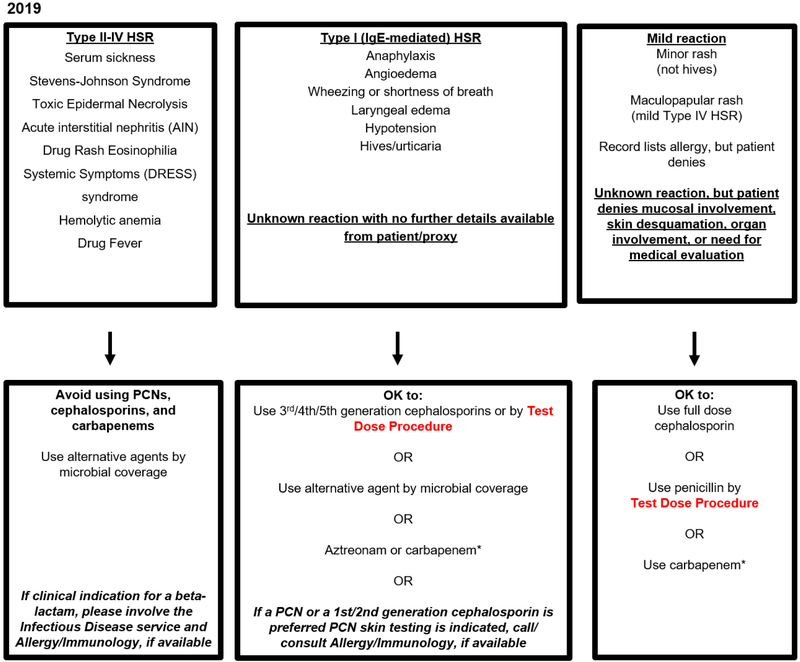
Figure 1.
Partners HealthCare System (PHS) Penicillin Hypersensitivity Pathway (Boston, MA, USA) 30,32,33-35
This penicillin allergy pathway algorithm was originally developed at MGH, modified and studied at BWH, and further adapted for use at community hospital sites of PHS including two without access to Allergy/Immunology or PST (2016, Figure 1A). The algorithm considers actions based on the generalist’s allergy history considering three groups: (1) Type II-IV HSR, (2) Type I, IgE-mediated HSR or unknown reaction, and (3) Mild reactions. The pathway applied to all adult and pediatric patients, including pregnant patients, but test doses were not considered appropriate for patients with unstable cardiac or respiratory status. After evaluating HSR frequencies, additional modifications were made to further promote antibiotic stewardship. These changes included full dose carbapenem use in Type I IgE penicillin allergy histories and full dose cephalosporin use in all mild penicillin allergy histories (2019, Figure 1B). There is a corresponding cephalosporin hypersensitivity pathway algorithm. Abbreviations: PHS, Partners HealthCare System; MGH, Massachusetts General Hospital; BWH, Brigham and Women’s Hospital; PST, Penicillin Skin Test; HSR, hypersensitivity reaction; PCN, penicillin
* Denotes antibiotics restricted by antibiotic stewardship programs at PHS sites
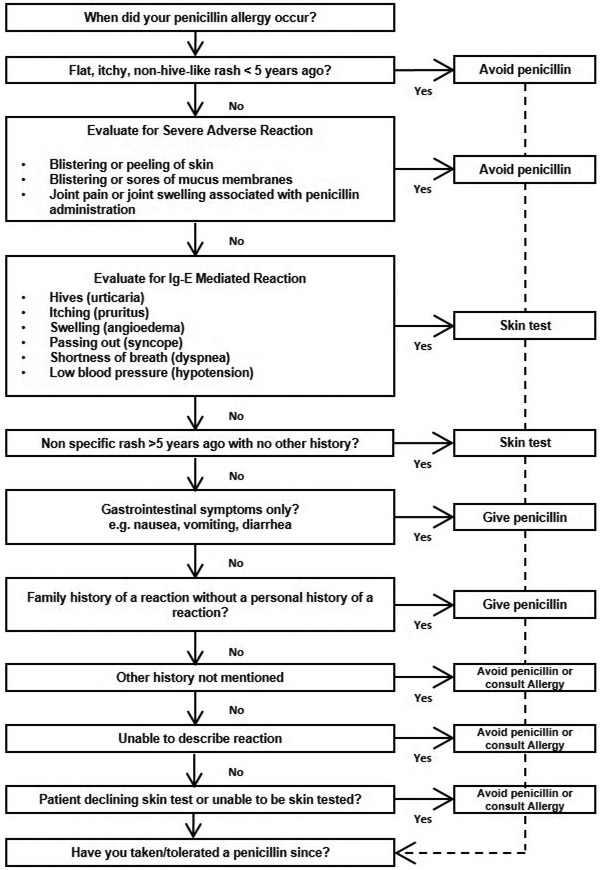
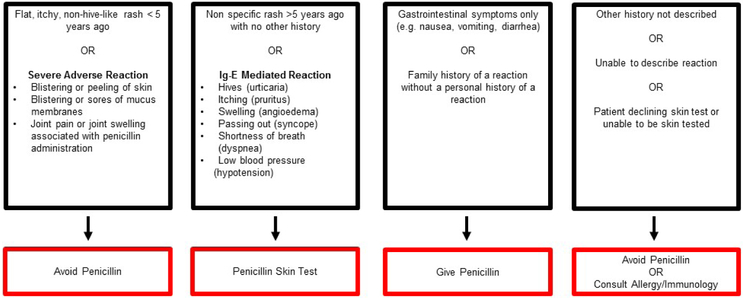
Another algorithmic approach, the penicillin allergy history algorithm (PAHA, Figure 2), was studied in two articles, and assisted in guiding generalists to avoid penicillin, use a full dose penicillin, call for PST, or call for Allergy/Immunology consultation.49 This algorithm was also used in a telemedicine consultation study where virtual visits were performed for all patients who received PST.51
Figure 2.
