Abstract
The lack of new antibiotics is among the most critical challenges facing medicine. The problem is particularly acute for Gram-negative bacteria. A novel antibiotic strategy is to target bacterial nutrition and metabolism. The metal gallium can disrupt bacterial iron metabolism as gallium can be taken up by bacteria, and replace iron. Here we performed pre-clinical work and a phase 1 human trial to investigate the antibiotic activity of gallium in people with cystic fibrosis (CF) and chronic Pseudomonas aeruginosa airway infections. We found that CF sputum was iron-limited, and that low micromolar concentrations of gallium inhibited P. aeruginosa growth in CF sputum. Ex vivo experiments indicated that gallium inhibited key iron-dependent enzymes, and increased bacterial sensitivity to oxidants. We also found that gallium resistance developed at low rates, its activity was synergistic with some antibiotics, and it did not affect P. aeruginosa killing by human macrophages. Finally, we tested parenteral gallium in murine lung infections, and in CF patients with chronic P. aeruginosa lung infections and found indications of safety and efficacy. These data represent a small step toward targeting iron metabolism, or other nutritional vulnerabilities of bacteria, to treat human infections.
One Sentence Summary:
The metal gallium disrupts the functioning of key iron-dependent enzymes in bacteria, effectively treats lethal animal infections and shows preliminary indications of efficacy in humans with chronic airway infections.
Introduction
In addition to treating primary infection, antibiotics provide a protective umbrella under which much of modern medicine operates. Patients undergoing surgery, invasive procedures, immune modulation, and cancer therapy all depend upon antibiotics. However, antibiotic effectiveness is threatened. Among the most pressing challenges are escalating resistance of both hospital and community acquired organisms (1, 2), and the increasing prevalence of pathogens with high-level intrinsic resistance (3). In addition, antibiotics work poorly against chronic infections, as the bacterial growth mode at chronic infection sites produces an antibiotic-tolerant phenotype (4). The problem is particularly acute for Gram-negative bacteria because of their low cell wall permeability, and effective and redundant efflux systems (5, 6).
A novel approach to combat infection is to exploit nutritional vulnerabilities of bacteria and bacterial iron (Fe) metabolism is a prime candidate. Iron is essential for almost all pathogens, as it is required in enzymes mediating DNA synthesis, electron transport, oxidative stress defense, and other key processes (7). Moreover, free iron levels are extremely low in vivo (~10−20 M) due to the insolubility of iron in aerobic environments and the multiple host defenses that sequester Fe (7). In addition, in vitro work indicates that iron metabolism may be a particular vulnerability for organisms in biofilm-like aggregates that cause chronic infections in people with wounds, cystic fibrosis (CF), and other conditions (8). Despite these factors, therapeutics targeting bacterial iron metabolism have been difficult to realize. So far, much of the focus has been on developing pharmacologic iron chelators, and a key problem is that some pathogens can liberate chelator-bound iron (9).
One alternative approach uses the metal gallium as a “Trojan horse” to disrupt iron metabolism. Gallium has a nearly identical ionic radius as Fe, and some bacterial uptake systems are unable to distinguish gallium from iron (10, 11). Gallium disrupts iron-dependent processes because Ga3+ cannot be reduced in physiological conditions, and iron’s biological functions involve redox cycling (11). Thus, gallium incorporation into iron-containing proteins disrupts their functioning. Previous work by others and us found that gallium compounds had antibacterial activity against a number of human pathogens including Pseudomonas aeruginosa (12), Fransella tulerensis (13), Acinetobacter baumanai (14), several mycobacterial species (15, 16), Klebsiella pneumoniae (17, 18), and other important pathogens (19–21). Work with P. aeruginosa showed that gallium was effective against bacteria grown as biofilms, in stationary phase cultures, and against multidrug-resistant CF clinical isolates (12).
Here we performed translational work to test gallium’s utility as an anti-infective treatment. We measured gallium’s activity in human CF sputum, performed experiments to better understand gallium’s mechanism of action and the potential for gallium resistance, and studied gallium’s combined activity with conventional antibiotics. We also tested gallium in murine infections, and report the results of a proof of principle human trial of intravenous (IV) gallium in people with CF and chronic P. aeruginosa lung infections.
Results and Discussion
Iron is a growth-limiting nutrient in CF sputum.
Previous work shows that high ambient iron levels can reduce gallium uptake by P. aeruginosa, and blunt gallium’s antibacterial activity (12). These finding suggest that gallium’s potency would be enhanced if iron was a growth-limiting nutrient for P. aeruginosa in CF sputum. We tested this in two ways. First, we investigated whether exogenous iron addition would increase P. aeruginosa growth in CF sputum. We prepared CF sputum for bacterial growth measurements by mixing freshly expectorated samples 1:1 with saline, and then removed solids and endogenous bacteria by centrifugation followed by filtration. Adding FeCl3 markedly increased both P. aeruginosa growth rate and cell yield, in sputum obtained from 4 patients with CF (Figs 1A, S1A, S2).
Figure 1. Expectorated Sputum from CF patients is iron limited.
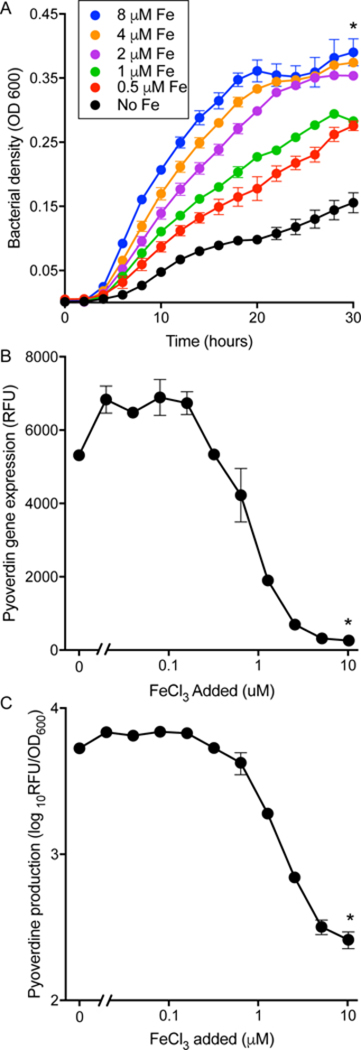
A. Addition of indicated concentrations of FeCl3 to sputum supernatants increased the growth rate and cell yield of inoculated P. aeruginosa. Results are representative of 5 sputum samples (see Figs S1 and S2); error bars indicate SEM; * indicates p<0.01 vs. no iron addition. B and C. P. aeruginosa incubated in CF sputum highly express the pyoverdine biosynthetic gene pvdA (as measured by a pvda-gfp reporter) (B), and produce high levels of pyoverdine indicating iron starvation (C). Addition of FeCl3 suppressed both effects. Results are mean of 3 replicates and representative of 3 experiments; error bars indicate SEM; * indicates p<0.01 vs. no iron addition. Also see Fig S1 for experiments with sputum from another subject.
Second, we investigated whether growth in sputum induced bacterial iron starvation genes using P. aeruginosa expressing a GFP reporter linked to the gene that encodes the key P. aeruginosa iron uptake protein pyoverdine, whose production is induced under iron-limited conditions (strainpvdA-gfp), and by directly measuring pyoverdine. As shown in Figs 1B and C and S1 B and C, pvdA and pyoverdine were highly induced during growth in sputum, and expression was repressed by adding exogenous iron. These findings, along with previous work measuring bacterial gene expression (22, 23), indicates that P. aeruginosa growing in CF sputum are likely iron limited.
Fe-limiting conditions in sputum could enhance gallium’s antibacterial effect.
We measured gallium’s antimicrobial activity in CF sputum with and without iron addition to determine if sputum iron-limitation contribute to gallium’s efficacy. In the absence of added iron, 4.0 or 5.0 μM gallium completely inhibited P. aeruginosa growth in all sputum sample we tested, and in some samples 10-fold lower gallium levels were effective (Figs 2A and S3). We tested the effect of adding growth-stimulatory concentrations of iron to sputum, and found that whereas the antibiotic effect of low gallium levels were blunted, gallium concentrations that strongly suppressed growth in the un-supplemented condition (4 and 6μM) were still effective after FeCl3 addition (Figs 2B).
Figure 2. Gallium inhibits P. aeruginosa growth in CF sputum.
