Summary
Olive anthracnose causes fruit rot leading to its drop or mummification, resulting in yield losses and the degradation of oil quality.
Taxonomy and distribution
The disease is caused by diverse species of Colletotrichum, mostly clustering in the C. acutatum species complex. Colletotrichum nymphaeae and C. godetiae are the prevalent species in the Northern Hemisphere, whereas C. acutatum sensu stricto is the most frequent species in the Southern Hemisphere, although it is recently and quickly emerging in the Northern Hemisphere. The disease has been reported from all continents, but it attains higher incidence and severity in the west of the Mediterranean Basin, where it is endemic in traditional orchards of susceptible cultivars.
Life cycle
The pathogens are able to survive on vegetative organs. On the fruit surface, infections remain quiescent until fruit maturity, when typical anthracnose symptoms develop. Under severe epidemics, defoliation and death of branches can also occur. Pathogen species differ in virulence, although this depends on the cultivar.
Control
The selection of resistant cultivars depends strongly on pathogen diversity and environmental conditions, posing added difficulties to breeding efforts. Chemical disease control is normally achieved with copper‐based fungicides, although this may be insufficient under highly favourable disease conditions and causes concern because of the presence of fungicide residues in the oil. In areas in which the incidence is high, farmers tend to anticipate harvest, with consequences in yield and oil characteristics.
Challenges
Olive production systems, harvest and post‐harvest processing have experienced profound changes in recent years, namely new training systems using specific cultivars, new harvest and processing techniques and new organoleptic market requests. Changes are also occurring in both the geographical distribution of pathogen populations and the taxonomic framework. In addition, stricter rules concerning pesticide use are likely to have a strong impact on control strategies. A detailed knowledge of pathogen diversity, population dynamics and host–pathogen interactions is basal for the deployment of durable and effective disease control strategies, whether based on resistance breeding, agronomic practices or biological or chemical control.
Keywords: Colletotrichum acutatum, Olea europaea, olive anthracnose, olive oil quality
Introduction
Olive anthracnose, caused by Colletotrichum spp., affects olive (Olea europaea L.) fruit at maturity. Symptoms are dark sunken lesions, covered with orange spore masses (Fig. 1). Rotten fruits either fall or mummify, resulting in important yield losses. Total crop losses are not uncommon, particularly under specific agroecological conditions, combining high humidity and rainfall during the autumn, the widespread use of susceptible varieties and the abundance of inoculum reservoirs (Talhinhas et al., 2011). If the diseased fruits are harvested, the quality of the olive oil produced is degraded (Moral et al., 2014). Defoliation and death of branches can also occur in severe epidemics, causing polyetic effects on the yield in subsequent years.
Figure 1.

Typical symptoms and signs of olive anthracnose on fruits at maturity (A), leading to fruit mummification (B) and, in severe epidemics, to defoliation (C).
Olive anthracnose is a common disease in the Western Mediterranean Basin, a situation illustrated by vernacular names in Portugal (‘gafa’), Spain (‘aceituna jabonosa’) and Italy (‘lebbra’). The disease was first described in scientific terms in 1899 in Portugal (Almeida, 1899), but reports that can be clearly linked to olive anthracnose symptoms have been known in southwestern Iberia since the 11th century by the Arab agronomist Bū'l‐Jayr (Moral et al., 2014). In Portugal, anthracnose rivals olive fruit fly, Bactrocera oleae (Gmelin.), as the most important aerial disease and pest of olives, respectively. The disease incidence in Portugal ranges between 30% and 50% of orchards surveyed, with an average severity of 14% (Talhinhas et al., 2011), whereas, in Spain, the disease is responsible for an average 2.6% crop losses country‐wide (Trapero and Blanco, 2008). In Italy, the disease seems to be more recent, as it has caused notorious epidemics since the 1940s, receding in the 1970s until recent years, when it has regained a foothold (Cacciola et al., 2012).
The disease has been reported from all continents (Fig. 2), attaining high economic importance in several olive‐producing regions. The area devoted to olive cultivation has increased markedly in recent years, together with an intensification in cultivation, prompted by an increase in olive oil consumption. The ability to predict the anthracnose risk in new olive‐growing areas is complex because of the multi‐faceted relationships between hosts and pathogens. Furthermore, as most Colletotrichum species are not host specific, the movement of olive groves to other regions may result in novel host–pathogen interactions, with local species affecting new hosts.
Figure 2.
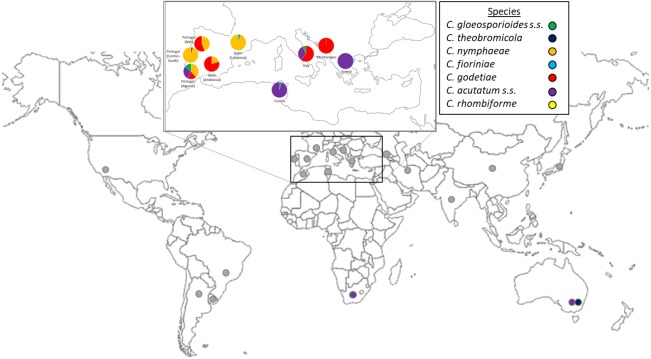
Geographical distribution of olive anthracnose reports, denoting (when possible) the relative frequency of Colletotrichum species populations: Abkhasia (Nagorny and Eristavi, 1929); Argentina (Brancher et al., 2008); Australia (Schena et al., 2014; Whitelaw‐Weckert et al., 2007); Brazil (Duarte et al., 2010); China (Margarita et al., 1986); Egypt (Embaby, 2014); France (Bompeix et al., 1988); Greece (Iliadi et al., 2018; Sarejanni, 1939); India (Mugnai et al., 1993); Iran (Sanei and Razavi, 2011, 2012); India (Sharma and Kaul, 1990); Italy (Agosteo et al., 2002; Ciccarone, 1950; Mosca et al., 2014); Japan (Hemmi and Kurata, 1935); Montenegro (Vučinić et al., 1999); Morocco (Achbani et al., 2013); Portugal (Talhinhas et al., 2009, 2011, 2015); South Africa (Gorter, 1956); Spain (Martín and García‐Figueres, 1999); Tunisia (Chattaoui et al., 2016; Rhouma et al., 2010); Uruguay (Acosta, 1932); USA (Pontis and Hansen, 1942).
Effect of Olive Anthracnose on Oil
Olive oil is the only vegetable fat that can be consumed in its crude form (Ranalli et al., 2000) and is a key element of the beneficiary effect on health recognized for the Mediterranean diet (Moral et al., 2014). Top quality olive oil is highly prized, and physico‐chemical and sensorial requirements are clearly defined by national and international authorities, including the International Olive Oil Council. Quality characteristics depend on agroecological traits, including climate, soil, agronomic practices and notably the cultivar and maturation stage, together with post‐harvest factors – transport and storage of fruits and oil extraction and storage. Pre‐harvest biotic stresses, including anthracnose and fruit fly, affect fruit because of tissue disruption and necrosis. Anthracnose causes sensorial defects, such as ‘soil’ and ‘mould’, and chemical detrimental effects, such as an increase in free acidity, the peroxide index and the content of aldehydes, a decrease in oxidative stability and the content of polyphenols and α‐tocoferol, and changes to the alkyl ester composition. The fatty acid composition, however, is not altered (Carvalho et al., 2008; Iannotta et al., 1999; Mincione et al., 2004; Moral et al., 2014; Runcio et al., 2008). Sensorial and chemical defects of olive oil are usually complex and the isolation of single effects is difficult. In addition to anthracnose, multiple biotic stresses can cause such defects, namely the olive fruit fly or the fungi Camarosporium dalmatica (Thüm.) Zachos & Tzav.‐Klon., Venturia oleaginea (Castagne) Rossman & Crous [=Spilocaea oleaginea (Castagne) S. Hughes] and Pseudocercospora cladosporioides (Sacc.) U. Braun. Studies on the detrimental effects of pests and diseases often do not account for the specific effect of each biotic stress (e.g. Bustan et al., 2014) and, even when focused only on anthracnose, normally do not account for the identity of the pathogen.
