Summary
Xanthomonas oryzae pv. oryzae (Xoo), the causal agent of bacterial blight (BB) of rice, uses transcription activator‐like effectors (TALEs) to interact with the basal transcription factor gamma subunit OsTFIIAγ5 (Xa5) and activates the transcription of host genes. However, how OsTFIIAγ1, the other OsTFIIAγ protein, functions in the presence of TALEs remains unclear. In this study, we show that OsTFIIAγ1 plays a compensatory role in the absence of Xa5. The expression of OsTFIIAγ1, which is activated by TALE PthXo7, increases the expression of host genes targeted by avirulent and virulent TALEs. Defective OsTFIIAγ1 rice lines show reduced expression of the TALE‐targeted susceptibility (S) genes, OsSWEET11 and OsSWEET14, which results in increased BB resistance. Selected TALEs (PthXo1, AvrXa7 and AvrXa27) were evaluated for interactions with OsTFIIAγ1, Xa5 and xa5 (naturally occurring mutant form of Xa5) using biomolecular fluorescence complementation (BiFC) and microscale thermophoresis (MST). BiFC and MST demonstrated that the three TALEs bind Xa5 and OsTFIIAγ1 with a stronger affinity than xa5. These results provide insights into the complex roles of OsTFIIAγ1 and OsTFIIAγ5 in TALE‐mediated host gene transcription.
Keywords: bacterial blight, Oryza sativa, OsTFIIAγ1, susceptibility, transcription activator‐like effector, Xanthomonas oryzae pv. oryzae
Introduction
Bacterial plant pathogens reduce the yield of many important crops of global importance, including rice, tomatoes, peppers and citrus. Xanthomonas is a widespread bacterial genus that contains approximately 30 pathogenic species known to cause disease in over 300 plant hosts (Boch etal., 2014; Schornack etal., 2013). One particularly important pathogen within the genus Xanthomonas is X. oryzae pv. oryzae (Xoo), which causes bacterial blight (BB) of rice, a devastating disease in rice production areas.
Many Xanthomonas spp. cause plant disease by injecting transcription activator‐like effectors (TALEs) directly into plant host cells via the Type III secretion system (T3SS) (Chen etal., 2010; Mak etal., 2013). TALEs are then translocated to the nucleus where they bind to specific promoter sequences in host genes, which are designated as TAL effector‐binding elements (EBEs) (Chen etal., 2010; Mak etal., 2013). The DNA‐binding domain of TALEs consists of repeat variable diresidues (RVDs) that bind to a predictable DNA recognition code in the promoter of the TALE gene target (Boch etal., 2009; Moscou and Bogdanove, 2009). TALE‐like proteins are not restricted to the genus Xanthomonas, as they are found in other plant pathogens and endosymbionts, including Ralstonia solanacearum and Burkholderia rhizoxinica, respectively (de Lange etal., 2014). Apart from their EBE‐binding ability, it remains unclear how TALEs function to promote the transcription of target genes cooperatively with other transcriptional factors.
Rice has developed an innate immune system to detect invading pathogens and trigger defensive responses to neutralize infection. As a counter‐offensive strategy, Xoo can deploy several different methods to interfere with the rice defence response. These include the use of interfering TALEs (iTALES) or truncated TALEs (truncTALEs), which can disrupt Xa1‐mediated defences that are triggered by archetypal TALEs (Ji etal., 2016; Read etal., 2016). Futhermore, Xoo can also deploy TALEs that promote the transcription of susceptibility (S) genes in the SWEET gene family (Streubel etal., 2013; Zhou etal., 2015). SWEET proteins are responsible for sugar transport in rice and their production can foster pathogen growth (Chen, 2014; Chen etal., 2010).
In response to TALEs and other Xoo virulence strategies, rice has co‐evolved counter‐measures, such as the utilization of recessive resistance (R) genes for many of the S gene targets. These recessive R genes can result in TALE mistargeting, reduced TALE binding and increased plant disease resistance (Boch etal., 2014; Hutin etal., 2015a). Three recessive R genes (xa13, xa25 and xa41(t)) have been identified in several rice varieties and are the EBE‐mutational alleles of OsSWEET11, OsSWEET13 and OsSWEET14, respectively (Chu etal., 2006; Hutin etal., 2015b; Liu etal., 2011; Yang etal., 2006; Zhou etal., 2015). Furthermore, some rice plants utilize a strategy that allows TALEs to recognize the promoters of dominantly inherited executor R genes, which trigger TAL effector‐triggered immunity (ETI) (Boch etal., 2014; Zhang etal., 2015). Executor R gene products have been divided into two groups (Zhang etal., 2015). Members of group 1 function in plant development and physiology; this group includes BS3, an R protein from pepper that belongs to the flavin mono‐oxygenase family (Expósito‐Rodríguez etal., 2011; Romer etal., 2007). Group 2 contains R proteins from rice, including XA10, XA27 and XA23, which are activated by the cognate effectors (AvrXa10, AvrXa27 and AvrXa23) (Gu etal., 2005; Tian etal., 2014; Wang etal., 2015). XA10, localized to endoplasmic reticulum (ER), is associated with ER Ca2+ cation depletion (Tian etal., 2014) and shares 50% identity with XA23 (Wang etal., 2015). In contrast, XA27‐mediated resistance depends on localization to the apoplast (Wu etal., 2008). Interestingly, these R genes have no obvious relationship with known R or S genes, suggesting that further complex defence responses are in play.
Several important studies have been conducted to understand how Xoo uses its collection of TALEs to activate plant transcriptional factors and modulate plant defence. Sugio etal. (2007) described how the rice gene OsTFX1, which encodes a bZIP transcription factor, was targeted by the TALE PthXo6 from Xoo strain PXO99A. In the same study, these authors also showed that PthXo7 induces the expression of the transcription factor OsTFIIAγ1 during rice infection by Xoo PXO99A (Sugio etal., 2007). Interestingly, in addition to OsTFIIAγ1, rice contains another gene, Xa5 (OsTFIIAγ5), that encodes the small (γ) subunit of the conserved general transcription factor TFIIA, which is important for polymerase II (Pol II)‐dependent transcription (Hoiby etal., 2007; Jiang etal., 2006). Recently, Xa5 and TFIIAγ proteins from rice, citrus, pepper and tomato have been shown to interact directly with a transcription factor binding (TFB) region in TALEs (Huang etal., 2017; Yuan etal., 2016). This is consistent with the hypothesis that TALEs may function as transcriptional activators by their involvement in the assembly of the transcription initiation complex at their target sites in plants (Boch and Bonas, 2010). However, information is lacking on how TALEs might specifically interact with the plant transcriptional machinery to modulate expression.
However, the rice recessive gene xa5, which is a natural allele of Xa5, contains a mutation in the 39th residue, in which the valine (V) residue is replaced with glutamine (E) (V39E) (Iyer and McCouch, 2004). It has been speculated that the missense mutation in xa5 may confer resistance by abolishing the interaction between DNA‐associated TALEs and the preinitiation complex, which could attenuate the transcription of TALE‐targeted genes (Schornack etal., 2006, 2013). Indeed, Yuan etal. (2016) reported that xa5 fails to interact with several tested TALEs. Furthermore, TALE‐mediated induction of R or S genes is attenuated in the xa5 background (Gu etal., 2009; Huang etal., 2016; Tian etal., 2014). However, there is no evidence supporting or negating the involvement of OsTFIIAγ1 in the assembly of the transcription initiation complex in rice plants.
To gain further insights into the fundamental roles of Xa5, xa5 and, especially, OsTFIIAγ1 in BB, we expressed avrXa7, pthXo1 and avrXa27 in Xoo strains PH and PE, which are tal‐free and pthXo7‐containing strains derived from PXO99A (Ji etal., 2016). These strains were evaluated for pathogenicity in rice lines IR24 (Xa5 and OsTFIIAγ1), IRBB5 (xa5 and OsTFIIAγ1), TF1 (xa5 and inactive OsTFIIAγ1), DH (Xa27, xa5 and OsTFIIAγ1) and 78‐1‐5 (Xa27, Xa5 and OsTFIIAγ1). The interaction and affinities of Xa5, xa5 and OsTFIIAγ1 with avrXa7, pthXo1 and avrXa27 were also examined. The results suggest that OsTFIIAγ1 has a role in BB and compensates for the absence of Xa5.
Results
IRBB5‐incompatible Xoo strains are unable to activate OsTFIIAγ1 expression
To investigate whether naturally occurring Xoo strains isolated from the environment have the ability to evade xa5‐mediated resistance, we examined the virulence of 65 Xoo strains isolated from 13 rice‐planting provinces in China. The well‐characterized Philippine strains PXO99A and PXO86 were included for comparative purposes (Table S1, see Supporting Information). The pathogenicity of Xoo strains was assessed in two near‐isogenic lines of rice, IR24 (Xa5) and IRBB5 (containing xa5 in the IR24 background). Xoo strains were inoculated using a tip‐cutting method, and the lesion length was measured at 14 days post‐inoculation (dpi) (see Experimental procedures). The 65 Chinese isolates of Xoo were pathogenic in IR24 rice, but were incompatible in xa5‐containing rice IRBB5. Lesions caused by eight of the 65 strains in both IR24 and IRBB5 are shown (Fig. 1A). We observed that PXO99A was compatible and PXO86 incompatible in IRBB5, which is consistent with previous results (Sugio etal., 2007). Previous work has shown that OsTFIIAγ1 expression is activated by the TALE PthXo7, which is present in PXO99A, but absent in PXO86 (Sugio etal., 2007). Therefore, we used quantitative real‐time reverse transcription‐polymerase chain reaction (qRT‐PCR) to examine whether the expression of OsTFIIAγ1 was altered during challenge with these Xoo strains. OsTFIIAγ1 expression was activated in IR24 rice inoculated with Xoo PXO99A, but not with PXO86 or the eight Chinese Xoo strains (Fig. 1B). These results suggest that the Chinese isolates lack pthXo7, which is the case with PXO86.
Figure 1.