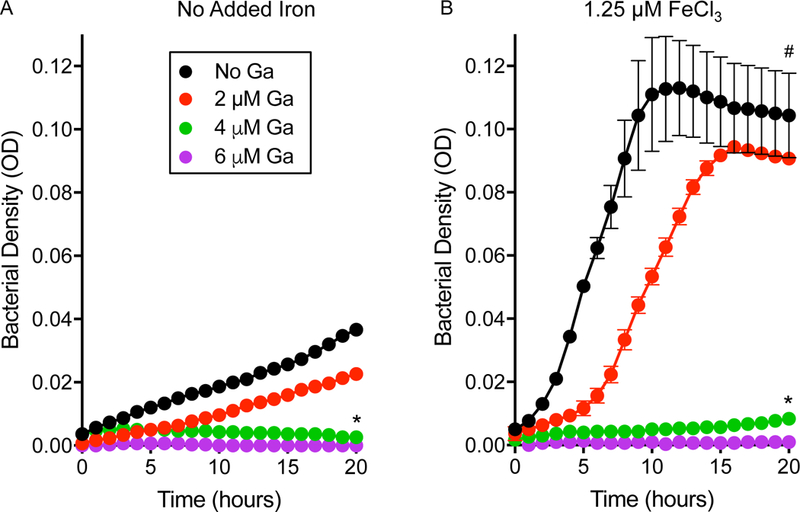
Gallium inhibited the growth of P. aeruginosa inoculated into CF sputum supernatants that were not (A) and were (B) supplemented with FeCl3. FeCl3 addition markedly increased P. aeruginosa growth rate and cell yield, but Ga(NO3)3 concentrations that effectively suppressed growth in the un-supplemented condition (4 and 6 μM) remained effective at suppressing P. aeruginosa growth. Results are mean of 3 replicates and representative of 3 experiments; error bars indicate SEM; * indicates p<0.01 vs. no gallium; # indicates p<0.01 vs. no added iron.
The findings that CF sputum is iron-limited, that low levels of gallium are inhibitory in sputum, and higher gallium can overcome the effects of growth-stimulating iron are encouraging. However, high in vivo iron levels likely occur in some circumstances and could blunt gallium’s activity. This may be a particular problem in severe CF, as previous work identified a negative correlation between total iron and Fe+2 levels, and lung function (24). Notably, our previous work in laboratory media shows that higher molar ratios of iron (than gallium) are required to restore growth of gallium-treated bacteria (12), and that gallium inhibits P. aeruginosa iron uptake (12). These factors could somewhat mitigate the effects of increased in vivo iron availability.
Gallium inhibits some Fe-containing enzymes in P. aeruginosa.
We performed studies to better understand gallium’s mechanism of action against P. aeruginosa by investigating gallium’s effect on key Fe-containing enzymes. The iron-dependent enzyme ribonucleotide reductase is essential for DNA synthesis, and gallium has been shown to inhibit M. tuberculosis growth by inhibiting cellular ribonucleotide reductase activity (16).
We investigated gallium’s effect in an iron-rich media in which gallium’s antimicrobial activity was inhibited, to reduce the chance that enzyme activity measurements were confounded by non-specific changes associated with bacterial death. We grew P. aeruginosa in gallium, then lysed cells and measured the activity of isolated ribonucleotide reductase. As shown in Fig 3A and consistent with our previous observations with M. tuberculosis (16), gallium progressively inhibited P. aeruginosa ribonucleotide reductase activity reaching a maximum of ~ 40% inhibition with 20 μM in this medium. However, further inhibition was not seen when the gallium concentration was increased (Fig. 3A). This result raises the possibility that gallium inhibits one of the two classes of P. aeruginosa ribonucleotide reductase (25), but not the other.
Figure 3. Gallium inhibits P. aeruginosa catalase and ribonucleotide reductase, but not SOD or aconitase activity.
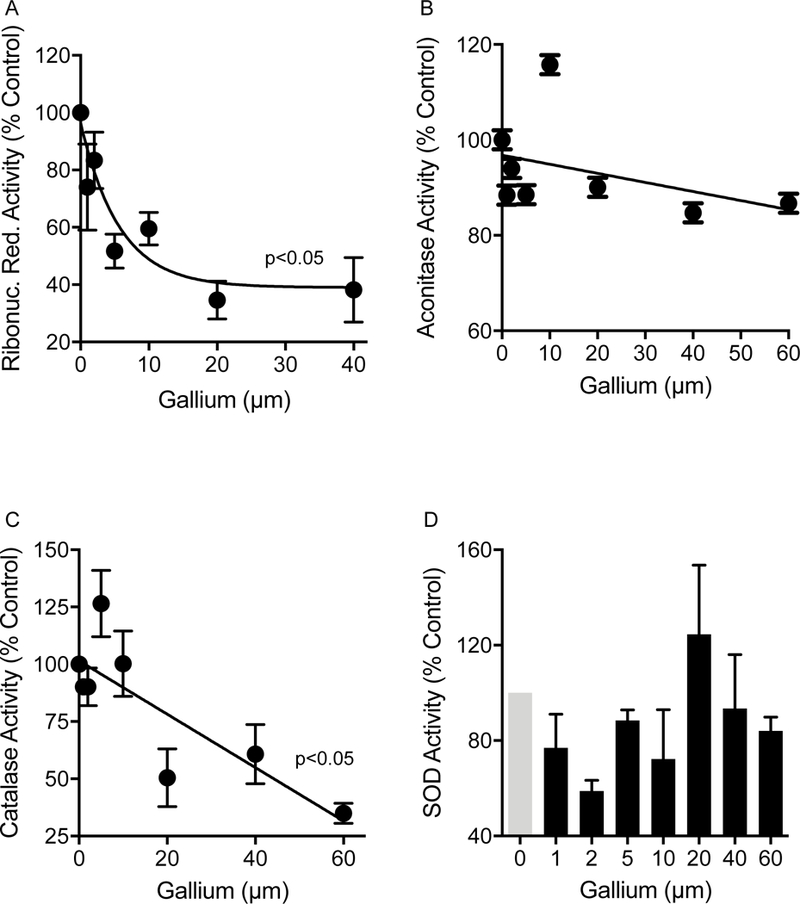
P. aeruginosa (strain PAO1) was incubated overnight in an iron-rich medium (10% TSB) containing increasing concentrations of Ga(NO3)3. The bacteria were harvested, lysed, and enzymatic activity determined for ribonucleotide reductase (A); aconitase (B); catalase (C); and superoxide dismutase (SOD) (D). Results shown are representative of 5 experiments and are mean enzyme activity relative to bacteria not treated with gallium; error bars indicate SEM. Gallium exposure significantly inhibited ribonucleotide reductase and catalase activity at gallium concentrations ≥2.5 μM and ≥ 20 μM, respectively (p<0.05, ANOVA). No significant effect of gallium on aconitase or SOD activity was observed.
We also exposed live bacteria to gallium to investigate gallium’s effect on the activity of aconitase, a Fe-sulfur enzyme that catalyzes the isomerization of citrate to isocitrate in the tricarboxylic acid cycle (16). Previous work indicated that exposure to gallium decreased M. tuberculosis aconitase activity (16). However, we found no effect of gallium on P. aeruginosa aconitase activity, even after a 24 hr incubation with up to 60 μM gallium (Fig 3B), which inhibited bacterial growth in this medium.
Catalase and Fe-superoxide dismutase (Fe-SOD) are key bacterial antioxidant enzymes that contain iron in their active sites. Consistent with our previous results with F. novicida (13), incubation of P. aeruginosa in the presence of increasing concentrations of gallium decreased P. aeruginosa catalase activity up to 70% (Fig 3C). However, in these assay conditions, we found no detectable inhibition of P. aeruginosa SOD activity (Fig 3D).
Gallium ability to generally substitute for iron suggests that gallium may inhibit or interfere with several iron-dependent processes. Consistant with this, our previous gene expression analysis (Table S1 and (12)) indicates that sub-inhibitory gallium disrupts several aspects of bacterial physiology. The most pronounced effects were on iron metabolism, and gallium produced some expression changes characteristic of Fe starvation and others that typify the Fe-replete state. Particularly notable was repression of the Haem/haemoglobin uptake receptor and the transcriptional regulator pvdS and genes under its control, which include genes mediating biosynthesis of pyoverdine, the pyoverdine receptor, and others (26). Gallium also altered the expression of genes involved in carbon utilization, reduced expression of ribosomal genes, and suppressed oxidative stress response genes.
The multiplicity of gallium effects could provide advantages of target redundancy, which could reduce the emergence of resistance (see below). In addition, it is notable that the bacterial functions that we identified as being gallium-inhibited are not targeted by antibiotics in clinical use. This could explain our previous finding that multidrug resistant P. aeruginosa isolates showed similar levels of gallium sensitivity as antibiotic-sensitive strains (12).
Gallium increases P. aeruginosa oxidant sensitivity.
