Summary
Deoxynivalenol (DON) is a mycotoxin produced in cereal crops infected with Fusarium graminearum. DON poses a serious threat to human and animal health, and is a critical virulence factor. Various environmental factors, including reactive oxygen species (ROS), have been shown to interfere with DON biosynthesis in this pathogen. The regulatory mechanisms of how ROS trigger DON production have been investigated extensively in F. graminearum. However, the role of the endogenous ROS‐generating system in DON biosynthesis is largely unknown. In this study, we genetically analysed the function of leucine zipper‐EF‐hand‐containing transmembrane 1 (LETM1) superfamily proteins and evaluated the role of the mitochondrial‐produced ROS in DON biosynthesis. Our results show that there are two Letm1 orthologues, FgLetm1 and FgLetm2, in F. graminearum. FgLetm1 is localized to the mitochondria and is essential for mitochondrial integrity, whereas FgLetm2 plays a minor role in the maintenance of mitochondrial integrity. The ΔFgLetm1 mutant demonstrated a vegetative growth defect, abnormal conidia and increased sensitivity to various stress agents. More importantly, the ΔFgLetm1 mutant showed significantly reduced levels of endogenous ROS, decreased DON biosynthesis and attenuated virulence in planta. To our knowledge, this is the first report showing that mitochondrial integrity and endogenous ROS production by mitochondria are important for DON production and virulence in Fusarium species.
Keywords: endogenous reactive oxygen species, FgLetm1, Fusarium graminearum, mitochondrial integrity, mycotoxin, virulence
Introduction
Deoxynivalenol (DON) is the most prevalent and economically important mycotoxin associated with grains infested by Fusarium species (Desjardins, 2006). Among Fusarium fungi, Fusarium graminearum is the main DON producer, which causes a devastating disease, known as Fusarium head blight (FHB), in cereal crops worldwide (Bennett and Klich, 2003; Desjardins, 2006; Kimura et al., 2007). The biosynthetic pathway of DON has been studied extensively, and nearly all genes involved in DON biosynthesis (TRI genes) have been identified (Desjardins et al., 1993; Kimura et al., 2001, 2007). The biosynthesis of secondary metabolites, including mycotoxins, is influenced by various environmental factors. Previous investigations on the regulation of DON biosynthesis in F. graminearum have revealed the influence on mycotoxin production of environmental or extracellular factors, such as nitrogen and carbon sources (Jiao et al., 2008; Miller and Greenhalgh, 1985; Oh et al., 2016), pH (Merhej et al., 2011), magnesium (Pinson‐Gadais et al., 2008), phenolic acids (Boutigny et al., 2009) and amines (Gardiner et al., 2009). A recent study has shown that methylation of histone H3K4 also contributes to DON production (Liu et al., 2015). In addition to these factors, reactive oxygen species (ROS) have been highlighted as a stimulator interfering with DON production (Audenaert et al., 2010; Jiang et al., 2015; Montibus et al., 2013; Ponts et al., 2006, 2007). Supplementation with hydrogen peroxide (H2O2) or the fungicide prothioconazole of liquid cultures of F. graminearum increases significantly the concentration of intracellular ROS, which subsequently stimulates TRI gene expression and induces DON production (Audenaert et al., 2010; Ponts et al., 2006, 2007). Relatively higher concentrations of H2O2 are observed in infection cushions, relative to runner hyphae, during the infection process of F. graminearum (Mentges and Bormann, 2015). However, the mechanism of the generation of endogenous ROS and the role of ROS in the regulation of DON biosynthesis remain relatively unknown in F. graminearum.
ROS are able to cause DNA damage, lipid peroxidation and protein oxidation (Beckman and Ames, 1998). Alternatively, ROS have been suggested to be a secondary messenger that transduces signals to regulate cellular functions, such as immunity, cell proliferation and ion transport, in mammals and plants. In microbial eukaryotes, ROS have been shown to be involved in the regulation of the life span (Osiewacz, 2002), host–pathogen interactions and other cellular functions (Missall et al., 2004; Nowikovsky et al., 2004). Mitochondria are the major source of endogenous ROS and produce about 95% of the total ROS during cellular oxidative metabolism (Liu, 1999). Meanwhile, several enzymatic and non‐enzymatic systems are also involved in intracellular ROS production (Grissa et al., 2010). The most important enzymatic ROS‐generating system is the NADPH (reduced form of nicotinamide adenine dinucleotide phosphate)‐dependent oxidase complex (Nox). The role of NADPH oxidases, NoxA and NoxB, and the regulator, NoxR, in ROS production have been investigated in F. graminearum (Wang et al., 2014; Zhang et al., 2016). However, the roles of mitochondria and the mitochondrial ROS‐generating system in the secondary metabolism and virulence of phytopathogenic fungi, including F. graminearum, have not been investigated.
Leucine zipper‐EF‐hand‐containing transmembrane 1 (LETM1), an inner mitochondrial membrane protein, has been identified as a protein associated with Wolf–Hirschhorn syndrome (WHS), a complex multigenic human disease caused by the partial deletion of the distal short arm of chromosome 4 (Endele et al., 1999; Zollino et al., 2003). Letm1 is evolutionarily conserved from yeast to mammals. The biological functions of the Letm1 orthologues have been investigated in various organisms (Dimmer et al., 2008; Hasegawa and van der Bliek, 2007; Hashimi et al., 2013; McQuibban et al., 2010; Nowikovsky et al., 2004; Zhang et al., 2012). However, the function of the Letm1 superfamily in filamentous fungi is still largely unknown. In this study, the Letm1 orthologues were selected as target proteins to investigate the biological function of ROS generated from mitochondria and mitochondrial integrity in DON biosynthesis, virulence and cell development. Our results showed that the deletion mutant ΔFgLetm1 had a vegetative growth defect and abnormal conidia. The mutant was also more sensitive to various stress agents. More importantly, ΔFgLetm1 significantly reduced the levels of cellular ROS, decreased DON biosynthesis and attenuated virulence in planta.
Results
Identification and sequence analysis of Letm1‐like proteins in F. graminearum
A blastp search using Saccharomyces cerevisiae Letm1 family proteins, Mdm38 and Ylh47, as queries in the F. graminearum genome revealed only one putative Letm1 gene in this fungus, FGSG_09158 (designated as FgLetm1). The FgLETM1 gene is predicted to encode a protein with 550 amino acids, sharing 47% and 45% sequence identity with Mdm38 and Ylh47, respectively. Meanwhile, we retrieved other genes with the LETM1 superfamily domain in the F. graminearum genome, and found that the FGSG_10063 locus (designated as FgLetm2) also contained a LETM1 superfamily domain. However, FgLetm2 shares very low sequence identity with Mdm38 and Ylh47 (9.8% and 11.4%, respectively). Similar to S. cerevisiae Letm1 orthologues, FgLetm1 contains a non‐canonical Letm1 protein structure with a Letm1 superfamily domain, a transmembrane (TM) domain and a coiled‐coil domain at the carboxyl terminus. The FgLetm2 protein harbours a truncated Letm1 superfamily domain after the TM domain at the carboxyl terminus (Fig. 1a). Both FgLetm1 and FgLetm2 lack the EF‐hand domain present in human Letm1 (NP_036450). This was consistent with a previous study, in which it was demonstrated that the EF‐hand domain was absent in lower eukaryotes, fungi and plasmodium (Nowikovsky et al., 2004).
Figure 1.
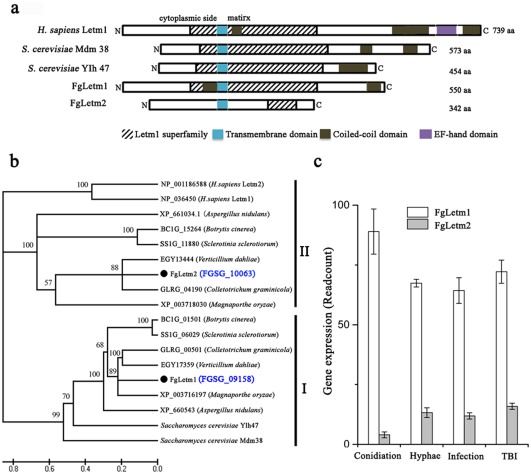
Identification of the Letm1‐like proteins in Fusarium graminearum. (a) Schematic architecture of the Letm1 superfamily proteins in F. graminearum: FgLetm1 and FgLetm2. The Homo sapiens Letm1 and Saccharomyces cerevisiae Mdm38 and Ylh47 were selected as references. Conserved domains are indicated. (b) Phylogenetic analysis of the putative Letm1‐like proteins from F. graminearum and six plant‐pathogenic fungi. Amino acid sequences of the Letm1 orthologues were aligned using ClustalW and a neighbour‐joining tree was generated by mega 5.0. (c) Transcriptional levels of the FgLETM1 and FgLETM2 genes in carboxymethylcellulose (CMC), hyphae, infected plant tissues and Trichothecene Biosynthesis Induction medium (TBI) by RNA‐seq.
To gain more insight into the Letm1 evolution in fungi, we retrieved all genes that encode proteins containing the Letm1 superfamily domain from 32 fungal genomes available in the National Center for Biotechnology Information (NCBI) Bioprojects and Broad Institute databases. The results indicated that genes for the Letm1‐like proteins are highly conserved in fungi, and the number of orthologues varies in different fungal species. Most fungal species (25 of 32) harboured two orthologues, albeit the representative fungi from Taphrinomycotina, Pucciniomycotina and Chytridiomycota contained only one Letm1‐like protein. Moreover, three different genes encoding the Letm1‐like proteins were retrieved from the Zygomycota fungi Rhizopus oryzae and Mucor circinelloides (Fig. S1, see Supporting Information). A phylogenetic analysis of the putative Letm1‐like proteins, which included F. graminearum and six filamentous phytopathogenic fungi, showed that the Letm1‐like proteins are significantly divided into two groups (Fig. 1b). Proteins in group II had a truncated Letm1 superfamily domain with a length of 58–75 amino acids (Table S1, see Supporting Information). The domain characteristic and the phylogenetic tree indicated that FgLetm1 might have similar biological functions to the Letm1 proteins Mdm38 and Yln47 in S. cerevisiae.
In addition, our in‐house RNA‐sequencing (RNA‐seq) data indicated that the transcriptional level of FgLETM1 was higher than that of FgLETM2, by a range of 5–20‐fold, in all four tested conditions, including in the conidiation medium (carboxymethylcellulose, CMC), in hyphae grown on potato dextrose agar (PDA), in plant infection and in Trichothecene Biosynthesis Induction medium (TBI) (Fig. 1c).
Disruption of FgLetm1 and FgLetm2
To characterize the function of FgLetm1 and FgLetm2, we generated single and double deletion mutants, ΔFgLetm1, ΔFgLetm2 and ΔΔFgLetm1/2, using the homologous recombination strategy. The single or double deletion mutants were confirmed by Southern hybridization assays (Fig. S2, see Supporting Information). To confirm that the phenotypic abnormalities of the mutants were directly related to the deletion, we complemented the deletion mutants with the gene fused with gfp for green fluorescent protein (GFP) at the carboxyl terminus under the native promoter, and generated the complemented strains ΔFgLetm1‐C (ΔFgLetm1+PLETM1FgLetm1‐GFP) and ΔFgLetm2‐C (ΔFgLetm2+PLETM2FgLetm2‐GFP). The complemented strains were also confirmed by Southern blot assays and polymerase chain reaction (PCR) amplification (Fig. S2).