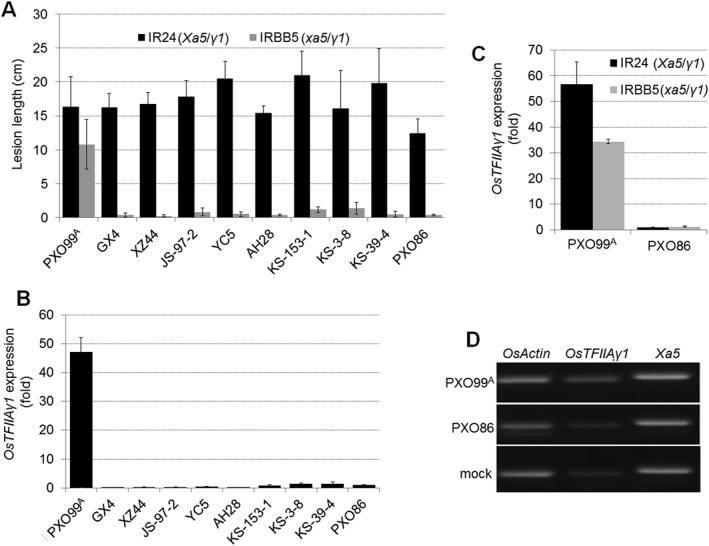
Virulence (lesion length) and OsTFIIAγ1 expression levels during Xanthomonas oryzae pv. oryzae (Xoo) infection in IR24 and IRBB5 rice lines. (A) Lesion lengths induced by Xoo PXO99A, PXO86 and eight Chinese strains (GX4, XZ44, JS‐97‐2, YC5, AH28, KS‐153‐1, KS‐3–8, KS‐39‐4) in IR24 and IRBB5 rice. Bacteria were inoculated by tip‐cutting and lesion lengths were measured at 14 days post‐inoculation (dpi). The mean lesion lengths ± standard deviation (SD) (n = 5) are shown. (B) OsTFIIAγ1 expression in IR24 rice seedlings inoculated with different Xoo strains. The expression of OsTFIIAγ1 was evaluated by quantitative real‐time reverse transcription‐polymerase chain reaction (qRT‐PCR) at 24h post‐infiltration (hpi). (C) qRT‐PCR analysis of OsTFIIAγ1 expression in IR24 and IRBB5 rice inoculated with Xoo PXO99A and PXO86 at 24 hpi. Values in (B) and (C) represent the mean ± SD (n = 3). (D) RT‐PCR analysis of OsTFIIAγ1 and Xa5 transcription in IR24 seedlings 24h after infection with PXO99A, PXO86 and water (mock control). The result shown is representative of three replicates. OsActin was used as the reference gene in both RT‐PCR and qRT‐PCR.
PXO99A caused significantly more disease in IR24 (lesion length, 16.3cm) than in IRBB5 (lesion length, 10.8cm) rice at 14 dpi (Fig. 1A). The reduced disease symptoms in the IRBB5 line could be partially due to xa5 (mutant form of Xa5). It is also important to note that lesions in the PXO99A/IRBB5 interaction might be modulated in part by OsTFIIAγ1, which is activated by PthXo7 in PXO99A, but not in the other Xoo strains. To confirm this possibility, we compared the expression of OsTFIIAγ1 in IR24 and IRBB5 by qRT‐PCR and RT‐PCR. Xoo PXO99A induced the expression of OsTFIIAγ1 in IRBB5, although the expression level was lower than in IR24 (Fig. 1C). Intriguingly, OsTFIIAγ1 expression in IR24 and IRBB5 seedlings was significantly lower than that of Xa5 (Fig. 1D) or xa5 (Fig. S1, see Supporting Information). Taken together, the lesion lengths caused by PXO99A in IRBB5 may be partially a result of the role of activated OsTFIIAγ1 in the presence of Xoo TALEs.
Activated OsTFIIAγ1 enhances the expression of TALE targets in rice
The availability of a set of PXO99A‐derived strains that are lacking specific tal genes (Ji etal., 2016) enabled the examination of potential overlapping functions for OsTFIIAγ1 and xa5 in rice during challenge with PthXo7 and other selected TALEs. In our experiments, two PXO99A‐derived strains were utilized (Fig. S2, see Supporting Information): Xoo PH (lacks genes encoding known TALEs) and Xoo PE; the latter strain contains pthXo7, which activates the expression of OsTFIIAγ1 (Fig. S2C). Xoo PH and PE were used to overexpress pthXo1, resulting in strains PH(pthXo1) and PE(pthXo1), as described in Methods S1 and S2, Figs S2 and S3, and Table S1 (see Supporting Information).
Xoo PH, PE, PH(pthXo1) and PE(pthXo1) were used to inoculate IR24 and IRBB5 rice. Lesion lengths in IR24 inoculated with PH(pthXo1) and PE(pthXo1) were significantly longer than those induced in IRBB5 rice (Fig. 2A,B). Xoo PE(pthXo1), which encodes endogenous pthXo7 combined with introduced pthXo1, resulted in more severe BB lesions than PH(pthXo1) in both IR24 and IRBB5 rice (Fig. 2A,B). Furthermore, the expression of the S gene OsSWEET11, which encodes a sucrose transporter targeted by PthXo1, was significantly higher in IR24 than IRBB5 (xa5 rice) (Fig. 2C). Xoo PE(pthXo1) induced higher levels of OsSWEET11 than Xoo PH(pthXo1) in IR24 and IRBB5 (Fig. 2C). These findings suggest that Xa5 in IR24 rice may foster PthXo1‐activated expression of the S gene OsSWEET11, which is attenuated by xa5 in IRBB5. In summary, we propose that the activation of OsTFIIAγ1 by PthXo7, which is suggested by Fig. S2C, leads to enhanced expression of OsSWEET11.
Figure 2.
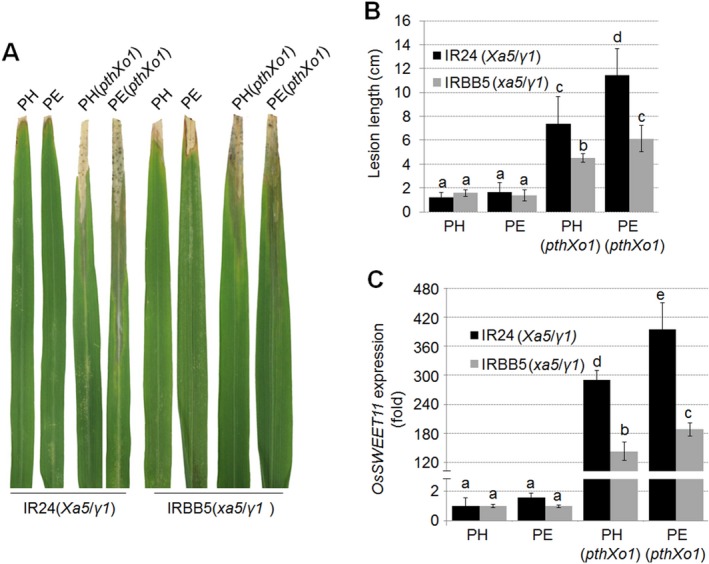
Effects of OsTFIIAγ1 on PthXo1‐induced lesion length and PthXo1‐activated OsSWEET11 expression. Disease phenotypes (A) and lesion lengths (B) in IR24 and IRBB5 rice inoculated with Xanthomonas oryzae pv. oryzae (Xoo) PH, PE, PH(pthXo1) and PE(pthXo1). Five rice leaves were inoculated by tip‐cutting; lesions were measured at 14 days post‐inoculation (dpi). One representative lesion of five is shown in (A). The mean values ± standard deviation (SD) (n = 5) are shown in (B). (C) OsSWEET11 expression in IR24 and IRBB5 inoculated with Xoo PH, PE, PH(pthXo1) and PE(pthXo1). The expression of OsSWEET11 was evaluated by quantitative real‐time reverse transcription‐polymerase chain reaction (qRT‐PCR) at 24h post‐infiltration (hpi). The mean values ± SD (n = 3) are shown. Experiments were repeated three times with similar results and a representative result is shown. Significant differences were detected using Student's t‐test at P < 0.05.
To further investigate the interplay between OsTFIIAγ1, TALEs and R/S gene targets, we introduced avrXa7 into Xoo PE and PH (Fig. S3; Table S1). The TALE AvrXa7 is a major virulence factor in Xoo that activates the expression of OsSWEET14, another known S gene in rice (Antony etal., 2010). Xoo PE and PH strains containing avrXa7 were inoculated to IR24 and IRBB5 rice, and lesions were observed at 14 dpi. BB lesions in IR24 rice inoculated with Xoo PH(avrXa7) were 6cm in length and dramatically shorter than those caused by PE(avrXa7); however, the lesion lengths in IRBB5 rice were less than 2cm (Fig. 3A,B). OsSWEET14 expression in rice was correlated with lesion length, e.g. higher levels of OsSWEET14 transcription were observed in IR24 rice inoculated with Xoo PE(avrXa7) than PH(avrXa7) (Fig. 3C). We also noticed significantly higher expression of OsSWEET14 in IRBB5 rice inoculated with Xoo PE(avrXa7) than PH(avrXa7) (Fig. 3C), suggesting that the endogenous copy of pthXo7 in Xoo PE may contribute to the enhanced OsSWEET14 expression that is activated by AvrXa7, perhaps via OsTFIIAγ1.
Figure 3.
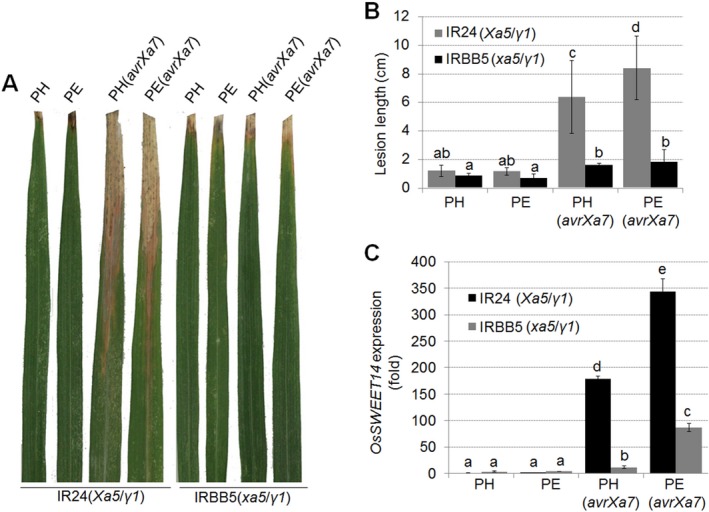
Effect of OsTFIIAγ1 on AvrXa7‐induced lesion length and AvrXa7‐activated OsSWEET14 expression. Disease phenotypes (A) and lesion length (B) in rice lines inoculated with Xanthomonas oryzae pv. oryzae (Xoo) PH, PE, PH(avrXa7) and PE(avrXa7). Five leaves were inoculated; lesions were measured at 14 days post‐inoculation (dpi). One representative lesion of five is shown in (A). The mean values ± standard deviation (SD) (n = 5) are shown in (B). (C) OsSWEET14 expression in IR24 and IRBB5 inoculated with Xoo PH, PE, PH(avrXa7) and PE(avrXa7). The expression of OsSWEET14 was evaluated by quantitative real‐time reverse transcription‐polymerase chain reaction (qRT‐PCR) at 24h post‐infiltration (hpi). The mean values ± SD (n = 3) are shown. Experiments were repeated three times with similar results and one representative result is shown. Significant differences were identified using Student's t‐test at P < 0.05.