Aetiology and Taxonomy
The causal agent of olive anthracnose was first identified as Gloeosporium olivarum Alm. (Almeida, 1899) and subsequently transferred to Colletotrichum gloeosporioides (Penz.) Penz. & Sacc. (von Arx, 1970) (Fig. 3). The latter species was then considered as a group species and, gradually, narrower species were described in the vicinity of C. gloeosporioides. The advent of molecular biology tools for identification and diagnostics, based mostly in the rDNA‐ITS region, further enabled such discrimination. Colletotrichum acutatum J.H. Simmonds was described in 1968, and it soon became a ‘popular species’ in the genus, with a growing number of fungi assigned to it (Baroncelli et al., 2017). Under this scenario, Martín and García‐Figueres (1999) recognized two causal agents associated with olive anthracnose: C. acutatum and C. gloeosporioides. Genetic diversity was identified among olive anthracnose pathogens assigned to C. acutatum (Talhinhas et al., 2005) based on the rDNA‐ITS region and β‐tubulin 2 nucleotide sequence analysis, and intraspecific groups (Sreenivasaprasad and Talhinhas, 2005; Whitelaw‐Weckert et al., 2007) were subsequently and gradually raised to species rank (Damm et al., 2012; Shivas and Tan, 2009). Currently, there are six species in the C. acutatum species complex (Baroncelli et al., 2017) and two in the C. gloeosporioides species complex (Mosca et al., 2014; Schena et al., 2014; Weir et al., 2012) considered to be causal agents of olive anthracnose (Table 1). In addition, fungi from four species in the C. gloeosporioides species complex and one in the C. boninense species complex have been isolated from olive fruits exhibiting anthracnose symptoms (Mosca et al., 2014; Schena et al., 2014), but their pathogenicity to olive fruit has not been confirmed.
Figure 3.
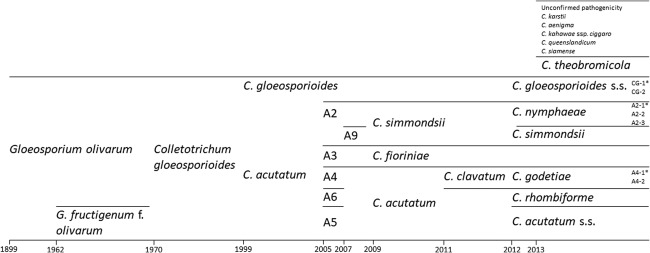
Historical perspective of the nomenclature of the olive anthracnose pathogens (Almeida, 1899; von Arx, 1970; Damm et al., 2012; Faedda et al., 2011; Gorter, 1962; Martín and García‐Figueres, 1999; Schena et al., 2014; Shivas and Tan, 2009; Talhinhas et al., 2005; Whitelaw‐Weckert et al., 2007). Groups A2–A9 (Talhinhas et al., 2005; Whitelaw‐Weckert et al., 2007) were subsequently and gradually assigned to species (Damm et al., 2012; Shivas and Tan, 2009). For a while (e.g. see Moral and Trapero, 2012), olive anthracnose pathogens belonging to the Colletotrichum acutatum species complex were assigned to C. simmondsii (comprising A2 and A9, i.e. C. nymphaeae and C. simmondsii), to C. fioriniae (A3) or to C. acutatum (comprising A4, A5 and A6, i.e. C. godetiae, C. acutatum sensu stricto and C. rhombiforme). Asterisks denote subgroups identified by Talhinhas et al. (2009).
Table 1.
Fungal species in the genus Colletotrichum associated with olive anthracnose
| Species complex | Species | Distribution | References |
|---|---|---|---|
| Pathogenicity confirmed | |||
| C. acutatum * | C. acutatum J.H. Simmonds sensu stricto (A5) | Australia, Brazil, Greece, Italy, Portugal, South Africa, Tunisia | Gorter (1956); Talhinhas et al. (2005); Sergeeva et al. (2008); Duarte et al. (2010); Mosca et al. (2014); Chattaoui et al. (2016); Iliadi et al. (2018) |
| C. fioriniae (Marcelino & Gouli) Pennycook (A3) | Portugal | Talhinhas et al. (2005) | |
| C. godetiae Neerg. (A4) | Greece, Italy, Montenegro, Portugal, Spain | Talhinhas et al. (2005); Faedda et al. (2011) | |
| C. nymphaeae (Pass.) Aa (A2) | Portugal, Spain | Martín and García‐Figueres (1999); Talhinhas et al. (2005) | |
| C. rhombiforme Damm, P.F. Cannon & Crous (A6) | Portugal | Talhinhas et al. (2005) | |
| C. simmondsii R.G. Shivas & Y.P. Tan (A9) | Australia | Whitelaw‐Weckert et al. (2007) | |
| C. gloeosporioides | C. gloeosporioides (Penz.) Penz. & Sacc. sensu stricto | Portugal, Spain, Italy, Tunisia, China | Martín and García‐Figueres (1999); Talhinhas et al. (2005); Rhouma et al. (2010); Schena et al. (2014) |
| C. theobromicola Delacr. | Australia | Schena et al. (2014) | |
| Pathogenicity not confirmed | |||
| C. boninense | C. karstii Y.L. Yang, Zuo Y. Liu, K.D. Hyde & L. Cai | Italy | Schena et al. (2014) |
| C. gloeosporioides | C. aenigma B.S. Weir & P.R. Johnston | Italy | Schena et al. (2014) |
| C. kahawae ssp. ciggaro B. Weir & P.R. Johnst. | Italy | Schena et al. (2014) | |
| C. queenslandicum B. Weir & P.R. Johnst. | Montenegro | Schena et al. (2014) | |
| C. siamense Prihastuti, L. Cai & K.D. Hyde | Australia | Schena et al. (2014) | |
There are currently 13 Colletotrichum species associated with olive anthracnose, many of which were identified as the result of a single study (Schena et al., 2014). It is therefore foreseeable that further species may be identified to be associated with olive anthracnose, either representing opportunistic or truly pathogenic interactions. Such an amalgam of distinct fungi may be a cradle for the evolution of new pathogens of global or regional relevance, as witnessed in the recent speciation process of C. kahawae J.M. Waller & Bridge, leading to the acquisition of the capacity to infect green coffee fruits from within a group of seemingly opportunistic fungi (Silva et al., 2012). In addition, Talhinhas et al. (2009) recognized three genetic groups within C. nymphaeae (Pass.) Aa (A2; groups named A2‐1, A2‐2 and A2–3), two within C. godetiae Neerg. [A4; groups named A4‐1 and A4‐2, which could correspond to the two vegetative compatibility groups reported by Agosteo et al. (1997)] and two within C. gloeosporioides sensu stricto (s.s.) (CG‐1 and CG‐2). No intraspecific diversity was found among C. acutatum s.s. populations either in Portugal or Tunisia (Chattaoui et al., 2016; Talhinhas et al., 2009), which may suggest a more recent introduction of this pathogen in the region. All of these seven entities are pathogenic to olives (Talhinhas et al., 2009, 2011, 2015), meaning that there are at least 12 genetically distinct fungal populations causing olive anthracnose. Considering that criteria for species delimitation, namely in Colletotrichum, are undergoing active adjustments (Marin‐Felix et al., 2017), it is possible that, in the future, the species delimitation threshold in Colletotrichum will descend to the point of recognition of these entities as species. A total of 17 species may thus be associated with olive anthracnose. Ascertaining the relative importance of these populations, both in terms of virulence and of prevalence, is therefore fundamental to devise better informed disease control strategies, including olive breeding programmes.
