Summary
The filamentous fungus Fusarium graminearum, a devastating pathogen of barley (Hordeum vulgare L.), produces mycotoxins that pose a health hazard. To investigate the surface interactions of F. graminearum on barley, we focused on barley florets, as the most important infection site leading to grain contamination. The fungus interacted with silica‐accumulating cells (trichomes and silica/cork cell pairs) on the host surface. We identified variation in trichome‐type cells between two‐row and six‐row barley, and in the role of specific epidermal cells in the ingress of F. graminearum into barley florets. Prickle‐type trichomes functioned to trap conidia and were sites of fungal penetration. Infections of more mature florets supported the spread of hyphae into the vascular bundles, whereas younger florets did not show this spread. These differences related directly to the timing and location of increases in silica content during maturation. Focal accumulation of cellulose in infected paleae of two‐row and six‐row barley indicated that the response is in part linked to trichome type. Overall, silica‐accumulating epidermal cells had an expanded role in barley, serving to trap conidia, provide sites for fungal ingress and initiate resistance responses, suggesting a role for silica in pathogen establishment.
Keywords: conidia, perithecia, prickle, resistance, silica, trichome
Introduction
Fusarium graminearum (Ascomycota, Sordariomycetes) is the primary causal agent of Fusarium head blight (FHB) in barley and wheat, and also causes stalk and ear rot of maize. Fungal biosynthesis of trichothecene mycotoxins, including deoxynivalenol (DON), significantly decreases grain quality independent of yield reduction (Escrivá et al., 2015). In the USA, declining plantings of wheat and barley are partly a result of FHB, which led to billions of dollars in losses of wheat and barley crops between 1993 and 2004 (McMullen et al., 2012). Worldwide, barley is used for food, feed and malt for beer production. Regulations on mycotoxin levels in malting barley are exceptionally stringent, and may be as low as 0.5 ppm (He et al., 2015), as the presence of the fungus can result in off‐flavours and gushing, which is the spontaneous and uncontrolled foaming of beer caused by the presence of fungal hydrophobins in the malt (Piacentini et al., 2015; Sarlin et al., 2012). FHB is partially controlled by fungicide applications. Although moderately resistant cultivars of wheat and barley are available, strong crop resistance has not been developed. However, progress has been made in combining available tools and strategies for more effective control (Wegulo et al., 2015; Willyerd et al., 2012). Understanding how the disease is initiated in barley heads would provide important knowledge for the development of new control measures to prevent infection.
In North America, two types of barley are bred, termed ‘two‐row’ and ‘six‐row’, based on the arrangement of the florets on the rachis. The types have different tolerances in adaptation to particular growing regions, and in grain quality and characteristics (Schwarz and Horsley, 1997). Both two‐row and six‐row barley are naturally resistant to the spread of F. graminearum from individual infection sites (Bushnell et al., 2003). However, in six‐row barley, the fungus spreads externally from one floret to the next without moving through the rachis, as is common in wheat (Langevin et al., 2004), highlighting the particular importance of surface penetration mechanisms for disease initiation and spread. Although, in wheat, DON is a virulence factor (Desjardins et al., 1996), in barley, F. graminearum strains mutated in the ability to produce DON have been found to be no less aggressive than wild‐type strains on the majority of varieties (Jansen et al., 2005; Langevin et al., 2004; Maier et al., 2006). Nevertheless, the elimination of DON in barley is essential from a human and animal safety perspective.
Fusarium graminearum is spread in the field by airborne spores formed on contaminated crop residues. Both sexual ascospores shot from perithecia (fruiting bodies) and asexual conidia formed on crop residues contribute to the disease cycle (Desjardins et al. 2006; Shaner, 2003). Spores released during host flowering require moisture to initiate infection of host floral tissue, hence the association of high disease with rain during grain flowering (Bushnell et al., 2003; De Wolf et al., 2003). Direct penetration of epidermal tissue of the inner palea and lemma, and ovaries, has been observed in wheat florets (Pugh et al., 1933; Wanjiru et al., 2002). In barley, researchers have observed hyphal growth across the surface of the floret prior to entry via natural crevices between the palea and lemma, and, once inside the floret, the fungus uses crevices at the base of the caryopsis to gain entrance into the rachis (Lewandowski et al., 2006). The TRI5 gene, encoding the trichodiene synthase for DON synthesis, is induced in these infection structures, implicating the involvement of DON in penetration (Boenisch and Schäfer, 2011). It is worth noting that the fungus has been observed on occasion to use stomates to enter the host tissue (Nguyen et al., 2016a; reviewed by Bushnell et al., 2003). In addition, anthers are known to be a primary tissue for the initiation of colonization, especially in wheat, as first described by Pugh et al. (1933). Similar infection processes in wheat have been observed for F. culmorum, causal agent of FHB in Europe and elsewhere (reviewed by Bushnell et al., 2003).
A variety of cell types occur on the epidermal landscape of the barley floret that eventually fill with silica. The terminology used to describe the morphology of these cells can be inconsistent amongst publications. We use the following terms and definitions for those cell types found in the barley varieties used in this study: silica/cork cell (flat silica‐filled cell associated with a cork cell) and trichome (general term for a cell with a protuberance). Trichomes are prickle‐like (those with a large pointed protuberance) or domed (those featuring a smaller, rounded protuberance). Silica‐containing cells are found in abundance in grasses and have been used for species identification, particularly in ancient sites, where the silica crystals, termed ‘phytoliths’, are all that remain of ancient grasses (Ball et al., 1999; Piperno, 2016). In addition, xylem vessels, long epidermal cells and stomates of the paleae and culms have been shown to harbour silica in barley (Hayward and Parry, 1980). Interestingly, mature paleae harbour the majority of silica at the trichomes, and have greater silica content overall than the culms (Hayward and Parry, 1980).
At the site of fungal ingress or attempted ingress into a host plant, the host cells may accumulate a number of defence‐associated compounds. The accumulation of lignin and the development of papillae (cell wall appositions that include cellulose), callose and phenolics have been observed at the site of fungal penetration in many plant–fungal interactions (Assaad, 2004; Chowdhury et al., 2014; Vance et al., 1980), where they have been associated with both compatible and incompatible responses (Assaad, 2004; Nishimura et al., 2003; Vance et al., 1980).
We have investigated the surface interactions of F. graminearum with barley florets to better understand the infection and colonization process, characterizing the ingress of barley by F. graminearum at the trichomes. In addition, we have identified a potential resistance response by trichomes and silica/cork cells, demonstrating that this response varies in magnitude between two‐ and six‐row barley. This information is essential to the development of more highly resistant lines, and can be used to harness the best characteristics of both types of barley. Parts of this work have been published previously in the form of a thesis (Afton, 2012). Specifically, the investigation of the later infection stages has been reported in the thesis, including Figs 3, 4, 5 and associated experiments.
Figure 3.
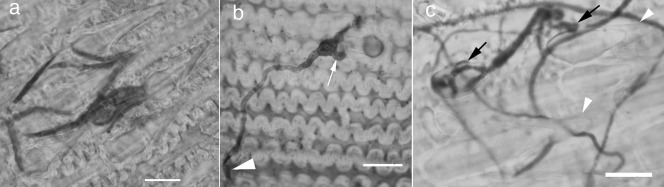
Early interactions of Fusarium graminearum with trichomes. (a) Conidia trapped on large prickles at 1 days post‐inoculation (dpi). (b) Germinating conidium on a small prickle with infection arm (arrow) and terminal end of hypha (arrowhead) penetrating the base of a neighbouring prickle at 3 dpi. (c) Bulbous hyphae on large prickles and colonized trichome base at 5 dpi (arrows). Large prickles visible (tips indicated by arrowheads). Bars, 20 µm.
