Summary
Protein phosphatases (PPs) counteract kinases in reversible phosphorylation events during numerous signal transduction pathways in eukaryotes. PP2Cs, one of the four major classes of the serine/threonine‐specific PP family, are greatly expanded in plants. Thus, PP2Cs are thought to play a specific role in signal transduction pathways. Some rice PP2Cs classified in subgroup K are responsive to infection by the compatible Xanthomonas oryzae pv. oryzae, the causal agent of bacterial blight. In Arabidopsis thaliana, orthologous PP2C genes (AtPP2C62 and AtPP2C26) classified to subgroup K are also responsive to Xanthomonas campestris pv. campestris (Xcc, causal agent of black rot) infection. To elucidate the function of these subgroup K PP2Cs, atpp2c62‐ and atpp2c26‐deficient A. thaliana mutants were characterized. A double mutant plant which was inoculated with a compatible Xcc showed reduced lesion development, as well as the suppression of bacterial multiplication. AtPP2C62 and AtPP2C26 localized to the chloroplast. Furthermore, the photosynthesis‐related protein, chaperonin‐60, was indicated as the potential candidate for the dephosphorylated substrate catalysed by AtPP2C62 and AtPP2C26 using two‐dimensional isoelectric focusing sodium dodecylsulfate‐polyacrylamide gel electrophoresis (2D‐IDF‐SDS‐PAGE). Taken together, AtPP2C62 and AtPP2C26 are suggested to be involved in both photosynthesis and suppression of the plant immune system. These results imply the occurrence of crosstalk between photosynthesis and the plant defence system to control productivity under pathogen infection.
Keywords: Arabidopsis thaliana; chloroplast; plant immunity; protein phosphatase 2C; type III secretion system, Xanthomonas campestris pv. campestris
Introduction
On perception of microbe‐associated molecular patterns (MAMPs) by their specific receptors (Boller and He, 2009; Jones and Dangl, 2006), plants trigger a series of defence responses, including the generation of reactive oxygen species (ROS), induction of mitogen‐activated protein kinases (MAPKs), pathogenesis‐related (PR) gene expression and callose deposition at the cell wall (Li et al., 2016; Segonzac and Zipfel, 2011). Because of MAMP‐triggered immunity (MTI), most bacteria are unable to cause disease (Boller and Felix, 2009). However, pathogens have acquired MTI‐interfering effector proteins to render certain plant species susceptible. In turn, plants have evolved disease resistance proteins that recognize the presence of individual effectors, resulting in effector‐triggered immunity (ETI). This conforms to the ‘gene‐for‐gene’ interaction model (Chisholm et al., 2006; Grant et al., 2006; Jones and Dangl, 2006).
Protein phosphatases (PPs), by reversing the action of protein kinases, provide modulations of protein phosphoregulation. Members of the PP2Cs, widely distributed in eukaryotes, have been implicated as modulators of signal transduction, and are known to be activated by diverse environmental stresses or developmental signalling cascades (Schweighofer et al., 2004). A comprehensive computational analysis identified 80 and 78 PP2C genes in Arabidopsis thaliana and Oryza sativa, respectively, which makes the PP2C gene family one of the largest identified in plants (Xue et al., 2008). Phylogenetic analysis divided PP2Cs in Arabidopsis and rice into 13 and 11 subgroups, respectively, with characteristic features in gene structures and protein motifs. Several Arabidopsis (Umezawa et al., 2010) and rice (Singh et al., 2015) PP2Cs classified into subgroup A have been described as negative regulators of abscisic acid‐mediated signalling and responses. Other PP2C genes, including AP2C1 (subgroup B) (Schweighofer et al., 2007), WIN2 (subgroup F) (Lee et al., 2008), KAPP (unclustered) (Gómez‐Gómez et al., 2001; Stone et al., 1994) and OsBIPP2C2 (subgroup K) (Hu et al., 2009), have been revealed to possess stress‐related functions. OsBIPP2C1 has been reported to be a rice gene up‐regulated by treatment with benzothiadiazole, a well‐known inducer of systemic acquired resistance (Hu et al., 2006). Furthermore, transgenic tobacco plants overexpressing the OsBIPP2C1 gene show enhanced disease resistance against Tobacco mosaic virus and Phytophthora parasitica. Interestingly, most of the PP2Cs belonging to subgroup K are predicted to be localized at the chloroplast. However, there has been no clear characterization of these genes in a Xanthomonas‐infected plant.
The primary reactions of photosynthesis occur in the thylakoid membranes of chloroplasts. These membranes harbour pigment–protein complexes called photosystem I (PSI) and photosystem II (PSII) which convert light energy into chemical energy [adenosine triphosphate (ATP) and nicotinamide adenine dinucleotide phosphate, reduced form (NADPH)]. The chloroplast is thought to contain around 3000 different proteins in Arabidopsis (Richly and Leister, 2004), and currently more than 40 proteins, such as kinases, phosphatases (including PP2C), proteases, transporters and chaperones, are known to associate with PS and have been shown to assist the PS repair cycle (Järvi et al., 2015). Moreover, PSII is crucial for plant immunity through the production of ROS, which not only damage the pathogen and components of the photosynthetic electron transfer chain, but also act as important retrograde signalling molecules (Rodríguez‐Herva et al., 2012; de Torres Zabala et al., 2015). Chloroplasts and mitochondria, in addition to being involved in biosynthetic pathways, energy production, redox homeostasis and retrograde signalling, also play key roles in plant immune responses (Maxwell et al., 2002; Stael et al., 2015). For instance, the chloroplast constitutes a platform for the synthesis of important defence hormones, such as salicylic acid (SA) and jasmonic acid (JA), and both chloroplasts and mitochondria are essential providers of redox resources to fight pathogen attack. Yang et al. (2011) reported that Xanthomonas oryzae pv. oryzae (Xoo) infection induced a changed distribution of Rubisco (ribulose‐1,5‐bisphosphate carboxylase/oxygenase) activase from the soluble stroma to the thylakoid membrane of chloroplasts. MAMP perception triggers the rapid, large‐scale suppression of nuclear‐encoded chloroplast‐targeted genes (NECGs). Virulent Pseudomonas syringae effectors can reprogram NECG expression in Arabidopsis, target the chloroplast and inhibit photosynthetic CO2 assimilation through the disruption of PSII (de Torres Zabala et al., 2015). As noted above, although the exact role of photosynthetic components in the sensing and signalling of pathogen infection has only just emerged, a wealth of information has accumulated during the past few years on the consequences of fluctuating light on the activity of the photosynthetic machinery (Allahverdiyeva et al., 2015; Grieco et al., 2012). Several P. syringae type III effectors (T3Es), including HopI1, HopN1, HopK1 and AvrRps4, target the chloroplast and suppress immune responses (Jelenska et al., 2007; Li et al., 2014; Rodríguez‐Herva et al., 2012).
Xanthomonas is one of the most agriculturally important pathogens and causes plant diseases on various plant species. Bacterial leaf blight, which is caused by Xoo, is one of the most devastating diseases in major rice production areas in tropical Asia (Niño‐Liu et al., 2006; Rajarajeswari and Muralidharan, 2006). Xanthomonas campestris pv. campestris (Xcc) is the causal agent of black rot, one of the most important diseases on cruciferous vegetables. The pathogen infects a wide range of brassica plants, including broccoli, mustard, cabbage, cauliflower, radish, turnip and the model plant A. thaliana (Meyer et al., 2005). Together with other bacterial pathogens, such as Pseudomonas, Xoo and Xcc pathogenicity are greatly attenuated when their type III secretion system (TTSS) is defective, indicating that T3Es are the major virulence factors (Dow and Daniels, 1994; Sun et al., 2011). Xanthomonas T3Es are translocated into the plant cytoplasm via the TTSS and are called Xanthomonas outer proteins (Xops). Many Xop proteins have been identified (Furutani et al., 2009) and characterized. The functions of XopR, XopN, XopZ, XopD, XopY, XopP, XopQ, AvrAC and AvrBs2 have been reported to be essential for bacterial virulence (Akimoto‐Tomiyama et al., 2012; Cheong et al., 2013; Gupta et al., 2015; Ishikawa et al., 2014; Li et al., 2015; Song and Yang, 2010; Wang et al., 2015).
In this study, to elucidate the function of chloroplast‐localized PP2Cs in plant immunity, we first used microarray analysis to identify novel PP2C genes of subgroup K in rice, which are down‐regulated in a TTSS‐dependent manner in response to inoculation with Xoo (http://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE63047, GSE63047). We further found the orthologous genes in Arabidopsis: AtPP2C62 and AtPP2C26. Through the analysis of the loss‐of‐function mutants, our results indicated that these two gene products are involved in the resistance to Xcc via action on the metabolic activities of the chloroplast.
