Summary
As a typical foliar pathogen, appressorium formation and penetration are critical steps in the infection cycle of Magnaporthe oryzae. Because appressorium formation and penetration are closely co‐regulated with the cell cycle, and Cdc14 phosphatases have an antagonistic relationship with cyclin‐dependent kinases (CDKs) on proteins related to mitotic exit and cytokinesis, in this study, we functionally characterized the MoCDC14 gene in M. oryzae. The Mocdc14 deletion mutant showed significantly reduced growth rate and conidiation. It was also defective in septum formation and nuclear distribution. Septation was irregular in Mocdc14 hyphae and hyphal compartments became multi‐nucleate. Mutant conidia often showed incomplete septa or lacked any septum. During appressorium formation, the septum delimiting appressoria from the rest of the germ tubes was often formed far away from the neck of the appressoria or not formed at all. Unlike the wild‐type, some mutant appressoria had more than one nucleus at 24 h. In addition to appressoria, melanization occurred on parts of the germ tubes and conidia, depending on the irregular position of the appressorium‐delimiting septum. The Mocdc14 mutant was also defective in glycogen degradation during appressorium formation and appressorial penetration of intact plant cells. Similar defects in septum formation, melanization and penetration were observed with appressorium‐like structures formed at hyphal tips in the Mocdc14 mutant. Often a long fragment of mutant hyphae was melanized, together with the apical appressorium‐like structures. These results indicate that MoCDC14 plays a critical role in septation, nuclear distribution and pathogenesis in M. oryzae, and correct septum formation during conidiogenesis and appressorium formation requires the MoCdc14 phosphatase.
Keywords: cell cycle, conidiation, cytokinesis, plant infection, Pyricularia oryzae, rice blast
Introduction
Magnaporthe oryzae (synonym: Pyricularia oryzae) is the causal agent of rice blast, one of the most important diseases threatening rice production worldwide, and a model system for the study of fungal–plant interactions (Dean et al., 2012; Ebbole, 2007; Xu et al., 2007). Although it also produces single‐celled microconidia (Kato et al., 1994; Zhang et al., 2014), pyriform macroconidia (commonly referred to as conidia) are the primary inoculum and main source of disease dissemination (Ebbole, 2007; Wilson and Talbot, 2009). Young conidia are single‐celled and contain one nucleus migrating from the conidiophore. After one round of mitosis and formation of a septum, developing conidia become two‐celled. The tip compartment will then undergo one more round of mitosis and cytokinesis to form mature, three‐celled conidia with one nucleus in each compartment (Howard and Valent, 1996; Liu et al., 2010). Various mutants with conidiogenesis defects have been identified in M. oryzae, and a number of them, such as the con1, con7, com1, cdc15 and chs1 mutants, are defective in plant infection (Goh et al., 2011; Kong et al., 2012; Shi and Leung, 1995; Yang et al., 2010).
Infection by M. oryzae begins with the attachment and germination of conidia on plant surfaces (Wilson and Talbot, 2009). Germ tubes emerging from conidia undergo one round of mitosis and develop dome‐shaped appressoria at the tip (Osés‐Ruiz et al., 2016). In M. oryzae, cytokinesis is spatially uncoupled from mitosis during appressorium formation (Saunders et al., 2010b). A special septum is formed at the neck of a developing appressorium after one daughter nucleus migrates into appressorium (Osés‐Ruiz et al., 2016). When appressoria mature, conidial compartments and germ tubes undergo autophagic cell death (Liu et al., 2007, 2012; Veneault‐Fourrey et al., 2006). Carbon storage in conidia is mobilized and degraded in appressoria to generate enormous turgor pressure, which is used by M. oryzae to physically penetrate the cuticle and underlying plant cells (Ebbole, 2007; Saunders et al., 2010a; Wilson and Talbot, 2009). Appressorium‐like structures can also be formed at the hyphal tips, which also involves the formation of a special septum at the neck for delimitation (Kong et al., 2013). Both appressoria formed by germ tubes and appressorium‐like structures formed at hyphal tips are heavily melanized, which is necessary for the generation of high intracellular turgor pressure and plant penetration (Howard and Valent, 1996; Martin‐Urdiroz et al., 2016; Wilson and Talbot, 2009). The septum that separates appressoria or appressorium‐like structures from the rest of the germ tubes or hyphae must be complete and have no septal pore when intracellular turgor builds up. Indeed, melanization occurs at this special septum, together with appressoria and appressorium‐like structures, but not in the rest of the germ tubes or hyphae.
In eukaryotic organisms, reversible phosphorylation of proteins by protein kinases and phosphatases is known to regulate various growth and developmental processes. As a model for the study of fungal–plant interactions, M. oryzae has been extensively studied with regard to the molecular mechanisms regulating plant infection processes. A number of protein kinases involved in well‐conserved signal transduction pathways have been shown to regulate surface recognition, appressorium formation, penetration and invasive hyphae in this important pathogen (Li et al., 2012; Zhao et al., 2007). Appressorium morphogenesis is also tightly regulated by the cell cycle in M. oryzae (Saunders et al., 2010a, 2010b; Veneault‐Fourrey et al., 2006). Premitotic DNA replication and one round of mitosis are essential for appressorium formation. Hydroxyl urea treatment before germination or expression of a temperature‐sensitive NimA kinase blocks appressorium development (Saunders et al., 2010a). Expression of a temperature‐sensitive allele of the SEP1 kinase gene that is involved in the determination of the position and frequency of cell division results in increased septation and nuclear division in the germ tubes and in defects in appressorium formation and plant infection (Saunders et al., 2010b). Protein kinases involved in cell cycle progression also probably play pivotal roles in the differentiation and growth of bulbous invasive hyphae inside plant cells in M. oryzae (Goh et al., 2011; Perez‐Martin et al., 2006; Saunders et al., 2010a). Similar observations of distinct cell cycle regulation during infectious growth have been observed in Ustilago maydis and Fusarium graminearum (Liu et al., 2015; Perez‐Martin et al., 2006; Sgarlata and Perez‐Martin, 2005).
Cdc14 belongs to a family of highly conserved dual‐specificity phosphatases that are present in fungi and animals, but absent in plants (Kerk et al., 2008; Mocciaro and Schiebel, 2010). Cdc14 enzymes play a direct role in the promotion of cytokinesis by acting on components of the contractile actomyosin ring and cell separation machineries (Meitinger et al., 2012). In the budding yeast Saccharomyces cerevisiae, CDC14 is an essential gene that regulates mitotic exit, cytokinesis and septation (Bardin and Amon, 2001; Meitinger et al., 2012). Dephosphorylation of Iqg1 by Cdc14 at its cyclin‐dependent kinase (CDK) phosphorylation sites promotes the formation of the actomyosin ring that is necessary for cytokinesis (Miller et al., 2015). Endoplasmic reticulum (ER) export of the Chs2 chitin synthase also requires its dephosphorylation by Cdc14 at the CDK phosphorylation sites to ensure septum formation following mitosis (Chin et al., 2012). In the fission yeast Schizosaccharomyces pombe, the Cdc14 orthologue Clp1 functions together with the septation initiation network (SIN) to coordinate cytokinesis with nuclear division, but is not essential for mitosis and growth (Trautmann et al., 2001). Clp1 reverses Cdk1‐mediated phosphorylation of the mitotic inducer Cdc25 and promotes its degradation by the anaphase‐promoting complex at the end of mitosis (Esteban et al., 2004; Wolfe and Gould, 2004). In the filamentous fungi Aspergillus nidulans, F. graminearum and Beauveria bassiana, the CDC14 orthologues are also dispensable for viability, but important for asexual development (Li et al., 2015; Son and Osmani, 2009; Wang et al., 2013). In the human pathogen Candida albicans, deletion of CaCDC14 has no effect on growth rate, but results in defects in cell separation, mitotic exit and morphogenesis (Clemente‐Blanco et al., 2006).
