Summary
Recent investigations have demonstrated that bacteria employ the volatile compounds they produce during interactions with other organisms, such as plants, fungi, nematodes and bacteria. However, studies focused on the antibacterial activity of plant growth‐promoting rhizobacteria (PGPR) volatiles against bacterial phytopathogens are still rare. In this study, Bacillus strain D13, which is antagonistic to Xanthomonas oryzae pv. oryzae (Xoo), was isolated and screened. Volatile compounds emitted from strain D13 reduced the colony diameter and cell motility of Xoo cultured in divided Petri plates. Transmission electron micrograph analysis showed concentration in cytoplasm and altered surface morphology in the majority of Xanthomonas cells after co‐cultivation with strain D13. Transcriptional expression of virulence‐associated genes in Xoo was repressed. Based on gas chromatography/mass spectrometry (GC/MS) analysis, 12 volatile compounds specifically produced by strain D13 were identified. Among them, decyl alcohol and 3,5,5‐trimethylhexanol inhibited the growth of Xoo at minimum inhibitory amounts of 0.48 and 2.4 mg, respectively. Furthermore, transcriptional expression of virulence‐associated genes was also repressed by decyl alcohol and 3,5,5‐trimethylhexanol. These results are useful for a better understanding of the biocontrol mechanisms of Bacillus.
Keywords: antibacterial activity; Bacillus; volatile compounds, Xanthomonas oryzae pv. oryzae
Introduction
Xanthomonas oryzae pv. oryzae (Xoo) is the causal agent of bacterial leaf blight, a vascular pathogen that grows in the xylem vessels, causing yellow/white lesions along the leaf veins. Bacterial leaf blight disease is one of the major diseases of rice in Asian countries, where high‐yielding rice cultivars are often susceptible to the disease, leading to 50%–60% failure in severely affected crops. Several virulence‐related factors have been identified, such as the hypersensitive response and pathogenicity (hrp) gene (Zhu et al., 2000), products depending on type II and III secretion systems (Ray et al., 2000), extracellular polysaccharides (EPSs) (Rajeshwari et al., 1997), motility (Yu et al., 2014), diffusible signal factors (DSFs) (He et al., 2010) and extracellular enzymes (Rajeshwari et al., 2005; Sun et al., 2005).
It has long been recognized that bacteria emit characteristic scents, and humans have exploited microbial volatiles as aroma components of cheese, yogurt and wine. Recent investigations have demonstrated that bacteria employ volatiles during interactions with other organisms, such as plants (Ryu et al., 2003; Velázquez‐Becerra et al., 2011; Zou et al., 2010), fungi (McCain, 1966; Zheng et al., 2013), nematodes (Dainty et al., 1985; Zechman and Labows, 1985) and bacteria (Kim et al., 2013). Ryu et al. (2003) demonstrated for the first time that volatiles released by bacteria can affect plant growth. Over the past decade, active volatiles have been identified as acetoin, 2,3‐butanediol (Ryu et al., 2003), dimethylhexadecylamine (Velázquez‐Becerra et al., 2011) and 2‐pentylfuran (Zou et al., 2010). In addition, volatiles released by plant growth‐promoting rhizobacteria (PGPR) have also been confirmed to induce plant resistance to abiotic and biotic stresses, including drought stress, salt stress and nutrient deficiency (Ryu et al., 2004; Yang et al., 2009). For example, bacterial volatile organic compounds (VOCs) help plants to increase iron uptake (Zhang et al., 2009) and modulate sodium homeostasis (Zhang et al., 2008). In addition, Ryu et al. (2004) first reported that VOCs released from PGPR induce systemic resistance in Arabidopsis. 2,3‐Butanediol from Bacillus subtilis GB03 and B. amyloliquefaciens IN937a significantly increase plant resistance against Pectobacterium carotovorum ssp. carotovorum (Ryu et al., 2004). Moreover, the tridecane produced by Paenibacillus polymyxa E681 also induces systemic resistance in plants (Lee et al., 2012).
Bacterial volatiles affecting fungal growth and development were reported as early as 1966 (McCain, 1966). This study demonstrated that volatiles from Streptomyces griseus reduced Gleosporium aridum sporulation and induced Rhizoctonia solani sclerotial formation. Numerous reports have demonstrated that fungal growth is inhibited by bacterial volatiles, including dimethyl disulfide, 1‐undecene, benzaldehyde, benzothiazole, dimethyl trisulfide, cyclohexanol, decanal, 2‐ethyl‐1‐hexanol, methyl pyrazine and some mid‐ and long‐chain alkanes, alkenes and alcohols (Kai et al., 2009).
Recently, a study has shown that VOCs emitted from B. subtilis reduce the motility and increase the antibiotic resistance of Escherichia coli (Kim et al., 2013). However, few studies have focused on the antibacterial activity of PGPR volatiles. The aim of this study was to select effective antibacterial volatile‐producing strains, and identify and screen the key volatile compounds produced by Bacillus spp. against Xoo.
Results
Screening and identification of antagonistic bacterial strains
Fifty Bacillus spp. strains isolated from rhizosphere soils in Malaysia and Tibet were tested for antagonistic activity mediated by volatile compounds, using divided Petri plates. The sources of the isolated strains are listed in Table S1 (see Supporting Information). Preliminary screening showed that strain D13 had the strongest antagonistic activity towards Xoo, and the inhibition rate was about 37.5% after incubation for 6 days (Fig. 1).
Figure 1.
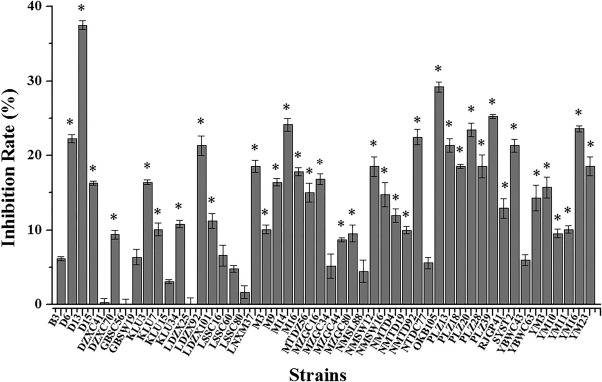
Antibacterial activity of Bacillus spp. volatiles against Xanthomonas oryzae pv. oryzae (Xoo). Each isolated Bacillus spp. strain was cultured in one compartment of the divided plates containing Luria broth (LB) medium. Xoo was cultured in a separate compartment containing nutrient agar (NA) medium. Control plates contained Xoo on one half and no Bacillus‐inoculated LB medium on the other half. After incubation for 6 days, the rates of Xoo growth inhibition by volatiles emitted from Bacillus were measured. Inhibition rate (%) = (average colony diameter of control – average colony diameter of treatment)/average colony diameter of control × 100%. The data were statistically evaluated using analysis of variance, followed by Fisher's least‐significant difference test (P < 0.05), employing SPSS software (SPSS, Chicago, IL, USA). All error bars represent standard deviations. Asterisks above the bars indicate a significant difference (P < 0.05).