Colletotrichum godetiae is the prevalent species in most countries in the Mediterranean Basin, whereas C. nymphaeae seems to be restricted to the southwest of the Iberian Peninsula (Fig. 2). Colletotrichum gloeosporioides s.s. occurs in several countries, although always presenting lower frequency than other species. Colletotrichum acutatum s.s. is the prevalent olive anthracnose pathogen in the Southern Hemisphere. This is inferred in the case of South African isolates because of the carmine colour of colonies described by Gorter (1956). Nevertheless, C. acutatum s.s. is emerging in several countries in the Mediterranean Basin. In Greece, C. acutatum s.s. was recorded for the first time in 2015 associated with fruit rots and flower and leaf necroses (Iliadi et al., 2018). In Tunisia, in a study involving 43 isolates collected in 2011, Chattaoui et al. (2016) assigned 41 isolates to C. acutatum s.s., whereas, in a sampling performed only 3 years before, Rhouma et al. (2010) reported olive anthracnose in Tunisia caused by a pathogen not exhibiting the typical carmine colony colour of C. acutatum s.s. Similarly, C. acutatum s.s. was not reported from Italy associated with olives until a 2012 sampling in Reggio Calabria. There, this pathogen rapidly went from undetected to very frequent, a particularly surprising situation as anthracnose is endemic in this area (Mosca et al., 2014), suggesting a rapid and strong change in the pathogen populations. Indeed, Faedda et al. (2011) reported the presence of C. acutatum s.s. in potted oleander (Nerium oleander L.) plants in 2001 in Sicily. Similarly, a metabarcoding study conducted in 2014 in Rizziconi (Calabria) on the fungal microbiota associated with the olive fruit fly enabled the detection of C. acutatum s.s. and C. gloeosporioides s.s., but not C. godetiae (Malacrinò et al., 2017). Colletotrichum acutatum s.s. is also commonly associated with anthracnose symptoms in oleander in Portugal (A. P. Ramos, personal communication, Instituto Superior de Agronomia, Universidade de Lisboa; Lisbon, Portugal), although, in olive, it is restricted to the southernmost region of this country, the Algarve (Talhinhas et al., 2005, 2009, 2011). This fungus also seems to be emerging as a causal agent of almond anthracnose in Andalucia (Spain), but not in olive (López‐Moral et al., 2017). Colletotrichum theobromicola Delacr., recently detected in Australia associated with olive anthracnose (Schena et al., 2014), could be an emergent olive pathogen in this country, where C. acutatum s.s. apparently was the dominant pathogen.
Additional species, such as C. fioriniae (Marcelino & Gouli) Pennycook and C. rhombiforme Damm, P.F. Cannon & Crous, are uncommon, and seem to be geographically confined. Colletotrichum fioriniae has been identified in different years and locations in Portugal, but very sporadically, although it is frequent on diverse perennial fruit crops in different parts of the world (Sreenivasaprasad and Talhinhas, 2005). Colletotrichum rhombiforme has been isolated only once (isolate PT250) in Trás‐os‐Montes (Portugal), and subsequent surveys in the same local and surrounding areas in following years led to the identification of C. godetiae and C. nymphaeae (Talhinhas et al., 2005, 2011). This isolate (PT250), together with a genetically similar isolate obtained from Vaccinium macrocarpon Aiton in the USA, gave rise to the species C. rhombiforme (Damm et al., 2012), recently also detected in China associated with apple bitter rot (Wu et al., 2017). It is noteworthy that such an infrequent fungus (at least according to the above‐mentioned Portuguese surveys) is found in different perennial plants in three distinct continents.
Virulence
Although several species of Colletotrichum isolated from anthracnose symptomatic olive trees could not be shown to be truly pathogenic to olives (Schena et al., 2014), at least eight species (Table 1) have been confirmed to be pathogenic, but differ in their virulence. In a study involving six Colletotrichum spp., Talhinhas et al. (2015) showed that C. acutatum s.s. and C. nymphaeae are more virulent, C. gloeosporioides s.s. and C. rhombiforme are less virulent, and C. godetiae and C. fioriniae are intermediate. Results obtained by Schena et al. (2014) also place C. acutatum s.s. as the most virulent, C. gloeosporioides as the least and C. godetiae in an intermediate position, together with C. simmondsii. Recently, the employment of a quantitative detection approach confirmed C. acutatum s.s. to be more virulent than C. godetiae in Italy (Schena et al., 2017). Schena et al. (2014) have also shown that C. theobromicola is as highly virulent as C. acutatum s.s., presumably resulting from the introduction of olive into new cropping areas. However, comparisons among different studies may not account for intraspecific variability or for differential responses by the host cultivars, suggesting the need for a study of global breadth.
Epidemiology and Life Cycle
Life styles of Colletotrichum spp. are highly variable, ranging from endophytic to necrotrophic, and have been the subject of extensive review (Peres et al., 2005; Perfect et al., 1999; de Silva et al., 2017). In the first description of the pathogen, Almeida (1899) pointed out some key aspects of the life cycle and epidemiology of olive anthracnose. Knowledge on olive anthracnose epidemiology and the life cycle has built up over the years and has been frequently revised (Cacciola et al., 2012; Moral et al., 2009b; Talhinhas et al., 2011) (Fig. 4). In recent years, research has contributed to improve the knowledge on specific aspects of this cycle, namely the provision of quantitative data to elucidate the relative importance of each stage. Regional factors, such as agronomical practices, preferred cultivars, climatic conditions and prevalent pathogen populations, are emerging as fine‐tuning variables that can contribute to detailed epidemiological models that can be applied both on a regional scale by plant breeders and extensionists, and on a farm scale to deploy short‐term disease management strategies.
Figure 4.
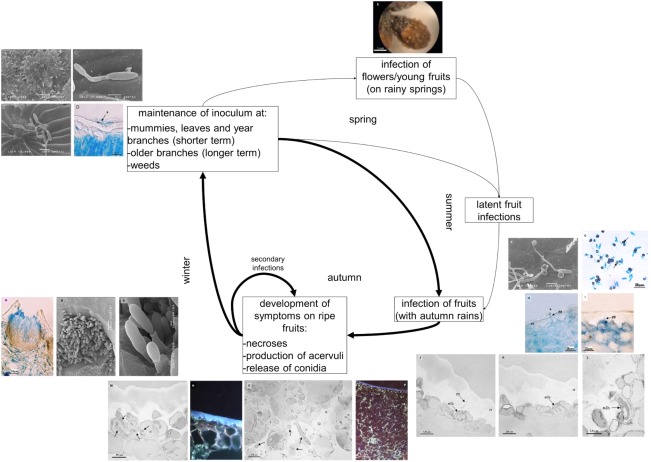
Disease cycle of olive anthracnose caused by Colletotrichum spp. (adapted from Talhinhas et al., 2011). Germination of Colletotrichum spp. conidia on olive leaf surface [A, long germination tubes; B, C, secondary conidiation; D, formation of melanized appressoria (a)]. (E) Spore masses formed on the surface of olive flower buds. Initial phases of olive fruit penetration [F, conidia germination and appressoria formation; G, internal light spots on melanized appressoria; H, penetration peg (pp) formed from appressoria (a) penetrating the fruit cuticle (ct); I, enlargement of the penetration peg when reaching an epidermal cell]. Colonization of olive fruit, with penetration, invasion and necrosis of the mesocarp cells [J–L, multilobed hyphae (mlh) in the first invaded host cell, beneath the penetration peg (pp)]. Proliferation of inter‐ and intracellular hyphae on mesocarp cells (M–P, arrows indicate examples). Production of acervuli, disruption of olive fruit cuticle and release of conidia (Q–S).