Figure 4.
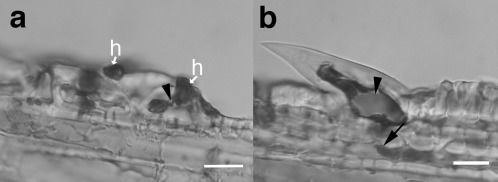
Penetration of trichomes by Fusarium graminearum at 5 days post‐inoculation (dpi). (a) Infection of trichome companion cell from (b) with external hyphopodia (h) appressed to outside and thin penetration hypha (arrowhead). (b) Large prickle near paleal margin with fungal growth into hypodermis (arrow). Note: staining reveals silica crystal inside trichome (arrowhead). Bars, 20 μm.
Figure 5.
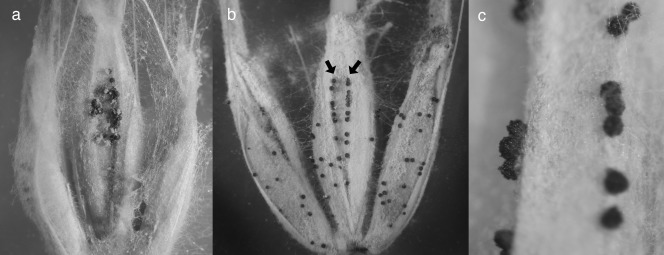
Perithecia emerging from the surface of inoculated barley florets. (a) Perithecia at the location of the inoculation droplet on the palea of a floret infected before fusion. (b) Perithecia emerging from the paleal vascular bundle ridges (arrows), including beyond the location of infection (compare with Fig. 1a). (c) Detail of perithecia emerging from vascular bundle ridges.
Results
Trichome variation and distribution amongst barley varieties
To understand the variation in surface morphology that impacts fungal–plant interactions, we examined the paleae of 11 randomly chosen barley varieties, plus two varieties chosen because their trichome type was not characteristic of their row type (Robust and CDC Harrington; Table 1). We characterized trichome morphology and distribution on the outer surface of the paleae of these 13 varieties. The epidermis of the paleae of all varieties was covered with elongated epidermal cells, and stomata appeared along the vascular bundles bordering the central furrow (Fig. 1). Trichomes and silica/cork cells were distributed amongst the epidermal cells (Fig. 2a,b). The distribution of trichomes and silica/cork cells across the paleal surface varied from sample to sample, but the entire surface of the vascular bundles was densely covered with trichomes in both two‐ and six‐row barley. Although they were present on all varieties, trichome shape and location were characteristic of the variety. Those with a round base and a domed peak appeared predominantly on two‐row barley (Fig. 2a,c). In contrast, most of the six‐row barley examined featured trichomes with a similar base, but a prickle‐like point that aligned with the direction of elongation of the epidermal cells (Fig. 2b,d; Table 1). There were two notable exceptions to these observations: the surface of Robust, a six‐row barley, featured the domed trichome observed primarily on two‐row barley; and CDC Harrington, a two‐row barley, featured the prickle‐like trichome observed in six‐row barley (Table 1). There were two types of prickle‐like trichomes, predominantly on six‐row barley. Small prickles covered the surface of the paleae (Fig. 2b) and large prickles (Fig. 3a) were present along the vascular traces and the margins, and at the tips.
Table 1.
Trichome type in paleae of two‐ and six‐row barley varieties.
| Variety | Row number | Trichome shape |
|---|---|---|
| Desperado | 6 | Prickle |
| Lacey | 6 | Prickle |
| Quest * | 6 | Prickle |
| Robust * | 6 | Domed |
| Stander * | 6 | Prickle |
| Aramir * | 2 | Domed |
| Bowman * | 2 | Domed |
| Conlon | 2 | Domed |
| CDC Harrington * | 2 | Prickle |
| CDC Kendall | 2 | Domed |
| Norman | 2 | Domed |
| Odyssey | 2 | Domed |
| Pinnacle | 2 | Domed |
*Varieties for which focal accumulation was assessed.
Figure 1.

Barley floret structure and placement of the inoculum droplet in the central furrow on the outer surface of the palea for the detached floret assay. (a) Intact florets with inoculation droplet in the furrow of the centre floret. White arrows indicate the location of the vascular bundles of the palea on either side of the central furrow. A row of stomates runs along the vascular bundle ridges. Black arrows indicate the edges of the lemma which is wrapped around the palea, (b) Cross‐section of the seed development stage, showing the fusion of the lemma and palea. Note that the right lemma edge is not yet fused. Arrowheads indicate the position of vascular bundle ridges on the palea.
Figure 2.
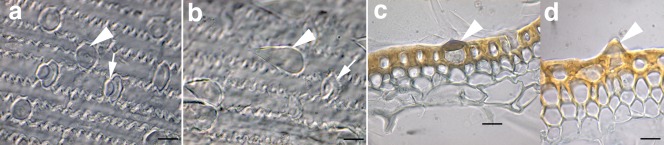
Epidermal cells of the outer surface of the barley cultivars Aramir (two‐row) and Stander (six‐row). (a) Aramir paleal surface. (b) Stander paleal surface. (c) Latitudinal cross‐section of the Aramir palea. (d) Latitudinal cross‐section of the Stander palea. The distinguishing domed trichomes of Aramir are indicated by arrowheads (a, c). The distinguishing small prickle‐like trichomes of Stander are indicated by arrowheads (b, d). Arrows indicate silica/cork cell combinations. Bars, 5 μm.
Fusarium graminearum–barley surface interactions in detached and intact florets
Fusarium graminearum–trichome interactions were studied in detail on the six‐row varieties Quest and Stander (Table 1), with Quest reported to harbour some FHB resistance (Smith et al., 2013). We were interested in determining whether the resistance would affect initial infection, thus possibly being associated with ingress through trichomes. Infection was investigated on both the surface of detached florets in culture dishes using the drop inoculation method, and on intact plants using spray inoculation. However, spray inoculation of intact plants resulted in drops rolling across the surface of the paleae, causing multiple conidia to become trapped by trichomes (Fig. 3a). In the detached floret assay, conidia settled to the bottom of the single inoculation droplet placed on the floret surface, and did not result in the accumulation of multiple conidia in association with single trichomes. Conidia on both spray‐inoculated plants and inoculated detached florets initiated disease.
The seed coat of barley is formed from the fusion of margins of the lemma and palea, which join as the florets mature (Legzdina and Buerstmayr, 2004). Spray inoculation necessitates the head being emerged from the flag leaf sheath; at this time point, the margins of the palea and lemma have fused. Spray‐inoculated florets were much harder to examine as a result of less synchronous conidial germination and infection. Therefore, we relied on detached florets for most work. Detached floret assays were performed both preceding and following lemma–palea fusion.
Trichomes on the surface of detached barley florets were frequent points of interaction with F. graminearum. Ingress of germlings into the host was observed to proceed in two phases. First, the hyphae formed bulbous structures prior to wrapping hyphal ‘arms’ (hyphopodia) around the trichome (Fig. 3b). Penetration hyphae then emerged from the hyphopodia (Figs 3c and 4a), and grew into trichomes and adjoining cells (Fig. 4a). Fusarium graminearum interacted with both the large prickles along the vascular bundles and the small prickles on the surface of the paleae. However, more extensive hyper‐branching of the hyphae occurred exclusively on the large prickles, which became completely enveloped by the hyphae at 5 days post‐inoculation (dpi) (Fig. 3c). Hyphae that had interacted with either type of prickle also continued to grow across the paleal surface, beyond the prickles, to interact with additional trichomes or to penetrate the paleal surface between surface cells (Fig. 3b). In several instances, attachment to one large prickle was followed by hyphal branching and penetration of another large prickle (Fig. 3b). Occasionally, the accumulation of silicic acid within the cell lumen resulted in a visible silica crystal (Fig. 4b). We were not able to determine when, during development, the crystals formed. Interactions of F. graminearum with barley varieties Stander and Quest were indistinguishable in the detached assays.