Both 16S rRNA and the gyrB gene of strain D13 were amplified and sequenced. blast analysis consistently showed 99% identity with Bacillus cereus when compared with the National Center for Biotechnology Information (NCBI) database. In addition, phylogenetic analysis performed using mega software indicated that strain D13 grouped with the B. cereus branch (Fig. 2). Based on these analyses, the strain D13 grouped with the B. cereus branch.
Figure 2.
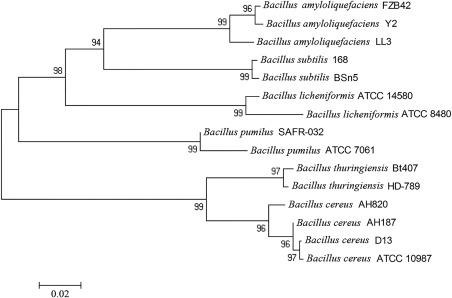
Phylogenetic analysis of Bacillus strain D13 based on gyrB gene sequences. The gyrB gene of Bacillus strain D13 was amplified and sequenced, and phylogenetic analysis was performed with the neighbour‐joining method using mega 4.0 software. Bars indicate the scales of genetic distances (0.02). Genetic distance measures are indicators of relatedness among species and are useful for reconstructing the historic and phylogenetic relationships among such groups.
Motility deficiency of Xoo after co‐cultivation with Bacillus strain D13
As shown in Fig. 3A, the swarming and swimming motilities of Xoo were significantly (P < 0.05) inhibited after exposure to Bacillus strain D13 volatiles for 3 days; the colony diameter and cell viability were unchanged in this time period (Fig. S1A,B, see Supporting Information). In addition, the transcriptional levels of motA and motC were correspondingly down‐regulated (Fig. 3B). The genes motA, encoding flagellar motor component MotA, and motC, encoding flagellar motor protein, are required for motility. Nevertheless, the number of viable cells declined sharply after exposure for 6 days, suggesting that the activity of Bacillus antibacterial volatiles requires a certain time to achieve lethal concentrations. These observations suggest that VOCs released by strain D13 may also inhibit Xoo swarming and swimming motility below the lethal concentration.
Figure 3.
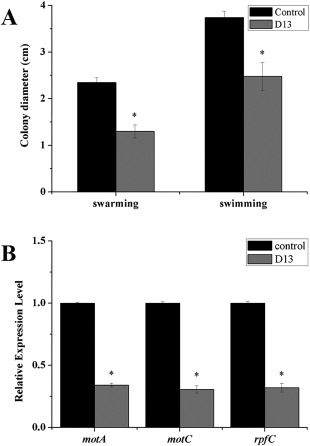
Effects of volatiles produced by Bacillus strain D13 on cell motility in Xanthomonas oryzae pv. oryzae (Xoo). Xoo cultures were spotted onto the compartments of divided plates containing 0.7% (w/v) or 0.3% (w/v) nutrient agar (NA) medium; strain D13 was cultured in a separate compartment containing Luria broth (LB) medium. Control plates contained Xoo on one half and no Bacillus‐inoculated LB medium on the other half. (A) Swarming and swimming haloes of Xoo exposed to strain D13 volatiles for 3 days. (B) Real‐time polymerase chain reaction (PCR) analysis of motA, motC and rpfC expression in Xoo cells after co‐cultivation with strain D13 for 36 h. Values were normalized to the levels of 16S rRNA, an internal reference gene. Three replicates were used for each treatment, and the experiment was repeated three times. Vertical bars represent standard errors. Asterisks above the bars indicate a significant difference (P < 0.05).
Assay of the effects of Bacillus strain D13 volatiles on Xoo cell virulence
The effect of strain D13 volatiles on Xoo virulence was also evaluated. As shown in Fig. 4, the lesion lengths observed in rice plants (cultivar IR24) inoculated with Xoo cells after co‐cultivation with strain D13 volatiles were significantly reduced (P < 0.05). In Xoo, the rpf (regulation of pathogenicity factor) gene cluster is required for biofilm formation and virulence, the core genes of which are rpfF, rpfC and rpfG (Chatterjee et al., 2008; Guo et al., 2012; He et al., 2006). rpfF encodes a protein similar to enoyl‐CoA hydratase which catalyses the synthesis of DSF. DSF is then sensed by a two‐component signal transduction system consisting of RpfC and RpfG. Real‐time polymerase chain reaction (PCR) showed that the transcriptional expression of rpfC was also repressed in the presence of volatile compounds (Fig. 3B).
Figure 4.
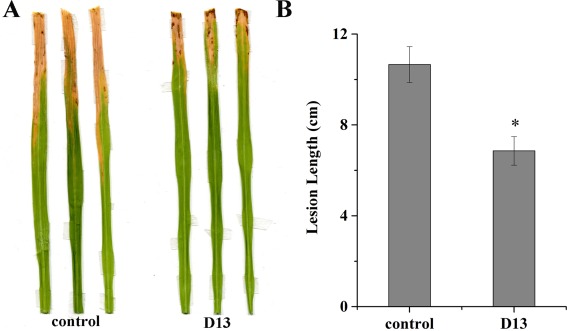
Effects of Bacillus sp. D13 volatiles on the virulence of Xanthomonas oryzae pv. oryzae (Xoo). (A) Representative lesion lengths in rice seedling leaves (cultivar IR24, 2 months old) following inoculation with control Xoo cells and Xoo cells after co‐cultivation with strain D13. (B) Analysis of lesion lengths. The y‐axis values represent the mean lesion length ± the standard deviation (n = 3). Asterisk indicates significant difference compared with the control (P < 0.05).
Ultrastructural changes in Xoo cells after co‐cultivation with Bacillus strain D13
To investigate the changes in shape and ultrastructure of Xanthomonas cells, cells were examined after co‐cultivation with strain D13 by transmission electron microscopy. As shown in Fig. 5A, the membrane of the non‐inoculated control cells was intact with uniformly distributed cytoplasm and electron density inside the cells. In contrast, more than 30% of Xoo cells showed concentrated cytoplasm and altered cell morphology after co‐cultivation with strain D13 (Fig. 5B). However, the effects of volatile treatment on Xoo cells were variable. In some cells, the walls and membranes were badly distorted, which may increase membrane permeability and cause leakage of intracellular substances. In other cases, the membrane permeability may decrease and result in concentrated cytoplasm.
Figure 5.
