Abstract
BACKGROUND
CD19-specific chimeric antigen receptor (CAR) T cells induce high rates of initial response among patients with relapsed B-cell acute lymphoblastic leukemia (ALL) and long-term remissions in a subgroup of patients.
METHODS
We conducted a phase 1 trial involving adults with relapsed B-cell ALL who received an infusion of autologous T cells expressing the 19–28z CAR at the Memorial Sloan Kettering Cancer Center (MSKCC). Safety and long-term outcomes were assessed, as were their associations with demographic, clinical, and disease characteristics.
RESULTS
A total of 53 adults received 19–28z CAR T cells that were manufactured at MSKCC. After infusion, severe cytokine release syndrome occurred in 14 of 53 patients (26%; 95% confidence interval [CI], 15 to 40); 1 patient died. Complete remission was observed in 83% of the patients. At a median follow-up of 29 months (range, 1 to 65), the median event-free survival was 6.1 months (95% CI, 5.0 to 11.5), and the median overall survival was 12.9 months (95% CI, 8.7 to 23.4). Patients with a low disease burden (<5% bone marrow blasts) before treatment had markedly enhanced remission duration and survival, with a median event-free survival of 10.6 months (95% CI, 5.9 to not reached) and a median overall survival of 20.1 months (95% CI, 8.7 to not reached). Patients with a higher burden of disease (≥5% bone marrow blasts or extramedullary disease) had a greater incidence of the cytokine release syndrome and neurotoxic events and shorter long-term survival than did patients with a low disease burden.
CONCLUSIONS
In the entire cohort, the median overall survival was 12.9 months. Among patients with a low disease burden, the median overall survival was 20.1 months and was accompanied by a markedly lower incidence of the cytokine release syndrome and neurotoxic events after 19–28z CAR T-cell infusion than was observed among patients with a higher disease burden. (Funded by the Commonwealth Foundation for Cancer Research and others; ClinicalTrials.gov number, NCT01044069.)
ADULTS WITH RELAPSED ACUTE LYMPHO-blastic leukemia (ALL) have a dismal prognosis. Standard chemotherapy regimens can induce a complete remission in 18 to 45% of patients, with a median overall survival of 3 to 9 months.1–5 Blinatumomab and inotuzumab ozogamicin have resulted in higher response rates and longer survival than conventional chemotherapy, but overall survival remains poor (median, 7.7 months).6,7
A new cellular immunotherapeutic approach involves the genetic modification of T cells to express a chimeric antigen receptor (CAR) specific for CD19.8 Multiple clinical trials of CD19-specific CAR T cells have shown complete remission rates of 70 to 90% among children and adults with relapsed B-cell ALL.9–16 However, most of the clinical trial results that have been published to date have focused primarily on initial clinical outcomes with relatively short-term follow-up, precluding the analysis of characteristics of the patients that may be predictive of long-term remission. Although relapse rates of 21 to 45% have already been reported in these trials despite limited follow-up,10–14,16 a subpopulation of patients in all the published trials appears to have had long-term sustained remissions. The clinical factors that are associated with these durable responses have not been identified.
We report the analysis of toxic effects and long-term follow-up results from a phase 1 clinical trial involving adult patients with relapsed B-cell ALL who were treated with CD19-specific CAR T cells. We focused on the incidence of the cytokine release syndrome and neurotoxic effects as safety outcomes and on the complete remission rate and overall survival and event-free survival as efficacy outcomes. We hypothesized that the safety and long-term efficacy of 19–28z CAR T cells may be associated with clinical characteristics of the patients, disease characteristics, the treatment regimen, and the kinetics of T-cell expansion.
METHODS
TRIAL DESIGN AND OVERSIGHT
We conducted a phase 1 clinical trial of 19–28z CAR T cells (i.e., T cells expressing a chimeric receptor composed of an anti-CD19 antibody binding site and intracellular domains from the T-cell coactivating receptors, CD28 and the CD3-zeta chain) in adult patients with CD19+ B-cell ALL at the Memorial Sloan Kettering Cancer Center (MSKCC). All the enrolled patients had relapsed or refractory disease in response to their previous treatment. The primary objective of the study was to assess the safety of 19–28z CAR T cells, and the secondary objective was to assess the efficacy.
The study protocol (available with the full text of this article at NEJM.org) was approved by the human studies review board at MSKCC and was granted breakthrough status by the Food and Drug Administration in 2014. The clinical investigation was conducted according to the principles of the Declaration of Helsinki. The study was designed by four of the authors, and the data were collected and analyzed by five of the authors, who vouch for the accuracy and completeness of the data and analyses and for the adherence of the study to the protocol. No one who is not an author contributed to the writing of the manuscript.
The study included three stages, which were designed to evaluate the safety and efficacy of two different doses of CAR T cells and conditioning chemotherapy regimens (Fig. S1 in the Supplementary Appendix, available at NEJM.org). After leukapheresis, patients received interim therapy at the discretion of the treating physician. All the patients underwent bone marrow evaluations after interim therapy and immediately before T-cell infusion. Additional details regarding the study design are provided in the Supplementary Appendix.