Gallium-mediated inhibition of catalase activity could increase bacterial sensitivity to oxidants, which are key effectors of epithelial and phagocyte-mediated bacterial killing (27). To explore this possibility, we exposed P. aeruginosa to sub-inhibitory concentrations of gallium and then measured P. aeruginosa killing by oxidants that are primarily detoxified by catalase. Gallium exposure increased sensitivity of P. aeruginosa to killing by H2O2 and to tert-Butyl hydroperoxide (tBuOOH) (Fig. 4A&B). In contrast, gallium did not increase sensitivity to paraquat (PQ) or phenazine methosulfate (PMS) (Fig 4C&D), which primarily generate superoxide. These finding are consistent with our findings that gallium inhibits catalase that catabolizes H2O2, but not superoxide dismutase that converts superoxide to H2O2.
Figure 4. Gallium sensitizes P. aeruginosa to killing by peroxides.
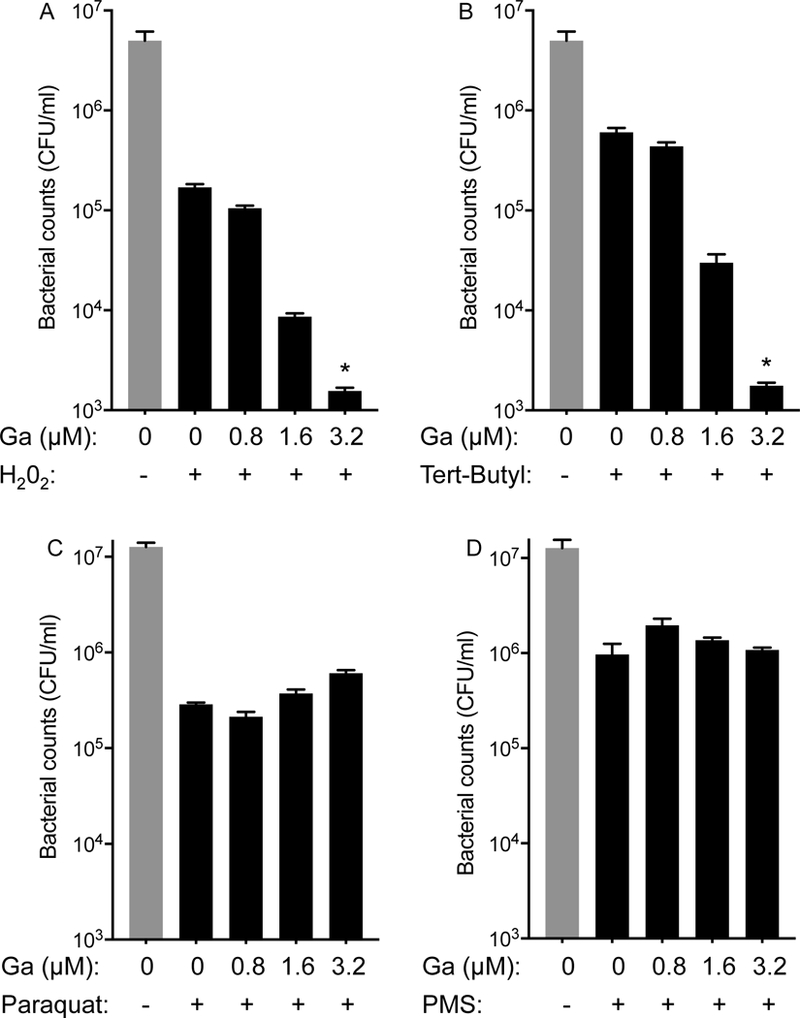
Bacteria were grown without and with the indicated sub-inhibitory concentrations of Ga(NO3)3 and then exposed to oxidants generating peroxide, including H2O2 (A) and tert-Butyl hydroperoxide (Tert-Butyl) (B); and superoxide, including paraquat (C) and phenazine methosulfate (PMS) (D). Sub-inhibitory Ga(NO3)3 increased sensitivity to killing by peroxides. Error bars indicate SEM, data are mean values of three replicate experiments and representative of three independent experiments; error bars indicate SEM; * indicates p<0.01.
P. aeruginosa develops gallium resistances at rates comparable to successful antibiotics.
Most successful antibiotics inhibit multiple essential bacterial functions and this may slow drug resistance (28). For example, ciprofloxacin targets DNA gyrase and topoisomerase, and β-lactam antibiotics target multiple penicillin-binding proteins (28). The fact that gallium can substitute for iron in many proteins and could interfere with multiple functions (Table S1) led us to hypothesize that P. aeruginosa may develop resistance to gallium at low rates, similar to successful antibiotics that have multiple targets.
We compared the frequency at which spontaneous P. aeruginosa mutants with resistance to gallium (and conventional anti-pseudomonal antibiotics) arise. Spontaneous resistance was defined as the heritable ability to grow in the presence of four times the minimal inhibitory concentration (MIC) of each agent. We used P. aeruginosa in which pyoverdine genes were inactivated (for all agents tested) as pyoverdin interfered with the assay (Fig S4). Approximately one in ten million P. aeruginosa cells spontaneously developed resistance to gallium and colistin. Spontaneous resistance to ciprofloxacin occurred approximately two times less frequently (~1 in 20 million cells), and resistance to tobramycin occurred approximately ten times more frequently (10 in ten million cells) (Table 1). A modified assay that reduced the interfering effects of pyoverdine showed that wild-type P. aeruginosa also exhibited similar spontaneous resistance frequencies to gallium and conventional antibiotics (Table S2).
Table 1.
Frequency of spontaneous P. aeruginosa mutants
| Antibacterial agent | Resistant Mutant Frequency |
|---|---|
| Gallium (20 μM) | 1.11 × 10−7 ± 5.18 × 10−8 |
| Tobramycin (4μg/ml) | 9.84 × 10−7 ± 1.12 × 10−7 |
| Colistin (2 μg/ml) | 1.17 × 10−7 ± 2.71 × 10−8 |
| Ciprofloxacin (2 μg/ml) | 0.51 × 10−7 ± 9.82 × 10−10 |
Stationary phase cultures of P. aeruginosa pyoverdine mutant (see Fig S$) were plated on agar containing four times the MIC of the indicated agents, and the number of resistant mutants were enumerated.
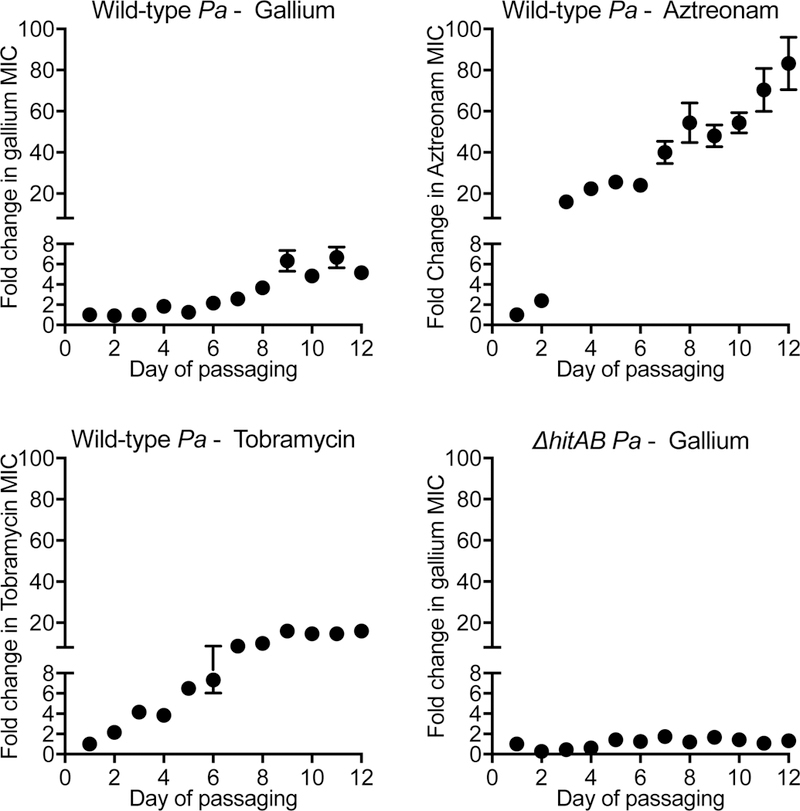
We also measured the rate at which mutations arise under selection by passaging 12 replicate cultures of wild-type P. aeruginosa in gallium, aztreonam, and tobramycin for 12 days. Increases in gallium resistance were modest, and were less that those seen with the tested anti-pseudomonal antibiotics (Fig 5). The relatively low rates of gallium resistance in both assays is consistent with our previous finding that except for one outlier (out of 115 strains tested), the most resistant P. aeruginosa clinical isolate we tested had an inhibitory concentration only 4-fold higher than the gallium-susceptible laboratory strain, PAO1 (12).