We also investigated how an executor R gene contributes to BB resistance when OsTFIIAγ1 is induced. For these experiments, we transferred avrXa27, which is the activator of Xa27 (Gu etal., 2005), into PH and PE strains (Fig. S3). Xoo strains PE, PH, PE(avrXa27) and PH(avrXa27) were then infiltrated into rice using needleless syringes. The three rice lines selected were 78‐1‐5 (containing Xa27, Xa5 and OsTFIIAγ1) (Hu etal., 2007), DH (Xa27, xa5 and OsTFIIAγ1) (Gu etal., 2009) and IRBB5 (xa5 and OsTFIIAγ1). Xoo strains containing avrXa27, e.g. PH(avrXa27), PE(avrXa27) and PXO99A, triggered a typical hypersensitive response (HR) in 78‐1‐5 rice; however, IRBB5 rice exhibited a water‐soaked, compatible interaction in response to all strains (Fig. 4A). Surprisingly, the HR in DH rice (xa5/Xa27/γ1) was not as robust as in 78‐1‐5 rice (Xa5/Xa27/γ1), although PE(avrXa27) did promote an obvious HR in DH rice (Fig. 4A,B). The findings suggest potential interplay between Xa5 and OsTFIIAγ1 in 78‐1‐5 rice that fosters resistance and promotes HR that is mediated by the AvrXa27–XA27 interaction.
Figure 4.
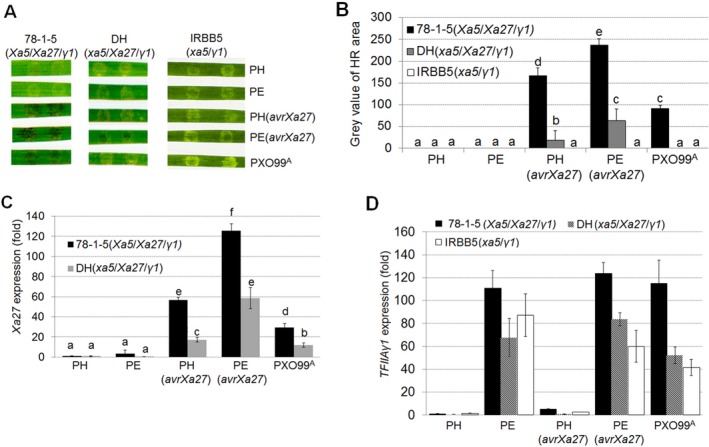
Effect of OsTFIIAγ1 on Xa27‐mediated resistance and Xa27 expression. (A) Phenotypes in rice lines 78‐1‐5, DH and IRBB5 infiltrated with Xanthomonas oryzae pv. oryzae (Xoo) strains PH, PE, PH(avrXa27), PE(avrXa27) and PXO99A. Each bacterial strain was infiltrated into three leaves with inoculated areas per leaf. One representative photograph was taken at 4 days post‐inoculation (dpi). (B) Quantification of the hypersensitive response (HR) in (A). Values represent the mean size of the grey (necrotic) regions ± standard deviation (SD) (n = 3). (C) Xa27 expression in rice lines 78‐1‐5 and DH inoculated with Xoo strains PH, PE, PH(avrXa27), PE(avrXa27) and PXO99A. The expression of Xa27 was evaluated by quantitative real‐time reverse transcription‐polymerase chain reaction (qRT‐PCR) at 24h post‐infiltration (hpi). (D) OsTFIIAγ1 expression levels in rice lines 78‐1‐5, DH and IRBB5 infiltrated with Xoo strains PH, PE, PH(avrXa27), PE(avrXa27) and PXO99A. The expression of OsTFIIAγ1 was evaluated by qRT‐PCR at 24 hpi. Values in (C) and (D) represent the mean ± SD (n = 3). Experiments were repeated three times with similar results, and one representative result is shown. Significant differences were detected using Student's t‐test at P < 0.05.
To build on these observations, we evaluated Xa27 expression in 78‐1‐5 and DH rice lines at 24h after infiltration with these bacterial strains. qRT‐PCR indicated that Xa27 expression was two‐ to three‐fold higher in 78‐1‐5 rice (Xa5/Xa27/γ1) than DH rice (xa5/Xa27/γ1) when inoculated with PH(avrXa27), PE(avrXa27) and PXO99A (Fig. 4B). The highest Xa27 expression levels were observed in 78‐1‐5 rice inoculated with PE(avrXa27) (Fig. 4B). These results suggest that Xa5 and OsTFIIAγ1 in 78‐1‐5 rice promote XA27‐mediated resistance; however, this resistance is attenuated in DH rice, potentially as a result of the absence of Xa5. We also observed elevated OsTFIIAγ1 expression in all three rice lines inoculated with Xoo PE, PE(avrXa27) and PXO99A (Fig. 4C); these three strains all encode a functional copy of pthXo7, a known activator of OsTFIIAγ1 expression.
The results presented above (Figs 2, 3, 4) lead us to speculate that OsTFIIAγ1 may partially compensate for the attenuated response to TALEs in xa5 rice (e.g. IRBB5 and DH). This hypothesis is based on several observations. First, Xoo PE, which contains an endogenous copy of pthXo7, activates OsTFIIAγ1 expression in IRBB5 (xa5) rice (Fig. S2C). Second, Xoo PE containing the three introduced TALEs (pthXo1, avrXa7 and avrXa27) induces higher levels of target gene expression (e.g OsSWEET11, OsSWEET14 and Xa27) than Xoo PH (Figs 2, 3, 4). Finally, in xa5 rice (IRBB5, DH), TALE target gene expression is lower than in Xa5 lines (IR24, 78‐1‐5) (Figs 2, 3, 4). However, we observed a modest increase in target gene expression in IRBB5 and DH rice inoculated with Xoo strains containing endogenous pthXo7, and this was correlated with an increase in OsTFIIAγ1 expression. Based on these observations, our next experiments were designed to determine whether OsTFIIAγ1 compensates for xa5, potentially by interacting with individual TALEs.
OsTFIIAγ1‐inactive rice plants are more resistant to BB
To directly test the hypothesis that OsTFIIAγ1 can compensate for the absence of Xa5, we constructed rice lines containing defective forms of OsTFIIAγ1. This was accomplished by editing OsTFIIAγ1 in IRBB5 using Clustered regularly interspaced short palin dromic repeats and CRISPR‐associated protein 9 (CRISPR/Cas9) technology (Zhou etal., 2014). Although the sequences of xa5 and OsTFIIAγ1 are similar, we designed a single‐guide RNA (sgRNA) sequence that specifically binds OsTFIIAγ1 (Fig. S4A,B, see Supporting Information). We generated 12 edited rice lines, and sequence analysis showed that they were genetically modified and homozygous (Fig. S4C). Two of these rice lines, designated TF1–2 and TF1–5, both had single nucleotide insertions (Fig. S4D) in OsTFIIAγ1 and were used in further studies.
To confirm that OsTFIIAγ1 was defective and not expressed as a functional protein in the TF1–2 and TF1–5 rice lines, the expression of the protein products was investigated. For these experiments, OsTFIIAγ1 and its defective derivatives, TF‐2 and TF‐5, were cloned in a yellow fluorescent protein (YFP) expression vector in which only functional proteins generate the fluorescence signal (Table S1). These YFP constructs were transiently expressed in Nicotiana benthamiana as described in Methods S3 (see Supporting Information). The OsTFIIAγ1::YFP fusion was clearly localized to the plasma membrane and nuclei; however, YFP was not expressed in tobacco transformed with TF‐2::YFP or TF‐5::YFP, which indicates that these modified forms of OsTFIIAγ1 were not expressed as functional proteins (Fig. S4E).
The impact of defective OsTFIIAγ1 on Xoo–rice interactions was investigated by inoculating PXO99A, PH(pthXo1) and PE(pthXo1) strains to TF1 rice lines and IRBB5 using the tip‐cutting method. Lesions in TF1 lines inoculated with wild‐type PXO99A were significantly smaller than those in IRBB5 rice (Fig. 5A,B). Interestingly, both PH(pthXo1) and PE(pthXo1) strains induced more severe symptoms in IRBB5 than in TF1 lines (Fig. 5A,B). No obvious differences in disease symptoms or lesion lengths were observed in TF1 lines inoculated with PH(pthXo1) or PE(pthXo1) strains.
Figure 5.