ASSESSMENT OF TOXIC EFFECTS
The cytokine release syndrome was graded according to the MSKCC cytokine release syndrome grading system (Table S1 in the Supplementary Appendix). The cytokine release syndrome was considered to be severe if it was of grade 3 or higher.
Neurotoxic effects were assessed according to the National Cancer Institute Common Terminology Criteria for Adverse Events, version 4.03. Severe neurotoxic effects were defined as a seizure of any grade or a toxic effect of grade 3 or higher.
ASSESSMENT OF RESPONSE
Complete remission was defined as less than 5% bone marrow blasts, the absence of circulating blasts, and no extramedullary sites of disease (as assessed by means of computed tomography or positron-emission tomography), regardless of cell-count recovery. A negative status for minimal residual disease was defined as less than 0.01% bone marrow blasts, as assessed by means of multiparameter flow cytometry.17,18 Relapsed disease was defined as the reappearance of blasts in blood or bone marrow or in an extramedullary site after a complete remission.
19–28Z CAR T-CELL MANUFACTURING AND EXPANSION ASSESSMENT
Details of the MSKCC manufacturing process with Cell Therapy Systems Dynabeads CD3/CD28 have been described previously.9,10,15,19 The transduced T-cell analysis and assays are described in the Supplementary Appendix.
STATISTICAL ANALYSIS
Exact methods (Clopper-Pearson 95% confidence intervals and Fisher’s exact tests) were used for categorical variables. Comparisons of continuous variables were made with the use of the Wil-coxon rank-sum test. Forest plots include confidence intervals both for individual groups and for the difference between the groups and the reference group. The time of the first CAR T-cell infusion was used as the origin in all the time-to-event analyses. The analysis of overall survival used death as the event, and the analysis of event-free survival used the earliest of no response, relapse, or death as the event. Patients who did not have an event had their data censored for the analyses at the date at which they were last known to be alive. The probabilities of overall survival and event-free survival were estimated by means of the Kaplan-Meier method and were compared with the use of the log-rank test. Multivariate analysis for toxic effects was performed with the use of logistic regression. All the reported P values are two-sided. Analyses were performed with the use of R software, version 3.3.1 (R Foundation for Statistical Computing).
RESULTS
PATIENTS
From February 2010 through June 2016, we enrolled 83 adult patients with relapsed or refractory B-cell ALL. A total of 53 patients were treated with 19–28z CAR T cells and included in the study analysis, and 30 patients did not receive treatment during the study for the reasons listed in Figure S2 in the Supplementary Appendix. A total of 78 patients underwent leukapheresis, 11 of whom did not undergo an attempt at cell production (owing to death or the receipt of alternative treatment), and 13 did not have cells infused (2 because of production failure and 11 owing to infection, alternative treatment, or death). The median absolute lymphocyte count at the time of leukapheresis was 0.8×l03 per cubic millimeter (range, 0.1 to 6.6). The protocol-specified CAR T-cell dose was successfully produced for 65 of 67 patients (success rate, 97%).
All 53 patients who received therapy had been heavily pretreated (Table 1). A total of 36 patients (68%) received CAR T-cell therapy as a third or later salvage treatment, 12 (23%) had primary refractory disease, 19 (36%) had undergone allogeneic hematopoietic stem-cell transplantation previously, and 13 (25%) had received blinatumomab previously. A total of 16 patients (30%) had Philadelphia chromosome (Ph)–positive ALL, including 5 patients with the T315I ABL kinase mutation. The median number of previous tyrosine kinase inhibitors that had been received was 2.5 (range, 1 to 4), and 10 of the 16 patients (62%) had disease that was refractory to ponatinib.
Table 1.
Characteristics of the 53 Patients at Baseline.*
| Characteristic | Value |
|---|---|
| Age | |
| Median (range) — yr | 44 (23–74) |
| Distribution — no. (%) | |
| 18–30 yr | 14 (26) |
| 31–60 yr | 31 (58) |
| >60 yr | 8 (15) |
| No. of previous therapies — no. (%) | |
| 2 | 21 (40) |
| 3 | 13 (25) |
| ≥4 | 19 (36) |
| Primary refractory disease — no. (%) | |
| Yes | 12 (23) |
| No | 41 (77) |
| Previous allogeneic HSCT — no. (%) | |
| Yes | 19 (36) |
| No | 34 (64) |
| Previous treatment with blinatumomab — no. (%) | |
| Yes | 13 (25) |
| No | 40 (75) |
| Pretreatment disease burden† | |
| Median bone marrow blasts (range) — % | 63 (5–97) |
| Bone marrow blasts — no. (%) | |
| ≥5% | 27 (51) |
| <5% with extramedullary disease | 5 (9) |
| ≥0.01% and <5% | 15 (28) |
| <0.01% | 6 (11) |
| Philadelphia chromosome-positive — no. (%) | |
| Yes | 16 (30) |
| No | 37 (70) |
Percentages may not total 100 because of rounding. HSCT denotes hematopoietic stem-cell transplantation.
