Summary
The chloroplast is one of the most dynamic organelles of a plant cell. It carries out photosynthesis, synthesizes major phytohormones, plays an active part in the defence response and is crucial for interorganelle signalling. Viruses, on the other hand, are extremely strategic in manipulating the internal environment of the host cell. The chloroplast, a prime target for viruses, undergoes enormous structural and functional damage during viral infection. Indeed, large proportions of affected gene products in a virus‐infected plant are closely associated with the chloroplast and the process of photosynthesis. Although the chloroplast is deficient in gene silencing machinery, it elicits the effector‐triggered immune response against viral pathogens. Virus infection induces the organelle to produce an extensive network of stromules which are involved in both viral propagation and antiviral defence. From studies over the last few decades, the involvement of the chloroplast in the regulation of plant–virus interaction has become increasingly evident. This review presents an exhaustive account of these facts, with their implications for pathogenicity. We have attempted to highlight the intricacies of chloroplast–virus interactions and to explain the existing gaps in our current knowledge, which will enable virologists to utilize chloroplast genome‐based antiviral resistance in economically important crops.
Keywords: chloroplast, defence, infection, interaction, replication, translation, virus
Introduction
Around 1.5 billion years ago, symbiotic inclusion of a photosynthetic cyanobacterium into another free‐living cell initiated one of the most important evolutions in the history of life: the establishment of the chloroplast as an indispensable part of a eukaryotic plant cell (Gray, 1989). In modern land plants, the chloroplast traps light energy and uses it to fix carbon via its photosynthetic machinery. Other key biochemical components, including amino acids, fatty acids, purine and pyrimidine, are also synthesized inside the chloroplast. Moreover, the chloroplast is also involved in the antipathogenic basal and systemic defence response of plants (Caplan et al., 2008; Zhao et al., 2013). Hence, for successful infections, microbial plant pathogens need to suppress the chloroplast‐mediated defence by employing pathogenicity factors, such as effector proteins (Jelenska et al., 2007; Petre et al., 2015). On the other hand, intracellular pathogens, such as plant viruses, manipulate the host cell in such a way that they can efficiently utilize host resources for propagation and transform the host cell into a ‘Trojan horse’ sheltering enemies inside.
The chloroplast of land plants is a lens‐like, double‐membrane organelle which contains stacked thylakoids floating in a semi‐solid stroma. During the process of evolution, much of its ancient prokaryotic genome was transferred into the nucleus of the host cell. The modern chloroplast genome contains around 100 genes and, interestingly, contains both prokaryote‐like operons and eukaryote‐like introns (McFadden, 2001). Most of the chloroplast‐encoded genes which are involved in photosynthesis and protein sorting are expressed by the chloroplast's own translation machinery. However, to transport large numbers of chloroplast‐related proteins that are encoded in the nuclear genome translated in the cytoplasm, complex protein import machinery has evolved. A tight coordination exists between the nucleus and the chloroplast through a two‐way signalling network. This network also plays a crucial role in defence and development (Beale, 2011; Beck, 2005). In response to unknown environmental and biochemical stimuli, stroma‐filled, tube‐shaped structures are extended from the chloroplast. These mysterious structures are hypothesized to be involved in the signalling network and metabolite exchange between chloroplasts and other cellular organelles, such as the nucleus. They also take part in innate immunity against plant pathogens and, according to recently emerging reports, possibly play a dynamic role during virus infection (Caplan et al., 2015).
Attachment to the chloroplast membrane is a signature infection pattern for many plant viruses (Prod'homme et al., 2003; Wei et al., 2010). They affect large numbers of chloroplast‐ and photosynthesis‐related genes (CPRGs) (Mochizuki et al., 2014b; Postnikova and Nemchinov, 2012). Indeed, broadly speaking, damage to the chloroplast is one of the pivotal steps in successful infection. For example, 2b mutant viruses of Cucumber mosaic virus (CMV) pepo strain have limited activity for the suppression of gene silencing, but a set of point mutations in the coat protein (CP), which enables it to repress CPRG expression, restores the virulence of the 2b mutant strain (Mochizuki et al., 2014a). Recent reports have shown that viruses can modify the retrograde signalling pathway transducing a signal from the chloroplast to the nucleus (Caplan et al., 2015; Fu et al., 2015). As an understanding of the mode of infection is crucial to combat the viral threat, it is extremely important to critically overview the different facets of chloroplast–virus interactions within the context of viral pathogenicity.
In the following sections, we highlight the striking sequence similarities between the chloroplast and viral genome, which indicate the probable displacement of plant genes (of cyanobacterial origin) by selection pressure from viruses. We then describe how various viruses adopt various strategic nuances to reach the chloroplast, and the involvement of several chloroplast proteins in this process of transportation. The mechanisms of virus‐induced structural and functional damage of the chloroplast are discussed with a brief account of the affected CPRGs. The complex network of the chloroplast‐induced defence response against viral pathogens is also discussed. Overall, we summarize the recent advances in our understanding of the chloroplast–virus interaction and provide a strategic opinion of how it can be exploited to generate disease resistance against different viruses in economically important crops.
Chloroplast and Virus: A Possible Case of Non‐Orthologous Horizontal Gene Transfer
Considering the chloroplast as the most favoured target for viruses, some sparse, yet intriguing, scientific findings have been reported that indicate a rather unique association between the two. Although no thorough study has been conducted, based on the limited genomic data available, we build a hypothesis which proposes that, similar to many other eukaryotic microbial pathogens, a ‘host–pathogen co‐evolution’‐like relationship exists, at least in the case of some chloroplast–virus interactions (Gluck‐Thaler and Slot, 2015).
During the course of evolution, present‐day chloroplasts, with their reduced genome size and compromised genetic autonomy, originated by gene transfer from the prokaryotic endosymbiont to the host nucleus. The similarities in the genome sequences and division processes of plastids and cyanobacteria provide evidence of this occurrence. An interesting observation was reported by Mayo and Jolly (1991), who showed that the sequence of Potato leaf roll virus RNA shared similarity with host plant RNA. In the following year, Masuta et al. (1992) reported that the symptom‐producing vernacular ‘yellow region’ of satellite RNA (Y‐Sat) of CMV contained an intriguing motif with significant sequence complementarity to a specific host tRNA‐GIu molecule, a chloroplast glutamate acceptor RNA. More than three decades ago, it was also found that chloroplast‐localizing viral CP encapsulated chloroplast DNA transcript, resulting in pseudo‐virion formation (Rochon and Siegel, 1984). Such reports call for a hypothetical approach to understand the basis of the unique relationship between chloroplasts and viruses from an evolutionary perspective.
Similar to plant–cyanobacterium gene transfer, it is possible that the events of exchange of genetic material between plants and viruses have played important roles in the co‐evolution of hosts and pathogens. Multiple copies of geminiviral (DNA virus) replication protein genes, as well as genetic material of partitiviruses and totiviruses [both double‐stranded RNA (dsRNA) viruses with no reverse transcriptase activity], have been found to be incorporated in the tobacco nuclear genome (Bejarano et al., 1996; Liu et al., 2010). Recently, both mitochondrial and chloroplastic DNA‐dependent RNA polymerases have been found to share homology with that of T3/T7 bacteriophage (Diray‐Arce et al., 2013). Such rare events of non‐orthologous displacement may explain the compatibility of the chloroplast and its machinery in supporting viral propagation. A contemporary study has revealed that a 22‐nucleotide stretch in the ‘yellow region’ of CMV Y‐Sat is complementary to a sequence in a chlorophyll biosynthetic gene (CHLI). As a result, during Y‐Sat infection, this similarity induced small interfering (siRNA)‐directed RNA silencing of host CHLI. Indeed, Nicotiana species lacking this complementary sequence similarity were found to be resistant to Y‐Sat RNA infection (Shimura et al., 2011; Smith et al., 2011).
Such examples of shared homology indicate a hitherto unexplored possibility of interspecies horizontal gene transfer (Fig. 1). It might also be possible that, during reductive evolution from bacterium to chloroplast, viruses imposed a selection pressure on the host and some of the cyanobacterial proteins were replaced by proteins of viral origin (Filée and Forterre, 2005). The fact that viruses prefer membranous structures and/or internal environments of chloroplasts for the replication of their genomes further supports this hypothesis.
Figure 1.
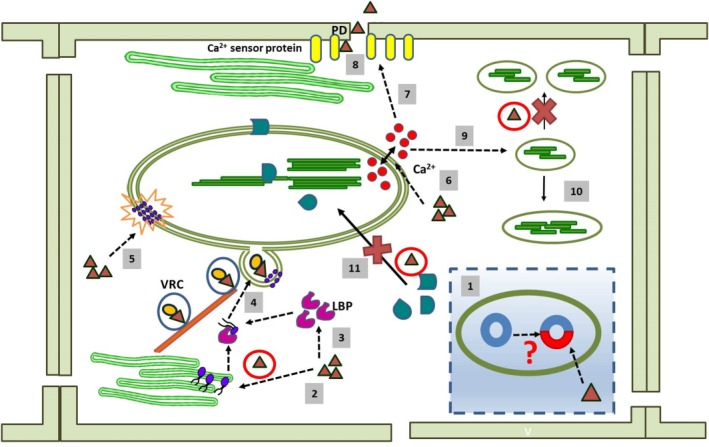
Conceptual depiction of different aspects of chloroplast–virus interaction. For simplified representation, the same symbols have been used for different viruses (filled red triangles). During the course of evolution, viruses have probably replaced some of the genes in the chloroplast with their own genetic material; this might be reflected by the plastid affinity of viruses (1). Viruses alter the lipid biosynthesis (lipid molecules are indicated by blue circles) (2) and lipid trafficking pathway by the overexpression of lipid‐binding proteins (LBPs) (3). These alterations aid in membrane invagination, vesicle formation (4) and rearrangement of the membrane lipid bilayer (5). Viral infection affects Ca2+ (Ca2+ ions indicated by red circles) signalling mediated by the chloroplast (6). This might affect the biochemical properties of synaptotagmin (SYTA)‐like Ca2+ sensor proteins (7) which, in turn, help the viral component to move through the plasmodesmata (PD) for cell‐to‐cell movement (8). A change in the Ca2+ level also hampers normal chloroplast division (9), and large, abnormally shaped chloroplasts are formed (10). Viral proteins sequester various chloroplast‐localized proteins (chloroplast‐localized proteins are indicated by green geometric shapes) in the cytosol (11); this again damages the organelle structurally and functionally. VRC, virus replication complex.
The Membranous Structure of the Chloroplast is Exploited by Viruses for Replication
Viruses, the master manipulators of the host cell environment, use different physiological and biochemical attributes of plants at an optimum level. In this section, we discuss how viruses exploit the chloroplast's double‐membrane structure for propagation.