FgLetm1 regulates hyphal growth, conidiation and conidial germination
The ΔFgLetm1 mutant demonstrated radial and hyphal growth defects. The rate of radial growth of ΔFgLetm1 was reduced on both PDA and minimal medium (MM) in comparison with that of wild‐type PH‐1. Moreover, the deletion mutant ΔFgLetm1 exhibited a reduction in the formation of aerial hyphae on solid agar plates (Fig. 2a).
Figure 2.
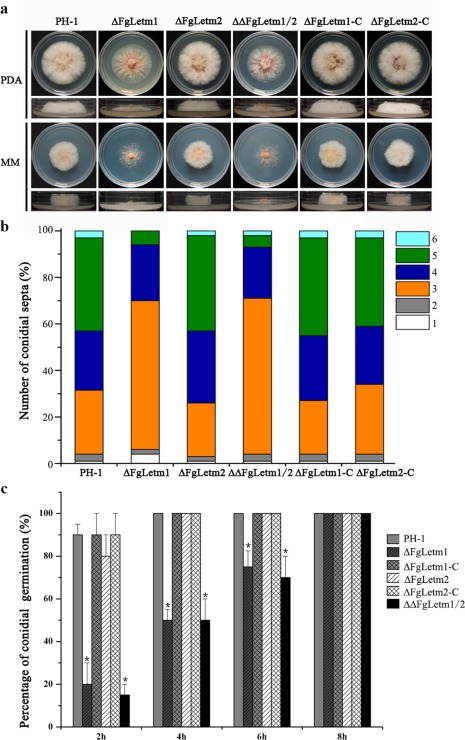
Phenotypes of the deletion mutants ΔFgLetm1, ΔFgLetm2 and ΔΔFgLetm1/2 in vegetative growth, conidiogenesis and germination. (a) Colony morphology of PH‐1, the mutants and the complemented strains grown on potato dextrose agar (PDA) and minimal medium (MM) at 25 °C for 3 days. (b) Ratio of the different number of conidial septa in PH‐1, mutants and complemented strains harvested from 5‐day‐old carboxymethylcellulose (CMC) cultures. (c) ΔFgLetm1 showed reduced conidial germination. Columns labelled with a star indicate a significant difference at P = 0.05.
ΔFgLetm1 produced fewer conidia than wild‐type PH‐1 after 4 days of incubation in CMC (Table 1). To further examine conidial morphology, calcofluor white staining assays were performed for individual mutants. The results were observed under a fluorescence microscope. As shown in Fig. S3 (see Supporting Information) and Table 1, the size of conidia produced by ΔFgLetm1 was shorter in comparison with those of the wild‐type. Moreover, the conidia of ΔFgLetm1 harboured fewer septa. Most of the conidia (65%) had only three septa in ΔFgLetm1, whereas the majority of conidia produced by the wild‐type had five septa (Fig. 2b). Meanwhile, the abnormal conidia of ΔFgLetm1 showed slower germination than those of the wild‐type in the present of 2% sucrose (Fig. 2c).
Table 1.
Vegetative growth and conidiation of Fusarium graminearum strains.
| Strain | Growth rate (cm/day) | Conidial production (×105) | Conidial length (µm) |
|---|---|---|---|
| PH‐1 | 2.43 ± 0.06a * | 6.15 ± 0.47a | 63.50 ± 6.10a |
| ΔFgLetm1 | 2.06 ± 0.03b | 4.65 ± 0.53b | 38.88 ± 4.39b |
| ΔFgLetm1‐C | 2.40 ± 0.04a | 6.28 ± 0.48a | 63.13 ± 8.04a |
| ΔFgLetm2 | 2.32 ± 0.02a | 6.03 ± 0.38a | 56.50 ± 5.07a |
| ΔFgLetm2‐C | 2.37 ± 0.02a | 5.98 ± 0.26a | 59.88 ± 2.10a |
| ΔΔFgLetm1/2 | 2.03 ± 0.03b | 5.20 ± 0.34b | 41.25 ± 3.57b |
*Values followed by the same letter are not significantly different at P = 0.05 for each treatment.
In contrast, the deletion mutant ΔFgLetm2 did not show visible phenotypic differences in vegetative growth, conidial formation and germination in comparison with the wild‐type. The ΔΔFgLetm1/2 double mutant demonstrated similar phenotypes to those of the ΔFgLetm1 single mutant. Phenotypic defects of ΔFgLetm1 were restored by complementation in the complemented strain ΔFgLetm1‐C (Fig. 2). Thus, our evidence confirms that the defects in the mutants were linked to the loss of the FgLETM1 gene. Taken together, the data presented here suggest that FgLetm1 is involved in hyphal growth, conidiation and conidial germination, and that FgLetm2 plays a dispensable role in these biological processes under the tested conditions.
Deletion mutant ΔFgLetm1 shows increased sensitivity towards osmotic stress, heat shock and fungicides
It has been reported that Mdm38 is involved in resistance to several biotic stresses in yeast (Dimmer et al., 2002; Frazier et al., 2006; Sinha et al., 2008). Therefore, we were interested in determining the susceptibility of the mutants of F. graminearum to various stresses, including osmotic stress, heat shock and fungicide treatment. The susceptibility assays showed that the ΔFgLetm1 mutant had a significantly increased sensitivity to osmotic stress generated by NaCl or KCl, whereas the ΔFgLetm2 mutant displayed the same susceptibility as that of the wild‐type towards osmotic stress (Fig. 3a,b). The heat tolerance of the mutants was examined at 15, 25 and 32 °C. As shown in Fig. 3c, all strains displayed a similar growth rate to that of the wild‐type at 15 °C. At 32 °C, notably, the ΔFgLetm1 mutant could not grow. The complemented strains showed a similar growth rate and colony morphology to the wild type PH‐1. Therefore, our results indicate that ΔFgLetm1 increased the sensitivity to heat shock stress. To further confirm the increase in sensitivity to high temperatures in ΔFgLetm1, we assayed the transcriptional levels of the FgHSP30 (Fg01158), FgHSP70 (Fg00838) and FgGSY2 (Fg06822) genes, whose products are involved in the heat shock tolerance in F. graminearum (Hu et al., 2014). When cultures were shifted from 25 to 32 °C for 1 h, the relative expression levels of FgHSP70 and FgHSP30 were 2.5‐ and two‐fold higher, respectively, in PH‐1 relative to ΔFgLetm1 (Fig. 3d). Therefore, the deletion mutant ΔFgLetm1 decreased the expression of the selected heat stress response genes in F. graminearum.
Figure 3.

ΔFgLetm1 increases the sensitivity towards osmotic stress, heat shock, fungicides and ion stress. (a) Growth phenotype of PH‐1, mutants and complemented strains grown on minimal medium (MM) with or without supplementation of NaCl or KCl after 4 days of incubation at 25 °C. (b) Statistical analysis of the growth inhibition rate of all strains under osmotic stress. (c) ΔFgLetm1 increased the sensitivity towards high temperature. Colony morphology is shown after 4 days of incubation on MM at 15 and 25 °C, and 7 days of incubation at 32 °C. (d) The transcriptional levels of the heat tolerant genes FgHSP30, FgHSP70 and FgGSY2 were decreased in the ΔFgLetm1 mutant in response to heat shock, in comparison with those in PH‐1. The expression levels of each gene at 25 °C for 16 h were set to unity. (e) The ΔFgLetm1 mutant was more sensitive towards the fungicides iprodione and phenamacril, and ion stress, than the wild‐type. Plates were incubated at 25 °C for 4 days before imaging. (f) Statistical analysis of the growth inhibition rate of strains towards the above stresses. Bars with the same letter indicate no significant difference at P = 0.05.
The susceptibility of the mutants towards ions and fungicides was also examined. ΔFgLetm1 displayed more sensitivity than the wild‐type and complemented strains to iprodione, phenamacril, FeSO4 and CaCl2 (Fig. 3e,f). In particular, the ΔFgLetm1 mutant was hypersensitive to Fe2+ and could not grow on MM amended with 10 mm Fe2+. This implies that FgLetm1 might regulate iron homeostasis in this fungus. The double mutant ΔΔFgLetm1/2 showed similar phenotypes to that of ΔFgLetm1 in all tested conditions.
FgLetm1 is localized to the mitochondria and is critical for mitochondrial integrity
The complemented strains ΔFgLetm1‐C and ΔFgLetm2‐C with GFP fusion proteins were rescued for the phenotypic defects seen in the mutants (Figs 2 and 3), indicating that the fusion proteins were functional. These strains were further used to observe the subcellular localization of FgLetm1 and FgLetm2. A filamentous network pattern of GFP signals was present in the vegetative mycelia of the ΔFgLetm1‐C strain (Fig. 4a). Co‐localization experiments were performed using dual labelling with FgLetm1‐GFP and the mitochondrial indicator Mito‐HcRed. As shown in Fig. 4a, the GFP and Mito‐HcRed signals clearly overlapped, suggesting that FgLetm1 was localized to the mitochondria. Using the same assay, we observed that FgLetm2‐GFP was also co‐localized with Mito‐HcRed (Fig. 4a). Therefore, both FgLetm1 and FgLetm2 were localized to the mitochondria.
Figure 4.
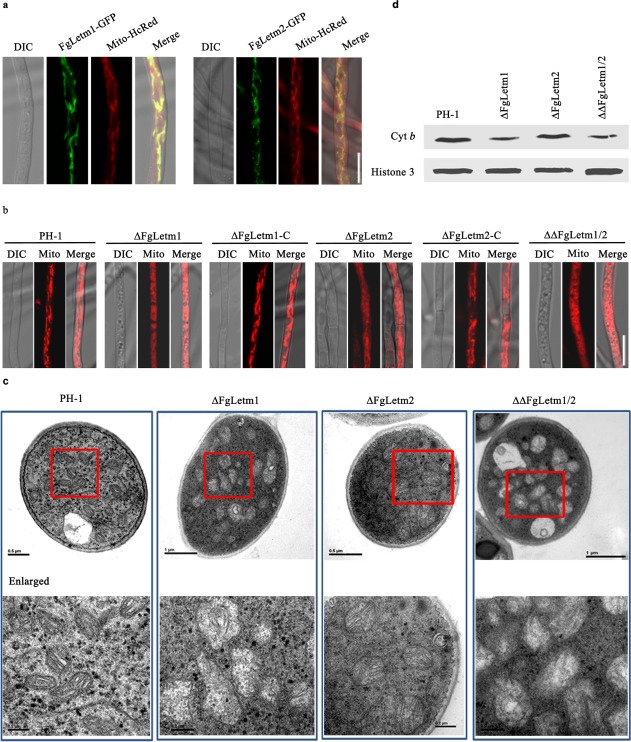
FgLetm1 is localized to the mitochondria and critical for mitochondrial integrity. (a) Both FgLetm1 and FgLetm2 are localized to the mitochondria. Mycelia of FgLetm1‐C and FgLetm2‐C were grown in Completed medium (CM) and stained with Mito‐HcRed. Images were taken by a confocal fluorescence microscope. Bar, 10 µm. DIC, Differential Interference Contrast (b) ΔFgLetm1 changed the mitochondrial structural patterns. Strains were grown in CM broth for 16 h at 25 °C, harvested and stained with Mito‐HcRed for observation. Typical patterns in individual strains are shown. Bar, 10 µm. (c) The ΔFgLetm1 mutant caused mitochondrial swelling. The ultrastructural morphology of mitochondria in each strain was visualized by transmission electron microscopy. Bar dimensions are shown in the images. (d) ΔFgLetm1 decreased the protein level of cytochrome b (Cyt b), an indicator protein of respiratory chain components. The protein abundance of Cyt b in PH‐1 and the mutants were analysed by immunoblot assays. Histone H3 was used as a reference protein.