Perithecium formation of inoculated florets
Because we observed perithecia forming on inoculated detached florets, we investigated the location and quantity of perithecium emergence during floret development. Florets were inoculated at four stages: early (E; Zadoks’ 5.5–5.7), middle (M; Zadoks’ 5.9–6.1); late (L; Zadoks’ 7–7.3) and very late (VL; Zadoks’ 7.7–7.8). For the florets with fused margins (stages L and VL), there was a significant reduction in the number of perithecia emerging from the paleal surface compared with the florets with unfused margins (stages E and M; F = 37.60454; P = 1.24 × 10−17) (Figs 5a,b and 6a). In addition, the florets inoculated at the E and M stages supported perithecium development almost exclusively within the area under the inoculation droplet, and very few outside of that area, in all florets examined (F = 133.0525; P = 8.51582−53) (Fig. 6a,b). Conversely, the florets inoculated at the L and VL stages supported perithecium development primarily along the vascular bundles, and in reduced numbers on the paleal surface (Figs 5b,c and 6b). Although perithecia emerged only in association with stomates on the vascular bundles, emergence on the remainder of the paleal surface was not observed to be linked to any specific cell type. In cross‐section, perithecial initials were observed in aleurone cells and mature perithecia emerged directly from this tissue.
Figure 6.
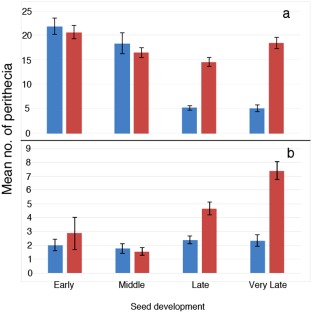
Effect of the developmental stage at inoculation on the location of perithecial emergence. (a) Mean perithecial emergence inside the inoculation droplet. (b) Mean perithecial emergence from locations outside of the inoculation droplet. Blue: at inoculation droplet. Red: outside of inoculation droplet. Error bars indicate standard deviation.
Fusarium graminearum–barley surface interactions in two‐row and six‐row varieties
To compare interactions of F. graminearum with two‐row versus six‐row barley, we used the detached floret assay of unfused paleae with representative barley varieties from each type (Quest, Robust, Stander, Aramir, Bowman and CDC Harrington) to compare host responses at the trichome site of infection. All varieties tested supported similar growth of F. graminearum regardless of previously characterized resistance. By 1 and 2 dpi, conidia had germinated and begun to spread across the surface of the palea, similar to those shown for the older inoculated palea (Fig. 3b). At 3–4 dpi, hyphae and conidiophores emerged via the stomata along the veins bordering the central furrow. This indicated that, under these conditions, successful entry and colonization of the host occurs between 1 and 3 dpi.
Focal accumulation of lignin and cellulose at trichomes
We used Chlorazol Black E (CBE) staining of infected paleae to investigate the plant response to infection. In the presence of CBE, plant cellulose and fungal chitin stain black, and lignin stains pink if CBE is contaminated with lignin pink (Cannon, 1941). Focal accumulation of lignin and cellulose was observed in paleal trichomes of infected florets stained with CBE (Fig. 7a–f), but was absent in mock‐inoculated florets (not shown). Foci were present in infected paleae, but not always associated with hyphae on or around the trichomes. This is partly a result of the physically disruptive staining and fixing protocol that can remove hyphae, but may also result from the trichomes responding to signals at some distance from infection. The staining revealed a high concentration of cellulose at trichomes (Fig. 7a–d) and some silica/cork cells (Fig. 7e,f) in infected tissue at 2 dpi, when conidia had germinated and spread across the plant surface, but had not yet emerged from the stomata. Pink coloration was observed at trichomes and silica/cork cells in tissues stained with CBE. The multicellular pink coloration response was highly visible in cells adjacent to the epidermis and hypodermis (Fig. 7d).
Figure 7.
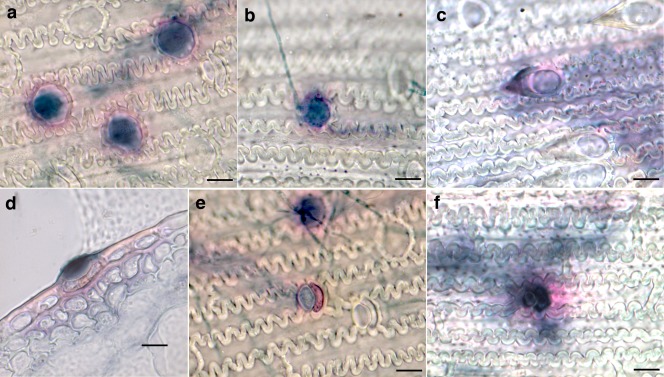
Focal accumulations of cellulose and lignin in trichomes in response to infection by Fusarium graminearum. Responses [at 2 days post‐inoculation (dpi)] of cultivars Aramir (a), Bowman (b) and Stander (c). Pink staining indicates lignification in tissue adjacent to the trichome in a latitudinal cross‐section of the Aramir palea (d). Staining can also be seen on silica and cork cells of Aramir (e) and Quest (f). Bars, 5 μm.
The number of foci accumulating lignin and cellulose in response to the presence of fungi on each palea varied greatly, but the numbers of foci were significantly higher in two‐row varieties with small, domed trichomes, where 24 Bowman paleae and 19 Aramir paleae were examined, than in the six‐row varieties with prickle‐like trichomes, where 29 Quest paleae and 24 Stander paleae were examined (Fig. 8). In these experiments, foci were rarely observed on inoculated Quest and Stander paleae, but, in handling this material, foci were never observed on uninoculated paleae, indicating that these varieties are capable of producing this response, but are not stimulated by the presence of the fungus. Foci were not observed on paleae of mock‐inoculated florets of any variety tested. Analysis of variance (ANOVA) indicated significant differences between six‐row and two‐row barley in their response (F = 22.45088; P = 5.411). However, in Robust paleae, a six‐row barley with small, domed trichomes, focal accumulations were detected in six of 38 paleae observed in two experiments. In the same experiments, foci were observed on 14 of 39 inoculated paleae of Aramir, a two‐row barley with small, domed trichomes, and no foci were observed on two varieties with prickle‐like trichomes, the six‐row variety Harrington (43 paleae) and the two‐row variety Stander (44 paleae). This indicates that the focal accumulation of cellulose and lignin is more likely to be linked to trichome morphology than to the arrangement of barley florets on the rachis.
Figure 8.
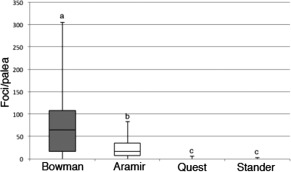
Number of foci [at 2 days post‐inoculation (dpi)] on the surface of infected paleae in two‐row (Bowman and Aramir) compared with six‐row (Quest and Stander) barley varieties. Letters that differ indicate varieties that show statistically significant differences in the number of foci per palea.