In brief, in the absence of ripe fruits, Colletotrichum fungi are able to survive on leaves and branches of olive and other plants as resting conidia. On such non‐target organs, the fungus is capable of epiphytic growth and sporulation (Fig. 4A–C; Talhinhas et al., 2011). On leaves and branches, conidia (both of C. nymphaeae and C. gloeosporioides) germinate (although at lower rates than on fruits) and produce long germ tubes (as opposed to short germ tubes on fruits), frequently leading to secondary conidiation. Alternatively, appressoria are formed, but without the formation of the internal light spot or host penetration (Fig. 4D; Talhinhas et al., 2011). In fact, the length of the germ tube and the readiness of appressorium formation also depend on nutrients and other microorganisms present on the organ surface (Agosteo et al., 2015). At bloom, flowers may be asymptomatically infected from the early stages of flowering until fruit set. Both C. acutatum sensu lato (s.l.) and C. gloeosporioides s.l. could be present in the calyx, petals, stamens and pistil (Iliadi et al., 2018; Sergeeva et al., 2008) and are capable of sporulation (Fig. 4E; Moral et al., 2009b; Talhinhas et al., 2011). If the flower is not destroyed, the infection may become quiescent until fruit ripening, although the impact of these infections on yield loss has not been estimated (Moral et al., 2009b). In a study conducted on two orchards during two growing seasons, Talhinhas et al. (2011) have shown that leaves have higher inoculum levels than branches and that inoculum decreases throughout the summer more markedly on leaves than on branches, suggesting that leaves can serve as short‐term inoculum reservoirs and branches as long‐term ones. This study also indicates that disease severity is more strongly correlated with the weather conditions in autumn than in spring, suggesting that infections of flowers and young fruits may not be relevant to explain the final disease outcome. To this end, this suggests that the cultivation of olive in non‐Mediterranean climates, namely sub‐tropical climates, may face an added risk, as summer rains favour inoculum maintenance and build‐up.
On the fruit surface, conidia germinate and differentiate appressoria (Fig. 4F). As opposed to leaves and branches, appressoria on unripe and ripe fruits present an internal light spot (Fig. 4G), which leads to the production of a penetration peg (7.5–8.1 µm in length) into the fruit cuticle (either for C. nymphaeae or C. gloeosporioides; Fig. 4H–I; Talhinhas et al., 2011). If the fruit is unripe, the infection does not develop any further until ripening. In ripe fruits, the fungus proceeds to differentiate a multi‐lobed primary hypha in the first invaded host cell, beneath the penetration peg, denoting a short biotrophic phase (Fig. 4J–L), followed by an extended necrotrophic stage, leading to disease symptoms and signs, namely necroses and the differentiation of acervuli (Fig. 4M–S; Talhinhas et al., 2011).
Lesions on diseased fruits thus generate profuse numbers of conidia, capable of originating secondary infections. Under laboratory conditions, at high humidity and 20–25 °C, symptoms can be reproduced 1–9 days after the inoculation of ripe fruits (Moral and Trapero, 2012; Talhinhas et al., 2011). Under very favourable conditions, particularly in mild autumns with frequent rain events and prolonged high humidity periods, disease severity can increase at a high rate. This severity increase rate, however, is also strongly dependent on the olive cultivar (Talhinhas et al., 2011, 2015) and presumably on the fungal species. The pathogen has been reported to produce a phytotoxin, aspergillomarasmin B (lycomarasmic acid) (Ballio et al., 1969), and a toxic substance has also been implied in branch die‐back and leaf senescence (Moral et al., 2009a, 2014). In addition, the fungus can reach the seed through the vascular tissues, causing seed rot or damping‐off of plantlets obtained from infected seeds (Moral et al., 2009a).
Diseased fruits desiccate as a result of tissue rotting and mummify. Mummies either remain on the tree or fall to the ground. The epidemiological importance of mummies in the soil seems low, as they are rapidly decomposed (Moral and Trapero, 2012; Talhinhas et al., 2011; Zachos and Makris, 1963). Mummies on the tree, however, are important inoculum reservoirs, as they are capable of releasing conidia at constant rates over at least 6 months (Moral and Trapero, 2012; Sergeeva, 2014). Nevertheless, mummies rarely remain on the tree for long. Moral and Trapero (2012) estimated that, on average, only one mummy per tree would remain on the canopy until next autumn, suggesting plant vegetative organs as the major inoculum reservoirs.
The optimal temperature for C. nymphaeae and C. godetiae conidial germination ranges between 20 and 25 °C (Moral et al., 2012), although, under autumn Mediterranean conditions, such values seldom occur concomitantly with rainfall and high humidity. The high variability of olive anthracnose incidence and severity can be related to the spatial and temporal variability in meteorological factors in the Mediterranean region, together with other factors, as emphasized previously (e.g. Almeida, 1899). Host infection requires free water or relative humidity higher than 98% (disease severity increases with the wetness period up to 48 h), with an optimal temperature of 17–20 °C, but infection can occur between 5 and 30 °C, although latent periods increase (Moral et al., 2012). Fruit cuticle wounds caused by pests, namely the Mediterranean olive fly and the Queensland fruit fly, Bactrocera tryoni (Froggatt), can serve as alternative pathways for pathogen entry, thus contributing to a higher disease severity (Graniti et al., 1993; Sergeeva and Spooner‐Hart, 2010).
Canopy density also plays an important role in disease epidemiology. Moral et al. (2012) reported higher severity in super‐high‐density orchards (c. 2000 trees/ha) relative to high‐density orchards (200–800 trees/ha), although there were no differences in total fruit infection (symptomatic plus latent infections). This shows that inoculum reservoirs and spreading agents were equivalent at both densities, but the lower wind/higher humidity conditions of the super‐high‐density orchards favoured disease progression and the development of symptoms.
With regard to nutrients, Xaviér et al. (2014) showed that calcium inhibits Colletotrichum sp. appressorial formation in vitro and that fruits with less than 0.8 ppm of Ca2+ were more susceptible, a situation that can be extrapolated to the calcium content in soil. This led Moral et al. (2014) to speculate on a relationship between high anthracnose incidence and low soil pH (associated with low calcium content in the soil) in some areas of Portugal and southwest Spain.
Epidemiological studies carried out in the field and supporting experiments conducted in the laboratory necessarily use the local pathogen populations and, indeed, it is commonly accepted that the pathogen populations causing epidemics in various olive‐growing countries are particularly adapted to both the host and the environment (Cacciola et al., 2012). Nevertheless, the rapidly evolving taxonomic framework of Colletotrichum spp. infecting olives, together with the pathogen population shifts recently witnessed, hampers the comparison of epidemiological studies conducted in different times and countries.
Host Resistance and Plant–Pathogen Interactions
The perennial nature of the crop and the genetic and geographical diversity of the pathogen render the comparison of olive anthracnose resistance studies conducted in different locations difficult. The normalization of procedures and the identification of standards are therefore of the utmost importance.
Moral and Trapero (2009) developed a 0–10 severity scale that reflects the percentage of affected fruits following a logistic curve [y = , where y is the percentage of affected fruit and x is a scale value]. The scale value of unity is considered to be the detection limit of visual assessments in the field (one affected fruit from 2500 observed fruits per tree], whereas 50% affected fruits correspond to a scale value of 7. As olives are small fruits, symptoms frequently evolve rapidly covering the entire fruit. Therefore, severity scales tend to reflect the percentage of affected fruits in a tree rather than the magnitude or extent of the lesion in a given fruit (Moral and Trapero, 2009; Talhinhas et al., 2015).
There are c. 2500 recognized olive cultivars worldwide (Caballero et al., 2006). The establishment of the Olive World Germplasm Bank in Córdoba (Spain) has enabled direct comparison of cultivars, contributing to the solution of homonymy and synonymy problems. It is a valuable source of germplasm which may help in the current expansion projects of the crop to other regions (Moral et al., 2017). It also enables the performance of olive anthracnose resistance screening experiments. For instance, Moral and Trapero (2009) tested 21 cultivars, classifying ‘Blanqueta’, ‘Empeltre’, ‘Frantoio’, ‘Koroneiki’, ‘Leccino’, ‘Morona‐D’, ‘Picual’ and ‘Razzola’ as resistant. Based on the same collection, Moral et al. (2017) analysed 308 cultivars from 21 countries, 21% of which were classified as highly susceptible, 27% as susceptible, 21% as moderately susceptible, 20% as resistant and 10% as highly resistant. These authors selected cultivars as representative of each resistance class: ‘Ocal’, highly susceptible; ‘Lechín de Sevilla’, susceptible; ‘Arbequina’, moderately susceptible; ‘Picual’, resistant; ‘Frantoio’, highly resistant. This selection prompts the use of these references in studies conducted in other regions, enabling better comparisons amongst studies.