The M. oryzae genome has one distinct orthologue of CDC14, but its functions during appressorium morphogenesis and invasive growth have not been characterized. Because of the relationship between appressorium morphogenesis and cell cycle regulation, and the function of Cdc14 phosphatases as major antagonists of CDKs (Mocciaro and Schiebel, 2010; Queralt and Uhlmann, 2008), in this study, we characterized the MoCDC14 phosphatase gene in M. oryzae. The Mocdc14 mutant showed significantly reduced growth rate and conidiation. It was also defective in septum formation and nuclear distribution during vegetative growth, conidiogenesis and appressorium formation. Mutant conidia often showed incomplete septa or lacked any septum, and produced appressoria with abnormal appressorium‐delimiting septation and melanization. In addition, the Mocdc14 mutant was defective in appressorial penetration and infection through wounds. Similar defects in septation, melanization and penetration were observed with appressorium‐like structures formed by Mocdc14 at hyphal tips. Taken together, our results show that MoCDC14 plays a critical role in septation, nuclear distribution and pathogenesis in M. oryzae, and correct septum formation during conidiogenesis and appressorium formation requires the MoCdc14 phosphatase.
Results
MoCDC14 is important for normal vegetative growth and nuclear distribution in hyphae
The MoCDC14 gene (MGG_04637) is orthologous to CDC14 of S. cerevisiae and clp1 of Sc. pombe, and has the typical HCKAGLGR catalytic sequence. To determine its function in M. oryzae, we generated the Mocdc14 deletion mutant by the split‐marker approach (Catlett et al., 2003). Putative Mocdc14 deletion mutants were identified by polymerase chain reaction (PCR). Four Mocdc14 mutants, M1, M49, M51 and M69 (Table 1), with the same phenotypes (described below) were confirmed by Southern blot analysis (Fig. S1, see Supporting Information).
Table 1.
Wild‐type and mutant strains of Magnaporthe oryzae used in this study.
| Strain | Brief description | Reference |
|---|---|---|
| Guy11 | Wild‐type | Chao and Ellingboe (1991) |
| M1 | Mocdc14 deletion mutant of Guy11 | This study |
| M49 | Mocdc14 deletion mutant of Guy11 | This study |
| M51 | Mocdc14 deletion mutant of Guy11 | This study |
| M69 | Mocdc14 deletion mutant of Guy11 | This study |
| C1 | Mocdc14/MoCDC14‐GFP transformant of M1* | This study |
| C2 | Mocdc14/MoCDC14‐GFP transformant of M1 | This study |
| HG1 | Transformant of Guy11 expressing H1‐GFP | This study |
| HG2 | Transformant of Guy11 expressing H1‐GFP | This study |
| HM1 | Transformant of M1 expressing H1‐GFP | This study |
| HM2 | Transformant of M1 expressing H1‐GFP | This study |
*All of the green fluorescent protein (GFP) fusion constructs were integrated ectopically in the M. oryzae genome.
The Mocdc14 mutant showed a reduced growth rate of approximately 75% (Table 2) and formed colonies with limited aerial hyphae on oatmeal agar (OTA) plates (Fig. 1A), indicating the importance of MoCDC14 during vegetative growth. To determine whether deletion of MoCDC14 affects cytokinesis, we transformed the H1‐GFP fusion construct (Luo et al., 2014) into Guy11 and the Mocdc14 mutant M1. In Guy11, septation occurred regularly in hyphae and each hyphal compartment had one nucleus (Fig. 1B). In the Mocdc14 mutant, hyphae had fewer and unevenly distributed septa, and individual hyphal compartments often contained multiple nuclei (Fig. 1B). These results suggest that the Mocdc14 mutant is defective in mitotic exit and nuclei continue to divide in the absence of cytokinesis in vegetative hyphae.
Table 2.
Defects of the Mocdc14 mutant in growth, conidiation and appressorium formation
| Strain | Growth rate (mm/day) | Conidiation (×103 spores/plate) | Germination (%)* | Appressorium formation (%) † |
|---|---|---|---|---|
| Guy11 | 3.4 ± 0.1A | 2475.0 ± 353.2A | 98.4 ± 0.5A | 95.8 ± 0.9A |
| M1 | 0.9 ± 0.1B | 2.2 ± 1.1B | 91.3 ± 2.0A | 79.0 ± 4.5B |
| C1 | 3.3 ± 0.1A | 2183.3 ± 222.9A | 99.1 ± 0.1A | 96.7 ± 1.0A |
*Percentage of conidia germinating by 24 h.
†Percentage of germ tubes forming appressoria by 24 h.
Means and standard deviations were calculated from three independent measurements. Data were analysed with the protected Fisher's least significant difference (LSD) test. Different letters indicate significant difference (P = 0.05).
Figure 1.
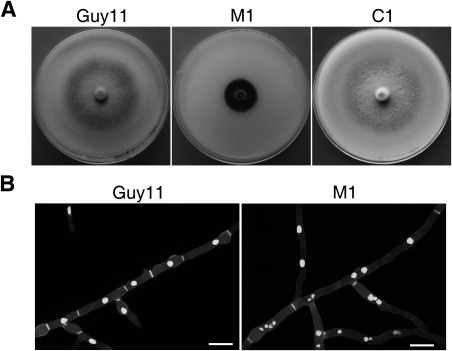
Growth and nuclear distribution defects of the Mocdc14 mutant. (A) Ten‐day‐old oatmeal cultures of the wild‐type (Guy11), Mocdc14 mutant (M1) and Mocdc14/MoCDC14 complemented transformant (C1). (B) Hyphae of transformants of Guy11 and Mocdc14 mutant expressing the H1‐GFP construct were stained with calcofluor white (CFW) and examined by epifluorescence microscopy. Bar, 10 μm.
The Mocdc14 mutant is defective in conidiogenesis
The Mocdc14 mutant also showed significantly reduced conidiation. The average number of conidia produced by the mutant on each OTA plate was (2.2 ± 1.1) × 103, which was reduced by more than 100‐fold in comparison with the wild‐type (Table 2). Microscopic examination showed that most of the mutant conidiophores were aberrant, bearing no conidia (Fig. 2A). Conidia formed by the Mocdc14 mutant were abnormal in morphology. Instead of forming typical three‐celled conidia, over 99% of mutant conidia were single‐celled (Fig. 2B). No septum or only incomplete septation was observed in almost all the conidia formed by the Mocdc14 mutant (Fig. 2C), further indicating the importance of MoCDC14 in septation in M. oryzae. Whereas wild‐type conidia had three nuclei (one nucleus in each compartment), mutant conidia had only one or two nuclei (Fig. 2C). No three‐celled conidia or conidia with three nuclei were produced by the Mocdc14 mutant, indicating that MoCDC14 plays a critical role in mitosis and cytokinesis in developing conidia.
Figure 2.
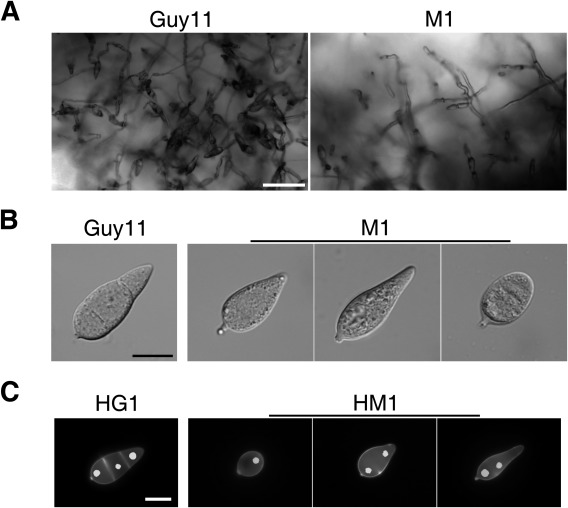
Defects of the Mocdc14 mutant in conidiogenesis. (A) Ten‐day‐old oatmeal cultures of the wild‐type (Guy11) and Mocdc14 mutant (M1) were examined for conidia and conidiophores under a dissection microscope. Bar, 50 μm. (B) Conidia of Guy11 and Mocdc14 mutant were examined by differential interference contrast (DIC) microscopy. Bar, 10 μm. (C) Conidia of transformants of Guy11 (HG1) and Mocdc14 mutant (HM1) expressing the H1‐GFP construct were stained with calcofluor white (CFW) and examined by epifluorescence microscopy. Bar, 10 μm.
