Abstract
Background:
Electronic cigarettes (e-cigarettes) have experienced a tremendous increase in use. Unlike cigarette smoking, the effects of e-cigarettes and their constituents on mediating vascular health remain understudied. However, given their increasing popularity, it is imperative to evaluate the health risks of e-cigarettes, including the effects of their ingredients, especially nicotine and flavorings.
Objectives:
To investigate the effects of flavored e-cigarette liquids (e-liquids) and serum isolated from e-cigarette users on endothelial health and endothelial cell dependent macrophage activation.
Methods:
We used human induced pluripotent stem cell-derived endothelial cells (iPSC-ECs) and a high-throughput screening approach to assess endothelial integrity following exposure to 6 different e-liquids with varying nicotine concentrations and to serum from e-cigarette users.
Results:
The cytotoxicity of the e-liquids varied considerably, with the cinnamon-flavored product being most potent and leading to significantly decreased cell viability, increased reactive oxygen species (ROS) levels, caspase 3/7 activity, and low-density lipoprotein uptake, activation of oxidative stress-related pathway, and impaired tube formation and migration, confirming endothelial dysfunction. Upon exposure of ECs to e-liquid, conditioned media induced macrophage polarization into a pro-inflammatory state, eliciting the production of interleukin-1β (IL-1β) and IL-6, leading to increased ROS. After exposure of iPSC-ECs to serum of e-cigarette users, we observed increased ROS linked to endothelial dysfunction, as indicated by impaired pro-angiogenic properties. We also noted an increase in inflammatory cytokine expression in serum of e-cigarette users.
Conclusions:
Acute exposure to flavored e-liquids or e-cigarette use exacerbates endothelial dysfunction, which often precedes cardiovascular diseases.
Keywords: E-cigarette aerosol, iPSC-ECs, e-liquid flavoring, endothelial dysfunction
Condensed Abstract:
E-cigarettes have seen a rapid increase in use although their effects on vascular health remain understudied. Here, we investigated the effects of flavored e-cigarette liquids (e-liquids) on endothelial health by exposing human induced pluripotent stem cell-derived endothelial cells (iPSC-ECs) to different e-liquids with varying nicotine concentrations. While cytotoxicity varied among the tested flavors, the cinnamon-flavored product was most potent leading to decreased cell viability, increased oxidative stress and caspase activity, and impaired tube formation confirming endothelial dysfunction; results which were further corroborated using e-cigarette users’ serum. Our results using iPSC-ECs implicate acute exposure to flavored e-liquids with endothelial dysfunction.
Introduction
Cigarette smoking causes one out of every 3 deaths that result from cardiovascular disease (CVD), leading to >480,000 premature deaths each year in the United States alone (1). While the detrimental effects of conventional cigarette smoking in CVD are well documented and smoking prevalence is declining, an explosive increase in the use of electronic cigarettes (e-cigarettes), especially among youth, is happening with scarce scientific evidence on their toxicity and health effects (2,3).
E-cigarettes are devices designed to deliver an aerosol containing nicotine by heating a liquid solution (often called e-liquid or e-juice) that typically uses propylene glycol (PG) and/or glycerol (glycerin) as a vehicle for nicotine and flavoring agents. Although e-cigarettes are relatively new to the market, they are an increasingly popular alternative to conventional tobacco cigarettes and encompass a wide range of product types and brands. As of 2014, there were >450 e-cigarette products with nearly 8,000 unique flavors available (4). While most of the flavoring chemicals in e-liquids may meet the generally recognized as safe (GRAS) standard for ingestion as food additives, they have not been adequately tested as inhalants. In fact, some popular flavorings are known to be toxic, including diacetyl (buttery flavor), acetyl propionyl (caramel or buttery flavor), and benzaldehyde (fruity taste). Recently, the Food and Drug Administration released a statement limiting the sales of flavored e-cigarettes except tobacco, menthol, and mint flavor in an effort to prevent a new generation of nicotine addicts (5). Despite the rapid increase in popularity, the potential for harmful cardiovascular effects following the use of inhaled e-cigarette flavoring chemicals has been largely unexplored.
To date, the toxicological studies conducted on e-cigarettes have been mostly limited to cytotoxicity studies using established cell lines. Early on, limited studies of e-cigarette vapor showed that e-cigarettes delivered much lower levels of carcinogens in aerosols when compared with conventional cigarette smoke (6,7). At this time, the cardiovascular risk of e-cigarettes is not clear; however, some e-liquid flavorings have been shown to be cytotoxic in cellular models including pulmonary fibroblasts, human embryonic stem cells, and neural stem cells (6,8).
The vascular endothelium plays an important role in vascular function through elaboration of paracrine factors that regulate vascular tone, cell adhesion, fibrinolysis, and blood flow (9). Smoking causes endothelial dysfunction, which is a risk factor for CVD (10). It has been hypothesized that the use of e-cigarettes is associated with endothelial cell damage leading to acute endothelial dysfunction (11), but further in-depth studies are warranted. Despite the extensive use of induced pluripotent stem cells (iPSCs) in other areas of research, no previous studies have leveraged the powerful platform of human iPSC-derived endothelial cells (iPSC- ECs) to assess the potential health risks of e-cigarettes on endothelial integrity. Here we used iPSC-ECs to evaluate the effects of flavored e-liquids on endothelial health and to understand the cross-talk between endothelial cells and macrophages. We further validated our results using serum collected from e-cigarette users and conventional cigarette smokers to assess the potential effects of e-cigarette use on cardiovascular function.
Methods
Detailed methods and supporting data are available in the Online Appendix.
Differentiation of iPSC-ECs
The iPSCs (over passage 20) from 3 healthy individuals were split at a 1:12 ratio using EDTA as described previously (12), and grown for 3–4 days until they reached ~75% confluence. For endothelial cell differentiation and characterization protocol, please refer to the Online Appendix.
E-cigarette liquids (e-liquids)
Six different e-liquids were purchased online: Freedom Smoke USA (Tucson, AZ), Johnson Creek (Johnson Creek, WI), and E Liquid Market (Birmingham, AL) in 0, 6, and 18 mg/ml of nicotine concentrations and stored at 4°C in the dark (Online Table 1). The bottles were chosen to represent the 3 most common vehicle types (50% PG:50% vegetable glycerin (VG), 80% PG:20% VG, and 100% VG) and a range of popular flavors.
Patients’ serum sample collection
The subject population consisted of 5 healthy non-smokers (non-smoker), 5 active cigarette smokers (cigarette), and 2 dual users of e-cigarettes and cigarettes and 2 sole users of e-cigarettes (e-cigarette). We included serum from both sole users of electronic cigarettes and dual users of electronic cigarettes and combustible cigarettes in our study because both patterns of use are common. We combined the 2 into a single group (e-cigarette) as most recruited participants were traditionally long-term cigarette smokers who started the use of e-cigarettes as an alternative to quitting the use of cigarettes. This is also due to the recruited population having a mean average age of 29, whereas most solely e-cigarette users with no prior smoking history tend to be younger. Depending on the population and the wording of the questions, 25–70% of electronic cigarette users describe themselves as dual users (13,14). The participants classified as e-cigarette users reported using their e-cigarettes an average of 27.5 ± 5.0 days a month, with 9.8 ± 3.3 e-cigarette sessions (>2 puffs) per day. Dual users smoked <2 packs of cigarettes a month. Cigarette smokers reported 29.8 ± 0.4 days of smoking in the past 30 days and 10.1 ± 3.4 cigarettes per day. All subjects were healthy individuals free of other major cardiovascular risk factors. Demographic and clinical characteristics of subjects are summarized in Online Table 2. Informed consent was obtained from all subjects and the conduct of the study was approved by the University of California, San Francisco Institutional Review Board.