Figure 5. Continuous passaging modestly increases gallium resistance.
Twelve replicate cultures of wild type (A-C) and AhitAB P. aeruginosa (D) were passaged in in gallium (A and D), aztreonam (B), and tobramycin (C). Bacteria were initially grown in the absence of drug, then subcultured into a range of drug concentrations. Each day, cells from the highest concentration of drug that supported growth was recorded, and cells from this condition were re-inoculated into a range of drug concentrations. The mean fold change in highest drug concentration that permitted growth (of 12 replicate cultures) is plotted as a function of the passaging day. Error bars indicate SEM. The highest drug concentration that permitted growth at in these conditions at day 0 was 1 μg/ml tobramycin and aztreonam, and 64 μg/ml gallium for wild type P. aeruginosa, and 128 μg/ml gallium for ΔhitAB P. aeruginosa.
Transposon mutagenesis identifies few gene inactivations producing gallium resistance.
Recent work using transposon mutagenesis in P. aeruginosa PA14 found that inactivation of the hitA gene, which encodes a periplasmic iron Fe+3 transporter, produced 4-fold reductions in gallium sensitivity (29). We used three approaches to determine if additional resistance-producing gene inactivation mutations could be identified.
First, because resistance elements may be strain-specific, we repeated transposon mutagenesis using the P. aeruginosa strain PA01, which is the reference strain most phylogenetically-related to CF clinical isolates, and is among the most divergent reference strain from PA14 (30, 31). Genome saturation-level transposon mutagenesis in PA01 (total of ~ 120,000 mutants screened) found no additional mutants (other than hitA, as found by (29)) to be associated with gallium resistance.
Second, we performed genome-saturating transposon mutagenesis in a PAO1 strain in which the hitAB genes had been deleted. This screen of ~240,000 transposon mutants found only 2 mutants with gallium resistance higher than the hitAB deletion strain. The transposon insertions were mapped to the open reading frame ofpa5248, which has homology to an inner membrane iron permease gene (the FTR1/Fip1/EfeU family), and to the intergenic region between the pvdA and fpvI genes, both of which are involved in iron acquisition (26). Notably the degree of resistance produced by adding transposon mutations in PA5248 or thepvdA-fpvI intergenic region to the hitAB deletion strain was modest (2–4 fold increases in resistance, Fig S5).
Third, we passaged 12 replicate cultures of the P. aeruginosa hitAB deletion strain in gallium for 12 days, and found that whereas this strain exhibited a higher starting inhibitory concentration than wild type, it developed minimal increases in gallium resistance after prolonged passaging (Fig 5D). Taken together this work suggests that marked gallium resistance is not likely to occur at high frequencies, and that inactivation of the hitAB Fe+3 transporter is the main pathway to resistance.
Gallium is synergistic with two anti-pseudomonal antibiotics.
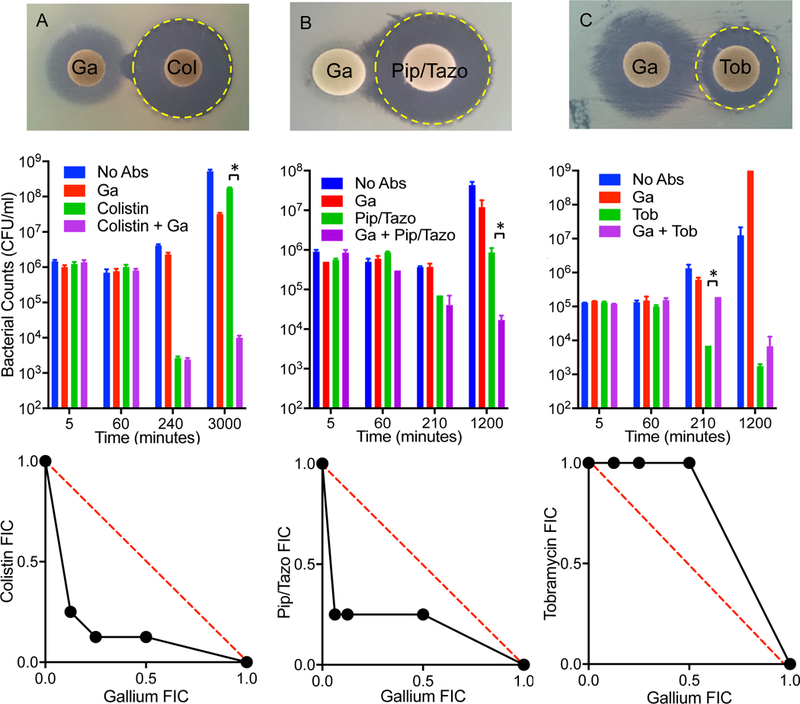
Gallium’s unique mechanism makes its combined activity with antibiotics difficult to predict. We used three independent assays to measure the combined activity of gallium and antibiotics. The agar disc diffusion method (32) measures combined activity by placing gallium-and antibiotic-containing disks in proximity to each other, and detecting either a concave (indicating antagonism) or convex (indicating synergism) inhibition zone between the disks. The time kill assay (32) measures the effect of a sub-inhibitory concentration of gallium in combination with bactericidal concentrations of other antibiotics. The checkerboard (isobologram) assay (32) measures the combined growth inhibitory activity of a range of gallium and antibiotic concentrations in liquid culture minimum inhibitory concentration (MIC)-type assays All three assays (disc diffusion, time kill, and checkerboard assays) detected synergistic interactions between gallium and colistin (polymyxin E), and gallium and piperacillin/tazobactam; and antagonistic interactions between gallium and tobramycin (Figs 6). Neither synergistic or antagonistic interactions effects were seen with ciprofloxacin, aztreonam, or ceftazadime (Fig S6). These finding could inform future clinical studies that combined gallium with conventional antibiotics.
Figure 6. Combined activity of gallium with antibiotics.
Gallium was synergistic with colistin (A) and piperacillin/tazobactam (B), but antagonistic to tobramycin (C). Photographs (top) show disc diffusion assays. Synergistic activity was indicated by the increased zone of inhibition produced by colistin and piperacillin/tazobactam in the proximity of the gallium-containing disc. Antagonistic activity was indicated by the decreased zone of inhibition produced by tobramycin in the proximity of the gallium-containing disc. The yellow dotted line represented the expected activity (in preventing P. aeruginosa growth) of the antibiotic in the absence of gallium. Graphs (middle) show time-kill assays using sub-inhibitory concentrations of gallium and inhibitory concentrations of antibiotics. Synergistic activity was indicated by the ability of sub- inhibitory gallium to enhance killing by colistin and piperacillin/tazobactam. Antagonistic activity was indicated by the ability of sub-inhibitory gallium to reduce killing by tobramycin. Error bars indicate SEM. Isobolograms (bottom) show results of checkerboard assays presented as the fractional inhibitory concentrations (FICs) of the 2 factors in combination. Calculations are described in methods. Experiments were repeated 2–4 times, each with similar results.
Gallium does not inhibit the antimicrobial activity of macrophages.
Gallium is used clinically to treat hypercalcemia of malignancy as it inhibits bone reabsorption by osteoclasts (11, 31), which are myeloid cells. Macrophages (also myeloid cells) are present in chronically-infected CF airways and take up gallium (33). These facts raise concern that Ga could negatively affect macrophage function. To test this, we isolated human monocytes, differentiated them to monocyte-derived macrophages (HMDMs), and treated them with vehicle or Ga. We used a long exposure (24h), and a gallium (100 μM) concentration that was ~20–200 times the level that inhibited P. aeruginosa in sputum (Figs 1, 2, S1 and S3), and ~10 times the level detected in sputum in the clinical trial (see below) for these experiments to maximize chances of detecting toxic effects.
Gallium treatment did not reduce HMDM viability (Fig S7A), but it did modestly effect the expression of some genes mediating bacterial uptake and killing (Fig S6B). We directly tested gallium’s effects on macrophage antimicrobial activity using HMDMs isolated from 2 healthy donors and found that continuous exposure to 100μM gallium for 24 hours did not reduce macrophage P. aeruginosa killing (Fig S6C).
Parenteral gallium effectively treats P. aeruginosa mouse lung infections.
Inhalation delivery of antibiotics can increase airway drug concentrations while reducing systemic toxicity, and both of these advantages could enhance gallium’s utility. However, testing gallium inhalation in humans would require a significant investment in pulmonary toxicology studies that may be difficult to realize given gallium’s unique mechanism and because the general concept of targeting bacterial nutrition is unproven. These factors led us to pursue proof of principle experiments in humans using intravenous gallium nitrate (see below) which is already FDA approved.