Penicillin Allergy History Algorithm (Rochester, NY, USA)49,51
This penicillin allergy history screening algorithm was used to screen hospitalized patients with a penicillin allergy (Figure 2A). The algorithm assesses and categorizes allergic reactions based on the Gell and Coombs classification scheme, time elapsed since the reported penicillin reaction, and whether a penicillin antibiotic had been subsequently tolerated. The algorithm did not apply to patients hospitalized in the cardiac, medical, or surgical intensive care unit, inability to provide informed consent, and pregnancy. There are structural similarities to the PHS pathways with recommendations: Avoidance, skin test, full dose penicillin, or allergy consultation (Figure 2B).
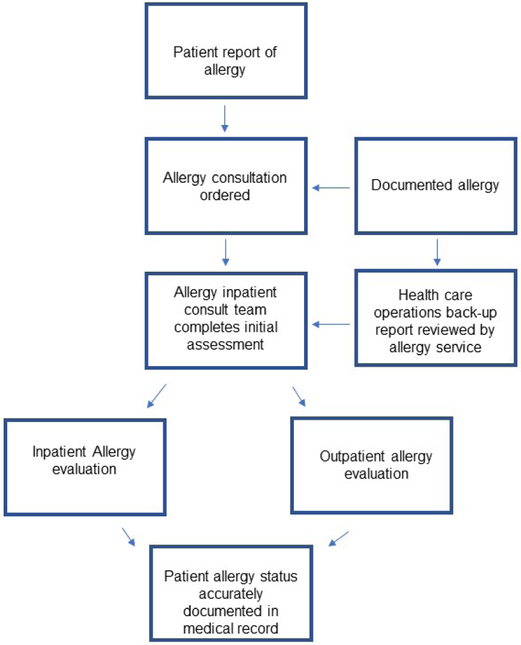
Comprehensive beta-lactam allergy pathways might alternatively consider the specialist at the center of evaluations. One hospital had Allergy/Immunology specialists triaging all inpatients with beta-lactam allergies on antibiotics (Figure 3).52 Another comprehensive pathway used a dedicated pharmacist and Infectious Diseases physician to perform comprehensive antibiotic allergy evaluations that extended to antibiotic reactions beyond beta-lactams and used both immediate and delayed hypersensitivity testing.54
Figure 3.
Proposed approach for specialist triage all inpatients with documented penicillin allergy (Bethesda, MD, US)52,53
This figure demonstrates the approach taken by a large military medical center and places the Allergy/Immunology consultants at the center of a broad penicillin allergy antibiotic stewardship initiative that identifies, assesses, and evaluates inpatients with a documented or reported allergy to penicillin or another beta-lactam.
Patient Selection
Targeting Antibiotics
Beta-lactam allergy pathways often targeted specific antibiotics or antibiotic classes important to antibiotic stewardship. The most common single antibiotic targeted for reduction was aztreonam.21,23,25,27,28,46,48 Other targeted antibiotics included carbapenems, vancomycin, linezolid, tigecycline, daptomycin, and moxifloxacin.22,30,32-35,46,49
Targeting Infections
One PST-based pathway relied on reviews of each patient’s infection and antibiotic data to select patients that would likely have a therapy change with a negative PST.37,41 One study developed antibiotic treatment guidelines along with their PST-based intervention, so that the patient note after PST contained standardized antibiotic guidance.41 Another group limited their guideline’s applicability to patients with infections that were Penicillin-sensitive or cellulitis.43 Methicillin-sensitive Staphylococcus aureus bacteremia was mentioned as a specific focus.6,51
Targeting Patients
Patients targeted for beta-lactam allergy interventions included patients with infective endocarditis45 and cancer.29 Pathways also targeted patients with planned antibiotic courses over 24 hours,41 or those without an imminent hospital discharge.40
Beta-lactam allergy pathways often included guidance for penicillin intolerances.37,49 All penicillin hypersensitivity types (I-IV) were included in the PHS guideline.30,32 PST-based pathways specified PST exclusions based on the allergy history (e.g. hemolytic anemia, toxic epidermal necrolysis, etc).34,40 Some PST pathways excluded patients with recent type I reaction or anaphylaxis history.38,48 Other PST-based pathway exclusions included cutaneous conditions, severe cardiac or pulmonary comorbidities, immunosuppression, pregnancy, altered mental status, and clinical instability.31,37,48
Implementation Teams
Implementation teams were often multidisciplinary with diverse perspectives and varied expertise. The PHS implementation team structure included a clinical champion (either an allergist, internist, pharmacist, or infectious diseases specialist) and team members, often from the site’s antibiotic stewardship program (ASP).33
Allergy and Immunology
Allergy specialists were leaders and key collaborators for many interventions.30,32,33,40,49,52 An antibiotic allergy nurse was important to the drug challenge pathway performed in Australia.29 Allergy/Immunology consultation performed PST for many pathways.30,34,42,44,46 An allergy nurse performed PST in the Kaiser Permanente study.36 The PHS pathway was led by an allergist, and both academic sites had an allergists as site clinical champions.33 Another pathway placed the Allergy/Immunology consultant at the center of the stewardship initiative.52,53
Allergist access was notably not needed for the history-based drug allergy pathways. Low-risk drug challenges without Allergy/Immunology consultation, based off of an electronic clinical decision support tool, were proposed in the UK.28 History-appropriate beta-lactam antibiotic challenges at PHS were performed largely without Allergy/Immunology specialist guidance (91%) in the largest study of beta-lactam test dose challenges performed on hospitalized patients.30,32-35
Infectious Diseases
Infectious Disease specialists were crucial to many acute care beta-lactam allergy pathways.25,37-39 Infectious Diseases specialists helped to create guidelines for antibiotic use and stewardship goals.26,41,43 Infectious Diseases trainees performed PST in two of the pathways.38,39 At PHS, there was an Infectious Diseases clinical lead for the system-wide project, and one community PHS site had an Infectious Diseases specialist as clinical champion.30,32-35 The PST-based pathway in Canada and the comprehensive antibiotic allergy pathway conducted in Australia were led by an Infectious Diseases physicians.20,54
Pharmacist
Pharmacists, particularly Infectious Diseases or ASP pharmacists, were instrumental to most beta-lactam allergy pathways. 32,33,37,38,45-47 Pharmacists designed and lead interventions.22-25,27 In the PHS pathway, a PHS community site had a pharmacist clinical champion.32 Pharmacists were also key collaborators with varied roles that included: creating patient tracking lists, intercepting antibiotic orders, educating patients/providers, taking allergy histories, and performing PST. 37,40 Pharmacy trainees and technicians were also involved. 26,28