Inside a plant cell, one of the most severe threats faced by viruses is the silencing of viral RNAs. To counter this, many viruses encode various silencing suppressor proteins in their genome (Pumplin and Voinnet, 2013). In addition, some viruses try to evade silencing by physically escaping the host RNAi surveillance (Ding and Voinnet, 2007; Schwartz et al., 2002). The establishment of the replication process within the vesicles and chloroplasts, which are considered to be devoid of silencing machinery, is likely to hamper the target accessibility of the host antiviral mechanism (Ahlquist et al., 2003; Laliberté and Sanfaçon, 2010; Prod'homme et al., 2001; Tabler and Tsagris, 2004). The virus replication complex (VRC), an assembly of viral proteins and viral genomic RNA with essential host factors, preferentially accumulates in the membranous structures of organelles, such as the endoplasmic reticulum (ER) and chloroplast. Turnip yellow mosaic virus (TYMV) of the Tymoviridae family and Turnip mosaic virus (TuMV) of the Potyviridae family are regularly associated with the chloroplast membrane during infection (Prod'homme et al., 2003; Wei et al., 2010). Indeed, TYMV infection is characteristically associated with the small vesicles in the periphery of the organelle (Matthews, 1991), manifesting the close relationship between chloroplasts and the virus life cycle. The 66‐ and 140‐kDa protein products of TYMV (containing RNA‐dependent RNA polymerase (RdRP), methyltransferase, proteinase domains and helicase motifs) are associated with the spherule‐like membranous structures of chloroplasts (Prod'homme et al., 2001). Moreover, during the infection of a positive‐stranded RNA virus, such a membranous scaffold spatially separates the translation and replication processes of the viral genome. As the genetic material of these viruses functions both as a replication template and mRNA, such a separation might enhance the specificity of template selection by host RdRP. Furthermore, it has been reported in the case of TuMV infection that the low‐pH condition of the intermembrane space may accelerate the interaction of viral CP and viral RNA (Rohozinski and Hancock, 1996).
As in the case of potyvirus TuMV, at ER exit sites, viral 6K protein induces vesicle formation. Viruses use the host's actomyosin motility system to follow the ER–Golgi transport track and to eventually reach the chloroplast (Fig. 2). ER‐localized soluble NSF (N‐ethylmaleimide‐sensitive fusion) attachment protein receptor (SNARE) proteins, such as Qc‐Syp71 and Vap27‐1, have been shown to be implicated in the process of vesicle attachment with the chloroplast outer membrane. Vesicles co‐localize with viral dsRNA‐rich foci, actively engaged in replication, on the chloroplast membrane (Wei et al., 2010, 2013). Once the vesicles reach the chloroplast, Syp71 mediates the formation of elongated tubular bridge‐like structures between two adjacent chloroplasts, causing the organelles to clump together (Kitajima and Costa, 1973; Wei et al., 2013). These tubular structures are characteristically similar to geminivirus‐induced stromules (Krenz et al., 2012) and are likely to facilitate the inter‐ and intracellular trafficking of virus particles. The clumping explains the earlier observation made by Kitajima and Costa (1973) in TuMV‐infected wild‐type Chenopodium quinoa. As the chloroplast membrane starts to invaginate, the vesicles are engulfed by the organelle. A detailed account of the significance of stromules is described later in this review.
Figure 2.
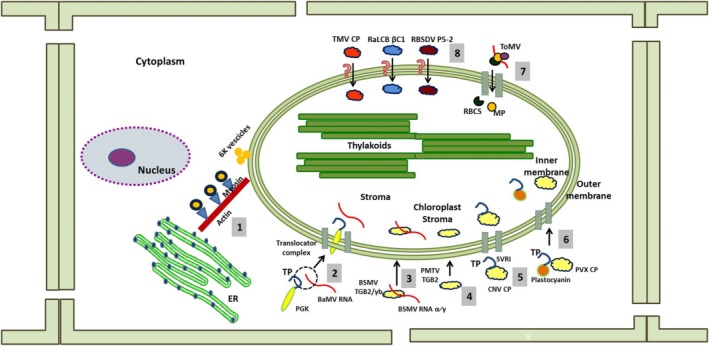
Different strategies adopted by different viruses to send their nucleic acid and/or protein products into the chloroplast. Vesicles induced by the 6K protein of Turnip mosaic virus (TuMV) follow the endoplasmic reticulum (ER)–Golgi vesicular transport pathway to reach the chloroplast using the actomyosin motility system (1). The 3′ untranslated region (UTR) of Bamboo mosaic virus (BaMV) RNA binds with the transit peptide of p51, a chloroplast phosphoglycerate kinase (chl‐PGK), to pass through the membranes of the chloroplast (2). TGB2, the movement protein of Barley stripe mosaic virus (BSMV) and Potato mop‐top virus (PMTV), shows different properties when localized inside the chloroplast in terms of being associated with or without the respective nucleic acids (3, 4). The arm domain of Cucumber necrosis virus (CNV) coat protein (CP) has an embedded sequence identical to the transit peptide (TP) of chloroplast proteins, which helps the viral protein to cross the translocation machinery (5). The Potato virus X (PVX) CP interacts with the TP of plastocyanine to reach the organelle (6). The movement protein (MP) of Tomato mosaic virus (ToMV) interacts with the small subunit of RuBisCO and localizes to the chloroplast (7). The mechanisms used by a few proteins, such as Tobacco mosaic virus (TMV) CP, Radish leaf curl betasatellite (RaLCB) βC1 and Rice black‐streaked dwarf virus (RBSDV) P5‐2, to pass through the translocation machinery are yet not known (8). RBCS, small subunit of RuBisCO; SVRI, R‐arm region plus the first 4 aa [SVRI] of the shell [S] domain of CNV coat protein.
Evidence suggests that, for replication, at least some plant RNA viruses, such as Brome mosaic virus, require an appropriate lipid composition, together with sufficient fluidity and plasticity of the membrane (Lee et al., 2001). Indeed, animal viruses extensively alter the lipid synthesis system of the host cell (Castro et al., 2016; Reiss et al., 2011). Massive rearrangement of the organellar membrane structure in plant cells is also related to a similar manipulation of the host lipid metabolism pathway (Fig. 1). Sterol has been shown previously to be involved in the replication of Tomato bushy stunt virus (Sharma et al., 2010). The enhanced expression of lipid transfer proteins (LTPs) has also been reported during chilli–Tomato leaf curl New Delhi virus interaction (Kushwaha et al., 2015). Such overexpressed LTPs may carry phospholipids and other fatty acids to the sites of membrane rearrangement. Both the outer and inner membranes of chloroplasts are lipid bilayers in which galactolipid is an important constituent. The lipid bilayer of the thylakoid membrane is involved in the biogenesis and maintenance of the membrane, as well as in photosynthesis (Kobayashi and Wada, 2016). The fatty acid and galactolipid composition of barley seedlings is severely altered by virus infection, and the levels of both non‐bilayer‐forming monogalactosyl‐diacylglycerol (MGDG) and bilayer‐forming digalactosyl‐diacylglycerol (DGDG) are reduced (Harsányi et al., 2006). Lipid‐mediated binding and transport of proteins, such as ribulose‐1,5‐bisphosphate carboxylase oxygenase (RuBisCO) and plastoquinone, may be directly affected by such changes in membrane structure. It would be interesting to explore the mode of plant virus‐induced alterations in lipid membrane structure.
Viral Proteins Exploit Both Canonical and Non‐Canonical Chloroplast Transport Pathways
As mentioned earlier, horizontal gene transfer has re‐localized a large number of chloroplast‐related genes into the nuclear genome (Richly and Leister, 2004). Many of these gene products possess N‐terminal chloroplast localization signals and use the Tic/Toc transportation system (Bedard and Jarvis, 2005). Most chloroplast transit peptides (TPs) are rich in serine, threonine and alanine residues (25–150 amino acids in length), and contain an uncharged N‐terminal and a basic internal region (May and Soll, 2000; Zhang and Glaser, 2002). Once the precursor protein enters into the stroma, TP undergoes specific proteolytic cleavage (Gary and Row, 1995; Hageman et al., 1990) and mature protein is released inside the organelle. In addition to this well‐known pathway, recently, several non‐canonical pathways of chloroplast localization have been mapped out. Such complexity in organelle localization is reflected by various strategies adopted by pathogens to send effector molecules into the chloroplast (Petre et al., 2015).
Plant viruses also manipulate the host cell's protein sorting mechanism by molecular mimicry (Fig. 2). One example of such a strategy is exhibited by the N‐terminal domain (called the arm domain) of CP of Cucumber necrosis virus (CNV). The arm sequence bears sequence similarity to typical chloroplast TPs (Xiang et al., 2006). However, not all viral proteins localizing to the thylakoid membrane, such as the CP of Tobacco mosaic virus (TMV) and Potato virus X (PVX), βC1 of Radish leaf curl betasatellite (RaLCB) or chloroplast targeting of P5‐2 of Rice black‐streaked dwarf virus (Bhattacharyya et al., 2015; Liu et al., 2015; Qiao et al., 2009; Reneiro and Beachy, 1986), possess such in‐built conventional TPs. They are likely to deploy different mechanisms to find their way to/into the chloroplast. In organello synthesis from the viral genome or interactions with host protein(s) may explain their chloroplast localization. However, efforts to identify in organello CP synthesis of TMV or PVX (Banerjee and Zaitlin, 1992; Qiao et al., 2009; Reneiro and Beachy, 1989) have not been successful to date. The analysis of mRNA from PVX‐infected plants could not detect the presence of PVX RNA in the chloroplast, indicating that PVX CP is not synthesized in the chloroplast from viral RNA, but interacts with the TP of the precursor of plastocyanin and localizes post‐translationally into the organelle (Qiao et al., 2009).
Protein import in the chloroplast is influenced by photosynthesis, light, redox state and developmental stage (Li and Chiu, 2010). The induction of a change in any of these states, e.g. reaction with redox‐related proteins, such as ferredoxin and Fe–S cluster proteins (Table 1), may assist viruses to target organelles. Moreover, specific translocons are available for the import of proteins; house‐keeping proteins use a different transport complex than photosynthetic proteins (Ivanova et al., 2004; Smith et al., 2004). In the presence of a high level of photosynthesis, viral proteins may use unique translocation machinery and directly or indirectly affect the transport of host proteins into the organelle. The translocation of nuclear‐encoded proteins into the plastid is largely dependent on the lipid‐mediated binding of the precursor protein on the chloroplast surface. NADPH:protochlorophyllide oxidoreductase (POR) is a nuclear‐encoded plastid inner membrane protein. Isoform B of POR translocates to the plastid through the canonical Tic/Toc pathway, whereas isoform A uses a non‐canonical translocation pathway (Schemenewitz et al., 2007). It has been suggested that Barley stripe mosaic virus (BSMV) infection alters the lipid composition of the etioplast membrane and thus affects the translocation of POR, causing structural and functional retardation of plastid development (Harsányi et al., 2006). Overall, further efforts are needed from plant biologists to decipher the exact mechanism of the chloroplast localization of viruses.
Table 1.