Results
Rice subgroup K PP2Cs are responsive to a compatible infection with Xoo in a TTSS‐dependent manner
According to the microarray analysis, three putative PP2C genes (OsBIPP2C1, Os03g0192500; OsPP2C71, Os10g370000; and OsPP2C01, Os01g0164600) were responsive to a compatible Xoo inoculation (http://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE63047). To confirm the results, we analysed the expression of the three rice PP2C genes in IR‐64, which was inoculated with wild‐type or TTSS mutant (hrcV deficient) of Xoo T7174R, by real‐time polymerase chain reaction (PCR) using specific primers. At 3 and 4 days post‐inoculation (dpi), the expression of each PP2C was suppressed following inoculation with wild‐type Xoo T7174R, but not suppressed following mock or TTSS mutant inoculation (Fig. 1), consistent with the microarray results. Gene expression induced by mock treatment at 3 dpi was a response to wounding in the inoculation process, as reported previously (Hu et al., 2006). The expression of OsPP2C01 induced by the TTSS mutant of Xoo increased significantly at 4 dpi, even though the expression level of mock treatment returned to the same level as at 1 or 2 dpi (Fig. 1). This indicates that OsPP2C01 is also responsive to MAMPs of the TTSS mutant as well as wounding.
Figure 1.
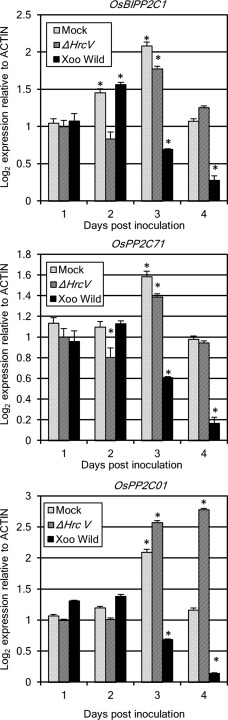
The expression of three rice clade K protein phosphatase 2Cs (PP2Cs) is down‐regulated by compatible Xanthomonas oryzae pv. oryzae (Xoo) inoculation. Wild‐type or hrcV (type III mutant) Xoo T7174R was inoculated to IR‐64 by leaf clipping, and collected at the indicated time point. Gene expression was obtained by real‐time reverse transcription‐polymerase chain reaction (RT‐PCR). Significant differences from 1‐day mock were identified by two‐way analysis of variance (ANOVA) followed by Tukey's multiple comparison test, and are indicated with an asterisk (*P < 0.01). Results are representative of three experiments.
These rice PP2C genes (OsBIPP2C1, OsPP2C71 and OsPP2C01) belong to subgroup K and encode for plastid proteins (Xue et al., 2008). The phylogenetic tree of subgroup K PP2Cs of rice and Arabidopsis is shown in Fig. 2 based on a previous report (Xue et al., 2008). The rice genes (OsPP2C71 and OsPP2C01) were predicted to be orthologous to AtPP2C62 and AtPP2C26 of Arabidopsis, respectively. Two other PP2Cs of subgroup K, AtPP2C55 and AtPP2C80, were not subjected to further analysis because they were predicted to localize to mitochondria (Fig. 2).
Figure 2.
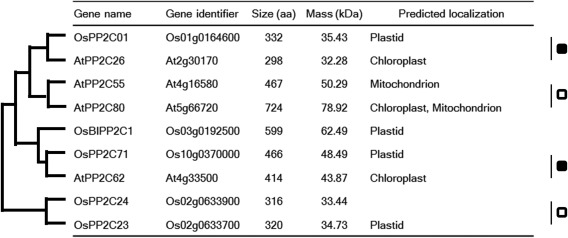
Rice and Arabidopsis subgroup K protein phosphatase 2Cs (PP2Cs). The phylogenetic relationships are indicated by the tree (shown on the left). The predicted localizations were determined for rice (http://rice.plantbiology.msu.edu/index.shtml) and Arabidopsis (http://www.arabidopsis.org/index.jsp). Putative orthologous and paralogous pairs are marked with black and white squares, respectively. aa, amino acid.
Arabidopsis subgroup K PP2Cs are responsive to a compatible infection with Xcc in a TTSS‐dependent manner
Next, we examined the expression pattern of the two Arabidopsis subgroup K PP2Cs in response to Xcc. Fully expanded A. thaliana ecotype Col‐0 leaves were inoculated with a compatible strain of Xcc MAFF106712 (Akimoto‐Tomiyama et al., 2014), and the expression of AtPP2C62 and AtPP2C26 was analysed by real‐time polymerase chain reaction (PCR) (Fig. 3). The hrcC deletion mutant of Xcc MAFF106712 was used as the TTSS loss‐of‐function mutant. AtPP2C62 gene expression was up‐regulated on inoculation with the TTSS mutant bacterium, but not on wild‐type inoculation. The expression of AtPP2C26 was down‐regulated at 1 day after mutant and wild‐type inoculation, which was not observed for AtPP2C62 expression. Although the induction of AtPP2C26 gene expression was observed following both mock and mutant inoculation, expression was down‐regulated by wild‐type inoculation at 3 dpi. At 5 dpi, AtPP2C26 gene expression was no different between mock and mutant inoculation, but was down‐regulated by wild‐type inoculation. These results indicate that the two Arabidopsis PP2Cs belonging to subgroup K are responsive to wounding and MAMPs. The gene AtPP2C26 is responsive to wounding and MAMPs, but the gene AtPP2C62 is responsive to MAMPs only.
Figure 3.
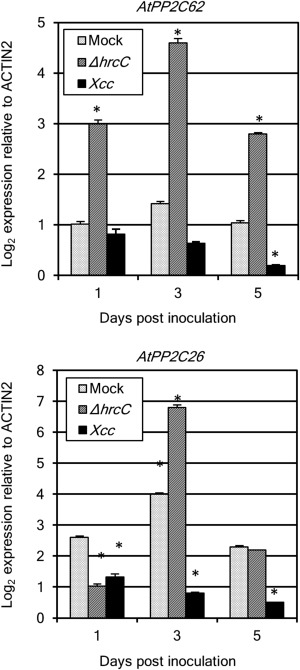
The expression of two Arabidopsis subgroup K protein phosphatase 2Cs (PP2Cs) is up‐regulated by the hrcC deletion mutant of Xanthomonas campestris pv. campestris (Xcc) MAFF106712 and down‐regulated by wild‐type Xcc. Five fully expanded leaves of each plant (Arabidopsis eco‐type, Col‐0) were inoculated by piercing three holes in the central vein with a needle dipped in a suspension of Xcc wild‐type and hrcC mutant bacteria [109 colony‐forming units (CFU)/mL]. Plant samples were harvested at 1, 3 and 5 days post‐inoculation (dpi) with the Xcc wild‐type, hrcC mutant (hrcC) or vehicle alone. Expression of the PP2C genes in the plants was obtained by real‐time reverse transcription‐polymerase chain reaction (RT‐PCR). Significant differences from 1‐day mock were identified by two‐way analysis of variance (ANOVA) followed by Tukey's multiple comparison test, and are indicated with an asterisk (*P < 0.01). The results are representative of three experiments.
AtPP2C62 and AtPP2C26 redundantly affect lesion development
To characterize AtPP2C62 and AtPP2C26, we obtained homozygous T‐DNA insertion lines of these genes (Col‐0 background; SALK_014358C and SALK_127920C, respectively, as shown in Fig. 4A) from the Arabidopsis Biological Resource Center. A double mutant (atpp2c62/atpp2c62) was created by crossing the single mutants, and its homozygous genotype was confirmed by PCR (data not shown). The expression of both AtPP2C62 and AtPP2C26 induced by the TTSS mutant of Xcc was abolished in the double mutant (Fig. S1, see Supporting Information).
Figure 4.
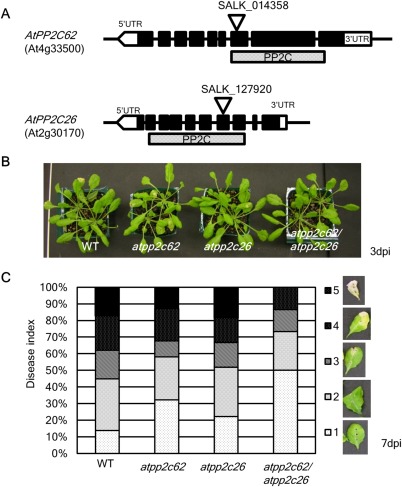
The lesions caused by compatible Xanthomonas campestris pv. campestris (Xcc) MAFF106712 inoculation are suppressed in the atpp2c62/atpp2c26 mutant. (A) Genomic structure of the two Arabidopsis PP2C genes analysed in this study. The black boxes show the exons and the dotted box shows the protein phosphatase 2C (PP2C) motif. The positions of the T‐DNA insertions are indicated by triangles. UTR, untranslated region. (B) Photograph of wild‐type (WT, Col‐0) and pp2cs mutant plants inoculated by Xcc MAFF106712 at 3 days post‐inoculation (dpi). (C) Percentage of inoculated leaves showing lesion development, categorized as disease index 1–5, at 7 dpi. Disease index examples are shown on the right.