The value for the median bone marrow blasts was assessed in patients who had bone marrow blasts of 5% or more. A high disease burden was defined as 5% or more bone marrow blasts or extramedullary disease, and a low disease burden as less than 5% bone marrow blasts. Minimal residual disease was assessed by means of multiparameter flow cytometry,17,18 and negative status with regard to minimal residual disease was defined as less than 0.01% bone marrow blasts.
The disease burden varied among patients at the time of T-cell infusion. A total of 32 patients (60%) had a high disease burden, with either 5% or more bone marrow blasts (27 patients) or extramedullary disease (5 patients). A total of 15 patients (28%) had minimal residual disease with bone marrow blasts in the range of 0.01% to less than 5%, and 6 (11%) had a negative status with respect to minimal residual disease (<0.01% bone marrow blasts) (Table 1).
RESPONSE RATES
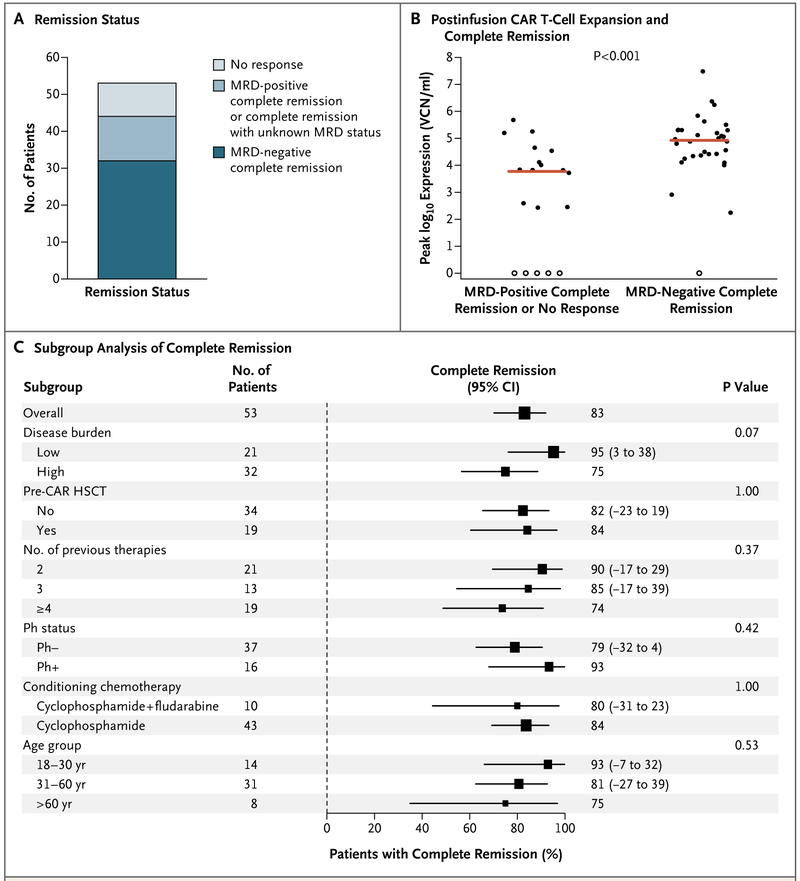
A total of 52 of 53 patients survived for 21 days or more and were evaluated for response. One patient who died from multiorgan failure and severe cytokine release syndrome on day 5 of treatment was considered not to have had a response. Of the 53 patients, 44 (83%; 95% confidence interval [CI], 70 to 92) had a complete remission. A total of 48 patients had sufficient bone marrow samples for the assessment of minimal residual disease, of whom 32 (67%; 95% CI, 52 to 80) had a minimal residual disease–negative complete remission (Fig. 1A).
Figure 1. Response to 19–28z T Cells.
Panel A shows the rate of minimal residual disease (MRD)–positive or MRD-negative complete remission and no response. Patients for whom MRD status was unknown were included with those who had MRD-positive complete remission. Panel B shows the correlation between chimeric antigen receptor (CAR) T-cell expansion after infusion and the occurrence of complete remission (P<0.00l). The red line indicates the median. Solid circles indicate patients with CAR T-cell proliferation, and open circles patients with no CAR T-cell proliferation. VCN denotes vector copy number. Panel C shows the rate of complete remission according to demographic and clinical characteristics of the patients and characteristics of the disease at baseline. Squares represent the observed proportions, and the lines extending from the squares are the 95% confidence intervals for these proportions. The confidence intervals that are reported as numbers to the right of the plot are 95% confidence intervals for the difference of proportions from the reference category. Reference categories are identified by the absence of 95% confidence intervals. HSCT denotes hematopoietic stem-cell transplantation, and Ph Philadelphia chromosome.