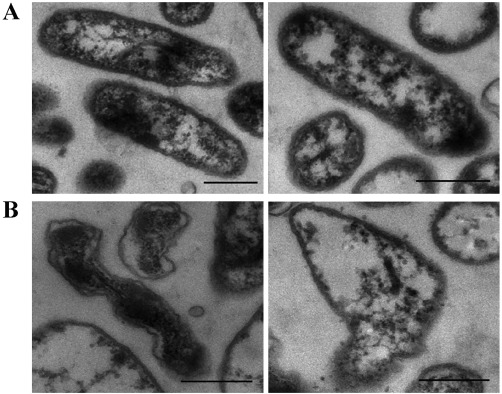
Transmission electron micrographs of Xanthomonas oryzae pv. oryzae (Xoo) co‐cultured with strain D13. The specimens were prepared from the samples taken directly from divided Petri plates after co‐cultivation for 6 days. (A) Untreated control Xoo cells. (B) Xoo cells during co‐cultivation. Scale bars indicate 500‐nm lengths.
Identification and antibacterial activity of volatile compounds produced by Bacillus strain D13
The volatiles released by strain D13 and Luria broth (LB) medium were analysed by solid‐phase microextraction‐gas chromatography/mass spectrometry (SPME‐GC/MS). More than 30 compounds produced by strain D13 cultured in LB medium were detected; nevertheless, LB medium can also produce many volatile compounds. Identical volatile compounds produced by D13 and LB medium were deducted, and 12 volatile compounds specifically produced by strain D13 were identified, including four acids, three alcohols, two nitrogen‐containing compounds, one benzene, one ketone and one sulfur‐containing compound (Table S2, see Supporting Information). Eight volatiles, including lauric acid (LA), tridecanoic acid (TA), myristic acid (MA), palmitic acid (PA), 3‐methyl‐1,2‐cyclopentanediol (MCP), 3,5,5‐trimethylhexanol (TMH), decyl alcohol (DA) and 2‐pentadecanone (PCO), were purchased and assayed for antibacterial activity in divided Petri dishes. The other four VOCs, including 1,4‐dihydro‐2‐methyl‐1,4‐ethenoisoquinolin‐3(2H)‐one, N,N‐dimethyl octanamide, 4‐octadecyl morpholine and bis(1,1,3,3‐tetramethylbutyl)disulfide, are unavailable as pure chemicals.
Among the eight volatile compounds mentioned above, only DA and TMH inhibited the growth of Xoo by 53.6% and 60.7%, respectively; the remaining compounds showed weak or no inhibitory activity (Fig. 6A). Different amounts of DA and TMH were tested for further investigation. The degree of inhibition varied depending on the amount of DA and TMH, and the minimum inhibitory amounts were 0.48 and 2.4 mg, respectively (Fig. 6B,C).
Figure 6.
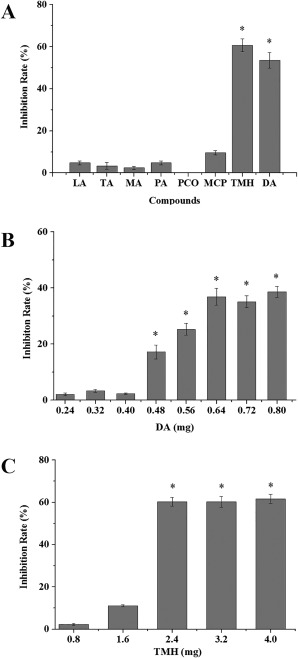
Antibacterial effects of selected volatile organic compounds (VOCs) on Xanthomonas oryzae pv. oryzae (Xoo) growth. (A) Xoo was incubated with eight volatile compounds, and the inhibition rates were measured after exposure for 6 days. (B) Xoo was incubated with DA at different amounts ranging from 0.24 to 0.8 mg, and the inhibition rates were measured after exposure for 6 days. (C) Xoo was incubated with TMH at different amounts ranging from 0.8 to 4 mg, and the inhibition rates were measured after exposure for 6 days. DA, decyl alcohol; LA, lauric acid; MA, myristic acid; MCP, 3‐methyl‐1,2‐cyclopentanediol; PA, palmitic acid; PCO, 2‐pentadecanone; TA, tridecanoic acid; TMH, 3,5,5‐trimethylhexanol. The y‐axis values represent the mean inhibition rate ± standard deviation (n = 3). *Significant difference compared with the control (P < 0.05).
Effects of DA and TMH on different pathogenic bacteria
The inhibitory effects on the growth of different pathogenic bacteria were also investigated. Xanthomonas oryzae pv. oryzicola (Xoc), Pseudomonas syringae pv. tomato DC3000 (Pst) and Ralstonia solanacearum (Rs) were inhibited by TMH, whereas DA inhibited only Xoc (Fig. 7). These results suggest that TMH may exert a general toxicity against pathogenic bacteria.
Figure 7.
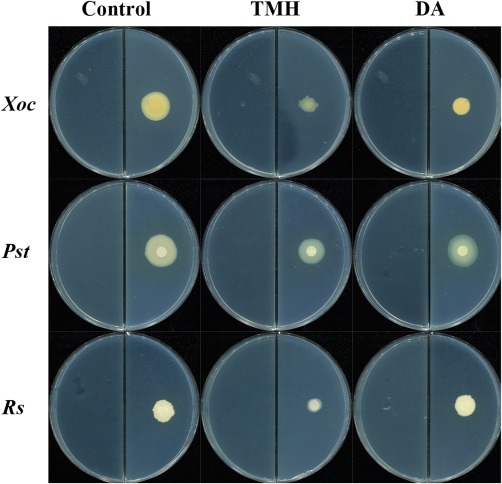
Antibacterial activities of decyl alcohol (DA) and 3,5,5‐trimethylhexanol (TMH) against Xanthomonas oryzae pv. oryzicola (Xoc), Pseudomonas syringae pv. tomato DC3000 (Pst) and Ralstonia solanacearum (Rs); 0.48 mg DA [dissolved in 100 µL dimethylsulfoxide (DMSO)], 2.4 mg TMH (dissolved in 100 µL DMSO) and DMSO were added to one compartment of the divided plates containing Luria broth (LB) medium; Xoc, Pst and Rs were cultured in a separate compartment containing nutrient agar (NA) or King's B (KB) medium. The diameters of Xoc, Pst and Rs colonies were measured after exposure for 14, 4 and 4 days, respectively. Three independent replicates of each experiment were performed.
Effects of DA and TMH on the transcriptional expression of virulence‐associated genes in Xoo
EPS production, motility and DSF have been confirmed to be associated with virulence in Xanthomonas oryzae (He et al., 2010; Jeong et al., 2008; Rai et al., 2012). In this study, transcriptional expression of gumD (EPS synthesis), motA and motC (motility) and rpfC (DSF) was investigated by real‐time PCR analysis. motA, motC and rpfC were all repressed following exposure to DA and TMH, whereas the expression of gumD was not affected (Fig. 8). The down‐regulated expression of motA, motC and rpfC was consistent with the reduced virulence in Xoo.