We began by testing parenteral gallium in a mouse model of P. aeruginosa lung infections, using a single dose of gallium nitrate administered 3 or 12 hours after mice were infected with P. aeruginosa. Gallium treatment increased mouse survival (Fig 7A) and reduced lung and blood P. aeruginosa counts (Fig 7B). To determine if disrupted iron metabolism explained gallium’s efficacy, we exogenously added an iron solution into mouse airways immediately prior to infection, and found iron addition reduced gallium’s therapeutic effect (Fig 7C). These data show that systemic gallium is effective in a model acute lung infection (even when administered well after the bacteria) and suggest that as seen in vitro, gallium’s in vivo activity results from disruption of Fe-dependent processes.
Figure 7. Parenteral gallium treats murine lung infections.
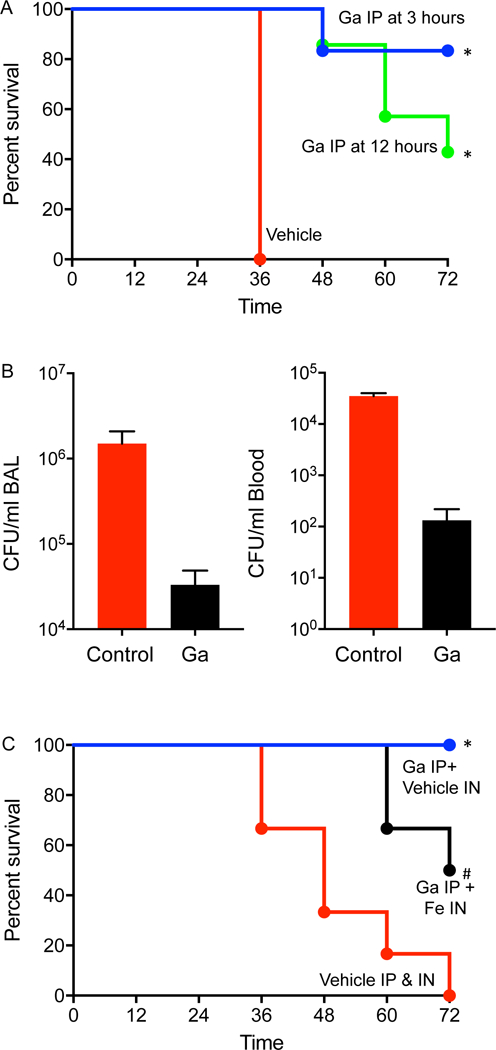
A. A single dose of gallium-free vehicle (red line) or gallium (50 μl of 250 mM Ga(NO3)3) was administered by the intraperitoneal (IP) route 3 hours (blue line) or 12 hours after (green line) intratracheal infection with P. aeruginosa (n=7 mice for Ga and 8 for vehicle); * indicates P< 0.001 vs. vehicle control.B. P. aeruginosa were enumerated in bronchoalveolar lavage fluid and blood sampled 12 hours after mice were infected by the intratracheal route and treated with gallium (IP) or vehicle (IP) 3 hours after infection (n=4 mice for vehicle alone and 5 for Ga). * indicates P< 0.001 vs. vehicle control. C. Administering intranasal (IN) FeCl3 (10 μl of 2 mM FeCl3) (black line) at the time of infection reduced the protective effect of intraperitoneal gallium, as compared intranasal iron-free vehicle (blue line) * indicates P< 0.001 vs. vehicle control; # indicates P< 0.05 vs. vehicle control; p=0.055 vs. no IN FeCl3. The red line shows mouse survival without gallium (vehicle administered IP and IN) (n=6 mice in each group).
A proof of principal phase 1 clinical trial of gallium in CF.
Given in vitro and in vivo preclinical data suggesting efficacy, the availability of an FDA-approved intravenous formulation (Ga(NO3)3 is FDA approved for hypercalcemia of malignancy), and the challenges inherent to inhaled treatment, we tested IV gallium in people with CF and chronic P. aeruginosa lung infections in a pilot Phase 1b non-randomized study (investigator initiated IND 104,363; ClinicalTrials.gov NCT01093521).
Patients were included if they were between 18 and 55 years of age; had a confirmed diagnosis of CF with chronic P. aeruginosa lung infection; and did not have severe lung dysfunction (forced expiratory volume of one second (FEV1) > 30% of predicted). In addition, subjects were required to be without clinically significant renal or liver disease, and not be experiencing acute disease flares (see Table S3 and S4 for inclusion and exclusion criteria and enrollment). We enrolled 20 patients, evenly divided between genders, with a median age of 32.8 yrs (range: 19 to 54.2), and mean forced expiratory volume in one second (FEV1) of 2.24 L (range: 1.06 to 4.59 L). The primary endpoints were safety, tolerability and pharmacokinetics of the drug, however we also measured efficacy endpoints including change in lung function as measured by FEV1 and forced vital capacity (FVC) (from baseline to days 7, 14, and 28) and change in sputum P. aeruginosa density (from baseline to days 7, 14 and 28).
Gallium can cause nephrotoxicity after large IV boluses because rapid infusion saturates the binding capacity of gallium-binding proteins (11, 34). CF patients frequently receive nephrotoxic drugs, so we administered Ga(NO3)3 by slow continuous IV infusion over 5 days, which has been shown to markedly reduce nephrotoxicity as compared to bolus infusion (11). Subjects were also instructed to consume 2 liters of fluid above their normal intake during the infusion period. In addition, we sequentially studied two dose regimes to enable early detection of adverse effects. Cohort 1 received a low dose, 100 mg/m2/day (9 subjects, 4 males and 5 females), and cohort 2 received 200 mg/m2/day (11 subjects, 6 males and 5 females).
IV gallium appears safe and pharmacokinetics indicates sustained sputum levels.
No serious adverse events were noted, and kidney function, electrolyte levels, and blood counts were all unaffected. See Table S5 and Fig S8 for these and additional safety parameters. Steady state plasma and sputum concentrations were achieved by 2 days (Figs 8A&B and S9). Gallium plasma and sputum area under the curve concentrations (AUC) did not change with increased dose (Table S6), likely because gallium’s saturable protein binding (e.g. to transferrin and albumin (35)) causes unbound gallium concentrations to increase at higher doses, which in turn increases renal and non-renal elimination (34).
Figure 8. IV gallium produces sustained blood and sputum levels, and improves lung function.
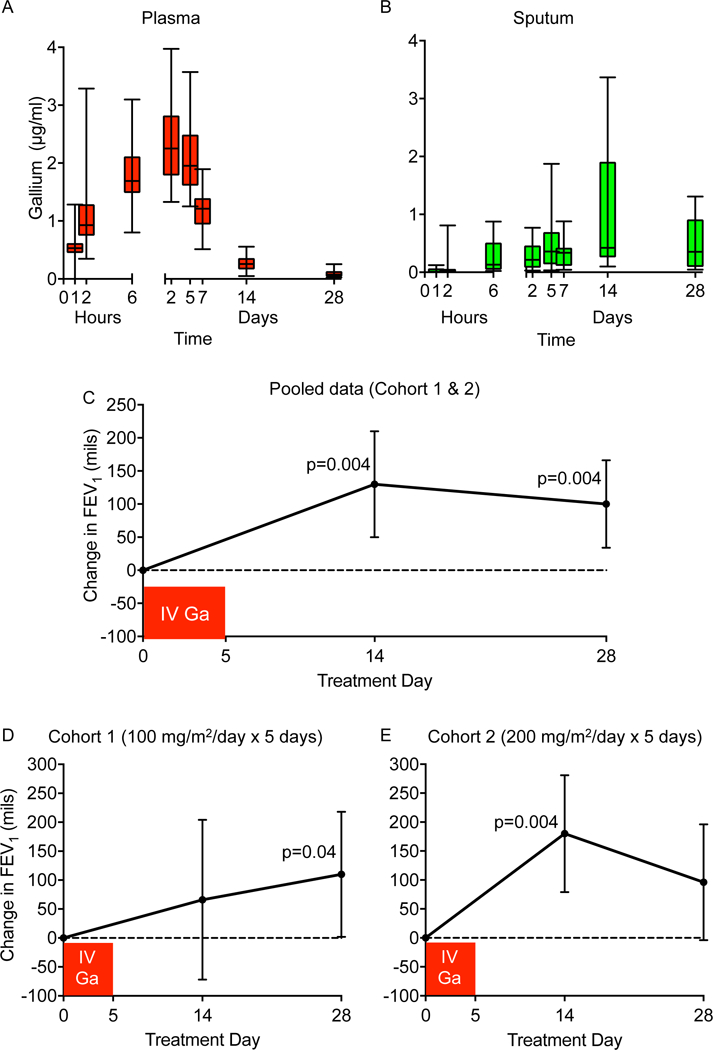
Plasma (A) and sputum (B) gallium levels in CF subjects treated with IV gallium for 5 days; boxes show 25th to 75th percentiles, hatches shows means, and whiskers show minimum and maximum values. Data shown are for cohort 1 and 2 (100 mg/m2 per day and 200 mg/m2 per day, respectively) combined. See Fig S9 for data from each cohort separated. Mean change in lung function (as measured by FEV1 in mls) by study day are for both cohorts pooled together (C), and cohort 1 (D) and 2 (E) separately (100 mg/m2 per day and 200 mg/m2 per day, respectively). Bars represent the 95% Confidence Intervals; * represents p values > 0.005.