Intervention Dissemination and Enforcement
Interventions were encouraged as formal hospital policies in many cases21,26,30,32-34 with coinciding educational programs. 21,25,26,30,32-34 One educational initiative had a mandatory exam with an 80% pass rate required.25 Posters and pocket cards were a common dissemination 25,27,30,32-34,43 Pharmacists intercepted antibiotic orders to enforce guidelines.30,32-34,51 EHR order sets were created to assist pathway implementation.25,30,32-34 Decision support tools were also described, including inserting beta-lactam cross-reactivity details into decision support22, an e-questionnaire to risk stratify patients for a penicillin challenge and PHS’ mobile-friendly website that was functionally an “app” used similarly on a phone, tablet, or desktop.31,33
BETA-LACTAM ALLERGY PATHWAYS: OUTCOMES
Acute care beta-lactam allergy pathway articles assessed an average of 3.5 (SD 1.6, range 0 to 6) outcomes. The most commonly reported outcomes were antibiotic utilization outcomes, such as use, doses, and/or days of therapy per 1000 patient days for beta-lactams and/or beta-lactam alternatives.21,25 Fewer studies assessed antibiotic appropriateness.6,49 Allergy safety outcomes, including frequency of allergy evaluations and adverse events, adverse drug reactions (ADRs) and/or hypersensitivity reactions (HSRs) were reported by most groups. PST-based pathways reported the frequency with which the test was positive, negative, and indeterminate.20,34,36-48 Other assessed outcomes included length of stay,21 mortality,29 cost,25,28,38,39,41,43,46-48,51 and allergy documentation.21,32,38,40,48,47,54
Antibiotic Use
Acute care beta-lactam allergy pathways resulted in changes to therapeutic antibiotics. The most frequently described antibiotics used before the interventions included vancomycin, fluoroquinolones, and aztreonam, and the most frequently described antibiotics used after the intervention were beta-lactams, both penicillins and cephalosporins.
History-based pathways noted decreased aztreonam days of therapy, 21,25,28 reduced time to aztreonam discontinuation,23 and lower frequency of aztreonam use (Table 1).27 The challenge-based pathway in Australia noted ASP-restricted antibiotics decreased from 55% to 23% (Table 2).29 Two PST-based pathways reported that their approach saved about 50 doses of vancomycin.39,41 Other PST-based pathways reported carbapenem and fluoroquinolone reduction (28% to 13%),20 and aztreonam reduction (2.54 to 1.47 administrations per 1000 patient-days).48
In the initial MGH pathway study, vancomycin, aztreonam, aminoglycosides and fluoroquinolones were switched to beta-lactams using test doses.30 One PHS community hospital reported an 85% reduction of aztreonam and 77% reduction in vancomycin.35 The modified PHS pathway used at Mayo Jacksonville reported a 14% decrease in vancomycin use.50 The comprehensive antibiotic allergy program in Australia decreased use of glycopeptide, carbapenem, lincosamide, and fluoroquinolones.54
The beta-lactam antibiotics replaced the beta-lactam alternative, often ASP-restricted antibiotics: 35-66% of patients on aztreonam were changed to a beta-lactam in history-based pathways,23,24 and penicillin use increased from 23% to 77% in one challenge-based pathway (Table 2).29
PST-based pathways largely reported high frequencies of subsequent beta-lactam use in patients with negative PST (Table 3): Chen40 (34%), Arroliga42 (48%), Ramsey49 (56%), Jones47 (75%), King46 (76%), Harris41 (82%), Arroliga44 (82%), Ward45 (83%), Heil38 (84%), Leis20 (92%), Forrest43 (93%), Rimawi39 (99%), Wall37 (100%). Studies with less patient selection for PST reported lower beta-lactam usages. Although some beta-lactams used after negative PST in some studies were later generation cephalosporins, broad-spectrum penicillins (e.g., piperacillin-tazobactam), and carbapenems,39,47 PST-based pathways nonetheless described penicillin use doubling (from 0.32 to 0.71 administrations per patient day)48 and tripling (from 11% to 32%).20 More modest changes were observed in the observational studies that used multivariable adjustment or matching. PST on internal medicine inpatients at BWH increased beta-lactam use from 50% to 60%; in the adjusted analysis compared to historical controls, PST patients had a 570% increased odds of beta-lactam use (adjusted OR 5.7 [95% CI 2.6 to 12.5]).34 Kaiser reported more penicillin (17% vs 7%) and cephalosporin (59% vs 48%) exposure for PST patients compared to matched controls.36
Implementation of the PHS pathway on internal medicine increased odds of receiving a penicillin or cephalosporin about 2-fold for internal medicine inpatients with a penicillin allergy history on antibiotics overall (adjusted OR 1.8 [95% CI 1.1 to 2.9]).34 A PHS community hospital reported a 42% increase in penicillins and 79% increase in cephalosporins.35 PHS test doses were performed most often to cephalosporins (77%); penicillin test doses comprised 14% and carbapenem test doses comprised 9%.32 Using a modified PHS pathway, Sacco et al. reported that penicillin use increased 250% and cephalosporin use increased 120%.50
Safety
Any medication use carries a risk of ADR or HSR. The risk is generally considered higher among inpatients because of patient factors (e.g., illness, comorbidities) and drug factors (e.g., duration, route). Despite this, beta-lactam pathways were safe, describing few reactions overall and expected reaction rates. Reported reactions were not significantly different in pathways that compared ADRs or HSRs to a control group: Phan (0.0% post-period vs 4.3% pre-period), Leis (3.0% post-period vs 4.0% pre-period), Blumenthal (4% post-period vs 6% pre-period), and Macy (0.7% PST-group vs 1.4% controls).20,27,30,36