Figure 8.
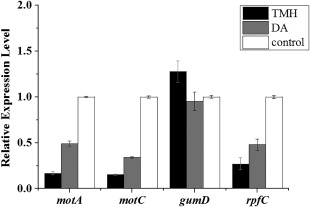
Real‐time polymerase chain reaction (PCR) analysis of motA, motC, gumD and rpfC expression in Xanthomonas oryzae pv. oryzae cells in response to 0.48 mg synthetic decyl alcohol (DA) or 2.4 mg 3,5,5‐trimethylhexanol (TMH) for 24 h. Values were normalized to the levels of 16S rRNA, an internal reference gene. The y‐axis values represent the mean expression ± standard deviation (n = 3) relative to the control.
Discussion
In this study, we screened a collection of Bacillus strains isolated from soils for their antagonistic activity against Xoo via secretion of volatile compounds. We identified one strain, D13, which showed strong antagonistic activity against this pathogen. Based on the sequence analysis of 16S rRNA and gyrB gene, D13 was grouped with B. cereus, which is a Gram‐positive bacterium belonging to Bacillus species. Several Bacillus spp., such as Quantum‐400 (Bacillus subtilis, strain GB03), Serenade (B. subtilis, strain QST713) and Rhizovital (Bacillus amyloliquefaciens, strain FZB42), have been widely studied and applied as efficient, stable biocontrol agents because of their ability to form heat‐ and desiccation‐resistant spores. Bacillus spp. have been shown to protect plants through antibiotic production, predation and parasitism, competition for ferric iron ions, competition for nutrients and niches, and induction of plant resistance (Pliego et al., 2008; Van et al., 1991). VOCs produced by B. amyloliquefaciens NJN‐6 inhibit the growth and spore germination of Fusarium oxysporum. Volatile compound 2,3‐butanediol from B. subtilis GB03 and B. amyloliquefaciens IN937a significantly increases plant resistance against Pectobacterium carotovorum ssp. carotovorum (Ryu et al., 2004).
Numerous reports of volatile compounds produced by bacteria have shown contrasting effects, including increased plant biomass (Ryu et al., 2003; Zou et al., 2010), improvement of plant resistance against abiotic and biotic stresses (Farag et al., 2013), nematode death (Gu et al., 2007) and inhibition of fungal mycelium growth and spore formation (Zou et al., 2007). Moreover, bacterial volatiles can also function as info‐chemicals for communication in and between organisms and cells (Kai et al., 2009). Bacteria use a cell‐to‐cell communication system to monitor their population density (Ryan and Dow, 2008) in a system that involves the synthesis, release and subsequent detection of small diffusible signal molecules, called autoinducers (Miller and Bassler, 2001; Uzureau et al., 2010). This system allows the coordinated behaviour of a group of organisms in order to regulate processes contributing to virulence, biofilm formation and other developmental processes (Miller and Bassler, 2001; Whitehead et al., 2001). Gram‐negative bacteria use N‐acylhomoserine lactones (N‐AHLs), fatty acid derivatives and cyclic dipeptides for communication. Many autoinducers are compounds with small molecular masses that might act as volatiles. However, little is known about the antibacterial activity of volatiles produced by Bacillus spp. strains. In the present study, we evaluated the antibacterial activity of volatiles emitted from Bacillus strain D13 against Xoo. The cell motility and virulence of Xoo were significantly reduced (P < 0.05). Transmission electron microscopy showed the concentration in cytoplasm and altered surface morphology in the majority of Xanthomonas cells after co‐cultivation with strain D13. In another study, transmission electron microscopy of fumigated and untreated Botrytis cinerea showed excessive vesiculation or thickened cell walls in exposed conidia, and increased vesiculation or marked retraction of the plasma membrane in exposed hyphae (Li et al., 2012). These results indicate that the mode of action of volatiles can be explained, at least in part, by their ability to cause leakage or impede the interchange of materials through a change in the membrane permeability. In addition, the rhizosphere is a relative closed environment. The small space seems favourable for volatile‐mediated communication, and volatiles are more likely to accumulate and reach their activity threshold than in the open environment. Moreover, the antibacterial effects of volatiles may inhibit Xoo infection via root wounds. These results may provide the basis of a potential method for the biocontrol of Xoo.
All of the active volatiles that have been reported so far can be grouped into alcohols, aldehydes, alkenes, alkanes, alkynes, esters, ketones, terpenoids and sulfur‐containing compounds (Corcuff et al., 2011; Fernando et al., 2005; Wan et al., 2008). For example, the growth of Fusarium culmorum mycelium is inhibited by dimethyl disulfide in a dose‐dependent manner, and small effects have been obtained with 1‐undecene (Kai et al., 2009). Furthermore, 2‐decanone, which is the second most dominant substance produced by Bacillus pumilus TB09 and B. thuringiensis T72, significantly inhibits the growth of Colletotrichun gloeosporioides mycelia (Zheng et al., 2013). 2‐Ethyl hexanol and n‐decanal, produced by bacteria, have been confirmed to inhibit sclerotia and ascospore germination, and the growth of Sclerotinia sclerotiorum mycelium (Fernando et al., 2005). Volatile compounds emitted from B. amyloliquefaciens NJN‐6 inhibit the growth of F. oxysporum (Yuan et al., 2012). Allyl alcohol inhibits carpogenic germination of sclerotia of S. sclerotiorum (Huang et al., 1997) and also increases the populations of beneficial bacteria, such as Pseudomonas fluorescens and P. putida (Altman and Lawlor, 1966; Domsch, 1959).
Our results demonstrate that DA and TMH are the volatile components responsible for antibacterial activity against Xoo (Fig. 6). DA is a straight chain fatty alcohol with 10 carbon atoms. It has been confirmed to inhibit and disrupt biofilms of Aspergillus fumigatus in a concentration‐dependent manner (Mowat et al., 2010). DA also shows antifungal activity against Zygosaccharomyces bailii (Fujita et al., 2008). TMH is a member of the fragrance structural group of branched chain saturated alcohols. TMH is a fragrance ingredient used in many fragrance compounds. It may be found in fragrances used in decorative cosmetics, fine fragrances, shampoos, toilet soaps and other toiletries, as well as in non‐cosmetic products, such as household cleaners and detergents (McGinty et al., 2010). Moreover, the proportion calculated from the relative peak areas (TMH : DA) was 1 : 3. The growth of Xoo was assessed after exposure to different amounts of artificial mixtures of volatiles: the minimum inhibitory amount was 0.4 mg (Fig. S2, see Supporting Information), which was smaller than that of the individual compounds. TMH also inhibited the growth of Xoc, Pst and Rs, suggesting that TMH has a broad‐spectrum resistance against pathogenic bacteria and has the potential for practical application.