In their study, Moral et al. (2017) evaluated disease severity at identical maturation stages across all cultivars. Although this approach enables a more accurate comparative rating of cultivars, it introduces an important bias because of the variability in meteorological conditions, as not all cultivars reach maturation at the same time. In a study involving eight cultivars, Talhinhas et al. (2015) employed fruits collected at a given date, and therefore at distinct maturation stages, enabling the comparison of susceptibility at typical harvest times. Avoidance of the meteorological bias brings in the maturation bias. This illustrates one of the difficulties of this type of study.
Although the centralization of germplasm evaluation in a single location has obvious advantages, field experiments are necessarily conducted with the local pathogen populations. The variability of olive anthracnose pathogens calls for regional/local studies, reflecting the prevalent local (and emergent) populations. In Italy, cultivars ‘Frantoio’ and ‘Santomauro’ are considered to be resistant (Cacciola et al., 2012), whereas, in Greece, ‘Koroneiki’ is also known as resistant (Cacciola et al., 2012; Xaviér, 2015). It is worth noting the recent outbreak of anthracnose in Greece, caused by C. acutatum s.s., which attained 50% severity on both ‘Kalamon’ fruits and ‘Koroneiki’ flowers. In Portugal, ‘Azeiteira’, ‘Blanqueta de Elvas’, ‘Carrasquenha’ and ‘Negrinha de Freixo’ are listed as resistant (Talhinhas et al., 2015; Xaviér, 2015). In most cases, however, field evaluation studies do not provide an accurate identification of the pathogen present, or otherwise its identity is blurred by the recent taxonomic volatility. Talhinhas et al. (2015) illustrated the occurrence of interaction between host cultivars and pathogen species, reinforcing the need to accurately identify local pathogen species and to conduct screening tests accordingly. A breeding programme conducted in Spain by the University of Córdoba has enabled the identification of two resistant cultivars, which, however, are not more resistant than their resistant parental ‘Frantoio’ (Moral et al., 2006). In Portugal, the highly susceptible, but highly appreciated and widely cultivated, ‘Galega Vulgar’, long recognized as a heterogeneous population, but with some unifying traits, encompasses important levels of genetic diversity (Gemas et al., 2002), among which higher levels of resistance are expected to be found. Oleaster (wild olive) is generally regarded as resistant (Moral et al., 2014), although its potential as a resistance donor to breeding programmes has not been exploited, presumably because of the difficulties arising from long breeding cycles.
Disease Control
The control of olive anthracnose is achieved through a combination of methods, from cultural practices to chemical or biological control. Concerning cultural practices, the selection of olive cultivars is of utmost relevance, as the susceptibility of cultivars to disease is very diverse (Cacciola et al., 2012; Moral et al., 2017) and dependent on the pathogen species (Talhinhas et al., 2015). In addition, appropriate cultivation techniques to maintain plant health are advisable, through balanced fertilization, irrigation and pruning (Moral et al., 2014; Sergeeva, 2011). Pruning can be effective in disease management by reducing inoculum (e.g. diseased twigs), by promoting unfavourable conditions to disease progress (e.g. sunlight infiltration and aeration within the tree canopy improves the drying of foliage and fruit surfaces) and by facilitating chemical treatments (Sergeeva, 2011). Infected pruning debris should be removed from the orchard or destroyed, as it is a source of inoculum.
In areas with high anthracnose incidence, disease control is mainly achieved by the employment of copper‐based fungicides and/or a tight selection of harvest dates balancing maturation (i.e. potential oil and phenolic compound contents), meteorological conditions (harvesting in advance of rainy/wet periods) and agronomic constraints (e.g. immature fruits are difficult to harvest mechanically). Copper‐based fungicides are recommended to be applied at the first autumnal rains, prior to the emergence of symptoms, and sprays should be repeated depending on the cultivar susceptibility, the maturity/harvest date and the frequency and intensity of rainfall (Moral et al., 2014). Nonetheless, under highly favourable conditions, such treatments may be ineffective because the product is easily leached by rain and the opportunity to repeat the treatments can be postponed for long periods if the autumnal rains persist. Moreover, the use of copper may have long‐term consequences because of its accumulation in the soil and water. The alternative use of organic fungicides allows adequate protection [as revised by Cacciola et al. (2012) and Moral et al. (2014)], but these are either not authorized for use in olive trees in European countries or are less cost‐effective.
In Australia, efforts to control olive anthracnose are directed more to the protection of the flowering stage than the fruit ripening period (Sergeeva, 2011). Two sprays, one before flowering and one in early fruit set, are recommended to protect trees before rain events and disease development. In addition, Cacciola et al. (2012) placed emphasis on spring sprays to prevent blossom blight and early fruit infections and to reduce inoculum for fruit infections in autumn. These treatments are also effective against peacock spot caused by Fusicladium oleagineum (Cast.) Ritsch. & Braun, another main aerial disease of olive trees. Even if these treatments are performed with copper‐based products, they have no adverse effects on flowering and fruit set (Roca et al., 2007).
The anticipation of harvest time may be one of the most important strategies to allow fruits to escape infection, as it is known that the susceptibility of olive fruits increases with fruit maturation. Studies have demonstrated that the total phenolic content of the oil obtained from olives harvested earlier is higher, and disease resistance is related to the constitutive phenolic content of fruits. This relationship has been stated for resistance to C. godetiae in Spain (Moral et al., 2015), but not in Portugal, for the highly and moderately susceptible cultivars ‘Galega Vulgar’ and ‘Cobrançosa’ (Gomes et al., 2012) against a C. acutatum s.l. isolate. It is possible that this discrepancy is related to the different characteristics of the cultivars (Moral et al., 2015), but may also be the result of the different characteristics of the pathogens.
Regulatory restrictions on the use of pesticides, together with public awareness concerning their impact on health and the environment, are directing research to the development of more eco‐friendly disease control alternatives (Landum et al., 2016; Preto et al., 2017). Recently, a safe and effective natural antifungal preparation to control olive anthracnose was obtained from pomegranate. The peel extract revealed a strong in vitro fungicidal activity against C. acutatum s.s. and was very effective in both preventative and curative trials with artificially inoculated fruit. Depending on the concentration of the product, the incidence of the disease was reduced by 77.6%, 57.0% and 51.8% through two treatments performed 30 and 15 days before the expected epidemic outbreak. In addition, induced resistance in treated olive tissues was registered (Pangallo et al., 2017).
Conclusion and Future Prospects
Modern molecular biology tools, including genomics, transcriptomics and metabolomics, are still in a relatively early stage in both Olea europaea and Colletotrichum spp. Anthracnose is not an easy subject for molecular biologists working with this difficult major crop, and olive is only one of a vast array of host plants of Colletotrichum spp. Acute and/or deadly diseases, such as those caused by Xylella fastidiosa Wells or Verticillium dahliae Kleb., easily call for more attention than a chronic disease such as anthracnose. Moreover, the relatively long breeding cycle of olive and the short time frame for in vivo anthracnose studies, limited to the autumn, do not fit the typical life cycle of research projects. Long‐standing research programmes, with clearly defined objectives, but sufficiently flexible to cope with changes that may occur over time, are therefore needed to cope with olive anthracnose. For example, C. acutatum s.s. is becoming a major olive anthracnose pathogen in several Mediterranean countries, replacing the less virulent C. godetiae. Breeding programmes must be planned in order to cope with such pathogen population shifts. Another example, more on the formal side but with practical implications, is the fact that Colletotrichum taxonomy has experienced major changes over the last 10–15 years. In part, these have helped to improve our understanding of olive anthracnose pathogens, but it is fair to say that research on the diversity of olive anthracnose pathogens has also contributed to spark such recent taxonomic changes, at least those in the C. acutatum species complex. Olive anthracnose research needs a stable taxonomic framework for its causal agents, so that studies can be conducted in such a way that they are informative to the international community.