Interactions between viral‐ and chloroplast‐related proteins.
| Virus(es) | Viral protein | Chloroplast protein | Implication of interaction | Reference |
|---|---|---|---|---|
| Alfalfa mosaic virus | CP | PsbP | PsbP is sequestered into the cytosol to prevent its repression effect on virus accumulation | Balasubramaniam et al. ( 2014) |
| Tomato mosaic virus (ToMV) | MP | RbcS | Cell‐to‐cell movement of virus and plant antiviral defence | Zhao et al. ( 2013) |
| CP | Ferredoxin | Regulates symptom development and pathogenicity of the virus | Sun et al. ( 2013) | |
| CP | IP‐L | Long‐distance movement of ToMV affecting chloroplast stability | Li et al. ( 2005) | |
| Plum pox virus | CI | Photosystem I PSI‐K | Regulates virus infection | Jiménez et al. ( 2006) |
| Cauliflower mosaic virus | P6 | CHUP1 | Intracellular movement of inclusion body | Harries et al. ( 2009) |
| Tobacco mosaic virus (TMV) | Replicase | ATP synthase γ‐subunit (AtpC), RuBisCO activase (RCA) | Regulation of defence response against TMV | Bhat et al. ( 2013) |
| Replicase | NRIP1 | Elicitation of effector‐triggered immunity | Caplan et al. ( 2008) | |
| RNA helicase domain of replicase | PsbO | Virus accumulation increases | Abbink et al. ( 2002) | |
| Cucumber mosaic virus (CMV) | CMV 1a | Tsi1‐interacting protein 1 | Regulation of viral replication | Huh et al. ( 2011) |
| CMV 2a | ||||
| Soybean mosaic virus | P1 | Rieske Fe/S | Translocation of the host protein into the chloroplast is hampered | Shi et al. ( 2007) |
| Sugarcane mosaic virus | HC‐Pro | Ferredoxin‐5 precursor | Translocation of the host protein into the chloroplast is hampered | Cheng et al. ( 2008) |
| Potato virus Y | CP | RuBisCO large subunit | Regulates symptom development and pathogenicity of the virus | Feki et al. ( 2005) |
| HC‐Pro | MinD | Interferes with chloroplast division | Jin et al. ( 2007) | |
| HC‐Pro | DXS | Increase in isoprenoid biosynthesis | Li et al. ( 2015) | |
| Shallot yellow stripe virus | P3 | RuBisCO | Affects the normal functions of RubisCO | Lin et al. ( 2011) |
| Alternanthera mosaic virus | TGB3 | PsbO | Symptom development and lethal damage under dark conditions | Jang et al. ( 2013) |
| TGB1L88 | βATPase | Elicits defence response | Seo et al. ( 2014) | |
| Potato virus X | CP | Plastocyanin transit peptide | Coat protein accumulation in chloroplasts and increased symptom severity | Qiao et al. ( 2009) |
| Rice stripe virus (RSV) | Disease‐specific protein | PsbP | Disruption of chloroplast leading to RSV accumulation | Kong et al. ( 2013) |
| Papaya ringspot virus | NIa‐pro | Methionine sulfoxide reductase B1 | Probable interference with PaMsrB1 localization from cytosol to the chloroplasts to scavenge ROS | Gao et al. ( 2012) |
CI, cylindrical inclusion protein; CP, coat protein; HC‐Pro, helper component proteinase; MP, movement protein; NIa‐pro, nuclear inclusion protein a protease; ROS, reactive oxygen species.
Association of Viruses and Chloroplasts is Important for Systemic Infection
Plant viruses can exploit the endogenous host trafficking system made up of the cytoskeleton, ER and Golgi network to move inside the susceptible host cell (Genoves et al., 2010; Laporte et al., 2003). In the absence of vesicle‐mediated transport, viral nucleic acids become associated with specific viral and/or host proteins to move through the hostile territory inside the cell. Being devoid of gene silencing machinery, chloroplasts may serve as a safe compartment and protect the viral genome from host defence molecules. Abutilon mosaic virus movement protein (MP) interacts with nucleus‐encoded, chloroplast‐targeted heat shock protein cpHSP70 and induces the formation of a tubular network of stromules containing cpHSP (Krenz et al., 2010). Such structures can create channels which may aid the local and systemic movement of viruses. Triple gene block proteins of many positive‐stranded RNA viruses are proteins that aid in the intracellular movement of the viral genome. A unique cistron encodes three proteins, namely TGB1, TGB2 and TGB3. Broadly, TGB1 interacts with plasmodesmata (PD), whereas TGB2 and TGB3 aid the viral nucleic acid to move through an ‘ER–Golgi’ tract (reviewed by Morozov and Solovyev, 2003). However, there exists a finer level of specificity in the mechanism for different viruses. TGB2 proteins of BSMV and Potato mop‐top virus (PMTV), two members of the hordei‐like group of viruses and both requiring chloroplast association for replication, direct their nucleic acid to the organelle in a different manner (Cowan et al., 2012; Torrance et al., 2006). Localization assay with green fluorescent protein (GFP)‐fused TGB2 showed that the presence of genomic RNA was important for TGB2 of BSMV to localize into the chloroplast, but not for TGB2 of PMTV (Torrance et al., 2006). The internal chloroplast localization sequence of TGB3 is indicative of a chloroplast‐targeted evolution. Interestingly, mutations in TGB3 of Alternanthera mosaic virus (AltMV) and in the single‐stranded, positive‐sense RNA genome of Bamboo mosaic virus (BaMV) caused impairments in replication and movement of the viruses (Lim et al., 2010; Lin et al., 2007). Geminivirus‐encoded βC1 is a chloroplast‐targeted protein which is required for systemic movement and symptom development of the helper virus (Bhattacharyya et al., 2015). As βC1 is deficient in the canonical chloroplast localization signal, we may infer that movement within the chloroplast is non‐canonical.
Inhibition in chloroplast localization of BaMV RNAs was observed when chloroplast p51 protein was mutated. Consequently, it also resulted in the decreased accumulation of viral CP (Cheng et al., 2013). Chloroplast localization of RNA can be explained by observations on the transport of non‐coding RNAs of viroids (Gómez and Pallás, 2010, 2012). This process requires a specific structural motif for nuclear import, together with a specific receptor via the cytoskeleton‐independent route. Although not observed to date in the context of viruses, viroid non‐coding RNA with a specific structural motif takes part in the chloroplast localization process (Gómez and Pallás, 2010, 2012). Although this observation represents a viroid‐specific intracellular RNA trafficking mechanism, it could act as a base model to explore the viral RNA movement in plants.
Involvement of Chloroplast Proteins in the Infection Process
Many chloroplast proteins assisting in viral propagation are localized in the chloroplast or functionally related to the photosynthetic machinery (Abbink et al., 2002; Bhat et al., 2013; Qiao et al., 2009; Seo et al., 2014). Chloroplast unusual positioning protein1 (CHUP1)–P6 interaction aids the movement of the Cauliflower mosaic virus (CaMV) inclusion body inside the cell along the microfilament to reach PD (Harries et al., 2009). Such interactions influence the accumulation, cell‐to‐cell and long‐distance movement of viruses or the defence response in plants. Various chloroplast and associated factors which are involved in the infection processes of specific viruses are listed in Table 1. Given the localization of the host protein, these interactions may take place in the thylakoid lumen, thylakoid membrane, stroma, chloroplast membrane or cytosol. As mentioned in the previous section, light can be a decisive factor in the localization of a host protein. During AltMV infection, TGB3–PsbO interaction in the cytosol is intensified in dark conditions, resulting in an increased symptom severity in plants (Jang et al., 2013). From a plant‐centric perspective, many of these interactions are involved in the activation of the antiviral defence response (Caplan et al., 2008). From a virus‐centric perspective, chloroplast proteins, such as PsbP (Balasubramaniam et al., 2014), facilitate the replication of viruses and are sequestered by the viral counterpart in order to minimize their negative effect on the pathogen (Fig. 1). In other words, such interactions are likely to maintain a dynamic equilibrium, which decides the fate of a certain chloroplast–virus interaction.
Virus Infection Massively Changes the Structure and Function of the Chloroplast
The presence of healthy photosynthetic plant tissues is often a prerequisite for virus infection. For their survival and propagation, viruses make use of the energy stored inside carbon compounds prepared by chloroplasts. Inside the host cell, the virus regulates diverse processes, such as sugar efflux, carbon partition and phloem transport of metabolites, increasing the need for photosynthesis (Balachandran et al., 1995; Chen et al., 2010; Olesinski et al., 1995). Nevertheless, the enhanced activity and vigour of the organelle also increases the threat of the antiviral immunity response.
A large number of recent studies, carried out using proteomic and transcriptomic approaches, have revealed how viral infection affects primarily the expression of CPRGs (Liu et al., 2014; Mochizuki et al., 2014a; Wu et al., 2013). RaLCB infection causes structural and functional damage to the chloroplast of Nicotiana benthamiana (Bhattacharyya et al., 2015) by selective suppression of gene expression; TMV flavum strain CP accumulates in and reduces the expression of tobacco chloroplast proteins, upsetting the efficiency of photosynthesis (Lehto et al., 2003); and TuMV infection suppresses the expression of the gene coding for chloroplast elongation factor Tu of Chinese cabbage (Peng et al., 2014) (Table 2). Virus‐infected plants manifest striking mosaic symptoms (Channarayappa et al., 1992; Esau, 1933) associated with swollen chloroplasts, large amounts of starch and plastoglobulin accumulation and disintegrated grana stacks (Bhattacharyya et al., 2015; Otulak et al., 2015). Physically, virus particles are often found to be associated with the chloroplasts of diseased hosts (Bhattacharyya et al., 2015; Lehto et al., 2003). Selective suppression of a set of genes (Bhattacharyya et al., 2015; Lehto et al., 2003) and/or targeted interaction with specific chloroplast proteins (Peng et al., 2014) can both produce massive changes in the organelle (Liu et al., 2014; Mochizuki and Ohki, 2011; Mochizuki et al., 2014b; Pérez‐Bueno et al., 2004; Pineda et al., 2010; Wu et al., 2013). For example, six distinct types of mosaic symptoms were manifested in different single point CP mutants of CMV‐infected tobacco plants. These symptoms were associated with the abnormal expression of large numbers of CPRGs (Mochizuki and Ohki, 2011; Mochizuki et al., 2014b) (Fig. 3). The CMV 2b mutant, which lacks a viral suppressor of RNA silencing, was unable to cause pale‐green chlorosis. Nevertheless, the same 2b mutant, when present with specific point mutant CP, caused mosaic symptoms (Mochizuki et al., 2014a). Such exactitude in affecting the host is probably attained by sequence‐specific mechanisms, such as interference with the miRNA‐mediated regulation of CPRGs. A brief account of the various CPRGs down‐regulated by virus infection is provided in Table 2. Such single point mutations may also affect the subcellular localization of CP and its mode of interference. Viral protein may directly inhibit the chloroplast translocation of host proteins (Tomato mosaic virus, ToMV) (Zhang et al., 2008), which may be important for specific functions, such as electron transport (Soybean mosaic virus) (Shi et al., 2007) or structural development. Damage of the chloroplast ultrastructure and/or function is the natural outcome of such inhibitions (Table 1).
Table 2.