Participants in the study were asked not to use combustible cigarettes for 7 days before all study visits and to abstain from the use of cigarettes, e-cigarettes, food, and caffeinated drinks for 12 hours prior to the study day. To ensure abstinence from tobacco products, carbon monoxide (CO) and nicotine levels were tested at the start of each visit. For the e-cigarette study, the subjects used an e-cigarette containing RY4-flavored e-liquid (Changning Dekang Ltd, Shenzhen, China) with 16 mg/ml nicotine and instructed to use it for a total of 10 minutes (1 puff every 30 seconds, each puff lasting 2 seconds). For the cigarette condition, subjects smoked a Marlboro cigarette for 10 minutes or until the cigarette went out, by taking 2 puffs per minute, each puff lasting 2 seconds.
Statistical analysis
Statistical analysis and graphs of data were performed with SigmaPlot 13.0 (SPSS Inc). An unpaired 2-tailed Student’s t-test for normal distributed variables and Mann-Whitney Test for non-normal distributed variables were used for 2-group comparisons. Differences of >2 groups were performed using analysis of variance (ANOVA) with Bonferroni post-hoc analysis. When >2 independent variables were present, a 2-way ANOVA with Bonferroni correction was used. To test for serial changes, a 1-way repeated-measures ANOVA was used. Normal distribution was tested with the Shapiro-Wilks test. All data were summarized as mean ± standard deviation (s.d.) or mean ± standard error mean (s.e.m.) and Bonferroni correction was used for multiple comparisons as indicated. P value <0.05 was considered statistically significant.
Results
Assessment of cell viability, ROS generation, and apoptosis in iPSC-ECs after exposure to e- cigarette liquids
Two iPSC lines per individual from 3 healthy individuals were differentiated into iPSC-ECs using chemically defined conditions (Online Figure 1A). Following CD144 purification, iPSC-ECs maintained their identity as demonstrated by a higher expression of endothelial-specific marker genes and proteins (Online Figure 1B–1E). Immunostaining showed an increase in the expression of intracellular adhesion molecule-1 (ICAM-1) upon cytokine tumor necrosis factor (TNF)-α stimulation. Lipid uptake was also confirmed using fluorescent acetylated-low density lipoprotein (Ac-LDL), demonstrating the in vitro functionality of these iPSC-ECs.
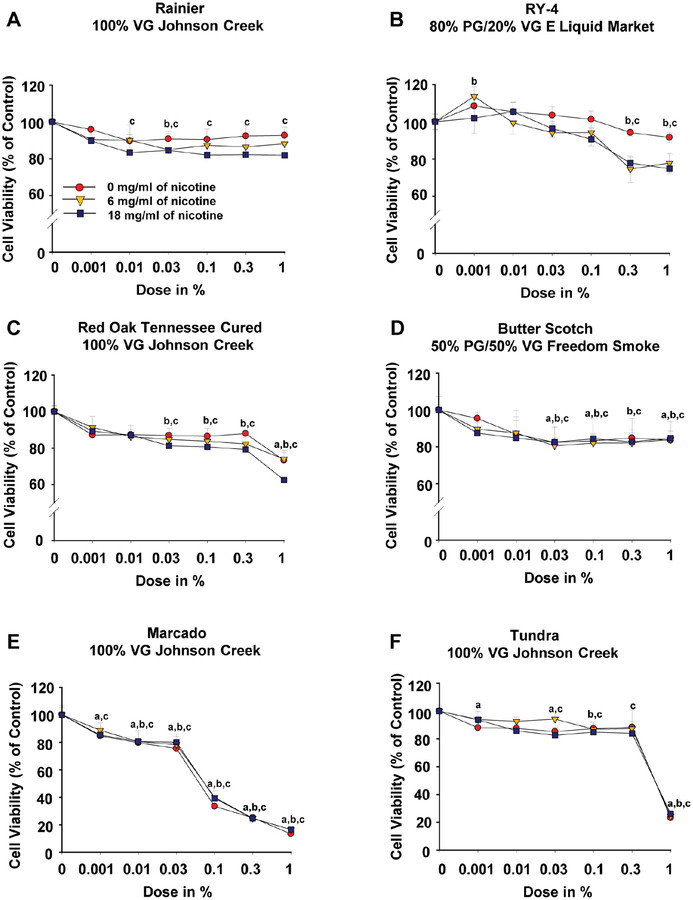
To examine the effects of e-liquids on cell viability, iPSC-ECs were treated with serial dilutions of 6 commercially available e-liquids at varying nicotine concentrations (0, 6, and 18 mg/ml) for 48 hours (Online Table 1). We observed that flavored e-liquids had varying effects on cell survival. While the fruit-flavored Rainier (Figure 1A), sweet tobacco with undertones of caramel and vanilla-flavored RY4 (Figure 1B), tobacco-flavored Red Oak Tennessee Cured (Figure 1C), and sweet-flavored Butter Scotch (Figure 1D) had moderate cytotoxic effects on iPSC-ECs, treatment with cinnamon-flavored Marcado led to a strong cytotoxic effect (Figure 1E). In addition, the menthol tobacco-flavored Tundra (Figure 1F) also had strong cytotoxic effects on iPSC-ECs at 1% dose of concentration with or without nicotine.
Figure 1. Assessment of e-liquid flavor-induced cytotoxicity in iPSC-ECs.
Effects of 6 e-liquid flavors with different nicotine concentrations on iPSC-EC viability after 48-hour treatment were determined using a luminescent CellTiter-Glo 2.0 assay. The data were obtained using iPSC-ECs from 3 biological donors and the assay was repeated twice. Data are represented as mean ± SD. ap < 0.05, compared to controls within 0 mg nicotine/ml group; bp < 0.05, compared to controls within 6 mg nicotine/ml group; cp < 0.05, compared to control within 18 mg nicotine/ml group. Statistically significant from controls (Bonferroni-adjusted P<0.05). VG: vegetable glycerin; PG: propylene glycol.
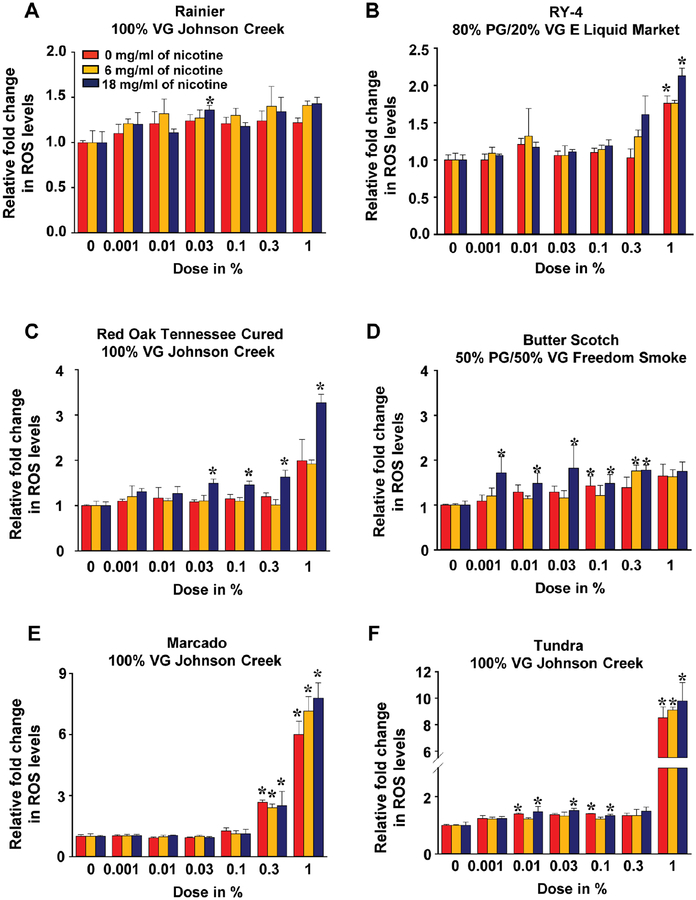
Oxidative stress has been widely implicated as a major factor in endothelial injury (15). To determine whether e-liquids regulate reactive oxygen species (ROS) production, H2O2 levels in iPSC-ECs were determined after exposure to increasing doses of flavored liquids. As shown in Figure 2, most e-liquid exposure for 48 hours, regardless of the flavor, led to increased H2O2 in a dose-dependent manner. This increase in ROS was especially marked when iPSC-ECs were exposed to the cinnamon-flavored Marcado e-liquid at 0.3% and 1% dose (Figure 2E) or the menthol tobacco-flavored Tundra e-liquid at 1% dose (Figure 2F).