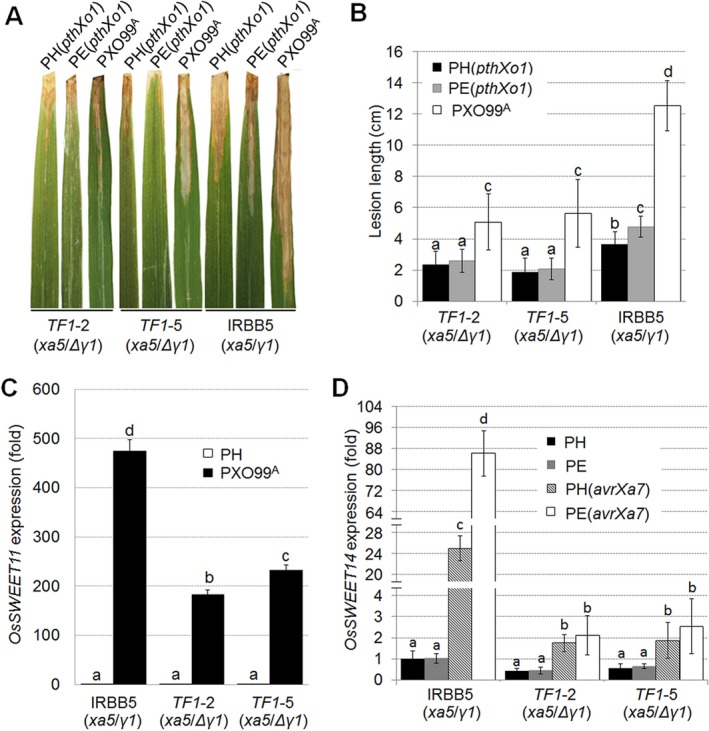
Disease phenotypes and S gene levels in IRBB5 (wild‐type OsTFIIAγ1), TF1‐2 and TF1‐5 rice (defective OsTFIIAγ1). Disease phenotypes (A) and lesion lengths (B) in TF1‐2, TF1‐5 and IRBB5 rice at 14 days post‐inoculation (dpi) with Xanthomonas oryzae pv. oryzae (Xoo) PH(pthXo1), PE(pthXo1) and PXO99A. A typical lesion (n = 4) is shown in (A); mean values ± standard deviation (SD) (n = 4) are shown in (B). (C) OsSWEET11 expression levels in IRBB5, TF1–2 and TF1–5 rice at 24h post‐infiltration (hpi) with Xoo PH and PXO99A. (D) OsSWEET14 expression in IRBB5, TF1–2 and TF1–5 rice inoculated with Xoo PH, PE, PH(avrXa7) and PE(avrXa7). Abbreviations: γ1, wild‐type OsTFIIAγ1; Δγ1, mutated OsTFIIAγ1. Values in (C) and (D) represent the mean ± SD (n = 3), and significant differences were detected using Student's t‐test at P < 0.05. One representative result of three biological replicates is shown.
Our results (Figs 2, 3, 4) suggest a complex interplay between OsTFIIAγ1 and the rice S genes encoded by OsSWEET11 and OsSWEET14. Thus, we examined the expression of these S genes in IRBB5 and TF1 rice inoculated with Xoo PXO99A, PH(avrXa7) and PE(avrXa7). Xoo PXO99A contains an endogenous copy of pthXo1, which activates OsSWEET11. When Xoo PXO99A was used as inoculum, the expression of OsSWEET11 was significantly lower in TF1 lines relative to IRBB5 rice (Fig. 5C). Thus, the defective OsTFIIAγ1 in TF1 lines had a direct, negative impact on expression of the S gene, OsSWEET11, and this was correlated with reduced virulence (Fig. 5A,B). We also evaluated OsSWEET14 expression in IRBB5 and TF1 rice inoculated with PH and PE containing avrXa7, which specifically activates this S gene. OsSWEET14 expression was significantly lower in TF1 lines inoculated with PH(avrXa7) and PE(avrXa7) than in IRBB5 rice (Fig. 5D). This is further evidence that the defective copy of OsTFIIAγ1 compromises the virulence of Xoo in the TF1 lines. Collectively, these results indicate that OsTFIIAγ1 promotes TALE‐mediated S gene transcription, and this function is more apparent in xa5 rice lines, such as IRBB5.
Xoo GX4 containing pthXo7 in trans causes disease in IRBB5 rice
OsTFIIAγ1 is activated by PthXo7 (Sugio etal., 2007) and can compensate for the absence of Xa5 in IRBB5 rice (Fig. 5C); thus, we speculated that the expression of pthXo7 in a Xoo strain lacking this gene might result in disease when inoculated to IRBB5 rice. Xoo GX4 was chosen for these experiments; this strain was non‐pathogenic when inoculated to IRBB5 rice (Fig. 1A) and did not induce OsTFIIAγ1 expression (Fig. 1B). Xoo GX4 was transformed with pHZWpthXo7 and the overproduction of PthXo7 was verified by immunoblotting (Fig. S3B). Xoo GX4 and GX4(pthXo7) were then inoculated to IR24 (Xa5/γ1), IRBB5 (xa5/γ1) and TF1‐2 (xa5/Δγ1). There was no obvious difference in lesion length or symptoms in IR24 rice inoculated with Xoo GX4 or GX4(pthXo7) (Fig. 6). Wild‐type Xoo GX4 did not cause disease in IRBB5 rice; however, Xoo GX4(pthXo7) gained the ability to induce small lesions (∼4.5cm) in IRBB5 (Fig. 6A,B). Intriguingly, both Xoo GX4 and GX4(pthXo7) were non‐pathogenic in TF1 rice (Fig. 6A,B), which lacks a functional copy of OsTFIIAγ1. These results suggest that the activation of OsTFIIAγ1 by PthXo7 contributes to lesion development in the IRBB5/GX4(pthXo7) interaction; we also speculate that OsTFIIAγ1 partially compensates for the lack of Xa5 in IRBB5 rice.
Figure 6.
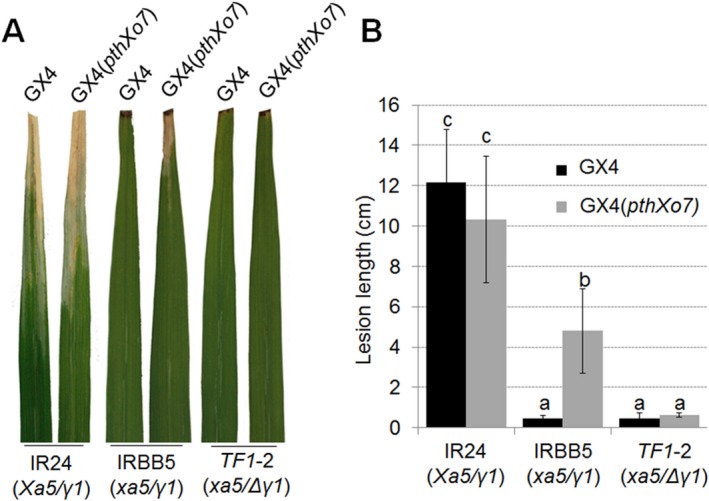
Disease symptoms in IR24, IRBB5 and TF1 rice lines inoculated with Xanthomonas oryzae pv. oryzae (Xoo) GX4 and GX4(pthXo7). Symptoms (A) and lesion lengths (B) at 14 days post‐inoculation (dpi) are shown. One representative leaf (n = 4) is shown in (A). Mean values ± standard deviation (SD) (n = 4) are shown in (B). One representative result from three biological repeats with similar results is shown. Abbreviations: γ1, OsTFIIAγ1; Δγ1, mutated OsTFIIAγ1.
TALEs interact with OsTFIIAγ, Xa5 and xa5 with different affinities
Bioinformatics analysis of OsTFIIAγ1, Xa5 and xa5 indicated that OsTFIIAγ1 shares the 39th valine residue with Xa5, but not with xa5 (Fig. S4A). Given that Xa5 interacts with several characterized TALEs (Yuan etal., 2016) and is highly similar to OsTFIIAγ1, we speculate that OsTFIIAγ1 also associates with a variety of TALEs. To address this hypothesis, we investigated the direct interaction of OsTFIIAγ1, Xa5 and xa5 with PthXo1, AvrXa7 and AvrXa27 using bimolecular fluorescence complementation (BiFC). For these experiments, OsTFIIAγ1, Xa5 and xa5 were fused with YN (N‐terminus of YFP), and PthXo1, AvrXa7 and AvrXa27 were fused with YC (C‐terminus of YFP), as detailed in Methods S3 and Table S1. Agrobacterium‐mediated transformation was used to introduce these constructs into N. benthamiana for transient expression and BiFC.
The co‐expression of Xa5::YN with the three YC‐tagged TALEs resulted in a fluorescent signal, indicating that PthXo1::YC, AvrXa27::YC and AvrXa7::YC form a complex with Xa5 in plant nuclei (Fig. 7A, see arrows). Similarly, the co‐expression of OsTFIIAγ1::YN (γ1::YN) with PthXo1::YC, AvrXa27::YC or AvrXa7::YC also resulted in fluorescent plant nuclei (Fig. 7A), which further supports the interaction of OsTFIIAγ1 with TALEs. However, the co‐expression of xa5::YN with PthXo1::YC, AvrXa27::YC or AvrXa7::YC resulted in weaker fluorescence relative to Xa5::YN and OsTFIIAγ1::YN (Fig. 7A). These results suggest that xa5 also associates with TALEs, but the affinity is much lower than that observed for Xa5 and OsTFIIAγ1.
Figure 7.