As both FgLetm1 and FgLemt2 were shown to be mitochondrial localized proteins, we were interested in testing whether the deletion mutants would show altered mitochondrial structures. First, the mitochondrial patterns were observed by Mito‐HcRed staining. The Mito‐HcRed signal in the mycelia of PH‐1 and the complemented strains predominantly showed filamentous network shapes (Fig. 4b; Video S1, see Supporting Information), whereas it displayed punctate patterns in the mycelia of the ΔFgLetm1 single and double mutants (Fig. 4b; Video S2, see Supporting Information). Interestingly, mitochondrial mobility was relatively slower in the ΔFgLetm1 mutant than in the wild‐type (Videos S1 and S2). This implied that the mitochondrial morphology and function might be different in PH‐1 and ΔFgLetm1. Next, transmission electron microscopy (TEM) was applied to visualize the details of the mitochondrial structures. The transmission electron micrographs revealed that deletion of the FgLETM1 gene caused mitochondrial swelling, an increase in mitochondrial volume and a lack of tubular‐shaped cristae structures (Fig. 4c). The mitochondrial morphologies in ΔFgLetm2 cells showed a similar shape and cristae structure to that in PH‐1, whereas the volume was increased slightly. The abnormal mitochondrial morphologies of the double mutant were consistent with those of the ΔFgLetm1 mutant (Fig. 4b,c). In yeast, Mdm38 is essential for the biosynthesis of the respiratory chain components (Frazier et al., 2006). To test whether FgLetm1 has a similar function in F. graminearum, we selected cytochrome b (Cyt b) as the indicator protein of respiratory chain components, and detected the protein level of Cyt b by western blotting. As shown in Fig. 4d, the abundance of the Cyt b protein was clearly decreased in ΔFgLetm1 and the double mutant. Our findings thus strongly suggest that FgLetm1 is a structural protein, which helps to maintain the mitochondrial structure and is essential for the biosynthesis or stability of the components in the respiratory chain, whereas FgLetm2 plays a minor role in these processes.
The deletion mutant ΔFgLetm1 decreases the production of endogenous ROS and reduces adenosine triphosphate (ATP) biosynthesis
Mitochondria are an important source of ATP synthesis and ROS production in eukaryotic cells. Given that the deletion mutant ΔFgLetm1 resulted in mitochondrial dysfunction, we next compared the concentration of intracellular ROS and ATP biosynthesis in all the strains. The ROS content was qualitatively analysed with H2DCFDA (2′,7′‐dichlorodihydrofluorescein diacetate) staining in MM and TBI. As indicated in Fig. 5a, the mycelia of the wild‐type, ΔFgLetm2 and complemented strains were all stained by the dye, and cells showed green signals under a fluorescence microscope. Interestingly, mycelia of the wild‐type, ΔFgLetm2 and complemented strains were swollen and formed ovoid toxigenic cells, and displayed noticeably stronger green signals in TBI (Fig. 5a, bottom panel) than in MM (Fig. 5a, top panel). These results suggest that ROS production was highly induced and ROS accumulated in cells during DON biosynthesis. However, limited fluorescence signals were detected in ΔFgLetm1 and the double mutant in both media (Fig. 5a). Quantification data also confirmed that endogenous ROS were significantly reduced in ΔFgLetm1 and ΔΔFgLetm1/2 (Table 2). Intracellular ROS levels are usually balanced by the activities of catalases and superoxide dismutases. Therefore, we performed quantitative reverse transcription‐polymerase chain reaction (qRT‐PCR) to measure the transcripts of seven putative catalase and superoxide dismutase genes in the mycelia of the wild‐type and the ΔFgLetm1 mutant after 3 days of incubation in TBI. As expected, the qRT‐PCR results indicated that all selected genes, except for FgZnSOD2, showed very low transcriptional levels in ΔFgLetm1 in response to limited intracellular ROS‐mediated oxidative stress, in comparison with their expression levels in the wild‐type (Fig. 5b). To determine whether the reduction in intercellular ROS in ΔFgLetm1 and the double mutant would lead to their higher tolerance towards extracellular ROS stress, we measured the sensitivity of all strains to oxidative stress on MM supplemented with 10 mm H2O2. The deletion mutant ΔFgLetm1 and the double mutant exhibited significantly increased tolerance to oxidative stress mediated by H2O2 relative to that of the wild‐type, ΔFgLetm2 and complemented strains (Fig. 5c,d).
Figure 5.
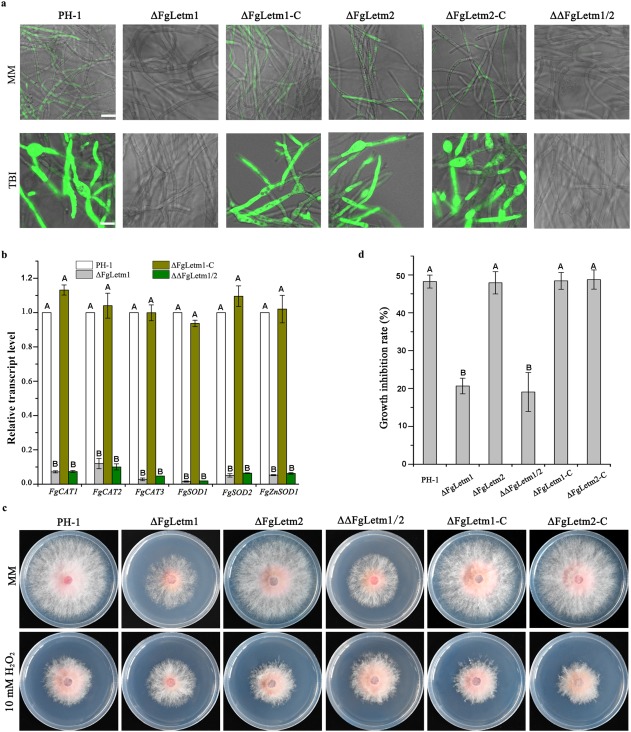
The deletion mutant ΔFgLetm1 decreases the concentration of endogenous reactive oxygen species (ROS). (a) ΔFgLetm1 strongly reduced endogenous ROS in minimal medium (MM) and Trichothecene Biosynthesis Induction medium (TBI). Hyphae grown in MM for 24 h, or in TBI for 3 days, were stained by the ROS indicator H2DCFDA (2′,7′‐dichlorodihydrofluorescein diacetate). Bar, 10 µm. (b) Mutants ΔFgLetm1 and ΔΔFgLetm1/2 decreased the transcriptional level of genes encoding catalases and superoxide dismutases in TBI. (c) Mutants ΔFgLetm1 and ΔΔFgLetm1/2 increased the resistance towards oxidative stress by H2O2. Strains were grown on MM with or without 10 mm H2O2 for 4 days at 25 °C. (d) Statistical analysis of the growth inhibition rate of PH‐1, mutants and complemented strains towards oxidative stress generated by H2O2. Bars with the same letter indicate no significant difference at P = 0.01.
Table 2.
Production of adenosine triphosphate (ATP), hydrogen peroxide (H2O2) and ethanol in PH‐1, mutants and complemented strains.
| ATP (µM) | H2O2 (mM) | Ethanol (mg/mL) | |||
|---|---|---|---|---|---|
| Strain | MM | TBI | MM | TBI | MM |
| PH‐1 | 1341.35 ± 29.24a * | 1360.05 ± 42.46a | 24.97 ± 2.14a | 133.94 ± 9.85a | 2.16 ± 0.07b |
| ΔFgLetm1 | 1008.54 ± 34.32b | 902.17 ± 23.46b | 3.26 ± 0.18b | 4.51 ± 0.36b | 4.32 ± 0.12a |
| ΔFgLetm1‐C | 1320.14 ± 13.11a | 1358.23 ± 35.23a | 20.67 ± 3.25a | 120.55 ± 20.11a | 2.3 ± 0.21b |
| ΔFgLetm2 | 1300.02 ± 24.53a | 1250.22 ± 48.37a | 19.66 ± 1.67b | 122.63 ± 15.37a | 2.43 ± 0.20b |
| ΔFgLetm2‐C | 1360.56 ± 15.21a | 1400.26 ± 68.70a | 21.79 ± 3.22a | 120.33 ± 18.54a | 2.1 ± 0.25b |
| ΔΔFgLetm1/2 | 953.56 ± 30.14b | 875.65 ± 50.32b | 2.34 ± 0.86b | 2.56 ± 0.25b | 4.17 ± 0.14a |
MM, minimal medium; TBI, Trichothecene Biosynthesis Induction medium.
*Values followed by the same letter are not significantly different at P = 0.05 for each treatment.
We next measured ATP production in the deletion mutants. The quantification data indicated that the production of ATP in ΔFgLetm1 and the double mutant was decreased by about 30% compared with that in the wild‐type. The ΔFgLetm2 mutant and the complemented strains produced ATP at a level similar to that of the wild‐type strain (Table 2). In mammals, LETM1 knock‐down led to mitochondrial malfunction and an induction of glycolysis in the cytoplasm to maintain the ATP supply in these cells (Dimmer et al., 2008; Hwang et al., 2010). To investigate whether the dysfunctional mitochondria in ΔFgLetm1 also increase glycolysis, we measured the concentration of ethanol, the byproduct of anaerobic respiration in fungi, in MM after 16 h of incubation. As expected, the ethanol concentration of the cell‐free supernatant from the ΔFgLetm1 mutant was significantly elevated compared with that of the wild‐type (Table 2), indicating that FgLETM1 deletion disrupts the respiratory chain and up‐regulates the glycolytic pathway in F. graminearum. Collectively, our data suggest that FgLetm1 is critical for normal mitochondrial function in ATP generation and ROS production.