Figure 2. Assessment of e-liquid flavor-induced ROS production in iPSC-ECs.
Effects of 6 e-liquid flavors with different nicotine concentrations on intracellular ROS production in iPSC-ECs after 48-hour treatment were determined using a ROS-GloTM H2O2 assay. The data were obtained using iPSC-ECs from 3 biological donors and the assay was repeated twice. Data are represented as mean ± SD. *p < 0.05 and **p < 0.001, compared to controls within each group. Statistically significant from controls (Bonferroni-adjusted P<0.05). VG: vegetable glycerin; PG: propylene glycol.
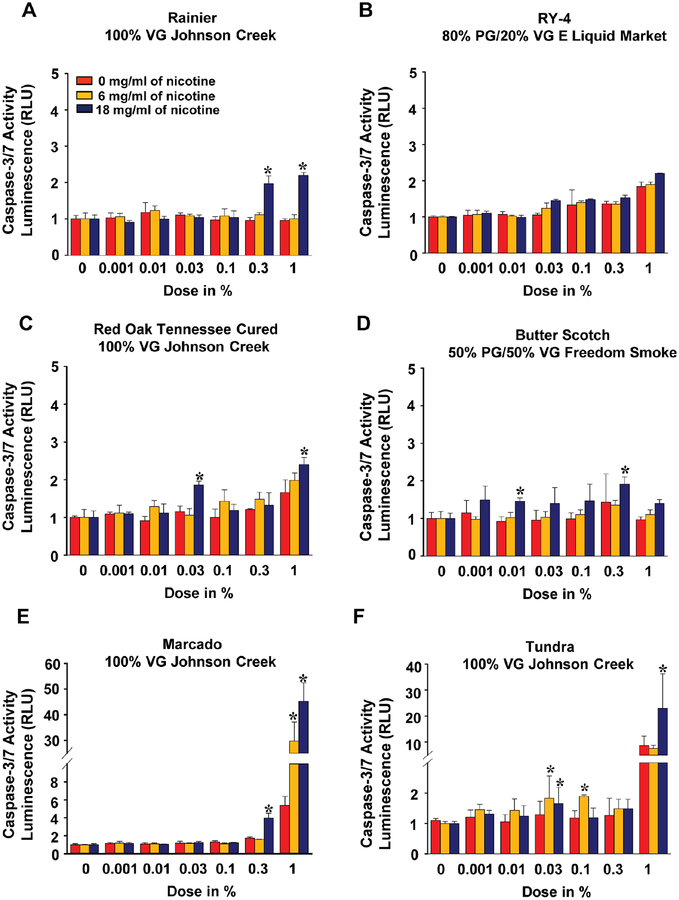
To elucidate the mechanisms underlying the reduction in iPSC-EC viability by e-liquid exposure, we then examined the activities of caspase-3 and caspase-7 in iPSC-ECs following e-liquids treatments. Both caspases were significantly more active in iPSC-ECs treated with most e-liquids compared to control, and the maximum response was observed when cells were treated with either Marcado- or Tundra-flavored e-liquids (Figure 3). In all measured parameters, including cytotoxicity, ROS generation, and apoptotic activities, we observed an increasing trend as the nicotine concentration increased, though the overall difference was not significant. Finally, 2 solvents that were used in all six flavored e-liquids, vegetable glycerin (VG) and propylene glycol (PG), did not affect cell viability, ROS level, or caspase activities (Online Figure 2).
Figure 3. Assessment of e-liquid flavor-induced caspase 3/7 activity in iPSC-ECs.
Effects of 6 e-liquid flavors with different nicotine concentration on caspase 3/7 activity for apoptosis in iPSC-ECs after 48-hour treatment were determined using a Caspase-Glo® 3/7. The data were obtained using iPSC-ECs from 3 biological donors and the assay was repeated twice. Data are represented as mean ± SD. *p < 0.05 and **p < 0.001, compared to controls within each group. Statistically significant from controls (Bonferroni-adjusted P<0.05). VG: vegetable glycerin; PG: propylene glycol.
Assessment of endothelial functions in iPSC-ECs after addition of e-cigarette liquids
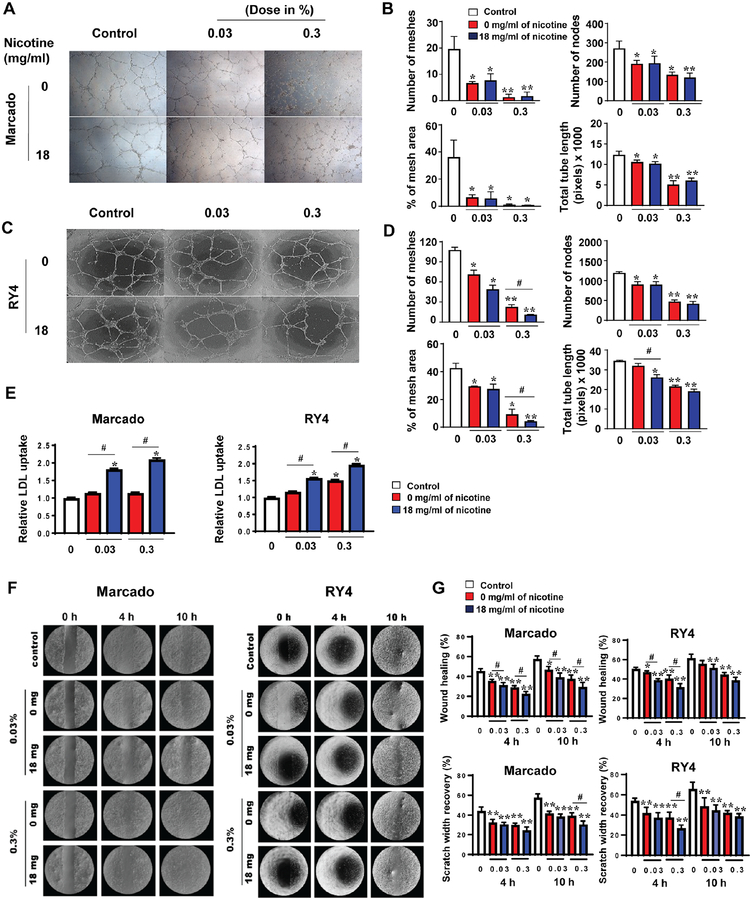
We investigated the influence of e-liquids on tube formation of iPSC-ECs, which reflects properties relevant to angiogenesis. Tubes were evaluated 16 hours after seeding in growth medium supplemented with 3 e-liquids: Marcado, RY4, and Tundra. Addition of the 2 most toxic e-liquids, cinnamon-flavored Marcado (Figure 4A and 4B) and menthol tobacco-flavored Tundra (Online Figure 3A and 3B), led to a significant decrease in the number of meshes and nodes, percent of mesh area, and the total tube length in iPSC-ECs, compared with controls. Similar findings were also observed in iPSC-ECs supplemented with the less toxic RY4 (Figure 4C and 4D).