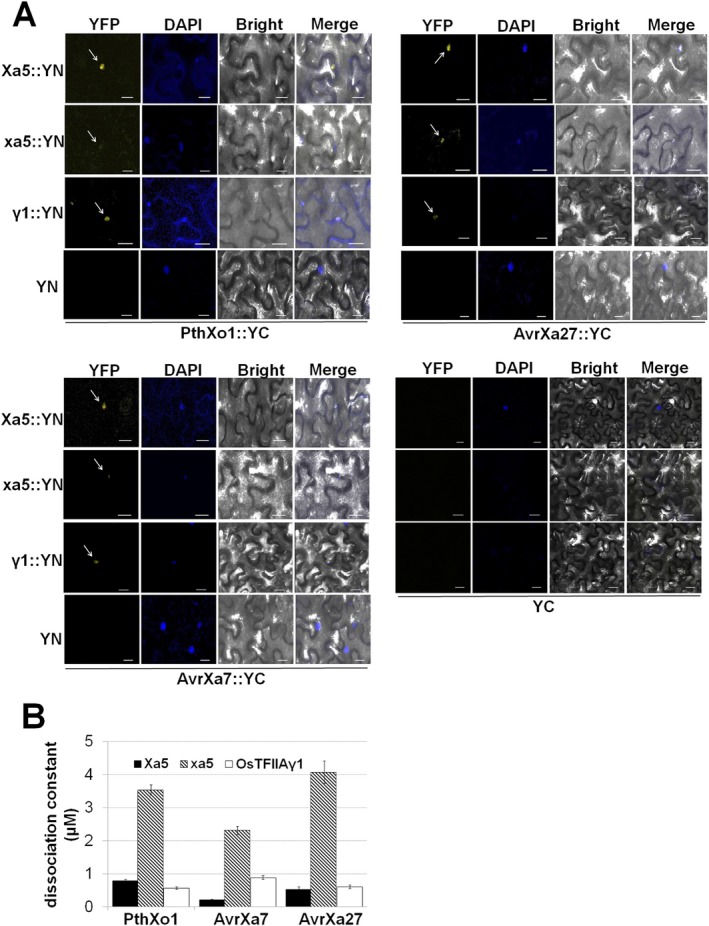
Interaction of Xa5, xa5 and OsTFIIAγ1 with PthXo1, AvrXa27 and AvrXa7 using biomolecular fluorescence complementation (BiFC) and microscale thermophoresis (MST). (A) BiFC visualization of the interaction between YN‐tagged TALEs and YC‐tagged OsTFIIAγ subunits in tobacco leaves. The fluorescence in the yellow fluorescent protein (YFP) panels occurred when YN‐labelled TALES interacted with YC‐labelled OsTFIIAγ subunits (see arrows). Controls included Nicotiana benthamiana transformed with empty YN vector and YC‐tagged TALEs, and empty YC vector and YN‐tagged OsTFIIAγ. Nuclei were stained by 4′,6‐diamidino‐2‐phenylindole (DAPI). Bars represent 20 μm. (B) Binding affinity of TALEs (PthXo1, AvrXa7 and AvrXa27) and labelled Xa5, xa5 and OsTFIIAγ1 as measured by MST. OsTFIIAγ subunits were labelled with the amine‐reactive, red fluorescent dye NT‐647 and mixed with 16 different concentrations of purified TALEs (Fig. S5, see Supporting Information). The affinity of the nine interactions is represented by the dissociation constant (K d). Values represent the means ± standard deviation (SD) (n = 3). Experiments were repeated twice with similar results.
To gain more information about the binding affinities of Xa5, xa5 and OsTFIIAγ1 and the three TALEs (PthXo1, AvrXa7 and AvrXa27), we used microscale thermophoresis (MST). Sixteen different concentrations of the purified TALE proteins (PthXo1, AvrXa7 and AvrXa27) were mixed with labelled OsTFIIAγ1, Xa5 and xa5, and subjected to MST (Fig. S5, see Supporting Information). When Xa5 and OsTFIIAγ1 were combined with PthXo1, AvrXa7 or AvrXa27, the K d values were relatively small (less than 1 μm; Fig. 7B), indicating strong affinity for the TALEs. Although xa5 interacted with the three TALES, the K d values were much higher (2.3–4.0 μm), indicating reduced affinity for the TALEs relative to Xa5 and OsTFIIAγ1 (Fig. 7B). Taken together, these results indicate that OsTFIIAγ1 and Xa5 strongly interact with PthXo1, AvrXa7 and AvrXa27 in vitro. Conversely, the interaction of these TALEs and xa5 is much weaker than observed with Xa5 and OsTFIIAγ1.
Discussion
A prerequisite for Pol II‐dependent transcription in eukaryotes is the recruitment of general transcription factors, e.g. TFIIA, TFIIB, TFIID, TFIIE, TFIIF and TFIIH, to the core promoter region of the target gene. This process begins with the recruitment of TFIIA and TFIID (Buratowski etal., 1989; Thomas and Chiang, 2006). TFIIA generally serves as a bridge between the TATA‐box binding protein and lobe B of TFIID, which facilitates TFIID binding to the TATA‐box (Louder etal., 2016). In Arabidopsis, TFIIA is composed of two subunits: the large subunit TFIIAαβ and the small subunit TFIIAγ (Li etal., 1999). Yuan etal. (2016) have recently demonstrated a role for TFIIAγ5 (OsTFIIAγ5, Xa5) in the Xoo–rice interaction. In their model, TALEs secreted by Xoo interact with Xa5 to facilitate activation of host susceptibility genes. It is also important to mention that xa5, a naturally occurring mutant allele of Xa5, confers a level of resistance to Xoo, which is presumably due to the reduced interaction between TALEs and the Pol II initiation complex when xa5 is present (Schornack etal., 2006, 2013).
In the current study, we examined the role of OsTFIIAγ1 in TALE‐mediated interactions. Sugio etal. (2007) have previously demonstrated that the TALE PthXo7 activates the expression of OsTFIIAγ1, which suggests a complex interplay between multiple transcriptional factors and TALEs, which can foster or impede the transcription of target R/S genes. In this study, we showed that the activation of OsTFIIAγ1 increased the TALE‐induced expression of target genes, especially in xa5‐containing rice (Figs 2C, 3C and 4C); thus, OsTFIIAγ1 plays a compensatory role in the absence of Xa5. It is important to note that the basal level of OsTFIIAγ1 expression is much lower than that of Xa5 in both seedlings and adult rice plants, regardless of pathogen infection (Figs 1D and S1). This observation is consistent with previous research (Iyer and McCouch, 2004) and supports the assumption that Xoo evolved or recruited PthXo7 to increase the transcription of OsTFIIAγ1, which can then promote TALE‐targeted R/S gene expression when Xa5 is mutated to xa5. Furthermore, our results showed that Xoo PE, which encodes pthXo7, induced a higher expression of target genes than Xoo PH when both strains contained the same set of introduced TALEs (Figs 2C and 3C). Taken together, these findings support the contention that the increased expression of OsTFIIAγ1 via PthXo7 can partially compensate for the attenuated expression of TALE‐targeted R/S genes in xa5 rice.
TALEs bind the EBEs of target genes near the TATA‐box, which generally activates transcription (Grau etal., 2013). Our findings indicate that TALEs interact with TFIIAγ subunits (Fig. 7) and form a complex with specific plant transcription factors. These TALE‐containing transcriptional complexes presumably promote target gene expression in planta. Recently, Yuan etal. (2016) used a yeast two‐hybrid system, and reported that 15 tested TALEs isolated from Xoo PXO99A interact with Xa5, but only PthXo1, Tal7a and Tal8a of the 15 TALEs interact with xa5. Interestingly, OsTFIIAγ1 did not interact with full‐length or truncated PthXo1 or the TFB site of 14 other Xoo TALEs (Yuan etal., 2016). As a result of the existence of a full set of transcription factors (Poss etal., 2013) and the self‐activating ability of TALEs in yeast, the yeast two‐hybrid system may not be the most robust system to test interactions between full‐length TALEs and OsTFIIAγ proteins. Thus, we used BiFC and MST assays to detect interactions in planta and in vitro, respectively. Both BiFC and MST indicated that three TALEs (PthXo1, AvrXa7 and AvrXa27) interacted with Xa5, xa5 and OsTFIIAγ1; however, the affinities of the three TALEs were significantly higher with Xa5 and OsTFIIAγ1 than with xa5 (Figs 7 and S5).
To better describe our observations and the potential roles of Xa5, xa5 and OsTFIIAγ1 in the activation of TALE‐targeted (R or S) genes, we present a model based on previous reports and the findings in our study (Fig. 8). In Xa5 rice, the expression of Xa5 is much higher than OsTFIIAγ1, which enables TALEs to function as transcription binding proteins (TBPs) in avirulent (Avr) or virulent (Vir) forms. The TALEs form a transcription complex together with Xa5 and other rice transcription factors, and this activates R or S gene expression, leading to disease resistance (Fig. 8A) or susceptibility (Fig. 8B). However, in xa5 rice, the reduced association of TALEs with xa5 results in a less effective transcription complex, leading to the suppression of TALE‐targeted gene expression. In this scenario, the BB resistance mediated by avirulent TALEs is neutralized (Fig. 8C) or the susceptibility mediated by virulent TALEs is suppressed and results in passive resistance (Fig. 8D), which is consistent with previous reports (Gu etal., 2009; Huang etal., 2016). An exception to this part of the model is the virulence of the PH(pthXo1) strain, which causes BB in xa5 rice and OsTFIIAγ1‐defective rice lines (TF1‐2 and TF1‐5) (Fig. 5). It should be noted that the PH(pthXo1) lesions are much smaller than those caused by PE(pthXo1) (Fig. 5A,B). The reason for this may be the weak affinity of xa5 with PthXo1 (Fig. 7), which is consistent with the findings reported by Yuan etal. (2016), who showed that xa5 interacts with PthXo1. Thus, PthXo1‐containing Xoo strains retain virulence and are compatible with xa5 rice.
Figure 8.