List of representative chloroplast‐ and photosynthesis‐related genes affected by virus infection.
| Virus–host | Gene | Reference |
|---|---|---|
| Cucumber mosaic virus–tobacco | Chlorophyll synthesis enzymes | Mochizuki et al. ( 2014b) |
| Glutamyl tRNA reductase (hemA gene) | ||
| Protoporphyrin IX oxidase | ||
| Mg protoporphyrin IX chelatase (ChlH) | ||
| Mg protoporphyrin chelatase subunit (ChlI) | ||
| S‐Adenosyl‐l‐methionine Mg‐protoporphyrin IX methyltranserase (ChlM) | ||
| NADPH:protochlorophyllide oxidoreductase | ||
| Chlorophyll synthase (ChlG) | ||
| Antenna proteins | ||
| Photosystem I (PSI) light‐harvesting chlorophyll a/b‐binding protein | ||
| Chloroplast pigment‐binding protein | ||
| Light‐harvesting chlorophyll a/b‐binding protein precursor | ||
| PSI‐related proteins | ||
| Photosystem I reaction centre subunit X psaK | ||
| Photosystem I psaH protein precursor | ||
| Photosystem I reaction centre subunit IV A | ||
| Photosystem I subunit XI | ||
| PSII‐related proteins | ||
| Chloroplast photosystem II PsbR | ||
| Photosystem II P680 chlorophyll A apoprotein | ||
| Photosystem II 23‐kDa polypeptide | ||
| Photosystem II 44‐kDa reaction centre protein | ||
| Oxygen‐evolving complex 33‐kDa photosystem II protein (PsbO) | ||
| Chloroplast oxygen‐evolving protein 16‐kDa subunit | ||
| Electron transport chain | ||
| Plastid quinol oxidase | ||
| Cytochrome b6 | ||
| RuBisCO proteins | ||
| Ribulose bisphosphate carboxylase large‐chain precursor | ||
| Ribulose bisphosphate carboxylase small chain | ||
| Ribulose‐1,5‐bisphosphate carboxylase | ||
| Chlorophyll catabolism | ||
| Chlorophyllase mRNA | ||
| Red chlorophyll catabolite reductase | ||
| Chloroplast differentiation | ||
| Plastid division regulator MinD mRNA | ||
| FtsZ‐like chloroplast protein | ||
| Antioxidant defence | ||
| Superoxide dismutase [Fe] | ||
| Stromal ascorbate peroxidase | ||
| Thylakoid‐bound ascorbate peroxidase | ||
| Glutathione reductase | ||
| Radish leaf curl betasatellite–Nicotiana benthamiana | Chlorophyll biosynthesis and photosystem | Bhattacharyya et al. ( 2015) |
| Chlorophyll synthase (ChlG) | ||
| Mg protoporphyrin IX chelatase (ChlH) | ||
| Mg protoporphyrin chelatase subunit (ChlI) | ||
| Glutamyl tRNA reductase (hemA gene) | ||
| Ferrochelatase | ||
| Photosystem II protein T | ||
| 33‐kDa protein of oxygen‐evolving complex (PsbO) | ||
| 23‐kDa protein of oxygen‐evolving complex (PsbP) | ||
| RuBisCO | ||
| Ribulose bisphosphate carboxylase large subunit | ||
| Ribulose bisphosphate carboxylase small subunit | ||
| Plastid development and chloroplast defence | ||
| ATP‐dependent ClpP1 protease | ||
| Maturase K | ||
| Allene oxide cyclase 3, chloroplast precursor | ||
| Tobamoviruses–Nicotiana benthamiana | 33‐kDa protein of oxygen‐evolving complex (PsbO) | Pérez‐Bueno et al. ( 2004) |
| 23‐kDa protein of oxygen‐evolving complex (PsbP) | ||
| Pepper mild mottle virus–Nicotiana benthamiana | Photosystem I PsaD protein | Pineda et al. ( 2010) |
| Ribulose bisphosphate carboxylase large subunit | ||
| Ribulose bisphosphate carboxylase small subunit | ||
| Ribulose 1,5‐bisphosphate carboxylase oxygenase activase | ||
| Cytochrome f | ||
| Ferredoxin‐NADP+ reductase | ||
| Glutamine synthetase | ||
| Phosphoribulokinase | ||
| Phosphoglycerate kinase | ||
| Sedoheptulose‐1,7‐bisphosphatase |
Figure 3.
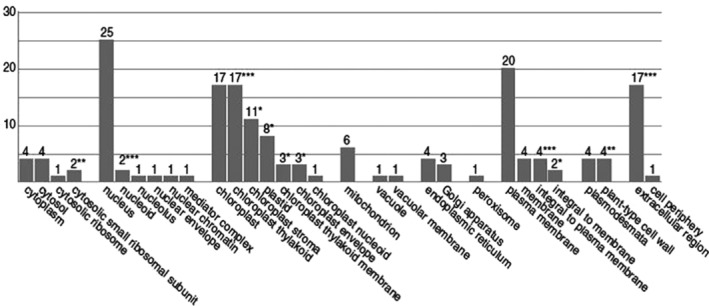
Organelle‐wise distribution of differentially expressed down‐regulated genes in tobacco during Cucumber mosaic virus infection. The graph shows that chloroplast‐related gene products comprise the highest percentage of differentially expressed genes. The figure is borrowed from the study of Mochizuki et al. (2014b) with permission.
Mosaic symptoms are often associated with an altered number and structure of chloroplasts (Bhattacharyya et al., 2015; Reuveni et al., 2015). Potyvirus‐encoded helper component proteinase (Hc‐Pro) interacts with MinD, a Ca2+‐dependent ATPase that controls the symmetric division of the plastid (Jin et al., 2007). The interaction inhibits the dimerization process and subsequent activities of MinD. The resulting chloroplasts increase in size (Fig. 1) (Tu et al., 2015). To protect the photosynthetic machinery from damage, at high light intensity, chloroplasts accumulate in the anticlinal position of the plasma membrane. CHUP1, a thylakoid membrane‐associated protein, aids in such movements by interacting with the microfilaments (Lehmann et al., 2011; Oikawa et al., 2008). Interaction of viral protein with CHUP1 helps the inclusion body of the virus to adhere to the microfilament and move inside the cell (Harries et al., 2009). However, the role of CHUP1 in plant–pathogen interaction has a rather complex implication. Interestingly, CHUP1‐silenced background plants constitutively induce the formation of stromules, a dynamic tubular structure involved in chloroplast–nucleus retrograde signalling in the plant's defence response against pathogens (Caplan et al., 2015). The stromule network is assumed to facilitate the systemic movement of geminiviruses (Krenz et al., 2012). During a plant–virus interaction, whether the chloroplast‐generated stromule network acts as a stretchable active ‘tract’ for viruses (Sattarzadeh et al., 2009), or contributes to chloroplast–nucleus defence signalling, requires detailed study. It is probable that the course of virus propagation will be determined depending on the specificity/stage of infection.
Chloroplast‐Mediated Defence Response Against Viruses
The accumulation of virus particles in living hosts depends on the result of the constant arms race between the host's defence response [e.g. hypersensitive response (HR) or post‐transcriptional gene silencing] and the pathogen's counter‐defence mechanisms (suppression of gene silencing). The organelle is a rich source of reactive oxygen species (ROS) and, when threatened by pathogens, including viruses, elicits effector‐triggered immunity (ETI) and HR, followed by programmed cell death (PCD) in the plant (Caplan et al., 2008). The organelle synthesizes salicylic acid, the primary defence hormone involved in both the local and systemic resistance of the plant. The synthesis of other plant hormones, such as jasmonic acid and abscisic acid, is closely regulated by the chloroplastic machinery. Moreover, a large pool of Ca2+ is stored in the chloroplast, whose level is changed in response to pathogen attack and immune signalling (Mur et al., 2008).
The expression of RNA‐dependent RNA polymerase1 (RDR1), an important component of antiviral RNA silencing, is increased by the exogenous application of phytohormones (Hunter et al., 2013; Pandey and Baldwin, 2007; Wang et al., 2010). Although the chloroplast lacks gene silencing machinery, this crosstalk between chloroplast‐derived hormones and RNA silencing is pertinent. The different aspects of the chloroplast‐mediated defence response are depicted in Fig. 4.
Figure 4.
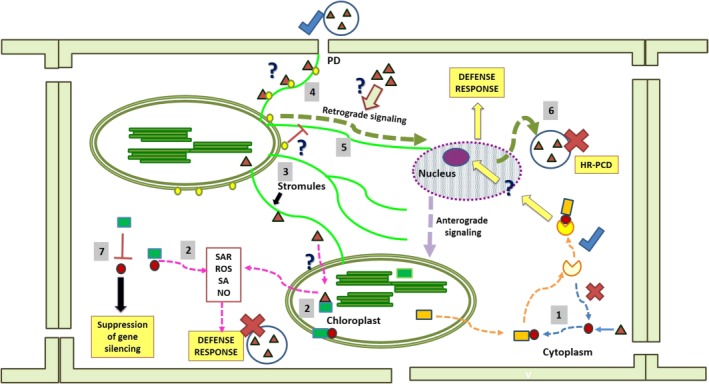
Conceptual depiction of the major lines of antiviral defence conferred by the chloroplast. For simplified representation, the same symbols have been used for different viruses (filled red triangles) and their gene products (filled red circles). Similarly filled green squares are used for interacting chloroplast proteins. Crosses are used to highlight an unfavourable reaction from the viewpoint of viruses, whereas blue ticks are used for favourable reactions. A detailed description is given in the text. N receptor‐interacting protein 1 (NRIP1) is originally located in the chloroplast and, on Tobacco mosaic virus (TMV) infection, it is recruited to the cytoplasm. The innate immunity of the plant against TMV is elicited by the activation of the cytoplasm/nucleus‐localized N‐immune receptor through the NRIP1–TMV p50 complex. The resultant active immune complex initiates the nucleus‐mediated defence response (1). Gene products of various viruses interact with different chloroplast proteins inside the organelle or cytoplasm and elicit the basal defence response (2). Virus infection causes the induction/enhanced accumulation of tubular channels of ‘stromules’ (3). Through chloroplast unusual positioning protein1 (CHUP1), viruses attach themselves to stromules and perform intra‐ and intercellular movements (4). Through stromules, the hypersensitive response‐mediated retrograde signal is transduced from the chloroplast to the nucleus (5) and programmed cell death takes place (6). By interacting with the viral suppressor of gene silencing, chloroplast proteins interfere with the virus counter‐defence mechanism (7). HR‐PCD, hypersensitive response‐programmed cell death; NO, nitric oxide; PD, plasmodesmata; ROS, reactive oxygen species; SA, salicylic acid; SAR, systemic acquired resistance.
The silencing of N. benthamiana photosystem I PSI‐K, a chloroplast thylakoid protein that interacts in vitro with the CI protein of Plum pox virus (PPV) of the Potyviridae family, enhances PPV accumulation (Jiménez et al., 2006). TGB1L88 (TGB1 protein with strong silencing suppressor activity) of AltMV selectively interacts with chloroplast βATPase and elicits the defence response (Seo et al., 2014). However, the involvement of chloroplast proteins in antiviral defence may cause the organelle to compromise its photosynthetic efficiency, as evident in the case of HR‐induced PCD in TMV‐infected resistant tobacco plants. PPV infection damages chloroplast metabolism, especially involving photosystem II (PSII), leading to the accumulation of ROS (Díaz‐Vivancos et al., 2008). To minimize such chloroplast‐derived stress signals, as seen in Alfalfa mosaic virus or Papaya ringspot virus, viral effectors may sequester proteins, such as PsbP or methionine sulfoxide reductase B1, in the cytosol, inhibiting their organelle localization and suppressing ROS production (Balasubramaniam et al., 2014; Gao et al., 2012). The amount of thylakoid‐bound protein DS9 decreases with increasing HR response and with decreasing photosynthetic electron transport rate (Seo et al., 2000). During PVY infection, HC‐Pro and a chloroplast protein interact, and the biosynthesis of isoprenoids is increased (Li et al., 2015). Isoprenoids exert a positive effect on the plant's defence, growth, metabolism and photosynthesis; hence, such an observation appears paradoxical.