Figure 4. Effect of e-liquid treatment on endothelial function of iPSC-ECs.
(A and C) The cells were incubated with each e-liquid flavor for 16 hours to allow the formation of capillary-like structures on Matrigel and then imaged by phase-contrast microscopy (x20). (B and D) Quantitative data from the tube-formation assay. (E) LDL uptake was measured in iPSC-ECs after treatment of the e-liquid for 2 days. (F) Representative images and (G) quantitative data from the migration assay at 0, 4, and 10 hours in the presence of the e-liquid flavors Marcado and RY4. The migration data were normalized to time point 0. Data are represented as mean ± SEM. *p < 0.05 and **p < 0.001, compared to controls; and #p<0.05, compared to groups treated with 0 mg nicotine/ml.
Next, we investigated the effect of Marcado or RY4 e-liquid on more specific endothelial functions, such as LDL and lipid uptake and cell migration. Our results indicate that incubation with e-liquid of both flavors led to increased uptake of both LDL (Figure 4E) and free fatty acids in iPSC-ECs (Online Figure 3C), an important link in the onset of inflammation and impaired endothelial function (16,17). The increase in LDL uptake was dependent on nicotine concentration within e-liquid. In addition, as shown in Figure 4F and 4G, exposure to each e-liquid caused significant reduction in iPSC-EC migration, as determined by the rate of wound closure and scratch width recovery. Similarly, using HUVECs as a positive control, we found free fatty acids to be significantly increased (Online Figure 3D), and migration rate to be severely compromised in the presence of e-liquids (Online Figure 3E and 3F). The results on cell migration were mostly independent of nicotine concentration, suggesting that the observed impairment of endothelial cell functions is associated with the combination of flavor additives and nicotine.
Cross-talk between e-liquid treated endothelial cells and macrophages
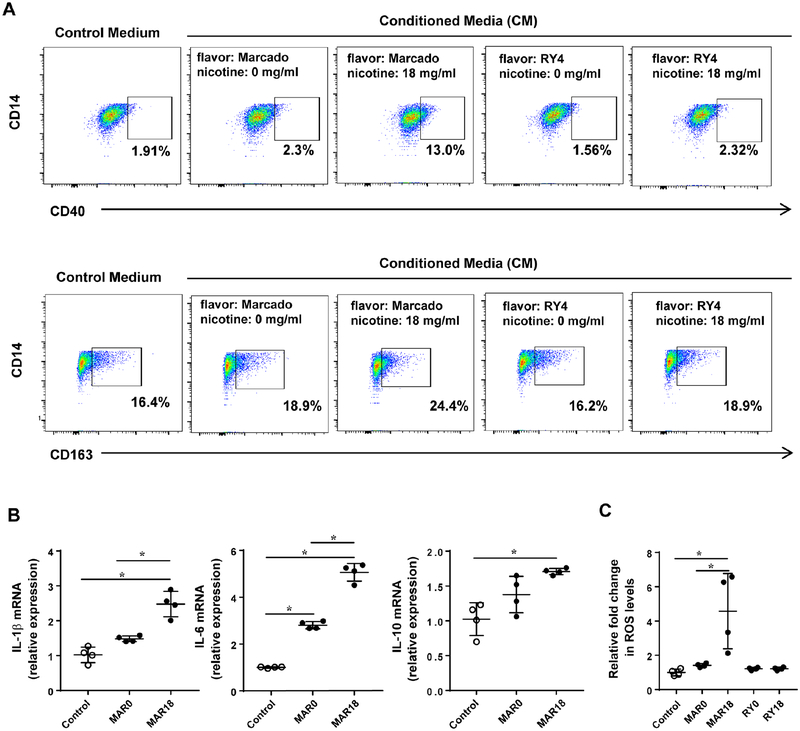
To understand the impact of e-liquid on the cross-talk between ECs and macrophages, we analyzed macrophages exposed to conditioned media from iPSC-ECs treated with e-liquid. Conditioned media from iPSC-ECs without e-liquid treatment was included as a control condition. As shown in Figure 5A, e-liquids, such as Marcado and RY4, triggered macrophage dual polarization compared with control groups. Especially for Marcado flavor, we found that the percentage of macrophages expressing CD40 (M1 marker) and CD163 (M2 marker) was increased with iPSC-EC-conditioned media treated with 18 mg/ml of nicotine compared to 0 mg/ml of nicotine (13.0±0.1% vs 2.3±0.2% and 24.4±1.8% vs 18.9±1.6%, respectively).
Figure 5. Effect of conditioned media from iPSC-ECs treated with e-liquid on macrophage polarization.
(A) Conditioned media was collected from iPSC-ECs after 48 hours of incubation with each e-liquid flavor, and then exposed to macrophage-like cells for 48 hours. Phenotype of macrophages was analyzed by flow cytometry for M1 (CD40) and M2 (CD163) markers. (B) After 48 hours incubation with conditioned media, the media was replaced with the fresh macrophage culture media for 16 hours to determine cytokine expression and ROS levels. Expression of cytokines produced by macrophages was determined by quantitative real-time RT-PCR. (C) Intracellular ROS levels produced by macrophages was determined using a ROS-Glo H2O2 assay. Data are represented as mean ± SEM. Statistically significant from controls (Bonferroni-adjusted P<0.05). MAR0: Marcado e-liquid without nicotine; MAR18: Marcado e-liquid with 18 mg/ml of nicotine; RY0: RY4 e-liquid without nicotine; RY18: RY4 e-liquid with 18 mg/ml of nicotine.
We also examined the effects of conditioned media collected from iPSC-ECs treated with e-liquid on pro-and anti-inflammatory cytokine production in macrophages. We found that exposure to conditioned media from iPSC-ECs treated with e-liquid Marcado significantly increased inflammatory factors produced by M1 macrophages, such as IL-1β and IL-6, and M2-related cytokine IL-10 (Figure 5B), whereas no significant changes in cytokine expression were found in those with e-liquid RY4 (data are not shown). We also found that intracellular ROS levels in the macrophages were significantly increased following treatment with conditioned media from iPSC-ECs treated with Marcado containing 18 mg/ml of nicotine (Figure 5C).
Effects of acute cigarette use and smoking on serum nicotine levels and circulating leukocyte populations
We then sought to gain a better understanding of the effects of e-cigarette use in vivo by recruiting nonsmokers, cigarette smokers, and e-cigarette users (sole or dual users of cigarettes). As nicotine is the key active ingredient in both e-cigarettes and conventional cigarettes, serial changes in the levels of nicotine and its major proximate metabolite, cotinine, were assessed in participants’ blood prior to (−1 hour), immediately after (0 hour), 1 hour, and 3 hours post-usage. Serum nicotine concentrations before tobacco product use (−1 hour) were below 1.5 ng/ml in both groups, demonstrating compliance with the overnight hold on tobacco products (Table 1). In both groups, there was a significant increase in serum nicotine concentrations immediately after product use. The uptake of nicotine from an e-cigarette was similar to a conventional cigarette, with maximal mean serum nicotine concentrations reaching 12.3 ng/ml and 12.6 ng/ml, respectively. Furthermore, results of total white blood cells (WBCs) and their subpopulations revealed no significant differences among nonsmokers, e-cigarette users, and smokers (Table 2). Similarly, there were no significant differences between e-cigarette and cigarette smoker groups pre- and post-smoking in terms of the mean platelet (PLT) count, although PLT at −1 hour was significantly lower (after adjustment for multiple comparisons) in both cigarette smoker and e-cigarette user groups in relation to non-smokers.
Table 1.