Neither the single mutants nor the double mutant showed morphological phenotypes during the 5 weeks following germination in our culture conditions (data not shown). We inoculated Xcc MAFF106712 on fully expanded leaves of 5‐week‐old Col‐0 and the two pp2c(s) knock‐out mutants, and observed lesion development in the inoculated leaves (Fig. 4B,C). The size of the lesions was categorized according to the disease index (Fig. 4C, right panels). At 3 dpi, visible lesions in the inoculated leaves emerged (Fig. 4B) and, at 7 dpi, the lesions on some inoculated leaves had spread to the whole leaf (Fig. 4C). In Col‐0 and the two single mutants, nearly 20% of total leaves had whole‐leaf lesions (disease index 5), whereas none of the inoculated leaves of the double mutants showed the phenotype. Furthermore, half of the inoculated leaves showed no symptoms (disease index 1) in the double mutant. Notably, lesion development caused by compatible Xcc inoculation was suppressed in the double pp2c mutant. These results strongly suggest that AtPP2C62 and AtPP2C26 work cooperatively in the suppression of lesion formation.
Bacterial growth is suppressed in the double mutant (atpp2c62/atpp2c26)
The changes in the visible symptoms shown in Fig. 4 were further quantified by measurement of bacterial growth in the host plant (Fig. 5). Although no significant difference was observed amongst the four lines of Arabidopsis at 3 dpi, bacterial growth in the double and atpp2c26 single mutant was reduced significantly (P < 0.01) compared with that of the wild‐type at 7 dpi. The bacterial growth in the atpp2c62 single mutant was reduced significantly (P < 0.5). Therefore, we conclude that the suppression of lesion development in the double mutant shown in Fig. 4C is accompanied by the inhibition of bacterial growth.
Figure 5.
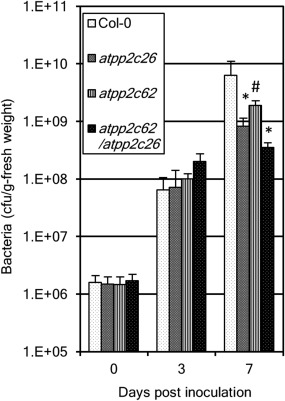
Bacterial multiplication in the double mutant (atpp2c62/atpp2c26) is suppressed. Bacterial multiplication was measured in Col‐0 (wild‐type), the single mutant of atpp2c62 or atpp2c26 and double mutant. Five fully expanded leaves of each plant were inoculated by piercing three holes in the central vein with a needle dipped in a suspension of Xanthomonas campestris pv. campestris (Xcc) MAFF106712 bacteria [109 colony‐forming units (CFU)/mL]. Significant differences from Col‐0 at each time point were identified by two‐way analysis of variance (ANOVA) followed by Tukey's multiple comparison test, and are indicated with an asterisk (*P < 0.01) or a hash (#P < 0.5). The results are representative of at least three experiments per condition.
AtPP2C62, as well as AtPP2C26, is localized to the chloroplast
One of the PP2Cs analysed in this study, AtPP2C26, has been reported as a chloroplast protein (Samol et al., 2012). To determine the localization of AtPP2C62, we generated citrine‐tagged full‐length AtPP2C62 and AtPP2C26 fusions expressed under the control of the oestrogen‐inducible promoter, and transiently expressed the constructs in Nicotiana benthamiana (Akimoto‐Tomiyama et al., 2012). Citrine fluorescence was observed by confocal laser scanning microscopy. One day after treatment with oestradiol, citrine fluorescence was observed at the chloroplast in AtPP2C62::citrine‐expressing (Fig. 6A) and AtPP2C26::citrine‐expressing (Fig. 6B) plants. In the absence of oestradiol, citrine fluorescence was not observed (data not shown). Notably, the fluorescence was observed only in chloroplasts of the inoculated part of the leaf, and not in every chloroplast.
Figure 6.
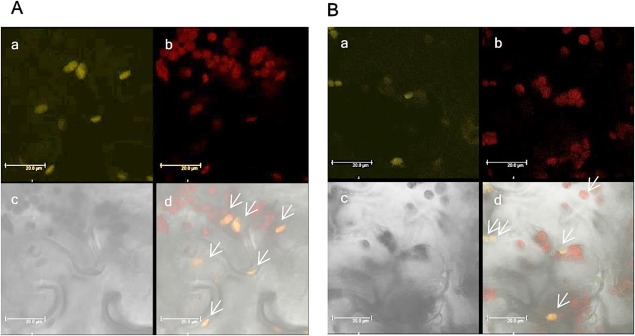
AtPP2C62::citrine localizes to the chloroplast. Agrobacterium tumefaciens EHA105, which mediates T‐DNA‐based transfer of AtPP2C62::citrine, was co‐infused into Nicotiana benthamiana leaves at an optical density at 600 nm of 1.0 with HC‐Pro in pMD1 at an optical density at 600 nm of 0.1. Samples were observed by confocal microscopy 1 day after oestradiol treatment. (A) AtPP2C62::citrine: a, citrine fluorescence is shown in yellow; b, chlorophyll autofluorescence is shown in red; c, bright field image; d, merged view. (B) AtPP2C26::citrine: a, citrine fluorescence is shown in yellow; b, chlorophyll autofluorescence is shown in red; c, bright field image; d, merged view. Arrows indicate the chloroplasts expressing protein phosphatase 2Cs (PP2Cs). Scale bars, 20.0 μm. The fluorescence was stable at least 3 days after oestradiol treatment. For microscopic observation: 514 nm excitation and 520–540 nm emission for citrine fluorescence; 514 nm excitation and 650–700 nm emission for chlorophyll autofluorescence.
Screening of the AtPP2C62 and AtPP2C26 substrate candidates
As described above, loss of the two chloroplast‐localized PP2Cs resulted in enhanced plant immunity. Thus, we hypothesized that phosphorylation of the target protein(s) of the PP2Cs positively affects the plant defence system. To screen candidates, we conducted protein analysis by combining two‐dimensional electrophoresis and liquid chromatography‐tandem mass spectrometry (LC‐MS/MS). Protein was extracted from the leaves inoculated with compatible Xcc MAFF106712 bacteria at 1, 2 and 3 dpi, and then separated by two‐dimensional electrophoresis. We selectively stained the phosphorylated proteins in polyacrylamide gels by Pro‐Q diamond (Thermo Fisher Scientific, Waltham, MA, USA) (Fig. 7A,B,D,E). As shown in Fig. 7A,D, many spots of phosphoprotein were detected in both Col‐0 and the double mutant. After comparison of the spot size and location for each gel, four spots which clearly changed on the basis of plant background were selected for further protein identification. By reference to the non‐selective Coomassie brilliant blue (CBB)‐ or silver‐stained spot (Fig. 7C,F), the changed spots were cut out and subjected to amino acid sequence analysis. The annotation of the four spots is shown in Table 1. Peptides derived from spot 1 of the mutant were identified as glycine decarboxylase P‐protein 1, whereas the peptides from spot 1 of Col‐0 were unknown. Spots 2 and 3 were physically close to each other and peptides from both spots 2 and 3 were annotated as chaperonin‐60α. The peptides from spot 4 were annotated to the large subunit of Rubisco. The spot size of spot 2 in the double mutant (Fig. 7B) was much larger than that in Col‐0 (Fig. 7A), suggesting abundant phosphorylated chaperonin‐60α. This is consistent with the predicted role of PP2C in the dephosphorylation of the target(s). However, the sizes of spots 1 and 4 in the double mutant were smaller than those in Col‐0 (Fig. 7A,B,D,F). This indicates that these are not direct target(s) of the PP2Cs.
Figure 7.
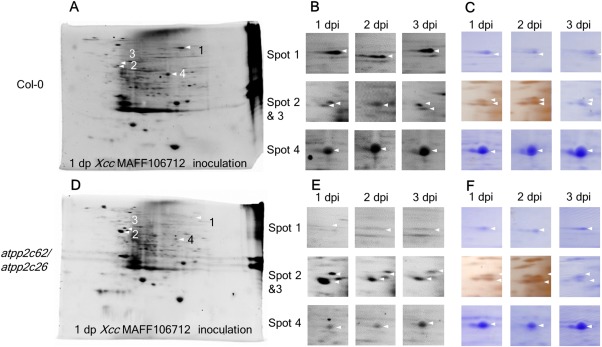
Loss of the protein phosphatase 2Cs (PP2Cs) impairs the protein phosphorylation triggered by compatible infection. Five fully expanded leaves of Col‐0 and the double mutant were inoculated by piercing three holes in the central vein with a needle dipped in a suspension of Xanthomonas campestris pv. campestris (Xcc) MAFF106712 bacteria [109 colony‐forming units (CFU)/mL]. Protein was extracted from an inoculated leaf and separated by two‐dimensional isoelectric focusing sodium dodecylsulfate‐polyacrylamide gel electrophoresis (2D‐IDF‐SDS‐PAGE). Protein was stained by Pro‐Q diamond and the spots were cut and then identified by liquid chromatography‐mass spectrometry (LC‐MS). Arrows indicate the spot changes in Col‐0 (A–C) compared with the double mutant (D–F). (A, D) Whole‐gel images stained by Pro‐Q diamond‐separated proteins extracted from Col‐0 (A) and the double mutant (D) at 1 day post‐inoculation (dpi) of Xcc MAFF106712. (B, E) High‐magnification image of (A, D); the spot number is given on the left and the time point is shown at the top. (C, F) Images of the protein stained by Coomassie brilliant blue (CBB) or silver.