Deletion of MoCDC14 affects septation to delimit appressoria on germ tubes
When assayed for appressorium formation on hydrophobic surfaces, although Mocdc14 showed defects in conidium morphology, mutant conidia were normal in germination and still produced melanized appressoria at the tips of germ tubes (Fig. 3A). However, appressorium formation was slightly reduced in the mutant. Whereas over 95% of wild‐type germ tubes formed appressoria after incubation for 24 h on hydrophobic surfaces, appressorium formation was observed in only 79% of the germ tubes in the Mocdc14 mutant under the same conditions (Table 2). Interestingly, parts of the germ tubes and conidia were often melanized in the Mocdc14 mutant (Fig. 3A). When stained with calcofluor white (CFW), the wild‐type formed a septum at the neck of appressoria. In the mutant, the septum that delimited the appressorium from the rest of the germ tubes was not formed, or formed on germ tubes far away from the appressoria (Fig. 3A). The part of the germ tubes delimited together with appressoria was also heavily melanized (Fig. 3A). If there was no septum formation or septation was incomplete in germ tubes and conidia, the entire germ tube together with conidial compartments became melanized (Fig. 3A). These results suggest that MoCDC14 is important for the formation of the septum delimiting the appressorium from the rest of the germ tubes.
Figure 3.
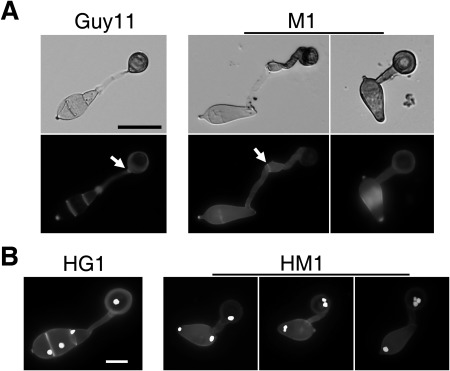
Appressorium formation assays with conidia of the Mocdc14 mutant. (A) Conidia of the wild‐type (Guy11) and Mocdc14 mutant (M1) were incubated on hydrophobic surfaces for 24 h and examined for appressorium formation after staining with calcofluor white (CFW). Septa in the germ tubes are marked with arrows. Bar, 20 μm. (B) Appressoria formed by the H1‐GFP transformants of Guy11 (HG1) and Mocdc14 (HM1) at 12 h were stained with CFW and examined by epifluorescence microscopy. Bar, 10 μm.
To determine the effects of MoCDC14 deletion on nuclear behaviour during appressorium formation, transformants expressing the H1‐GFP construct were stained with CFW. In the wild‐type, one nucleus moved back into the conidia after a single round of mitosis had occurred in the germ tube, and the other moved into developing appressoria (Saunders et al., 2010b). Each wild‐type appressorium contained a single nucleus (Fig. 3B). However, approximately 10.4 ± 3.0% of the appressoria formed by the Mocdc14 mutant had two or more nuclei (Fig. 3B). Therefore, deletion of MoCDC14 affects mitotic division and cytokinesis (septation) during appressorium formation in M. oryzae.
MoCDC14 is also important for the delimitation of appressoria at hyphal tips
In addition to the formation of appressoria at the tips of germ tubes, M. oryzae also forms appressorium‐like structures at hyphal tips (Kong et al., 2013). On artificial hydrophobic surfaces, hyphal tips of the Mocdc14 mutant still developed appressorium‐like structures. However, fragments of hyphae were often delimited and melanized together with appressorium‐like structures in the Mocdc14 mutant (Fig. 4A). Unlike the wild‐type, septation in the mutant occurred far away from the neck of appressorium‐like structures along the hyphae. Therefore, similar to its septation defects during appressorium formation on germ tubes, the Mocdc14 mutant was defective in the formation of the septum to delimit appressorium‐like structures at the hyphal tips (Fig. 4B). MoCDC14 must also be important for the formation of the septum delimiting the appressorium‐like structures on hyphae.
Figure 4.
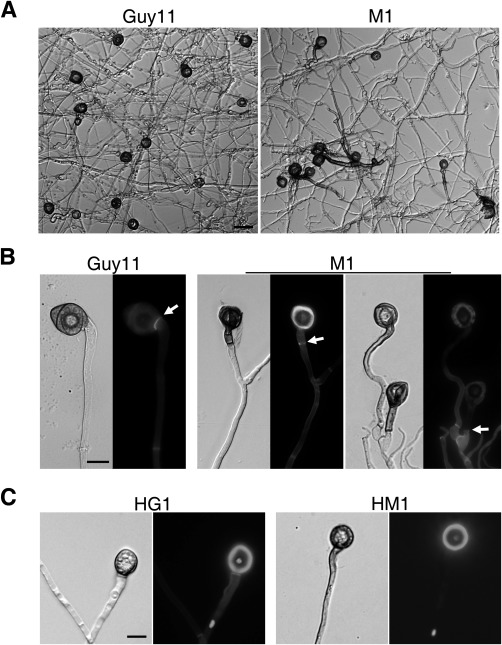
Formation of appressorium‐like structures at hyphal tips. (A) Appressorium‐like structures formed on glass cover slips by hyphal tips of Guy11 and Mocdc14 mutant M1. Bar, 20 μm. (B) Appressorium‐like structures formed at hyphal tips were stained with calcofluor white (CFW) and examined by differential interference contrast (DIC) (left) and epifluorescence (right) microscopy. Septa are marked with arrows. Bar, 10 μm. (C) Hyphae and appressorium‐like structures formed by the H1‐GFP transformants of Guy11 (HG1) and Mocdc14 (HM1) were examined by DIC and epifluorescence microscopy. Bar, 10 μm.
We also examined the number of nuclei in appressorium‐like structures formed at the hyphal tips of transformants expressing the H1‐GFP construct. Like the wild‐type, the Mocdc14 mutant had a single nucleus in appressorium‐like structures (Fig. 4C).
MoCDC14 is important for plant infection
To determine the effect of MoCDC14 deletion on virulence, 2‐week‐old seedlings of rice cultivar CO‐39 were used for spray infection assays. At 7 days post‐inoculation (dpi), numerous typical blast lesions were observed on leaves inoculated with Guy11 (Fig. 5A). Under the same conditions, 63.0 ± 6.4% of the leaves sprayed with the Mocdc14 mutant had no typical blast lesions. Although some leaves had rare small black spots, blast lesions with extensive necrotic zones were never observed on leaves inoculated with the Mocdc14 mutant (Fig. 5A), indicating that MoCDC14 plays a critical role in plant infection and lesion development.
Figure 5.
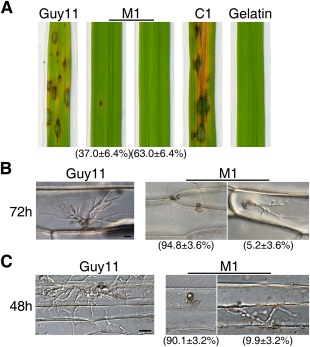
Infection and penetration assays with conidia. (A) Leaves of 2‐week‐old rice seedlings were sprayed with conidia of Guy11, the Mocdc14 deletion mutant M1 and the complemented transformant C1. Inoculation with gelatin solution was used as the negative control. (B) Onion epidermal cells inoculated with conidia of Guy11 and mutant M1 were examined at 72 h post‐inoculation (hpi). Only 5.2 ± 3.6% of the appressoria formed by the Mocdc14 mutant penetrated onion cells. (C) Barley epidermal cells inoculated with conidia from Guy11 and the mutant M1 were examined at 48 hpi. Only 9.9 ± 3.2% of the appressoria formed by the Mocdc14 mutant penetrated barley cells. Bars, 20 μm.
MoCDC14 is important for appressorium penetration and invasive growth
In penetration assays with onion epidermal cells, whereas 67.3 ± 4.2% of Guy11 appressoria penetrated and formed invasive hyphae by 72 hpi, only 5.2 ± 3.6% of the appressoria formed by the Mocdc14 mutant penetrated onion epidermal cells (Fig. 5B). The majority of Mocdc14 appressoria appeared to be melanized, together with parts of or entire germ tubes on onion epidermis, and failed to penetrate underlying plant cells. Furthermore, in comparison with the wild‐type, invasive hyphae formed by the Mocdc14 mutant in rare onion epidermal cells penetrated by mutant appressoria were narrower and less bulbous (Fig. 5B).
Similar results were obtained in penetration assays with barley epidermal cells. Whereas 74.6 ± 5.1% of Guy11 appressoria penetrated and formed invasive hyphae by 48 hpi, only 9.9 ± 3.2% of the appressoria formed by the Mocdc14 mutant penetrated barley epidermal cells (Fig. 5C). The Mocdc14 mutant also showed delayed spread to neighbouring cells. By 48 hpi, wild‐type invasive hyphae had spread from the penetrated cells to neighbouring barley epidermal cells. Under the same conditions, invasive hyphae of the mutant were limited to the penetrated cell (Fig. 5C). These results indicate that the Mocdc14 mutant is defective in penetration and infectious growth after penetration.