The ΔFgLetm1 mutant is significantly attenuated in virulence
On wheat heads inoculated with PH‐1, ΔFgLetm2 or the complemented strains, scab symptoms first developed on the inoculated spikelets and rapidly spread to the whole wheat head after 15 days of inoculation. In contrast, the hyphal growth of ΔFgLetm1 or the ΔΔFgLetm1/2 double mutant failed to spread from the inoculated floret to the rachis, and subsequently caused scab symptoms only in the inoculated spikelet (Fig. 6a, top panel). Moreover, almost all grains in wheat ears infected by wild‐type PH‐1, ΔFgLetm2 and complemented strains were shrivelled and bleached, whereas only the grain at the inoculated site was shrunken on treatment with ΔFgLetm1 and ΔΔFgLetm1/2 (Fig. 6a, bottom panel). As ΔFgLetm1 and ΔΔFgLetm1/2 mutants grew well on the wheat head tissue medium (WA) (Fig. S4, see Supporting Information), the attenuated virulence of ΔFgLetm1 and ΔΔFgLetm1/2 was not likely to be a result of a growth defect. Next, we investigated whether the deletion mutations affected the penetration process. We examined the infection structures of strains during infection using scanning electron microscopy (SEM). Ultrastructural examination showed that the hyphae of the wild‐type formed typical infection cushions on the glumes at 48 h post‐inoculation with conidia, but such penetration structures were not observed on the glumes inoculated with conidia of either ΔFgLetm1 or ΔΔFgLetm1/2 under the same conditions. Both ΔFgLetm2 and the two complemented strains showed similar infection structures on plant tissues to those of the wild‐type (Fig. 6b). Notably, the ΔFgLetm1 mutant was capable of penetrating the spikelet and resulted in scab symptoms after 2 weeks at the inoculated sites (Figs 6a and S5, see Supporting Information). These results suggest that the ΔFgLetm1 mutant delays penetration structure formation and is defective in spreading from the inoculation site to nearby spikelets via the rachis. We conclude that FgLetm1 is important in the virulence of F. graminearum.
Figure 6.
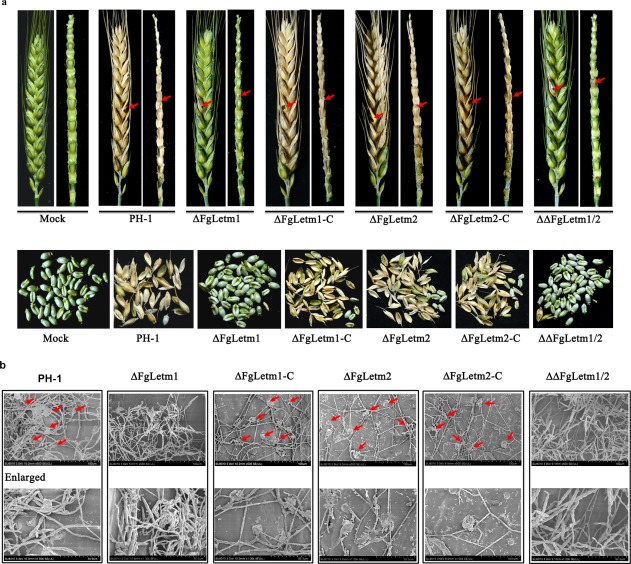
Deletion mutants ΔFgLetm1 and ΔΔFgLetm1/2 are attenuated in virulence in planta. (a) Dissection of infected wheat heads caused by PH‐1, mutants and complemented strains. Inoculated ears were dissected at 15 days post‐inoculation (dpi). Inoculated sites are indicated by red arrows. (b) Infection structures on glumes infected by PH‐1, mutants and complemented strains. The inoculated glumes were collected after 2 dpi with conidia, and observed by scanning electron microscopy (SEM). The infection structures are indicated by red arrows, and the details are enlarged.
FgLetm1 plays a critical role in DON biosynthesis
DON biosynthesis in the mutants was evaluated both in vitro and in planta. First, the transcriptional levels of TRI genes in the mutants were assayed by qRT‐PCR in TBI. All selected TRI genes were strongly down‐regulated in the ΔFgLetm1 mutant, but expression was not affected in ΔFgLetm2, compared with that in the wild‐type (Fig. 7a). Next, toxisome formation (Boenisch et al., 2017) for DON biosynthesis in the wild‐type and mutants was observed using Tri1‐GFP as an indicator in TBI cultures. As shown in Fig. 7b, Tri1‐GFP was highly induced and formed spherical and crescent‐shaped toxisomes in the mycelia of the wild‐type and ΔFgLetm2 mutant after 3 days of incubation in TBI. However, no visible green fluorescence signals were observed in the mycelia of ΔFgLetm1 under the same conditions. Consistent with the expression of TRI genes and toxisome formation, the amount of DON biosynthesis in ΔFgLetm1 was strongly reduced by 18‐fold when compared with that in the wild‐type in TBI liquid medium (Table 3). DON production was also significantly reduced in the ΔFgLetm1 deletion mutant in wheat grain cultures and infested wheat kernels in planta. The complemented strain ΔFgLetm1‐C was completely restored in DON production (Table 3). Collectively, our results suggest that FgLetm1 is important for TRI gene expression and DON production in F. graminearum.
Figure 7.
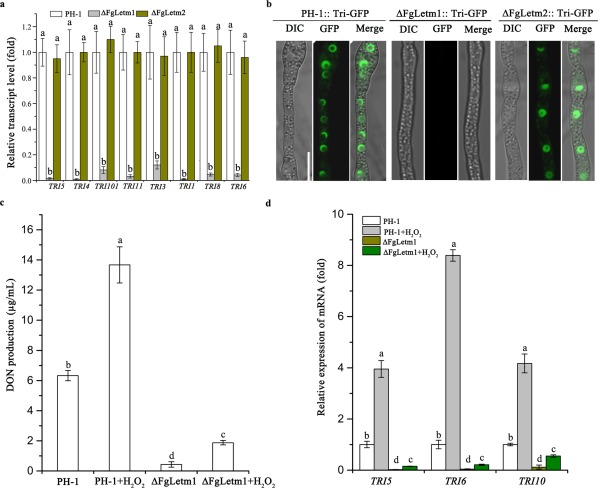
Deletion mutants ΔFgLetm1 and ΔΔFgLetm1/2 reduce deoxynivalenol (DON) biosynthesis in vitro and in planta. (a) ΔFgLetm1 significantly decreased the transcriptional level of TRI genes in Trichothecene Biosynthesis Induction medium (TBI) medium. (b) Toxisome formation of PH‐1, ΔFgLetm1 and ΔFgLetm2. Strains were labelled with Tri1‐GFP, incubated in TBI for 3 days and toxisomes were observed by confocal fluorescence microscopy. Bar, 10 µm. DIC, Differential Interference Contrast; GFP, green fluorescent protein. (c) Induction of DON biosynthesis by H2O2 in wild‐type and ΔFgLetm1 grown in Ammonium Trichothecene Biosynthesis Induction medium (ATBI) medium. H2O2 was added to ATBI daily, and the supernatant after 7 days of incubation was used for the quantification of DON production. (d) Relative expression levels of TRI5, TRI6 and TRI10 in PH‐1 and ΔFgLetm1 with or without H2O2 treatment. The relative expression level of each gene in the wild‐type without H2O2 treatment was arbitrarily set to unity. Bars with the same letter indicate no significant difference at P = 0.05.
Table 3.
Deoxynivalenol (DON) production of the wild‐type, mutants and complemented strains in Trichothecene Biosynthesis Induction medium (TBI), wheat kernel medium and infected spikelets.
| TBI | Wheat kernel | Infected spikelet | |
|---|---|---|---|
| Strain | DON production (µg/g dried mycelia) | DON production (µg/mg ergosterol) | DON production (mg/mg ergosterol) |
| PH‐1 | 650.75 ± 7.07a * | 398.88 ± 13.07a | 581.53 ± 30.28a |
| ΔFgLetm1 | 38.24 ± 2.81b | 11.81 ± 1.40b | 228.61 ± 20.25b |
| ΔFgLetm1‐C | 630.80 ± 12.86a | 428.56 ± 14.59a | 536.27 ± 17.36a |
| ΔFgLetm2 | 635.07 ± 13.80a | 420.97 ± 15.58a | 566.54 ± 30.85a |
| ΔFgLetm2‐C | 660.46 ± 8.96a | 394.22 ± 15.08a | 605.03 ± 35.18a |
| ΔΔFgLetm1/2 | 35.93 ± 3.53b | 9.43 ± 2.89b | 188.10 ± 19.51b |
*Values followed by the same letter are not significantly different at P = 0.05 for each treatment.
To determine whether DON reduction is caused by decreased ATP and ROS production in ΔFgLetm1, we conducted DON rescue assays by supplying exogenous ATP or H2O2 to the liquid cultures induced by ammonium, as described previously (Gardiner et al., 2009). Treatment with H2O2 clearly increased DON biosynthesis in PH‐1, and partially recovered DON biosynthesis in ΔFgLetm1 (Fig. 7c). However, the final production of DON after induction by H2O2 was still less than in the wild‐type (Fig. 7c). We next assayed the expression of TRI5, TRI6 and TRI10 with RNA samples isolated from hyphae of the wild‐type and ΔFgLetm1 mutant with or without treatment with 0.5 mm H2O2 in Ammonium Trichothecene Biosynthesis Induction medium (ATBI) at day 3. In the wild‐type strain, the expression levels of TRI5, TRI6 and TRI10 were 4.0‐, 10.4‐ and 5.2‐fold higher in H2O2‐treated samples than in untreated samples (Fig. 7d). Treatment with H2O2 also induced the expression of these TRI genes in the ΔFgLetm1 mutant (Fig. 7d). However, the addition of exogenous ATP in TBI was unable to rescue the decrease in DON biosynthesis in the ΔFgLetm1 mutant (Fig. S6, see Supporting Information). Therefore, our evidence implies that the diminished endogenous ROS might be partly responsible for the reduction in DON biosynthesis in ΔFgLetm1.
Wild‐type F. graminearum produces less DON and is strongly attenuated in virulence under hypoxic conditions
Given that dysfunctional mitochondria in the ΔFgLetm1 mutant increased the activity of glycolysis, but decreased DON biosynthesis and virulence, we speculated that DON biosynthesis might be suppressed under hypoxic conditions. Therefore, we investigated the DON biosynthesis and virulence of F. graminearum under limited O2 conditions (1%). Surprisingly, the radial growth rate of wild‐type PH‐1 under hypoxic conditions was similar to that under open air conditions (Fig. 8a). Compared with the expression of TRI5, TRI6 and TRI10 in TBI under open air conditions, the expression of these genes in the wild‐type was strongly reduced under hypoxic conditions (Fig. 8b), and was similar to the levels in the ΔFgLetm1 mutant (Fig. 7a). However, the transcriptional level of the control gene AURJ for pigment formation was increased under hypoxic conditions, indicating that the reduction in TRI gene expression was somehow specific under hypoxic conditions. Meanwhile, Tri1‐GFP‐labelled toxisome formation was also completely abolished under hypoxic conditions (Fig. 8c). Finally, DON production under hypoxic conditions decreased by 40‐fold compared with that in open air conditions (Fig. 8d). The pathogenicity assay was also conducted under both conditions. At 7 days post‐inoculation, the mycelia were able to infect the inoculated site, cause necrotic symptoms and spread to nearby spikelets under open air conditions (Fig. 8e, left‐hand panel). Remarkably, wild‐type PH‐1 failed to infect the inoculate spikelet under hypoxic conditions (Fig. 8e, right‐hand panel). Taken together, DON biosynthesis and virulence were suppressed in F. graminearum under low oxygen conditions, similar to the effect of dysfunctional mitochondria caused by deletion of the FgLETM1 gene.
Figure 8.