Most pathways reported no or few ADRs or HSRs.22-25,27-29,34,36,39,42,44,47,51 The most commonly reported reaction was rash, largely those that were transient, delayed, and/or benign.25,34,35,38,45,49,52 Not surprisingly given the overall patient numbers, severe reactions also occurred. Severe IgE-mediated reactions were described by King et al., who reported an anaphylactic reaction to an amoxicillin challenge that occurred after negative PST.46 Of HSRs resulting from the PHS pathway (n=40), 3 patients (8% of HSR patients) were treated with epinephrine.32 Severe delayed reactions were also described to beta-lactams after PHS test doses: two patients developed severe cutaneous adverse reactions and one patient developed acute interstitial nephritis.32 However, considering over 1,000 beta-lactam test doses performed across five acute care PHS sites, just 40 patients (3.8%) had HSRs confirmed by allergy specialist review.32
The HSR frequencies from the PHS pathway multi-site safety analysis provided insight into acute care beta-lactam cross-reactivity in patients with defined penicillin allergy histories, including patients with severe IgE-mediated histories. These data indicated that the later generation cephalosporins (3rd/4th/5th) overall had a low 2.6% HSR rate in patients with IgE-mediated or unknown penicillin allergy histories (see algorithm middle box), but with the cefepime HSR rate higher (4.4% [95%CI 2.1% to 8.0%]),32 the multidisciplinary PHS team opted to continue to initiate these cephalosporins with a test dose. However, of 56 carbapenem test doses administered to patients with IgE penicillin allergy histories (including almost half with severe IgE histories), there were no HSRs. This motivated a structural modification to the penicillin hypersensitivity pathway to indicate that carbapenems be administered by a full dose (Figure 1B). For mild penicillin allergy histories (see algorithm right-most box), full dose challenges for 1st/2nd generation cephalosporins were also a safe modification given an observed low (2-4%) HSRs for those patients (Figure 1B).32
Antibiotic Appropriateness
Antibiotic appropriateness was not a routinely considered outcome. However, King, et al. reported more effective therapy prescribed to patients who had PST, and the PHS pathway at MGH was associated with an increased use of first-line therapy for patients with MSSA bacteremia (41% to 88%, p=0.02).6,46 Trubiano et al. reported that guideline-preferred therapy increased from 12%-18% to 83%.54
Costs
While costs were included as outcomes for many pathway analyses, to date, no comprehensive cost assessments for acute care beta-lactam pathways have been performed. A comprehensive cost analysis must consider all costs related to the patient’s infection treatment, including drug costs, drug monitoring costs (e.g., vancomycin trough), hospital length of stay, discharge location (e.g., rehabilitation center) as well as that associated length of stay, treatment failures and their associated healthcare visits and/or readmissions, and ADRs/HSRs resulting from use of beta-lactam or beta-lactam alternatives.56,57 The start-up and maintenance costs related to the pathway implementation would need to be considered. For PST-based pathways, the cost of PST materials and personnel would be considered.58 For the PHS pathways, the cost would include those of involved personnel, such as the clinical champions and the computer programmers who created, tested, and disseminated the “app,” alerts, and order set.
Many pathway studies limited their cost analyses to drug costs or projected cost savings. However, cost data are nonetheless encouraging that pathways might be cost-saving: Estep et al. reported an annual acquisition cost savings of $28,134,23 Clark et al reported a cost savings of $12,88928, and Staicu et al. reported estimated annual direct cost avoidance from $60,000 to $100,00.25 Assessed cost savings per patient considering drug costs were modest: Ramsey49 ($70), Harris41 ($103), Rimawi39 ($225), King46 ($297), Jones47 ($315), Staicu ($350)51, Lambl35 ($630), and Chen48 ($674). However, the savings per-patient must be considered given the scale of patients impacted, which is large. For example, the PHS pathway implementation paper estimated system-wide cost savings with full implementation would be from 8.9 to 13.7 million dollars in the first year.33
Allergy Documentation
Allergy documentation is a crucial part of acute care beta-lactam allergy interventions. Without clear documentation reflecting allergy status changes, pathways will have limited effectiveness. Swearingen et al. reported that 84% of charts were updated.21 Although the PST-based initiative in Texas reported initially reported a similar value (91%), this improved to 100% in their subsequent study.40,48 Implementation of the modified PHS guideline in Florida was associated with improved overall penicillin allergy documentation (5% to 65%).50 However, at PHS where generalists order test doses largely without allergist involvement, allergy records were updated for only 474 (45%) patients receiving beta-lactam test doses.32 In order to improve allergy documentation, PHS implemented a targeted EHR alert to the team that ordered the beta-lactam test doses. The specialist team performing comprehensive antibiotic allergy assessments not surprisingly achieved great documentation outcomes, with 94% of the allergy labels were revised and 83% of labels were removed.54
CONCLUSIONS
We identified and reviewed various acute care beta-lactam allergy interventions that included pathways exclusively reliant on the allergy history, and those that used the allergy history to guide allergy procedures including drug challenges, PST, or both. Interventions used different personnel (e.g., pharmacists, allergists) and protocols (history tools, PST inclusion/exclusion criteria, test dose protocols). In PST evaluations, patients were selected based on factors beyond allergy history, often targeting specific patients or infections to appropriately shepherd limited resources and improve outcomes. The PHS beta-lactam allergy pathways also targeted PST for use when indicated and institutionally available, but commonly used non-specialist directed test dose and full dose challenges. Interventions described traditional methods for education and dissemination, such didactics, testing, pocket cards, posters, and guidelines. Some pathways described electronic support and EHR integration.