MoCDC14 is required for appressorium‐like structure‐mediated penetration and infection
We also conducted infection assays with culture blocks (Liu et al., 2010) to determine the effect of MoCDC14 deletion on penetration by appressorium‐like structures formed at hyphal tips. On intact barley leaves inoculated with the Mocdc14 mutant, only limited necrosis was observed directly beneath culture blocks (Fig. 6A). On wounded leaves, the Mocdc14 mutant also caused only limited blast lesions outside the wounding sites (Fig. 6A). Under the same conditions, Guy11 caused extensive necrosis on both intact and wounded leaves surrounding the inoculation sites (Fig. 6A).
Figure 6.
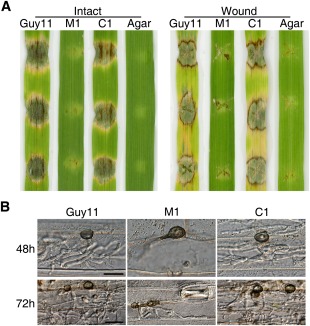
Infection and penetration assays with culture blocks. (A) Intact and wounded barley leaves were inoculated with culture blocks of the wild‐type Guy11, Mocdc14 deletion mutant M1 and Mocdc14/MoCDC14 complemented strain C1. Inoculation with oatmeal agar blocks (Agar) was used as the negative control. Typical leaves were photographed at 5 days post‐inoculation (dpi). (B) Barley leaves inoculated with hyphal blocks of Guy11, M1 and C1 were examined for invasive hyphae at 48 and 72 h post‐inoculation (hpi). Bars, 20 μm.
In barley epidermal cell penetration assays with culture blocks, whereas the wild‐type penetrated into host cells through appressorium‐like structures and developed invasive hyphae by 48 hpi (Fig. 6B), the majority of the appressorium‐like structures formed by Mocdc14 failed to penetrate under the same conditions. Even at 72 hpi, only less than 2% of the appressorium‐like structures formed by the Mocdc14 mutant were able to penetrate and showed limited invasive growth in the penetrated cells. Under the same conditions, Guy11 showed extensive invasive growth in the initial penetrated and neighbouring cells (Fig. 6B). These results indicate that the Mocdc14 mutant is defective in penetration by appressorium‐like structures and MoCDC14 is also important for invasive growth after penetration.
Complementation and localization of MoCdc14
For complementation assays, the MoCDC14‐GFP fusion construct was generated by the yeast gap repair approach (Zhou et al., 2011a) and transformed into the Mocdc14 deletion mutant. The resulting Mocdc14/MoCDC14‐GFP transformant C1 (Table 1) showed normal growth (Fig. 1A), conidiation (Table 2) and plant infection (Figs 5A and 6A). When examined by epifluorescence microscopy, green fluorescent protein (GFP) signals were found mainly in the nucleus in conidia and hyphae of transformant C1 (Fig. 7). Each nucleus had one bright spot probably caused by the localization of MoCdc14‐GFP proteins to the spindle pole body (SPB). These results indicate that the expression of MoCDC14‐GFP complements the Mocdc14 mutant and MoCdc14 is localized to the nucleus.
Figure 7.
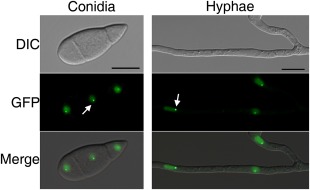
Subcellular localization of the MoCdc14‐GFP fusion proteins. Conidia and vegetative hyphae of the Mocdc14/MoCDC14‐GFP transformant C1 were examined by differential interference contrast (DIC) and epifluorescence microscopy. Arrows point to the putative spindle pole body (SPB) as spots with stronger green fluorescent protein (GFP) signals. Bars, 20 μm.
Mocdc14 mutants show altered mobilization of glycogens
Defects in conidium morphology and appressorium melanization may affect appressorium turgor generation. To test this hypothesis, we assayed the mobilization and degradation of glycogen stored in conidia. Like the wild‐type, the Mocdc14 mutant had abundant glycogen in conidia (Fig. 8A). However, the mobilization of glycogen to developing appressoria was notably delayed in the Mocdc14 mutant after incubation on hydrophobic surfaces for 8 h (Fig. 8A). At 24 h, a significantly higher percentage of mutant conidia still contained glycogen (Fig. 8B). At 48 h, many appressoria formed by the Mocdc14 mutant still contained glycogen (Fig. 8C). These data indicate that the mobilization and degradation of glycogen are affected by the deletion of MoCDC14.
Figure 8.
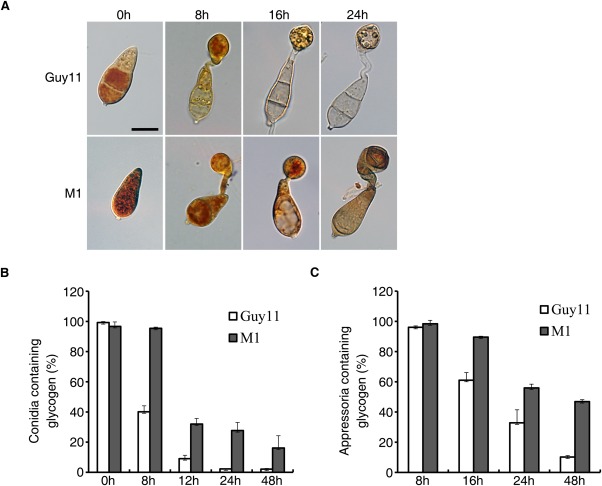
Mobilization and degradation of glycogens during appressorium morphogenesis. (A) Conidia of Guy11 and Mocdc14 mutant M1 were incubated on hydrophobic surfaces for the indicated time and examined for glycogens (yellowish‐brown deposits) after staining with iodine. Bar, 10 μm. (B) Percentage of conidia containing glycogen at each time point. (C) Percentage of appressoria containing glycogen at each time point. The Mocdc14 mutant was defective in the mobilization of glycogen from conidia to developing appressoria and glycogen degradation in appressoria.
Deletion of MoCDC14 reduces the expression levels of CON1 and CON7
Because the Mocdc14 mutant produced morphologically abnormal conidia and showed significantly reduced conidiation, we assayed the expression of several genes that are known to be important for conidiogenesis in M. oryzae, including CON1, CON2, CON7, COM1 and HTF1 (Liu et al., 2010; Shi and Leung, 1995; Shi et al., 1998; Yang et al., 2010), using RNA isolated from 7‐day‐old OTA cultures incubated at 25°C under fluorescent light. Whereas the expression of CON1, CON2, CON7 and COM1 was down‐regulated, the expression of HTF1 was up‐regulated in the Mocdc14 mutant (Fig. S2, see Supporting Information). Nevertheless, in comparison with Guy11, only the expression levels of CON1 and CON7 were reduced over two‐fold in the Mocdc14 mutant (Fig. S2). Reduced expression of CON1 and CON7 may contribute to the conidiation defects of the mutant, because these genes are important for the development of conidiophores and normal conidium morphology (Odenbach et al., 2007; Shi and Leung, 1995; Zhou et al., 2009).
Discussion
The Cdc14 protein phosphatases are well conserved in fungi for the promotion of mitotic exit and cytokinesis by dephosphorylation of their substrates at sites phosphorylated by CDKs (Bloom et al., 2011; Chen et al., 2008). However, whether or not the single‐copy CDC14 gene is essential for growth varies among different fungi. In S. cerevisiae, CDC14 is an essential gene, but its orthologue in Sc. pombe is not. In M. oryzae, MoCDC14 encodes a typical Cdc14 phosphatase protein, with the HCX5R catalytic site sequence (Mocciaro and Schiebel, 2010). The Mocdc14 mutant is viable, but shows a significantly reduced growth rate, which is similar to the cdc14 mutants in F. graminearum and B. bassiana (Li et al., 2015; Wang et al., 2013). However, deletion of the CDC14 orthologue produces no obvious defects in growth rate in A. nidulans (Son and Osmani, 2009) or C. albicans (Clemente‐Blanco et al., 2006). In M. oryzae, in addition to a reduced growth rate, the Mocdc14 mutant produces colonies with enhanced pigmentation and reduced aerial hyphae. Vegetative hyphae show fewer and unevenly distributed septa and individual hyphal compartments often contain multiple nuclei. Therefore, it is likely that deletion of MoCDC14 affects cytokinesis and nuclear distribution in vegetative hyphae, which is similar to the defects of the Fgcdc14 mutant in F. graminearum (Li et al., 2015). MoCdc14 may also be important for cell division and septum formation by counteracting CDK phosphorylation on its substrate in M. oryzae during vegetative growth.