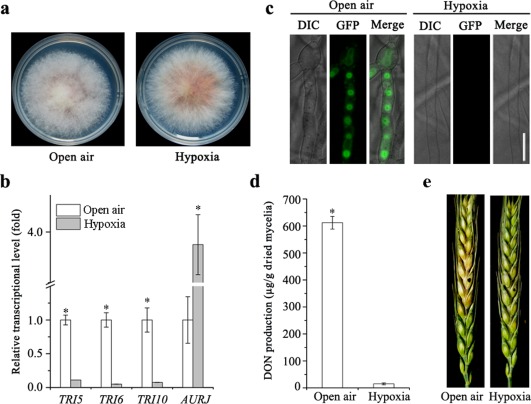
Deoxynivalenol (DON) biosynthesis and virulence are reduced under hypoxic conditions. (a) Colony morphology of wild‐type grown on potato dextrose agar (PDA) in open air and hypoxic conditions. (b) Relative expression levels of TRI5, TRI6 and TRI10 in the mycelium of PH‐1 under open air and hypoxic conditions. Strains were grown in Trichothecene Biosynthesis Induction medium (TBI) for 3 days. The pigment biosynthesis gene, AURJ, was used as a control. (c) Toxisome formation of the wild‐type under open air and hypoxic conditions. Tri1‐GFP was observed after 3 days of incubation. DIC, Differential Interference Contrast; GFP, green fluorescent protein. (d) DON production of the wild‐type under open air and hypoxic conditions after 7 days of incubation. (e) Virulence of the wild‐type under open air and hypoxic conditions. Scab symptoms were observed at 7 days post‐inoculation.
Discussion
Mdm38 in yeast is critical for the maintenance of mitochondrial morphology. The lack of, or RNAi silencing of, Letm1 orthologues leads to the disruption of the mitochondrial network and apparent swelling of the mitochondria in various organisms (Dimmer et al., 2002; Hasegawa and van der Bliek, 2007; Hashimi et al., 2013; McQuibban et al., 2010; Nowikovsky et al., 2004; Schlickum et al., 2004; Sickmann et al., 2003). To date, no study has been conducted to investigate the biological roles of the Letm1 orthologues in filamentous fungi. In this study, we showed that the ΔFgLetm1 mutant of the filamentous fungus F. graminearum lacks tubular‐shaped cristae structures and shows increased mitochondrial volumes (Fig. 4b,c). Interestingly, for the first time, we also showed that FgLetm1 plays an important role in mitochondrial mobility, as the ΔFgLetm1 mutation slows down the dynamic change in the mitochondria (Videos S1 and S2). Mdm38 in S. cerevisiae also plays a critical role in the biogenesis of the respiratory chain by coupling ribosome function to protein transport across the inner membrane (Frazier et al., 2006; Tamai et al., 2008). Here, we found that the protein synthesis of Cyt b decreased in the ΔFgLetm1 mutant (Fig. 4d). To support this idea, we conducted an affinity capture assay with the FgLetm1‐GFP fusion protein as a bait. Protein mass spectrometry data showed that 18 proteins of the mitochondrial ribosome were associated with FgLetm1 (Table S2, see Supporting Information). Among them, 12 homologous proteins were also found to be complexed with Mdm38 and Ylh47 by affinity purification in yeast (Frazier et al., 2006). Taken together, the roles of the Letm1 superfamily proteins in mitochondrial integrity and the biogenesis of the respiratory chain seem to be highly conserved from yeast to mammals, although their amino acid sequences and motif features differ.
In addition, we also found that FgLetm1 in F. graminearum possesses certain distinct features from its orthologues in other organisms. For instance, Mdm38 is required for efficient growth on non‐fermentable carbon sources, such as glycerol, in yeast (Frazier et al., 2006). In contrast, the ΔFgLetm1 mutant of F. graminearum shows a comparable growth phenotype on agar medium supplemented with either glucose or glycerol as the sole carbon source (Fig. S7, see Supporting Information). Deletion of ΔFgLetm1 strongly decreases the number of conidia and septa of conidia, and reduces the stress response towards fungicides, ions and oxidative stress. These results imply that FgLetm1 is critical for fitness in certain environmental niches. Therefore, FgLetm1 may have species‐specific activities, in addition to the conserved function of Letm1 orthologues, in maintaining the integrity of mitochondria.
ROS play a major role in pathogen–plant interactions during which the host plant rapidly triggers an oxidative burst to suppress pathogen infection. Pathogens must cope with plant‐released ROS during a successful infection (Apel and Hirt, 2004; Heller and Tudzynski, 2011). It is probable that all organisms have evolved oxidative stress response (OSR) mechanisms to scavenge elevated intracellular ROS levels. The ROS scavenging system is important for the detoxification of ROS in cells, and the OSR must be tightly regulated. In budding yeast, several signal components are involved in the regulation of the OSR at the transcriptional level, including the Hog1 cascade and the transcription factors Yap1, Atf1 and Skn7 (He and Fassler, 2005; Kim and Hahn, 2013; Raitt et al., 2000). In F. graminearum, all three stress‐related transcription factor genes, FgAP1, FgATF1 and FgSKN7, play a role in tolerance to oxidative stress. Moreover, F. graminearum has evolved its OSR system to transduce oxidative stress as a signal for the induction of DON biosynthesis, which is a critical virulence factor during the infection process (Jiang et al., 2015; Montibus et al., 2013; Van Nguyen et al., 2013). The addition of H2O2 to the cell culture medium of F. graminearum stimulated TRI gene expression and increased DON accumulation, in a manner largely dependent on the OSR transcription factor FgSKN7 (Jiang et al., 2015). In addition to treatment with exogenous ROS, endogenous ROS also modulate DON production. H2O2 was shown to constitutively accumulate in the DON induction medium in the culture of F. graminearum. Moreover, the time course curve of H2O2 accumulation followed the pattern of DON production (Ponts et al., 2006, 2007). Consistent with the above findings, we found that ROS were highly accumulated in the mycotoxin induction medium (TBI), and exogenous H2O2 stimulated DON production (Figs 5 and 7). We also found that deletion of FgLETM1 almost completely abolished ROS production in mitochondria in MM and TBI media (Fig. 5a, Table 2), and caused a reduction in DON biosynthesis both in vitro and in planta (Table 3). The expression of TRI genes and DON production in ΔFgLetm1 were partially rescued in LTB medium supplemented with H2O2 (Fig. 7c,d). Therefore, mitochondrial‐derived ROS are important for DON biosynthesis in F. graminearum, although other factors related to mitochondrial dysfunction might also be involved in this process (Bonnighausen et al., 2015).
The concentration of oxygen in the atmosphere is important for the biosynthesis of mycotoxins. Penicillium griseofulvum produced less patulin toxin in a 1% or 5% O2 environment than in the open air (20% O2) (Paster and Lisker, 1985). In another example, only trace amounts of T‐2 toxin were detected in Fusarium sporotrichioides under 40% CO2/5% O2, in comparison with a much greater amount of T‐2 toxin under 40% CO2/20% O2 (Paster et al., 1986). Fungal growth in these gaseous environments was identical to that under open air conditions, even in O2 concentrations of <1% (Hocking, 1989). Here, we found that F. graminearum showed normal growth patterns in the 1% O2 condition as in the open air condition (Fig. 8a), but low levels of oxygen strongly reduced DON production and virulence (Fig. 8b,e), similar to the phenotypes seen in the ΔFgLetm1 mutant (Figs 6 and 7). However, disruption of the mitochondrial integrity in ΔFgLetm1 significantly reduced DON production (Fig. 7, Table 3). We infer that fungicides targeting the mitochondria might play a potential role in controlling DON biosynthesis and FHB. We thus evaluated the effect of three mitochondrial targeting fungicides, boscalid, pyraclostrobin and py‐diflumetofen, on DON production. As expected, all three tested fungicides strongly reduced DON production (Fig. S8, see Supporting Information). Taken together, our results suggest that the storage of grain under hypoxia, and fungicides targeting mitochondria, might provide potential approaches for DON management.
Experimental Procedures
Fungal strains and culture conditions
Fusarium graminearum strain PH‐1 (NRRL 31084) was used as the progenitor for the construction of gene deletion mutants. The wild‐type and transformants generated in this study were grown at 25 °C on PDA, MM and WA for mycelial growth tests (Liu et al., 2015). CMC medium was used for sporulation assays (Cappelli and Peterson, 1965). For the quantification of DON production, strains were grown in liquid TBI medium (Menke et al., 2012). To evaluate the effect of H2O2 on the induction of DON biosynthesis, wild‐type or mutants were cultured in LTB as described previously (Jiang et al., 2016), and H2O2 was supplemented at a final concentration of 0.5 mm. For hypoxic conditions, the inoculated TBI or wheat heads were statically incubated in a modular incubator chamber (Billups‐Rothenberg, Inc., San Diego, CA, USA) filled with a mixture of gases (1% O2, 99% N2).
The sensitivity of the strains towards stress agents was determined as described previously (Liu et al., 2015). The final concentrations of NaCl, KCl, fungicides, Fe2+, CaCl2 and H2O2 in MM are indicated in the figures. For testing the temperature sensitivity of the mutants, cells were grown at 15, 25 and 32 °C. The mycelial growth inhibition rate (MGIR) was calculated using the formula MGIR% = [(N – C )/C ] × 100, where C is the colony diameter of the control without treatment and N is that with treatment. Each experiment was repeated three times independently.
Construction of gene deletion mutants and complemented strains
The construction of gene deletion and complementation vectors and the subsequent transformation of F. graminearum were carried out using the protocols described previously (Jiang et al., 2011). In order to generate the double mutant of FgLETM1 and FgLETM2, FgLETM1 was knocked out in the FgLETM2 deletion mutant (ΔFgLETM2). The primers used to amplify the flanking sequences of each gene are listed in Table S3 (see Supporting Information). Deletion candidates were identified by PCR with the designated primers (Table S3), and further analysed by Southern blotting. Three independent transformants for each mutant were used in all experiments. FgLetm1‐GFP, FgLetm2‐GFP and Tri1‐GFP fusion constructs were generated as described previously (Gu et al., 2015a).
Plant infection and DON production assays
A 10‐μL aliquot of conidial suspension (1 × 105 conidia/mL) was injected into a floret in the central section spikelet of a single flowering wheat head of a susceptible cultivar grown in the field. There were 10 replicates for each strain. Fifteen days after inoculation, the infected spikelets in each inoculated wheat head were recorded. The experiment was repeated four times, and typical symptoms were shown. Infectious hyphae developed in wheat tissue cells were examined at 48 h post‐inoculation by SEM.