Discussion
Most grasses are covered with silica‐filled trichomes that evolved to discourage grazing by animals (Hartley et al., 2015). Indeed, the evolution of hypsodont teeth, to combat wear when animals feed on abrasive material, is thought to have co‐evolved with high silica levels in grasses (Prasad et al., 2005). Non‐silica‐accumulating trichomes, characteristic of some dicots, include both ‘non‐glandular’ and ‘glandular’ trichomes. The latter host a diversity of secondary metabolites and volatiles that have roles in protection from herbivory, fungal infection and abiotic stresses (Hauser, 2014; Horton et al., 2014; Kottb et al., 2015; Mandal et al., 2015; Steiner et al., 2015). To our knowledge, the silica‐associated trichomes of grasses have not been associated previously with plant resistance responses to biotic stress, at least not a cellular response to infection. A study by Sherwood and Vance (1980) examined fungal penetration and host response in a survey of 11 species of wild grasses, and failed to find resistance responses in penetration associated with trichomes. A study by Peraldi et al. (2011) compared resistance responses in different ecotypes of Brachypodium distachyon after infection through trichomes; however, the results were based on symptoms and subsequent sporulation, not on responses of host cells. Interactions between maize trichomes and F. graminearum were first reported by Stephens et al. (2008). In maize seedlings, F. graminearum germlings were shown to infect two‐celled (not found in barley) and prickle‐like trichomes (Nguyen et al., 2016b). Germlings adhere to the trichomes, and may form hyphae that wrap or form appressoria and then penetrate the trichome at its base, but, in both maize studies, the host reaction was not explored. In a study by Dupont et al. (2015), infection of perennial ryegrass by the endophyte Epichloe fustucae was shown to alter trichome development, such that leaf trichomes of infected plants were significantly larger than those of uninfected plants. Here, we show that focal accumulation of cellulose in infected paleae of two‐ and six‐row barley is, in part, linked to trichome type, but we could not determine that it was linked to resistance, as our detached floret assay enhanced the susceptibility of all cultivars. Further experimentation on intact heads is essential to link foci formation with reduced disease.
Surface interactions of trichomes with the conidia of F. graminearum begin with the trapping of conidia. The lunate shape of the conidia promotes trapping by the prickle‐like trichomes: conidial‐laden water droplets roll down the plant surface and the trichomes rake the conidia from the droplets. The hydrophilic conidial surface facilitates their spread by water (Deacon, 2005). Interestingly, the domed trichomes of two‐row barley would not trap conidia, as there is nothing to hold them back from washing off, and thus may reduce the inoculum available on the surface of the paleae, reducing disease. In six‐row barley, the fungus spreads externally as hyphae grow from one floret to the next without moving through the rachis (Langevin et al., 2004), suggesting a role for the prickle‐like trichomes in the surface spread of the fungus. The formation of infection arms that embrace the trichomes provides stability for the growing germling and, once penetration occurs, can provide a source of nutrients to support growth of the hyphae across the surface of the floret to the paleal margins.
Trichomes provide direct routes for the penetration of the floral paleae at developmental stages that occur after fusion of the paleal margins. Vascular bundles on the lemma and palea run parallel to the margins. The lemma, which is thicker than the palea, contains three vascular bundles, whereas the palea contains two (Fig. 1). The vascular bundles create ‘furrows’ between them on the surface of the paleae, and these furrows, together with the paleal margins, are often the location of lesion initiation in the field (Lewandowski et al., 2006). The same authors showed that infection of barley occurred predominantly via paleal margins, but their study was based on early‐stage infections and gave no mechanism for the establishment of these infections. Trichomes located over vascular bundles are important as hyphal conduits to the endosperm (Kirby and Rymer, 1974). Our results suggest that the stage of grain development of barley at inoculation dictates the mode of colonization by F. graminearum, as examined in six‐row barley. Infection at early stages of grain development, prior to fusion of the lemma and palea, resulted in the penetration of small prickles on the surface of the palea that assisted in surface spread towards the margins. These infections also led to the formation of perithecia localized to the initial point of inoculation. At later stages of grain development, after lemma and palea fusion, direct penetration of large prickles led to the colonization of the vasculature, and the region of perithecium development extended beyond the point of inoculation. Thus, trichomes play a vital and different role in facilitating fungal ingress in early and late stages of grain development. However, it is still not understood why early‐stage infections do not lead to spread through the vascular bundles. It may be that, in young florets, the vascular bundles are not fully formed conduits. Previous work using Brachypodium distachyon showed strong evidence supporting the importance of trichomes to F. graminearum infection of cereal grains and suggested access to the vasculature as an outcome of these infections (Peraldi et al., 2011). Our study confirms this interaction in barley, and extends it by linking access to the vasculature with stages of development in the fungus important to sexual and asexual sporulation.
Silica is known to accumulate in some plant species in response to pathogens, including bacteria (Dannon and Wydra, 2004), soybean rust (Arsenault‐Labrecque et al., 2012) and powdery mildew of Arabidopsis (Ghanmi et al., 2004). Studies on the infection of cucumber by powdery mildew showed that silica was associated with increased deposition of phenolic compounds (Fawe et al., 1998). A recent report has demonstrated the attenuation of infection of rice by Pyricularia oryzae with applications of silica (Domiciano et al., 2015). Silica can increase plant resistance by increasing the activity of reactive oxygen species (ROS)‐associated enzymes (Liang et al., 2005). Jasmonic acid, induced by wounding, infection by necrotrophic fungi, and herbivore damage, has been shown to increase in expression in silica‐treated plants challenged with Ralstonia sp. (Ghareeb et al., 2011). There are now products on the market encouraging the supplementation of crops with silica to improve resistance to both biotic and abiotic stress, and so a possible negative effect of silica on resistance to F. graminearum is important to understand.
Silica is found in abundance in the lemma and palea of wheat and barley, where it is thought to provide physical protection against fungal pathogen ingress (Hayward and Parry, 1972; Hodson and Sangster, 1988, 1989). In grasses, trichomes function as sinks of monosilicic acid and accumulate silicon dioxide in the form of biogenic silica, as do vascular bundles, stomates, small prickles on the epidermal surface, various silica cells, bulliform cells, xylem vessels and certain intercellular locations (Hayward and Parry, 1972; Kaufman et al., 1985; Ma and Yamaji, 2006; Parry et al., 1984; Sangster et al., 1983). Many of these cell types have been implicated in F. graminearum infection pathways in graminaceous host species (Boenisch and Schäfer, 2011; Guenther and Trail, 2005; Jansen et al., 2005; Lewandowski et al., 2006; Ma and Yamaji, 2006; Maier et al., 2006; Peraldi et al., 2011; Pritsch et al., 2000; Rittenour and Harris, 2010; Walter et al., 2010), suggesting that the fungus may be attracted to areas of high silica. Boenisch and Schäfer (2011) observed that green fluorescent protein (GFP) induction from Tri‐5 promoter‐GFP expression strains was often visible in association with silica cells, indicating that, although these cells are not essential for penetration, they are a preferred site. More specifically, silica cells were implicated as sites of hyphal differentiation, penetration and formation of toxin‐producing infection structures in wheat (Boenisch and Schäfer, 2011; Rittenour and Harris, 2010). Also, in wheat, xylem vessels have been shown to harbour ‘runner hyphae’ that spread through the rachis and stalk, and stomates and silica cell/cork cells have been associated with sexual and asexual sporulation (Guenther and Trail, 2005; Nguyen et al., 2016b). However, the silica content of these cells at the time of infection is not known, and the process and timing of phytolith formation have not been elucidated.