Plasma nicotine and cotinine levels at baseline (1 hour prior), immediately after (0 hour), 1 hour, and 3 hours after smoking.
| (ng/ml) | Baseline | Immediately After | 1 hour After | 3 hours After | P-value* | P-valueϯ | |
|---|---|---|---|---|---|---|---|
| Mean ± SEM | |||||||
| E-cig | Nicotine | 1.0 ± 0.6 | 12.3 ± 3.3* | 4.5 ± 0.8 | 2.3 ± 0.4 | 0.001 | - |
| Cotinine | 127.5 ± 16.7 | 116.8 ± 16.0 | 91.6 ± 30.4 | 82.0 ±26.5* | 0.01 | - | |
| Cig | Nicotine | 0.6 ± 0.1 | 12.6± 1.9* | 5.8 ± 0.8ϯ | 2.8 ± 0.4 | <0.001 | 0.04 |
| Cotinine | 99.4 ± 33.9 | 94.1 ± 32.5 | 96.9 ± 29.8 | 91.0 ± 27.7 | - | - | |
Data are presented as mean ± SD.
Statistically significant from baseline.
Table 2.
Peripheral blood counts and lymphocyte subpopulation in 5 nonsmokers, 4 e-cigarette users, and 5 cigarette smokers before and after exposure to smoke.
| Nonsmokers | E-cig user | Cig smokers | |||
|---|---|---|---|---|---|
| Before | After | Before | After | ||
| WBC, k/μl | 5.5 ± 0.9 | 5.5 ± 0.6 | 5.6 ± 0.8 | 6.0 ± 0.7 | 6.4 ± 0.5 |
| Lymphocytes, % | 33.7 ± 0.7 | 35.7 ± 4.7 | 33.7 ± 3.9 | 31.0 ± 3.9 | 26.1 ± 2.5 |
| Neutrophils, % | 54.9 ± 0.5 | 51.7 ± 4.6 | 54.5 ± 3.9 | 59.5 ± 3.2 | 63.2 ± 2.3 |
| Monocytes, % | 6.2 ± 0.4 | 6.2 ± 0.6 | 6.5 ± 0.3 | 6.6 ± 0.6 | 6.2 ± 0.7 |
| Eosinophils, % | 3.1 ± 0.5 | 3.4 ± 0.6 | 2.7 ± 0.7 | 3.0 ± 0.7 | 2.4 ± 0.8 |
| Basophils, % | 0.5 ± 0.1 | 0.5 ± 0.1 | 0.5 ± 0.1 | 0.3 ± 0.1 | 0.4 ± 0.1 |
| RBC, mil/μl | 4.7 ± 0.5 | 4.6 ± 0.1 | 4.6 ± 0.2 | 4.3 ± 0.1 | 4.2 ± 0.1 |
| HGB, g/dl | 13.7 ± 1.4 | 13.8 ± 0.3 | 14.0 ± 0.3 | 13.0 ± 0.4 | 12.8 ± 0.4 |
| HCT, % | 41.2 ± 4.1 | 41.1 ± 0.8 | 41.4 ± 1.0 | 39.5 ± 1.2 | 38.3 ± 1.1 |
| MCV, fl | 87.2 ± 1.8 | 89.9 ± 2.3 | 90.7 ± 2.1 | 91.9 ± 1.3* | 91.4 ± 1.2 |
| PLT, k/ μl | 304.3 ± 4.2 | 213.3 ± 21.8* | 208.5 ± 25.1 | 206.8 ± 17.0** | 201.3 ± 19.1 |
Data are presented as mean ± SD. WBC: white blood cells, RBC: red blood cells, HGB: hemoglobin, HCT: hematocrit, MCV: mean volume of red blood cells, PLT: platelets.
Statistically significant from nonsmokers (*p<0.05 and **p<0.001).
Acute effects of tobacco product use on intracellular ROS and tube formation of iPSC-ECs and inflammatory cytokines in serum
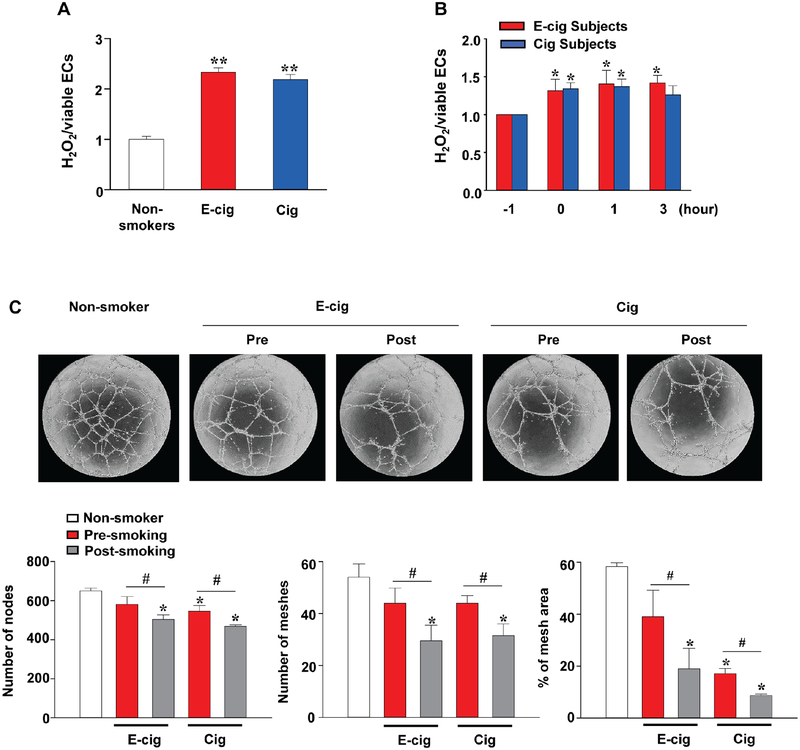
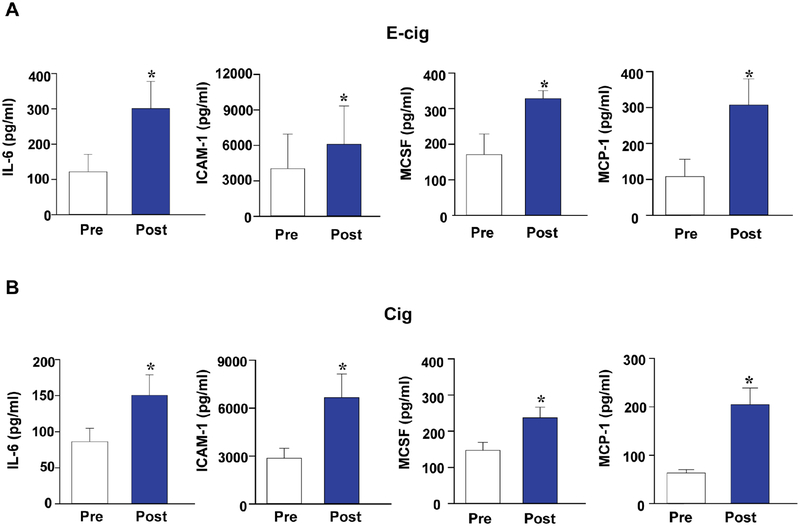
To explore how the use of tobacco products affects ROS production and angiogenic signaling in vivo, we next incubated iPSC-ECs with serum derived from nonsmokers, e-cigarette users, and cigarette smokers, as described above. In the −1 hour samples collected after an overnight hold on food, alcohol, and tobacco, we observed significantly higher intracellular ROS in the cells incubated with serum from e-cigarette users and smokers as compared with serum from nonsmokers (Figure 6A), further heightened by the serum collected immediately after (0 hour), 1 hour, and 3 hours post-usage of a single e-cigarette or cigarette (Figure 6B). Treatment of iPSC-ECs with serum collected before and 3 hours after smoking also revealed impairment of the tube-formation capability compared to controls treated with serum from nonsmokers (Figure 6C). We subsequently measured 62 human inflammatory cytokines in the serum from e-cigarette and cigarette users pre- and post-smoking (Online Table 3). Levels of interleukin-6 (IL-6), ICAM-1, macrophage colony-stimulating factor (MCSF), and monocyte chemoattractant protein-1 (MCP-1) in serum of e-cigarette users and cigarette smokers after smoking were significantly higher than prior to smoking (Figure 7) but lost statistical significance after adjustment for multiple comparisons. In addition, a trend towards increased levels of all other cytokines was observed in the serum of both e-cigarette users and cigarette smokers after smoking compared with the levels before smoking, and no cytokine level showed a significant difference between e-cigarette users and cigarette users.