Table 1.
List of the identified proteins.
| Spot name | GI | Possible function | Score | No. of peptides | Peptides |
|---|---|---|---|---|---|
| #1 Col‐0 | gi|21592723 | Unknown | 12 | 1 | K.QVASVIPVTRK.L |
| #1 atpp2c62/atpp2c26 | gi|14596025 | Glycine dehydrogenase | 41 | 1 | K.CSDAHAIADAASK.S |
| #2 Col‐0 | gi|15226314 | Chaperonin‐60α | 73 | 3 | K.VGAATETELEDR.K |
| K.HGLLSVTSGANPVSLK.R | |||||
| K.DSTTLIADAASKDELQAR.I | |||||
| #2 atpp2c62/atpp2c26 | gi|15226314 | Chaperonin‐60α | 152 | 5 | K.VGAATETELEDR.K |
| K.ITAIKDIIPILEK.T | |||||
| K.HGLLSVTSGANPVSLK.R | |||||
| K.TNDSAGDGTTTASILAR.E | |||||
| K.DSTTLIADAASKDELQAR.I | |||||
| #3 Col‐0 | gi|15226314 | Chaperonin‐60α | 145 | 3 | K.VGAATETELEDR.K |
| K.TNDSAGDGTTTASILAR.E | |||||
| K.DSTTLIADAASKDELQAR.I | |||||
| #3 atpp2c62/atpp2c26 | gi|15227717 | UDP‐glycosyltransferase 87A1 | 0 | 1 | R.VGMGIER.K |
| #4 Col‐0 | gi|7525041 | Ribulose‐1,5‐bisphosphate carboxylase/oxygenase large subunit | 99 | 10 | K.LGLSAK.N |
| K.ALAALR.L | |||||
| R.GGLDFTK.D | |||||
| R.AVYECLR.G + propionamide (C) | |||||
| R.VALEACVQAR.N + propionamide (C) | |||||
| K.LTYYTPEYETK.D | |||||
| R.LEDLRIPPAYTK.T | |||||
| R.LSGGDHIHAGTVVGK.L | |||||
| R.LSGGDHIHAGTVVGK.L | |||||
| R.LSGGDHIHAGTVVGK.L | |||||
| K.TFQGPPHGIQVER.D | |||||
| K.EITFNFPTIDKLDGQE.‐ | |||||
| #4 atpp2c62/atpp2c26 | gi|7525041 | Ribulose‐1,5‐bisphosphate carboxylase/oxygenase large subunit | 64 | 5 | K.ASVGFK.A |
| R.AVYECLR.G + propionamide (C) | |||||
| K.DTDILAAFR.V | |||||
| R.LSGGDHIHAGTVVGK.L | |||||
| K.TFQGPPHGIQVER.D |
The mass spectrometry data were obtained by one‐time analysis using the spots excised from the five or six gels.
Discussion
The two chloroplast‐localized PP2Cs are wounding and/or MAMP responsive, and their induction is abrogated by successful pathogen infection. This strongly suggests that the induction of PP2Cs is required for plant defence. Indeed, in previous work, the overexpression of OsBIPP2C1 in tobacco plants led to enhanced disease resistance against Tobacco mosaic virus and Phytophthora parasitica (Hu et al., 2006). However, surprisingly, the bacterial growth in the PP2C knock‐out plants was significantly reduced in this study, indicating that the loss of PP2Cs enhances Arabidopsis immunity. This implies that the constant dephosphorylated status of the potential substrate of these PP2Cs acts in a physiological or physical negative manner with regard to defence. Although no visible phenotype change was observed in the experiment, the atpp2c26 single mutant has been reported to show a concomitant reduction in the number of layers in the grana stacks (Samol et al., 2012), and further analysis of the thylakoid membrane protein composition of this mutant characterized its smaller PSII antenna, whereas cytochrome (Cyt) b 6 f complexes and PSI were similar to those of the wild‐type (Puthiyaveetil et al., 2014). Thus, the attenuation of the PSII antenna may lead to the enhancement of the basal defence system. From a physiological viewpoint, we observed PR1 gene expression in the double mutant. The PR1 gene is induced by both MTI (Silipo et al., 2005) and ETI (Rong et al., 2010) triggered by Xanthomonas or Pseudomonas infection (Hamdoun et al., 2013), and leads to the enhancement of plant immunity. The induction of PR1 gene expression in the double mutant was observed for both Xcc wild‐type and the TTSS‐deficient mutant (Fig. S2, see Supporting Information). The induction of PR1 was concomitant with the decrease in bacterial growth. Notably, the expression level of PR1 by mock treatment was not impaired by the loss of PP2Cs, indicating that they are not responsible for PR1 induction by wounding. It has been shown that photosynthesis is involved in the activation of SA biosynthesis, PR1 gene expression and the hypersensitive response (HR) against infection by pathogens (Jelenska et al., 2007; Kangasjärvi et al., 2012; Kim et al., 2012). We found that PP2Cs are not involved in HR development triggered by AvrRpm1 under continuous light conditions (Fig. S3, see Supporting Information). In addition, the bacterial growth of the TTSS mutant was not impaired in the double mutant plant (Fig. S4, see Supporting Information), suggesting that the PP2Cs are responsible for T3E‐mediated virulence. It could be hypothesized that the PP2Cs are virulence targets of an Xcc effector and, in its absence, the pathogen no longer has such an advantage. As light is required for PR1 induction and HR development (Zeier et al., 2004), a detailed analysis of thylakoid protein composition and examination of PR1 induction and HR development under various light intensities in the double mutant may elucidate this phenomenon. Through an analysis of protein phosphorylation by bacterial infection in pp2cs knock‐out plants, three photorespiratory cycle‐related proteins were identified as potentially differentially phosphorylated proteins in the pp2c double mutant during Xcc MAFF106712 infection: chaperonin‐60α2 (Cpn60α2), glycine decarboxylase P‐protein 1 and the large subunit of Rubisco (Fig. 7, Table 1). Amongst them, Cpn60α2 is a probable potential substrate candidate for the PP2Cs. Cpn60 plays a critical role in Rubisco assembly (Cannon et al., 1986; Milos and Roy, 1984) and, moreover, the reduction of AtCpn60 results in impaired Arabidopsis plastid division and reduction of chlorophylls (Suzuki et al., 2009). OsCpn60α1 has been shown to be essential for the folding of the Rubisco large subunit in rice (Kim et al., 2013). In addition, AtCpn60α2 has been identified as a phosphoprotein (Reiland et al., 2009), but its internal regulation and the effect of its phosphorylated status are still obscure. As there is no evidence that the maintenance of the phosphorylated status of Cpn60α2 alters or affects the photorespiratory activity or interaction between the photorespiratory activity and plant immunity, further elucidation is needed.
Following pathogen infection, the expression of the chloroplast‐localized PP2Cs in subgroup K decreased dramatically in a TTSS‐dependent manner for both rice–Xoo wild‐type and Arabidopsis–Xcc wild‐type interactions at the late stage (3–5 dpi, Figs 1 and 3). Furthermore, AtPP2C62 and AtPP2C26 expression is induced by the TTSS mutant within 1 or 3 dpi, respectively. This suggests that the PP2Cs are potential targets of T3Es. In support of this idea, previous microarray analysis with a different bacterial pathogen, Arabidopsis–Pseudomonas syringae pv. tomato (Pst) DC3000, revealed that both AtPP2C62 and AtPP2C26 were significantly down‐regulated within 12 hpi in a TTSS‐dependent manner (Truman et al., 2006). Furthermore, many chloroplast proteins essential for plant defence have been suggested to be targets for pathogen effectors (de Torres Zabala et al., 2015). In the case of the Arabidopsis–Xcc TTSS mutant interaction, AtPP2C62 is induced within 1 dpi. This strongly indicates that AtPP2C62 is responsive to MAMPs. Indeed, both AtPP2C62 and AtPP2C26 were induced by flg22 for 30 min in a light‐dependent manner (Sano et al., 2014). Thus, T3Es which impair the MAMP receptor itself or downstream signal transduction may indirectly control PP2C expression. Based on the analysis of the whole‐genome information of Xoo (KACC10331, MAFF311018, PXO99A) and Xcc (ATCC33913, 8004, B100) strains, they share at least 13 T3E proteins, including AvrBs2, XopF, XopG, XopK, XopL, XopN, XopP, XopQ, XopR, XopX, XopZ, XopA and HpaA. Although the genome sequence of Xcc MAFF106712 is not available so far, it harbours at least XopN, XopK, XopQ, XopZ and XopR (Akimoto‐Tomiyama et al., 2014). Single mutations of XopN, XopQ, XopZ (Cheong et al., 2013; Gupta et al., 2015; Song and Yang, 2010) and XopR (Akimoto‐Tomiyama et al., 2012) have already been reported to affect virulence. Further analysis is needed to determine the T3E(s) involving PP2C expression and those T3Es with the potential to attenuate photosynthesis.