The rate of complete remission did not differ significantly in analyses performed according to previous transplantation, number of previous therapies, conditioning chemotherapy (cyclophosphamide vs. cyclophosphamide plus fludarabine), age of the patient, and CAR T-cell dose (Fig. 1C, and Fig. S3 in the Supplementary Appendix). Confidence intervals for the differential response rates suggest an association with disease burden and with Philadelphia chromosome status, although these results did not reach statistical significance (Fig. 1C). Among the 16 patients who had bone marrow blasts of 50% or more, 11 had a complete remission. A status of complete remission with negative minimal residual disease was significantly associated with a higher peak CAR T-cell expansion (P<0.001 for the comparison with no response or minimal residual disease-positive remission) (Fig. 1B). This association remained significant when the analysis was repeated with the exclusion of patients with no T-cell proliferation (data not shown).
TOXIC EFFECTS
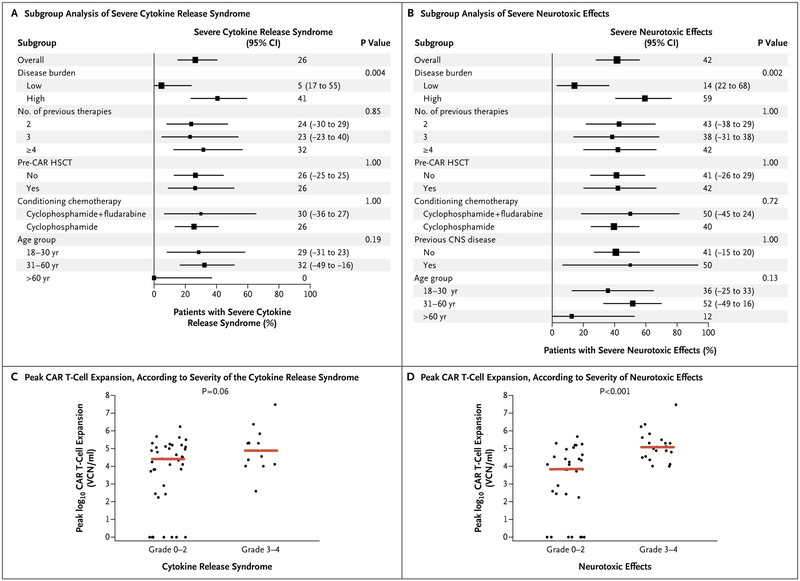
The two most common treatment-related adverse events that were observed were the cytokine release syndrome and neurotoxic effects. The cytokine release syndrome manifested as fever, tachycardia, hypotension, respiratory distress, or hypoxemia. Neurologic adverse events included confusion, disorientation, aphasia, encephalopathy, and seizure. Overall, the cytokine release syndrome of any grade occurred in 45 of 53 patients (85%; 95% CI, 72 to 93) and was severe (grade ≥3) in 14 (26%; 95% CI, 15 to 40). One patient died from severe cytokine release syndrome and multiorgan failure on day 5 of treatment in this trial before the implementation of dose modification according to the pretreatment disease burden (stage 1 in Fig. S1 in the Supplementary Appendix). The cytokine release syndrome was managed according to our management guideline (Fig. S4 in the Supplementary Appendix). A total of 22 patients received supportive care alone, 6 received the anti-interleukin-6 receptor monoclonal antibody tocilizumab alone, 13 received tocilizumab plus a glucocorticoid, and 4 received glucocorticoids alone. Grade 2 neurotoxic effects were observed in 1 patient (2%), grade 3 in 19 (36%), and grade 4 in 3 (6%). No case of grade 5 neurotoxic effect or cerebral edema was observed.
Although the number of previous therapies, previous transplantation, previous central nervous system disease, and conditioning chemotherapy regimen did not correlate with toxic effects, our analyses suggested a possible association with the age of the patient, although these results did not reach significance (Fig. 2A and 2B). However, as compared with a lower disease burden (<5% bone marrow blasts), a higher disease burden (≥5% bone marrow blasts or extramedullary disease) was associated with a higher risk of severe cytokine release syndrome (P = 0.004) and neurotoxic effects (P = 0.002) (Fig. 2A and 2B). Severe cytokine release syndrome occurred in 41% (95% CI, 25 to 61) of the patients with a high disease burden, as compared with 5% (95% CI, 0 to 25) of those with a low disease burden. Neurotoxic effects occurred in 59% of the patients with a high disease burden (95% CI, 39 to 75), as compared with 14% (95% CI, 3 to 38) of those with a low disease burden.
Figure 2 (facing page). Cytokine Release Syndrome and Neurotoxic Effects after Infusion of 19–28z T Cells.