Beta-lactam allergy pathways provided safe frameworks to challenge penicillins or indicated beta-lactams to immediately improve antibiotic choice in acutely ill patients with penicillin allergy histories. All pathways reported improvements in antibiotic stewardship outcomes with appropriate safety profiles. Beta-lactam pathways in acute care are likely to improve the quality of allergy documentation, and be cost saving, depending on the details of the intervention, patient selection, time horizon, and perspective for cost determination. Although no systematic review nor meta-analysis was performed, we reviewed a variety of approaches trialed by groups throughout the US and internationally, and consider that adoption of any previously vetted beta-lactam allergy pathway might meaningfully facilitate antibiotic stewardship, and improve quality of care for inpatients with penicillin allergy histories.
LEARNING OBJECTIVES.
At the conclusion of this activity, participants should be able to:
Identify the benefits of inpatient beta-lactam allergy assessments
Describe different approaches to beta-lactam allergy pathways including the methods used to identify patients
QUESTIONS.
Q1. Which of the following would be an effective acute care beta-lactam allergy pathway?
Inpatients with no history of drug allergy with unknown need for antibiotics are penicillin skin tested.
Inpatients with no history of drug allergy who need a beta-lactam are penicillin skin tested.
An Allergy/Immunology consultation is called for an inpatient with multiple antibiotic allergies
Patients with any reported history of beta-lactam allergy with clear indication for beta-lactam are penicillin skin tested.
-
A decision-support tool is embedded into the electronic health record to help with antibiotic prescribing.
Q1 Answer: D, Patients with any reported history of beta-lactam allergy with clear indication for beta-lactam are given penicillin skin testing.
Rationale: An acute care beta-lactam allergy pathway is defined as a coordinated inpatient program for beta-lactam allergy assessments as a tool of antibiotic stewardship. (D.) describes an acute care beta-lactam allergy pathway that used the penicillin skin test as the intervention. Both (A.) and (B.) describe allergy interventions in patients without a drug allergy history. (C.) describes an intervention at a patient level, not a coordinated system-level intervention. (E.) describes a system-level intervention that applies to patients with and without drug allergies.
References:
Blumenthal KG, Shenoy ES, Wolfson AR, Berkowitz DN, Carballo VA, Balekian DS, et al. Addressing Inpatient Beta-Lactam Allergies: A Multihospital Implementation. J Allergy Clin Immunol Pract. 2017;5(3):616-25 e7.
Chiriac AM, Banerji A, Gruchalla RS, Thong BYH, Wickner P, Mertes PM, et al. Controversies in Drug Allergy: Drug Allergy Pathways. J Allergy Clin Immunol Pract. 2019;7(1):46-60 e4.
Q2. Which of the following groups was a target of an acute care beta-lactam allergy pathway?
Patients with cancer.
Pediatric inpatients.
Cardiology inpatients.
Surgery outpatients.
-
Pediatric outpatients.
Q2 answer: A, Patients with cancer.
Rationale: Patients might have been targeted based on their hospital location (e.g., intensive care unit), active infection (e.g., Methicillin-sensitive Staphylococcus aureus bacteremia), or disease status (e.g. cancer). Patients with cancer (A.) were a specific target of an oral challenge program in Australia because of their high prevalence of antibiotic allergy and common need for antibiotics because of infections that result from being immunocompromised. No acute care beta-lactam allergy pathway covered in this review described an intervention in pediatric inpatients (B.) or cardiology inpatients (C.). The review focused on inpatient assessments, so (D.) and (E.) do not apply.
References:
Trubiano JA, Smibert O, Douglas A, et al. The Safety and Efficacy of an Oral Penicillin Challenge Program in Cancer Patients: A Multicenter Pilot Study. Open Forum Infect Dis. 2018;5(12):ofy306.
Huang KG, Cluzet V, Hamilton K, Fadugba O. The impact of reported beta-lactam allergy in hospitalized patients with hematologic malignancies requiring antibiotics. Clin Infect Dis 2018: In press. doi: 10.1093/cid/ciy037
Q3. PST-based pathways reported subsequent use of a beta-lactam in patients with negative testing at least which percent of the time:
5%
10%
15%
25%
-
35%
Q3 answer: E, 35%.
Rationale: The range in this Review article was from 35-100% (Table 3). While most inpatients with a reported penicillin allergy are not allergic and would ultimately benefit from evaluation and delabeling, it is important to realize than not all hospitalized patients with a beta-lactam allergy and an infection require a beta-lactam antibiotic. Additionally, the indicated beta-lactams are cephalosporins for common infections such as pneumonia, urinary tract infection, and sepsis. Given the time and resources needed to accomplish inpatient penicillin allergy skin testing, patient selection for need may be advisable. Structures to ensure that patients receive indicated cephalosporins should also be considered.
References:
Sacco KA, Bates A, Brigham TJ, Imam JS, Burton MC. Clinical outcomes following inpatient penicillin allergy testing: A systematic review and meta-analysis. Allergy. 2017;72(9):1288-96.
Chen JR, Tarver SA, Alvarez KS, Tran T, Khan DA. A Proactive Approach to Penicillin Allergy Testing in Hospitalized Patients. J Allergy Clin Immunol Pract. 2017;5(3):686-93.
Q4. Which of the following were instrumental to the success of most acute care beta-lactam allergy pathways?
Hospitalist
Information technology experts
Pharmacists
Hospital leadership
-
Quality improvement specialists
Q4 Answer: C, Pharmacists
Rationale: Pharmacists, particularly Infectious Diseases or ASP pharmacists, were instrumental to most beta-lactam allergy pathways. Pharmacists designed and lead interventions and were key collaborators with varied roles that ranged from creating patient tracking lists to educating patients/providers to performing skin testing. Fewer interventions relied on hospitalists (A.), hospital leadership (D.), or quality improvement specialists (E.). Although electronic health record support was instrumental to implementation success, some pathways did not have electronic support (B.)