The chloroplast‐mediated defence response is largely light regulated (Kangasjarvi et al., 2012). Light influences resistance gene‐induced HR during virus infection in Arabidopsis (Chandra‐Shekara et al., 2006). The expression of the RuBisCO small subunit, another chloroplast protein involved in defence against viruses, is light dependent. In RbCS‐silenced N. benthamiana, the expression of the PR1 gene was suppressed and ToMV was able to induce local necrosis (Zhao et al., 2013). As an avirulence factor, ToMV MP is recognized by tomato (Solanum lycopersicum) proteins encoded by resistance genes, namely Tm‐2 and Tm‐22. Tm‐22 confers resistance against both TMV and ToMV in tomato and tobacco (N. tabacum) plants, whereas RbCS plays an important role in resistance (Lanfermeijer et al., 2004; Zhao et al., 2013). MP of TMV acts as an enhancer of gene silencing, and hence the RbCS‐mediated defence response in tobamovirus infection is likely to contribute to the maintenance of the host silencing apparatus. Alternatively, RbcS may act together with any other component of the photosynthesis machinery, such as the oxygen‐evolving complex (OEC), to elicit the defence response in plants. OEC is one of the most vulnerable protein complexes in plants and is extremely susceptible to biotic and abiotic stress conditions (Ashraf and Harris, 2013; Bhattacharyya et al., 2015; Lehto et al., 2003). OEC 33 (alternatively named PsbO) binds to the helicase domain of TMV replicase. As, in OEC 33‐silenced plants, TMV accumulation is increased significantly (Abbink et al., 2002), OEC 33 may be involved in the basal defence response against TMV in tobacco.
Tobacco stress‐induced 1 (Tsip1), which is mainly localized on the chloroplast surface, is capable of diffusing into the cytoplasm and nucleus with phytohormone‐responsive transcription factors, such as ethylene‐response factor (ERF). By interacting directly with the CMV replicase complex associated with the vacuole‐like membrane, Tsip1 affects the replication of the virus (Huh et al., 2011). By forming a tripartite complex involving viral replicase‐associated protein (CMV 1a) and RNA‐dependent RNA polymerase protein (CMV 2a), host Tsip1 interferes with both the replication and movement process of the virus.
Nuclear‐encoded chloroplast proteins, such as ATP synthase γ‐subunit (AtpC) and Rubisco activase (RCA), are co‐purified with VRC during TMV infection. By interacting with TMV replicase, both AtpC and RCA play an active role in the plant's defence against the spread of TMV and Turnip vein clearing virus. However, neither AtpC nor RCA could influence PVX or CMV infection (Bhat et al., 2013).
Plant innate immunity is based on the recognition of specific domains or motifs present in pathogen effector molecules by nucleotide‐binding leucine‐rich repeat (NB‐LRR) immune receptor families (DeYoung and Innes, 2006). The chloroplast‐mediated defence response through pathogen recognition, as in the case of ETI, posits a scenario of a two‐step challenge: first, it requires either the receptor or the elicitor protein component to be localized into the organelle; second, it requires the retrograde signal to be sent to the nucleus to elicit the subsequent defence response. An intensive search using advanced software, such as ‘LocTree’ and ‘ChloroP’, reported that around 22 Toll interleukin receptor (TIR)‐NB‐LRRs from different families contained putative chloroplast localization signals, indicating the possibility of an extensive network of chloroplast‐based pathogen recognition systems. However, receptors that are not chloroplast localized can recognize their pathogenic counterparts through the mediation of chloroplast proteins. During TMV infection, the 50‐kDa helicase domain of TMV replicase (p50) was recognized indirectly by the TIR domain of N, an immune receptor belonging to the TIR‐NB‐LRR class (Burch‐Smith et al., 2007). The recognition process takes place through N receptor‐interacting protein 1 (NRIP1), which is localized in the chloroplast, but, in the presence of p50, is released from the organelle. The association of NRIP1 with both the TIR domain of N and the p50 effector, forming a tripartite complex, activates defence signalling (Caplan et al., 2008). On pathogen recognition, the chloroplast structure undergoes general alteration and NRIP1 is released to the cytoplasm. As a chloroplast‐to‐nucleus retrograde signalling molecule, it reprograms the transcriptional blueprint of host defence. Indeed, in mutant gun1 and abi4 Arabidopsis plants compromised in plastid signalling, CMV infection was found to be more severe than in wild‐type plants (Fu et al., 2015). As CMV infection alters chloroplast‐to‐nucleus retrograde signalling, the differential CPRG suppression by CP mutants, which was observed in tobacco, may be related to the different level of CP‐mediated interference with retrograde signalling (Mochizuki et al., 2014b).
In a recent study, the formation of an array of tubular structures full of stroma (stromules) spanning the chloroplast to nucleus during the TMV p50‐induced, N‐mediated defence response was observed (Caplan et al., 2015). These stroma‐filled double‐membrane channels have a strong association with the elicitation of HR‐PCD, and have been proposed to be responsible for the transduction of the pro‐defence signal from the chloroplast to the nucleus. The actin cytoskeleton is important for stromule formation, and thus may be involved in the trafficking of viruses. During the HR following virus infection, plant cells undergo rapid death, leading to the formation of necrotic lesions (Goodman and Novacky, 1994). The presence of the resistant N gene affiliates the HR in tobacco against TMV infection (Whitham et al., 1994). The expression of DSC9, an FtsH protease which is the only ATP‐dependent metalloprotease present in thylakoid membranes (Lindahl et al., 1996), is diminished post‐TMV infection. Being a protease, FtsH is essentially involved in the degradation of denatured and unfolded chloroplastic proteins to maintain homeostasis (Andersson and Aro, 1997). As FtSH speculatively ‘cleans up’ the damaged D1 protein of the PSII reaction centre in virus‐infected plant cells, a down‐regulation of the FtsH level following TMV infection (Seo et al., 2000) causes photosynthetic inhibition and subsequent cell death, resulting in HR and necrosis on virus infection.
Conclusion and Future Perspectives
Given the dynamic role of the chloroplast in the signalling network, much is unknown about the implications of chloroplast–virus interactions. The major future prospect in the field lies in the manipulation of chloroplast proteins or genetic properties to control the threat of plant‐infecting viruses. The special attributes of chloroplasts, such as the high copy number genome and the absence of gene silencing machinery, should be used to produce transplastomic plants with boosted antiviral activity. Furthermore, as discussed in this review, an understanding of the central aspects of virus infection involving chloroplasts will lead to engineering strategies to increase antiviral resistance. For example, the regulation of the expression level of chloroplast‐bound proteins, such as CHUP1 involved in viral movement or chloroplast membrane‐bound SNARE proteins involved in vesicle trafficking, is a promising tool to control the subsequent infection cascade of viruses. The overexpression of PsbP negatively regulates Alfalfa mosaic virus accumulation. Such a PsbP‐mediated antiviral state might be mimicked in the case of other host–virus systems. HR elicited during a virus infection causes a change in cellular Ca2+ ion homeostasis. MPs of both DNA and RNA viruses have been shown to interact with the Arabidopsis Ca2+ sensor synaptotagmin (SYTA), prior to their genome trafficking through PD (Uchiyama et al., 2014). As shown in Fig. 1, the hypothesis is worth exploring if the plastid‐localized Ca2+ sensor(s), such as CAS, is(are) involved in the subsequent movement of the virus in/out of the chloroplast or PD. Targeted modification of chloroplast genome editing, with technologies such as Transcription Activator‐Like Effector Nucleases (TALENs) and Clustered Regulatory Interspaced Short Palindromic Repeats/Cas9 system (CRISPR‐Cas9), represents a powerful tool for the design of such next‐generation, virus‐resistant plants. For example, a chloroplast protein involved in virus movement can be knocked out using a guide RNA sharing sequence similarity to the specific RNA to direct the CAS9 nuclease to disrupt the target region. However, careful selection of candidate chloroplast proteins is important for gene editing to minimize the disruptive effect on photosynthesis or other cellular pathways. Finally, a critical breakthrough in the area of plant‐pathogenic viruses can be achieved by exploring the chloroplast–nucleus signalling network. The role of stromules in chloroplast‐regulated intercellular trafficking of viruses through PD can be understood from mutant lines with affected stromule formation. Overall, the identification of the relevant chloroplast proteins as candidates for the development of antiviral resistance in important crop species represents a very promising research area to explore in the future.
Conflicts of Interest
The authors declare that they do not have any conflicts of interest.
Acknowledgements
We gratefully acknowledge the financial support from the Department of Science and Technology, Government of India (Grant no: SERB/SB/SO/PS/107/2013).