Panels A and B show the rate of severe cytokine release syndrome and neurotoxic effects according to demographic and clinical characteristics of the patients and characteristics of the disease at baseline. A high disease burden was defined as 5% or more bone marrow blasts or extramedullary disease, and a low disease burden as less than 5% bone marrow blasts. P values were not adjusted for multiple comparisons. Squares represent the observed proportions and the lines extending from the squares are the 95% confidence intervals for these proportions. The confidence intervals reported as numbers to the right of the plot are 95% confidence intervals for the difference of proportions from the reference category. Reference categories are identified by the absence of the 95% confidence intervals. The peak CAR T-cell expansion after infusion significantly correlated with the incidence of neurotoxic effects (P<0.001) (Panel D) but not with severe cytokine release syndrome (P = 0.06) (Panel C). The red line indicates the median. CNS denotes central nervous system.
We also found that the peak CAR T-cell expansion values were significantly higher in patients with neurotoxic effects of grade 3 or 4 than in those with toxic effects of grade 2 or lower (P<0.001); there was no significant difference with regard to the severity grades of the cytokine release syndrome (P=0.06) (Fig. 2C and 2D). We explored whether the correlation between peak CAR T-cell expansion and severe neurotoxic effects could be explained by pretreatment disease burden in a multivariate logistic-regression model with disease burden and peak CAR T-cell expansion as covariates. Both factors retained their significance (P = 0.01 for both comparisons), which suggests that they are independent predictors of severe neurotoxic effects.
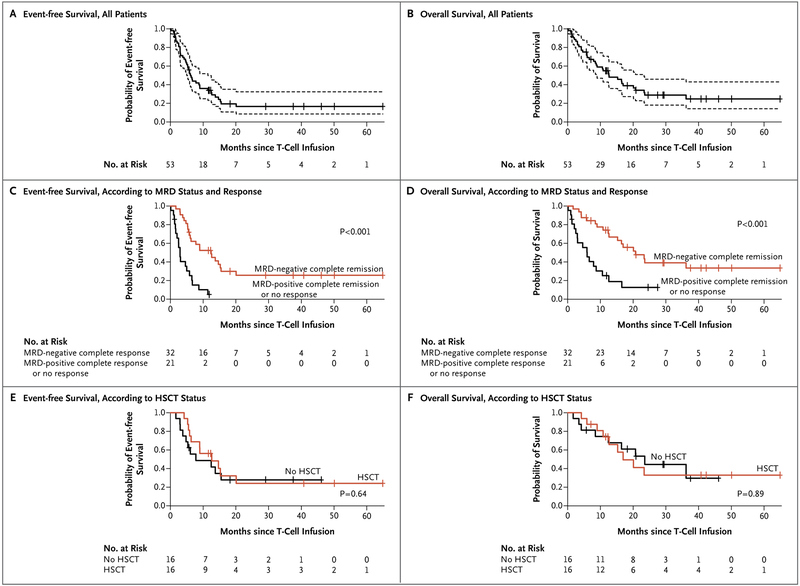
LONG-TERM SURVIVAL
At a median follow-up of 29 months (range, 1 to 65), the median event-free survival among the 53 treated patients was 6.1 months (95% CI, 5.0 to 11.5) and the median overall survival was 12.9 months (95% CI, 8.7 to 23.4) (Fig. 3A and 3B). Event-free and overall survival were significantly longer among the 32 patients who had a minimal residual disease-negative complete remission than among the 21 patients with either minimal residual disease-positive complete remission or no response after the infusion of 19–28z CAR T cells (Fig. 3C and 3D). All 9 patients who had a minimal residual disease-positive complete remission after CAR T-cell therapy had a relapse with CD19+ blasts, and 16 of the 32 patients who had a minimal residual disease-negative complete remission had a relapse, including 6 patients who had a relapse after transplantation and 4 who had a relapse with loss of CD19 detection.
Figure 3 (facing page). Event-free Survival and Overall Survival.
Panels A and B show event-free survival and overall survival, respectively, among all the patients. The median event-free survival was 6.1 months (95% CI, 5.0 to 11.5), and the median overall survival was 12.9 months (95% CI, 8.7 to 23.4). Tick marks indicate censored data, and dashed lines 95% confidence intervals. Panels C and D show event-free survival and overall survival, respectively, according to status with regard to minimal residual disease (MRD) and response. Among patients who had an MRD-negative complete remission after the infusion of 19–28z T cells, the median event-free survival was 12.5 months (95% CI, 6.3 to 20.1) and the median overall survival was 20.7 months (95% CI, 15.3 to not reached). Among patients who had an MRD-positive complete remission or no response, the median event-free survival was 3.1 months (95% CI, 2.5 to 6.7) and the median overall survival was 6.6 months (95% CI, 3.0 to not reached). Panels E and F show event-free survival and overall survival, respectively, among patients who had an MRD-negative complete remission after the infusion of 19–28z CAR T cells according to whether the patient subsequently underwent allogeneic HSCT or did not.