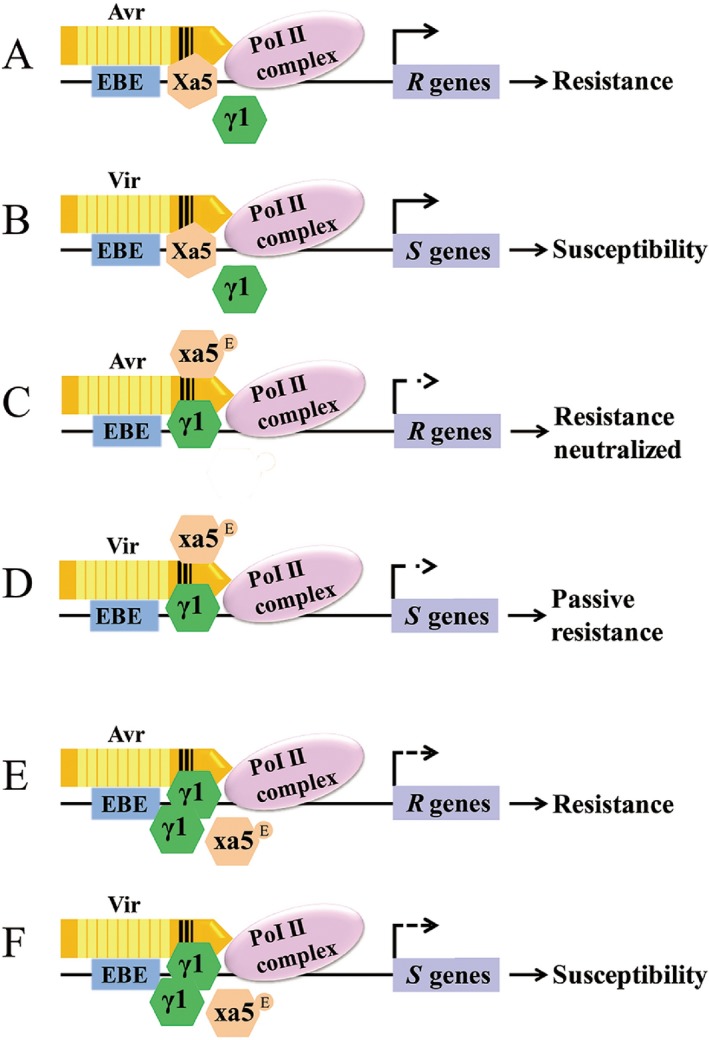
Theoretical model showing how OsTFIIAγ subunits (Xa5, OsTFIIAγ1 and xa5) modulate transcription activator‐like effector (TALE)‐activated host gene transcription and disease development. (A, B) Avirulent (Avr) and virulent (Vir) TALEs associate with Xa5 to form a transcription factor complex for the initiation of the expression of R (A) or S (B) genes. Through interaction with TALEs, the low expression level of OsTFIIAγ1 makes it play a minor role in the activation of R or S genes. (C, D) Rice lines are homozygous for xa5. The weaker affinity of the xa5–TALE association facilitates OsTFIIAγ1 binding (green hexagons); however, the relatively low level of OsTFIIAγ1 prevents TALE‐mediated R or S gene activation and leads to neutralized (C) or passive (D) resistance. (E, F) The elevated copy number of OsTFIIAγ1 when transcription is enhanced by PthXo7 (not shown). In this scenario, OsTFIIAγ1 plays a compensatory role for Xa5 in the xa5 background, and this leads to R or S gene expression and an enhanced level of resistance (E) or susceptibility (F). Arrows with a single dash (  ) indicate transcriptional inhibition and failure to express the target R or S gene. Arrows with multiple dashes (
) indicate transcriptional inhibition and failure to express the target R or S gene. Arrows with multiple dashes (  ) indicate elevated transcription, which results in an enhanced level of resistance or susceptibility. Abbreviations: Avr, avirulent TALE; EBE, effector‐binding element; Pol II, polymerase II; Vir, virulent TALE; γ1, OsTFIIAγ1.
) indicate elevated transcription, which results in an enhanced level of resistance or susceptibility. Abbreviations: Avr, avirulent TALE; EBE, effector‐binding element; Pol II, polymerase II; Vir, virulent TALE; γ1, OsTFIIAγ1.
To overcome xa5‐mediated resistance, Xoo strains, such as PXO99A, use PthXo7 to increase the transcription of OsTFIIAγ1 (Fig. 1C). Furthermore, it is important to mention that the affinities of OsTFIIAγ1 and Xa5 with the three tested TALE proteins were similar and much higher than the affinity of TALEs for xa5 (Fig. 7). Thus, we speculate that the binding of OsTFIIAγ1 can cause the formation of a transcriptional complex that facilitates TALE‐activated target gene expression. It is important to consider the elevated copy number of OsTFIIAγ1 that occurs when transcription is enhanced by PthXo7. In this scenario, OsTFIIAγ1 can play a compensatory role for Xa5 in the xa5 background, and this leads to R or S gene expression and some level of resistance (Fig. 8E) or susceptibility (Fig. 8F). In some cases, an R rice line may show elevated resistance to the pathogen carrying the cognate avirulent TALE (Fig. 8E). Conversely, an S rice line may show enhanced susceptibility to Xoo strains harbouring the associated virulent TALE (Fig. 8F). An example of the complex interplay between transcription factors can also be observed with Xoo GX4, which activates transcription of OsSWEET14 (Fig. S6, see Supporting Information) and is avirulent (incompatible) in xa5 rice (Fig. 6). However, Xoo GX4(pthXo7) was able to induce some disease symptoms in xa5 rice. Future studies are underway to clarify the association of TALEs with Xa5, xa5 andOsTFIIAγ1, and how TALEs specifically activate R or S gene expression.
In this study, we also generated an inactive form of OsTFIIAγ1 in xa5 rice using CRISPR/Cas9 technology (Fig. 4). The genetically modified TF1 rice lines retained resistance to GX4 (lacks pthXo7) and enhanced resistance to PXO99A and GX4(pthXo7) (Figs 5 and 6). These results suggest that TF1 lines will be valuable in future efforts to evade Xoo and reduce BB symptoms in rice breeding programmes.
Experimental procedures
Bacterial strains, plasmids and plant materials
The bacterial strains and plasmids used in this study are listed in Table S1. Escherichia coli strains were cultivated in Luria–Bertani medium at 37°C (Chong, 2001). Xanthomonas strains were cultured in nutrient broth (NB) or NB amended with agar at 28°C (Li etal., 2011). Agrobacterium was cultured in Luria–Bertani medium containing rifampicin at 28°C. Antibiotics were used at the following final concentrations: ampicillin, 100 µg/mL; rifampicin, 75 µg/mL; kanamycin, 25 µg/mL; spectinomycin, 50 µg/mL.
Indica rice IRBB5 (harbouring xa5) and IR24 were obtained from the International Rice Research Institute. DH, the rice line containing the two homozygous resistance genes Xa27 and xa5, was kindly provided by Zhongchao Yin (Gu etal., 2009). Rice line 78‐1‐5, containing Xa27, was obtained from Chaozu He (Hu etal., 2007). All rice plants were grown at 28°C in a glasshouse at Shanghai Jiao Tong University with a 12‐h photoperiod.
Plant infection and HR assays
HR assays were carried out as described previously (Hopkins etal., 1992). Briefly, three to five leaves of 3‐week‐old rice plants were infiltrated with bacterial suspensions [optical density at 600nm (OD600) = 0.6] using a needleless syringe. The quantification of HR was analysed by measuring the grey, necrotic regions in leaf tissue using Fiji software (Schindelin etal., 2012; Sekulska‐Nalewajko etal., 2016). For measurement of lesion lengths, three to five leaves from 6–8‐week‐old rice plants were inoculated with bacterial suspensions (OD600 = 0.6) using a tip‐cutting method (Kauffman etal., 1973). Both disease and HR assays were performed at least three times, and Student's t‐test was used for significance (P < 0.05).
RNA extraction and gene expression analysis
At 24h post‐infiltration (hpi), leaves of inoculated rice seedlings were selected and frozen in liquid nitrogen. For RNA extraction, frozen samples were pulverized, suspended in 1ml of RNAiso Plus (Takara, Dalian, China) and precipitated with isopropanol. RNA (1 μg) was then added for cDNA synthesis using EasyScript® One Step gDNA Removal and cDNA Synthesis Supermix (TransGen, Beijing, China). Synthesized cDNA (20 μL) was diluted to 100 μL and used for qRT‐PCR employing TransStart® Tip Green qPCR SuperMix (TransGen). qRT‐PCR was performed using an ABI 7500 quantitative PCR system. Fold change in gene expression was measured using the 2–△△Ct method (Livak and Schmittgen, 2001). The primer sequences are provided in Table S2 (see Supporting Information).
Modification of IRBB5 rice line using the CRISPR/Cas9 system
IRBB5 rice was genetically modified using CRISPR/Cas9 technology as described previously (Zhou etal., 2014). Briefly, the sgRNA targeted a 20‐bp region (5′‐GACCATGTCGTCCAGCGTGT‐3′, minus strand) in the first exon of OsTFIIAγ1; this sequence was driven by the rice U6.2 promoter. The sgRNA and Cas9 constructs were transferred into IRBB5 callus cells using Agrobacterium‐mediated transformation (Hiei etal., 1994), which was a service by Wuhan Biorun Bio‐Tech Co. Ltd., Wuhan, China. Genomic DNA was isolated from leaves of transgenic rice using the Cetyltrimethyl Ammonium Bromide method (Zhou etal., 2014). Genomic DNA was employed for PCR amplification of the OsTFIIAγ1 region using the primer pair TFIIAγ1‐YN‐F(XbaI)/TFIIAγ1‐test‐R (Table S2). The resulting amplicons were cloned into the pMD18‐T vector (Takara) using the TA cloning method; clones with confirmed inserts were then sequenced.
BiFC experiments
For BiFC experiments, Xa5, xa5 and OsTFIIAγ1 were amplified using the primer pairs Xa5‐YN‐F(XbaI)/Xa5‐YN‐R(SmaI) and TFIIAγ1‐YN‐F(XbaI)/TFIIAγ1‐YN‐R(SmaI). Xa5, xa5 and OsTFIIAγ1 were inserted into the N‐terminus of the YFP (YN) vector using XbaI and SmaI (see Methods S3), resulting in Xa5::YN, xa5::YN and OsTFIIAγ1::YN, respectively.
BiFC assays were performed as described previously with minor modifications (Walter etal., 2004). Briefly, Agrobacterium GV3101 strains containing YN and YC constructs were cultured to OD600 = 1.5, harvested by centrifugation and resuspended in inducing buffer (10mm MgCl2, 0.2mm acetosyringone and 200 mm 2‐(4‐Morpholino) ethanesulfonic acid (MES), pH 5.6) to OD600 = 1.0. Buffer‐supplemented Agrobacterium strains containing the YN constructs (Xa5::YN, xa5::YN and OsTFIIAγ1::YN) and YC constructs (pthXo1::YC, avrXa7::YC and avrXa27::YC) were mixed in a ratio of 1 : 1 and incubated at 25°C for 1h. Controls included N. benthamiana transformed with empty YN vector and YC‐tagged TALE, and empty YC vector and YN‐tagged OsTFIIAγ. The induced Agrobacterium mixtures were infiltrated into N. benthamiana leaves for transient expression. At 48 hpi, fluorescence was imaged with a confocal laser fluorescence microscope and 4′,6‐diamidino‐2‐phenylindole (DAPI, 100 μg/mL) was used for nuclei staining.