Plasma and sputum gallium levels remained detectable for prolonged periods (Figs 8A&B and S9). The average plasma and sputum elimination half-life (T1/2β) of gallium exceeded 100 and 220 hours, respectively, in both cohorts (Table S6). Sputum concentration continued to increase after the end of the infusion, increasing on average over 2 fold by day 14, and only decreased by ~ 50% on Day 28 (Fig. 8B and Table S6). These findings suggest that a depot compartment exists, which could explain the prolonged improvement in lung function we observed (see below).
IV gallium may improve lung function.
Analysis of pooled data (from cohorts 1 and 2) showed statistically significant increases in lung function (both FEV1 and FVC) 14 and 28 days after a single infusion of gallium (Fig. 8C, Tables 2 and S7). Data from the individual cohorts are shown in Fig 8D and E and Table S7. When defined by the proportion of subjects achieving a 5% FEV1 improvement, 73% and 45% of cohort 2 were responders at day 14 and day 28, respectively compared to 22% and 44% of cohort 1 at day 14 and day 28, respectively. However, we found no correlation between lung function response and peak or area under the curve sputum gallium levels.
Table 2:
Lung function and microbiology in CF patients at baseline, day 14 and day 28.
| Endpoint | Mean and Median Baseline Day 0 |
Mean and Median Change from Day 0–14 |
P Value |
Mean and Median Change from Day 0–28 |
P Value |
|---|---|---|---|---|---|
| All Patients N=20 | |||||
| FEV1 (L) Mean (SD) Median (IQR) |
2.24 (0.84) 2.1 (1.68 to 2.58) |
0.13 (0.17) 0.075 (0.015 to 0.22) |
0.0041 0.0064 |
0.10 (0.14) 0.085 (0.00 to 0.18) |
0.0041 0.0042 |
| Proportion with 5% change in FEV1 (95% CI) | Na | 50% (27% to 73%) | 45% (23% to 69%) | ||
| FVC (L) Mean (SD) Median (IQR) |
3.58 (1.0) 3.53 (3.14 to 4.04) |
0.13 (0.15) 0.12 (0.01 to 0.23) |
0.0010 0.0007 |
0.16 (0.15) 0.15 (0.045 to 0.24) |
0.0001 0.0001 |
| Sputum P. aeruginosa (million CFU/gm) Mean (SD) Median (IQR) |
N=19 117 (200) 48.4 (5.5 to 137.5) |
N=19 −5.51 (218) −1.83 (−87 to 74) |
0.9134 0.5949 |
N=17 −29.8 (171) −3.9 (−105 to 55) |
0.4825 0.5171 |
Table notes both mean and median values with standard deviation (SD) and interquartile range (IQR). Lung function is measured by both forced expiratory volume in one second (FEV1) and forced vital capacity (FVC) both in liters. Quantitative sputum P. aeruginosa concentrations are measured in (million CFU/gm). Given 5% improvement in FEV1 is considered a meaningful improvement, we report the proportion of patients with a 5% improvement in FEV1. The change in proportion is noted by the 95% Confidence Intervals (95% CI) of that change.
The magnitude of gallium-associated lung function improvement is similar to that produced by approved antibiotics in CF. For example, 14 day-long treatment using a combination of two IV anti-pseudomonal antibiotics chosen based on the antimicrobial susceptibility of subject’s P. aeruginosa strains improved FEV1 by ~ 7%, when measured immediately (12–48 hours) after the last antibiotics dose (36). Likewise, high doses of tobramycin and aztreonam administered by inhalation twice or three times daily for month-long courses improved FEV1 by 2.7–10.3%. Notably, the lung function improvements produced by conventional antibiotics in these studies waned 2–4 weeks after the drug was stopped (37–40), whereas gallium-associated changes persisted to the last time point we measured (day 28) (Fig 8C).
Sputum P. aeruginosa counts declined but did not reach significance.
Gallium treatment reduced sputum P. aeruginosa concentrations. Mean and median P. aeruginosa density declined by 5.5 million and 1.8 million CFU/gm between day 0 and 14; and by 29.8 million and 3.9 million CFU/gm between day 0 and 28 (Table 2). While the change was in the appropriate direction of a treatment response, they were not statistically significant.
Study limitations.
This study had several limitations. First, the sputum activity, mechanism of action, resistance and synergy, and animal experiments were performed with the P. aeruginosa strain, PA01, and it is possible that different strains could give different results. This limitation is mitigated somewhat because PA01 is the reference strain most phylogenetically related to CF isolates (30). Furthermore, our previous work testing 120 CF clinical P. aeruginosa isolates (12) (including many multi-drug resistant strains) found these generally gallium susceptible. In addition, the encouraging results of our phase 1 human trial suggest that gallium activity is not dependent on the presence of a particular P. aeruginosa strain as CF subjects are generally infected by different environmental strains.
Second, while we assayed resistance using three independent assays (i.e. spontaneous mutants, passaging under selection, and transposon mutagenesis) and combined action with antibiotics using three independent assays (disc diffusion, time kill, and checkerboard) and found concordant results, it is possible that these do not reflect in vivo activity.
Third, while gallium treatment did produce statistically-significant improvements in lung function (despite the small “n”), declines in P. aeruginosa burden did not reach significance. The absence of statistically-significant reductions in P. aeruginosa counts could be due to the variability of enumerating bacterial counts in sputum, or if gallium’s beneficial effect on lung function was due to its previously-described anti-biofilm (12) or anti-inflammatory (41–44) effects, or activity against other bacteria that may co-inhabit CF airways. In addition, subjects were studied during disease quiescence, and antibiotics and anti-inflammatories generally show greater clinical effects during CF disease flares (45, 46).
Fourth, parenteral treatment may be impractical long-term. Our objective here was to exploit the FDA-approved formulation to rapidly perform a proof-of-principal study in humans in urgent need of new treatment approaches. In addition, parenteral gallium’s unusual sputum pharmacokinetics produces uncertainty about when lung function and microbiology responses to gallium should be assessed in future studies.
Fifth, and most importantly, the clinical trial was small, un-blinded, did not have a placebo control, and was limited to subjects with mild CF lung disease. Thus, the promising safety and efficacy results need to be confirmed in a larger randomized study, and in subjects with more advanced disease.
Concluding remarks.
The public health crisis of antibiotic resistance has spurred studies of non-conventional antimicrobial approaches. While the idea of disrupting bacterial nutrition as an antimicrobial strategy was raised by Louis Pasteur in the 1800’s (47), therapeutic approaches that exploit bacterial nutrient vulnerabilities have been difficult to develop. Our work provides preliminary proof of concept evidence that using gallium as a chemical mimic to disrupt P. aeruginosa iron metabolism may be useful.
It will be critical in future work to perform larger, randomized human studies, study the safety of repeated administration, investigate combined use with antibiotics in humans, and to determine if oral or inhaled treatment is possible. In addition, gallium’s broad-spectrum activity against many extracellular and intracellular pathogens raises the possibility that gallium or other iron- disrupting strategies may also be useful in infections caused by other resistant organisms.
Finally, these findings suggest a renewed focus on targeting bacterial nutrition and metabolism to treat infectious diseases in the era of antibiotic resistance.
Abbrevated Methods
Ex vivo assays
Sputum was collected from CF patients and used for growth studies using strains PAO1 and PAO1-pvdA-gfp. BM2 media was used for oxidant sensitivity, time-kill, checkerboard, spontaneous and selected mutation rate assays, and for selection of transposon mutants. LB agar plates were used in dic diffusion assays. Enzyme activity assays were preformed in 10% TSB medium. Transposon mutagenesis was performed using the mini-Tn5-pro delivered on pUT from E. coli S17–1. See supplement for additional information.