Figure 6. Effects of sera from active e-cigarette users and cigarette smokers on ROS generation and tube formation of iPSC-ECs in vitro.
iPSC-ECs were incubated with 10% serum from nonsmokers (n=5), e-cigarette users (n=4), and cigarette smokers (n=5) for 48 hours. (A) Intracellular ROS production in iPSC-ECs treated with sera collected from nonsmokers, e-cigarette users, or cigarette smokers at baseline. (B) Serial changes of ROS production in iPSC- ECs treated with serum collected from smokers before (−1 hours; shown in detail in A) and after (0, 1, and 3 hours) smoking. (C) iPSC-ECs (1 × 104) treated with serum samples from nonsmokers or smokers were seeded onto μ-Slide Angiogenesis containing Matrigel® Basement Membrane Matrix for 16 hours, and the formation of capillary-like structures were then imaged by phase-contrast microscopy (x 20) and quantified. Data are represented as mean ± SD. *p < 0.05 and **p < 0.001, compared to nonsmokers or baseline; and #p < 0.05, compared to pre-smoking within each group.
Figure 7. Serum cytokine levels in active e-cigarette users and cigarette smokers obtained before and after smoking.
Concentration of IL-6, ICAM-1, MCSF, and MCP-1 in serum from e-cigarette users (A) or cigarette smokers (B) were compared before (Pre) and 3 hours after smoking (Post). Data are represented as mean ± SEM. *p < 0.05, compared to pre-smoking. IL-6 = interleukin-6; ICAM-1 = intracellular adhesion molecule-1; MCSF = macrophage colony- stimulating factor; MCP-1 = monocyte chemoattractant protein-1.
Gene and functional enrichment analysis with the addition of e-cigarette liquids
Based on the results from our in vitro experiments, we next investigated the effects of 2 flavored e-liquids on the transcriptomic profile of iPSC-ECs (GEO accession number: GSE125217). We chose an e-liquid with high cytotoxicity, Marcado (MAR, 0.03% concentration), and one with low cytotoxicity, RY4 (RY, 0.3% concentration), both with and without nicotine. Optimal concentrations for each e-liquid flavor were set at 80% cell viability of iPSC-ECs after 24 hours of exposure in vitro. Using principal component analysis (PCA), we observed that principal component 1 (PC1) reflected the differences between iPSC-ECs that were derived from 2 different individuals (Online Figure 4A), whereas PC2 identified distinct clusters corresponding to MAR-treated samples from RY-treated samples, and PC3 further separated RY18-treated samples from either RY0-treated or control (CTR) samples (Online Figure 4B). Collectively, these data demonstrate that both MAR0 and MAR18 treatment markedly affect the transcriptome of iPSC-ECs, whereas RY treatment has little influence at either nicotine concentration. Gene Ontology (GO) analysis using a false positive discovery rate (pFDR) cutoff of < 0.05 identified a total of 104 DEGs (including 64 up-regulated DEGs and 40 down-regulated DEGs) in iPSC-ECs after exposure to either MAR0 or MAR18 compared with controls (Online Figure 5A and Online Table 4). GO enrichment analysis found that the most preserved expression profiles between MAR-treated samples and controls were related to oxidation-reduction process, extracellular matrix organization, and response to toxic substances (Online Figure 5B and 5C). Chord diagram for top 5 overrepresented pathways (P-value < 0.05) included differential expression of genes involved in response to toxic substances, membrane bounded vesicles, growth factor binding, pentose biosynthetic process, and oxidation reduction process (Online Figure 5D). Interestingly, when we compared differential gene expression in iPSC-ECs exposed to MAR0 or MAR18, no significant enriched annotation cluster was detected (data not shown).
Discussion
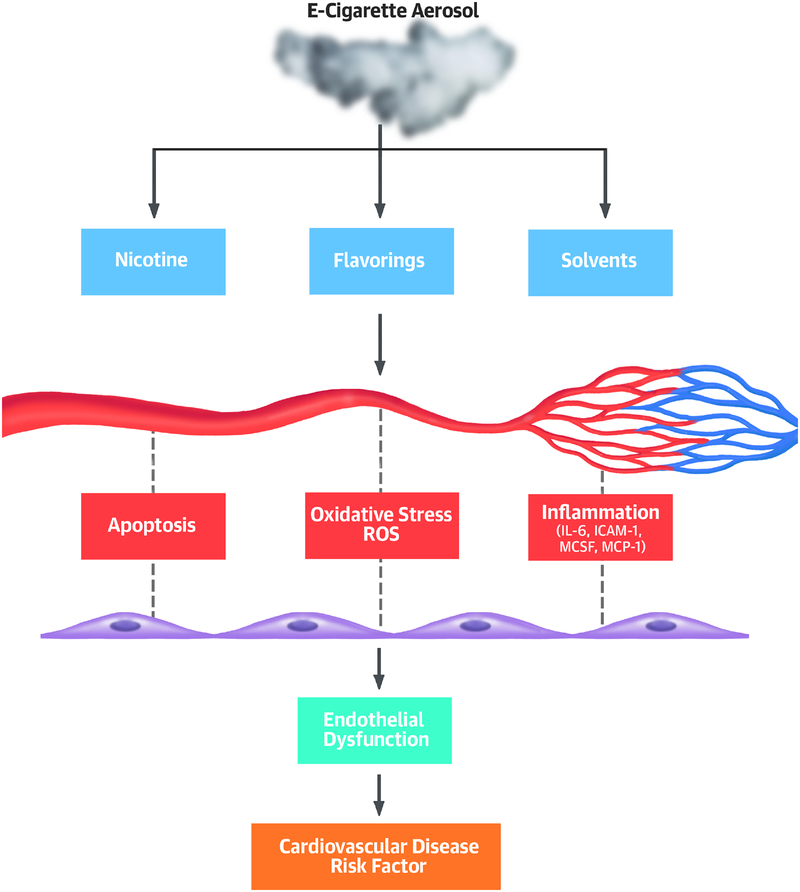
Understanding the health effects of e-liquids, especially flavorings, is important for establishing short- and long-term safety of e-cigarettes. In this study, we compared the biological effects of flavored e-liquids (both with and without nicotine) on iPSC-ECs and found that some flavorings had toxic effects on endothelial cell viability and function (Central Illustration). The cinnamon-flavored product (Marcado) was the most toxic sample tested, producing strong cytotoxicity in iPSC-ECs that led to decreased cell survival, impaired angiogenic responses, and increased ROS levels and caspase 3/7 activity. Our findings are concordant with the results of recent studies showing that cinnamon-flavored e-liquids and aerosols are highly volatile, cytotoxic, and genotoxic to human embryonic cells and adult lung cells (8,18). Notably, our results showed that the effects of e-liquid flavorings on endothelial phenotypes and function including cytotoxicity, ROS level, caspase 3/7 activity, pro-angiogenic properties, and migration, were stronger than those of nicotine concentration. Similar biological effects of e-liquid flavoring on iPSC-ECs were also observed in a gene set enrichment and GO enrichment analyses. We identified changes in the expression of genes involved in multiple biological functions, including response to toxic substances, membrane-bounded vesicle, growth factor binding, pentose biosynthetic process, and oxidation reduction process 24 hours post-exposure to e-liquid flavor, Marcado, with or without nicotine. Some of these enriched functional categories from our study were also highlighted in previously reported studies performed on a primary 3D airway cell system and mouse lungs exposed to e-cigarette aerosol or cigarette smoking, respectively (19,20).