In this study, we suggest that two chloroplast PP2Cs are responsible for plant immunity. One of these, AtPP2C26, has been reported previously as a PSII core phosphatase (PBCP) required for the efficient dephosphorylation of PSII (the first protein complex in the light‐dependent reactions of oxygenic photosynthesis) proteins involved in light acclimation (Samol et al., 2012). By contrast, AtPP2C62 has not been shown to be required for the dephosphorylation of PSII (Samol et al., 2012). We screened potentially differentially phosphorylated proteins in the pp2c double mutant, such as glycine decarboxylase P‐protein 1, chaperonin‐60α and the large subunit of Rubisco, which were all related to the photorespiratory cycle of light‐independent reactions, but not light‐dependent reactions. It is suggested that the functions of AtPP2C62 and AtPP2C26 in plant immunity relate to the light‐independent reactions (photorespiratory cycle) of photosynthesis. In support of this idea, Järvi et al. (2016) have indicated the interconnection between light acclimation and plant immunity, where the appropriate repair cycle of PSII plays a key role in the process. Moreover, a rice leaf colour mutant, which is defective in chlorophyll synthesis and photosynthesis, conferred resistance to Xoo (Chen et al., 2016), and the white sector of an Arabidopsis variegation mutant promoted susceptibility to the pathogen P. syringae (Pogorelko et al., 2016). These results strongly indicate that the chloroplast is relevant to immunity. Further experiments are needed to elucidate the function of PP2Cs in plant defence, especially the point of tradeoff of carbon.
In previous work, the pbcp mutant, which is exactly the same genotype as the atpp2c26 mutant used in this study, showed retarded growth under normal light conditions (illumination for 9 h with 220 µmol quanta/m2/s at 23 °C; dark temperature, 21 °C) in two of three experiments (Puthiyaveetil et al., 2014). With our experimental conditions (illumination for 8 h with 300 µmol quanta/m2/s at 23 °C; dark temperature, 21 °C), in contrast, biomass loss of the atpp2c26 mutant was not observed. Considering the inconsistent results with previous studies, we conclude that there is a low correlation between AtPP2C26 (or Pbcp) and biomass gain. The variability may be a result of the difference in culture conditions, especially light conditions.
Interestingly, the PP2Cs focused on here belong to the ‘PP2C7s’, which are almost universally distributed in Eukaryotes (Kerk et al., 2015). The ‘PP2C7s’ are distributed in most crops, vegetables and fruits, which are potential hosts for Xanthomonas. Therefore, to establish a disease‐tolerant variety, breeding for PP2Cs may be effective. Interestingly, the ‘PP2C7s’ have been suggested to originate from Bacteria group II PP2Cs. In addition, Xanthomonas contains group II PP2Cs. How could this horizontal gene transfer have resulted in the acquisition of effector‐triggered susceptibility? Furthermore, how is the host protein selected as the target by pathogen effectors? Evolutionary analysis may help to provide an answer to these questions.
Experimental Procedures
Bacterial strains and media
Xoo strain T7174R and Xcc strain MAFF106712 (Akimoto‐Tomiyama et al., 2014) were grown at 28 °C in nutrient broth–yeast extract (NBY) medium. Agrobacterium tumefaciens was grown in Luria broth at 28 °C. For solid medium, agar was added at a final concentration of 1.5% (w/v). All media were supplemented with antibiotics at the following concentrations: for Xcc and Xoo, 50 μg/mL rifampin, 50 μg/mL kanamycin and 40 μg/mL spectinomycin; for Agrobacterium, 50 μg/mL ampicillin, 25 μg/mL kanamycin and 40 μg/mL spectinomycin.
Plant material, growth conditions and infection tests
Rice cv. IR‐64 (susceptible to Xoo MAFF311018) was grown in a growth chamber set at 28 °C with a 14‐h photoperiod and 24 °C with a 10‐h dark period. The fully expanded upper leaves of 5‐week‐old rice were inoculated by the clipping method (Kauffman et al., 1973). Arabidopsis plants were grown on soil in pots, as described previously (Akimoto‐Tomiyama et al., 2012). Homozygous Arabidopsis T‐DNA insertion lines SALK_014358C and SALK_127920C (Col‐0 background) were obtained from the Arabidopsis Biological Resource Center. A double mutant (atpp2c62/atpp2c26) was created by crossing the single mutants, and its homozygous genotype was confirmed by PCR (data not shown). Xcc pathogenicity was assayed on A. thaliana by piercing inoculation of a bacterial suspension at 109 colony‐forming units (CFU)/mL, as described previously (Akimoto‐Tomiyama et al., 2012). Disease development was scored at 7 dpi using a disease index ranging from 1 (no symptoms) to 5 (full leaf necrosis). For assays of bacterial growth in leaves, four leaves were weighed, ground in 1 mL of 10 mm MgCl2 and mixed with serial dilutions of bacteria on appropriate media to calculate bacterial numbers.
Transient expression of AtPP2C62::citrine protein in N. benthamiana
The open reading frame of AtPP2C62 was cloned into the pENTR/d‐TOPO vector (Thermo Fisher Scientific), and AtPP2C62::citrine and AtPP2C26::citrine in pMDC7 (Akimoto‐Tomiyama et al., 2012) were generated by LR clonase II (Thermo Fisher Scientific) according to the manufacturer's instructions. Transient expression in N. benthamiana was conducted as reported previously (Akimoto‐Tomiyama et al., 2012). In brief, A. tumefaciens EHA105 transformed with either AtPP2C62::citrine or AtPP2C26::citrine in pMDC7 or HC‐Pro of Potato virus Y (a silencing suppressor protein) in pMD1 (Kubota et al., 2003) was cultivated overnight at 28 °C in the presence of appropriate antibiotics. Bacterial cells were collected by centrifugation and resuspended in induction buffer (10 mm MgCl2 and 10 mm MES, pH 5.6, supplemented with 0.5 mm acetosyringone) and incubated for 2 h with gentle mixing. Bacteria were diluted with induction buffer to a final optical density at 600 nm of 1.0 for AtPP2C62::citrine and AtPP2C26::citrine and 0.1 for Hc‐Pro, and then co‐infused into the leaves of 4–6‐week‐old N. benthamiana using a needleless syringe. Two days after infiltration, leaves were sprayed with a combination of 30 μm 17‐oestradiol (Sigma‐Aldrich, St. Louis, MO, USA) and 0.01% Silwet L‐77 to induce protein expression. After 1 day, the expression and accumulation of AtPP2C62::citrine and AtPP2C26::citrine protein in plant cells were observed using a TCS SP5 confocal microscope (Leica Microsystems, Solms, Germany).
Quantitative real‐time PCR
Total RNA was extracted using a RNeasy Plant Mini Kit (Qiagen, Germantown, MD, USA). First‐strand cDNA was synthesized from 500 ng of total RNA using a PrimeScript RT reagent Kit (Takara Bio Inc., Otsu, Japan) with an oligo (dT) primer and a random 6‐mer primer, according to the manufacturer's instructions. For quantitative real‐time PCR, 20 ng of cDNA were combined with SYBR premix Ex Taq (Takara Bio Inc.). PCRs were performed in triplicate with an MX 3000P (Agilent Technologies, Santa Clara, CA, USA), with actin RNA as an internal control. Primers for the genes were as follows: OsBIPP2C1, 5′‐TGCCTGGATGTCCGTGTTTTG‐3′ and 5′‐TCAGCGCCATCCTCAGAGCACA‐3′; OsPP2C71, 5′‐GTCTCGAAATCTTTACAAGCCGATCT‐3′ and 5′‐CGGCTGCAGATCTACCAACTT‐3′; OsPP2C01, 5′‐TTCGCGCCGCCAAGTTGGAA‐3′ and 5′‐AGCTGAGGATCATGATTGACCTCCT‐3′; AtPP2C62, 5′‐TCGCAGCAACTGATGGGCTC‐3′ and 5′‐GCTGCGTCAGCAAATGGCGT‐3′; AtPP2C26, 5′‐ GGTTGGGCTGAACAAGATGT‐3′ and 5′‐CCCACTTCCTCAAGCATAGC‐3′; actin, 5′‐AGTGGTCGTACAACCGGTATTGT‐3′ and 5′‐GAGGAAGAGCATTCCCCTCGTA‐3′; actin (Os03g50890), 5′‐CTCCCCCATGCTATCCTTCG‐3′ and 5′‐TGAATGAGTAACCACGCTCCG‐3′; PR1, 5′‐ TCATGGCTAAGTTTGCTTCC‐3′ and 5′‐AATACACACGATTTAGCACC‐3′.