Of the 44 patients who had a complete remission after the infusion of CAR T cells, 26 (59%) were observed with no further therapy, including 9 who were alive and 17 who had a relapse or died; 1 patient (2%) received alternative treatment for minimal residual disease-positive disease; and 17 patients (39%) proceeded to trans-plantation. The median time from the CAR T-cell infusion to transplantation was 74 days (range, 44 to 312). Of the 17 patients who underwent allogeneic transplantation after the CAR T-cell infusion, 5 patients were alive and had a complete remission, 6 had a relapse, and 6 died from transplant-related toxic effects. Among the 32 patients who had a minimal residual disease-negative complete remission, we found no significant difference in event-free and overall survival between patients who underwent transplantation and those who did not (P = 0.64 for event-free survival and P = 0.89 for overall survival by the log-rank test) (Fig. 3E and 3F).
CORRELATION BETWEEN DISEASE BURDEN AND LONG-TERM SURVIVAL
The CAR T-cell dose, Ph-positive status, and conditioning chemotherapy regimen were not associated with overall survival (P=0.65, P=0.95, and P=0.13, respectively) (Fig. S5A in the Supplementary Appendix). The median duration of CAR T-cell detection — a surrogate measure for T-cell persistence — was 14 days (range, 7 to 138), and we found that neither the duration of CAR T-cell detection nor the absolute magnitude of peak CAR T-cell expansion was associated with longer survival (P = 0.28 and P=0.21, respectively) (Fig. S5B and S5C in the Supplementary Appendix).
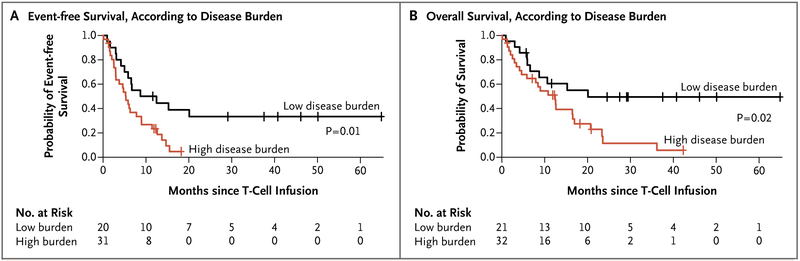
We observed that despite an overall lower degree of CAR T-cell expansion, patients with a low disease burden had significantly longer event-free survival and overall survival than did patients with a high disease burden (Fig. 4A and 4B). The median event-free survival among patients with a low disease burden was 10.6 months (95% CI, 5.9 to not reached), as compared with 5.3 months (95% CI, 3.0 to 9.0) among patients with a high disease burden (P = 0.01). The median overall survival among patients with a low disease burden was 20.1 months (95% CI, 8.7 to not reached), as compared with 12.4 months (95% CI, 5.9 to 20.7) among patients with a high disease burden (P = 0.02). The significant difference in overall survival according to disease burden remained unaffected even when allogeneic transplantation after CAR T-cell infusion was included in the analysis (Fig. S5D in the Supplementary Appendix).
Figure 4. Event-free Survival and Overall Survival, According to Pretreatment Disease Burden.
Patients with a low disease burden (<5% bone marrow blasts) at the time of T-cell infusion had significantly longer event-free survival (Panel A) and overall survival (Panel B) than did those with a high disease burden (≥5% bone marrow blasts or extramedullary disease). The median event-free survival among patients with a low disease burden was 10.6 months (95% CI, 5.9 to not reached), as compared with 5.3 months (95% CI, 3.0 to 9.0) among patients with a high disease burden (P = 0.01). The median overall survival among patients with a low disease burden was 20.1 months (95% CI, 8.7 to not reached), as compared with 12.4 months (95% CI, 5.9 to 20.7) among those with a high disease burden (P = 0.02).
DISCUSSION
In this phase 1 clinical trial involving adult patients with relapsed B-cell ALL who were treated with 19–28z CAR T cells, we analyzed data from 53 treated patients with a median follow-up of 29 months (range, 1 to 65). This approach allowed for the assessment of long-term outcomes relevant to understanding the nature and durability of response to CAR T cells in patients with ALL.
We found the in vivo peak CAR T-cell expansion to be the best predictor of short-term response and toxic effects. In contrast, for the analysis of long-term outcomes, we found that the pretreatment disease burden was a useful predictor of remission duration and survival. Patients with a low disease burden had a significantly longer event-free survival and overall survival than did those with a high disease burden. Despite previous reports from other studies of CAR T-cell persistence correlating with remissions,11,16 we did not find a significant correlation between the persistence of CAR T cells and survival in all the subgroups of patients, which indicates that 19–28z CAR T-cell persistence is not requisite for durable remissions. Our findings corroborate those from preclinical studies that showed the high effector function and self-limited expansion of CD28-based CARs,20,21 which allow for rapid tumor elimination and explain the lack of correlation with T-cell persistence beyond the induction of complete remission. Finally, we found that disease characteristics, number of previous therapies, conditioning chemotherapy regimen, CAR T-cell expansion, and hematopoietic stem-cell transplantation during remission after the infusion of CAR T cells were not predictive of long-term survival. The absence of these associations may be investigated in larger cohorts, since our study was not powered to detect moderate effects.