Central Illustration. Human Induced Pluripotent Stem Cell-Derived Endothelial Cells for evaluating e-cigarette risk.
Mechanistic overview by which e-cigarette use might case acute endothelial dysfunction. Exposure of endothelial cells to e-cigarette flavorings or serum of e-cigarette users leads to endothelial dysfunction associated with increased apoptosis, ROS, and inflammation. IL-6 = interleukin-6; ICAM-1 = intracellular adhesion molecule-1; MCSF = macrophage colony-stimulating factor; MCP-1 = monocyte chemoattractant protein-1.
Nicotine, one of the major active constituents in most smoking products, is primarily metabolized by the liver into cotinine (21). Although it has been reported that nicotine absorption rate and plasma nicotine levels are lower from e-cigarette use compared to conventional cigarette smoking, it could still reach a delayed but comparable level compared to a tobacco cigarette (21). As intensive puffing (i.e., more puffs and greater puff volume) may influence nicotine delivery, we applied a similar study design for e-cigarette users and smokers (one 2 second puff every 30 seconds for 10 minutes). We found that the effect of e-cigarette use on mean plasma nicotine levels was similar to tobacco cigarette smoking. Peak plasma nicotine concentration for both conventional cigarette and e-cigarette users was observed immediately after smoking, which is consistent with previous reports (21,22), indicating that e-cigarettes provide nicotine via rapid pulmonary absorption.
Although studies with conventional cigarettes have shown that acute and long-term exposure to smoking increases the risk of CVD via enhanced oxidative stress (9,23), inflammation (9), endothelial dysfunction (9,10), and elevated blood pressure (24), studies on the effects of e-cigarette nicotine and liquid flavors have been somewhat limited. In the present study, we observed that pro-inflammatory markers such as IL-6, ICAM-1, MCSF, and MCP-1 were elevated in the serum of users of e-cigarettes and conventional cigarettes 3 hours after use compared with serum collected prior to smoke, highlighting the danger of even short-term exposure to e-cigarette aerosol in vivo. These inflammatory markers are known to play a critical role in the pathogenesis of vascular disease.
We also demonstrated that e-cigarette and cigarette users’ serum expressed elevated basal ROS when compared to nonsmokers, which may be linked to activated inflammatory mediators. The already elevated basal ROS levels in e-cigarette users’ serum increased rapidly after smoking. Accumulating evidence suggests that ROS plays an important role in physiological and pathological angiogenesis (9,25). In this study, we found that conditioned media from iPSC-ECs with exposure to e-liquid treatment promoted polarization of both M1 and M2 macrophages, with a higher tendency of M1 polarization. These M1 polarized macrophages may be, in part, responsible for strong pro-inflammatory profiles, which are linked to increased ROS generation. This is consistent with previous studies demonstrating that macrophages produce high levels of ROS, which also acts in a feedback-loop regulating macrophage phenotype (25,26). However, the exact role of ROS in macrophages activation still requires further investigation. Importantly, our data further validated that the presence of ROS in e-cigarette users’ serum after vaping flavored e-liquids was associated with endothelial cell dysfunction, as shown by altered tube formation in iPSC-ECs. Consistent with this finding, Carnevale et al. (23) observed negative effects on markers of oxidative stress (i.e., soluble NOX2-derived peptide and 8-iso- prostaglandin F2α) and flow-mediated dilation after a single use of e-cigarette, raising concerns regarding vascular safety of e-cigarettes. The results from these studies lend further support to the harmful effects of e-cigarette use and cigarette smoking in increasing oxidative stress and endothelial dysfunction.
Finally, we performed a characterization of leukocyte responses in smokers, as it has been reported that acute and chronic cigarette smoking is followed by a transient elevation of WBC count, considered to be a well-established cardiovascular risk factor in epidemiological studies (27). In our study, there were no statistically significant differences in either total or sub-population leukocyte counts between nonsmokers and smokers of either e-cigarettes or conventional cigarettes, which is consistent with a previous study (28). However, we did find a significantly lower PLT count in smokers compared with nonsmokers which could be due to the decreased thrombopoietic activity induced by nicotine commonly seen in chronic smokers (29). Regardless of the underlying mechanisms, these changes are important indications of how e-cigarette use could compromise host immunity.
Despite its many strengths, our study has several limitations. First, as we were interested in assessing the direct effects of these liquids on vascular health, the results from our in vitro evaluation of e-liquid flavoring using iPSC-ECs were restricted to e-liquids and not their aerosols, as no heating or combustion was involved. We did, however, add serum from e-cigarette users into culture media of iPSC-ECs to mimic cellular exposure to e-cigarette aerosol that occurs in vivo, including exposure to toxicants that have undergone metabolic activation or deactivation. The presence or absence of aerosols may explain the difference observed in nicotine effects whereby nicotine present in e-liquids seems to only have a slight effect on ROS production and no effect on tube formation, whereas serum collected from both e-cigarette and cigarette smokers after smoking, which contains increased levels of nicotine, significantly increased ROS production and impaired tube formation. These effects may be due to the lack of combustion used in the in vitro settings, which could make e-cigarettes potentially more toxic compared with serum collected from both e-cigarette and cigarette smokers which involves combustion. Another factor that may contribute to the differential responses is the increased molecular complexity of serum collected from patients due to systemic effects of other cell types beyond endothelial cells that may be affected by e-cigarettes or cigarettes. Further studies exploring the chemical toxicants in the e-liquids, e-cigarette aerosol, and serum in relation to the endothelial cytotoxicity and function, are needed to better understand the potential risk from toxic compounds generated by e-cigarette products. Second, a fixed puffing protocol used in our study at an experimental setting may not reflect actual user puff practice. However, this fixed protocol was necessary to allow us to directly compare the results between different subjects across separate studies in a standardized manner. It should be noted that our results on 6 selected flavors may not be universally applicable to all e-cigarettes, given the wide variety of e-liquids in the market that can vary in composition by brand. Third, the relatively small volume and sample size of sera available precluded the possibility of performing additional assays, and additional sample collection may be needed to extrapolate the findings of our study to the general population of e-cigarette users.
Conclusions
Our data demonstrated that selected e-liquid flavorings have detrimental effects on endothelial cell viability and function, changes that are accompanied by increased ROS and caspase 3/7 activity. We also showed that the use of e-cigarettes alone is capable of increasing plasma nicotine concentrations comparable to levels achieved via conventional cigarette, indicating that e-cigarettes provide effective and measurable nicotine delivery. In addition, our results show increased ROS generation and inflammatory cytokines present in serum was observed in concert with acute e-cigarette use-induced endothelial dysfunction, as indicated by impaired tube formation of iPSC-ECs. As e-cigarette use becomes more widespread, additional studies of their health effects become more urgent as this understanding could inform both public health policy and regulation. Nonetheless, our current findings are an important first step in filling this gap by providing mechanistic insights on how e-cigarettes cause endothelial injury and dysfunction, which are an important risk factors for the development of CVD.
Supplementary Material
CLINICAL PERSPECTIVES.
Competency in Medical Knowledge:
Human induced pluripotent stem cell-derived endothelial cells exposed to the compounds in e-cigarettes or serum from e-cigarette users develop dysfunction associated with decreased viability, accumulation of reactive oxygen species, and impaired proangiogenic properties.