References
- Abbink, T.E. , Peart, J.R. , Mos, T.N. , Baulcombe, D.C. and Bol, J.F. (2002) Silencing of a gene encoding a protein component of the oxygen‐evolving complex of photosystem II enhances virus replication in plants. Virology, 295, 307–319. [DOI] [PubMed] [Google Scholar]
- Ahlquist, P. , Noueiry, A.O. , Lee, W.M. , Kushner, D.B. and Dye, B.T. (2003) Host factors in positive‐strand RNA virus genome replication. J. Virol. 77, 8181–8186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersson, B. and Aro, E.M. (1997) Proteolytic activities and proteases of plant chloroplasts. Physiol. Plant. 100, 780–793. [Google Scholar]
- Ashraf, M. and Harris, P.J.C. (2013) Photosynthesis under stressful environments: an overview. Photosynthetica, 51, 163–190. [Google Scholar]
- Balachandran, S. , Hull, R.J. , Vaadia, Y. , Wolf, S. and Lucas, W.J. (1995) Alteration in carbon partitioning induced by the movement protein of Tobacco mosaic virus originates in the mesophyll and is independent of change in the plasmodesmal size exclusion limit. Plant Cell Environ. 18, 1301–1310. [Google Scholar]
- Balasubramaniam, M. , Kim, B.S. , Hutchen‐Williams, H.M. and Loesch‐Fries, L.S. (2014) The photosystem II oxygen evolving complex protein, PsbP, interacts with the coat protein of Alfalfa mosaic virus and inhibits virus replication. Mol. Plant–Microbe Interact. 27, 1107–1118. [DOI] [PubMed] [Google Scholar]
- Banerjee, N. and Zaitlin, M. (1992) Import of Tobacco Mosaic Virus coat protein into intact chloroplast in vivo. Mol. Plant–Microbe Interact. 5, 466–471. [Google Scholar]
- Beale, S.I. (2011) Chloroplast signaling: retrograde regulation revelations. Curr. Biol. 21, 391–393. [DOI] [PubMed] [Google Scholar]
- Beck, C.F. (2005) Signaling pathways from the chloroplast to the nucleus. Planta, 222, 743–756. [DOI] [PubMed] [Google Scholar]
- Bedard, J. and Jarvis, P. (2005) Recognition and envelope translocation of chloroplast preproteins. J. Exp. Bot. 56, 2287–2320. [DOI] [PubMed] [Google Scholar]
- Bejarano, E.R. , Khashoggi, A. , Witty, M. and Lichtenstein, C. (1996) Integration of multiple repeats of geminiviral DNA into the nuclear genome of tobacco during evolution. Proc. Natl. Acad. Sci. USA, 93, 759–764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhat, S. , Folimonova, S.Y. , Cole, A.B. , Ballard, K.D. and Lei, Z. (2013) Influence of host chloroplast proteins on Tobacco mosaic virus accumulation and intercellular movement. Plant Physiol. 161, 134–147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhattacharyya, D. , Prabu, G. , Reddy, K.K. , Kushwaha, N.K. , Sharma, V.K. , Yusuf, M.A. and Chakraborty, S. (2015) A geminivirus betasatellite damages the structural and functional integrity of chloroplasts leading to symptom formation and inhibition of photosynthesis. J. Exp. Bot. 66, 5881–5895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burch‐Smith, T.M. , Schiff, M. , Caplan, J.L. , Tsao, J. , Czymmek, K. and Dinesh‐Kumar, S.P. (2007) A novel role for the tir domain in association with pathogen‐derived elicitors. PLoS Biol. 5, e68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caplan, J.L. , Mamillapalli, P. , Burch‐Smith, T.M. , Czymmek, K. and Dinesh‐Kumar, S.P. (2008) Chloroplastic protein NRIP1 mediates innate immune receptor recognition of a viral effector. Cell, 132, 449–462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caplan, J.L. , Kumar, A.S. , Park, E. , Padmanabhan, M.S. , Hoban, K. , Modla, S. , Czymmek, K. and Dinesh‐Kumar, S.P. (2015) Chloroplast stromules function during innate immunity. Dev. Cell, 34, 45–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castro, I.F. , Raquel, T. and Risco, C. (2016) Virus assembly factories in a lipid world. Curr. Opin. Virol. 18, 20–26. [DOI] [PubMed] [Google Scholar]
- Chandra‐Shekara, A.C. , Gupte, M. , Navarre, D. , Raina, S. , Raina, R. , Klessig, D. and Kachroo, P. (2006) Light‐dependent hypersensitive response and resistance signalling against Turnip crinkle virus in Arabidopsis . Plant J. 45, 320–334. [DOI] [PubMed] [Google Scholar]
- Channarayappa, V.M. , Schwegler‐Berry, D. and Shivashankar, G. (1992) Ultrastructural changes in tomato infected with tomato leaf curl virus, a whitefly‐transmitted geminivirus. Can. J. Bot. 70, 1747–1753. [Google Scholar]
- Chen, L.Q. , Hou, B.H. , Lalonde, S. , Takanaga, H. , Hartung, M.L. , Qu, X.Q. , Guo, W.J. , Kim, J.G. , Underwood, W. , Chaudhuri, B. , Chermak, D. , Antony, G. , White, F.F. , Somerville, S.C. , Mudgett, M.B. and Frommer, W.B. (2010) Sugar transporters for intercellular exchange and nutrition of pathogens. Nature, 468, 527–532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng, Y.Q. , Liu, Z.M. , Xu, J. , Zhou, T. , Wang, M. , Chen, Y.T. , Li, H.F. and Fan, Z.F. (2008) HC‐Pro protein of sugar cane mosaic virus interacts specifically with maize ferredoxin‐5 in vitro and in planta. J. Gen. Virol. 89, 2046–2054. [DOI] [PubMed] [Google Scholar]
- Cheng, S.F. , Huang, Y.P. , Chen, L. , Hsu, Y. and Tsai, C.H. (2013) Chloroplast phosphoglycerate kinase is involved in the targeting of Bamboo mosaic virus to chloroplasts in Nicotiana benthamiana plants. Plant Physiol. 163, 1598–1608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cowan, G.H. , Roberts, A.G. , Chapman, S.N. , Ziegler, A. , Savenkov, E.I. and Torrance, L. (2012) The Potato mop‐top virus TGB2 protein and viral RNA associate with chloroplasts and viral infection induces inclusions in the plastids. Front Plant Sci. 3, 290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeYoung, B.J. and Innes, R.W. (2006) Plant NBS‐LRR proteins in pathogen sensing and host defense. Nature Immunol. 7, 1243–1249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Díaz‐Vivancos, P. , Clemente‐Moreno, M.J. , Rubio, M. , Olmos, E. , García, J.A. , Martínez‐Gómez, P. and Hernández, J.A. (2008) Alteration in the chloroplastic metabolism leads to ROS accumulation in pea plants in response to Plum pox virus . J. Exp. Bot. 59, 2147–2160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding, S.W. and Voinnet, O. (2007) Antiviral immunity directed by small RNAs. Cell, 130, 413–426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diray‐Arce, J. , Liu, B. , Cupp, J.D. , Hunt, T. and Nielsen, B.L. (2013) The Arabidopsis At1g30680 gene encodes a homologue to the phage T7 gp4 protein that has both DNA primase and DNA helicase activities. BMC Plant Biol. 13, 36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esau, K. (1933) Pathologic changes in the anatomy of leaves of the sugar beet, Beta vulgaris L., affected by curly top. Phytopathology, 23, 679–712. [Google Scholar]
- Feki, S. , Lotikili, M.J. , Triki‐Marrakchi, R. , Karimova, G. , Ounouna, H. , Nato, A. , Nato, F. , Guesdon, J.L. , Lafaye, P. , Ben, A. and Elgaaied, A. (2005) Interaction between tobacco Ribulose‐l,5‐biphosphate Carboxylase/Oxygenase large subunit (RubisCO‐LSU) and the PVY Coat Protein (PVY‐CP). Eur. J. Plant Pathol. 112, 221–234. [Google Scholar]
- Filée, J. and Forterre, P. (2005) Viral proteins functioning in organelles: a cryptic origin? Trends Microbiol. 13, 510–513. [DOI] [PubMed] [Google Scholar]
- Fu, F.Q. , Zhang, D.W. , Deng, S.G. , Li, J.Y. , Peng, X.J. , Tang, H. and Lin, H.‐H. (2015) Role of plastid signals in modulating Arabidopsis responses to Cucumber mosaic virus . Plant Growth Regul. 75, 761–769. [Google Scholar]
- Gao, L. , Shen, W. , Yan, P. , Tuo, D. , Li, X. and Zhou, P. (2012) NIa‐pro of Papaya ringspot virus interacts with papaya methionine sulfoxide reductase B1. Virology, 434, 78–87. [DOI] [PubMed] [Google Scholar]
- Gary, J.C. and Row, P.E. (1995) Protein translocation across chloroplast envelope membranes. Trends Cell Biol. 5, 243–247. [DOI] [PubMed] [Google Scholar]
- Genoves, A. , Navarro, J.A. and Pallas, V. (2010) The intra‐ and intercellular movement of Melon necrotic spot virus (MNSV) depends on an active secretory pathway. Mol. Plant–Microbe Interact. 23, 263–272. [DOI] [PubMed] [Google Scholar]
- Gluck‐Thaler, E. and Slot, J.C. (2015) Dimensions of horizontal gene transfer in eukaryotic microbial pathogens. PLoS Pathog. 11, e1005156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gómez, G. and Pallás, V. (2010) Can the import of mRNA into chloroplasts be mediated by a secondary structure of a small non‐coding RNA? Plant Signal Behav. 5, 1517–1519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gómez, G. and Pallás, V. (2012) A pathogenic non coding RNA that replicates and accumulates in chloroplasts traffics to this organelle through a nuclear‐dependent step. Plant Signal Behav. 7, 882–884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodman, R.N. and Novacky, A.J. (1994) The Hypersensitive Reaction in Plants to Pathogens: A Resistance Phenomenon. St. Paul, MN: APS Press. [Google Scholar]
- Gray, M.W. (1989) The evolutionary origins of organelles. Trends Genet. 5, 294–299. [DOI] [PubMed] [Google Scholar]
- Hageman, J. , Baecke, C. , Ebskamp, M. , Pilon, R. , Smeekens, S. and Weisbeek, P. (1990) Protein import into and sorting inside the chloroplast are independent processes. Plant Cell, 2, 479–494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harries, P. , Palanichelvam, K. , Yu, W. , Schoelz, J.E. and Nelson, R.S. (2009) The Cauliflower mosaic virus protein P6 forms motile inclusions that traffic along actin microfilaments and stabilize microtubules. Plant Physiol. 149, 1005–1016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harsányi, A. , Ryberg, M. , Andersson, M.X. , Bóka, K. , László, L. , Botond, G. , Böddi, B. and Gáborjányi, R. (2006) Alterations of NADPH:protochlorophyllide oxidoreductase quantity and lipid composition in etiolated barley seedlings infected by Barley stripe mosaic virus (BSMV). Mol. Plant Pathol. 7, 533–541. [DOI] [PubMed] [Google Scholar]
- Huh, S.U. , Kim, M.J. , Ham, B.K. and Paek, K.H. (2011) A zinc finger protein Tsip1 controls Cucumber mosaic virus infection by interacting with the replication complex on vacuolar membranes of the tobacco plant. New Phytol. 191, 746–762. [DOI] [PubMed] [Google Scholar]