Acknowledgements
The research unit LEAF – Linking Landscape, Environment, Agriculture and Food – is supported by the Fundação para a Ciência e a Tecnologia (FCT), Portugal (reference UID/AGR/04129/2013). The authors declare no conflicts of interest.
References
- Achbani, E.A. , Benbouazza, A. and Douira, A. (2013) First report of olive anthracnose, caused by Colletotrichum gloeosporioides, in Morocco. Atlas J. Biol. 2, 171–174. [Google Scholar]
- Acosta, D.R. (1932) Investigaciones fitopatológicas. Min. Industrias, Dir. Agronomía, Publicación Mensual 4, 1–18. [Google Scholar]
- Agosteo, G.E. , Cacciola, S.O. , Pane, A. and Frisullo, S. (1997) Vegetative compatibility groups of Colletotrichum gloeosporioides from olive in Italy. In: Proceedings of the 10th Congress of the Mediterranean Phytopathological Union, Montpellier, France, pp. 95–99. Florence, Italy: Mediterranean Phytopathological Union. [Google Scholar]
- Agosteo, G.E. , Magnano di San Lio, G. , Frisullo, S. and Cacciola, S.O. (2002) Characterisation of the causal agent of olive anthracnose in southern Italy. Acta Hort. 586, 713–716. [Google Scholar]
- Agosteo, G.E. , Sanzani, S.M. , Macri, C. , Cacciola, S.A. , Li Destri Nicosia, M.G. and Schena, L. (2015) Olive leachates affect germination of Colletotrichum godetiae conidia and the development of appressoria. Phytopathol. Mediterr. 54, 35–44. [Google Scholar]
- Almeida, J.V. (1899) La gaffa des olives en Portugal. Bull. Soc. Mycol. Fr. 15, 90–94. [Google Scholar]
- von Arx, J.A. (1970) A revision of the fungi classified as Gloeosporium . Bibl. Mycol. 24, 1–203. [Google Scholar]
- Ballio, A. , Bottalico, A. , Bounocore, V. , Carilli, A. , Di Vittorio, V. and Graniti, A. (1969) Production and isolation of aspergillomarasmin B (lycomarasmic acid) from cultures of Colletotrichum gloeosporioides (Gloeosporium olivarum). Phytopathol. Mediterr. 8, 187–196. [Google Scholar]
- Baroncelli, R. , Talhinhas, P. , Pensec, F. , Sukno, S.A. , Le Floch, G. and Thon, M. (2017) The Colletotrichum acutatum species complex as a model system to study evolution and host specialization in plant pathogens. Front. Microbiol. 8, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bompeix, G. , Julio, E.V.R. and Phillips, D.H. (1988) Glomerella cingulata (Stoneman) Spaulding et V. Schrenk In: European Handbook of Plant Diseases (Smith I. M., Dunez J., Lelliot R.A., Phillips D.H. and Archer S.A., eds), pp. 325–327. Oxford: Blackwell Scientific Publications. [Google Scholar]
- Brancher, N. , Pérez, B.A. , Matías, C. , Otero, L. , Oriolani, E. , Aybar, V.E. and Roca, M. (2008) Olive (Olea europea L.) pathologies and pests in Catamarca province, Argentina. Acta Hort. 949, 317–321. [Google Scholar]
- Bustan, A. , Kerem, Z. , Yermiyahu, U. , Ben‐Gal, A. , Lichter, A. , Droby, S. , Zchori‐Fein, E. , Orbach, D. , Zipori, I. and Dag, A. (2014) Preharvest circumstances leading to elevated oil acidity in ‘Barnea’ olives. Sci. Hort. 176, 11–21. [Google Scholar]
- Caballero, J.M. , del Río, C. , Barranco, D. and Trujillo, I. (2006) The olive world germplasm bank of Córdoba, Spain. Olea, 25, 14–19. [Google Scholar]
- Cacciola, S.A. , Faedda, R. , Sinatra, F. , Agosteo, G.E. , Schena, L. , Frisullo, S. and Magnano di San Lio, G. (2012) Olive anthracnose. J. Plant Pathol. 94, 29–44. [Google Scholar]
- Carvalho, M.T. , Simões‐Lopes, P. and Silva, M.J.M. (2008) Influence of different olive infection rates of Colletotrichum acutatum on some important olive oil chemical parameters. Acta Hort. 791, 555–559. [Google Scholar]
- Chattaoui, M. , Raya, M.C. , Bouri, M. , Moral, J. , Perez‐Rodriguez, M. , Trapero, A. , Msallem, M. and Rhouma, A. (2016) Characterization of a Colletotrichum population causing anthracnose disease on olive in northern Tunisia. J. Appl. Microbiol. 120, 1368–1381. [DOI] [PubMed] [Google Scholar]
- Ciccarone, A. (1950) Considerazioni biologiche e sistematiche sull'agente della “lebbra” delle olive, recentemente osservata nel Leccese. Boll. Regia Staz. Patol. Veg. Roma, Ser. III. 5, 143–165. [Google Scholar]
- Damm, U. , Cannon, P.F. , Woundenberg, J.H.C. and Crous, P.W. (2012) The Colletotrichum acutatum species complex. Stud. Mycol. 73, 27–113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duarte, H.S.S. , Cabral, P.G.C. , Pereira, O.L. , Zambolim, L. , Gonçalves, E.D. , Vieira Neto, J. , Zambolim, E.M. and Sergeeva, V. (2010) First report of anthracnose and fruit mummification of olive fruit (Olea europaea) caused by Colletotrichum acutatum in Brazil. Plant Pathol. 59, 1170. [Google Scholar]
- Embaby, E.‐S. (2014) Anthracnose disease (Colletotrichum sp.) affecting olive fruit quality and its control in Egypt. J. Agric. Technol. 10, 1289–1306. [Google Scholar]
- Faedda, R. , Agosteo, G.E. , Schena, L. , Mosca, S. , Frisullo, S. , Magnano di San Lio, G. and Cacciola, S.A. (2011) Colletotrichum clavatum sp. nov. identified as the causal agent of olive anthracnose in Italy. Phytopathol. Mediterr. 50, 283–302. [Google Scholar]
- Gemas, V.J.V. , Rijo‐Johansen, M.J. and Fevereiro, P. (2002) Intra‐variability of the Portuguese olive cultivar Galega Vulgar expressed by RAPD, ISSR and SPAR. Acta Hort. 586, 175–178. [Google Scholar]
- Gomes, S. , Bacelar, E. , Martins‐Lopes, P. , Carvalho, T. and Guedes‐Pinto, H. (2012) Infection process of olive fruits by Colletotrichum acutatum and the protective role of the cuticle and epidermis. J. Agric. Sci. 4, 101–110. [Google Scholar]
- Gorter, G.J.M.A. (1956) Anthracnose fungi of olives. Nature, 178, 1129–1130. [Google Scholar]
- Gorter, G.J.M.A. (1962) The identity of the fungus causing anthracnose of olives in South Africa. Bothalia, 7, 769–778. [Google Scholar]
- Graniti, A. , Frisullo, S. , Pennisi, A.M. and Magnano di San Lio, G. (1993) Infections of Glomerella cingulata on olive in Italy. EPPO Bull. 23, 457–465. [Google Scholar]
- Hemmi, T. and Kurata, S. (1935) Contributions to the knowledge of anthracnose of plants II, on Gloeosporium olivarum Alm. causing the olive anthracnose. J. Soc. Trop. Agric. Taiwan, 6, 573–583. [Google Scholar]
- Iannotta, I. , Perri, E. , Siriani, R. and Tocci, C. (1999) Influence of Colletotrichum gloeosporioides (Penzing) and Camarosporium dalmatica (Thum) attacks on olive oil quality. Acta Hort. 474, 399–401. [Google Scholar]
- Iliadi, M.K. , Tjamos, E. , Antoniou, P. and Tsitsigiannis, D.I. (2018) First report of Colletotrichum acutatum causing anthracnose on olives in Greece. Plant Dis, 102, 820. [DOI] [PubMed] [Google Scholar]
- Landum, M.C. , Félix, M.R. , Alho, J. , Garcia, R. , Cabrita, M.J. , Rei, F. and Varanda, C. (2016) Antagonistic activity of fungi of Olea europaea L. against Colletotrichum acutatum . Microbiol. Res. 183, 100–108. [DOI] [PubMed] [Google Scholar]
- López‐Moral, A. , Raya‐Ortega, M.C. , Agustí‐Brisach, C. , Roca, L.F. , Lovera, M. , Luque, F. , Arquero, O. and Trapero, A. (2017) Morphological, pathogenic, and molecular characterization of Colletotrichum acutatum isolates causing almond anthracnose in Spain. Plant Dis. 101, 2034–2045. [DOI] [PubMed] [Google Scholar]
- Malacrinò, A. , Schena, L. , Campolo, O. , Laudani, F. , Mosca, S. , Giunti, G. , Strano, C.P. and Palmeri, V. (2017) A metabarcoding survey on the fungal microbiota associated to the olive fruit fly. Microb. Ecol. 73, 677–684. [DOI] [PubMed] [Google Scholar]