In previous research, the antimicrobial mechanisms of B. cereus strains have been demonstrated. For example, B. cereus UW85 inhibits the growth of Pseudopeziza medicaginis by producing zwittermicin A and kanosamine (Emmert et al., 2004; Milner et al., 1996). Two antifungal chitinases (ChiCW and ChiCH) excreted by B. cereus 28‐9 show inhibitory activity on the conidial germination of Botrytis elliptica (Huang et al., 2005). The results of this study provide a better understanding of the mode of action of B. cereus as a biocontrol agent. Furthermore, unlike traditional chemical fungicides or bactericides, DA emitted by Bacillus strain D13 is generally non‐phytotoxic (Kim et al., 2004), thus strengthening its potential application for plant disease management.
Experimental Procedures
Microorganisms and culture conditions
Fifty Bacillus spp. strains isolated from soils in Malaysia and Tibet were tested for antagonistic activity using divided Petri plates. The sources of the isolated strains are listed in Table S1. These strains were grown on LB medium incubated at 37 °C overnight. Bacillus strain D13 was stored in the China General Microbiological Culture Center (CGMCC, Beijing, China) under the registration number CGMCC No. 10243. The pathogenic bacteria Xanthomonas oryzae pv. oryzae PXO99A (Xoo) (Hopkins et al., 1992), Xanthomonas oryzae pv. oryzicola RS105 (Xoc) (Zou et al., 2006), Pseudomonas syringae pv. tomato DC3000 (Pst) (Whalen et al., 1991) and Ralstonia solanacearum (Rs) (provided by Dr Qiao from Jiangsu‐Provincial Academy of Agricultural Sciences, China) were used as target bacteria. Rs and Xoo were grown on nutrient agar (NA) medium at 28 °C for 24 and 48 h, respectively. Pst was grown on King's B (KB) medium at 28 °C for 24 h.
Antagonistic assay of VOCs against bacteria
One compartment of the divided plates containing LB medium was incubated with each of the isolated Bacillus spp. stains [30 µL; 1 × 108 colony‐forming units (cfu)/mL]; the other compartment containing NA medium was cultured with 10 µL (108 cfu/mL) Xoo to test growth inhibition. Control plates contained Xoo on one half and no Bacillus‐inoculated LB medium on the other half. The divided Petri plates were sealed and incubated for 6 days at 28 °C before the diameter of Xoo colonies was measured.
Motility assays
Xoo motility after co‐cultivation with strain D13 was tested using divided Petri plates. Xoo cultures were normalized to an optical density at 600 nm (OD600) of 1.0, and then 2 µL of the culture was spotted onto the compartment of the divided plates containing 0.7% (w/v) or 0.3% (w/v) NA medium. Bacillus strain D13 (30 µL; 108 cfu/mL) was cultured in another compartment containing LB medium. The motility halo was examined after incubation at 28 °C for 72 h.
Pathogenicity assays
Xoo cells were collected after co‐cultivation with Bacillus strain D13 VOCs for 3 days, resuspended in distilled water (final concentration, 108 cfu/mL) and inoculated into 2‐month‐old rice cultivar IR24 (Mazzola et al., 1994) by the leaf clipping method (Kauffman et al., 1973). After 14 days, the lesion lengths were measured. Twelve plants were inoculated with the Xoo strain in each treatment, and the experiment was repeated three times.
Transmission electron microscopy
Xoo cells were co‐cultured with Bacillus strain D13 VOCs to investigate the changes in shape and ultrastructure. After 6 days, Xoo cells were collected and washed three times with sterilized water and then fixed with 2.5% glutaraldehyde. Fixed cells were rinsed three times for 10 min with 100 mm phosphate buffer, post‐fixed for 3 h in 1% osmium tetroxide and dehydrated through an ethanol gradient. Samples were then embedded in Epon 812, sectioned with an ultramicrotome and observed using a Hitachi H‐600 transmission electron microscope (Hitachi, Tokyo, Japan).
Collection of VOCs with SPME‐GC/MS analysis
Volatiles were collected by a 2‐cm, 50/30 μm divinylbenzene/carboxen on polydimethylsiloxane fibre (57348‐U, Supelco, Bellefonte, PA, USA). For VOC extraction, the Bacillus strain (20 µL) was inoculated onto 30 ml LB medium in a 100‐mL vial. After incubation at 28 °C for 6 days, the SPME fibre was inserted into the headspace of the vial and then placed at 50 °C for 30 min. GC/MS analysis was performed using a Bruker 450‐GC gas chromatograph (Bruker diatonic, MA, USA) in combination with a Bruker 320‐MS mass spectrometer, and helium gas was used as carrier (flow rate, 1 mL/min). The SPME fibres were desorbed at 220 °C for 5 min and the oven temperature was programmed as follows: 35 °C for 3 min at the beginning, increasing to 180 °C (10 °C/min), further increasing to 240 °C (4 °C/min) and held for 5 min (Yuan et al., 2012). The mass spectrometer was operated in the electron ionization mode at 70 eV with a source temperature of 220 °C, and a continuous scan from 50 to 500 m/z was used. Mass spectral data of the volatile compounds were compared with data in the National Institute of Standards and Technology/Environmental Protection Agency/National Institutes of Health (NIST/EPA/NIH) Mass Spectrum Library.
Assessment of the effects of volatile compounds on Xoo growth
Eight high‐purity individual VOCs (>98%) identified through GC/MS analysis were purchased and assayed for antibacterial activity in divided Petri dishes. These volatile compounds included LA (L110737, Aladdin, Shanghai, China), TA (T114032, Aladdin), MA (M108284, Aladdin), PA (P101053, Aladdin), MCP (S352810, Sigma‐Aldrich, St. Louis, MO, USA), TMH (289485, Sigma‐Aldrich), DA (74723, Sigma‐Aldrich) and PCO (76535, Sigma‐Aldrich). Liquid‐state volatile compounds (100 µL) or solid‐state volatile compounds [100 µL; final concentration, 1 g/mL dissolved in dimethylsulfoxide (DMSO)] were added to LB plates and 10 µL (108 cfu/mL) Xoo cultures were spotted onto another plate containing NA medium. Equivalent amounts of DMSO were used as control. After incubation at 28 °C for 6 days, the colony diameter was assessed.