References:
Ramsey A, Staicu ML. Use of a Penicillin Allergy Screening Algorithm and Penicillin Skin Testing for Transitioning Hospitalized Patients to First-Line Antibiotic Therapy. J Allergy Clin Immunol Pract. 2018;6(4):1349-55.
Blumenthal KG, Li Y, Hsu JT, Wolfson AR, Berkowitz DN, Carballo VA, et al. Outcomes from an inpatient beta-lactam allergy guideline across a large US health system. Infect Control Hosp Epidemiol. 2019:1-8.
Q5. Which of the following accounts for the variation in cost-savings reported by acute care beta-lactam allergy pathway studies?
Personnel costs.
Drug costs.
Readmission rates.
Program start-up costs.
-
Hospital length of stay.
Q5 answer: B, Drug costs.
Rationale: To date, no comprehensive cost assessments for acute care beta-lactam pathways have been performed. A comprehensive cost analysis would must consider personnel costs (A.), drug costs (B.), readmission costs (C.), program start-up costs (D.) and hospital length of stay (E.), but assessments have focused on drug costs to date (B.) Antibiotic costs are different for different institutions and so, it was not surprising that different groups reported different drug cost types (drug acquisition costs, average wholesale prices).
References:
Li Y, Minhas JS, Blumenthal KG. Economic Impact of Drug Allergy. In: Khan DA, Banerji A, eds. Drug Allergy Testing. St. Louis, MO: Elsevier; 2018.
Blumenthal KG, Li Y, Banerji A, Yun BJ, Long AA, Walensky RP. The Cost of Penicillin Allergy Evaluation. J Allergy Clin Immunol Pract. 2018;6(3):1019-27 e2.
Acknowledgements
The authors wish to thank Yu Li, MS and Christian Mancini for their research assistance.
Funding Source: This work was supported by NIH K01AI125631, the American Academy of Allergy Asthma and Immunology Foundation, and the MGH Claflin Distinguished Scholars Award. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Abbreviations:
- MSSA
methicillin-sensitive Staphylococcus aureus bacteremia
- US
United States
- PST
penicillin skin test
- PHS
Partners HealthCare System
- EHR
electronic health record
- UK
United Kingdom
- MGH
Massachusetts General Hospital
- ASP
Antibiotic Stewardship Program
- ADR
adverse drug reaction
- HSR
hypersensitivity reaction
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest: Dr. Blumenthal reports a licensed clinical decision support tool used for beta-lactam allergy evaluations in hospitalized patients at Partners HealthCare System.
Clinical Trail Registration: Not applicable.
REFERENCES
- 1.Zhou L, Dhopeshwarkar N, Blumenthal KG, et al. Drug allergies documented in electronic health records of a large healthcare system. Allergy. 2016;71(9):1305–1313. [DOI] [PubMed] [Google Scholar]
- 2.Picard M, Begin P, Bouchard H, et al. Treatment of patients with a history of penicillin allergy in a large tertirary care academic hospital. J Allergy Clin Immunol Pract. 2013;1(3):252–257. [DOI] [PubMed] [Google Scholar]
- 3.Lee CE, Zembower TR, Fotis MA, et al. The incidence of antimicrobial allergies in hospitalized patients: implication regarding prescribing patterns and emerging bacterial resistance. Arch Intern Med. 2000;160:2819–2822. [DOI] [PubMed] [Google Scholar]
- 4.Sacco KA, Bates A, Brigham TJ, Imam JS, Burton MC. Clinical outcomes following inpatient penicillin allergy testing: A systematic review and meta-analysis. Allergy. 2017;72(9):1288–96. [DOI] [PubMed] [Google Scholar]
- 5.Blumenthal KG, Ryan EE, Li Y, Lee H, Kuhlen JL, Shenoy ES. The impact of a reported penicillin allergy on surgical site infection risk. Clin Infect Dis. 2018;66(3):329–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Blumenthal KG, Shenoy ES, Huang M, et al. The impact of reporting a prior penicillin allergy on the treatment of methicillin-sensitive Staphylococcus aureus bacteremia. PLoS One. 2016;11(7):e0159406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Blumenthal KG, Lu N, Zhang Y, Li Y, Walensky RP, Choi HK. Risk of meticillin resistant Staphylococcus aureus and Clostridium difficile in patients with a documented penicillin allergy: population based matched cohort study. BMJ. 2018;361:k2400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.MacFadden DR, LaDelfa A, Leen J, et al. Impact of Reported Beta-Lactam Allergy on Inpatient Outcomes: A Multicenter Prospective Cohort Study. Clin Infect Dis. 2016;63(7):904–910. [DOI] [PubMed] [Google Scholar]
- 9.Blumenthal KG, Parker RA, Shenoy ES, Walensky RP. Improving clinical outcomes in patients with methicillin-sensitive Staphylococcus aureus bacteremia and reported penicillin allergy. Clin Infect Dis. 2015;61(5):741–749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mattingly TJ 2nd, Meninger S, Heil EL. Penicillin skin testing in methicillin-sensitive staphylococcus aureus bacteremia: A cost-effectiveness analysis. PLoS One. 2019;14(1):e0210271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jeffres MN, Narayanan PP, Shuster JE, Schramm GE. Consequences of avoiding beta-lactams in patients with beta-lactam allergies. J Allergy Clin Immunol. 2016;137(4):1148–1153. [DOI] [PubMed] [Google Scholar]