The Mocdc14 mutant shows reduced conidiation and the majority of mutant conidia contain only one or two nuclei without a septum or with only one incomplete septum. In the entomopathogenic fungus B. bassiana, the Cdc14 orthologue also acts as a positive regulator of asexual development (Wang et al., 2013, 2016). In addition to reduced conidiation, the Mocdc14 mutant is also defective in conidium morphology. In M. oryzae, young conidia formed at the tip of conidiophores are single‐celled and contain a single nucleus. Mature three‐celled conidia with uni‐nucleate compartments are formed by two asymmetric mitotic divisions and septation (Liu et al., 2010). The Mocdc14 mutant may be defective in septation after the first asymmetrical division, which results in blocking of the second asymmetrical nuclear division in developing conidia. In F. graminearum, conidia normally have more than five septa and uni‐nucleate compartments. The conidia of the Fgcdc14 mutant show reduced septum formation and an increased number of nuclei per conidial compartment (Li et al., 2015). Nevertheless, the majority of Fgcdc14 mutant conidia still contain three or more septa. It seems that CDC14 plays a more critical role in nuclear division and septum formation in M. oryzae than in F. graminearum during conidiogenesis.
In S. cerevisiae, temporal control of actin ring assembly by CDK and Cdc14 may help to ensure that cytokinesis onset occurs after nuclear division is complete (Miller et al., 2015). In S. pombe, Clp1 regulates entry into mitosis and septum formation (Trautmann and McCollum, 2005) and interacts with the contractile ring (CR) scaffold protein Mid1 for cytokinesis (Clifford et al., 2008). In C. albicans, deletion of CaCDC14 does not interfere with cytokinesis, but results in defects in cell separation (Clemente‐Blanco et al., 2006). Unlike the unicellular ascomycete species, septation in M. oryzae does not result in the cleavage of adjacent cells in vegetative hyphae and conidia. Deletion of MoCDC14 does not abolish septum formation and mitosis, although nuclear distribution and septation become irregular. The Mocdc14 mutant still produces normal hyphae with unevenly distributed septa, but fails to produce normal three‐celled, uni‐nucleate conidia with three nuclei. These results indicate that MoCdc14 may have slightly different functions in septation during hyphal growth and conidiogenesis. In M. oryzae, nuclear division mainly occurs in the hyphal tip compartment, but the formation of three‐celled conidia requires two asymmetrical mitotic divisions followed by cytokinesis. The defects of the Mocdc14 mutant in the completion of septation after the first asymmetrical mitotic division may affect the distinct identity of the two nuclei in developing conidia, which may block the second mitotic division of the nucleus in the tip compartment.
In the rice blast fungus, appressorium morphogenesis is regulated by the cell cycle (Saunders et al., 2010a; Wilson and Talbot, 2009). In the wild‐type, after one mitosis has occurred in the germ tube, one daughter nucleus migrates to the developing appressoria, but the other returns to the germinating conidium. The nucleus which moves into the developing appressoria is assumed to be arrested in G1 and mature appressoria have a single nucleus. In the Mocdc14 mutant, 10.4 ± 3.0% of 24‐h appressoria had two or more nuclei. One possibility is that the nucleus entering the developing appressorium may continue to divide without cytokinesis as a result of defects in mitotic exit associated with MoCDC14 deletion. Because the Mocdc14 mutant is defective in the formation of the septum that delimits the appressoria from the rest of the germ tubes, it is also possible that more than one nucleus migrates into the developing appressoria. Furthermore, when mature appressoria are melanized, conidia and germ tubes undergo autophagic cell death in the wild‐type (Veneault‐Fourrey et al., 2006). However, conidial compartments often still contain nuclei after appressoria have been melanized in the Mocdc14 mutant when appressorium‐delimiting septa are not properly formed. Complete septation of appressoria from the rest of the germ tubes by the appressorium‐delimiting septum may be a prerequisite for the triggering of autophagic cell death.
In M. oryzae, appressorium melanization is important for appressorium turgor generation (deJong et al., 1997). The Mocdc14 mutant still forms melanized appressoria on germ tubes, but parts of the germ tubes and sometimes conidia are also melanized. Similar defects in melanization have been observed in the Mocdc14 mutant when assayed for the formation of appressorium‐like structures at hyphal tips. Fragments of hyphae are often melanized together with appressorium‐like structures, depending on the position of the septum that delimits the appressorium‐like structures. These results indicate that the formation of these special septum‐delimiting appressoria or appressorium‐like structures from the rest of the germ tubes or hyphae plays a critical role in defining the boundary of melanin deposition. Enzymes involved in melanin biosynthesis may be only expressed or active after the completion of the appressorium‐delimiting septum. In M. oryzae, deletion of the MoAND1 gene, an orthologue of A. nidulans ApsA, results in septation defects in hyphae, but has no effect on the formation of appressoria and the appressorium‐delimiting septum (Jeon et al., 2014). An earlier study in M. oryzae with hydroxyl urea treatment and NimA mutations has also shown that the differentiation of appressoria requires a cytokinetic event that is distinct from cell divisions within hyphae (Saunders et al., 2010a). Our studies show that MoCdc14 is involved in deciding the occurrence and position of this special septum during appressorium formation.
In infection assays, the Mocdc14 mutant is almost non‐pathogenic. The defects of the Mocdc14 mutant in plant infection can be related directly to its defects in growth and appressorium morphogenesis. In addition, the Mocdc14 mutant is defective in glycogen mobilization and degradation, which is important for appressorium turgor generation and penetration. However, mutants with deletion of MoCDC14 are also defective in infection through wounding, suggesting a critical role of MoCdc14 during invasive growth. In rare plant cells penetrated by the Mocdc14 mutant, only limited growth of invasive hyphae is seen and the mutant fails to spread into neighbouring cells. Rare invasive hyphae formed by the mutant inside plant cells are less branching than those of the wild‐type. In M. oryzae, bulbous invasive hyphae are considered to show pseudohyphal‐like growth in plant cells (Kankanala et al., 2007; Yi and Valent, 2013). It is possible that MoCdc14 is important for constriction or septum formation in invasive hyphae with pseudohyphal growth.
Experimental Procedures
Strains and culture conditions
The M. oryzae wild‐type strain Guy11 and mutants used in this study (Table 1) were cultured on OTA or complete medium (CM) plates at 25°C as described previously (Xu and Hamer, 1996; Zhou et al., 2011b). For fungal transformation, protoplast preparation and polyethylene glycol (PEG)‐mediated transformation of M. oryzae were performed as described previously (Park et al., 2006). Hygromycin B (Calbiochem, La Jolla, CA, USA) and geneticin (MP Biochemicals, Santa Ana, CA, USA) were added to final concentrations of 300 and 500 μg/mL, respectively, for transformant selection. For DNA isolation, vegetative hyphae were harvested from 2‐day‐old liquid CM cultures (Zhao et al., 2005). Measurements of growth rate and conidiation were performed as described previously (Li et al., 2004; Park et al., 2004).
Generation of the Mocdc14 deletion mutants
To delete the MoCDC14 gene, the double‐joint PCR method (Yu et al., 2004) was used to generate the CDC14 gene replacement construct (Fig. S1A). The 1080‐bp upstream and 992‐bp downstream flanking sequences of MoCDC14 were amplified with the primer pairs C1F/C2R and C3F/C4R (Table S1, see Supporting Information), respectively, and ligated with the hph cassette amplified with primers HYG/F and HYG/R from pCB1003 (Carroll et al., 1994). The products of double‐joint PCR were amplified with primers CCF and CCR (Table S1) and transformed into protoplasts of Guy11. Hygromycin‐resistant transformants were screened by PCR and putative gene replacement mutants were confirmed by Southern blot analysis.