Our analyses show that, whereas robust CAR T-cell expansion rapidly leads to high rates of complete remission, it may not always be sufficient to prevent relapse, especially when the tumor burden is high. When we examined the ratio of peak CAR T-cell expansion to baseline tumor burden with regard to survival, we found that a higher ratio of peak CAR T-cell expansion to tumor burden significantly correlated with event-free survival and overall survival and was a better predictor of long-term survival than was the absolute magnitude of T-cell expansion or disease burden (Fig. S6 in the Supplementary Appendix). This observation raises the hypothesis that an effective ratio of CAR T cells to target CD19+ leukemia cells is more likely to occur in patients with a low disease burden than in those with a high disease burden, despite a smaller number of expanded T cells in patients with a low disease burden. This in turn may explain how patients with a low disease burden can have a long-term survival benefit without having high-grade short-term toxic effects and also explains why high peak proliferation alone does not correlate with long-term outcomes. This model is consistent with a recent study that showed that the degree of T-cell reinvigoration relative to pretreatment disease burden correlated with clinical response in patients with melanoma who were treated with the programmed death 1 (PD-1) antibody pembrolizumab.22
The median overall survival of 12.9 months in this study compares favorably with the median overall survival of 7.7 months among adult patients with relapsed ALL who were treated with blinatumomab.7 The present trial was a phase 1 single-institution study that included patients with more heavily pretreated disease (68% of the patients in this study received protocol treatment as a third or later salvage therapy vs. 24% of the patients in the blinatumomab study). Furthermore, patients with Ph-positive ALL and relapsed disease with less than 5% bone marrow blasts were included in our trial but were excluded from the blinatumomab study. Although CD19 CAR T cells and blinatumomab target the same antigen, blinatumomab activity is constrained by the number and function of endogenous T cells, in contrast to CAR T cells, which constitutively express a CD19-specific receptor and undergo prompt and robust in vivo expansion. This may explain the rapid tumor eradication15 and the higher rate of complete remission among patients with 50% or more bone marrow blasts in response to 19–28z CAR therapy in this study (69%) than among those in the blinatumomab study (34%).7 Of the 13 patients in our study whose disease was refractory to or progressed after blinatumomab treatment, 9 (69%) had a complete remission after receipt of 19–28z CAR T cells. Owing to the differences in trial design, patient populations, and post-treatment consolidation, the long-term efficacy of CD19 CAR T cells versus blinatumomab in patients with a high or low burden of ALL cannot be compared across trials.23,24 However, the observation of patients with durable remissions in these two studies highlights the potential of CD19-targeted immunotherapies.
In conclusion, long-term follow-up of our patients confirmed a potent antitumor efficacy of 19–28z CAR T cells that induced a high rate of minimal residual disease-negative complete remission among heavily pretreated adults with relapsed or refractory B-cell ALL, regardless of disease burden. With a median follow-up of 29 months, we found that 19–28z CAR T-cell therapy had favorable long-term remission rates in a population of patients with a low disease burden, who had significantly longer event-free survival and overall survival with a markedly lower incidence of toxic effects than did those with a high disease burden.
Supplementary Material
Acknowledgments
Supported by Mr. William H. Goodwin and Mrs. Alice Goodwin and the Commonwealth Foundation for Cancer Research, the Carson Family Charitable Trust, the Center for Experimental Therapeutics at Memorial Sloan Kettering Cancer Center, the Emerald Foundation, the Annual Terry Fox Run for Cancer Research organized by the Canada Club of New York, Kate’s Team, the William Lawrence and Blanche Hughes Foundation, the Lake Road Foundation, and Juno Therapeutics and by a support grant (P30 CA008748) from the Memorial Sloan Kettering Cancer Center. Dr. Park is supported by grants from the American Society of Clinical Oncology, the Leukemia and Lymphoma Society, and the National Comprehensive Cancer Network.
Disclosure forms provided by the authors are available with the full text of this article at NEJM.org.
We thank the patients who participated in this trial and their families, the staff of the Cell Therapy and Cell Engineering Facility, and Elizabeth Halton, Claudia Diamonte, and Yvette Bernal of the Cellular Therapeutics Center at the Memorial Sloan Kettering Cancer Center for help with clinical trial management.