Mouse infections.
All experiments were approved in advance by the U. Washington Animal Care and Use Committee and used 8–12 wk old C57Bl/6 pathogen-free mice (Jackson). Animals were euthanized if they became moribund, distressed, or unable to eat or drink. See supplement for additional information.
Human studies and statistical analysis
Macrophage studies were approved by the U. Chicago Institutional Biosafety Committee, and the trial of IV gallium nitrate (ClinicalTrials.gov NCT01093521) was preformed under IND #104,363 with IRB approval from all three study sites (UW IRB 35876, UI IRB-01 201002774, JHU IRB5 NA_00044996). Subjects received a single 5-day infusion of gallium nitrate (2 dosing cohorts noted above). Cohort 2 did not commence until review of Cohort 1 safety data by the Data Safety Monitoring Committee. Inferential analyses of the lung function changes tested the null hypothesis that the change was equal to zero using paired t-tests with normally distributeddata with 95% confidence intervals (CI) and Wilcoxon signed-rank tests with non-normally distributed data with interquartile range (IQR). See supplement for additional information.
Supplementary Material
Acknowledgements.
We thank the participants who made this study possible. This study was supported by NIH (R01 HL085868, P30DK089507, K24HL102246, CTSA ITHS IGNITION Award), the Cystic Fibrosis foundation (G0SS09A0, and SINGH15R0), the Arcadia Foundation, the Burroughs Wellcome Fund (BWF1006700), and the University of Washington’s Institute of Translational Health Sciences. Study drug was donated by Genta, Inc.
References
- 1.Flamm RK, Weaver MK, Thornsberry C, Jones ME, Karlowsky JA, Sahm DF, Factors associated with relative rates of antibiotic resistance in Pseudomonas aeruginosa isolates tested in clinical laboratories in the United States from 1999 to 2002. Antimicrob Agents Chemother 48, 2431–2436 (2004); published online EpubJul ( [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Marston HD, Dixon DM, Knisely JM, Palmore TN, Fauci AS, Antimicrobial Resistance. Jama 316, 1193–1204 (2016); published online EpubSep 20 ( 10.1001/jama.2016.11764). [DOI] [PubMed] [Google Scholar]
- 3.Skovgaard N, New trends in emerging pathogens. Int J Food Microbiol 120, 217–224 (2007); published online EpubDec 15 ( [DOI] [PubMed] [Google Scholar]
- 4.Stewart PS, Mechanisms of antibiotic resistance in bacterial biofilms. Int. J. Med. Microbiol. 292, 107–113 (2002). [DOI] [PubMed] [Google Scholar]
- 5.Silver LL, A Gestalt approach to Gram-negative entry. Bioorganic & medicinal chemistry 24, 6379–6389 (2016); published online EpubDec 15 ( 10.1016/j.bmc.2016.06.044). [DOI] [PubMed] [Google Scholar]
- 6.Singh SB, Young K, Silver LL, What is an “ideal” antibiotic? Discovery challenges and path forward. Biochemical pharmacology 133, 63–73 (2017); published online EpubJun 1 ( 10.1016/j.bcp.2017.01.003). [DOI] [PubMed] [Google Scholar]
- 7.Bullen JJ, Rogers HJ, Spalding PB, Ward CG, Iron and infection: the heart of the matter. FEMS Immunol Med Microbiol 43, 325–330 (2005); published online EpubMar 1 ( [DOI] [PubMed] [Google Scholar]
- 8.Singh PK, Parsek MR, Greenberg EP, Welsh MJ, A component of innate immunity prevents bacterial biofilm development. nature 417, 552–555 (2002). [DOI] [PubMed] [Google Scholar]
- 9.Thompson MG, Corey BW, Si Y, Craft DW, Zurawski DV, Antibacterial activities of iron chelators against common nosocomial pathogens. Antimicrob Agents Chemother 56, 5419–5421 (2012); published online EpubOct ( 10.1128/AAC.01197-12). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chitambar CR, Narasimhan J, Targeting iron-dependent DNA synthesis with gallium and transferrin-gallium. Pathobiology 59, 3–10 (1991). [DOI] [PubMed] [Google Scholar]
- 11.Apseloff G, Therapeutic uses of gallium nitrate: past, present, and future. American journal of therapeutics 6, 327–339 (1999); published online EpubNov ( [DOI] [PubMed] [Google Scholar]
- 12.T. M. Kaneko Y, Olakanmi O, Britigan BE, Singh PK, The transition metal gallium disrupts iron metabolism in P. aeruginosa and has nti-microbial and anti-biofilm action. J. Clin. Invest. 177, 887–888 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Olakanmi O, Gunn JS, Su S, Soni S, Hassett DJ, Britigan BE, Gallium disrupts iron uptake by intracellular and extracellular Francisella strains and exhibits therapeutic efficacy in a murine pulmonary infection model. Antimicrob Agents Chemother 54, 244–253 (2010); published online EpubJan ( 10.1128/AAC.00655-09). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Antunes LC, Imperi F, Minandri F, Visca P, In vitro and in vivo antimicrobial activities of gallium nitrate against multidrug-resistant Acinetobacter baumannii. Antimicrob Agents Chemother 56, 5961–5970 (2012); published online EpubNov ( 10.1128/AAC.01519-12). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Olakanmi O, Britigan BE, Schlesinger LS, Gallium disrupts iron metabolism of mycobacteria residing within human macrophages. Infection and immunity 68, 5619–5627 (2000); published online EpubOct ( [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Olakanmi O, Kesavalu B, Pasula R, Abdalla MY, Schlesinger LS, Britigan BE, Gallium nitrate is efficacious in murine models of tuberculosis and inhibits key bacterial Fe-dependent enzymes. Antimicrob Agents Chemother 57, 6074–6080 (2013); published online EpubDec ( 10.1128/AAC.01543-13). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Thompson MG, Truong-Le V, Alamneh YA, Black CC, Anderl J, Honnold CL, Pavlicek RL, Abu-Taleb R, Wise MC, Hall ER, Wagar EJ, Patzer E, Zurawski DV, Evaluation of Gallium Citrate Formulations against a Multidrug-Resistant Strain of Klebsiella pneumoniae in a Murine Wound Model of Infection. Antimicrob Agents Chemother 59, 6484–6493 (2015); published online EpubOct ( 10.1128/AAC.00882-15). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kelson AB, Carnevali M, Truong-Le V, Gallium-based anti-infectives: targeting microbial iron-uptake mechanisms. Current opinion in pharmacology 13, 707–716 (2013); published online EpubOct ( 10.1016/j.coph.2013.07.001). [DOI] [PubMed] [Google Scholar]
- 19.Richter K, Thomas N, Zhang G, Prestidge CA, Coenye T, Wormald PJ, Vreugde S, Deferiprone and Gallium-Protoporphyrin Have the Capacity to Potentiate the Activity of Antibiotics in Staphylococcus aureus Small Colony Variants. Frontiers in cellular and infection microbiology 7, 280 (2017) 10.3389/fcimb.2017.00280). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Coleman M, Kuskie K, Liu M, Chaffin K, Libal M, Giguere S, Bernstein L, Cohen N, Corrigendum to: “In vitro antimicrobial activity of gallium maltolate against virulent Rhodococcus equi “ [Veterinary Microbiology 146 (2010) 175–178]. Veterinary microbiology 195, 165 (2016); published online EpubNov 15 ( 10.1016/j.vetmic.2016.09.004). [DOI] [PubMed] [Google Scholar]