In barley, high silica accumulation correlates with developmental stages that occur during grain maturation after fusion of the paleal margins (Hayward and Parry, 1972; Kirby and Rymer, 1974). In rice, vascular bundles are the conduit bringing silicic acid to the trichomes and contain the highest concentration of non‐polymerized silicic acid (Ma and Yamaji, 2006). In wheat and barley, vascular bundles are an important link to the endosperm (Kirby and Rymer, 1974; Rittenour and Harris, 2010) and the rachis, and colonization spreads outwards to the epidermis from the vascular system (Guenther and Trail, 2005). In the present study, perithecium emergence spread from the point of inoculation in more mature florets through vascular bundles and across the paleal surface. The differences found in perithecium emergence between the developmentally dependent infection pathways relate directly to the timing and location of increases in silica content of the paleae (Hayward and Parry, 1972). Previous work has shown that perithecium emergence occurred preferentially from silica cells on stem nodes in wheat, and suggested a link between the two, which our work substantiates (Guenther and Trail, 2005). These findings strongly implicate silicic acid/silica as playing a role in infection which leads to sexual development.
In light of the ability of F. graminearum to penetrate trichomes, the focal accumulation of defence‐associated compounds at these cells, which are associated with the presence of the fungus, is intriguing. Cellulose and lignin are both components of plant cell walls, but are also known to accumulate in plant–pathogen interactions (Chowdhury et al., 2014; Vance et al., 1980). The accumulation of lignin and cellulose at the site of fungal interaction and in adjacent cells has been noted in other plant–pathogen systems and, although generally associated with defence, can be seen in both compatible and incompatible interactions (Sherwood and Vance, 1980; Vance et al., 1980). Therefore, this response could indicate that some barley trichomes are more likely to mount a defence response, or could indicate that small, domed trichomes are more frequently the site of successful fungal infection. Although the accumulation of cellulose and lignin is associated with a plant defence response, there was no notable difference in the development of disease symptoms in plants which showed this response and those which did not. The use of the detached floret assay increased the susceptibility of all cultivars. Further testing in a more natural setting will be necessary to determine the association with resistance in the field.
In conclusion, we have shown that trichomes and silica cells are important targets for F. graminearum ingress into barley paleae, supporting the initiation and spread of FHB. Furthermore, we have shown that different types of trichome are characteristic of two‐ and six‐row barley. Droplets of water carrying conidia provide a medium for the spores to associate with the prickle‐like trichomes common to the paleal surface of six‐row barley. It has been suggested that the six‐row lines have increased susceptibility to FHB because of the increase in numbers of florets packed around the rachis, thus raising humidity for enhanced fungal growth (McCallum et al., 2004; Tekauz et al., 2000). However, these prickle‐like trichomes, covering the surface of the paleae, are likely to play a role in this increased susceptibility, compared with the two‐row domed trichomes which cannot serve to trap conidia in the same manner. Trichomes are a front line in the sensing of pathogen invasion and the initiation of a resistance response.
We have demonstrated the importance of trichomes in the later stages of disease development, resulting in the ability of F. graminearum to overcome resistance to spread in barley, by enabling the fungus to generate new infections. This serial trichome infection route occurs on more mature florets later in the season. Other work has suggested the infection of trichomes of the rachis (Jansen et al., 2005), which would also serve as a route for F. graminearum to overcome resistance to pathogen spread in barley. As the fungus cannot spread through the rachis node of barley (Jansen et al., 2005), trichomes remain a significant entry route to late‐stage infections. Together, these results provide new information on the role of trichomes and silica/cork cells in both fungal invasion and the resistance response of barley. Furthermore, this work suggests that the use of silica soil enrichment practices for enhanced resistance may not be advisable in this interaction.
Experimental Procedures
Strains and culture conditions
Wild‐type F. graminearum PH‐1 (FGSC 9075; NRRL 31084) (Trail and Common, 2000) was used for all studies. Fusarium graminearum stocks were maintained on colonized pieces of V8 agar in sterile 35% glycerol at −80 °C. Macroconidia were generated in carboxymethylcellulose liquid medium, as described previously (Cappellini and Peterson, 1965), and stored as 2.5 × 105 conidia/mL 35% glycerol stocks at −80 °C.
Plant growth and inoculation conditions
Barley seeds were sown in Suremix growing medium (Michigan Grower Products, Inc., Galesburg, MI, USA) and grown in the glasshouse under supplemental lighting with a 16‐h day at approximately 22 °C. To study the interactions between F. graminearum and the plant surface, two inoculation techniques were used. As a convenient and consistent inoculation method, a detached floret technique was adapted from Lewandowski et al. (2006) for the analysis of F. graminearum establishment and spread on florets for ease of histological analysis. In addition, a spray inoculation technique was also used on whole plants to better mimic the natural infection processes (McCallum and Tekauz, 2002). For the detached floret assay, three to four of the lowest florets on a head were taken for inoculation with F. graminearum from at least five different heads of flowering barley. Florets were removed following the opening of the flag leaf sheath, but before head emergence (Zadoks’ stage 47; Zadoks et al., 1974). Florets were removed from the head by cutting the base of the rachilla, and placed upright, with the cut end in fresh 0.9% water agar, in a culture dish (diameter, 100 mm; depth, 20 mm). Inoculum from F. graminearum (5 μL macroconidial stock) was situated in the central furrow of the palea (Fig. 1). Covered culture dishes with excised inoculated florets were incubated under continuous white fluorescent light at room temperature (22–24 °C). The detached floret assay was also used to study the host reaction and infection of different types of surface cell.
The spray inoculation technique was performed in the glasshouse at head emergence from the flag leaf sheath (Zadoks’ stage 60; Zadoks et al., 1974). Prior to inoculation, plants were misted with sterile water to imitate the wet plant surface that spores would probably encounter in the field during an infection period. Inoculations were accomplished using a spray bottle to mist barley florets with a conidial suspension in sterile water (5 × 103 spores/mL). Following inoculation, heads were covered with a waxed paper bag, which was loosely closed at the base for 72 h to maintain humidity for infection.
The detached floret assay was also used for investigations into the location of perithecium emergence. Florets were inoculated as above and incubated until perithecia formed on the surface of the palea. Florets were inoculated at four stages of development: early (Zadoks’ 5.5–5.7; partial head emergence from the flag leaf sheath); middle (Zadoks’ 5.9–6.1; full head emergence through the initiation of flowering); late (Zadoks’ 7–7.3; early milk stage in kernel); and very late (Zadoks’ 7.7–7.8; late milk stage to early dough stage). For each of the four developmental stages, 34 florets were inoculated in each of the two separate experiments. Lesion observations were made at 3 dpi (McCallum and Tekauz, 2002). Numbers of perithecia were tallied at 12 dpi.
Light microscopy
Inoculated florets were removed from whole plants or culture dishes, placed in FAA (10% formalin, 5% glacial acetic acid, 50% ethanol) and subjected to vacuum infiltration for 30 min. Fixed samples were stored in FAA in the dark at room temperature until further processing.
For the analysis of the outer palea surface, florets were removed from FAA and transferred to an aqueous solution of 0.1% CBE for at least 15 h. Florets were subjected to a four‐step ethanol dehydration series (ethanol–water, 25 : 75, 50 : 50, 75 : 25, 100 : 0), after which paleae were removed from the florets. Detached paleae were placed in xylene prior to permanent mounting on glass slides with Cytoseal 60 (Richard Allen Scientific, Kalamazoo, MI, USA). Using this method, trichome morphology for the outer side of the palea was assessed for 13 varieties (Table 1), and the focal accumulation of defence compounds was measured in six of the 13 varieties (Quest, Robust, Stander, Aramir, Bowman and CDC Harrington).