Generation of the MoCDC14‐GFP fusion construct
To generate the MoCDC14‐GFP fusion construct, the entire MoCDC14 gene, including its promoter region, was amplified with primers 14GFP‐F and 14GFP‐R (Table S1) and cloned into XhoI‐digested pFL2 (Zhou et al., 2011b) by the yeast gap repair approach (Zhou et al., 2011a). The MoCDC14‐GFP fusion construct recovered from yeast Trp+ transformants was confirmed by sequencing analysis and transformed into the Mocdc14 deletion mutant M1 (Table 1). Geneticin‐resistant transformants expressing the MoCDC14‐GFP construct were verified by PCR and examined for GFP signals.
Appressorium formation, penetration and plant infection assays
Conidia were harvested from 10‐day‐old OTA cultures and resuspended to a concentration of 5 × 104 conidia/mL (Zhou et al., 2012). Appressorium formation by germ tubes on artificial surfaces was assayed as described previously (Wang et al., 2015; Zhou et al., 2011b). Appressorial penetration and invasive hyphal development were assayed with barley and onion epidermal cells (Chi et al., 2009; Kong et al., 2013). For spray infection assays, conidia were adjusted to 1 × 105 conidia/mL in 0.25% gelatin and used for inoculation of 14‐day‐old seedlings of rice cultivar CO‐39 (Kong et al., 2013).
Assays for the formation and penetration of appressorium‐like structures
Assays for appressorium‐like structures at hyphal tips were performed as described previously (Liu et al., 2010). For infection assays with culture blocks, the second leaves of 8‐day‐old seedlings of barley cultivar Golden Promise were inoculated with 1–2‐mm2 blocks of 10‐day‐old OTA cultures as described previously (Liu et al., 2010). Penetration, invasive growth and lesion development were examined as described previously (Liu et al., 2010; Yang et al., 2010).
Cell wall and glycogen staining
The cell wall was stained with 10 µg/mL CFW (Sigma‐Aldrich, St. Louis, MO, USA), as described previously (Zhou et al., 2011b), to visualize septa in hyphae and during appressorium formation. Conidia incubated at room temperature on hydrophobic surfaces were stained for glycogens with 60 mg of KI and 10 mg of I2 (Thines et al., 2000; Zhang et al., 2014). Glycogen mobilization and degradation during appressorium formation were examined with an Olympus BX51 epifluorescence microscope (Olympus Corporation, Tokyo, Japan).
Quantitative reverse transcription‐polymerase chain reaction (qRT‐PCR) analysis
Hyphae harvested from 7‐day‐old OTA cultures of the wild‐type strain Guy11 and Mocdc14 mutant M1 were used for RNA isolation with TRIzol reagent (Invitrogen, Carlsbad, CA, USA). After treatment with the DNA‐free kit (Promega, Madison, WI, USA), purified RNA samples were used for cDNA synthesis with the Fermentas first cDNA synthesis kit (Hanover, MD, USA). The resulting first cDNA was used for qRT‐PCR assays as described previously (Ding et al., 2010) with the primers listed in Table S1. Data from three biological replicates were used to estimate the relative expression levels of CON1, CON2, CON7, COM1 and HTF1 with the 2–ΔΔCt method (Livak and Schmittgen, 2001). The M. oryzae actin gene MGG_03982.6 was used as the endogenous control for normalization.
Supporting information
Additional Supporting Information may be found in the online version of this article at the publisher's website:
Fig. S1 The MoCDC14 gene replacement construct and deletion mutants. (A) The MoCDC14 locus and gene replacement construct. The MoCDC14 and hph genes are marked with white and black arrows, respectively. H, HindIII. (B) Southern blot analysis with the wild‐type (Guy11) and Mocdc14 transformants (M1, M49, M51 and M69). All the DNA samples were digested with HindIII. The blots were hybridized with probe A (left) amplified with primers C5F and C6R and probe B (right) amplified with H852 and H850.
Fig. S2 Assays of the expression levels of CON1, CON2, CON7, COM1 and HTF1 by quantitative reverse transcription‐polymerase chain reaction (qRT‐PCR). For each gene, the relative expression level in Guy11 was set to unity. The mean and standard deviation were calculated using data from three independent replicates.
Table S1 Polymerase chain reaction (PCR) primers used in this study.
Acknowledgements
We thank Xuli Gao for assistance with appressorium formation and penetration assays. We also thank Drs Huiquan Liu, Cong Jiang and Guanghui Wang for fruitful discussions. This work was supported by grants from the United States Department of Agriculture‐National Institute of Food and Agriculture (USDA‐NIFA) (2012‐67013‐19381), Specialized Research Cultivation Fund for Excellent Young Scholars of Northwest Agricultural and Forestry University (NWSUAF) and Nature Science Foundation of China (No. 31271989; No. 31201464).
References
- Bardin, A.J. and Amon, A. (2001) Men and sin: what's the difference? Nat. Rev. Mol. Cell Biol. 2, 815–826. [DOI] [PubMed] [Google Scholar]
- Bloom, J. , Cristea, I.M. , Procko, A.L. , Lubkov, V. , Chait, B.T. , Snyder, M. and Cross, F.R. (2011) Global analysis of Cdc14 phosphatase reveals diverse roles in mitotic processes. J. Biol. Chem. 286, 5434–5445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carroll, A. , Sweigard, J. and Valent, B. (1994) Improved vectors for selecting resistance to hygromycin. Fungal Genet. Newsl. 41, 135–143. [Google Scholar]
- Catlett, N. , Lee, B.N. , Yoder, O. and Turgeon, B.G. (2003) Split‐marker recombination for efficient targeted deletion of fungal genes. Fungal Genet. Newsl. 9–11. [Google Scholar]
- Chao, C.‐C.T. and Ellingboe, A.H. (1991) Selection for mating competence in Magnaporthe grisea pathogenic to rice. Canadian Journal of Botany 69, 2130–2134. [Google Scholar]
- Chen, C.T. , Feoktistova, A. , Chen, J.S. , Shim, Y.S. , Clifford, D.M. , Gould, K.L. and McCollum, D. (2008) The SIN kinase Sid2 regulates cytoplasmic retention of the S. pombe Cdc14‐like phosphatase Clp1. Curr. Biol. 18, 1594–1599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chi, M.H. , Park, S.Y. , Kim, S. and Lee, Y.H. (2009) A novel pathogenicity gene is required in the rice blast fungus to suppress the basal defenses of the host. PLoS Pathog. 5, e1000401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chin, C.F. , Bennett, A.M. , Ma, W.K. , Hall, M.C. and Yeong, F.M. (2012) Dependence of Chs2 ER export on dephosphorylation by cytoplasmic Cdc14 ensures that septum formation follows mitosis. Mol. Biol. Cell. 23, 45–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clemente‐Blanco, A. , González‐Novo, A. , Machín, F. , Caballero‐Lima, D. , Aragón, L. , Sánchez, M. , de Aldana, C.R.V. , Jiménez, J. and Correa‐Bordes, J. (2006) The Cdc14p phosphatase affects late cell‐cycle events and morphogenesis in Candida albicans . J. Cell Sci. 119, 1130–1143. [DOI] [PubMed] [Google Scholar]
- Clifford, D.M. , Wolfe, B.A. , Roberts‐Galbraith, R.H. , McDonald, W.H. , Yates, J.R., 3rd . and Gould, K.L. (2008) The Clp1/Cdc14 phosphatase contributes to the robustness of cytokinesis by association with anillin‐related Mid1. J. Cell Biol. 181, 79–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dean, R. , Van Kan, J.A. , Pretorius, Z.A. , Hammond‐Kosack, K.E. , Di Pietro, A. , Spanu, P.D. , Rudd, J.J. , Dickman, M. , Kahmann, R. , Ellis, J. and Foster, G.D. (2012) The top 10 fungal pathogens in molecular plant pathology. Mol. Plant Pathol. 13, 414–430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- deJong, J.C. , MCCormack, B.J. , Smirnoff, N. and Talbot, N.J. (1997) Glycerol generates turgor in rice blast. Nature, 389, 244–245. [Google Scholar]