According to the results of initial screening assays, TMH and DA were chosen for further investigation of their antibacterial activity against Xoo. The effects of different amounts of TMH and DA, ranging from 0.08 to 4 mg, against Xoo were evaluated. In addition, assays of the antibacterial activity of TMH and DA against Xoc, Pst and Rs were carried out. Amounts of 0.48 mg DA (dissolved in 100 µL DMSO), 2.4 mg TMH (dissolved in 100 µL DMSO) or DMSO were added to one compartment of the divided plates containing LB medium; 10 µL (108 cfu/mL) of Xoc, Pst or Rs were cultured in a separate compartment containing NA or KB medium. The diameters of Xoc, Pst and Rs colonies were measured after exposure for 14, 4 and 4 days, respectively. Three independent replicates of each experiment were performed.
Real‐time PCR
Xoo cells were harvested after exposure to 2.4 mg TMH or 0.48 mg DA. Total RNA was extracted using the Bacterial RNA Kit (Omega Bio‐Tek, Norcross, GA, USA) according to the manufacturer's instructions. First‐strand cDNA was obtained using reverse transcriptase (TaKaRa Bio, Dalian, China) with random hexamer primers. Real‐time PCR was performed with SYBR Premix Ex Taq™ (TaKaRa Bio) using an ABI PRISM 7500 Real‐time PCR System (Applied Biosystems, Foster City, CA, USA). 16S rRNA was used as an internal reference, and the transcriptional levels of motA, motC, gumD and rpfC were detected. The specific primers used are listed in Table S3 (see Supporting Information).
Statistical analysis
Three independent experiments were performed for each assay. The data were statistically evaluated using analysis of variance, followed by a Fisher's least‐significant difference test (P < 0.05), employing SPSS software (SPSS, Chicago, IL, USA).
Supporting information
Additional Supporting Information may be found in the online version of this article at the publisher's website:
Table S1 Bacillus spp. isolated from different regions in Malaysia and Tibet.
Table S2 Gas chromatography/mass spectrometry (GC/MS) volatile profile of Bacillus strain D13.
Table S3 Oligonucleotide DNA primers used in this study.
Fig. S1 Colony diameters (A) and cell viability (B) of Xanthomonas oryzae pv. oryzae (Xoo) exposed to Bacillus strain D13. Bacillus strain D13 [30 µL; 1 × 108 colony‐forming units (cfu)/mL] was cultured in one compartment of the divided Petri dishes containing Luria broth (LB) medium, except for control plates. Another compartment containing nutrient agar (NA) medium was used for Xoo (10 µL; 108 cfu/mL) to test the growth inhibition and cell viability. The colony diameters of Xoo were measured and viable cells were counted by the dilution method at 24‐h intervals during exposure to volatiles. The experiment was repeated three times with three replicates each time.
Fig. S2 The colony diameters of Xanthomonas oryzae pv. oryzae (Xoo) exposed to different amounts of the artificial mixture of volatile compounds. The proportion calculated from the relative peak areas [3,5,5‐trimethylhexanol : decyl alcohol (TMH : DA)] was 1 : 3. The diameters of Xoo colonies were measured after exposure to 0.08, 0.2, 0.4, 0.6, 0.8, 1, 3 and 6 mg of an artificial mixture of volatiles for 6 days. The experiment was repeated three times with three replicates each time.
Acknowledgements
This work was supported by grants from the National Natural Science Foundation of China (grant number 31471811), Agro‐scientific Research in the Public Interest (grant number 20130315) and the National High‐tech R&D Program of China (grant number 2012AA101504). The authors declare no competing financial interests.
References
- Altman, J. and Lawlor, S. (1966) The effects of some chlorinated hydrocarbons on certain soil bacteria. J. Appl. Bacteriol. 29, 260–265. [DOI] [PubMed] [Google Scholar]
- Chatterjee, S. , Newman, K.L. and Lindow, S.E. (2008) Cell‐to‐cell signaling in Xylella fastidiosa suppresses movement and xylem vessel colonization in grape. Mol. Plant–Microbe Interact. 21, 1309–1315. [DOI] [PubMed] [Google Scholar]
- Corcuff, R. , Mercier, J. , Tweddell, R. and Arul, J. (2011) Effect of water activity on the production of volatile organic compounds by Muscodor albus and their effect on three pathogens in stored potato. Fungal Biol. 115, 220–227. [DOI] [PubMed] [Google Scholar]
- Dainty, R. , Edwards, R. and Hibbard, C. (1985) Time course of volatile compound formation during refrigerated storage of naturally contaminated beef in air. J. Appl. Bacteriol. 59, 303–309. [DOI] [PubMed] [Google Scholar]
- Domsch, K. (1959) Die wirkung von bodenfungiciden III. Quantitative varenderungen der bodenflora. Z. Pflanzenkr. Pflanzenschutz, 66, 17–26. [Google Scholar]
- Emmert, E.A. , Klimowicz, A.K. , Thomas, M.G. and Handelsman, J. (2004) Genetics of zwittermicin A production by Bacillus cereus . Appl. Environ. Microbiol. 70, 104–113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farag, M.A. , Zhang, H. and Ryu, C.M. (2013) Dynamic chemical communication between plants and bacteria through airborne signals: induced resistance by bacterial volatiles. J. Chem. Ecol. 39, 1007–1018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernando, W. , Ramarathnam, R. , Krishnamoorthy, A.S. and Savchuk, S.C. (2005) Identification and use of potential bacterial organic antifungal volatiles in biocontrol. Soil Biol. Biochem. 37, 955–964. [Google Scholar]
- Fujita, K. , Fujita, T. and Kubo, I. (2008) Antifungal activity of alkanols against Zygosaccharomyces bailii and their effects on fungal plasma membrane. Phytother. Res. 22, 1349–1355. [DOI] [PubMed] [Google Scholar]
- Gu, Y.Q. , Mo, M.H. , Zhou, J.P. , Zou, C.S. and Zhang, K.Q. (2007) Evaluation and identification of potential organic nematicidal volatiles from soil bacteria. Soil Biol. Biochem. 39, 2567–2575. [Google Scholar]
- Guo, Y. , Zhang, Y. , Li, J.L. and Wang, N. (2012) Diffusible signal factor‐mediated quorum sensing plays a central role in coordinating gene expression of Xanthomonas citri subsp. citri . Mol. Plant–Microbe Interact. 25, 165–179. [DOI] [PubMed] [Google Scholar]