Protein analysis
Three inoculated leaves were dissociated in lysis buffer (10 mg of tissue/300 μL of buffer) containing 7 m urea, 2 m thiourea, 4% 3‐[(3‐Cholamidopropyl)dimethylammonio]propanesulfonate, 2% Nonidet P‐40, 5% 2‐mercaptoethanol, 0.2% ampholine (pH 3.5–10) and ethylenediaminetetraacetic acid (EDTA)‐free protease inhibitor. The dissociated tissue was subjected to repeated freeze–thaw cycles in liquid nitrogen and then stored at −80 °C until use for electrophoresis. The two‐dimensional polyacrylamide gel electrophoresis (PAGE) gels were stained with CBB or Pro‐Q Diamond Phosphoprotein Gel Stain (Thermo Fisher Scientific) following the manufacturer's instructions. MS and data analysis were performed largely as described previously (Kajiwara et al., 2009). Spots were excised from five or six gels, and amaZon SL (Bruker Daltonics, Bremen, Germany) was used for MS analysis. The MS data were analysed with Mascot software (Perkins et al., 1999) using the amino acid sequence data in the National Center for Biotechnology Information data bank (www.ncbi.nlm.nih.gov/).
Supporting information
Additional Supporting Information may be found in the online version of this article at the publisher's website:
Fig. S1 The double mutant (atpp2c62/atpp2c26) was a null mutant. Five fully expanded leaves of Col‐0 and the double mutant were inoculated by piercing three holes in the central vein with a needle dipped in a suspension of Xanthomonas campestris pv. campestris (Xcc) MAFF106712 hrcC mutant bacteria [109 colony‐forming units (CFU)/mL]. Expression of the AtPP2C62 and AtPP2C26 genes in the plants harvested at 1 day post‐inoculation (dpi) was obtained by real‐time reverse transcription‐polymerase chain reaction (RT‐PCR). *P < 0.001 represents the significance vs. the expression level in Col‐0 by Student's t‐test. Results are representative of three experiments.
Fig. S2 Pathogenesis‐related 1 (PR1) expression was up‐regulated by either Xcc MAFF106712 or the hrcC deletion mutant after inoculation of the plant double mutant (atpp2c62/atpp2c26). Five fully expanded leaves of Col‐0 and the double mutant were inoculated by piercing three holes in the central vein with a needle dipped in a suspension of Xanthomonas campestris pv. campestris (Xcc) wild‐type and hrcC mutant bacteria [109 colony‐forming units (CFU)/mL] or vehicle alone. Expression of the PR1 gene in the plants harvested at 1 day post‐inoculation (dpi) was obtained by real‐time reverse transcription‐polymerase chain reaction (RT‐PCR). Significant differences from Col‐0 mock were identified by two‐way analysis of variance (ANOVA) followed by Tukey's multiple comparison test, and are indicated with an asterisk (*P < 0.05). The results are representative of three experiments.
Fig. S3 Hypersensitive response (HR) caused by AvrRPM1 is not changed in the double mutant (atpp2c26/atpp2c62). The HR phenotypes of the plant lines indicated following inoculation with 5 × 107 colony‐forming units (CFU/mL) of Pseudomonas syringae pv. tomato (Pst) DC3000 carrying avrRpm1 or empty vector (EV). Representative leaves are shown at 20 h (hpi, left panel) and 3 days (dpi, right panel) post‐inoculation. Beneath each leaf is the number of leaves showing HR from the total number of leaves infiltrated. Black and red marks indicate the inoculated leaves. WT, wild‐type.
Fig. S4 The multiplication of type III secretion system (TTSS) mutant bacteria is not changed in the double mutant. Bacterial multiplication was measured in Col‐0 (wild‐type) and the double mutant. Five fully expanded leaves of each plant were inoculated by piercing three holes in the central vein with a needle dipped in a suspension of Xanthomonas campestris pv. campestris (Xcc) hrcC bacteria [109 colony‐forming units (CFU)/mL]. Results are representative of three experiments. Significant differences from Col‐0 were not identified by two‐way analysis of variance (ANOVA) followed by Tukey's multiple comparison test.
Acknowledgements
Dr Derek Lundberg helped us to prepare the manuscript. This work was supported by a grant from the Ministry of Agriculture, Forestry, and Fisheries of Japan (Genomics for Agricultural Innovation, RTR‐0003).
References
- Akimoto‐Tomiyama, C. , Furutani, A. , Tsuge, S. , Washington, E.J. , Nishizawa, Y. , Minami, E. and Ochiai, H. (2012) XopR, a type III effector secreted by Xanthomonas oryzae pv. oryzae, suppresses microbe‐associated molecular pattern‐triggered immunity in Arabidopsis thaliana . Mol. Plant–Microbe Interact. 25, 505–514. [DOI] [PubMed] [Google Scholar]
- Akimoto‐Tomiyama, C. , Furutani, A. and Ochiai, H. (2014) Real time live imaging of phytopathogenic bacteria Xanthomonas campestris pv. campestris MAFF106712 in “plant sweet home”. PLoS One, 9, e94386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allahverdiyeva, Y. , Suorsa, M. , Tikkanen, M. and Aro, E.M. (2015) Photoprotection of photosystems in fluctuating light intensities. J. Exp. Bot. 66, 2427–2436. [DOI] [PubMed] [Google Scholar]
- Boller, T. and Felix, G. (2009) A renaissance of elicitors: perception of microbe‐associated molecular patterns and danger signals by pattern‐recognition receptors. Annu. Rev. Plant Biol. 60, 379–406. [DOI] [PubMed] [Google Scholar]
- Boller, T. and He, S.Y. (2009) Innate immunity in plants: an arms race between pattern recognition receptors in plants and effectors in microbial pathogens. Science, 324, 742–744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cannon, S. , Wang, P. and Roy, H. (1986) Inhibition of ribulose bisphosphate carboxylase assembly by antibody to a binding protein. J. Cell Biol. 103, 1327–1335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen, H. , Li, C. , Liu, L. , Zhao, J. , Cheng, X. , Jiang, G. and Zhai, W. (2016) The Fd‐GOGAT1 mutant gene lc7 confers resistance to Xanthomonas oryzae pv. oryzae in rice. Sci. Rep. 6, 26 411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheong, H. , Kim, C.Y. , Jeon, J.S. , Lee, B.M. , Sun Moon, J. and Hwang, I. (2013) Xanthomonas oryzae pv. oryzae type III effector XopN targets OsVOZ2 and a putative thiamine synthase as a virulence factor in rice. PLoS One, 8, e73346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chisholm, S.T. , Coaker, G. , Day, B. and Staskawicz, B.J. (2006) Host–microbe interactions: shaping the evolution of the plant immune response. Cell, 124, 803–814. [DOI] [PubMed] [Google Scholar]
- Dow, J. and Daniels, M. (1994) Pathogenicity determinants and global regulation of pathogenicity of Xanthomonas campestris pv. campestris . Curr. Top. Microbiol. Immunol. 192, 29–41. [DOI] [PubMed] [Google Scholar]
- Furutani, A. , Takaoka, M. , Sanada, H. , Noguchi, Y. , Oku, T. , Tsuno, K. , Ochiai, H. and Tsuge, S. (2009) Identification of novel Type III secretion effectors in Xanthomonas oryzae pv. oryzae . Mol. Plant–Microbe Interact. 22, 96–106. [DOI] [PubMed] [Google Scholar]
- Gómez‐Gómez, L. , Bauer, Z. , Boller, T. (2001) Both the extracellular leucine‐rich repeat domain and the kinase activity of FSL2 are required for flagellin binding and signaling in Arabidopsis . Plant Cell, 13, 1155–1163. [PMC free article] [PubMed] [Google Scholar]
- Grant, S.R. , Fisher, E.J. , Chang, J.H. , Mole, B.M. and Dangl, J.L. (2006) Subterfuge and manipulation: type III effector proteins of phytopathogenic bacteria. Annu. Rev. Microbiol. 60, 425–449. [DOI] [PubMed] [Google Scholar]
- Grieco, M. , Tikkanen, M. , Paakkarinen, V. , Kangasjarvi, S. and Aro, E.‐M. (2012) Steady‐state phosphorylation of light‐harvesting complex II proteins preserves Photosystem I under fluctuating white light. Plant Physiol. 160, 1896–1910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta, M.K. , Nathawat, R. , Sinha, D. , Haque, A.S. , Sankaranarayanan, R. and Sonti, R.V. (2015) Mutations in the predicted active site of Xanthomonas oryzae pv. oryzae XopQ differentially affect virulence, suppression of host innate immunity, and induction of the HR in a nonhost plant. Mol. Plant–Microbe Interact. 28, 195–206. [DOI] [PubMed] [Google Scholar]