Fixed tissue for sectioning was carried through a tert‐butyl alcohol (TBA)–ethanol dehydration series (ethanol–TBA–water, 50 : 10 : 40, 50 : 20 : 30, 50 : 35 : 15) at 4 °C for 2 h each, followed by an ethanol–TBA series (50 : 50, 25 : 75 and 0 : 100; 2–15 h each) at room temperature, and was then infiltrated and embedded in Tissue Prep paraffin wax (Fisher Scientific, Fair Lawn, NJ, USA; Cat#T555). Paleae of mature spray‐inoculated barley florets were punctured with an insect pin at the base of the palea to allow infiltration of the wax. Dehydrated tissue in TBA was incubated at 68 ºC and wax was added as the TBA evaporated until a final change to fresh molten wax was performed immediately prior to embedding. Paraffin‐embedded tissue was sectioned to 12–15 µm thick with a rotary microtome (American Optical Spencer). Sections were placed on glass slides, dewaxed with xylene and rehydrated through an ethanol series (100% xylene for 5 min and 100%, 95%, 80%, 60% and 30% ethanol for 3 min each). Sections were stained in a 1% aqueous solution of toluidine blue for 3 h for the differentiation of plant cell types and fungal hyphae. Sections were de‐stained in 30% ethanol for 1–3 h, mounted in Cytoseal 60 permanent mount mixture (Richard Allen Scientific) and allowed to dry overnight in a chemical fume hood.
Samples were observed with a Leica DM LB microscope (Leica Microsystems, Wetzlar, Germany) and images were recorded with an AxioCam Hrc (ZEISS Group, Oberkochen, Germany). Images were captured and processed with Axiovision Rel. 4.6 software and Adobe Photoshop Elements version 11.
Statistical analyses
ANOVA was performed with Microsoft Excel software Version 15.32 to evaluate the interaction of the location of the perithecial emergence and the grain developmental stage at the time of inoculation. The location was divided into four categories: perithecia emerging from vascular bundles at the point of inoculation; perithecia emerging from the paleal surface at the point of inoculation; perithecia emerging from the vascular bundles beyond the point of inoculation; and perithecia emerging on the paleal surface beyond the point of inoculation. The developmental stage of the grain at inoculation was divided into four categories for statistical analyses: early (E; Zadoks’ 5.5–5.7; Zadoks et al., 1974; partial head emergence from the flag leaf sheath through the initiation of flowering), middle (M; Zadoks’ 5.9–6.1; partial head emergence from the flag leaf sheath through the initiation of flowering); late (L; Zadoks’ 7–7.3; early milk stage in the kernels); and very late (VL; Zadoks’ 7.7–7.8; early dough stage in the kernels). ANOVA was also performed to evaluate the interaction of perithecium development for the areas under the inoculation droplet and outside the droplet for the florets inoculated at the E and M stages.
ANOVA was performed to evaluate the interaction between the formation of foci and barley type (six‐row and two‐row). For specific comparisons of focal formation between individual barley cultivars, Student's t‐test was applied using Microsoft Excel software Version 15.32.
Acknowledgements
This material is based on work supported by the US Department of Agriculture under Agreement No. 59‐0206‐6‐004. This is a cooperative project with the US Wheat & Barley Scab Initiative. Any opinions, findings, conclusions or recommendations expressed in this publication are those of the authors and do not necessarily reflect the view of the US Department of Agriculture. This research was also supported by MSU AgBioResearch. This work has been accepted as part of the thesis of an MS degree by D.A.
References
- Afton, D.E. (2012) Mechanism of surface establishment and colonization of barley florets by Fusarium graminearum. Master's thesis, Michigan State University, East Lansing, MI.
- Arsenault‐Labrecque, G. , Menzies, J.G. and Bélanger, R.R. (2012) Effect of silicon absorption on soybean resistance to Phakopsora pachyrhizi in different cultivars. Plant Dis. 96, 37–42. [DOI] [PubMed] [Google Scholar]
- Assaad, F.F. (2004) The PEN1 syntaxin defines a novel cellular compartment upon fungal attack and is required for the timely assembly of papillae. Mol. Biol. Cell. 15, 5118–5129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ball, T.B. , Gardner, J.S. and Anderson, N. (1999) Identifying inflorescence phytoliths from selected species of wheat (Triticum monococcum, T. dicoccon, T. dicoccoides and T. aestivum) and barley (Hordeum vulgare and H. spontaneum) (Gramineae). Am. J. Bot. 86, 1615–1623. [PubMed] [Google Scholar]
- Boenisch, M.J. and Schäfer, W. (2011) Fusarium graminearum forms mycotoxin producing infection structures on wheat. BMC Plant Biol. 11, 110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bushnell, W.R. , Hazen, B.E. and Pritsch, C. (2003) Histology and physiology of Fusarium Head Blight In: Fusarium Head Blight of Wheat and Barley (Leonard K.J. and Bushnell W.R., eds), pp. 44–83. St. Paul, Minnesota, USA: American Phytopathological Society. [Google Scholar]
- Cannon, H.G. (1941) VIII.‐On Chorazol Black E and some other new stains. J. R. Microsc. Soc. 61, 88–94. [Google Scholar]
- Cappellini, R.A. and Peterson, J.L. (1965) Macroconidium formation in submerged cultures by a non‐sporulating strain of Gibberella zeae . Mycologia, 57, 962–966. [Google Scholar]
- Chowdhury, J. , Henderson, M. , Schweizer, P. , Burton, R.A. , Fincher, G.B. and Little, A. (2014) Differential accumulation of callose, arabinoxylan and cellulose in nonpenetrated versus penetrated papillae on leaves of barley infected with Blumeria graminis f. sp. hordei . New Phytol. 204, 650–660. [DOI] [PubMed] [Google Scholar]
- Dannon, E.A. and Wydra, K. (2004) Interaction between silicon amendment, bacterial wilt development and phenotype of Ralstonia solanacearum in tomato genotypes. Physiol. Mol. Plant Pathol. 64, 233–243. [Google Scholar]
- De Wolf, E.D. , Madden, L.V. and Lipps, P.E. (2003) Risk assessment models for wheat Fusarium Head Blight epidemics based on within‐reason weather data. Phytopathology, 93, 428–435. [DOI] [PubMed] [Google Scholar]
- Deacon, J. (2005) Fungal spores, spore dormancy, and spore dispersal In: Fungal Biology, 4th Ed., pp. 184–212. Massachusetts, USA: Wiley‐Blackwell, Malden. [Google Scholar]
- Desjardins, A.E. , Proctor, R.H. , Bai, G. , McCormick, S.P. , Shaner, G. , Buechley, G. and Hohn, T.M. (1996) Reduced virulence of trichothecene‐nonproducing mutants of Gibberella zeae in wheat field tests. Mol. Plant–Microbe Interact. 9, 775–781. [Google Scholar]