- 12.Hogan M, Bridgeman MB, Min GH, Dixit D, Bridgeman PJ, Narayanan N. Effectiveness of empiric aztreonam compared to other beta-lactams for treatment of Pseudomonas aeruginosa infections. Infect Drug Resist. 2018;11:1975–1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Micromedex. Greenwood Village, CO: Truven Health Analytics LLC; 2016. [Google Scholar]
- 14.Mattingly TJ 2nd, Fulton A, Lumish RA, et al. The Cost of Self-Reported Penicillin Allergy: A Systematic Review. J Allergy Clin Immunol Pract. 2018;6(5):1649–1654 e1644. [DOI] [PubMed] [Google Scholar]
- 15.Bates DW. Drugs and adverse drug reactions: how worried should we be? JAMA. 1998;279(15): 1216–1217. [DOI] [PubMed] [Google Scholar]
- 16.Bates DW, Leape LL, Cullen DJ, et al. Effect of computerized physician order entry and a team intervention on prevention of serious medication errors. JAMA. 1998;280(15):1311–1316. [DOI] [PubMed] [Google Scholar]
- 17.Topaz M, Seger DL, Slight SP, et al. Rising drug allergy alert overrides in electronic health records: an observational retrospective study of a decade of experience. J Am Med Inform Assoc. 2016;23(3):601–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Barlam TF, Cosgrove SE, Abbo LM, et al. Executive Summary: Implementing an Antibiotic Stewardship Program: Guidelines by the Infectious Diseases Society of America and the Society for Healthcare Epidemiology of America. Clin Infect Dis. 2016;62(10):1197–1202. [DOI] [PubMed] [Google Scholar]
- 19.Shenoy ES, Macy E, Rowe T, Blumenthal KG. Evaluation and Management of Penicillin Allergy: A Review. JAMA. 2019;321(2):188–199. [DOI] [PubMed] [Google Scholar]
- 20.Leis JA, Palmay L, Ho G, et al. Point-of-Care beta-Lactam Allergy Skin Testing by Antimicrobial Stewardship Programs: A Pragmatic Multicenter Prospective Evaluation. Clin Infect Dis. 2017;65(7):1059–65. [DOI] [PubMed] [Google Scholar]
- 21.Swearingen SM, White C, Weidert S, Hinds M, Narro JP, Guarascio AJ. A multidimensional antimicrobial stewardship intervention targeting aztreonam use in patients with a reported penicillin allergy. Int J Clin Pharm. 2016;38(2):213–217. [DOI] [PubMed] [Google Scholar]
- 22.Caplinger C, Smith G, Remington R, Madaras-Kelly K. Evaluation of a Computerized Decision Support Intervention to Decrease Use of Anti-Pseudomonal Carbapenems in Penicillin Allergic Patients. Antibiotics (Basel, Switzerland). 2016;5(1). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Estep PM, Ferreira JA, Dupree LH, Aldridge PJ, Jankowski CA. Impact of an antimicrobial stewardship initiative to evaluate beta-lactam allergy in patients ordered aztreonam. Am J Health Syst Pharm. 2016;73(5 Suppl 1):S8–13. [DOI] [PubMed] [Google Scholar]
- 24.Sigona NS, Steele JM, Miller CD. Impact of a pharmacist-driven beta-lactam allergy interview on inpatient antimicrobial therapy: A pilot project. J Am Pharm Assoc (2003). 2016;56(6):665–669. [DOI] [PubMed] [Google Scholar]
- 25.Staicu ML, Brundige ML, Ramsey A, et al. Implementation of a penicillin allergy screening tool to optimize aztreonam use. Am J Health Syst Pharm. 2016;73(5):298–306. [DOI] [PubMed] [Google Scholar]
- 26.Krey SC, Waise J, Skrupky LP. Confronting the Challenge of Beta-Lactam Allergies: A Quasi-Experimental Study Assessing Impact of Pharmacy-Led Interventions. J Pharm Pract. 2017:897190017743154. [DOI] [PubMed] [Google Scholar]
- 27.Phan A, Allen B, Epps K, Alikhil M, Kamataris K, Tucker C. Initiative to reduce aztreonam use in patients with self-reported penicillin allergy: Effects on clinical outcomes and antibiotic prescribing patterns. Am J Health Syst Pharm. 2018;75(17 Supplement 3):S58–S62. [DOI] [PubMed] [Google Scholar]
- 28.Clark KE, Briand ME, Kapoor O, Pirasteh A. Impact of a Standardized Beta-Lactam Allergy Questionnaire on Aztreonam Use. J Pharm Pract. 2018:897190018758557. [DOI] [PubMed] [Google Scholar]
- 29.Trubiano JA, Smibert O, Douglas A, et al. The Safety and Efficacy of an Oral Penicillin Challenge Program in Cancer Patients: A Multicenter Pilot Study. Open Forum Infect Dis. 2018;5(12):ofy306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Blumenthal KG, Shenoy ES, Varughese CA, Hurwitz S, Hooper DC, Banerji A. Impact of a clinical guideline for prescribing antibiotics to inpatients reporting penicillin or cephalosporin allergy. Ann Allergy Asthma Immunol. 2015;115(4):294–300 e292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Krishna MT, Huissoon AP, Li M, et al. Enhancing antibiotic stewardship by tackling "spurious" penicillin allergy. Clin Exp Allergy. 2017;47(11):1362–1373. [DOI] [PubMed] [Google Scholar]
- 32.Blumenthal KG, Li Y, Hsu JT, et al. Outcomes from an inpatient beta-lactam allergy guideline across a large US health system. Infect Control Hosp Epidemiol. 2019, In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Blumenthal KG, Shenoy ES, Wolfson AR, et al. Addressing inpatient beta-lactam allergies: A multihospital implementation. J Allergy Clin Immunol Pract. 2017;5(3):616–625 e617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Blumenthal KG, Wickner PG, Hurwitz S, et al. Tackling inpatient penicillin allergies: Assessing tools for antimicrobial stewardship. J Allergy Clin Immunol. 2017;140(1):154–161 e156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lambl BB, Reyes-Dassum S, Oommen VV, et al. Abstract 1960. Antibiotic Challenge Dose Testing Improves Patient Care and Lowers Costs in a Community Hospital: A 2-Year Prospective Study. Open Forum Infect Dis 2018;5(suppl_1):S568. [Google Scholar]