- He, Y.W. , Xu, M. , Lin, K. , Ng, Y.J.A. , Wen, C.M. and Wang, L.H. (2006) Genome scale analysis of diffusible signal factor regulon in Xanthomonas campestris pv. campestris: identification of novel cell–cell communication‐dependent genes and functions. Mol. Microbiol. 59, 610–622. [DOI] [PubMed] [Google Scholar]
- He, Y.W. , Wu, J.E. , Cha, J.S. and Zhang, L.H. (2010) Rice bacterial blight pathogen Xanthomonas oryzae pv. oryzae produces multiple DSF‐family signals in regulation of virulence factor production. BMC Microbiol. 10, 187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hopkins, C.M. , White, F. , Choi, S. , Guo, A. and Leach, J. (1992) Identification of a family of avirulence genes from Xanthomonas oryzae pv. oryzae . Mol. Plant–Microbe Interact. 5, 451–459. [DOI] [PubMed] [Google Scholar]
- Huang, C. , Wang, T. , Chung, S. and Chen, C. (2005) Identification of an antifungal chitinase from a potential biocontrol agent, Bacillus cereus 28‐9. J. Biochem. Mol. Biol. 38, 82. [DOI] [PubMed] [Google Scholar]
- Huang, H. , Huang, J. , Saindon, G. and Erickson, R. (1997) Effect of allyl alcohol and fermented agricultural wastes on carpogenic germination of sclerotia of Sclerotinia sclerotiorum and colonization by Trichoderma spp. Can. J. Plant Pathol. 19, 43–46. [Google Scholar]
- Jeong, K.S. , Lee, S.E. , Han, J.W. , Yang, S.U. , Lee, B.M. , Noh, T.H. and Cha, J.S. (2008) Virulence reduction and differing regulation of virulence genes in rpf mutants of Xanthomonas oryzae pv. oryzae . Plant Pathol. J. 24, 143–151. [Google Scholar]
- Kai, M. , Haustein, M. , Molina, F. , Petri, A. , Scholz, B. and Piechulla, B. (2009) Bacterial volatiles and their action potential. Appl. Microbiol. Biotechnol. 81, 1001–1012. [DOI] [PubMed] [Google Scholar]
- Kauffman, H. , Reddy, A. , Hsieh, S. and Merca, S. (1973) Improved technique for evaluating resistance of rice varieties to Xanthomonas oryzae . Plant Dis. Rep. 57, 537–541. [Google Scholar]
- Kim, K.S. , Lee, S. and Ryu, C.M. (2013) Interspecific bacterial sensing through airborne signals modulates locomotion and drug resistance. Nat. Commun. 4, 1809. [DOI] [PubMed] [Google Scholar]
- Kim, K.W. , Jeong, H.C. and Kim, Y.Y. (2004) Effect of Decyl Alcohol EC on Tobacco Sucker Control. J. Kor. Soc. Tob. Sci. 26, 7–12. [Google Scholar]
- Lee, B. , Farag, M.A. , Park, H.B. , Kloepper, J.W. , Lee, S.H. and Ryu, C.M. (2012) Induced resistance by a long‐chain bacterial volatile: elicitation of plant systemic defense by a C13 volatile produced by Paenibacillus polymyxa . PLoS One, 7, 48 744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li, Q. , Ning, P. , Zheng, L. , Huang, J. , Li, G. and Hsiang, T. (2012) Effects of volatile substances of Streptomyces globisporus JK‐1 on control of Botrytis cinerea on tomato fruit. Biol. Control, 61, 113–120. [Google Scholar]
- Mazzola, M. , Leach, J.E. , Nelson, R. and White, F.F. (1994) Analysis of the interaction between Xanthomonas oryzae pv. oryzae and the rice cultivars IR24 and IRBB21. Phytopathology, 84, 392–397. [Google Scholar]
- McCain, A. (1966) A volatile antibiotic produced by Streptomyces griseus . Phytopathology, 56, 150. [Google Scholar]
- McGinty, D. , Scognamiglio, J. , Letizia, C.S. and Api, A.M. (2010) Fragrance material review on 3,5,5‐trimethyl‐1‐hexanol. Food Chem. Toxicol. 48, 47–50. [DOI] [PubMed] [Google Scholar]
- Miller, M.B. and Bassler, B.L. (2001) Quorum sensing in bacteria. Annu. Rev. Microbiol. 55, 165–199. [DOI] [PubMed] [Google Scholar]
- Milner, J.L. , Silo‐Suh, L. , Lee, J.C. , He, H. , Clardy, J. and Handelsman, J. (1996) Production of kanosamine by Bacillus cereus UW85. Appl. Environ. Microbiol. 62, 3061–3065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mowat, E. , Rajendran, R. , Williams, C. , McCulloch, E. , Jones, B. , Lang, S. and Ramage, G. (2010) Pseudomonas aeruginosa and their small diffusible extracellular molecules inhibit Aspergillus fumigatus biofilm formation. FEMS Microbiol. Lett. 33, 96–102. [DOI] [PubMed] [Google Scholar]
- Pliego, C. , Wheert, S.D. , Lamers, G. , De, V.A. , Bloemberg, G. , Cazorla, F.M. and Ramos, C. (2008) Two similar enhanced root‐colonizing Pesudomonas strains differ largely in their colonization strategies of avocado roots and Rosellinia necatrix hyphae. Environ. Microbiol. 10, 3295–3304. [DOI] [PubMed] [Google Scholar]
- Rai, R. , Ranjan, M. , Pradhan, B.B. and Chatterjee, S. (2012) Atypical regulation of virulence‐associated functions by a diffusible signal factor in Xanthomonas oryzae pv. oryzae . Mol. Plant–Microbe Interact. 25, 789–801. [DOI] [PubMed] [Google Scholar]
- Rajeshwari, R. , Yashitola, J. , Sonti, R. and Reddy, A. (1997) Characteristics of stationary‐phase variation affecting virulence in Xanthomonas oryzae pv. oryzae . Can. J. Microbiol. 43, 862–867. [Google Scholar]
- Rajeshwari, R. , Jha, G. and Sonti, R.V. (2005) Role of an in planta‐expressed xylanase of Xanthomonas oryzae pv. oryzae in promoting virulence on rice. Mol. Plant–Microbe Interact. 18, 830–837. [DOI] [PubMed] [Google Scholar]
- Ray, S.K. , Rajeshwari, R. and Sonti, R.V. (2000) Mutants of Xanthomonas oryzae pv. oryzae deficient in general secretory pathway are virulence deficient and unable to secrete xylanase. Mol. Plant–Microbe Interact. 13, 394–401. [DOI] [PubMed] [Google Scholar]
- Ryan, R.P. and Dow, J.M. (2008) Diffusible signals and interspecies communication in bacteria. Microbiology, 154, 1845–1858. [DOI] [PubMed] [Google Scholar]
- Ryu, C.M. , Farag, M.A. , Hu, C.H. , Reddy, M.S. , Wei, H.X. and Paré, P.W. (2003) Bacterial volatiles promote growth in Arabidopsis . Proc. Natl. Acad. Sci. USA, 100, 4927–4932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryu, C.M. , Farag, M.A. , Hu, C.H. , Reddy, M.S. , Kloepper, J.W. and Pare, P.W. (2004) Bacterial volatiles induce systemic resistance in Arabidopsis . Plant Physiol. 134, 1017–1026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun, Q. , Hu, J. , Huang, G. , Ge, C. , Fang, R. and He, C. (2005) Type‐II secretion pathway structural gene xpsE, xylanase‐ and cellulase secretion and virulence in Xanthomonas oryzae pv. oryzae . Plant Pathol. 54, 15–21. [Google Scholar]