MST experiments
To assess interactions between TALEs and OsTFIIAγ subunits, MST was performed as described previously (Cai etal., 2017; Wienken etal., 2010). His‐tagged TALEs and OsTFIIAγ subunits were purified from the pET30a constructs (Methods S4, see Supporting Information) with Ni‐NTA His‐Bind resin. The purified protein buffer was exchanged for MST buffer [50mm Tris‐HCl (pH 7.8) with 150mm NaCl, 10mm MgCl2 and 0.05% Tween‐20], and protein concentrations were determined using the Bradford method. OsTFIIAγ proteins were labelled with the amine‐reactive, red fluorescent dye NT‐647 using the Monolith NT.115 Protein Labeling Kit as recommended by the manufacturer (NanoTemper Technologies, Germany), and then eluted with MST buffer. Sixteen different concentrations of TALE proteins starting from 10 μm were made by two‐fold serial dilutions. Different concentrations of TALEs were mixed with 1 μm labelled OsTFIIAγ proteins in a 1 : 1 (v/v) ratio. After a 10‐min incubation at room temperature, the samples were loaded into silica capillaries. Measurements were performed at 25°C using 35% LED power and 80% IR laser power. MST was performed with a Monolith NT.115T (NanoTemper Technologies), and data were analysed using NTAnalysis v. 1.5.41.
Author contributions
W.M. and G.C. designed the experiments; W.M. performed the experiments; L.Z., Z.J. and X.X. contributed materials; Z.X. provided technical assistance for sgRNA design; Y.Y. isolated Xoo strains from China rice fields; W.M. and G.C. wrote the paper; J.R.A. revised the manuscript; all authors read, commented on and approved the manuscript.
Conflicts of interest
The authors declare no conflicts of interest.
Supporting information
Additional supporting information may be found in the online version of this article at the publisher's web‐site
Fig. S1 Basal expression level of OsTFIIAγ1 and Xa5/xa5 in IR24 and IRBB5 rice lines as analysed by real‐time= reverse transcription‐polymerase chain reaction (RT‐PCR). OSActin was used as an internal control. NTC, no template control.
Fig. S2 Functional map showing the two tal‐deletion mutants [Xanthomonas oryzae pv. oryzae (Xoo) PE and PH] derived from PXO99A and the expression of OsTFIIAγ1.
Fig. S3 Western blot analysis of transcription activator‐like effector (TALE) production in various Xanthomonas oryzae pv. oryzae (Xoo) strains.
Fig. S4 Modification of OsTFIIAγ1 in IRBB5 rice by Clustered regularly interspaced short palin dromic repeats and CRISPR‐associated protein 9 editing.
Fig. S5 The affinity of PthXo1, AvrXa7 and AvrXa27 for Xa5, xa5 and OsTFIIAγ using microscale thermophoresis (MST).
Fig. S6 Xanthomonas oryzae pv. oryzae (Xoo) strain GX4 induces the expression of OsSWEET14, but not OsSWEET11, in IR24 rice.
Methods S1 DNA manipulation and plasmid construction.
Methods S2 Immunoblotting assays.
Methods S3 Assembly of yellow fluorescent protein (YFP)‐tagged constructs.
Methods S4 Protein production and purification.
Table S1 Strains and plasmids used in this study.
Table S2 Primers used in this study.
Acknowledgements
We thank Dr Chaozu He (Hainan University, Haikou, China) for providing rice cv. 78‐1‐5, Dr Zhongchao Yin (National University of Singapore, Singapore) for providing rice line DH, Dr Wei Qian (Chinese Academy of Sciences, Beijing, China) for assistance with the MST experiments and Dr Bing Yang (Iowa State University, Ames, IA, USA) for providing the CRISPR/Cas9 system. This study was supported by the National Key Research and Development Program of China (2016YFD0100601), the National Natural Science Foundation of China (31471742, 31230095) and the National Transgenic Major Program (2016ZX08001‐002).
References
- Antony, G., Zhou, J. H., Huang, S., Li, T., Liu, B., White, F. and Yang, B. (2010) Rice xa13 recessive resistance to bacterial blight is defeated by induction of disease susceptibility gene Os-11N3. Plant Cell. 22, 3864–3876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boch, J. and Bonas, U. (2010) Xanthomonas AvrBs3 family‐type III effectors: discovery and function. Annu. Rev. Phytopathol. 48, 419–436. [DOI] [PubMed] [Google Scholar]
- Boch, J., Scholze, H., Schornack, S., Landgraf, A., Hahn, S., Kay, S., Lahaye, T., Nickstadt, A. and Bonas, U. (2009) Breaking the code of DNA binding specificity of TAL‐type III effectors. Science, 326, 1509–1512. [DOI] [PubMed] [Google Scholar]
- Boch, J., Bonas, U. and Lahaye, T. (2014) TAL effectors–pathogen strategies and plant resistance engineering. New Phytol. 204, 823–832. [DOI] [PubMed] [Google Scholar]
- Buratowski, S., Hahn, S., Guarente, L. and Sharp, P.A. (1989) Five intermediate complexes in transcription initiation by RNA polymerase II. Cell, 56, 549–561. [DOI] [PubMed] [Google Scholar]
- Cai, L., Cao, Y., Xu, Z., Ma, W., Zakria, M., Zou, L., Cheng, Z. and Chen, G. (2017) A transcription activator‐like effector Tal7 of Xanthomonas oryzae pv. oryzicola activates rice gene Os09g29100 to suppress rice immunity. Sci. Rep. 7, 5089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen, L.Q. (2014) SWEET sugar transporters for phloem transport and pathogen nutrition. New Phytol. 201, 1150–1155. [DOI] [PubMed] [Google Scholar]
- Chen, L.‐Q., Hou, B.‐H., Lalonde, S., Takanaga, H., Hartung, M.L., Qu, X.‐Q., Guo, W.‐J., Kim, J.‐G., Underwood, W., Chaudhuri, B., Chermak, D., Antony, G., White, F.F., Somerville, S.C., Mudgett, M.B. and Frommer, W.B. (2010) Sugar transporters for intercellular exchange and nutrition of pathogens. Nature, 468, 527–532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chong, L. (2001) Molecular cloning – a laboratory manual, 3rd edition. Science, 292, 446. [Google Scholar]
- Chu, Z., Yuan, M., Yao, J., Ge, X., Yuan, B., Xu, C., Li, X. , Fu, B. , Li, Z. , Bennetzen, J.L. , Zhang, Q. and Wang, S. (2006) Promoter mutations of an essential gene for pollen development result in disease resistance in rice. Genes Dev. 20, 1250–1255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Expósito‐Rodríguez, M., Borges, A.A., Borges‐Pérez, A. and Pérez, J.A. (2011) Gene structure and spatiotemporal expression profile of tomato genes encoding YUCCA‐like flavin monooxygenases: the ToFZY gene family. Plant Physiol. Biochem. 49, 782–791. [DOI] [PubMed] [Google Scholar]
- Grau, J., Wolf, A., Reschke, M., Bonas, U., Posch, S. and Boch, J. (2013) Computational predictions provide insights into the biology of TAL effector target sites. PLoS Comput. Biol. 9, e1002962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu, K., Yang, B., Tian, D., Wu, L., Wang, D., Sreekala, C., Yang, F., Chu, Z., Wang, G.‐L., White, F.F. and Yin, Z. (2005) R gene expression induced by a type‐III effector triggers disease resistance in rice. Nature, 435, 1122–1125. [DOI] [PubMed] [Google Scholar]
- Gu, K., Tian, D., Qiu, C. and Yin, Z. (2009) Transcription activator‐like type III effector AvrXa27 depends on OsTFIIAgamma5 for the activation of Xa27 transcription in rice that triggers disease resistance to Xanthomonas oryzae pv. oryzae . Mol. Plant Pathol. 10, 829–835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hiei, Y., Ohta, S., Komari, T. and Kumashiro, T. (1994) Efficient transformation of rice (Oryza sativa L.) mediated by Agrobacterium and sequence analysis of the boundaries of the T‐DNA. Plant J. 6, 271–282. [DOI] [PubMed] [Google Scholar]
- Hoiby, T., Zhou, H., Mitsiou, D.J. and Stunnenberg, H.G. (2007) A facelift for the general transcription factor TFIIA. Biochim. Biophys. Acta, 1769, 429–436. [DOI] [PubMed] [Google Scholar]
- Hopkins, C.M., White, F.F., Choi, S.H., Guo, A. and Leach, J.E. (1992) Identification of a family of avirulence genes from Xanthomonas oryzae pv. oryzae . Mol. Plant–Microbe Interact. 5, 451–459. [DOI] [PubMed] [Google Scholar]
- Hu, J., Zhang, Y., Qian, W. and He, C. (2007) Avirulence gene and insertion element‐based RFLP as well as RAPD markers reveal high levels of genomic polymorphism in the rice pathogen Xanthomonas oryzae pv. oryzae . Syst. Appl. Microbiol. 30, 587–600. [DOI] [PubMed] [Google Scholar]
- Huang, R., Hui, S., Zhang, M., Li, P., Xiao, J., Li, X., Yuan, M. and Wang, S. (2017) A conserved basal transcription factor is required for the function of diverse TAL effectors in multiple plant hosts. Front. Plant Sci. 8, 1919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang, S., Antony, G., Li, T., Liu, B., Obasa, K., Yang, B. and White, F.F. (2016) The broadly effective recessive resistance gene xa5 of rice is a virulence effector‐dependent quantitative trait for bacterial blight. Plant J. 86, 186–194. [DOI] [PubMed] [Google Scholar]
- Hutin, M., Perez‐Quintero, A.L., Lopez, C. and Szurek, B. (2015a) MorTAL Kombat: the story of defense against TAL effectors through loss‐of‐susceptibility. Front. Plant Sci. 6, 535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutin, M., Sabot, F., Ghesquiere, A., Koebnik, R. and Szurek, B. (2015b) A knowledge‐based molecular screen uncovers a broad‐spectrum OsSWEET14 resistance allele to bacterial blight from wild rice. Plant J. 84, 694–703. [DOI] [PubMed] [Google Scholar]
- Iyer, A.S. and McCouch, S.R. (2004) The rice bacterial blight resistance gene xa5 encodes a novel form of disease resistance. Mol. Plant–Microbe Interact. 17, 1348–1354. [DOI] [PubMed] [Google Scholar]