- Desjardins, A.E. , Plattner, R.D. , Shaner, G. , Brown, D.W. , Buechley, G. , Proctor, R.H. and Turgeon, G.G. (2006) Field release of Gibberella zeae genetically modified to lack ascospores In: Fusarium Head Blight Forum (Van Sanford D., Canty S.M. and Clark A, eds), pp. 39–44. Research Triangle Park, NC: University of Kentucky. [Google Scholar]
- Domiciano, G.P. , Cacique, I.S. , Chagas Freitas, C. , Filippi, M.C.C. , DaMatta, F.M. , Vale, F.X.R.D. and Rodrigues, F.Á. (2015) Alterations in gas exchange and oxidative metabolism in rice leaves infected by Pyricularia oryzae are attenuated by silicon. Phytopathology, 105, 738–747. [DOI] [PubMed] [Google Scholar]
- Dupont, P.‐Y. , Eaton, C.J. , Wargent, J.J. , Fechtner, S. , Solomon, P. , Schmid, J. , Day, R.C. , Scott, B. and Cox, M.P. (2015) Fungal endophyte infection of ryegrass reprograms host metabolism and alters development. New Phytol. 208, 1227–1240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Escrivá, L. , Font, G. and Manyes, L. (2015) In vivo toxicity studies of fusarium mycotoxins in the last decade: a review. Food Chem. Toxicol. 78, 185–206. [DOI] [PubMed] [Google Scholar]
- Fawe, A. , Abou‐Zaid, M. , Menzies, J.G. and Bélanger, R.R. (1998) Silicon‐mediated accumulation of flavonoid phytoalexins in cucumber. Phytopathology, 88, 396–401. [DOI] [PubMed] [Google Scholar]
- Ghanmi, D. , McNally, D.J. , Benhamou, N. , Menzies, J.G. and Bélanger, R.R. (2004) Powdery mildew of Arabidopsis thaliana: a pathosystem for exploring the role of silicon in plant–microbe interactions. Physiol. Mol. Plant Pathol. 64, 189–199. [Google Scholar]
- Ghareeb, H. , Bozsó, Z. , Ott, P.G. , Repenning, C. , Stahl, F. and Wydra, K. (2011) Transcriptome of silicon‐induced resistance against Ralstonia solanacearum in the silicon non‐accumulator tomato implicates priming effect. Physiol. Mol. Plant Pathol. 75, 83–89. [Google Scholar]
- Guenther, J.C. and Trail, F. (2005) The development and differentiation of Gibberella zeae (anamorph: Fusarium graminearum) during colonization of wheat. Mycologia, 97, 229–237. [DOI] [PubMed] [Google Scholar]
- Hartley, S.E. , Fitt, R.N. , McLarnon, E.L. and Wade, R.N. (2015) Defending the leaf surface: intra‐ and inter‐specific differences in silicon deposition in grasses in response to damage and silicon supply. Front. Plant Sci. 6, 35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hauser, M.‐T. (2014) Molecular basis of natural variation and environmental control of trichome patterning. Front. Plant Sci. 5, 320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayward, D.M. and Parry, D.W. (1972) Electron microanalysis studies of silica distribution in barley (Hordeum sativum L.). Ann. Bot. 37, 579–591. [Google Scholar]
- Hayward, D.M. and Parry, D.W. (1980) Scanning electron microscopy of silica deposits in the culms, floral bracts and awns of barley (Hordeum sativum Jess.). Ann. Bot. 46, 541–548. [Google Scholar]
- He, X. , Xinyao, H. , Mohamed, O. , James, H. , Flavio, C. and Singh, P.K. (2015) Evaluation of Canadian barley breeding lines for Fusarium head blight resistance. Can. J. Plant Sci. 95, 923–929. [Google Scholar]
- Hodson, M.J. and Sangster, A.G. (1988) Silica deposition in the inflorescence bracts of wheat (Triticum aestivum). I. Scanning electron microscopy and light microscopy. Can. J. Bot. 66, 829–838. [Google Scholar]
- Hodson, M.J. and Sangster, A.G. (1989) Subcellular localization of mineral deposits in the roots of wheat (Triticum aestivum L.). Protoplasma, 151, 19–32. [Google Scholar]
- Hoiles, H.H.K. (1978) Nature and genesis of the Afton copper deposit, Kamloops, British Columbia. Master of Science Thesis, Department of Geology, University of Alberta, British Columbia. doi: 10.7939/R39Z90N7M. 221 pp. [DOI]
- Horton, M.W. , Bodenhausen, N. , Beilsmith, K. , Meng, D. , Muegge, B.D. , Subramanian, S. , Vetter, M.M. , Vilhjálmsson, B.J. , Nordborg, M. , Gordon, J.I. and Bergelson, J. (2014) Genome‐wide association study of Arabidopsis thaliana leaf microbial community. Nat. Commun. 5, 5320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jansen, C. , Wettstein, D. V. , Schäfer, W. , Kogel, K.‐H. , Felk, A. and Maier, F.J. (2005) Infection patterns in barley and wheat spikes inoculated with wild‐type and trichodiene synthase gene disrupted Fusarium graminearum . Proc. Natl. Acad. Sci. USA, 102, 16 892–16 897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaufman, P.B. , Dayanandan, P. , Franklin, C.I. and Takeoka, Y. (1985) Structure and function of silica bodies in the epidermal system of grass shoots. Ann. Bot. 44, 487–507. [Google Scholar]
- Kirby, E.J.M. and Rymer, J.L. (1974) The vascular anatomy of the barley spikelet. Ann. Bot. 39, 205–211. [Google Scholar]
- Kottb, M. , Gigolashvili, T. , Großkinsky, D.K. and Piechulla, B. (2015) Trichoderma volatiles effecting Arabidopsis: from inhibition to protection against phytopathogenic fungi. Front. Microbiol. 6, 995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langevin, F. , François, L. , François, E. and André, C. (2004) Effect of trichothecenes produced by Fusarium graminearum during Fusarium Head Blight development in six cereal species. Eur. J. Plant Pathol. 110, 735–746. [Google Scholar]
- Legzdina, L. and Buerstmayr, H. (2004) Comparison of infection with Fusarium Head Blight and accumulation of mycotoxins in grain of hulless and covered barley. J. Cereal Sci. 40, 61–67. [Google Scholar]
- Lewandowski, S.M. , Bushnell, W.R. and Evans, C.K. (2006) Distribution of mycelial colonies and lesions in field‐grown barley inoculated with Fusarium graminearum . Phytopathology, 96, 567–581. [DOI] [PubMed] [Google Scholar]
- Liang, Y.C. , Sun, W.C. , Si, J. and Romheld, V. (2005) Effects of foliar‐ and root‐applied silicon on the enhancement of induced resistance to powdery mildew in Cucumis sativus . Plant Pathol. 54, 678–685. [Google Scholar]
- Maier, F.J. , Miedaner, T. , Hadeler, B. , Felk, A. , Salomon, S. , Lemmens, M. , Kassner, H. and Schäfer, W. (2006) Involvement of trichothecenes in fusarioses of wheat, barley and maize evaluated by gene disruption of the trichodiene synthase (Tri5) gene in three field isolates of different chemotype and virulence. Mol. Plant Pathol. 7, 449–461. [DOI] [PubMed] [Google Scholar]
- Ma, J.F. and Yamaji, N. (2006) Silicon uptake and accumulation in higher plants. Trends Plant Sci. 11, 392–397. [DOI] [PubMed] [Google Scholar]
- Mandal, S. , Upadhyay, S. , Wajid, S. , Ram, M. , Jain, D.C. , Singh, V.P. , Abdin, M.Z. and Kapoor, R. (2015) Arbuscular mycorrhiza increase artemisinin accumulation in Artemisia annua by higher expression of key biosynthesis genes via enhanced jasmonic acid levels. Mycorrhiza, 25, 345–357. [DOI] [PubMed] [Google Scholar]
- McCallum, B.D. and Tekauz, A. (2002) Influence of inoculation method and growth stage on Fusarium Head Blight in barley. Can. J. Plant Pathol. 24, 77–80. [Google Scholar]