The strain expressing Tri1‐GFP was used as the fluorescent reporter strain for toxisome formation, and toxisome formation was observed after 3 days of incubation in TBI. DON production in the wild‐type, mutants and complemented strains was quantified under several conditions, including TBI, LTB, wheat kernel medium and inoculated spikelets. The supernatant of TBI after 7 days of incubation was collected for the quantification of DON. DON production in wheat kernel medium was conducted as described previously (Ji et al., 2014; Liu et al., 2015). The inoculated spikelets were harvested after 15 days, and DON was extracted as described previously (Jiang et al., 2015). The total amount of ergosterol was extracted from infected spikelets as described previously (Liu et al., 2013). For ROS induction assay, LTB was used for the replacement of TBI, as DON biosynthesis was already extremely induced. The recipe of LTB medium was modified from TBI, replacing putrescine with ammonium nitrate at a final concentration of 5 mm. H2O2 was added daily to LTB to a final concentration of 0.5 mm. After 7 days of incubation, the supernatant was collected for DON quantification. DON samples were quantified by liquid chromatography‐tandem mass spectrometry (LC‐MS/MS), as described previously (Dong et al., 2016). The experiment was repeated three times.
qRT‐PCR assays
RNA samples of the wild‐type, mutants and complemented strains were isolated as described previously (Liu et al., 2015). For the induction of TRI genes by H2O2, the hyphae of wild‐type or mutant were harvested for RNA extraction after 48 h of treatment. TAKARA (Dalian, Liaoning, China) SYBR Premix Ex Taq was used for qRT‐PCR assays with the CFX96 Real‐Time System as described previously (Bio‐Rad, Hercules, CA, USA). The actin gene of F. graminearum was used as the internal control. Relative expression levels of each gene were calculated with the 2–ΔΔCt method (Livak and Schmittgen, 2001).
Western blotting hybridization
The protein extraction and western blot analysis were performed as described previously (Yun et al., 2015). Anti‐MT‐CYB antibody (Abcam, ab103405, Cambridge, MA, USA) was used to detect Cyt b for the analysis of the biosynthesis of the respiratory chain. The samples were also detected with monoclonal anti‐H3 antibody (Abcam, ab1791) as a reference. The samples were also detected with monoclonal anti‐GAPDH (Glyceraldehyde‐3‐phosphate dehydrogenase) antibody EM1101 (Hangzhou HuaAn Biotechnology Co., Ltd., HuaAn Biotechnology, Hangzhou, Zhejiang, China) as a reference. All experiments were conducted three times.
Microscopic imaging
The localization of green fluorescent‐labelled proteins and Mito‐HcRed (Thermo Fisher, M7512, Rockford, IL, USA) staining signals was visualized using a Zeiss LSM780 confocal microscope (Carl Zeiss AG, Oberkochen, Germany). The microstructure of the mitochondria in the wild‐type or mutants was treated as described previously (Yun et al., 2015), and observed by TEM (JEOL JEM‐1230, Otemachi, Chiyoda, Tokyo). For the observation of infection structures on wheat glumes, the glumes were treated as described previously (Gu et al., 2015b), and observed using a Hitachi Model TM‐1000 scanning electron microscope (Hitachi, Tokyo, Japan).
Quantification of ATP and H2O2 production
The mycelia grown in MM for 24 h and in TBI for 3 days were used for the quantification of H2O2 and ATP. H2O2 and ATP production was assayed using a Hydrogen Peroxide Assay Kit (Beyotime Institute of Biotechnology, Shanghai, China, S0038) and an ATP Assay Kit (Beyotime, S0026), respectively. Briefly, mycelia (0.05 g) were added to 200 µL of lysis buffer in the H2O2 detection kit or 500 µL of lysis buffer in the ATP detection kit. After lysis of mycelia, quantification of H2O2 or ATP production was conducted following the instructions provided by the manufacturer. The experiments were repeated three times.
Supporting information
Additional Supporting Information may be found in the online version of this article at the publisher's website:
Fig. S1 Phylogenetic tree of the Letm1 superfamily orthologues from 32 fungal genomes available in the National Center for Biotechnology Information (NCBI) Bioprojects and Broad Institute databases. Orthologues were retrieved with the yeast Letm1 proteins, Mdm38 and Ylh47, and FgLetm1 and FgLetm2 protein sequences as queries. The phylogenetic tree was constructed by the neighbour‐joining method using mega 5.0. Numbers at the nodes represent the results of 1000 bootstrap replications. The GenBank or organism‐specific accession numbers are indicated in the figure.
Fig. S2 Identification of deletion mutants and complemented strains. (a) Southern blot hybridization analysis of the deletion mutants ΔFgLetm1 and ΔΔFgLetm1/2, and the complemented strain ΔFgLetm1+PLETM1FgLetm1‐GFP, using downstream DNA fragments of FgLETM1 as the probe. Both ΔFgLetm1 and ΔΔFgLetm1/2 had an anticipated 4594‐bp band, but both lacked the 2219‐bp band present in wild‐type PH‐1, when probed with a 725‐bp downstream DNA fragment of FgLETM1. (b) Southern blot hybridization analysis of the deletion mutants ΔFgLetm2 and ΔΔFgLetm1/2, and the complemented strain ΔFgLetm2+PLETM2FgLetm2‐GFP, using a downstream DNA fragment of FgLETM2 as the probe. Using an 800‐bp upstream DNA fragment of FgLETM2 as the probe, the ΔFgLetm2 mutant had an anticipated 3253‐bp band, when the chromosomal DNA of ΔFgLetm2 was digested with NdeI. ΔΔFgLetm1/2 presented a 4098‐bp band, but lacked the 1585‐bp band present in the wild‐type PH‐1, when the chromosomal DNA of ΔΔFgLetm1/2 was digested with EcoRV and blotted with the same probe. (c) PCR verification of the complemented strains ΔFgLetm1+PLETM1FgLetm1‐GFP and ΔFgLetm2+PLETM2FgLetm2‐GFP. The whole cassette, including the promoter, open reading frame (ORF) and gfp, was amplified.
Fig. S3 Conidial morphology of the wild‐type, ΔFgLetm1, ΔFgLetm2, ΔΔFgLetm1/2 and complemented strains. The septa were stained with calcofluor white and observed using a fluorescence microscope. Bar, 20 µm.
Fig. S4 Colony morphology of PH‐1, mutants and complemented strains grown on wheat head tissue medium (WA) at 25 °C for 3 days.
Fig. S5 Infection structures of wild‐type and deletion mutant ΔFgLetm1 on the inoculated glumes at 14 days post‐inoculation. Samples were collected at 2 weeks post‐inoculation and observed by scanning electron microscopy. Bar dimensions are indicated on the images.
Fig. S6 Induction of deoxynivalenol (DON) biosynthesis by exogenous adenosine triphosphate (ATP) in the wild‐type and mutants grown in TBI medium. ATP was added to Trichothecene Biosynthesis Induction medium (TBI) at a final concentration of 10 µm, and the cell‐free supernatant after 7 days of incubation was used for the quantification of DON production. Error bars denote the standard deviation from three repeated experiments.
Fig. S7 The growth phenotype of the deletion mutant ΔFgLetm1 was not changed by the utilization of non‐fermentable carbon. Colony morphology of the wild‐type, ΔFgLetm1, ΔFgLetm2 and ΔΔFgLetm1/2 on minimal medium supplemented with glucose or glycerol as sole carbon source. Plates were photographed after incubation at 25 °C for 3 days.
Fig. S8 Fungicides targeting mitochondria are able to inhibit toxisome formation and deoxynivalenol (DON) biosynthesis. (a) The growth inhibition of the tested fungicides at 0.3 µg/mL. (b) Toxisome formation in the presence of fungicides at 0.3 µg/mL. The ΔTri1:Tri1‐GFP strain was grown in Trichothecene Biosynthesis Induction medium (TBI) for 24 h, individual fungicides were added at a final concentration at 0.3 µg/mL and incubation was performed for another 24 h before observation. (c) DON production of each treatment. DON was extracted from 7‐day‐cultured TBI in each treatment and quantified by liquid chromatography‐mass spectrometry (LC‐MS). Columns with different letters indicate a significant difference at P = 0.05. DIC, Differential Interference Contrast; DMSO, Dimethyl sulphoxide; GFP, green fluorescent protein.
Table S1 The Letm1 superfamily domain in the Letm1 orthologues of six filamentous plant‐pathogenic fungi and Saccharomyces cerevisiae.
Table S2 Proteins of the mitochondrial ribosome complexed with FgLetm1 by affinity purification and mass spectrometry assay.
Table S3 Oligonucleotide primers used in this study.
Video S1 Mitochondrial patterns stained by Mito‐HcRed in the wild‐type.
Video S2 Mitochondrial patterns stained by Mito‐HcRed in the deletion mutant ΔFgLetm1.
Acknowledgements
We thank Dr Jiang Cong and Dr Huiquan Liu (Northwest A&F University, Xianyang, China) for DON quantification and bioinformatics analyses, and Professor Yunrong Chai (Northeastern University, Boston, MA, USA) and Kevin Gozzi (Massachusetts Institute of Technology, Cambridge, MA, USA) for manuscript editing. The research was supported by the Natural Science Foundation of Zhejiang Province (LR17C140001), National Natural Science Foundation of China (31672064), International Science & Technology Cooperation Program of China (2016YFE0112900) and China Agriculture Research System (CARS‐3‐1‐15). The authors declare no conflicts of interest.