REFERENCES
- 1.Tavernier E, Boiron JM, Huguet F, et al. Outcome of treatment after first relapse in adults with acute lymphoblastic leukemia initially treated by the LALA-94 trial. Leukemia 2007;21:1907–14. [DOI] [PubMed] [Google Scholar]
- 2.Fielding AK, Richards SM, Chopra R, et al. Outcome of 609 adults after relapse of acute lymphoblastic leukemia (ALL); an MRC UKALL12/ECOG 2993 study. Blood 2007;109:944–50. [DOI] [PubMed] [Google Scholar]
- 3.Gökbuget N, Stanze D, Beck J, et al. Outcome of relapsed adult lymphoblastic leukemia depends on response to salvage chemotherapy, prognostic factors, and performance of stem cell transplantation. Blood 2012;120:2032–41. [DOI] [PubMed] [Google Scholar]
- 4.Kantarjian HM, Thomas D, Ravandi F, et al. Defining the course and prognosis of adults with acute lymphocytic leukemia in first salvage after induction failure or short first remission duration. Cancer 2010;116:5568–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.O’Brien S, Thomas D, Ravandi F, et al. Outcome of adults with acute lymphocytic leukemia after second salvage therapy. Cancer 2008;113:3186–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kantarjian HM, DeAngelo DJ, Stelljes M, et al. Inotuzumab ozogamicin versus standard therapy for acute lymphoblastic leukemia. N Engl J Med 2016;375:740–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kantarjian H, Stein A, Gökbuget N, et al. Blinatumomab versus chemotherapy for advanced acute lymphoblastic leukemia. N Engl J Med 2017;376:836–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Brentjens RJ, Latouche JB, Santos E, et al. Eradication of systemic B-cell tumors by genetically targeted human T lymphocytes co-stimulated by CD80 and interleukin-15. Nat Med 2003;9:279–86. [DOI] [PubMed] [Google Scholar]
- 9.Brentjens RJ, Rivière I, Park JH, et al. Safety and persistence of adoptively transferred autologous CD19-targeted T cells in patients with relapsed or chemotherapy refractory B-cell leukemias. Blood 2011; 118:4817–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Davila ML, Riviere I, Wang X, et al. Efficacy and toxicity management of 19–28z CART cell therapy in B cell acute lymphoblastic leukemia. Sci Transi Med 2014; 6:224ra25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Maude SL, Frey N, Shaw PA, et al. Chimeric antigen receptor T cells for sustained remissions in leukemia. N Engl J Med 2014;371:1507–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lee DW, Kochenderfer JN, Stetler-Stevenson M, et al. T cells expressing CD19 chimeric antigen receptors for acute lymphoblastic leukaemia in children and young adults: a phase 1 dose-escalation trial. Lancet 2015;385:517–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gardner R, Wu D, Cherian S, et al. Acquisition of a CD19-negative myeloid phenotype allows immune escape of MLL-rearranged B-ALL from CD19 CAR-T-cell therapy. Blood 2016;127:2406–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Park JH, Geyer MB, Brentjens RJ. CD19-targeted CAR T-cell therapeutics for hematologic malignancies: interpreting clinical outcomes to date. Blood 2016; 127:3312–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Brentjens RJ, Davila ML, Riviere I, et al. CD19-targeted T cells rapidly induce molecular remissions in adults with chemo-therapy-refractory acute lymphoblastic leukemia. Sci Transl Med 2013;5:177ra38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Turtle CJ, Hanafi LA, Berger C, et al. CD 19 CAR-T cells of defined CD4+:CD8+ composition in adult B cell ALL patients. J Clin Invest 2016;126:2123–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Theunissen P, Mejstrikova E, Sedek L, et al. Standardized flow cytometry for highly sensitive MRD measurements in B-cell acute lymphoblastic leukemia. Blood 2017; 129:347–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.van Dongen JJ, van der Velden VH, Brüggemann M, Orfao A. Minimal residual disease diagnostics in acute lymphoblastic leukemia: need for sensitive, fast, and standardized technologies. Blood 2015; 125:3996–4009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hollyman D, Stefanski J, Przybylowski M, et al. Manufacturing validation of biologically functional T cells targeted to CD19 antigen for autologous adoptive cell therapy. J Immunother 2009;32:169–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.van der Stegen SJ, Hamieh M, Sade-lain M. The pharmacology of second-generation chimeric antigen receptors. Nat Rev Drug Discov 2015;14:499–509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhao Z, Condomines M, van der Stegen SJC, et al. Structural design of engineered costimulation determines tumor rejection kinetics and persistence of CAR T cells. Cancer Cell 2015;28:415–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Huang AC, Postow MA, Orlowski RJ, et al. T-cell invigoration to tumour burden ratio associated with anti-PD-1 response. Nature 2017;545:60–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Topp MS, Kufer P, Gökbuget N, et al. Targeted therapy with the T-cell-engaging antibody blinatumomab of chemotherapy-refractory minimal residual disease in B-lineage acute lymphoblastic leukemia patients results in high response rate and prolonged leukemia-free survival. J Clin Oncol 2011;29:2493–8. [DOI] [PubMed] [Google Scholar]
- 24.Gökbuget N, Zugmaier G, Klinger M, et al. Long-term relapse-free survival in a phase 2 study of blinatumomab for the treatment of patients with minimal residual disease in B-lineage acute lymphoblastic leukemia. Haematologica 2017;102(4):el32–el35. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.