Translational Outlook:
Since endothelial dysfunction often precedes clinical manifestations of disease, clinical studies are needed to examine the long-term effects of e-cigarettes on cardiovascular outcomes.
Funding:
This work was supported by the American Heart Association Scientist Development Grant 16SDG27560003 (W.H.L.), a Pilot Award from the Stanford Diabetes Research Center from a grant sponsored by NIH P30DK116074 (W.H.L.), National Institutes of Health (NIH) R00 HL130416 (S.G.O.), University of California Tobacco Related Disease Research Program 27IR-0012 (J.C.W.), American Heart Association 17MERIT33610009 (J.C.W), P50-CA-180890–01 from the National Heart, Lung, and Blood Institute (NHLBI) at the NIH and the US Food and Drug Administration Center for Tobacco Products (FDA CTP) (S.F.S.), University of California Tobacco Related Disease Research Program 24RT-0039 (S.F.S.), and R01 HL120062 from the NIH NHLBI and FDA CTP (M.L.S.).
Abbreviations
- CRP
c-reactive protein
- CVD
cardiovascular disease
- E-cigarette
electronic cigarette
- E-liquids
electronic cigarette liquids
- iPSC-ECs
induced pluripotent stem cell-derived endothelial cells
- PG
propylene glycol
- PLT
platelet
- ROS
reactive oxygen species
- VG
vegetable glycerin
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures: The authors have nothing to disclose.
References
- 1.The health consequences of smoking-50 years of progress: A report of the surgeon general. Atlanta (GA): U.S. Department of Health and Human Services, 2014. [Google Scholar]
- 2.General. OotS. E-cigarette use among youth and young adults: A report of the surgeon general. Atlanta (GA): U.S. Department of Health and Human Services, 2016. [Google Scholar]
- 3.Arrazola RA, Singh T, Corey CG et al. Tobacco use among middle and high school students - United States, 2011–2014. MMWR Morb Mortal Wkly Rep 2015;64:381–5. [PMC free article] [PubMed] [Google Scholar]
- 4.Zhu SH, Sun JY, Bonnevie E et al. Four hundred and sixty brands of e-cigarettes and counting: implications for product regulation. Tob Control 2014;23 Suppl 3:iii3–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Scott G Proposed new steps to protect youth by preventing access to flavored tobacco products and banning menthol in cigarettes. FDA Statement. November 2018. Availabe at: https://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/UCM625884.htm. [Google Scholar]
- 6.Goniewicz ML, Knysak J, Gawron M et al. Levels of selected carcinogens and toxicants in vapour from electronic cigarettes. Tob Control 2014;23:133–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Grana R, Benowitz N, Glantz SA. E-cigarettes: a scientific review. Circulation 2014;129:1972–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bahl V, Lin S, Xu N, Davis B, Wang YH, Talbot P. Comparison of electronic cigarette refill fluid cytotoxicity using embryonic and adult models. Reprod Toxicol 2012;34:529–37. [DOI] [PubMed] [Google Scholar]
- 9.Messner B, Bernhard D. Smoking and cardiovascular disease: mechanisms of endothelial dysfunction and early atherogenesis. Arterioscler Thromb Vasc Biol 2014;34:509–15. [DOI] [PubMed] [Google Scholar]
- 10.Celermajer DS, Sorensen KE, Georgakopoulos D et al. Cigarette smoking is associated with dose-related and potentially reversible impairment of endothelium-dependent dilation in healthy young adults. Circulation 1993;88:2149–55. [DOI] [PubMed] [Google Scholar]
- 11.Fetterman JL, Weisbrod RM, Feng B et al. Flavorings in tobacco products induce endothelial cell dysfunction. Arterioscler Thromb Vasc Biol 2018;38:1607–1615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kodo K, Ong SG, Jahanbani F et al. iPSC-derived cardiomyocytes reveal abnormal TGF-beta signalling in left ventricular non-compaction cardiomyopathy. Nat Cell Biol 2016;18:1031–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Coleman B, Rostron B, Johnson SE et al. Transitions in electronic cigarette use among adults in the Population Assessment of Tobacco and Health (PATH) Study, Waves 1 and 2 (2013–2015). Tob Control 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Delnevo CD, Giovenco DP, Steinberg MB et al. Patterns of electronic cigarette use among adults in the United States. Nicotine Tob Res 2016;18:715–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Schnabel R, Blankenberg S. Oxidative stress in cardiovascular disease: successful translation from bench to bedside? Circulation 2007;116:1338–40. [DOI] [PubMed] [Google Scholar]
- 16.Ghosh A, Gao L, Thakur A, Siu PM, Lai CWK. Role of free fatty acids in endothelial dysfunction. J Biomed Sci 2017;24:50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kim F, Tysseling KA, Rice J et al. Free fatty acid impairment of nitric oxide production in endothelial cells is mediated by IKKbeta. Arterioscler Thromb Vasc Biol 2005;25:989–94. [DOI] [PubMed] [Google Scholar]
- 18.Behar RZ, Luo W, Lin SC et al. Distribution, quantification and toxicity of cinnamaldehyde in electronic cigarette refill fluids and aerosols. Tob Control 2016;25:ii94–ii102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Miller MA, Danhorn T, Cruickshank-Quinn CI et al. Gene and metabolite time-course response to cigarette smoking in mouse lung and plasma. PLoS One 2017;12:e0178281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Haswell LE, Baxter A, Banerjee A et al. Reduced biological effect of e-cigarette aerosol compared to cigarette smoke evaluated in vitro using normalized nicotine dose and RNA-seq-based toxicogenomics. Sci Rep 2017;7:888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Marsot A, Simon N. Nicotine and cotinine levels with electronic cigarette: a review. Int J Toxicol 2016;35:179–85. [DOI] [PubMed] [Google Scholar]
- 22.Dawkins L, Corcoran O. Acute electronic cigarette use: nicotine delivery and subjective effects in regular users. Psychopharmacology (Berl) 2014;231:401–7. [DOI] [PubMed] [Google Scholar]
- 23.Carnevale R, Sciarretta S, Violi F et al. Acute impact of tobacco vs electronic cigarette smoking on oxidative stress and vascular function. Chest 2016;150:606–12. [DOI] [PubMed] [Google Scholar]
- 24.Nakamura K, Barzi F, Lam TH et al. Cigarette smoking, systolic blood pressure, and cardiovascular diseases in the Asia-Pacific region. Stroke 2008;39:1694–702. [DOI] [PubMed] [Google Scholar]
- 25.Tan HY, Wang N, Li S, Hong M, Wang X, Feng Y. The reactive oxygen species in macrophage polarization: reflecting its dual role in progression and treatment of human diseases. Oxid Med Cell Longev 2016;2016:2795090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.He C, Carter AB. The metabolic prospective and redox regulation of macrophage polarization. J Clin Cell Immunol 2015;6:371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wannamethee SG, Lowe GD, Shaper AG, Rumley A, Lennon L, Whincup PH. Associations between cigarette smoking, pipe/cigar smoking, and smoking cessation, and haemostatic and inflammatory markers for cardiovascular disease. Eur Heart J 2005;26:1765–73. [DOI] [PubMed] [Google Scholar]
- 28.Lymperaki E, Makedou K, Iliadis S, Vagdatli E. Effects of acute cigarette smoking on total blood count and markers of oxidative stress in active and passive smokers. Hippokratia 2015;19:293–7. [PMC free article] [PubMed] [Google Scholar]
- 29.Nair S, Kulkarni S, Camoens HM, Ghosh K, Mohanty D. Changes in platelet glycoprotein receptors after smoking--a flow cytometric study. Platelets 2001;12:20–6. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.