- Hamdoun, S. , Liu, Z. , Gill, M. , Yao, N. and Lu, H. (2013) Dynamics of defense responses and cell fate change during Arabidopsis–Pseudomonas syringae interactions. PLoS One, 8, e83219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu, X. , Song, F. and Zheng, Z. (2006) Molecular characterization and expression analysis of a rice protein phosphatase 2C gene, OsBIPP2C1, and overexpression in transgenic tobacco conferred enhanced disease resistance and abiotic tolerance. Physiol. Plant, 127, 225–236. [Google Scholar]
- Hu, X. , Zhang, H. , Li, G. , Yang, Y. , Zheng, Z. and Song, F. (2009) Ectopic expression of a rice protein phosphatase 2C gene OsBIPP2C2 in tobacco improves disease resistance. Plant Cell Rep. 28, 985–995. [DOI] [PubMed] [Google Scholar]
- Ishikawa, K. , Yamaguchi, K. , Sakamoto, K. , Yoshimura, S. , Inoue, K. , Tsuge, S. , Kojima, C. and Kawasaki, T. (2014) Bacterial effector modulation of host E3 ligase activity suppresses PAMP‐triggered immunity in rice. Nat. Commun. 5, 5430. [DOI] [PubMed] [Google Scholar]
- Järvi, S. , Suorsa, M. and Aro, E.M. (2015) Photosystem II repair in plant chloroplasts—regulation, assisting proteins and shared components with photosystem II biogenesis. Biochim. Biophys. Acta, 1847, 900–909. [DOI] [PubMed] [Google Scholar]
- Järvi, S. , Isojärvi, J. , Kangasjärvi, S. , Salojärvi, J. , Mamedov, F. , Suorsa, M. and Aro, E.‐M. (2016) Photosystem II repair and plant immunity: lessons learned from Arabidopsis mutant lacking the THYLAKOID LUMEN PROTEIN 18.3. Front. Plant Sci. 7, 1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jelenska, J. , Yao, N. , Vinatzer, B.A. , Wright, C.M. , Brodsky, J.L. and Greenberg, J.T. (2007) A J‐domain virulence effector of Pseudomonas syringae remodels host chloroplasts and suppresses defenses. Curr Biol. 17, 499–508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones, J. and Dangl, J. (2006) The plant immune system. Nature, 444, 323–329. [DOI] [PubMed] [Google Scholar]
- Kajiwara, H. , Atsue, I. , Masatoshi, N. , Kazuei, M. , Qingyu, X. and Masami, I. (2009) Proteome analysis of silkworm 1. Fat body. J. Electroph. 53, 19–26. [Google Scholar]
- Kangasjärvi, S. , Neukermans, J. , Li, S. , Aro, E. and Noctor, G. (2012) Photosynthesis, photorespiration, and light signalling in defence responses. J. Exp. Bot. 63, 1619–1636. [DOI] [PubMed] [Google Scholar]
- Kauffman, H.E. , Reddy, A.P.K. , Hsieh, S.P.Y. and Merca, S.D. (1973) An improved technique for evaluating resistance to rice varieties of Xanthomonas oryzae . Plant Dis. Rep. 57, 537–541. [Google Scholar]
- Kerk, D. , Silver, D. , Uhrig, R.G. and Moorhead, G.B.G. (2015) “PP2C7s”, genes most highly elaborated in photosynthetic organisms, reveal the bacterial origin and stepwise evolution of PPM/PP2C protein phosphatases. PLoS One, 10, e0132863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim, C. , Meskauskiene, R. , Zhang, S. , Lee, K.P. , Ashok, M.L. , Blajecka, K. , Herrfurth, C. , Feussner, I. and Apel, K. (2012) Chloroplasts of Arabidopsis are the source and a primary target of a plant‐specific programmed cell death signaling pathway. Plant Cell, 24, 3026–3039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim, S.R. , Yang, J.I. and An, G. (2013) OsCpn60α1, encoding the plastid chaperonin 60α subunit, is essential for folding of rbcL. Mol. Cells, 35, 402–409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kubota, K. , Tsuda, S. , Tamai, A. and Meshi, T. (2003) Tomato mosaic virus replication protein suppresses virus‐targeted posttranscriptional gene silencing. J. Virol. 77, 11016–11026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee, M.W. , Jelenska, J. and Greenberg, J.T. (2008) Arabidopsis proteins important for modulating defense responses to Pseudomonas syringae that secrete HopW1‐1. Plant J. 54, 452–465. [DOI] [PubMed] [Google Scholar]
- Li, B. , Meng, X. , Shan, L. and He, P. (2016) Transcriptional regulation of pattern‐triggered immunity in plants. Cell Host Microbe, 19, 641–650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li, G. , Froehlich, J.E. , Elowsky, C. , Msanne, J. , Ostosh, A.C. , Zhang, C. , Awada, T. and Alfano, J.R. (2014) Distinct Pseudomonas type‐III effectors use a cleavable transit peptide to target chloroplasts. Plant J. 77, 310–321. [DOI] [PubMed] [Google Scholar]
- Li, S. , Wang, Y. , Wang, S. , Fang, A. , Wang, J. , Liu, L. , Zhang, K. , Mao, Y. and Sun, W. (2015) The type III effector AvrBs2 in Xanthomonas oryzae pv. oryzicola suppresses rice immunity and promotes disease development. Mol. Plant–Microbe Interact. 28, 869–880. [DOI] [PubMed] [Google Scholar]
- Maxwell, D.P. , Nickels, R. and McIntos, L. (2002) Evidence of mitochondrial involvement in the transduction of signals required for the induction of genes associated with pathogen attack and senescence. Plant J. 29, 269–279. [DOI] [PubMed] [Google Scholar]
- Meyer, D. , Lauber, E. , Roby, D. , Arlat, M. and Kroj, T. (2005) Optimization of pathogenicity assays to study the Arabidopsis thaliana–Xanthomonas campestris pv. campestris pathosystem. Mol. Plant Pathol. 6, 327–333. [DOI] [PubMed] [Google Scholar]
- Milos, P. and Roy, H. (1984) ATP‐released large subunits participate in the assembly of RuBP carboxylase. J. Cell Biochem. 24, 153–162. [DOI] [PubMed] [Google Scholar]
- Niño‐Liu, D.O. , Ronald, P.C. and Bogdanove, A.J. (2006) Xanthomonas oryzae pathovars: model pathogens of a model crop. Mol. Plant Pathol. 7, 303–324. [DOI] [PubMed] [Google Scholar]
- Perkins, D. , Pappin, D. , Creasy, D. and Cottrell, J. (1999) Probability‐based protein identification by searching database using mass spectrometry data. Electrophoresis, 20, 3551–3567. [DOI] [PubMed] [Google Scholar]
- Pogorelko, G.V. , Kambakam, S. , Nolan, T. , Foudree, A. , Zabotina, O.A. and Rodermel, S.R. (2016) Impaired chloroplast biogenesis in immutans, an Arabidopsis variegation mutant, modifies developmental programming, cell wall composition and resistance to Pseudomonas syringae . PLoS One, 11, e0150983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puthiyaveetil, S. , Woodiwiss, T. , Knoerdel, R. , Zia, A. , Wood, M. , Hoehner, R. and Kirchhoff, H. (2014) Significance of the photosystem II core phosphatase PBCP for plant viability and protein repair in thylakoid membranes. Plant Cell Physiol. 55, 1245–1254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajarajeswari, N.V.L. and Muralidharan, K. (2006) Assessments of farm yield and district production loss from bacterial leaf blight epidemics in rice. Crop Protect. 25, 244–252. [Google Scholar]
- Reiland, S. , Messerli, G. , Baerenfaller, K. , Gerrits, B. , Endler, A. , Grossmann, J. , Gruissem, W. and Baginsky, S. (2009) Large‐scale Arabidopsis phosphoproteome profiling reveals novel chloroplast kinase substrates and phosphorylation networks. Plant Physiol. 150, 889–903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richly, E. and Leister, D. (2004) An improved prediction of chloroplast proteins reveals diversities and commonalities in the chloroplast proteomes of Arabidopsis and rice. Gene, 329, 11–16. [DOI] [PubMed] [Google Scholar]
- Rodríguez‐Herva, J.J. , González‐Melendi, P. , Cuartas‐Lanza, R. , Antúnez‐Lamas, M. , Río‐Alvarez, I. , Li, Z. , López‐Torrejón, G. , Díaz, I. , Del Pozo, J.C. , Chakravarthy, S. and Collmer, A. (2012) A bacterial cysteine protease effector protein interferes with photosynthesis to suppress plant innate immune responses. Cell. Microbiol. 14, 669–681. [DOI] [PubMed] [Google Scholar]