References
- Apel, K. and Hirt, H. (2004) Reactive oxygen species: metabolism, oxidative stress, and signal transduction. Annu. Rev. Plant Biol. 55, 373–399. [DOI] [PubMed] [Google Scholar]
- Audenaert, K. , Callewaert, E. , Hofte, M. , De Saeger, S. and Haesaert, G. (2010) Hydrogen peroxide induced by the fungicide prothioconazole triggers deoxynivalenol (DON) production by Fusarium graminearum . BMC Microbiol. 10, 112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beckman, K.B. and Ames, B.N. (1998) The free radical theory of aging matures. Physiol Rev. 78, 547–581. [DOI] [PubMed] [Google Scholar]
- Bennett, J.W. and Klich, M. (2003) Mycotoxins. Clin. Microbiol. Rev. 16, 497–516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boenisch, M.J. , Broz, K.L. , Purvine, S.O. , Chrisler, W.B. , Nicora, C.D. , Connolly, L.R. , Freitag, M. , Baker, S.E. and Kistler, H.C. (2017) Structural reorganization of the fungal endoplasmic reticulum upon induction of mycotoxin biosynthesis. Sci. Rep. 7, 44 296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonnighausen, J. , Gebhard, D. , Kroger, C. , Hadeler, B. , Tumforde, T. , Lieberei, R. , Bergemann, J. , Schafer, W. and Bormann, J. (2015) Disruption of the GABA shunt affects mitochondrial respiration and virulence in the cereal pathogen Fusarium graminearum . Mol. Microbiol. 98, 1115–1132. [DOI] [PubMed] [Google Scholar]
- Boutigny, A.L. , Barreau, C. , Atanasova‐Penichon, V. , Verdal‐Bonnin, M.N. , Pinson‐Gadais, L. and Richard‐Forget, F. (2009) Ferulic acid, an efficient inhibitor of type B trichothecene biosynthesis and Tri gene expression in Fusarium liquid cultures. Mycol. Res. 113, 746–753. [DOI] [PubMed] [Google Scholar]
- Cappelli, R. and Peterson, J.L. (1965) Macroconidium formation in submerged cultures by a non‐sporulating strain of Gibberella Zeae . Mycologia, 57, 962. [Google Scholar]
- Desjardins, A.E. (2006) Fusarium mycotoxins: chemistry, genetics, and biology. St. Paul, MN: APS Press. [Google Scholar]
- Desjardins, A.E. , Hohn, T.M. and McCormick, S.P. (1993) Trichothecene biosynthesis in Fusarium species – chemistry, genetics, and significance. Microbiol. Rev. 57, 595–604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dimmer, K.S. , Fritz, S. , Fuchs, F. , Messerschmitt, M. , Weinbach, N. and Neupert, W. (2002) Genetic basis of mitochondrial function and morphology in Saccharomyces cerevisiae . Mol. Biol. Cell, 13, 847–853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dimmer, K.S. , Navoni, F. , Casarin, A. , Trevisson, E. , Endele, S. , Winterpacht, A. , Salviati, L. and Scorrano, L. (2008) LETM1, deleted in Wolf–Hirschhorn syndrome is required for normal mitochondrial morphology and cellular viability. Hum. Mol. Genet. 17, 201–214. [DOI] [PubMed] [Google Scholar]
- Dong, F. , Qiu, J. , Xu, J. , Yu, M. , Wang, S. , Sun, Y. , Zhang, G. and Shi, J. (2016) Effect of environmental factors on Fusarium population and associated trichothecenes in wheat grain grown in Jiangsu province, China. Int. J. Food Microbiol. 230, 58–63. [DOI] [PubMed] [Google Scholar]
- Endele, S. , Fuhry, M. , Pak, S.J. , Zabel, B.U. and Winterpacht, A. (1999) LETM1, a novel gene encoding a putative EF‐hand Ca2+‐binding protein, flanks the Wolf–Hirschhorn syndrome (WHS) critical region and is deleted in most WHS patients. Genomics, 60, 218–225. [DOI] [PubMed] [Google Scholar]
- Frazier, A.E. , Taylor, R.D. , Mick, D.U. , Warscheid, B. , Stoepel, N. , Meyer, H.E. , Ryan, M.T. , Guiard, B. and Rehling, P. (2006) Mdm38 interacts with ribosomes and is a component of the mitochondrial protein export machinery. J. Cell Biol. 172, 553–564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gardiner, D.M. , Kazan, K. and Manners, J.M. (2009) Nutrient profiling reveals potent inducers of trichothecene biosynthesis in Fusarium graminearum . Fungal Genet. Biol. 46, 604–613. [DOI] [PubMed] [Google Scholar]
- Grissa, I. , Bidard, F. , Grognet, P. , Grossetete, S. and Silar, P. (2010) The Nox/Ferric reductase/Ferric reductase‐like families of Eumycetes. Fungal Biol. 114, 766–777. [DOI] [PubMed] [Google Scholar]
- Gu, Q. , Chen, Y. , Liu, Y. , Zhang, C. and Ma, Z.H. (2015a) The transmembrane protein FgSho1 regulates fungal development and pathogenicity via the MAPK module Ste50‐Ste11‐Ste7 in Fusarium graminearum . New Phytol. 206, 315–328. [DOI] [PubMed] [Google Scholar]
- Gu, Q. , Zhang, C. , Liu, X. and Ma, Z.H. (2015b) A transcription factor FgSte12 is required for pathogenicity in Fusarium graminearum . Mol. Plant Pathol. 16, 1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasegawa, A. and van der Bliek, A.M. (2007) Inverse correlation between expression of the Wolfs Hirschhorn candidate gene Letm1 and mitochondrial volume in C. elegans and in mammalian cells. Hum. Mol. Genet. 16, 2061–2071. [DOI] [PubMed] [Google Scholar]
- Hashimi, H. , McDonald, L. , Stribrna, E. and Lukes, J. (2013) Trypanosome Letm1 protein is essential for mitochondrial potassium homeostasis. J. Biol. Chem. 288, 26 914–26 925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He, X.J. and Fassler, J.S. (2005) Identification of novel Yap1p and Skn7p binding sites involved in the oxidative stress response of Saccharomyces cerevisiae . Mol. Microbiol. 58, 1454–1467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heller, J. and Tudzynski, P. (2011) Reactive oxygen species in phytopathogenic fungi: signaling, development, and disease. Annu. Rev. Phytopathol. 49, 369–390. [DOI] [PubMed] [Google Scholar]
- Hocking, A.D. (1989) Responses of fungi to modified atmospheres In: Champ, B.R., Highley, E., Banks, H.J. (Eds.), Fumigation and Controlled Atmosphere Storage of Grain: Proceedings of an International Conference, Singapore, 14–18 February 1989. ACIAR Proceedings No. 25. [Google Scholar]
- Hu, S. , Zhou, X.Y. , Gu, X.Y. , Cao, S.L. , Wang, C.F. and Xu, J.R. (2014) The cAMP‐PKA pathway regulates growth, sexual and asexual differentiation, and pathogenesis in Fusarium graminearum . Mol. Plant–Microbe Interact. 27, 557–566. [DOI] [PubMed] [Google Scholar]
- Hwang, S.‐K. , Piao, L. , Lim, H.‐T. , Minai‐Tehrani, A. , Yu, K.‐N. , Ha, Y.‐C. , Chae, C.‐H. , Lee, K.‐H. , Beck, G.R. , Park, J. and Cho, M.‐H. (2010) Suppression of lung tumorigenesis by Leucine Zipper/EF Hand‐containing transmembrane‐1. PLoS One, 5, e12535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ji, F. , Xu, J. , Liu, X. , Yin, X. and Shi, J. (2014) Natural occurrence of deoxynivalenol and zearalenone in wheat from Jiangsu province, China. Food Chem. 157, 393–397. [DOI] [PubMed] [Google Scholar]
- Jiang, C. , Zhang, S.J. , Zhang, Q. , Tao, Y. , Wang, C.F. and Xu, J.R. (2015) FgSKN7 and FgATF1 have overlapping functions in ascosporogenesis, pathogenesis and stress responses in Fusarium graminearum . Environ. Microbiol. 17, 1245–1260. [DOI] [PubMed] [Google Scholar]
- Jiang, C. , Zhang, C. , Wu, C. , Sun, P. , Hou, R. , Liu, H. and Xu, J.R. (2016) TRI6 and TRI10 play different roles in the regulation of deoxynivalenol (DON) production by cAMP signalling in Fusarium graminearum . Environ. Microbiol. 18, 3689–3701. [DOI] [PubMed] [Google Scholar]
- Jiang, J. , Liu, X. , Yin, Y. and Ma, Z. (2011) Involvement of a velvet protein FgVeA in the regulation of asexual development, lipid and secondary metabolisms and virulence in Fusarium graminearum . PLoS One, 6, e28291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiao, F. , Kawakami, A. and Nakajima, T. (2008) Effects of different carbon sources on trichothecene production and Tri gene expression by Fusarium graminearum in liquid culture. FEMS Microbiol. Lett. 285, 212–219. [DOI] [PubMed] [Google Scholar]
- Kim, D. and Hahn, J.S. (2013) Roles of the Yap1 transcription factor and antioxidants in Saccharomyces cerevisiae's tolerance to furfural and 5‐hydroxymethylfurfural, which function as thiol‐reactive electrophiles generating oxidative stress. Appl. Environ. Microbiol. 79, 5069–5077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimura, M. , Anzai, H. and Yamaguchi, I. (2001) Microbial toxins in plant–pathogen interactions: biosynthesis, resistance mechanisms, and significance. J. Gen. Appl. Microbiol. 47, 149–160. [DOI] [PubMed] [Google Scholar]
- Kimura, M. , Tokai, T. , Takahashi‐Ando, N. , Ohsato, S. and Fujimura, M. (2007) Molecular and genetic studies of Fusarium trichothecene biosynthesis: pathways, genes, and evolution. Biosci. Biotechnol. Biochem. 71, 2105–2123. [DOI] [PubMed] [Google Scholar]
- Liu, S.S. (1999) Cooperation of a “reactive oxygen cycle” with the Q cycle and the proton cycle in the respiratory chain – superoxide generating and cycling mechanisms in mitochondria. J. Bioenerg. Biomembr. 31, 367–376. [DOI] [PubMed] [Google Scholar]
- Liu, X. , Jiang, J.H. , Yin, Y.N. and Ma, Z.H. (2013) Involvement of FgERG4 in ergosterol biosynthesis, vegetative differentiation and virulence in Fusarium graminearum . Mol. Plant Pathol. 14, 71–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu, Y. , Liu, N. , Yin, Y.N. , Chen, Y. , Jiang, J.H. and Ma, Z.H. (2015) Histone H3K4 methylation regulates hyphal growth, secondary metabolism and multiple stress responses in Fusarium graminearum . Environ. Microbiol. 17, 4615–4630. [DOI] [PubMed] [Google Scholar]
- Livak, K.J. and Schmittgen, T.D. (2001) Analysis of relative gene expression data using real‐time quantitative PCR and the 2− ΔΔCT method. Methods, 25, 402–408. [DOI] [PubMed] [Google Scholar]
- McQuibban, A.G. , Joza, N. , Megighian, A. , Scorzeto, M. , Zanini, D. , Reipert, S. , Richter, C. , Schweyen, R.J. and Nowikovsky, K. (2010) A Drosophila mutant of LETM1, a candidate gene for seizures in Wolf–Hirschhorn syndrome. Hum. Mol. Genet. 19, 987–1000. [DOI] [PubMed] [Google Scholar]
- Menke, J. , Dong, Y. and Kistler, H.C. (2012) Fusarium graminearum Tri12p influences virulence to wheat and trichothecene accumulation. Mol. Plant–Microbe Interact. 25, 1408–1418. [DOI] [PubMed] [Google Scholar]
- Mentges, M. and Bormann, J. (2015) Real‐time imaging of hydrogen peroxide dynamics in vegetative and pathogenic hyphae of Fusarium graminearum . Sci Rep. 5, 14 980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merhej, J. , Richard‐Forget, F. and Barreau, C. (2011) The pH regulatory factor Pac1 regulates Tri gene expression and trichothecene production in Fusarium graminearum . Fungal Genet Biol. 48, 275–284. [DOI] [PubMed] [Google Scholar]
- Miller, J.D. and Greenhalgh, R. (1985) Nutrient effects on the biosynthesis of trichothecenes and other metabolites by Fusarium graminearum . Mycologia, 77, 130–136. [Google Scholar]