- Uzureau, S. , Lemaire, J. , Delaive, E. , Dieu, M. , Gaigneaux, A. and Raes, M. (2010) Global analysis of quorum sensing targets in the intracellular pathogen Brucella melitensis 16 M. J. Proteome Res. 9, 3200–3217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van, P.R. , Niemann, G. and Schippers, B. (1991) Induced resistance and phytoalexin accumulation in biological control of Fusarium wilt of carnation by Pseudomonas sp. strain WCS 417r. Phytopathology, 81, 728–734. [Google Scholar]
- Velázquez‐Becerra, C. , Macías‐Rodríguez, L.I. , López‐Bucio, J. , Altamirano‐Hernández, J. , Flores‐Cortez, I. and Valencia‐Cantero, E. (2011) A volatile organic compound analysis from Arthrobacter agilis identifies dimethylhexadecylamine, an amino‐containing lipid modulating bacterial growth and Medicago sativa morphogenesis in vitro. Plant Soil, 339, 329–340. [Google Scholar]
- Wan, M. , Li, G. , Zhang, J. , Jiang, D. and Huang, H.C. (2008) Effect of volatile substances of Streptomyces platensis F‐1 on control of plant fungal diseases. Biol. Control, 46, 552–559. [Google Scholar]
- Whalen, M.C. , Innes, R.W. , Bent, A.F. and Staskawicz, B.J. (1991) Identification of Pseudomonas syringae pathogens of Arabidopsis and a bacterial locus determining avirulence on both Arabidopsis and soybean. Plant Cell, 3, 49–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitehead, N.A. , Barnard, A.M. , Slater, H. , Simpson, N.J. and Salmond, G.P. (2001) Quorum‐sensing in Gram‐negative bacteria. FEMS Microbiol. Rev. 25, 365–404. [DOI] [PubMed] [Google Scholar]
- Yang, J. , Kloepper, J.W. and Ryu, C.M. (2009) Rhizosphere bacteria help plants tolerate abiotic stress. Trends Plant Sci. 14, 1–4. [DOI] [PubMed] [Google Scholar]
- Yu, C. , Chen, H. , Tian, F. , Leach, J.E. ,. and He, C. (2014) Differentially‐expressed genes in rice infected by Xanthomonas oryzae pv. oryzae relative to a flagellin‐deficient mutant reveal potential functions of flagellin in host–pathogen interactions. Rice, 7, 20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan, J. , Raza, W. , Shen, Q. and Huang, Q. (2012) Antifungal activity of Bacillus amyloliquefaciens NJN‐6 volatile compounds against Fusarium oxysporum f. sp. cubense . Appl. Environ. Microbiol. 78, 5942–5944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zechman, J.M. and Labows Jr, J.N. (1985) Volatiles of Pseudomonas aeruginosa and related species by automated headspace concentration‐gas chromatography. Can. J. Microbiol. 31, 232–237. [DOI] [PubMed] [Google Scholar]
- Zhang, H. , Kim, M.S. , Sun, Y. , Dowd, S.E. , Shi, H. and Paré, P.W. (2008) Soil bacteria confer plant salt tolerance by tissue‐specific regulation of the sodium transporter HKT1. Mol. Plant–Microbe Interact. 21, 737–744. [DOI] [PubMed] [Google Scholar]
- Zhang, H. , Sun, Y. , Xie, X. , Kim, M.S. , Dowd, S.E. and Paré, P.W. (2009) A soil bacterium regulates plant acquisition of iron via deficiency‐inducible mechanisms. Plant J. 58, 568–577. [DOI] [PubMed] [Google Scholar]
- Zheng, M. , Shi, J. , Shi, J. , Wang, Q. and Li, Y. (2013) Antimicrobial effects of volatiles produced by two antagonistic Bacillus strains on the anthracnose pathogen in postharvest mangos. Biol. Control, 65, 200–206. [Google Scholar]
- Zhu, W. , MaGbanua, M.M. and White, F.F. (2000) Identification of two novel hrp‐associated genes in the hrp gene cluster of Xanthomonas oryzae pv. oryzae . J. Bacteriol. 182, 1844–1853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zou, C.S. , Mo, M.H. , Gu, Y.Q. , Zhou, J.P. and Zhang, K.Q. (2007) Possible contributions of volatile‐producing bacteria to soil fungistasis. Soil Biol. Biochem. 39, 2371–2379. [Google Scholar]
- Zou, C. , Li, Z. and Yu, D. (2010) Bacillus megaterium strain XTBG34 promotes plant growth by producing 2‐pentylfuran. J. Microbiol. 48, 460–466. [DOI] [PubMed] [Google Scholar]
- Zou, L.F. , Wang, X.P. , Xiang, Y. , Zhang, B. , Li, Y.R. and Xiao, Y.L. (2006) Elucidation of the hrp clusters of Xanthomonas oryzae pv. oryzicola that control the hypersensitive response in nonhost tobacco and pathogenicity in susceptible host rice. Appl. Environ. Microbiol. 72, 6212–6224. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional Supporting Information may be found in the online version of this article at the publisher's website:
Table S1 Bacillus spp. isolated from different regions in Malaysia and Tibet.
Table S2 Gas chromatography/mass spectrometry (GC/MS) volatile profile of Bacillus strain D13.
Table S3 Oligonucleotide DNA primers used in this study.
Fig. S1 Colony diameters (A) and cell viability (B) of Xanthomonas oryzae pv. oryzae (Xoo) exposed to Bacillus strain D13. Bacillus strain D13 [30 µL; 1 × 108 colony‐forming units (cfu)/mL] was cultured in one compartment of the divided Petri dishes containing Luria broth (LB) medium, except for control plates. Another compartment containing nutrient agar (NA) medium was used for Xoo (10 µL; 108 cfu/mL) to test the growth inhibition and cell viability. The colony diameters of Xoo were measured and viable cells were counted by the dilution method at 24‐h intervals during exposure to volatiles. The experiment was repeated three times with three replicates each time.
Fig. S2 The colony diameters of Xanthomonas oryzae pv. oryzae (Xoo) exposed to different amounts of the artificial mixture of volatile compounds. The proportion calculated from the relative peak areas [3,5,5‐trimethylhexanol : decyl alcohol (TMH : DA)] was 1 : 3. The diameters of Xoo colonies were measured after exposure to 0.08, 0.2, 0.4, 0.6, 0.8, 1, 3 and 6 mg of an artificial mixture of volatiles for 6 days. The experiment was repeated three times with three replicates each time.