- McCallum, B.D. , Tekauz, A. and Gilbert, J. (2004) Reaction of a diverse collection of barley lines to Fusarium Head Blight. Plant Dis. 88, 167–174. [DOI] [PubMed] [Google Scholar]
- McMullen, M. , Marcia, M. , Gary, B. , De Wolf, E. , Ruth, D.‐M. , Don, H. , Greg, S. and Van Sanford, D. (2012) A unified effort to fight an enemy of wheat and barley: Fusarium head blight. Plant Dis. 96, 1712–1728. [DOI] [PubMed] [Google Scholar]
- Nguyen, T.T.X. , Dehne, H.‐W. and Steiner, U. (2016a) Histopathological assessment of the infection of maize leaves by Fusarium graminearum, F. proliferatum, and F. verticillioides . Fungal Biol. 120, 1094–1104. [DOI] [PubMed] [Google Scholar]
- Nguyen, T.T.X. , Dehne, H.‐W. and Steiner, U. (2016b) Maize leaf trichomes represent an entry point of infection for Fusarium species. Fungal Biol. 120, 895–903. [DOI] [PubMed] [Google Scholar]
- Nishimura, M.T. , Stein, M. , Hou, B.‐H. , Vogel, J.P. , Edwards, H. and Somerville, S.C. (2003) Loss of a callose synthase results in salicylic acid‐dependent disease resistance. Science, 301, 969–972. [DOI] [PubMed] [Google Scholar]
- Parry, D.W. , Hodson, M.J. , Sangster, A.G. , Jones, W.C. and O'Neill, C.H. (1984) Some recent advances in studies of silicon in higher plants [and discussion]. Philos. Trans. R. Soc. London B: Biol. Sci. 304, 537–549. [Google Scholar]
- Peraldi, A. , Beccari, G. , Steed, A. and Nicholson, P. (2011) Brachypodium distachyon: a new pathosystem to study Fusarium head blight and other Fusarium diseases of wheat. BMC Plant Biol. 11, 100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piacentini, K.C. , Savi, G.D. , Pereira, M.E.V. and Scussel, V.M. (2015) Fungi and the natural occurrence of deoxynivalenol and fumonisins in malting barley (Hordeum vulgare L.). Food Chem. 187, 204–209. [DOI] [PubMed] [Google Scholar]
- Piperno, D.R. (2016) Phytolith radiocarbon dating in archaeological and paleoecological research: a case study of phytoliths from modern Neotropical plants and a review of the previous dating evidence. J. Archaeol. Sci. 68, 54–61. [Google Scholar]
- Prasad, V. , Strömberg, C.A.E. , Alimohammadian, H. and Sahni, A. (2005) Dinosaur coprolites and the early evolution of grasses and grazers. Science, 310, 1177–1180. [DOI] [PubMed] [Google Scholar]
- Pritsch, C. , Muehlbauer, G.J. , Bushnell, W.R. , Somers, D.A. and Vance, C.P. (2000) Fungal development and induction of defense response genes during early infection of wheat spikes by Fusarium graminearum . Mol. Plant–Microbe. Interact. 13, 159–169. [DOI] [PubMed] [Google Scholar]
- Pugh, G.W. , Johann, H. and Dickson, J.G. (1933) Factors affecting infection of wheat heads by Gibberella saubinetii . J. Agric. Res. 46, 771–797. [Google Scholar]
- Rittenour, W.R. and Harris, S.D. (2010) An in vitro method for the analysis of infection‐related morphogenesis in Fusarium graminearum . Mol. Plant Pathol. 11, 361–369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sangster, A.G. , Hodson, M.J. , Parry, D.W. and Rees, J.A. (1983) A developmental study of silicification in the trichomes and associated epidermal structures of the inflorescence bracts of the grass Phalaris canariensis L. Ann. Bot. 52, 171–187. [Google Scholar]
- Sarlin, T. , Kivioja, T. , Kalkkinen, N. , Linder, M.B. and Nakari‐Setälä, T. (2012) Identification and characterization of gushing‐active hydrophobins from Fusarium graminearum and related species. J. Basic Microbiol. 52, 184–194. [DOI] [PubMed] [Google Scholar]
- Schwarz, P.B. and Horsley, R.D. (1997) A comparison of North American two‐row and six‐row malting barley. The Brewers’ Market Guide Available at: https://www.morebeer.com/brewingtechniques/bmg/schwarz.html. [accessed on Sept 2, 2017].
- Shaner, G.E. (2003) Epidemiology of Fusarium head blight of small grain cereals in North America In: Fusarium Head Blight of Wheat and Barley (Leonard K.J. and Bushnell W.R., eds), pp. 84–119. St. Paul, MN: American Phytopathological Society. [Google Scholar]
- Sherwood, R.T. and Vance, C.P. (1980) Resistance to fungal penetration in Gramineae. Phytopathology, 70, 273–279. [Google Scholar]
- Smith, K.P. , Budde, A. , Dill‐Macky, R. , Rasmusson, D.C. , Schiefelbein, E. , Steffenson, B. , Wiersma, J.J. , Wiersma, J.V. and Zhang, B. (2013) Registration of “Quest” spring malting barley with improved resistance to Fusarium Head Blight. J. Plant Regist. 7, 125–129. [Google Scholar]
- Steiner, U. , Kucht, S.H.N. , Ahimsa‐Müller, M.A. , Grundmann, N. , Li, S.‐M. , Drewke, C. and Leistner, E. (2015) The key role of peltate glandular trichomes in symbiota comprising clavicipitaceous fungi of the genus Periglandula and their host plants. Toxins, 7, 1355–1373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stephens, A.E. , Gardiner, D.M. , White, R.G. , Munn, A.L. and Manners, J.M. (2008) Phases of infection and gene expression of Fusarium graminearum during crown rot disease of wheat. Mol. Plant–Microbe Interact. 21, 1571–1581. [DOI] [PubMed] [Google Scholar]
- Tekauz, A. , McCallum, B. and Gilbert, J. (2000) Review: Fusarium head blight of barley in western Canada. Can. J. Plant Pathol. 22, 9–16. [Google Scholar]
- Trail, F. and Common, R. (2000) Perithecial development by Gibberella zeae: a light microscopy study. Mycologia, 92, 130–138. [Google Scholar]
- Vance, C.P. , Kirk, T.K. and Sherwood, R.T. (1980) Lignification as a mechanism of disease resistance. Annu. Rev. Phytopathol. 18, 259–288. [Google Scholar]
- Walter, S. , Nicholson, P. and Doohan, F.M. (2010) Action and reaction of host and pathogen during Fusarium head blight disease. New Phytol. 185, 54–66. [DOI] [PubMed] [Google Scholar]
- Wanjiru, M.W. , Kang, Z. and Heinrich, B. (2002) Importance of cell wall degrading enzymes produced by Fusarium graminearum during infection of wheat heads. Eur. J. Plant Pathol. 108, 803–810. [Google Scholar]
- Wegulo, S.N. , Stephen Baenziger, P. , Nopsa, J.H. , Bockus, W.W. and Hallen‐Adams, H. (2015) Management of Fusarium head blight of wheat and barley. Crop Prot. 73, 100–107. [Google Scholar]
- Willyerd, K.T. , Li, C. , Madden, L.V. , Bradley, C.A. , Bergstrom, G.C. , Sweets, L.E. , McMullen, M. , Ransom, J.K. , Grybauskas, A. , Osborne, L. , Wegulo, S.N. , Hershman, D.E. , Wise, K. , Bockus, W.W. , Groth, D. , Dill‐Macky, R. , Milus, E. , Esker, P.D. , Waxman, K.D. , Adee, E.A. , Ebelhar, S.E. , Young, B.G. and Paul, P.A. (2012) Efficacy and stability of integrating fungicide and cultivar resistance to manage Fusarium Head Blight and deoxynivalenol in wheat. Plant Dis. 96, 957–967. [DOI] [PubMed] [Google Scholar]
- Zadoks, J.C. , Chang, T.T. and Konzak, C.F. (1974) A decimal code for the growth stages of cereals. Weed Res. 14, 415–421. [Google Scholar]