- Rong, W. , Feng, F. , Zhou, J. and He, C. (2010) Effector‐triggered innate immunity contributes Arabidopsis resistance to Xanthomonas campestris . Mol. Plant Pathol. 11, 783–793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samol, I. , Shapiguzov, A. , Ingelsson, B. , Fucile, G. , Crevecoeur, M. , Vener, A. V. , Rochaix, J.D. and Goldschmidt‐Clermont, M. (2012) Identification of a photosystem II phosphatase involved in light acclimation in Arabidopsis . Plant Cell, 24, 2596–2609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sano, S. , Aoyama, M. , Nakai, K. , Shimotani, K. , Yamasaki, K. , Sato, M.H. , Tojo, D. , Suwastika, I.N. , Nomura, H. and Shiina, T. (2014) Light‐dependent expression of flg22‐induced defense genes in Arabidopsis . Front. Plant Sci. 5, 531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schweighofer, A. , Hirt, H. and Meskiene, I. (2004) Plant PP2C phosphatases: emerging functions in stress signaling. Trends Plant Sci. 9, 236–243. [DOI] [PubMed] [Google Scholar]
- Schweighofer, A. , Kazanaviciute, V. , Scheikl, E. , Teige, M. , Doczi, R. , Hirt, H. , Schwanninger, M. , Kant, M. , Schuurink, R. , Mauch, F. and Buchala, A. (2007) The PP2C‐type phosphatase AP2C1, which negatively regulates MPK4 and MPK6, modulates innate immunity, jasmonic acid, and ethylene levels in Arabidopsis . Plant Cell, 19, 2213–2224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Segonzac, C. and Zipfel, C. (2011) Activation of plant pattern‐recognition receptors by bacteria. Curr. Opin. Microbiol. 14, 54–61. [DOI] [PubMed] [Google Scholar]
- Silipo, A. , Molinaro, A. , Sturiale, L. , Dow, J.M. , Erbs, G. , Lanzetta, R. , Newman, M.A. and Parrilli, M. (2005) The elicitation of plant innate immunity by lipooligosaccharide of Xanthomonas campestris . J. Biol. Chem. 280, 33 660–33 668. [DOI] [PubMed] [Google Scholar]
- Singh, A. , Jha, S.K. , Bagri, J. and Pandey, G.K. (2015) ABA inducible rice protein phosphatase 2C confers ABA insensitivity and abiotic stress tolerance in Arabidopsis . PLoS One, 10, e0125168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song, C. and Yang, B. (2010) Mutagenesis of 18 type III effectors reveals virulence function of XopZ(PXO99) in Xanthomonas oryzae pv. oryzae . Mol. Plant–Microbe Interact. 23, 893–902. [DOI] [PubMed] [Google Scholar]
- Stael, S. , Kmiecik, P. , Willems, P. , Kelen, K. , Van Der, Coll, N.S. , Teige, M. , and Van Breusegem, F. , (2015) Plant innate immunity—sunny side up? Trends Plant Sci. 20, 3–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stone, J. , Collinge, M. , Smith, R. , Horn, M. and Walker, J. (1994) Interaction of a protein phosphatase with an Arabidopsis serine–threonine receptor kinase. Science, 266, 793–795. [DOI] [PubMed] [Google Scholar]
- Sun, W. , Liu, L. and Bent, A.F. (2011) Type III secretion‐dependent host defense elicitation and type III secretion‐independent growth within leaves by Xanthomonas campestris pv. campestris . Mol. Plant Pathol. 12, 731–745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki, K. , Nakanishi, H. , Bower, J. , Yoder, D.W. , Osteryoung, K.W. and Miyagishima, S. (2009) Plastid chaperonin proteins Cpn60α and Cpn60β are required for plastid division in Arabidopsis thaliana . BMC Plant Biol. 9, 38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Torres Zabala, M. , Littlejohn, G. , Jayaraman, S. , Studholme, D. , Bailey, T. , Lawson, T. , Tillich, M. , Licht, D. , Bölter, B. , Delfino, L. , Truman, W. and Mansfield, J. (2015) Chloroplasts play a central role in plant defense and are targeted by pathogen effectors. Nat. Plants, 1, 15 074. [DOI] [PubMed] [Google Scholar]
- Truman, W. , Zabala, M.T. and De Grant, M. (2006) Type III effectors orchestrate a complex interplay between transcriptional networks to modify basal defense responses during pathogenesis and resistance. Plant J. 46, 14–33. [DOI] [PubMed] [Google Scholar]
- Umezawa, T. , Nakashima, K. , Miyakawa, T. , Kuromori, T. , Tanokura, M. , Shinozaki, K. and Yamaguchi‐Shinozaki, K. (2010) Molecular basis of the core regulatory network in ABA responses: sensing, signaling and transport. Plant Cell Physiol. 51, 1821–1839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, G. , Roux, B. , Feng, F. , Guy, E. , Li, L. , Li, N. , Zhang, X. , Lautier, M. , Jardinaud, M.F. , Chabannes, M. and Arlat, M. (2015) The decoy substrate of a pathogen effector and a pseudokinase specify pathogen‐induced modified‐self recognition and immunity in plants. Cell Host Microbe, 18, 285–295. [DOI] [PubMed] [Google Scholar]
- Xue, T. , Wang, D. , Zhang, S. , Ehlting, J. , Ni, F. , Jakab, S. , Zheng, C. and Zhong, Y. (2008) Genome‐wide and expression analysis of protein phosphatase 2C in rice and Arabidopsis. BMC Genomics, 9, 550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang, Y. , Yu, C.L. , Wang, X.M. , Yan, C.Q. , Cheng, Y. and Chen, J.P. (2011) Inoculation with Xanthomonas oryzae pv. oryzae induces thylakoid membrane association of Rubisco activase in Oryza meyeriana . J. Plant Physiol. 168, 1701–1704. [DOI] [PubMed] [Google Scholar]
- Zeier, J. , Pink, B. , Mueller, M.J. and Berger, S. (2004) Light conditions influence specific defense responses in incompatible plant–pathogen interactions: uncoupling systemic resistance from salicylic acid and PR‐1 accumulation. Planta, 219, 673–683. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional Supporting Information may be found in the online version of this article at the publisher's website:
Fig. S1 The double mutant (atpp2c62/atpp2c26) was a null mutant. Five fully expanded leaves of Col‐0 and the double mutant were inoculated by piercing three holes in the central vein with a needle dipped in a suspension of Xanthomonas campestris pv. campestris (Xcc) MAFF106712 hrcC mutant bacteria [109 colony‐forming units (CFU)/mL]. Expression of the AtPP2C62 and AtPP2C26 genes in the plants harvested at 1 day post‐inoculation (dpi) was obtained by real‐time reverse transcription‐polymerase chain reaction (RT‐PCR). *P < 0.001 represents the significance vs. the expression level in Col‐0 by Student's t‐test. Results are representative of three experiments.
Fig. S2 Pathogenesis‐related 1 (PR1) expression was up‐regulated by either Xcc MAFF106712 or the hrcC deletion mutant after inoculation of the plant double mutant (atpp2c62/atpp2c26). Five fully expanded leaves of Col‐0 and the double mutant were inoculated by piercing three holes in the central vein with a needle dipped in a suspension of Xanthomonas campestris pv. campestris (Xcc) wild‐type and hrcC mutant bacteria [109 colony‐forming units (CFU)/mL] or vehicle alone. Expression of the PR1 gene in the plants harvested at 1 day post‐inoculation (dpi) was obtained by real‐time reverse transcription‐polymerase chain reaction (RT‐PCR). Significant differences from Col‐0 mock were identified by two‐way analysis of variance (ANOVA) followed by Tukey's multiple comparison test, and are indicated with an asterisk (*P < 0.05). The results are representative of three experiments.
Fig. S3 Hypersensitive response (HR) caused by AvrRPM1 is not changed in the double mutant (atpp2c26/atpp2c62). The HR phenotypes of the plant lines indicated following inoculation with 5 × 107 colony‐forming units (CFU/mL) of Pseudomonas syringae pv. tomato (Pst) DC3000 carrying avrRpm1 or empty vector (EV). Representative leaves are shown at 20 h (hpi, left panel) and 3 days (dpi, right panel) post‐inoculation. Beneath each leaf is the number of leaves showing HR from the total number of leaves infiltrated. Black and red marks indicate the inoculated leaves. WT, wild‐type.
Fig. S4 The multiplication of type III secretion system (TTSS) mutant bacteria is not changed in the double mutant. Bacterial multiplication was measured in Col‐0 (wild‐type) and the double mutant. Five fully expanded leaves of each plant were inoculated by piercing three holes in the central vein with a needle dipped in a suspension of Xanthomonas campestris pv. campestris (Xcc) hrcC bacteria [109 colony‐forming units (CFU)/mL]. Results are representative of three experiments. Significant differences from Col‐0 were not identified by two‐way analysis of variance (ANOVA) followed by Tukey's multiple comparison test.