- Hunter, L.J. , Westwood, J.H. , Heath, G. , Macaulay, K. , Smith, A.G. , Macfarlane, S.A. , Palukaitis, P. and Carr, J.P. (2013) Regulation of RNA‐dependent RNA polymerase1 and isochorismate synthase gene expression in Arabidopsis. PLoS One, 8, e66530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ivanova, Y. , Smith, M.D. , Chen, K. and Schnell, D.J. (2004) Members of the Toc159 import receptor family represent distinct pathways for protein targeting to plastids. Mol. Biol. Cell, 15, 3379–3392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jang, C. , Seo, E. , Nam, J. , Bae, H. and Gim, Y. (2013) Insights into Alternanthera mosaic virus TGB3 functions: interactions with Nicotiana benthamiana PsbO correlate with chloroplast vesiculation and veinal necrosis caused by TGB3 over‐expression. Front. Plant Sci. 4, 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jelenska, J. , Yao, N. , Vinatzer, B.A. , Wright, C.M. , Brodsky, J.L. and Greenberg, J.T. (2007) A J domain virulence effector of Pseudomonas syringae remodels host chloroplasts and suppresses defenses. Curr. Biol. 17, 499–508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiménez, I. , López, J.M. , Valli, A. and Gracia, J.A. (2006) Identification of a plum pox virus CI‐interacting protein from chloroplast that has a negative effect in virus infection. Mol. Plant–Microbe Interact. 19, 350–358. [DOI] [PubMed] [Google Scholar]
- Jin, Y. , Ma, D. , Dong, J. , Li, D. , Deng, C. , Jin, J. and Wang, T. (2007) The HC‐Pro protein of Potato Virus Y interacts with NtMinD of tobacco. Mol. Plant–Microbe Interact. 20, 1505–1511. [DOI] [PubMed] [Google Scholar]
- Kangasjarvi, S. , Neukermans, J. , Li, S. , Aro, E.M. and Noctor, G. (2012) Photosynthesis, photorespiration, and light signalling in defence responses. J. Exp. Bot. 63, 1619–1636. [DOI] [PubMed] [Google Scholar]
- Kitajima, E.W. and Costa, A.S. (1973) Aggregates of chloroplasts in local lesions induced in Chenopodium quinoa Wild. by Turnip mosaic virus . J. Gen. Virol. 20, 413–416. [Google Scholar]
- Kobayashi, K. and Wada, H. (2016) Role of lipids in chloroplast biogenesis In: Lipids in Plant and Algae Development (Nakamura Y. and Li‐Beisson Y., eds), pp. 103–125. Dordrecht: Springer International Publishing. [DOI] [PubMed] [Google Scholar]
- Kong, L. , Wu, J. , Lu, L. , Xu, Y. and Zhou, X. (2013) Interaction between Rice stripe virus disease‐specific protein and host PsbP enhances virus symptoms. Mol. Plant. 7, 691–708. [DOI] [PubMed] [Google Scholar]
- Krenz, B. , Windeisen, V. , Wege, C. , Jeske, H. and Kleinow, T. (2010) A plastid‐targeted heat shock cognate 70kDa protein interacts with the Abutilon mosaic virus movement protein. Virology, 401, 6–17. [DOI] [PubMed] [Google Scholar]
- Krenz, B. , Jeske, H. and Kleinow, T. (2012) The induction of stromule formation by a plant DNA‐virus in epidermal leaf tissues suggests a novel intra‐ and intercellular macromolecular trafficking route. Front. Plant Sci. 3, 291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kushwaha, N. , Singh, A.K. , Basu, S. and Chakraborty, S. (2015) Differential response of diverse solanaceous hosts to tomato leaf curl New Delhi virus infection indicates coordinated action of NBS‐LRR and RNAi‐mediated host defense. Arch. Virol. 160, 1499–1509. [DOI] [PubMed] [Google Scholar]
- Laliberté, J.F. and Sanfaçon, H. (2010) Cellular remodeling during plant virus infection. Annu. Rev. Phytopathol. 48, 69–91. [DOI] [PubMed] [Google Scholar]
- Lanfermeijer, F.C. , Sturre, M.J. , de Hann, P. and Hille, J. (2004) Cloning and characterization of durable Tomato mosaic virus resistance gene Tm‐22 from Lycoperscion esculentum . Plant Mol. Biol. 52, 1037–1049. [DOI] [PubMed] [Google Scholar]
- Laporte, C. , Vetter, G. , Loudes, A.M. , Robinson, D.G. , Hillmer, S. , Stussi‐Garaud, C. and Ritzenthaler, C. (2003) Involvement of the secretory pathway and the cytoskeleton in intracellular targeting and tubule assembly of Grapevine fanleaf virus movement protein in tobacco BY‐2 cells. Plant Cell, 15, 2058–2075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee, W.M. , Ishikawa, M. and Ahlquist, P. (2001) Mutation of host delta9 fatty acid desaturase inhibits brome mosaic virus RNA replication between template recognition and RNA synthesis. J. Virol. 75, 2097–2106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehmann, P. , Bohnsack, M.T. and Schleiff, E. (2011) The functional domains of the chloroplast unusual positioning protein1. Plant Sci. 180, 650–654. [DOI] [PubMed] [Google Scholar]
- Lehto, K. , Tikkanen, M. , Hiriart, J.B. , Paakkarinen, V. and Aro, E.M. (2003) Depletion of the photosystem II core complex in mature tobacco leaves infected by the flavum strain of Tobacco mosaic virus . Mol. Plant–Microbe Interact. 16, 1135–1144. [DOI] [PubMed] [Google Scholar]
- Li, H. and Chiu, C.C. (2010) Protein transport into chloroplasts. Annu. Rev. Plant Biol. 61, 157–180. [DOI] [PubMed] [Google Scholar]
- Li, H. , Ma, D. , Jin, Y. , Tu, Y. , Liu, L. , Leng, C. , Dong, J. and Wang, T. (2015) Helper component‐proteinase enhances the activity of 1‐deoxy‐D‐xylulose‐5‐phosphate synthase and promotes the biosynthesis of plastidic isoprenoids in Potato virus Y‐infected tobacco. Plant Cell Environ. 38, 2023–2034. [DOI] [PubMed] [Google Scholar]
- Li, Y. , Wu, M.Y. , Song, H.H. , Hu, X. and Qiu, B.S. (2005) Identification of a tobacco protein interacting with Tomato mosaic virus coat protein and facilitating long‐distance movement of virus. Arch. Virol. 150, 1993–2008. [DOI] [PubMed] [Google Scholar]
- Lim, H.S. , Vaira, A.M. , Bae, H. , Bragg, J.N. , Ruzin, S.E. and Bauchan, G.R. (2010) Mutation of a chloroplast targeting signal in Alternanthera mosaic virus TGB3 impairs cell‐to‐cell movement and eliminates long distance virus movement. J. Gen. Virol. 91, 2102–2115. [DOI] [PubMed] [Google Scholar]
- Lin, J.W. , Ding, M.P. , Hsu, Y.H. and Tsa, C.H. (2007) Chloroplast phosphoglycerate kinase, a gluconeogenetic enzyme, is required for efficient accumulation of Bamboo mosaic virus . Nucleic Acids Res. 35, 424–432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin, L. , Luo, Z. , Yan, F. , Lu, Y. , Zheng, H. and Chen, J. (2011) Interaction between potyvirus P3 and ribulose‐1,5‐bisphosphate carboxylase/oxygenase (RubisCO) of host plants. Virus Genes, 43, 90–92. [DOI] [PubMed] [Google Scholar]
- Lindahl, M. , Tabak, S. , Cseke, L. , Pichersky, E. , Andersson, B. and Adam, Z. (1996) Identification, characterization, and molecular cloning of a homologue of the bacterial FtsH protease in chloroplasts of higher plants. J. Biol. Chem. 271, 29 329–29 334. [DOI] [PubMed] [Google Scholar]
- Liu, H. , Fu, Y. , Jiang, D. , Li, G. , Xie, J. , Cheng, J. , Peng, Y. , Ghabrial, S.A. and Yi, X. (2010) Widespread horizontal gene transfer from double‐stranded RNA viruses to eukaryotic nuclear genomes. J. Virol. 84, 11 876–11 887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu, J. , Yang, J. , Bi, H. and Zhang, P. (2014) Why mosaic? Gene expression profiling of African cassava mosaic virus‐infected cassava reveals the effect of chlorophyll degradation on symptom development. J. Integr. Plant Biol. 56, 122–132. [DOI] [PubMed] [Google Scholar]
- Liu, X.Y. , Yang, J. , Xie, L. , Li, J. , Song, X.J. , Chen, J.P. and Zhang, H.M. (2015) P5‐2 of rice black‐streaked dwarf virus is a non‐structural protein targeted to chloroplasts. Arch. Virol. 160, 1211–1217. [DOI] [PubMed] [Google Scholar]
- Masuta, C. , Kuwata, S. , Matzuzaki, T. , Takanami, Y. and Koiwai, A. (1992) A plant virus satellite RNA exhibits a significant sequence complementarity to a chloroplast tRNA. Nucleic Acids Res. 20, 2885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthews, R.E.F. (1991) Plant Virology. New York: Academic Press. [Google Scholar]
- May, T. and Soll, J. (2000) 14‐3‐3 Proteins form a guidance complex with chloroplast precursor proteins in plants. Plant Cell, 12, 53–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayo, M.A. and Jolly, C.A. (1991) The 5'‐terminal sequence of Potato leaf roll virus RNA: evidence of recombination between virus and host RNA. J. Gen. Virol. 72, 2591–2595. [DOI] [PubMed] [Google Scholar]
- McFadden, G.I. (2001) Chloroplast origin and integration. Plant Physiol. 125, 50–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mochizuki, T. and Ohki, S.T. (2011) Single amino acid substitutions at residue 129 in the coat protein of cucumber mosaic virus affect symptom expression and thylakoid structure. Arch. Virol. 156, 881–886. [DOI] [PubMed] [Google Scholar]
- Mochizuki, T. , Yamazaki, R. , Wada, T. and Ohki, S. (2014a) Coat protein mutations in an attenuated Cucumber mosaic virus encoding mutant 2b protein that lacks RNA silencing suppressor activity induces chlorosis with photosynthesis gene repression and chloroplast abnormalities in infected tobacco plants. Virology, 456–457, 292–299. [DOI] [PubMed] [Google Scholar]
- Mochizuki, T. , Ogata, Y. , Hirata, Y. and Ohki, S.T. (2014b) Quantitative transcriptional changes associated with chlorosis severity in mosaic leaves of tobacco plants infected with Cucumber mosaic virus . Mol. Plant Pathol. 15, 242–254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morozov, S.Y. and Solovyev, A.G. (2003) Triple gene block: modular design of a multifunctional machine for plant virus movement. J. Gen. Virol. 84, 1351–1366. [DOI] [PubMed] [Google Scholar]
- Mur, L.A. , Kenton, P. , Lloyd, A.J. , Ougham, H. and Prats, E. (2008) The hypersensitive response; the centenary is upon us but how much do we know? J. Exp. Bot. 59, 501–520. [DOI] [PubMed] [Google Scholar]
- Oikawa, K. , Yamasato, A. , Kong, S.G. , Kasahara, M. , Nakai, M. , Takahashi, F. , Ogura, Y. , Kagawa, T. and Wada, M. (2008) Chloroplast outer envelope protein CHUP1 is essential for chloroplast anchorage to the plasma membrane and chloroplast movement. Plant Physiol. 148, 829–842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olesinski, A.A. , Lucas, W.J. , Galun, E. and Wolf, S. (1995) Pleiotropic effects of Tobacco mosaic virus movement protein on carbon metabolism in transgenic tobacco plants. Planta, 197, 118–126. [Google Scholar]
- Otulak, K. , Choudaa, M. , Bujarskib, J. and Garbaczewska, G. (2015) The evidence of Tobacco rattle virus impact on host plant organelles ultrastructure. Micron, 70, 7–20. [DOI] [PubMed] [Google Scholar]
- Pandey, S.P. and Baldwin, I.T. (2007) RNA‐directed RNA polymerase1 (RdR1) mediates the resistance of Nicotiana attenuata to herbivore attack in nature. Plant J. 50, 40–53. [DOI] [PubMed] [Google Scholar]
- Peng, H.T. , Li, Y.X. , Zhang, C.W. , Li, Y. and Hou, X.L. (2014) Chloroplast elongation factor BcEF‐Tu responds to turnip mosaic virus infection and heat stress in non‐heading Chinese cabbage. Biol. Plant. 58, 561–566. [Google Scholar]