- Ding, S.L. , Liu, W. , Iliuk, A. , Ribot, C. , Vallet, J. , Tao, A. , Wang, Y. , Lebrun, M.H. and Xu, J.R. (2010) The Tig1 histone deacetylase complex regulates infectious growth in the rice blast fungus Magnaporthe oryzae . Plant Cell, 22, 2495–2508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ebbole, D.J. (2007) Magnaporthe as a model for understanding host–pathogen interactions. Annu. Rev. Phytopathol. 45, 437–456. [DOI] [PubMed] [Google Scholar]
- Esteban, V. , Blanco, M. , Cueille, N. , Simanis, V. , Moreno, S. and Bueno, A. (2004) A role for the Cdc14‐family phosphatase Flp1p at the end of the cell cycle in controlling the rapid degradation of the mitotic inducer Cdc25p in fission yeast. J. Cell Sci. 117, 2461–2468. [DOI] [PubMed] [Google Scholar]
- Goh, J. , Kim, K.S. , Park, J. , Jeon, J. , Park, S.Y. and Lee, Y.H. (2011) The cell cycle gene MoCDC15 regulates hyphal growth, asexual development and plant infection in the rice blast pathogen Magnaporthe oryzae . Fungal Genet. Biol. 48, 784–792. [DOI] [PubMed] [Google Scholar]
- Howard, R.J. and Valent, B. (1996) Breaking and entering: host penetration by the fungal rice blast pathogen Magnaporthe grisea . Annu. Rev. Microbiol. 50, 491–512. [DOI] [PubMed] [Google Scholar]
- Jeon, J. , Rho, H. , Kim, S. , Kim, K.S. and Lee, Y.H. (2014) Role of MoAND1‐mediated nuclear positioning in morphogenesis and pathogenicity in the rice blast fungus, Magnaporthe oryzae . Fungal Genet. Biol. 69, 43–51. [DOI] [PubMed] [Google Scholar]
- Kankanala, P. , Czymmek, K. and Valent, B. (2007) Roles for rice membrane dynamics and plasmodesmata during biotrophic invasion by the blast fungus. Plant Cell, 19, 706–724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kato, H. , Mayama, R. , Sekine, R. and Urashima, A. (1994) Microconidium formation in Magnaporthe grisea . Annu. Phytopathol. Soc. Jpn. 60, 175–185. [Google Scholar]
- Kerk, D. , Templeton, G. and Moorhead, G.B. (2008) Evolutionary radiation pattern of novel protein phosphatases revealed by analysis of protein data from the completely sequenced genomes of humans, green algae, and higher plants. Plant Physiol. 146, 351–367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kong, L.A. , Yang, J. , Li, G.T. , Qi, L.L. , Zhang, Y.J. , Wang, C.F. , Zhao, W.S. , Xu, J.R. and Peng, Y.L. (2012) Different chitin synthase genes are required for various developmental and plant infection processes in the rice blast fungus Magnaporthe oryzae . PLoS Pathog. 8, e1002526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kong, L.A. , Li, G.T. , Liu, Y. , Liu, M.G. , Zhang, S.J. , Yang, J. , Zhou, X.Y. , Peng, Y.L. and Xu, J.R. (2013) Differences between appressoria formed by germ tubes and appressorium‐like structures developed by hyphal tips in Magnaporthe oryzae . Fungal Genet. Biol. 56, 33–41. [DOI] [PubMed] [Google Scholar]
- Li, C.H. , Melesse, M. , Zhang, S.J. , Hao, C.F. , Wang, C.F. , Zhang, H.C. , Hall, M.C. and Xu, J.R. (2015) FgCDC14 regulates cytokinesis, morphogenesis, and pathogenesis in Fusarium graminearum . Mol. Microbiol. 98, 770–786. [DOI] [PubMed] [Google Scholar]
- Li, G.T. , Zhou, X.Y. and Xu, J.R. (2012) Genetic control of infection‐related development in Magnaporthe oryzae . Curr. Opin. Microbiol. 15, 678–684. [DOI] [PubMed] [Google Scholar]
- Li, L. , Xue, C.Y. , Bruno, K. , Nishimura, M. and Xu, J.R. (2004) Two PAK kinase genes, CHM1 and MST20, have distinct functions in Magnaporthe grisea . Mol. Plant–Microbe Interact. 17, 547–556. [DOI] [PubMed] [Google Scholar]
- Liu, H.Q. , Zhang, S.J. , Ma, J.W. , Dai, Y.F. , Li, C.H. , Lyu, X. , Wang, C.F. and Xu, J.R. (2015) Two Cdc2 kinase genes with distinct functions in vegetative and infectious hyphae in Fusarium graminearum . PLoS Pathog. 11, e1004913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu, W.D. , Xie, S.Y. , Zhao, X.H. , Chen, X. , Zheng, W.H. , Lu, G.D. , Xu, J.R. and Wang, Z.H. (2010) A homeobox gene is essential for conidiogenesis of the rice blast fungus Magnaporthe oryzae . Mol. Plant–Microbe Interact. 23, 366–375. [DOI] [PubMed] [Google Scholar]
- Liu, X.H. , Lu, J.P. and Lin, F.C. (2007) Autophagy during conidiation, conidial germination and turgor generation in Magnaporthe grisea . Autophagy, 3, 472–473. [DOI] [PubMed] [Google Scholar]
- Liu, X.H. , Gao, H.M. , Xu, F. , Lu, J.P. , Devenish, R.J. and Lin, F.C. (2012) Autophagy vitalizes the pathogenicity of pathogenic fungi. Autophagy, 8, 1415–1425. [DOI] [PubMed] [Google Scholar]
- Livak, K.J. and Schmittgen, T.D. (2001) Analysis of relative gene expression data using real‐time quantitative PCR and the 2(–Delta Delta C(T)) method. Methods, 25, 402–408. [DOI] [PubMed] [Google Scholar]
- Luo, Y.P. , Zhang, H.C. , Qi, L.L. , Zhang, S.J. , Zhou, X.Y. , Zhang, Y.M. and Xu, J.R. (2014) FgKin1 kinase localizes to the septal pore and plays a role in hyphal growth, ascospore germination, pathogenesis, and localization of Tub1 beta‐tubulins in Fusarium graminearum . New Phytol. 204, 943–954. [DOI] [PubMed] [Google Scholar]
- Martin‐Urdiroz, M. , Oses‐Ruiz, M. , Ryder, L.S. and Talbot, N.J. (2016) Investigating the biology of plant infection by the rice blast fungus Magnaporthe oryzae . Fungal Genet. Biol. 90, 61–68. [DOI] [PubMed] [Google Scholar]
- Meitinger, F. , Palani, S. and Pereira, G. (2012) The power of MEN in cytokinesis. Cell Cycle, 11, 219–228. [DOI] [PubMed] [Google Scholar]
- Miller, D.P. , Hall, H. , Chaparian, R. , Mara, M. , Mueller, A. , Hall, M.C. and Shannon, K.B. (2015) Dephosphorylation of Iqg1 by Cdc14 regulates cytokinesis in budding yeast. Mol. Biol. Cell, 26, 2913–2926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mocciaro, A. and Schiebel, E. (2010) Cdc14: a highly conserved family of phosphatases with non‐conserved functions? J. Cell Sci. 123, 2867–2876. [DOI] [PubMed] [Google Scholar]
- Odenbach, D. , Breth, B. , Thines, E. , Weber, R.W. , Anke, H. and Foster, A.J. (2007) The transcription factor Con7p is a central regulator of infection‐related morphogenesis in the rice blast fungus Magnaporthe grisea . Mol. Microbiol. 64, 293–307. [DOI] [PubMed] [Google Scholar]
- Osés‐Ruiz, M. , Sakulkoo, W. and Talbot, N.J. (2016) Septation and cytokinesis in pathogenic fungi In: Growth, Differentiation and Sexuality, Wendland, J. (ed.) pp. 67–79. Berlin: Springer. [Google Scholar]
- Park, G. , Bruno, K.S. , Staiger, C.J. , Talbot, N.J. and Xu, J.R. (2004) Independent genetic mechanisms mediate turgor generation and penetration peg formation during plant infection in the rice blast fungus. Mol. Microbiol. 53, 1695–1707. [DOI] [PubMed] [Google Scholar]
- Park, G. , Xue, C. , Zhao, X. , Kim, Y. , Orbach, M. and Xu, J.R. (2006) Multiple upstream signals converge on the adaptor protein Mst50 in Magnaporthe grisea . Plant Cell, 18, 2822–2835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perez‐Martin, J. , Castillo‐Lluva, S. , Sgarlata, C. , Flor‐Parra, I. , Mielnichuk, N. , Torreblanca, J. and Carbo, N. (2006) Pathocycles: Ustilago maydis as a model to study the relationships between cell cycle and virulence in pathogenic fungi. Mol. Genet. Genomics, 276, 211–229. [DOI] [PubMed] [Google Scholar]