- Margarita, L. , Porta‐Puglia, A. and Quacquarelli, A. (1986) Colletotrichum acutatum, nuovo patogeno dell'olivo in Cina e confronto con l'agente della “lebbra” dell'olivo. Ann. Ist. Sperim. Patol. Veg. 11, 125–133. [Google Scholar]
- Marin‐Felix, Y. , Groenewald, J.Z. , Cai, L. , Chen, Q. , Marincowitz, S. , Barnes, I. , Bensch, K. , Braun, U. , Camporesi, E. , Damm, U. , de Beer, Z.W. , Dissanayake, A. , Edwards, J. , Giraldo, A. , Hernández‐Restrepo, M. , Hyde, K.D. , Jayawardena, R.S. , Lombard, L. , Luangsa‐Ard, J. , McTaggart, A.R. , Rossman, A.Y. , Sandoval‐Denis, M. , Shen, M. , Shivas, R.G. , Tan, Y.P. , van der Linde, E.J. , Wingfield, M.J. , Wood, A.R. , Zhang, J.Q. , Zhang, Y. and Crous, P. (2017) Genera of phytopathogenic fungi: GOPHY 1. Stud. Mycol. 86, 99–216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martín, M.P. and García‐Figueres, F. (1999) Colletotrichum acutatum and C. gloeosporioides cause anthracnose on olives. Eur. J. Plant Pathol. 105, 733–741. [Google Scholar]
- Mincione, A. , Valenzise, M. , Runcio, A. , Poiana, M. , Agosteo, G.E. and Taccone, P.L. (2004) Ricerche sugli oli di oliva vergini calabresi. Influenza delle fitopatie sulle caratteristiche qualitative degli oli. Nota I‐Effetti diretti degli attacchi di Antracnosi. Riv. It. Sost. Grasse. 81, 9–17. [Google Scholar]
- Moral, J. and Trapero, A. (2009) Assessing the susceptibility of olive cultivars to anthracnose caused by Colletotrichum acutatum . Plant Dis. 93, 1028–1036. [DOI] [PubMed] [Google Scholar]
- Moral, J. and Trapero, A. (2012) Mummified fruit as a source of inoculum and disease dynamics of olive anthracnose caused by Colletotrichum spp. Phytopathology, 102, 982–989. [DOI] [PubMed] [Google Scholar]
- Moral, J. , Alsalimiya, M. , Muñoz‐Díez, C. , León, L. , de la Rosa, R. and Trapero, A. (2006) Evaluación de preselecciones de olivo por su resistencia a Repilo y Antracnosis. Act. Hort. 45, 177–178. [Google Scholar]
- Moral, J. , Cherifi, F. , Muñoz‐Díez, C. , Xaviér, C.J. and Trapero Casas, A. (2009a) Infection of olive seeds by Colletotrichum acutatum and its effect on germination. Phytopathology, 99, S88. [Google Scholar]
- Moral, J. , de Oliveira, R. and Trapero, A. (2009b) Elucidation of disease cycle of olive anthracnose caused by Colletotrichum acutatum . Phytopathology, 99, 548–556. [DOI] [PubMed] [Google Scholar]
- Moral, J. , Jurado‐Bello, J. , Sánchez, M.I. , Oliveira, R. and Trapero, A. (2012) Effect of temperature, wetness duration, and planting density on olive anthracnose caused by Colletotrichum spp. Phytopathology, 102, 974–981. [DOI] [PubMed] [Google Scholar]
- Moral, J. , Xaviér, C. , Roca, L.F. , Romero, J. , Moreda, W. and Trapero, A. (2014) La Antracnosis del olivo y su efecto en la calidad del aceite. Grasas y Aceites, 65, e028. [Google Scholar]
- Moral, J. , Alsalimiya, M. , Roca, L.F. , Díez, C.M. , León, L. , de la Rosa, R. , Barranco, D. , Rallo, L. and Trapero, A. (2015) Relative susceptibility of new olive cultivars to Spilocaea oleagina, Colletotrichum acutatum, and Pseudocercospora cladosporioides . Plant Dis. 99, 58–64. [DOI] [PubMed] [Google Scholar]
- Moral, J. , Xaviér, C. , Roca, L.F. , Viruega, J.R. , Roca, L.F. , Caballero, J. and Trapero, A. (2017) Variability in susceptibility to anthracnose in the world collection of olive cultivars of Cordoba (Spain). Front. Plant Sci. 8, 1892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mosca, S. , Li Destri Nicosia, M.G. , Cacciola, S.O. and Schena, L. (2014) Molecular analysis of Colletotrichum species in the carposphere and phyllosphere of olive. PLoS One, 9, e114031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mugnai, L. , Surico, G. and Ragazzi, A. (1993) Glomerella cingulata on olive in India: morphological and pathological notes. Bull. OEPP, 23, 449–455. [Google Scholar]
- Nagorny, P.I. and Eristavi, E.M. (1929) A brief survey of plant diseases in Abkhasia in 1928. Pub. Agr. Exp. St. Abkhasia. 38, 1–28. [Google Scholar]
- Pangallo, S. , Nicosia, M.G.L.D. , Agosteo, G.E. , Abdelfattah, A. , Romeo, F.V. , Cacciola, S.O. , Rapisarda, P. and Schena, L. (2017) Evaluation of a pomegranate peel extract as an alternative means to control olive anthracnose. Phytopathology, 107, 1462–1467. [DOI] [PubMed] [Google Scholar]
- Peres, N.A. , Timmer, L.W. , Adaskaveg, J.E. and Correll, J.C. (2005) Life styles of Colletotrichum acutatum . Plant Dis. 89, 784–796. [DOI] [PubMed] [Google Scholar]
- Perfect, S.E. , Hughes, H.B. , O'Connell, R.J. and Green, J.R. (1999) Colletotrichum: a model genus for studies on pathology and fungal–plant interactions. Fungal Genet. Biol. 27, 186–198. [DOI] [PubMed] [Google Scholar]
- Pontis, R.E. and Hansen, H.N. (1942) Olive anthracnose in the United States. Phytopathology, 32, 642–644. [Google Scholar]
- Preto, G. , Martins, F. , Pereira, J.A. and Baptista, P. (2017) Fungal community in olive fruits of cultivars with different susceptibilities to anthracnose and selection of isolates to be used as biocontrol agents. Biol. Control. 110, 1–9. [Google Scholar]
- Ranalli, A. , Modesti, G. , Patumi, M. and Fontanazza, G. (2000) The compositional quality and sensory properties of virgin olive oil from a new olive cultivar I‐77. Food Chem. 69, 37–46. [Google Scholar]
- Rhouma, A. , Triki, M.A. and Msallem, M. (2010) First report of olive anthracnose caused by Colletotrichum gloeosporioides in Tunisia. Phytopathol. Mediterr. 49, 95–98. [Google Scholar]
- Roca, L.F. , Moral, J. , Viruega, J.R. , Ávila, A. , Oliveira, R. and Trapero, A. (2007) Copper fungicides in the control of olive diseases. Olea, 26, 48–50. [Google Scholar]
- Runcio, A. , Sorgonà, L. , Mincione, A. , Santacaterina, S. and Poiana, M. (2008) Volatile compounds of virgin olive oil obtained from Italian cultivars grown in Calabria. Effect of processing methods, cultivar, stone removal, and anthracnose attack. Food Chem. 106, 735–740. [Google Scholar]
- Sanei, S.J. and Razavi, S.E. (2011) Differentiation of olive Colletotrichum gloeosporioides populations on the basis of vegetative compatibility and pathogenicity. Afr. J. Agric. Res. 6, 2099–2107. [Google Scholar]
- Sanei, S.J. and Razavi, S.E. (2012) Survey of olive fungal disease in north of Iran. Annu. Rev. Res. Biol. 2, 27–36. [Google Scholar]
- Sarejanni, J.A. (1939) Catalogue commenté des champignons rencontrés sur les plantes cultivées en Grèce. Ann. Inst. Phytopathol. Benaki. 3, 60. [Google Scholar]
- Schena, L. , Mosca, S. , Cacciola, S.O. , Faedda, R. , Sanzani, S.M. , Agosteo, G.E. , Sergeeva, V. and Magnano di San Lio, G. (2014) Species of the Colletotrichum gloeosporioides and C. boninense complexes associated with olive anthracnose. Plant Pathol. 63, 437–336. [Google Scholar]
- Schena, L. , Abdelfattah, A. , Mosca, S. , Li Destri Nicosia, M.G. , Agosteo, G.E. and Cacciola, S.O. (2017) Quantitative detection of Colletotrichum godetiae and C. acutatum sensu stricto in the phyllosphere and carposphere of olive during four phenological phases. Eur. J. Plant Pathol. 149, 337–347. [Google Scholar]
- Sergeeva, V. (2011) Anthracnose in olives: symptoms, disease cycle and management. In: 4th International Conference for Olive Tree and Olive Products – OLIVEBIOTEQ (Chartzoulakis, Kostas S. ed), pp. 269–274. Chania, Greece: NAGREF Institute for Olive Tree and Subtropical Plants of Chania.