- 21.Baldoni D, Steinhuber A, Zimmerli W, Trampuz A, In vitro activity of gallium maltolate against Staphylococci in logarithmic, stationary, and biofilm growth phases: comparison of conventional and calorimetric susceptibility testing methods. Antimicrob Agents Chemother 54, 157–163; published online EpubJan (AAC.00700–09 [pii] 10.1128/AAC.00700-09). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Palmer KL, Mashburn LM, Singh PK, Whiteley M, Cystic fibrosis sputum supports growth and cues key aspects of Pseudomonas aeruginosa physiology. Journal of bacteriology 187, 5267–5277 (2005); published online EpubAug ( 10.1128/JB.187.15.5267-5277.2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Son MS, Matthews WJ Jr., Kang Y, Nguyen DT, Hoang TT, In vivo evidence of Pseudomonas aeruginosa nutrient acquisition and pathogenesis in the lungs of cystic fibrosis patients. Infection and immunity 75, 5313–5324 (2007); published online EpubNov ( 10.1128/IAI.01807-06). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hunter RC, Asfour F, Dingemans J, Osuna BL, Samad T, Malfroot A, Cornelis P, Newman DK, Ferrous iron is a significant component of bioavailable iron in cystic fibrosis airways. mBio 4, (2013); published online EpubAug 20 ( 10.1128/mBio.00557-13). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jordan A, Torrents E, Sala I, Hellman U, Gibert I, Reichard P, Ribonucleotide reduction in Pseudomonas species: simultaneous presence of active enzymes from different classes. Journal of bacteriology 181, 3974–3980 (1999); published online EpubJul ( [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Vasil ML, Ochsner UA, The response of Pseudomonas aeruginosa to iron: genetics, biochemistry and virulence. Mol. Microbiol. 34, 399–413 (1999). [DOI] [PubMed] [Google Scholar]
- 27.Miller RA, Britigan BE, Role of oxidants in microbial pathophysiology. Clinical microbiology reviews 10, 1–18 (1997); published online EpubJan ( [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Silver LL, Challenges of antibacterial discovery. Clinical microbiology reviews 24, 71–109 (2011); published online EpubJan ( 10.1128/CMR.00030-10). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Garcia-Contreras R, Lira-Silva E, Jasso-Chavez R, Hernandez-Gonzalez IL, Maeda T, Hashimoto T, Boogerd FC, Sheng L, Wood TK, Moreno-Sanchez R, Isolation and characterization of gallium resistant Pseudomonas aeruginosa mutants. International journal of medical microbiology : IJMM 303, 574–582 (2013); published online EpubDec ( 10.1016/j.ijmm.2013.07.009). [DOI] [PubMed] [Google Scholar]
- 30.Freschi L, Jeukens J, Kukavica-Ibrulj I, Boyle B, Dupont MJ, Laroche J, Larose S, Maaroufi H, Fothergill JL, Moore M, Winsor GL, Aaron SD, Barbeau J, Bell SC, Burns JL, Camara M, Cantin A, Charette SJ, Dewar K, Deziel E, Grimwood K, Hancock RE, Harrison JJ, Heeb S, Jelsbak L, Jia B, Kenna DT, Kidd TJ, Klockgether J, Lam JS, Lamont IL, Lewenza S, Loman N, Malouin F, Manos J, McArthur AG, McKeown J, Milot J, Naghra H, Nguyen D, Pereira SK, Perron GG, Pirnay JP, Rainey PB, Rousseau S, Santos PM, Stephenson A, Taylor V, Turton JF, Waglechner N, Williams P, Thrane SW, Wright GD, Brinkman FS, Tucker NP, Tummler B, Winstanley C, Levesque RC, Clinical utilization of genomics data produced by the international Pseudomonas aeruginosa consortium. Frontiers in microbiology 6, 1036 (2015) 10.3389/fmicb.2015.01036). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bockman R, The effects of gallium nitrate on bone resorption. Semin Oncol 30, 5–12 (2003); published online EpubApr ( [DOI] [PubMed] [Google Scholar]
- 32.Amsterdam D. Antibiotics in Laboratory Medicine (Wolters Kluwer Health, Philadelphia, PA, ed. 6th, 2014). [Google Scholar]
- 33.Kennedy SM, Walker DC, Belzberg AS, Hogg JC, Macrophage accumulation of inhaled gallium-67 citrate in normal lungs. Journal of nuclear medicine : official publication, Society of Nuclear Medicine 26, 1195–1201 (1985); published online EpubOct ( [PubMed] [Google Scholar]
- 34. Leyland-Jones B, Pharmacokinetics and therapeutic index of gallium nitrate. Semin Oncol 18, 16–20 (1991); published online EpubAug ( [PubMed] [Google Scholar]
- 35.Rudnev AV, Foteeva LS, Kowol C, Berger R, Jakupec MA, Arion VB, Timerbaev AR, Keppler BK, Preclinical characterization of anticancer gallium(III) complexes: solubility, stability, lipophilicity and binding to serum proteins. Journal of inorganic biochemistry 100, 1819–1826 (2006); published online EpubNov ( 10.1016/j.jinorgbio.2006.07.003). [DOI] [PubMed] [Google Scholar]
- 36.Moskowitz SM, Emerson JC, McNamara S, Shell RD, Orenstein DM, Rosenbluth D, Katz MF, Ahrens R, Hornick D, Joseph PM, Gibson RL, Aitken ML, Benton WW, Burns JL, Randomized trial of biofilm testing to select antibiotics for cystic fibrosis airway infection. Pediatric pulmonology 46, 184–192 (2011); published online EpubFeb ( 10.1002/ppul.21350). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wainwright CE, Quittner AL, Geller DE, Nakamura C, Wooldridge JL, Gibson RL, Lewis S, Montgomery AB, Aztreonam for inhalation solution (AZLI) in patients with cystic fibrosis, mild lung impairment, and P. aeruginosa. Journal of cystic fibrosis : official journal of the European Cystic Fibrosis Society 10, 234–242 (2011); published online EpubJul ( 10.1016/j.jcf.2011.02.007). [DOI] [PubMed] [Google Scholar]
- 38.Retsch-Bogart GZ, Quittner AL, Gibson RL, Oermann CM, McCoy KS, Montgomery AB, Cooper PJ, Efficacy and safety of inhaled aztreonam lysine for airway pseudomonas in cystic fibrosis. Chest 135, 1223–1232 (2009); published online EpubMay ( 10.1378/chest.08-1421). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ramsey BW, Dorkin HL, Eisenberg JD, Gibson RL, Harwood IR, Kravitz RM, Schidlow DV, Wilmott RW, Astley SJ, McBurnie MA, et al. , Efficacy of aerosolized tobramycin in patients with cystic fibrosis. The New England journal of medicine 328, 1740–1746 (1993); published online EpubJun 17 ( 10.1056/NEJM199306173282403). [DOI] [PubMed] [Google Scholar]
- 40.Ramsey BW, Pepe MS, Quan JM, Otto KL, Montgomery AB, Williams-Warren J, Vasiljev KM, Borowitz D, Bowman CM, Marshall BC, Marshall S, Smith AL, Intermittent administration of inhaled tobramycin in patients with cystic fibrosis. Cystic Fibrosis Inhaled Tobramycin Study Group. The New England journal of medicine 340, 23–30 (1999); published online EpubJan 7 ( 10.1056/NEJM199901073400104). [DOI] [PubMed] [Google Scholar]
- 41.Lobanoff MC, Kozhich AT, Mullet DI, Gerber N, Gery I, Chan CC, Whitcup SM, Effect of gallium nitrate on experimental autoimmune uveitis. Exp Eye Res 65, 797–801 (1997); published online EpubDec ( [DOI] [PubMed] [Google Scholar]
- 42.Apseloff G, Hackshaw KV, Whitacre C, Weisbrode SE, Gerber N, Gallium nitrate suppresses lupus in MRL/lpr mice. Naunyn Schmiedebergs Arch Pharmacol 356, 517–525 (1997); published online EpubOct ( [DOI] [PubMed] [Google Scholar]
- 43.Krecic-Shepard ME, Shepard DR, Mullet D, Apseloff G, Weisbrode SE, Gerber N, Gallium nitrate suppresses the production of nitric oxide and liver damage in a murine model of LPS-inducrd septic shock. Life sci 65, 1359–1371 (1999). [DOI] [PubMed] [Google Scholar]
- 44.L. B. Apseloff G, Weisbrode SE, et al. :, Gallium ntrate ameliorates asthma in B6D2F1/J mice. FASEB J 10, A441 (1996). [Google Scholar]
- 45.West NE, Beckett VV, Jain R, Sanders DB, Nick JA, Heltshe SL, Dasenbrook EC, VanDevanter DR, Solomon GM, Goss CH, Flume PA, investigators S, Standardized Treatment of Pulmonary Exacerbations (STOP) study: Physician treatment practices and outcomes for individuals with cystic fibrosis with pulmonary Exacerbations. Journal of cystic fibrosis : official journal of the European Cystic Fibrosis Society 16, 600–606 (2017); published online EpubSep ( 10.1016/j.jcf.2017.04.003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Quittner AL, Modi AC, Wainwright C, Otto K, Kirihara J, Montgomery AB, Determination of the minimal clinically important difference scores for the Cystic Fibrosis Questionnaire-Revised respiratory symptom scale in two populations of patients with cystic fibrosis and chronic Pseudomonas aeruginosa airway infection. Chest 135, 1610–1618 (2009); published online EpubJun ( 10.1378/chest.08-1190). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Brown SA, Palmer KL, Whiteley M, Revisiting the host as a growth medium. Nature reviews. Microbiology 6, 657–666 (2008); published online EpubSep ( 10.1038/nrmicro1955). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