- Pérez‐Bueno, M.L. , Rahoutei, J. , Sajnani, C. , García‐Luque, I. and Barón, M. (2004) Proteomic analysis of the oxygen‐evolving complex of photosystem II under biotic stress: studies on Nicotiana benthamiana infected with tobamoviruses. Proteomics, 4, 418–425. [DOI] [PubMed] [Google Scholar]
- Petre, B. , Lorrain, C. , Saunders, D.G. , Win, J. , Sklenar, J. , Duplessis, S. and Kamoun, S. (2015) Rust fungal effectors mimic host transit peptides to translocate into chloroplasts. Cell. Microbiol. 18, 453–465. [DOI] [PubMed] [Google Scholar]
- Pineda, M. , Sajnani, C. and Barón, M. (2010) Changes induced by the Pepper mild mottle tobamovirus on the chloroplast proteome of Nicotiana benthamiana . Photosynth. Res. 103, 31–45. [DOI] [PubMed] [Google Scholar]
- Postnikova, O.A. and Nemchinov, L.G. (2012) Comparative analysis of microarray data in Arabidopsis transcriptome during compatible interactions with plant viruses. Virology J. 9, 101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prod'homme, D. , Le Panse, S. , Drugeon, G. and Jupin, I. (2001) Detection and subcellular localization of the turnip yellow mosaic virus 66K replication protein in infected cells. Virology, 281, 88–101. [DOI] [PubMed] [Google Scholar]
- Prod'homme, D. , Jakubiec, A. , Tournier, V. , Drugeon, G. and Jupin, I. (2003) Targeting of the Turnip Yellow Mosaic Virus 66K replication protein to the chloroplast envelope is mediated by the 140K protein. J. Virol. 77, 9124–9135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pumplin, N. and Voinnet, O. (2013) RNA silencing suppression by plant pathogens: defence, counter‐defence and counter‐counter‐defence. Nat. Rev. Microbiol. 11, 745–760. [DOI] [PubMed] [Google Scholar]
- Qiao, Y. , Li, H.F. , Wong, S.M. and Fan, Z.F. (2009) Plastocyanin transit peptide interacts with Potato virus X coat protein, while silencing of plastocyanin reduces coat protein accumulation in chloroplasts and symptom severity in host plants. Mol. Plant–Microbe Interact. 22, 1523–1534. [DOI] [PubMed] [Google Scholar]
- Reiss, S. , Rebhan, I. , Backes, P. , Romero‐Brey, I. , Erfle, H. , Matula, P. , Kaderali, L. , Poenisch, M. , Blankenburg, H. , Hiet, M.S. , Longerich, T. , Diehl, S. , Ramirez, F. , Balla, T. , Rohr, K. , Kaul, A. , Bühler, S. , Pepperkok, R. , Lengauer, T. , Albrecht, M. , Eils, R. , Schirmacher, P. , Lohmann, V. and Bartenschlager, R. (2011) Recruitment and activation of a lipid kinase by hepatitis C virus NS5A is essential for integrity of the membranous replication compartment. Cell Host Microbe, 9, 32–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reneiro, A. and Beachy, R.N. (1986) Association of TMV coat protein with chloroplast membranes in virus infected leaves. Plant Mol. Biol. 6, 291–301. [DOI] [PubMed] [Google Scholar]
- Reneiro, A. and Beachy, R.N. (1989) Reduced photosystem II activity and accumulation of viral coat protein in chloroplasts of leaves infected with Tobacco mosaic virus . Plant Physiol. 89, 111–116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reuveni, M. , Debbi, A. , Kutsher, Y. , Gelbart, D. , Zemach, H. , Belausov, E. , Levin, I. and Lapidot, M. (2015) Tomato yellow leaf curl virus effects on chloroplast biogenesis and cellular structure. Physiol. Mol. Plant Pathol. 92, 51–58. [Google Scholar]
- Richly, E. and Leister, D. (2004) An improved prediction of chloroplast proteins reveals diversities and commonalities in the chloroplast proteomes of Arabidopsis and rice. Gene, 329, 11–16. [DOI] [PubMed] [Google Scholar]
- Rochon, D. and Siegel, A. (1984) Chloroplast DNA transcripts are encapsidated by Tobacco mosaic virus coat protein. Proc. Natl. Acad. Sci. USA, 81, 1719–1723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rohozinski, J. and Hancock, J.M. (1996) Do light‐induced pH changes within the chloroplast drive Turnip yellow mosaic virus assembly? J. Gen. Virol. 77, 163–165. [DOI] [PubMed] [Google Scholar]
- Sattarzadeh, A. , Krahmer, J. , Germain, A.D. and Hanson, M.R. (2009) A Myosin XI tail domain homologous to the yeast myosin vacuole‐binding domain interacts with plastids and stromules in Nicotiana benthamiana . Mol. Plant, 2, 1351–1358. [DOI] [PubMed] [Google Scholar]
- Schemenewitz, A. , Pollmann, S. , Reinbothe, C. and Reinbothe, S. (2007) A substrate‐independent, 14:3:3 protein mediated plastid import pathway of NADPH:protochlorophyllide oxidoreductase A. Proc . Natl. Acad. Sci. USA, 104, 8538–8543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz, M. , Chen, J. , Janda, M. , Sullivan, M. , den Boon, J. and Ahlquist, P. (2002) A positive‐strand RNA virus replication complex parallels form and function of retrovirus capsids. Mol. Cell, 9, 505–514. [DOI] [PubMed] [Google Scholar]
- Seo, E.Y. , Nam, J. , Kim, H.S. , Park, Y.H. , Hong, S.M. , Lakshman, D. , Bae, H. , Hammond, J. and Lim, H.S. (2014) Selective interaction between chloroplast β‐ATPase and TGB1L88 retards severe symptoms caused by Alternanthera mosaic virus infection. Plant Pathol. J. 30, 58–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seo, S. , Okamoto, M. , Iwai, T. , Iwano, M. , Fukui, K. , Isogai, A. , Nakajima, N. and Ohashi, Y. (2000) Reduced levels of chloroplast FtsH protein in Tobacco mosaic virus‐infected tobacco leaves accelerate the hypersensitive reaction. Plant Cell, 12, 917–932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma, M. , Sasvari, Z. and Nagy, P.D. (2010) Inhibition of sterol biosynthesis reduces tombusvirus replication in yeast and plants. J. Virol. 84, 2270–2281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi, Y. , Chen, J. , Hong, X. , Chen, J. and Adams, M.J. (2007) A potyvirus P1 protein interacts with the Rieske Fe/S protein of its host. Mol. Plant Pathol. 8, 785–790. [DOI] [PubMed] [Google Scholar]
- Shimura, H. , Pantaleo, V. , Ishihara, T. , Myojo, N. , Inaba, J. , Sueda, K. , Burgyán, J. and Masuta, C. (2011) A viral satellite RNA induces yellow symptoms on tobacco by targeting a gene involved in chlorophyll biosynthesis using the RNA silencing machinery. PLoS Pathog. 7, e1002021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith, M.D. , Rounds, C.M. , Wang, F. , Chen, K. , Afitlhile, M. and Schnell, D.J. (2004) AtToc159 is a selective transit peptide receptor for the import of nucleus‐encoded chloroplast proteins. J. Cell Biol. 165, 323–334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith, N.A. , Eamens, A.L. and Wang, M.B. (2011) Viral small interfering RNAs target host genes to mediate disease symptoms in plants. PLoS Pathog. 7, e1002022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun, X. , Li, Y. , Shi, M. , Zhang, N. and Wu, G. (2013) In vitro binding and bimolecular fluorescence complementation assays suggest an interaction between Tomato mosaic virus coat protein and tobacco chloroplast ferredoxin I. Arch. Virol. 158, 2611–2615. [DOI] [PubMed] [Google Scholar]
- Tabler, M. and Tsagris, M. (2004) Viroids: petite RNA pathogens with distinguished talents. Trends Plant Sci. 9, 339–348. [DOI] [PubMed] [Google Scholar]
- Torrance, L. , Cowan, G.H. , Gillespie, T. , Ziegler, A. and Lacomme, C. (2006) Barley stripe mosaic virus‐encoded proteins triple gene block 2 and γb localize to chloroplasts in virus‐infected monocot and dicot plants, revealing hitherto‐unknown roles in virus replication. J. Gen. Virol. 87, 2403–2411. [DOI] [PubMed] [Google Scholar]
- Tu, Y. , Zhang, Z. , Li, D. , Li, H. , Dong, J. and Wang, T. (2015) Potato virus Y HC‐Pro reduces the ATPase activity of NtMinD, which results in enlarged chloroplasts in HC‐Pro transgenic tobacco. PLoS One, 10, e0136210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uchiyama, A. , Shimada‐Beltran, H. , Levy, A. , Zheng, J.Y. , Javia, P.A. and Lazarowitz, S.G. (2014) The Arabidopsis synaptotagmin SYTA regulates the cell‐to‐cell movement of diverse plant viruses. Front. Plant Sci. 5, 584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, X.B. , Wu, Q. , Ito, T. , Cillo, F. , Li, W.X. , Chen, X. , Yu, Z.L. and Ding, S.W. (2010) RNAi‐mediated viral immunity requires amplification of virus‐derived siRNAs in Arabidopsis thaliana . Proc. Natl. Acad. Sci. USA, 107, 484–489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei, T. , Huang, T.S. , McNeil, J. , Laliberte, J.F. , Hong, J. , Nelson, R.S. and Wang, A. (2010) Sequential recruitment of the endoplasmic reticulum and chloroplasts for plant potyvirus replication. J. Virol. 84, 799–809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei, T. , Zhang, C. , Hou, X. , Sanfacon, H. and Wang, A. (2013) The SNARE Protein Syp71 is essential for Turnip mosaic virus infection by mediating fusion of virus‐induced vesicles with chloroplasts. PLoS Pathog. 9, e1003378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitham, S. , Dinesh‐Kumar, S.P. , Choi, D. , Hehl, R. , Corr, C. and Baker, B. (1994) The product of the tobacco mosaic virus resistance gene N: similarity to toll and the interleukin‐1 receptor. Cell, 78, 1101–1115. [DOI] [PubMed] [Google Scholar]
- Wu, L. , Han, Z. and Wang, S. (2013) Comparative proteomic analysis of the plant–virus interaction in resistant and susceptible ecotypes of maize infected with sugarcane mosaic virus. J. Prot. 89, 124–140. [DOI] [PubMed] [Google Scholar]
- Xiang, Y. , Kakani, K. , Reade, R. , Hui, E. and Rochon, D.A. (2006) A 38‐amino‐acid sequence encompassing the arm domain of the Cucumber necrosis virus coat protein functions as a chloroplast transit peptide in infected plants. J. Virol. 80, 7952–7964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao, J. , Liu, Q. , Zhang, H. , Jia, Q. , Hong, Y. and Liu, Y. (2013) The rubisco small subunit is involved in Tobamovirus movement and Tm‐22‐mediated extreme resistance. Plant Physiol. 161, 374–383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang, C.Z. , Liu, Y.Y. , Sun, X.C. , Qian, W.D. , Zhang, D.D. and Qiu, B.S. (2008) Characterization of a specific interaction between IP‐L, a tobacco protein localized in the thylakoid membranes, and Tomato mosaic virus coat protein. Biochem. Biophys. Res. Commun. 374, 253–257. [DOI] [PubMed] [Google Scholar]
- Zhang, X.P. and Glaser, E. (2002) Interaction of plant mitochondrial and chloroplast signal peptides with the Hsp70 molecular chaperone. Trends Plant Sci. 7, 14–21. [DOI] [PubMed] [Google Scholar]