- Sergeeva, V. (2014) The role of epidemiology data in developing integrated management of anthracnose in olives: a review. Acta Hortic. 1057, 163–168. [Google Scholar]
- Sergeeva, V. and Spooner‐Hart, R. (2010) Anthracnose and Queensland fruit fly in olives. Olive Press. 16, 23–24. [Google Scholar]
- Sergeeva, V. , Spooner‐Hart, R. and Nair, N.G. (2008) First report of Colletotrichum acutatum and C. gloeosporioides causing leaf spots of olives (Olea europaea) in Australia. Australas. Plant Dis. Notes, 3, 143–144. [Google Scholar]
- Sharma, R.L. and Kaul, J.L. (1990) Field evaluation of fungicide for control of olive anthracnose. Indian J. Mycol. Plant Pathol. 20, 185–187. [Google Scholar]
- Shivas, R.G. and Tan, Y.P. (2009) A taxonomic re‐assessment of Colletotrichum acutatum in Australia, introducing C. fioriniae comb. nov. and C. simmondsii sp. nov. Fungal Divers, 39, 111–122. [Google Scholar]
- de Silva, D.D. , Crous, P.W. , Ades, P.K. , Hyde, K.D. and Taylor, P.W.J. (2017) Life styles of Colletotrichum species and implications for plant biosecurity. Fungal Biol. Rev. 31, 155–168. [Google Scholar]
- Silva, D.N. , Talhinhas, P. , Cai, L. , Manuel, L. , Guichuru, E.K. , Loureiro, A. , Várzea, V. , Paulo, O.S. and Batista, D. (2012) Host‐jump drives rapid and recent ecological speciation of the emergent fungal pathogen Colletotrichum kahawae . Mol. Ecol. 21, 2655–2670. [DOI] [PubMed] [Google Scholar]
- Sreenivasaprasad, S. and Talhinhas, P. (2005) Genotypic and phenotypic diversity in Colletotrichum acutatum, a cosmopolitan pathogen causing anthracnose on a wide range of hosts. Mol. Plant Pathol. 6, 361–378. [DOI] [PubMed] [Google Scholar]
- Talhinhas, P. , Sreenivasaprasad, S. , Neves‐Martins, J. and Oliveira, H. (2005) Molecular and phenotypic analyses reveal the association of diverse Colletotrichum acutatum groups and a low level of C. gloeosporioides with olive anthracnose. Appl. Environ. Microbiol. 71, 2987–2998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talhinhas, P. , Neves‐Martins, J. , Oliveira, H. and Sreenivasaprasad, S. (2009) The distinctive population structure of Colletotrichum species associated with olive anthracnose in the Algarve region of Portugal reflects a host–pathogen diversity hot spot. FEMS Microbiol. Lett. 296, 31–38. [DOI] [PubMed] [Google Scholar]
- Talhinhas, P. , Mota‐Capitão, C. , Martins, S. , Ramos, A.P. , Neves‐Martins, J. , Guerra‐Guimarães, L. , Várzea, V. , Silva, M.C. , Sreenivasaprasad, S. and Oliveira, H. (2011) Epidemiology, histopathology and aetiology of olive anthracnose caused by Colletotrichum acutatum and C. gloeosporioides in Portugal. Plant Pathol. 60, 483–495. [Google Scholar]
- Talhinhas, P. , Gonçalves, E. , Sreenivasaprasad, S. and Oliveira, H. (2015) Virulence diversity of anthracnose pathogens (Colletotrichum acutatum and C. gloeosporioides complexes) on eight olive cultivars commonly grown in Portugal. Eur. J. Plant Pathol. 142, 73–83. [Google Scholar]
- Trapero, A. and Blanco, M.A. (2008) Enfermedades In: El Cultivo de Olivo (Barranco D., Fernández‐Escobar R. and Rallo L., eds), pp. 595–656. Madrid: Junta de Andalucía/Mundi‐Prensa. [Google Scholar]
- Vučinić, Z. , Latinović, J. , Metzidakis, I.T. and Voyiatzis, D.G. (1999) Colletotrichum gloeosporioides, a new olive (Olea europaea L.) parasite in Yugoslavia. Acta Hortic. 474, 577–579. [Google Scholar]
- Weir, B. , Johnston, P.R. and Damm, U. (2012) The Colletotrichum gloeosporioides species complex. Stud. Mycol. 73, 115–180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitelaw‐Weckert, M. , Curtin, S.J. , Huang, R. , Steel, C.C. , Blanchard, C.L. and Roffey, P.E. (2007) Phylogenetic relationships and pathogenicity of Colletotrichum acutatum isolates from grape in subtropical Australia. Plant Pathol. 56, 448–463. [Google Scholar]
- Wu, W.X. , Huang, X.Q. , Liu, Y. and Zhang, L. (2017) First report of apple bitter rot caused by Colletotrichum rhombiforme in China. Plant Dis. 101, 1033. [Google Scholar]
- Xaviér, C.J. (2015) Resistencia y Control Químico en la Antracnosis del Olivo causada por Colletotrichum spp. PhD Thesis. Córdoba: Universidad de Córdoba, p. 168.
- Xaviér, C.J. , Moral, J. , Pérez, M. , Agalliu, G. , Alcántara, E. and Trapero, A. (2014) El calcio como herramienta para el control de la antracnosis del olivo causada por Colletotrichum spp. In: Libro de resúmenes del XVII Congreso de la Sociedad Española de Fitopatología, 7–19 octubre 2014, Lleida, Spain, p. 314. Chania, Greece: Sociedad Española de Fitopatologia and Universidad de Lleida. [Google Scholar]
- Zachos, D.G. and Makris, S.A. (1963) Recherches sur le Gloeosporium olivarum Alm. en Grèce III. Épidémiologie de la maladie. Ann. Inst. Phytopathol. Benaki, Nouv. Ser. 5, 238–259. [Google Scholar]