- Queralt, E. and Uhlmann, F. (2008) Cdk‐counteracting phosphatases unlock mitotic exit. Curr. Opin. Cell Biol. 20, 661–668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saunders, D.G. , Aves, S.J. and Talbot, N.J. (2010a) Cell cycle‐mediated regulation of plant infection by the rice blast fungus. Plant Cell, 22, 497–507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saunders, D.G. , Dagdas, Y.F. and Talbot, N.J. (2010b) Spatial uncoupling of mitosis and cytokinesis during appressorium‐mediated plant infection by the rice blast fungus Magnaporthe oryzae . Plant Cell, 22, 2417–2428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sgarlata, C. and Perez‐Martin, J. (2005) Inhibitory phosphorylation of a mitotic cyclin‐dependent kinase regulates the morphogenesis, cell size and virulence of the smut fungus Ustilago maydis . J. Cell Sci. 118, 3607–3622. [DOI] [PubMed] [Google Scholar]
- Shi, Z.X. and Leung, H. (1995) Genetic analysis of sporulation in Magnaporthe grisea by chemical and insertional mutagenesis. Mol. Plant–Microbe Interact. 8, 949–959. [Google Scholar]
- Shi, Z.X. , Christian, D. and Leung, H. (1998) Interactions between spore morphogenetic mutations affect cell types, sporulation, and pathogenesis in Magnaporthe grisea . Mol. Plant–Microbe Interact. 11, 199–207. [DOI] [PubMed] [Google Scholar]
- Son, S. and Osmani, S.A. (2009) Analysis of all protein phosphatase genes in Aspergillus nidulans identifies a new mitotic regulator, Fcp1. Eukaryot. Cell, 8, 573–585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thines, E. , Weber, R.W. and Talbot, N.J. (2000) MAP kinase and protein kinase A‐dependent mobilization of triacylglycerol and glycogen during appressorium turgor generation by Magnaporthe grisea . Plant Cell, 12, 1703–1718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trautmann, S. and McCollum, D. (2005) Distinct nuclear and cytoplasmic functions of the S. pombe Cdc14‐like phosphatase Clp1p/Flp1p and a role for nuclear shuttling in its regulation. Curr. Biol. 15, 1384–1389. [DOI] [PubMed] [Google Scholar]
- Trautmann, S. , Wolfe, B.A. , Jorgensen, P. , Tyers, M. , Gould, K.L. and McCollum, D. (2001) Fission yeast Clp1p phosphatase regulates G2/M transition and coordination of cytokinesis with cell cycle progression. Curr. Biol. 11, 931–940. [DOI] [PubMed] [Google Scholar]
- Veneault‐Fourrey, C. , Barooah, M. , Egan, M. , Wakley, G. and Talbot, N.J. (2006) Autophagic fungal cell death is necessary for infection by the rice blast fungus. Science, 312, 580–583. [DOI] [PubMed] [Google Scholar]
- Wang, G.H. , Li, G.T. , Zhang, S.J. , Jiang, C. , Qin, J. and Xu, J.R. (2015) Activation of the signalling mucin MoMsb2 and its functional relationship with Cbp1 in Magnaporthe oryzae . Environ. Microbiol. 17, 2969–2981. [DOI] [PubMed] [Google Scholar]
- Wang, J. , Liu, J. , Hu, Y. , Ying, S.H. and Feng, M.G. (2013) Cytokinesis‐required Cdc14 is a signaling hub of asexual development and multi‐stress tolerance in Beauveria bassiana . Sci. Rep. 3, 3086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, Z.K. , Wang, J. , Liu, J. , Ying, S.H. , Peng, X.J. and Feng, M.G. (2016) Proteomic and phosphoproteomic insights into a signaling hub role for Cdc14 in asexual development and multiple stress responses in Beauveria bassiana . PLoS One, 11, e0153007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson, R.A. and Talbot, N.J. (2009) Under pressure: investigating the biology of plant infection by Magnaporthe oryzae . Nat. Rev. Microbiol. 7, 185–195. [DOI] [PubMed] [Google Scholar]
- Wolfe, B.A. and Gould, K.L. (2004) Fission yeast Clp1p phosphatase affects G2/M transition and mitotic exit through Cdc25p inactivation. EMBO J. 23, 919–929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu, J.R. and Hamer, J.E. (1996) MAP kinase and cAMP signaling regulate infection structure formation and pathogenic growth in the rice blast fungus Magnaporthe grisea . Genes Dev. 10, 2696–2706. [DOI] [PubMed] [Google Scholar]
- Xu, J.R. , Zhao, X.H. and Dean, R.A. (2007) From genes to genomes: a new paradigm for studying fungal pathogenesis in Magnaporthe oryzae . Adv. Genet. 57, 175–218. [DOI] [PubMed] [Google Scholar]
- Yang, J. , Zhao, X.Y. , Sun, J. , Kang, Z.S. , Ding, S.L. , Xu, J.R. and Peng, Y.L. (2010) A novel protein Com1 is required for normal conidium morphology and full virulence in Magnaporthe oryzae . Mol. Plant–Microbe Interact. 23, 112–123. [DOI] [PubMed] [Google Scholar]
- Yi, M. and Valent, B. (2013) Communication between filamentous pathogens and plants at the biotrophic interface. Annu. Rev. Phytopathol. 51, 587–611. [DOI] [PubMed] [Google Scholar]
- Yu, J.H. , Hamari, Z. , Han, K.H. , Seo, J.A. , Reyes‐Dominguez, Y. and Scazzocchio, C. (2004) Double‐joint PCR: a PCR‐based molecular tool for gene manipulations in filamentous fungi. Fungal Genet. Biol. 41, 973–981. [DOI] [PubMed] [Google Scholar]
- Zhang, H.L. , Wu, Z.S. , Wang, C.F. , Li, Y. and Xu, J.R. (2014) Germination and infectivity of microconidia in the rice blast fungus Magnaporthe oryzae . Nat Commun. 5, 4518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao, X.H. , Kim, Y. , Park, G. and Xu, J.R. (2005) A mitogen‐activated protein kinase cascade regulating infection‐related morphogenesis in Magnaporthe grisea . Plant Cell, 17, 1317–1329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao, X.H. , Mehrabi, R. and Xu, J.R. (2007) Mitogen‐activated protein kinase pathways and fungal pathogenesis. Eukaryot. Cell, 6, 1701–1714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou, X.Y. , Li, G.T. and Xu, J.R. (2011a) Efficient approaches for generating GFP fusion and epitope‐tagging constructs in filamentous fungi. Methods Mol. Biol. 722, 199–212. [DOI] [PubMed] [Google Scholar]
- Zhou, X.Y. , Liu, W.D. , Wang, C.F. , Xu, Q.J. , Wang, Y. , Ding, S.L. and Xu, J.R. (2011b) A MADS‐box transcription factor MoMcm1 is required for male fertility, microconidium production and virulence in Magnaporthe oryzae . Mol. Microbiol. 80, 33–53. [DOI] [PubMed] [Google Scholar]
- Zhou, X.Y. , Zhang, H.F. , Li, G.T. , Shaw, B. and Xu, J.R. (2012) The cyclase‐associated protein Cap1 is important for proper regulation of infection‐related morphogenesis in Magnaporthe oryzae . PLoS Pathog. 8, e1002911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou, Z.Z. , Li, G.H. , Lin, C.H. and He, C.Z. (2009) Conidiophore stalk‐less1 encodes a putative zinc‐finger protein involved in the early stage of conidiation and mycelial infection in Magnaporthe oryzae . Mol Plant–Microbe Interact. 22, 402–410. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional Supporting Information may be found in the online version of this article at the publisher's website:
Fig. S1 The MoCDC14 gene replacement construct and deletion mutants. (A) The MoCDC14 locus and gene replacement construct. The MoCDC14 and hph genes are marked with white and black arrows, respectively. H, HindIII. (B) Southern blot analysis with the wild‐type (Guy11) and Mocdc14 transformants (M1, M49, M51 and M69). All the DNA samples were digested with HindIII. The blots were hybridized with probe A (left) amplified with primers C5F and C6R and probe B (right) amplified with H852 and H850.
Fig. S2 Assays of the expression levels of CON1, CON2, CON7, COM1 and HTF1 by quantitative reverse transcription‐polymerase chain reaction (qRT‐PCR). For each gene, the relative expression level in Guy11 was set to unity. The mean and standard deviation were calculated using data from three independent replicates.
Table S1 Polymerase chain reaction (PCR) primers used in this study.