- Missall, T.A. , Lodge, J.K. and McEwen, J.E. (2004) Mechanisms of resistance to oxidative and nitrosative stress: implications for fungal survival in mammalian hosts. Eukaryot. Cell, 3, 835–846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montibus, M. , Ducos, C. , Bonnin‐Verdal, M.‐N. , Bormann, J. , Ponts, N. , Richard‐Forget, F. and Barreau, C. (2013) The bZIP transcription factor Fgap1 mediates oxidative stress response and trichothecene biosynthesis but not virulence in Fusarium graminearum . PLoS One, 8, e83377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nowikovsky, K. , Froschauer, E.M. , Zsurka, G. , Samaj, J. , Reipert, S. , Kolisek, M. , Wiesenberger, G. and Schweyen, R.J. (2004) The LETM1/YOL027 gene family encodes a factor of the mitochondrial K+ homeostasis with a potential role in the Wolf–Hirschhorn syndrome. J. Biol. Chem. 279, 30 307–30 315. [DOI] [PubMed] [Google Scholar]
- Oh, M. , Son, H. , Choi, G.J. , Lee, C. , Kim, J.C. , Kim, H. and Lee, Y.W. (2016) Transcription factor ART1 mediates starch hydrolysis and mycotoxin production in Fusarium graminearum and F. verticillioides . Mol. Plant Pathol. 17, 755–768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osiewacz, H.D. (2002) Mitochondrial functions and aging. Gene, 286, 65–71. [DOI] [PubMed] [Google Scholar]
- Paster, N. and Lisker, N. (1985) Effect of controlled atmospheres on Penicillium patulum growth and patulin production In: J. Lacey. (Ed.), Tricothecenes and Other Mycotoxins, pp. 233–234. John Wiley and Sons Ltd. [Google Scholar]
- Paster, N. , Barkai‐Golan, R. and Calderon, M. (1986) Control of T‐2 toxin production using atmospheric gases. J. Food Protect. 49, 615–617. [DOI] [PubMed] [Google Scholar]
- Pinson‐Gadais, L. , Richard‐Forget, F. , Frasse, P. , Barreau, C. , Cahagnier, B. , Richard‐Molard, D. and Bakan, B. (2008) Magnesium represses trichothecene biosynthesis and modulates Tri5, Tri6, and Tri12 genes expression in Fusarium graminearum . Mycopathologia, 165, 51–59. [DOI] [PubMed] [Google Scholar]
- Ponts, N. , Pinson‐Gadais, L. , Verdal‐Bonnin, M.N. , Barreau, C. and Richard‐Forget, F. (2006) Accumulation of deoxynivalenol and its 15‐acetylated form is significantly modulated by oxidative stress in liquid cultures of Fusarium graminearum . FEMS Microbiol. Lett. 258, 102–107. [DOI] [PubMed] [Google Scholar]
- Ponts, N. , Pinson‐Gadais, L. , Barreau, C. , Richard‐Forget, F. and Ouellet, T. (2007) Exogenous H2O2 and catalase treatments interfere with Tri genes expression in liquid cultures of Fusarium graminearum . FEBS Lett. 581, 443–447. [DOI] [PubMed] [Google Scholar]
- Raitt, D.C. , Johnson, A.L. , Erkine, A.M. , Makino, K. , Morgan, B. , Gross, D.S. and Johnston, L.H. (2000) The Skn7 response regulator of Saccharomyces cerevisiae interacts with Hsf1 in vivo and is required for the induction of heat shock genes by oxidative stress. Mol. Biol. Cell, 11, 2335–2347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlickum, S. , Moghekar, A. , Simpson, J.C. , Steglich, C. , O'Brien, R.J. , Winterpacht, A. and Endele, S.U. (2004) LETM1, a gene deleted in Wolf–Hirschhorn syndrome, encodes an evolutionarily conserved mitochondrial protein. Genomics, 83, 254–261. [DOI] [PubMed] [Google Scholar]
- Sickmann, A. , Reinders, J. , Wagner, Y. , Joppich, C. , Zahedi, R. , Meyer, H.E. , Schonfisch, B. , Perschil, I. , Chacinska, A. , Guiard, B. , Rehling, P. , Pfanner, N. and Meisinger, C. (2003) The proteome of Saccharomyces cerevisiae mitochondria. Proc. Natl. Acad. Sci. USA, 100, 13 207–13 212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinha, H. , David, L. , Pascon, R.C. , Clauder‐Munster, S. , Krishnakumar, S. , Nguyen, M. , Shi, G. , Dean, J. , Davis, R.W. , Oefner, P.J. , McCusker, J.H. and Steinmetz, L.M. (2008) Sequential elimination of major‐effect contributors identifies additional quantitative trait loci conditioning high‐temperature growth in yeast. Genetics, 180, 1661–1670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamai, S. , Iida, H. , Yokota, S. , Sayano, T. , Kiguchiya, S. and Ishihara, N. (2008) Characterization of the mitochondrial protein LETM1, which maintains the mitochondrial tubular shapes and interacts with the AAA‐ATPase BCS1L. J. Cell Sci. 121, 2588–2600. [DOI] [PubMed] [Google Scholar]
- Van Nguyen, T. , Kroger, C. , Bonnighausen, J. , Schafer, W. and Bormann, J. (2013) The ATF/CREB transcription factor Atf1 is essential for full virulence, deoxynivalenol production, and stress tolerance in the cereal pathogen Fusarium graminearum . Mol. Plant–Microbe Interact. 26, 1378–1394. [DOI] [PubMed] [Google Scholar]
- Wang, L. , Mogg, C. , Walkowiak, S. , Joshi, M. and Subramaniam, R. (2014) Characterization of NADPH oxidase genes NoxA and NoxB in Fusarium graminearum . Can. J. Plant Pathol. 36, 12–21. [Google Scholar]
- Yun, Y.Z. , Liu, Z.Y. , Yin, Y.N. , Jiang, J.H. , Chen, Y. , Xu, J.R. and Ma, Z.H. (2015) Functional analysis of the Fusarium graminearum phosphatome. New Phytol. 207, 119–134. [DOI] [PubMed] [Google Scholar]
- Zhang, B. , Carrie, C. , Ivanova, A. , Narsai, R. , Murcha, M.W. , Duncan, O. , Wang, Y. , Law, S.R. , Albrecht, V. , Pogson, B. , Giraud, E. , Van Aken, O and Whelan, J. (2012) LETM proteins play a role in the accumulation of mitochondrially encoded proteins in Arabidopsis thaliana and AtLETM2 displays parent of origin effects. J. Biol. Chem. 287, 41 757–41 773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang, C. , Lin, Y. , Wang, J. , Wang, Y. , Chen, M. , Norvienyeku, J. , Li, G. , Yu, W. and Wang, Z.H. (2016) FgNoxR, a regulatory subunit of NADPH oxidases, is required for female fertility and pathogenicity in Fusarium graminearum . FEMS Microbiol Lett. 363, fnv223, 1–8. [DOI] [PubMed] [Google Scholar]
- Zollino, M. , Lecce, R. , Fischetto, R. , Murdolo, M. , Faravelli, F. , Selicorni, A. , Butte, C. , Memo, L. , Capovilla, G. and Neri, G. (2003) Mapping the Wolf–Hirschhorn syndrome phenotype outside the currently accepted WHS critical region and defining a new critical region, WHSCR‐2. Am. J. Hum. Genet. 72, 590–597. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional Supporting Information may be found in the online version of this article at the publisher's website:
Fig. S1 Phylogenetic tree of the Letm1 superfamily orthologues from 32 fungal genomes available in the National Center for Biotechnology Information (NCBI) Bioprojects and Broad Institute databases. Orthologues were retrieved with the yeast Letm1 proteins, Mdm38 and Ylh47, and FgLetm1 and FgLetm2 protein sequences as queries. The phylogenetic tree was constructed by the neighbour‐joining method using mega 5.0. Numbers at the nodes represent the results of 1000 bootstrap replications. The GenBank or organism‐specific accession numbers are indicated in the figure.
Fig. S2 Identification of deletion mutants and complemented strains. (a) Southern blot hybridization analysis of the deletion mutants ΔFgLetm1 and ΔΔFgLetm1/2, and the complemented strain ΔFgLetm1+PLETM1FgLetm1‐GFP, using downstream DNA fragments of FgLETM1 as the probe. Both ΔFgLetm1 and ΔΔFgLetm1/2 had an anticipated 4594‐bp band, but both lacked the 2219‐bp band present in wild‐type PH‐1, when probed with a 725‐bp downstream DNA fragment of FgLETM1. (b) Southern blot hybridization analysis of the deletion mutants ΔFgLetm2 and ΔΔFgLetm1/2, and the complemented strain ΔFgLetm2+PLETM2FgLetm2‐GFP, using a downstream DNA fragment of FgLETM2 as the probe. Using an 800‐bp upstream DNA fragment of FgLETM2 as the probe, the ΔFgLetm2 mutant had an anticipated 3253‐bp band, when the chromosomal DNA of ΔFgLetm2 was digested with NdeI. ΔΔFgLetm1/2 presented a 4098‐bp band, but lacked the 1585‐bp band present in the wild‐type PH‐1, when the chromosomal DNA of ΔΔFgLetm1/2 was digested with EcoRV and blotted with the same probe. (c) PCR verification of the complemented strains ΔFgLetm1+PLETM1FgLetm1‐GFP and ΔFgLetm2+PLETM2FgLetm2‐GFP. The whole cassette, including the promoter, open reading frame (ORF) and gfp, was amplified.
Fig. S3 Conidial morphology of the wild‐type, ΔFgLetm1, ΔFgLetm2, ΔΔFgLetm1/2 and complemented strains. The septa were stained with calcofluor white and observed using a fluorescence microscope. Bar, 20 µm.
Fig. S4 Colony morphology of PH‐1, mutants and complemented strains grown on wheat head tissue medium (WA) at 25 °C for 3 days.
Fig. S5 Infection structures of wild‐type and deletion mutant ΔFgLetm1 on the inoculated glumes at 14 days post‐inoculation. Samples were collected at 2 weeks post‐inoculation and observed by scanning electron microscopy. Bar dimensions are indicated on the images.
Fig. S6 Induction of deoxynivalenol (DON) biosynthesis by exogenous adenosine triphosphate (ATP) in the wild‐type and mutants grown in TBI medium. ATP was added to Trichothecene Biosynthesis Induction medium (TBI) at a final concentration of 10 µm, and the cell‐free supernatant after 7 days of incubation was used for the quantification of DON production. Error bars denote the standard deviation from three repeated experiments.
Fig. S7 The growth phenotype of the deletion mutant ΔFgLetm1 was not changed by the utilization of non‐fermentable carbon. Colony morphology of the wild‐type, ΔFgLetm1, ΔFgLetm2 and ΔΔFgLetm1/2 on minimal medium supplemented with glucose or glycerol as sole carbon source. Plates were photographed after incubation at 25 °C for 3 days.
Fig. S8 Fungicides targeting mitochondria are able to inhibit toxisome formation and deoxynivalenol (DON) biosynthesis. (a) The growth inhibition of the tested fungicides at 0.3 µg/mL. (b) Toxisome formation in the presence of fungicides at 0.3 µg/mL. The ΔTri1:Tri1‐GFP strain was grown in Trichothecene Biosynthesis Induction medium (TBI) for 24 h, individual fungicides were added at a final concentration at 0.3 µg/mL and incubation was performed for another 24 h before observation. (c) DON production of each treatment. DON was extracted from 7‐day‐cultured TBI in each treatment and quantified by liquid chromatography‐mass spectrometry (LC‐MS). Columns with different letters indicate a significant difference at P = 0.05. DIC, Differential Interference Contrast; DMSO, Dimethyl sulphoxide; GFP, green fluorescent protein.
Table S1 The Letm1 superfamily domain in the Letm1 orthologues of six filamentous plant‐pathogenic fungi and Saccharomyces cerevisiae.
Table S2 Proteins of the mitochondrial ribosome complexed with FgLetm1 by affinity purification and mass spectrometry assay.
Table S3 Oligonucleotide primers used in this study.
Video S1 Mitochondrial patterns stained by Mito‐HcRed in the wild‐type.
Video S2 Mitochondrial patterns stained by Mito‐HcRed in the deletion mutant ΔFgLetm1.