- Ji, Z., Ji, C., Liu, B., Zou, L., Chen, G. and Yang, B. (2016) Interfering TAL effectors of Xanthomonas oryzae neutralize R‐gene‐mediated plant disease resistance. Nat. Commun. 7, 13 435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang, G.‐H., Xia, Z.‐H., Zhou, Y.‐L., Wan, J., Li, D.‐Y., Chen, R.‐S., Zhai, W.‐X. and Zhu, L.‐H. (2006) Testifying the rice bacterial blight resistance gene xa5 by genetic complementation and further analyzing xa5 (Xa5) in comparison with its homolog TFIIAgamma1. Mol. Genet. Genomics, 275, 354–366. [DOI] [PubMed] [Google Scholar]
- Kauffman, H.E., Reddy, A.P.K., Hsieh, S.P.Y. and Merca, S.D. (1973) Improved technique for evaluating resistance of rice varieties to Xanthomonas oryzae . Plant Dis. Rep. 56, 537–541. [Google Scholar]
- de Lange, O. D., Wolf, C., Dietze, J., Elsaesser, J., Morbitzer, R. and Lahaye, T. (2014) Programmable DNA‐binding proteins from Burkholderia provide a fresh perspective on the TALE‐like repeat domain. Nucleic Acids Res. 42, 7436–7449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li, Y.F., Le Gourierrec, J., Torki, M., Kim, Y.J., Guerineau, F. and Zhou, D.X. (1999) Characterization and functional analysis of Arabidopsis TFIIA reveal that the evolutionarily unconserved region of the large subunit has a transcription activation domain. Plant Mol. Biol. 39, 515–525. [DOI] [PubMed] [Google Scholar]
- Li, Y.‐R., Zou, H.‐S., Che, Y.‐Z., Cui, Y.‐P., Guo, W., Zou, L.‐F., Chatterjee, S., Biddle, E.M., Yang, C.‐H. and Chen, G.‐Y. (2011) A novel regulatory role of HrpD6 in regulating hrp‐hrc‐hpa genes in Xanthomonas oryzae pv. oryzicola . Mol. Plant–Microbe Interact. 24, 1086–1101. [DOI] [PubMed] [Google Scholar]
- Liu, Q., Yuan, M., Zhou, Y., Li, X., Xiao, J. and Wang, S. (2011) A paralog of the MtN3/saliva family recessively confers race‐specific resistance to Xanthomonas oryzae in rice. Plant Cell Environ. 34, 1958–1969. [DOI] [PubMed] [Google Scholar]
- Livak, K.J. and Schmittgen, T.D. (2001) Analysis of relative gene expression data using real‐time quantitative PCR and the 2(T)(–Delta Delta C) method. Methods, 25, 402–408. [DOI] [PubMed] [Google Scholar]
- Louder, R.K., He, Y., López‐Blanco, J.R., Fang, J., Chacón, P. and Nogales, E. (2016) Structure of promoter‐bound TFIID and model of human pre‐initiation complex assembly. Nature, 531, 604–609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mak, A.N., Bradley, P., Bogdanove, A.J. and Stoddard, B.L. (2013) TAL effectors: function, structure, engineering and applications. Curr. Opin. Struct. Biol. 23, 93–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moscou, M.J. and Bogdanove, A.J. (2009) A simple cipher governs DNA recognition by TAL effectors. Science, 326, 1501. [DOI] [PubMed] [Google Scholar]
- Poss, Z.C., Ebmeier, C.C. and Taatjes, D.J. (2013) The mediator complex and transcription regulation. Crit. Rev. Biochem. Mol. Biol. 48, 575–608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Read, A.C., Rinaldi, F.C., Hutin, M., He, Y.Q., Triplett, L.R. and Bogdanove, A.J. (2016) Suppression of Xo1‐mediated disease resistance in rice by a truncated, non‐DNA‐binding TAL effector of Xanthomonas oryzae . Front. Plant Sci. 7, 1516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romer, P., Hahn, S., Jordan, T., Strauss, T., Bonas, U. and Lahaye, T. (2007) Plant pathogen recognition mediated by promoter activation of the pepper Bs3 resistance gene. Science, 318, 645–648. [DOI] [PubMed] [Google Scholar]
- Schindelin, J., Arganda‐Carreras, I., Frise, E., Kaynig, V., Longair, M., Pietzsch, T., Preibisch, S., Rueden, C., Saalfeld, S., Schmid, B., Tinevez, J.‐Y., White, D.J., Hartenstein, V., Eliceiri, K., Tomancak, P. and Cardona, A. (2012) Fiji: an open‐source platform for biological‐image analysis. Nat. Methods, 9, 676–682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schornack, S., Meyer, A., Romer, P., Jordan, T. and Lahaye, T. (2006) Gene‐for‐gene‐mediated recognition of nuclear‐targeted AvrBs3‐like bacterial effector proteins. J. Plant Physiol. 163, 256–272. [DOI] [PubMed] [Google Scholar]
- Schornack, S., Moscou, M.J., Ward, E.R. and Horvath, D.M. (2013) Engineering plant disease resistance based on TAL effectors. Annu. Rev. Phytopathol. 51, 383–406. [DOI] [PubMed] [Google Scholar]
- Sekulska‐Nalewajko, J., Gocławski, J., Chojak‐Koźniewska, J. and Kuźniak, E. (2016) Automated image analysis for quantification of reactive oxygen species in plant leaves. Methods, 109, 114–122. [DOI] [PubMed] [Google Scholar]
- Streubel, J., Pesce, C., Hutin, M., Koebnik, R., Boch, J. and Szurek, B. (2013) Five phylogenetically close rice SWEET genes confer TAL effector‐mediated susceptibility to Xanthomonas oryzae pv. oryzae . New Phytol. 200, 808–819. [DOI] [PubMed] [Google Scholar]
- Sugio, A., Yang, B., Zhu, T. and White, F.F. (2007) Two type III effector genes of Xanthomonas oryzae pv. oryzae control the induction of the host genes OsTFIIAgamma1 and OsTFX1 during bacterial blight of rice. Proc. Natl. Acad. Sci. USA, 104, 10 720–10 725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas, M.C. and Chiang, C.M. (2006) The general transcription machinery and general cofactors. Crit. Rev. Biochem. Mol. Biol. 41, 105–178. [DOI] [PubMed] [Google Scholar]
- Tian, D., Wang, J., Zeng, X., Gu, K., Qiu, C., Yang, X., Zhou, Z., Goh, M., Luo, Y., Murata‐Hori, M., White, F.F. and Yin, Z. (2014) The rice TAL effector‐dependent resistance protein XA10 triggers cell death and calcium depletion in the endoplasmic reticulum. Plant Cell, 26, 497–515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walter, M., Chaban, C., Schutze, K., Batistic, O., Weckermann, K., Nake, C., Blazevic, D.,Grefen, C.,Schumacher, K.,Oeching, C.,Harter, K. and Kudla, J. (2004) Visulization of protein interactions in living cells using bimolecular fluorescence complementation. Plant J. 40, 428–438. [DOI] [PubMed] [Google Scholar]
- Wang, C., Zhang, X., Fan, Y., Gao, Y., Zhu, Q., Zheng, C., Qin, T., Li, Y., Che, J., Zhang, M., Yang, B., Liu, Y. and Zhao, K. (2015) XA23 is an executor R protein and confers broad‐spectrum disease resistance in rice. Mol. Plant. 8, 290–302. [DOI] [PubMed] [Google Scholar]
- Wienken, C.J., Baaske, P., Rothbauer, U., Braun, D. and Duhr, S. (2010) Protein‐binding assays in biological liquids using microscale thermophoresis. Nat. Commun. 1, 100. [DOI] [PubMed] [Google Scholar]
- Wu, L., Goh, M.L., Sreekala, C. and Yin, Z. (2008) XA27 depends on an amino‐terminal signal‐anchor‐like sequence to localize to the apoplast for resistance to Xanthomonas oryzae pv oryzae . Plant Physiol. 148, 1497–1509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang, B., Sugio, A. and White, F.F. (2006) Os8N3 is a host disease‐susceptibility gene for bacterial blight of rice. Proc. Natl. Acad. Sci. USA, 103, 10 503–10 508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan, M., Ke, Y., Huang, R., Ma, L., Yang, Z., Chu, Z., Xiao, J., Li, X. and Wang, S. (2016) A host basal transcription factor is a key component for infection of rice by TALE‐carrying bacteria. eLife, 5, e 19605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang, J., Yin, Z. and White, F. (2015) TAL effectors and the executor R genes. Front. Plant Sci. 6, 641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou, H., Liu, B., Weeks, D.P., Spalding, M.H. and Yang, B. (2014) Large chromosomal deletions and heritable small genetic changes induced by CRISPR/Cas9 in rice. Nucleic Acids Res. 42, 10 903–10 914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou, J., Peng, Z., Long, J., Sosso, D., Liu, B., Eom, J.‐S., Huang, S., Liu, S., Vera Cruz, C., Frommer, W.B., White, F.F. and Yang, B. (2015) Gene targeting by the TAL effector PthXo2 reveals cryptic resistance gene for bacterial blight of rice. Plant J. 82, 632–643. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional supporting information may be found in the online version of this article at the publisher's web‐site
Fig. S1 Basal expression level of OsTFIIAγ1 and Xa5/xa5 in IR24 and IRBB5 rice lines as analysed by real‐time= reverse transcription‐polymerase chain reaction (RT‐PCR). OSActin was used as an internal control. NTC, no template control.
Fig. S2 Functional map showing the two tal‐deletion mutants [Xanthomonas oryzae pv. oryzae (Xoo) PE and PH] derived from PXO99A and the expression of OsTFIIAγ1.
Fig. S3 Western blot analysis of transcription activator‐like effector (TALE) production in various Xanthomonas oryzae pv. oryzae (Xoo) strains.
Fig. S4 Modification of OsTFIIAγ1 in IRBB5 rice by Clustered regularly interspaced short palin dromic repeats and CRISPR‐associated protein 9 editing.
Fig. S5 The affinity of PthXo1, AvrXa7 and AvrXa27 for Xa5, xa5 and OsTFIIAγ using microscale thermophoresis (MST).
Fig. S6 Xanthomonas oryzae pv. oryzae (Xoo) strain GX4 induces the expression of OsSWEET14, but not OsSWEET11, in IR24 rice.
Methods S1 DNA manipulation and plasmid construction.
Methods S2 Immunoblotting assays.
Methods S3 Assembly of yellow fluorescent protein (YFP)‐tagged constructs.
Methods S4 Protein production and purification.
Table S1 Strains and plasmids used in this study.
Table S2 Primers used in this study.