- 36.Macy E, Roppe LB, Schatz M. Routine Penicillin Skin Testing in Hospitalized Patients with a History of Penicillin Allergy. Perm J. 2004;8(3):20–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wall GC, Peters L, Leaders CB, Wille JA. Pharmacist-managed service providing penicillin allergy skin tests. Am J Health Syst Pharm. 2004;61(12): 1271–1275. [DOI] [PubMed] [Google Scholar]
- 38.Heil EL, Bork JT, Schmalzle SA, et al. Implementation of an Infectious Disease Fellow-Managed Penicillin Allergy Skin Testing Service. Open Forum Infect Dis. 2016;3(3):ofw155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rimawi RH, Cook PP, Gooch M, et al. The impact of penicillin skin testing on clinical practice and antimicrobial stewardship. J Hosp Med. 2013;8(6):341–345. [DOI] [PubMed] [Google Scholar]
- 40.Chen JR, Tarver SA, Alvarez KS, Tran T, Khan DA. A Proactive Approach to Penicillin Allergy Testing in Hospitalized Patients. J Allergy Clin Immunol Pract. 2017;5(3):686–693. [DOI] [PubMed] [Google Scholar]
- 41.Harris AD, Sauberman L, Kabbash L, Greineder DK, Samore MH. Penicillin skin testing: a way to optimize antibiotic utilization. Am J Med. 1999;107(2): 166–168. [DOI] [PubMed] [Google Scholar]
- 42.Arroliga ME, Wagner W, B MB, Hoffman-Hogg L, Gordon SM, Arroliga AC. A pilot study of penicillin skin testing in patients with a history of penicillin allergy admitted to the medical ICU. Chest. 2000;118:1106–1108. [DOI] [PubMed] [Google Scholar]
- 43.Forrest DM, Schellenberg RR, Thien VV, King S, Anis AH, Dodek PM. Introduction of a practice guideline for penicillin skin testing improves the appropriateness of antibiotic therapy. Clin Infect Dis. 2001;32(12):1685–1690. [DOI] [PubMed] [Google Scholar]
- 44.Arroliga ME, Radojicic C, Gordon SM, et al. A prospective observational study of the effect of penicillin skin testing on antibiotic use in the intensive care unit. Infect Control Hosp Epidemiol. 2003;24(5):347–350. [DOI] [PubMed] [Google Scholar]
- 45.Ward C, Kasternow B, Haque R, Klein J. Inpatient allergy testing in patients with infective endocarditis: an un-met need? J Infect. 2015;70(2):206–207. [DOI] [PubMed] [Google Scholar]
- 46.King EA, Challa S, Curtin P, Bielory L. Penicillin skin testing in hospitalized patients with beta-lactam allergies: Effect on antibiotic selection and cost. Ann Allergy Asthma Immunol. 2016;117(1):67–71. [DOI] [PubMed] [Google Scholar]
- 47.Jones BM, Bland CM. Penicillin skin testing as an antimicrobial stewardship initiative. Am J Health Syst Pharm. 2017;74(4):232–237. [DOI] [PubMed] [Google Scholar]
- 48.Chen JR, Tarver SA, Alvarez KS, Wei W, Khan DA. Improving Aztreonam Stewardship and Cost Through a Penicillin Allergy Testing Clinical Guideline. Open Forum Infect Dis. 2018;5(6):ofy106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ramsey A, Staicu ML. Use of a Penicillin Allergy Screening Algorithm and Penicillin Skin Testing for Transitioning Hospitalized Patients to First-Line Antibiotic Therapy. J Allergy Clin Immunol Pract. 2018;6(4):1349–1355. [DOI] [PubMed] [Google Scholar]
- 50.Sacco KA, Cochran BP, Epps K, Parkulo M, Gonzalez-Estrada A. Inpatient beta-lactam test-dose protocol and antimicrobial stewardship in patients with a history of penicillin allergy. Ann Allergy Asthma Immunol. 2019;122(2):184–188. [DOI] [PubMed] [Google Scholar]
- 51.Staicu ML, Holly AM, Conn KM, Ramsey A. The Use of Telemedicine for Penicillin Allergy Skin Testing. J Allergy Clin Immunol Pract. 2018;6(6):2033–2040. [DOI] [PubMed] [Google Scholar]
- 52.Banks TA, Ressner RA, Gada SM. Antibiotic reclamation: penicillin allergy, antibiotic stewardship, and the allergist. Ann Allergy Asthma Immunol. 2015;115(5):451–452. [DOI] [PubMed] [Google Scholar]
- 53.Ressner RA, Gada SM, Banks TA. Antimicrobial Stewardship and the Allergist: Reclaiming our Antibiotic Armamentarium. Clin Infect Dis. 2016;62(3):400–401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Trubiano JA, Thursky KA, Stewardson AJ, et al. Impact of an Integrated Antibiotic Allergy Testing Program on Antimicrobial Stewardship: A Multicenter Evaluation. Clin Infect Dis. 2017;65(1):166–174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Vaisman A, McCready J, Powis J. Clarifying a "Penicillin" Allergy: A Teachable Moment. JAMA Intern Med. 2017;177(2):269–270. [DOI] [PubMed] [Google Scholar]
- 56.Li Y, Minhas JS, Blumenthal KG. Economic Impact of Drug Allergy In: Khan DA, Banerji A, eds. Drug Allergy Testing. St. Louis, MO: Elsevier; 2018. [Google Scholar]
- 57.Scott RD. The Direct Medical costs of Healthcare-Associated Infections in U.S. Hospitals and the Benefits of Prevention. March 2009. Available at: https://www.cdc.gov/hai/pdfs/hai/scottcostpaper.pdf Accessed March 2, 2019.
- 58.Blumenthal KG, Li Y, Banerji A, Yun B, Long AA, Walensky RP. The cost of penicillin allergy evaluation. J Allergy Clin Immunol Pract. 2018;6(3):1019–27 e2. [DOI] [PMC free article] [PubMed] [Google Scholar]